Abstract
A significant proportion of the world’s population suffers from insomnia, a disorder characterized by complications in initiating and maintaining sleep. Many medications used to treat insomnia target the γ-aminobutyric acid (GABA) neurotransmitter system. However, these substances, such as benzodiazepines, induce significant adverse consequences, including dependence and memory impairment, after prolonged use. Thus, current studies are aimed at developing therapeutic hypnotics derived from natural sources that may cause less severe side effects. Heukharang is a variety of lettuce from Korea that was discovered to contain sleep-promoting compounds. Therefore, we investigated the potential effects of sub-chronic administration of Heukharang extract (FSD-LS) on sleep behavior (pentobarbital-induced sleeping test), brain wave activity and sleep architecture (electroencephalography), and physiological behavior (open-field test and rota-rod) in mice, along with radioligand binding assays (GABAA, adenosine A1 and A2A receptors). We found that FSD-LS prolonged the total sleep duration and reduced the onset time of sleep, and enhanced delta wave power and non-rapid eye movement (NREM) sleep duration, all indicating persistent sleep-enhancing effects. FSD-LS lacked adverse effects on the spontaneous locomotor activity and motor coordination of mice, unlike diazepam. Pharmacological blocking using caffeine and bicuculline supported the possible involvement of adenosine receptors in the sleep-promoting effects of FSD-LS, with partial contribution from GABA receptor activity. Overall, our study recommends FSD-LS as a potential source for the development of sleep-aiding therapeutics.
Keywords: FSD-LS, Sleep, Adenosine A1 receptor, Adenosine A2A receptor, EEG, NREM
Introduction
Insomnia, the difficulty in falling or staying asleep, is a phenomenon experienced by society that can be caused by environmental triggers. Surveys showed that 10–15% of individuals suffer from sleeplessness manifested by dysregulated sleeping patterns [1]. Cognitive impairment, fatigue, and poor work performance are commonly reported consequences of insomnia that negatively affect personal and social functioning [2, 3], which may also lead to the development of comorbidities, including Alzheimer’s disease and schizophrenia [4, 5]. Many hypnotics and off-label sleep-improving medications may exert their effects by activating γ-aminobutyric acid (GABA)-related neuronal activity, aside from current drugs for insomnia mainly target neurotransmitter systems such as GABA, melatonin, serotonin, dopamine, orexin, and histamine [6]. However, GABAergic drugs such as synthetic benzodiazepines possess various adverse effects, including addiction, cognitive impairment, and cardiac problems [7]. Therefore, current research is focused on developing safer and more effective therapeutics with herbal origins.
Natural sources of potential therapeutics, such as lettuce (Lactuca sativa L.), were found to possess beneficial properties against adverse physical and psychological symptoms [8–10]. Extracts of a Korean variety of lettuce, Heukharang (LS), were found to contain components potentially responsible for its sleep-potentiating effect in rodents [11, 12]. Furthermore, acute administration of LS in rodents lacked adverse effects on locomotor activity, motor coordination, short-term memory, or anxiety levels, while increasing sleep quantity and non-rapid eye movement (NREM) sleep duration during electroencephalography (EEG). These findings underscore the necessity for investigating the impact of LS on sleep and overall behavior, with emphasis on elucidating the underlying mechanisms of their effects. Determining the effects of repeated exposure to potential sleep-promoting substances is imperative, given their therapeutic potential might be reduced by adverse effects or tolerance. The involvement of adenosine receptor activity in their effects may also support other mechanisms of action besides GABA neurotransmission, which is associated with unwanted side effects [13].
In this study, we examined the effects of acute and/or sub-chronic administration of Heukharang extract (FSD-LS) on sleep behavior, EEG signals, spontaneous locomotor activity, and motor coordination in mice. We then determined the potential mechanism mediating the effect of FSD-LS on sleep through radioligand binding assays and pharmacological blocking during the pentobarbital-induced sleeping test and EEG using adenosine and GABA receptor antagonists.
Materials and methods
Materials
FSD-LS extract obtained from FromBIO (Yongin, Korea) was prepared according to a previous study [12]. Pentobarbital was obtained from Hanlim Pharm. Co. Ltd. (Seoul, Korea), caffeine (A1 and A2A antagonist) and bicuculline (GABAA antagonist) were purchased from Sigma-Aldrich (St. Louis, MO, USA), and diazepam (DZP) was procured from Myungjin Pharm. Co. Ltd. (Seoul, Korea). FSD-LS (50, 100, and 150 mg/kg) and caffeine (50 mg/kg) were administered orally, whereas pentobarbital (45 mg/kg), bicuculline (1 mg/kg), and DZP (1 mg/kg) were administered intraperitoneally. The doses herein were based on previous reports [14–17] showing their efficacy in sleep-related behaviors (DZP and caffeine) or GABA antagonism (bicuculline).
Animals
Male ICR mice (5–6 weeks old, 25–30 g) were purchased from Hanlim Animal Laboratory Co. (Hwasung, Korea). Five mice were housed in each cage for a week before use and were provided with food and water ad libitum. The cages were kept in a room with a 12 h light/dark cycle (lights on 07:00–19:00) at a constant temperature (22 ± 2 °C) and humidity (55 ± 5%). Animal maintenance and treatments were performed in accordance with the Animal Care and Use Guidelines issued by Sahmyook University, Republic of Korea (SYUIACUC 2022–005).
Pentobarbital-induced sleeping test
This test was performed using a slightly modified version of previously described methods [18]. Mice were administered with FSD-LS 1 h before (DZP: 30 min before) pentobarbital administration. Sleep onset time and sleep duration were measured immediately after pentobarbital injection. Different groups of mice were used for acute and 5-day treatments. Additionally, separate cohorts of mice were pre-treated with a single dose of caffeine or bicuculline 15 min before distilled water (DW) or FSD-LS treatment.
EEG and sleep–wake cycle
Headmount implantation, data acquisition, and analyses were conducted according to previous studies [19], except that recording was only done for 4 h. After surgery recovery, the mice were treated with DW, FSD-LS, or DZP acutely or sub-chronically (5 days), using separate cohorts of mice for acute or sub-chronic treatments. A day before the 1st or 5th treatment, the mice were allowed to acclimatize to the EEG apparatus for 4 h without recording. On the 1st or 5th day of treatment, mice were connected to the apparatus (lights on, 09:50–14:00), with a 10-min habituation period, and EEG was recorded for 4 h (Pinnacle Technology, Inc., Lawrence, KS, USA). At 10:00, the mice were immediately treated with DW, FSD-LS, or DZP. Mean absolute power (μV2) was determined for delta (0.5–4 Hz) and alpha (8.5–14 Hz) wave frequencies. EEG and electromyography (EMG) recordings were then divided into 10-s epochs. Each epoch was scored as awake (WAKE), NREM, or REM sleep, based on the major waveform that occupied the epoch, following the criteria indicated by previous studies [20, 21]. The duration (min) of each waveform was determined. Similarly, separate cohorts of mice were pre-treated with a single dose of caffeine or bicuculline following a 10-min habituation on the 5th day of FSD-LS treatment. After 10 min, the mice were treated with DW or FSD-LS, and EEG was recorded for 4 h.
Open field test (OFT) and rota-rod
Locomotor activity was evaluated using a square Plexiglas open-field arena in accordance with a previous study [22], except that different mice strains were used and the behavior was recorded for 30 min. Separate cohorts of mice were used for acute and 5-day treatments. The mice were placed at the center of the arena 1 h after FSD-LS or immediately after DZP treatment and recording for 30 min on the 1st or 5th day of treatment. An automated system (EthoVision, Noldus, Netherlands) recorded the total distance moved (cm) and movement duration (s) of each mouse. Immediately following OFT, the mice were placed on a rota-rod and parameters were measured using a rotating rod (Ugo Basile, Varese, Italy) at a fixed speed of 36 rpm, as described in previous studies [22]. The mice were trained to run on the rota-rod for 3 min (2 days prior), and then for 10 min on the actual experiment day. The falling latency and frequency were recorded.
Radioligand binding assay
Radioligand binding assays against Cl− channel (GABA-gated), adenosine A1 and A2A were performed (Custom Screen by Eurofins Cerep, Le Bois I’Eveque, France) based on previous studies [23–25]. The assay measured the binding of [35S] t-butylbicyclophosphorothionate (TBPS) to the Cl− channel (GABA-gated), [3H] 8-cyclopentyl-1,3-dipropylxanthine (DPCPX) to A1, and [3H] CGS-21680 to A2A receptors. Non-specific binding was estimated in the presence of picrotoxinin (100 µM), R(-)-phenylisopropyladenosine (100 µM), and 5'-nethylcarboxamideadenosine (50 µM) for Cl− channel (GABA-gated), A1, and A2A, respectively. Membranes were filtered, washed 3 times, and the filters were counted to determine specifically bound [35S] TBPS, [3H] DPCPX, or [3H] CGS-21680. FSD-LS was screened at 10, 30, 100, 300, and 1000 µg/ml. The half-maximal inhibitory concentration (IC50) values were determined using GraphPad Prism 8.0 software (La Jolla, CA, USA).
Statistical analyses
Experimental data were analyzed using one- or two-way analysis of variance (ANOVA), followed by Tukey’s or Dunnett’s post-hoc analysis, or t-test. P-values < 0.05 were considered statistically significant. Values are expressed as the mean ± standard error of mean (SEM). All statistical analyses were performed using the GraphPad Prism 8.0 software.
Results
Sleep onset and duration
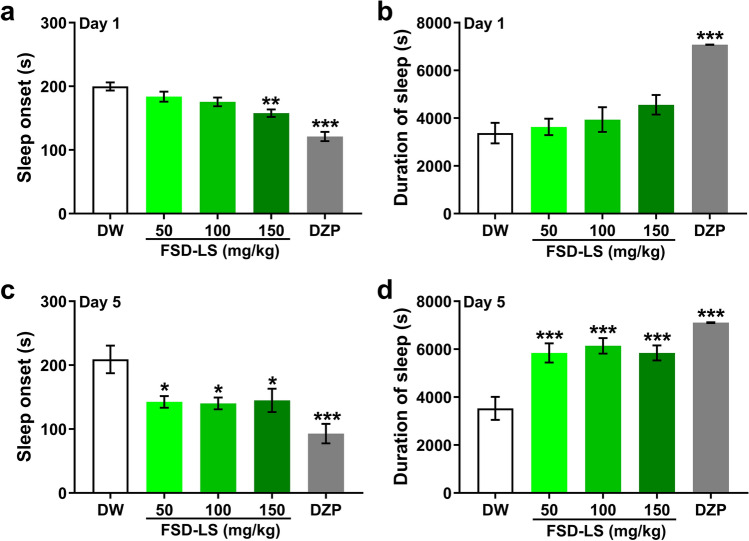
Sleep-enhancing effects may be demonstrated by improvements in pentobarbital-induced sleep [26]. One-way ANOVA revealed significant differences between treatment groups following sleeping test in terms of sleep onset (Fig. 1a, Day 1: F [4, 44] = 18.7, P < 0.001; Fig. 1c, Day 5: F [4, 70] = 7.13, P < 0.001) and duration (Fig. 1b, Day 1: F [4, 44] = 15.4, P < 0.001; Fig. 1d, Day 5: F [4, 70] = 14.6, P < 0.001). Specifically, acute FSD-LS treatment only at 150 mg/kg reduced sleep onset (P < 0.01), while sub-chronic administration of FSD-LS (50, 100, and 150 mg/kg) significantly reduced sleep onset (P < 0.05) and increased sleep duration (P < 0.001), similar to DZP (P < 0.001), as shown by Tukey’s post-hoc analysis.
Fig. 1.
Sleep onset (a, c) and duration (b, d) in seconds, resulting from pentobarbital-induced sleep effect (42 mg/kg) after acute and sub-chronic treatment with FSD-LS extracts (50, 100, and 150 mg/kg, p.o.) and diazepam (DZP, 1 mg/kg, i.p.) administration in ICR mice. Values are mean ± SEM. n = 9–10 (acute), 10–15 (sub-chronic) animals per group. *P < 0.05, **P < 0.01, and ***P < 0.001 vs. DW (one-way ANOVA followed by Tukey’s post-hoc analysis)
Delta and alpha power
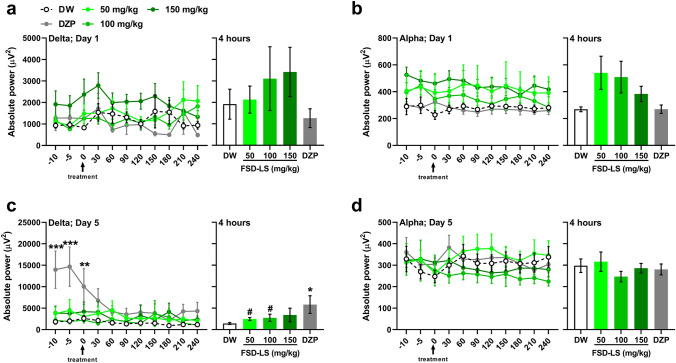
Because enhanced delta wave generation generally occurs during sleep [27], and is usually accompanied by the reduction of alpha wave activity [28], we also determined the absolute power of delta and alpha waves in treated mice. While no significant differences were found among groups in acutely treated mice (Figs. 2a-b), two-way ANOVA exhibited significant time (F [10, 501] = 4.97, P < 0.001) and treatment (F [4, 52] = 2.76, P < 0.05) differences in delta absolute power every 30 min, while t-test revealed a significant difference in the average delta power generated by mice in 4 h between DW and sub-chronic FSD-LS treatment (Fig. 2c, 50 mg/kg: t [2.68] = 22, P < 0.05; 100 mg/kg: t [1.89] = 22, P < 0.05). However, significantly higher delta power in DZP-treated mice (Dunnett, P < 0.001) was only observed prior to treatment (-10 to 0 min), but was sustained over the 4-h recording (Tukey, P < 0.05). No significant differences were observed in the alpha wave power of the sub-chronically treated mice.
Fig. 2.
Delta and alpha wave absolute power (µV2) generated after acute (a, b) and sub-chronic (c, d) treatment with FSD-LS extract (50, 100, and 150 mg/kg, p.o.) and diazepam (DZP, 1 mg/kg, i.p.) administration in ICR mice. Values are mean ± SEM. n = 5–7 (acute), 9–16 (sub-chronic) animals per group. *P < 0.05, **P < 0.01 and ***P < 0.001 vs. DW (one- or two-way ANOVA followed by Tukey’s post-hoc analysis). #P < 0.05 vs. DW (t-test)
WAKE, NREM, and REM
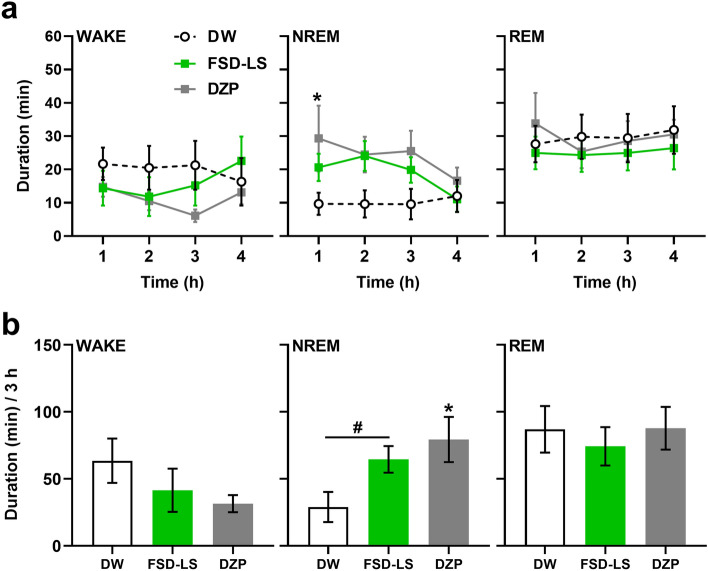
Sleep-promoting effects can also be represented by changes in sleep architecture [29]. Herein, the sleep–wake cycles of mice treated with 100 mg/kg FSD-LS were further investigated, as they exhibited maximal delta power increase and a decreased tendency of alpha power. Significant alterations were observed in the hourly duration (Fig. 3a, two-way ANOVA, subject: F [27, 81] = 5.10, P < 0.001) and average 3-h duration (Fig. 3b, one-way ANOVA, F [2, 27] = 3.95, P < 0.05) of NREM sleep in mice during EEG recording, while no changes were observed in the overall WAKE and REM sleep duration. Although only sub-chronic DZP treatment increased NREM sleep duration (P < 0.05) in the first hour of EEG recording, sub-chronic administration of DZP (P < 0.05) and FSD-LS (t [2.38] = 18, P < 0.05) increased the total duration of NREM sleep over 3 h of EEG recording.
Fig. 3.
Hourly average (a) and 3-h total (b) WAKE, NREM, and REM durations (min) of ICR mice after 5-day treatment of FSD-LS extract (100 mg/kg, p.o.) and diazepam (DZP, 1 mg/kg, i.p.) administration. Values are mean ± SEM. n = 10 animals per group. *P < 0.05 vs. DW (one- or two-way ANOVA followed by Tukey’s post-hoc analysis). #P < 0.05 vs. DW (t-test)
Locomotor activity and motor balance
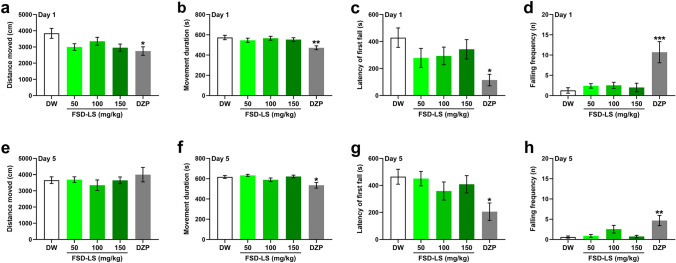
Given that some sleeping/hypnotic agents have unwanted side effects, FSD-LS-treated mice were also subjected to the OFT (Figs. 4a-b, e, f) and rota-rod test (Figs. 4c, d, g, h). One-way ANOVA showed significant differences in the distance moved (F [4, 67] = 2.90, P < 0.05), movement duration (F [4, 67] = 4.12, P < 0.01), fall latency (F [4, 61] = 2.83, P < 0.05), and falling frequency (F [4, 63] = 7.97, P < 0.001) between the treatment groups after acute treatment. However, Tukey’s post-test revealed that only DZP significantly reduced locomotor activity (P < 0.01) and first-fall latency (P < 0.05), with a significantly higher falling frequency (P < 0.001). Similarly, only sub-chronic treatment with DZP induced changes in movement duration (F [4, 47] = 4.76, P < 0.01), latency of first fall (F [4, 70] = 2.94, P < 0.05), and falling frequency (F [4, 70] = 5.55, P < 0.001) in mice. Tukey’s post-hoc analysis revealed similar trends in sub-chronic and acute DZP treatments.
Fig. 4.
Behavior of ICR mice in the open field test and rota-rod after acute (a, b, c, d) and sub-chronic (e, f, g, h) treatment with FSD-LS extract (50, 100, and 150 mg/kg, p.o.) and diazepam (DZP, 1 mg/kg, i.p.) administration. Values are mean ± SEM. n = 10–15 animals per group. *P < 0.05, **P < 0.01, and ***P < 0.001 vs. DW (one-way ANOVA followed by Tukey’s post-hoc analysis)
Adenosine and GABA receptor binding assay
Figure 5 shows the specific binding of FSD-LS at various concentrations. FSD-LS exhibited an affinity with IC50 values of 348 µg/mL, 376 µg/mL, and 124 µg/mL for A1, A2A, and GABAA receptors, respectively. Concentration curves show that FSD-LS could inhibit 50% of GABAA receptors at a lower concentration than A1 or A2A receptors.
Fig. 5.

Percentage of inhibition and concentration-inhibition relationship of FSD-LS on adenosine and GABA receptors at varying concentrations (10, 30, 100, 300, and 1000 µg/mL). IC50 and Ki values are expressed in µg/mL
Bicuculline pre-treatment
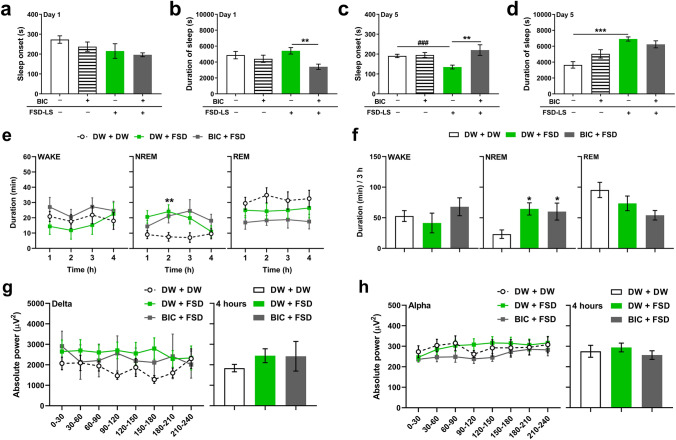
One-way ANOVA revealed significant differences in the sleep duration of acutely treated mice (Fig. 6b; F [3, 38] = 4.02, P < 0.05), but not in sleep onset (Fig. 6a). However, significant differences in sleep onset (Fig. 6c; F [3, 40] = 5.11, P < 0.01) and sleep duration (Fig. 6d; F [3, 39] = 11.8, P < 0.001) of sub-chronically treated mice were detected. Bicuculline pre-treatment exhibited shorter sleep duration than DW pre-treatment following acute FSD-LS administration (P < 0.01), while bicuculline pre-treatment exhibited longer sleep onset than DW pre-treatment following sub-chronic FSD-LS administration (P < 0.01). We also determined the effect of bicuculline on sleep–wake cycle (Figs. 6e-f) and EEG waves (Figs. 6g-h). Two-way ANOVA showed significant differences in hourly NREM sleep duration among groups (treatment: F [2, 32] = 4.02, P < 0.05; time × treatment: F [6, 96] = 2.83, P < 0.05), while one-way ANOVA revealed significant differences among groups in terms of NREM sleep duration over a 3-h period (F [2, 28] = 4.85, P < 0.05). However, statistical analysis did not show any differences between DW- and bicuculline-treated mice administered with FSD-LS in terms of NREM sleep duration or delta/alpha wave power.
Fig. 6.
Effect of bicuculline (BIC, 1 mg/kg, i.p.) pre-treatment on sleep onset and duration (s) resulting from pentobarbital-induced sleep (42 mg/kg, i.p.) after acute (a, b) and sub-chronic (c, d) treatment of FSD-LS extract (100 mg/kg, p.o.) and diazepam (DZP, 1 mg/kg, i.p.) administration in ICR mice. Hourly average (e) and 3-h total (f) WAKE, NREM, and REM durations (min), along with delta (g) and alpha (h) wave absolute power (µV2) generated, after 5-day treatment with FSD-LS extract and DZP administration in mice pre-treated with BIC. Values are mean ± SEM. n = 10–14 animals per group. *P < 0.05, **P < 0.01, and ***P < 0.001 (one- or two-way ANOVA followed by Tukey’s post-hoc analysis or Dunnett’s post-hoc analysis). ###P < 0.05 (t-test)
Caffeine pre-treatment
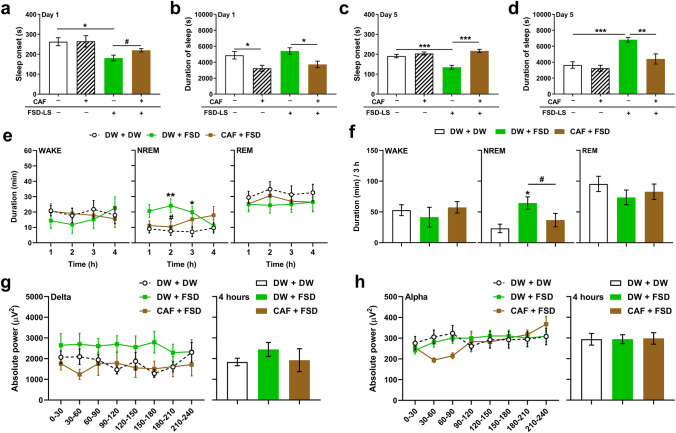
A previous study demonstrated the efficacy of LS against caffeine-induced sleep arousal in mice [11]. Herein, significant differences were observed in mice following both acute (Figs. 7a, b, onset: F [3, 38] = 4.15, P < 0.05; duration: F [3, 38] = 5.70, P < 0.01) and sub-chronic (Figs. 7c, d, onset: F [3, 38] = 18, P < 0.001; duration: F [3, 38] = 12, P < 0.001) administration of FSD-LS, through one-way ANOVA. The effect of caffeine was evident in sleep onset (t [2.32] = 18, P < 0.05) and duration (P < 0.05) following acute FSD-LS treatment. Caffeine also inhibited the effects of sub-chronic FSD-LS treatment on both sleep onset (P < 0.001) and duration (P < 0.01). We then determined the effect of caffeine pre-treatment on sleep architecture (Figs. 7e, f) and EEG (Figs. 7g, h). Two-way ANOVA revealed significant differences in the hourly duration (time × treatment: F [9, 123] = 2.92, P < 0.01), while one-way ANOVA showed differences in the 3-h total duration of NREM sleep in mice (F [2, 28] = 5.14, P < 0.05). Caffeine reduced the effect of FSD-LS on the duration of NREM sleep in mice (t [1.88] = 18, P < 0.05). Furthermore, no significant differences among the groups were observed in the average absolute delta or alpha wave power over 4 h.
Fig. 7.
Effect of caffeine (CAF, 50 mg/kg, p.o.) pre-treatment on sleep onset and duration (s) resulting from pentobarbital-induced sleep (42 mg/kg, i.p.) after acute (a, b) and sub-chronic (c, d) treatment of FSD-LS extract (100 mg/kg, p.o.) and diazepam (DZP, 1 mg/kg, i.p.) administration in ICR mice. Hourly average (e) and 3-h total (f) WAKE, NREM, and REM durations (min), along with delta (g) and alpha (h) wave absolute power (µV2) generated, after 5-day treatment with FSD-LS extract and DZP administration in mice pre-treated with CAF. Values are mean ± SEM. n = 10–16 animals per group. *P < 0.05, **P < 0.01, and ***P < 0.001 (one- or two-way ANOVA followed by Tukey’s post-hoc analysis or Dunnett’s post-hoc analysis). #P < 0.05 (t-test)
Discussion
We observed that varying doses of sub-chronically administered FSD-LS significantly shortened the sleep onset and prolonged the sleeping time of mice following pentobarbital adminstration, similar to DZP. This suggests that FSD-LS may potentiate the hypnotic effects of pentobarbital and increase sleep quantity. These findings coincide with previous studies that also investigated the effects of LS [11, 12], even though only acute administrations were performed. Nevertheless, our observations, along with previous findings, exhibit the sleep quality-enhancing capabilities of FSD-LS. Because sleep quantity only constitutes a fraction of overall sleep quality (i.e. depth of sleep and subjective experience of sleep) [30], it could also be characterized by changes in brain wave activity and sleep architecture. Current results revealed increased delta wave power in mice repeatedly exposed to FSD-LS and DZP, while sleep–wake analysis revealed that both FSD-LS (100 mg/kg) and DZP enhanced the total duration of NREM sleep within a 3-h EEG recording, similar to a previous study that exhibited increased NREM duration and delta activity, along with decreased stages of wake [11]. Our current findings may suggest that FSD-LS could improve the quality of sleep by increasing delta activity during NREM sleep. Previous studies have indicated that slow rhythm waves, such as delta waves, are generally more abundant during NREM sleep than wakefulness [31]. In clinical settings, enhanced NREM sleep and delta power were implicated in sleep quality [32, 33]. Thus, the observed increased delta wave power in our study might be correlated with the enhancement of NREM sleep. Taken together, although previous studies only employed acute administration, the current and previous studies are comparable, underlining the efficacy of FSD-LS on sleep enhancement regardless of treatment frequency.
Benzodiazepines, such as DZP, potentiate GABA neurotransmission by increasing GABAA receptor binding affinity in thalamocortical networks, which generate delta oscillations [34]. This mechanism was thought to possibly associate GABA receptor activation with shorter sleep onset, longer sleep duration, and improvement of NREM sleep via delta wave power enhancement [35, 36]. Thus far, the results demonstrate the sleep-promoting effects of FSD-LS, although the high GABA receptor affinity of DZP might seem to render it less effective than DZP. However, acute and sub-chronic FSD-LS administration lacked adverse effects on mice motor balance and locomotor activity, unlike DZP, suggesting that the sleep-promoting doses of FSD-LS might be comparable to 1 mg/kg DZP but lack lethargic effects and motor impairments in mice. Furthermore, the effective dose used from sleep cycle analysis (100 mg/kg) lacking significant effects on locomotor activity and motor balance suggests that the sleep-enhancing doses may have no drowsy or lethargic effects during wakefulness. Therefore, FSD-LS might be a potential therapeutic agent against sleep disorders with possibly fewer side effects.
Both GABA and adenosine receptors have been implicated in the sleep-promoting effects of anti-insomnia therapies. Such treatments may act as positive allosteric modulators of GABAA receptors and enhance GABA-associated inhibitory action, thus profoundly influencing sleep induction and maintenance [37]. Moreover, some sleeping agents targeting adenosine receptors may activate A1 receptors, inhibiting arousal and inducing sleep, while some may activate A2A receptors, inducing an excitatory effect in sleep-promoting neurons found in the hypothalamus [19, 36]. Herein, FSD-LS may possess affinity for A1 and A2A adenosine receptors (A1 ≈ A2A), with considerable affinity for GABAA vs. adenosine receptors. Upon pre-treatment of appropriate antagonists prior to FSD-LS treatment, it was observed that bicuculline may have only partly inhibited the effect of sub-chronic FSD-LS administration, thereby only affecting sleep onset, but not sleep duration. Moreover, no significant differences between DW and bicuculline pre-treatment in sleep–wake cycle and EEG power were detected (Fig. 6). Taken together, these inconsistent observations suggest that GABAA receptor activation may only partially contribute to the sleep-enhancing effect of FSD-LS. Therefore, a different neurobiological mechanism might more accurately mediate the effects of FSD-LS on sleep behavior, although GABAA receptor modulation is still considerable. Alternatively, caffeine competitively binds to A1 and A2A adenosine receptors [38], exerting neuromodulatory effects on the brain and affecting sleep. Herein, caffeine pre-treatment blocked the enhanced sleep caused by FSD-LS administration and inhibited FSD-LS-induced NREM sleep duration enhancement (Fig. 7). Overall, our results demonstrate that adenosine receptor antagonism abolishes the sleep-enhancing effects of FSD-LS, implying adenosine receptor activation to primarily mediate the positive effects of FSD-LS.
In summary, our findings strongly suggest that FSD-LS elicits sleep-promoting effects in mice, potentially through the activation of adenosine A1 and A2A receptors. It may be considered as an alternative to benzodiazepine-based treatments for sleep disorders, as FSD-LS lacks negative effects on locomotor activity and coordination. Despite these findings, the current study does contain some limitations. First, the specific compounds responsible for the sleep-enhancing effects of FSD-LS has not yet been specified, although certain compounds with potential sleep-aiding capabilities has already been identified in LS. Moreover, further studies distinguishing the roles of A1 from A2A receptors in the effects of FSD-LS are still warranted. Nevertheless, the present study proposes that FSD-LS could be used in the form of sleeping pills and/or supplements with fewer adverse effects as a novel therapeutic strategy against sleeping disorders.
Acknowledgements
This study was supported by the FromBIO Company of South Korea (FromBIO 2022).
Author contributions
Conceptualization: Mikyung Kim, Hee Jin Kim; Methodology: Mikyung Kim, Hee Jin Kim; Formal analysis and investigation: Leandro Val Sayson, Darlene Mae Ortiz, Hyun Jun Lee, Nicole Bon Campomayor; Writing—original draft preparation: Leandro Val Sayson, Se Jin Jeon; Funding acquisition: Mikyung Kim; Supervision: Mikyung Kim.
Declarations
Conflict of interest
The authors have no conflicts of interest to declare.
Ethical approval
Animal maintenance and treatments were performed in accordance with the Animal Care and Use Guidelines issued by Sahmyook University, Republic of Korea (SYUIACUC 2022–005).
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Leandro Val Sayson and Se Jin Jeon should be considered joint first author.
Contributor Information
Hee Jin Kim, Email: hjkim@syu.ac.kr.
Mikyung Kim, Email: mkkim@syu.ac.kr.
References
- 1.Soehner AM, Kaplan KA, Harvey AG. Insomnia comorbid to severe psychiatric illness. Sleep Med Clin. 2013;8:361. doi: 10.1016/j.jsmc.2013.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Calhoun SL, Fernandez-Mendoza J, Vgontzas AN, Liao D, Bixler EO. Prevalence of insomnia symptoms in a general population sample of young children and preadolescents: gender effects. Sleep Med. 2014;15:91–95. doi: 10.1016/j.sleep.2013.08.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yaffe K, Falvey CM, Hoang T. Connections between sleep and cognition in older adults. Lancet Neurol. 2014;13:1017–28. doi: 10.1016/S1474-4422(14)70172-3. [DOI] [PubMed] [Google Scholar]
- 4.Dauvilliers Y. Insomnia in patients with neurodegenerative conditions. Sleep Med. 2007;8:S27–34. doi: 10.1016/S1389-9457(08)70006-6. [DOI] [PubMed] [Google Scholar]
- 5.Spiegelhalder K, Regen W, Nanovska S, Baglioni C, Riemann D. Comorbid sleep disorders in neuropsychiatric disorders across the life cycle. Curr Psychiatry Rep. 2013;15:1–6. doi: 10.1007/s11920-013-0364-5. [DOI] [PubMed] [Google Scholar]
- 6.Neubauer DN, Pandi-Perumal SR, Spence DW, Buttoo K, Monti JM. Pharmacotherapy of Insomnia. J Cent Nerv Syst Dis. 2018;10:117957351877067. doi: 10.1177/1179573518770672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shi Y, Dong J-W, Zhao J-H, Tang L-N, Zhang J-J. Herbal Insomnia Medications that Target GABAergic Systems: A Review of the Psychopharmacological Evidence. Curr Neuropharmacol. 2014 [cited 2023 Sep 18];12:289–302. Available from: http://www.eurekaselect.com/openurl/content.php?genre=article&issn=1570-159X&volume=12&issue=3&spage=289 [DOI] [PMC free article] [PubMed]
- 8.Kim HD, Hong KB, Noh DO, Suh HJ. Sleep-inducing effect of lettuce (Lactuca sativa) varieties on pentobarbital-induced sleep. Food Sci Biotechnol. 2017;26:807–14. doi: 10.1007/s10068-017-0107-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sayyah M, Hadidi N, Kamalinejad M. Analgesic and anti-inflammatory activity of Lactuca sativa seed extract in rats. J Ethnopharmacol. 2004;92:325–329. doi: 10.1016/j.jep.2004.03.016. [DOI] [PubMed] [Google Scholar]
- 10.Shi M, Gu J, Wu H, Rauf A, Emran T Bin, Khan Z, et al. Phytochemicals, Nutrition, Metabolism, Bioavailability, and Health Benefits in Lettuce—a Comprehensive Review. Antioxidants 2022;11: 1158. 2022 [cited 2023 Sep 18]. Available from: https://www.mdpi.com/2076-3921/11/6/1158/htm [DOI] [PMC free article] [PubMed]
- 11.Ahn Y, Lee HH, Kim BH, Park SJ, Kim YS, Suh HJ, et al. Heukharang lettuce (Lactuca sativa L.) leaf extract displays sleep-promoting effects through GABAA receptor. J Ethnopharmacol. 2023;314:116602. doi: 10.1016/j.jep.2023.116602. [DOI] [PubMed] [Google Scholar]
- 12.Ortiz DM, Oh H, Kwon S, Jeon E, Kamal Hossain M, Kim HJ, et al. Sleep-potentiating effects of Passiflora incarnata and ‘Heukharang’, a Novel Korean Lactuca sativa, on pentobarbital-induced sleep in mice. Nat Product Sci. 2023;29:138–45. doi: 10.20307/nps.2023.29.3.138. [DOI] [Google Scholar]
- 13.Uemura SI, Kanbayashi T, Wakasa M, Satake M, Ito W, Shimizu K, et al. Residual effects of zolpidem, triazolam, rilmazafone and placebo in healthy elderly subjects: a randomized double-blind study. Sleep Med. 2015;16:1395–1402. doi: 10.1016/j.sleep.2015.05.021. [DOI] [PubMed] [Google Scholar]
- 14.Houston AJ, Wong JCL, Ebenezer IS. Effects of subcutaneous administration of the γ-aminobutyric acidA receptor agonist muscimol on water intake in water-deprived rats. Physiol Behav. 2002;77:445–450. doi: 10.1016/S0031-9384(02)00876-4. [DOI] [PubMed] [Google Scholar]
- 15.Vohs JL, Andrew Chambers R, Krishnan GP, O’Donnell BF, Berg S, Morzorati SL. GABAergic modulation of the 40 Hz auditory steady-state response in a rat model of schizophrenia. Int J Neuropsychopharmacol. 2010;13:487–97. doi: 10.1017/S1461145709990307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yoon M, Jung J, Kim M, Lee C, Cho S, Um M. Effect of black pepper (piper nigrum) extract on caffeine-induced sleep disruption and excitation in mice. Nutrients. 2022;14:2249. doi: 10.3390/nu14112249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Silva MIG, de Aquino Neto MR, Teixeira Neto PF, Moura BA, do Amaral JF, de Sousa DP, et al. Central nervous system activity of acute administration of isopulegol in mice. Pharmacol Biochem Behav. 2007;88:141–7. doi: 10.1016/j.pbb.2007.07.015. [DOI] [PubMed] [Google Scholar]
- 18.DelaPeña IJI, Kim HJ, de la Peña JB, Kim M, Botanas CJ, You KY, et al. A tryptic hydrolysate from bovine milk αs1-casein enhances pentobarbital-induced sleep in mice via the GABAA receptor. Behaviour Brain Res. 2016;313:184–90. doi: 10.1016/j.bbr.2016.07.013. [DOI] [PubMed] [Google Scholar]
- 19.Kim TH, Custodio RJ, Cheong JH, Kim HJ, Jung YS. Sleep promoting effect of luteolin in mice via adenosine A1 and A2A receptors. Biomol Ther (Seoul). 2019;27:584. doi: 10.4062/biomolther.2019.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim D, Yoon M, Kim S, Um MY, Cho S. Effects of green kiwifruit peel extract on sleep-wake profiles in mice: a polysomnographic study based on electroencephalogram and electromyogram recordings. Nutrients. 2022;14:4732. doi: 10.3390/nu14224732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Manor R, Kumarnsit E, Samerphob N, Rujiralai T, Puangpairote T, Cheaha D. Characterization of pharmaco-EEG fingerprint and sleep-wake profiles of Lavandula angustifolia Mill. essential oil inhalation and diazepam administration in rats. J Ethnopharmacol. 2021;276:114193. doi: 10.1016/j.jep.2021.114193. [DOI] [PubMed] [Google Scholar]
- 22.Custodio RJP, Botanas CJ, de la Peña JB, Dela Peña IJ, Kim M, Sayson LV, et al. Overexpression of the thyroid hormone-responsive (THRSP) gene in the striatum leads to the development of inattentive-like phenotype in mice. Neuroscience. 2018;390:141–50. doi: 10.1016/j.neuroscience.2018.08.008. [DOI] [PubMed] [Google Scholar]
- 23.Atack JR, Ohashi Y, McKernan RM. Characterization of [35S]t-butylbicyclophosphorothionate ([35S]TBPS) binding to GABAA receptors in postmortem human brain. Br J Pharmacol. 2007;150:1066–74. doi: 10.1038/sj.bjp.0707186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Olah ME, Ren H, Ostrowski J, Jacobson KA, Stiles GL. Cloning, expression, and characterization of the unique bovine A1 adenosine receptor. Studies on the ligand binding site by site-directed mutagenesis. J Biol Chem. 1992;267:10764–70. doi: 10.1016/S0021-9258(19)50084-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Varani K, Gessi S, Dalpiaz A, Borea PA. Pharmacological and biochemical characterization of purified A2a adenosine receptors in human platelet membranes by [3H]-CGS 21680 binding. Br J Pharmacol. 1996;117:1693–701. doi: 10.1111/j.1476-5381.1996.tb15341.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Choi HS, Hong KB, Han SH, Suh HJ. Valerian/Cascade mixture promotes sleep by increasing non-rapid eye movement (NREM) in rodent model. Biomed Pharmacother. 2018;99:913–920. doi: 10.1016/j.biopha.2018.01.159. [DOI] [PubMed] [Google Scholar]
- 27.Uygun DS, Basheer R. Circuits and components of delta wave regulation. Brain Res Bull. 2022;188:223–32. doi: 10.1016/j.brainresbull.2022.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Adamantidis AR, Gutierrez Herrera C, Gent TC. Oscillating circuitries in the sleeping brain. Nat Rev Neurosci. 2019;20:746–762. doi: 10.1038/s41583-019-0223-4. [DOI] [PubMed] [Google Scholar]
- 29.Yue XF, Wang AZ, Hou YP, Fan K. Effects of propofol on sleep architecture and sleep–wake systems in rats. Behav Brain Res. 2021;411:113380. doi: 10.1016/j.bbr.2021.113380. [DOI] [PubMed] [Google Scholar]
- 30.Bin YS. Is sleep quality more important than sleep duration for public health? Sleep. 2016;39:1629–1630. doi: 10.5665/sleep.6078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dang-Vu TT, Desseilles M, Laureys S, Degueldre C, Perrin F, Phillips C, et al. Cerebral correlates of delta waves during non-REM sleep revisited. Neuroimage. 2005;28:14–21. doi: 10.1016/j.neuroimage.2005.05.028. [DOI] [PubMed] [Google Scholar]
- 32.Krystal AD, Edinger JD. Measuring sleep quality. Sleep Med. 2008;9:10–11. doi: 10.1016/S1389-9457(08)70011-X. [DOI] [PubMed] [Google Scholar]
- 33.Kao CH, D’Rozario AL, Lovato N, Wassing R, Bartlett D, Memarian N, et al. Insomnia subtypes characterised by objective sleep duration and NREM spectral power and the effect of acute sleep restriction: an exploratory analysis. Sci Rep. 2021;11:S1–S13. doi: 10.1038/s41598-021-03564-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McKillop LE, Fisher SP, Milinski L, Krone LB, Vyazovskiy VV. Diazepam effects on local cortical neural activity during sleep in mice. Biochem Pharmacol. 2021;191:114515. doi: 10.1016/j.bcp.2021.114515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cho S, Yoon M, Pae AN, Jin YH, Cho NC, Takata Y, et al. Marine polyphenol phlorotannins promote non-rapid eye movement sleep in mice via the benzodiazepine site of the GABAA receptor. Psychopharmacology (Berl). 2014;231:2825–37. doi: 10.1007/s00213-014-3445-1. [DOI] [PubMed] [Google Scholar]
- 36.Kim TH, Bormate KJ, Custodio RJP, Cheong JH, Lee BK, Kim HJ, et al. Involvement of the adenosine A1 receptor in the hypnotic effect of rosmarinic acid. Biomed Pharmacother. 2022;146:112483. doi: 10.1016/j.biopha.2021.112483. [DOI] [PubMed] [Google Scholar]
- 37.Kim YR, Lee SY, Lee SM, Shim I, Lee MY. Effect of hibiscus syriacus linnaeus extract and its active constituent, saponarin, in animal models of stress-induced sleep disturbances and pentobarbital-induced sleep. Biomed Pharmacother. 2022;146:112301. doi: 10.1016/j.biopha.2021.112301. [DOI] [PubMed] [Google Scholar]
- 38.Aranda JV, Beharry KD. Pharmacokinetics, pharmacodynamics and metabolism of caffeine in newborns. Semin Fetal Neonatal Med. 2020;25:101183. doi: 10.1016/j.siny.2020.101183. [DOI] [PubMed] [Google Scholar]