Abstract
Hypoalbuminaemia (serum albumin levels ≤3.5 g/dl) is associated with poor outcomes among patients with heart failure (HF). This narrative review includes original articles and reviews published over the past 20 years and retrieved from PubMed using the following search terms (or their combination): ‘heart failure’, ‘hypoalbuminaemia’, ‘heart failure with reduced ejection fraction’, ‘heart failure with preserved ejection fraction’, ‘all‐cause mortality’, ‘in‐hospital mortality’, ‘hospitalization’, ‘prognosis’. The aims of this review are to provide an overview on the prevalence of hypoalbuminaemia in HF, its impact on clinical outcomes, and potential mechanisms that may suggest future therapeutic strategies. Hypoalbuminaemia is frequent in HF patients, especially among the elderly. However, data about the exact epidemiology of hypoalbuminaemia are scant due to different definitions, and prevalence is estimated between 5% and 70% across the whole spectrum of ejection fraction. Current evidence points to hypoalbuminaemia as a marker of poor outcomes in HF, irrespective of the ejection fraction, and in other cardiovascular diseases. Among patients who suffered from acute coronary syndrome, those with hypoalbuminaemia had an increased risk of new‐onset HF and in‐hospital mortality. Albumin, however, might also play a role in the natural history of such diseases due to its antioxidant, anti‐inflammatory, and antithrombotic properties. Whether albumin supplementation or nutritional support in general would be beneficial in improving clinical outcomes in HF is not completely clear and should be evaluated in adequately designed studies.
Keywords: Hypoalbuminaemia, Albumin, Heart failure, Mortality, Hospitalization, Prognosis
Introduction
Prevalence of heart failure (HF) is increasing over time. 1 HF is associated with morbidity and mortality and often requires hospitalization. Current strategies rely on serum biomarkers to support diagnosis and prognosis in both hospital and outpatient settings, 2 such as B‐type natriuretic peptide (BNP) or N‐terminal proBNP (NT‐proBNP) and cardiac troponins (high‐sensitivity cardiac troponin I or T). 3 , 4 , 5
Serum albumin is associated with adverse events in patients with medical conditions. It has indeed been added to the Acute Physiology and Chronic Health Evaluation III (APACHE III) score to determine in‐hospital mortality in critically ill patients 6 and various studies have confirmed its short‐ and long‐term prognostic role in the acute setting, irrespective of the underlying disease. 7 , 8 Hypoalbuminaemia – defined as serum albumin levels ≤3.5 g/dl – is common in patients with HF, especially in older patients with multiple comorbidities. 9 , 10 , 11 , 12 Mild hypoalbuminaemia is also encountered in patients with advanced HF causing congestive liver disease, usually associated with malnutrition or protein‐losing enteropathy. 13 Different studies identified hypoalbuminaemia as an independent predictive factor for cardiovascular (CV) and all‐cause mortality 14 , 15 , 16 and incident HF. 10 Importantly, hypoalbuminaemia was independently associated with other CV diseases, such as coronary artery disease, atrial fibrillation, stroke and venous thromboembolism, and has emerged as a prognostic parameter in these conditions. 17 , 18 , 19 , 20 , 21 , 22 , 23 , 24
In this narrative review, we aim at providing an overview on the prevalence of hypoalbuminaemia in HF, its impact on clinical outcomes, and potential causes that may suggest future therapeutic strategies. In particular, we will discuss the pathophysiology of hypoalbuminaemia, present available data regarding its epidemiology in HF, and its link with frailty. We will then review the existing literature investigating the prognostic role of albumin in HF patients by considering different clinical phenotypes. Finally, we will present data about albumin supplementation and discuss potential benefits of implementing dedicated studies to assess this knowledge gap.
Search criteria
This narrative review is based on original articles and reviews published over the past 20 years and retrieved from PubMed using the following search terms (or combination of terms): ‘heart failure’, ‘hypoalbuminaemia’, ‘pathophysiology’, ‘heart failure with reduced ejection fraction’, ‘heart failure with preserved ejection fraction’, ‘all‐cause mortality’, ‘in‐hospital mortality’, ‘hospitalization’, ‘prognosis’. Only English‐language papers were included in the literature search. Other papers identified from the reference list of the retrieved articles were also considered.
General properties of albumin in health and disease
Albumin represents more than 50% of total plasma protein concentration. In adults, normal albumin levels ranges between 3.5 and 5.5 g/dl. To date, no age‐related cut‐offs for albumin are available, however serum albumin levels decrease with age in both sexes, especially among individuals aged ≥90 years. 25
Albumin is synthetized by hepatocytes and regulated by different stimuli, mainly nutrient intake. Albumin has several different properties, the most important being the contribution to oncotic pressure. 9 In addition, albumin is a carrier of fatty acids, inactivates toxins, affects drug activity and has antioxidant capacity and many enzymatic properties. 26 General features of albumin and its metabolism are summarized in Figure 1 . Albumin leaks into the extravascular space for up to 5% of its concentration through the so‐called transcapillary exchange/escape rate (TER), that is dependent on the integrity of the endothelium and the glycocalyx, the latter being injured by inflammation. Once leaked, albumin is able to return into the bloodstream through the lymphatic system at a rate similar to TER and this is particularly evident for pulmonary vessels that own increased permeability for albumin. 27 Hence, the development of pulmonary oedema is linked to the balance between the transcapillary difference of intravascular and interstitial oncotic pressure, interstitial pressure, and lymphatic flow rate. Arques et al. 28 , 29 described that in acutely decompensated HF (ADHF) hypoalbuminaemia is a promoting factor for pulmonary oedema among patients with diastolic dysfunction presenting with a moderate increase in left ventricular filling pressure. Consequently, in such conditions, restoration of the transcapillary oncotic pressure gradient through albumin supplementation might increase intravascular oncotic pressure. 27 In ADHF patients, hypoalbuminaemia is associated with enteric losses caused by splanchnic congestion, as occurs in systemic venous hypertension resulting from tricuspid incompetence, pulmonary stenosis, constrictive pericardial disease, or dilated cardiomyopathy. 30 , 31 , 32 , 33 , 34 As a consequence, within the villi, the increase in interstitial fluid impairs capillary permeability, leading to the loss of macromolecules (including albumin) and lymphocytes from the lamina propria into the lumen of the gastrointestinal system, that results in hypoalbuminaemia and lymphopenia. 34 , 35 , 36 In this view, lower albumin levels may partially explain diuretic resistance in some patients as loop diuretics bind to albumin, 37 with albumin that increases their volume of distribution and decreases their renal delivery, as clearly outlined in experiments with analbuminaemic rats. 38 There is growing evidence supporting a role of inflammation in the underlying pathophysiology of HF. 39 , 40 , 41 The most commonly understood mechanisms include production of cytokines from myocardial cells as a reaction to fluid overload, 41 tissue hypoxia, 42 and intestinal congestion that could promote bacterial translocation and production of elevated levels of endotoxins. 43 In ADHF, all of these mechanisms become more intense. A meta‐analysis of 48 studies demonstrated a link between hypoalbuminaemia in HF and persistent inflammation, reflected by the elevation of C‐reactive protein (CRP) levels 44 as the inflammatory activity is higher in patients with low levels of albumin. Bonilla‐Palomas et al. 45 found that inflammatory activation was the most important aetiologic factor associated with hypoalbuminaemia in ADHF patients. From a pathophysiological standpoint, interleukin‐2 (IL‐2) and interleukin‐6 (IL‐6) inhibit the hepatic synthesis of albumin and, together with interferon‐α (IFN‐α), increase vascular endothelial permeability, promoting the transcapillary albumin leakage from the intravascular to the extravascular compartment. 33
Figure 1.
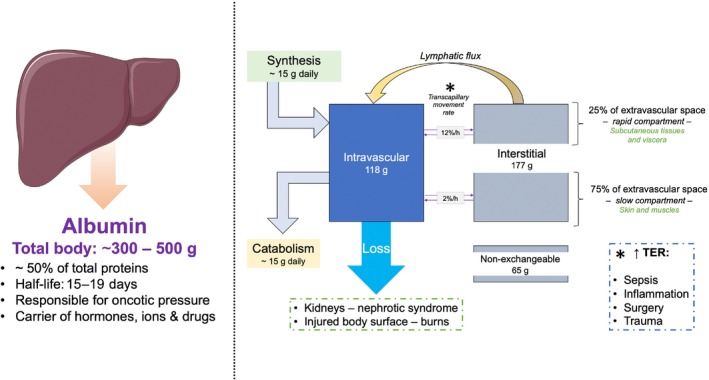
Properties and metabolism of albumin. Albumin is the most abundant protein in the body and plays important roles, such as regulation of oncotic pressure and carrier of various compounds. Its metabolism relies on a balance between synthesis and catabolism. Importantly, transcapillary escape rate (TER) – an average of two rates, fast (12%, into the viscera) and slow (2%, into the skin and muscles) – may be increased in case of inflammation, infections, and trauma. TER, transcapillary escape rate. This figure has been partially created using Servier Medical Art templates, which are licensed under a Creative Commons Attribution 3.0 Unported License; https://smart.servier.com.
In sum, current evidence points to hypoalbuminaemia as a marker of poor outcomes in HF and other CV diseases, due to its association with coexisting conditions – chronic congestion, 46 chronic low‐grade inflammation, 47 malnutrition, 48 , 49 and renal failure 50 – that have a detrimental effect on the prognosis of patients with HF. This makes hypoalbuminaemia an inclusive prognostic factor, since its interpretation encompasses a greater number of pathophysiological processes and helps identifying patients with a more severe disease who are at higher risk of worse outcomes. On the other side, it could be tempting to speculate that low serum albumin levels play a direct role in the natural history of chronic HF. Indeed, hypoalbuminaemia may trigger an acute worsening of HF through a reduction in oncotic pressure 28 and a predisposition to diuretic resistance. 38 However, if a role in the pathophysiology of acute and chronic HF were true, albumin supplementation should be beneficial in improving outcomes, while currently available observational studies have not shown a substantial improvement of HF outcomes, neither in acute nor in the chronic setting. 51 , 52 This leads to the final consideration that the association between hypoalbuminaemia and HF is explained by the presence of several supporting mechanisms and hypoalbuminaemia itself should be considered, at present, as a marker of advanced disease that predisposes to episodes of acute decompensation and long‐term mortality.
Epidemiology of hypoalbuminaemia in patients with heart failure
Hypoalbuminaemia is frequently observed in HF patients, and it is prevalent with increasing age and number of chronic diseases. 9 , 25 , 53 However, information about the exact epidemiology of hypoalbuminaemia is lacking because of different working definitions, different study designs, and diverse populations considered in the studies (ADHF, chronic HF, HF with reduced [HFrEF] and preserved ejection fraction [HFpEF]) (Table 1 ). 10 , 15 , 16 , 45 , 54 , 55 , 56 , 57 , 58 , 59 , 60 , 61 , 62 , 63 , 64 , 65 Hypoalbuminaemia is encountered both in HFrEF and HFpEF 45 as well as in acute and chronic HF patients, 29 , 54 , 58 , 66 with a higher prevalence in the acute setting (38.5% vs. 19.7%). 44 , 65 A meta‐analysis of 48 studies estimated the prevalence of hypoalbuminaemia among HF patients between 12% and 54%, with a pooled prevalence of 32%. 44 Around 15% of ambulatory HF patients suffer from hypoalbuminaemia, irrespective of their ejection fraction 64 (Table 1 ).
Table 1.
Main observational studies investigating the epidemiology of hypoalbuminaemia in patients with heart failure
| Author | Study design | No. of patients | HF classification | Age, years | HypA cut‐off value (g/dl) | Prevalence of HypA |
|---|---|---|---|---|---|---|
| Bonilla‐Palomas et al. 45 | Prospective, cohort study | 362 | All types | 78.5 ± 8 | ≤3.4 | 29.8% |
| Liu et al. 58 | Prospective, cohort study | 576 | HFpEF | 77 ± 10 | 3.4 | 28% |
| Prenner et al. 59 | Prospective, cohort study | 118 | HFpEF | 66 ± 10 | 3.7 | 28% |
| Yatsu et al. 60 | Prospective, cohort study | 551 | All types | 69.6 ± 14 | <3.4 | 56.4% |
| Filippatos et al. 10 | Prospective, cohort study | 5450 | Not available | 74.1 ± 6 | ≤3.5 | 11% |
| Horwich et al. 61 | Retrospective, cohort study | 1726 | HFrEF | 52 ± 12 | ≤3.4 | 25% |
| Uthamalingam et al. 54 | Retrospective, cohort study | 438 | All types | 75 ± 14 | 3.4 | 54% |
| Gotsman et al. 62 | Retrospective, cohort study | 5779 | All types | 75 ± 13 | 3.5 | 12% |
| Grodin et al. 63 | Retrospective, cohort study | 456 | All types | 68 (57.5–78) | 3.5 | 44.7% |
| Bavishi et al. 64 | Retrospective, cohort study | 9437 | All types | – | 3.5 | 15% |
| Polat et al. 15 | Retrospective, cohort study | 135 | HFrEF | 67 ± 14.0 | ≤3.4 | 69.6% |
| Karki et al. 65 | Retrospective, cohort study | 1 365 529 | All types | – | <3.5 | 88% |
HF, heart failure; HFpEF, heart failure with preserved ejection fraction; HFrEF, heart failure with reduced ejection fraction; HypA, hypoalbuminaemia.
Hypoalbuminaemia is prevalent in the elderly population due to the concurrent presence of aging, 25 , 53 frailty 16 , 29 , 66 and HFpEF. 28 , 58 Frailty (which includes cognitive, social and psychological frailty) in HF is associated with poor prognosis and increases the risk of death and hospitalization. 67 Due to a pro‐inflammatory state, pathological changes in muscle composition are responsible for reduced muscle mass and strength, i.e. sarcopenia. 67 Sarcopenia itself could be a cause of acute decompensation in HF and is associated with haemodynamic changes that could worsen symptoms and duration of the event. 68 As elderly patients are more likely to carry a greater number of comorbidities, the vicious cycle of inflammation, malnutrition (due to reduced intake and reduced absorption of nutrients due to intestinal oedema), cachexia, and frailty are responsible for reduced albumin levels in HF patients 69 (Figure 2 ).
Figure 2.

Predisposing factors to hypoalbuminaemia. Different factors contribute to low levels of serum albumin. Hypoalbuminaemia is more frequently encountered in elderly people and generally increases all‐cause mortality and negatively impacts on heart failure (HF) outcomes. ADHF, acutely decompensated heart failure. This figure has been partially created using Servier Medical Art templates, which are licensed under a Creative Commons Attribution 3.0 Unported License; https://smart.servier.com.
Hypoalbuminaemia derives from different mechanisms – reduced synthesis or increased catabolism, kidney and intestine loss, or increased vascular permeability, 70 although the single contribution of each factor was not studied in depth in patients with HF. It is, however, clear that inflammation and malnutrition play a major role, 71 , 72 along with liver disease especially in patients with long‐standing congestive HF 9 , 70 (Figure 2 ).
Prognostic role of albumin in heart failure
Impact of hypoalbuminaemia on clinical outcomes in patients with heart failure
In the past decade, a large number of observational studies and sub‐analyses of randomized controlled trials were published that investigated the role of hypoalbuminaemia as a prognostic marker. In many of them, HF was considered across the whole spectrum of the ejection fraction (Table 2 ). 10 , 14 , 54 , 55 , 58 , 63 , 73 , 74 , 75 , 76
Table 2.
Observational studies investigating the prognostic role of hypoalbuminaemia in patients with heart failure
| Author | No. of patients | HF classification | Main findings |
|---|---|---|---|
| Liu et al. 58 | 576 | HFpEF | Hypoalbuminaemia was as an independent predictor of all‐cause mortality at 1 year (HR 3.18, 95% CI 2.77–4.45; p < 0.001). |
| Uthamalingam et al. 54 | 438 | All types |
Hypoalbuminemia independently predicted 1‐year mortality (HR 2.05, 95% CI 1.10–3.81; p = 0.001). In HFrEF patients, hypoalbuminaemia carried a higher independent risk of 1‐year mortality (HR 5.00, 95% CI 2.17–11.5; p < 0.001). |
| Grodin et al. 63 | 443 | All types | Hypoalbuminaemia was not associated with the composite outcome (all‐cause mortality, all‐cause rehospitalization, and unscheduled ER visit). |
| Clarke et al. 73 | 177 | All types | Baseline hypoalbuminaemia was an independent predictor of WRF (HR 2.87, 95% CI 1.60–5.16; p = 0.0004) and in‐hospital mortality among those with WRF (OR 2.86, 95% CI 1.24–6.65; p = 0.011). |
| Prenner et al. 55 | 372 | HFpEF |
Albumin was independently associated with the primary outcome including CV death, aborted cardiac arrest, or HF hospitalization after correction for multiple comorbidities (HR 0.72, 95% CI 0.67–0.78, and HR 0.78, 95% CI 0.71–0.85; p < 0.001 for both models). Albumin was also independently associated with the composite outcome of all‐cause death or HF hospitalization (HR 0.72, 95% CI 0.67–0.78, and HR 0.78, 95% CI 0.72–0.84; p < 0.001 for both models). |
| Cardoso et al. 74 | 204 | EF not assessed | Increasing levels of albumin independently reduced the risk of 1‐year all‐cause mortality (OR 0.41, 95% CI 0.22–0.75; p = 0.004). |
| Yatsu et al. 60 | 551 | All types | Hypoalbuminaemia did not predict all‐cause mortality. |
| Filippatos et al. 10 | 5450 | Not available |
Among older adults aged <73 years, hypoalbuminaemia was associated with an increased risk of incident HF (HR 2.09, 95% CI 1.41–3.10; p < 0.001). Hypoalbuminaemia was found to increase the composite outcome of incident HF or all‐cause mortality (HR 1.33, 95% CI 1.11–1.61; p = 0.002) and all‐cause mortality (HR 1.23, 95% CI 1.02–1.49; p = 0.035). |
| Gopal et al. 14 | 2907 | All types | Hypoalbuminaemia was associated with an increased risk for HF hospitalization and readmission up to 6 years (baseline HR 1.13 per −0.1 g/dl, 95% CI 1.05–1.22; p = 0.001). |
| Su et al. 75 | 385 | HFrEF | Serum albumin independently predicted CV death or WHF (HR 0.96, 95% CI 0.94–0.99; p = 0.02). |
| Feng et al. 76 | 559 | All types | Hypoalbuminaemia increased the risk for all‐cause mortality (HR 1.34, 95% CI 1.02–1.74; p = 0.032) as well as did baseline serum albumin levels as a continuous variable (HR 1.53 per 1 g/dl decrease, 95% CI 1.15–2.04; p = 0.003). |
ADHF, acutely decompensated heart failure; CI, confidence interval; CV, cardiovascular; EF, ejection fraction; ER, emergency room; HD, haemodialysis; HF, heart failure; HR, hazard ratio; OR, odds ratio; WHF, worsening heart failure; WRF, worsening renal function.
The COAPT (Cardiovascular Outcomes Assessment of the MitraClip Percutaneous Therapy for Heart Failure Patients with Functional Mitral Regurgitation) trial demonstrated that transcatheter edge‐to‐edge repair (TEER) with the MitraClip™ system in addition to guideline‐directed medical therapies reduced hospitalization for HF and improved survival in patients with moderate‐to‐severe or severe secondary mitral regurgitation. 77 A sub‐analysis of the COAPT trial recently focused on serum albumin levels. 76 In this study, 13.6% of patients had a baseline albumin <3.5 g/dl. Patients with albumin levels <4.0 g/dl were older and suffered from a larger number of diseases and more frequently experienced a hospitalization in the previous 12 months before enrolment. Baseline hypoalbuminaemia and baseline albumin levels expressed as a continuous variable were both found to increase the risk of all‐cause mortality, but not hospitalization for HF, all‐cause hospitalization or the composite of all‐cause mortality or hospitalization for HF. 76 A recent report including more than 1 million patients hospitalized for ADHF showed that 88% of them had hypoalbuminaemia and died during hospitalization or experienced acute kidney injury, sepsis, and blood transfusions more frequently than those with normal albumin levels. 65 Interestingly, Peterson et al. 78 reported about the importance of hypoalbuminaemia assessed by the nadir – and not the baseline – albumin level. Indeed, they showed that nadir serum albumin levels were observed in up to 50% of patients, were associated with in‐hospital mortality, and provided a two‐fold increased risk to develop acute worsening of kidney function. In the AURORA (Acute Heart Failure Registry in the Osaka Rosai Hospital) study conducted in Japan, hypoalbuminaemia increased the risk of 1‐year all‐cause mortality in patients aged ≥80 years, but not in those aged <80 years. 79
However, not all studies are concordant on the role of hypoalbuminaemia in HF. A post‐hoc analysis of two double‐blind trials – DOSE‐AHF (Diuretic Optimization Strategies Evaluation Acute Heart Failure) and ROSE‐AHF (Renal Optimization Strategies Evaluation Acute Heart Failure) – designed to test the effectiveness and consequences of different decongestive therapies indicated that baseline albumin levels were associated with net fluid loss, but not with weight change or lower diuretic efficiency. 63 In addition, baseline hypoalbuminaemia was not likely to increase the incidence of worsening HF or worsening kidney function. At discharge, the composite endpoint of death, rehospitalization and unscheduled emergency room visit over a follow‐up of 60 days occurred in 43.4% of patients, however no correlation with baseline serum albumin levels was found. Although hypoalbuminaemia could be associated with increased peripheral congestion, but not with central congestion, its baseline levels were not correlated with prognosis. 63 The authors, however, recognized various biases in the post‐hoc analysis, such as selection bias due to the inclusion criteria of the two studies and the inadequate power of the two studies to detect clinical endpoints based upon serum albumin levels. On the other hand, an interesting approach to the problem has been proposed by Nakayama et al. 80 In a study on 115 patients, a rising trend of serum albumin values during hospitalization was observed in 61% of patients and was independently associated with a good prognosis. 80
Two meta‐analyses were performed on the prognostic role of serum albumin in HF. Peng et al. 81 studied both acute and chronic HF: in both groups the pooled risk for all‐cause mortality was higher in patients with hypoalbuminaemia (relative risk [RR] 1.75, 95% confidence interval [CI] 1.35–2.27, pooled p = 0.048, and RR 3.50, 95% CI 1.29–9.73, pooled p = 0.002, respectively). El Iskandarani et al. 44 confirmed that mortality in HF patients was ~4 times higher in those with lower serum albumin, both in the inpatient (odds ratio [OR] 3.77, 95% CI 1.96–7.23) and outpatient settings (OR 2.44, 95% CI 2.05–2.91). Baseline hypoalbumineamia had a pooled area under the curve of 0.79, which was comparable to that of elevated BNP levels at admission. Long‐term mortality was also significantly increased in patients with hypoalbuminaemia and this was more evident in the first year of follow‐up (OR 1.5, 95% CI 1.36–1.64). Moreover, hypoalbuminaemia in HF patients was correlated with a longer hospital stay, coupled with a non‐significant higher rehospitalization rate (OR 1.22, 95% CI 0.96–1.55). 44
Heart failure with preserved ejection fraction
Among the patients included in the TOPCAT (Treatment of Preserved Cardiac Function Heart Failure With an Aldosterone Antagonist Trial) trial, that studied spironolactone in patients with HFpEF, 82 , 83 Prenner et al. 55 evaluated the phenotypic effects of serum albumin levels in patients with HFpEF on measures of target organ damage (glomerular filtration rate, echocardiographic data, arterial wall stiffness). In this study including 3265 patients from the original trial, the prevalence of hypoalbuminaemia was 10.7% and was associated with older age, black race, advanced HF, peripheral arterial disease, atrial fibrillation, and diabetes. Patients in the lower tertile of serum albumin levels (serum albumin between 1 and 3.9 g/dl) had a higher prevalence of markers of micro‐ and macrovascular dysfunction. 55 In another study by Prenner et al. 59 conducted in 118 HFpEF patients with a median follow‐up of 57.6 months, serum albumin was proved as an independent predictor of mortality and hospital readmission for HF after adjustment for several clinical variables, including NT‐proBNP. In this regard, a number of studies confirmed the prognostic value of albumin irrespective of natriuretic peptides. 57 , 75 , 84 , 85 , 86 , 87 Among 100 elderly patients, Arques et al. 28 reported that lower serum albumin was more prevalent in patients with HFpEF than with HFrEF or pulmonary disease and that oncotic pressure, of which albumin is the main leading force, is the first pathophysiological determinant in pulmonary oedema, especially in HFpEF patients. In the JASPER (Japanese Heart Failure Syndrome With Preserved Ejection Fraction) registry, lower serum albumin levels at hospitalization were predictive of all‐cause mortality and worsening HF across a 2‐year period after discharge among HFpEF patients irrespective of New York Heart Association class III or IV at discharge, systolic blood pressure, sodium and BNP on admission. 86
A post‐hoc analysis of the EMPEROR‐Preserved (Empagliflozin Outcome Trial in Patients With Chronic Heart Failure with Preserved Ejection Fraction) trial evaluating liver function abnormalities and CV outcomes found that albumin levels in the lowest tertile increased the risk for the composite of time to first adjudicated hospitalization for HF or CV death and this was particularly true for albumin levels <4.2 g/dl. 88 A significant increase in albumin levels from week 4 to week 120 for empagliflozin versus placebo was observed, 88 and this might depend, at least in part, from a reduction in albuminuria, although regarded as a not clinically meaningful change by the authors of the paper. Another post‐hoc analysis of the EMPEROR‐Preserved trial included albumin in biomarker‐driven prognostic models and confirmed that lower albumin levels increased the risk of both all‐cause and CV mortality (hazard ratio [HR] 1.07, 95% CI 1.05–1.10 and HR 1.07, 95% CI 1.03–1.10, respectively). 89
Heart failure with reduced ejection fraction
In a sub‐analysis of the VERITAS (Value of Endothelin Receptor Inhibition with Tezosentan in Acute Heart Failure Studies) study, lower serum albumin levels were predictive of the composite endpoint of death, worsening HF or HF rehospitalization within 30 days only in patients younger than 72 years, while hypoalbuminaemia was associated with 90‐day mortality only among those older than 72 years. 90 A post‐hoc analysis of the EVEREST (Efficacy of Vasopressin Antagonism in Heart Failure Outcome Study with Tolvaptan) trial found that participants in the lowest quartile of serum albumin at baseline (between 1.4 and 3.4 g/dl) experienced a 36% increased risk for all‐cause mortality (HR 1.36, 95% CI 1.02–1.82) compared to those in the highest quartile (serum albumin levels at baseline between 4.1 and 5.6 g/dl), but had no influence on CV mortality of first HF hospitalization. 84
Heart failure associated with other conditions
A couple of studies evaluated the prognostic role of lower levels of albumin in patients with acute coronary syndrome. Among such patients, hypoalbuminaemia independently predicted new‐onset HF and in‐hospital mortality and this trend was inversely correlated with progressively low albumin levels. 17 Another report described increased in‐hospital mortality, HF development, and major bleeding in patients with hypoalbuminaemia. In addition, hypoalbuminaemia was found to predict long‐term mortality and worsening HF. 18
Hypoalbuminaemia is also important in rare forms of CV diseases, such as congenital heart diseases (CHD), and is a strong independent predictor of death even after adjustment for disease complexity. 70 The Fontan palliative procedure often fails because of the onset of protein‐losing enteropathy which causes severe hypoalbuminaemia. 91 The prognostic role of serum albumin was evaluated in adult HF patients with CHD listed for heart transplantation. Serum albumin levels <3.2 g/dl were found to almost double the risk of the primary outcome – death or delisting for clinical worsening within 1 year – among CHD patients irrespective of lower estimated glomerular filtration rate, admission to the intensive care unit, and need for mechanical ventilation. 92
Biventricular congestive HF, which causes fluid retention and increased vascular permeability, contributes to the onset and perpetuation of hypoalbuminaemia. The latter is a prognostic marker for patients referred to cardiac interventional therapies, such as transcatheter aortic valve replacement, left ventricular assist device, cardiac resynchronization therapy, and in heart transplant recipients. 93 , 94 , 95 , 96
Role of albumin in prognostic scores in heart failure
Some prognostic models, that include albumin in their algorithms, were proposed over the past years in patients with acute HF. A retrospective analysis of the REALITY‐AHF (Registry Focused on Very Early Presentation and Treatment in Emergency Department of Acute Heart Failure) study, which compared the MELD XI (Model for End‐stage Liver Disease excluding INR) score with the ALBI (albumin‐bilirubin) score, showed that the latter performed better than the MELD XI score to predict 1‐year mortality in ADHF patients. 97 Shibata et al. 98 demonstrated that the Fibrosis‐4 (FIB4) index was an independent predictor for all‐cause mortality and HF readmission at 3, 6 and 12 months; on the other hand, liver function tests at admission, which are part of FIB4, could not per se predict adverse events. In a report from the PROTECT (Placebo‐Controlled Randomized Study of the Selective A1 Adenosine Receptor Antagonist Rolofylline for Patients Hospitalized with Acute Decompensated Heart Failure and Volume Overload to Assess Treatment Effect on Congestion and Renal Function) study, a model with eight clinical parameters including serum albumin was found to predict a number of adverse outcomes (30‐day mortality or rehospitalization for any reason; 30‐day death or rehospitalization for CV or renal reasons; 30‐ and 180‐day all‐cause mortality) with similar accuracy to more complex models, although with moderate accuracy for non‐fatal events. 99 The CRP/albumin ratio was proposed as a marker of chronic inflammation in HF with contrasting results. 100 , 101 , 102 An elevation of the Glasgow prognostic score (GPS) or modified GPS (mGPS) – a well‐established nutritional and inflammatory assessment tool in patients with cancer including a combination of albumin and CRP – was associated with poor survival in HF patients. 103 , 104 Elevated fibrinogen‐to‐albumin ratio was independently associated with major adverse cardiac and cerebrovascular events in patients with ADHF and diabetes. 105
Almost 70% of HF patients, independently of age, fulfil frailty diagnostic criteria and almost one third of younger HF patients are diagnosed as frail. 106 Assessment of frailty is crucial in all HF patients and different tools are available. 107 , 108 In a prospective, single‐centre study, serum albumin concentration was measured in frail, institutionalized patients aged >70 years. Hypoalbuminaemia, together with age, blood urea nitrogen and BNP, was an independent predictor of in‐hospital mortality. 57 Albumin concentrations <3 g/dl were found to predict in‐hospital mortality with a modest specificity (68%) and a good sensitivity (94%). 29 Finally, the Charlson comorbidity index, which was developed in 1987 to predict 10‐year survival in patients with multiple comorbidities, 109 was found higher in HF patients with hypoalbuminaemia than in those with normoalbuminaemia. 65
Impact of albumin supplementation in patients with heart failure and hypoalbuminaemia
An important matter of debate deals with the rationale for nutritional interventions in patients diagnosed with hypoalbuminaemia and acute HF. Low prognostic nutritional index (including albumin and lymphocytes) values were associated with short‐ and long‐term mortality in elderly patients hospitalized for ADHF. 110 A metanalysis by Vincent et al. 6 resulted inconclusive in supporting administration of albumin in target patient cohorts. Despite the detrimental effect of hypoalbuminaemia on mortality, morbidity and length of hospitalization, no evidence was found for a threshold above which albumin was no longer affecting these areas. A subgroup analysis of the PICNIC (Nutritional Intervention Program in Hospitalized Patients with Heart Failure who are Malnourished) study, in which a nutritional intervention reduced all‐cause mortality in hospitalized patients with acute HF and readmission for worsening HF, 85 focused specifically on hypoalbuminaemia with a primary endpoint of all‐cause mortality and readmission for HF. Patients were divided between normo‐ and hypoalbuminaemia (serum albumin <3.4 g/dl, with hypoalbuminaemia prevalence of 49.2%). At 12‐month follow‐up, the number of events for the primary endpoint were comparable between the two subgroups. 111 The authors concluded that the cause of hypoalbuminaemia in HF patients was multifactorial and, therefore, in this type of population albumin loses its value as a nutritional parameter and should not be used solely for this purpose.
Hypoalbuminaemia may reduce oncotic pressure and contribute to diuretic resistance, as observed in nephrotic syndrome. 112 While albumin supplementation is established for some conditions (Table 3 ), 113 , 114 , 115 , 116 , 117 , 118 , 119 , 120 , 121 its effectiveness in HF is not known and was poorly studied. In a randomized controlled cross‐over trial in patients with hypoalbuminaemia and chronic kidney disease, the addition of albumin to furosemide provided a short‐term advantage in terms of water and sodium diuresis over furosemide alone, 122 supporting previous pre‐clinical findings in analbuminaemic rats. 38 While convincing data about albumin supplementation emerged from the infectious disease field (severe sepsis and septic shock and in spontaneous bacterial peritonitis), 116 , 123 , 124 scant evidence is available for albumin supplementation in patients with ADHF. In a retrospective cohort study of 1038 ADHF patients, albumin supplementation did not show any advantage in terms of reduction of the primary endpoint (a composite of intubation, emergency renal replacement, or mortality). 125 Despite the retrospective nature of the study, a slight, non‐significant signal towards an increased harm for albumin supplementation was observed. A retrospective study including 162 ADHF patients, with 20.3% of them presenting hypoalbuminaemia, found that average net urine output over a 2‐day study period and diuretic doses (expressed as furosemide equivalent/24 h) were similar irrespective of albumin levels. 126 In another study, a reduced urinary output was observed when co‐administering albumin (12.5 g over 24 h) and furosemide (continuous infusion of 250 mg over 24 h, dose of 0.1 mg/kg/h) compared to continuous infusion of furosemide for 24 h alone (initial dose of 1 mg/kg, followed by 0.1 mg/kg/h). 127 In the retrospective LILAC‐HF (Levels of Albumin and Impact on Loop Diuretic and Albumin Co‐administration in Heart Failure) study, concomitant administration of intravenous loop diuretics and albumin in 276 patients hospitalized for ADHF was effective in improving median diuretic output and weight within 72 h of treatment, but no association between baseline serum albumin level and 72‐h urine output was found. 128 Taken together, these findings underline the need for randomized trials testing albumin supplementation in HF patients.
Table 3.
Randomized clinical trials that tested the effect of albumin supplementation in different conditions
| Author | No. of patients | Condition | Main findings |
|---|---|---|---|
| Lee et al.113 | 203 | AKI | Administration of albumin immediately before off‐pump coronary artery bypass surgery in patients with a preoperative albumin level <4 g/dl was associated with a reduced risk of AKI after surgery (RR 0.56, 95% CI 0.33–0.92) compared to saline. |
| Macedo et al.114 | 65 | Intermittent KRT | Among AKI or ESKD patients with hypoalbuminaemia (albumin <3 g/dl) undergoing KRT, pre‐dialysis albumin supplementation reduced the number of episodes of hypotension compared to saline (7% vs. 15%, p = 0.002). |
| O'Brien et al.115 | 1508 | Continuos KRT | The administration of 20% albumin (either alone or in combination with 4% albumin) did not increase 90‐day mortality or KRT dependence. |
| Caironi et al.116 | 1810 | Severe sepsis | In patients with severe sepsis, albumin replacement in addition to crystalloids did not improve survival rate at 28 and 90 days compared to crystalloids alone. |
| Park et al.117 | 360 | Severe sepsis or septic shock | Albumin supplementation together with lactated Ringer's in cancer patients with sepsis did not improve 7‐day survival compared to lactated Ringer's alone. |
| Philips et al.118 | 308 | Sepsis‐induced hypotension | Albumin supplementation was able to reverse sepsis‐induced hypotension at 3 h compared to normal saline in patients with cirrhosis (11.7% vs. 3.2%, p = 0.008; OR 3.9, 95% CI 1.42–10.9). |
| Martin et al.119 | 40 | ARDS | Combination therapy with albumin and furosemide resulted in improved oxygenation compared to placebo and furosemide (OR 19.3, 95% CI 2.9–127.3). |
| China et al.120 | 777 | Cirrhosis | Among hospitalized patients with decompensated cirrhosis, no differences were observed between albumin infusions aimed at maintaining an albumin level ≥3.5 g/dl compared to standard care. |
| Finfer et al.121 | 6997 | Critically ill patients in the ICU | No differences in the rate of 28‐day death from any cause was found between patients receiving albumin or normal saline. |
AKI, acute kidney injury; ARDS, acute respiratory distress syndrome; CI, confidence interval; ESKD, end‐stage kidney disease; ICU, intensive care unit; KRT, kidney replacement therapy; OR, odds ratio; RR, relative risk.
Charokopos et al. 72 evaluated serum and urine albumin in two independent populations including outpatients with HF and inpatients admitted for ADHF. Among outpatients, serum albumin inversely correlated with NT‐proBNP, loop diuretic dose, and plasma IL‐6. However, when controlling for IL‐6, the association between diuretic efficiency and serum albumin disappeared. In addition, outpatients with larger amounts of albuminuria needed higher doses of loop diuretic and albuminuria was inversely associated with serum albumin and positively correlated with baseline NT‐proBNP and IL‐6. Similar results were confirmed in an inpatient cohort, suggesting that serum and urine albumin are not associated with diuretic resistance in patients with HF. 72
Albuminuria and heart failure
Albuminuria – the loss of albumin in the urine – represents an earliest marker of glomerular damage, that could present before the estimated glomerular filtration rate reduction <60 ml/min/1.73 m2. 129 Pathophysiological mechanisms linking albuminuria to HF are multifactorial. They depend on the damage to the kidney filtration barrier causing systemic inflammation and increased activation of the renin–angiotensin–aldosterone system, finally leading to volume overload mediated by water and salt retention, as discussed elsewhere in more detail. 130 In patients with chronic HF, albuminuria is found in up to 44% patients 131 , 132 and more frequently in patients with HFpEF. 131 , 133 , 134 Data from the BIOSTAT‐CHF (A Systems Biology Study to Tailored Treatment in Chronic Heart Failure) reported that albuminuria was associated with clinical, echocardiographic, and circulating biomarkers of congestion in HF patients. 135
Albuminuria was found to predict incident HF in high‐risk subjects, irrespective of the presence or absence of type 2 diabetes and history of CV disease, as found in the RENAAL (Reduction in Endpoints in Non‐insulin dependent diabetes mellitus with the Angiotensin II Antagonist Losartan) trial, the Framingham Heart Study, and the MESA (Multi‐Ethnic Study of Atherosclerosis) study. 136 , 137 , 138 In a prospective analysis of the ARIC (Atherosclerosis Risk in Communities) study, including patients without HF at baseline, a progressive increase in the degree of albuminuria was associated with a progressively increased risk of HF across time. 139 The association between albuminuria and risk of incident HF across different HF subtypes is controversial, 137 , 140 given different study design and cohorts that make comparisons difficult.
Albuminuria was found as an independent predictor of HF hospitalization and mortality among HF patients included in the SOLVD (Studies of Left Ventricular Dysfunction) trial. 141 Similar results were described in the CHARM (Candesartan in Heart Failure Assessment of Reduction in Mortality and Morbidity) trial, 131 where albuminuria conferred a 60–80% increased risk of death and a 30–70% risk of HF hospitalization in HFpEF patients. Importantly, a direct relationship was proved between an increase in albuminuria and an augmented mortality risk. 142 In addition, in the GISSI‐HF (Gruppo Italiano per lo Studio della Streptochinasi nell'Infarto) and CHARM trials, albuminuria was a strong independent predictor of poor outcomes in HF patients, irrespective of hypertension and diabetes, 131 , 132 that are commonly associated with albuminuria and reduced kidney function. As for incident HF, specific associations of albuminuria with HFrEF versus HFpEF remain limited and are worthy of further investigation.
Given the availability of therapeutic agents that could reduce albuminuria, it might be plausible that reducing albuminuria could reduce the risk of developing HF or of its complications among HF patients. Limited, although encouraging, data are available to this regard. Losartan was able to reduce albuminuria in the RENAAL trial and this was associated with a 27% reduced risk of HF among patients without HF at baseline. 136 Data from the FIGARO‐DKD (Finerenone in Reducing Cardiovascular Mortality and Morbidity in Diabetic Kidney Disease) trial showed that finerenone was able to decrease new‐onset HF, CV death, and hospitalizations for HF in patients with chronic kidney disease and type 2 diabetes, irrespective of a history of HF. 143 Similar results apply to chronic kidney disease patients on sodium–glucose cotransporter 2 inhibitors, as outlined in the DAPA‐CKD (Dapagliflozin and Prevention of Adverse Outcomes in Chronic Kidney Disease) and EMPEROR‐Pooled (Empagliflozin Outcome Trial in Patients With Chronic Heart Failure With Reduced Ejection Fraction [EMPEROR‐Reduced] and EMPEROR‐Preserved) trials. 144 , 145
An exhaustive review on the role of albuminuria in HF can be found elsewhere. 130
Open questions about the role of albumin in heart failure
Following an exhaustive review of the literature, additional investigation is needed to overcome the presence of multiple working definitions for hypoalbuminaemia, that makes comparisons of findings among studies extremely difficult. In addition, the presence of studies with different designs (retrospective and prospective cohorts but lack of randomized clinical studies) and populations with age varying from 50 to 80 years points to a complexity that is larger than previously expected and to knowledge gaps claiming for future studies. This is closely linked to the current cut‐off of albumin levels, which is generally fixed between 3.4 and 3.5 g/dl. We believe that albumin levels <3.5 g/dl should initially raise some degree of attention in clinicians treating patients with both acute and chronic HF as this could highlight the phenotype of a more frail patients with worse outcomes compared to similar patients with albumin levels ≥3.5 g/dl. However, it is documented that a physiological decline in hepatic synthesis of albumin occurs with aging, with a potential impact on congestion, but this belief is for now only speculative and somewhat limited to clinical observation. A meta‐analysis has recently shown that patients with albumin levels <2.5 g/dl benefit the most from co‐administration of furosemide and albumin in terms of diuretic and natriuretic response, 52 suggesting that lowest cut‐offs for albumin should be studied to assess their clinical impact in the management of fluid congestion. In addition, what is not completely clear is whether hypoalbuminaemia is always due to a true decrease in albumin levels (caused by increased loss and/or reduced production) or rather a pseudo‐hypoalbuminaemia explained by dilution of albumin following volume increase, as already described in patients on haemodialysis. 146 To date, this aspect has not been investigated in clinical studies and, to the best of our knowledge, neither in pre‐clinical studies, but could certainly represent a knowledge gap to be filled in the future, especially if this may represent a tool to improve decongestive therapies in ADHF.
Studies are needed in the next future in order to depict the ideal phenotype of HF patients who could benefit from albumin infusion. Based on the available evidence in the literature and on the clinical experience of the authors, older patients with severe fluid retention (as those with pleural and pericardial effusion and ascites) and/or with poor response to high‐dose loop diuretics within 48 h while still symptomatic might represent the best patients to be included in trials of albumin supplementation. An additional feature that should be taken into consideration deals with the nutritional status of the patient, as malnutrition – that could underlie lower albumin levels – may negatively impact on the response to diuretics and on patient prognosis during hospitalization for ADHF.
At present, clinical management of patients with acute HF and hypoalbuminaemia should follow guidelines, 2 including diuretics, as fluid decongestion reduces albumin TER and contributes to correcting hypoalbuminaemia, once splanchnic venous congestion resolved. 31 , 34 The same applies to chronic HF with coexisting hypoalbuminaemia, since guideline‐directed medical therapies are able to improve the dysregulation of mechanisms causing the clinical syndrome and indirectly decrease biomarkers of inflammation. 39
Conclusion
Hypoalbuminaemia is common in HF patients and is explained by multiple pathophysiological processes. Several studies showed that hypoalbuminaemia could be used as a prognostic biomarker for patients hospitalized due to HF or to predict HF events across the full spectrum of ejection fraction, irrespective of potential confounders. In the opinion of the authors, this is the current and more helpful role of albumin in such patients. Whether albumin supplementation in ADHF or chronic HF patients would be beneficial to improve outcomes is not known. Indeed, adequately designed, multicentre randomized clinical trials, as done for iron supplementation in HF, are warranted to fill this knowledge gap, considering the beneficial properties of albumin that have been demonstrated in other settings. In this view, it would be helpful to consider both inpatients and outpatients, different HF phenotypes, and acute versus chronic HF in order to gather information about the best time, if any, to supplement albumin.
Acknowledgement
Open access publishing facilitated by Aziende Socio Sanitarie Territoriale dei Sette Laghi, as part of the Wiley – SBBL agreement. [Correction added on 22 July 2024, after first online publication: SBBL funding statement has been added.]
Conflict of interest: A.B. received honoraria from Effetti s.r.l. (Milan, Italy) to collaborate on the medical website www.inflammology.org in the past 36 months, outside the present work. All other authors have nothing to disclose.
References
- 1. Metra M, Teerlink JR. Heart failure. Lancet 2017;390:1981–1995. 10.1016/S0140-6736(17)31071-1 [DOI] [PubMed] [Google Scholar]
- 2. McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Böhm M, et al.; ESC Scientific Document Group . 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: Developed by the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). With the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail 2022;24:4–131. 10.1002/ejhf.2333 [DOI] [PubMed] [Google Scholar]
- 3. Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr, Drazner MH, et al. 2013 ACCF/AHA Guideline for the management of heart failure: A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2013;62:e147–e239. 10.1016/j.jacc.2013.05.019 [DOI] [PubMed] [Google Scholar]
- 4. Clerico A, Zaninotto M, Passino C, Aspromonte N, Piepoli MF, Migliardi M, et al. Evidence on clinical relevance of cardiovascular risk evaluation in the general population using cardio‐specific biomarkers. Clin Chem Lab Med 2020;59:79–90. 10.1515/cclm-2020-0310 [DOI] [PubMed] [Google Scholar]
- 5. Clerico A, Zaninotto M, Aimo A, Cardinale DM, Dittadi R, Sandri MT, et al. Variability of cardiac troponin levels in normal subjects and in patients with cardiovascular diseases: Analytical considerations and clinical relevance. Clin Chem Lab Med 2023;61:1209–1229. 10.1515/cclm-2022-1285 [DOI] [PubMed] [Google Scholar]
- 6. Vincent JL, Dubois MJ, Navickis RJ, Wilkes MM. Hypoalbuminemia in acute illness: Is there a rationale for intervention? A meta‐analysis of cohort studies and controlled trials. Ann Surg 2003;237:319–334. 10.1097/01.SLA.0000055547.93484.87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Jellinge ME, Henriksen DP, Hallas P, Brabrand M. Hypoalbuminemia is a strong predictor of 30‐day all‐cause mortality in acutely admitted medical patients: A prospective, observational, cohort study. PLoS One 2014;9:e105983. 10.1371/journal.pone.0105983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Oster HS, Dolev Y, Kehat O, Weis‐Meilik A, Mittelman M. Serum hypoalbuminemia is a long‐term prognostic marker in medical hospitalized patients, irrespective of the underlying disease. J Clin Med 2022;11:1207. 10.3390/jcm11051207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Arques S, Ambrosi P. Human serum albumin in the clinical syndrome of heart failure. J Card Fail 2011;17:451–458. 10.1016/j.cardfail.2011.02.010 [DOI] [PubMed] [Google Scholar]
- 10. Filippatos GS, Desai RV, Ahmed MI, Fonarow GC, Love TE, Aban IB, et al. Hypoalbuminaemia and incident heart failure in older adults. Eur J Heart Fail 2011;13:1078–1086. 10.1093/eurjhf/hfr088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Monzo L, Kotrc M, Benes J, Sedlacek K, Jurcova I, Franekova J, et al. Clinical and humoral determinants of congestion in heart failure: Potential role of adiponectin. Kidney Blood Press Res 2019;44:1271–1284. 10.1159/000502975 [DOI] [PubMed] [Google Scholar]
- 12. Weaving G, Batstone GF, Jones RG. Age and sex variation in serum albumin concentration: An observational study. Ann Clin Biochem 2016;53:106–111. 10.1177/0004563215593561 [DOI] [PubMed] [Google Scholar]
- 13. Sundaram V, Fang JC. Gastrointestinal and liver issues in heart failure. Circulation 2016;133:1696–1703. 10.1161/CIRCULATIONAHA.115.020894 [DOI] [PubMed] [Google Scholar]
- 14. Gopal DM, Kalogeropoulos AP, Georgiopoulou VV, Tang WW, Methvin A, Smith AL, et al.; Health ABC Study . Serum albumin concentration and heart failure risk the health, aging, and body composition study. Am Heart J 2010;160:279–285. 10.1016/j.ahj.2010.05.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Polat N, Aydin M, Yildiz A, Acet H, Akil MA, Bilik MZ, et al. The prognostic significance of serum albumin in patients with acute decompensated systolic heart failure. Acta Cardiol 2014;69:648–654. 10.1080/ac.69.6.1000007 [DOI] [PubMed] [Google Scholar]
- 16. Ancion A, Allepaerts S, Oury C, Gori AS, Piérard LA, Lancellotti P. Serum albumin level and hospital mortality in acute non‐ischemic heart failure. ESC Heart Fail 2017;4:138–145. 10.1002/ehf2.12128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. González‐Pacheco H, Amezcua‐Guerra LM, Sandoval J, Martínez‐Sánchez C, Ortiz‐León XA, Peña‐Cabral MA, et al. Prognostic implications of serum albumin levels in patients with acute coronary syndromes. Am J Cardiol 2017;119:951–958. 10.1016/j.amjcard.2016.11.054 [DOI] [PubMed] [Google Scholar]
- 18. Oduncu V, Erkol A, Karabay CY, Kurt M, Akgün T, Bulut M, et al. The prognostic value of serum albumin levels on admission in patients with acute ST‐segment elevation myocardial infarction undergoing a primary percutaneous coronary intervention. Coron Artery Dis 2013;24:88–94. 10.1097/MCA.0b013e32835c46fd [DOI] [PubMed] [Google Scholar]
- 19. van Beek DEC, Kuijpers YAM, Konigs MHH, van der Horst ICC, Scheeren TWL. Low serum albumin levels and new‐onset atrial fibrillation in the ICU: A prospective cohort study. J Crit Care 2020;56:26–30. 10.1016/j.jcrc.2019.11.011 [DOI] [PubMed] [Google Scholar]
- 20. Dziedzic T, Pera J, Slowik A, Gryz‐Kurek EA, Szczudlik A. Hypoalbuminemia in acute ischemic stroke patients: Frequency and correlates. Eur J Clin Nutr 2007;61:1318–1322. 10.1038/sj.ejcn.1602643 [DOI] [PubMed] [Google Scholar]
- 21. Zhou H, Wang A, Meng X, Lin J, Jiang Y, Jing J, et al. Low serum albumin levels predict poor outcome in patients with acute ischaemic stroke or transient ischaemic attack. Stroke Vasc Neurol 2021;6:458–466. 10.1136/svn-2020-000676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Chi G, Gibson CM, Liu Y, Hernandez AF, Hull RD, Cohen AT, et al. Inverse relationship of serum albumin to the risk of venous thromboembolism among acutely ill hospitalized patients: Analysis from the APEX trial. Am J Hematol 2019;94:21–28. 10.1002/ajh.25296 [DOI] [PubMed] [Google Scholar]
- 23. Gyamlani G, Molnar MZ, Lu JL, Sumida K, Kalantar‐Zadeh K, Kovesdy CP. Association of serum albumin level and venous thromboembolic events in a large cohort of patients with nephrotic syndrome. Nephrol Dial Transplant 2017;32:157–164. 10.1093/ndt/gfw227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Arques S. Serum albumin and cardiovascular disease: State‐of‐the‐art review. Ann Cardiol Angeiol (Paris) 2020;69:192–200. 10.1016/j.ancard.2020.07.012 [DOI] [PubMed] [Google Scholar]
- 25. Gom I, Fukushima H, Shiraki M, Miwa Y, Ando T, Takai K, et al. Relationship between serum albumin level and aging in community‐dwelling self‐supported elderly population. J Nutr Sci Vitaminol (Tokyo) 2007;53:37–42. 10.3177/jnsv.53.37 [DOI] [PubMed] [Google Scholar]
- 26. De Simone G, di Masi A, Ascenzi P. Serum albumin: A multifaced enzyme. Int J Mol Sci 2021;22:10086. 10.3390/ijms221810086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Joannidis M, Wiedermann CJ, Ostermann M. Ten myths about albumin. Intensive Care Med 2022;48:602–605. 10.1007/s00134-022-06655-8 [DOI] [PubMed] [Google Scholar]
- 28. Arques S, Ambrosi P, Gelisse R, Luccioni R, Habib G. Hypoalbuminemia in elderly patients with acute diastolic heart failure. J Am Coll Cardiol 2003;42:712–716. 10.1016/s0735-1097(03)00758-7 [DOI] [PubMed] [Google Scholar]
- 29. Arques S, Roux E, Sbragia P, Gelisse R, Pieri B, Ambrosi P. Usefulness of serum albumin concentration for in‐hospital risk stratification in frail, elderly patients with acute heart failure. Insights from a prospective, monocenter study. Int J Cardiol 2008;125:265–267. 10.1016/j.ijcard.2007.07.094 [DOI] [PubMed] [Google Scholar]
- 30. Peterson VP, Ottosen P. Albumin turnover and thoracic‐duct lymph in constrictive pericarditis. Acta Med Scand 1964;176:335–344. 10.1111/j.0954-6820.1964.tb00941.x [DOI] [PubMed] [Google Scholar]
- 31. Hesse B, Parving HH, Lund‐Jacobsen H, Noer I. Transcapillary escape rate of albumin and right atrial pressure in chronic congestive heart failure before and after treatment. Circ Res 1976;39:358–362. 10.1161/01.res.39.3.358 [DOI] [PubMed] [Google Scholar]
- 32. Carr JG, Stevenson LW, Walden JA, Heber D. Prevalence and hemodynamic correlates of malnutrition in severe congestive heart failure secondary to ischemic or idiopathic dilated cardiomyopathy. Am J Cardiol 1989;63:709–713. 10.1016/0002-9149(89)90256-7 [DOI] [PubMed] [Google Scholar]
- 33. Ballmer PE. Causes and mechanisms of hypoalbuminaemia. Clin Nutr 2001;20:271–273. 10.1054/clnu.2001.0439 [DOI] [PubMed] [Google Scholar]
- 34. Battin DL, Ali S, Shahbaz AU, Munir A, Davis RC Jr, Newman KP, et al. Hypoalbuminemia and lymphocytopenia in patients with decompensated biventricular failure. Am J Med Sci 2010;339:31–35. 10.1097/MAJ.0b013e3181bfc83f [DOI] [PubMed] [Google Scholar]
- 35. Wilkinson P, Pinto B, Senior JR. Reversible protein‐losing enteropathy with intestinal lymphangiectasia secondary to chronic constrictive pericarditis. N Engl J Med 1965;273:1178–1181. 10.1056/NEJM196511252732202 [DOI] [PubMed] [Google Scholar]
- 36. Valberg LS, Corbett WE, McCorriston JR, Parker JO. Excessive loss of plasma protein into the gastrointestinal tract associated with primary myocardial disease. Am J Med 1965;39:668–673. 10.1016/0002-9343(65)90088-4 [DOI] [PubMed] [Google Scholar]
- 37. Wilcox CS, Testani JM, Pitt B. Pathophysiology of diuretic resistance and its implications for the management of chronic heart failure. Hypertension 2020;76:1045–1054. 10.1161/HYPERTENSIONAHA.120.15205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Inoue M, Okajima K, Itoh K, Ando Y, Watanabe N, Yasaka T, et al. Mechanism of furosemide resistance in analbuminemic rats and hypoalbuminemic patients. Kidney Int 1987;32:198–203. 10.1038/ki.1987.192 [DOI] [PubMed] [Google Scholar]
- 39. Murphy SP, Kakkar R, McCarthy CP, Januzzi JL Jr. Inflammation in heart failure: JACC state‐of‐the‐art review. J Am Coll Cardiol 2020;75:1324–1340. 10.1016/j.jacc.2020.01.014 [DOI] [PubMed] [Google Scholar]
- 40. Adamo L, Rocha‐Resende C, Prabhu SD, Mann DL. Reappraising the role of inflammation in heart failure. Nat Rev Cardiol 2020;17:269–285. 10.1038/s41569-019-0315-x [DOI] [PubMed] [Google Scholar]
- 41. Maekawa Y, Anzai T, Yoshikawa T, Asakura Y, Takahashi T, Ishikawa S, et al. Prognostic significance of peripheral monocytosis after reperfused acute myocardial infarction: A possible role for left ventricular remodeling. J Am Coll Cardiol 2002;39:241–246. 10.1016/s0735-1097(01)01721-1 [DOI] [PubMed] [Google Scholar]
- 42. Hasper D, Hummel M, Kleber FX, Reindl I, Volk HD. Systemic inflammation in patients with heart failure. Eur Heart J 1998;19:761–765. 10.1053/euhj.1997.0858 [DOI] [PubMed] [Google Scholar]
- 43. Niebauer J, Volk HD, Kemp M, Dominguez M, Schumann RR, Rauchhaus M, et al. Endotoxin and immune activation in chronic heart failure: A prospective cohort study. Lancet 1999;353:1838–1842. 10.1016/S0140-6736(98)09286-1 [DOI] [PubMed] [Google Scholar]
- 44. El Iskandarani M, El Kurdi B, Murtaza G, Paul TK, Refaat MM. Prognostic role of albumin level in heart failure: A systematic review and meta‐analysis. Medicine (Baltimore) 2021;100:e24785. 10.1097/MD.0000000000024785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Bonilla‐Palomas JL, Gámez‐López AL, Moreno‐Conde M, López‐Ibáñez MC, Anguita‐Sánchez M, Gallego de la Sacristana A, et al. Hypoalbuminemia in acute heart failure patients: Causes and its impact on hospital and long‐term mortality. J Card Fail 2014;20:350–358. 10.1016/j.cardfail.2014.01.016 [DOI] [PubMed] [Google Scholar]
- 46. Nohria A, Tsang SW, Fang JC, Lewis EF, Jarcho JA, Mudge GH, et al. Clinical assessment identifies hemodynamic profiles that predict outcomes in patients admitted with heart failure. J Am Coll Cardiol 2003;41:1797–1804. 10.1016/s0735-1097(03)00309-7 [DOI] [PubMed] [Google Scholar]
- 47. Anand IS, Latini R, Florea VG, Kuskowski MA, Rector T, Masson S, et al. C‐reactive protein in heart failure: Prognostic value and the effect of valsartan. Circulation 2005;112:1428–1434. 10.1161/CIRCULATIONAHA.104.508465 [DOI] [PubMed] [Google Scholar]
- 48. Jia X, Yu XL, Lu B, Shang YY, Shen LF, Li YL, et al. Malnutrition and infection lead to poor prognosis and heavy financial burden of patients with chronic heart failure. Front Cardiovasc Med 2022;9:1045262. 10.3389/fcvm.2022.1045262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Sharma Y, Horwood C, Shahi R, Hakendorf P, Thompson C. Impact of malnutrition on clinical outcomes of acutely hospitalised heart failure patients at two tertiary hospitals in Australia: An observational study. Heart Lung Circ 2023;32:330–337. 10.1016/j.hlc.2022.10.011 [DOI] [PubMed] [Google Scholar]
- 50. Damman K, Masson S, Lucci D, Gorini M, Urso R, Maggioni AP, et al. Progression of renal impairment and chronic kidney disease in chronic heart failure: An analysis from GISSI‐HF. J Card Fail 2017;23:2–9. 10.1016/j.cardfail.2016.09.006 [DOI] [PubMed] [Google Scholar]
- 51. Kitsios GD, Mascari P, Ettunsi R, Gray AW. Co‐administration of furosemide with albumin for overcoming diuretic resistance in patients with hypoalbuminemia: A meta‐analysis. J Crit Care 2014;29:253–259. 10.1016/j.jcrc.2013.10.004 [DOI] [PubMed] [Google Scholar]
- 52. Lee TH, Kuo G, Chang CH, Huang YT, Yen CL, Lee CC, et al. Diuretic effect of co‐administration of furosemide and albumin in comparison to furosemide therapy alone: An updated systematic review and meta‐analysis. PLoS One 2021;16:e0260312. 10.1371/journal.pone.0260312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Salive ME, Cornoni‐Huntley J, Phillips CL, Guralnik JM, Cohen HJ, Ostfeld AM, et al. Serum albumin in older persons: Relationship with age and health status. J Clin Epidemiol 1992;45:213–221. 10.1016/0895-4356(92)90081-w [DOI] [PubMed] [Google Scholar]
- 54. Uthamalingam S, Kandala J, Daley M, Patvardhan E, Capodilupo R, Moore SA, et al. Serum albumin and mortality in acutely decompensated heart failure. Am Heart J 2010;160:1149–1155. 10.1016/j.ahj.2010.09.004 [DOI] [PubMed] [Google Scholar]
- 55. Prenner SB, Kumar A, Zhao L, Cvijic ME, Basso M, Spires T, et al. Effect of serum albumin levels in patients with heart failure with preserved ejection fraction (from the TOPCAT trial). Am J Cardiol 2020;125:575–582. 10.1016/j.amjcard.2019.11.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Jabbour R, Ling HZ, Norrington K, Amaral N, Zaman N, Aggarwal S, et al. Serum albumin changes and multivariate dynamic risk modelling in chronic heart failure. Int J Cardiol 2014;176:437–443. 10.1016/j.ijcard.2014.07.096 [DOI] [PubMed] [Google Scholar]
- 57. Arques S, Roux E, Stolidi P, Gelisse R, Ambrosi P. Usefulness of serum albumin and serum total cholesterol in the prediction of hospital death in older patients with severe, acute heart failure. Arch Cardiovasc Dis 2011;104:502–508. 10.1016/j.acvd.2011.06.003 [DOI] [PubMed] [Google Scholar]
- 58. Liu M, Chan CP, Yan BP, Zhang Q, Lam YY, Li RJ, et al. Albumin levels predict survival in patients with heart failure and preserved ejection fraction. Eur J Heart Fail 2012;14:39–44. 10.1093/eurjhf/hfr154 [DOI] [PubMed] [Google Scholar]
- 59. Prenner SB, Pillutla R, Yenigalla S, Gaddam S, Lee J, Obeid MJ, et al. Serum albumin is a marker of myocardial fibrosis, adverse pulsatile aortic hemodynamics, and prognosis in heart failure with preserved ejection fraction. J Am Heart Assoc 2020;9:e014716. 10.1161/JAHA.119.014716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Yatsu S, Kasai T, Matsumoto H, Shitara J, Shimizu M, Murata A, et al. Relationship between hypoalbuminemia on admission and long‐term mortality in patients with acute decompensated heart failure. Intern Med 2019;58:1695–1702. 10.2169/internalmedicine.1716-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Horwich TB, Kalantar‐Zadeh K, MacLellan RW, Fonarow GC. Albumin levels predict survival in patients with systolic heart failure. Am Heart J 2008;155:883–889. 10.1016/j.ahj.2007.11.043 [DOI] [PubMed] [Google Scholar]
- 62. Gotsman I, Shauer A, Zwas DR, Tahiroglu I, Lotan C, Keren A. Low serum albumin: A significant predictor of reduced survival in patients with chronic heart failure. Clin Cardiol 2019;42:365–372. 10.1002/clc.23153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Grodin JL, Lala A, Stevens SR, DeVore AD, Cooper LB, AbouEzzeddine OF, et al. Clinical implications of serum albumin levels in acute heart failure: Insights from DOSE‐AHF and ROSE‐AHF. J Card Fail 2016;22:884–890. 10.1016/j.cardfail.2016.01.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Bavishi C, Ather S, Bozkurt B, Deswal A. Prognostic significance of hypoalbuminemia among ambulatory heart failure patients with reduced and preserved ejection fraction [abstract]. Circulation 2013;128:A16960. 10.1161/circ.128.suppl_22.A16960 [DOI] [Google Scholar]
- 65. Karki S, Gajjar R, Bittar‐Carlini G, Jha V, Yadav N. Association of hypoalbuminemia with clinical outcomes in patients admitted with acute heart failure. Curr Probl Cardiol 2023;48:101916. 10.1016/j.cpcardiol.2023.101916 [DOI] [PubMed] [Google Scholar]
- 66. Novack V, Pencina M, Zahger D, Fuchs L, Nevzorov R, Jotkowitz A, et al. Routine laboratory results and thirty day and one‐year mortality risk following hospitalization with acute decompensated heart failure. PLoS One 2010;5:e12184. 10.1371/journal.pone.0012184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Talha KM, Pandey A, Fudim M, Butler J, Anker SD, Khan MS. Frailty and heart failure: State‐of‐the‐art review. J Cachexia Sarcopenia Muscle 2023;14:1959–1972. 10.1002/jcsm.13306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Zuo X, Li X, Tang K, Zhao R, Wu M, Wang Y, et al. Sarcopenia and cardiovascular diseases: A systematic review and meta‐analysis. J Cachexia Sarcopenia Muscle 2023;14:1183–1198. 10.1002/jcsm.13221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Araujo JP, Lourenco P, Rocha‐Goncalves F, Ferreira A, Bettencourt P. Nutritional markers and prognosis in cardiac cachexia. Int J Cardiol 2011;146:359–363. 10.1016/j.ijcard.2009.07.042 [DOI] [PubMed] [Google Scholar]
- 70. Arques S. Human serum albumin in cardiovascular diseases. Eur J Intern Med 2018;52:8–12. 10.1016/j.ejim.2018.04.014 [DOI] [PubMed] [Google Scholar]
- 71. Don BR, Kaysen G. Serum albumin: Relationship to inflammation and nutrition. Semin Dial 2004;17:432–437. 10.1111/j.0894-0959.2004.17603.x [DOI] [PubMed] [Google Scholar]
- 72. Charokopos A, Griffin M, Rao VS, Inker L, Sury K, Asher J, et al. Serum and urine albumin and response to loop diuretics in heart failure. Clin J Am Soc Nephrol 2019;14:712–718. 10.2215/CJN.11600918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Clarke MM, Dorsch MP, Kim S, Aaronson KD, Koelling TM, Bleske BE. Baseline albumin is associated with worsening renal function in patients with acute decompensated heart failure receiving continuous infusion loop diuretics. Pharmacotherapy 2013;33:583–588. 10.1002/phar.1241 [DOI] [PubMed] [Google Scholar]
- 74. Cardoso A, Branco C, Sant'Ana M, Costa C, Silva B, Fonseca J, et al. Hypoalbuminaemia and one‐year mortality in haemodialysis patients with heart failure: A cohort analysis. J Clin Med 2021;10:4518. 10.3390/jcm10194518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Su W, An T, Zhou Q, Huang Y, Zhang J, Zhang Y, et al. Serum albumin is a useful prognostic indicator and adds important information to NT‐proBNP in a Chinese cohort of heart failure. Clin Biochem 2012;45:561–565. 10.1016/j.clinbiochem.2012.02.010 [DOI] [PubMed] [Google Scholar]
- 76. Feng KY, Ambrosy AP, Zhou Z, Li D, Kong J, Zaroff JG, et al.; COAPT Trial Investigators . Association between serum albumin and outcomes in heart failure and secondary mitral regurgitation: The COAPT trial. Eur J Heart Fail 2023;25:553–561. 10.1002/ejhf.2809 [DOI] [PubMed] [Google Scholar]
- 77. Stone GW, Lindenfeld J, Abraham WT, Kar S, Lim DS, Mishell JM, et al.; COAPT Investigators . Transcatheter mitral‐valve repair in patients with heart failure. N Engl J Med 2018;379:2307–2318. 10.1056/NEJMoa1806640 [DOI] [PubMed] [Google Scholar]
- 78. Peterson EJ, Ng TMH, Patel KA, Lou M, Elkayam U. Association of admission vs. nadir serum albumin concentration with short‐term treatment outcomes in patients with acute heart failure. J Int Med Res 2018;46:3665–3674. 10.1177/0300060518777349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Nishino M, Egami Y, Kawanami S, Abe M, Ohsuga M, Nohara H, et al. Prognostic comparison of octogenarian vs. non‐octogenarian with acute decompensated heart failure – AURORA study. Circ J 2023;88:103–109. 10.1253/circj.CJ-23-0470 [DOI] [PubMed] [Google Scholar]
- 80. Nakayama H, Koyama S, Kuragaichi T, Shiba M, Fujiwara H, Takatsu Y, et al. Prognostic value of rising serum albumin during hospitalization in patients with acute heart failure. Am J Cardiol 2016;117:1305–1309. 10.1016/j.amjcard.2016.01.030 [DOI] [PubMed] [Google Scholar]
- 81. Peng W, Zhang C, Wang Z, Yang W. Prediction of all‐cause mortality with hypoalbuminemia in patients with heart failure: A meta‐analysis. Biomarkers 2019;24:631–637. 10.1080/1354750X.2019.1652686 [DOI] [PubMed] [Google Scholar]
- 82. Desai AS, Lewis EF, Li R, Solomon SD, Assmann SF, Boineau R, et al. Rationale and design of the Treatment of Preserved Cardiac Function Heart Failure with an Aldosterone Antagonist Trial: A randomized, controlled study of spironolactone in patients with symptomatic heart failure and preserved ejection fraction. Am Heart J 2011;162:966–972.e10. 10.1016/j.ahj.2011.09.007 [DOI] [PubMed] [Google Scholar]
- 83. Pitt B, Pfeffer MA, Assmann SF, Boineau R, Anand IS, Claggett B, et al.; TOPCAT Investigators . Spironolactone for heart failure with preserved ejection fraction. N Engl J Med 2014;370:1383–1392. 10.1056/NEJMoa1313731 [DOI] [PubMed] [Google Scholar]
- 84. Ambrosy AP, Vaduganathan M, Huffman MD, Khan S, Kwasny MJ, Fought AJ, et al.; EVEREST Trial Investigators . Clinical course and predictive value of liver function tests in patients hospitalized for worsening heart failure with reduced ejection fraction: An analysis of the EVEREST trial. Eur J Heart Fail 2012;14:302–311. 10.1093/eurjhf/hfs007 [DOI] [PubMed] [Google Scholar]
- 85. Bonilla‐Palomas JL, Gámez‐López AL, Castillo‐Domínguez JC, Moreno‐Conde M, López Ibáñez MC, Alhambra Expósito R, et al. Nutritional intervention in malnourished hospitalized patients with heart failure. Arch Med Res 2016;47:535–540. 10.1016/j.arcmed.2016.11.005 [DOI] [PubMed] [Google Scholar]
- 86. Nagai T, Yoshikawa T, Saito Y, Takeishi Y, Yamamoto K, Ogawa H, et al.; JASPER Investigators . Clinical characteristics, management, and outcomes of Japanese patients hospitalized for heart failure with preserved ejection fraction – a report from the Japanese Heart Failure Syndrome with Preserved Ejection Fraction (JASPER) Registry. Circ J 2018;82:1534–1545. 10.1253/circj.CJ-18-0073 [DOI] [PubMed] [Google Scholar]
- 87. Chien SC, Lo CI, Lin CF, Sung KT, Tsai JP, Huang WH, et al. Malnutrition in acute heart failure with preserved ejection fraction: Clinical correlates and prognostic implications. ESC Heart Fail 2019;6:953–964. 10.1002/ehf2.12501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Böhm M, Butler J, Krawczyk M, Mahfoud F, Haring B, Filippatos G, et al.; EMPEROR‐Preserved Trial Committees and Investigators . Liver tests, cardiovascular outcomes and effects of empagliflozin in patients with heart failure and preserved ejection fraction: The EMPEROR‐Preserved trial. Eur J Heart Fail 2023;25:1375–1383. 10.1002/ejhf.2922 [DOI] [PubMed] [Google Scholar]
- 89. Pocock SJ, Ferreira JP, Packer M, Zannad F, Filippatos G, Kondo T, et al. Biomarker‐driven prognostic models in chronic heart failure with preserved ejection fraction: The EMPEROR‐Preserved trial. Eur J Heart Fail 2022;24:1869–1878. 10.1002/ejhf.2607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Metra M, Cotter G, El‐Khorazaty J, Davison BA, Milo O, Carubelli V, et al. Acute heart failure in the elderly: Differences in clinical characteristics, outcomes, and prognostic factors in the VERITAS study. J Card Fail 2015;21:179–188. 10.1016/j.cardfail.2014.12.012 [DOI] [PubMed] [Google Scholar]
- 91. Udink Ten Cate FE, Hannes T, Germund I, Khalil M, Huntgeburth M, Apitz C, et al. Towards a proposal for a universal diagnostic definition of protein‐losing enteropathy in Fontan patients: A systematic review. Heart 2016;102:1115–1119. 10.1136/heartjnl-2015-308823 [DOI] [PubMed] [Google Scholar]
- 92. Alshawabkeh LI, Hu N, Carter KD, Opotowsky AR, Light‐McGroary KA, Cavanaugh JE, et al. Wait‐list outcomes for adults with congenital heart disease listed for heart transplantation in the U.S. J Am Coll Cardiol 2016;68:908–917. 10.1016/j.jacc.2016.05.082 [DOI] [PubMed] [Google Scholar]
- 93. Hermiller JB Jr, Yakubov SJ, Reardon MJ, Deeb GM, Adams DH, Afilalo J, et al. Predicting early and late mortality after transcatheter aortic valve replacement. J Am Coll Cardiol 2016;68:343–352. 10.1016/j.jacc.2016.04.057 [DOI] [PubMed] [Google Scholar]
- 94. Cowger J, Sundareswaran K, Rogers JG, Park SJ, Pagani FD, Bhat G, et al. Predicting survival in patients receiving continuous flow left ventricular assist devices: The HeartMate II risk score. J Am Coll Cardiol 2013;61:313–321. 10.1016/j.jacc.2012.09.055 [DOI] [PubMed] [Google Scholar]
- 95. Uchikawa T, Shimano M, Inden Y, Murohara T. Serum albumin levels predict clinical outcomes in chronic kidney disease (CKD) patients undergoing cardiac resynchronization therapy. Intern Med 2014;53:555–561. 10.2169/internalmedicine.53.1209 [DOI] [PubMed] [Google Scholar]
- 96. Kato TS, Cheema FH, Yang J, Kawano Y, Takayama H, Naka Y, et al. Preoperative serum albumin levels predict 1‐year postoperative survival of patients undergoing heart transplantation. Circ Heart Fail 2013;6:785–791. 10.1161/CIRCHEARTFAILURE.111.000358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Matsue Y, Kagiyama N, Yamaguchi T, Kuroda S, Okumura T, Kida K, et al. Clinical and prognostic values of ALBI score in patients with acute heart failure. Heart Lung Circ 2020;29:1328–1337. 10.1016/j.hlc.2019.12.003 [DOI] [PubMed] [Google Scholar]
- 98. Shibata N, Kondo T, Kazama S, Kimura Y, Oishi H, Arao Y, et al. Impact of predictive value of Fibrosis‐4 index in patients hospitalized for acute heart failure. Int J Cardiol 2021;324:90–95. 10.1016/j.ijcard.2020.09.056 [DOI] [PubMed] [Google Scholar]
- 99. Cleland JG, Chiswell K, Teerlink JR, Stevens S, Fiuzat M, Givertz MM, et al. Predictors of postdischarge outcomes from information acquired shortly after admission for acute heart failure: A report from the Placebo‐Controlled Randomized Study of the Selective A1 Adenosine Receptor Antagonist Rolofylline for Patients Hospitalized with Acute Decompensated Heart Failure and Volume Overload to Assess Treatment Effect on Congestion and Renal Function (PROTECT) Study. Circ Heart Fail 2014;7:76–87. 10.1161/CIRCHEARTFAILURE.113.000284 [DOI] [PubMed] [Google Scholar]
- 100. Karaman E, Ozturk Demir T, Sinem Akgun F. Efficacy of ProBNP and CRP/albumin ratio in patients with acute heart failure in the emergency department. Ann Med Res 2021;27:1241–1245. 10.5455/annalsmedres.2019.11.770 [DOI] [Google Scholar]
- 101. Lima PC, Rios DM, de Oliveira FP, Passos LR, Ribeiro LB, Serpa RG, et al. Inflammation as a prognostic marker in heart failure. Cureus 2022;14:e28605. 10.7759/cureus.28605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Sonsöz MR, Karadamar N, Yılmaz HÇ, Eroğlu Z, Şahin KK, Özateş Y, et al. C‐reactive protein to albumin ratio predicts in‐hospital mortality in patients with acute heart failure. Turk Kardiyol Dern Ars 2023;51:174–181. 10.5543/tkda.2022.27741 [DOI] [PubMed] [Google Scholar]
- 103. Cho A, Arfsten H, Goliasch G, Bartko PE, Wurm R, Strunk G, et al. The inflammation‐based modified Glasgow prognostic score is associated with survival in stable heart failure patients. ESC Heart Fail 2020;7:654–662. 10.1002/ehf2.12625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Itagaki T, Motoki H, Otagiri K, Machida K, Takeuchi T, Kanai M, et al. Inflammation‐based assessment for the risk stratification of mortality in patients with heart failure. Sci Rep 2021;11:14989. 10.1038/s41598-021-94525-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Huang R, Dai Q, Chang L, Wang Z, Chen J, Gu R, et al. The association between fibrinogen‐to‐albumin ratio (FAR) and adverse prognosis in patients with acute decompensated heart failure at different glucose metabolic states. Cardiovasc Diabetol 2022;21:241. 10.1186/s12933-022-01662-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Richter D, Guasti L, Walker D, Lambrinou E, Lionis C, Abreu A, et al. Frailty in cardiology: Definition, assessment and clinical implications for general cardiology. A consensus document of the Council for Cardiology Practice (CCP), Association for Acute Cardio Vascular Care (ACVC), Association of Cardiovascular Nursing and Allied Professions (ACNAP), European Association of Preventive Cardiology (EAPC), European Heart Rhythm Association (EHRA), Council on Valvular Heart Diseases (VHD), Council on Hypertension (CHT), Council of Cardio‐Oncology (CCO), Working Group (WG) Aorta and Peripheral Vascular Diseases, WG e‐Cardiology, WG Thrombosis, of the European Society of Cardiology, European Primary Care Cardiology Society (EPCCS). Eur J Prev Cardiol 2022;29:216–227. 10.1093/eurjpc/zwaa167 [DOI] [PubMed] [Google Scholar]
- 107. Sze S, Pellicori P, Zhang J, Weston J, Clark AL. Which frailty tool best predicts morbidity and mortality in ambulatory patients with heart failure? A prospective study. Eur Heart J Qual Care Clin Outcomes 2023;9:731–739. 10.1093/ehjqcco/qcac073 [DOI] [PubMed] [Google Scholar]
- 108. Vitale C, Jankowska E, Hill L, Piepoli M, Doehner W, Anker SD, et al. Heart Failure Association/European Society of Cardiology position paper on frailty in patients with heart failure. Eur J Heart Fail 2019;21:1299–1305. 10.1002/ejhf.1611 [DOI] [PubMed] [Google Scholar]
- 109. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J Chronic Dis 1987;40:373–383. 10.1016/0021-9681(87)90171-8 [DOI] [PubMed] [Google Scholar]
- 110. Candeloro M, Di Nisio M, Balducci M, Genova S, Valeriani E, Pierdomenico SD, et al. Prognostic nutritional index in elderly patients hospitalized for acute heart failure. ESC Heart Fail 2020;7:2479–2484. 10.1002/ehf2.12812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Ramiro‐Ortega E, Bonilla‐Palomas JL, Gámez‐López AL, Moreno‐Conde M, López‐Ibáñez MC, Alhambra‐Expósito R, et al. Nutritional intervention in acute heart failure patients with undernutrition and normalbuminemia: A subgroup analysis of PICNIC study. Clin Nutr 2018;37:1762–1764. 10.1016/j.clnu.2017.07.009 [DOI] [PubMed] [Google Scholar]
- 112. Kirchner KA, Voelker JR, Brater DC. Binding inhibitors restore furosemide potency in tubule fluid containing albumin. Kidney Int 1991;40:418–424. 10.1038/ki.1991.228 [DOI] [PubMed] [Google Scholar]
- 113. Lee EH, Kim WJ, Kim JY, Chin JH, Choi DK, Sim JY, et al. Effect of exogenous albumin on the incidence of postoperative acute kidney injury in patients undergoing off‐pump coronary artery bypass surgery with a preoperative albumin level of less than 4.0 g/dl. Anesthesiology 2016;124:1001–1011. 10.1097/ALN.0000000000001051 [DOI] [PubMed] [Google Scholar]
- 114. Macedo E, Karl B, Lee E, Mehta RL. A randomized trial of albumin infusion to prevent intradialytic hypotension in hospitalized hypoalbuminemic patients. Crit Care 2021;25:18. 10.1186/s13054-020-03441-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. O'Brien Z, Finnis M, Gallagher M, Bellomo R; RENAL Study Investigators and the Australian and New Zealand Intensive Care Clinical Trials Group . Hyperoncotic albumin solution in continuous renal replacement therapy patients. Blood Purif 2022;51:590–599. 10.1159/000517957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Caironi P, Tognoni G, Masson S, Fumagalli R, Pesenti A, Romero M, et al.; ALBIOS Study Investigators . Albumin replacement in patients with severe sepsis or septic shock. N Engl J Med 2014;370:1412–1421. 10.1056/NEJMoa1305727 [DOI] [PubMed] [Google Scholar]
- 117. Park CHL, de Almeida JP, de Oliveira GQ, Rizk SI, Fukushima JT, Nakamura RE, et al. Lactated Ringer's versus 4% albumin on lactated Ringer's in early sepsis therapy in cancer patients: A pilot single‐center randomized trial. Crit Care Med 2019;47:e798–e805. 10.1097/CCM.0000000000003900 [DOI] [PubMed] [Google Scholar]
- 118. Philips CA, Maiwall R, Sharma MK, Jindal A, Choudhury AK, Kumar G, et al. Comparison of 5% human albumin and normal saline for fluid resuscitation in sepsis induced hypotension among patients with cirrhosis (FRISC study): A randomized controlled trial. Hepatol Int 2021;15:983–994. 10.1007/s12072-021-10164-z [DOI] [PubMed] [Google Scholar]
- 119. Martin GS, Moss M, Wheeler AP, Mealer M, Morris JA, Bernard GR. A randomized, controlled trial of furosemide with or without albumin in hypoproteinemic patients with acute lung injury. Crit Care Med 2005;33:1681–1687. 10.1097/01.ccm.0000171539.47006.02 [DOI] [PubMed] [Google Scholar]
- 120. China L, Freemantle N, Forrest E, Kallis Y, Ryder SD, Wright G, et al.; ATTIRE Trial Investigators . A randomized trial of albumin infusions in hospitalized patients with cirrhosis. N Engl J Med 2021;384:808–817. 10.1056/NEJMoa2022166 [DOI] [PubMed] [Google Scholar]
- 121. Finfer S, Bellomo R, Boyce N, French J, Myburgh J, Norton R; SAFE Study Investigators . A comparison of albumin and saline for fluid resuscitation in the intensive care unit. N Engl J Med 2004;350:2247–2256. 10.1056/NEJMoa040232 [DOI] [PubMed] [Google Scholar]
- 122. Phakdeekitcharoen B, Boonyawat K. The added‐up albumin enhances the diuretic effect of furosemide in patients with hypoalbuminemic chronic kidney disease: A randomized controlled study. BMC Nephrol 2012;13:92. 10.1186/1471-2369-13-92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Charpentier J, Mira JP. Efficacy and tolerance of hyperoncotic albumin administration in septic shock patients: The EARSS study [abstract]. Intensive Care Med 2011;37:S115. [Google Scholar]
- 124. Salerno F, Navickis RJ, Wilkes MM. Albumin infusion improves outcomes of patients with spontaneous bacterial peritonitis: A meta‐analysis of randomized trials. Clin Gastroenterol Hepatol 2013;11:123–130.e1. 10.1016/j.cgh.2012.11.007 [DOI] [PubMed] [Google Scholar]
- 125. Wang L, Zhao YT. Albumin infusion in hospitalized patients with acute heart failure: A retrospective cohort study. BMC Cardiovasc Disord 2022;22:358. 10.1186/s12872-022-02797-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Bleske BE, Clark MM, Wu AH, Dorsch MP. The effect of continuous infusion loop diuretics in patients with acute decompensated heart failure with hypoalbuminemia. J Cardiovasc Pharmacol Ther 2013;18:334–337. 10.1177/1074248412474347 [DOI] [PubMed] [Google Scholar]
- 127. Makhoul N, Riad T, Friedstrom S, Zveibil FR. Frusemide in pulmonary oedema: Continuous versus intermittent. Clin Intensive Care 2011;8:273–276. 10.3109/tcic.8.6.273.276 [DOI] [Google Scholar]
- 128. Lai M, Lam JC, Radosevich JJ, Patanwala AE, Vijayakrishnan R. Levels of albumin and impact on loop diuretic and albumin co‐administration in heart failure. J Cardiovasc Pharmacol 2024;83:271–275. 10.1097/FJC.0000000000001513 [DOI] [PubMed] [Google Scholar]
- 129. Kidney Disease: Improving Global Outcomes . CKD evaluation and management. https://kdigo.org/guidelines/ckd‐evaluation‐and‐management/. Accessed 21 February 2024
- 130. Khan MS, Shahid I, Anker SD, Fonarow GC, Fudim M, Hall ME, et al. Albuminuria and heart failure: JACC state‐of‐the‐art review. J Am Coll Cardiol 2023;81:270–282. 10.1016/j.jacc.2022.10.028 [DOI] [PubMed] [Google Scholar]
- 131. Jackson CE, Solomon SD, Gerstein HC, Zetterstrand S, Olofsson B, Michelson EL, et al.; CHARM Investigators and Committees . Albuminuria in chronic heart failure: Prevalence and prognostic importance. Lancet 2009;374:543–550. 10.1016/S0140-6736(09)61378-7 [DOI] [PubMed] [Google Scholar]
- 132. Masson S, Latini R, Milani V, Moretti L, Rossi MG, Carbonieri E, et al.; GISSI‐HF Investigators . Prevalence and prognostic value of elevated urinary albumin excretion in patients with chronic heart failure: Data from the GISSI‐Heart Failure trial. Circ Heart Fail 2010;3:65–72. 10.1161/CIRCHEARTFAILURE.109.881805 [DOI] [PubMed] [Google Scholar]
- 133. Katz DH, Burns JA, Aguilar FG, Beussink L, Shah SJ. Albuminuria is independently associated with cardiac remodeling, abnormal right and left ventricular function, and worse outcomes in heart failure with preserved ejection fraction. JACC Heart Fail 2014;2:586–596. 10.1016/j.jchf.2014.05.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Miura M, Shiba N, Nochioka K, Takada T, Takahashi J, Kohno H, et al.; CHART‐2 Investigators . Urinary albumin excretion in heart failure with preserved ejection fraction: An interim analysis of the CHART 2 study. Eur J Heart Fail 2012;14:367–376. 10.1093/eurjhf/hfs001 [DOI] [PubMed] [Google Scholar]
- 135. Boorsma EM, Ter Maaten JM, Damman K, Damman K, van Essen BJ, Zannad F, et al. Albuminuria as a marker of systemic congestion in patients with heart failure. Eur Heart J 2023;44:368–380. 10.1093/eurheartj/ehac528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. de Zeeuw D, Remuzzi G, Parving HH, Keane WF, Zhang Z, Shahinfar S, et al. Albuminuria, a therapeutic target for cardiovascular protection in type 2 diabetic patients with nephropathy. Circulation 2004;110:921–927. 10.1161/01.CIR.0000139860.33974.28 [DOI] [PubMed] [Google Scholar]
- 137. Nayor M, Larson MG, Wang N, Santhanakrishnan R, Lee DS, Tsao CW, et al. The association of chronic kidney disease and microalbuminuria with heart failure with preserved vs. reduced ejection fraction. Eur J Heart Fail 2017;19:615–623. 10.1002/ejhf.778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Bahrami H, Bluemke DA, Kronmal R, Bertoni AG, Lloyd‐Jones DM, Shahar E, et al. Novel metabolic risk factors for incident heart failure and their relationship with obesity: The MESA (Multi‐Ethnic Study of Atherosclerosis) study. J Am Coll Cardiol 2008;51:1775–1783. 10.1016/j.jacc.2007.12.048 [DOI] [PubMed] [Google Scholar]
- 139. Blecker S, Matsushita K, Kottgen A, Loehr LR, Bertoni AG, Boulware LE, et al. High‐normal albuminuria and risk of heart failure in the community. Am J Kidney Dis 2011;58:47–55. 10.1053/j.ajkd.2011.02.391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Brouwers FP, de Boer RA, van der Harst P, Voors AA, Gansevoort RT, Bakker SJ, et al. Incidence and epidemiology of new onset heart failure with preserved vs. reduced ejection fraction in a community‐based cohort: 11‐year follow‐up of PREVEND. Eur Heart J 2013;34:1424–1431. 10.1093/eurheartj/eht066 [DOI] [PubMed] [Google Scholar]
- 141. Capes SE, Gerstein HC, Negassa A, Yusuf S. Enalapril prevents clinical proteinuria in diabetic patients with low ejection fraction. Diabetes Care 2000;23:377–380. 10.2337/diacare.23.3.377 [DOI] [PubMed] [Google Scholar]
- 142. Shuvy M, Zwas DR, Lotan C, Keren A, Gotsman I. Albuminuria: Associated with heart failure severity and impaired clinical outcomes. Can J Cardiol 2020;36:527–534. 10.1016/j.cjca.2019.09.001 [DOI] [PubMed] [Google Scholar]
- 143. Filippatos G, Anker SD, Agarwal R, Ruilope LM, Rossing P, Bakris GL, et al.; FIGARO‐DKD Investigators . Finerenone reduces risk of incident heart failure in patients with chronic kidney disease and type 2 diabetes: Analyses from the FIGARO‐DKD trial. Circulation 2022;145:437–447. 10.1161/CIRCULATIONAHA.121.057983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Heerspink HJL, Stefansson BV, Correa‐Rotter R, Chertow GM, Greene T, Hou FF, et al.; DAPA‐CKD Trial Committees and Investigators . Dapagliflozin in patients with chronic kidney disease. N Engl J Med 2020;383:1436–1446. 10.1056/NEJMoa2024816 [DOI] [PubMed] [Google Scholar]
- 145. Ferreira JP, Zannad F, Butler J, Filippatos G, Pocock SJ, Brueckmann M, et al. Association of empagliflozin treatment with albuminuria levels in patients with heart failure: A secondary analysis of EMPEROR‐Pooled. JAMA Cardiol 2022;7:1148–1159. 10.1001/jamacardio.2022.2924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Kalantar‐Zadeh K, Ficociello LH, Bazzanella J, Mullon C, Anger MS. Slipping through the pores: Hypoalbuminemia and albumin loss during hemodialysis. Int J Nephrol Renovasc Dis 2021;14:11–21. 10.2147/IJNRD.S291348 [DOI] [PMC free article] [PubMed] [Google Scholar]


