Abstract
Aim
Periodontitis is an inflammatory disease driven by opportunistic bacteria including Porphyromonas gingivalis and Fusobacterium nucleatum, where T‐cell and NKT‐cell responses to these bacteria in patients with periodontitis grade B or C are not fully elucidated. The objective is to determine if exaggerated proinflammatory Th‐cell responses to periodontitis‐associated bacteria, but not commensal bacteria, is a characteristic of increased periodontitis grade.
Methods
Mononuclear cells from patients with periodontitis grade C (n = 26) or grade B (n = 33) and healthy controls (HCs; n = 26) were stimulated with P. gingivalis, F. nucleatum or the commensal bacteria, Staphylococcus epidermidis and Cutibacterium acnes. Cytokine production by different T‐cell populations and FOXP3‐expression by regulatory T cells were assessed by flow cytometry.
Results
Compared to HCs, grade C patients had decreased frequencies of interleukin (IL)‐10‐producing CD4+ T cells before stimulation (p = .02) and increased frequencies of IFN‐y‐producing CD4+ T cells after stimulation with P. gingivalis (p = .0019). Grade B patients had decreased frequencies of FOXP3+ CD4+ T cells before (p = .030) before and after stimulation with anti‐CD2/anti‐CD3/anti‐CD28‐loaded beads (p = .047), P. gingivalis (p = .013) and S. epidermidis (p = .018). Clinical attachment loss correlated with the frequencies of IFN‐y‐producing Th1 cells in P. gingivalis‐ and F. nucleatum‐stimulated cultures in grade B patients (p = .023 and p = .048, respectively) and with the frequencies of Th17 cells in P. gingivalis‐stimulated cultures (p = .0062) in grade C patients. Patients with periodontitis grade C or grade B showed lower frequencies of IL‐10‐producing NKT cells than HCs in unstimulated cultures (p = .0043 and p = .027 respectively).
Conclusions
Both periodontitis groups showed decreased frequencies of immunoregulatory T‐cell and NKT cell subsets at baseline. Clinical attachment loss correlated with P. gingivalis‐induced Th17‐responses in grade C patients and with Th1‐responses in grade B patients when cells were stimulated with P. gingivalis, supporting that dysregulated pro‐inflammatory T‐cell responses to periodontitis‐associated bacteria contribute to the pathogenesis of periodontitis.
Keywords: cytokines, Fusobacterium Nucleatum, NKT cells, periodontitis, Porphyromonas gingivalis, T‐helper cells
Dysregulated pro‐inflammatory T‐cell responses to periodontitis associated bacteria contribute to the pathogenesis of periodontitis.

Clinical Relevance.
Background
Periodontitis is an inflammatory disease driven by opportunistic bacteria including Porphyromonas gingivalis and Fusobacterium nucleatum, where T‐cell and NKT‐cell responses to these bacteria in patients with periodontitis grade B or C are not fully elucidated.
Added Value of this Study
Decreased frequencies of IL‐10‐producing T cells and NKT cells were observed in the two periodontitis groups compared to the healthy controls, while in cell cultures stimulated with P. gingivalis, frequencies of CD4+ T cells producing the proinflammatory cytokines IL‐17 and IFN‐y correlated with attachment loss (AL) in patients with grade C and grade B periodontitis, respectively.
Clinical Implication
PBMC cultures from grade C patients harboring P. gingivalis in their oral cavity showed enhanced Th1‐cell and Th17‐cell responses after stimulation with this bacterium, with the frequency of Th17 cells correlating with disease severity as assessed by AL. Grade B patients showed lower frequencies of Tregs than HCs, and in this patient group, AL correlated with Th1 frequencies in cultures stimulated with P. gingivalis and F. nucleatum, respectively. Taken together, the data suggests that skewing toward pro‐inflammatory T‐cell responses to periodontitis‐associated bacteria contributes to the pathogenesis of periodontitis, which may lead to new therapeutic targets in the future.
1. INTRODUCTION
Periodontitis is a highly prevalent chronic inflammatory disease characterized by the breakdown of the tooth‐supporting tissues including alveolar bone, as a result of self‐destructive immuno‐inflammatory responses against opportunistic bacteria residing in biofilms formed on tooth surfaces. 1 , 2 CD4+ T helper (Th) cells are considered to play a pathogenic role in periodontitis, whereas regulatory T cells (Tregs) are thought to play a protective role by limiting tissue‐destructive inflammatory responses. 3 , 4
Th17 cells, in particular, have been linked directly to osteoclastogenesis and bone destruction, 5 and their signature cytokine, interleukin (IL)‐17, is elevated in the inflamed periodontium 6 , 7 , 8 , 9 , 10 and gingival crevicular fluid from patients with periodontitis. 11 The concentration of IL‐17 in the periodontium correlates with the severity of alveolar bone loss and decreases upon antibiotic treatment in mice with ligature‐induced periodontitis. 10 IL‐17 plays an important role in recruitment of neutrophils 12 , 13 that are centrally involved in the tissue destruction observed in PD. 14 , 15 Accordingly, increased IL‐17 responses by CD4+ T cells and increased numbers of neutrophils have been documented locally in periodontitis lesions. 16
The role of Th1 cells in periodontitis is controversial, and increased proportions of Th1 cells have been associated with bone breakdown in periodontitis, 17 , 18 but Th1 cells have also been shown to suppress osteoclasts and inhibit osteoclastogenesis in vitro, indicating a protective role in periodontitis. 19 , 20 IFN‐γ promotes the maturation of osteoclasts, activates macrophages, upregulates major histocompatibility complex (MHC) class II expression on antigen‐presenting cells, and activates RANKL‐ and TNF‐α‐producing T cells, 21 which likely enhances bone destruction. 21 In the periodontium, Tregs act as attenuators of inflammation. 22 , 23 , 24 , 25 , 26 , 27 Over recent years, it has been demonstrated that a high degree of plasticity exists between different CD4+ T‐cell subsets and that transdifferentiation between FOXP3+ Tregs and Th17 cells occurs. 4 , 28 , 29
Innate T cells comprise γδ T cells, NKT cells, and mucosa‐associated invariant T cells (MAIT). 30 , 31 , 32 , 33 NKT cells, MAIT and γδ T cells respond to antigens represented on CD1d, MR1, and CD1c, respectively, and recognize microbial lipids, vitamin B metabolites, and lipopeptides. CD1d‐restricted NKT cells, comprising around 0.01–1% of T cells, 33 are the only subset of innate T cells that have been investigated in PD, being more abundant in this disease than in gingivitis. 34 , 35 Moreover, they appear to play an essential role in alveolar bone destruction in mice. 36
Although infection with Porphyromonas gingivalis and Fusobacterium nucleatum is strongly associated with periodontitis, only a few studies have investigated T‐cell responses against these bacteria. In patients with grade B periodontitis, supernatants from antigen‐presenting cells stimulated with different strains of P. gingivalis induced extensive Th17 polarization and a modest increase in Th1 responses. 37 On the other hand, F. nucleatum was shown to induce Th1 responses. 38 The T‐cell and NKT‐cell responses induced by these bacteria in grade C periodontitis remain to be elucidated.
We hypothesized that exaggerated proinflammatory Th‐cell responses to periodontitis‐associated bacteria, but not commensal bacteria, is a characteristic of increased periodontitis grade. Therefore, we examined the pro‐ and anti‐inflammatory cytokine responses of T cells and NKT cells induced by two periodontitis‐associated bacteria, P. gingivalis and F. nucleatum, and two commensal bacteria, S. epidermidis and C. acnes, in patients with periodontitis, grade B, − C, and in healthy controls. Furthermore, we determined the corresponding induction of FOXP3+ Tregs.
2. METHODS
2.1. Study population
This study included 90 participants recruited from 2017 to 2018 at the Department of Odontology, Faculty of Health and Medical Sciences, University of Copenhagen, as described in three previous studies of the same cohort. 39 , 40 , 41 One participant left the study, and three samples were excluded due to technical problems. The remaining 85 participants included 26 periodontally healthy controls (HCs) (n = 26), 33 patients with periodontitis, grade B (grade B group), and 26 patients with periodontitis, grade C (grade C group). All patients were medically healthy and originally classified in accordance with the 1999 criteria for periodontal diseases defined by the World Workshop and American Academy of Periodontology, 42 but later reclassified in line with the 2017 criteria for periodontal diseases by the American Academy of Periodontology (AAP) and the European Federation of Periodontology. 2
The study was approved by the regional ethical committee (The Capital Region of Denmark, protocol no.: H‐1602473), the Danish Data Authorization (Approval no.: P‐2019‐18), and registered at clinicaltrials.gov no. NCT03225950. All participants were properly informed and gave their written consent before enrolment.
2.2. Clinical examination and collection of blood and saliva sample
Sex, age, smoking status, and medical history were registered together with probing pocket depth (PPD), bleeding on probing (BoP), plaque index (PI), and clinical attachment loss (AL). The registrations were performed at six sites per tooth (excluding 3rd molars), and six intraoral X‐rays were taken to confirm bone loss and record decayed, missing, and filled teeth (DMFT). Periodontitis was defined by: interproximal AL >1 mm and BoP on ≥2 nonadjacent teeth (excluding 3rd molars), generalized in extent. Grading was determined by AL at the most affected tooth in relation to patient age and case phenotype. Smoking was considered a grade‐modifying risk factor. 2 A total of 57 of 59 patients were categorized as stage III or IV according to interdental AL at the most affected site, tooth loss, and maximum PPD in accordance with the 2017 classification system. 2
Exclusion criteria were <20 teeth, pregnancy or breastfeeding, antibiotic treatment within 6 months immunosuppressive medication within the latest 2 weeks, or any systemic disease affecting the immune system (e.g., diabetes), cancer, and hematological diseases.
We collected 2 mL of unstimulated saliva in Oragene DNA tubes (cat.# OG‐500; DNA Genotek Inc., Ontario, Canada), 30 mL of venous blood in BD Vacutainer™ heparin plasma tubes (cat. #368480; Thermo Fisher Scientific Inc., Gothenburg, Sweden), and 4 mL of venous blood in Vacutainer Clot Activator tubes (cat. # 369032; Becton Dickinson [BD] Biosciences, Heidelberg, Germany).
2.3. Bacterial strains
Four strains of viable bacteria were prepared and used as previously described in detail. 41 In brief, purchased Porphyromonas gingivalis (W50) (State Serum Institute (SSI), Copenhagen, Denmark) and Fusobacterium nucleatum (ATCC 49256) (American Type Culture Collection, Manassas, VA) strains and Cutibacterium acnes and S. epidermidis strains, isolated from medically healthy donors and identified via eHOMD as described previously, 45 were grown in an anaerobic incubator (P. gingivalis, F. nucleatum, and C. acnes) or aerobically (S. epidermidis), for around 7 days, and appearing colonies were harvested and resuspend in sterile DPBS. C. acnes and S. epidermidis were used as negative controls since they are considered general oral commensal bacteria 44 , 45 and appear to be relatively well tolerated, as evidenced by their low‐titer persistence in blood units from donors with periodontitis. 45
2.4. Stimulation of mononuclear cells with bacteria
Peripheral blood mononuclear cells (PBMCs) were frozen, thawed, and prepared as described. 41 In short, PBMCs were purified by density gradient centrifugation for 30 min at 1000 g over Lymphoprep (cat. #1114547; Alere Technologies, Oslo, Norway), then washed three times, counted, and aliquoted in cryotubes with a freezing medium consisting of 60% RPMI 1640, 30% fetal bovine serum (FBS), and 10% dimethyl sulfoxide (DMSO) solution (Merck KGaA, Darmstadt, Germany) containing 5 million PBMCs. Thereafter, frozen using a cryogenic CoolCell container (CORNING, Corning, NY) for 24–48 h at −80°C and later stored in liquid nitrogen. Upon thawing, PBMCs were resuspended in RPMI 1640 medium (cat. #14‐490E; Lonza, Basel, Switzerland) containing 10% (v/v) normal human serum (NHS) washed, counted, and resuspended to the required concentration in the same medium. and left at 37°C for 1 h before stimulation with bacteria.
One million PBMCs were distributed into each well of a 48‐well Nunc™ – multidish flat bottom plate (cat. #161441; Thermo Fisher Scientific Inc., Gothenburg, Sweden) and left at 37°C, 5% CO2 for 1 h before stimulation with bacteria. Subsequently, cells were either left unstimulated or co‐cultured with with 104 P. gingivalis, 104 F. nucleatum, 104 S. epidermidis, and 104 C. acnes for 19 h under aerobic conditions. Although P. gingivalis, F. nucleatum, and C. acnes are anaerobic bacteria, they all possess the ability to survive in aerobic cultures. 46 , 47 , 48 Similarly, samples from 16 HCs, 18 grade B patients, and 15 grade C patients were stimulated with MACSiBead beads loaded with anti‐CD2, anti‐CD3, and anti‐CD28 antibodies (cat #130–091‐441; Miltenyi Biotec Inc, Auburn, CA), for 19 h. Brefeldin A solution (cat. #420601; Biolegend, San Diego, CA), a protein transport inhibitor for intracellular cytokine staining, was added for the last 4 h of incubation. Thereafter, the plates were centrifuged, supernatants were discarded, and the cells were resuspended and transferred to 96‐well Nunc™‐MicroWell round bottom plates (cat. #162747; Thermo Fisher Scientific Inc., Gothenburg, Sweden). Finally, the cells were centrifuged, supernatants were removed, and the plates were gently shaken and kept on ice during the staining, fixation, and permeabilization procedure.
2.5. Staining of cells for flow cytometry
Prior to staining for surface markers and intracellular markers, immunoglobulin suspension for intravenous use (IVIg) (CSL Behring AG, Bern, Switzerland) and mouse serum (Staten Serum Institute [SSI], Copenhagen, Denmark) were added to all wells to block Fc receptors. PBMCs were then stained with the surface markers live/dead fixable viability stain 780 (FVS780) (cat. #565388; Becton Dickinson [BD] Biosciences), APC‐R700 mouse anti‐human CD3 (cat. #565119; Becton Dickinson [BD] Biosciences), BUV395 mouse anti‐human CD4 (cat. #563550; Becton Dickinson [BD] Biosciences), BV510 mouse anti‐human CD8 (cat. #563256; Becton Dickinson [BD] Biosciences), BV421™ mouse anti‐human CD56 (cat. #562752; Becton Dickinson [BD] Biosciences), brilliant stain buffer (cat. #563794; Becton Dickinson [BD] Biosciences) and incubated 30 min under gentle shaking. The PBMCs were centrifuged and washed in a sterile RPMI 1640 medium containing 2% FBS (Sigma‐Aldrich, St. Louis, MO). PBMCs were resuspended in fixation and permeabilization buffer BD CytoFix/CytoPerm™ (Becton Dickinson [BD] Biosciences) and incubated for 45 min in the dark. After two washes in a 1:10 diluted permeabilization and wash buffer BD Perm/Wash™ (Becton Dickinson [BD] Biosciences), the supernatant was removed, and IVIg (Promega, Madison, WI) plus mouse serum (State Serum Institute (SSI), Copenhagen, Denmark) were added again. For intracellular staining, the following antibodies were used: BV605 mouse anti‐human interferon (IFN)‐γ (cat. #562974; Becton Dickinson [BD] Biosciences), BV711 mouse anti‐human IL‐10 (cat. #564050)‡, BV786 mouse anti‐human IL‐17A (cat. #563745; Becton Dickinson [BD] Biosciences), Alexa Flour 488 mouse anti‐human transforming growth factor (TGF)‐β1 (cat. #562545; Becton Dickinson [BD] Biosciences), BV650 mouse anti‐human tumor necrosis factor (TNF) (cat. #563418; Becton Dickinson [BD] Biosciences) and PerCP‐Cy5.5 anti‐human FOXP3 (cat. #561493; Becton Dickinson [BD] Biosciences). The antibodies were added to brilliant stain buffer (cat. #563794; Becton Dickinson [BD] Biosciences) and incubated with the cells for 45 min under slow shaking in the dark. Finally, the cell suspensions were washed twice and resuspended in the 1:10 diluted permeabilization and wash buffer. The stained PBMCs were left overnight in the dark at 4°C before flow cytometric analysis the next day.
Samples were analyzed on a FACS Fortessa (5 lasers) (Becton Dickinson [BD] Biosciences) with a blue laser (488 nm), red laser (640 nm), violet laser (405 nm), yellow/green laser (561 nm), and UV laser (355 nm). The gating was performed using FlowJo 10.6.1 (Tree Star, Ashland, OR). Conventional T cells were identified as CD3 + CD56‐ events within a morphologic lymphocyte and singlet gate. Dead cells were excluded based on viability staining with 780 (FVS780) (Becton Dickinson [BD] Biosciences). Tregs were identified as CD4 + FOXP3+ T cells within the CD3 + CD56‐ T‐cell subset. CD4+ T cells producing IFN‐γ, IL‐17A, TNF‐α, TGF‐β1, and IL‐10 were identified as CD3 + CD4+ T cells.
2.6. Measurement of P. gingivalis in saliva
DNA was extracted from saliva as previously described. 41 In brief, DNA was extracted using Maxwell 16 Cell DNA Purification Kit (cat. #AS1020; Promega, Madison, WI) and the DNA concentration was measured with a Qubit dsDNA high‐sensitivity kit (cat. #Q32854; Thermo Fischer, Roskilde, Denmark).
A probe‐based quantitative polymerase chain reaction (qPCR) assay for the detection of P. gingivalis DNA was designed with Primer‐BLAST 51 and purchased from Integrated DNA Technologies, Coralville, IA. Sequences for primers and probes are available in Table S1. Serial dilutions of a DNA oligonucleotide perfectly matching the target sequence (standard template) were included in every amplification plate, allowing to set the threshold for positivity at 40 copies/well of the standard template. Samples were run in duplicates, and randomly selected samples were run twice for confirmation of the results. Amplification reactions were performed on a Stratagene Mx3000P thermocycler (Agilent Technologies, Santa Clara, CA).
2.7. Statistics
GraphPad Prism 8 (GraphPad, San Diego, CA) and R (version 3.6.1; R Core Team (2019), Vienna, Austria) with Rstudio (Rstudio team (2019), Boston, MA) were used for the statistical analysis. Data were assessed for normal distribution and log‐transformed accordingly. Descriptive statistics in Table S1 were performed with the R package “Publish” (R package version 2020.12.23). Mixed‐effects analysis or two‐way ANOVA with uncorrected Fishers LSD test were performed to compare the three groups. Of note, between groups comparisons of the frequencies of FOXP3 + CD4+ T cells were done with Kruskal–Wallis with uncorrected Dunn's test. The correlation test was performed with Spearman's rank correlation test. p‐values <.05 were considered significant.
3. RESULTS
The study population characteristics are shown in Table S1. In accordance with the inclusion criteria, the grade C and HC groups were younger and contained fewer smokers than the grade B group. Grades B and C patients also differed in numbers of DMFT. Irrespective of periodontitis grade, more than half of the patients with periodontitis carried P. gingivalis in saliva (Table S1).
After incubation with or without bacteria, the frequency of CD3+ T cells, CD4+ T cells and CD8+ T cells did not differ significantly between HCs, grade B and grade C patients under any experimental conditions (Table S1).
3.1. CD4+ T‐cell cytokine responses
We examined the frequencies of cytokine‐producing CD4+ T cells in grade B patients, grade C patients, and HCs before and after stimulation of PBMCs with the periodontitis‐associated bacteria P. gingivalis or F. nucleatum, or with the commensal bacteria S. epidermidis or C. acnes. The flow cytometric gating strategy used for identification of CD4+ T cells is shown in Figure S1, while the gating for cytokine production is shown in Figure 1A.
FIGURE 1.

Induction of cytokine‐producing CD4+ T cells by periodontitis‐associated bacteria and commensal bacteria. Peripheral blood mononuclear cells from 26 periodontally healthy controls (HCs; white bars), 33 periodontitis grade B patients (light grey bars) and 26 periodontitis grade C patients (dark grey bars) were stimulated with P. gingivalis, F. nucleatum, S. epidermidis, C. acnes or left unstimulated for 19 h and assessed for intracellular cytokines by flow cytometry. In addition, T cells from 16 HCs, 18 grade B patients, and 16 grade C patients were stimulated with anti‐CD2/CD3/CD28 beads. (A) Gates defining IFN‐γ‐ and IL‐17‐producing and IL‐10‐producing CD4+ T cells from a representative patient with periodontitis grade C in unstimulated cultures and after stimulation with P. gingivalis. (B) Frequencies of CD4+ T cells producing IFN‐γ, IL‐17A, or IL‐10 in unstimulated samples.1 (C–E) Frequencies of CD4+ T cells producing IFN‐γ, IL‐17A, or IL‐10 in peripheral blood mononuclear cell cultures stimulated with bacteria. The corresponding frequencies observed in unstimulated cultures have been subtracted. Statistical analysis was performed on log‐transformed data. Mean baseline frequencies of cytokine‐producing cells were compared between groups with repeated measures of one‐way ANOVA (B). Comparisons between groups after stimulation with bacteria were carried out with mixed‐effects analysis with uncorrected Fishers LSD test (C–E). Bars and error bars represent means and SEMs. 1Data on Th17 cells from two HCs, five periodontitis grade B patients and two grade C patients and on S. epidermidis‐stimulated cells from one grade B and two grade C patients were missing.
The three study groups showed similar frequencies of IFN‐γ‐producing Th1 cells and IL‐17‐producing Th17 cells at baseline (Figure 1B).
On the other hand, patients with grade C periodontitis had on average, 35% lower baseline frequencies of IL‐10‐producing CD4+ T cells than HCs (p = .02) and 32% lower baseline frequencies than patients with grade B (p = .06). Of note, the proportions of IL‐10‐producing CD4+ T cells were generally one order of magnitude higher than those of Th1 cells and Th17 cells (Figure 1B).
We estimated the frequencies of bacteria‐specific T cells by subtracting the baseline frequency of cytokines‐producing CD4+ T cells from that of the corresponding bacteria‐stimulated cells. Assessed in this manner, CD4+ T cells from grade C patients showed a significantly higher degree of differentiation into Th1 cells after stimulation with P. gingivalis than CD4+ T cells from HCs or grade B patients (p = .0019, and p = .021 respectively, Figure 1C). In analogy, CD4+ T cells from grade C patients showed a higher degree of differentiation into Th17 cells than CD4+ T cells from grade B patients after stimulation with S. epidermidis (p = .046; Figure 1D). Moreover, stimulation with C. acnes caused a decrease in the frequency of IL‐10 producing CD4+ T cells in the HCs group, compared to the grade C group (p = .041; Figure 1E).
Neither the baseline frequency of TNF‐α‐ and TGF‐β‐producing CD4+ T cells nor the bacteria‐induced frequencies differed between the three study groups (data not shown).
3.2. CD4+ T‐cell cytokine responses in carriers and non‐carriers of P. gingivalis
Since the cytokine responses are likely to depend on previous exposure to the bacterium, we divided each of the three subject categories into those whose saliva contained the bacterium and those whose saliva did not, as assessed by qPCR (Figure 2). Only three out of 26 HCs (12%) were carriers of P. gingivalis, compared to 18 out of 33 grade B patients (55%) and 18 out of 26 grade C patients (69%) (Table S1).
FIGURE 2.
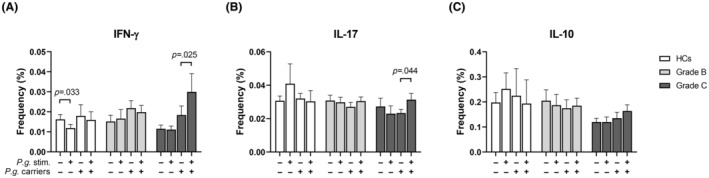
Frequencies of cytokine‐producing CD4+ T‐cell in response to P. gingivalis according to carriage of the bacterium. Peripheral blood mononuclear cells from healthy controls (HCs; 3 carriers of P. gingivalis and 23 non‐carriers) (white bars), patients with periodontitis grade B (18 carriers and 15 non‐carriers) (light grey bars) and patients with periodontitis grade C (18 carriers and 8 non‐carriers) (dark grey bars) were stimulated with P. gingivalis for 19 h or left unstimulated and assessed by flow cytometry for the frequencies of CD4+ T cells producing (A) IFN‐γ, (B) IL‐17A or (C) IL‐10.1 Mixed‐effects model with uncorrected Fisher LSD test was used for statistical analysis. Bars and error bars indicate mean and SEM. 1Data on IL‐17‐producing cells were missing for two HCs, five grade B patients and two grade C patients.
Within the HCs group, we observed a decrease in the proportion of IFN‐γ‐producing CD4+ cells after challenge with P. gingivalis in noncarriers of the bacterium (p = .033) (Figure 2A). P. gingivalis did not induce significant changes in the frequency of cytokine‐producing CD4+ T cells from grade B patients, irrespective of carrier status (Figure 2A–C). Th1 cells from grade C patients who harbored P. gingivalis increased in frequency after challenge with the bacterium (p = .025, Figure 2A), and a similar pattern was observed for Th17 cells (p = .044 Figure 2B).
Carriers of P. gingivalis did not differ from non‐carriers with respect to induction of cytokine producing CD4+ T cells by F. nucleatum, S. epidermidids, or C. acnes in any of the study groups (data not shown).
3.3. Association between CD4+ T‐cell cytokine production and clinical attachment loss
We next examined the frequencies of cytokine‐producing CD4+ T cells in relation to severity and level of periodontal destruction expressed as AL (Figure 3 and Table 1).
FIGURE 3.
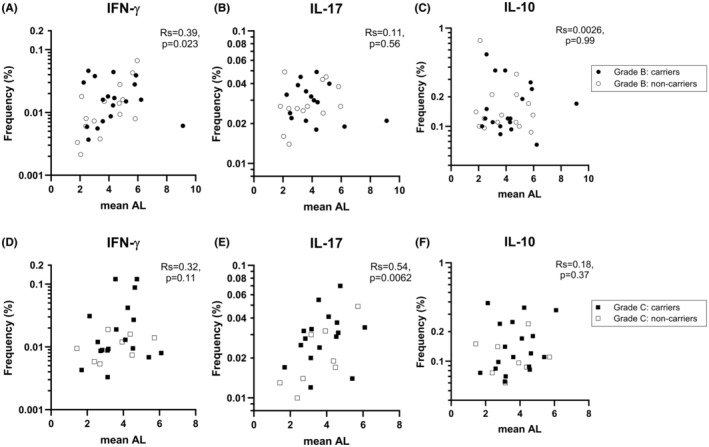
Correlation between tooth attachment loss and frequencies of CD4+ T cells producing IFN‐γ, IL‐17, or IL‐10 after stimulation with P. gingivalis. (A–C) Association within the periodontitis grade B group between mean attachment loss (AL) and the frequencies of IFN‐γ‐, IL‐17‐ or IL‐10‐producing CD4+ T cells in cultures of peripheral blood mononuclear cells stimulated with P. gingivalis. (D–F) The corresponding associations within the grade C group. Closed and open symbols indicate patients with and without P. gingivalis in saliva, respectively; i.e. carriers vs. non‐carriers. Correlation coefficients were calculated using Spearman's rank correlation test.
TABLE 1.
Correlation between clinical attachment loss and frequencies of cytokine‐producing CD4+ T‐cells in PBMC cultures unstimulated or stimulated with bacteria.
| Periodontitis grade B (n = 33) | Periodontitis grade C (n = 26) | |||||
|---|---|---|---|---|---|---|
| IFN‐γ Rs, p‐value | IL‐17 Rs, p‐value | IL‐10 Rs, p‐value | IFN‐γ Rs, p‐value | IL‐17 Rs, p‐value | IL‐10 Rs, p‐value | |
| Unstimulated | 0.19, p = .30 | −0.066, p = .74 | −0.12, p = .52 | 0.25, p = .21 | 0.16, p = .46 | −0.069, p = .74 |
| P. gingivalis | 0.39, p = .023 | 0.11, p = .56 | 0.0026, p = .99 | 0.32, p = .11 | 0.54, p = .0062 | 0.18, p = .37 |
| F. nucleatum | 0.35, p = .048 | 0.18, p = .35 | −0.019, p = .92 | 0.24, p = .24 | 0.26, p = .22 | 0.078, p = .71 |
| S. epidermidis | 0.26, p = .15 | 0.16, p = .42 | −0.036, p = .85 | 0.15, p = .47 | 0.23, p = .29 | 0.09, p = .67 |
| C. acnes | 0.33, p = .059 | 0.056, p = .78 | 0.018, p = .92 | 0.48, p = .014 | 0.12, p = .57 | 0.17, p = .41 |
Note: Correlation test was performed with Spearman's rank correlation test. p‐values <.05 were considered significant (Bold).
In the grade B group, AL correlated positively with the proportion of Th1 cells observed after stimulation with P. gingivalis (p = .023; Figure 3A), but not with the proportion of Th17 cells (Figure 3B) or IL‐10‐producing CD4+ T cells (Figure 3C). In this patient group, AL also correlated with the proportion of Th1 cells observed after stimulation with F. nucleatum (p = .048), but not after stimulation with the commensal bacteria S. epidermidis or C. acnes (Table 1).
In the grade C group, AL showed a weak tendency to correlate with the proportion Th1 cells observed after stimulation with P. gingivalis but correlated strongly with the proportion of Th17 cells under the same stimulation (p = .0062) (Figure 3C,D, respectively). In the grade C group, AL also correlated with the proportion of Th1 cells in C. acnes‐stimulated cultures (p = .0142) (Table 1).
No significant correlations were observed between AL and frequencies of CD4+ T cells producing IL‐10 in either patient group under different bacterial stimulations (Figure 3C,F and Table 1).
3.4. Induction of Tregs after challenge with bacteria
Expression of FOXP3 by Tregs was assessed by flow cytometry. Grade B patients had lower frequencies of Tregs than HCs both at baseline (p = .030) and after stimulation with anti‐CD2/anti‐CD3/anti‐CD28‐loaded beads (p = .047), as well as after stimulation with the bacteria P. gingivalis (p = .013) and S. epidermidis (p = .018) (Figure 4A). The frequencies of Tregs from grade C patients were not significantly different from those of HCs or grade B patients. Subtracting the baseline frequency from that of bead‐stimulated cells showed that grade B patients had significantly lower frequencies of inducible FOXP3 + CD4+ T cells than HCs (p = .045, Figure 4B).
FIGURE 4.
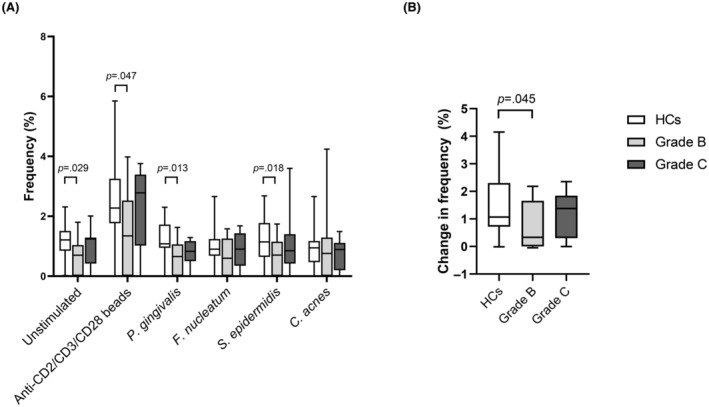
FOXP3+ expression by CD4+ T cells. (A) Peripheral blood mononuclear cells from 16 periodontally healthy controls (HCs; white bars), 18 patients with periodontitis grade B (light grey bars), and 15 patients with periodontitis grade C (dark grey bars) were stimulated with P. gingivalis, F. nucleatum, S. epidermidis, or C. acnes or left unstimulated for 19 h and assessed for FOXP3 content. Stimulation with anti‐CD2/anti‐CD3/anti‐CD28‐loaded MACSiBeads was used as positive control. Frequencies of FOXP3 + CD4+ T cells are shown as mean and SEM. (B) Net increase in the frequency of FOXP3 + CD4+ T cells after stimulation with the beads. Kruskal–Wallis with uncorrected Dunn's test was used to compare the three groups.
3.5. NKT cell responses to PD‐associated bacteria and commensal bacteria
We next focused on cytokine‐producing CD3 + CD56+ NKT cells (Figure S1A). As shown in Figure 5A, the two patient groups did not differ from HCs regarding their frequencies of IFN‐γ‐ and IL‐17‐producing NKT cells at baseline. On the other hand, both grade B and grade C patients had lower frequencies of IL‐10‐producing NKT cells than HCs at baseline (p = .028 and p = .0043, respectively).
FIGURE 5.
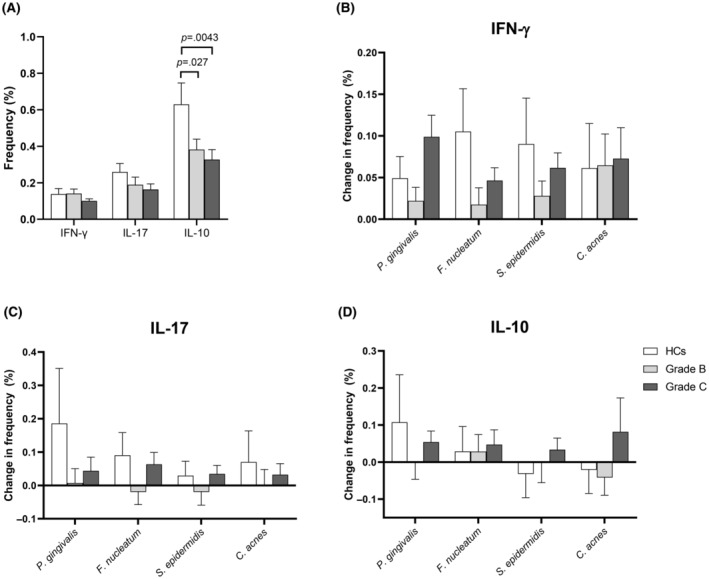
Induction of cytokine‐producing NKT cell in response to periodontitis‐associated bacteria and commensal bacteria. Peripheral blood mononuclear cells from 26 periodontally healthy controls (HCs), 33 patients with periodontitis grade B, and 26 patients with periodontitis grade C were stimulated with P. gingivalis, F. nucleatum, S. epidermidis, or C. acnes or left unstimulated for 19 h at 37°C in the presence of 10% normal human serum. The intracellular content of cytokines in CD3 + CD56+ NKT cells was assessed by flow cytometry. (A) Frequencies of CD3+ CD56+ NKT cells producing IFN‐γ, IL‐17A or IL‐10 in unstimulated samples, shown as mean and SEM. Statistical analysis was performed on log‐transformed data using repeated measures one‐way ANOVA. (B–D) Frequencies of CD3+ CD56+ T cells producing IFN‐γ, IL‐17A, or IL‐10 in cultures stimulated with bacteria after subtraction of the corresponding frequencies in unstimulated cell cultures. Comparisons between groups were carried out using mixed‐effects analysis with uncorrected Fishers LSD test (B–D). 1Data from S. epidermidis‐stimulated samples from one grade B and two grade C patients were excluded due to insufficient CD3 staining, and data on IL‐17 were missing for two HCs, five grade B patients, and two grade C patients.
Stimulation with bacteria generally induced a larger increase in the frequency of IFN‐γ‐producing and IL‐17‐producing NKT cells in the HCs group and in the group of patients with periodontitis grade C than in the group of patients with grade B periodontitis, although the specific differences between the groups were not significant (Figure 5B,C). No clear pattern was observed regarding the bacteria‐induced induction of IL‐10‐producing NKT cells (Figure 5D).
4. DISCUSSION
Th17 cells and Th1 cells have been implicated in the alveolar bone destruction observed in periodontitis, 5 , 10 , 17 , 18 while Tregs are thought to attenuate inflammation. 22 , 23 , 24 , 25 , 26 , 27 Several studies have quantified these T‐cell subpopulations in gingival tissues or peripheral blood, but to our knowledge, their antigen specificity has not been comprehensively examined previously, and it is not known whether periodontitis grades B and C differ with respect to T‐cell phenotype characteristics and excitability. Nor have NKT‐cell responses to periodontal opportunistic bacteria been examined before.
A main finding of our study was that grade C patients showed generally increased proinflammatory Th1 and Th17 responses to stimulation with bacteria compared to HCs and grade B patients. In particular, elevated Th1 responses were observed in the grade C patient group following stimulation with P. gingivalis. Notably, the increased IFN‐γ (Th1) and IL‐17 (Th17) responses observed in grade C patients were driven by the subgroup harboring P. gingivalis in the oral cavity. In particular, the frequency of Th17 cells in PBMCs stimulated with P. gingivalis correlated with mean AL in this patient group. A similar trend was observed for the corresponding frequency of Th1 cells. In addition, grade C patients exhibited reduced baseline frequencies of anti‐inflammatory, IL‐10‐producing CD4+ T cells compared to both HCs and grade B patients. Taken together, these results suggest that exaggerated proinflammatory Th‐cell responses elicited by P. gingivalis, and to a lower extent by other bacteria, are related to the stage and hence the disease severity in periodontitis grade C.
Grade B patients, as a group, showed no significant upregulation of CD4+ T cells producing IFN‐γ or IL‐17 after bacterial stimulation, but in individual patients, the frequencies of IFN‐γ‐producing CD4+ T cells in cultures stimulated with P. gingivalis or F. nucleatum correlated with AL in the grade B group, indicating a relation between Th1 responses to oral pathogenic bacteria and the severity of periodontitis in this group.
The P. gingivalis W50 strain used in this study, has been shown to induce increased mRNA expression and increased proportions of Th1 and Th17 cells in periodontal lesions and in peripheral lymph nodes, respectively, in a murine model of periodontitis, and this response resulted in increased alveolar bone loss compared to both non‐infected mice and mice infected with other P. gingivalis strains. 52 Indeed, the role of IL‐17 and IFN‐γ in inflammation‐induced bone breakdown is controversial, and both protective and destructive effects have been shown. 53 There is ample evidence to suggest a harmful role for the increased Th1‐ and Th17‐cell responses in periodontitis, but we cannot rule out that these reflect appropriate protective responses against the bacteria.
PBMCs from grade B patients contained fewer FOXP3+ Tregs than PBMCs from HCs, both at baseline and after challenge with bacteria and polyclonal activation through CD2, CD3, and CD28, suggesting an impaired general ability to downregulate T‐cell responses in this patient group. Accordingly, the frequency of Tregs is diminished in the bone resorption lesion of periodontal disease compared to healthy gingival tissue, 54 and fewer Tregs can be found in the cervical lymph nodes of mice with experimental periodontitis than in healthy mice. 4 On the other hand, increased Treg frequency has been found in the peripheral blood of patients with chronic periodontitis compared to HCs. 55 Notably, we also observed a relative lack of IL‐10‐producing cells within the NKT cell population in both grade B and grade C patients. Like Tregs, these cells, referred to as NKT10 cells, are believed to exert an immunoregulatory function. 56
Some limitations apply to this study. First, the group of patients with periodontitis grade B contained more smokers than both the HCs and the group with periodontitis grade C. The percentage of smokers among HCs (15%) and patients with grade C periodontitis (19%) were comparable. In general, grade B patients were older than the HCs and, by definition, older than the grade C patients. Although age and smoking per se did not influence the observed T‐ and NKT‐cell responses within groups, we cannot rule out that the differences between HCs and grade B patients were influenced by age and smoking. Like smoking, diabetes may potentially impact grading. However, in the present study, none of the participants were diabetic, allowing comparisons across groups. Further studies in larger cohorts and with a more appropriately matched HC population are warranted.
5. CONCLUSIONS
In summary, PBMC cultures from grade C patients harboring P. gingivalis in their oral cavity showed enhanced Th1‐cell and Th17‐cell responses after stimulation with this bacterium, with the frequency of Th17 cells correlating with disease severity as assessed by AL. Grade B patients showed lower frequencies of Tregs than HCs, and in this patient group, AL correlated with Th1 frequencies in cultures stimulated with P. gingivalis and F. nucleatum, respectively. Both patient groups showed signs of reduced IL‐10 production by CD4+ T cells and NKT cells. Taken together, the data suggests that skewing toward pro‐inflammatory T‐cell responses to periodontitis‐associated bacteria contributes to the pathogenesis of periodontitis.
AUTHOR CONTRIBUTIONS
C.D., P.H., A.K.D, and C.H.N. conceived the research question and designed the study. C.D., P.H., C.H.N., Li.M. were involved in planning and supervision of the work. A.K.D., C.D., and La.M. recruited and collected the clinical registrations and or the samples. A.K.D. performed the flow cytometry. La.M. performed the qPCR assays. Preparations of bacteria were performed by P.Ø., C.D., and A.K.D. A.K.D. and C.H.N. analyzed the data and took the lead in drafting the manuscript and designing the figures. C.D., La.M., and Li.M. aided in interpreting the results. P.H., La.M., Li.M., P.R.H., and P.Ø. provided valuable criticism to the final version of the manuscript. All authors gave their final approval and agreed to be accountable for all aspects of the work.
FUNDING INFORMATION
This study was supported by the Danish Dental Association, the International Association of Dental Research (IADR), and the Clinical Academic Group Greater Copenhagen Research Centre for Systemic Low‐grade inflammation (CAG LOGINFLAM).
CONFLICT OF INTEREST STATEMENT
All authors declare no financial conflicts of interest.
Supporting information
Figure S1.
Table S1.
ACKNOWLEDGEMENTS
We thank for the assistance and use of the flow cytometer at the Core Facility for Flow Cytometry, Faculty of Health and Medical Sciences, University of Copenhagen. The graphical abstract was partially created using images from smart.servier.com and is licensed under a Creative Commons Attribution 4.0 license (CC BY 4.0).
Danielsen AK, Massarenti L, Minculescu L, et al. Cytokine responses of CD4+ T cells and NKT cells to periodontitis‐associated bacteria in individuals with or without periodontitis. J Periodont Res. 2025;60:177‐188. doi: 10.1111/jre.13317
Anne Katrine Danielsen and Laura Massarenti contributed equally to this study.
Christian Damgaard and Claus Henrik Nielsen contributed equally to this study.
Clinical Trial Registration: This study was registered at clinicaltrials.gov no. NCT03225950.
DATA AVAILABILITY STATEMENT
The datasets are archived at Rigshospitalet, Copenhagen University Hospital in line with the Danish Data Authorization (Approval no.: P‐2019‐18). Access to all data will be shared upon reasonable request (chrd@sund.ku.dk).
REFERENCES
- 1. Page RC, Schroeder HE. Pathogenesis of inflammatory periodontal disease. A summary of current work. Lab Investig. 1976;34(3):235‐249. [PubMed] [Google Scholar]
- 2. Papapanou PN, Sanz M, Buduneli N, et al. Periodontitis: consensus report of workgroup 2 of the 2017 world workshop on the classification of periodontal and Peri‐implant diseases and conditions. J Clin Periodontol. 2018;45(Suppl 20):S162‐S170. [DOI] [PubMed] [Google Scholar]
- 3. Baker PJ, Dixon M, Evans RT, Dufour L, Johnson E, Roopenian DC. CD4(+) T cells and the proinflammatory cytokines gamma interferon and interleukin‐6 contribute to alveolar bone loss in mice. Infect Immun. 1999;67(6):2804‐2809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Alvarez C, Suliman S, Almarhoumi R, et al. Regulatory T cell phenotype and anti‐osteoclastogenic function in experimental periodontitis. Sci Rep. 2020;10(1):19018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sato K, Suematsu A, Okamoto K, et al. Th17 functions as an osteoclastogenic helper T cell subset that links T cell activation and bone destruction. J Exp Med. 2006;203(12):2673‐2682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Johnson RB, Wood N, Serio FG. Interleukin‐11 and IL‐17 and the pathogenesis of periodontal disease. J Periodontol. 2004;75(1):37‐43. [DOI] [PubMed] [Google Scholar]
- 7. Takahashi K, Azuma T, Motohira H, Kinane DF, Kitetsu S. The potential role of interleukin‐17 in the immunopathology of periodontal disease. J Clin Periodontol. 2005;32(4):369‐374. [DOI] [PubMed] [Google Scholar]
- 8. Lester SR, Bain JL, Johnson RB, Serio FG. Gingival concentrations of interleukin‐23 and ‐17 at healthy sites and at sites of clinical attachment loss. J Periodontol. 2007;78(8):1545‐1550. [DOI] [PubMed] [Google Scholar]
- 9. Oda T, Yoshie H, Yamazaki K. Porphyromonas gingivalis antigen preferentially stimulates T cells to express IL‐17 but not receptor activator of NF‐kappaB ligand in vitro. Oral Microbiol Immunol. 2003;18(1):30‐36. [DOI] [PubMed] [Google Scholar]
- 10. Dutzan N, Kajikawa T, Abusleme L, et al. A dysbiotic microbiome triggers TH17 cells to mediate oral mucosal immunopathology in mice and humans. Sci Transl Med. 2018;10(463). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Vernal R, Dutzan N, Chaparro A, Puente J, Antonieta Valenzuela M, Gamonal J. Levels of interleukin‐17 in gingival crevicular fluid and in supernatants of cellular cultures of gingival tissue from patients with chronic periodontitis. J Clin Periodontol. 2005;32(4):383‐389. [DOI] [PubMed] [Google Scholar]
- 12. Eskan MA, Jotwani R, Abe T, et al. The leukocyte integrin antagonist Del‐1 inhibits IL‐17‐mediated inflammatory bone loss. Nat Immunol. 2012;13(5):465‐473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zenobia C, Hajishengallis G. Basic biology and role of interleukin‐17 in immunity and inflammation. Periodontol 2000. 2015;69(1):142‐159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lee W, Aitken S, Sodek J, McCulloch CA. Evidence of a direct relationship between neutrophil collagenase activity and periodontal tissue destruction in vivo: role of active enzyme in human periodontitis. J Periodontal Res. 1995;30(1):23‐33. [DOI] [PubMed] [Google Scholar]
- 15. Landzberg M, Doering H, Aboodi GM, Tenenbaum HC, Glogauer M. Quantifying oral inflammatory load: oral neutrophil counts in periodontal health and disease. J Periodontal Res. 2015;50(3):330‐336. [DOI] [PubMed] [Google Scholar]
- 16. Dutzan N, Konkel JE, Greenwell‐Wild T, Moutsopoulos NM. Characterization of the human immune cell network at the gingival barrier. Mucosal Immunol. 2016;9(5):1163‐1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kawai T, Eisen‐Lev R, Seki M, Eastcott JW, Wilson ME, Taubman MA. Requirement of B7 costimulation for Th1‐mediated inflammatory bone resorption in experimental periodontal disease. J Immunol. 2000;164(4):2102‐2109. [DOI] [PubMed] [Google Scholar]
- 18. Kotake S, Nanke Y, Mogi M, et al. IFN‐gamma‐producing human T cells directly induce osteoclastogenesis from human monocytes via the expression of RANKL. Eur J Immunol. 2005;35(11):3353‐3363. [DOI] [PubMed] [Google Scholar]
- 19. Gowen M, Mundy GR. Actions of recombinant interleukin 1, interleukin 2, and interferon‐gamma on bone resorption in vitro. J Immunol. 1986;136(7):2478‐2482. [PubMed] [Google Scholar]
- 20. Horwood NJ, Elliott J, Martin TJ, Gillespie MT. IL‐12 alone and in synergy with IL‐18 inhibits osteoclast formation in vitro. J Immunol. 2001;166(8):4915‐4921. [DOI] [PubMed] [Google Scholar]
- 21. Tang M, Tian L, Luo G, Yu X. Interferon‐gamma‐mediated Osteoimmunology. Front Immunol. 2018;9:1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Nakajima T, Ueki‐Maruyama K, Oda T, et al. Regulatory T‐cells infiltrate periodontal disease tissues. J Dent Res. 2005;84(7):639‐643. [DOI] [PubMed] [Google Scholar]
- 23. Dutzan N, Gamonal J, Silva A, Sanz M, Vernal R. Over‐expression of forkhead box P3 and its association with receptor activator of nuclear factor‐kappa B ligand, interleukin (IL) ‐17, IL‐10 and transforming growth factor‐beta during the progression of chronic periodontitis. J Clin Periodontol. 2009;36(5):396‐403. [DOI] [PubMed] [Google Scholar]
- 24. Cardoso CR, Garlet GP, Moreira AP, Junior WM, Rossi MA, Silva JS. Characterization of CD4+CD25+ natural regulatory T cells in the inflammatory infiltrate of human chronic periodontitis. J Leukoc Biol. 2008;84(1):311‐318. [DOI] [PubMed] [Google Scholar]
- 25. Garlet GP, Cardoso CR, Mariano FS, et al. Regulatory T cells attenuate experimental periodontitis progression in mice. J Clin Periodontol. 2010;37(7):591‐600. [DOI] [PubMed] [Google Scholar]
- 26. Glowacki AJ, Yoshizawa S, Jhunjhunwala S, et al. Prevention of inflammation‐mediated bone loss in murine and canine periodontal disease via recruitment of regulatory lymphocytes. Proc Natl Acad Sci USA. 2013;110(46):18525‐18530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Araujo‐Pires AC, Vieira AE, Francisconi CF, et al. IL‐4/CCL22/CCR4 axis controls regulatory T‐cell migration that suppresses inflammatory bone loss in murine experimental periodontitis. J Bone Miner Res. 2015;30(3):412‐422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Komatsu N, Okamoto K, Sawa S, et al. Pathogenic conversion of Foxp3+ T cells into TH17 cells in autoimmune arthritis. Nat Med. 2014;20(1):62‐68. [DOI] [PubMed] [Google Scholar]
- 29. Gagliani N, Amezcua Vesely MC, Iseppon A, et al. Th17 cells transdifferentiate into regulatory T cells during resolution of inflammation. Nature. 2015;523(7559):221‐225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Nörenberg J, Jaksó P, Barakonyi A. Gamma/Delta T cells in the course of healthy human pregnancy: cytotoxic potential and the tendency of CD8 expression make CD56+ γδT cells a unique lymphocyte subset. Front Immunol. 2021;11:Article 596489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Van Acker HH, Capsomidis A, Smits EL, Van Tendeloo VF. CD56 in the immune system: more than a marker for cytotoxicity? Front Immunol 2017;8:Article 892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Dias J, Boulouis C, Sobkowiak MJ, et al. Factors influencing functional heterogeneity in human mucosa‐associated invariant T cells. Front Immunol. 2018;9:Article 1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Gao Y, Williams AP. Role of innate T cells in anti‐bacterial immunity. Front Immunol. 2015;6:Article 302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Yamazaki K, Ohsawa Y, Yoshie H. Elevated proportion of natural killer T cells in periodontitis lesions: a common feature of chronic inflammatory diseases. Am J Pathol. 2001;158(4):1391‐1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Amanuma R, Nakajima T, Yoshie H, Yamazaki K. Increased infiltration of CD1d and natural killer T cells in periodontal disease tissues. J Periodontal Res. 2006;41:73‐79. [DOI] [PubMed] [Google Scholar]
- 36. Aoki‐Nonaka Y, Nakajima T, Miyauchi S, et al. Natural killer T cells mediate alveolar bone resorption and a systemic inflammatory response in response to oral infection of mice with Porphyromonas gingivalis. J Periodontal Res. 2014;49:69‐76. [DOI] [PubMed] [Google Scholar]
- 37. Moutsopoulos NM, Kling HM, Angelov N, et al. Porphyromonas gingivalis promotes Th17 inducing pathways in chronic periodontitis. J Autoimmun. 2012;39(4):294‐303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Shin J, Kho SA, Choi YS, Kim YC, Rhyu IC, Choi Y. Antibody and T cell responses to Fusobacterium nucleatum and Treponema denticola in health and chronic periodontitis. PLoS One. 2013;8(1):e53703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Damgaard C, Danielsen AK, Enevold C, et al. Porphyromonas gingivalis in saliva associates with chronic and aggressive periodontitis. J Oral Microbiol. 2019;11(1):1653123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Damgaard C, Danielsen AK, Enevold C, et al. Circulating antibodies against leukotoxin a as marker of periodontitis grades B and C and oral infection with Aggregatibacter actinomycetemcomitans. J Periodontol. 2021;92(12):1795‐1804. [DOI] [PubMed] [Google Scholar]
- 41. Danielsen AK, Damgaard C, Massarenti L, et al. B‐cell cytokine responses to Porphyromonas gingivalis in periodontitis patients and healthy individuals. J Periodontol. 2023;94:997‐1007. [DOI] [PubMed] [Google Scholar]
- 42. Armitage GC. Development of a classification system for periodontal diseases and conditions. Ann Periodontol. 1999;4(1):1‐6. [DOI] [PubMed] [Google Scholar]
- 43. Damgaard C, Saekmose SG, Nilsson M, Kilian M, Nielsen CH, Holmstrup P. Periodontitis increases risk of viable bacteria in freshly drawn blood donations. Blood Transfus. 2021;19:376‐383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Segata N, Haake SK, Mannon P, et al. Composition of the adult digestive tract bacterial microbiome based on seven mouth surfaces, tonsils, throat and stool samples. Genome Biol. 2012;13(6):R42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Eren AM, Borisy GG, Huse SM, Mark Welch JL. Oligotyping analysis of the human oral microbiome. Proc Natl Acad Sci USA. 2014;111(28):E2875‐E2884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Mayslich C, Grange PA, Dupin N. Cutibacterium acnes as an opportunistic pathogen: an update of its virulence‐associated factors. Microorganisms. 2021;9(2):Article 303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Diaz PI, Zilm PS, Rogers AH. The response to oxidative stress of Fusobacterium nucleatum grown in continuous culture. FEMS Microbiol Lett. 2000;187(1):31‐34. [DOI] [PubMed] [Google Scholar]
- 48. Lewis JP, Iyer D, Anaya‐Bergman C. Adaptation of Porphyromonas gingivalis to microaerophilic conditions involves increased consumption of formate and reduced utilization of lactate. Microbiology (Reading). 2009;155(Pt 11):3758‐3774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Ye J, Coulouris G, Zaretskaya I, Cutcutache I, Rozen S, Madden TL. Primer‐BLAST: a tool to design target‐specific primers for polymerase chain reaction. BMC Bioinformatics. 2012;13:134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Monasterio G, Fernández B, Castillo F, et al. Capsular‐defective Porphyromonas gingivalis mutant strains induce less alveolar bone resorption than W50 wild‐type strain due to a decreased Th1/Th17 immune response and less osteoclast activity. J Periodontol. 2019;90:522‐534. [DOI] [PubMed] [Google Scholar]
- 51. Garlet GP. Destructive and protective roles of cytokines in periodontitis: a re‐appraisal from host defense and tissue destruction viewpoints. J Dent Res. 2010;89(12):1349‐1363. [DOI] [PubMed] [Google Scholar]
- 52. Ernst CW, Lee JE, Nakanishi T, et al. Diminished forkhead box P3/CD25 double‐positive T regulatory cells are associated with the increased nuclear factor‐kappaB ligand (RANKL+) T cells in bone resorption lesion of periodontal disease. Clin Exp Immunol. 2007;148(2):271‐280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Sabarish R, Rao SR, Lavu V. Natural T regulatory cells (n Treg) in the peripheral blood of healthy subjects and subjects with chronic periodontitis – a pilot study. J Clin Diagn Res. 2016;10(3):ZC36‐39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Sag D, Krause P, Hedrick CC, Kronenberg M, Wingender G. IL‐10‐producing NKT10 cells are a distinct regulatory invariant NKT cell subset. J Clin Invest. 2014;124(9):3725‐3740. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1.
Table S1.
Data Availability Statement
The datasets are archived at Rigshospitalet, Copenhagen University Hospital in line with the Danish Data Authorization (Approval no.: P‐2019‐18). Access to all data will be shared upon reasonable request (chrd@sund.ku.dk).


