Abstract
Introduction
Adherence to cardiovascular drug treatment can significantly benefit from a reduced pill burden, but data on this matter derived from real-life settings are currently scanty. This analysis assessed the possible changes in adherence in patients treated with rosuvastatin and ezetimibe (ROS/EZE) as free multi-pill combination who switched to ROS/EZE as single-pill combination in the setting of real clinical practice in Italy.
Methods
A retrospective analysis was conducted on the administrative databases for a catchment area of about seven million health-assisted residents. Adults receiving ROS/EZE as a single-pill combination from January 2010 to June 2020 (followed up to 2021) were identified. The date of the first prescription of single-pill combination of ROS/EZE was considered as the index date. The analysis included the users of ROS/EZE as a free combination during the year before the index date. Baseline demographic and clinical characteristics were collected during the period of data availability prior to the index date. Adherence to therapy was evaluated as proportion of days covered (PDC), namely the percentage of days during which a patient had access to medication, in the 12-month interval preceding or following the index date (PDC < 25% non-adherence; PDC = 25–75% partial adherence; PDC > 75% adherence).
Results
A total of 1219 patients (61.1% male, aged 66.2 ± 10.4 years) were included. Cardiovascular comorbidities were found in 83.3% of them, diabetes in 26.4%, and a combination of both in 16.2%. Single-pill combination of ROS/EZE was associated with a higher proportion of adherent patients compared to free-pill combination (75.2% vs 51.8%, p < 0.001).
Conclusions
This real-world analysis suggested that switching from a regimen based on separate pills to one based on a single-pill combination resulted in improved adherence to ROS/EZE therapy.
Keywords: Adherence, Rosuvastatin, Ezetimibe, Single-pill combination
Plain Language Summary
Lipid-lowering therapy to control low-density lipoprotein (LDL) cholesterol levels is essential for cardiovascular risk prevention. Successful therapy depends on the type of lipid-lowering therapy, i.e., low or high statin intensity and combination of statins with ezetimibe or proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors, and adherence to therapy, i.e., whether the patient actually takes their pills as prescribed. If there are fewer pills to be taken, this can help patients to follow their treatment. Single-pill combinations of two drugs could facilitate adherence and thus the chances of reaching the recommended lipid targets. Here, we analyzed a sample of Italian patients with dyslipidemia to examine whether the switch from a free combination of two separate pills of rosuvastatin and ezetimibe to a single-pill combination of the same drugs could improve adherence to therapy. We found that the proportion of adherent patients increased from about just over half (51.8%) to about three-fourths (75.1%) when switching from two-pill to single-pill combination of rosuvastatin and ezetimibe. These findings suggest that simplifying therapy can help improve patient adherence, which is essential for reaching lipid targets and ultimately for alleviating atherosclerotic cardiovascular disease.
Key Summary Points
| Why carry out this study? |
| Low-density lipoprotein cholesterol (LDL-C) levels represent a key driver in the development of atherosclerotic cardiovascular disease (ASCVD), thus their therapeutic control by lipid-lowering therapy is a cornerstone of cardiovascular prevention. |
| The success in achievement of lipid targets in patients at high and very high cardiovascular risk is largely dependent on the type of therapy, i.e., statin intensity and combination therapy with ezetimibe or proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors, and adherence to therapy. |
| Drug regimens based on single-pill combinations can simplify treatment and increase the chances of reaching lipid targets. The present analysis in a real-world clinical setting in Italy, evaluated the changes in adherence in patients treated with rosuvastatin and ezetimibe (ROS/EZE) as free combination of separate pills who switched to ROS/EZE as single-pill combination. |
| What was learned from the study? |
| In a dyslipidemic population previously treated with ROS/EZE as free pill combination, the proportion of adherent patients significantly increased (from 51.8% to 75.1%) after switching to single-pill combination. |
| These findings confirm that simplifying lipid-lowering therapy through single-pill combination of ROS/EZE represents a valid strategy to improve medication adherence, which is essential for increasing the likelihood of reaching the recommended LDL-C targets to improve cardiovascular outcomes, with the ultimate goal of alleviating the clinical and economic burden related to ASCVD. |
Introduction
There is a large body of evidence from genetic, epidemiologic, and clinical studies to indicate that low-density lipoprotein cholesterol (LDL-C) levels are a major culprit in the complex and multifactorial pathophysiology of atherosclerotic cardiovascular disease (ASCVD) [1, 2]. Hence, the control of LDL-C represents a cornerstone in the prevention and treatment of ASCVD [3]. Lipid-lowering therapy with statins has been shown to significantly reduce ASCVD events in several randomized trials [4]. Furthermore, high-intensity statin regimens reduce LDL-C by 50% or more, regardless of the initial baseline levels, ultimately leading to a substantially decreased risk of ASCVD compared to low-intensity statin [5]. For this reason, the latest guidelines for the management of dyslipidemia recommend the use of high-intensity statin therapy in secondary prevention for patients with established ASCVD and in primary prevention for those without ASCVD but with cardiovascular risk factors (i.e., diabetes, severe hypercholesterolemia, metabolic syndrome, chronic kidney disease) [6–9]. Also, given the results of recent placebo-controlled clinical trials [10], the 2019 guidelines of the European Society of Cardiology and European Atherosclerosis Society (ESC/EAS 2020 guidelines) [9] indicated that, compared to statin alone [11], the addition of the cholesterol absorption inhibitor ezetimibe to statin therapy can provide further protection from ASCVD risk, which is directly correlated with the incremental reduction of LDL-C. Of note, in poor responders to statin monotherapy who failed to achieve LDL-C target level, the addition of ezetimibe to statins was more effective in reducing LDL-C than doubling the dose of the statin [10]. Thus, upfront use of high intensity statin and ezetimibe combination therapy for primary and secondary prevention is recommended by different consensus documents for a faster and more effective achievement of LDL-C target levels [12–18].
Despite the growing availability of novel cardiovascular drugs, experience from clinical practice has shown that problems in medication adherence can often occur in several asymptomatic conditions, including dyslipidemia [19]. Adherence to lipid-lowering therapy is still suboptimal, particularly during primary prevention, as more than half of patients discontinue statins within 6 months from treatment initiation, resulting in higher cardiovascular morbidity and mortality [20].
Complex medication regimens, defined by the number of different pills (polypharmacy) and number of doses (multiple dosing schedules) to be taken every day, are more likely to be associated with poor adherence, and therapeutic failure [21, 22]. On the other hand, simplifying cardiovascular drug treatment by alleviating pill burden could lead to better adherence and more chances to achieve the lipid target [23, 24]. In Italy, a single-pill combination of rosuvastatin and ezetimibe (ROS/EZE) has been launched during 2018 [25].
To address the issue of lipid-lowering regimen complexity in a real-world clinical setting in Italy, this analysis evaluated the changes in adherence in patients treated with ROS/EZE as free combination who switched to ROS/EZE as single-pill combination.
Methods
Data Source and Administrative Databases
A retrospective analysis was conducted using administrative data from a sample of Local Health Units (LHU) covering approximately seven million health-assisted Italian subjects. Specifically, administrative flows contain all the information regarding the healthcare resources/services covered by the Italian National Healthcare Service (INHS), such as beneficiaries’ database for patients’ demographic data, pharmaceutical database for data on all drug dispensed and reimbursed identified by their Anatomical Therapeutic Chemical (ATC code), hospitalization database for all hospitalization data, like discharge diagnosis codes classified according to the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM), and outpatient specialist service database for information on specialist visits or diagnostic/laboratory tests. To guarantee patients’ privacy, an anonymous univocal numerical code was assigned to each subject included in the analysis, in full compliance with the European General Data Protection Regulation (GDPR) (2016/679). This code allowed the electronic linkage between all different databases. All the results of the analyses were produced and presented as aggregated summaries, which are not possible to assign, either directly or indirectly, to individual patients. In line with the pronouncement of the Data Privacy Guarantor Authority (General Authorization for personal data treatment for scientific research purposes—n.9/2014), informed consent was waived as its collection would be impossible for organizational reasons. The study was approved by the following institutional review boards (or ethics committees): Comitato Etico Regionale Liguria (protocol number 0179046/2020, approval date 14/06/2021); Comitato Etico “Lazio 2” (protocol number 0216084/2020, approval date 16/12/2020); Comitato Indipendente di Etica Medica (protocol number 48144, approval date 28/05/2021); Comitato Etico per le Sperimentazioni Cliniche (CESC) della Provincia di Vicenza (protocol number 1627, approval date 28/10/2020); Comitato etico interprovinciale Area I (protocol number 63/CE/20, approval date 3/12/2020); Comitato Etico Inter-aziendale Campania Sud (protocol number 51, approval date 02/09/2020); Comitato Etico “Lazio 1” (protocol number 1079/CE Lazio 1, approval date 23/09/2020); Comitato Etico Inter-aziendale Campania Sud (protocol number 64, approval date 03/11/2020); Comitato Etico per la Sperimentazione Clinica della provincia di Venezia e IRCCS S.Camillo (28/07/2020); Comitato Etico per le province di L’Aquila e Teramo (protocol number 11, approval date 24/03/2021); Comitato Etico Regionale Umbria (protocol number 19414/20/ON, approval date 16/09/2020); Comitato Etico “Lazio 1” (protocol number 1080/CE Lazio 1, approval date 23/09/2020). The authors affialiated with CliCon S.r.l., Società Benefit-Health were authorized for data treatment and analysis by the involved healthcare entities (Local Health Units).
Study Design and Population Selection Criteria
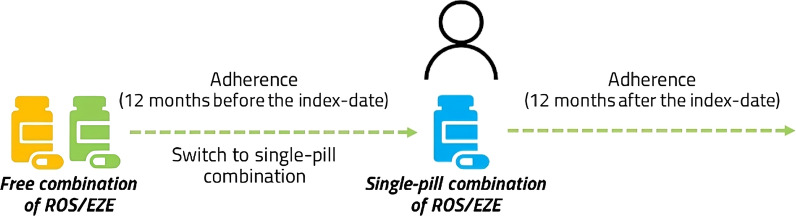
All adults prescribed ROS/EZE as a single-pill combination from January 2010 up to June 2020 (and followed up to June 2021) were identified through the ATC code C10BA06. The date of inclusion (index date) was defined as the date of the first prescription of ROS/EZE as single-pill combination. Among the eligible patients, those utilizing ROS/EZE free combination in the year preceding the index date were included in the analysis (switchers from free/multi-pill to single-pill combination). The characterization period was defined as the all available period before the index date, and the follow-up as the 1-year period after the index date (Fig. 1).
Fig. 1.
Scheme for the evaluation of drug utilization. ROS/EZE rosuvastatin and ezetimibe
Baseline Patient Characteristics
At index date, demographic characteristics (age at inclusion and gender distribution) were collected. During the characterization period (all available period), the comorbidity profile was investigated using hospitalization discharge codes (ICD-9-CM) and prescriptions (other than rosuvastatin and ezetimibe) identified by ATC codes as a proxy of diagnosis. Specifically, among concomitant conditions, the frequency of hypertension (identified by ICD-9-CM codes 401–405), chronic obstructive pulmonary disease (COPD, identified by at least two prescriptions for ATC R03), ischemic heart disease (identified by ICD-9-CM codes 410–414), heart failure (identified by ICD-9-CM code 428), cerebrovascular diseases (identified by ICD-9-CM codes 430–438), peripheral vascular diseases (identified by ICD-9-CM codes 440–442), chronic kidney disease (CKD, identified by ICD-9-CM codes 585.3–5), and diabetes (identified by ICD-9-CM code 250 or at least two prescriptions of antidiabetic drugs, ATC code A10).
The following medications (at least two prescriptions) were investigated during the characterization period: statins other than rosuvastatin (ATC C10AA), antihypertensives (ATC C03, C07, C08, C09), antithrombotic agents (ATC B01), antiarrhythmics (ATC C01B), anti-inflammatory agents (ATC M01), and antidepressants (ATC N06A).
Drug Utilization Outcomes: Treatment Adherence
Adherence was calculated as the proportion of days covered (PDC) using the following cutoffs: PDC < 25% (non-adherence); PDC = 25–75% (partial adherence); PDC > 75% (adherence).
Among the included patients (starting the single-pill combination), treatment adherence was evaluated at 12 months of follow-up (estimating the adherence to single-pill combination) and during 12 months of the characterization period (estimating the adherence to free combination) (Fig. 1).
Specifically, adherence to the free combination was assessed as the number of days covered by the separate drug ROS and EZE, assuming the consumption of two pills (one for each drug) daily during 12 months before the index date, while adherence to single-pill combination was calculated as the number of days covered by single-pill combination, assuming the consumption of one pill daily, during 12 months of follow-up.
Statistical Analysis
Continuous variables are reported as mean ± standard deviation (SD), whereas categorical variables are expressed as frequencies and percentages. For comparative analyses of percentages, chi-square test was applied and a p value < 0.05 was considered for statistical significance. All analyses were performed using Stata SE version 17.0 (StataCorp, College Station, TX, USA).
Results
Overall, 1219 patients starting ROS/EZE single-pill combination from January 2010 to June 2020 were included in the analysis. As shown in Table 1, the mean (± SD) age at initiation of ROS/EZE as single-pill combination was 66.2 (± 10.4) years, and 61.1% were male. The most frequent comorbidities were diabetes (26.4%), COPD (10.3%), and ischemic heart disease (9.0%), among others. During the all available period before ROS/EZE single-pill combination initiation, 42.9% of patients had received other statins (i.e., not rosuvastatin), 82.8% antihypertensives, 13.6% anti-inflammatory agents, and 10.0% antidepressants (Table 1). Overall, the cardiovascular comorbidities were present in 83.2% of patients, diabetes in 26.4%, and 16.2% of the included patients had a combination of cardiovascular comorbidities and diabetes (Fig. 2). Figure 3 shows the proportion of patients stratified by level of adherence, before and after the switch from ROS/EZE free combination to single-pill formulation. A significantly higher percentage of patients were adherent (PDC > 75%) to single-pill compared to free combination (75.2% vs 51.8%, p < 0.001). Consistently, the proportion of partially adherent patients (PDC = 25–75%) and non-adherent patients (PDC < 25%) was significantly lower with the single-pill combination compared to free combination (Fig. 3).
Table 1.
Baseline demographic characteristics, comorbidity profile, and presence of drug prescription during the characterization period in patients who initiated the single-pill combination of ROS/EZE
| Patients starting the single-pill combination of ROS/EZE (N = 1219) | |
|---|---|
| Age, years, mean (± SD) | 66.2 (± 10.4) |
| Male | 745 (61.1%) |
| Female | 474 (38.9%) |
| Comorbidity profile | |
| COPD | 126 (10.3%) |
| Diabetes | 322 (26.4%) |
| Ischemic heart disease | 110 (9.0%) |
| Heart failure | 13 (1.1%) |
| Cerebrovascular disease | 16 (1.3%) |
| Peripheral vascular disease | 12 (1.0%) |
| CKD disease | 4 (0.3%) |
| Co-medications | |
| Statinsa (different than rosuvastatin) | 523 (42.9%) |
| Antihypertensive treatment | 1009 (82.8%) |
| ACE inhibitors | 451 (37.0%) |
| Angiotensin II receptor blockers | 399 (32.7%) |
| Beta-blockers | 662 (54.3%) |
| Calcium channel blockers | 208 (17.1%) |
| Antithrombotic agents | 74 (6.1%) |
| Antiarrhythmics | 62 (5.1%) |
| Diureticsb | 123 (10.1%) |
| Digoxin | 10 (0.8%) |
| Ivabradine | 51 (4.2%) |
| Anti-inflammatory treatment | 166 (13.6%) |
| Antidepressants | 122 (10.0%) |
Data are presented as n (%) unless otherwise stated
ACE angiotensin-converting enzyme, CKD chronic kidney disease, COPD chronic pulmonary disease, ROS/EZE rosuvastatin and ezetimibe, SD standard deviation
aStatins taken before rosuvastatin
bDiuretics analyzed comprised thiazides, high-ceiling diuretics, spironolactone
Fig. 2.
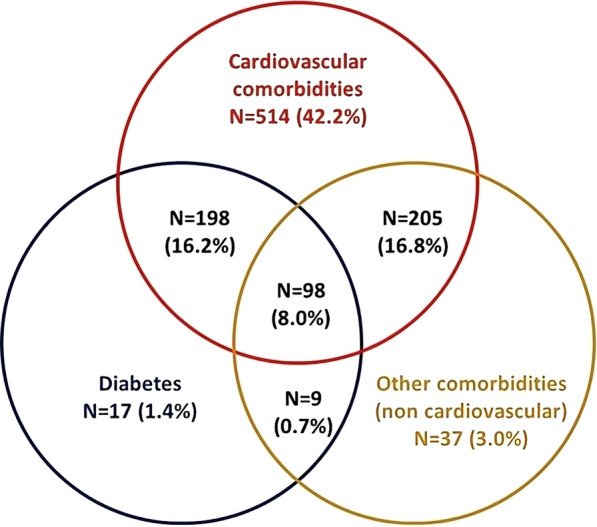
Combinations of comorbidities and co-medications of patients starting ROS/EZE as a single-pill between January 2010 and June 2020. Cardiovascular comorbidities identified by the presence of cardiovascular hospitalization (ischemic heart, heart failure, cerebrovascular disease, peripheral vascular disease); cardiovascular drugs (other antihypertensive treatment, lipid-lowering treatment). Other comorbidities identified by the presence of anti-inflammatory treatments, psychiatric conditions (hospitalization/drugs), CKD, COPD. CKD chronic kidney disease, COPD chronic pulmonary disease, ROS/EZE rosuvastatin and ezetimibe
Fig. 3.
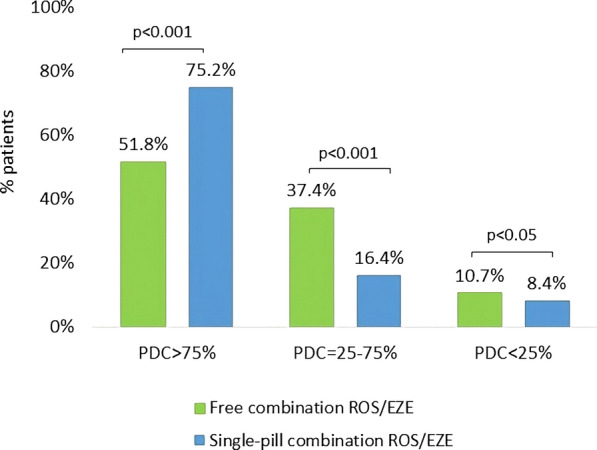
Proportion of patients by level of adherence, before and after the switch to ROS/EZE single-pill combination. PDC proportion of days covered, ROS/EZE rosuvastatin and ezetimibe
Discussion
The current observational analysis, carried out in an Italian clinical practice setting, corroborates the view that simplifying patients’ medication self-management through single-pill combination regimens can improve adherence to lipid-lowering therapy with ROS/EZE. Although the LDL-cholesterol levels were not evaluated in our population, there is extensive evidence in the literature to indicate that higher adherence can increase the chances of achieving the lipid target, ultimately resulting in better cardiovascular protection [20–22].
The patients included in this analysis revealed a very high-risk clinical profile, with more than 80% of them having ASCVD comorbidities, 26% diabetes, and 16% both conditions. Moreover, 82.8% of them received antihypertensive therapy and 42.9% had been prescribed statins other than rosuvastatin in the all available period before inclusion. These findings are unsurprising in view of the complex and multifaceted clinical picture of dyslipidemic subjects who are known to be commonly burdened by several major cardiovascular risk factors [1, 26, 27]. Consistent with our data, a previous real-world analysis conducted in Italy reported that among patients treated with the single-pill combination, 41.5% had ASCVD, 81.5% and 60.5% were under treatment with antihypertensives and antithrombotic agents, respectively, and 25.1% had a diagnosis of diabetes [28]. It is important to underline that in view of the presence of multiple comorbidities, patients on lipid-lowering therapy are often treated with polypharmacy regimens, which in turn represents a further factor leading to poor adherence [29]. Similarly, real-world data from the Japanese population showed that age ≤ 54 years and ≥ 75 years, concomitant use of certain drug classes (i.e., those for the treatment of mental disorders like depression/anxiety), and secondary prevention were correlated with worse adherence and persistence to separate pill combination of statin-ezetimibe [30]. Hence, the objective of improved drug utilization of cardiovascular therapy might greatly benefit from the support of polypills (i.e., a combination pill including aspirin, a beta-blocker, a statin, and an ACE inhibitor) in certain high-risk groups, including patients with a previous major cardiovascular event, diabetics, hypertensive, potentially delivering positive returns in terms of lower costs and improved adherence [31–33].
As extensively reported, our results provide evidence that in real-life conditions of clinical practice, adherence to lipid-lowering medication is scarce [24, 28, 30, 34], as we found that just over half (51.8%) of patients had a PDC < 75% with the free combination and this proportion rose to about three-fourths (75.1%) when switching to the single-pill ROS/EZE regimen. These findings are consistent with a previous Italian retrospective population-based study by Guglielmi et al. [35]. Those authors investigated adult patients at very high cardiovascular risk and newly prescribed statin, ezetimibe, or their combination; they reported that, despite the previous history of major cardiovascular events in almost all of them (99.9%), only 61% and 55.14% resulted adherent 3 and 6 months of follow-up, respectively [35]. Another study conducted in Finland among new users of statins between 1995 and 2005 revealed that about 56% of patients discontinued statin therapy over the decade of observation [34].
Poor adherence thus remains a challenge for clinicians, as it represents one of the main underlying reasons beyond the failure in achieving the recommended lipid targets in several patients. This discouraging scenario was recently confirmed by the SANTORINI study [36], an international multicenter observational study, reporting that a considerable proportion of subjects at high and very high cardiovascular risk on lipid-lowering therapy fail to reach the new LDL-C goals established by the 2019 ESC/EAS guidelines (< 70 and < 55 mg/dL, respectively) [9]. However, besides poor adherence, the lack of intensification of lipid-lowering therapy also contributes to low LDL-C control rates. The ACS EuroPath IV project recently investigated the 2019 ESC/EAS dyslipidemia guidelines on LDC-C goal achievement between years 2022 and 2018 in patients with acute coronary syndromes. The results highlighted that prescribing attitudes changed over time, with a rising, although still limited, trend towards more aggressive lipid-lowering therapeutic interventions: the proportion of patients receiving statin plus ezetimibe combination therapy increased in 2022 vs 2018 (34% vs 13%) and fewer patients were prescribed high (44% vs 59%) or low/moderate intensity statin monotherapy (12% vs 25%) alone [37]. Thus, taken together, the data from the present analysis and previous literature support the notion that both intensity and adherence contribute to successful lipid-lowering therapy. A Swedish study on adult patients who had experienced myocardial infarction or had undergone coronary revascularization between 2012 and 2018 and started lipid-lowering therapy showed that good adherence reduced the risk of major adverse cardiovascular events (MACE); high intensity statins were more effective over low-moderate statins in LDL-C goal attainment, while presenting a similar MACE risk [38].
The current analysis revealed that switching from free to single-pill combination is associated with a significant improvement of about 30% in the proportion of patients adherent to lipid-lowering treatment, over a 12-month observational period. These data are in line with a previous Italian study by Rea and colleagues who reported that, compared to patients taking a free combination of statin and ezetimibe, those treated with a single-pill therapy were more likely to be highly adherent, resulting in an adjusted cardiovascular risk reduction of 55% [28]. This analysis confirms the point that efficient interventions are required for patients with poor adherence, especially when dealing with clinically silent conditions like dyslipidemia or hypertension. Simplification of therapy by a reduced number of daily pills represents a valid strategy to improve medication adherence, thus leading to higher chances to achieve the therapeutic targets, improve outcomes and ultimately clinical and economic burden for chronic conditions requiring life-long therapy [22, 39].
The present analysis based on data extrapolated from administrative databases has advantages and pitfalls. The main strength lies in the availability of prescription data on a large number of patients, primarily collected for reimbursement purposes. In Italy, reimbursable drugs are classified into two classes: class A, which includes essential drugs and drugs for chronic diseases, delivered through community pharmacies; and class H, for drugs dispensed in-hospital only [40]. Class A drugs, including the lipid-lowering therapies analyzed here, are free of charge for the patients, whatever the formulation, so we can assume that all the prescriptions of ROS/EZE, either as single-pill or as free combination, were traced.
On the other hand, some limitations should be acknowledged. Administrative databases might partly lack certain clinical information on comorbidities and other confounders potentially influencing the results. Since the comorbidity profile was assessed using a proxy of diagnosis on data extrapolated from administrative flows before inclusion, information about patients’ clinical status might be incomplete. Moreover, another intrinsic flaw when using this kind of approach is due to the representativeness of the sample, which depicts only part of the national population. Here, we accessed the administrative database of healthcare bodies covering about seven million health-assisted residents (corresponding to 11% of the Italian inhabitants), and this prevents generalizability of the results to the whole country population or to other countries. Nevertheless, in an effort to increase the representativeness of our sample and the reliability of the data on a national scale, the participating units were selected from North, Central, and South Italy. Data on pharmacological treatments (medication adherence) were collected from medical prescriptions and dispensing information. Thus, the reasons behind treatment non-adherence could not be ascertained. Indeed, this is an unavoidable flaw of administrative databases that cannot supply data on non-measurable variables potentially related to adherence, including lifestyle habits, education, social status, and the patient’s attitude toward medication self-management. Moreover, the prescriptions of ROS/EZE as single-pill combination were identified using the ATC code C10BA06 which is comprehensive of all the available doses: ROS/EZE 5 mg/10 mg, ROS/EZE 10 mg/10 mg, and ROS/EZE 20 mg/10 mg, so we were not able to evaluate the possible role of ROS dose in adherence. Lastly, the present analysis did not assess the effect of adherence to lipid-lowering therapy on LDL-C blood levels and the rate of lipid target achievement.
Conclusion
This analysis carried out in the setting of Italian clinical practice provides real-world evidence on the adherence of patients to ROS/EZE in free or in single-pill combination, suggesting that switching from separate pills to single-pill combination could positively impact the level of adherence to lipid-lowering medications. This strategy may have positive implications for ASCVD prevention.
Acknowledgments
Medical Writing/Editorial Assistance
Medical writing assistance was provided by Melania Dovizio and Maria Cappuccilli of CliCon S.r.l. Società Benefit and funded by Servier, France.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICJME) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Author Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by Leopoldo Perez de Isla, Evangelos Liberopoulos, Alberto Zambon, Chiara Veronesi, and Luca Degli Esposti. The first draft of the manuscript was written by Melania Dovizio and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Funding
The study report was developed by CliCon S.r.l. Società Benefit and funded by Servier, France. The journal’s Rapid Service Fee and Open Access fee were funded by Servier, France.
Data Availability
All data used for the current study are available upon reasonable request from CliCon Società Benefit S.r.l. which is the body entitled of data treatment and analysis by Local Health Units.
Declarations
Conflict of Interest
Leopoldo Perez de Isla reports research grants, speaker fees and consultant fees from Servier, Sanofi, Amgen, Daiichi-Sankyo and Merk. Evangelos Liberopoulos reports grants from Hellenic Atherosclerosis Society; personal fees and non-financial support from Amgen, personal fees from Sanofi, personal fees from Lilly, personal fees from Bayer, personal fees from Novo Nordisk, grants and personal fees from AstraZeneca, personal fees from Boehringer Ingelheim, personal fees from Novartis, personal fees from Servier, grants and personal fees from Viatris, outside the submitted work. Alberto Zambon reports speaker fees and consultant fees from Amgen, Amarin, Amryt, AlfaSigma, Abbott, Viatris, Novartis, Sanofi, Sobi, Servier, Daiichi Sankyo. Melania Dovizio, Chiara Veronesi, Luca Degli Esposti have no competing interests to disclose.
Ethical Approval
The study was approved by the following institutional review boards (or ethics committees): Comitato Etico Regionale Liguria (protocol number 0179046/2020, approval date 14/06/2021); Comitato Etico “Lazio 2” (protocol number 0216084/2020, approval date 16/12/2020); Comitato Indipendente di Etica Medica (protocol number 48144, approval date 28/05/2021); Comitato Etico per le Sperimentazioni Cliniche (CESC) della Provincia di Vicenza (protocol number 1627, approval date 28/10/2020); Comitato etico interprovinciale Area I (protocol number 63/CE/20, approval date 3/12/2020); Comitato Etico Inter-aziendale Campania Sud (protocol number 51, approval date 02/09/2020); Comitato Etico “Lazio 1” (protocol number 1079/CE Lazio 1, approval date 23/09/2020); Comitato Etico Inter-aziendale Campania Sud (protocol number 64, approval date 03/11/2020); Comitato Etico per la Sperimentazione Clinica della provincia di Venezia e IRCCS S.Camillo (28/07/2020); Comitato Etico per le province di L’Aquila e Teramo (protocol number 11, approval date 24/03/2021); Comitato Etico Regionale Umbria (protocol number 19414/20/ON, approval date 16/09/2020); Comitato Etico “Lazio 1” (protocol number 1080/CE Lazio 1, approval date 23/09/2020). The authors affialiated with CliCon S.r.l., Società Benefit-Health were authorized for data treatment and analysis by the involved healthcare entities (Local Health Units).
Footnotes
Prior Presentation: Parts of these results were presented at the ESC Preventive Cardiology Congress 2023, Malaga (Spain), 13–15 April 2023.
References
- 1.Borén J, Chapman MJ, Krauss RM, et al. Low-density lipoproteins cause atherosclerotic cardiovascular disease: pathophysiological, genetic, and therapeutic insights: a consensus statement from the European Atherosclerosis Society Consensus Panel. Eur Heart J. 2020;41(24):2313–30. 10.1093/eurheartj/ehz962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ference BA, Ginsberg HN, Graham I, et al. Low-density lipoproteins cause atherosclerotic cardiovascular disease. 1. Evidence from genetic, epidemiologic, and clinical studies. A consensus statement from the European Atherosclerosis Society Consensus Panel. Eur Heart J. 2017;38:2459–72. 10.1093/eurheartj/ehx144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Michos ED, McEvoy JW, Blumenthal RS. Lipid management for the prevention of atherosclerotic cardiovascular disease. N Engl J Med. 2019;381(16):1557–67. 10.1056/NEJMra1806939 [DOI] [PubMed] [Google Scholar]
- 4.Fulcher J. Cholesterol Treatment Trialists’ (CTT) Collaboration Efficacy and safety of LDL-lowering therapy among men and women: meta-analysis of individual data from 174,000 participants in 27 randomised trials. Lancet. 2015;385:1397–405. 10.1016/S0140-6736(14)61368-4 [DOI] [PubMed] [Google Scholar]
- 5.Grundy SM, Feingold KR. Guidelines for the management of high blood cholesterol. [Updated 2022 May 28]. In: Feingold KR, Anawalt B, Blackman MR, et al., editors. Endotext [Internet]. South Dartmouth (MA): MDText.com; 2000. https://www.ncbi.nlm.nih.gov/books/NBK305897/. Accessed 15 Apr 2024. [PubMed]
- 6.Stone NJ, Robinson JG, Lichtenstein AH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129(25 Suppl 2):S1-45. [DOI] [PubMed] [Google Scholar]
- 7.Stone NJ, Robinson JG, Lichtenstein AH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63(25 Pt B):2889–934. 10.1016/j.jacc.2013.11.002 [DOI] [PubMed] [Google Scholar]
- 8.Grundy SM, Stone NJ, Guideline Writing Committee for the 2018 Cholesterol Guidelines. 2018 cholesterol clinical practice guidelines: synopsis of the 2018 American Heart Association/American College of Cardiology/Multisociety Cholesterol Guideline. Ann Intern Med. 2019;170(11):779–83. 10.7326/M19-0365 [DOI] [PubMed] [Google Scholar]
- 9.Mach F, Baigent C, Catapano AL, et al. 2019 ESC/EAS guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. Eur Heart J. 2020;41(1):111–88. 10.1093/eurheartj/ehz455. 10.1093/eurheartj/ehz455 [DOI] [PubMed] [Google Scholar]
- 10.Morrone D, Weintraub WS, Toth PP, et al. Lipid-altering efficacy of ezetimibe plus statin and statin monotherapy and identification of factors associated with treatment response: a pooled analysis of over 21,000 subjects from 27 clinical trials. Atherosclerosis. 2012;223:251–61. 10.1016/j.atherosclerosis.2012.02.016 [DOI] [PubMed] [Google Scholar]
- 11.Cannon CP, Blazing MA, Giugliano RP, et al. Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med. 2015;372(25):2387–97. 10.1056/NEJMoa1410489 [DOI] [PubMed] [Google Scholar]
- 12.Poli A, Catapano AL, Corsini A, et al. LDL-cholesterol control in the primary prevention of cardiovascular diseases: an expert opinion for clinicians and health professionals. Nutr Metab Cardiovasc Dis. 2023;33(2):245–57. 10.1016/j.numecd.2022.10.001 [DOI] [PubMed] [Google Scholar]
- 13.Krychtiuk KA, Ahrens I, Drexel H, et al. Acute LDL-C reduction post ACS: strike early and strike strong: from evidence to clinical practice. A clinical consensus statement of the Association for Acute CardioVascular Care (ACVC), in collaboration with the European Association of Preventive Cardiology (EAPC) and the European Society of Cardiology Working Group on Cardiovascular Pharmacotherapy. Eur Heart J Acute Cardiovasc Care. 2022;11(12):939–49. 10.1093/ehjacc/zuac123 [DOI] [PubMed] [Google Scholar]
- 14.Amarenco P, Kim JS, Labreuche J, et al. Yield of dual therapy with statin and ezetimibe in the treat stroke to target trial. Stroke. 2022;53(11):3260–7. 10.1161/STROKEAHA.122.039728 [DOI] [PubMed] [Google Scholar]
- 15.Averna M, Banach M, Bruckert E, et al. Practical guidance for combination lipid-modifying therapy in high- and very-high-risk patients: a statement from a European Atherosclerosis Society Task Force. Atherosclerosis. 2021;325:99–109. 10.1016/j.atherosclerosis.2021.03.039 [DOI] [PubMed] [Google Scholar]
- 16.Schiele F, Sabouret P, Puymirat E, et al. French expert group proposal for lipid-lowering therapy in the first 3 months after acute myocardial infarction. Panminerva Med. 2023;65(4):521–26. [DOI] [PubMed]
- 17.Schiele F, Farnier M, Krempf M, Bruckert E, Ferrières J, French Group. A consensus statement on lipid management after acute coronary syndrome. Eur Heart J Acute Cardiovasc Care. 2018;7(6):532–43. 10.1177/2048872616679791 [DOI] [PubMed] [Google Scholar]
- 18.Escobar C, Anguita M, Arrarte V, et al. Recommendations to improve lipid control. Consensus document of the Spanish Society of Cardiology. Rev Esp Cardiol (Engl Ed). 2020;73(2):161–7. 10.1016/j.recesp.2019.07.024 [DOI] [PubMed] [Google Scholar]
- 19.Schiele F, Pérez de Isla L, Arca M, Vlachopoulos C. Is it time for single-pill combinations in dyslipidemia? Am J Cardiovasc Drugs. 2022;22(3):239–49. 10.1007/s40256-021-00498-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Muir AJ, Sanders LL, Wilkinson WE, Schmader K. Reducing medication regimen complexity: a controlled trial. J Gen Intern Med. 2001;16(2):77–82. 10.1046/j.1525-1497.2001.016002077.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liberopoulos EN, Florentin M, Mikhailidis DP, Elisaf MS. Compliance with lipid-lowering therapy and its impact on cardiovascular morbidity and mortality. Expert Opin Drug Saf. 2008;7(6):717–25. 10.1517/14740330802396984 [DOI] [PubMed] [Google Scholar]
- 22.Borghi C, Jayagopal PB, Konradi A, et al. Adherence to triple single-pill combination of perindopril/indapamide/amlodipine: findings from real-world analysis in Italy. Adv Ther. 2023;40(4):1765–72. 10.1007/s12325-023-02451-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schedlbauer A, Davies P, Fahey T. Interventions to improve adherence to lipid lowering medication. Cochrane Database Syst Rev. 2010;(3):CD004371. [DOI] [PubMed]
- 24.Corrao G, Monzio Compagnoni M, Franchi M, et al. Good adherence to therapy with statins reduces the risk of adverse clinical outcomes even among very elderly. Evidence from an Italian real-life investigation. Eur J Intern Med. 2018;47:25–31. 10.1016/j.ejim.2017.09.023 [DOI] [PubMed] [Google Scholar]
- 25.Agenzia Italiana del Farmaco (AIFA) - DETERMINA 2 marzo 2018 - Riclassificazione del medicinale per uso umano «Cholecomb». https://www.gazzettaufficiale.it/atto/serie_generale/caricaDettaglioAtto/originario?atto.dataPubblicazioneGazzetta=2018-03-19&atto.codiceRedazionale=18A01848&elenco30giorni=true. Accessed 15 Apr 2024.
- 26.Kennard L, O’Shaughnessy KM. Treating hypertension in patients with medical comorbidities. BMJ. 2016;16(352): i101. 10.1136/bmj.i101 [DOI] [PubMed] [Google Scholar]
- 27.Sandström YK, Ljunggren G, Wändell P, Wahlström L, Carlsson AC. Psychiatric comorbidities in patients with hypertension—a study of registered diagnoses 2009–2013 in the total population in Stockholm County. Sweden J Hypertens. 2016;34(3):414–20. 10.1097/HJH.0000000000000824 [DOI] [PubMed] [Google Scholar]
- 28.Rea F, Savaré L, Corrao G, Mancia G. Adherence to lipid-lowering treatment by single-pill combination of statin and ezetimibe. Adv Ther. 2021;38(10):5270–85. 10.1007/s12325-021-01892-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Desai NR, Farbaniec M, Karalis DG. Nonadherence to lipid-lowering therapy and strategies to improve adherence in patients with atherosclerotic cardiovascular disease. Clin Cardiol. 2023;46(1):13–21. 10.1002/clc.23935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Umeda T, Hayashi A, Fujimoto G, Piao Y, Matsui N, Tokita S. Medication adherence/persistence and demographics of Japanese dyslipidemia patients on statin-ezetimibe as a separate pill combination lipid-lowering therapy—an observational pharmacy claims database study. Circ J. 2019;83(8):1689–97. 10.1253/circj.CJ-18-1344 [DOI] [PubMed] [Google Scholar]
- 31.Ray KK, Ference BA, Séverin T, Blom D, et al. World Heart Federation Cholesterol Roadmap 2022. Glob Heart. 2022;17(1):75. 10.5334/gh.1154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Joseph P, Roshandel G, Gao P, et al. Fixed-dose combination therapies with and without aspirin for primary prevention of cardiovascular disease: an individual participant data meta-analysis. Lancet. 2021;398(10306):1133–46. 10.1016/S0140-6736(21)01827-4 [DOI] [PubMed] [Google Scholar]
- 33.Huffman MD, Yusuf S. Polypills: essential medicines for cardiovascular disease secondary prevention? J Am Coll Cardiol. 2014;63(14):1368–70. 10.1016/j.jacc.2013.08.1665 [DOI] [PubMed] [Google Scholar]
- 34.Helin-Salmivaara A, Lavikainen P, Korhonen MJ, et al. Long-term persistence with statin therapy: a nationwide register study in Finland. Clin Ther. 2008;30(Pt 2):2228–40. 10.1016/j.clinthera.2008.12.003 [DOI] [PubMed] [Google Scholar]
- 35.Guglielmi V, Bellia A, Pecchioli S, et al. Effectiveness of adherence to lipid lowering therapy on LDL-cholesterol in patients with very high cardiovascular risk: a real-world evidence study in primary care. Atherosclerosis. 2017;263:36–41. 10.1016/j.atherosclerosis.2017.05.018 [DOI] [PubMed] [Google Scholar]
- 36.Ray KK, Haq I, Bilitou A, et al. Evaluation of contemporary treatment of high- and very high-risk patients for the prevention of cardiovascular events in Europe—methodology and rationale for the multinational observational SANTORINI study. Atheroscler Plus. 2021;13(43):24–30. 10.1016/j.athplu.2021.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Laufs U, Catapano AL, De Caterina R, et al. The effect of the 2019 ESC/EAS dyslipidaemia guidelines on low-density lipoprotein cholesterol goal achievement in patients with acute coronary syndromes: the ACS EuroPath IV project. Vascul Pharmacol. 2023;148:107141. 10.1016/j.vph.2023.107141 [DOI] [PubMed] [Google Scholar]
- 38.Mazhar F, Hjemdahl P, Clase CM, et al. Intensity of and adherence to lipid-lowering therapy as predictors of major adverse cardiovascular outcomes in patients with coronary heart disease. J Am Heart Assoc. 2022;11(14):e025813. 10.1161/JAHA.122.025813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Parati G, Kjeldsen S, Coca A, Cushman WC, Wang J. Adherence to single-pill versus free-equivalent combination therapy in hypertension: a systematic review and meta-analysis. Hypertension. 2021;77(2):692–705. 10.1161/HYPERTENSIONAHA.120.15781 [DOI] [PubMed] [Google Scholar]
- 40.Garattini L, Curto A, Padula A. Reimbursable drug classes and ceilings in Italy: why not only one? Eur J Health Econ. 2016;17(8):923–6. 10.1007/s10198-016-0808-z [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data used for the current study are available upon reasonable request from CliCon Società Benefit S.r.l. which is the body entitled of data treatment and analysis by Local Health Units.