SUMMARY
The human pathogen Streptococcus pneumoniae (Spn) encodes several cell-cell communication systems, notably multiple members of the Rgg/SHP and the Tpr/Phr families. Until now, members of these diverse communication systems were thought to work independently. Our study reveals that the ABC transporter PptAB and the transmembrane enzyme Eep act as a molecular link between Rgg/SHP and TprA/PhrA systems. We demonstrate that PptAB/Eep activates the Rgg/SHP systems and represses the TprA/PhrA system. Specifically, they regulate the respective precursor peptides (SHP and PhrA) before these leave the cell. This dual mode of action leads to temporal coordination of these systems, producing an overlap between their respective regulons during host cell infection. Thus, we have identified a single molecular mechanism that targets diverse cell-cell communication systems in Spn. Moreover, these molecular components are encoded by many gram-positive bacteria, suggesting that this mechanism may be broadly conserved.
Graphical Abstract
In brief
Mueller Brown et al. show that diverse cell-cell communication systems in the human pathogen Streptococcus pneumoniae are coordinated by the PptAB/Eep transporter-protease pair. They demonstrate that PptAB/Eep targets the precursor form of several signaling peptides, thereby controlling peptide signaling in producing cells without interfering with signals received from neighboring cells.
INTRODUCTION
Bacterial populations respond to fluctuations in their environment through the release of signaling molecules, a process referred to as quorum sensing (QS).1 In gram-positive bacteria, these signaling molecules are typically peptides, which bind to extracellular or intracellular receptors, triggering gene expression changes and ultimately phenotypic responses.2,3 Many gram-positive species encode multiple cell-cell communication systems in their genomes, suggesting that these are likely coordinated to optimize phenotypic responses. Understanding this coordination is the focus of this study.
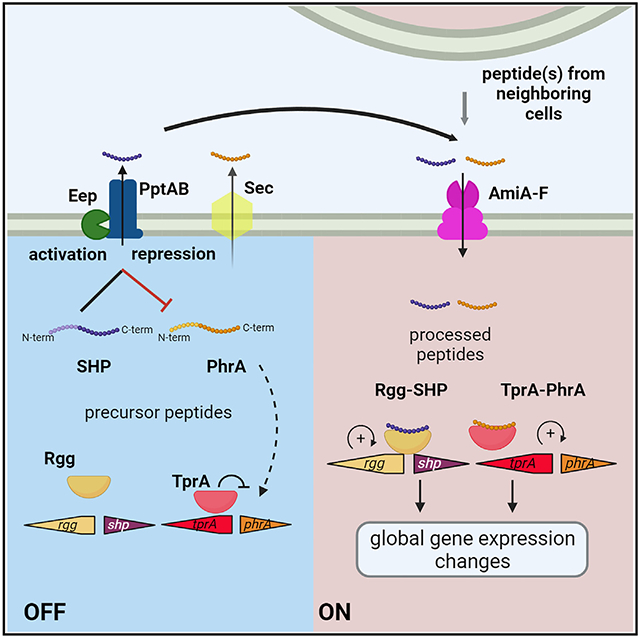
Extensive studies in Enterococci, Streptococci, and Bacilli have characterized cell-cell communication systems within the Rgg/SHP family and the PlcR/PapR family (also known as Tpr/Phr), subsets of the expansive RRNPPA (Rap, Rgg, NprR, PlcR, PrgX and AimR)-type quorum-sensing proteins.2-6 The SHP (small hydrophobic peptide) and Phr (phosphatase regulator) peptides undergo a maturation process involving export, enzymatic modifications, and subsequent import into producing and neighboring cells wherein the processed peptides bind to cytosolic Rgg or Tpr, respectively. Export mechanisms encompass the Sec system for Phr and the ABC (ATP synthase-binding cassette) transporter PptAB for SHPs.2,7 Enzymatic modifications are facilitated by proteases, most notably the transmembrane enzyme Eep for SHPs. Finally, import for both SHPs and Phr is mediated by the oligopeptide permease (Opp; also referred to as Ami).2,8,9 These peptide-processing components are broadly distributed and well conserved across species encoding these signaling systems.2,3
The human pathobiont Streptococcus pneumoniae (Spn) is a common colonizer of the nasopharynx. Spn can also disseminate into other body sites causing mild to severe disease, including otitis media, pneumonia, sepsis, and meningitis.10 A recent large-scale epidemiological study on 33 bacterial pathogens, acquired from over 10 million people across 204 countries, concluded that Spn was the leading cause of death in children younger than 5 years of age. Further, when considering overall death rates, Spn placed third, with an estimated 829,000 annual deaths.11 To sense and respond to the diverse and fluctuating environment in the host, Spn utilizes multiple cell-cell communication systems.12 For example, it encodes the Rgg/SHP and Tpr/Phr signaling systems, which contribute to Spn colonization and virulence.12 Nutrients in the host environment largely contribute to activation and/or inhibition of these systems and their respective regulatory networks.13-22 Moreover, emerging evidence highlights that the Rgg/SHP systems comprise a larger network with shared key nodes.18,20,23 While the Rgg/SHP and Tpr/Phr systems have been studied independently, little is known regarding their coordination within the cell.
In this study, we uncovered a molecular process that facilitates the coordination of the Rgg/SHP and TprA/PhrA systems in Spn. Specifically, our findings demonstrate a dual function of the transporter PptAB and the transmembrane enzyme Eep. They not only activate the Rgg/SHP systems but also repress the TprA/PhrA system. This bimodal action leads to temporal coordination of these systems and their respective regulons both during infection of lung epithelial cells in vitro and in a murine model of pneumonia in vivo. Moreover, the coordination is mediated through conserved N-terminal domains on the precursor peptides, ensuring that PptAB and Eep act exclusively on precursor peptides produced intracellularly, rather than on processed peptides (C-terminal ends) present in the extracellular environment. Thus, this study demonstrates that PptAB and Eep govern the production of signaling molecules within a population without impeding the cell’s ability to sense and respond to peptides already present in the environment.
RESULTS
PptAB, Eep, and AmiA-F activate the Rgg/SHP systems in Spn
A central molecule in this study is PptAB, an ABC-type transporter that acts as an exporter of SHPs in several streptococcal species such as S. pyogenes, S. mutans, S. agalactiae, and S. thermophilus.7,24,25 Once outside the cell, the SHPs are imported via the oligopeptide transporter AmiA-F (Figure 1A), where they bind their respective intracellular Rgg-type transcriptional regulators. In Spn, four Rgg/SHP systems (namely RtgR/RtgS1, Rgg/SHP144, Rgg/SHP939, and Rgg/SHP1518) have been characterized,18-20 yet only the SHP from the RtgR/RtgS1 system has been shown to be transported by PptAB and AmiA-F.19 Our first objective was to establish whether PptAB and AmiA-F mediate the transport of multiple Spn SHPs.
Figure 1. The transporters PptAB and AmiA-F and the protease Eep are required for activation of Rgg/SHP systems in Spn strain D39.
(A) Schematic displaying SHP processing and signaling for selected Rgg/SHP systems in Spn strain D39* (*Rgg/SHP939 is not active in the conditions tested, Figures S1B, S1E, and S1F), which has been described in other streptococcal species.2,3 The model displays the precursor SHP being exported via the transporter PptAB and processed via the membrane protease Eep. The processed SHP enters the cells via the transporter AmiA-F and binds its cognate Rgg regulator to activate gene expression of adjacent genes and in the genome (indicated by “+”). Gene sizes are not to scale. Image created with BioRender.com.
(B) WT and peptide maturation molecule mutants (ΔpptAB, Δeep, ΔamiA-F) were grown in CDM-glucose (55 mM) until early log phase (OD600 = 0.3), and gene expression of target genes that are regulated by the described Rgg/SHP systems was measured via RT-qPCR. Gene expression is plotted relative to WT.
(C) Plot of Pshp144:lux reporter over time in diverse backgrounds for cells grown in CDM-glucose (55 mM) in the presence of 1 μM final concentration (f.c.) synthetic SHP144-C12 peptide (EWVIVIPFLTNL).
(D) To indirectly quantify SHP144 export, we used the Pshp144:lux reporter strain lacking endogenous shp144. The WT, ΔpptAB, or ΔamiA-F strains were grown in CDM-glucose (55 mM) and mixed with the reporter at a 1 to 1 ratio, and activity of the reporter was measured at the indicated time points. All experiments were carried out in triplicate, graphs depict values with standard error of the mean (SEM), and statistical analysis was performed using two-way ANOVA for multiple comparisons (*p < 0.05, ****p < 0.0001; ns, not significant).
To evaluate the roles of PptAB and AmiA-F in SHP transport, we utilized Spn strain D39, which encodes all four Rgg/SHP systems (Figure S1A), and tested whether deletions of these transporters influenced signaling of Rgg/SHPs. One common feature of the Spn Rgg/SHP systems is the positive regulation of the loci located immediately downstream in the genome (Figures 1A and S1A).17-21 Thus, we selected a set of these genes as markers for Rgg/SHP activation, after validating that they are upregulated by their neighboring Rgg in an SHP-dependent manner (Figures S1A and S1B). We collected samples for RT-qPCR analysis from cultures of wild-type (WT) and transporter deletion mutants grown in chemically defined medium (CDM)-glucose (55 mM). While the Rgg/SHP939 system remained inactive in all conditions tested, preventing us from investigating the role of the transporters for SHP939 (Figures S1B, S1E, and S1F), we found that deletion of either pptAB or amiA-F resulted in a significant decrease in gene expression of selected genes (rtgA [~250-fold], vpoB1 [~15-fold], and SPD1517 [~12-fold]) for the other three Rgg/SHP systems (Figure 1B, blue and pink bars). These differences in gene expression are not a result of differing growth rates between WT and mutants (Figures S1C and S1D) and are thus in agreement with a critical role of these transporters in SHP activation.
To further distinguish between the import and the export of SHPs, we focused on the Rgg/SHP144 system and employed a bioluminescent promoter reporter for the autoinducing SHP144 (Pshp144:lux). To differentiate between importer and exporter, we added synthetic SHP144 (the C-terminal 12 residues [SHP144-C12])20,22 to the extracellular environment. This rescued signaling in the ΔpptAB strains to WT levels (Figure 1C, blue and black lines) yet failed to induce signaling in the ΔamiA-F strain, consistent with the role of the latter as an importer (Figure 1C, pink line). Next, to verify that PptAB acts as an SHP144 exporter, the reporter strain Pshp144:luxΔshp144 was co-cultured in a 1:1 ratio with WT, ΔpptAB, or ΔamiA-F strains (Figure 1D). As expected, the reporter displayed high activation of the shp144 promoter when co-cultured with WT. When co-cultured with the ΔamiA-F mutant, the reporter was activated, albeit at lower levels compared to WT, suggesting that the failure to import SHP144 in the ΔamiA-F mutant results in reduced overall SHP144 levels in the supernatant. Finally, when the reporter was co-cultured with the ΔpptAB strain, we did not detect any signal implicating PptAB as the SHP144 exporter. These data indicate that, akin to findings in other streptococcal species, PptAB acts as an SHP exporter and AmiA-F as an SHP importer in Spn.
In some streptococcal species, SHP activation requires not only export through the PptAB transporter but also processing by the associated membrane zinc protease Eep.8,9,24-26 The role of Eep relative to SHPs in Spn has not yet been studied. Thus, we explored its function in SHP activation by assessing transcriptional changes in the eep deletion mutant (Δeep). We found that the RT-qPCR data in Δeep mirrored those of the transporter deletion mutants, suggesting that the Spn Eep protease functions in processing of SHPs (Figures 1A and 1B, green bars).
PptAB, AmiA-F, and Eep all display high conservation in the Spn pangenome regarding both prevalence in the population (over 99.8%) and sequence similarity as determined by in silico analysis (Table S2). Thus, we propose that the respective roles of Eep in SHP processing, PptAB in SHP export, and AmiA-F in SHP import are conserved across Spn strains.
PptAB and Eep repress the TprA/PhrA system in Spn
To investigate whether the role of PptAB extends beyond SHPs, we conducted an RNA-sequencing (RNA-seq) analysis comparing gene expression profiles between WT and ΔpptAB strains. Strains were grown in CDM-glucose, where we had already observed differences in SHP activation (Figure 1B; Table S3). Removal of pptAB led to significant decreases in the expression of the loci adjacent to RtgR/RtgS, Rgg/SHP144, and Rgg/SHP1518, validating the role of PptAB in promoting Rgg/SHP signaling. Furthermore, the RNA-seq data revealed differential gene expression of a second Spn cell-cell communication system: genes encoding the TprA/PhrA system and its adjacent operon were highly upregulated (~5- to 32-fold) when pptAB was deleted (Table S3). This led us to investigate the role of PptAB in regulation of the TprA/PhrA cell-cell communication signaling system.
The TprA/PhrA system has an architecture similar to that of Rgg/SHPs, being composed of an intracellular transcriptional regulator (TprA) and a cognate autoinducing peptide (PhrA). PhrA is predicted to be secreted via the Sec pathway and imported into producing or neighboring cells via the importer AmiA-F.12 Once imported into the cytosol, PhrA binds TprA, releasing repression of the regulon, including a predicted lantibiotic biosynthesis operon immediately downstream (including the lanM gene) (Figure 2A).13 In contrast to the observed downregulation of Rgg/SHP-regulated loci, the TprA/PhrA system and its adjacent locus were highly upregulated in the absence of the PptAB transporter (Table S3). Thus, we hypothesized that PptAB has a dual mode of action: activation of Rgg/SHP systems on one hand and repression of the TprA/PhrA system on the other.
Figure 2. The Spn TprA/PhrA locus is negatively regulated by PptAB and Eep in an Rgg/SHP-system-independent manner.
(A) Schematic displaying PhrA peptide processing and signaling in Spn strain D39. The precursor form is predicted to be exported via the Sec pathway. The processed form is then imported via AmiA-F into producing or neighboring cells, where it binds TprA, its cognate regulator, releasing repression and activating expression of genes in the adjacent locus (predicted lantibiotic gene cluster) and elsewhere in the genome.13 Gene size is not to scale. Image created with BioRender.com.
(B) PphrA:lux reporter in WT and mutant backgrounds was grown in CDM-glucose (55 mM), and bioluminescence was quantified at indicated optical densities.
(C) WT and mutants (ΔtprA, ΔpptAB, Δeep, Δrgg/shpall, and Δrgg/shpallΔpptAB) were grown in CDM-glucose (55 mM) until early log phase (OD600 = 0.3), and gene expression of the regulator tprA, the signaling peptide phrA, and the target gene lanM was measured via RT-qPCR. Gene expression is plotted relative to WT (dotted line).
(D) PphrA:lux double-deletion mutant reporter strains were grown in CDM-glucose (55 mM), and bioluminescence was quantified at the indicated optical densities. Dotted line indicates highest bioluminescence levels for ΔpptAB.
(E) Schematic showing inhibition of TprA/PhrA, but not TprB/PhrB or TprC/PhrC, via PptAB that is independent of the Rgg/SHP144 system in Spn strain TIGR4.
(F) TIGR4 WT and mutants were grown in CDM-glucose (55 mM) until early log phase (OD600 = 0.3), and gene expression of tpr regulators and phr peptides was quantified via RT-qPCR relative to WT. All experiments were carried out in triplicate, graphs depict values with standard error of the mean (SEM), and statistical analysis was performed using two-way ANOVA for multiple comparisons (**p < 0.01, ***p < 0.001, ****p < 0.0001).
We used a genetic approach to confirm whether PptAB inhibits the TprA/PhrA system. In our first set of experiments, we quantified the activity of the phrA promoter (PphrA:lux) using a bioluminescent reporter of the promoter (Figure 2B, black and orange lines). We noted that the PphrA:lux reporter induction levels are comparable between WT and ΔphrA, suggesting the system is not naturally active in this condition and revealing a phrA-independent basal level of promoter activity. In accordance, the RNA-seq data showed no transcriptional changes between WT and ΔphrA (except for phrA), indicating that TprA/PhrA signaling activity is low in CDM-glucose (Table S3). As a positive control, we deleted the negative transcriptional regulator tprA. Consistent with the current model, this triggered an increase in PphrA activity compared to the WT (Figure 2B, red line, 3-fold increase) and set the limit for PphrA activity levels in the absence of the transcriptional inhibitor. Strikingly, deletion of pptAB mimicked the PphrA activity observed for ΔtprA and went back to WT levels in the pptAB-complemented strain, confirming that this transporter is involved in phrA inhibition (Figure 2B, blue and blue dotted lines).
Now that we implicated PptAB in TprA/PhrA regulation and uncovered that both PptAB and the Eep influence SHP activation, we next tested Eep’s role in inhibiting the TprA/PhrA system. Indeed, we found that the PphrA activity in the Δeep strain mimicked levels observed for ΔtprA and ΔpptAB but could be reversed in the eep-complemented strain (Figure 2B, green and green dotted line). Furthermore, the double-deletion strain ΔpptABΔeep was indistinguishable from the single mutants, suggesting that both PptAB and Eep are required for phrA repression (Figure 2B, gray line). As a secondary validation, RT-qPCR analyses of genes in the TprA/PhrA locus (tprA, phrA, and lanM) demonstrated that gene products from pptAB and eep negatively regulate TprA/PhrA signaling (Figure 2C). Specifically, levels of tprA, phrA, and lanM were up to 40-fold higher in ΔpptAB or Δeep compared to WT (Figure 2C, blue and green bars). Previous work has shown that expression of PphrA is higher in CDM-galactose than in CDM-glucose,13-15 which we also observed in our assays (Figure S2A). Importantly, in both glucose and galactose conditions the deletions of ΔtprA, ΔpptAB, or Δeep significantly released inhibition of the TprA/PhrA system as measured both with the PphrA:lux reporter (Figure S2A) and by RT-qPCR (Figure S2B). Thus, these data demonstrate that PptAB and Eep negatively regulate the TprA/PhrA system in Spn.
One molecular mechanism that may explain how deletions of either PptAB or Eep abrogate repression of the TprA/PhrA system is that this inhibition is mediated by Rgg/SHP systems or their effectors. To investigate this hypothesis, we tested mutants with individual SHP deletions and a quadruple Rgg/SHP mutant and used RT-qPCR to measure gene expression of tprA, phrA, and lanM (Figures S2C, S2D, and 2C, respectively). We did not observe gene expression differences in the TprA/PhrA locus, neither in single shp deletion mutants (Figures S2C and S2D) nor in a quadruple mutant Δrgg/shpall (Figure 2C, dark purple bars), suggesting Rgg/SHP systems are not inhibitors of the TprA/PhrA system. Moreover, when all Rgg/SHP systems were deleted in a ΔpptAB background (Δrgg/shpallΔpptAB), the expression levels were comparable to those of the ΔpptAB single mutant (Figure 2C, light purple bars). These findings indicate that the PptAB-mediated repression of the TprA/PhrA system is Rgg/SHP independent.
We next asked whether PptAB and Eep inhibit the phrA promoter activity through effects on the PhrA peptide. To this end, we generated a strain where phrA is deleted in the background of the ΔpptAB or Δeep strain. Indeed, we found that these double mutants displayed significantly reduced PphrA activity compared to their respective single mutants (Figure 2D). These data suggest that PptAB and Eep negatively regulate the TprA/PhrA system in a phrA-dependent manner.
To explore whether the negative regulation of theTpr/Phr family via PptAB is conserved across diverse Spn strains, we conducted RT-qPCR analysis in Spn strain TIGR4. This model strain encodes three Phr paralogs (tprA/phrA, tprB/phrB, and tprC/phrC) and a single Rgg/SHP system (rgg/shp144) (Figure 2E). Given the functional overlap between PptAB and Eep, the subsequent work was performed only with ΔpptAB strains. As observed in strain D39, deletion of pptAB in TIGR4 resulted in significantly increased expression of tprA and phrA, with phrA levels comparable to those in ΔtprA (Figure 2F). Furthermore, the process was also phrA dependent, as tprA expression decreased in ΔpptABΔphrA compared to the ΔpptAB strain. Importantly, deletion of rgg/shp144 did not induce significant gene expression changes of tprA or phrA. Together, these data suggest that PptAB represses TprA/PhrA signaling in a manner that is phrA dependent and rgg/shp independent in strain TIGR4, which is consistent with conservation of this mechanism across multiple Spn strains. In contrast to the tprA/phrA system, levels of the paralogs tprB/phrB and tprC/phrC were unchanged between WT and ΔpptAB strains, indicating that the negative effect is specific to the TprA/PhrA system (Figure 2F). While the PhrB and PhrC peptides share about 80% sequence identity, they share less than 25% sequence identity with the PhrA peptide (Figure S3D). Given that the PptAB effect is phrA dependent, the low similarity between paralogs may explain the specificity of PptAB inhibition to the TprA/PhrA system.
In summary, we have captured a novel function for PptAB and Eep: the transporter and the protease are negative regulators of the TprA/PhrA system. This mechanism of action requires phrA and is independent of rgg/shp systems. Finally, we observed that this function extends to an additional and phylogenetically distinct Spn strain, suggesting that this mechanism may be broadly relevant across Spn strains.
PptAB represses the TprA/PhrA system by targeting the intracellular precursor PhrA
Our data demonstrate that PptAB inhibition is phrA dependent; our next goal was to establish where in the PhrA processing cycle this effect takes place. First, we tested whether secreted PhrA is influenced by PptAB. To indirectly quantify secreted PhrA levels, we made use of a reporter strain encoding PphrA:lux in a background where phrA is deleted (PphrA:luxΔphrA) and co-cultured this reporter 1:1 with WT, ΔtprA, or ΔpptAB strains (Figure 3A). When the reporter strain was in co-culture with the ΔpptAB strain, it displayed significantly higher signal induction over time compared to WT, suggesting that in WT cells, PptAB decreases levels of secreted PhrA in the supernatant. Surprisingly, when the reporter was co-cultured with the ΔtprA strain, it did not show significantly increased induction, even though phrA is transcribed at high levels in this background (Figures 2C and 3A). These results suggest that PptAB inhibits the secretion of PhrA in the ΔtprA strain. Together, these data are consistent with PptAB inhibition acting on the precursor form of the PhrA peptide before it is secreted from the cell.
Figure 3. PptAB negatively regulates the PhrA precursor peptide.
(A) To indirectly quantify PhrA secretion, we used the PphrA:lux reporter strain lacking endogenousphrA. The WT, ΔtprA, or ΔpptAB strains were grown in CDM-glucose (55 mM) and mixed with the reporter at a 1 to 1 ratio, and activity of the reporter was measured at the indicated time points.
(B) Strains containing the PphrA:lux reporter were grown in CDM-glucose (55 mM), and promoter activity was measured at the indicated optical densities. Dotted line indicates the highest bioluminescent signal in the ΔpptAB strain.
(C) Schematic displaying PptAB-mediated repression of precursor PhrA (created with BioRender.com).
(D) Representative confocal microscopy images of WT and mutant strains that were incubated with 1 μM synthetic PhrA-C10 that has an N-terminally conjugated Alexa 546 fluorophore. Images were taken at 100× original magnification and processed with ImageJ as described in the STAR Methods. Scale bar, 10 μm.
(E) Quantification of phrA promoter activity as measured with the PphrA:lux reporter in WT (i), ΔpptAB (ii), ΔamiA-F (iii), and ΔpptABΔamiA-F (iv) incubated with 1 μM synthetic PhrA-C10 (blue line) or Alexa 546PhrA-C10 (red line). Measurements were taken at indicated time points and compared to DMSO control (black line). All experiments were carried out in triplicate, graphs depict values with standard error of the mean (SEM), and statistical analysis was performed using two-way ANOVA for multiple comparisons (*p < 0.05, ****p < 0.0001).
Secreted PhrA is imported into the cell via AmiA-F.13 Thus, a strain lacking amiA-F (ΔamiA-F) provides a tool to evaluate whether PptAB acts on imported PhrA. If AmiA-F is not required, it would confirm that PptAB acts on the precursor PhrA. We made use of the PphrA:lux reporter and assessed induction in ΔamiA-F and ΔpptABΔamiA-F backgrounds. As expected, the ΔamiA-F strain displayed low activity for the phrA promoter. In contrast, the ΔpptABΔamiA-F strain displayed an intermediate phenotype, where the bioluminescent signal was lower than in the ΔpptAB strain but significantly higher than in ΔamiA-F (Figure 3B). We also captured this intermediate phenotype when comparing TprA/PhrA system gene expression changes in the double mutant, WT, and ΔpptAB using RT-qPCR (Figure S2E) and RNA-seq (Table S3). These data suggest that TprA/PhrA signaling occurs even without import of the processed PhrA through AmiA-F and that this TprA/PhrA signaling is repressed by PptAB. Thus, we hypothesized that the precursor form of PhrA may be the target of PptAB regulation and that the precursor peptide, when not repressed by PptAB, can bind the TprA regulator and activate the system without going through a maturation process. Indeed, this intermediate phenotype was dependent on functional PhrA, as neither the triple mutant ΔpptABΔa-miA-FΔphrA nor the ΔpptABΔamiA-F-phrAΔaa47-56 mutant with deletion of the previously reported functional C-terminal end (amino acids 47–56)13 showed signals higher than ΔamiA-F (Figure 3B). Ultimately, these data indicate that both the precursor and the processed forms of PhrA can activate the TprA/PhrA system and that PptAB represses this process through inhibition of precursor PhrA.
This model required us to rule out the possibility of an alternative PhrA importer, which could explain the intermediate phenotype in the ΔpptABΔamiA-Fstrain. To visualize PhrA import, we applied a fluorescently tagged synthetic PhrA peptide (Alexa 546PhrA-C10) that comprises the 10 C-terminal amino acids, which robustly activate the TprA/PhrA system.13 Utilizing confocal microscopy, we found that WT and ΔpptAB strains imported the labeled peptide (Figure 3D, first and second images), while strains where the amiA-F transporter was deleted (ΔamiA-F and ΔpptABΔamiA-F) did not (Figure 3D, third and fourth images). To confirm that phrA promoter activity corresponded to the PhrA internalization data, we assessed promoter activity using the PphrA:lux reporter. As expected, the WT displayed high signal induction levels upon addition of labeled or unlabeled extracellular PhrA-C10 (Figure 3Ei). In contrast, induction of the reporter was absent in the ΔamiA-F background (Figure 3Eiii), highlighting its critical role as a PhrA importer. The activity in the ΔpptAB strain was already saturated and did not respond to synthetic peptide (Figure 3Eii). Finally, strain ΔpptABΔamiA-Fdisplayed the intermediate phenotype, confirming that in the absence of PptAB the PhrA signal is elevated independent of PhrA import, albeit not to the same degree as the peptide-treated WT or ΔpptAB (Figure 3Eiv). Together, these data demonstrate that amiA-F is required for PhrA import and support a model where PptAB acts on the precursor form of PhrA.
PptAB’s dual mode of action provides coordination of Spn cell-cell communication systems in the presence of host cells
Our data suggest a mechanism through which PptAB activates the Rgg/SHP systems and represses the TprA/PhrA system in Spn. Thus, we hypothesized that PptAB may serve to coordinate these peptide-mediated signaling systems. To investigate this hypothesis, we first had to identify a condition where these signaling systems are naturally induced. Previous work from our group and others suggested that these systems are induced in vivo and in the presence of host cells.15,17,18,20,27 Thus, we tested induction of phrA when cells were exposed to A549 lung epithelial cells.
In our initial experimental design, we spun down Spn cells onto a monolayer of host cells in the presence of cell-culture medium. Using RT-qPCR, we observed a moderate increase in phrA expression (2- to 5-fold) in WT cells after exposure to host cells in medium compared to medium alone (Figure S2F). The ΔpptAB strain, however, maintained significantly higher induction of the TprA/PhrA system with or without host cells (Figure S2F), suggesting that PptAB inhibition of the TprA/PhrA system was not fully released under these conditions in the WT. We postulated that the presence of cell culture medium allowed continued PptAB repression. Thus, we altered our experimental design, accordingly, replacing the medium with only PBS and allowing Spn infection of host cells to proceed for up to 4 h. In this condition, WT cells displayed far greater induction of genes associated with TprA/PhrA and the three Rgg/SHP systems (RtgR/RtgS1, Rgg/SHP144, and Rgg/SHP1518) (Figures 4A-4F, black lines). We concluded that these cell-cell communication systems are upregulated when bacterial cells are exposed to host cells in the absence of cell culture medium.
Figure 4. PptAB coordinates Spn cell-cell communication systems in the presence of host cells.
(A–F) WT, ΔpptAB, ΔamiA-F, and WT + synthetic peptides (cocktail of 1 μM each PhrA-C10, RtgS1-C10, SHP144-C12, and SHP1518-C12) cells were incubated with A549 lung epithelial cells (MOI 1:100), and gene expression changes of relevant genes were measured. Fold changes are plotted relative to the WT 0 h time point, and mutant/treated WT were compared to WT at individual time points.
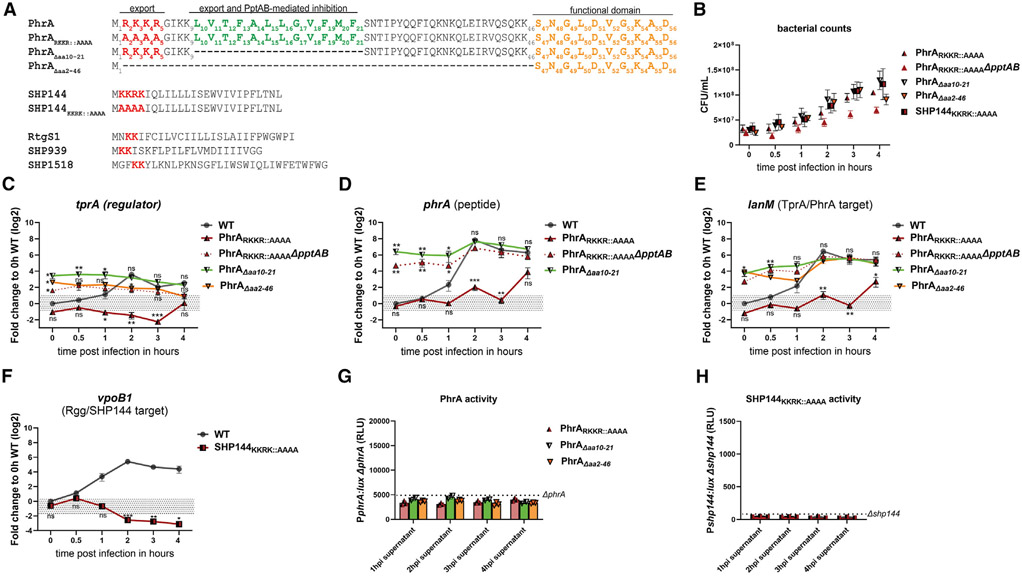
(G) Bacterial counts at indicated time points measured as colony forming units (CFU) per milliliter.
(H and I) Bioluminescent peptide reporters lacking endogenous peptide were treated with cell-free supernatants from WT, ΔpptAB, ΔamiA-F, or peptide-deletion mutants harvested at multiple time points during A549 cell infection. Final time point for PhrA activity is displayed at 3.5 h post treatment (H) and final time point for SHP144 is displayed at 1 h post treatment (I). All experiments were carried out in triplicate, graphs depict values with standard error of the mean (SEM), and statistical analysis was performed using two-way ANOVA for multiple comparisons (*p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001).
We used this setup to measure the dynamics of gene expression for TprA/PhrA and Rgg/SHP signaling as well as the role of PptAB in this process. Importantly, pptAB gene expression remained stable throughout infection (Figure S2G). At early time points (0.5 and 1 h post infection [hpi]), the expression of tprA, phrA, and lanM was significantly higher in the ΔpptAB cells than in WT cells. By 2 hpi the expression of these genes was the same between WT and ΔpptAB, demonstrating that PptAB inhibition of the TprA/PhrA system is relieved by 2 hpi when bacteria are cultured in the presence of epithelial cells (Figures 4A-4C, black and blue lines). These findings indicate that PptAB dampens the onset of PhrA signaling—serving to control the timing of TprA/PhrA system activation. Moreover, in the ΔpptAB strain, the expression of Rgg/SHP system targets was significantly lower than in WT throughout the time course (Figures 4D-4F, blue lines) but WT cells reached maximal expression by 2 hpi (Figures 4D-4F, black lines), similar to the TprA/PhrA system. Together these findings indicate that PptAB delays the onset of TprA/PhrA signaling while activating the Rgg/SHP systems. This regulation ultimately serves to temporally coordinate Spn cell-cell communication.
To directly assess the importance of signaling peptide cycling in these autoinducing systems for signal amplification across the population, we compared dynamics between the WT and the ΔamiA-F strain, since AmiA-F serves as a peptide importer for both systems. Relative to WT, expression of target genes in this mutant was lower across all systems (Figures 4A-4F, pink lines) starting as early as 0.5 hpi (Figure 4D). The WT and mutants maintained comparable bacterial counts throughout the experiment, demonstrating that our results are not confounded by growth differences (Figure 4G). This result emphasizes the importance of processing, circulation, and amplification of signaling peptides for population-level responses.
Finally, we tested whether these signaling dynamics are reflected in the amount of signaling peptide cycling in the environment. To this end, we assessed the activity of the cell-free supernatants from WT, ΔpptAB, ΔamiA-F, and ΔphrA or Δshp144 strains grown in the same conditions. We utilized reporter strains lacking their respective endogenous signaling peptide (PphrA:luxΔphrA and Pshp144:luxΔshp144) and indirectly measured activity of PhrA and SHP144 in these supernatants (Figures 4H and 4I). Analysis of TprA/PhrA signaling revealed that treatment with cell-free supernatants from WT infections displayed increased PphrA signal over time compared to the ΔphrA control and ΔamiA-F, whereas supernatants from ΔpptAB infections consistently induced higher PphrA signal. These findings are consistent with PptAB’s role in repression of PhrA secretion. Analysis of Rgg/SHP144 signaling revealed that cell-free supernatants from WT infections induced Pshp144 signaling, while the signals from ΔpptAB and Δshp144 supernatants were both low, consistent with PptAB’s required role as the SHP144 exporter. Thus, this quantification of activity of secreted peptides is aligned with the transcriptional measurements. Overall, our analyses of transcriptional changes and functional secreted peptides revealed that PptAB temporally coordinates two families of Spn cell-cell communication systems in the presence of host cells.
PptAB does not interfere with Spn’s ability to sense extracellular peptide signals
In our model, PptAB acts on the precursor forms of PhrA and SHPs such that this transporter should not affect the cell’s ability to respond to exogenous peptide. To test this deduction, we incubated WT cells with a cocktail of synthetic peptides containing each of the systems studied (PhrA-C10, RtgS1-C10, SHP144-C12, and SHP1518-C12) during the time course of host cell infection. Indeed, all four systems were induced early during infection (Figures 4A-4F, black dotted lines). Thus, we conclude that, while PptAB mediates coordination of Spn cell-cell communication systems by regulating release of signaling peptides, it does not interfere with a cell’s ability to sense or respond to peptides in the extracellular environment.
The N-terminal domain of PhrA mediates its export as well as its interaction with PptAB
To provide a deeper mechanistic understanding of how PhrA is influenced by PptAB, we set out to identify the domain(s) in PhrA required for PptAB-mediated inhibition. To this end, we searched for motifs shared among PhrA and SHPs. An amino acid sequence alignment of the D39 peptide alleles revealed a series of up to four positively charged residues (lysine [K] and arginine [R]) at the N-terminal start site of all these sequences (red in Figure 5A). In addition, PhrA contains a stretch of hydrophobic residues in a region upstream of its C-terminal functional domain, which is notable given that sequences of hydrophobic residues are the hallmark of SHPs (green in Figures 5A and S3A). To investigate whether these motifs are conserved across PhrA alleles, we carried out an in silico analysis utilizing a dataset of 7,548 well-curated Spn genomes (Figure S3B). First, we established that PhrA is encoded within 78% of the genomes and can be organized into five common alleles and other rare alleles (less than 3% of the set for any one of the alleles) (Figure S3C). The alignment revealed that the N-terminal positively charged residues and the hydrophobic stretch are both highly conserved across PhrA alleles. Having identified two regions with similarities to SHPs as well as conservation across PhrA alleles, we set out to test whether PptAB’s ability to influence peptide signaling required these regions.
Figure 5. The N-terminal domain of PhrA mediates its secretion as well as its interaction with PptAB.
(A) Alignment of D39 signaling peptides PhrA (and respective mutants), RtgS1, SHP144 (and mutant), SHP939, and SHP1518, highlighting conserved N-terminal positively charged residues (red) as well as a hydrophobic stretch in PhrA (green) and C-terminal functional domain (orange).
(B) Bacterial counts at indicated time points measured as colony forming units (CFU) per milliliter.
(C–F) PhrARKKR::AAAA, PhrARKKR::AAAAΔpptAB, PhrAΔaa10-21, PhrAΔaa2-46, and SHP144KKRK::AAAA cells were incubated with A549 epithelial cells (MOI 1:100), and gene expression changes of tprA, phrA (except PhrAΔaa2-46, sequence too short), lanM, and vpoB1 were measured. Fold changes are plotted relative to the WT 0 h time point, and mutants were compared to WT (replotted from Figure 4) at individual time points.
(G and H) Bioluminescent reporters for secreted PhrA at 3.5 h post treatment (G) and SHP144 at 1 h post treatment (H) were treated with cell-free supernatants of respective mutants from selected time points of A549 infection. Dotted lines indicate maximum levels from supernatants of cells lacking endogenous peptide. All experiments were carried out in triplicate, graphs depict values with standard error of the mean (SEM), and statistical analysis was performed using two-way ANOVA for multiple comparisons (*p < 0.05, **p < 0.01, ***p < 0.001).
We started by testing the role of the positively charged residues in targeting SHPs and PhrA to PptAB. To test the former, we modified the native shp144 to replace these residues with alanines (termed SHP144KKRK::AAAA, for details see Star Methods). Analysis with RT-qPCR revealed that this mutated SHP144 failed to induce the Rgg/SHP144 system as quantified by the absence of vpoB1 expression during infection of A549 cells (Figure 5F). Moreover, cell-free supernatants from strain SHP144KKRK::AAAA did not promote induction of Pshp144 in a reporter strain (Pshp144:lux Δshp144) (Figure 5H). We conclude that the region encoding the N-terminal positively charged residues is required for presence of an active SHP144 in the supernatant. Next, we examined whether these residues contribute to PptAB-mediated inhibition of PhrA. To this end, we modified the native phrA to replace these positively charged residues with alanines (termed PhrARKKR:AAAA). The cell-free supernatants from this PhrA mutant failed to induce the PphrA:lux ΔphrA reporter (Figure 5G), indicating that PhrARKKR:AAAA is not exported. Further, this mutant displayed reduced expression of tprA, phrA, and lanM compared to WT during infection of host cells (Figures 5C-5E). To confirm that this was due to PptAB inhibition, we tested the activity of PhrARKKR::AAAA in a background where pptAB is deleted. This strain showed high expression of the TprA/PhrA system (Figures 5C-5E) at early time points, indicating that this PhrA variant is recognized and inhibited by PptAB. Overall, these data suggest that the N-terminal positively charged residues are required for SHP144 export and PhrA secretion but not PptAB-mediated inhibition of PhrA.
To determine whether the N terminus is involved in PptAB inhibition, we removed the entire N-terminal region in the endogenous phrA (PhrAΔaa2-46). While we found that this mutation prevents export (Figure 5G), it led to significantly higher expression of tprA and lanM during early infection time points compared to WT, suggesting a lack of the PptAB-interacting domain (Figures 5A-5C and 5E). Thus, we returned to the second N-terminal feature conserved between SHPs and PhrA (hydrophobic stretch) and tested whether its deletion prevents PptAB inhibition of PhrA. The cell-free supernatant from this strain (PhrAΔaa10-21) lacked the ability to induce a reporter strain, indicating that the hydrophobic stretch is required for PhrA secretion (Figure 5G). Further, and in contrast to the PhrARKKR::AAAA, the RT-qPCR showed that this PhrA variant induced the TprA/PhrA system early in infection similar in magnitude to the ΔpptAB (Figures 5C-5E), suggesting that this hydrophobic region is required to interface with PptAB. Together, these data indicate that the N terminus of PhrA is required both for secretion by the Sec pathway and for interaction with PptAB.
PptAB is a virulence factor that mediates coordination of cell-cell communication systems in vivo
Spn cell-cell communication systems play a critical role in colonization, survival, and translocation from the lungs to the blood.15,17,18,20 To specifically investigate the role of PptAB in virulence, we compared WT and ΔphrA to ΔpptAB and ΔpptABΔphrA mutants that have not yet been investigated in vivo. We infected mice intranasally to induce pneumonia and tested translocation of Spn to the blood at 16 and 24 hpi. Compared to the WT, the ΔpptABΔphrA double mutant displayed significantly lower colony-forming units at 16 hpi (1.7 vs. 4.1 log10 CFU/mL), whereas all the mutants (ΔpptABΔphrA, ΔpptAB, and ΔphrA) displayed lower bacterial counts at 24 hpi (Figures 6A and 6B). These results confirmed that phrA is a virulence determinant and demonstrate that PptAB contributes to Spn virulence.
Figure 6. PptAB is a virulence factor that coordinates Spn cell-cell communication signals in vivo.
(A and B) Mice (n = 10 per group) were infected with 4 × 106 CFU of WT, ΔphrA, ΔpptAB, or ΔpptABΔphrA using the pneumonia model. Bacterial counts were determined in the blood at 16 and 24 h post infection. Individual data points and median are plotted. Differences in CFU counts were calculated using one-way ANOVA (*p < 0.05, **p < 0.01, ****p < 0.0001).
(C and D) Utilizing the pneumonia mouse model of infection, PphrA::CBRluc (C) and Pshp144::CBRluc (D) reporters in WT and ΔpptAB backgrounds were imaged at 2 h post infection as shown on the right (n = 10 animals per group, except for the mouse marked with “+” in (D), which was excluded due to lack of signal). Quantifications on the left were carried out using luminescence over lung normalized to CFU/mg of lung tissue. Differences between strains were determined using the Mann-Whitney test (*p < 0.05).
Finally, we aimed to capture PptAB’s role in temporal coordination of cell-cell communication systems during infection in vivo by monitoring the timing of phrA and shp144 promoter induction. We developed luciferase reporter strains for PphrA and Pshp144 in either WT or ΔpptAB background and monitored luminescence in a mouse model of pneumonia.28 Prior to in vivo experiments, the reporters were tested in vitro. The Pshp144 induction was abrogated and the PphrA induction was amplified in the ΔpptAB compared to WT, in accordance with our other in vitro data (Figure S2H). Our model predicts that deletion of pptAB will lead to early induction of PphrA. To test this, reporter activity was quantified at 2 hpi using an IVIS imager, and the signal was normalized to CFU/mg of lung tissue (Figures 6C and 6D). The 2 h time point is the earliest time point allowed in our animal protocol and likely mitigates clearance and/or attenuation phenotypes. Consistent with the model, we observed significantly higher PphrA induction in ΔpptAB than in WT (Figure 6C). We detected promoter activity in the Pshp144 ΔpptAB mutant, which was absent during in vitro growth (Figure S2H), indicating either an increase in basal promoter activity or pptAB-independent promoter regulation that is associated with the in vivo condition (Figure 6D). However, Pshp144 induction was still significantly lower in ΔpptAB compared to WT. Overall, our in vivo data support a model where PptAB not only plays a role in activating SHPs but also temporally regulates the TprA/PhrA system. In conclusion, our findings reveal that the peptide exporter PptAB controls and coordinates cell-cell communication by regulating precursor peptides and their role in population level behaviors.
DISCUSSION
In gram-positive bacteria, several cell-cell communication systems utilize signaling peptides to mediate population-level responses.2,3,6 These autoinducing peptides are exported and imported by designated transporters and often processed by proteases.2 This study addresses the question of how multiple cell-cell communication systems of the human pathogen Spn are coordinated. We demonstrate that the transporter PptAB and the protease Eep activate peptides from the Rgg/SHP signaling family and delay the activation of the peptide from the TprA/PhrA system. PptAB/Eep are conserved across species and related streptococci, and the mechanism was tested in two distinct strains, suggesting this mechanism of signaling peptide regulation may be widespread.
Regulators of peptide signaling can exert their effects at different stages within the peptide maturation cycle, from initial transcription to final receptor binding and all steps in between. Consistent with studies in other streptococci,7,24,25 our data confirmed that PptAB stimulates the precursor form of SHP by acting as an exporter, such that deletion of pptAB is rescued by addition of synthetic peptide to the extracellular environment. Moreover, we show that PptAB influences the precursor form of PhrA before it leaves the producing cell. Specifically, in the absence of inhibition of PptAB and import of processed PhrA, the PhrA precursor is active on the TprA/PhrA locus, indicating that PhrA maturation is not a prerequisite for signaling and that PptAB maintains tight control on the precursor peptides. There is precedence for such intracellular signaling in streptococci, as has been reported for the competence comRS system in S. mutans.29,30 Together, these data show that the PptAB transporter activates precursor SHPs by acting as exporter and inhibits precursor PhrA by preventing intracellular signaling and secretion. This maturation process is imperative to both Rgg/SHP and TprA/PhrA systems, as in WT cells, both Rgg/SHP and TprA/PhrA systems require import of their respective signaling peptides via AmiA-F on host cells for optimal population-level signaling. Thus, we have uncovered that both systems are regulated at the level of precursor peptides via PptAB and at the level of processed peptides via AmiA-F.
The enzymatic modification driving peptide maturation may correspond to another level of regulation. The standing model predicts that the transmembrane enzyme Eep acts as a protease to process substrates of PptAB and that these molecules likely work together, as the Eep cleavage site has a transmembrane localization.2 In agreement with this model, our data show that Eep and PptAB are required for Spn SHP activation as well as inhibition of PhrA. While we tested single mutants in parallel only in a subset of experiments, our findings showed that phenotypic changes created by the single mutants are similar to the double mutant ΔpptABΔeep. Thus, our results strongly indicate that both molecules are required to regulate Rgg/SHPs and TprA/PhrA systems.
The multistep processing of signaling peptides provides several entryways for regulatory molecules to exert their effects. Deletion of pptAB results in increased secreted PhrA. In contrast, deletion of tprA leads to a significant increase in PphrA activity, albeit having only a minor effect on the levels of secreted PhrA. This difference suggests that PptAB-mediated inhibition of PhrA provides a second layer of negative regulation at the post-translational level. This finding is highly consistent with previous work on the TprA/PhrA locus, which reported increased activity of PphrA and gene expression of the TprA/PhrA locus in the absence of tprA.13-15 Moreover, previous reports showed that addition of synthetic peptide or cell-free supernatants of high-density cultures activates the TprA/PhrA system, consistent with our observation that peptides imported from the environment are not targets of PptAB-mediated repression. Thus, we propose that the PhrA precursor is regulated at the transcriptional level by TprA (and other regulators binding to PphrA) and at the post-translational level by PptAB.
To investigate how PptAB may interact with the precursor peptides, we screened for shared features. Peptides from the PlcR family, such as Tpr/Phr, are predicted to be exported via the Sec system.2 As such, PhrA contains N-terminal basic/positive residues, a hydrophobic region, and a polar region. While SHPs harbor the first two features, they are missing the polar region associated with Sec export, consistent with Sec-independent export. Indeed, we found that SHPs and PhrA alleles display conserved positive residues encoded within the first five amino acids and that these are required for SHP export and PhrA secretion. A recent in -silico study identified the double-lysine (KK) motif as a conserved feature in SHPs across several streptococcal species, suggesting high conservation of this motif.31 In addition, alignment of PhrA alleles revealed a conserved stretch of N-terminal hydrophobic residues, which our experimental work demonstrated to be involved in both PhrA secretion and PptAB-mediated inhibition. Whether these divergent fates are mediated by distinct residues within this domain and can be decoupled from one another remains to be investigated. Overall, our studies uncover functional domains at the N termini of signaling peptides and provide the basis for future studies to evaluate peptide maturation processes in more detail.
This work unveils an exciting question regarding the regulation of PptAB. During planktonic growth, we observed that PptAB both continuously activated Rgg/SHP systems and repressed the TprA/PhrA system. Similarly, when bacteria were incubated with A549 lung epithelial cells in the presence of cell culture medium (high-glucose condition), PptAB repression of the TprA/PhrA system remained high. Repression in WT cells was fully overcome only when the cell culture medium was switched to PBS. Thus, PptAB inhibition is likely linked to nutrients. The gene expression levels of pptAB were stable during A549 infection, even as repression of PhrA was released, suggesting that transcriptional regulation of PptAB is not driving PhrA regulation. Instead, we propose three other general possibilities that will be explored in future studies: post-translational effects on PptAB’s function, changes to trafficking of the precursor peptides to PptAB, or substrate saturation of PptAB that allows PhrA to overcome PptAB inhibition. Our data on A549 cells and a murine model of infection are consistent with a cellular factor(s) and/or nutrient sources modulating PptAB regulation to change the levels and kinetics of peptide signaling in response to the environment.
Last, regulation of Spn cell-cell communication systems via PptAB has potential implications beyond the individual strain/population level. Multistrain Spn infections are common,32,33 and while PptAB, Eep, and AmiA-F are highly conserved across the Spn population, the peptide regulators and their associated regulons are variable, displaying polymorphisms as well as differences in gene possession.12,19,22 Moreover, commensal species that occupy the same niche may harbor similar or identical systems and pathways (e.g., S. mitis).34 In our proposed pathway, bacterial cells coordinate and/or synchronize diverse families of signaling systems at the precursor peptide level while being responsive to external signaling peptides. Phenotypic outcomes are likely to differ depending on which systems are harbored by the individual population and whether signals are exchanged between co-existing populations. When Spn establishes itself in the human nasopharynx, it likely competes and co-exists with resident microbes; thus, incorporating signals into the existing circuit and maintaining responsiveness to external signals could be critical to survival.
This study provides insights into the mechanistic intricacies that underlie the coordination of population-level behaviors by showing that Spn utilizes highly conserved peptide maturation molecules to coordinate multiple cell-cell communication systems. Given the widespread distribution of components from this pathway beyond species boundaries, this mechanism is likely conserved in closely related species and/or other gram-positive bacteria.
Limitations of the study
Our study demonstrates that PptAB functions to temporally regulate the Rgg/SHP and TprA/PhrA systems in vitro and in vivo and that PptAB is a virulence determinant. However, we have not yet determined what important phenotypic consequences arise from this coordination. To determine this, decoupling of PptAB’s dual function is likely required. We were already able to demonstrate that PptAB requires the PhrA N terminus for inhibition, but so does the Sec system for PhrA secretion. Future work will establish whether PhrA signaling can remain active without PptAB inhibition and, if so, provide a tool to understand how temporal regulation of these communication systems by PptAB influences phenotypes that contribute to Spn virulence.
STAR★METHODS
RESOURCE AVAILABILITY
Lead contact
Further information and request for resources and reagents should be directed to Dr. N. Luisa Hiller (lhiller@andrew.cmu.edu).
Materials availability
Bacterial strains and new recombinant DNA constructs are available upon request.
Data and code availability
RNAseq data have been deposited at GEO database and are publicly available as of the date of publication. The accession number is GSE268602.
This paper does not report original code.
Any additional information required to reanalyze the data reported in this work paper is available from the lead contact upon request.
EXPERIMENTAL MODEL AND STUDY PARTICIPANT DETAILS
Bacteria culture
All Spn strains used in this study can be found in the key resources table. For all experiments, Spn strains were streaked from frozen glycerol stocks onto TSA-II agar plates supplemented with 5% sheep blood (BD, BBL, New Jersey, USA) and incubated at 37°C and 5% CO2 overnight. Cultures from these plates were inoculated the next day into either Columbia broth (Cat. #R452972, Remel, Thermo Scientific, USA) for transformations or chemically defined media (CDM) supplemented with 55mM glucose or galactose20 for either growth curves, transcriptional assays, or reporter assays. Cultures were grown at 37°C and 5% CO2 until desired OD600.
KEY RESOURCES TABLE
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Bacterial and virus strains | ||
| Streptococcus pneumoniae strain D39 | Paixao et al. | N/A |
| Streptococcus pneumoniae strain TIGR4 | Winger et al. | N/A |
| D39 Pshp144:lux | This study | N/A |
| D39 Pshp144:lux ΔpptAB | This study | N/A |
| D39 Pshp144:lux ΔamiA-F | This study | N/A |
| D39 Pshp144:lux Δshp144 | This study | N/A |
| D39 PphrA:lux | This study | N/A |
| D39 PphrA:lux ΔpptAB | This study | N/A |
| D39 PphrA:lux Δeep | This study | N/A |
| D39 PphrA:lux ΔamiA-F | This study | N/A |
| D39 PphrA:lux ΔphrA | This study | N/A |
| D39 PphrA:lux ΔtprA | This study | N/A |
| D39 PphrA:lux ΔpptAB::pptAB | This study | N/A |
| D39 PphrA:lux Δeep::eep | This study | N/A |
| D39 PphrA:lux ΔpptABΔeep | This study | N/A |
| D39 PphrA:lux ΔpptABΔamiA-F | This study | N/A |
| D39 PphrA:lux ΔpptAB ΔphrA | This study | N/A |
| D39 PphrA:lux Δeep ΔphrA | This study | N/A |
| D39 PphrA:lux Δrgg/shpall | This study | N/A |
| D39 PphrA:lux Δrgg/shpall ΔpptAB | This study | N/A |
| TIGR4 ΔtprA | This study | N/A |
| TIGR4 ΔpptAB | This study | N/A |
| TIGR4 ΔpptABΔphrA | This study | N/A |
| TIGR4 Δrgg/shp144 | This study | N/A |
| D39 PphrA:lux ΔpptABΔamiA-FΔphrA | This study | N/A |
| D39 PphrA:lux ΔpptABΔamiA-F-phrAΔaa47-56 | This study | N/A |
| D39 ΔpptAB | This study | N/A |
| D39 ΔtprA | This study | N/A |
| D39 ΔamiA-F | This study | N/A |
| D39 ΔphrA | This study | N/A |
| D39 Δshp144 | This study | N/A |
| D39 ΔpptABΔamiA-F | This study | N/A |
| D39 ΔpptABΔphrA | This study | N/A |
| D39 PhrARKKR::AAAA | This study | N/A |
| D39 PhrARKKR::AAAA ΔpptAB | This study | N/A |
| D39 PhrAΔaa10-21 | This study | N/A |
| D39 SHP144KKRK::AAAA | This study | N/A |
| D39 PhrAΔaa2-46 | This study | N/A |
| D39 PphrA::CBRluc | This study | N/A |
| D39 PphrA::CBRluc ΔpptAB | This study | N/A |
| D39 Pshp144::CBRluc | This study | N/A |
| D39 Pshp144::CBRluc ΔpptAB | This study | N/A |
| D39 PphrA:lux ΔrtgS1 | This study | N/A |
| D39 PphrA:lux Δshp144 | This study | N/A |
| D39 PphrA:lux Δshp939 | This study | N/A |
| D39 PphrA:lux Δ1518 | This study | N/A |
| Chemicals, peptides, and recombinant proteins | ||
| PhrA-C10 (SNGLDVGKAD) | GenScript | Custom |
| CSP-1 (EMRLSKFFRDFILQRKK) | GenScript | Custom |
| CSP-2 (EMRISRIILDFLFLRKK) | GenScript | Custom |
| SHP144-C12 (EWVIVIPFLTNL) | GenScript | Custom |
| Alexa 546PhrA-C10 (Alexa546-SNGLDVGKAD) | Thermo Scientific | Custom |
| Critical commercial assays | ||
| Quick-RNA™ MiniPrep Kit (200 Preps) | Zymo Research Corporation | Cat. # R1055 |
| DNA Clean & Concentrator-5™ | Zymo Research Corporation | Cat. # D4003 |
| Applied Biosystems™ PowerUp™ SYBR™ Green Master Mix for qPCR | Thermo Scientific | Cat. # A25742 |
| LunaScript® RT SuperMix Kit | NEB | Cat. # E3010L |
| Q5® High-Fidelity DNA Polymerase | NEB | Cat. # M0491L |
| Deposited data | ||
| RNAseq data | GEO database | GEO database: GSE268602 |
| Experimental models: Cell lines | ||
| A549 cell line | ATCC | ATTC® CCL-185 |
| Experimental models: Organisms/strains | ||
| Female CD1 outbred mice | bred in-house, University of Leicester (UK) | CD1 outbred |
| Oligonucleotides | ||
| Primers for qRT-PCR, see Table S1 | This study | N/A |
| Primers for S. pneumoniae mutant generation, see Data S2 | This study | N/A |
| Recombinant DNA | ||
| pPP3phrA:luxABCDE | This study | N/A |
| pPP3phrA:luc | This study | N/A |
| pPP3shp144:luc | This study | N/A |
| Software and algorithms | ||
| Prism 10 | GraphPad Software | https://www.graphpad.com/features |
| Adobe Illustrator | illustration software | https://www.adobe.com/products/illustrator.html |
| BioRender | illustration software | https://www.biorender.com/ |
| Snapgene | cloning software | https://www.snapgene.com/ |
| ImageJ/Fiji | Imaging software | https://fiji.sc/ |
| Galaxy | data analysis software | https://usegalaxy.org/ |
Cell line
Low passage A549 human type II alveolar epithelial cells (Cat. #CCL-185) from ATCC (Manassas, VA) were obtained in DMEM+10% FBS (Cat #: 25-501, Genesee Scientific, USA) without antibiotics at 37°C and 5% CO2. Cells were split using trypsin (Cat #: 25-510, Genesee Scientific, USA) to either maintain confluent cultures or seed plates for infections.
Ethics statement
Mouse experiments were performed under permit no. PP0757060 according to United Kingdom Home Office guidelines under the Animals Scientific Procedures Act 1986 and University of Leicester ethics committee approval. The protocol used was approved by both the UK Home Office and the University of Leicester ethics committee. Where indicated, the procedures were carried out under anesthesia with isoflurane. Animals were housed in individually ventilated cages in a controlled environment and frequently monitored after infection to minimize suffering.
Animals
This study used 8–10-week-old female CD1 outbred mice, which were bred in house at the Leicester University, UK.
METHOD DETAILS
Bacterial cloning
Fragments for construct generation were amplified from templates using Q5 2x Master Mix (NEB, USA), verified by gel electrophoresis and subsequently cleaned up via DNA Clean & Concentrator-5 kit (Zymo Research, USA). Fragment assembly was carried out using an in-house Gibson assembly mix. Following transformation, strains were verified via PCR and Sanger sequencing. All primers used for construct generation are listed in Data S2. Deletions: Deletion constructs were generated utilizing site-directed homologous recombination to replace the region of interest with the kanamycin-resistant gene (kan) or the spectinomycin-resistant gene (aad9) as previously described35. Fluorescent removable antibiotic cassette (FRANC): In a subset of strains, constructs were generated with a two-step transformation approach using the fluorescent removable antibiotic cassette (FRANC), which contains the spectinomycin-resistance gene aad9, the sucrose-sensitivity gene sacB and mCardinal as a fluorescent marker. For the first transformation FRANC was inserted to replace the region desired for deletion or modification. Following transformation, colonies were screened for spectinomycin resistance, mCardinal fluorescence and verified via PCR. Positive clones from the first round of transformation where then transformed again to remove the FRANC cassette by replacing it with either the two flanking regions (unmarked deletion) or the two flanking regions and a desired modifications (e.g., amino acid substitutions). Colonies were then selected for sucrose resistance. Complementation: Complements were constructed using either aad9 or the kan without a terminator sequence resulting in constitutive expression of the complemented genes (pptAB or eep) downstream. The marker and genes of interest were inserted downstream of the bgaA region without disrupting bgaA35. Bioluminescent reporter: The plasmid containing the bioluminescent PphrA:luxABCDE promoter reporter construct and a tetracycline resistance marker (tet3) was generously provided by Dr. Hasan Yesilkaya. The plasmid carries flanks for the bgaA region that were used for insertion into the genome. The plasmid was extracted via plasmid preparation from an E. coli producing strain and directly used for transformations. To generate the Pshp144:luxABCDE reporter, the bioluminescent reporter region in the PphrA:luxABCDE strains were replaced with the spectinomycin resistance gene (aad9) and the shp144 promoter. PphrA:luc and Pshp144:luc were constructed using Ppp3 as previously described28. Promoter sequences can be found in the Data S1.
Bacterial transformations
Strains were inoculated in Columbia broth (CB) and grown to an OD600 of 0.05. Then a 1 mL culture were incubated with the either the Gibson mix, a PCR product from existing constructs or plasmid and 125 ng CSP-1 peptide (EMRLSKFFRDFILQRKK, GenScript, USA) for strain D39 or CSP-2 peptide (EMRISRIILDFLFLRKK, GenScript, USA) for strain TIGR4. After a 2-hour incubation period, cultures were plated on respective CB agar plates with antibiotics (final concentrations: spectinomycin 100 μg/mL; kanamycin 150 μg/mL, tetracycline 1 μg/mL) or CB agar sucrose plates (final concentration 10% (w/v)). After overnight incubation, resistant colonies were picked, regrown in CB media with respective antibiotics or 10% sucrose (w/v) and frozen stocks were generated. Mutants were verified via PCR and Sanger sequencing.
Growth curves
Specified strains were inoculated into 30 mL of either CB or CDM-glucose. Once the cultures reached an OD600 of 0.05, measurements were taken every 30 minutes until the cultures reached stationary phase.
RNA extraction and qRT-PCR
Bacterial strains were grown in CDM-glucose to early-log phase (OD600 = 0.3) or in presence of A549 epithelial cells (see below). RNA extraction was performed as described previously35. Purity of RNA was assessed via PCR for gapdh gene product to check for DNA contamination. High quality mRNA was then transcribed into cDNA using the LunaScript RT SuperMix kit (NEB, USA). qRT-PCR was carried out using PowerUp SYBR Green Master Mix reagent (Applied BiosystemsTM, Thermo Scientific, USA) and the QuantStudio3 (Applied Biosystems, USA). 16S rRNA was used for normalization. Primers can be found in Table S1.
Bioluminescent assays
Growth curves: Strains were inoculated in 20 mL CDM-glucose from overnight plates. Once the cultures reached OD600 of 0.05 measurements were taken every 45 minutes to 1 hour with the TECAN (Infinite M Plex, USA). Briefly, 150 μL of culture were assessed for luminescence in white 96-well plates (Greiner, USA) at 1000 ms. In addition, 700 μL of culture were taken to measure the optical density for each timepoint. Treatment with synthetic peptide: Strains were grown in CDM-glucose until they reached an OD600 of 0.05. Then 4 mL of culture were inoculated with either DMSO (control) or 1 μM final concentration (f.c.) of synthetic peptide (SHP144-C12 [EWVIVIPFLTNL, GenScript, USA], PhrA-C10 [SNGLDVGKAD, GenScript, USA] or Alexa546PhrA-C10 [Alexa546SNGLDVGKAD, Thermo Scientific, USA]). Measurements were taken every hour as described above. Co-culture: Reporter and donor strains were grown separately in CDM-glucose until they reached an OD600 of 0.05. Then 2 mL of each culture was combined for a 1:1 (v/v) ratio and co-cultures were incubated. Measurements were taken every hour as described above. Treatment with supernatants from A549 infections: Strains were grown in CDM-glucose from overnight plates. Once the cultures reached an OD600 of 0.05, 2 mL of culture were mixed 1:1 (v/v) with the respective supernatant. Measurements were taken as described above.
RNAseq
Strains were grown in CDM-glucose to early-log phase (OD600 = 0.3). RNA was extracted as described above. RNA was purified from bacterial ribosomal RNA and then run through a pipeline. RNAseq libraries were generated as previously described36. RNAseq data analysis was performed using GALAXY37. All tools were located and used on the GALAXY platform. For alignment of the RNAseq data to the reference genome, the D39 Spn reference genome file38 and GTF annotation file were imported into Galaxy.
Confocal microscopy
Strains were grown in CDM-glucose until they reached an OD600 of 0.05. Then 1 mL of each culture was incubated with 1 μM f.c. of labeled PhrA (Alexa546-SNGLDVGKAD, Thermo Fisher, USA) for 2 hours. Cells were spun down at 8,000 xg for 5 minutes, washed twice with PBS and intermittent spins and then fixed with 4% PFA (Thermo Scientific, USA) for 20 minutes at room temperature (RT). Cells were subsequently washed twice with PBS and stored at 4°C until imaging. For imaging, microscope slides (VWR, USA) were coated with Poly-L-lysine (Advanced Bio Matrix, USA). Next, 10μL of cell suspension was added and coverslips were applied. Zeiss LSM880 was used with a 100x objective and at least 3 pictures were taken per sample. Images were processed with the same setting using Fiji39. DIC pictures were utilized to create edges-only images and then channels were merged.
A549 cell infection model and supernatant collection
For infections, A549 cells were seeded into 6-well plates and grown to at least 90% confluency. The appropriate bacterial strains were grown in CB until an OD600 of 0.05 and then the appropriate volume to maintain around 108 CFUs was spun down, media was aspirated, and the bacterial pellet resuspended in 200 μL PBS (Genesee Scientific, USA) for infection. Meanwhile, cell culture media was aspirated and substituted for PBS. Bacteria were added to each well at an MOI of 1:100 and gently spun down. Incubation for indicated timepoints was done at 37°C and 5% CO2. For mRNA collection, cells were harvested using cell scrapers (VWR, USA) and the solution was transferred into tubes with 1:1 (v/v) of in-house RNA Later solution, spun down and the pellets were stored at−20°C until further processing. To obtain cell-free supernatants, samples were collected without scraping the plates. The samples were then spun down and filtered through a 0.2 μm syringe filter (VWR, USA) and stored at −80°C until further use.
Mouse infection model and in vivo luminescent reporter assays
Pneumonia model. In vivo studies were carried out using 8–10-week-old female CD1 outbred mice (bred in-house at the Leicester University, UK) that were lightly anesthetized. Bacterial strains were grown in BHI broth (Oxoid, Thermo Scientific, UK) with calf serum (4:1, v/v) to prepare the inocula. A 50 μl inoculum containing approximately 4 × 106 CFUs in PBS was administered into the nostrils, dropwise20. Mice were monitored for clinical signs (progressively starry coat, hunched appearance, and lethargy). To investigate the development of bacteremia in each mouse, approximately 20 μl of venous blood was collected at 16- and 24-hours post infection and viable counts were determined by plating serial dilutions on blood agar plates. In vivo imaging. Prior to infection, the reporters were tested in vitro for activity by growing them in BHI, CDM-glucose, or CDM-galactose, respectively (Figure S2H) For imaging, mice were infected with approximately 5x106 CFU/mouse in 50 ml PBS, under light anesthesia using 2.5% (v/v) isoflurane over oxygen (1.4–1.6 liters/min). At 2 hours post infection, mice were anesthetized, and luciferin (150 mg/kg) administered subcutaneously. The animals were imaged using the IVIS® Spectrum in vivo imaging system (Perkin Elmer) over 20 minutes at one-minute intervals. The peak signal was determined, and data were analyzed. Immediately after imaging, mice were euthanized to dissect their lungs and the bacterial counts were determined in the lung homogenates to normalize the signal levels against the colony-forming units28.
In silico analysis of amiA, pptAB, eep and phrA in the Spn population
To uncover the distribution and conservation of peptide processing components and the signaling peptide PhrA, we carried out a tBLASTn search in the GoldenSet (n=7548) 22 using the sequences of pptA (SPD_0464, SPD_RS02470), pptB (SPD_0465, SPD_RS02475), eep (SPD_0245, SPD_RS01315), amiA (SPD_1671, SPD_RS08840) and phrA (SPD_1746, SPD_RS09235) from Spn strain D39. The parameters were set to 70% identity over 90% of length for the processing molecules and 50% similarity over 50% length for the peptide to account for high variability. The output from the search was used as input to blastParser.py, which outputs Excel sheet of hits based on BLAST parameters (E-value, minimum similarity percentage and length). To determine the allelic distribution of PhrA, we organized the 5157 hits into 58 unique PhrA alleles out of which 36 had a frequency count that was higher than 1 genome (Figure S3).
QUANTIFICATION AND STATISTICAL ANALYSIS
Two-way ANOVA for multiple comparisons was used for most experiments unless otherwise stated. For RNAseq analysis, differential expression between groups was considered significant when the FDR value was <0.05 and absolute log2 values higher than 1. Most graphs depict standard error mean (SEM) unless otherwise specified. Details regarding sample size and p-values can be found in the figure legends.
Supplementary Material
Highlights.
The transporter-protease pair PptAB/Eep coordinates signaling peptides in Spn
PptAB/Eep targets the precursor forms of communication peptides via N-terminal domains
PptAB/Eep delays PhrA signaling and induces SHP signaling
In vivo tracking of peptide promoter activity captured the coordination in a pneumonia model
ACKNOWLEDGMENTS
We would like to thank Dr. Lydia Eutsey Perkins for contributions to the confocal microscopy imaging and image processing, Dr. Jon Minden for his expertise and support regarding peptide biochemistry, and Dr. Qidong Jia for assistance with RNA-seq. Further, we are grateful to Dr. Surya Dev Aggarwal, Shaw Camphire, Pratyush Madapuji Ravi, and Bailey Smith for constructive feedback on data and the manuscript. We greatly acknowledge Ms. Lucy Onion’s contribution to the in vivo imaging experiments. Special thanks to Franc the cat for providing inspiration for the development of an improved gene modification system. We are grateful for the support from the NIH (R01 AI139077 to N.L.H.) and the Glen de Vries fellowship (awarded to K.M.B.).
Footnotes
DECLARATION OF INTERESTS
The authors declare no competing interests.
SUPPLEMENTAL INFORMATION
Supplemental information can be found online at https://doi.org/10.1016/j.celrep.2024.114432.
REFERENCES
- 1.Federle MJ, and Bassler BL (2003). Interspecies communication in bacteria. J. Clin. Invest 112, 1291–1299. 10.1172/JCI20195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Neiditch MB, Capodagli GC, Prehna G, and Federle MJ (2017). Genetic and Structural Analyses of RRNPP Intercellular Peptide Signaling of Gram-Positive Bacteria. Annu. Rev. Genet 51, 311–333. 10.1146/annurev-genet-120116-023507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cook LC, and Federle MJ (2014). Peptide pheromone signaling in Streptococcus and Enterococcus. FEMS Microbiol. Rev 38, 473–492. 10.1111/1574-6976.12046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rocha-Estrada J, Aceves-Diez AE, Guarneros G, and De La Torre M (2010). The RNPP family of quorum-sensing proteins in Gram-positive bacteria. Appl. Microbiol. Biotechnol 87, 913–923. 10.1007/s00253-010-2651-y. [DOI] [PubMed] [Google Scholar]
- 5.Monnet V, and Gardan R (2015). Quorum-sensing regulators in Gram-positive bacteria: “cherchez le peptide.”. Mol. Microbiol 97, 181–184. 10.1111/mmi.13060. [DOI] [PubMed] [Google Scholar]
- 6.Shanker E, and Federle MJ (2017). Quorum sensing regulation of competence and bacteriocins in Streptococcus pneumoniae and mutans. Genes 8, 15. 10.3390/genes8010015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chang JC, and Federle MJ (2016). PptAB exports Rgg quorum-sensing peptides in Streptococcus. PLoS One 11, e0168461. 10.1371/journal.pone.0168461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fleuchot B, Gitton C, Guillot A, Vidic J, Nicolas P, Besset C, Fontaine L, Hols P, Leblond-Bourget N, Monnet V, and Gardan R (2011). Rgg proteins associated with internalized small hydrophobic peptides: a new quorum-sensing mechanism in streptococci. Mol. Microbiol 80, 1102–1119. 10.1111/j.1365-2958.2011.07633.x. [DOI] [PubMed] [Google Scholar]
- 9.Chang JC, LaSarre B, Jimenez JC, Aggarwal C, and Federle MJ (2011). Two group a streptococcal peptide pheromones act through opposing rgg regulators to control biofilm development. PLoS Pathog. 7, e1002190. 10.1371/journal.ppat.1002190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Weiser JN, Ferreira DM, and Paton JC (2018). Streptococcus pneumoniae: Transmission, colonization and invasion. Nat. Rev. Microbiol 16, 355–367. 10.1038/s41579-018-0001-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ikuta KS, Swetschinski LR, Robles Aguilar G, Sharara F, Mestrovic T, Gray AP, Davis Weaver N, Wool EE, Han C, Gershberg Hayoon A, et al. (2022). Global mortality associated with 33 bacterial pathogens in 2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet 400, 2221–2248. 10.1016/S0140-6736(22)02185-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Aggarwal SD, Yesilkaya H, Dawid S, and Hiller NL (2020). The pneumococcal social network. PLoS Pathog. 16, 10089311–e1009020. 10.1371/journal.ppat.1008931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hoover SE, Perez AJ, Tsui HCT, Sinha D, Smiley DL, Dimarchi RD, Winkler ME, and Lazazzera BA (2015). A new quorum-sensing system (TprA/PhrA) for Streptococcus pneumoniaeD39 that regulates a lantibiotic biosynthesis gene cluster. Mol. Microbiol 97, 229–243. 10.1111/mmi.13029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Motib A, Guerreiro A, Al-Bayati F, Piletska E, Manzoor I, Shafeeq S, Kadam A, Kuipers O, Hiller L, Cowen T, et al. (2017). Modulation of Quorum Sensing in a Gram-Positive Pathogen by Linear Molecularly Imprinted Polymers with Anti-infective Properties. Angew. Chem., Int. Ed. Engl 56, 16555–16558. 10.1002/anie.201709313. [DOI] [PubMed] [Google Scholar]
- 15.Motib AS, Al-Bayati FAY, Manzoor I, Shafeeq S, Kadam A, Kuipers OP, Hiller NL, Andrew PW, and Yesilkaya H (2019). TprA/PhrA Quorum Sensing System Has a Major Effect on Pneumococcal Survival in Respiratory Tract and Blood, and Its Activity Is Controlled by CcpA and GlnR. Front. Cell. Infect. Microbiol 9, 326–411. 10.3389/fcimb.2019.00326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kadam A, Eutsey RA, Rosch J, Miao X, Longwell M, Xu W, Woolford CA, Hillman T, Motib AS, Yesilkaya H, et al. (2017). Promiscuous signaling by a regulatory system unique to the pandemic PMEN1 pneumococcal lineage. PLoS Pathog. 13, 10063399–e1006427. 10.1371/journal.ppat.1006339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cuevas RA, Eutsey R, Kadam A, West-Roberts JA, Woolford CA, Mitchell AP, Mason KM, and Hiller NL (2017). A novel streptococcal cell-cell communication peptide promotes pneumococcal virulence and biofilm formation. Mol. Microbiol 105, 554–571. 10.1111/mmi.13721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shlla B, Gazioglu O, Shafeeq S, Manzoor I, Kuipers OP, Ulijasz A, Hiller NL, Andrew PW, and Yesilkaya H (2021). The Rgg1518 transcriptional regulator is a necessary facet of sugar metabolism and virulence in Streptococcus pneumoniae. Mol. Microbiol 116, 996–1008. 10.1111/mmi.14788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang CY, Medlin JS, Nguyen DR, and Disbennett WM (2020). Molecular Determinants of Substrate Selectivity of a Pneumococcal Rgg-Regulated Peptidase-Containing ABC Transporter. MBio 11, 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhi X, Abdullah IT, Gazioglu O, Manzoor I, Shafeeq S, Kuipers OP, Hiller NL, Andrew PW, and Yesilkaya H (2018). Rgg-Shp regulators are important for pneumococcal colonization and invasion through their effect on mannose utilization and capsule synthesis. Sci. Rep 8, 6369–6415. 10.1038/s41598-018-24910-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Junges R, Salvadori G, Shekhar S, Åmdal HA, Periselneris JN, Chen T, Brown JS, and Petersen C (2017). Crossm Biofilm Formation and Surface Polysaccharide Production, 2, pp. 1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Abdullah IT, Ulijasz AT, Girija UV, Tam S, Andrew P, Hiller NL, Wallis R, and Yesilkaya H (2022). Structure-function analysis for the development of peptide inhibitors for a Gram-positive quorum sensing system. Mol. Microbiol 117, 1464–1478. 10.1111/mmi.14921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hu D, Laczkovich I, Federle MJ, and Morrison DA (2023). Identification and Characterization of Negative Regulators of Rgg1518 Quorum Sensing in Streptococcus pneumoniae. J. Bacteriol 205, e00087–23. 10.1128/jb.00087-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lingeswaran A, Metton C, Henry C, Monnet V, Juillard V, and Gardan R (2020). Export of RGG quorum sensing peptides is mediated by the PPTAB ABC transporter in streptococcus thermophilus strain LMD-Genes 11, 1096–1122. 10.3390/genes11091096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pérez-Pascual D, Gaudu P, Fleuchot B, Besset C, Rosinski-Chupin I, Guillot A, Monnet V, and Gardan R (2015). RovS and its associated signaling peptide form a cell-to-cell communication system required for Streptococcus agalactiae pathogenesis. mBio 6. 10.1128/mBio.02306-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gardan R, Besset C, Gitton C, Guillot A, Fontaine L, Hols P, and Monnet V (2013). Extracellular life cycle of ComS, the competence-stimulating peptide of Streptococcus thermophilus. J. Bacteriol 195, 1845–1855. 10.1128/JB.02196-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Aprianto R, Slager J, Holsappel S, and Veening JW (2018). High-resolution analysis of the pneumococcal transcriptome under a wide range of infection-relevant conditions. Nucleic Acids Res. 46, 9990–10006. 10.1093/nar/gky750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Glanville DG, Gazioglu O, Marra M, Tokars VL, Kushnir T, Habtom M, Croucher NJ, Nebenzahl YM, Mondragón A, Yesilkaya H, and Ulijasz AT (2023). Pneumococcal capsule expression is controlled through a conserved, distal cisregulatory element during infection. PLoS Pathog. 19, 10110355–e1011129. 10.1371/journal.ppat.1011035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Underhill SAM, Shields RC, Kaspar JR, Haider M, Burne RA, and Hagen SJ (2018). Intracellular Signaling by the comRS System in Streptococcus mutans Genetic Competence. mSphere 3, e00444–18. 10.1128/msphere.00444-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Son M, Ahn SJ, Guo Q, Burne RA, and Hagen SJ (2012). Microfluidic study of competence regulation in Streptococcus mutans: Environmental inputs modulate bimodal and unimodal expression of comX. Mol. Microbiol 86, 258–272. 10.1111/j.1365-2958.2012.08187.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Felipe-Ruiz A, Marina A, and Rocha EPC (2022). Structural and Genomic Evolution of RRNPPA Systems and Their Pheromone Signaling. mBio 13, e0251422. 10.1128/mbio.02514-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Turner P, Hinds J, Turner C, Jankhot A, Gould K, Bentley SD, Nosten F, and Goldblatt D (2011). Improved detection of nasopharyngeal cocolonization by multiple pneumococcal serotypes by use of latex agglutination or molecular serotyping by microarray. J. Clin. Microbiol 49, 1784–1789. 10.1128/JCM.00157-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Valente C, Hinds J, Gould KA, Pinto FR, de Lencastre H, and Sá-Leão R (2016). Impact of the 13-valent pneumococcal conjugate vaccine on Streptococcus pneumoniae multiple serotype carriage. Vaccine 34, 4072–4078. 10.1016/j.vaccine.2016.06.017. [DOI] [PubMed] [Google Scholar]
- 34.Junges R, Salvadori G, Chen T, and Petersen FC (2019). Characterization of a Signaling System in Streptococcus Mitis, 85, pp. 1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Aggarwal SD, Eutsey R, West-Roberts J, Domenech A, Xu W, Abdullah IT, Mitchell AP, Veening JW, Yesilkaya H, and Hiller NL (2018). Function of BriC peptide in the pneumococcal competence and virulence portfolio. PLoS Pathog. 14, e1007328. 10.1371/journal.ppat.1007328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Warrier I, Ram-Mohan N, Zhu Z, Hazery A, Echlin H, Rosch J, Meyer MM, and van Opijnen T (2018). The Transcriptional landscape of Streptococcus pneumoniae TIGR4 reveals a complex operon architecture and abundant riboregulation critical for growth and virulence. PLoS Pathog. 14, 10074611–e1007525. 10.1371/journal.ppat.1007461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jalili V, Afgan E, Gu Q, Clements D, Blankenberg D, Goecks J, Taylor J, and Nekrutenko A (2020). The Galaxy platform for accessible, reproducible and collaborative biomedical analyses: 2020 update. Nucleic Acids Res. 48, W395–W402. 10.1093/nar/gkac247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lanie JA, Ng WL, Kazmierczak KM, Andrzejewski TM, Davidsen TM, Wayne KJ, Tettelin H, Glass JI, and Winkler ME (2007). Genome sequence of Avery’s virulent serotype 2 strain D39 of Streptococcus pneumoniae and comparison with that of unencapsulated laboratory strain R6. J. Bacteriol 189, 38–51. 10.1128/JB.01148-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T, Preibisch S, Rueden C, Saalfeld S, Schmid B, et al. (2012). Fiji: An open-source platform for biological-image analysis. Nat. Methods 9, 676–682. 10.1038/nmeth.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
RNAseq data have been deposited at GEO database and are publicly available as of the date of publication. The accession number is GSE268602.
This paper does not report original code.
Any additional information required to reanalyze the data reported in this work paper is available from the lead contact upon request.