Highlights
-
•
USP9X expression was negatively associated with metastasis in patients with PSC.
-
•
USP9X regulates EMT, angiogenesis and immunoinfiltration of PSC by inhibiting MMP9.
-
•
Targeting USP9X/MMP9 may be a promising strategy for inhibiting PSC metastasis.
Keywords: Pulmonary sarcomatoid carcinomas, Metastasis, Epithelial-to-mesenchymal transition, Angiogenesis, Immune infiltration, Ubiquitin-specific peptidase 9X
Abstract
Background
Pulmonary sarcomatoid carcinoma (PSC) is a highly invasive pulmonary malignancy with an extremely poor prognosis. The results of previous studies suggest that ubiquitin-specific peptidase 9X (USP9X) contributes to the progression of numerous types of cancer. Nevertheless, there is little knowledge about the molecular mechanisms and functions of USP9X in the metastasis of PSC.
Methods
Immunohistochemistry and western blotting were used to detect USP9X expression levels in PSC tissues and cells. Wound healing, transwell, enzyme-linked immunosorbent assay (ELISA), tube formation, and aortic ring assays were used to examine the function and mechanism of USP9X in the metastasis of PSC.
Results
Expression of USP9X was markedly decreased and significantly correlated with metastasis and prognosis of patients with PSC. Then we revealed that USP9X protein levels were negatively associated with the levels of epithelial-mesenchymal transition (EMT) markers and the migration of PSC cells. It was confirmed that USP9X in PSC cells reduced VEGF secretion and inhibited tubule formation of human umbilical vein endothelial cells (HUVEC) in vitro. USP9X was detected to downregulate MMP9. Meanwhile, MMP9 was positively related to EMT, angiogenesis and was negatively related to immune infiltration in the public databases. USP9X was significantly negatively associated with the expression of MMP9, EMT markers, CD31, and positively associated with CD4, and CD8 in PSC tissues.
Conclusion
The present study reveals the vital role of USP9X in regulating EMT, angiogenesis and immune infiltration and inhibiting metastasis of PSC via downregulating MMP9, which provides a new effective therapeutic target for PSC.
Introduction
Non-small cell lung cancer (NSCLC), the main pathological type of which is adenocarcinoma, accounts for about 85% of all lung cancers [1], which has high incidence mortality for its complex pathogenic mechanism [2]. PSC, a highly invasive pulmonary malignancy with a poor prognosis, accounts for 0.1-0.4% of NSCLC [3,4]. PSC patients exhibit low response rates to traditional treatments such as chemotherapy, radiotherapy, and neoadjuvant chemotherapy due to the highly aggressive character and propensity for frequent metastasis of this disease. The exploration of the molecular mechanisms involved in the progression of PSC metastasis and identification of new targets are urgently needed.
PSC is a typically biphasic neoplasm that includes both epithelial and fusiform components. The fusiform components of PSC may originate from the epithelial-mesenchymal transition (EMT) of epithelial cancer cells [5]. EMT is initially observed in early development and describes the transdifferentiation of stationary epithelial cells to a mesenchymal motile phenotype [6]. Pathological reactivation of the EMT has been suggested to play a fundamental role in PSC metastasis [7]. Previous studies have demonstrated that the metastasis of PSC cell line analogs, which originate from NSCLC cell lines treated with transforming growth factor β1 (TGFβ1), results in significant EMT-related morphological changes related to EMT and angiogenesis [8,9].
Moreover, PSC metastasis is caused by genome instability, during which cancer cells can reprogram tumor metabolism, resist cell death, avoid immune destruction, and construct the tumor microenvironment through various mechanisms, such as angiogenesis and immune infiltration [10,11]. Pathologic angiogenesis, which is principally initiated by chemoattractants and proliferative cytokines such as vascular endothelial growth factor (VEGF) [12], is essential for malignant tumor metastasis. The VEGF/VEGFR pathway is reportedly associated with NSCLC metastasis [13]. In addition, immune infiltration has been shown to be closely related to tumor metastasis. Remark et al. revealed that the immune contexture was present and organized in primary lung tumors, liver metastases from lung cancer and lung metastases of various origins (colorectal, renal, and breast cancers as well as melanoma) [14]. There was a significant correlation between the density of immune cell infiltration with tumor metastasis [15]. However, the current evidence has paid little attention to the mechanisms of EMT, angiogenesis and immune infiltration in the metastasis of PSC.
Ubiquitination and deubiquitylation are the two main types of posttranslational modifications and rely on ubiquitin ligases and deubiquitinating enzymes (DUBs) to maintain protein homeostasis during biological processes [16]. It has been reported that E3 ubiquitin ligases and deubiquitinating enzymes, which are ubiquitination system components, play the prominent roles in modulating tumor metastasis [17]. We have proven that ubiquitin-specific protease 5 (USP5), a DUB, promotes metastasis in NSCLC by inducing EMT [18], suggesting that DUBs are closely related to NSCLC metastasis. USP9X, a member of the DUBs, regulates numerous signaling pathways by deubiquitinating essential proteins. It plays vital roles in the processes of cell survival, invasion, and migration in cancers. Genetic inactivation of USP9X was found to enhance the expression of the oncogenic gene K-ras, accelerating tumorigenesis and cancer progression [19]. Although USP9X is a differentially expressed gene in lung cancer, its precise role in PSC progression and metastasis remains unclear. Matrix metalloproteinase 9 (MMP9) is a widely studied extracellular matrix enzyme that degrades various matrix biomolecules. MMP9 has been found to play roles in tumor invasion, metastasis, angiogenesis, and tumor microenvironment regulation [20,21]. However, whether MMP9 can be regulated by deubiquitylation to participate in PSC metastasis is not yet known.
Here, we found that reducing the expression of USP9X could enhance PSC cells migration and EMT. USP9X downregulation could also exert proangiogenic effects by inducing VEGF secretion and microvessel sprouting. We then found that MMP9 signaling was activated in USP9X-knockdown PSC cells. Furthermore, USP9X was negatively associated with the expression of MMP9, EMT biomarkers, angiogenesis factors and positively associated with immune infiltration in PSC tissues. Therefore, USP9X plays an important role in inhibiting PSC metastasis and may be a promising pharmaceutical target for treating PSC metastasis.
Materials and methods
PSC specimens
Primary PSC and metastatic PSC tissue samples were obtained after informed consent was obtained from patients at the Affiliated Suzhou Hospital of Nanjing Medical University (Suzhou, China) between March 2014 and February 2020. None of the patients had received either chemotherapy or radiotherapy before surgery. All the experimental protocols were approved by the Ethics Committee of the Affiliated Suzhou Hospital of Nanjing Medical University and were conducted in accordance with the Helsinki Declaration.
Immunohistochemistry
An ABC (Avidin/Biotin) detection system (Benchmark, Angleton, Texas, USA) was used for immunostaining according to the manufacturer's protocol, as described previously [22]. The following specific primary antibodies were used: anti-E-cadherin (Gene Tech, Shanghai, China), anti-USP9X (Abcam, Cambridge, UK), anti-MMP9 (Abcam), anti-CD4 (Gene Tech), anti-CD8 (Gene Tech), and anti-CD31 (Gene Tech). The negative control slides, which were not incubated in the primary antibodies, did not exhibit nonspecific staining. In this study, all the slides were examined by two pathologists in a blinded fashion.
Western blotting
Western blotting was conducted as previously described [23]. The primary antibodies used in this study were as follows: anti-USP9X (Cell Signaling Technology, Boston, MA, USA), anti-MMP9 (ABclonal, Wuhan, China), anti-E-cadherin (Cell Signaling Technology), and anti-Vimentin (Absin, Shanghai, China). Finally, the primary antibodies were probed with rabbit or mouse secondary antibodies labeled with DyLight 800 (KPL) and scanned with an Odyssey Infrared Imaging System (LI-COR).
Cell lines and culture conditions
Human NSCLC cell lines (PC9, A549, H1299, 95-D) were purchased from the Type Culture Collection of the Chinese Academy of Sciences (Shanghai, China), and cultured under the recommended conditions. The cells were grown in a humidified incubator with 5% CO2 at 37°C. The cells were incubated in medium supplemented with 10 ng/mL human recombinant TGFβ1 (MedChamExpress, New Jersey, USA) in a humidified 5% CO2 incubator at 37°C for 15 days to transform into PSC cells [7].
Lentivirus transfection and isolation of stable cell clones
HEK293T cells were used to produce recombinant lentiviral stocks by cotransfecting the transfer vector, packaging plasmid pMD2.G (Addgene, Cambridge, UK) and pSPAX2 (Addgene) using Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA) according to the manufacturer's protocol. Lentiviruses carrying short hairpin RNA (shRNA) were prepared and added to NSCLC cells in the presence of polybrene (Life Technologies, Darmstadt, Germany) according to the manufacturer's protocol. The medium was replaced with fresh culture medium after 24 h. The medium was subsequently replaced with fresh RPMI-1640 medium supplemented with 10% fetal bovine serum (FBS) and 2 μg/mL puromycin (MedChemExpress). After the dilution culture was limited by the pressure of puro, several independent clones from each transfection group were selected and screened for USP9X protein silencing by western blotting.
Cell migration assay
The protocol was performed as previously described [24]. For the wound healing assay, PSC cells were grown in 6-well plates. When a confluent layer was achieved, a sterile 200-μL pipette tip was used to create a scratch on the cell monolayer, and the culture was subsequently further incubated with fresh medium containing 1% FBS. The status of the scratch wound closure was observed after 24 h or 48 h by considering the distance ratio of the scratch wound at 24 h or 48 h to the scratch wound at 0 h. The migration of PSC cells was measured by the cells that moved into the scraped site using an inverted phase contrast microscope. For the transwell assay, PSC cells subjected to different treatments were added to the top chamber. The bottom chamber was filled with complete medium. After being cultured at 37°C for 3 h, the cells from the interiors of the inserts were removed, and the membranes were washed 3 times with phosphate-buffered saline (PBS), fixed in 4% paraformaldehyde, and stained with crystal violet. The cells were counted by photographing the membrane under a microscope.
Immunofluorescence staining
The cells were grown on glass coverslips and then fixed with 4% paraformaldehyde for 20 min. Following a PBS wash, the cells were permeabilized with 0.1% Triton X-100, blocked with bovine serum albumin (2%), and further incubated overnight at 4°C with primary antibodies against E-cadherin (Cell Signaling Technology) and Vimentin (Absin). Thereafter, Alexa Fluor 488 (Invitrogen) was added as a fluorescent conjugated secondary antibody for 1 h at 37°C in the dark. DAPI (Sigma Aldrich, St. Louis, MO, USA) was used to stain the nuclei of the cells. Finally, the coverslips were mounted onto slides with fluorescent mounting medium and immediately observed using a fluorescence microscope (Carl Zeiss, Jena, Germany).
Tube formation assay
The 96-well plate was coated with 50 μL of matrigel mixed with an equal volume of FBS-free medium, which was allowed to polymerize at 37°C for 30 min. The cellular matrix (CM) was collected from PC9 cells pretreated with TGFβ1. After pretreatment with endothelial cell medium (ECM) or ECM containing 50% CM for 24 h, the human microvascular endothelial cell line (HMEC) cells were inoculated on matrigel. After 6 h of incubation, the formation of tubes was observed using a Nikon C2 microscope.
Aortic ring assay
The aortic ring assay was performed as previously described [25]. Briefly, aortas isolated from male Sprague-Dawley rats were cut into sections of 1- to 1.5-mm long rings and immersed individually in matrigel in 96-well plates. Approximately 1 h later, the aortic rings were treated with 50 μL of regular medium and incubated at 37°C for 7 days. The sprouted microvessels were observed and photographed using a Nikon C2 microscope.
Statistical analysis
Data were shown as mean ± standard error of mean (SEM) of three independent experiments. GraphPad Prism 6.0 Software were employed to perform data. The data were compared by Student's t test between two groups and ordinary one-way analysis of variance (ANOVA) for three or more groups. The TIMER2.0 database was utilized to obtain immune infiltration data for USP9X and MMP9 in NSCLC. Subsequently, Spearman's correlation analysis was used to assess the relationships between two genes. The log-rank test was used to determine the statistical significance of Kaplan–Meier survival curves. P values of less than 0.05 were classified as statistically significant.
Results
Low USP9X expression was positively associated with metastasis in PSC
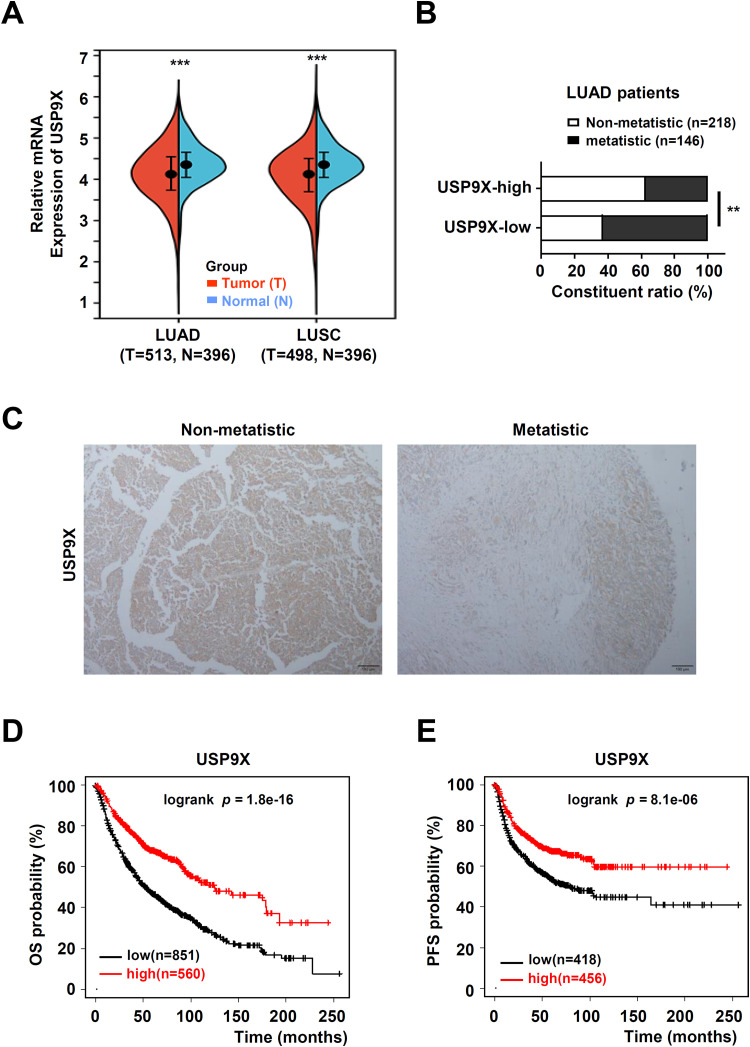
At present, there are very few databases of patients with PSC. We analyzed USP9X expression in NSCLC and normal tissues from the TCGA database. The expression of USP9X in NSCLC tissues was lower than that in normal tissues (Fig. 1A). USP9X expression was significantly negatively correlated with tumor metastasis in samples from 364 patients with lung adenocarcinoma from the TCGA dataset (Fig. 1B). Consistent with these findings, we detected strong moderate USP9X staining in non-metastatic PSC tissues, whereas USP9X staining was considerably weaker in PSC metastatic tissues (Fig. 1C).
Fig. 1.
Low USP9X expression was positively associated with metastasis in PSC. (A) USP9X expression in 909 lung adenocarcinoma (LUAD)tissues and normal tissues, and in 894 lung squamous cell carcinoma (LUSC) tissues and normal tissues from the TCGA dataset. (B) Comparison of metastatic status of 364 LUADsamples from the TCGA dataset with different levels of USP9X expression. (C) Representative image of USP9X immunohistochemical staining in non-metastatic and metastatic PSC tissues. Black bar, 100 μm. (D) OS and PFS were also determined according to USP9X expression in NSCLC samples via Kaplan-Meier plotter. ** P < 0.01, *** P < 0.001.
To further evaluate USP9X as a potential prognostic factor in NSCLC, the relationship between USP9X and survival rate in patients with NSCLC was analyzed. The results from the Kaplan-Meier plotter dataset showed that patients with high USP9X mRNA levels had better 20-year overall survival (OS, n = 1411, Fig. 1D) and progression-free survival (PFS, n = 874, Fig. 1E) rates. These data suggested that USP9X may play a vital role in the metastasis of PSC.
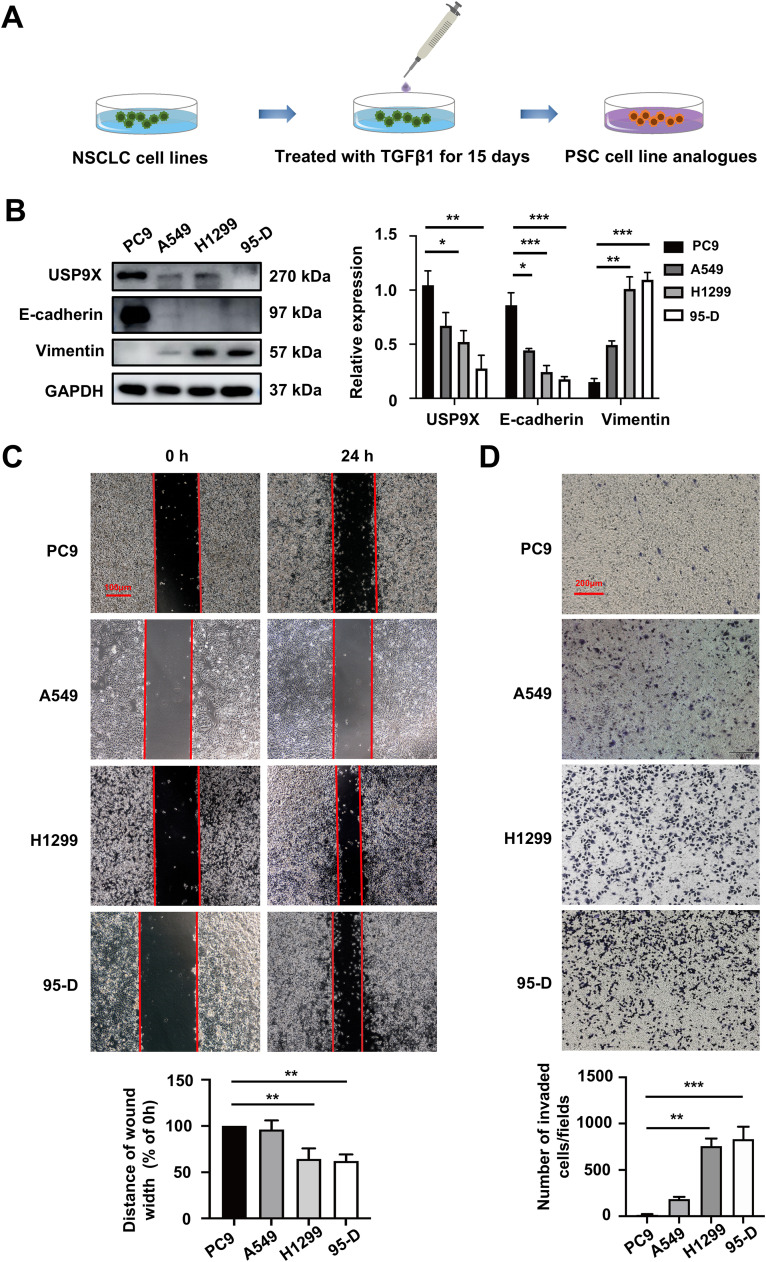
USP9X was low-expressed in PSC cells which were prone to EMT and migration
To the best of our knowledge, no PSC-derived cell lines are currently available. We used TGFβ1 to simulate NSCLC cell lines (PC9, A549, H1299 and 95-D) to obtain in vitro PSC cell line analogs [7] (Fig. 2A). Next, we examined the level of USP9X expression in several PSC cell lines. The results showed that USP9X expression was lower in TGFβ1-treated H1299 and 95-D cells than in PC9 and A549 cells. TGFβ1-treated H1299 and 95-D cells had a higher EMT profile than PC9 and A549 cells, as indicated by the decreased expression of the epithelial marker E-cadherin and increased expression of the mesenchymal marker Vimentin in H1299 and 95-D cells at the protein level (Fig. 2B). As expected, the migration ability was greater in TGFβ1-treated H1299 and 95-D cells according to the results of migration assays (Fig. 2C, D). These results indicated that low USP9X expression was positively correlated with EMT and migration in PSC cells.
Fig. 2.
USP9X was low-expressed in PSC cells which were prone to EMT and migration. (A) NSCLC cells were treated with TGFβ1 for 15 days to establish in vitro PSC cell line analogs. (B) Western blot analysis of USP9X and EMT marker expression in PSC cell lines. (C) The migration of PSC cells was determined via a wound healing assay. Red bar, 100 μm. (D) Cell migration was determined via a transwell migration assay. Red bar, 200 μm. * P < 0.05, ** P < 0.01, *** P < 0.001.
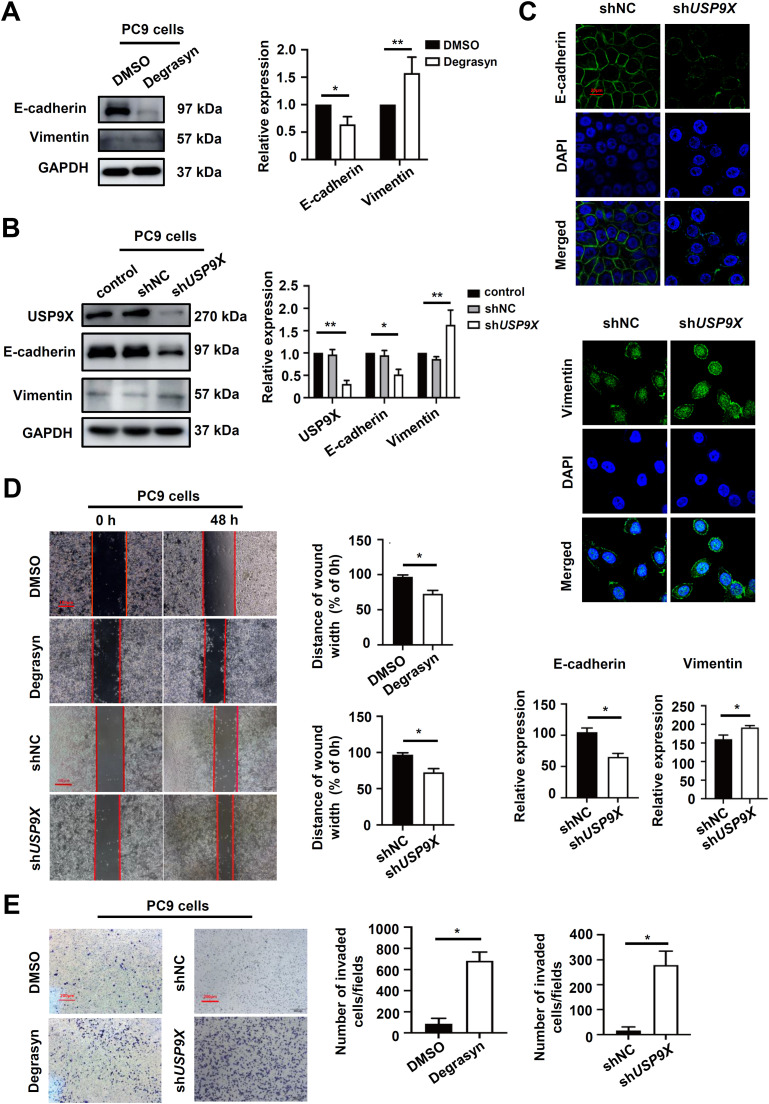
USP9X inhibits the EMT and migration of PSC cells
As USP9X expression was significantly reduced in metastatic PSC tissues, we inhibited USP9X with Degrasyn in TGFβ1-pretreated PC9 cells, which express high levels of USP9X. Moreover, USP9X inhibition reduced E-cadherin protein levels and elevated Vimentin protein levels (Fig. 3A). Consistently, TGFβ1-pretreated PC9 cells treated with the shUSP9X lentivirus exhibited significant downregulation of E-cadherin and upregulation of Vimentin (Fig. 3B).
Fig. 3.
USP9X inhibits the EMT and migration of PSC cells. (A) PSC cells were treated with Degrasyn or DMSO, after which western blotting was used to measure the protein levels of EMT markers. (B) PSC cells were treated with the shUSP9X or shNC lentivirus, and western blotting was subsequently used to assess the protein levels of EMT markers. (C) The epithelial phenotype marker E-cadherin (green) and mesenchymal marker Vimentin (green) were detected via IF staining. Nuclei were stained with DAPI (blue). Red bar, 20 μm. (D) Cell migration was determined via wound healing assays. Red bar, 100 μm. (E) Cell migration was determined via a transwell migration assay. * P < 0.05, ** P < 0.01.
Moreover, immunofluorescence analysis illustrated that TGFβ1-pretreated PC9-shUSP9X cells exhibited lower E-cadherin protein expression and greater Vimentin protein expression in the cytoplasm, compared with TGFβ1-pretreated PC9-shNC cells (Fig. 3C). To determine whether USP9X has the potential to inhibit migration, wound healing and transwell assays were performed using PSC cells. The wound healing revealed that the widths of the wound scratches in PC9-shNC cell cultures were much greater than those in the PC9-shUSP9X cell cultures at 48 h, indicating that USP9X knockdown has the potential to promote the migration of PSC cells (Fig. 3D). The cell transwell showed that the number of migrating cells was increased in the TGFβ1-pretreated PC9-shUSP9X group compared with the control group (Fig. 3E). These data indicated that USP9X could inhibit EMT and migration in PSC cells.
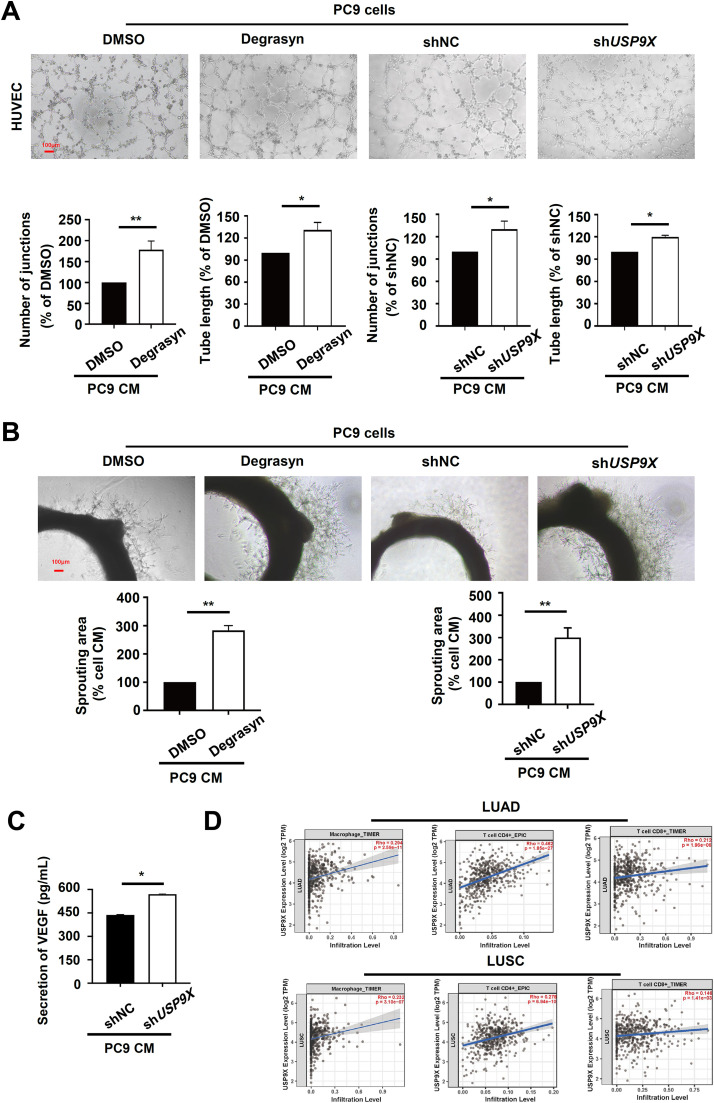
USP9X inhibits angiogenesis and induces immune infiltration in PSC
Tumor-associated angiogenesis provides the necessary conditions for tumor progression and metastasis [26]. To confirm a direct role of USP9X in angiogenesis during PSC metastasis in vitro, we first prepared new blood vessels from the existing vasculature through the extra CM. Then, we collected CM from TGFβ1-pretreated PC9 cells treated with Degrasyn, DMSO, shUSP9X or shNC lentivirus and evaluated its effect on HUVEC tubule formation. CM from USP9X-depleted PC9 cells induced HUVEC tubule formation, resulting in elongated and robust capillary-like structures and longer tube lengths (Fig. 4A). Endothelial cell proliferation, migration, and tubular formation are several key stages of angiogenesis that can be simulated by the rat aortic ring experiment [25]. In this study, aortic rings embedded in matrigel and cultured under chemically defined conditions produced dendritic vascular growths, which can be stimulated or inhibited by angiogenesis modulators [27]. The effect of USP9X on microvessel sprouting was examined in the rat aortic ring angiogenesis model. As shown in Fig. 4B, compared with that in the control group, the formation of microvascular networks surrounding the aortic ring in the PC9 CM was markedly triggered by depletion of USP9X. Consequently, the emergence of new vessels from the aortic ring induced by PC9 CM was significantly promoted by depletion of USP9X. Next, we examined the correlation between the expression of USP9X and the canonical proangiogenic factor VEGF. ELISA revealed that USP9X negatively regulated VEGF secretion in PC9 cells (Fig. 4C). These findings suggested that inhibition of USP9X could induce angiogenesis in PSC cells.
Fig. 4.
USP9X inhibits angiogenesis and induces immune infiltration in PSC. (A) The tube formation ability of HMEC-1 cells cultured with CM collected from PSC cells pretreated with Degrasyn or DMSO, shUSP9X or shNC lentiviruses was tested via an endothelial cell tube formation assay. Red bar, 100 μm. (B) Rat aortic ring microvessel sprouting induced by CM, collected from PSC cells pretreated with Degrasyn or DMSO, shUSP9X or shNC was tested by a rat aortic ring assay. (C) USP9X knockdown in PSC cells increased VEGF secretion into the supernatant, as determined by ELISA. Tumor cells were cultured in serum-containing medium for 48 h, after which the CM was collected to determine the concentration of VEGF. (D) USP9X expression is positively associated with macrophage, CD4+ T cell, and CD8+ T-cell infiltration in NSCLC. * P < 0.05, ** P < 0.01.
Emerging studies have suggested that immune infiltration cells play important roles in tumor metastasis [28]. Therefore, we continued to explore the relationship between USP9X levels and immune infiltrates in the tumor microenvironment (TME). The results from the TIMER database showed a relationship between the expression level of USP9X and the infiltration of immune cells (Figure S1A), and we consistently observed that the expression level of USP9X was positively correlated with the infiltration of macrophages, CD4+ T cells, CD8+ T cells in lung adenocarcinoma (LUAD) and lung squamous cell carcinoma (LUSC) (Fig. 4D). Our analysis also revealed positive correlations between USP9X and immune checkpoint genes in LUAD and LUSC (Figure S1B). In summary, these results indicated that USP9X inhibited angiogenesis and induced immune infiltration in PSC.
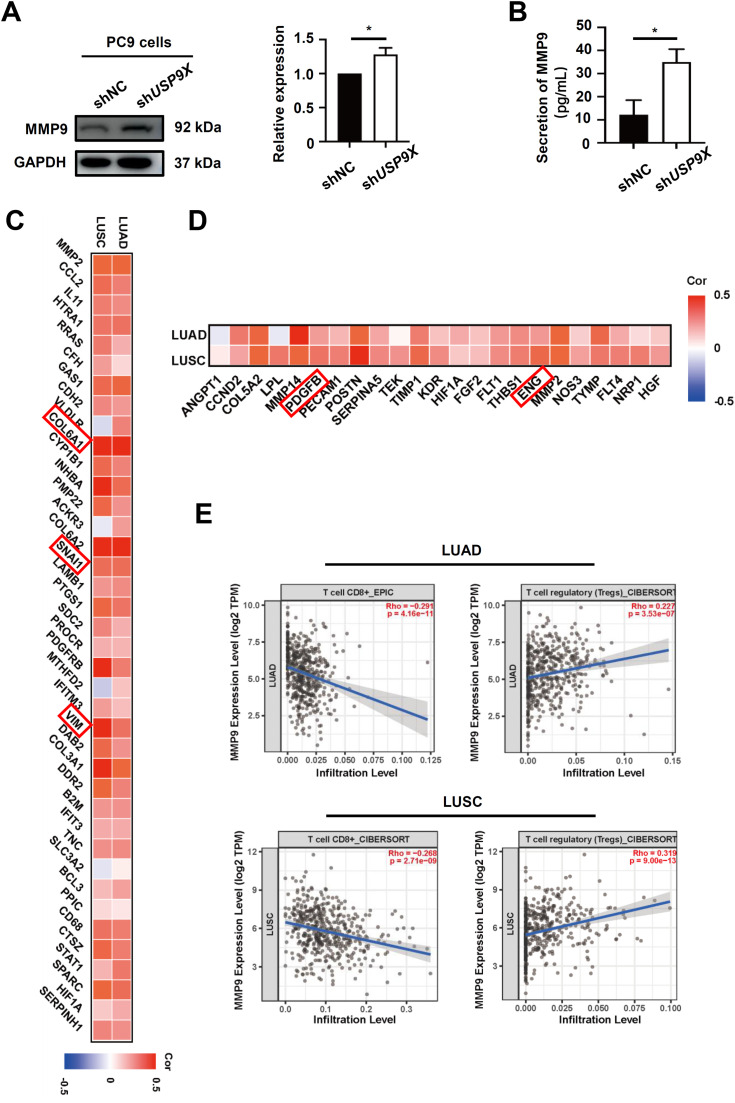
USP9X regulates EMT, angiogenesis and immune infiltration through suppressing MMP9
Since MMP9 plays an important role in tumorigenesis, invasion and metastasis [29], we evaluated the effect of USP9X on the cellular and secreted levels of MMP9 in TGFβ1-pretreated PC9 cells. The western blot results indicated that knocking down USP9X in PC9 cells significantly increased MMP9 protein levels (Fig. 5A). By using ELISA, we also found that USP9X negatively regulated MMP9 secretion in TGFβ1-pretreated PC9 cells (Fig. 5B). We subsequently explored the correlation of MMP9 with EMT, angiogenesis and immune infiltration in the NSCLC dataset. Notably, a relatively high positive correlation was observed between the mRNA expression levels of MMP9 and those of EMT-related molecules, such as COL6A1, Snail, and Vimentin (Fig. 5C). It was also found that most angiogenesis-related genes, such as Platelet-derived growth factor B chain, and Endothelin were positively correlated with MMP9 in NSCLC (Fig. 5D). Furthermore, we performed a correlation analysis of the TIMER database, which showed that the expression level of MMP9 was negatively correlated with the infiltration of CD8+ T cells but positively correlated with immunosuppressive regulatory T cells (Treg cells) in LUAD and LUSC (Fig. 5E). These results indicated that USP9X inhibited EMT, angiogenesis and induced immune infiltration through suppressing the level of MMP9.
Fig. 5.
USP9X regulates EMT, angiogenesis and immune infiltration through suppressing MMP9. (A) The protein level of MMP9 was determined using a western blot assay. (B) The levels of MMP9 in the culture media were quantified via ELISA. (C) Coexpression analysis of MMP9 and EMT-related molecules, angiogenesis-related molecules and infiltrating immune cells in NSCLC. (D) The mRNA level of MMP9 was positively correlated with angiogenesis-related genes in NSCLC. (E) MMP9 expression was positively associated with Treg cells, and negatively related to CD8+ T-cell infiltration in NSCLC. * P < 0.05.
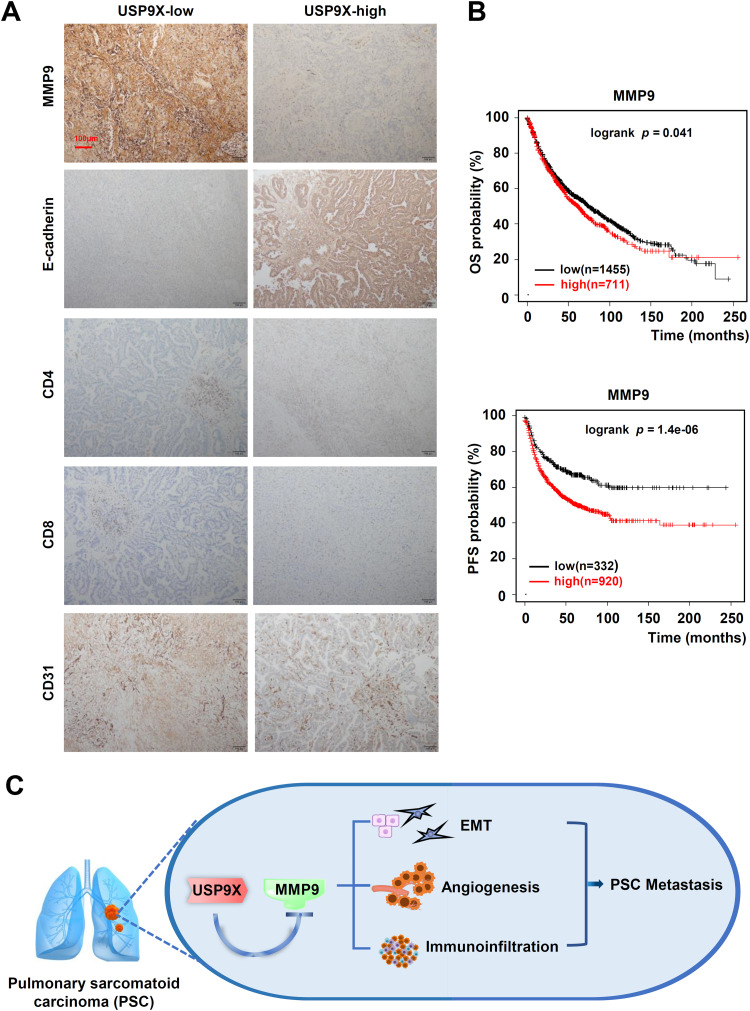
Correlations between USP9X/MMP9 and EMT, angiogenesis, and immune infiltration in PSC tissues
To determine the correlations between USP9X/MMP9 and EMT, angiogenesis, and immune infiltration, we detected the EMT markers, MMP9 expression, and immune cells numbers in PSC tissues using Immunohistochemistry (Fig. 6A). The expression of MMP9 in USP9X-high tissues was markedly lower than this in USP9X-low tissues, whereas the expression of E-cadherin was markedly higher. We also found that the numbers of CD4+ T cells and CD8+ T cells were higher in USP9X-high PSC tissues than in USP9X-low tissues.
Fig. 6.
Correlations between USP9X/MMP9 and EMT, angiogenesis, and immune infiltration in PSC tissues. (A) Representative IHC staining for MMP9, E-cadherin, CD4, CD8, and CD31 in PSC tissues of patients, which were divided into USP9X high-expression subgroup and USP9X low-expression subgroup. Red bar, 100 μm. (B) Kaplan-Meier analysis of OS and PFS in patients with NSCLC stratified by MMP9 expression. (C) Schematic diagram of the mechanism of USP9X in PSC metastasis. USP9X inhibits metastasis in pulmonary sarcomatoid carcinomas by regulating epithelial-mesenchymal transition, the tumor vascular system and immune infiltration.
CD31 is expressed on all vascular endothelial cells and is involved in cell adhesion and signal transduction. It has been reported that tumor-associated endothelial cells express typical endothelial cell markers, including CD31 [30]. Therefore, we evaluated the number of CD31-positive vessels in USP9X-high- and low-expression PSC tissues. Within the USP9X low-expression tissues, numerous CD31-positive vessels were observed, and the staining intensity was strong (Fig. 6A). Kaplan-Meier survival analysis revealed that patients in the MMP9-high subgroup had significantly worse outcomes than patients in the MMP9-low subgroup (Fig. 6B), indicating that MMP9 expression was positively associated with poor prognosis among PSC patients.
Discussion
PSC is a typically biphasic neoplasm that is highly invasive and has a poor prognosis compared with other subtypes of NSCLC [3,4]. Multiple molecular interactions regulated by complex key genes are involved in the process of PSC metastasis. Hence, there is an urgent need to identify the regulatory mechanisms of these key genes to provide promising strategies for the precise treatment of patients with PSC. Currently, growing evidence suggests that USP9X may serve as a potential therapeutic target in human cancers [31]. In this study, we first revealed the critical role of USP9X in the metastasis of PSC and its underlying mechanisms. Furthermore, we confirmed that USP9X is an important prognostic indicator for patients with PSC.
The main reason for poor outcome of patients with PSC is high rate of tumor metastasis. Accumulating evidence indicates that aberrant expression or function of DUBs in patients with cancer represents molecular signatures that not only account for tumor recurrence but also are involved in tumor metastasis [17]. USP9X has been reported to play vital roles in the progression of tumors, including breast cancer, lung cancer and glioblastoma [31]. However, the relationship between USP9X and PSC metastasis has not been determined. This study firstly explored the expression level and biological function of USP9X in PSC. Herein, we found that USP9X was expressed at significantly lower levels in PSC tissues than in adjacent normal tissues and that a low expression level of USP9X was significantly associated with poor prognosis in patients with NSCLC according to public datasets. In addition, we demonstrated that knockdown of USP9X induced migration and enhanced angiogenesis in PSC cells, indicating that low USP9X expression was significantly positively associated with tumor metastasis in PSC, revealing a new function of USP9X in tumor progression.
Distant metastasis involves the separation of tumor cells from their primary location, and EMT plays a significant role in this process, including promoting migration and invasion, which mainly occur in the circulatory or lymphatic system and the establishment of these tumor cells in new locations [32]. Among them, EMT is characterized by the downregulation of epithelial cell markers (E-cadherin and Claudin-1) and the upregulation of mesenchymal cell markers (N-cadherin and Vimentin) [33], and is an early event in tumor metastasis. A previous study shown that an EMT transcription factor Twist1 has been suggested to play a fundamental role in PSC metastasis [7]. However, the relationship between USP9X and EMT in PSC cells remains unclear. In this study, we found that USP9X resulted in an increase in E-cadherin expression and a decrease in Vimentin expression, inhibiting migration in PSC cells. Several studies have shown that MMP9 plays roles in tumor invasion, metastasis and tumor microenvironment regulation [20,21]. Here, we also found a positive relationship between MMP9 and EMT-related molecules in NSCLC in public databases. We confirmed that USP9X downregulated the expression of MMP9, while high expression of MMP9 was significantly positively associated with poor prognosis in patients with NSCLC. Furthermore, MMP9 was highly expressed in patients with USP9X-low PSC. These data indicate that USP9X inhibits the EMT and metastasis of PSC by downregulating MMP9.
Moreover, tumor metastasis can also be induced by angiogenesis, which exploits the endothelium by triggering the formation of abnormal, leaky vasculature [34]. In this study, we first examined the antiangiogenic effect of USP9X in PSC by using in vitro models. In tube formation experiments, we found that USP9X effectively interfered with the formation of tubular structures and new blood vessels around the aortic ring that was pretreated with CM from PSC cells. We also examined the negative correlation between USP9X expression and the expression of the canonical proangiogenic factor VEGF. In addition, CD31 was found to be highly expressed in patients with USP9X-low PSC. Moreover, studies have shown that MMP9 can regulate angiogenesis and participate in tumor metastasis [35]. In this study, we found a positive relationship between MMP9 and angiogenesis-related genes in NSCLC in a public database. The aforementioned results suggest that USP9X/MMP9 is a potential signaling pathway for inhibiting PSC metastasis angiogenesis.
The growth, invasion and metastasis of cancer are complex and dynamic processes that involve interactions with immune cells within the local microenvironment [14]. Approximately two-thirds of lung tumor-infiltrating immune cells are composed of T and B cells and other a fraction of immune cells [36]. These tumor-infiltrating lymphocytes are involved in the antitumor response within the PSC niche [37]. In this study, we found that USP9X expression was positively associated with immune cell infiltration in NSCLC. In addition, the numbers of CD4+ T cells and CD8+ T cells were higher in USP9X-high PSC tissues. Some studies have confirmed that MMP9 was related to immune infiltration in many diseases, such as hepatocellular carcinoma and spinal tuberculosis [38,39]. In the present study, the level of MMP9 was negatively correlated with immune cell infiltration in NSCLC via the TIMER database. These data indicate that USP9X participates in inducing immune infiltration by downregulating MMP9, which may be another important factor involved in inhibiting tumor metastasis.
The invasion and metastasis of cancer cells are complex and dynamic processes and are closely related with the tumor microenvironment [11,37]. In the present study, the role of USP9X on metastasis of PSC was firstly comprehensively demonstrated from EMT, angiogenesis and immune infiltration, providing a broad idea for solving PSC metastasis. However, our research has certain limitations. First, when exploring the relationship between USP9X and metastasis in PSC, the number of samples for Immunohistochemistry was insufficient. Second, we were unable to assess the benefit of targeting USP9X in patients with PSC owing to a lack of PSC patients in public databases. Therefore, patients with NSCLC were used as an alternative, and a small number of patients with PSC in our hospital were employed. Finally, the detailed mechanism by which USP9X regulates MMP9X has not been determined. It was needed to conduct further research on the indirectly or directly regulatory effects of USP9X on MMP9 in the future.
In summary, our results indicate that USP9X is significantly negatively associated with the metastasis of PSC. USP9X inhibits EMT, angiogenesis and boosts immune infiltration by downregulating MMP9 in PSC cells, and there have negative correlations between USP9X with MMP9, EMT, and angiogenesis in metastasis in PSC tissues, while USP9X is positively associated with immune infiltration. Therefore, USP9X may serve as a novel prognostic biomarker and provide a potential antimetastatic therapeutic target for the treatment of PSC.
Funding information
This study was supported by the grants from the National Natural Science Foundation of China (82272871 and 82172840), Gusu Health Talents Project of Suzhou Municipal Health Commission (Grant No. GSWS2021033 and GSWS2021037), the Natural Science Foundation of the Jiangsu Higher Education Institutions of China (Grant No. 21KJB320017), Jiangsu Key Laboratory of Neuropsychiatric Diseases (Grant No. KJS2111), Bethune Oncology Basic Research Program (Grant No. BCF-XD-JC-20221205-08), Jiangsu Research Hospital Association for Precision Medication (Grant No. JY202202 and JY202225).
Availability of data and materials
Data will be made available on request and the public databases referenced in the methods section were all open access.
Ethics approval statement
Informed consent was obtained from all patients and all the experimental protocols were approved by the Ethics Committee of Nanjing Medical University and conducted in accordance with the Helsinki Declaration. The animal experiments were authorized by the Institutional Animal Care and Use Committee of Nanjing Medical University and were strictly implemented in compliance with the ARRIVE guidelines.
CRediT authorship contribution statement
Qin Feng: Writing – original draft, Validation, Methodology. Qian Liu: Visualization, Methodology. Zi Liu: Writing – review & editing, Methodology, Data curation. Jianyu Xu: Visualization, Methodology, Data curation. Yang Yang: Visualization, Methodology. Ying Zhu: Visualization, Methodology. Guangxian Lu: Methodology. Guangjuan Xu: Funding acquisition, Data curation. Dan Wu: Visualization, Methodology. Feng Wang: Visualization, Methodology. Biao Liu: Methodology. Wenjuan Wang: Supervision, Conceptualization. Xinyuan Ding: Writing – review & editing, Supervision, Project administration, Funding acquisition, Data curation, Conceptualization.
Declaration of competing interest
The authors have no relevant financial or non-financial interests to disclose.
Acknowledgements
The authors thank the public databases, including TIMER2.0, GEPIA2, and Kaplan-Meier plotter for providing their platforms and the contributors for uploading their valuable datasets.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.tranon.2024.101950.
Contributor Information
Biao Liu, Email: georgebliu@126.com.
Wenjuan Wang, Email: wangwenjuan1110@163.com.
Xinyuan Ding, Email: aladdine@163.com.
Appendix. Supplementary materials
References
- 1.Santucci C, Carioli G, Bertuccio P, Malvezzi M, Pastorino U, Boffetta P, Negri E, Bosetti C, La Vecchia C. Progress in cancer mortality, incidence, and survival: a global overview [J] Eur. J. Cancer Prev. 2020;29(5):367–381. doi: 10.1097/CEJ.0000000000000594. [DOI] [PubMed] [Google Scholar]
- 2.Li Y, Yan B. He S. Advances and challenges in the treatment of lung cancer [J] Biomed. PharmacOther. 2023;169 doi: 10.1016/j.biopha.2023.115891. [DOI] [PubMed] [Google Scholar]
- 3.Li X, Wu D, Liu H, Chen J. Pulmonary sarcomatoid carcinoma: progress, treatment and expectations [J] Ther. Adv. Med. Oncol. 2020;12 doi: 10.1177/1758835920950207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blandin Knight S, Crosbie P A, Balata H, Chudziak J, Hussell T, Dive C. Progress and prospects of early detection in lung cancer [J] Open. Biol. 2017;7(9) doi: 10.1098/rsob.170070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Manzotti G, Torricelli F, Benedetta D, Lococo F, Sancisi V, Rossi G, Piana S, Ciarrocchi A. An Epithelial-to-Mesenchymal Transcriptional Switch Triggers Evolution of Pulmonary Sarcomatoid Carcinoma (PSC) and Identifies Dasatinib as New Therapeutic Option [J] Clin. Cancer Res. 2019;25(7):2348–2360. doi: 10.1158/1078-0432.CCR-18-2364. [DOI] [PubMed] [Google Scholar]
- 6.Jiang K, Zhao T, Shen M, Zhang F, Duan S, Lei Z, Chen Y. MiR-940 inhibits TGF-beta-induced epithelial-mesenchymal transition and cell invasion by targeting Snail in non-small cell lung cancer [J] J. Cancer. 2019;10(12):2735–2744. doi: 10.7150/jca.31800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu T, Zhao X, Zheng X, Zheng Y, Dong X, Zhao N, Liao S, Sun B. The EMT transcription factor, Twist1, as a novel therapeutic target for pulmonary sarcomatoid carcinomas [J] Int. J. Oncol. 2020;56(3):750–760. doi: 10.3892/ijo.2020.4972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang F, Li T, Han L, Qin P, Wu Z, Xu B, Gao Q, Song Y. TGFbeta1-induced down-regulation of microRNA-138 contributes to epithelial-mesenchymal transition in primary lung cancer cells [J] Biochem. Biophys. Res. Commun. 2018;496(4):1169–1175. doi: 10.1016/j.bbrc.2018.01.164. [DOI] [PubMed] [Google Scholar]
- 9.Yuan R, Fan Q, Liang X, Han S, He J, Wang Q Q, Gao H, Feng Y, Yang S. Cucurbitacin B inhibits TGF-beta1-induced epithelial-mesenchymal transition (EMT) in NSCLC through regulating ROS and PI3K/Akt/mTOR pathways [J] Chin. Med. 2022;17(1):24. doi: 10.1186/s13020-022-00581-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pantazaka E, Vardas V, Roumeliotou A, Kakavogiannis S, Kallergi G. Clinical Relevance of Mesenchymal- and Stem-Associated Phenotypes in Circulating Tumor Cells Isolated from Lung Cancer Patients [J] Cancers. (Basel) 2021;(9):13. doi: 10.3390/cancers13092158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xie S, Wu Z, Qi Y, Wu B, Zhu X. The metastasizing mechanisms of lung cancer: Recent advances and therapeutic challenges [J] Biomed. PharmacOther. 2021;138 doi: 10.1016/j.biopha.2021.111450. [DOI] [PubMed] [Google Scholar]
- 12.Carmeliet P, Jain R K. Molecular mechanisms and clinical applications of angiogenesis [J] Nature. 2011;473(7347):298–307. doi: 10.1038/nature10144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhao Y, Guo S, Deng J, Shen J, Du F, Wu X, Chen Y, Li M, Chen M, Li X, Li W, Gu L, Sun Y, Wen Q, Li J, Xiao Z. VEGF/VEGFR-Targeted Therapy and Immunotherapy in Non-small Cell Lung Cancer: Targeting the Tumor Microenvironment [J] Int. J. Biol. Sci. 2022;18(9):3845–3858. doi: 10.7150/ijbs.70958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Remark R, Becker C, Gomez J E, Damotte D, Dieu-Nosjean M C, Sautes-Fridman C, Fridman W H, Powell C A, Altorki N K, Merad M, Gnjatic S. The non-small cell lung cancer immune contexture. A major determinant of tumor characteristics and patient outcome [J] Am. J. Respir. Crit. Care Med. 2015;191(4):377–390. doi: 10.1164/rccm.201409-1671PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Remark R, Alifano M, Cremer I, Lupo A, Dieu-Nosjean M C, Riquet M, Crozet L, Ouakrim H, Goc J, Cazes A, Flejou J F, Gibault L, Verkarre V, Regnard J F, Pages O N, Oudard S, Mlecnik B, Sautes-Fridman C, Fridman W H, Damotte D. Characteristics and clinical impacts of the immune environments in colorectal and renal cell carcinoma lung metastases: influence of tumor origin [J] Clin. Cancer Res. 2013;19(15):4079–4091. doi: 10.1158/1078-0432.CCR-12-3847. [DOI] [PubMed] [Google Scholar]
- 16.Reyes-Turcu F E, Ventii K H, Wilkinson K D. Regulation and cellular roles of ubiquitin-specific deubiquitinating enzymes [J] Annu. Rev. Biochem. 2009;78:363–397. doi: 10.1146/annurev.biochem.78.082307.091526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Han S, Wang R, Zhang Y, Li X, Gan Y, Gao F, Rong P, Wang W, Li W. The role of ubiquitination and deubiquitination in tumor invasion and metastasis [J] Int. J. Biol. Sci. 2022;18(6):2292–2303. doi: 10.7150/ijbs.69411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xue S, Wu W, Wang Z, Lu G, Sun J, Jin X, Xie L, Wang X, Tan C, Wang Z, Wang W, Ding X. USP5 Promotes Metastasis in Non-Small Cell Lung Cancer by Inducing Epithelial-Mesenchymal Transition via Wnt/beta-Catenin Pathway [J] Front. Pharmacol. 2020;11:668. doi: 10.3389/fphar.2020.00668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Perez-Mancera P A, Rust A G, van der Weyden L, Kristiansen G, Li A, Sarver A L, Silverstein K A, Grutzmann R, Aust D, Rummele P, Knosel T, Herd C, Stemple D L, Kettleborough R, Brosnan J A, Li A, Morgan R, Knight S, Yu J, Stegeman S, Collier L S, ten Hoeve J J, de Ridder J, Klein A P, Goggins M, Hruban R H, Chang D K, Biankin A V, Grimmond S M, Australian Pancreatic Cancer Genome I, Wessels L F, Wood S A, Iacobuzio-Donahue C A, Pilarsky C, Largaespada D A, Adams D J, Tuveson D A. The deubiquitinase USP9X suppresses pancreatic ductal adenocarcinoma [J] Nature. 2012;486(7402):266–270. doi: 10.1038/nature11114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pego E R, Fernandez I, Nunez M.J. Molecular basis of the effect of MMP-9 on the prostate bone metastasis: A review [J] Urol. Oncol. 2018;36(6):272–282. doi: 10.1016/j.urolonc.2018.03.009. [DOI] [PubMed] [Google Scholar]
- 21.Kalali D. The Role of the Matrix Metalloproteinase-9 Gene in Tumor Development and Metastasis: A Narrative Review [J] Glob. Med. Genet. 2023;10(2):48–53. doi: 10.1055/s-0043-1768166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sullivan S, Tosetto M, Kevans D, Coss A, Wang L, O'Donoghue D, Hyland J, Sheahan K, Mulcahy H, O'Sullivan J. Localization of nuclear cathepsin L and its association with disease progression and poor outcome in colorectal cancer [J] Int. J. Cancer. 2009;125(1):54–61. doi: 10.1002/ijc.24275. [DOI] [PubMed] [Google Scholar]
- 23.Wang W J, Long L M, Yang N, Zhang Q Q, Ji W J, Zhao J H, Qin Z H, Wang Z, Chen G, Liang Z Q. NVP-BEZ235, a novel dual PI3K/mTOR inhibitor, enhances the radiosensitivity of human glioma stem cells in vitro [J] Acta Pharmacol. Sin. 2013;34(5):681–690. doi: 10.1038/aps.2013.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang W, Xiong Y, Ding X, Wang L, Zhao Y, Fei Y, Zhu Y, Shen X, Tan C, Liang Z. Cathepsin L activated by mutant p53 and Egr-1 promotes ionizing radiation-induced EMT in human NSCLC [J] J. Exp. Clin. Cancer Res. 2019;38(1):61. doi: 10.1186/s13046-019-1054-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huang, Fang J, Lu W, Wang Z, Wang Q, Hou Y, Jiang X, Reizes O, Lathia J, Nussinov R, Eng C, Cheng F. A Systems Pharmacology Approach Uncovers Wogonoside as an Angiogenesis Inhibitor of Triple-Negative Breast Cancer by Targeting Hedgehog Signaling [J] Cell Chem. Biol. 2019;26(8):1143–1158. doi: 10.1016/j.chembiol.2019.05.004. e1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kanugula A K, Adapala R K, Jamaiyar A, Lenkey N, Guarino B D, Liedtke W, Yin L, Paruchuri S, Thodeti C.K. Endothelial TRPV4 channels prevent tumor growth and metastasis via modulation of tumor angiogenesis and vascular integrity [J] Angiogenesis. 2021;24(3):647–656. doi: 10.1007/s10456-021-09775-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nicosia R.F. The aortic ring model of angiogenesis: a quarter century of search and discovery [J] J. Cell Mol. Med. 2009;13(10):4113–4136. doi: 10.1111/j.1582-4934.2009.00891.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wei C, Yang C, Wang S, Shi D, Zhang C, Lin X, Liu Q, Dou R, Xiong B. Crosstalk between cancer cells and tumor associated macrophages is required for mesenchymal circulating tumor cell-mediated colorectal cancer metastasis [J] Mol. Cancer. 2019;18(1):64. doi: 10.1186/s12943-019-0976-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jiang W G, Sanders A J, Katoh M, Ungefroren H, Gieseler F, Prince M, Thompson S K, Zollo M, Spano D, Dhawan P, Sliva D, Subbarayan P R, Sarkar M, Honoki K, Fujii H, Georgakilas A G, Amedei A, Niccolai E, Amin A, Ashraf S S, Ye L, Helferich W G, Yang X, Boosani C S, Guha G, Ciriolo M R, Aquilano K, Chen S, Azmi A S, Keith W N, Bilsland A, Bhakta D, Halicka D, Nowsheen S, Pantano F, Santini D. Tissue invasion and metastasis: Molecular, biological and clinical perspectives [J] Semin. Cancer Biol. 2015;35:S244–S275. doi: 10.1016/j.semcancer.2015.03.008. Suppl. [DOI] [PubMed] [Google Scholar]
- 30.Maishi N, Hida K. Tumor endothelial cells accelerate tumor metastasis [J] Cancer Sci. 2017;108(10):1921–1926. doi: 10.1111/cas.13336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Meng Y, Hong C, Yang S, Qin Z, Yang L, Huang Y. Roles of USP9X in cellular functions and tumorigenesis (Review) [J] Oncol. Lett. 2023;26(6):506. doi: 10.3892/ol.2023.14093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Huang Y, Hong W, Wei X. The molecular mechanisms and therapeutic strategies of EMT in tumor progression and metastasis [J] J. Hematol. Oncol. 2022;15(1):129. doi: 10.1186/s13045-022-01347-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Polyak K, Weinberg R.A. Transitions between epithelial and mesenchymal states: acquisition of malignant and stem cell traits [J] Nat. Rev. Cancer. 2009;9(4):265–273. doi: 10.1038/nrc2620. [DOI] [PubMed] [Google Scholar]
- 34.Jiang X, Wang J, Deng X, Xiong F, Zhang S, Gong Z, Li X, Cao K, Deng H, He Y, Liao Q, Xiang B, Zhou M, Guo C, Zeng Z, Li G, Li X, Xiong W. The role of microenvironment in tumor angiogenesis [J] J. Exp. Clin. Cancer Res. 2020;39(1):204. doi: 10.1186/s13046-020-01709-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yang L Q, Yu S P, Yang Y T, Zhao Y S, Wang F Y, Chen Y, Li Q H, Tian P, Zhu Y Y, Zhang J G, Lin G.Q. Muscone derivative ZM-32 inhibits breast tumor angiogenesis by suppressing HuR-mediated VEGF and MMP9 expression [J] Biomed. PharmacOther. 2021;136 doi: 10.1016/j.biopha.2021.111265. [DOI] [PubMed] [Google Scholar]
- 36.Gajewski T F, Schreiber H, Fu Y X. Innate and adaptive immune cells in the tumor microenvironment [J] Nat. Immunol. 2013;14(10):1014–1022. doi: 10.1038/ni.2703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang C, Li Z, Zhang Y, Zhao C, Wang H, Lin J, Liu C, Wang X, Wang H. Genomic Variations and Immune-Related Features of TMB, PD-L1 Expression and CD8(+) T Cell Infiltration in Chinese Pulmonary Sarcomatoid Carcinoma [J] Int. J. Gen. Med. 2022;15:4209–4220. doi: 10.2147/IJGM.S357659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ding H, Hu H, Tian F, Liang H. A dual immune signature of CD8+ T cells and MMP9 improves the survival of patients with hepatocellular carcinoma [J] Biosci. Rep. 2021;(3):41. doi: 10.1042/BSR20204219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhou C, Liang T, Jiang J, Chen J, Chen T, Huang S, Chen L, Sun X, Chen W, Zhu J, Wu S, Fan B, Liu C, Zhan X. MMP9 and STAT1 are biomarkers of the change in immune infiltration after anti-tuberculosis therapy, and the immune status can identify patients with spinal tuberculosis [J] Int. Immunopharmacol. 2023;116 doi: 10.1016/j.intimp.2022.109588. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request and the public databases referenced in the methods section were all open access.