SUMMARY
Increased values of arsenic in potable water in eastern Croatia has been a matter of scientific interest for the past two decades due to numerous health effects, including carcinogenic ones. This study investigated whether prolonged exposure to increased arsenic from water could be detectable through increased arsenic in urine, and whether it influenced the incidence of kidney and bladder cancer in Osijek-Baranja County. Inductively coupled plasma mass spectrometry (ICP-MS) was used for analysis of water samples from available water sources (wells, aqueducts). In addition, examinees from Osijek, Našice, Vladislavci, Čepin and Dalj gave their urine samples for analysis. Data on cancer incidence were obtained from the Institute for Public Health Registry and cumulative incidence of kidney and bladder cancer was calculated for the period between January 1, 2000 and December 31, 2018. Elevated arsenic concentration in drinking water was recorded in Vladislavci, Čepin and Osijek area with values above the allowed maximum according to the EU standards (10 µg L-1) and as a result, arsenic levels in urine of the inhabitants were also elevated. Cumulative incidence for bladder cancer showed correlation between increased arsenic in water and urine in the areas affected by increased arsenic in water. Epidemiologic data suggest a conclusion that elevated arsenic could be considered at least as a cofounding factor for urinary tract cancer.
Key words: Water, Urine, Arsenic, Kidney, Bladder
Introduction
Arsenic (As) represents one of the most dangerous geocontaminants of modern times. Presence of elevated arsenic in local water supplies poses significant public health problems, raising public concern. For the past two decades, arsenic in the east Slavonian water supply, in particular wider Osijek and Vinkovci area, was investigated and published, causing great concern among local inhabitants (1, 2). Arsenic contaminated water sources have been reported across Europe, from Hungary, Romania, northern Serbia to Greece, from Slovakia to Spain and United Kingdom.
Arsenic contaminated water (with measured arsenic in water above 10 µg L-1) is still being used as drinking water in local aqueducts for public use, with local exemptions such as Zrenjanin, Vojvodina, which has banned using drinking water from local aqueduct since 2004 (3-6). In the Osijek wider area, using arsenic filtered water started in 2021.
Groundwater (and surface water) can be contaminated mainly because of the natural contamination and, rarely, due to anthropogenic activities. Natural contamination can be considered as natural hydrogeological characteristics of wider Pannonian region.
Increased concentrations of arsenic in water sources of eastern Croatia are considered as a hydrogeochemical quality of the soil in aqueducts of Drava and Danube basin. Recent geological surveys suggest that water sources around Sava have 10 times lower arsenic concentrations than Danube or Drava basins (7). Environmental arsenic pollution due to human activities includes metal industry, fossil fuels, wood, pharmaceutical or glass industry. Use of pesticides, herbicides and veterinary antibiotics based on arsenic also is an important source, as well as improper disposal of contaminated agricultural or industrial waste (8, 9).
The problem of elevated arsenic in waterways from parts of eastern Croatia represents a significant health problem and a potential risk to the health of the population. First reports of arsenic in drinking water of eastern Croatia were published in water and hair of the population of Vinkovci and Osijek area in 2005 (1, 2). According to previous research, elevated arsenic in the soil and green leafy vegetables (cabbage) was recorded mainly because of crop irrigation with arsenic contaminated water (10, 11).
In a study by Ćavar et al. in 2005 (1), the measured values of arsenic in the hair of examinees from Vinkovci and Osijek ranged from 1.74 to 4.31 µg kg-1, and those examinees were using water with arsenic levels ranging from 17.60 up to 611.89 µg L-1, respectively. In 2005, state regulations prescribed maximum allowed concentration of arsenic in water of 50 µg L-1, while the European Union (EU) allows 10 µg L-1 (12).
In a recently published paper, the authors correlated elevated arsenic in contaminated water in eastern Croatia with elevated arsenic concentrations in hair as an indicator of long-term exposure (13).
Studying arsenic levels is mainly important for its possible health effects, which are many. Health effects can be divided into acute and chronic. Arsenic has multiorgan toxic effects, including lung, bladder and skin cancers, gastrointestinal, cardiovascular and cerebrovascular disease, respiratory and vision systems (8, 14-16). Peripheral polyneuropathy is a common consequence of acute oral arsenic poisoning. Peripheral neuritis, cognitive and memory impairment have also been reported (17).
A special clinical entity and the most striking consequence of chronic arsenic exposure is blackfoot disease. This rare condition consists of circulatory impairment in the arms and legs, finally resulting in ischemia and gangrene. It is an endemic disease that occurs exclusively in Taiwan, where inhabitants are using water with high arsenic concentrations which usually range from 170 to 800 µg L-1 (an equivalent to a daily dose of 0.014 to 0.065 mg kg-1) (18, 19).
Arsenic is carcinogenic and prolonged exposure is linked with bladder and kidney cancer, particularly uroepithelium (20, 21). Winemakers from the Moselle area who consumed wine contaminated with pesticides containing arsenic from grapes had increased mortality from bladder cancer (22). Environmental studies have confirmed a correlation between mortality from bladder and kidney cancer and elevated arsenic in water of Taiwan. Subjects with signs of chronic arsenic exposure (skin cancer, blackfoot disease, etc.) also have a higher risk of bladder cancer, as do workers in copper smelters in the United States (US) and Japan (23-25).
Materials and Methods
Area of investigations
This research was part of the project entitled Investigation of the Long-Term Consequences of the War on the Health of the Population, launched by the Ministry of Science and Education, Republic of Croatia. The area of research is shown in Figure 1. Water and urine samples were collected from the locations in two towns, Osijek and Našice, and five neighboring municipalities/villages of Čepin, Dalj and Vladislavci (including Hrastin and Dopsin). In previous studies, some of these locations had elevated arsenic values in drinking water (1, 2, 7, 13).
Fig. 1.
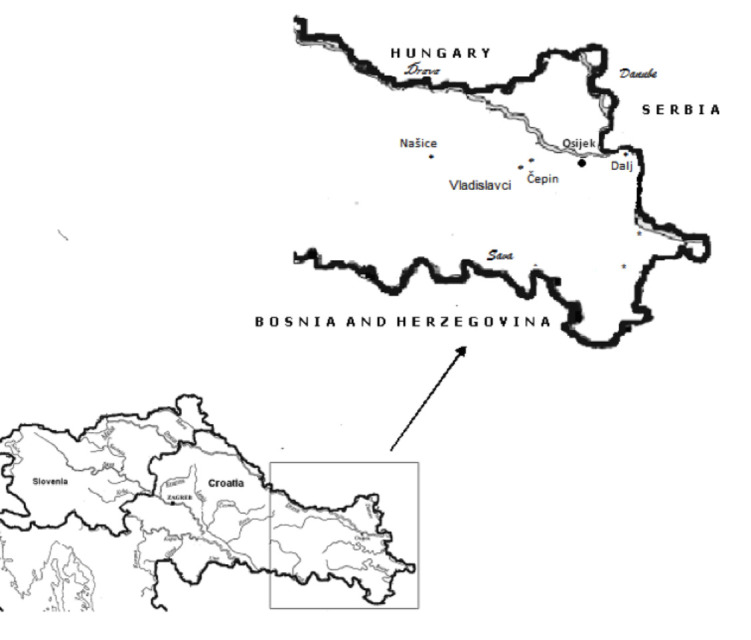
Map of sampling sites (author: Assist. Prof. V. Gvozdić, MD, PhD).
Urine sampling
Urine samples of 385 subjects were collected by a nurse and analyzed using inductively coupled plasma mass spectrometry (ICP-MS) with standardized protocol for arsenic (2, 13).
Water sampling
According to standardized protocol, water samples were taken from local aqueducts or wells used for drinking water and analyzed using ICP-MS. Sampling was performed during a one week period (2, 13).
Epidemiological data
Epidemiological data on the number and location of uropoietic cancer cases (kidney and bladder cases, in situ cancer of the bladder) were obtained from the National Cancer Registry kept at the Croatian Institute of Public Health in Zagreb, for the period from January 1, 2000 to December 31, 2018, for the examined locations. The number of inhabitants was determined using the national 2011 Census and total incidence rate was calculated per 100 000 inhabitants for a 19-year period giving cumulative incidence for the period (27).
Data analysis
Besides results on the mean arsenic concentrations in water and urine, data on cumulative incidence of uropoietic malignancies according to site per 100 000 inhabitants over the 19-year period (Table 1) were calculated by the principal component analysis method (PCA). Statistica software package, version 14.0.0.15 was used on data analysis.
Table 1. Mean arsenic concentrations in water and urine and cumulative incidence of uropoietic malignancies according to site per 100 000 inhabitants over a 19-year period (ICD 10).
| Parameter | N | Water (mean) µg L-1 |
N | Urine (mean) µg L-1 |
Urine (median) µg L-1 |
Urine (max) µg L-1 |
C64 | C67 | D09.01 |
|---|---|---|---|---|---|---|---|---|---|
| Location | |||||||||
| Osijek | 16 | 26.3 | 64 | 38.8 | 32.4 | 130.4 | 241 | 438 | 32.4 |
| Naπice | 10 | 0.4 | 80 | 23.3 | 19.8 | 73.2 | 160 | 277 | 6.2 |
| »epin | 13 | 184.5 | 52 | 114.8 | 70.2 | 351 | 172 | 370 | 17.2 |
| Dalj | 19 | 1.5 | 106 | 27.3 | 29.5 | 86.3 | 123 | 328 | 13.7 |
| Vladislavci | 12 | 16.3 | 83 | 34.5 | 24.2 | 108.8 | 158 | 371 | 0 |
C64 = kidney cancer; C67 = bladder cancer; D09.01 = urinary bladder in situ carcinoma
Results
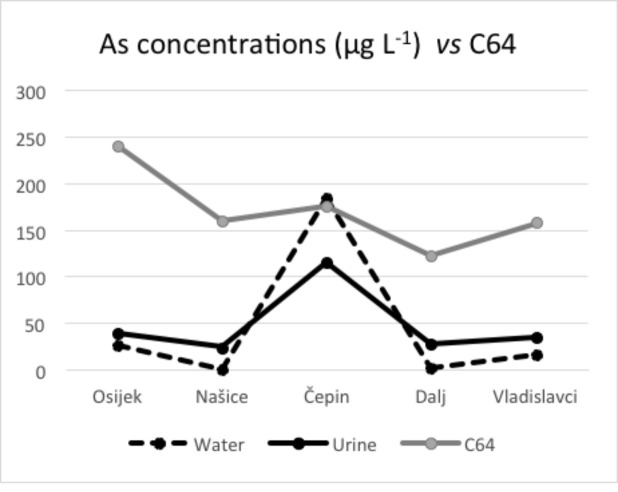
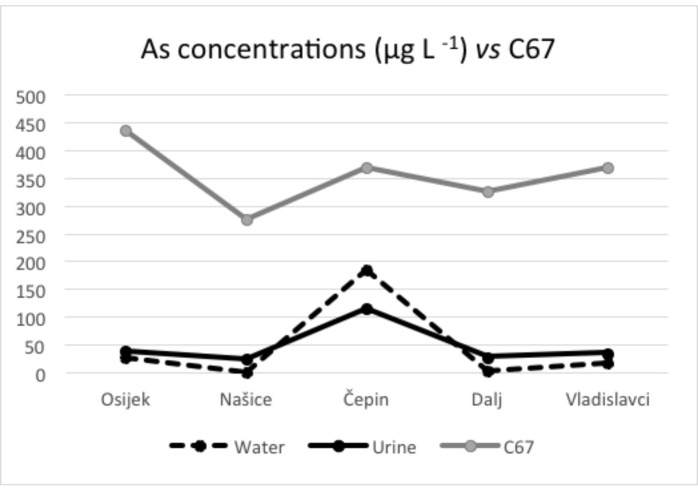
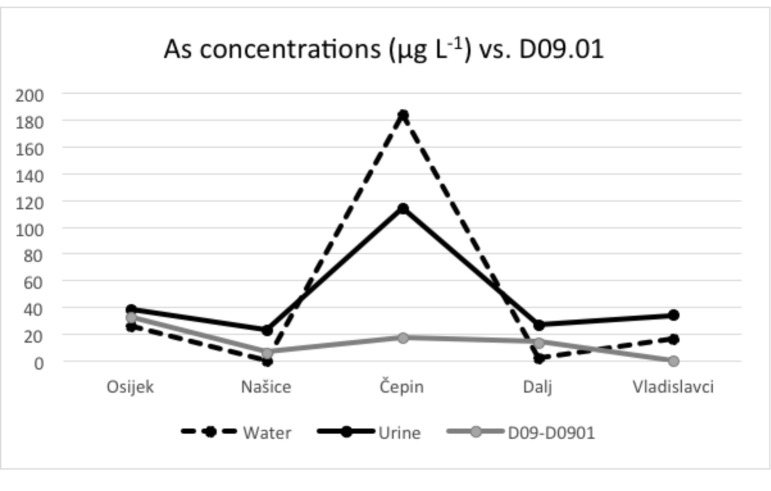
Arsenic concentrations in urine and drinking water presented in Figures 2-4 show similar pattern, thus leading to the conclusion that water and urine concentrations are strongly correlated. When these data are compared with the 19-year incidence of bladder cancer and in situ bladder cancer, it is visible that locations with higher arsenic concentrations in both urine and water have a higher incidence of these cancers, which is depicted in Figures 3 and 4. Kidney cancer, on the other hand, shows weaker pattern that could indicate possible relation with other carcinogenic sources (Fig. 2).
Fig. 2.
Mean arsenic concentrations in water and urine according to kidney cancer cases.
Fig. 3.
Mean arsenic concentrations in water and urine according to bladder cancer cases.
Fig. 4.
Mean arsenic concentrations in water and urine according to in situ bladder cancer cases.
Principal component analysis
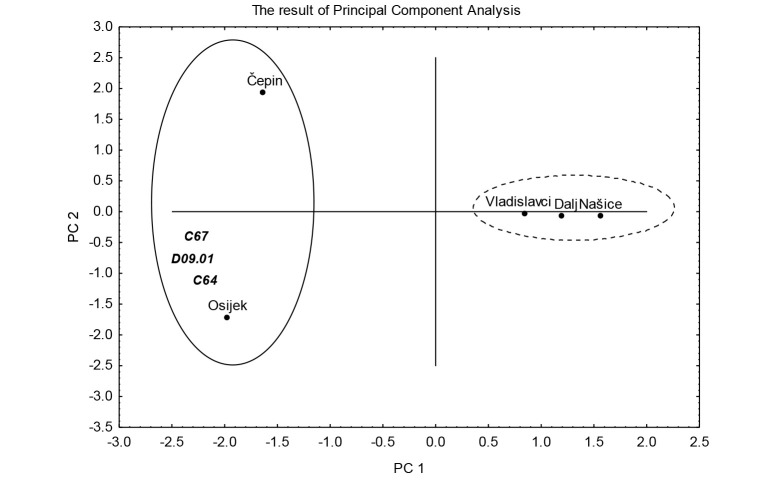
Principal component analysis results are shown in Figure 5. A combination of variables allowed 88% of variance, highly sufficient for explanation of data variations. Sampling sites are divided into two clusters; the right part of Figure 5 depicts the locations of Našice, Vladislavci and Dalj with low arsenic values in water and urine and lower cumulative incidence of uropoietic malignancies, whereas the two-member elongated cluster in the left part of Figure 5 contains the locations of Osijek and Čepin with higher arsenic concentrations and higher incidence of uropoietic malignancies.
Fig. 5.
Principal component analysis (PCA) biplot in the PC1/PC2 coordinate system. The result of PCA shows relationship between arsenic concentrations in water and urine according to kidney cancer, bladder and in situ bladder cancer cases at five locations in Osijek-Baranja County, Croatia.
Discussion
Renal and bladder cancer are the 13th and 14th most common newly detected cancer cases in Croatian population according to data from the 2018 Cancer Registry in Croatia, accounting for approximately 10% of all cancer cases in males (5% of renal and 5% of bladder cases). In Croatia, 873 new cases of renal and 1019 new cases of bladder cancer were registered in 2018. The standardized incidence rates were 24.9/100 000 for bladder cancer, (38.1 male vs. 12.6 female) and 21.4/100 000 for renal cancer (29.7 male vs.13.6 female). In Osijek-Baranja County, 53 new cases of bladder cancer and 49 new cases of renal cancer were reported in 2018, yielding the standardized incidence rate of 17.7/100 000 (male 24.0 vs. 11.8 female) and 19.1/100 000 (24.7 male vs. 13.9 female), respectively (28).
Renal cancer accounts for 2.4% of all cancer cases, with annual incidence of 330.000 new cases in the world, making it the 13th most common cancer worldwide (29). It is more common in the United States, Europe, Australia and Japan, twice more often in male than in female, and it is the 7th most common cancer in developed countries (30). The age standardized incidence rate for male is 15.6/100 000 in the US, 21.9/100 000 in Czech Republic and 18.7/100 000 in Lithuania (31). Croatia, however, has the renal cancer age standardized incidence rate of 21.4/100 000 for general population, with a higher incidence in males of 29.7/100 000 (28). Renal cancer is associated with tobacco smoking, alcohol consumption, occupational exposure to trichloroethylene, hypertension, chronic kidney disease, and diabetes mellitus (32).
Bladder cancer has annual incidence of 430 000 cases worldwide, making it the 9th most often cancer (33), predominantly in male (7th most common male cancer in the world) (34). Bladder cancer is considered as the 4th most common in US male population. In developed western countries, it has a 3-fold higher incidence as compared with developing countries of the third world (35). The US, Europe and Asia have the highest incidence rates, but mortality is significantly higher in developing countries, mainly due to the low level of health care (35). The risk factors for bladder cancer are tobacco and opium smoking, professional exposure (metal and dye industry, tobacco companies), alcohol consumption or presence of Schistosoma in endemic areas (36, 37).
In Croatia, bladder cancer has the incidence of 24.9/100 000 (male 38.1 and female 12.6/100 000) (28), higher than Latvia (12.5/100 000) but lower than China (35.1/100 000) (38, 39). Arsenic toxicity and carcinogenicity from arsenic in water is well documented but only at very high levels >200 µg L-1. At lower levels, especially under 50 µg L-1 or even 10 µg L-1, the risks and potential mechanisms are not so clear, especially from epidemiological perspective and it still needs to be established. Epidemiological studies suggest the possible relationship of bladder, liver, kidney and lung cancer with arsenic exposure (40, 41).
The maximal allowable concentration (MAC) of arsenic in drinking water is still a matter of discussion. In 2001, the United States Environmental Protection Agency (USEPA) declared it to be 10 µg L-1, but recently the same institution has proposed even lower dose-response slope for carcinogenic risk (5, 26). Meta regression analysis performed by Lynch et al. presented even lower values for risk assessment than the one proposed by USEPA, based on bladder and lung cancer epidemiological studies (42, 43).
In their comprehensive study, Tsuji et al. (44) performed a dose-response study to determine the risk of cancer and its relationship with arsenic in water. Based on animal models and in vitro study, they established a threshold for water at 65 µg L-1 and found it to correlate to in vivo effects that can cause bladder, lung and skin cancer. Finally, they suggest, with strong evidence, that the threshold for arsenic in potable water is ranging from 50 to 150 µg L-1 44.
Jayasumana et al. investigated chronic kidney disease in Sri Lanka and found that fertilizers based on phosphates were the main sources of arsenic and important cause of the disease (45). Bofetta and Borron (46) in their meta-analysis determined that, even in measured arsenic concentrations of up to 150 µg L-1, there was no evidence for increased lung or bladder cancer risk.
The population of east Croatia, based on water arsenic levels that exceed 10 µg L-1, are under prolonged exposure, and therefore, are at risk of potential toxic effects that arsenic causes (20, 47). Capak estimated that 74 177 men and 83 295 women from Osijek- Baranja County were to be considered at risk due to elevated arsenic in potable water, if applying MAC of 10 µg L-1 (48).
As shown in Figure 5, correlation between two locations known for higher arsenic in water (Čepin and Osijek) and arsenic concentrations in drinking water and urine, when correlated with the incidence of malignant tumors of uropoietic tract, clearly shows that higher arsenic concentrations in water and urine are linked with the increased incidence of renal and bladder cancer, and in situ cancer of the bladder.
Similar to this, Smith et al. explored cancer mortality in northern Chile, wider Antofagasta region, 40 years after the incident with arsenic in drinking water (49). They concluded even after reduction and 40-year period, there still was elevated incidence of cancer mortality, demonstrating long latency of arsenic carcinogenicity (49). Saint-Jacques et al. in their study investigated the relationship between measured arsenic in water under 50 µg L-1 and bladder cancer. They concluded that there was an 83% likelihood of elevated incidence and 74% likelihood of higher mortality. At higher values (150 µg L-1), mortality rates were about 30% higher than those at 10 µg L-1 for bladder and renal cancer. According to their study, even exposure to 10 µg L-1 (MAC according to Croatian, EU and US legislation) can elevate the risk of bladder cancer by 40% (50).
Conclusion
Cumulative incidence of bladder and renal cancer indicates the possible relationship between elevated arsenic in water and urine over the 19-year period. Despite the fact that standardized and total incidence rates per year in Osijek-Baranja County do not exceed other national and international levels, a long-term epidemiological study still indicates the possible link between elevated values of arsenic in potable water and bladder cancer in larger, or kidney cancer in smaller extent for the population of Čepin, Vladislavci and Osijek. With the introduction of arsenic filtered water in public water supply, trends in the number and incidence of arsenic related urinary tract cancer should be reduced over a longer period of time. All this should be implemented in a broader frame of public health measures, preventive actions at the levels of family medicine, primary health care and population of these areas in general. Further extended epidemiological research is needed.
References
- 1.Ćavar S, Klapec T, Grubešić RJ, Valek M. High exposure to arsenic from drinking water at several localities in eastern Croatia. Sci Total Environ. 2005. March;339(1-3):277–82. 10.1016/j.scitotenv.2004.12.013 [DOI] [PubMed] [Google Scholar]
- 2.Jergović M, Miškulin M, Puntarić D, Gmajnić R, Milas J, Sipos L. Cross-sectional Biomonitoring of metals in adult populations in post-war eastern Croatia: differences between areas of moderate and heavy combat. Croat Med J. 2010. October;51(5):451–60. 10.3325/cmj.2010.51.451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jovanovic D, Jakovljević B, Rašić-Milutinović Z, Paunović K, Peković G, Knežević T. Arsenic occurrence in drinking water supply systems in ten municipalities in Vojvodina Region, Serbia. Environ Res. 2011;111(2):315–8. 10.1016/j.envres.2010.11.014 [DOI] [PubMed] [Google Scholar]
- 4.Kostik V. Distribution of the total arsenic content in drinking water obtained from different water sources in the Republic of Macedonia. J Food Nutr Sci. 2014;2(4):146. 10.11648/j.jfns.20140204.18 [DOI] [Google Scholar]
- 5.Hough RL, Fletcher T, Leonardi GS, Goessler W, Gnagnarella P, Clemens F, et al. Lifetime exposure to arsenic in residential drinking water in Central Europe. Int Arch Occup Environ Health. 2010;83(5):471–81. 10.1007/s00420-010-0519-1 [DOI] [PubMed] [Google Scholar]
- 6.Middleton DR, Watts MJ, Hamilton EM, Fletcher T, Leonardi GS, Close RM, et al. Prolonged exposure to arsenic in UK private water supplies: toenail, hair and drinking water concentrations. Environ Sci Process Impacts. 2016. May 18;18(5):562–74. 10.1039/C6EM00072J [DOI] [PubMed] [Google Scholar]
- 7.Ujević M, Duić Ž, Casiot C, Sipos L, et al. Occurrence of geochemistry of arsenic in the groundwater of eastern Croatia. Appl Geochem. 2010 Apr;(25)1017-29:55-66. doi: 10.1016/j.apgeochem.2010.04.008 10.1016/j.apgeochem.2010.04.008 [DOI]
- 8.ATSDR, Agency for Toxic Substances and Disease Registry. Toxicological Profile for Arsenic. Draft for public comment. Atlanta, GA, USA; 2005. [PubMed] [Google Scholar]
- 9.Benramdane L, Bressolle F, Vallon JJ. Arsenic speciation in humans and food products: a review. J Chromatogr Sci. 1999. September 1;37(9):330–44. 10.1093/chromsci/37.9.330 [DOI] [PubMed] [Google Scholar]
- 10.Vidosavljević D, Puntarić D, Gvozdić V, Jergović M, Juričev-Savičević A, Puntarić E, et al. Trace metals in the environment and population as possible long term consequence of war in Osijek-Baranja County, Croatia. Coll Antropol. 2014;38:925–32. [PubMed] [Google Scholar]
- 11.Bijelić L, Puntarić D, Gvozdić V, Vidosavljević D, Jurić D, Lončarić Z, et al. Presence of war related elements in dandelion (Taraxacum officinale) as a possible consequence of military activities in east Croatia. Acta Agricult Scand – B Soil. Plant Sci. 2018. April 3;68(3):264–72. 10.1080/09064710.2017.1394485 [DOI] [Google Scholar]
- 12.Republic of Croatia Official Gazette. Regulation on drinking water health safety. Narodne Novine, Zagreb; 2008. (in Croatian) [Google Scholar]
- 13.Vidosavljevic M, Puntaric D, Gvozdic V, Vidosavljevic D, Juric D, Begovic L. Assessment of arsenic in hair of the inhabitants of east Croatia – relationship to arsenic concentrations in drinking water. Water. 2022;14:1558. 10.3390/w14101558 [DOI] [Google Scholar]
- 14.Cohen SM, Arnold LL, Beck BD, Lewis AS, Eldan M. Evaluation of the carcinogenicity of inorganic arsenic. Crit Rev Toxicol. 2013. October 16;43(9):711–52. 10.3109/10408444.2013.827152 [DOI] [PubMed] [Google Scholar]
- 15.Navas-Acien A, Sharrett AR, Silbergeld EK, Schwartz BS, Nachman KE, Burke TA, et al. Arsenic exposure and cardiovascular disease: a systematic review of the epidemiologic evidence. Am J Epidemiol. 2005;162:1037–49. 10.1093/aje/kwi330 [DOI] [PubMed] [Google Scholar]
- 16.Kaufman JA, Mattison C, Fretts AM, Umans JG, Cole SA, Voruganti VS, et al. Arsenic, blood pressure, and hypertension in the Strong Heart Family Study. Environ Res. 2021. April;195:110864. 10.1016/j.envres.2021.110864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen Y, Parvez F, Gamble M, Islam T, Ahmed A, Argos M, et al. Arsenic exposure at low-to-moderate levels and skin lesions, arsenic metabolism, neurological functions, and biomarkers for respiratory and cardiovascular diseases: review of recent findings from the Health Effects of Arsenic Longitudinal Study (HEALS) in Bangladesh. Toxicol Appl Pharmacol. 2009. September 1;239(2):184–92. 10.1016/j.taap.2009.01.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tseng WP. Effects and dose-response relationships of skin cancer and blackfoot disease with arsenic. Environ Health Perspect. 1977;19:109–19. 10.1289/ehp.7719109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chiou HY, Hsueh YM, Liaw KF, Horng SF, Chiang MH, Pu YS, et al. Incidence of internal cancers and ingested inorganic arsenic: a seven-year follow-up study in Taiwan. Cancer Res. 1995. March 15;55(6):1296–300. [PubMed] [Google Scholar]
- 20.IARC Working Group on the Evaluation of Carcinogenic Risks to Humans . Some drinking-water disinfectants and contaminants, including arsenic. IARC Monogr Eval Carcinog Risks Hum. 2004;84:1–477. [PMC free article] [PubMed] [Google Scholar]
- 21.Schuhmacher-Wolz U, Dieter HH, Klein D, Schneider K. Oral exposure to inorganic arsenic: evaluation of its carcinogenic and non-carcinogenic effects. Crit Rev Toxicol. 2009;39(4):271–98. 10.1080/10408440802291505 [DOI] [PubMed] [Google Scholar]
- 22.Lüchtrath H. The consequences of chronic arsenic poisoning among Moselle wine growers: pathoanatomical investigations of post-mortem examinations performed between 1960 and 1977. J Cancer Res Clin Oncol. 1983;105:173–82. 10.1007/BF00406929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tsai SM, Wang TN, Ko YC. Mortality for certain diseases in areas with high levels of arsenic in drinking water. Arch Environ Health. 1999;54:186–93. 10.1080/00039899909602258 [DOI] [PubMed] [Google Scholar]
- 24.Tsuda T, Babazono A, Yamamoto E, Kurumatani N, Mino Y, Ogawa T, et al. Ingested arsenic and internal cancer: a historical cohort study followed for 33 years. Am J Epidemiol. 1995;141(3):198–209. 10.1093/oxfordjournals.aje.a117421 [DOI] [PubMed] [Google Scholar]
- 25.Lamm SH, Ferdosi H, Dissen E, Li J, Ahn J. A systematic review and meta-regression analysis of lung cancer risk and inorganic arsenic in drinking water. Int J Environ Res Public Health. 2015. December 7;12(12):15498–515. 10.3390/ijerph121214990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.U.S. Environmental Protection Agency. National primary drinking water regulations: arsenic and clarifications to compliance and new source contaminants monitoring Final Rule. Fed Reg 66:6976-7066 USA: USEPA; 2011. [Google Scholar]
- 27.National bureau of Statistics. Republic of Croatia. National Census for 2011. Available at: www.dzs.hr; accessed Mar 11, 2022.
- 28.Croatian Institute of Public Health. Cancer Registry. Cancer incidence for 2018. Bulletin 43, Zagreb, Croatia, 2020. [Google Scholar]
- 29.Ferlay J, Soerjomataram I, Ervik M, Dikshit R, Eser S, Mathers C, et al., editors. IARC: GLOBOCAN 2012: Estimated Cancer Incidence, Mortality and Prevalence Worldwide in 2012 v1. 0, Base No. 11. IARC Scientific Publications, Lyon, IARC. [Google Scholar]
- 30.Forman D, Bray F, Brewster DH, Gombe Mbalawa C, Kohler B, Piñeros M, et al., editors. Cancer Incidence in Five Continents, Vol. X. IARC Scientific Publications, No. 164, Lyon, IARC, 2013. [Google Scholar]
- 31.Antoni S, Ferlay J, Soerjomataram I, Znaor A, Jemal A, Bray F. Bladder cancer incidence and mortality: a global overview and recent trends. Eur Urol. 2017;71(1):96–108. 10.1016/j.eururo.2016.06.010 [DOI] [PubMed] [Google Scholar]
- 32.Scelo G, Larose TL. Epidemiology and risk factors for kidney cancer. J Clin Oncol. 2018. October 29;36(36):JCO2018791905. 10.1200/JCO.2018.79.1905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018. January;68(1):7–30. 10.3322/caac.21442 [DOI] [PubMed] [Google Scholar]
- 34.Greiman AK, Rosoff JS, Prasad SM. Association of human development index with global bladder, kidney, prostate and testis cancer incidence and mortality. BJU Int. 2017;120(6):799–807. 10.1111/bju.13875 [DOI] [PubMed] [Google Scholar]
- 35.Dy GW, Gore JL, Forouzanfar MH, Naghavi M, Fitzmaurice C. Global burden of urologic cancers, 1990-2013. Eur Urol. 2017;71(3):437–46. 10.1016/j.eururo.2016.10.008 [DOI] [PubMed] [Google Scholar]
- 36.Cumberbatch MGK, Jubber I, Black PC, Esperto F, Figueroa JD, Kamat AM, et al. Epidemiology of bladder cancer: a systematic review and contemporary update of risk factors in 2018. Eur Urol. 2018. December;74(6):784–95. 10.1016/j.eururo.2018.09.001 [DOI] [PubMed] [Google Scholar]
- 37.Rambau PF, Chalya PL, Jackson K. Schistosomiasis and urinary bladder cancer in north western Tanzania: a retrospective review of 185 patients. Infect Agent Cancer. 2013;8(1):19. 10.1186/1750-9378-8-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bitiņa-Barlote Ē, Plonis J, Cauce V, Vjaters E, Gardovskis J, Miklaπevi ËE. Nakazawa-MiklaπeviË M. Bladder cancer trends in Latvia during 1990-2017: incidence, mortality, and survival rates. Cent European J Urol. 2021;74(1):14–23. 10.5173/ceju.2021.0266.R2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li HZ, Zheng RS, Du LB, Zhang SW, Zhu C, Wei WW, et al. Bladder cancer incidence, mortality and temporal trends in China. Zhonghua Zhong Liu Za Zhi. 2021. March 23;43(3):293–8. 10.3760/cma.j.cn112152-20200421-00362 [DOI] [PubMed] [Google Scholar]
- 40.Thornton I, Farago M. The geochemistry of arsenic. In: Abernathy CO, Calderon RL, Chappell WR, editors. Arsenic: Exposure and Health Effects. London: Chapman and Hall, Kluwer Academic Publishers, 1997; p. 1-16. [Google Scholar]
- 41.Cohen SM, Arnold LL, Beck BD, Lewis AS, Eldan M. Evaluation of the carcinogenicity of inorganic arsenic. Crit Rev Toxicol. 2013. October 16;43(9):711–52. 10.3109/10408444.2013.827152 [DOI] [PubMed] [Google Scholar]
- 42.Lynch HN, Zu K, Kennedy EM, Lam T, Liu X, Pizzurro DM, et al. Quantitative assessment of lung and bladder cancer risk and oral exposure to inorganic arsenic: meta-regression analyses of epidemiological data. Environ Int. 2017. September;106:178–206. 10.1016/j.envint.2017.04.008 [DOI] [PubMed] [Google Scholar]
- 43.Lynch HN, Zu K, Kennedy EM, Lam T, Liu X, Pizzurro DM, et al. Corrigendum to “Quantitative assessment of lung and bladder cancer risk and oral exposure to inorganic arsenic: meta-regression analyses of epidemiological data”. Environ Int. 2017;106:178-206. Environ Int. 2017. December;109:195–6. 10.1016/j.envint.2017.10.004 [DOI] [PubMed] [Google Scholar]
- 44.Tsuji JS, Chang ET, Gentry PR, Clewell HJ, Boffetta P, Cohen SM. Dose-response for assessing the cancer risk of inorganic arsenic in drinking water: the scientific basis for use of a threshold approach. Crit Rev Toxicol. 2019;49(1):36–84. 10.1080/10408444.2019.1573804 [DOI] [PubMed] [Google Scholar]
- 45.Jayasumana C, Fonseka S, Fernando A, Jayalath K, Amarasinghe M, Siribaddana S, et al. Phosphate fertilizer is a main source of arsenic in areas affected with chronic kidney disease of unknown etiology in Sri Lanka. Springerplus. 2015. February 24;4(1):90. 10.1186/s40064-015-0868-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Boffetta P, Borron C. Low-level exposure to arsenic in drinking water and risk of lung and bladder cancer: a systematic review and dose-response meta-analysis. Dose Response. 2019. July 22;17(3):1559325819863634. 10.1177/1559325819863634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mink PJ, Alexander DD, Barraj LM, Kelsh MA, Tsuji JS. Low-level arsenic exposure in drinking water and bladder cancer: a review and meta-analysis. Regul Toxicol Pharmacol. 2008. December;52(3):299–310. 10.1016/j.yrtph.2008.08.010 [DOI] [PubMed] [Google Scholar]
- 48.Capak K. Exposure to arsenic in drinking water and its health effects. Doctoral dissertation. Rijeka: University of Rijeka, Croatia; 2012. (in Croatian) [Google Scholar]
- 49.Smith AH, Marshall G, Roh T, Ferreccio C, Liaw J, Steinmaus C. Lung, bladder, and kidney cancer mortality 40 years after arsenic exposure reduction. J Natl Cancer Inst. 2018. March 1;110(3):241–9. 10.1093/jnci/djx201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Saint-Jacques N, Parker L, Brown P, Dummer TJ. Arsenic in drinking water and urinary tract cancers: a systematic review of 30 years of epidemiological evidence. Environ Health. 2014. June 2;13:44. 10.1186/1476-069X-13-44 [DOI] [PMC free article] [PubMed] [Google Scholar]