Abstract
Type 1 diabetes (T1D) is a specific autoimmune disease related to genetic and autoimmune factors. Recent studies have found that the intestinal flora is one of the important environmental factors in the development of T1D. The gut microbiota is the largest microbiota in the human body and has a significant impact on material and energy metabolism. Related studies have found that the intestinal floras of T1D patients are unbalanced. Compared with normal patients, the abundance of beneficial bacteria is reduced, and various pathogenic bacteria are significantly increased, affecting the occurrence and development of diabetes. Medicinal and food homologous traditional Chinese medicine (TCM) has a multicomponent, multitarget, and biphasic regulatory effect. Its chemical composition can increase the abundance of beneficial bacteria, improve the diversity of the intestinal flora, reduce blood sugar, and achieve the purpose of preventing and treating T1D by regulating the intestinal flora and its metabolites. Therefore, based on a review of T1D, intestinal flora, and TCM derived from medicine and food, this review describes the relationship between T1D and the intestinal flora, as well as the research progress of TCM interventions for T1D through regulation of the intestinal flora. Medicine and food homologous TCM has certain advantages in treating diabetes and regulating the intestinal flora. It can be seen that there is still great research space and broad development prospects for the treatment of diabetes by regulating the intestinal flora with drug and food homologous TCM.
Keywords: type 1 diabetes, intestinal flora, mechanism, traditional Chinese medicine with same origin as medicine and food, beneficial bacteria
INTRODUCTION
Type 1 diabetes (T1D) is a specific autoimmune disease related to genetic and autoimmune factors. According to the latest statistics of the International Alliance for Diabetes, the incidence rate of T1D is on the rise year by year worldwide. There are about 1.1 million children and adolescents (0–19 years old) suffering from diabetes, and more than 50% of them have not been diagnosed with diabetes [1]. T1D is the main disease of childhood diabetes, and its pathogenesis is not yet clear. It is mainly caused by genetic and environmental factors. At present, the treatment methods for T1D include lifestyle intervention, local insulin injection, and oral hypoglycemic drugs. However, the main group of T1DM patients is adolescents and children, and blood sugar usually fluctuates in this group, as it is usually poorly controlled through diet and exercise. In addition, long-term use of insulin can cause a series of side effects, such as obesity, hypoglycemia, hyperinsulinemia, and injection site pain. Importantly, it can also lead to unhealthy psychology in adolescent patients [2]. Traditional Chinese medicine (TCM) has the advantages of low toxicity and adverse reactions, as well as mild effects. The idea that “medicine and food are homologous” has a long history and is a concept introduced by TCM [3, 4]. This is because our ancestors believed that TCM and food were interwoven. This is mainly due to the common root causes for which TCM products and food, which may bring multiple health benefits if combined, are used [4]. Nowadays, the concept of homology between medicine and food has gradually been accepted by the public. A large number of experiments have proved that TCM, which is derived from medicine and food, has a significant positive role in the treatment of T1D. These experimental results show that the TCM derived from food and medicine can effectively reduce the level of blood sugar and increase the secretion and sensitivity of insulin, thereby alleviating the symptoms of T1D [5]. Some studies have found that intestinal ecological imbalance is related to T1D [6]. The metabolites secreted by gut microbiota can regulate immune responses and slow down the development of T1D [7, 8]. In particular, the potential pathogenesis of T1D may occur via the reduction of pathogenic bacteria and the increase of probiotics. It can be seen that drug interventions targeting gut microbiota will provide new strategies for disease prevention. This article elaborates on the relationship between T1D and gut microbiota based on analysis of T1D, gut microbiota, and medicinal and food homologous TCM, as well as the research progress of TCM interventions for T1D via regulation of gut microbiota.
EXPLANATION OF T1D BY TRADITIONAL CHINESE AND WESTERN MEDICINE AND THE TREATMENT CONCEPT OF HOMOLOGOUS MEDICINE AND FOOD
Mechanism of Western medicine in the treatment of T1D
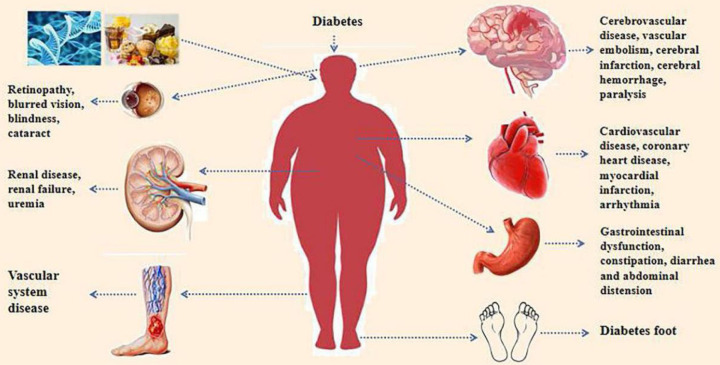
Diabetes mellitus (DM) is a metabolic disease with elevated blood sugar levels. Diabetes can lead to a variety of complications, including ischemic heart disease, peripheral vascular disease, cerebrovascular disease and other cardiovascular diseases, as well as retinopathy, nephropathy, neuropathy, and other diseases [9]. As shown in Fig. 1, diabetes can be divided into T1D, type 2 diabetes (T2D), gestational diabetes (GDM), and specific types of diabetes (American diabetes Association 2017) [10]. T1D is an autoimmune disease mainly mediated by effector T cells, which are activated by autoantigens, leading to islet destruction and insulin deficiency [11]. Western medicine usually adopts comprehensive measures, such as dietary control, moderate exercise, medication interventions, and blood pressure and lipid management to treat and prevent complications. During this period, there will inevitably be some treatment deficiencies and side effects, such as the poor effect of Western medicine in regulating overall body function. Many patients taking increasing doses and types of hypoglycemic drugs can prolong their condition, easily damage the liver and kidneys, and develop drug resistance, toxic side effects, and various complications [12]. Compared with Western medicine treatment, TCM treatment can improve the structural composition of intestinal microbiota and balance gray energy supply and demand and have the advantages of small side effects and strong safety and control [13].
Fig. 1.
Complications caused by diabetes.
Understanding and treatment concept of Xiao Ke disease in TCM
In TCM, DM belongs to the category of disease called “Xiao Ke” [14]. The meaning of the word Xiao Ke focuses on the word “Xiao”. Xiao refers to the function of the spleen and stomach “Xiaoshuigu” under normal conditions, as well as to the abnormal hyperactivity of the function under pathological conditions, resulting in thirst, which is also called “Xiao Ke”. In a broad sense, Xiao Ke disease is equivalent to diabetes, diabetes insipidus, mental polydipsia, polyuria, and other diseases in modern medicine. The main causes of diabetes include eating inappropriately, heat accumulation and injury yin, insufficient endowment, asthenia of five viscera, emotional depression, excessive will hurt, exertion internal injury, two qi of dryness and dampness, and six exogenous causes. In addition, there is also the pathogenesis of diabetes, including spleen dysfunction, deficiency of vital energy, phlegm, and blood stasis [15].
Under the influence of the abovementioned causes, phlegm and dampness form directly or indirectly in the body, causing massive depletion of the patient’s fluids. The deficiency of yin and fluid in the body makes it difficult to moisten the tendons and internal organs. This results in a deficiency of both qi and yin, causing the person to urinate more frequently, consume more food when eating, and drink more when thirsty. Therefore, the disease is both symptomatic and real, with dry heat as the symptom and yin deficiency as the basis. The clinical principles of TCM treatment are to nourish yin and clear heat, invigorate blood, and remove dampness and phlegm [16]. Diabetes has been treated using TCM for thousands of years. It focuses on regulation of the patient’s overall body functions and makes full use of the characteristics of TCM, such as mild and long-lasting therapeutic effects, fewer toxic side effects, and lower chance of produce producing drug resistance, thus providing the key to prevention and treatment of diabetes.
Concept of homology between medicine and food
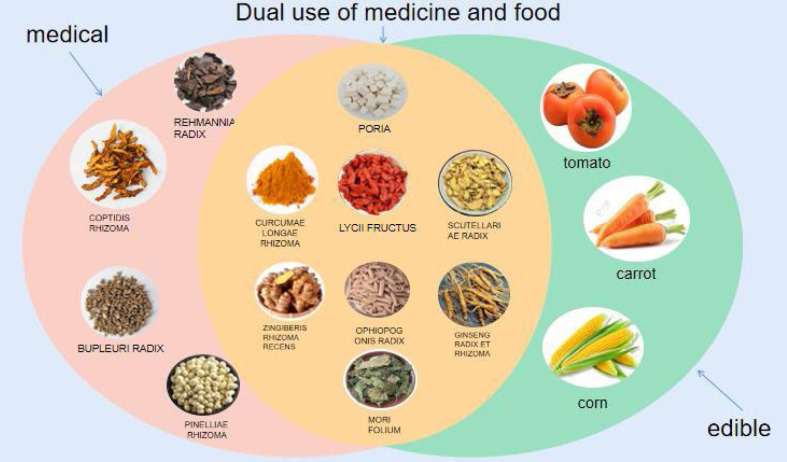
Traditional herbal therapies with multifunctional nutrients, known as “medicinal food homology”, have developed from ancient treatment systems [17, 18]. This concept can be traced back to 475–221 BC, and the “Huangdi Neijing” is China’s first medical theory monograph that collected many dietary remedies. In 500 AD, food therapy entered an important stage. At that time, the medical expert Sun Simiao wrote a book called “Thousand Golden Prescriptions”, which was a monograph on “dietary therapy”. The book systematically expounded the theory of dietary therapy for the first time and comprehensively elaborated on how to combine food and drugs to treat diseases. Therefore, from a development perspective, Chinese food and medicine were homologous in ancient times [17]. In addition to the ability to treat specific diseases, TCM with the same origin as medicine and food is also widely used for disease prevention due to its easy inclusion in our daily diets [19]. The concept of medicinal and food homologous TCM is shown in Fig. 2. Nowadays, the viewpoint of “medicine and food are of the same origin” has deeply rooted in people’s hearts.
Fig. 2.
Concept of homology between medicine and food.
Pink: medicinal herbs; Blue: food; Orange: traditional Chinese medicine with the same origin as medicine and food.
HUMAN BODY AND INTESTINAL FLORA
Overall relationship between the human body and intestinal flora
TCM believes that the various parts of the human body are organically connected; that is, the human body is an organic whole [20]. As a relatively complete medical system, TCM is based on several theories, including the theory of yin and yang, the theory of five elements (five elements), the theory of visceral states (zang fu), and the meridian system (referred to as meridians) [21]. It advocates maintaining the coordination and balance of the functions of various organs and systems in the human body, including the gut microbiota. The human gut microbiota is a rich microecosystem [22] that has developed together with its host through the process of evolution. TCM improves the body’s metabolism and immune function by regulating the gut microbiota. The gut microbiota can trigger the pharmacological activity or reduce the toxicity of drugs by regulating metabolism, thereby enabling TCM formulations to achieve the best therapeutic effect [23].
Overview of intestinal flora
In the human digestive system, there are many symbiotic microorganisms, approximately 1014 germs and about 1,000 bacterial species [24], which are collectively referred to as the “intestinal microbiota” [25]. Also known as the “second genome of the human body” [26], the intestinal microbiota is crucial for maintaining the integrity of the intestinal mucosal barrier function, absorbing nutrients, and regulating energy. The intestinal flora is mainly composed of 99% bacteria, as well as a small number of archaea, fungi, viruses, and protozoa [27]. These bacteria, which can be divided into beneficial bacteria, harmful bacteria, and neutral bacteria according to their functions, compete and restrict each other and maintain an average dynamic balance in healthy individuals [28,29,30,31,32]. Beneficial bacteria account for 30% of them, and they can promote intestinal peristalsis, excrete harmful substances, and prevent the invasion of pathogens, such as lactic acid bacteria and Pasteurella. Harmful bacteria account for 10% of them and can produce harmful substances. They can also increase the reabsorption of harmful substances in the intestines, resulting in slow intestinal peristalsis and colonization by pathogenic bacteria easier, such as Staphylococcus and Clostridium tetani. Finally, neutral bacteria account for 60% of them and are also known as conditional pathogenic bacteria. They are beneficial to health under normal conditions, but when their proliferation is out of control, they are invasive and harmful to the human body. They include bacteria such as Escherichia coli, Enterococcus, and Candida albicans. The main physiological functions of the intestinal microbiota are as follows: (1) It maintains the integrity and position of the gastrointestinal tract, including maintaining the protein structure and resisting pathogens. (2) It regulates immune and inflammatory responses. The intestinal microbiota not only regulates host immunity by producing a variety of metabolites but also regulates its cellular components, such as lipopolysaccharide (LPS), lipoteichoic acid (LTA), peptidoglycan, flagellin, and DNA. It can also participate in the immune pathways of the body by preventing the colonization of pathogens in the intestine, regulating the inflammatory state, activating the complement bypass pathway, neutralizing metabolic waste, and other functions. (3) It regulates metabolism, mainly by helping to decompose plant polysaccharides and resistant starch. It also produces metabolites, such as short-chain fatty acids (SCFAs), to provide various vitamins and essential amino acids to the body and reduces the accumulation of body toxins [33,34,35]. As shown in Table 1. The human intestinal flora undergoes a dynamic colonization and development process at different stages of life, and its composition is affected by anatomical location, time, and personal health status [36, 37]. The gene pool of the microbial inhabitants is considerably diverse and almost 100 times larger than the gene pool of the host. Intestinal microorganisms contribute functional genes and metabolites that affect host metabolism, immunity, and endocrine and other physiological processes. Therefore, it is increasingly recognized as a necessary and critical factor for maintaining health [38].
Table 1. Functional classification of gut microbiota.
| Beneficial bacteria | Neutral bacteria (conditional pathogenic bacteria) | Harmful bacteria | |
|---|---|---|---|
| Bifidobacterium | Escherichia coli | Staphylococcus sp. | |
| Lactobacillus beijerinck | Candida albicans | Shigella castellani | |
| Enterococcus | Enterococcus mundtii | Clostridium perfringen | |
| Akkermanisa | Bacteroides vulgatus | Pseudomonas aeruginosa | |
| Coprococcus | Actinomycetes | Salmonella minnesota | |
| Ruminococcus | |||
| Lactobacillus | |||
| Bifidobacterium | |||
| Butyric acid bacteria | |||
| Pectinolytic bacteria | |||
| Enterococcus | |||
| Pseudomonas aeruginosa | |||
| Role | Can promote intestinal peristalsis, excrete harmful substances, and prevent pathogen invasion. | Beneficial to health under normal circumstances. Nevertheless, once proliferation is out of control, they will be invasive and harmful to the human body. | May produce harmful substances. It can increase the reabsorption of harmful substances in the intestinal tract, resulting in slow intestinal peristalsis and vulnerability to pathogenic bacteria. |
RELATIONSHIP BETWEEN INTESTINAL FLORA IMBALANCE AND DISEASES
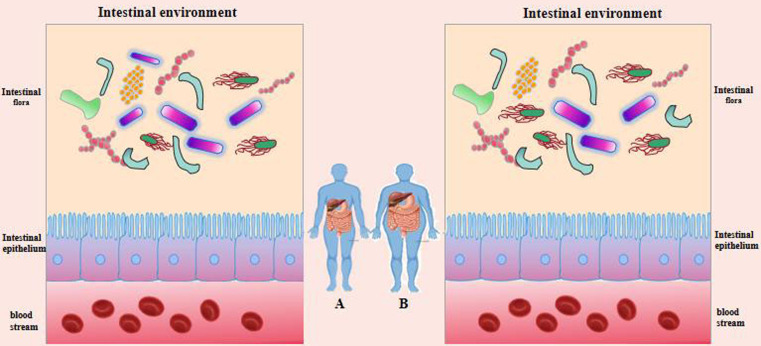
In most cases, gut bacteria thrive in a dynamically balanced environment that is rather stable [39]. By occupying and competing for nutrients in bacterial membrane barriers, Bifidobacterium spp., for instance, can prevent the colonization and proliferation of harmful bacteria, preserving human health. By secreting rhodobactin, Lactobacillus can prevent enterohemorrhagic E. coli, Salmonella, Shigella, and Vibrio cholerae [40]. Once this equilibrium is upset, dysbiosis will follow, which will cause pathological changes in the host [39]. As shown in Fig. 3, dysbiosis is caused by an imbalance in the type, amount, proportion, and location of gut bacteria. For example, the intestinal flora provides energy for the body and affects fat absorption by regulating enzymes and regulatory factors that play an important role in lipid metabolism. When the intestinal environment changes, the growth of normal bacteria, such as bifidobacteria, lactobacilli, and butyric acid-producing bacteria, is inhibited, while the number of enterobacteria increases, resulting in an imbalance of the intestinal flora. This disorder can lead to abnormal lipid metabolism, which in turn can further aggravate the imbalance of the intestinal flora and form a vicious circle [41, 42]. Other studies have found a close relationship between obesity and gut microbiota imbalance. Intestinal microbiota may obtain energy from the diet and participate in energy metabolism by regulating energy storage, fat generation, and fatty acid oxidation, thereby affecting the development of obesity [43]. Ortega et al. [44] pointed out that obesity and T2D have consistent risk factors and pathophysiological mechanisms, among which those of the gut microbiota play a core role in the occurrence of obesity-related T2D.
Fig. 3.
Distribution of intestinal flora in healthy people and diabetes patients.
Purple: beneficial bacteria, Other colors: pathogenic and harmful bacteria (A: healthy people, B:diabetes patients).
At the same time, the gut is the largest immune organ in the human body, with a large number of microorganisms coexisting with the host. The immune system and gut microbiota interact and co-evolve to maintain the immune homeostasis of the body. The immune system plays an important role in the formation and remodeling of gut microbiota [45]. When the homeostasis between microorganisms and the immune system is disrupted, autoimmune diseases may occur. More and more studies have found that gut microbiota disorders are associated with various autoimmune diseases, including inflammatory bowel disease, rheumatoid arthritis, systemic lupus erythematosus, allergic asthma, and T1D [46]. In addition, the development of some diseases can lead to imbalances in the gut microbiota, which can also trigger the occurrence of such diseases. For example, in ischemic stroke [47], animal and clinical studies have found that gut microbiota imbalance is an important pathophysiological outcome of ischemic stroke [48, 49]. The imbalance of the intestinal flora may be one of the causes of stroke caused by abnormal glucose metabolism, atherosclerosis, and other risk factors [50, 51].
RELATIONSHIP BETWEEN T1D AND IMBALANCE OF THE INTESTINAL FLORA
In populations with certain genetic backgrounds and environmental factors, disruption of microbiota balance can trigger autoimmune diseases [52]. Many studies have found that ecological imbalances are also involved in the pathogenesis of T1D [53]. This means that the onset and development of T1D are jointly driven by genetic susceptibility and environmental factors [54]. These environmental factors are all related to the construction of the gut microbiota during the development of infants and young children. A series of studies have shown an imbalance of the gut microbiota in T1D patients and animal models. Biobreeding diabetes-prone (BBDP) rats (biologically screened diabetes rats) are an important animal model for studying spontaneous diseases of T1D. There are many similarities between diabetes in BBDP rats and human T1D, including gene susceptibility and the impact of environmental factors on the disease. Compared with BBDR (biobreeding diabetes-resistant rat) rats, BBDP rats showed a decrease in Lactobacillus, Bryantella, Bifidobacterium, and Tubriciber, while Bacteroides, Eubacterium, and Ruminococcus increased. Consistent with animal experimental results, there are also differences in the gut microbiota between T1D patients and healthy controls, characterized by a decrease in the Firmicutes/Bacteroidetes ratio and an increase in Clostridium, Bacteroides, and Veillonella [55]. Moreover, the diversity and stability of gut microbiota in T1D patients also decreased. Demirci et al. [56] tested the levels of Bacillus and Bacteroidetes in 53 fecal samples from T1D patients. The results showed that compared with healthy individuals, the levels of Bacteroidetes in the gut microbiota of T1D patients increased. However, the level of Firmicutes decreased, and the Firmicutes/Bacteroidetes ratio significantly decreased. This indicates that there is an imbalance in the gut microbiota in the body before and after the onset of T1D. Buassoni et al. [57] compared the gut microbiota of 31 T1D children and 25 healthy children using 16s RNA sequencing technology. Their results showed that the abundance of Bacteroides and Proteus increased in children with T1D, while the abundance of Proteus decreased. Soyucen et al. [58] found that compared with healthy children, the number of bifidobacteria that colonized the intestines of T1D children was decreased, while the number of C. albicans and Enterobacteriaceae (excluding E. coli) was increased. Huang et al. [59] examined the intestinal microbiota in the feces of 12 Han (the main ethnic group in China and representing the vast majority of Chinese) children with T1D and compared it with that in healthy children. They found that the ratio of Bacteroidetes to Firmicutes in the intestinal microbiota of T1D children increased and that the abundance of fecal Bacteroidetes was negatively correlated with HbA1c levels. The abundance of Bacteroidetes was positively correlated with anti-pancreatic island autoantibodies. The above research indicates that intestinal microbiota imbalance is closely related to the onset of T1D. The gut microbiota can metabolize substances in the human diet that are difficult or impossible to digest and absorb and can produce metabolites that can be absorbed by the body, such as SCFAs, among which acetic acid, propionic acid, butyric acid, and their salts occupy a dominant position in the human gut [60, 61].
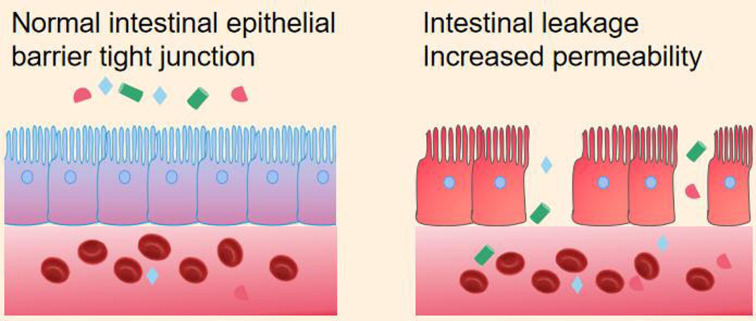
Sun et al. [62] found that compared with healthy controls, non-obese diabetic (NOD) mice showed a decrease in SCFAs, especially butyric acid. In T1D patients, there is a decrease in butyrate-producing bacteria compared with healthy individuals. Therefore, butyric acid-producing bacteria play an important role in T1D. Butyric acid plays an important role in maintaining tight junctions between intestinal epithelial cells by inducing mucin synthesis, such as affecting the barrier function of the intestinal epithelium. Its function is to limit the content of intestinal contents (water, chyme, intestinal microbiota) and regulate immune responses. Disruption of the integrity of the intestinal epithelium can lead to “gut leakage”, thereby activating Toll-like receptor 4 (TLR4) and participating in the occurrence and development of T1D. From this, it can be concluded that the intestinal epithelial barrier requires a tightly connected cell layer (Fig. 4).
Fig. 4.
Comparison of intestinal epithelial barriers.
On the left is the intact intestinal epithelium; on the right is the intestinal epithelium that flows out of the damaged intestinal content.
RESEARCH ON THE MECHANISM OF TCM, WHICH HAS THE SAME ORIGIN AS MEDICINE AND FOOD, REGULATING THE INTESTINAL FLORA TO INTERFERE WITH T1D
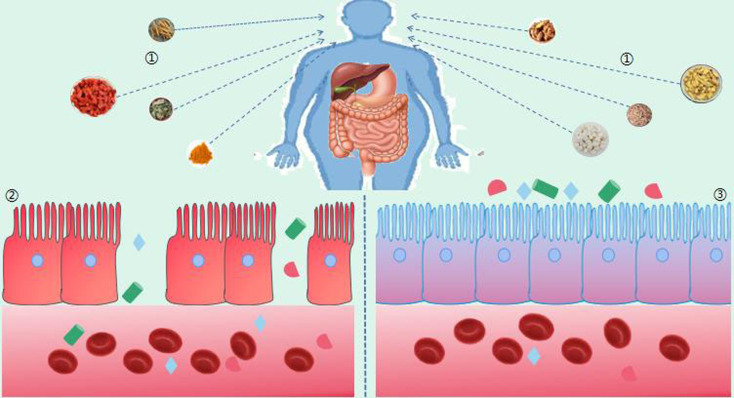
TCM has a long history of preventing and treating diabetes and has also achieved remarkable results in the field of medical care. In the process of searching for food, ancient sages discovered that various foods and medicines have the same taste and efficacy, and many foods can be used for both medicinal and edible purposes; that is, “medicine and food have the same origin”. Oral administration is the most important mode of administration in TCM. After entering the intestine, TCM inevitably comes into contact and interacts with the gut microbiota [63]. Research found that medicine and food homologous TCM can intervene and treat the development of T1D by regulating the intestinal flora. For example, Poria cocos, turmeric, ginger, Lycium chinense, Scutellaria baicalensis, Ophiopogon japonicus, ginseng, mulberry leaves, and other active ingredients of TCM can regulate the composition of the intestinal microbiota, increase beneficial bacteria, improve the intestinal microbiota, and further promote the conversion and utilization of bioactive ingredients of TCM. As shown in Table 2 and Fig. 5, it can be seen that there is a two-way relationship between the intestinal flora and TCM and that the intestinal flora is also a potential target of TCM for diabetes [61, 64].
Table 2. Regulatory effects of medicinal and food homologous traditional Chinese medicine components on intestinal microbiota associated with T1DM (Ginkgo leaf content has been replaced).
| Chinese herbal medicinal ingredient | Related Traditional Chinese Medicine | Regulating gut microbiota | Disease indicators | |
|---|---|---|---|---|
| Increase | Reduce | |||
| Pachyman pachymic acid | Poria cocos | Lactobacillus, Ruminococcus | Bacteroidetes | Reduce blood sugar and induce insulin like growth factor |
| curcumin | turmeric | Actinobacteria, Collinsella, Streptococcus, Sutterella, Gemella, Thalassospira, Gordonibacter, Actinomyces | Spirochaetae, Tenericutes, Elusimicrobia | The body weight, liver index, blood ALT, LPS, HOMA - IR, intestinal coliform bacteria and the number of beneficial bacteria in the intestinal tract were significantly reduced. |
| Ginger polysaccharides | ginger | Oscillospira, Adlercreutzia, Akkermansia, Lactobacillus | Prevotella, Oscillospira, Adlercreutzia | Bacteria that produce short chain fatty acids and have anti-inflammatory effects, enhancing intestinal barrier function. |
| Lycium barbarum polysaccharides | Lycium chinensis | Bacteroides, Ruminococcaceae_UCG-014, Mucispirillum, nestiimonas, Ruminococcaceae_UCG-009 | Allobaculum, Dubosiella, Romboutsia | Relieve hyperglycemia and insulin resistance, significantly alter intestinal composition, significantly increase levels of SCFAs, accompanied by an increase in SCFAs production genera |
| Baicalin | Scutellaria baicalensis | Akkermanisa, Coprococcus, Ruminococcus | Odoribacter, Parabacteroides | Increase the production of SCFAs and improve abnormal glucose and lipid metabolism. |
| Ophiopogon japonicus polysaccharides | Ophiopogon japonicus | Lactobacillus, Bifidobacterium | Escherichia coli,Streptococcus thermophilus | Improve dietary habits and lower blood sugar |
| ginsenoside | ginseng | Phylum Firmicutes, Bacteroidetes | Proteobacteria, Actinomycesbovis | Regulating intestinal immunity and promoting the repair of intestinal mucosa. |
| polysaccharide of mulberry leaves | mulberry leaves | Proteus hauser | Bacteroides vulgatus | Suppress the proliferation of harmful bacteria, restore intestinal function, and lower blood sugar. |
ALT: alanine transaminase; LPS: lipopolysaccharide; HOMA: homeostatic model assessment; IR: infrared; SCFAs: short-chain fatty acids.
Fig. 5.
Treatment of diabetes with TCM with the same origin as medicine and food by regulating the intestinal flora.
(1.TCM with same origin in medicine and food, 2. Intestinal leakage increased permeability, 3. Normal intestinal epithelial barrier tight junction).
Poria cocos
The dried sclerotia of the porous fungus P. cocos have functions such as beneficial effects on urination, spleen strengthening, and calming the nerves. The bioactive components in P. cocos include polysaccharides, triterpenes, fatty acids, sterols, and enzymes [65]. Consumption of P. cocos in China has a history of over two thousand years. The “Shennong Materia Medica Classic” states that it “soothes the soul and nourishes the soul for a long time, and does not starve and prolong the year” [66]. As a medicinal and edible homologous herb, P. cocos has medicinal tonifying properties but is not harsh, and its main components include triterpenes, polysaccharides, and fatty acids. In their research on the effects of P. cocos triterpenoids on intestinal epithelial integrity, scholars have found that P. cocos triterpenoids can improve intestinal barrier function [67]. Zhu et al. [68] found that P. cocos oligosaccharides (PCOs) can reduce blood sugar and insulin levels in high-fat diet (HFD)-induced obese mice. They also alleviated the damage to the gut barrier in HFD-fed mice, partially restored the imbalance of the gut microbiota in HFD-fed mice,including bile acid (BA), SCFA, and tryptophan metabolites. In recent years, studies have found that a P. cocos extract can treat T1D by regulating the intestinal flora. Liu et al. [69] found that after an intervention with a Poria cocos extract (PCE), PCOs can promote glucose consumption in Hep G2 cells of T1D model mice, exerting hypoglycemic effects. Triterpenoids can delay cell aging in mice, stimulate insulin secretion from pancreatic islet cells, and exert hypoglycemic effects. After the PCE intervention, the proportion of lactobacilli and rumen bacilli in the intestinal microbiota increased, improving the imbalance of the intestinal microbiota structure in the mice, enhancing immune regulation, inhibiting the development of intestinal inflammation, and thus alleviating progression of the disease. Wu et al. [70] found that administration of different extracts from Poria cocos effectively improved lipid metabolism in mouse intestinal microbiota dysbiosis models, repaired intestinal barriers, decreased the relative abundance of Firmicutes, and increased the relative abundance of Bacteroidetes, regulating the structure of the intestinal microbiota and increasing potential beneficial bacteria, such as Lactobacillus.
Turmeric
Turmeric is a perennial tuberous herbaceous plant with yellow flowers and wide leaves. It belongs to the ginger family and grows in tropical climates [71, 72]. TCM believes that turmeric is a type of TCM that has the effects of promoting qi and breaking blood, relieving pain through meridians, clearing the heart, and relieving depression [73]. Curcumin is a lipophilic polyphenol substance [74] that accounts for 2–5% of turmeric powder [74]. It has antioxidant, antibacterial, anti-inflammatory, antidiabetic, antiplatelet aggregation, and other characteristics [73, 75,76,77]. In some experimental animal models of diabetes, oral curcumin can reduce body weight, blood sugar, and glycosylated hemoglobin levels and improve insulin sensitivity. In patients with diabetes, curcumin treatment can reduce HbA1c and fasting blood glucose and improve pancreatic beta cell function. Some studies found that after treatment with curcumin, the abundances of Spirochaetae, Tenericutes, and Elusimicrobia at the phylum level in diabetes patients were significantly reduced. The abundances of Actinobacteria microbiota were significantly increased, with significant increases at the genus level in Collinsella, Streptococcus, Sutterella, Gemella, Thalassospira, Gordonibacter, and Actinomyces microbiota [78]. In the study of Liu et al. [79], as well as other studies, it was found that the flora structures of the normal control group and curcumin treatment group showed low Chlamydia/Bacteroides ratios and that the flora compositions and structures were similar. There was also a significantly increased proportion of Melainabacteria in the rectal contents of the diabetes model group, and this increase could be inhibited by curcumin perfusion. Hou et al. [80] found that the weight, liver index, and blood alanine transaminase (ALT), LPS, and HOMA-IR of rats fed a high-fat diet were significantly reduced when the rats subjected to a curcumin intervention. The coliform bacteria in the intestinal tract decreased, and the number of beneficial bacteria in the intestinal tract increased. This shows that curcumin can improve the imbalance of the intestinal flora in rats fed a high-fat diet.
Ginger
Ginger is a perennial herbaceous plant belonging to the family Zingiberaceae, order Zingiberales. Its rhizome is thick and branched, and it has a fragrant and spicy taste. It can be used for medicinal purposes, as a cooking ingredient, or in beverages and cosmetics. The Analects of Confucius state, “do not remove ginger food, do not eat too much”, and the Records of Famous Doctors state, “ginger can eliminate wind evil, cold and heat, typhoid fever, headache, nasal congestion... and stop vomiting” [81]. This indicates that since ancient times, ginger has been widely used as a food and medicine homologous material. Ginger polysaccharides are important components in ginger, with various biological activities, such as antioxidant activity [82], immune regulation [83], antitumor activity [84], anticoagulant activity [85], and hypoglycemic activity [86]. Wang et al. [87] found that ginger polysaccharide effectively reduced the blood sugar level of diabetic mice, increased the abundances of the beneficial bacteria Oscillospira, Adlercreutzia, Akkermansia, and Lactobacillus, and reduced the abundance of the harmful bacteria Prevotella. Among them, the genus Oscillospira is associated with obesity; Adlercreutzia is a bacterium that can produce SCFAs and has anti-inflammatory effects [88]. Akkermansia is a kind of mucin-degrading bacteria that colonizes the intestinal mucosa and can enhance the intestinal barrier function. It is negatively related to many diseases, such as obesity and diabetes [89]. Prevotella has been proven to induce insulin resistance [90]. To sum up, ginger polysaccharide can change the composition of the intestinal flora in diabetic mice and increase the abundance of relevant beneficial bacteria.
Lycium chinense
Lycium chinense is a member of the Solanaceae family and a famous Chinese herbal medicine with various benefits, including anti-inflammatory effects [91], immune regulatory effects [92], anticancer effects [93], blood sugar and lipid lowering effects [94], and antiaging effects [95]. Its fruit, goji berries, contains abundant endogenous molecules, such as polysaccharides, organic acids, alkaloids, flavonoids, and polyphenols, which are key to its biological effects [96, 97]. Research has shown [98,99,100] that polysaccharides may help regulate the composition of the gut microbiota and selectively increase the proportion of beneficial bacteria. Polysaccharides are important functional components of goji berries, with biological activities such as metabolism, antioxidant activity, and immune regulation [101]. Some reports suggest that Lycium barbarum polysaccharide (LBP) can affect the composition and structure of the gut microbiota [102,103,104,105]. Ma et al. [106] found that LBP significantly reduced the intake of food and water in diabetic mice and alleviated hyperglycemia and insulin resistance by increasing the intake and utilization of blood sugar. Significantly altered intestinal composition, reduced Allobaculum, Dubosiella, and Romboutsia, and increased Bacteroides and Ruminococcaceae_UCG-014, Mucispirillum, Intestinimonas, and Ruminococcaceae_ UCG-009. The levels of SCFAs in mice treated with LBP significantly increased, accompanied by an increase in SCFA-producing genera. L. barbarum polysaccharides may alleviate hyperglycemia and hyperlipidemia by regulating intestinal microbiota.
Scutellaria baicalensis
S. baicalensis is a commonly used TCM and a perennial herbaceous plant belonging to the Lamiaceae family. Its roots are the main part used as medicine. It is widely distributed in China, Japan, South Korea, and Russia [107]. So far, more than 40 compounds have been isolated and identified from S. baicalensis, including flavonoids, terpenoids, volatile oils, and polysaccharides. The compounds and extracts isolated from S. baicalensis have a wide range of pharmacological activities, including nervous system, immune system, antitumor, antibacterial, antiviral, antioxidant, and other pharmacological effects [108]. Xu [109] measured the effect of an S. baicalensis extract on the growth of intestinal flora in normal and diabetes rats by microplate turbidimetry. The results showed that S. baicalensis promoted the growth of beneficial Bifidobacterium and Lactobacillus bacteria and inhibited the growth of harmful bacteria such as Enterococcus and Enterobacteriaceae. Ju [110] found that a baicalin intervention increased the abundances of Akkermansia, Coprococcus, and Ruminococcus in high-fat model mice, while the abundances of Odoribacter and Parabacteroides decreased. Baicalin increases the production of SCFAs, indicating that it may improve glucose and lipid metabolism abnormalities caused by a high-fat diet by regulating intestinal microbiota disorder and increasing the production of SCFAs.
Ophiopogon japonicus
O. japonicus is a dry tuber in the lily family. It has a sweet and slightly bitter taste and the functions of generating fluid, moistening the lungs, nourishing yin, and clearing heat. It is mainly used in clinical practice for conditions such as heat damage, restlessness, and thirst [111]. O. japonicus is a homogeneous molecular weight β-D-fructan obtained by isolation and purification from O. japonicus tubers. It has a certain effect on lowering blood sugar and improving insulin resistance, while also improving and regulating the glucose tolerance and intestinal microbiota of normal mice. Wang et al. [112] found that different doses of O. japonicus can improve the symptoms of polydipsia and polydipsia in spontaneous diabetic mice. High doses of O. japonicus can reduce the fasting blood sugar of diabetic mice, reduce the numbers of E. coli and Streptococcus, and increase the numbers of lactic acid bacteria and bifidobacteria.
Ginseng
Ginseng is a dried root and rhizome of the genus Panax, family Araliaceae. It has a long history of application and is an essential medicine for restoring yang, relieving adversity, tonifying qi, and promoting fluid production; tonifying the kidneys and blood; and calming the mind and improving eyesight [113, 114]. Sun et al. [115] used 200 mg/kg of white ginseng and red ginseng water extracts by gavage as an intervention in an HFD-induced diabetes model. They found that white ginseng and red ginseng water extracts could inhibit lipid metabolism disorder and insulin resistance in obese mice. White ginseng and red ginseng water extracts increased the relative abundances of Firmicutes and Bacteroides, reduced the relative abundances of Proteobacteria and Actinomycetes, improved the structure of intestinal microbiota, and promoted the growth of probiotics, balancing metabolic processes. Sun et al. [116] used an obesity-induced diabetes model and administered 150 mg/kg of a white ginseng extract by gavage for 12 weeks. It was found that the white ginseng extract could regulate liver glucose and lipid metabolism disorder and improve insulin sensitivity. The mechanism may be related to regulation of the intestinal flora by up-regulating acidophilus bacteria. Kim et al. [117] found that the glycosyl group of ginsenoside Rg3 stimulated the secretion of glucagon like peptide-1 (GLP-1) by intestinal cells through the sweet taste receptor–mediated signal transduction pathway and showed a strong antihyperglycemic effect in diabetic mice. To sum up, ginseng extract can improve the hyperglycemia level of diabetes by influencing intestinal cell metabolism, protecting the intestinal mucosa and regulating the intestinal flora.
Mulberry Leaves
Mulberry leaf is the dried leaf of the mulberry plant, family Moraceae, which is sweet, bitter, and cooling in nature and mainly affects the lung and liver meridians. It is used for dispersing wind-heat, clearing the lungs and moistening dryness, and clearing the liver and brightening the eyes and for wind-heat colds, lung-heat and dry cough, dizziness and headache, and redness and faintness of the eyes [118]. Mulberry leaves are rich in flavonoids and polysaccharide compounds, which can promote the absorption of nutrients in the intestines, thereby improving the intestinal function of the human body. At the same time, mulberry leaves are also rich in a large amount of organic acids, which can alter the intestinal environment and make it acidic. This effectively inhibits the proliferation of harmful bacteria, prevent the imbalance of the gut microbiota, restores normal intestinal function, and enhances the body’s ability to absorb nutrients [119]. Chen et al. [120] induced intestinal flora imbalance in mice using lincomycin hydrochloride. After 7 days of intragastric administration of 0.6 mg/mL mulberry leaf polysaccharide, 16S rDNA was used as the target sequence for DNA amplification. Denaturation gradient gel electrophoresis (DGGE) and visual image analysis showed that the type and quantity of mouse flora in the mulberry leaf polysaccharide group were significantly increased compared with the natural recovery group but that the flora could not be restored to their original levels. It was proved that mulberry leaf polysaccharide could regulate intestinal flora disorder in mice.
SUMMARY
In summary, a large number of experimental studies have shown that intestinal flora disorders are closely related to the occurrence and development of diabetes. Homologous TCM can improve the symptoms of T1D by regulating the composition of the intestinal flora and increasing the abundances of beneficial bacteria. As the gut microbiota is further studied, the number and mechanisms of diseases caused by microbiota imbalance will become clearer. In the study of disease prevention and treatment, effective interventions for disease treatment by regulating the balance of the intestinal flora will become an important research direction. In recent years, with the increasing prosperity of TCM health, “medicine comes from food, food with medicine function, medicine with food”, it has been found that some homologous TCM can support the growth of intestinal probiotics and inhibit the proliferation of harmful bacteria, thus maintaining the balance of the intestinal flora, improving the metabolic function of the body, and achieving the purpose of preventing and treating T1D. It is believed that with the deepening of relevant research, the relationship between the intestinal flora and diabetes will become clearer and that this clearer understanding will provide new directions for research and new treatment measures for the prevention and treatment of diabetes and its complications.
DATA AVAILABILITY STATEMENT
Data availability is not applicable to this article as no new data were created or analyzed in this study.
ETHICS APPROVAL
This article is a comprehensive paper and does not require ethical approval.
AUTHOR CONTRIBUTIONS
All authors contributed to the study conception and design. Yang Ping and Jianing Liu drafted of the manuscript. Huilin Wang and Yan Wang reviewed the previous literature, and Hongbin Qiu and Yu Zhang designed the study and revised the manuscript. All authors contributed to manuscript revision and read and approved the submitted version.
FUNDING
This work was supported by the National Fund Cultivation Program of Jiamusi University (JMSUGPZR2022-006), North Medicine and Functional Food Characteristic Subject Project in Heilongjiang Province (No. HLJTSXK-2022-03), Basic Research Support Program for Excellent Young Teachers in Heilongjiang Province (YQJH2023216), and Basic Scientific Research Funds for Higher Education Institution in Heilongjiang Province (2022-KYYWF-0611).
CONFLICT OF INTEREST
The authors have no relevant financial or non-financial interests to disclose.
REFERENCES
- 1.Cho NH, Shaw JE, Karuranga S, Huang Y, da Rocha Fernandes JD, Ohlrogge AW, Malanda B. 2018. IDF Diabetes Atlas: global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Res Clin Pract 138: 271–281. [DOI] [PubMed] [Google Scholar]
- 2.Rosen CJ, Ingelfinger JR. 2019. Traveling down the long road to type 1 diabetes mellitus prevention. N Engl J Med 381: 666–667. [DOI] [PubMed] [Google Scholar]
- 3.Su Y, Bai Q, Tao H, Bin X. 2023. Application prospect of traditional Chinese medicine network pharmacology in food science research. J Sci Food Agric 103: 5183–5200. [DOI] [PubMed] [Google Scholar]
- 4.He C, Zhao X, Yao R, Xiao P. 2022. Food-medicine can promote cross-culture communication between East and West. Chin Herb Med 15: 3–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang D, Li C, Fan W, Yi T, Wei A, Ma Y. 2019. Hypoglycemic and hypolipidemic effects of a polysaccharide from Fructus Corni in streptozotocin-induced diabetic rats. Int J Biol Macromol 133: 420–427. [DOI] [PubMed] [Google Scholar]
- 6.Yuan X, Wang R, Han B, Sun C, Chen R, Wei H, Chen L, Du H, Li G, Yang Y, et al. 2022. Functional and metabolic alterations of gut microbiota in children with new-onset type 1 diabetes. Nat Commun 13: 6356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cambuli VM, Incani M, Cossu E, Congiu T, Scano F, Pilia S, Sentinelli F, Tiberti C, Cavallo MG, Loche S, et al. 2010. Prevalence of type 1 diabetes autoantibodies (GADA, IA2, and IAA) in overweight and obese children. Diabetes Care 33: 820–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Da Rosa LC, Boldison J, De Leenheer E, Davies J, Wen L, Wong FS. 2018. B cell depletion reduces T cell activation in pancreatic islets in a murine autoimmune diabetes model. Diabetologia 61: 1397–1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.UPDS Group 1991. UK Prospective Diabetes Study (UKPDS). VIII. Study design, progress and performance. Diabetologia 34: 877–890. [PubMed] [Google Scholar]
- 10.Nie Q, Chen H, Hu J, Fan S, Nie S. 2019. Dietary compounds and traditional Chinese medicine ameliorate type 2 diabetes by modulating gut microbiota. Crit Rev Food Sci Nutr 59: 848–863. [DOI] [PubMed] [Google Scholar]
- 11.Wang Y, Xie Q, Liang CL, Zeng Q, Dai Z. 2017. Chinese medicine Ginseng and Astragalus granules ameliorate autoimmune diabetes by upregulating both CD4+FoxP3+ and CD8+CD122+PD1+ regulatory T cells. Oncotarget 8: 60201–60209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bener A, Kim EJ, Mutlu F, Eliyan A, Delghan H, Nofal E, Shalabi L, Wadi N. 2014. Burden of diabetes mellitus attributable to demographic levels in Qatar: an emerging public health problem. Diabetes Metab Syndr 8: 216–220. [DOI] [PubMed] [Google Scholar]
- 13.Guo K, Yan Y, Zeng C, Shen L, He Y, Tan Z. 2022. Study on baohe pills regulating intestinal microecology and treating diarrhea of high-fat and high-protein diet mice. BioMed Res Int 2022: 6891179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Di YM, Sun L, Lu C, Guo XF, Tang X, Zhang AL, Fan G, Xue CC. 2022. Benefits of herbal formulae containing Poria cocos (Fuling) for type 2 diabetes mellitus: a systematic review and meta-analysis. PLoS One 17: e0278536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang J. 2013. Experience in traditional Chinese medicine treatment and dietary regulation of diabetes. Front Med 9: 111–111 (in Chinese). [Google Scholar]
- 16.Zhang S, Li J, Xie J. 2015. Traditional Chinese medicine treatment and nursing of diabetes (Diabetes) III. Med Infant 28: 119–120 (in Chinese). [Google Scholar]
- 17.Hou Y, Jiang JG. 2013. Origin and concept of medicine food homology and its application in modern functional foods. Food Funct 4: 1727–1741. [DOI] [PubMed] [Google Scholar]
- 18.Diling C, Xin Y, Chaoqun Z, Jian Y, Xiaocui T, Jun C, Ou S, Yizhen X. 2017. Extracts from Hericium erinaceus relieve inflammatory bowel disease by regulating immunity and gut microbiota. Oncotarget 8: 85838–85857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chau CF, Wu SH. 2006. The development of regulations of Chinese herbal medicines for both medicinal and food uses. Trends Food Sci Technol 17: 313–323. [Google Scholar]
- 20.Maciocia G. 2005. The foundations of Chinese medicine: a comprehensive text for acupuncturists and herbalists, 2nd ed. Churchill Livingstone Press, London. [Google Scholar]
- 21.Chen KJ, Xu H. 2003. The integration of traditional Chinese medicine and western medicine. Eur Rev 11: 225–235 (in Chinese). [Google Scholar]
- 22.Wu Z, Chen Y, Zhu D, Zheng Y, Ali KB, Hou K. 2022. Advancement of traditional Chinese medicine in regulation of intestinal flora: mechanism-based role in disease management. Recent Pat Anticancer Drug Discov 17: 136–144. [DOI] [PubMed] [Google Scholar]
- 23.Qin J, Li R, Raes J, Arumugam M, Burgdorf KS, Manichanh C, Nielsen T, Pons N, Levenez F, Yamada T, et al. MetaHIT Consortium 2010. A human gut microbial gene catalogue established by metagenomic sequencing. Nature 464: 59–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.He L, Long C, Liu Y, Guo Y, Xiao N, Tan Z. 2017. Effects of Debaryomyces hansenii treatment on intestinal microorganisms in mice with antibiotics-induced diarrhea. 3 Biotech 7: 347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li X, Peng X, Guo K, Tan Z. 2021. Bacterial diversity in intestinal mucosa of mice fed with Dendrobium officinale and high-fat diet. 3 Biotech 11: 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yuan Z, Zhang C, Peng X, Shu L, Long C, Tan Z. 2020. Intestinal microbiota characteristics of mice treated with Folium senna decoction gavage combined with restraint and tail pinch stress. 3 Biotech 10: 180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sender R, Fuchs S, Milo R. 2016. Are we really vastly outnumbered? Revisiting the ratio of bacterial to host cells in humans. Cell 164: 337–340. [DOI] [PubMed] [Google Scholar]
- 28.Ventura M, Turroni F, Motherway MO, MacSharry J, van Sinderen D. 2012. Host-microbe interactions that facilitate gut colonization by commensal bifidobacteria. Trends Microbiol 20: 467–476. [DOI] [PubMed] [Google Scholar]
- 29.Mandy L. Corrigan, Roberts K, Steiger Z. 2019. Gut microbiome. Adult short bowel syndrome: nutritional, medical, and surgical management, Chapter 4. Gail A.M. Cresci and Kristin Izzo. Academic Press 45: 54. [Google Scholar]
- 30.van den Bogert B, Erkus O, Boekhorst J, de Goffau M, Smid EJ, Zoetendal EG, Kleerebezem M. 2013. Diversity of human small intestinal Streptococcus and Veillonella populations. FEMS Microbiol Ecol 85: 376–388. [DOI] [PubMed] [Google Scholar]
- 31.Wampach L, Heintz-Buschart A, Hogan A, Muller EEL, Narayanasamy S, Laczny CC, Hugerth LW, Bindl L, Bottu J, Andersson AF, et al. 2017. Colonization and succession within the human gut microbiome by archaea, bacteria, and microeukaryotes during the first year of life. Front Microbiol 8: 738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lim ES, Rodriguez C, Holtz LR. 2018. Amniotic fluid from healthy term pregnancies does not harbor a detectable microbial community. Microbiome 6: 87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bäumler AJ, Sperandio V. 2016. Interactions between the microbiota and pathogenic bacteria in the gut. Nature 535: 85–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gensollen T, Iyer SS, Kasper DL, Blumberg RS. 2016. How colonization by microbiota in early life shapes the immune system. Science 352: 539–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Aron-Wisnewsky J, Clément K. 2016. The gut microbiome, diet, and links to cardiometabolic and chronic disorders. Nat Rev Nephrol 12: 169–181. [DOI] [PubMed] [Google Scholar]
- 36.Zhao J, Xiang X. 2020. Changes of intestinal microflora and its influencing factors at different stages of life. Health Research 49: 155–159. [Google Scholar]
- 37.Ruan W, Engevik MA, Spinler JK, Versalovic J. 2020. Healthy human gastrointestinal microbiome: composition and function after a decade of exploration. Dig Dis Sci 65: 695–705. [DOI] [PubMed] [Google Scholar]
- 38.Shao H, Zhang C, Xiao N, Tan Z. 2020. Gut microbiota characteristics in mice with antibiotic-associated diarrhea. BMC Microbiol 20: 313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Long C, Shao H, Luo C, Yu R, Tan Z. 2020. Bacterial diversity in the intestinal mucosa of dysbiosis diarrhea mice treated with qiweibaizhu powder. Gastroenterol Res Pract 2020: 9420129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wu Y, Zhang C, Shao H, Luo H, Tan Z. 2021. Characteristics of intestinal microbiota and enzyme activities in mice fed with lily bulb. 3 Biotech 11: 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tilg H, Zmora N, Adolph TE, Elinav E. 2020. The intestinal microbiota fuelling metabolic inflammation. Nat Rev Immunol 20: 40–54. [DOI] [PubMed] [Google Scholar]
- 42.Qin J, Li Y, Cai Z, Li S, Zhu J, Zhang F, Liang S, Zhang W, Guan Y, Shen D, et al. 2012. A metagenome-wide association study of gut microbiota in type 2 diabetes. Nature 490: 55–60. [DOI] [PubMed] [Google Scholar]
- 43.Guo K, Xu S, Zhang Q, Peng M, Yang Z, Li W, Tan Z. 2020. Bacterial diversity in the intestinal mucosa of mice fed with Asparagus extract under high-fat diet condition. 3 Biotech 10: 228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ortega MA, Fraile-Martínez O, Naya I, García-Honduvilla N, Álvarez-Mon M, Buján J, Asúnsolo Á, de la Torre B. 2020. Type 2 diabetes mellitus associated with obesity (diabesity). The central role of gut microbiota and its translational applications. Nutrients 12: 2749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mao K, Baptista AP, Tamoutounour S, Zhuang L, Bouladoux N, Martins AJ, Huang Y, Gerner MY, Belkaid Y, Germain RN. 2018. Innate and adaptive lymphocytes sequentially shape the gut microbiota and lipid metabolism. Nature 554: 255–259. [DOI] [PubMed] [Google Scholar]
- 46.Mathis D, Benoist C. 2011. Microbiota and autoimmune disease: the hosted self. Cell Host Microbe 10: 297–301. [DOI] [PubMed] [Google Scholar]
- 47.Wang W, Jiang B, Sun H, Ru X, Sun D, Wang L, Wang L, Jiang Y, Li Y, Wang Y, et al. NESS-China Investigators 2017. Prevalence, incidence, and mortality of stroke in China: results from a nationwide population-based survey of 480 687 adults. Circulation 135: 759–771. [DOI] [PubMed] [Google Scholar]
- 48.Singh V, Roth S, Llovera G, Sadler R, Garzetti D, Stecher B, Dichgans M, Liesz A. 2016. Microbiota dysbiosis controls the neuroinflammatory response after stroke. J Neurosci 36: 7428–7440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Xu N, Kan P, Yao X, Yang P, Wang J, Xiang L, Zhu Y. 2018. Astragaloside IV reversed the autophagy and oxidative stress induced by the intestinal microbiota of AIS in mice. J Microbiol 56: 838–846. [DOI] [PubMed] [Google Scholar]
- 50.Ji W, Zhu Y, Kan P, Cai Y, Wang Z, Wu Z, Yang P. 2017. Analysis of intestinal microbial communities of cerebral infarction and ischemia patients based on high throughput sequencing technology and glucose and lipid metabolism. Mol Med Rep 16: 5413–5417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kiouptsi K, Reinhardt C. 2018. Contribution of the commensal microbiota to atherosclerosis and arterial thrombosis. Br J Pharmacol 175: 4439–4449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mokhtari P, Metos J, Anandh Babu PV. 2021. Impact of type 1 diabetes on the composition and functional potential of gut microbiome in children and adolescents: possible mechanisms, current knowledge, and challenges. Gut Microbes 13: 1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Qi CJ, Zhang Q, Yu M, Xu JP, Zheng J, Wang T, Xiao XH. 2016. Imbalance of fecal microbiota at newly diagnosed type 1 diabetes in Chinese children. Chin Med J (Engl) 129: 1298–1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.He L, Chen R, Zhang B, Zhang S, Khan BA, Zhu D, Wu Z, Xiao C, Chen B, Chen F, et al. 2022. Fecal microbiota transplantation treatment of autoimmune-mediated type 1 diabetes mellitus. Front Immunol 13: 930872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Murri M, Leiva I, Gomez-Zumaquero JM, Tinahones FJ, Cardona F, Soriguer F, Queipo-Ortuño MI. 2013. Gut microbiota in children with type 1 diabetes differs from that in healthy children: a case-control study. BMC Med 11: 46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Demirci, Mehmet NT, Hrisi B, ZeynepNK, Fatma E, Penbe B, Yesim O, Mucahit K, Nuri K, Bekir S. 2019. Bacteroidetes and Firmicutes levels in gut microbiota and effects of hosts TLR2/TLR4 gene expression levels in adult T1DM patients in Istanbul, Turkey. J Diabetes Complications, 34: 107449. [DOI] [PubMed] [Google Scholar]
- 57.Biassoni R, Di Marco E, Squillario M, Barla A, Piccolo G, Ugolotti E, Gatti C, Minuto N, Patti G, Maghnie M, et al. 2020. Gut microbiota in T1DM-onset pediatric patients: machine-learning algorithms to classify microorganisms as disease linked. J Clin Endocrinol Metab 105: dgaa407. [DOI] [PubMed] [Google Scholar]
- 58.Soyucen E, Gulcan A, Aktuglu-Zeybek AC, Onal H, Kiykim E, Aydin A. 2014. Differences in the gut microbiota of healthy children and those with type 1 diabetes. Pediatr Int 56: 336–343. [DOI] [PubMed] [Google Scholar]
- 59.Huang Y, Li S, Hu J, Ruan H, Guo H, Zhang H, Wang X, Pei Y, Pan Y, Fang C. Gut microbiota profiling in Han Chinese with type 1 diabetes. Diabetes Res Clin Pract 141: 256–263. [DOI] [PubMed] [Google Scholar]
- 60.Parada Venegas D, De la Fuente MK, Landskron G, González MJ, Quera R, Dijkstra G, Harmsen HJM, Faber KN, Hermoso MA. 2019. Short Chain Fatty Acids (SCFAs)-mediated gut epithelial and immune regulation and its relevance for inflammatory bowel diseases. Front Immunol 10: 277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ping Y, Liu J, Wang L, Qiu H, Zhang Y. 2024. Research progress on the mechanism of TCM regulating intestinal microbiota in the treatment of DM mellitus. Front Endocrinol (Lausanne) 15: 1308016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sun J, Furio L, Mecheri R, van der Does AM, Lundeberg E, Saveanu L, Chen Y, van Endert P, Agerberth B, Diana J. 2015. Pancreaticβ-cells limit autoim mune diabetes via an immunoregulatory antimicrobial peptide ex pressed under the influence of the gut microbiota. Immunity 43: 304–317. [DOI] [PubMed] [Google Scholar]
- 63.Niu T, Smith DL, Yang Z, Gao S, Yin T, Jiang ZH, You M, Gibbs RA, Petrosino JF, Hu M. 2013. Bioactivity and bioavailability of ginsenosides are dependent on the glycosidase activities of the A/J mouse intestinal microbiome defined by pyrosequencing. Pharm Res 30: 836–846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhang M, Gao X, Sun X. 2008. Pharmacological effects and research progress of Poria cocos. Journal of Beihua University 9: 63–65 (Natural Science Edition). [Google Scholar]
- 65.Li X, Ma L, Zhang L. 2019. Molecular basis for Poria cocos mushroom polysaccharide used as an antitumor drug in China. Prog Mol Biol Transl Sci 163: 263–296. [DOI] [PubMed] [Google Scholar]
- 66.Zheng C. 2010. Experimental study on the anti diabetes effect of Poria cocos polysaccharide. Chin Med J 5: 12–13 (in Chinese). [Google Scholar]
- 67.Xu H, Wang Y, Jurutka PW, Wu S, Chen Y, Cao C, Chen G, Tian B, Wang S, Cheng S. 2019. 16α-Hydroxytrametenolic Acid from Poria cocos improves intestinal barrier function through the glucocorticoid receptor-mediated PI3K/Akt/NF-κB pathway. J Agric Food Chem 67: 10871–10879. [DOI] [PubMed] [Google Scholar]
- 68.Zhu L, Ye C, Hu B, Xia H, Bian Q, Liu Y, Kong M, Zhou S, Liu H. 2022. Regulation of gut microbiota and intestinal metabolites by Poria cocos oligosaccharides improves glycolipid metabolism disturbance in high-fat diet-fed mice. J Nutr Biochem 107: 109019. [DOI] [PubMed] [Google Scholar]
- 69.Liu L, Li I, Zheng H, Chen Q. 2022. The regulatory effect of Poria cocos extract on blood glucose and intestinal flora in type 1 diabetes mice. J Northwest Pharmacy 37: 89–94 (in Chinese). [Google Scholar]
- 70.Wu Z, Qi L, Chen D. 2020. The effect of Poria cocos extract on intestinal microbiota imbalance in mice induced by a high-fat diet. Chinese J Modern Chinese Med 22: 1822–1829 (in Chinese). [Google Scholar]
- 71.Akpolat M, Tarladac Y, Uz Y, Metin M, Kòzòlay G. 2010. Kanser tedavisinde curcuminin yeri. Yeni Tòp Dergisi 27: 142–147. [Google Scholar]
- 72.Prasad S, Gupta SC, Tyagi AK, Aggarwal BB. 2014. Curcumin, a component of golden spice: from bedside to bench and back. Biotechnol Adv 32: 1053–1064. [DOI] [PubMed] [Google Scholar]
- 73.Qin Y, Fei C, Zhang W, Li Y, Xu Z, Soviet U, De J, Mao C, Lu T. 2022. Research progress on the related substances of Curcuma traditional Chinese medicine in promoting blood circulation and resolving blood stasis. Chinese J Traditional Chinese Medicine 47: 24–35 (in Chinese). [Google Scholar]
- 74.Jurenka JS. 2009. Anti-inflammatory properties of curcumin, a major constituent of Curcuma longa: a review of preclinical and clinical research. Altern Med Rev 14: 141–153. [PubMed] [Google Scholar]
- 75.Deogade S, Ghate S. 2015. Curcumòn: therapeutòc applòcatòons in systemòc and oral health. Int J Biol Pharm Res 6: 281–290. [Google Scholar]
- 76.Patil BS, Jayaprakasha GK, Chidambara Murthy KN, Vikram A. 2009. Bioactive compounds: historical perspectives, opportunities, and challenges. J Agric Food Chem 57: 8142–8160. [DOI] [PubMed] [Google Scholar]
- 77.Shehzad A, Rehman G, Lee Y. 2013. Curcumin in inñammatory diseases. Int Union Biochem Mol Biology Inc. 39: 69–77. [Google Scholar]
- 78.Bao B, Chen YG, Zhang L, Na Xu YL, Wang X, Liu J, Qu W. 2013. Momordica charantia (Bitter Melon) reduces obesity-associated macrophage and mast cell infiltration as well as inflammatory cytokine expression in adipose tissues. PLoS One 8: e84075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Liu X, Xia N, Liang Y. 2014. Curcumin can improve lipopolysaccharide induced diabetes by regulating intestinal flora. Jiyinzuxue Yu Yingyong Shengwuxue 33: 970–974 (in Chinese). [Google Scholar]
- 80.Hou H, Qiu Y, Hu Y, Zhang J, Li Y. 2017. The effect of curcumin on the gut microbiota of rats fed with high-fat feed. Modern Preventive Med 44: 3310–3312+3341 (in Chinese). [Google Scholar]
- 81.Gao Z, Di N. 2022. The medicinal value and application prospects of ginger. Special Economic Animals and Plants 25: 65–68+122 (in Chinese). [Google Scholar]
- 82.Zhao W, Zhang R, Yu Z, Wang X, Li J, Liu J. 2016. Research progress in ginger chemical composition and biological activity. Science and Technology of Food Industry 37: 383–389 (in Chinese). [Google Scholar]
- 83.Hou M, Gao J, Liu Z, Shan Y, Liu S. 2021. Antioxidant and immun omodulatory activities in vitro of a neutral polysaccharide from gin ger (Zingiber officinale). Stärke 73: 2100048. [Google Scholar]
- 84.Liao DW, Cheng C, Liu JP, Zhao LY, Huang DC, Chen GT. 2020. Characterization and antitumor activities of polysaccharides obtained from ginger (Zingiber officinale) by different extraction methods. Int J Biol Macromol 152: 894–903. [DOI] [PubMed] [Google Scholar]
- 85.Wang C, He Y, Tang X, Li N. 2020. Sulfation, structural analysis, and anticoagulant bioactivity of ginger polysaccharides. J Food Sci 85: 2427–2434. [DOI] [PubMed] [Google Scholar]
- 86.Chen X, Wang Z, Kan J. 2021. Polysaccharides from ginger stems and leaves: effects of dual and triple frequency ultra sound assisted extraction on structural characteristics and biological activities. Food Biosci 42: 101166. [Google Scholar]
- 87.Wang N, Chen M, Meng F, Zhou L, Lu Z. 2023. Extraction of ginger polysaccharide and its regulation on intestinal flora of diabetes mice. Food Industry Science and Technology 44: 278–286 (in Chinese). [Google Scholar]
- 88.Wei X, Tao J, Xiao S, Jiang S, Shang E, Zhu Z, Qian D, Duan J. 2018. Xiexin Tang im proves the symptom of type 2 diabetic rats by modulation of the gut microbiota. Sci Rep 8: 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ottman N, Geerlings SY, Aalvink S, de Vos WM, Belzer C. 2017. Action and function of Akkermansia muciniphila in microbiome ecology, health and disease. Best Pract Res Clin Gastroenterol 31: 637–642. [DOI] [PubMed] [Google Scholar]
- 90.Pedersen HK, Gudmundsdottir V, Nielsen HB, Hyotylainen T, Nielsen T, Jensen BA, Forslund K, Hildebrand F, Prifti E, Falony G, et al. MetaHIT Consortium 2016. Human gut microbes impact host serum metabolome and insulin sensitivity. Nature 535: 376–381. [DOI] [PubMed] [Google Scholar]
- 91.Oh YC, Cho WK, Im GY, Jeong YH, Hwang YH, Liang C, Ma JY. 2012. Anti-inflammatory effect of Lycium Fruit water extract in lipopolysaccharide-stimulated RAW 264.7 macrophage cells. Int Immunopharmacol 13: 181–189. [DOI] [PubMed] [Google Scholar]
- 92.Gan L, Hua Zhang S, Liang Yang X, Bi Xu H. 2004. Immunomodulation and antitumor activity by a polysaccharide-protein complex from Lycium barbarum. Int Immunopharmacol 4: 563–569. [DOI] [PubMed] [Google Scholar]
- 93.Mao F, Xiao B, Jiang Z, Zhao J, Huang X, Guo J. 2011. Anticancer effect of Lycium barbarum polysaccharides on colon cancer cells involves G0/G1 phase arrest. Med Oncol 28: 121–126. [DOI] [PubMed] [Google Scholar]
- 94.Luo Q, Cai Y, Yan J, Sun M, Corke H. 2004. Hypoglycemic and hypolipidemic effects and antioxidant activity of fruit extracts from Lycium barbarum. Life Sci 76: 137–149. [DOI] [PubMed] [Google Scholar]
- 95.Chang RCC, So KF. 2008. Use of anti-aging herbal medicine, Lycium barbarum, against aging-associated diseases. What do we know so far? Cell Mol Neurobiol 28: 643–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Amagase H, Farnsworth RN. 2011. A review of botanical characteristics, phytochemistry, clinical relevance in efffcacy and safety of Lycium barbarum fruit (Goji). Food Res 44: 1702–1717. [Google Scholar]
- 97.Qian D, Zhao Y, Yang G, Huang L. 2017. Systematic review of chemical constituents in the genus Lycium (Solanaceae). Molecules 22: 911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Zhao F, Liu Q, Cao J, Xu Y, Pei Z, Fan H, Yuan Y, Shen X, Li C. 2020. A sea cucumber (Holothuria leucospilota) polysaccharide improves the gut microbiome to alleviate the symptoms of type 2 diabetes mellitus in Goto–Kakizaki rats. Food Chem Toxicol 135: 110886. [DOI] [PubMed] [Google Scholar]
- 99.Chang CJ, Lin CS, Lu CC, Martel J, Ko YF, Ojcius DM, Tseng SF, Wu TR, Chen YM, Young JD, et al. 2017. Corrigendum: Ganoderma lucidum reduces obesity in mice by modulating the composition of the gut microbiota. Nat Commun 8: 16130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Xu S, Dou Y, Ye B, Wu Q, Wang Y, Hu M, Ma F, Rong X, Guo J. 2017. Ganoderma lucidum polysaccharides improve insulin sensitivity by regulating inñammatory cytokines and gut microbiota composition in mice. J Funct Foods 38: 545–552. [Google Scholar]
- 101.Jin M, Huang Q, Zhao K, Shang P. 2013. Biological activities and potential health benefit effects of polysaccharides isolated from Lycium barbarum L. Int J Biol Macromol 54: 16–23. [DOI] [PubMed] [Google Scholar]
- 102.Zhu J, Liu W, Yu J, Zou S, Wang J, Yao W, Gao X. 2013. Characterization and hypoglycemic effect of a polysaccharide extracted from the fruit of Lycium barbarum L. Carbohydr Polym 98: 8–16. [DOI] [PubMed] [Google Scholar]
- 103.Zhang XR, Zhou WX, Zhang YX, Qi CH, Yan H, Wang ZF, Wang B. 2011. Macrophages, rather than T and B cells are principal immunostimulatory target cells of Lycium barbarum L. polysaccharide LBPF4-OL. J Ethnopharmacol 136: 465–472. [DOI] [PubMed] [Google Scholar]
- 104.Ding Y, Yan Y, Peng Y, Chen D, Mi J, Lu L, Luo Q, Li X, Zeng X, Cao Y. 2019. In vitro digestion under simulated saliva, gastric and small intestinal conditions and fermentation by human gut microbiota of polysaccharides from the fruits of Lycium barbarum. Int J Biol Macromol 125: 751–760. [DOI] [PubMed] [Google Scholar]
- 105.Masci A, Carradori S, Casadei MA, Paolicelli P, Petralito S, Ragno R, Cesa S. 2018. Lycium barbarum polysaccharides: extraction, purification, structural characterisation and evidence about hypoglycaemic and hypolipidaemic effects. A review. Food Chem 254: 377–389. [DOI] [PubMed] [Google Scholar]
- 106.Ma Q, Zhai R, Xie X, Chen T, Zhang Z, Liu H, Nie C, Yuan X, Tu A, Tian B, et al. 2022. Hypoglycemic effects of Lycium barbarum polysaccharide in type 2 diabetes mellitus mice via modulating gut microbiota. Front Nutr 9: 916271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Wang ZL, Wang S, Kuang Y, Hu ZM, Qiao X, Ye M. 2018. A comprehensive review on phytochemistry, pharmacology, and flavonoid biosynthesis of Scutellaria baicalensis. Pharm Biol 56: 465–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Zhao T, Tang H, Xie L, Zheng Y, Ma Z, Sun Q, Li X. 2019. Scutellaria baicalensis Georgi. (Lamiaceae): a review of its traditional uses, botany, phytochemistry, pharmacology and toxicology. J Pharm Pharmacol 71: 1353–1369. [DOI] [PubMed] [Google Scholar]
- 109.Xu J. 2014. Study on the Interaction between Huangqin Huanglian Pairs and Intestinal Microflora. Master’s Thesis of Nanjing University of Traditional Chinese Medicine (03), 129 (in Chinese).
- 110.Ju M. 2019. Mechanism study on baicalin improving glucose and lipid metabolism in high-fat diet mice by regulating intestinal microbiota. Southeast University 70. (in Chinese). [Google Scholar]
- 111.Qi J, Tao G, Guo X, Feng H, Zhang J. 2008. Research progress on the biological activity of polysaccharides from Ophiopogon japonicus Journal of Xi’an University of Arts and Sciences (Natural Science Edition). 11: 44–46 Impact Chinese. J Appl Physiol 25: 160–161 (in Chinese). [Google Scholar]
- 112.Wang L, Wang S, Wang Y, Ruan K, Feng Y. 2011. Effects of ophiopogon polysaccharide MDG-1 on glucose tolerance and intestinal flora in diabetes mice. World Chinese Journal of Digestion 19: 2058–2062 (in Chinese). [Google Scholar]
- 113.de Oliveira Zanuso B, de Oliveira Dos Santos AR, Miola VFB, Guissoni Campos LM, Spilla CSG, Barbalho SM. 2022. Panax ginseng and aging related disorders: a systematic review. Exp Gerontol 161: 111731–111752. [DOI] [PubMed] [Google Scholar]
- 114.Lee IS, Kang KS, Kim SY. 2019. Panax ginseng pharmacopuncture: current status of the research and future challenges. Biomolecules 10: 33–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Sun YF, Zhang X, Wang XY, Jia W. 2018. [Effect of long-term intake of ginseng extracts on gut microbiota in rats] . Zhongguo Zhongyao Zazhi 43: 3927–3932 (in Chinese). [DOI] [PubMed] [Google Scholar]
- 116.Sun RX, Huang WJ, Xiao Y, Wang DD, Mu GH, Nan H, Ni BR, Huang XQ, Wang HC, Liu YF, et al. 2022. Shenlian (SL) decoction, a traditional Chinese medicine compound, may ameliorate blood glucose via mediating the gut microbiota in db/db mice. J Diabetes Res 2022: 7802107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Kim K, Yang H, Lee I, Kim K, Jang H. 2015. The aglycone of ginsenosideRg3 enables glucagon-like peptide-1 secretion in enteroendocrine cells and alleviates hyperglycemia in type 2 diabetic mice. Sci Rep 5: 20–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Liu F. 2018. Research progress on the determination of main components in traditional Chinese medicine mulberry leaves. Tianjin Pharm 30: 53–57 (in Chinese). [Google Scholar]
- 119.Nie K. 2012. Study on the therapeutic effect of mulberry leaf water extract on type 1 diabetes mice. Hunan Agricultural University 56. (in Chinese). [Google Scholar]
- 120.Chen L, Zhang X, Sun S, Wang L, Che Y, Zhao J, Song C. 2015. Study on the regulation of intestinal microbiota imbalance in mice by mulberry leaf polysaccharides. Modern Medicine and Clinical Journal 30: 633–636 (in Chinese). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data availability is not applicable to this article as no new data were created or analyzed in this study.