ABSTRACT
BACKGROUND:
Post-ischemia reperfusion can lead to oxidative stress and an increase in oxidative markers. Employing preventive strategies and antioxidant agents may help mitigate ischemia-reperfusion injury (IRI). The use of a tourniquet in extremity surgery has been associated with IRI. This study aims to investigate the impact of three different approaches— brachial plexus block, total intravenous anesthesia (TIVA), and inhalation anesthesia—on IRI during upper extremity surgery using a tourniquet.
METHODS:
Patients aged 18 to 45 with American Society of Anesthesiologists (ASA) I-II scores were randomly assigned to one of three groups: Group A received an axillary block with bupivacaine; Group I underwent inhalation anesthesia with sevoflurane; and Group T received TIVA with propofol and remifentanil infusion. Blood samples were collected to measure glucose, lactate, total antioxidant status (TAS), total oxidant status (TOS), and ischemia-modified albumin (IMA) levels at various time points: before anesthesia (t1), 1 minute before tourniquet release (t2), 20 minutes after tourniquet release (t3), and 4 hours after tourniquet release (t4).
RESULTS:
In Group I, lactate levels at t3, and glucose levels at t2 and t3, were higher compared to the other groups. Group A exhibited lower IMA levels at t2, t3, and t4 than the other groups. Additionally, Group I had lower IMA levels at t2, t3, and t4 compared to Group T. TAS levels were higher in Group I at t2, t3, and t4 compared to the other groups. TOS levels at t2 and t3 were lower in Group A than in Group I.
CONCLUSION:
Axillary anesthesia results in a sympathetic block, promoting better perfusion of the upper extremity. This study demonstrated lower levels of oxidative stress markers with axillary plexus block. Therefore, these results suggest that the axillary block has the potential to mitigate IRI.
Keywords: Ischemia-reperfusion injury, axillary block, inhalation anesthesia, total intravenous anesthesia, oxidative stress
INTRODUCTION
Upper extremity injuries, frequently encountered in industrial accidents, necessitate various anesthesia methods during surgical intervention. Methods that offer additional benefits beyond merely providing adequate anesthesia for the procedure are favored. The use of a proximal tourniquet is a standard practice in upper and lower limb surgeries to achieve a bloodless surgical field.[1-3] However, tourniquet use can result in significant ischemia in the affected limb. When arterial perfusion is restored by deflating the tourniquet, it can lead to ischemia-reperfusion injury (IRI).[4-6] IRI is a complex sterile inflammatory response characterized by disturbances in perfusion, causing a severe imbalance between metabolic supply and demand, ultimately resulting in tissue hypoxia.[7,8]
IRI involves oxidative damage, where reactive oxygen species (ROS) are generated and released into the systemic circulation after reperfusion and reoxygenation of the ischemic area. These ROS contribute to oxidative damage, including lipid peroxidation, protein oxidation, and DNA damage at the cellular level.[9,10] Oxidative stress produces toxic metabolites such as ischemia-modified albumin (IMA) and malondialdehyde (MDA) levels, which can cause cellular injury.[5,6,11,12] As a result of IRI, the levels of oxidant metabolites increase while antioxidant enzymes such as glutathione peroxidase (GPX), superoxide dismutase (SOD), and catalase (CAT) decrease, creating an imbalance between the total oxidant status (TOS) and total antioxidant status (TAS) levels. The production of ROS and alterations in the TAS/TOS ratio levels can impact distant organs and systems (13), and may result in hemodynamic complications due to IRI.[14]
Numerous studies have been conducted to prevent IRI, with a focus on anesthesia methods and anesthetic agents. Neuraxial block has been identified as a useful approach for preventing IRI.[15] Additionally, both intravenous and inhalational anesthetic agents have demonstrated potential preventive effects against IRI. Propofol, known for its antioxidant properties, is commonly used for anesthesia induction and maintenance to mitigate IRI.[16] Similarly, sevoflurane, an inhalational anesthetic agent, has shown preventive effects against IRI.[17]
Previous research has established ischemic damage from tourniquet use through biochemical parameters.[3,18]
This study aims to compare the effects of axillary block, inhalational anesthesia, and total intravenous anesthesia (TIVA) on upper extremity surgery concerning tourniquet-induced ischemia-reperfusion injury. The assessment will be carried out by measuring lactate, glucose, TAS, TOS, and IMA levels.
MATERIALS AND METHODS
Patients and Groups
This prospective, randomized study was conducted following approval from the local ethics committee of Karadeniz Technical University, Faculty of Medicine, with the date 21. 02. 2014 and number 17522305/607. All participants provided written informed consent prior to participation. The study included 99 male subjects, categorized as American Society of Anesthesiologists physical status I and II, aged between 18 and 45 years, scheduled for unilateral hand or forearm surgery using a tourniquet.
Ninety-nine patients were randomly assigned to three groups using a sealed envelope method. Three different anesthesia techniques were compared: axillary block with bupivacaine (Group A, n=33), inhalation anesthesia with sevoflurane (Group I, n=33), and total intravenous anesthesia with propofol and remifentanil (Group T, n=33).
To maintain consistency in muscle mass, only male patients with a body mass index (BMI) between 18.5 and 29.5 were selected. Patients with severe cardiopulmonary, metabolic, renal, hepatic, central nervous system, or psychiatric disorders, as well as those with diabetes or other peripheral neuropathies, alcohol or drug abuse, and smokers, were excluded from the study.
In the operating room, an intravenous catheter was inserted, and an infusion of 0.9% NaCl solution was initiated. After monitoring heart rate (HR), noninvasive blood pressure (BP), and peripheral oxygen saturation (SpO2), all patients received sedation with 1 mg of midazolam. Arterial cannulation was performed on the contralateral limb for blood sample collection.
In Group A, an axillary block was administered for upper-limb anesthesia using a combination of 0.5% bupivacaine (20 ml) and 2% lidocaine (10 ml), guided by an ultrasound probe and a nerve stimulator.
For Group I, inhalation anesthesia was induced with 1.5-2 mg/kg of propofol, 1 µg/kg of fentanyl, and 0.1 mg/kg of vecuronium. Anesthesia was maintained with 1-3% sevoflurane in a mixture of 40% oxygen and 60% nitrous oxide.
In Group T, anesthesia induction involved 1.5-2 mg/kg of propofol, 1 µg/kg of fentanyl, and 0.1 mg/kg of vecuronium. Maintenance of anesthesia was achieved through intravenous (i.v.) infusion of 100 µg/kg/min of propofol and 0.25 µg/kg/min of remifentanil. The depth of anesthesia in both Group I and Group T was monitored using the bispectral index (BIS).
Plasma Samples
Blood samples were collected at various time points to measure glucose, lactate, TAS, TOS, and IMA levels. These time points included before anesthesia (t1), 1 minute before tourniquet release (t2), 20 minutes after tourniquet release (t3), and 4 hours after tourniquet release (t4).
The blood samples were collected without the use of anticoagulants and processed by centrifugation at 1800 g for 10 minutes. Subsequently, plasma samples were obtained and divided into aliquots, which were then stored in Eppendorf tubes at -80 °C until they were ready for biochemical analysis.
Biochemical Assessments
Plasma IMA Levels
The assessment of reduced cobalt-to-albumin binding capacity—IMA level—was carried out using the rapid and colorimetric method described by Bar-Or et al. (19). Two hundred milliliters (mL) of patient serum were placed in glass tubes. To this, 50 mL of 0.1% cobalt chloride (Sigma, CoCl2.6H2O) in H2O was added. After gently shaking the mixture, it was left undisturbed for 10 minutes to ensure adequate cobalt albumin binding. Subsequently, 50 microliters (µL) of dithiothreitol (DTT) (Sigma, USA 1.5 mg/mL H2O) was added as a colorizing agent. The reaction was quenched 2 minutes later by adding 1.0 mL of 0.9% NaCl. For preoperative and postoperative serum samples, a colorimetric control was prepared using 50 mL of distilled water instead of 50 mL of 1.5 mg/mL DTT. The absorbencies of the specimens were analyzed at 470 nm using a spectrophotometer (Shimadzu UV1601, Australia). The color of the specimens containing DTT was compared with that of the colorimetric control tubes. The results were reported in absorbance units (ABSUs).
Plasma TAS Levels
The antioxidants present in the sample act to reduce the dark blue-green colored ABTS radical, transforming it into the colorless reduced ABTS form. The change in absorbance at 660 nm is indicative of the total antioxidant content within the sample. To calibrate the assay, a stable antioxidant standard solution known as Trolox equivalent, an analog of vitamin E (20), is employed.
Plasma TOS Levels
In the sample, oxidants are responsible for oxidizing the ferrous ion-chelator complex into the ferric ion. This oxidation is enhanced by molecules present in the reaction medium. The ferric ion then forms a colored complex with a chromogen under acidic conditions. The intensity of this color, measurable via spectrophotometry, is directly proportional to the total quantity of oxidant molecules within the sample. To standardize the assay, hydrogen peroxide is used, and the results are expressed in terms of micromolar hydrogen peroxide equivalent per liter (μmol H2O2 Equiv./L) (20).
Glucose and Lactate Levels
Glucose and lactate levels were documented through arterial blood gas analyses.
Statistical Analysis
Data analysis was conducted using SPSS version 23.0 software (SPSS Inc., Chicago, IL, USA). The normality of data distribution was assessed using the Kolmogorov-Smirnov test. Descriptive statistics were presented as mean ± SD. For parametric data with a normal distribution among the three groups, comparisons were made using one-way analysis of variance (ANOVA). The homogeneity of variances was evaluated using the Levene test. Post-hoc comparisons were conducted using Tukey and Thamhan’s T2 tests. Nonparametric data, which did not exhibit a normal distribution among the three groups, were compared using the Kruskal-Wallis test. Pairwise comparisons were conducted using the Mann-Whitney U-test, with a Bonferroni correction applied. A p-value below 0.05 was considered statistically significant.
RESULTS
One patient failed to receive the axillary block (Group A), three patients experienced hemolysis of their blood samples (one patient in each group), and three patients had a tourniquet time of less than 30 minutes (one patient in Group I and two patients in Group T). Consequently, these seven individuals were excluded from the study. This resulted in a total of 92 patients who successfully completed the study, with no missing samples (Fig. 1).
Figure 1.
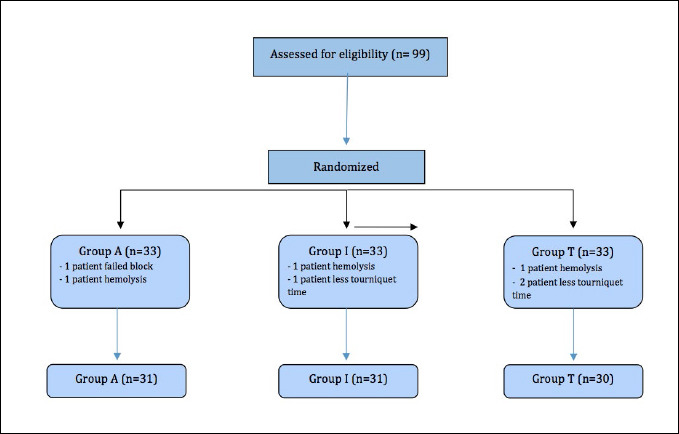
Flow diagram for patients.
There were no significant differences among the groups in terms of age, body mass index, American Society of Anesthesiologists (ASA) classification, hemoglobin levels, and tourniquet duration (p>0.05) (Table 1). Baseline measurements of glucose, lactate, IMA, TAS, and TOS levels did not exhibit statistically significant differences among the patient groups (p>0.05) (Table 2, Figures 2 and 3). Additionally, no significant variations were observed among the groups regarding HR, BP, and SpO2.
Table 1.
Patient characteristics data and tourniquet duration
| Group A (n=31) | Group I (n=31) | Group T (n=30) | p-value | |
|---|---|---|---|---|
| Age (years) | 30.13±7.54 | 30.55±8.45 | 31.60±9.44 | 0.805 |
| BMI (kg/m2) | 25.53±2.94 | 26.13±2.19 | 25.93±3.13 | 0.686 |
| ASA (I/II) | 28/3 | 29/2 | 22/3 | 0.772 |
| Hb (g/dL) | 15.14±0.95 | 15.36±1.20 | 15.18±1.03 | 0.688 |
| Tourniquet Duration (minutes) | 45.81±17.42 | 46.29±15.92 | 45.56±14.98 | 0.985 |
Data are expressed as mean±standard deviation or number of patients. BMI: Body mass index; ASA: American society of anesthesiologists.
Table 2.
Plasma glucose, lactate, TAS, TOS, and IMA levels (mean ± SD)
| Group A | Group I | Group T | p-value | |
|---|---|---|---|---|
| Glucose t1 Levels | 105.35±24.00 | 109.16±22.94# | 102.20±18.64 | 0.323 |
| Glucose t2 Levels | 101.35±16.70 | 113.48±22.35# | 100.04±11.59 | 0.008 |
| Glucose t3 Levels | 96.32±11.29 | 112.45±20.76 | 101.20±12.98 | 0.000 |
| Glucose t4 Levels | 101.06±22.47 | 102.10±21.18 | 106.28±28.88 | 0.651 |
| Lactate t1 Levels | 0.875±0.36 | 1.017±0.37 | 1.080±0.38 | 0.074 |
| Lactate t2 Levels | 0.989±0.56 | 1.284±0.48 | 1.064±0.50 | 0.074 |
| Lactate t3 Levels | 0.971±0.48 | 1.268±0.40# | 1.004±0.26 | 0.016 |
| Lactate t4 Levels | 0.897±0.36 | 1.021±0.40 | 1.002±0.44 | 0.349 |
| TAS t1 Levels | 1.005±0.139 | 1.044±0.109# | 1.001±0.066 | 0.345 |
| TAS t2 Levels | 0.976±0.115 | 1.084±0.118# | 0.970±0.066 | 0.000 |
| TAS t3 Levels | 0.988±0.106 | 1.070±0.147# | 0.991±0.086 | 0.014 |
| TAS t4 Levels | 0.989±0.097 | 1.081±0.123 | 0.984±0.104 | 0.001 |
| TOS t1 Levels | 11.84±4.04& | 15.14±6.58 | 12.89±4.70 | 0.125 |
| TOS t2 Levels | 10.28±4.77& | 14.70±7.19 | 11.50±2.78 | 0.006 |
| TOS t3 Levels | 11.05±7.77 | 17.53±9.01 | 13.29±8.55 | 0.012 |
| TOS t4 Levels | 13.43±6.78 | 13.65±4.77 | 15.23±4.53 | 0.449 |
| IMA t1 Levels | 0.462±0.066& | 0.485±0.068# | 0.485±0.087 | 0.498 |
| IMA t2 Levels | 0.421±0.106& | 0.494±0.081# | 0.631±0.100 | 0.000 |
| IMA t3 Levels | 0.395±0.114& | 0.476±0.089# | 0.610±0.116 | 0.000 |
| IMA t4 Levels | 0.416±0.128 | 0.488±0.093 | 0.610±0.078 | 0.000 |
TAS: Total Antioxidant Status; TOS: Total Oxidant Status; IMA: Ischemia Modified Albumin.
: p<0.05 when glucose levels in Group I are compared to those in Group A and Group T at t2 and t3.
#: p<0.05 when lactate levels in Group I are compared to those in Group A and Group T at t3.
#: p<0.05 when TAS levels in Group I are compared to those in Group A and Group T at t2, t3, and t4.
: p<0.05 when TOS levels in Group A are compared to those in Group I at t2 and t3. &: p<0.05 when IMA levels in Group A are compared to those in Group I and Group T at t2, t3, and t4.
#: p<0.05 when IMA levels in Group I are compared to those in Group T at t2, t3, and t4.
Figure 2.
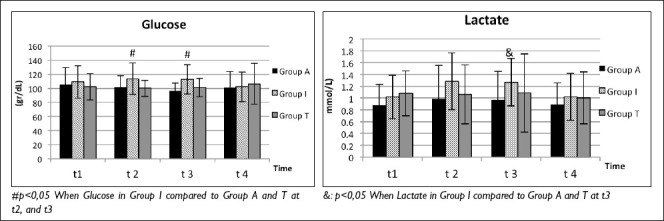
Plasma Glucose and Lactate levels.
Figure 3.
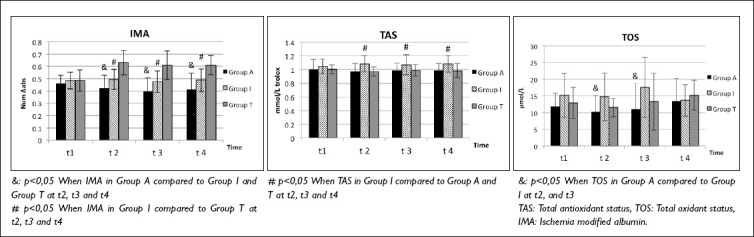
IMA, TAS and TOS changes.
Biochemical Assessments
Alterations in Glucose and Lactate Levels
Glucose levels were notably higher in Group I at t2 and t3 compared to both Group A and Group T (t2: p=0.024, p=0.017; t3: p=0.000, p=0.025) (Table 2, Fig. 2).
Lactate levels were significantly higher in Group I at t3 compared to both Group A and Group T (p=0.016) (Table 2, Fig. 2).
Alterations in IMA Levels
Plasma IMA levels exhibited significant differences between the groups. Group A had significantly lower IMA levels compared to both Group I and Group T at t2 (p=0.013; p<0.001, respectively), t3 (p=0.011; p<0.001, respectively), and t4 (p=0.023; p<0.001, respectively) (Table 2, Fig. 3).
Additionally, Group I had significantly lower plasma IMA levels compared to Group T at t2 (p<0.001), t3 (p<0.001), and t4 (p<0.001) (Table 2, Fig. 3).
Alterations in TAS Levels
Plasma TAS levels exhibited a significant increase in Group I compared to both Group A and Group T at t2 (p=0.002; p<0.001, respectively), t3 (p=0.023; p=0.047, respectively), and t4 (p=0.004; p=0.004, respectively) (Table 2, Fig. 3).
Alterations in TOS Levels
Plasma TOS levels were notably lower in Group A compared to Group I at t2 (p=0.019) and at t3 (p=0.009) (Table 2, Fig. 3).
DISCUSSION
This study demonstrated that the axillary block method provides better protection against reperfusion injury in patients undergoing upper extremity surgery with surgical tourniquets compared to TIVA with propofol and inhalation anesthesia with sevoflurane. Oxidative stress markers such as TOS and IMA levels were lower in patients treated with axillary block, and biochemical stress markers such as lactate and glucose levels were lower compared to those treated with inhalation anesthesia. This study also revealed that all anesthetic agents and methods had a positive effect on ischemia-reperfusion injury. Inhalation anesthesia was effective in maintaining TAS levels and limiting the increase in IMA, while propofol anesthesia limited the increase in stress markers such as lactate and glucose levels.
To investigate the effects of anesthesia methods and drugs used in this study on IRI, numerous studies have analyzed antioxidant enzymes including glutathione peroxidase, superoxide dismutase, and catalase, as well as oxidative intermediates including malondialdehyde, ischemia-modified albumin, advanced oxidation protein products (AOPP), and nitrite/nitrate (N/N).[17,21] Although methods for measuring separate oxidant/antioxidant species in blood serum are available, they are time-consuming, labor-intensive, technically challenging, and costly. Therefore, TAS and TOS measurement levels were used in this study instead of evaluating each of the oxidative stress markers separately. The total oxidant status of a sample is preferred over measuring each oxidant species individually due to the cumulative nature of their oxidant effects. Total antioxidant capacity (TAC) can be determined as a measurement of the overall protective effect of antioxidants at the cellular level, in body fluids, and in other cellular components against oxidative injury. TAC information indicates both the rate of antioxidant consumption and the capacity for oxidation during acute oxidative stress.[5,22]
Inhalation anesthetics have long been recognized for their protective role against ischemia-reperfusion injury, exhibiting these effects by inhibiting and regulating ischemia-induced polymorphonuclear neutrophil adhesion and preventing endothelial dysfunction.[23-26] Studies have shown that sevoflurane synchronizes endothelial dysfunction and reperfusion damage that occur after tourniquet release by activating the protein kinase A (PKA) pathway.[23] Erturk et al.[17] compared the effects of inhalation anesthesia using sevoflurane and TIVA with propofol on IRI in a model they created for thoracic surgeries performed with one-lung ventilation (OLV). IMA levels were lower in the inhalation anesthesia group at the sixth hour after reperfusion, highlighting the protective effects of sevoflurane. In our study, IMA levels were found to be lower in the propofol group, consistent with the findings in the literature. Additionally, the higher TAS levels in the TIVA and axillary block groups may be considered an indicator of the protective effect of inhalation anesthetics against IRI. However, glucose and lactate levels were higher in the inhalation group, and both TOS and oxidative stress index (OSI) levels were also elevated in the inhalation group. This suggests that inhalation anesthetics may increase oxidative damage. Mass et al. compared spinal and general anesthesia and found that plasma isofurans values after tourniquet release were higher in the general anesthesia group using sevoflurane. They reported that increased isofurans during general anesthesia reflect higher oxidative stress, attributed to elevated oxygen concentrations from intubation, resulting in higher PO2 levels.[27]
Propofol is well-known for its antioxidant properties, similar to those of vitamin D, and for its favorable effects against reperfusion damage.[10,28] It has been utilized in clinical studies involving tourniquet-induced IRI models due to these properties and is reported to improve reperfusion injury by enhancing tissue saturation.[5,17] Turan et al. reported that propofol restored antioxidant enzymes, inhibited lipid peroxidation, and reduced the formation of toxic metabolites such as malondialdehyde after reperfusion.[29]
Regional anesthesia has been found to limit reperfusion injury due to its beneficial effects on anaerobic glucose metabolism levels and oxidative stress levels, thus reducing the rate of postoperative complications.[6] Shin et al.[30] noted that epidural anesthesia reduced the increase in oxidative stress markers by blocking the sympathetic nervous system, thereby providing a protective effect against oxidative stress. Vasodilatation caused by a sympathetic block decreases vascular resistance, increases the density of the capillary vascular bed, and promotes microcirculatory recruitment. This improves oxidative damage by reducing the inflammatory response, lipid peroxidation, and mucosal apoptosis.[15] However, the effects of peripheral nerve blocks, which affect a more limited area, on oxidative damage remain controversial. Lumbar plexus and sciatic nerve blocks have been shown to reduce the inflammatory response,[31] while the rectus sheath block reportedly has no positive effects on oxidative stress.[32] Oksuz et al.[33] studied the effects of general anesthesia and interscalene block application on pain and oxidative stress, finding lower pain scores and oxidative stress markers in patients who underwent block application. They reported that interscalene block application provided more stable hemodynamics in the perioperative period.
In addition to the sensory block provided by the axillary plexus block, it may enhance tissue perfusion and accelerate cell metabolism in the arm and forearm region by creating a sympathetic block, which may allow the rapid removal of reperfusion intermediates from the tissues. In our study, oxidative intermediates such as IMA, lactate, and TOS levels, which reflect oxidative stress, were found to be lower in patients with axillary block than in those with TIVA and inhalation anesthesia. These results suggest that the increase of oxidative products after reperfusion is limited by axillary block application or that these products are removed from plasma more rapidly than with other anesthetic methods. Therefore, the axillary block appears to offer better protection against reperfusion injury than TIVA and inhalation anesthesia in upper extremity surgeries with tourniquet application.
Limitations
Although the positive effects of the drugs and methods used against ischemia-reperfusion (IR) damage have been demonstrated, this study has some limitations. Primarily, a control group could not be established because each of the anesthetic agents and methods used had effects on IRI. Secondly, although the favorable effects of brachial plexus block on biochemical markers of reperfusion were documented, these effects could not be clinically demonstrated in patients. Detailed electrocardiogram (ECG) analysis and cardiopulmonary examination were not conducted in the perioperative period, and patient follow-up in the later postoperative period was not performed. Therefore, it is not possible to conclusively assert that clinical results will be favorable based solely on biochemical markers. Furthermore, since the patients included in the study were not in the high ASA risk group, the effects on high-risk patient groups, where reperfusion injury may lead to more serious consequences, are unknown.
CONCLUSION
As a result, axillary block application in upper extremity surgeries performed using a tourniquet shows a protective effect against ischemia-reperfusion injury by decreasing oxidative stress markers. However, further studies involving high-risk patient groups are needed to reveal these favorable effects on biochemical markers at the clinical level.
Footnotes
Ethics Committee Approval: This study was approved by the Karadeniz Technical University Ethics Committee (Date: 24.02.2014, Decision No: 2014/7).
Peer-review: Externally peer-reviewed.
Authorship Contributions: Concept: D.K., A.A., E.E.; Design: E.E., A.A., D.K.; Supervision: D.K., A.A., A.B.; Resource: A.A., E.E., A.B.; Materials: H.K., A.A., D.K.; Data collection and/or processing: S.D.K., A.A., E.E.; Analysis and/or interpretation: H.K., A.A., M.Y.; Literature search: A.A., E.E., A.B.; Writing: H.K., A.A., D.K.; Critical review: H.K., A.A., D.K.
Conflict of Interest: None declared.
Use of AI for Writing Assistance: Not declared.
Financial Disclosure: The author declared that this study has received no financial support.
REFERENCES
- 1.Estebe JP, Davies JM, Richebe P. The pneumatic tourniquet:mechanical, ischaemia-reperfusion and systemic effects. Eur J Anaesthesiol. 2011;28:404–11. doi: 10.1097/EJA.0b013e328346d5a9. [DOI] [PubMed] [Google Scholar]
- 2.Sarfani S, Cantwell S, Shin AY, Kakar S. Challenging the dogma of tourniquet pressure requirements for upper extremity surgery. J Wrist Surg. 2016;5:120–3. doi: 10.1055/s-0036-1571281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yagmurdur H, Ozcan N, Dokumaci F, Kilinc K, Yilmaz F, Basar H. Dexmedetomidine reduces the ischemia-reperfusion injury markers during upper extremity surgery with tourniquet. J Hand Surg Am. 2008;33:941–7. doi: 10.1016/j.jhsa.2008.01.014. [DOI] [PubMed] [Google Scholar]
- 4.Alagöz A, Küçükgüçlü S, Boztaş N, Hancı V, Yuluğ E, Şişman AR. Effects of sugammadex on ischemia reperfusion in a rat extremity model. Ulus Travma Acil Cerrahi Derg. 2020;26:509–16. doi: 10.14744/tjtes.2019.12524. [DOI] [PubMed] [Google Scholar]
- 5.Erturk E, Cekic B, Geze S, Kosucu M, Coskun I, Eroglu A, et al. Comparison of the effect of propofol and N-acetyl cysteine in preventing ischaemia-reperfusion injury. Eur J Anaesthesiol. 2009;26:279–84. doi: 10.1097/EJA.0b013e32831c87c7. [DOI] [PubMed] [Google Scholar]
- 6.Kosucu M, Coskun I, Eroglu A, Kutanis D, Mentese A, Karahan SC, et al. The effects of spinal, inhalation, and total intravenous anesthetic techniques on ischemia-reperfusion injury in arthroscopic knee surgery. Biomed Res Int. 2014;2014:846570. doi: 10.1155/2014/846570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eltzschig HK, Eckle T. Ischemia and reperfusion--from mechanism to translation. Nat Med. 2011;17:1391–401. doi: 10.1038/nm.2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zarbock A, Eroglu A, Erturk E, Ince C, Westphal M. Ischemia-reperfusion injury and anesthesia. Biomed Res Int. 2014;2014:980318. doi: 10.1155/2014/980318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Collard CD, Gelman S. Pathophysiology, clinical manifestations, and prevention of ischemia-reperfusion injury. Anesthesiology. 2001;94:1133–8. doi: 10.1097/00000542-200106000-00030. [DOI] [PubMed] [Google Scholar]
- 10.Akyol A, Ulusoy H, Imamoglu M, Cay A, Yulug E, Alver A, et al. Does propofol or caffeic acid phenethyl ester prevent lung injury after hindlimb ischaemia-reperfusion in ventilated rats? Injury. 2006;37:380–7. doi: 10.1016/j.injury.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 11.Peker K, Ökesli S, Kıyıcı A, Deyişli C. The effects of ketamine and lidocaine on free radical production after tourniquet-induced ischemia-reperfusion injury in adults. The effects of ketamine and lidocaine on free radical production after tourniquet-induced ischemia-reperfusion injury in adults. Ulus Travma Acil Cerrahi Derg. 2019;25:111–17. doi: 10.5505/tjtes.2018.63439. [DOI] [PubMed] [Google Scholar]
- 12.Chen H, Wei JQ, Wang YW, Zhou KP, He Y, Liu H, et al. Protective effects of rocuronium bromide on ischemia-reperfusion injury in skeletal muscle induced by tourniquet in patients undergoing elective unilateral total knee arthroplasty:a prospective, double blind, randomized, controlled study. Drug Des Devel Ther. 2020;14:3373–84. doi: 10.2147/DDDT.S252546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baysal Z, Togrul T, Aksoy N, Cengiz M, Celik H, Boleken ME, et al. Evaluation of total oxidative and antioxidative status in pediatric patients undergoing laparoscopic surgery. J Pediatr Surg. 2009;44:1367–70. doi: 10.1016/j.jpedsurg.2008.11.031. [DOI] [PubMed] [Google Scholar]
- 14.Oxman T, Arad M, Klein R, Avazov N, Rabinowitz B. Limb ischemia preconditions the heart against reperfusion tachyarrhythmia. Am J Physiol. 1997;273:1707–12. doi: 10.1152/ajpheart.1997.273.4.H1707. [DOI] [PubMed] [Google Scholar]
- 15.Bedirli N, Akyürek N, Kurtipek O, Kavutcu M, Kartal S, Bayraktar AC. Thoracic epidural bupivacaine attenuates inflammatory response, intestinal lipid peroxidation, oxidative injury, and mucosal apoptosis induced by mesenteric ischemia/reperfusion. Anesth Analg. 2011;113:1226–32. doi: 10.1213/ANE.0b013e31822b8984. [DOI] [PubMed] [Google Scholar]
- 16.Arnaoutoglou H, Vretzakis G, Souliotis D, Cambili M, Galaris D, Papadopoulos G. The effects of propofol or sevoflurane on free radical production after tourniquet induced ischaemia-reperfusion injury during knee arthroplasty. Acta Anaesthesiol Belg. 2007;58:3–6. [PubMed] [Google Scholar]
- 17.Erturk E, Topaloglu S, Dohman D, Kutanis D, Besir A, Demirci Y, et al. The comparison of the effects of sevoflurane inhalation anesthesia and intravenous propofol anesthesia on oxidative stress in one lung ventilation. Biomed Res Int. 2014;2014:360936. doi: 10.1155/2014/360936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wakai A, Wang JH, Winter DC, Street JT, O'Sullivan RG, Redmond HP. Tourniquet-induced systemic inflammatory response in extremity surgery. J Trauma. 2001;51:922–26. doi: 10.1097/00005373-200111000-00016. [DOI] [PubMed] [Google Scholar]
- 19.Bar-Or D, Lau E, Winkler JV. A novel assay for cobalt-albumin binding and its potential as a marker for myocardial ischemia-a preliminary report. J Emerg Med. 2000;19:311–15. doi: 10.1016/s0736-4679(00)00255-9. [DOI] [PubMed] [Google Scholar]
- 20.Erel O. A novel automated method to measure total antioxidant response against potent free radical reactions. Clin Biochem. 2004;37:112–9. doi: 10.1016/j.clinbiochem.2003.10.014. [DOI] [PubMed] [Google Scholar]
- 21.Kutanis D, Erturk E, Besir A, Demirci Y, Kayir S, Akdogan A, et al. Dexmedetomidine acts as an oxidative damage prophylactic in rats exposed to ionizing radiation. J Clin Anesth. 2016;34:577–85. doi: 10.1016/j.jclinane.2016.06.031. [DOI] [PubMed] [Google Scholar]
- 22.Koca K, Yurttas Y, Cayci T, Bilgic S, Kaldirim U, Durusu M, et al. The role of preconditioning and N-acetylcysteine on oxidative stress resulting from tourniquet-induced ischemia-reperfusion in arthroscopic knee surgery. J Trauma. 2011;70:717–23. doi: 10.1097/TA.0b013e3181f30fb0. [DOI] [PubMed] [Google Scholar]
- 23.Erturk E. Ischemia-reperfusion injury and volatile anesthetics. Biomed Res Int. 2014;2014:526301. doi: 10.1155/2014/526301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Budic I, Pavlovic D, Kocic G, Cvetkovic T, Simic D, Basic J, et al. Biomarkers of oxidative stress and endothelial dysfunction after tourniquet release in children. Physiol Res. 2011;60:S137–45. doi: 10.33549/physiolres.932170. [DOI] [PubMed] [Google Scholar]
- 25.Li T, Han Y, Guo B, Chen P, Wan Y, Ye B. Sevoflurane protects against ischemia-reperfusion injury in mice after total knee arthroplasty via facilitating RASD1-mediated protein kinase A pathway activation. Aging (Albany NY) 2021;13:13333–48. doi: 10.18632/aging.103899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xu X, Deng R, Zou L, Pan X, Sheng Z, Xu D, et al. Sevoflurane participates in the protection of rat renal ischemia-reperfusion injury by down-regulating the expression of TRPM7. Immun Inflamm Dis. 2023;11:e753. doi: 10.1002/iid3.753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mas E, Barden AE, Corcoran TB, Phillips M, Roberts LJ, 2nd, Mori TA. Effects of spinal or general anesthesia on F₂-isoprostanes and isofurans during ischemia/reperfusion of the leg in patients undergoing knee replacement surgery. Free Radic Biol Med. 2011;50:1171–6. doi: 10.1016/j.freeradbiomed.2011.01.021. [DOI] [PubMed] [Google Scholar]
- 28.Ozkan F, Senayli Y, Ozyurt H, Erkorkmaz U, Bostan B. Antioxidant effects of propofol on tourniquet-induced ischemia-reperfusion injury:an experimental study. J Surg Res. 2012;176:601–7. doi: 10.1016/j.jss.2011.10.032. [DOI] [PubMed] [Google Scholar]
- 29.Turan R, Yagmurdur H, Kavutcu M, Dikmen B. Propofol and tourniquet induced ischaemia reperfusion injury in lower extremity operations. Eur J Anaesthesiol. 2007;24:185–9. doi: 10.1017/S0265021506001347. [DOI] [PubMed] [Google Scholar]
- 30.Shin S, Bai SJ, Rha KH, So Y, Oh YJ. The effects of combined epidural and general anesthesia on the autonomic nervous system and bioavailability of nitric oxide in patients undergoing laparoscopic pelvic surgery. Surg Endosc. 2013;27:918–26. doi: 10.1007/s00464-012-2536-5. [DOI] [PubMed] [Google Scholar]
- 31.Bagry H, de la Cuadra Fontaine JC, Asenjo JF, Bracco D, Carli F. Effect of a continuous peripheral nerve block on the inflammatory response in knee arthroplasty. Reg Anesth Pain Med. 2008;33:17–23. doi: 10.1016/j.rapm.2007.06.398. [DOI] [PubMed] [Google Scholar]
- 32.Purdy M, Kärkkäinen J, Kokki M, Anttila M, Aspinen S, Juvonen P, et al. Does rectus sheath block analgesia alter levels of the oxidative stress biomarker glutathione peroxidase:a randomised trial of patients with cancer and benign disease. Anticancer Res. 2017;37:897–902. doi: 10.21873/anticanres.11396. [DOI] [PubMed] [Google Scholar]
- 33.Oksuz M, Abitagaoglu S, Kaciroglu A, Koksal C, Ozturk BY, Erel O, et al. Effects of general anaesthesia and ultrasonography-guided interscalene block on pain and oxidative stress in shoulder arthroscopy:A randomised trial. Int J Clin Pract. 2021;75:e14948. doi: 10.1111/ijcp.14948. [DOI] [PubMed] [Google Scholar]


