Abstract
Outbreaks of methicillin-resistant Staphylococcus aureus (MRSA) are well described in the neonatal intensive care unit (NICU) setting. Genomics has revolutionized the investigation of such outbreaks; however, to date, this has largely been completed retrospectively and has typically relied on short-read platforms. In 2022, our laboratory established a prospective genomic surveillance system using Oxford Nanopore Technologies sequencing for rapid outbreak detection. Herein, using this system, we describe the detection and control of an outbreak of sequence-type (ST)97 MRSA in our NICU. The outbreak was identified 13 days after the first MRSA-positive culture and at a point where there were only two known cases. Ward screening rapidly defined the extent of the outbreak, with six other infants found to be colonized. There was minimal transmission once the outbreak had been detected and appropriate infection control measures had been instituted; only two further ST97 cases were detected, along with three unrelated non-ST97 MRSA cases. To contextualize the outbreak, core-genome single-nucleotide variants were identified for phylogenetic analysis after de novo assembly of nanopore data. Comparisons with global (n=45) and national surveillance (n=35) ST97 genomes revealed the stepwise evolution of methicillin resistance within this ST97 subset. A distinct cluster comprising nine of the ten ST97-IVa genomes from the NICU was identified, with strains from 2020 to 2022 national surveillance serving as outgroups to this cluster. One ST97-IVa genome presumed to be part of the outbreak formed an outgroup and was retrospectively excluded. A second phylogeny was created using Illumina sequencing, which considerably reduced the branch lengths of the NICU isolates on the phylogenetic tree. However, the overall tree topology and conclusions were unchanged, with the exception of the NICU outbreak cluster, where differences in branch lengths were observed. This analysis demonstrated the ability of a nanopore-only prospective genomic surveillance system to rapidly identify and contextualize an outbreak of MRSA in a NICU.
Keywords: antibiotic resistance, genomic surveillance, infection control, outbreak detection, phylogenetic analysis
Impact Statement
This research emphasizes the significance of utilizing genomics for prospective monitoring to promptly identify methicillin-resistant Staphylococcus aureus (MRSA) outbreaks in a neonatal intensive care unit. An MRSA outbreak was detected only 13 days from the initial positive sample being collected, enabling prompt implementation of infection prevention and control measures, thus limiting potential negative impacts on the infants on the unit and the functioning of the unit as a whole. In addition, the research showed diverse lineage categorizations, underscoring the importance of genomics in comprehending antibiotic resistance evolution. The results show that nanopore sequencing is a reliable platform for studying outbreaks because it is cost-effective and provides quick results. In addition to healthcare, the study also points out the relationship between human and animal sources of S. aureus, which could have implications for veterinary medicine and agricultural systems. The study enhances our understanding of antibiotic resistance development and dissemination by examining the evolutionary history of MRSA strains.
Data Summary
The study sequences are available in the National Center for Biotechnology Information (NCBI) under BioProject accession number PRJNA1046639. The raw sequence read data generated in this study have been deposited to the NCBI sequence read archive (SRA, https://www.ncbi.nlm.nih.gov/sra) under accession numbers SRR26992863 to SRR26992891. The complete assembly for strain 23MR1425 has been deposited to GenBank under accession numbers CP143800 and CP143801. The software used to analyse raw sequence reads for polymorphism discovery and whole-genome sequencing-based phylogenetic reconstruction are available as described in the Methods. The authors confirm that all supporting data protocols have been provided in the article or supplementary data files.
Introduction
Staphylococcus aureus is a Gram-positive pathogen that can lead to severe opportunistic infections in clinical settings [1]. Despite advancements in combating such pathogens [2,3], the emergence of antibiotic-resistant strains, particularly methicillin-resistant S. aureus (MRSA), has posed formidable challenges globally [4,8]. MRSA and methicillin-susceptible S. aureus (MSSA) are common causes of hospital-associated and community-associated infections [4,8].
MRSA outbreaks in healthcare settings, especially in neonatal intensive care units (NICUs), have become a well-recognized global concern [9]. Neonates, especially preterm and low-birthweight infants, face higher risks of MRSA colonization and infection due to their underdeveloped protective microbiota and immature immune systems [10]. This increased vulnerability is exacerbated by evidence of nosocomial and neonatal transmission of S. aureus involving environmental reservoirs as well as colonized parents and healthcare workers [9].
Such outbreaks threaten the ability to safely deliver healthcare to this vulnerable population and are highly disruptive to models of care, especially if prolonged [11,13]. Genomics has improved the understanding of the transmission dynamics of MRSA in NICU settings and has been used to identify and control outbreaks [13,15]. This has been in the form of retrospective or reactive investigations instigated when outbreaks have reached sufficient size to be detected based on standard surveillance [13,14]. To our knowledge, the utility of prospective/proactive genomic surveillance in this setting is less well described, as is the use of Oxford Nanopore Technologies (ONT) sequencing as a standalone sequencing platform [16].
In 2022 we instituted a prospective genomic surveillance programme using ONT sequencing to target several common hospital pathogens, one of which was MRSA isolated from patients in the NICU of our institution [17]. The programme aimed to detect potential outbreaks early to enable effective infection prevention and control (IPC) interventions and limit the negative impacts of such outbreaks [18]. Here, we describe the rapid detection and control of an outbreak of sequence type (ST)97 MRSA in our NICU using this surveillance programme. As a proof-of-concept, we explored an alternative approach to the conventional Illumina sequencing used in New Zealand’s genomic surveillance landscape [19]. By integrating nanopore sequencing, our study offers a fresh perspective in the broader phylogeny and evolution of ST97 MRSA.
Additionally, S. aureus ST97 is a predominant pathogen in bovine cattle worldwide [20]. Given New Zealand’s isolated environment and heavy dependence on agriculture as a fundamental pillar of its economy and export industry [21], it is crucial to understand whether there are clear genomic differences between ST97 strains isolated from humans and bovine hosts. Studies on MRSA in New Zealand cattle are limited, with only preliminary data available [22,23]. By comparing agricultural samples, our research investigates potential zoonotic links, emphasizing the importance of a One Health approach in managing MRSA across different reservoirs. This shift underscores the importance of adopting advanced genomic technologies to enhance our understanding and control of infectious diseases in healthcare settings.
Methods
Setting
New Zealand (known as Aotearoa in the Māori language) is an island nation in the Southwest Pacific, with a population of around 5.27 million. Wellington Regional Hospital (WRH) provides tertiary services to the lower North Island/Te Ika-a-Māui of New Zealand, serving a population of around 500 000. The WRH NICU is a Level 3 unit resourced for 37 infants. It provides advanced care for extremely premature infants (born as early as 24 weeks of gestation) and for infants in need of ventilation, intravenous feeding, and other forms of intensive care monitoring and treatment. Routine MRSA screening is not performed.
Awanui Laboratories Wellington, formerly Southern Community Laboratories (SCL), is a medium-sized laboratory that provides clinical diagnostic services to WRH and the local region. The microbiology and molecular departments process around 300 000 samples yearly. The analysis and reporting of this outbreak constituted an ‘audit or related activity’ as per New Zealand Health and Disability Ethics Committees, so it did not require review.
Genomic surveillance programme
From the beginning of 2022, all MRSA isolated from infants in the unit were prospectively sequenced at Awanui Laboratories [17]. Isolates for sequencing are batched and run on a weekly or fortnightly basis. Details describing sampling and extraction of DNA can be found in the Supplementary Materials. Methicillin resistance was determined phenotypically for all isolates using the Vitek II instrument (bioMerieux) and the AST-P656 card, according to European Committee on Antimicrobial Susceptibility Testing (EUCAST) methods [24].
For isolates sequenced before March 2023 (sa220609barcode87 and sa230215barcode55), libraries were constructed using 50 ng of genomic DNA with the ONT rapid barcoding kit 96 (SQK-RBK110-96) as per the manufacturer’s instructions. Subsequently, the entire library was loaded onto an R9.4 flow cell (FLO-MIN106) and run on a MinION device for approximately 20–40 h (using MinKNOW v22.10.10). After March 2023 (i.e. including when the outbreak occurred), sequencing used Q20+ chemistry; libraries were created using 50–100 ng of genomic DNA, prepared using the rapid barcoding kit 96 (SQK-RBK114-96) and sequenced on an R10.4.1 flow cell (FLO-MIN114) with MinKNOW v23.04.5. Krocus v1.0.3 [25] was used to generate a rapid multi-locus sequence type (MLST) from the raw FASTQ files. After each sequencing run, MLSTs were compared to other recent MRSA isolates in the NICU. Awanui Laboratories does not have next-generation sequencing bioinformatics expertise, so if greater than expected numbers of a given MLST were observed, further investigations were instigated by the IPC team and data were transferred to the Institute of Environmental Science and Research (ESR) for more granular analysis.
Retrospective genome analyses
Original Fast5 sequence files were converted to Pod5 using pod5 v0.3.2 (https://github.com/nanoporetech/pod5-file-format, accessed on 18 March 2024) and then basecalled using Dorado v0.3.4 (https://github.com/nanoporetech/dorado, accessed on 18 March 2024). The base-calling process was carried out using the ‘super accuracy’ models, with a batch size of 2008 and a chunk size of 1000, while parameters were kept at their default settings. Further details of laboratory methods including: nanopore read quality control, Illumina sequencing, genome assembly, multilocus sequence typing, virulence and antibiotic resistance gene genotyping, and public data curation, are available in the Supplementary Materials.
Genome annotation for 23MR1425
In the absence of an available ST97 reference genome representing New Zealand S. aureus strains, we selected 23MR1425, a clinical isolate collected from a neonatal eye swab, as our reference genome (index case on the WRH NICU identified in June 2023). The assembly representing strain 23MR1425 was annotated using Prokka v1.14.6 [26]. Prophage regions were identified using PHASTER [27,28] and then annotated using Pharokka v1.5.1 [29]. Mobile genetic elements were identified using IslandViewer 4 [30] and ISsaga v2.0 [31] (ISfinder platform [32]), followed by manual curation using Artemis v18.2.0 [33].
Assembly-based variant detection and ST97 phylogenetic analyses
Further details of public data curation and de novo assembly are available in the Supplementary Materials. A core-genome alignment was generated from 679 S. aureus ST97 genomes using Parsnp v1.7.4 [34] with the 23MR1425 chromosome serving as the reference to call single-nucleotide variants (SNVs) (index ST97 MRSA case, date sample received June 2023). Resulting SNV alignments were used to reconstruct phylogenies. Maximum-likelihood phylogenetic trees were reconstructed using RaxML v8.2.12 [35] (GTR-GAMMA correction) by optimizing 20 distinct, randomized maximum-parsimony trees before adding 1000 bootstrap replicates. The resulting phylogenetic trees were visualized using FigTree v1.4.4 (http://tree.bio.ed.ac.uk/software/figtree/, accessed on 18 March 2024). To further identify robust phylogenetic groups within the ST97 phylogeny, we used the core-genome SNV alignment as input into rhierBAPS v1.0.1 [36] [an R v4.3.2 [37] implementation of hierarchical Bayesian Analysis of Population Structure (BAPS) [38]] with one level of clustering, allowing up to ten initial clusters.
High-resolution cluster phylogeny
High-resolution analyses of genetic variants were performed using Burrows-Wheeler Aligner (BWA) v0.7.17 [39]; BEDTools v2.28.0 [40]; seqtk v1.3-r106 (https://github.com/lh3/seqtk, accessed on 15 September 2023); Trimmomatic v0.36 [41]; pindel [42]; Mosdepth [43]; SAMtools v1.9 [44]; Picard v2.7.1 (https://github.com/broadinstitute/picard, accessed on 18 March 2024); the Genome Analysis Tool Kit v4.3.0.0 (GATK) [45,46]; and SNPEff v4.3.1t [47], as implemented in SPANDx v4.0 [48]. Resulting SNV alignments were used to reconstruct phylogenies. The pairwise SNV distances were determined using snp-dist v0.6.3 (https://github.com/tseemann/snp-dists, accessed on 18 March 2024). Maximum-parsimony trees were reconstructed using the heuristic search feature of PAUP v4.0a [49]. The resulting phylogenetic trees were visualized using FigTree v1.4.4. Notably, this analysis defines a core genome as regions estimated to the nearest 100 bp with ≥95 % coverage across one or more genomes in the given population.
Divergence estimates of a subset of Clade 1.1 S. aureus ST97
To calibrate the phylogeny, we used tip-dating approaches using TempEst v1.5.3 [50] and BEAST2 v2.7.1 [51,52]. For this analysis, we utilized Illumina sequence data due to their higher accuracy and lower error rates compared to Nanopore sequencing. This choice ensures robust calibration of the phylogeny and improves the reliability of evolutionary timeline estimations. For the Bayesian approach, we first determined whether the strict or optimized relaxed clock (with a log-normal distributed rate) model best fits our dataset. Using the tip date’s function, six models representative of a strict clock model and an optimized relaxed log-normal clock model were set up. The Bayesian skyline, coalescent constant and exponential growth population size change models were compared for each clock model to ensure the selection of the best-fit model. The Gamma Site Model Category Count was set to four, and the GTR substitution model rates determined from jModelTest v2.1.10 [53] were included (i.e. rate AC=0.93, AG=3.20, AT=0.83, CG=0.21, CT=3.39 and GT=1.00). The initial clock rate was set to 5.67×10−4 substitutions per site per year (estimated from the root-to-tip regression analysis in TempEst) with a uniform distribution and an upper bound of 0.1. All other priors were left as default. All models were tested with the Nested Sampling Bayesian computation algorithm v1.2.1 within the BEAST2 package with a particle count of 32, sub-chain length of 5000 and Epsilon of 1.0×10−12.
Once the best-fitting tree model was determined, three independent Markov chain Monte Carlo generations were conducted for 100 million generations for each analysis. Trees were sampled every 1000 generations, resulting in triplicate samples of 100 000 trees for each model test. To assess statistics, all BEAST2 runs were imported into Tracer v1.7.2 (http://github.com/beast-dev/tracer/, accessed on 18 March 2024). LogCombiner v2.7.1 (BEAST2 package) then combined the replicated analyses for each model with a 10 % burn-in to assess convergence. Finally, TreeAnnotator v2.7.1 (BEAST2 package) removed the 10 % burn-in and generated maximum clade credibility trees for each run (established from 243 million trees), reporting median values with a posterior probability limit set at 0.5. The resulting phylogenetic trees were visualized using FigTree v1.4.4.
Results
Outbreak detection
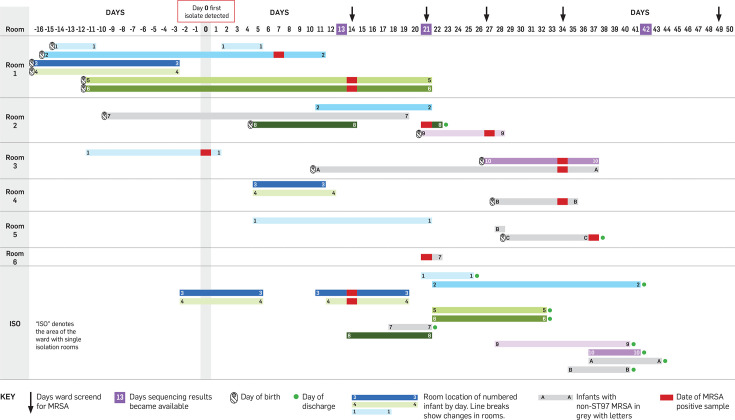
During a routine weekly sequencing run in June 2023, two NICU MRSA isolates recovered from eye swabs were identified as ST97. This was an uncommon ST for the NICU, and the infants had spent time in the same room, so a possible transmission event was suspected (Fig. 1). The following day, all infants who had been cared for in the same rooms as the two index cases were screened for MRSA (combined nasal, axilla, umbilicus and perineum swabs), which detected a further four infants colonized with MRSA (two sets of twins; infants 3–4 and 5–6, Fig. 1). These infants were immediately placed in contact isolation. The following weekly sequencing run was brought forward, and these four isolates were also shown to be ST97. At this point, all infants in the NICU were screened for MRSA, and this detected two more cases. Three additional rounds of ward screening were undertaken over 4 weeks, detecting a further two cases of ST97 MRSA, after which the outbreak was declared closed. Screening also revealed three infants with non-ST97 MRSA (infants A–C, Fig. 1), who could be rapidly excluded from the outbreak. The outbreak totalled ten infants, none of whom developed invasive infection with MRSA (Table S1, available in the online version of this article). The outbreak was identified 13 days after the collection of the initial MRSA-positive sample from infant 1 and closed 38 days later.
Fig. 1. Line chart of neonatal intensive care unit MRSA ST97 outbreak. Days are displayed on the x-axis, with the day the first isolate was collected denoted as day 0 (actual dates have been omitted for patient privacy). Each horizontal shaded bar represents the days spent by the numbered infant in the room as indicated on the y-axis. Infants with non-ST97 MRSA are shown in lighter shaded bars and denoted with letters instead of numbers. Infant 7 was ST97 but was excluded from the outbreak based on the phylogenetic analysis, so is also shown in light grey.
A high-quality reference genome was generated for the index S. aureus ST97 case
We performed initial nanopore whole-genome sequencing (WGS) on 23MR1425, which yielded 102 227 single-ended reads, providing complete genome coverage at an average depth of 133×. The median read length was 3 160 bp (N50=10 546 bp), and the median read quality score was 15.4. The de novo nanopore assembly, further refined with Illumina reads, revealed that 23MR1425 possesses a circular chromosome with a length of 2 753 159 bp and an average GC content of 32.86 % (Fig. S1a). In silico analysis determined that 23MR1425 is spa type t359. Additionally, the chromosome of 23MR1425 was predicted to harbour a type IVa(2B) staphylococcal cassette chromosome mec (SCCmec) which carried the mecA gene (encoding penicillin-binding protein 2a). We comprehensively characterized the chromosomal attributes of 23MR1425 through BLASTn comparison with 18 other complete S. aureus genomes downloaded from GenBank (Fig. S2). Notably, the chromosome of 23MR1425 contained a conserved prophage, designated 23MR1425_prophage1, which was also found in six other publicly available ST97 genomes. In contrast, 23MR1425_prophage2, housing the sak gene responsible for encoding staphylokinase (protease III), a virulence factor involved in clot dissolution, appeared to be absent from the ST97 chromosome of MOK063 (GenBank: CP029629). Remarkably, the chromosome of 23MR1425 represents the first complete ST97 chromosome displaying the integration of the type IVa(2B) SCCmec element. The complete genome of 23MR1425 included the plasmid p23MR1425A (20 403 bp, rep20 plasmid), which houses a bla operon putatively responsible for penicillin resistance. This bla operon is positioned downstream of an ISSau6 insertion sequence element on p23MR1425A (Fig. S1b). A concise overview of genes encoding antibiotic resistance and virulence factors in the chromosome or plasmid of 23MR1425 is shown in Table S2.
Genomic diversity and lineage classification of S. aureus ST97 reveals two primary clades with distinct origins
In this study, we analysed 679 genomes in total. These included: six complete genomes from NCBI, 468 draft genomes from the PathogenWatch platform (https://pathogen.watch/, accessed on 25 August 2023), 127 bovine genomes from Livestock Improvement Corporation (LIC) trials and 13 human genomes sourced from the Pathlab (Supplementary Materials), 35 from ESR national staphylococcal surveillance surveys, 18 draft genomes from bovine mastitis-causing S. aureus in New Zealand [54] and 12 ST97 genomes from WRH NICU. The nanopore sequence read data quality metrics (Table S3) and de novo assembly quality metrics (Table S4) for the 12 ST97 genomes from WRH are presented in the Supplementary Materials. Additionally, the assembly metrics for the 492 publicly available genomes are outlined in Table S5.
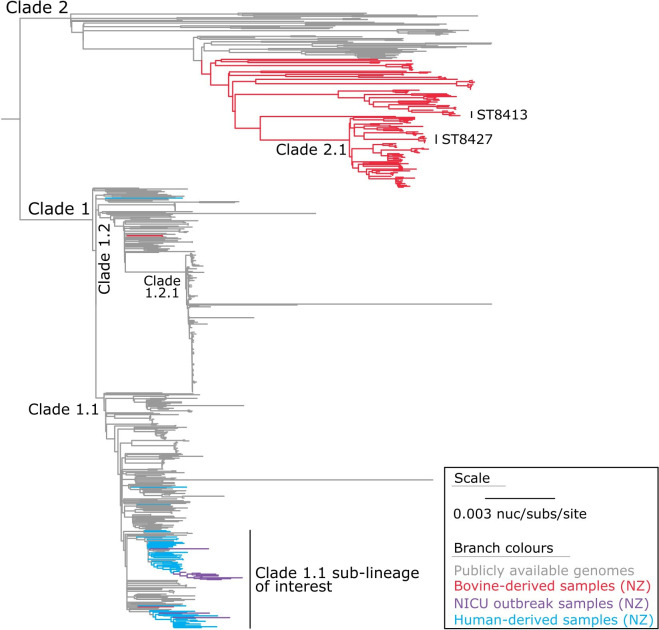
When using nanopore-only data (for the NICU outbreak genome), we identified 28 634 core-genome SNVs from the 679 genomes (Fig. 2). These SNVs were called against the chromosome of sample 23MR1425 using assembly-based variant detection (see Methods). The phylogenetic tree is rooted at the midpoint (Fig. 2), which corresponds to the actual root by S. aureus ST834 strain 70017 (SRA accession: DRR291698) (Fig. S3).
Fig. 2. Maximum-likelihood phylogeny of S. aureus ST97. The phylogeny was inferred from 28 634 core-genome single-nucleotide variants (SNVs) from 679 assembled genomes. SNVs were derived from a core-genome alignment of 2 087 573 bp and are called against the chromosome of sample 23MR1425. The phylogenetic tree is rooted at the midpoint, which corresponds to the actual root by S. aureus ST834 strain 70017 (SRA accession: DRR291698), which has been omitted for visualization.
Considering our findings, S. aureus ST97 can be broadly classified into two primary lineages: Clade 1, predominantly consisting of genomes sourced from humans, and Clade 2, primarily consisting of genomes from bovine origins. Notably, within Clade 1, we observed two bovine-derived genomes, B791 and B792, which cluster within sub-clade 1.2, and another genome, B371 (collected from a non-aseptic bulk milk tank in the North Island/Te Ika-a-Māui in 2021), which clusters in sub-clade 1.1. Of particular interest, a substantial portion of the S. aureus genomes obtained through ESR national surveillance surveys (n=35) and a separate group of genomes from the Pathlab (n=10) and a genome from an LIC trial (n=1) coalesce within a sub-lineage of sub-clade 1.1, alongside the NICU outbreak genomes (n=12) (Fig. 2).
Genomic comparison of S. aureus ST97 sub-clade 1.1 reveals consistency between nanopore and Illumina sequencing
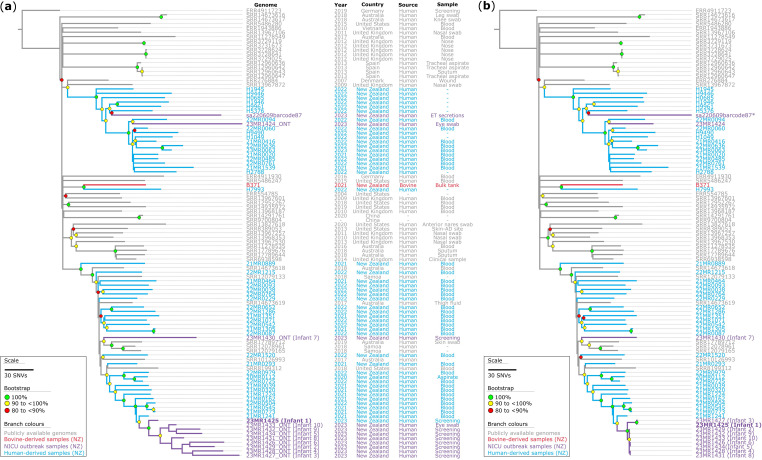
Utilizing the assembly-based ST97 phylogeny above, we could narrow our analysis to the genomic data of strains within the specific sub-clade 1.1 sub-lineage of interest (Fig. 2). This analysis assessed the genomic diversity of 12 high-quality S. aureus genomes from neonatal hosts in New Zealand, comparing them with 45 publicly available draft S. aureus ST97 Clade 1.1 sub-lineage genomes (Fig. 3). The analysis also included 35 ST97 genomes from ESR national staphylococcal surveillance surveys, ten genomes from the Pathlab and one LIC trial isolate. The initial objective was to compare the genomes sequenced using nanopore sequencing to determine if the genomes could be grouped together using nanopore-only sequencing data without Illumina data and achieve comparable results (Fig. 3a). To do this, we generated a maximum-parsimony phylogenetic tree based on 4797 core-genome SNVs from 103 genomes, using 23MR1425 as the reference genome (see Methods). We then repeated the experiment using the same genomic data; however, we replaced the nanopore-only assemblies from 12 ST97 genomes with Illumina sequence read data. We generated another maximum-parsimony phylogenetic tree based on 4653 core-genome SNVs from 103 genomes (Fig. 3b). These phylogenetic analyses revealed notable differences with different sequencing technologies. In both phylogenetic trees, we observed robustly supported branches, reaffirming the accuracy of our evolutionary conclusions. Notably, one sample, sa220609barcode87, exclusively relied on nanopore sequencing due to the unavailability of an isolate for Illumina sequencing (collected in June 2022).
Fig. 3. Maximum-parsimony phylogeny of a subset of Clade 1.1 S. aureus ST97 isolates. (a) The phylogeny is based on nanopore data for the neonatal intensive care unit (NICU) isolates (denoted by ONT in taxon labels). The phylogeny was inferred from 4797 core-genome single-nucleotide variants (SNVs) from 103 genomes. SNVs were derived from a core-genome alignment of ~2 560 000 bp and were called against the chromosome of sample 23MR1425. The consistency index for the tree was 0.96. (b) The phylogeny is based on Illumina data for the NICU isolates. The phylogeny was inferred from 4651 core-genome SNVs from 103 genomes. SNVs were derived from a core-genome alignment of ~2 602 000 bp and are called against the chromosome of sample 23MR1425. The genome for sample sa220609barcode87 (*) represents nanopore-only sequence data. The consistency index for the tree is 0.99. SNV density filtering in SPANDx (excluded regions with three or more SNVs in a 10 bp window). Both phylogenetic trees were rooted according to the ERR4911723 outgroup. Bootstrap values >80 % (1000 replicates) are shown.
The sa220609barcode87 and 23MR1424 genomes were methicillin-susceptible and clustered with 18 other MSSA ST97 Clade 1.1 sub-lineage genomes from New Zealand. On the other hand, the ten MRSA ST97 genomes collected from the NICU clustered with 27 other MRSA genomes collected from New Zealand as part of the ESR surveillance. The genome of 23MR1430/infant 7 was ST97 and thought to be part of the outbreak; however, it was shown to be an outgroup to the other ST97 isolates, so was retrospectively excluded from the outbreak (Fig. 3a and b). Both phylogenetic trees were nearly identical, except for the NICU outbreak cluster (Fig. 3a and b). The nanopore-only genomes exhibited elongated and potentially spurious branches in the phylogenetic tree (Fig. 3a), with a median pairwise SNV distance of 65 [interquartile range (IQR): 51–75; range: 37–94] observed within the main outbreak cluster. In contrast, the Illumina genomes yielded shorter branches (Fig. 3b), and a median pairwise SNV distance of 2 (IQR: 1–13; range: 0–14), which is within previously proposed cutoffs [55]. This difference is particularly notable for isolates 23MR1425/infant 1 and 23MR1427/infant 3, where the Illumina data indicate they may share ten additional SNVs, separating them from the main outbreak cluster.
It is worth noting that the genomes for B371 (collected in 2021 from a bovine non-aseptic bulk milk tank in the North Island/Te Ika-a-Māui) and H7993 (collected in 2022 from a human sample in the North Island/Te Ika-a-Māui) clustered together, albeit with a pairwise SNV distance, determined using snp-dist v0.6.3 (https://github.com/tseemann/snp-dists, accessed on 31 May 2024), of 148 core-genome SNVs separating them (Fig. 3).
Evolutionary timeline of a subset of Clade 1.1 ST97 from New Zealand
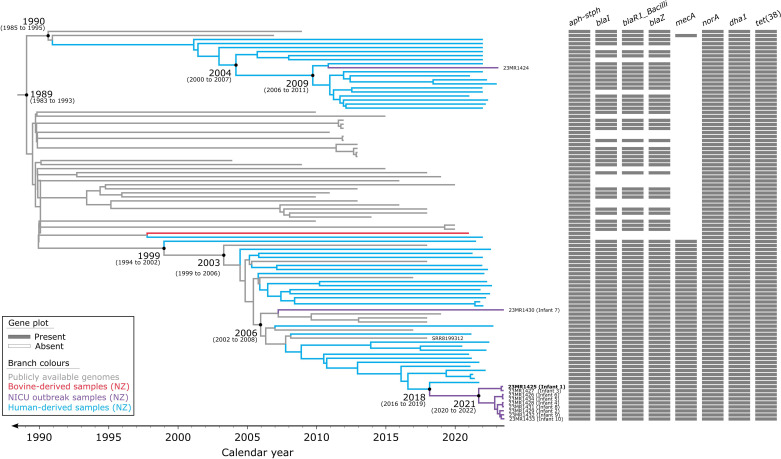
The evolutionary timeline of a subset of Clade 1.1 ST97 from New Zealand was examined in this study. First, we assessed the temporal signal of the dataset by constructing a maximum-likelihood phylogeny in TempEst (Fig. S4). The analysis revealed that the ST97 genomes (n=97) exhibited a linear relationship (correlation coefficient=0.72) between divergence time and evolutionary distance. Regression analysis in TempEst indicated that these genomes accumulated mutations at a rate of 5.67×10−4 substitutions per site per year (R2=0.52) (Fig. S4b). This analysis, while exploratory, suggested that this dataset exhibits a clock-like behaviour. According to the root-to-tip divergence analysis, the most recent common ancestor (MRCA) of this subset of Clade 1.1 ST97 lineage was estimated to have emerged in 1979 (95 % confidence interval: 1968–1988). Additionally, an SNV density plot (Fig. S5) showing the number of SNVs across a 1 000 bp sliding window did not reveal any regions in the core-genome SNV alignment with >15 SNVs in a 1 000 bp window relative to the reference genome (23MR1425), supporting the absence of any substantial recombination in this particular phylogenetic analysis. This was later confirmed with additional recombination filtering (Supplementary Materials), which identified seven putative recombinogenic regions (relative to the reference genome 2314MR25) containing only five of the total 4189 core-genome SNVs (Table S6). Excluding these regions had minimal effects on the phylogeny (Fig. S6), and therefore it was decided not to exclude these five SNVs in downstream analyses [56].
Once we confirmed the presence of a temporal signal in the ST97 dataset (n=97), we employed the Nested Sampling Bayesian computation algorithm to determine the best-fitting tree model and generate a time-calibrated phylogeny. The results of the Nested Sampling algorithm favoured the combination of a strict clock model and the Bayesian skyline population size change model, with a marginal likelihood estimate of −33 211.28 (sd: ±4.14) (Table S7). A BEAST2-based phylogenetic analysis revealed fine-detail relationships among the subset of ST97 strains, leading to the identification of two major sub-lineages. The MRCA of these two major sub-lineages was estimated to have emerged around 1989 [95 % highest posterior density (HPD): 1983–1993] (Fig. 4). The median evolutionary rate determined by BEAST2 is 8.18×10−4 substitutions per site per year (95 % HPD: 6.54×10−4 to 9.51×10−4). However, ascertainment bias occurs when the data collected do not accurately represent the population due to biased collection methods, particularly in SNV analyses. This bias can lead to incorrect conclusions about the frequency and distribution of genetic variants. Correcting for this bias provides a more accurate depiction of genetic diversity. We corrected for this bias using the equation below:
Fig. 4. Evolutionary reconstruction of a subset of Clade 1.1 S. aureus ST97 isolates. A time-calibrated maximum clade credibility tree was inferred from 4189 core-genome single-nucleotide variants (SNVs) from 97 ST97 genomes. SNVs were derived from a core-genome alignment of ~2 605 600 bp and were called against the chromosome of sample 23MR1425 (bold). SNV density filtering in SPANDx (excluded regions with three or more SNVs in a 10 bp window). The x-axis represents the emergence time estimates.
Where mu' is the clock rate corrected for ascertainment bias with the estimated proportion of invariant sites pinv=2 601 411/2 605 600. mu is the estimated clock rate. This translates to a genome-wide mutation rate of 1.32×10−6 mutations per year per site (Table S7), relative to genome size, implying that across the core-genome, approximately 3.4 new mutations arise each year for this subset of Clade 1.1 of S. aureus ST97, in concordance with previous studies [57,58].
Further detection of ST97 MRSA after outbreak declared closed
Around 2 months after the closure of the outbreak a further ST97 MRSA from a clinical sample was detected on a routine weekly sequencing run from an infant who had not been present in the NICU at the time of the original outbreak. This caused significant concern in the IPC team regarding a possible ongoing reservoir of ST97 MRSA on the unit. Pod5 files for the isolate were cloud transferred to ESR later that day. By the next morning the isolate had been contextualized with the ST97 phylogeny, revealing that it did not cluster with the other outbreak isolates (Fig. S7). Plans for further ward screening and other control measures were withdrawn.
Discussion
Genomics has emerged as a practical frontline tool for investigating healthcare-associated infections (HCAIs) with the capacity to conduct WGS within clinically relevant timeframes [15,59]. Such investigations often focus on tracing MRSA transmission using SNV distances as indicators [15,16, 60]. By integrating genomics with traditional hospital epidemiological methods, transmission routes for HCAIs can be effectively identified [15]. This integration can guide infection prevention strategies and optimize hospital resource allocation [18]. Other studies of S. aureus HCAIs have similarly demonstrated the superior resolution of WGS compared to traditional approaches, such as the conventional S. aureus-specific staphylococcal protein A (spa) typing, which often fails to detect transmission events and erroneously attributed unrelated isolates as HCAIs [61]. Recognizing these advantages, we implemented a prospective genomic surveillance system for S. aureus in the NICU starting in 2022 [17].
This analysis has demonstrated the rapid detection and control of an outbreak of ST97 MRSA on a NICU via prospective/proactive genomic surveillance using nanopore sequencing. The outbreak was detected when there were only two known cases, which was 13 days after collection of the first positive sample for MRSA. This early detection facilitated prompt screening of other infants on the ward, which rapidly revealed the extent of the outbreak and enabled colonized infants to be managed appropriately to prevent onwards transmission. This probably limited further transmission events to only two infants (infants 9 and 10, Fig. 1) and the outbreak was declared closed 38 days later. Because the outbreak was contained at an early stage with only a small number of infants involved, there was relatively little disruption to the overall functioning of the ward. Colonized infants could be managed within existing isolation rooms, meaning the need to close rooms or services for the purposes of cohorting colonized infants did not arise.
MRSA outbreaks in the neonatal setting are well described in the literature [9]. Many of these have reached considerable size, have persisted over long time periods and have been highly disruptive to services [11,13]. Many outbreaks occurred at a time when genomic sequencing was less available, and for others genomic sequencing was utilized but in a reactive fashion once the outbreak had already been suspected based on increased incidence [13,14]. A critical factor influencing the impact of an outbreak is speed of recognition [62]. Here we have shown that prospective/proactive genomic surveillance offers the ability to detect outbreaks at a very early stage (days compared to weeks–months [13,15]), which in this case probably limited the size of the outbreak, meaning relatively few infants were affected and the NICU could avoid some of the draconian and highly disruptive IPC interventions required to control larger outbreaks.
Nanopore sequencing offers several practical advantages over other platforms, particularly for smaller clinical laboratories where capital costs and laboratory space constraints are key considerations [17]. However, it has not typically been used as a standalone platform for investigation of outbreaks such as described in this study due to concerns regarding lower raw read accuracy [63]. Here we have shown that the addition of high-quality epidemiological information and historical sequence data help mitigate this lower accuracy, allowing clinically useful conclusions to be drawn. Strain clusters could be identified, and some isolates thought to be part of the outbreak could be excluded. The ability to rapidly exclude a subsequent ST97 case from the outbreak was of particular use (Fig. S7), because it avoided further invasive and costly IPC investigations. Whilst we could identify strain clusters through comparison with publicly available data, it is important to note that Illumina data remain essential for exploring more intricate transmission dynamics. The presence of elongated branches in nanopore-only assemblies, in contrast to shorter branches seen in short-read Illumina genomes (Fig. 3), may be attributed to the higher error rate in nanopore sequencing. These errors could introduce artificial genetic variations, potentially compromising the accuracy of the phylogenetic trees and evolutionary relationships. However, from a clinical decision-making perspective, the level of detail was sufficient for the purposes of outbreak identification, which was the primary driver of the IPC response, and fine-scale analysis of transmission dynamics was not required. Although it was not a specific aim of our analysis, another advantage of a long-read approach is the ability to better contextualize mobile antimicrobial resistance genes (e.g. on plasmids), which has particular potential advantages for hospital IPC [64].
This analysis has also demonstrated the application and potential value of a distributed sequencing approach, which may be an option for many front-line clinical microbiology laboratories that lack bioinformatics expertise but can perform the wet lab aspects of sequencing. Simple, relatively non-discriminatory sequence typing was performed in a front-line clinical laboratory. In this case, this was sufficient to identify the outbreak due to the rarity of ST97 on the NICU. However, to further define the outbreak, sequence data were transferred to ESR for more granular analysis, which contextualized the outbreak and revealed one ST97 case was unrelated to the outbreak strains. This approach proved to be extremely valuable when a further case of ST97 MRSA was detected on the unit after the closure of the outbreak and was rapidly shown to be unrelated, meaning further investigations could be avoided. Had this rapid rule-out not been possible, a persistent undetected reservoir of MRSA would have been suspected by the IPC team (e.g. a colonized staff member, or other infants who had gone undetected for an extended period). This would have probably triggered extensive screening of all infants on the unit, as well as possible screening of staff, which would have caused considerable anxiety amongst parents and staff, and generated further work for ward staff, the IPC team and the microbiology laboratory.
While our primary focus lies within healthcare settings, we observed that genomes from B371, sourced from a bovine milk tank, and H7993, obtained from a human sample, clustered together. Despite a notable distance of 148 core-genome SNVs between them, this clustering underscores intriguing connections between human and animal reservoirs of S. aureus (Fig. 3). Extending beyond healthcare settings, S. aureus impacts a range of livestock, causing complications such as mastitis in dairy-producing animals [55,65, 66], skin abscesses in rabbits [67], septicaemia and skeletal problems in broiler chickens [68], and exudative epidermitis in pigs [69]. This animal association of S. aureus threatens veterinary medicine, agricultural systems and food production, critical sectors for the economy in New Zealand [70]. The ST97 lineage is a prime instance of epidemic community-associated S. aureus in humans. The emergence of ST97 in humans can be traced back to independent jumps from cattle between 1894 and 1977 [71]. The estimated emergence of the ST97 Clade 1.1 sub-lineage around 1989 (95 % HPD: 1983–1993) suggests the MRCA probably existed post-exchange between cattle and humans, and after the introduction of methicillin in 1959 [4,72, 73], and the later emergence of resistance to methicillin [74,75].
Conclusion
While this study focused on the detection and control of an outbreak within an NICU setting, the implications extend beyond this specific context. Prospective genomic surveillance holds promise for enhancing infection control practices across various healthcare settings, facilitating early intervention and containment of emerging threats posed by multidrug-resistant pathogens. Despite its rapid and cost-effective nature, nanopore data alone may not suffice for identifying transmissions accurately; however, by combining sequence data with epidemiological data, clinically useful conclusions can still be drawn. Continued advancements in sequencing technologies and bioinformatics tools will further bolster the capacity for timely and effective outbreak detection, ultimately safeguarding patient safety and minimizing the burden on healthcare systems.
supplementary material
Acknowledgements
We acknowledge the facilities, and the scientific and technical assistance of staff at Awanui Labs, formerly known as Southern Community Laboratories (SCL) (Wellington, New Zealand). We are grateful to the diagnostic microbiology laboratories across New Zealand for contributing isolates and associated data to the surveillance programmes at the Institute of Environmental Science and Research (ESR). We also acknowledge the facilities and the scientific and technical assistance of staff at the Livestock Improvement Corporation (Newstead, New Zealand). Additionally, the Livestock Improvement Corporation (LIC) would like to thank Murray Robinson, Michael Addidle and Claudio Turilli from Pathlab for the human S. aureus isolates. We thank the Next Generation Sequencing Team and the Antibiotic Reference and Nosocomial Infections Laboratories at ESR (Porirua, New Zealand) for their valuable scientific and technical assistance. We acknowledge and thank the following ESR staff for their valuable feedback: Hilary Miller (Kenepuru Science Centre), William Taylor (Christchurch Science Centre), Xiaoyun Ren (Kenepuru Science Centre) and Jenny Ralston (Kenepuru Science Centre). This research was made possible by the Computational Science Team and the High-Performance Compute (HPC) platform at ESR, with specific thanks to Russell Smithies and Shane Sturrock for HPC support and helpful discussions about software pipelines. Finally, we thank the New Zealand Ministry of Health/Manatū Hauora for funding this work.
Abbreviations
- BAPS
Bayesian Analysis of Population Structure
- BWA
Burrows-Wheeler Aligner
- ESR
Institute of Environmental Science and Research
- EUCAST
European Committee on Antimicrobial Susceptibility Testing
- GATK
Genome Analysis Tool Kit
- HCAI
healthcare-associated infection
- HPC
High-Performance Compute
- HPD
highest posterior density
- IPC
infection prevention and control
- IQR
interquartile range
- LIC
Livestock Improvement Corporation
- MBIE
Ministry of Business Innovation and Employment
- MLST
multi-locus sequence type
- MRCA
most recent common ancestor
- MRSA
methicillin-resistant Staphylococcus aureus
- MSSA
methicillin-susceptible S. aureus
- NCBI
National Center for Biotechnology Information
- NICU
neonatal intensive care unit
- ONT
Oxford Nanopore Technologies
- SCCmec
staphylococcal cassette chromosome mec
- SCL
Southern Community Laboratories
- SNV
single nucleotide variant
- SRA
sequence read archive
- ST
sequence type
- WGS
whole genome sequencing
- WRH
Wellington Regional Hospital
Footnotes
Funding: This study was supported by internal departmental funds at Awanui Laboratories Wellington, the Institute of Environmental Science and Research (ESR), New Zealand Ministry of Health/Manatū Hauora, and by Genomics Aotearoa through funding from the Ministry of Business Innovation and Employment (MBIE). The collection of bovine and human-associated isolates by the Livestock Improvement Corporation (LIC) was made possible through support from the New Zealand Ministry of Primary Industries, specifically the SFF Futures Programme under the project titled ‘Resilient Dairy – Innovative Breeding for a sustainable dairy future’ (Grant Number: PGP06-17006).
Author contributions: Conceptualization: R.T.W. and M.B.l.; investigation: R.T.W. and M.B.l.; funding acquisition: D.M.C., C.C. and M.B.l.; formal analysis: R.T.W.; wet-lab experiments: S.B., M.Bu., A.E., S.H., C.T., C.V., E.M.V. and J.W.; data analysis: R.T.W.; data curation: R.T.W., E.M.V. and M.B.l.; writing (original draft preparation): R.T.W.; writing (review and editing): R.T.W., S.B., M.bu., M.L.C., C.C., K.D., A.E., C.H., S.H., D.M.C., C.T., C.V., E.M.V., J.W. and M.B.l. All authors have read and agreed to the published version of the manuscript.
Ethical statement: The analysis and reporting of this outbreak constituted an ‘audit or related activity’ as per New Zealand Health and Disability Ethics Committees, so did not require review.
Accession No: National Center for Biotechnology Information (NCBI) BioProject accession number PRJNA1046639. NCBI sequence read archive (SRA) accession numbers SRR26992863 to SRR26992891. GenBank accession numbers CP143800 and CP143801. More information about sequence data is in the data summary section below.
Contributor Information
Rhys T. White, Email: rhys.white@esr.cri.nz.
Sarah Bakker, Email: sarah.bakker@esr.cri.nz.
Megan Burton, Email: megan.burton@awanuilabs.co.nz.
M. Leticia Castro, Email: leticia.castro@esr.cri.nz.
Christine Couldrey, Email: christine.couldrey@lic.co.nz.
Kristin Dyet, Email: kristin.dyet@esr.cri.nz.
Alexandra Eustace, Email: alexandra.eustace@esr.cri.nz.
Chad Harland, Email: chad.harland@lic.co.nz.
Samantha Hutton, Email: samantha.hutton@awanuilabs.co.nz.
Donia Macartney-Coxson, Email: donia.macartney-coxson@esr.cri.nz.
Claire Tarring, Email: claire.tarring@esr.cri.nz.
Charles Velasco, Email: charlesvincent.velasco@mpi.govt.nz.
Emma M. Voss, Email: emma.voss@lic.co.nz.
John Williamson, Email: John.Williamson@lic.co.nz.
Max Bloomfield, Email: maxim.bloomfield@ccdhb.org.nz.
References
- 1.Diekema DJ, Pfaller MA, Schmitz FJ, Smayevsky J, Bell J, et al. Survey of infections due to Staphylococcus species: frequency of occurrence and antimicrobial susceptibility of isolates collected in the United States, Canada, Latin America, Europe, and the Western Pacific region for the SENTRY Antimicrobial Surveillance Program, 1997-1999. Clin Infect Dis. 2001;32:S114–S132. doi: 10.1086/320184. [DOI] [PubMed] [Google Scholar]
- 2.Abraham EP, Chain E, Fletcher CM, Gardner AD, Heatley NG, et al. Further observations on penicillin. Lancet. 1941;238:177–189. doi: 10.1016/S0140-6736(00)72122-2. [DOI] [PubMed] [Google Scholar]
- 3.Flemming P. The medical aspects of the Mediæval monastery in England. Proc R Soc Med. 1929;22:771–782. doi: 10.1177/003591572902200627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lowy FD. Antimicrobial resistance: the example of Staphylococcus aureus. J Clin Invest. 2003;111:1265–1273. doi: 10.1172/JCI18535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chambers HF, Deleo FR. Waves of resistance: Staphylococcus aureus in the antibiotic era. Nat Rev Microbiol. 2009;7:629–641. doi: 10.1038/nrmicro2200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Klein EY, Sun L, Smith DL, Laxminarayan R. The changing epidemiology of methicillin-resistant Staphylococcus aureus in the United States: a national observational study. Am J Epidemiol. 2013;177:666–674. doi: 10.1093/aje/kws273. [DOI] [PubMed] [Google Scholar]
- 7.Tong SYC, Davis JS, Eichenberger E, Holland TL, Fowler VG., Jr Staphylococcus aureus infections: epidemiology, pathophysiology, clinical manifestations, and management. Clin Microbiol Rev. 2015;28:603–661. doi: 10.1128/CMR.00134-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Turner NA, Sharma-Kuinkel BK, Maskarinec SA, Eichenberger EM, Shah PP, et al. Methicillin-resistant Staphylococcus aureus: an overview of basic and clinical research. Nat Rev Microbiol. 2019;17:203–218. doi: 10.1038/s41579-018-0147-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Johnson J, Quach C. Outbreaks in the neonatal ICU: a review of the literature. Curr Opin Infect Dis. 2017;30:395–403. doi: 10.1097/QCO.0000000000000383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dong Y, Glaser K, Speer CP. New threats from an old foe: methicillin-resistant Staphylococcus aureus infections in neonates. Neonatology. 2018;114:127–134. doi: 10.1159/000488582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Andersen BM, Lindemann R, Bergh K, Nesheim BI, Syversen G, et al. Spread of methicillin-resistant Staphylococcus aureus in a neonatal intensive unit associated with understaffing, overcrowding and mixing of patients. J Hosp Infect. 2002;50:18–24. doi: 10.1053/jhin.2001.1128. [DOI] [PubMed] [Google Scholar]
- 12.Hensel KO, van den Bruck R, Klare I, Heldmann M, Ghebremedhin B, et al. Nursing staff fluctuation and pathogenic burden in the NICU - effective outbreak management and the underestimated relevance of non-resistant strains. Sci Rep. 2017;7:45014. doi: 10.1038/srep45014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Madera S, McNeil N, Serpa PH, Kamm J, Pak C, et al. Prolonged silent carriage, genomic virulence potential and transmission between staff and patients characterize a neonatal intensive care unit (NICU) outbreak of methicillin-resistant Staphylococcus aureus (MRSA) Infect Control Hosp Epidemiol. 2023;44:40–46. doi: 10.1017/ice.2022.48. [DOI] [PubMed] [Google Scholar]
- 14.Madigan T, Cunningham SA, Patel R, Greenwood-Quaintance KE, Barth JE, et al. Whole-genome sequencing for methicillin-resistant Staphylococcus aureus (MRSA) outbreak investigation in a neonatal intensive care unit. Infect Control Hosp Epidemiol. 2018;39:1412–1418. doi: 10.1017/ice.2018.239. [DOI] [PubMed] [Google Scholar]
- 15.Köser CU, Holden MTG, Ellington MJ, Cartwright EJP, Brown NM, et al. Rapid whole-genome sequencing for investigation of a neonatal MRSA outbreak. N Engl J Med. 2012;366:2267–2275. doi: 10.1056/NEJMoa1109910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Worley JN, Crothers JW, Wolfgang WJ, Venkata SLG, Hoffmann M, et al. Prospective genomic surveillance reveals cryptic MRSA outbreaks with local to international origins among NICU patients. J Clin Microbiol. 2023;61:e0001423. doi: 10.1128/jcm.00014-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bloomfield M, Hutton S, Velasco C, Burton M, Benton M, et al. Oxford nanopore next generation sequencing in a front-line clinical microbiology laboratory without on-site bioinformaticians. Pathology. 2024;56:444–447. doi: 10.1016/j.pathol.2023.07.014. [DOI] [PubMed] [Google Scholar]
- 18.Bloomfield M, Hutton S, Burton M, Tarring C, Velasco C, et al. Early identification of a ward-based outbreak of Clostridioides difficile using prospective multilocus sequence type-based Oxford nanopore genomic surveillance. Infect Control Hosp Epidemiol. 2024;6:1–7. doi: 10.1017/ice.2024.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Heffernan H, Bakker S. Porirua, New Zealand: Institute of Environmental Science and Research Ltd; 2018. [4-April-2024]. 2017 survey of methicillin-resistant Staphylococcus aureus (MRSA)https://www.esr.cri.nz/digital-library/2017-annual-survey-of-mrsa/ accessed. [Google Scholar]
- 20.Campos B, Pickering AC, Rocha LS, Aguilar AP, Fabres-Klein MH, et al. Diversity and pathogenesis of Staphylococcus aureus from bovine mastitis: current understanding and future perspectives. BMC Vet Res. 2022;18:115. doi: 10.1186/s12917-022-03197-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ballingall J, Lattimore R. Wellington, New Zealand: New Zealand Institute of Economic Research (NZIER); 2004. [6-June-2024]. Farming in New Zealand: the state of play and key issues for the backbone of the New Zealand economy.https://hdl.handle.net/10419/66092 accessed. [Google Scholar]
- 22.Grinberg A, Lopez-Villalobos N, Lawrence K, Nulsen M. Prediction of penicillin resistance in Staphylococcus aureus isolates from dairy cows with mastitis, based on prior test results. N Z Vet J. 2005;53:332–335. doi: 10.1080/00480169.2005.36569. [DOI] [PubMed] [Google Scholar]
- 23.Grinberg A, Kingsbury DD, Gibson IR, Kirby BM, Mack HJ, et al. Clinically overt infections with methicillin-resistant Staphylococcus aureus in animals in New Zealand: a pilot study. N Z Vet J. 2008;56:237–242. doi: 10.1080/00480169.2008.36840. [DOI] [PubMed] [Google Scholar]
- 24.European Committee on Antimicrobial Susceptibility Testing . Växjö, Sweden: The European Committee on Antimicrobial Susceptibility Testing; 2023. [6-June-2024]. Breakpoint tables for interpretation of MICs and zone diameters. Version 13.1.https://www.eucast.org/clinical_breakpoints accessed. [Google Scholar]
- 25.Page AJ, Keane JA. Rapid multi-locus sequence typing direct from uncorrected long reads using Krocus. PeerJ. 2018;6:e5233. doi: 10.7717/peerj.5233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Seemann T. Prokka: rapid prokaryotic genome annotation. Bioinformatics. 2014;30:2068–2069. doi: 10.1093/bioinformatics/btu153. [DOI] [PubMed] [Google Scholar]
- 27.Zhou Y, Liang Y, Lynch KH, Dennis JJ, Wishart DS. PHAST: a fast phage search tool. Nucl Acids Res. 2011;39:W347–W352. doi: 10.1093/nar/gkr485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Arndt D, Grant JR, Marcu A, Sajed T, Pon A, et al. PHASTER: a better, faster version of the PHAST phage search tool. Nucleic Acids Res. 2016;44:W16–W21. doi: 10.1093/nar/gkw387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bouras G, Nepal R, Houtak G, Psaltis AJ, Wormald PJ, et al. Pharokka: a fast scalable bacteriophage annotation tool. Bioinformatics. 2023;39:btac776. doi: 10.1093/bioinformatics/btac776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bertelli C, Laird MR, Williams KP, Lau BY, Hoad G, et al. IslandViewer 4: expanded prediction of genomic islands for larger-scale datasets. Nucl Acids Res. 2017;45:W30–W35. doi: 10.1093/nar/gkx343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Varani AM, Siguier P, Gourbeyre E, Charneau V, Chandler M. ISsaga is an ensemble of web-based methods for high throughput identification and semi-automatic annotation of insertion sequences in prokaryotic genomes. Genome Biol. 2011;12:R30. doi: 10.1186/gb-2011-12-3-r30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Siguier P, Perochon J, Lestrade L, Mahillon J, Chandler M. ISfinder: the reference centre for bacterial insertion sequences. Nucl Acids Res. 2006;34:D32–D36. doi: 10.1093/nar/gkj014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rutherford K, Parkhill J, Crook J, Horsnell T, Rice P, et al. Artemis: sequence visualization and annotation. Bioinformatics. 2000;16:944–945. doi: 10.1093/bioinformatics/16.10.944. [DOI] [PubMed] [Google Scholar]
- 34.Treangen TJ, Ondov BD, Koren S, Phillippy AM. The harvest suite for rapid core-genome alignment and visualization of thousands of intraspecific microbial genomes. Genome Biol. 2014;15:524. doi: 10.1186/s13059-014-0524-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stamatakis A. RAxML version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics. 2014;30:1312–1313. doi: 10.1093/bioinformatics/btu033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tonkin-Hill G, Lees JA, Bentley SD, Frost SDW, Corander J. RhierBAPS: an R implementation of the population clustering algorithm hierBAPS. Wellcome Open Res. 2018;3:93. doi: 10.12688/wellcomeopenres.14694.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.R Core Team . Vienna, Austria: R Foundation for Statistical Computing; 2023. [4-April-2024]. R: a language and environment for statistical computing.https://www.r-project.org/ accessed. [Google Scholar]
- 38.Cheng L, Connor TR, Sirén J, Aanensen DM, Corander J. Hierarchical and spatially explicit clustering of DNA sequences with BAPS software. Mol Biol Evol. 2013;30:1224–1228. doi: 10.1093/molbev/mst028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li H, Durbin R. Fast and accurate short read alignment with Burrows–Wheeler transform. Bioinformatics. 2009;25:1754–1760. doi: 10.1093/bioinformatics/btp324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Quinlan AR, Hall IM. BEDTools: a flexible suite of utilities for comparing genomic features. Bioinformatics. 2010;26:841–842. doi: 10.1093/bioinformatics/btq033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bolger AM, Lohse M, Usadel B. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics. 2014;30:2114–2120. doi: 10.1093/bioinformatics/btu170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ye K, Schulz MH, Long Q, Apweiler R, Ning Z. Pindel: a pattern growth approach to detect break points of large deletions and medium sized insertions from paired-end short reads. Bioinformatics. 2009;25:2865–2871. doi: 10.1093/bioinformatics/btp394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pedersen BS, Quinlan AR. Mosdepth: quick coverage calculation for genomes and exomes. Bioinformatics. 2018;34:867–868. doi: 10.1093/bioinformatics/btx699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li H, Handsaker B, Wysoker A, Fennell T, Ruan J, et al. The sequence alignment/map format and SAMtools. Bioinformatics. 2009;25:2078–2079. doi: 10.1093/bioinformatics/btp352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.DePristo MA, Banks E, Poplin R, Garimella KV, Maguire JR, et al. A framework for variation discovery and genotyping using next-generation DNA sequencing data. Nat Genet. 2011;43:491–498. doi: 10.1038/ng.806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.McKenna A, Hanna M, Banks E, Sivachenko A, Cibulskis K, et al. The genome analysis toolkit: a mapreduce framework for analyzing next-generation DNA sequencing data. Genome Res. 2010;20:1297–1303. doi: 10.1101/gr.107524.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cingolani P, Platts A, Wang LL, Coon M, Nguyen T, et al. A program for annotating and predicting the effects of single nucleotide polymorphisms, SnpEff: SNPs in the genome of Drosophila melanogaster strain w1118; iso-2; iso-3. Fly. 2012;6:80–92. doi: 10.4161/fly.19695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sarovich DS, Price EP. SPANDx: a genomics pipeline for comparative analysis of large haploid whole genome re-sequencing datasets. BMC Res Notes. 2014;7:618. doi: 10.1186/1756-0500-7-618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wilgenbusch JC, Swofford D. Inferring evolutionary trees with PAUP. Curr Protoc Bioinform. 2003;6 doi: 10.1002/0471250953.bi0604s00. [DOI] [PubMed] [Google Scholar]
- 50.Rambaut A, Lam TT, Max Carvalho L, Pybus OG. Exploring the temporal structure of heterochronous sequences using TempEst (formerly Path-O-Gen) Virus Evol. 2016;2:vew007. doi: 10.1093/ve/vew007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bouckaert R, Heled J, Kühnert D, Vaughan T, Wu C-H, et al. BEAST 2: a software platform for Bayesian evolutionary analysis. PLOS Comput Biol. 2014;10:e1003537. doi: 10.1371/journal.pcbi.1003537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bouckaert R, Vaughan TG, Barido-Sottani J, Duchêne S, Fourment M, et al. BEAST 2.5: an advanced software platform for Bayesian evolutionary analysis. PLOS Comput Biol. 2019;15:e1006650. doi: 10.1371/journal.pcbi.1006650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Darriba D, Taboada GL, Doallo R, Posada D. jModelTest 2: more models, new heuristics and parallel computing. Nat Methods. 2012;9:772. doi: 10.1038/nmeth.2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nesaraj J, Grinberg A, Laven R, Biggs P. Genomic epidemiology of bovine mastitis-causing Staphylococcus aureus in New Zealand. Vet Microbiol. 2023;282:109750. doi: 10.1016/j.vetmic.2023.109750. [DOI] [PubMed] [Google Scholar]
- 55.Coll F, Raven KE, Knight GM, Blane B, Harrison EM, et al. Definition of a genetic relatedness cutoff to exclude recent transmission of meticillin-resistant Staphylococcus aureus: a genomic epidemiology analysis. Lancet Microbe. 2020;1:e328–e335. doi: 10.1016/S2666-5247(20)30149-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gorrie CL, Da Silva AG, Ingle DJ, Higgs C, Seemann T, et al. Key parameters for genomics-based real-time detection and tracking of multidrug-resistant bacteria: a systematic analysis. Lancet Microbe. 2021;2:e575–e583. doi: 10.1016/S2666-5247(21)00149-X. [DOI] [PubMed] [Google Scholar]
- 57.Nübel U, Dordel J, Kurt K, Strommenger B, Westh H, et al. A timescale for evolution, population expansion, and spatial spread of an emerging clone of methicillin-resistant Staphylococcus aureus. PLoS Pathog. 2010;6:e1000855. doi: 10.1371/journal.ppat.1000855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Young BC, Golubchik T, Batty EM, Fung R, Larner-Svensson H, et al. Evolutionary dynamics of Staphylococcus aureus during progression from carriage to disease. Proc Natl Acad Sci U S A. 2012;109:4550–4555. doi: 10.1073/pnas.1113219109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Harris SR, Cartwright EJ, Török ME, Holden MT, Brown NM, et al. Whole-genome sequencing for analysis of an outbreak of meticillin-resistant Staphylococcus aureus: a descriptive study. Lancet Infect Dis. 2013;13:130–136. doi: 10.1016/S1473-3099(12)70268-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sansom SE, Logan LK, Green SJ, Moore NM, Hayden MK. Whole-genome sequencing for neonatal intensive care unit outbreak investigations: insights and lessons learned - ADDENDUM. Antimicrob Steward Healthc Epidemiol. 2021;1:e18. doi: 10.1017/ash.2021.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Price JR, Golubchik T, Cole K, Wilson DJ, Crook DW, et al. Whole-genome sequencing shows that patient-to-patient transmission rarely accounts for acquisition of Staphylococcus aureus in an intensive care unit. Clin Infect Dis. 2014;58:609–618. doi: 10.1093/cid/cit807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Smolinski MS, Crawley AW, Olsen JM. Finding outbreaks faster. Health Secur. 2017;15:215–220. doi: 10.1089/hs.2016.0069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Delahaye C, Nicolas J. Sequencing DNA with nanopores: troubles and biases. PLoS One. 2021;16:e0257521. doi: 10.1371/journal.pone.0257521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhao W, Zeng W, Pang B, Luo M, Peng Y, et al. Oxford nanopore long-read sequencing enables the generation of complete bacterial and plasmid genomes without short-read sequencing. Front Microbiol. 2023;14:1179966. doi: 10.3389/fmicb.2023.1179966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bradley AJ, Leach KA, Breen JE, Green LE, Green MJ. Survey of the incidence and aetiology of mastitis on dairy farms in England and Wales. Vet Rec. 2007;160:253–257. doi: 10.1136/vr.160.8.253. [DOI] [PubMed] [Google Scholar]
- 66.Peton V, Le Loir Y. Staphylococcus aureus in veterinary medicine. Infect Genet Evol. 2014;21:602–615. doi: 10.1016/j.meegid.2013.08.011. [DOI] [PubMed] [Google Scholar]
- 67.Viana D, Comos M, McAdam PR, Ward MJ, Selva L, et al. A single natural nucleotide mutation alters bacterial pathogen host tropism. Nat Genet. 2015;47:361–366. doi: 10.1038/ng.3219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.McNamee PT, Smyth JA. Bacterial chondronecrosis with osteomyelitis ('femoral head necrosis’) of broiler chickens: a review. Avian Pathol. 2000;29:253–270. doi: 10.1080/03079450050118386. [DOI] [PubMed] [Google Scholar]
- 69.van Duijkeren E, Jansen MD, Flemming SC, de Neeling H, Wagenaar JA, et al. Methicillin-resistant Staphylococcus aureus in pigs with exudative epidermitis. Emerg Infect Dis. 2007;13:1408–1410. doi: 10.3201/eid1309.061268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ballingall J, Lattimore R. Farming in New Zealand: the state of play and key issues for the backbone of the New Zealand economy. Farm Policy J. 2004;1:1–11. [Google Scholar]
- 71.Spoor LE, McAdam PR, Weinert LA, Rambaut A, Hasman H, et al. Livestock origin for a human pandemic clone of community-associated methicillin-resistant Staphylococcus aureus. mBio. 2013;4:e00356-13. doi: 10.1128/mBio.00356-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Knox R. A new penicillin (BRL 1241) active against penicillin-resistant staphylococci. Br Med J. 1960;2:690–693. doi: 10.1136/bmj.2.5200.690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Woodford N. Biological counterstrike: antibiotic resistance mechanisms of Gram-positive cocci. Clin Microbiol Infect. 2005;11 Suppl 3:2–21. doi: 10.1111/j.1469-0691.2005.01140.x. [DOI] [PubMed] [Google Scholar]
- 74.Jevons MP, Rolinson GN, Knox R. “Celbenin” - resistant staphylococci. Br Med J. 1961;1:124–125. doi: 10.1136/bmj.1.5219.124-a. [DOI] [Google Scholar]
- 75.Eriksen KR, Erichsen I. Resistance to methicillin, isoxazolyl penicillins, and cephalothin in Staphylococcus aureus. Acta Pathol Microbiol Scand. 1964;62:255–275. doi: 10.1111/apm.1964.62.2.255. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.