Abstract
With an increasing prevalence, metabolic dysfunction–associated steatotic liver disease (MASLD) has become a major global health problem. MASLD is well-known as a multifactorial disease. Mitochondrial dysfunction and alterations in the gut bacteria are 2 vital events in MASLD. Recent studies have highlighted the cross-talk between microbiota and mitochondria, and mitochondria are recognized as pivotal targets of the gut microbiota to modulate the host's physiological state. Mitochondrial dysfunction plays a vital role in MASLD and is associated with multiple pathological changes, including hepatocyte steatosis, oxidative stress, inflammation, and fibrosis. Metabolites are crucial mediators of the gut microbiota that influence extraintestinal organs. Additionally, regulation of the composition of gut bacteria may serve as a promising therapeutic strategy for MASLD. This study reviewed the potential roles of several common metabolites in MASLD, emphasizing their impact on mitochondrial function. Finally, we discuss the current treatments for MASLD, including probiotics, prebiotics, antibiotics, and fecal microbiota transplantation. These methods concentrate on restoring the gut microbiota to promote host health.
INTRODUCTION
Metabolic dysfunction–associated steatotic liver disease (MASLD) is characterized by excessive accumulation of fat in the liver tissue in the absence of reasons for secondary hepatic fat accumulation, such as genetic disorders, viral hepatitis, and excessive alcohol consumption.1 Actually, the overload of fat in the liver reflects the hepatic manifestation of systemic lipid metabolism disorder; thus, the name of the disease has recently been redefined from NAFLD to MASLD.2,3 MASLD is currently the most common chronic liver disease worldwide.4 The prevalence of MASLD in Mainland China was ~30% in 2020,5 and the prevalence of MASLD ranged from 29.5% in 1999–2000 to 40.3% in 2015-2016 in the American adult population.6 Concerningly, with disease progression, a substantial portion of benign metabolic dysfunction–associated steatotic liver may evolve into metabolic dysfunction–associated steatohepatitis (MASH), cirrhosis, and hepatocellular cancer.7 Therefore, prior treatment and early prevention are vital in patients with MASLD. However, the underlying mechanism of MASLD remains unclear, and definitive treatments for MASLD and MASH are lacking.8
With advances in culture-independent techniques, intestinal bacteria have been found to play a crucial role in MASLD and are closely associated with the development of hepatic steatosis, fibrosis, and inflammation.9 Unfortunately, there is no conclusive mechanism that illustrates how the gut microbiota contributes to the pathogenesis of MASLD. Recently, the interdependence of the intestinal flora and mitochondria has received particular attention in many studies.10,11,12 According to the endosymbiotic theory, mitochondria in eukaryotic cells have been described as endosymbiotic organelles evolved from α-proteobacteria, sharing significant similarities in structural components and metabolic pathways with bacteria.13 Based on the metabolic reconstruction model Recon2, Thiele et al identified 437 mitochondria-related metabolites, 325 of which overlapped with gut microbiota metabolites, implying strong cross-talk between these 2 components.14 Additionally, metabolites of gut bacteria are reported to modulate energy metabolism, oxidative stress, and inflammation through mitochondrial metabolism–related pathways in animal models.12 Accurately, mitochondrial effects have been proposed as a vital mechanism by which the gut flora can affect health and disease.15 Mitochondrial dysfunction has also emerged as a pivotal pathological feature in MASLD, and literature suggests that compromised hepatic mitochondrial fatty acid oxidation and mitochondrial turnover are intimately related to increasing MASLD severity in patients with obesity.16 This review will concentrate on the cross-talk between microbiota and mitochondria in MASLD and discuss treatments for MASLD associated with the manipulation of the gut microbiota.
THE ROLE OF MITOCHONDRIAL DYSFUNCTION IN MASLD
Each hepatocyte contains 1000–2000 mitochondria, and the liver is a mitochondrion-rich organ.17 Mitochondria are the most important sites for converting nutrients into energy. Thus, mitochondrial dysfunction plays a vital role in the pathogenesis of diseases related to metabolic syndrome.8,10,18 Additionally, there is accumulating evidence for the crucial role of mitochondrial dysfunction in the formation of multiple events, including hepatocyte steatosis, oxidative stress, inflammation, and fibrosis, during the progression of MASLD.18 Hence, MASLD is considered to be a mitochondrial disease.19 Nevertheless, mitochondria have complex and dynamic structures and functions, revealing their dysfunction in multiple ways, including deficient mitochondrial oxidative metabolism, oxidative stress, and mitochondrial quality control imbalance.
Defect in mitochondria fatty acid oxidation
Mitochondrial fatty acid oxidation (mtFAO) is an important method to reduce fat accumulation in the liver.20 In the classical mtFAO pathway, free fatty acids (FFAs) are converted to acyl acyl-CoA in the cytoplasm, which can be gradually metabolized to water, carbon dioxide, and a large amount of ATP through β-oxidation, the tricarboxylic acid cycle, and oxidative phosphorylation (OXPHOS) in mitochondria (Figure 1).18 In the liver, fatty acids that are not metabolized in time are stored as triglycerides (TG) in lipid droplets. Even though a part of TG is packaged into VLDL for secretion from the liver to circulation, the effect size with regard to liver fat accumulation is extremely weak,21 and high serum VLDL levels have been considered an independent risk factor for cardiovascular disease.22 However, long and persistent accumulation of FFAs and chronic acyl-CoA by mtFAo defects separate the function of the tricarboxylic acid cycle from mitochondrial respiration, resulting in excess reactive oxygen species(ROS) production.23,24
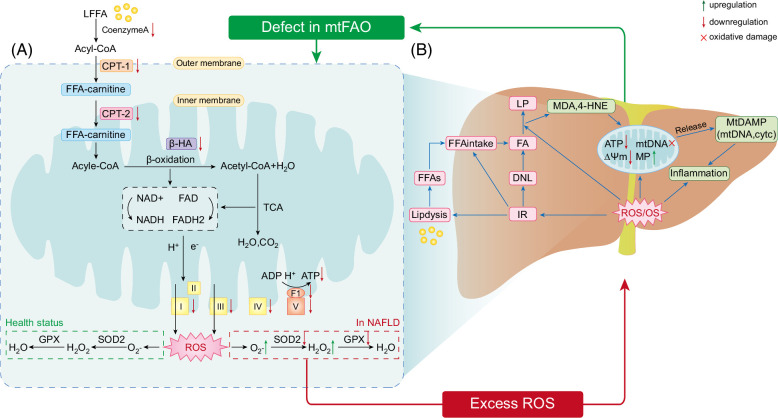
FIGURE 1.
(A) MtFAO is a vital way for the liver to reduce fat accumulation. In the classical pathway of mtFAO, FFAs are converted to acyl-CoA in the cytoplasm, which can be gradually metabolized to water, CO2, and a large number of ATP by way of β-oxidation, tricarboxylic acid cycle and OXPHOS in mitochondria. However, mtFAO function is defective in subjects with MASLD and animal models, which is manifested as decreased expression of β-oxidation and OXPHOS-related genes and protein, including CoA, CPTI, CPT2, and β-HAD, and mitochondrial complex (Ⅰ, Ⅱ, Ⅲ, Ⅳ, and Ⅴ). Deficient ETC function could result in the production of ROS, which could further impair mitochondrial function. (B) Excessive ROS can cause OS and play an important role in mitochondrial dysfunction, inflammation, and lipid peroxidation. ROS will generate oxidative damage to mtDNA and be associated with decreased MMP and increased MP. Increased MP enables mtDAMP release into the cytosol, promoting an inflammatory response. Apparently, ROS can directly activate inflammatory pathways, promoting MASLD. Additionally, OS can contribute to insulin resistance, facilitating fat accumulation in the liver by promoting lipolysis, DNL, and hepatic uptake of FFAs. Besides, elevated ROS levels will lead to increases in LP, which can produce toxic byproducts (such as 4-HNE and MDA), thereby inducing mitochondrial dysfunction, more ROS production and inflammation. In conclusion, mitochondrial dysfunction can lead to increased mitochondrial production of ROS, which causes a vicious cycle of mitochondrial dysfunction, leading to more ROS production. Abbreviations: β-HA, β-hydroxyacyl-CoA-dehydrogenase; CPT1, carnitine palmitoyl transferase 1; CPT2, carnitine palmitoyl transferase 2; DNL, de novo lipogenesis; ETC, electron transport chain; FFA, free fatty acid; 4-HNE, 4-hydroxynonenal; LP, lipid peroxidation; MDA, malondialdehyde; MASLD, metabolic dysfunction–associated steatotic liver disease; MMP, mitochondrial membrane potential; MP, mitochondrial permeability; mtDAMP, mitochondrial damage-associated molecular patterns; mtFAO, mitochondrial fatty acid oxidation; OS, oxidative stress; OXPHOS, oxidative phosphorylation; ROS, reactive oxygen species; TCA cycle, tricarboxylic acid cycle.
Liver fat metabolism is highly dependent on mtFAO, and defects in mitochondrial respiration are important factors that can facilitate the development of MASLD.25 β-hydroxyacyl-CoA-dehydrogenase is one of the most important enzymes involved in β-oxidation. In addition, impaired mitochondrial β oxidation also causes alternative peroxisomal and cytochrome oxidation of FFA, producing peroxide products and amounts of ROS.26 Mary et al reported that β-hydroxyacyl-CoA-dehydrogenase decreased by 40%–50% in patients with MASH when compared with controls, suggesting impaired β-oxidation.27 Interestingly, their study also identified that the degree of liver fibrosis was associated with a loss of OXPHOS complexes CⅡ, CⅢ, and CⅣ.27 Carnitine palmitoyl transferase Ⅰ (CPT1) and Carnitine palmitoyl transferaseⅡ 2 (CPT2) are located in outer and inner mitochondrial membranes, respectively. Together with carnitine, they are required for the movement of long-chain fatty acyl-CoAs into the mitochondrial matrix for β-oxidation. Moreover, CPT1 and CPT2 are always used as indicators of the β-oxidation capacity.28,29,30,31 In addition, multiple studies have revealed a reduction in CPT1 and CPT2 in high-fat diet (HFD)-induced animal models.28,29,30,31 Correspondingly, the administration of syringic acid to MASLD mice exhibited anti-hepatic steatosis effects by upregulating CPT1 and CPT2 expression.31 CoA is another enzyme that catalyzes the conversion of LFAs into long-chain acyl-CoA. Additionally, CoA was decreased upon mtFAO defects in MASLD mice (Figure 1).31 Mitochondrial cristae are an important part of the inner mitochondrial membrane, including 5 major membrane protein complexes, the mitochondrial complexes I–V, which are responsible for OXPHOS and ATP synthesis (Figure 1).18 However, evidence has shown that the abundance of mitochondrial cristae is decreased in brown adipose tissue of obese mice, providing supportive evidence that mitochondrial respiration is impaired in metabolic diseases.32 Additionally, mitochondrial complex (I, II, III, and V) activity has also been reported to be decreased in MASLD mice.30,33 Notably, deficient mitochondrial complex function could contribute to the production of ROS, which in turn damages mitochondrial function by oxidizing a variety of macromolecules, including proteins, lipids, and DNA. AMP-activated protein kinase (AMPK) is a key regulator of fatty acid metabolism in the liver, and sirtuins (SIRTs) and proliferator-activated receptorγ coactivator (PGC)-1x are its downstream mediators. In MASLD, the expression of PGC-1α mRNA and sirt expression in the liver are reduced.34 Studies have shown that the activation of the AMPK/PGC-1α or AMPK/Sirt3 signaling pathway in hepatocytes can regulate lipid metabolism, improve mitochondrial respiratory capacity and oxidation-reduction reaction homeostasis, reduce hepatic lipid accumulation and oxidative stress, and thus improve mitochondrial dysfunction.35,36
Based on the above discussion, mtFAO deficiency is an important factor in lipid accumulation in the liver. MtFAO is a complex metabolic process, and its defects are not only manifested by reduced β-oxidation, OXPHOS-related gene or protein expression, and ATP synthesis, but also by the massive production of ROS and the resulting oxidative damage (Figure 1). Therefore, enhancing the mtFAO capacity is a promising therapeutic strategy.
ROS and mitochondrial dysfunction
Physiologically, ROS are produced as secondary messengers in several important biological processes.37 Meanwhile, excess ROS can be detoxified by antioxidant defense enzymes (ie, peroxiredoxins, glutathione peroxidases, catalase, and superoxide dismutases) to maintain redox homeostasis.38 When high levels of ROS overwhelm the antioxidant defense system, they lead to a state of oxidative stress (OS) in vivo,38 which is a well-known key causative factor in initiating inflammation, lipid peroxidation, and insulin resistance (IR) events. Certainly, besides a consequence of fat accumulation in the liver, IR is a vital facilitator of fat accumulation in the liver by promoting lipolysis, de novo lipogenesis in the liver, and hepatic uptake of FFAs.39,40 In addition to their ability to generate ROS, mitochondria are vulnerable to oxidative damage.18 As mitochondrial DNA (mtDNA) lacks protective histones and is close to the ROS source, mtDNA is susceptible to ROS-mediated oxidative damage, which affects mtDNA replication and transcription, causing further mitochondrial functional impairment. Oxidative damage to mtDNA has been demonstrated in animal models and patients with MASLD, accompanied by the inhibition of mitochondrial complex activity.41,42 Moreover, the increased level of ROS might contribute to a decrease in ΔΨm, which in turn increases mitochondrial membrane permeability, leading to the release of mitochondrial damage-associated molecular pattern (eg, mtDNA, cytochrome c, cardiolipin, and heat shock protein 60) into the cytoplasm, which are key stimulators of the inflammatory response pathway.43,44 Using a rodent model and primary mouse KCs, Pan et al found that the release of mtDNA can activate KCs and upregulate the expression of NLRP3, which has been proposed as a potential cause of progression from simple steatosis to MASH.44 Noticeably, in MASLD rats, high levels of ROS can directly activate the NLRP3 inflammasome and promote the expression of pro-inflammatory cytokines (eg, TNFα, IL-6, and NF-kβ), contributing to liver inflammation and fibrosis progression.45,46 In human MASLD, another adverse effect of OS is the promotion of hepatic lipid peroxidation, which can produce toxic byproducts,39 including 4-hydroxynonenal and malondialdehyde (MDA).42,47,48 As a result, this can induce mitochondrial dysfunction, more ROS production, and inflammation. In addition, decreased antioxidant enzyme (such as superoxide dismutase [SOD] and glutathione peroxidase) activity is common in patients with MASLD, and animal models provide an opportunity for disease progression.49,50
Excessive ROS production is a hallmark of mitochondrial dysfunction. Oxidative stress caused by ROS can trigger IR, lipid peroxidation, inflammation, and mtDNA oxidative damage, thereby exacerbating mitochondrial function and liver damage during MASLD progression (Figure 1).
Aberrant mitochondrial quality control
Mitochondrial quality control (mitoQC) is a complex set of mechanisms that have evolved to maintain a healthy mitochondrial network and cellular homeostasis in dynamic host environments. These mechanisms include mitochondrial redox regulation, proteosis, mitophagy, mitochondrial dynamics, and biogenesis.51 The maintenance of normal mitochondrial morphology and volume is also dependent on mitoQC; however, in rodent models of MASLD, fragmented cristae, swelling, and outer member disruption of mitochondria are frequent pathological features,52,53,54 indicating that aberrant mitoQC is involved in the pathogenesis of MASLD. In this chapter, we discuss how mitophagy, mitochondrial dynamics, and mitochondrial biogenesis contribute to MASLD development.
Mitophagy
A dynamic cellular process called mitophagy helps cells eliminate extraneous, damaged, or defective mitochondria. This is a type of specialized autophagy. “PINK/PARKIN” is referred to as a canonical mitophagy pathway.55 Evidence shows that Pink and Parkin protein levels are lowered in MASLD animal models, along with apoptosis, mitophagy blockade, and altered mitochondrial architecture.56,57,58 Liraglutide has a solid track record of its protective effects against MASLD.59,60 Yu et al found that liraglutide inhibited the production of ROS, exerted anti-inflammatory effects, reduced lipid accumulation, and inhibited the activation of NLRP3 inflammasome and cell pyroptosis by activating PINK expression, suggesting that liraglutide ameliorates MASLD by enhancing mitophagy.61 The Bcl-2 family member Bnip3 is connected in the control of mitophagy. Both patients with MASH and the MASLD mouse model had lower levels of Bnip3 expression,27,62 and the liver mitochondria of BNIP3-deficient mice had a lower ∆Ψm value and reduced oxygen consumption.63 Macrophage stimulating 1 (Mst1) is a regulatory factor that can affect the apoptosis of cancer cells by inhibiting mitochondrial phagocytosis, and BNIP3 plays an essential role in the induction of mitochondrial autophagy under Mst1 and Mst2-mediated mitochondrial stress.64 In addition, Mst1 can regulate the expression of Parkin through the AMPK pathway, inhibit the phagocytosis of Parkin-related mitophagy, and restore the apoptosis of hepatocyte mitochondria, thus promoting the improvement of MASLD.65
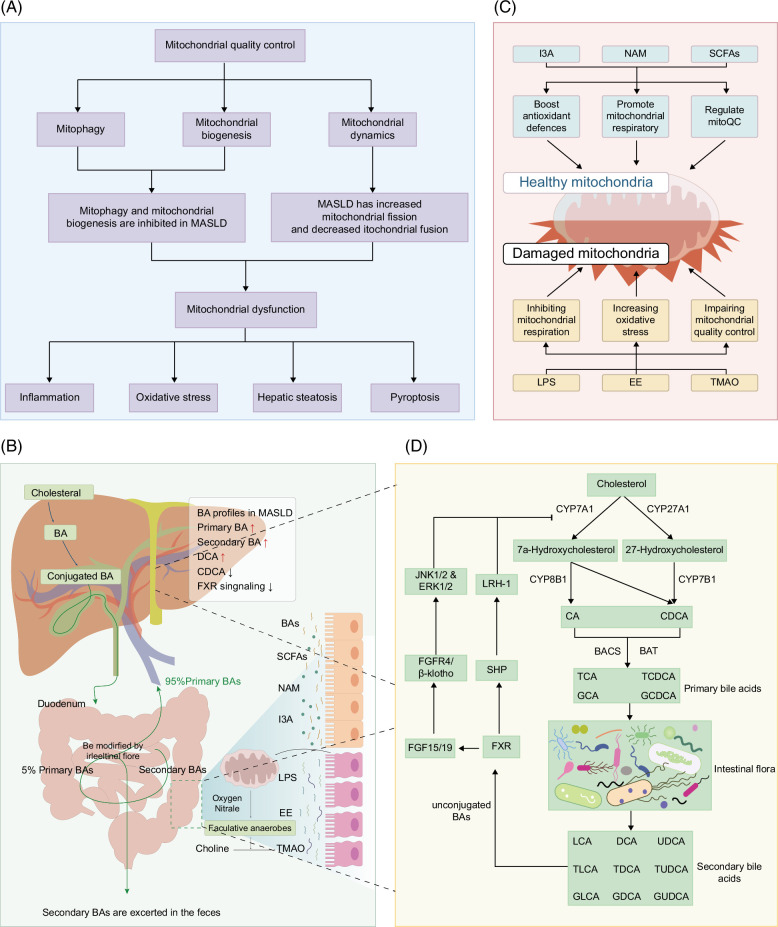
Together, these findings indicate that MASLD evolution is aided by poor mitophagy (Figure 2A).
FIGURE 2.
(A) The roles of mitophagy, mitochondrial dynamics, and mitochondrial biogenesis play in MASLD progression. (B) The gut-liver axis is altered in the development of MASLD. When patients with MASLD were compared with healthy subjects, the differences in BA profiles persisted, which can be mainly reflected in the production of both primary and secondary bile acids. Meanwhile, according to the changes in the content of different BA species, it can be inferred that FXR signaling is inhibited in MASLD. Additionally, intestinal epithelial barrier damage is also one of the key factors promoting MASLD, which can result in an increased risk of metabolites of bacterial origin and microbial translocation from the gut to the periphery. (C) The intestinal flora is rich in metabolites, including both beneficial and unfavorable components. From the bacteria and mitochondria interaction perspective, LPS, EE, and TMAO can exert dramatic detrimental effects on mitochondrial function, including inhibiting mitochondrial respiration, increasing oxidative stress, and impairing mitochondrial quality control. Conversely, mitochondrial dysfunction was beneficially impacted by I3A, NAM, and SCFAs. However, their expression was reduced in patients with MASLD. Notably, decreased mitochondrial respiratory function in the gut epithelium affects the structure of the gut microbiota, generating an increase in harmful metabolites, including TMAO. (D) Gut microbiota-involved biosynthesis and metabolism of bile acids. Primary BAs are synthesized in hepatocytes from cholesterolviaclassical or alternative pathways. In the classical pathway, cholesterol is initiated by CYP7A1 and converted into 2 primary BAs, CA, and CDCA. The alternative pathway is initiated by CYP27A1 and forms mostly CDCA. They are further conjugated with taurine (in mice) or glycine (in humans) and transformed into conjugated BAs (eg, TCA, GCA, TCDCA, and GCDCA). Part of BAs is converted to secondary bile acids(eg, UDCA, DCA, LCA, TUDCA, TDCA, TLCA, GUDCA, GDCA, and GLCA) by various organisms by intestinal flora. FXR is activated by unconjugated BAs, thus stimulating the expression of SHP, which further binds to LRH1 to inhibit CYP7A1 transcription. In addition, FGF19/FGF15 released by intestinal epithelial cells also induces JNK1/2 and ERK1/2 signaling by binding to FGFR4/β-klotho complexes. Abbreviations: BA, bile acid; DCA, deoxycholic acid; EE, endogenous ethanol; FGFR4, FGF receptor 4; FXR, farnesoid X-activated receptor; GCA, glycocholic acid; GCDCA, glycochenodeoxycholic acid; I3A, indol-3-acetic acid; LCA, lithocholic acid; LPS, lipopolysaccharide; LRH1, liver receptor homolog 1; MASLD, metabolic dysfunction–associated steatotic liver disease; NAM, nicotinamide; SCFAs, short-chain fatty acids; SHP, small heterodimer partner; TCA, taurocholic acid; TCDCA, taurochenodeoxycholic acid; TMAO, trimethylamine N-oxide; UDCA, ursodeoxycholic acid.
Mitochondrial biogenesis
Mitochondrial biogenesis is an important mediator of mitoQC because of its ability to synthesize new mitochondria, which helps cells to adapt to their energy requirements. PGC-1α is a pivotal regulator of mitochondrial fatty acid oxidation and biogenesis.66 A low level of PGC-1 has been reported to be associated with decreased antioxidant mechanisms in a mouse model of diet-induced MASLD.41 Besides, In an in vitro study, Zhang et al showed that Lycium barbarum polysaccharides increase PGC-1-dependent mitochondrial biogenesis in an MASLD cell model, which contributes to improving liver energy metabolism and attenuating intracellular lipid accumulation.67 Likewise, a 2020 study in mice revealed that supplementation with neohesperidin could mitigate diet-induced insulin resistance and hepatic steatosis, which are associated with upregulated mitochondrial biogenesis.68 Collectively, mitochondrial biosynthesis was inhibited during MASLD development (Figure 2A).
Mitochondrial dynamics
Mitochondrial dynamics encompasses 2 complementary processes: mitochondrial fission and fusion. Mitochondrial fission is mainly mediated by dynamin-related protein 1 (Drp1), which can seriously damage mitochondria in healthy mitochondrial networks and function in mitophagy to eliminate mitochondrial garbage. In addition, mitochondrial fusion can promote the exchange of mitochondrial components (eg, proteins, lipids, and genomes) to maintain mitochondrial health, and optic atrophy 1 and mitofusins promote fusion of the mitochondrial inner and outer membranes, respectively.51 Indeed, mitochondrial fission has been shown to be excessive in mice fed a choline-deficient, L-amini-acid-defined diet. The same study revealed that mdivi1, a chemical mitochondrial fission inhibitor, is capable of rebalancing mitochondrial dynamics and attenuating fibrosis and inflammation in the livers of MASLD mice.52 Similarly, several ongoing studies have shown an elevation of Drp1 and downregulation of Mfn2 and Opa1 in the MASLD disease model induced by HFD feeding, suggesting that disrupted mitochondrial fusion-fission balance is involved in MASLD progression.53,54 In addition, Han et al revealed that excessive mitochondrial fission seems to be associated with AMPK pathway inhibition.53
THE GUT-LIVER AXIS AND MASLD
Both the liver and gut are involved in the digestion and absorption of food. Due to the existence of portal circulation and the biliary tree, there are many connections between these 2 organs, including metabolism, neuroendocrine, and immune. The gut provides ~70% of the blood for the liver through the portal vein, enterogenic substances such as gut microbial products, exogenous toxins, and fragments of digested food enter the liver through the portal vein.69,70 The liver responds to these intestinal signals by excreting bile acids, cytokines, and other bioactive substances into the gut, a 2-way link often referred to as the “gut-liver axis” (Figure 2B).70
Association of MASLD pathogenesis and gut dysbiosis
Gut microbiome dysbiosis has attracted the attention of researchers in metabolic disorders. The human gastrointestinal tract is colonized by a complex microbial community, gut microbiota (GM), consisting of bacteria, archaea, protists, fungi, and viruses.71 GM is characterized by highly dynamic changes that vary with age, gender, diet, hormonal changes, and medication.72 In addition to absorbing host energy, GM actively participates in human physiological activities, including digestion, immunity, neurotrophy, and inflammation. Gut bacteria have a powerful metabolic capacity. They ferment hard-to-digest dietary fiber and produce short-chain fatty acids (SCFAs), which provide the body with an energy source. Endogenous substances, such as bile acids (BAs) and vitamins, are also metabolized by gut bacteria.73 The composition and abundance of GM is accepted as a barometer of overall health.16,70 Obvious changes in the composition of the fecal microbiota have been reported in patients with MASLD. For example, 2 studies74,75 have recently reported an upregulation of Firmicutes/Bacteroidetes ratio in obese youths with MASLD and adults with MASLD, respectively, indicating that the Firmicutes/Bacteroidetes ratio is associated with steatosis and obesity. Apart from this, the gut microbiome is an essential component of the immune barrier. Host bacteria attach to the surface of intestinal epithelial cells to form the intestinal barrier. They can also secrete antimicrobial substances such as microproteins or lower local pH by releasing metabolites to prevent pathogenic bacteria from colonizing them.76 Moreover, Metabolites produced by specific microbes in the gut microbiome, such as SCFAs, tryptophan, and BAs, can help regulate host immune responses and homeostasis.4,77
In addition to reduced richness and diversity of the microbiome, the breakdown of the intestinal barrier can also lead to intestinal dysbiosis.78 The intestinal barrier plays a vital role in the gut-liver axis, including physical, immune, and biochemical components.79 Intestinal blood vessels and a single layer of epithelial cells, connected by tight junction proteins, together with the mucus layer and microorganisms, form a physical barrier. Biochemical barriers are maintained and mediated by molecules with antimicrobial properties, such as BAs and antimicrobial proteins. The main components of the immune barrier include secreted Ig A and lymphoid follicles containing a variety of immune cells.80 A complex relationship exists between the gut microbiota and the immune system. Most gut microbiome-mediated diseases are associated with impaired immune responses.81 Certainly, the intestinal barrier constitutes the body’s first line of defense for immunity, intact intestinal barrier prevents noxiousness from entering the circulation by permselective and active transport in a healthy state.69 However, under pathological conditions, the stimuli (eg, dysbiosis, gut inflammation, and toxins) can disrupt the intestinal barrier and increase permeability, leading to the translocation of intestinal bacteria and endotoxin entry into the portal vein system,82 allowing more bacteria and bacteria-derived components and metabolites to escape to the liver, stimulating the activation of immune cells in the liver, the release of inflammatory cytokines, and the progression of fibrosis,83 thus causing an imbalance in enterohepatic homeostasis, which is a pathological basis for various chronic liver diseases.84 Repairing the intestinal microbiota may be an effective strategy for MASLD treatment. It is well known that unhealthy diet is an important driver of MASLD. An animal study found that energy-dense diets could successfully induce MASLD, which is simultaneously accompanied by elevated gut permeability.85 Another study found that Lactobacillus plantarum NA136 treatment could regulate gut microbial dysbiosis, thus strengthening the intestinal microbiota and reducing inflammation in the liver.86
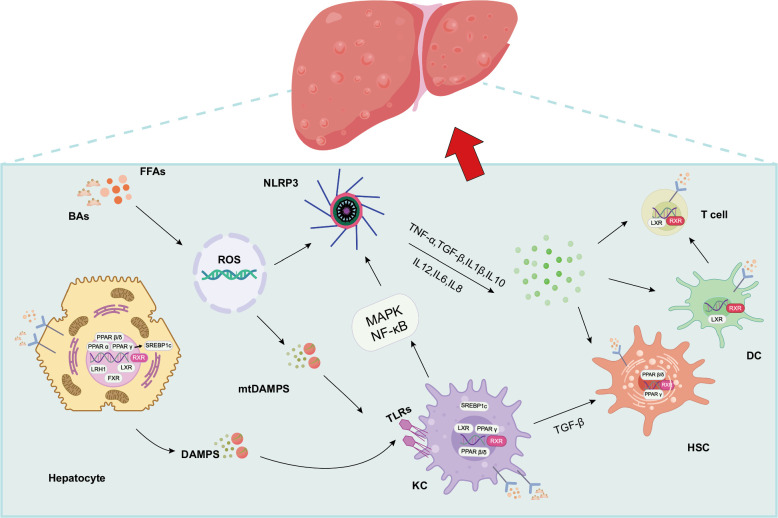
The liver has the function of fine-tuning inflammation and immune effector cells and can control the inflammatory response of the GM and its degradation products, which reach the liver through portal vein blood.87,88 The liver provides a second line of defense against disease-causing agents that evade the intestinal mucosal immune defense.89 Excessive accumulation of FFA can lead to the transfer of lipopolysaccharide (LPS) to the liver and interaction with Toll-like receptors (TLRs) (mainly TLR4-CD14 complexes) on macrophages and stellate cells, thereby enabling hepatocytes, KCS, and other pro-inflammatory cells to activate downstream MAPK and NF-κB signaling pathways. Ultimately, it promotes the release of pro-inflammatory cytokines.90 TNF, IL-6, and IL-1β have been found to be highly expressed in MASLD.91 TNF is one of the earliest cytokines to be studied. In addition, IL-1 family members have been shown to affect insulin-glucose metabolism and regulate metabolic dysfunction in patients with MASLD. Moreover, IL-1β is one of the key cytokines leading to the induction and persistence of liver inflammation.92 IL-6 is also expressed at increased concentrations in the liver and adipose tissue of patients with MASLD.93 Loss of IL-17 can promote MASLD/MASH progression, and recent evidence has demonstrated that IL-17 can exert a gut microbiota-mediated restoring function of the gut barrier, thereby inhibiting hepatocyte damage.94 Furthermore, the role of inflammasomes and TLRs in MASLD has been reported several times. NLRP3 and various TLRS affect key pathways involved in MASLD, including inflammatory infiltration of the liver and adipose tissue and regulation of insulin sensitivity.95 Actually, regulating the gut microbiome through the activity of certain TLRs or inflammatory mediators may lead to alterations in microbiome-related inflammation, which in turn may worsen liver inflammation and MASLD96 (Figure 3).
FIGURE 3.
Schematic diagram of liver immune changes in MASLD. After excessive accumulation of FFAs, hepatic steatosis causes the release of DAMPs and other substances and stimulates TLRs, which enables hepatocytes, KCS, and other pro-inflammatory cells to activate downstream MAPK and NF-κB signaling pathways, thereby activating NLRP3 and ultimately promotes the release of pro-inflammatory cytokines (including IL-β, TNF-α, IL-6, IL-8, TGF-β, etc.). Immune activation and hepatocyte injury will affect HSC activation by upregulating pro-fibrotic and pro-inflammatory cytokines such as TGF-β1 and IL-β, further aggravating MASLD. In addition, activated DC coordinates the T-cell immune response. Moreover, the increased level of ROS might contribute to the release of mtDAMP release, which can activate KCs and upregulate the NLRP3 expression. Abbreviations: BA, bile acid; DC, dendritic cell; FFA, free fatty acid; MASLD, metabolic dysfunction–associated steatotic liver disease; TLR, toll-like receptors.
Collectively, the gut-liver axis is a pivotal player in MASLD pathogenesis, and alterations in the gut-liver axis primarily result from gut bacterial dysbiosis (Figure 2B). Thus, a full understanding of the structural characteristics of the gut microbiota in MASLD is required to develop effective treatment strategies for improving MASLD.
THE LINK BETWEEN MICROBIAL METABOLITES AND MITOCHONDRIAL DYSFUNCTION IN MASLD
Multiple lines of evidence suggest that the adverse effects of the gut microbiota on many nondigestive systems are achieved through various bacterial metabolites. Changes in microbial metabolites may reflect compositional alterations in the gut microbiota during MASLD development. Recently, the interaction between mitochondria and microbiota has attracted particular attention, and evidence has shown that damaging factors resulting from gut-flora-induced mitochondrial dysfunction act as vital regulators of lipid accumulation in the liver.97,98,99,100 A review in this chapter aims to summarize several microbial metabolites linked to MASLD as well as their impact on mitochondrial dysfunction (Figure 2C).
Bile acids
Bile acids (BAs) act as vital mediators for communication between the gut and the liver.101 The gut microbiota can biotransform and modify BAs, substantially influencing the synthesis and metabolism of BAs.102 Catalyzed by cholesterol 7α-monooxygenase or sterol 27-hydroxylase, cholesterol can be converted to primary BA in pericentral hepatocytes that can be further conjugated to taurine or glycine to produce primary conjugated BAs, which are stored in the gallbladder and spilled into the small intestine after feeding the host.103 However, 95% of BAs are actively reabsorbed and transported back to the liver through the portal vein; the remaining BAs are converted into secondary BAs by bacterial-dependent modifications and lost into feces (Figure 2D).104 Furthermore, gut microbiota can not only directly biotransform primary BA into secondary BA, but also control the BA synthesis by regulating the bile acids synthetase through dehydroxyisomerization, oxidative desulfurization, and esterification, including deconjugation, 7α-Dehydroxyisomerization, oxidation, isomerization, desulfurization, and esterification.105,106 In turn, in addition to promoting the absorption of lipids and lipid-soluble vitamins, BAs can also regulate diverse immunological and metabolic pathways by activating bile acid receptors, including farnesoid X-activated receptor (FXR) and G protein-coupled BA receptor 1, and play a beneficial role in maintaining gut microbiota homeostasis and intestinal microbiota integrity.69 Therefore, gut microbiota disorder may lead to BA synthesis and metabolism disorder, which may affect lipid metabolism, hepatobiliary function, and intestinal health. The imbalance in BA homeostasis plays a critical role in MASLD progression. Clinical cohort studies have shown the plasma BA profiles differ between patients with MASLD/MASH and healthy controls. For example, Jiao et al found an elevated production of both primary and secondary BAs in patients with MASLD.107 Nevertheless, the same study revealed that deoxycholic acid (DCA), an FXR antagonist, was increased, whereas chenodeoxycholic acid, an FXR agonist, was decreased in MASLD, suggesting that FXR signaling is inhibited in MASLD, which could promote obesity and steatosis in the liver (Figure 2D).107 In another study, researchers proved that the alteration of circulating BA composition was associated with the histological features of MASH.108 In addition, BA has an effect on the antioxidant capacity of mitochondria. In liver conditions with biliary stasis, the antioxidant capacity of mitochondria is reduced.109 Studies have found that ursodeoxycholic acid, taurine deoxycholic acid, and lipophilic BA, such as DCA, chenodeoxycholic acid, and lithocholic acid, can inhibit electron transport chain in isolated mitochondria of rat liver.110 In addition, recent studies have proved that CA and DCA can reduce mitochondrial potential, and the levels of OXPHOS complexes I, II and III also inhibit mitochondrial biogenesis and affect mitochondrial function, thus increasing ROS production.111
Overall, as a major mediator of enterohepatic circulation, BAs, play a pivotal role in nutrient absorption and signal transduction by regulating the gut microbiota and activating different BA receptors, and it is of great interest to understand the effect of changes in bile acid metabolism in the gut and mitochondrial cross-talk in MASLD.
Short-chain fatty acids
Short-chain fatty acids (SCFAs) are bacterial metabolites derived from indigestible dietary fibers and saccharides.100 The ample evidence has demonstrated that SCFAs exert beneficial effects on the systemic glycolipid metabolism and intestinal mucosal barrier function.16,18,112 Clearly, SCFAs have been accepted as beneficial regulators for mitochondrial metabolism.10 However, patients with MASLD show a depletion of SCFA-producing bacteria in stool samples, including Prevotella-9, Lachnospiraceae, and Ruminococcus.113 Interestingly, in vivo studies of HFD-fed mice revealed that sodium butyrate supplementation could mitigate hepatic steatosis and inflammation by upregulating the abundance of SCFA-producing bacteria, although the pathogenic mechanism remains unclear.114 Based on this observation, Maria et al found that butyrate reduced the lipid content and OS levels in the liver of an obese mouse model.99 Notably, these effects were mainly related to improved mitochondrial respiration through the activation of the AMPK-acetyl-CoA carboxylase pathway.99 In another study, a significant increase in propionic acid and isobutyric acid concentrations was found in the feces of Methylation-controlled J protein knockout mice fed CDA-HFD.115 In the hyperinsulin-induced dysfunction of HepG2 cells, sodium butyrate can significantly increase the mitochondrial DNA content of HepG2 cells, increase the membrane potential function, and enhance the activities of SOD and glutathione peroxidase. It depletes levels of pro-oxidant NADPH oxidase 2, ROS, and MDA, thereby improving mitochondrial.116 In addition to these beneficial effects, butyrate was shown to elevate the expression of Mfn1, Mfn2, and Opa1 protein mRNAs in the liver, promoting mitochondrial fusion.99 Interestingly, sodium acetate has been reported to prevent HSC activation and alleviate liver fibrosis by activating the AMPK/PPARγ pathway.117 However, the association between sodium acetate and the mitochondrial function of HSC requires further study. Additionally, at the cellular level, the addition of propionate to FFA-treated calf hepatocytes reversed mitochondrial dysfunction, OS, and apoptosis, which was associated with the upregulation of PGC-1α, which is specific for propionate.118 Similarly, Deng et al demonstrated that SCFAs, particularly butyrate, can prevent mitochondrial dysfunction by lowering ROS production and caspase-3 protein expression in THLE-2 Cells after exposure to hippuric acid.119 Besides, studies in obese rodent models have revealed that butyrate can lead to the upregulation of CPT-1b and cytochrome c oxidase I gene expression, as well as the downregulation of histone deacetylase activity in skeletal muscle cells. Changes in these mechanisms can improve mitochondrial function and contribute to increased energy expenditure and insulin sensitivity.120 Notably, histone deacetylases have been associated with mitochondrial energy metabolism and quality control.121,122 SCFA treatment exhibits beneficial effects by altering histone deacetylase mRNA expression and attenuating systemic inflammation in obese patients.123
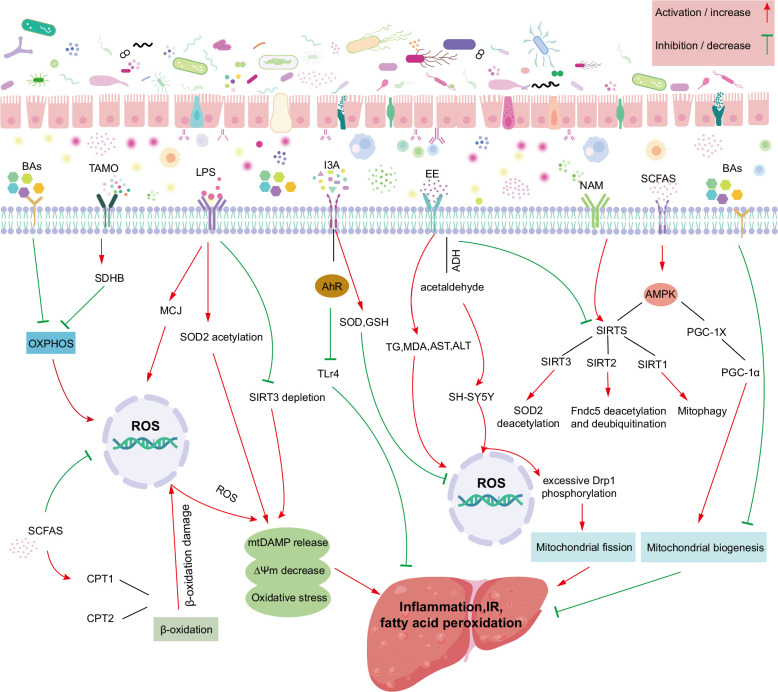
Collectively, SCFA therapy has been shown to have antioxidant effects by downregulating pro-inflammatory mediators, maintaining the integrity of the intestinal microbiota, and increasing mitochondrial respiratory capacity.124 These data indicated that SCFAs exert a protective role in MASLD by optimizing mitochondrial function (Figure 4).
FIGURE 4.
Microbiota-derived metabolites impact mitochondrial dysfunction in MASLD. Gut microbiota and its metabolites affect the structure and function of hepatocyte mitochondria through enterohepatic circulation, and the interaction between microbiota and mitochondria completes the progression of gut microbiota to promote MASLD. Abbreviations: ADH, alcohol dehydrogenase; AhR, aryl hydrocarbon receptor; ALT, alanine aminotransferase; AST, aspartate aminotransferase; AMPK, AMP-activated protein kinase; BA, bile acids; CPT1, carnitine palmitoyl transferase Ⅰ; CPT2, carnitine palmitoyl transferase Ⅱ; Drp1, dynamin-related protein 1; EE, endogenous ethanol; Fndc5, Fibronectin type III domain-containing protein 5; FXR, farnesoid X-activated receptor; GSH, glutathione; I3A, indol-3-acetic acid; LPS, lipopolysaccharide; MASLD, metabolic dysfunction–associated steatotic liver disease; MCJ, methylation-controlled J protein; MDA, malondialdehyde; NAM, nicotinamide; OXPHOS, oxidative phosphorylation; PGC-1α, proliferator-activated receptorγ coactivator-1α; SCFAs, short-chain fatty acids; SDHB, succinate dehydrogenase complex subunit B; SH-SY5Y, human neuroblastoma cell line; SIRTs, sirtuins; SOD2, superoxide dismutase; TG, triglycerides; TLR4, toll-like receptors 4; TMAO, trimethylamine N-oxide.
Endogenous ethanol
Endogenous ethanol (EE) is produced by gut bacteria through carbohydrate metabolism.125 Several studies have reported that ethanol-producing bacteria (including Clostridium, Escherichia, Klebsiella pneumoniae, and Proteobacteria) in MASLD have a higher representation, contributing to the high serum level of ethanol in the absence of alcohol ingestion.98,126,127 Thus, some researchers have described MASLD as an endogenous alcohol-associated fatty liver disease.128 Additionally, MASLD and alcohol-associated liver disease share similar disease phenotypes, including hepatic steatosis, inflammation, and fibrosis, as well as a common mechanistic background, such as mitochondrial dysfunction.125 Ethanol may cause liver damage by inducing intestinal cytochrome P450 2E1 and iNOS to increase oxidative stress and steatosis, and eventually dysregulate tight junction proteins and damage the intestinal microbiota.129,130 Liver mitochondria play an important role in ethanol metabolism. Following the conversion of ethanol to acetaldehyde by cytosolic alcohol dehydrogenase in hepatocytes, mitochondrial acetaldehyde dehydrogenase, and cytochrome P450 2E1 catalyze the conversion of hepatocytes into acetate.131 Apparently, acetaldehyde, a potentially toxic molecule, was reported to upregulate the levels of ROS and Ca2+ in SH-SY5Y cells, thereby promoting excessive Drp1 phosphorylation and mitochondrial fragmentation.132 In addition, FLD has been directly induced in mice by the inadministration of Klebsiella pneumoniae with high-yield alcohol, showing upregulation of genes associated with cytochrome P450 2E1 and lipogenesis, suggesting that endogenous alcohol produced by the intestinal bacterium HiAlc-Kpn may be sufficient to cause the liver pathogenesis observed in mice.98 Additionally, SIRT has been demonstrated to promote mitochondrial fatty acid oxidation and induce stress tolerance.133 Nevertheless, SIRT1 and SIRT3 expression was downregulated and functionally insufficient in a mouse model of alcohol-induced fatty liver, along with reduced mitochondrial content and inadequate ATP production.134 Moreover, similar results were reported in a study by Fan et al, in which alcohol-dependent mitochondrial dysfunction was associated with downregulation of SIRT1.135 A preclinical study has shown that endogenous ethanol produced by gut bacteria can induce mitochondrial damage in liver cells. Yuan and his colleagues considered Klebsiella pneumoniae as a contributor to MASLD.98 Their study further verified the induction of MASLD by Klebsiella pneumoniae in mice and the effect of EE on the liver in vivo, and the results showed that the content of EE in blood was positively correlated with the levels of TG and MDA in liver tissue, and the activity of AST and ALT in serum. Moreover, EE induced by Klebsiella pneumoniae could impair mitochondrial integrity, decrease ATP content, and increase mitochondrial ROS accumulation and DNA damage.97,98 Furthermore, it should be noted that their study also demonstrated that EE could reduce the expression of Mfn1 but upregulate the expression of Drp1 and Fis1, generating mitochondrial disintegration.98
Collectively, although the underlying molecular mechanisms of endogenous ethanol in MASLD have rarely been specifically studied, there is no denying that EE is probably produced by the microbiota in the colon and is largely involved in the highly complex pathogenesis of MASLD, leading to liver damage and steatosis. However, the combination and interaction of these factors remain to be clarified, and the association between EE and mitochondrial dysfunction in MASLD is also worth further exploration.
Nicotinamide and nicotinamide adenine dinucleotide
The gut microbiota-derived metabolite nicotinamide (NAM) serves as a precursor of NAD+.136 NAD+ is a classic coenzyme that exerts its biological role through REDOX and non-redox reactions.137 In mammals, NAD+ is synthesized through 3 main pathways: the de novo synthesis pathway of tryptophan, the recovery pathway of NAM, and the Preiss-Handler pathway of niacin (NA).138 In addition, the role of gut microbiota in NAD+ synthesis has received significant attention in recent years. Evidence shows that Bacteroidetes, Clostridium, Proteobacteria, Actinobacteria, and Firmicutes are capable of synthesizing niacin in the healthy human intestines.139 Using 16S rRNA sequencing technology, significant differences were discovered in the phenotypes of amino acid metabolism of gut microbiota between the MASLD and healthy control groups.140 Studies have shown that normal gut microbiota transplantation can not only restore gut microbiota imbalance but also induce an increase in serum NMN levels and enhance the biosynthesis of NAD+ in the pancreas.141 In addition, Shats et al demonstrated the importance of gut microbiota in promoting NAD+ with oral nicotinamide riboside (NR) supplementation, and found that partially labeled NR in normal mice was converted to NAM in the colonic lumen at a rate 3 times greater than that of NR in germ-free mice, suggesting that the conversion of NR to NAM is mainly done by the gut microbiota. Furthermore, this study also found that conventional mice treated with oral pyridine-15NNR had significantly increased levels of NA and NAR in portal vein blood, and the increase in NAM in germ-free mice was also significantly diminished, suggesting that both amidated and deamidated NAD+ precursors produced by the gut microbiota from NR reach the circulatory system.142
Notably, the gut microbiome has a unique NAD+ metabolic pathway, which may help improve the metabolic flexibility of the host.143 In contrast to the liver, the gut microbiota, such as Clostridium, Firmicutes, and Proteus, which regulate key enzymes NAMNAT and NAD+, synthesize NAD+ precursors into NAD+ through a specific de novo synthesis pathway. At the same time, some Firmicutes convert quinolinic acid to NAMN and synthesize NAD+ through NMNS and NMNAT. In addition, gut microbiota supplementation of NAD+ precursors NAM, NA, and NR also plays a crucial role in the synthesis of NAD+. Only Actinomyces, Firmicutes, and Proteobacteria can absorb NAM and NA, while the salvage of NR is only found in Firmicutes and Proteobacteria.139 It has also been reported that there is another important pathway in the gut microbiota, namely the catalytic conversion of NAM to NA through PncA11, which combines the recycling and Preiss-handler pathways, which is known as deamidation.142 Moreover, Kim et al also confirmed the pathway whereby orally isotopically labeled NMN in rats was partially deaminated to NAMN by intestinal flora before absorption. Although the extent was significantly lower than that of the amidation metabolites NMN and NAD+, the level of nicotinate adenine dinucleotide in the liver was increased.144 The deamidation process is considered to be an important step in the regulation of NAM and NR-enhanced NAD+ levels by intestinal flora.145 Shen et al reported a liver-protective effect of nicotinamide; that is, nicotinamide pretreatment protects HepG2 cells from palmitate-induced lipotoxicity and cell death through SIRT1-mediated induction of autophagy.146 Indeed, mitochondria harbor a large number of oxidoreduction reactions in which NAD+ is a vital player acting as an electron transfer carrier.147 NAD+ not only plays a key role in energy metabolism and promotes mitochondrial function but is also a precursor to NADPH, an important component of the antioxidant defense system.148 NAD+ can accept electrons for reduction to NADH, which can further transfer electrons into the respiratory chain and oxidize to NAD+.149 Principally, NAD+ deletion results in mitochondrial dysfunction, which is an established cause of metabolic disorders.150 Furthermore, the concentration of NAD+ within the mitochondria was significantly decreased in animal models of MASLD and obesity.32,50 Of note, a study in mice revealed that oral administration of NR, a precursor of NAD+, could rescue the MASLD phenotype (eg, fatty liver, low insulin sensitivity, fibrosis, and inflammation) through the increase of mitochondrial respiratory and content.32 Besides, it should also be noted that the effect of NR on mitochondrial function is mediated through SIRT1-dependent and SIRT3-dependent mitochondrial unfolded proteins.32 Similarly, in obese mice, Carles et al found that supplementation with NR reversed mitochondrial dysfunction in obese mice, thereby enhancing the oxidation capacity in skeletal muscle and brown adipose tissue, which could contribute to weight loss in obese mice.50 Moreover, mitochondrial SOD2 is an important antioxidant enzyme, and NAD+ has been reported to facilitate the deacetylation of SOD2 by triggering SIRT3 activity to increase its antioxidant properties.50 Interestingly, NAD+-boosting therapy has been reported to increase mitochondrial biogenesis and autophagy by modulating SIRT2-mediated Fndc5 deacetylation and deubiquitination.151 The above evidence demonstrates that the therapeutic potential of NAM in MASLD models is related to mitochondrial dysfunction (Figure 4).
Lipopolysaccharide
LPS, also known as an endotoxin, is a potent inducer of inflammation.152 LPS expression was found to be increased in liver tissue and serum in patients with MASLD as well as in an HFD model.153 Moreover, LPS has been demonstrated to increase with MASLD disease severity154 and is proposed to be a potential serum biomarker for MASLD and MASH.155 An interesting animal study found that fatty liver formation was associated with high serum endotoxin levels triggered by gut flora disturbances.156 Inflammation has been associated with oxidative stress, and the treatment of THLE-2 cells with LPS can successfully induce oxidative stress.157 Using this cellular model, Li et al found that LPS contributed to the expression of MCJ, an endogenous inhibitor of mitochondrial complex I and mitochondrial membrane potential, increased ROS levels, and reduced mitochondrial membrane potential.157 Notably, Wei et al revealed that LPS treatment could promote hepatocyte apoptosis by stimulating the release of cytochrome C from the mitochondria into the cytosol.158 Moreover, LPS significantly reduced the copy number of mtDNA in the liver and further affected the activity of mitochondrial complexes IV and V, ultimately generating impaired mitochondrial OXPHOS and decreasing ATP production.159 An additional study reported that LPS could induce sepsis in C57BL/6 mice and damage the liver, leading to increased SOD2 acetylation, SIRT3 depletion, mitochondrial morphological changes, and upregulation of heat shock protein 60 in the liver.160 Collectively, LPS is a vital player in MASLD, which can impair mitochondrial function and lead to hepatocyte apoptosis, OS, and inflammation (Figure 4).
Indole-3-acetic acid
Over the past few years, the potential preventive role of indol-3-acetic acid (I3A) in metabolic diseases has attracted considerable attention.161 I3A is a tryptophan metabolite that has been repeatedly reported as an aryl hydrocarbon receptor (AhR) ligand,162,163 the AhR agonist in a variety of cells, including hepatocytes.164,165,166 Moreover, the effect of I3A on AhR target genes is mediated by ligand activation of nuclear receptors.167 This AhR can sense a wide range of gut signals and maintain homeostasis between the gut microbiota and the host.168,169 In addition, I3A can alleviate cytokine-mediated lipogenesis in hepatocytes by activating AhR.167 Therefore, I3A can maintain intestinal homeostasis through AHR ligands and regulate the gut-liver axis.170 However, the abundance of I3A-producing bacteria, such as Bcteroides, Alistipes, and Prevotellaceae, decreased in HFD-induced mouse models. Correspondingly, the expression of indole-related metabolites decreased in the feces of patients with MASLD and model mice, as confirmed by metabolic studies.113 Notably, in HFD-induced MASLD mice, oral administration of I3A alleviated the MASLD phenotype, mainly driven by the repair of mitochondrial respiration defects.159 This effect was related to the increased expression of beta-oxidation and mitochondrial complex genes and decreased expression of lipogenesis genes in the liver. Moreover, the present study found that I3A treatment restored the level of ATP5A1, a component of ATP synthase, in an MASLD mouse model.171 Additionally, I3A has the ability to scavenge free radicals.172 It has been reported that macrophages pretreated with I3A were successively exposed to palmitic acid and LPS, which significantly reduced the mRNA levels of TNF-α, IL-1β, and McP-1, suggesting that I3A can reduce the expression of pro-inflammatory cytokines in macrophages and induce liver synthesis of FFAs. The study also demonstrated that I3A significantly reduced macrophage migration in response to MCP-1 in a dose-dependent manner. In addition, the study also demonstrated that I3A acts on hepatocytes to attenuate the upregulation of cell kinematics during adipogenesis, thereby attenuating free fatty acid-induced adipogenesis of hepatocytes.167 Furthermore, I3A is reported to mitigate the upregulation of lipogenic genes involved in Srebf1, Scd1, PPARγ, Acaca, and Gpam, thereby reducing the accumulation of liver triglycerides after HFD induction in mice. In addition, it alleviates the increase in F4/80, MCP-1, and TNF-α expression and inhibits the infiltration of macrophages and reducing liver inflammation. The study also measured the levels of ROS and lipid peroxidation products, and the results showed that I3A can increase the expression of antioxidant enzymes (ie, SOD and GSH), reduce ROS levels, and protect the liver from oxidative stress.171 It has also been reported that I3A treatment increases the expression of Ahr and decreases the expression of Tlr4 mRNA that recognizes and activates LPS, thereby inhibiting macrophage activation and NF-κB signaling pathway, and ultimately improving the inflammatory response.170 Melatonin, an indoleamine small molecular substance inside the human body, is not only a microbiota metabolite in the gut but also an endocrine secretion produced by the pineal gland, retina, skin, and others.173 Type 2 diabetes is characterized by low serum melatonin serum levels, which may be associated with gut microbiota-mediated melatonin signaling.173 Melatonin is a powerful antioxidant molecule that declines with age. Evidence shows that melatonin treatment can increase the expression of Mfn2, Opa1, and Drp1 in aging mice, thereby recovering mitochondrial dynamics altered by aging and maintaining mitochondrial morphology and number, which contributes to maintaining cardiac function.174 In addition, in a mouse model of ochratoxin A-induced liver inflammation, melatonin treatment was found to regulate mitophagy, ameliorate oxidative stress, regulate gut microbiota disturbances, and repair intestinal microbiota functions.175 These animal data provide evidence for the protective effect of I3A on MASLD under the protection of mitochondrial function (Figure 4), and targeting I3A may be an effective therapy for MASLD.
Trimethylamine N-oxide
A positive correlation between elevated serum trimethylamine N-oxide (TMAO) level and body weight, Visceral Adiposity Index, and Fatty Liver Index has been well described in overweight or obese subjects.176 The importance of choline in maintaining hepatic lipid homeostasis has been recognized for several years.177 However, under MASLD-associated dysbiosis, choline is overconsumed or metabolized to trimethylamine by anaerobic bacteria, followed by conversion of TMA to TMAO in the liver by choline-TMA lyase.178 A recent study suggested that TMAO aggravates liver steatosis by suppressing BA-mediated hepatic FXR signaling.179 Besides, it has been shown that long-term administration of 1.5% TMAO in drinking water induces oxidative stress in liver tissues.180 Facultative anaerobic Enterobacteriaceae is closely associated with a pathological increase in TMAO in the gut.178 Normally, the colonic epithelium depends on mitochondria to consume sufficient oxygen to maintain physiological hypoxia, forming an anoxic condition in the intestine, which restricts the growth of facultative anaerobic bacteria.181 However, a prolonged high-fat diet is reported to impair the host control mechanism, causing an increased number of respiratory electron acceptors, such as oxygen and nitrate, from the mucosal surface, supporting E. coli respiration and choline catabolism.178 Succinate dehydrogenase, located on the inner membrane of mitochondria, is involved in OXPHOS and is closely linked to mitochondrial ROS production.182Wu et al found that TMAO was capable of increasing succinate dehydrogenase complex subunit B expression and could lead to mitochondrial damage and high levels of ROS, resulting in endothelial cell pyroptosis and the progression of atherosclerotic lesions.183 Another study demonstrated that TMAO reduces both pyruvate and fatty acid oxidation in cardiac mitochondria.184 Based on these data, it is clear that TMAO negatively affects mitochondrial function (Figure 4). However, the association between TMAO and mitochondrial metabolism in hepatocytes requires further investigation.
TREATMENT OPTIONS FOR MASLD BASED ON MODULATION OF GUT MICROBIOTA
Optimizing the composition of the intestinal flora has been considered a vital factor in preventing or reversing the progression of MASLD in recent years because dysbiosis of the gut microbiota plays an essential role in the disease. Appropriate measures based on gut microbiota have been used in the clinical treatment of MASLD, such as the administration of probiotics, prebiotics, and antibiotics as well as the use of fecal microbiota transplantation (FMT). The beneficial effects of these measures have been validated in several animal and clinical studies, offering promising prospects for the treatment of MASLD. In addition to targeting the gut microbiota, these therapeutic strategies play a role in improving mitochondrial function.
Probiotics
Probiotics are a range of microbial food supplements that confer substantial health benefits to the host. It is reported that lactic acid bacteria cause Firmicutes/Bacteroidetes ratio and SCFA content changes in MASLD mice and, therefore, have an impact on energy and lipid metabolism and restrict the development of MASLD.185 Notably, lactic acid bacteria fermentation enhanced the antioxidant capacity of black tea, and the lactic acid bacteria-fermented BT sample maintained mitochondrial membrane potential and inhibited ROS production in H2O2-treated CCD841 cells.186 Additionally, a mouse model induced by HFD treated with probiotics containing Limosilactobacillus fermentum MG4231 and MG4244 exhibited enhanced lipid metabolism and reduced synthesis and liver inflammation, and these changes were associated with increased phosphorylation of AMPK and acetyl-CoA carboxylase, which aid mtFAO.187 In another study, Bifidobacterium animalis subsp. lactis A6 conferred a relieving effect on obesity development in mice by regulating the gut bacterial community, normalizing the serum LPS level, improving mitochondrial biosynthesis, and upregulating the expression of uncoupling protein-1, which could increase the uncoupling mitochondrial OXPHOS to generate more heat.8,188 Likewise, Dong et al found that Lactobacillus plantarum fermented barley extract restricts the development of obesity by repairing mitochondrial function.189,190,191 Overall, these preclinical studies provide evidence for the potential anti-MASLD effects of probiotics. Notably, various probiotic strains have been developed and used in the food industry, making it difficult to choose appropriate probiotics for patients with MASLD. In addition, the effectiveness of probiotics in MASLD has been inconsistent in clinical studies. For example, several recent clinical studies have demonstrated the therapeutic effect of probiotics in patients with MASLD, including the reduction of liver fat and inflammatory cytokine levels.192,193 In contrast, a 6-month study found that probiotics failed to alter important parameters of MASLD, such as blood lipid metabolism, hepatic steatosis, and fibrosis levels in patients with MASLD, but improved intestinal mucosal barrier function.194 Therefore, the real benefits of probiotics in MASLD need to be explored in adequately designed clinical trials.
Prebiotics
Prebiotics can be fermented by gut microbiota to provide health benefits to the host.195 Polyphenols are known for their prebiotic effects and are ubiquitous in plants. Li et al revealed that blueberry leaves contain 8 polyphenols, chlorogenic acid, D-catechin, L-epicatechin, rutin, isoquercitrin, cyanidin-3-O-glucoside, iridin, and quercetin.196 The administration of polyphenols in blueberry leaves enhances antioxidant capacity in the liver and prevents the development of MASLD in mice by decreasing ROS and MDA levels and upregulating SOD2 expression in the liver. Polyphenols in blueberry leaves also repair mitochondrial dysfunction by increasing MMP, augmenting cellular ATP content, and reversing the decrease in mtDNA in the liver.196 Rafiei et al found that several plant polyphenols that protect against steatosis were mediated by various mitochondria-related mechanisms in a cell model of MASLD. For instance, while cyanidin improves mitochondrial function primarily by restoring MMP levels to normal, kuromanin improves mitochondrial function primarily by increasing the mRNA levels of CPT1 and deacetylated PGC-1 and inhibiting the upregulation of FAS.197 Likewise, evidence has shown that the polyphenol-rich foods Taurisolo and cocoa polyphenols may also attenuate the development of MASLD by restoring optimal mitochondrial function.198,199 Moreover, using a zebrafish model and human hepatocytes, Zhang et al showed that Lycii fructus polysaccharides restore redox balance and resist mitochondrial-mediated apoptosis, and therefore affect the development of MASLD.200 Resistant dextrin is another form of a prebiotic. A recent study showed that the anti-MASLD capability of resistant dextrin contributes to endothelial function improvement and intestinal flora structure optimization, in addition to maintaining mitochondrial integrity and function.201 Taken together, the protective effect of prebiotics on mitochondria is crucial for preventing MASLD progression.
The beneficial effects of probiotics on MASLD have been supported by clinical studies. The combined use of camelina sativa oil and resistant dextrin could help patients with MASLD with metabolic risk factors and mental wellness.202 Additionally, a study on obese individuals found that inulin-propionate ester administered to patients at doses of 10 mg daily for 24 weeks decreased weight gain, abdominal fat distribution, and liver fat content.203 It is noteworthy that a different investigation carried out by the same research group found that oral administration of inulin-propionate ester (20 mg/day) for 6 weeks did not alter intrahepatocellular lipid levels.204
Antibiotics
In a published article, Yuan et al described an intriguing case of MASH with auto-brewing syndrome and attributed the morbidity of the patient to the high level of endogenous alcohol induced by gut microbial dysbiosis, and the blood alcohol concentration and severity of MASH were alleviated after dietary changes and antibiotic treatment.98 In mice fed with HFD, neomycin has been shown to reduce intestinal inflammation, hepatic steatosis, and atrophic intestinal lining.205 Additionally, rifaximin is poorly absorbed in the intestine and is recommended for the treatment of functional gastrointestinal disorders and hepatic encephalopathy.206 Its reported that the administration of rifaximin for 4 weeks could mitigate the disease phenotype of MASH in mice, accompanied by alterations in the intestinal microecology and decreased DCA in the ileum.207 In addition, a recent small clinical study, which focused on the effects of rifaximin on MASH, also found that rifaximin had positive effects on liver function, LPS, TLR4, IL-6, and MASLD-liver fat score in patients with MASH but was ineffective in lowering TG and BMI.206 Nevertheless, a prospective clinical study of 15 subjects found no effect of rifaximin therapy of 6-week duration on either hepatic inflammation, hepatic lipid content, or hepatic and peripheral insulin sensitivity in patients with MASH.208 Notably, another interesting study reported that inulin supplementation for 12 weeks after brief metronidazole therapy for 1 week could enhance the beneficial metabolic effects of short-term, very-low-calorie diets for 4 weeks among patients with MASLD.209
Overall, antibiotics may have a limited ability to treat MASLD, and it is important to consider any potential negative consequences they may have on human health, including the resistance of microorganisms to antibiotics and the reduction of beneficial bacteria by antibiotics, along with liver and kidney damage to the host.207,210 The total impact of antibiotics on MASLD should be the focus of future studies.
Fecal microbiota transplantation
Fecal microbiota transplantation (FMT) aims to reconstitute a healthy gut microbiota with a “physiological” microbiome obtained from fecal material from a healthy donor.211 FMT has been clinically applied to treat a variety of metabolic syndromes, including MASLD, obesity, and diabetes.212,213 Six-week FMT treatment has been reported to promote beta cell regeneration, lower endotoxin burden, and promote pathological damage in the intestine and liver in a mouse model of type 2 diabetes mellitus.214 Another interesting study showed that transplanting microbiota from healthy people could reduce body weight and liver triglyceride levels in HFD-fed mice compared with transplanting microbiota from patients with MASLD.215 Similarly, a randomized clinical study conducted by Xue et al216 revealed that FMT treatment delayed the progression of MASLD by altering the gut microecology. Additionally, the authors claimed that the effect of FMT was more pronounced in lean patients with MASLD than in patients with obesity. Conversely, another study reported that FMT reduced small intestinal permeability but failed to improve IR and hepatic proton density fat fraction in patients with MASLD.217 Siew et al reported that a combination of lifestyle intervention and FMT alleviated hyperlipidemia and liver stiffness in patients with obesity with type 2 diabetes mellitus.218
CONCLUSIONS AND FUTURE PERSPECTIVES
The gut microbiota of patients with MASLD shows an obvious alteration in composition and functionality, and in recent years, researchers have been exploring how the gut microbiota alters host homeostasis. In this review, the summarized data demonstrate a clear causal relationship between the gut microbiota and mitochondrial dysfunction during the development of MASLD. Oxygen consumption in gut epithelial mitochondria modulates the structure of the gut microbiota. Furthermore, altered gut microbiota and their metabolites affect the structure and function of hepatocyte mitochondria through enterohepatic circulation, which appears to be a key mechanism responsible for hepatocyte steatosis and imbalance in homeostasis. In conclusion, the interaction between the microbiota and mitochondria completes the storyline of the gut microbiota, promoting MASLD progression. Metabolites of the gut microbiota are diverse and can play either beneficial or detrimental roles in human physiological functions. Nevertheless, in this review, we summarized only 7 metabolites of gut microbiota, including BAs, SCFAs, ethanol, NAM, LPS, I3A, and TMAO, which may not be sufficient to generalize the overall impact of gut microbiota on mitochondrial function.Other metabolites (eg, H2S, NO, and p-cresols) should also be taken into consideration. In future studies, the correlation between gut microbiota and mitochondria warrants comprehensive and in-depth study. “Supplement the good ones, reduce the bad ones” appears to be the core strategy for therapeutic approaches targeting the modulation of the gut microbiota. In addition, probiotics, prebiotics, antibiotics, and FMT have been used to regulate the composition of the gut microbiota in patients with MASLD and to inhibit disease development. These treatments have been shown to be effective for treating MASLD in experimental animal models. However, the safety and efficacy of these therapeutic strategies should be demonstrated in future large-scale, randomized clinical studies. A better understanding of these mechanisms will aid in the development of potent therapeutic approaches to prevent MASLD.
Supplementary Material
AUTHOR CONTRIBUTIONS
Ruhan Zhang and Zhaobo Yan took the lead in writing the manuscript; Huan Zhong and Weiai Liu contributed to manuscript preparation and revision; Rong Luo and Shulin Xiong provided assistance regarding illustrations; Mi Liu and Qianyan Liu contributed to the conception and design of the article as well as revised the important intellectual content in this manuscript. All authors have read and approved the final manuscript.
ACKNOWLEDGMENTS
The authors thank the editor and the anonymous reviewers for the insightful suggestions and comments. And give heartfelt thanks to all the people who have ever helped with this paper.
FUNDING INFORMATION
The National Natural Science Foundation of China (No. 81774438 and 81904097 ), Natural Science Foundation of Hunan Province (No. 2023JJ30457 and 2023JJ60338), Natural Science Foundation of Changsha (no. kq2208183), Project of Chinese Medicine Research in Hunan Province (No. C2022027, D2022043, and A2023022), State Administration of Traditional Chinese Medicine 2022 Youth Qihuang Scholars Training Program (National Letter of Traditional Chinese Medicine Education [2022] 256), Graduate Student Research Innovation Project of Hunan University of Chinese Medicine(No. 2023CX166)
CONFLICTS OF INTEREST
The authors have no conflicts to report.
Footnotes
Abbreviations: AhR, aryl hydrocarbon receptor; AMPK, AMP-activated protein kinase; BA, bile acids; CPT, carnitine palmitoyl transferase; DCA, deoxycholic acid; Drp1, dynamin-related protein 1; EE, endogenous ethanol; FFA, free fatty acid; FMT, fecal microbiota transplant; FXR, farnesoid X-activated receptor; GM, gut microbiota; HFD, high-fat diet; I3A, indol-3-acetic acid; IR, insulin resistance; LPS, lipopolysaccharide; MASH, metabolic dysfunction–associated steatohepatitis; MASLD, metabolic dysfunction–associated steatotic liver disease; Mst, macrophage stimulating; MDA, malondialdehyde; mitoQC, mitochondrial quality control; mtDNA, mitochondrial DNA; mtFAO, Mitochondrial fatty acid oxidation; NA, niacin; NAM, nicotinamide; NR, nicotinamide riboside; OS, oxidative stress; OXPHOS, oxidative phosphorylation; PGC, proliferator-activated receptorγ coactivator; ROS, reactive oxygen species; SCFA, short-chain fatty acid; SIRT, Sirtuin; SOD, superoxide dismutase; TG, triglycerides; TLRs, Toll-like receptors; TMAO, trimethylamine N-oxide.
Ruhan Zhang and Zhaobo Yan provided equal contributions to this work.
Contributor Information
Ruhan Zhang, Email: 1411319231@qq.com.
Zhaobo Yan, Email: 2671790233@qq.com.
Huan Zhong, Email: 359653775@qq.com.
Rong Luo, Email: 529488363@qq.com.
Weiai Liu, Email: 55999630@qq.com.
Shulin Xiong, Email: 241160064@qq.com.
Qianyan Liu, Email: 157476218@qq.com.
Mi Liu, Email: 7417091@qq.com.
REFERENCES
- 1. Chalasani N, Younossi Z, Lavine JE, Charlton M, Cusi K, Rinella M, et al. The diagnosis and management of nonalcoholic fatty liver disease: Practice guidance from the American Association for the Study of Liver Diseases. Hepatology. 2018;67:328–357. [DOI] [PubMed] [Google Scholar]
- 2. Malhi H, Brown RS, Jr, Lim JK, Reau N, Tapper EB, Wong CC, et al. Precipitous changes in nomenclature and definitions-NAFLD becomes SLD: Implications for and expectations of AASLD journals. Hepatology. 2023;78:1680–1681. [DOI] [PubMed] [Google Scholar]
- 3. Rinella ME, Lazarus JV, Ratziu V, Francque SM, Sanyal AJ, Kanwal F, et al. A multisociety Delphi consensus statement on new fatty liver disease nomenclature. Hepatology. 2023;78:1966–1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Huang DQ, El-Serag HB, Loomba R. Global epidemiology of NAFLD-related HCC: Trends, predictions, risk factors and prevention. Nat Rev Gastroenterol Hepatol. 2021;18:223–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wu Y, Zheng Q, Zou B, Yeo YH, Li X, Li J, et al. The epidemiology of NAFLD in Mainland China with analysis by adjusted gross regional domestic product: A meta-analysis. Hepatol Int. 2020;14:259–269. [DOI] [PubMed] [Google Scholar]
- 6. Golabi P, Paik JM, Harring M, Younossi E, Kabbara K, Younossi ZM. Prevalence of high and moderate risk nonalcoholic fatty liver disease among adults in the United States, 1999-2016. Clin Gastroenterol Hepatol. 2022;20:2838–2847.e7. [DOI] [PubMed] [Google Scholar]
- 7. Vuppalanchi R, Siddiqui MS, Van Natta ML, Hallinan E, Brandman D, Kowdley K, et al. Performance characteristics of vibration-controlled transient elastography for evaluation of nonalcoholic fatty liver disease. Hepatology. 2018;67:134–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Goedeke L, Shulman GI. Therapeutic potential of mitochondrial uncouplers for the treatment of metabolic associated fatty liver disease and NASH. Mol Metab. 2021;46:101178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zhuge A, Li S, Lou P, Wu W, Wang K, Yuan Y, et al. Longitudinal 16S rRNA sequencing reveals relationships among alterations of gut microbiota and nonalcoholic fatty liver disease progression in mice. Microbiol Spectr. 2022;10:e0004722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Vezza T, Abad-Jiménez Z, Marti-Cabrera M, Rocha M, Víctor VM. Microbiota-mitochondria inter-talk: A potential therapeutic strategy in obesity and type 2 diabetes. Antioxidants (Basel). 2020;9:848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Rodrigues RR, Gurung M, Li Z, García-Jaramillo M, Greer R, Gaulke C, et al. Transkingdom interactions between Lactobacilli and hepatic mitochondria attenuate western diet-induced diabetes. Nat Commun. 2021;12:101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mach N, Moroldo M, Rau A, Lecardonnel J, Le Moyec L, Robert C, et al. Understanding the holobiont: Crosstalk between gut microbiota and mitochondria during long exercise in horse. Front Mol Biosci. 2021;8:656204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Saint-Georges-Chaumet Y, Edeas M. Microbiota-mitochondria inter-talk: Consequence for microbiota-host interaction. Pathog Dis. 2016;74:ftv096. [DOI] [PubMed] [Google Scholar]
- 14. Thiele I, Swainston N, Fleming RM, Hoppe A, Sahoo S, Aurich MK, et al. A community-driven global reconstruction of human metabolism. Nat Biotechnol. 2013;31:419–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bajpai P, Darra A, Agrawal A. Microbe-mitochondrion crosstalk and health: An emerging paradigm. Mitochondrion. 2018;39:20–25. [DOI] [PubMed] [Google Scholar]
- 16. Canfora EE, Meex R, Venema K, Blaak EE. Gut microbial metabolites in obesity, NAFLD and T2DM. Nat Rev Endocrinol. 2019;15:261–273. [DOI] [PubMed] [Google Scholar]
- 17. Wiesner RJ, Rüegg JC, Morano I. Counting target molecules by exponential polymerase chain reaction: Copy number of mitochondrial DNA in rat tissues. Biochem Biophys Res Commun. 1992;183:553–559. [DOI] [PubMed] [Google Scholar]
- 18. Ramanathan R, Ali AH, Ibdah JA. Mitochondrial dysfunction plays central role in nonalcoholic fatty liver disease. Int J Mol Sci. 2022;23:7280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Pessayre D, Fromenty B. NASH: A mitochondrial disease. J Hepatol. 2005;42:928–940. [DOI] [PubMed] [Google Scholar]
- 20. Nassir F, Ibdah JA. Role of mitochondria in nonalcoholic fatty liver disease. Int J Mol Sci. 2014;15:8713–8742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Begriche K, Igoudjil A, Pessayre D, Fromenty B. Mitochondrial dysfunction in NASH: Causes, consequences and possible means to prevent it. Mitochondrion. 2006;6:1–28. [DOI] [PubMed] [Google Scholar]
- 22. Heidemann BE, Koopal C, Bots ML, Asselbergs FW, Westerink J, Visseren F. The relation between VLDL-cholesterol and risk of cardiovascular events in patients with manifest cardiovascular disease. Int J Cardiol. 2021;322:251–257. [DOI] [PubMed] [Google Scholar]
- 23. Koliaki C, Szendroedi J, Kaul K, Jelenik T, Nowotny P, Jankowiak F, et al. Adaptation of hepatic mitochondrial function in humans with non-alcoholic fatty liver is lost in steatohepatitis. Cell Metab. 2015;21:739–746. [DOI] [PubMed] [Google Scholar]
- 24. Dewidar B, Mastrototaro L, Englisch C, Ress C, Granata C, Rohbeck E, et al. Alterations of hepatic energy metabolism in murine models of obesity, diabetes and fatty liver diseases. EBioMedicine. 2023;94:104714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Mato JM, Alonso C, Noureddin M, Lu SC. Biomarkers and subtypes of deranged lipid metabolism in non-alcoholic fatty liver disease. World J Gastroenterol. 2019;25:3009–3020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Li Z, Li Y, Zhang HX, Guo JR, Lam C, Wang CY, et al. Mitochondria-mediated pathogenesis and therapeutics for non-alcoholic fatty liver disease. Mol Nutr Food Res. 2019;63:e1900043. [DOI] [PubMed] [Google Scholar]
- 27. Moore MP, Cunningham RP, Meers GM, Johnson SA, Wheeler AA, Ganga RR, et al. Compromised hepatic mitochondrial fatty acid oxidation and reduced markers of mitochondrial turnover in human NAFLD. Hepatology. 2022;76:1452–1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Zhou W, Deng X, Zhu X, Yan Q, Zhou N, Du S, et al. HtrA2/Omi mitigates NAFLD in high-fat-fed mice by ameliorating mitochondrial dysfunction and restoring autophagic flux. Cell Death Discov. 2022;8:218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lin JJ, Liu YC, Chang CJ, Pan MH, Lee MF, Pan BS. Hepatoprotective mechanism of freshwater clam extract alleviates non-alcoholic fatty liver disease: Elucidated in vitro and in vivo models. Food Funct. 2018;9:6315–6325. [DOI] [PubMed] [Google Scholar]
- 30. Sangineto M, Bukke VN, Bellanti F, Tamborra R, Moola A, Duda L, et al. A novel nutraceuticals mixture improves liver steatosis by preventing oxidative stress and mitochondrial dysfunction in a NAFLD Model. Nutrients. 2021;13:652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ham JR, Lee HI, Choi RY, Sim MO, Seo KI, Lee MK. Anti-steatotic and anti-inflammatory roles of syringic acid in high-fat diet-induced obese mice. Food Funct. 2016;7:689–697. [DOI] [PubMed] [Google Scholar]
- 32. Gariani K, Menzies KJ, Ryu D, Wegner CJ, Wang X, Ropelle ER, et al. Eliciting the mitochondrial unfolded protein response by nicotinamide adenine dinucleotide repletion reverses fatty liver disease in mice. Hepatology. 2016;63:1190–1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lee K, Haddad A, Osme A, Kim C, Borzou A, Ilchenko S, et al. Hepatic mitochondrial defects in a nonalcoholic fatty liver disease mouse model are associated with increased degradation of oxidative phosphorylation subunits. Mol Cell Proteomics. 2018;17:2371–2386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Buler M, Aatsinki SM, Izzi V, Uusimaa J, Hakkola J. SIRT5 is under the control of PGC-1α and AMPK and is involved in regulation of mitochondrial energy metabolism. FASEB J. 2014;28:3225–3237. [DOI] [PubMed] [Google Scholar]
- 35. Nam H, Lim JH, Kim TW, Kim EN, Oum SJ, Bae SH, et al. Extracellular superoxide dismutase attenuates hepatic oxidative stress in nonalcoholic fatty liver disease through the adenosine monophosphate-activated protein kinase activation. Antioxidants (Basel). 2023;12:2040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Wang D, Cao L, Zhou X, Wang G, Ma Y, Hao X, et al. Mitigation of honokiol on fluoride-induced mitochondrial oxidative stress, mitochondrial dysfunction, and cognitive deficits through activating AMPK/PGC-1α/Sirt3. J Hazard Mater. 2022;437:129381. [DOI] [PubMed] [Google Scholar]
- 37. Tsubata T. Involvement of reactive oxygen species (ROS) in BCR signaling as a second messenger. Adv Exp Med Biol. 2020;1254:37–46. [DOI] [PubMed] [Google Scholar]
- 38. Staerck C, Gastebois A, Vandeputte P, Calenda A, Larcher G, Gillmann L, et al. Microbial antioxidant defense enzymes. Microb Pathog. 2017;110:56–65. [DOI] [PubMed] [Google Scholar]
- 39. Chen Z, Tian R, She Z, Cai J, Li H. Role of oxidative stress in the pathogenesis of nonalcoholic fatty liver disease. Free Radic Biol Med. 2020;152:116–141. [DOI] [PubMed] [Google Scholar]
- 40. Koh JH, Johnson ML, Dasari S, LeBrasseur NK, Vuckovic I, Henderson GC, et al. TFAM enhances fat oxidation and attenuates high-fat diet-induced insulin resistance in skeletal muscle. Diabetes. 2019;68:1552–1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Chimienti G, Orlando A, Russo F, D’Attoma B, Aragno M, Aimaretti E, et al. The mitochondrial trigger in an animal model of nonalcoholic fatty liver disease. Genes (Basel). 2021;12:1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Pirola CJ, Garaycoechea M, Flichman D, Castaño GO, Sookoian S. Liver mitochondrial DNA damage and genetic variability of Cytochrome b - a key component of the respirasome - drive the severity of fatty liver disease. J Intern Med. 2021;289:84–96. [DOI] [PubMed] [Google Scholar]
- 43. Khwaja B, Thankam FG, Agrawal DK. Mitochondrial DAMPs and altered mitochondrial dynamics in OxLDL burden in atherosclerosis. Mol Cell Biochem. 2021;476:1915–1928. [DOI] [PubMed] [Google Scholar]
- 44. Pan J, Ou Z, Cai C, Li P, Gong J, Ruan XZ, et al. Fatty acid activates NLRP3 inflammasomes in mouse Kupffer cells through mitochondrial DNA release. Cell Immunol. 2018;332:111–120. [DOI] [PubMed] [Google Scholar]
- 45. Fanaei H, Mard SA, Sarkaki A, Goudarzi G, Khorsandi L. Gallic acid treats dust-induced NAFLD in rats by improving the liver’s anti-oxidant capacity and inhibiting ROS/NFκβ/TNFα inflammatory pathway. Iran J Basic Med Sci. 2021;24:240–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Zhang H, Gao X, Chen P, Wang H. Protective effects of tiaoganquzhi decoction in treating inflammatory injury of nonalcoholic fatty liver disease by promoting CGI-58 and inhibiting expression of NLRP3 inflammasome. Front Pharmacol. 2022;13:851267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Yun MR, Park HM, Seo KW, Lee SJ, Im DS, Kim CD. 5-Lipoxygenase plays an essential role in 4-HNE-enhanced ROS production in murine macrophages via activation of NADPH oxidase. Free Radic Res. 2010;44:742–750. [DOI] [PubMed] [Google Scholar]
- 48. Tavakoli T, Zarban A, Hooshyar R, Salmani F, Tajik H. Improvement of thiol groups and total antioxidant capacity in patients with non-alcoholic fatty liver after treatment with pioglitazone. Arch Physiol Biochem. 2022;128:1591–1595. [DOI] [PubMed] [Google Scholar]
- 49. Li J, Wang T, Liu P, Yang F, Wang X, Zheng W, et al. Hesperetin ameliorates hepatic oxidative stress and inflammation via the PI3K/AKT-Nrf2-ARE pathway in oleic acid-induced HepG2 cells and a rat model of high-fat diet-induced NAFLD. Food Funct. 2021;12:3898–3918. [DOI] [PubMed] [Google Scholar]
- 50. Cantó C, Houtkooper RH, Pirinen E, Youn DY, Oosterveer MH, Cen Y, et al. The NAD(+) precursor nicotinamide riboside enhances oxidative metabolism and protects against high-fat diet-induced obesity. Cell Metab. 2012;15:838–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Li R, Toan S, Zhou H. Role of mitochondrial quality control in the pathogenesis of nonalcoholic fatty liver disease. Aging. 2020;12:6467–6485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Miyao M, Kawai C, Kotani H. Altered mitochondrial dynamics in hepatocytes induces nonalcoholic fatty liver disease progression. Faseb J. 2022;36(Suppl 1):R2918. [Google Scholar]
- 53. Han H, Li X, Guo Y, Zheng M, Xue T, Wang L. Plant sterol ester of α-linolenic acid ameliorates high-fat diet-induced nonalcoholic fatty liver disease in mice: Association with regulating mitochondrial dysfunction and oxidative stress via activating AMPK signaling. Food Funct. 2021;12:2171–2188. [DOI] [PubMed] [Google Scholar]
- 54. Geng Y, Wang Y, Sun R, Kang X, Zhao H, Zhu M, et al. Carnosol alleviates nonalcoholic fatty liver disease by inhibiting mitochondrial dysfunction and apoptosis through targeting of PRDX3. Toxicol Appl Pharmacol. 2021;432:115758. [DOI] [PubMed] [Google Scholar]
- 55. Matsuda N. Phospho-ubiquitin: Upending the PINK-Parkin-ubiquitin cascade. J Biochem. 2016;159:379–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Dong Y, Yu M, Wu Y, Xia T, Wang L, Song K, et al. Hydroxytyrosol promotes the mitochondrial function through activating mitophagy. Antioxidants (Basel). 2022;11:893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Liu P, Lin H, Xu Y, Zhou F, Wang J, Liu J, et al. Frataxin-mediated PINK1-Parkin-dependent mitophagy in hepatic steatosis: The protective effects of Quercetin. Mol Nutr Food Res. 2018;62:e1800164. [DOI] [PubMed] [Google Scholar]
- 58. Cai J, Huang J, Yang J, Chen X, Zhang H, Zhu Y, et al. The protective effect of selenoprotein M on non-alcoholic fatty liver disease: The role of the AMPKα1-MFN2 pathway and Parkin mitophagy. Cell Mol Life Sci. 2022;79:354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Armstrong MJ, Gaunt P, Aithal GP, Barton D, Hull D, Parker R, et al. Liraglutide safety and efficacy in patients with non-alcoholic steatohepatitis (LEAN): A multicentre, double-blind, randomised, placebo-controlled phase 2 study. Lancet. 2016;387:679–690. [DOI] [PubMed] [Google Scholar]
- 60. Petit JM, Cercueil JP, Loffroy R, Denimal D, Bouillet B, Fourmont C, et al. Effect of liraglutide therapy on liver fat content in patients with inadequately controlled type 2 diabetes: The Lira-NAFLD Study. J Clin Endocrinol Metab. 2017;102:407–415. [DOI] [PubMed] [Google Scholar]
- 61. Yu X, Hao M, Liu Y, Ma X, Lin W, Xu Q, et al. Liraglutide ameliorates non-alcoholic steatohepatitis by inhibiting NLRP3 inflammasome and pyroptosis activation via mitophagy. Eur J Pharmacol. 2019;864:172715. [DOI] [PubMed] [Google Scholar]
- 62. Sheldon RD, Meers GM, Morris EM, Linden MA, Cunningham RP, Ibdah JA, et al. eNOS deletion impairs mitochondrial quality control and exacerbates Western diet-induced NASH. Am J Physiol Endocrinol Metab. 2019;317:E605–E616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Gonçalves IO, Passos E, Diogo CV, Rocha-Rodrigues S, Santos-Alves E, Oliveira PJ, et al. Exercise mitigates mitochondrial permeability transition pore and quality control mechanisms alterations in nonalcoholic steatohepatitis. Appl Physiol Nutr Metab. 2016;41:298–306. [DOI] [PubMed] [Google Scholar]
- 64. Jeong DJ, Um JH, Kim YY, Shin DJ, Im S, Lee KM, et al. The Mst1/2-BNIP3 axis is required for mitophagy induction and neuronal viability under mitochondrial stress. Exp Mol Med. 2024;56:674–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Zhou T, Chang L, Luo Y, Zhou Y, Zhang J. Corrigendum to “Mst1 inhibition attenuates non-alcoholic fatty liver disease via reversing Parkin-related mitophagy” [Redox Biol. 21 (2019 Feb) 101120]. Redox Biol. 2020;28:101299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Ducheix S, Vegliante MC, Villani G, Napoli N, Sabbà C, Moschetta A. Is hepatic lipogenesis fundamental for NAFLD/NASH? A focus on the nuclear receptor coactivator PGC-1β. Cell Mol Life Sci. 2016;73:3809–3822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Zhang YN, Guo YQ, Fan YN, Tao XJ, Gao QH, Yang JJ. Lycium barbarum polysaccharides promotes mitochondrial biogenesis and energy balance in a NAFLD cell model. Chin J Integr Med. 2022;28:975–982. [DOI] [PubMed] [Google Scholar]
- 68. Wang SW, Sheng H, Bai YF, Weng YY, Fan XY, Lou LJ, et al. Neohesperidin enhances PGC-1α-mediated mitochondrial biogenesis and alleviates hepatic steatosis in high fat diet fed mice. Nutr Diabetes. 2020;10:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Tripathi A, Debelius J, Brenner DA, Karin M, Loomba R, Schnabl B, et al. The gut-liver axis and the intersection with the microbiome. Nat Rev Gastroenterol Hepatol. 2018;15:397–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Tranah TH, Edwards LA, Schnabl B, Shawcross DL. Targeting the gut-liver-immune axis to treat cirrhosis. Gut. 2021;70:982–994. [DOI] [PubMed] [Google Scholar]
- 71. Kolodziejczyk AA, Zheng D, Shibolet O, Elinav E. The role of the microbiome in NAFLD and NASH. EMBO Mol Med. 2019;11:e9302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Gratz SW, Mykkanen H, El-Nezami HS. Probiotics and gut health: A special focus on liver diseases. World J Gastroenterol. 2010;16:403–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Ahmad F, Saha P, Singh V, Wahid M, Mandal RK, Nath Mishra B, et al. Diet as a modifiable factor in tumorigenesis: Focus on microbiome-derived bile acid metabolites and short-chain fatty acids. Food Chem. 2023;410:135320. [DOI] [PubMed] [Google Scholar]
- 74. Jasirwan C, Muradi A, Hasan I, Simadibrata M, Rinaldi I. Correlation of gut Firmicutes/Bacteroidetes ratio with fibrosis and steatosis stratified by body mass index in patients with non-alcoholic fatty liver disease. Biosci Microbiota Food Health. 2021;40:50–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Monga Kravetz A, Testerman T, Galuppo B, Graf J, Pierpont B, Siebel S, et al. Effect of gut microbiota and PNPLA3 rs738409 variant on nonalcoholic fatty liver disease (NAFLD) in obese youth. J Clin Endocrinol Metab. 2020;105:e3575–e3585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Luo L, Chang Y, Sheng L. Gut-liver axis in the progression of nonalcoholic fatty liver disease: From the microbial derivatives-centered perspective. Life Sci. 2023;321:121614. [DOI] [PubMed] [Google Scholar]
- 77. Zhang X, Coker OO, Chu ES, Fu K, Lau H, Wang YX, et al. Dietary cholesterol drives fatty liver-associated liver cancer by modulating gut microbiota and metabolites. Gut. 2021;70:761–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Sookoian S, Salatino A, Castaño GO, Landa MS, Fijalkowky C, Garaycoechea M, et al. Intrahepatic bacterial metataxonomic signature in non-alcoholic fatty liver disease. Gut. 2020;69:1483–1491. [DOI] [PubMed] [Google Scholar]
- 79. Peterson LW, Artis D. Intestinal epithelial cells: Regulators of barrier function and immune homeostasis. Nat Rev Immunol. 2014;14:141–153. [DOI] [PubMed] [Google Scholar]
- 80. He LH, Yao DH, Wang LY, Zhang L, Bai XL. Gut microbiome-mediated alteration of immunity, inflammation, and metabolism involved in the regulation of non-alcoholic fatty liver disease. Front Microbiol. 2021;12:761836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Vemuri R, Gundamaraju R, Shastri MD, Shukla SD, Kalpurath K, Ball M, et al. Gut microbial changes, interactions, and their implications on human lifecycle: An ageing perspective. Biomed Res Int. 2018;2018:4178607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. De Santis S, Cavalcanti E, Mastronardi M, Jirillo E, Chieppa M. Nutritional keys for intestinal barrier modulation. Front Immunol. 2015;6:612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Szabo G. Gut-liver axis in alcoholic liver disease. Gastroenterology. 2015;148:30–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Plaza-Díaz J, Solís-Urra P, Rodríguez-Rodríguez F, Olivares-Arancibia J, Navarro-Oliveros M, Abadía-Molina F, et al. The gut barrier, intestinal microbiota, and liver disease: Molecular mechanisms and strategies to manage. Int J Mol Sci. 2020;21:8351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Gart E, Souto Lima E, Schuren F, de Ruiter C, Attema J, Verschuren L, et al. Diet-independent correlations between bacteria and dysfunction of gut, adipose tissue, and liver: A comprehensive microbiota analysis in feces and mucosa of the ileum and colon in obese mice with NAFLD. Int J Mol Sci. 2018;20:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Zhao Z, Chen L, Zhao Y, Wang C, Duan C, Yang G, et al. Lactobacillus plantarum NA136 ameliorates nonalcoholic fatty liver disease by modulating gut microbiota, improving intestinal barrier integrity, and attenuating inflammation. Appl Microbiol Biotechnol. 2020;104:5273–5282. [DOI] [PubMed] [Google Scholar]
- 87. Ludwig J, Viggiano TR, McGill DB, Oh BJ. Nonalcoholic steatohepatitis: Mayo Clinic experiences with a hitherto unnamed disease. Mayo Clin Proc. 1980;55:434–438. [PubMed] [Google Scholar]
- 88. Eslam M, Newsome PN, Sarin SK, Anstee QM, Targher G, Romero-Gomez M, et al. A new definition for metabolic dysfunction-associated fatty liver disease: An international expert consensus statement. J Hepatol. 2020;73:202–209. [DOI] [PubMed] [Google Scholar]
- 89. Martens EC, Neumann M, Desai MS. Interactions of commensal and pathogenic microorganisms with the intestinal mucosal barrier. Nat Rev Microbiol. 2018;16:457–470. [DOI] [PubMed] [Google Scholar]
- 90. Ferro D, Baratta F, Pastori D, Cocomello N, Colantoni A, Angelico F, et al. New insights into the pathogenesis of non-alcoholic fatty liver disease: Gut-derived lipopolysaccharides and oxidative stress. Nutrients. 2020;12:2762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Cirmi S, Maugeri A, Russo C, Musumeci L, Navarra M, Lombardo GE. Oleacein attenuates lipopolysaccharide-induced inflammation in THP-1-derived macrophages by the inhibition of TLR4/MyD88/NF-κB pathway. Int J Mol Sci. 2022;23:1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Mirea AM, Tack CJ, Chavakis T, Joosten L, Toonen E. IL-1 family cytokine pathways underlying NAFLD: Towards new treatment strategies. Trends Mol Med. 2018;24:458–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Whitham M, Pal M, Petzold T, Hjorth M, Egan CL, Brunner JS, et al. Adipocyte-specific deletion of IL-6 does not attenuate obesity-induced weight gain or glucose intolerance in mice. Am J Physiol Endocrinol Metab. 2019;317:E597–E604. [DOI] [PubMed] [Google Scholar]
- 94. He S, Cui S, Song W, Jiang Y, Chen H, Liao D, et al. Interleukin-17 Weakens the NAFLD/NASH process by facilitating intestinal barrier restoration depending on the gut microbiota. mBio. 2022;13:e0368821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Tilg H, Adolph TE, Dudek M, Knolle P. Non-alcoholic fatty liver disease: The interplay between metabolism, microbes and immunity. Nat Metab. 2021;3:1596–1607. [DOI] [PubMed] [Google Scholar]
- 96. Spruss A, Kanuri G, Wagnerberger S, Haub S, Bischoff SC, Bergheim I. Toll-like receptor 4 is involved in the development of fructose-induced hepatic steatosis in mice. Hepatology. 2009;50:1094–1104. [DOI] [PubMed] [Google Scholar]
- 97. Chen X, Zhang Z, Li H, Zhao J, Wei X, Lin W, et al. Endogenous ethanol produced by intestinal bacteria induces mitochondrial dysfunction in non-alcoholic fatty liver disease. J Gastroenterol Hepatol. 2020;35:2009–2019. [DOI] [PubMed] [Google Scholar]
- 98. Yuan J, Chen C, Cui J, Lu J, Yan C, Wei X, et al. Fatty liver disease caused by high-alcohol-producing Klebsiella pneumoniae. Cell Metab. 2019;30:675–688.e7. [DOI] [PubMed] [Google Scholar]
- 99. Mollica MP, Mattace Raso G, Cavaliere G, Trinchese G, De Filippo C, Aceto S, et al. Butyrate regulates liver mitochondrial function, efficiency, and dynamics in insulin-resistant obese mice. Diabetes. 2017;66:1405–1418. [DOI] [PubMed] [Google Scholar]
- 100. Schönfeld P, Wojtczak L. Short- and medium-chain fatty acids in energy metabolism: The cellular perspective. J Lipid Res. 2016;57:943–954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Winston JA, Theriot CM. Diversification of host bile acids by members of the gut microbiota. Gut Microbes. 2020;11:158–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Li N, Zhan S, Tian Z, Liu C, Xie Z, Zhang S, et al. Alterations in bile acid metabolism associated with inflammatory bowel disease. Inflamm Bowel Dis. 2021;27:1525–1540. [DOI] [PubMed] [Google Scholar]
- 103. Wang LX, Frey MR, Kohli R. The role of FGF19 and MALRD1 in enterohepatic bile acid signaling. Front Endocrinol (Lausanne). 2021;12:799648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Schneider KM, Albers S, Trautwein C. Role of bile acids in the gut-liver axis. J Hepatol. 2018;68:1083–1085. [DOI] [PubMed] [Google Scholar]
- 105. Thomas JP, Modos D, Rushbrook SM, Powell N, Korcsmaros T. The emerging role of bile acids in the pathogenesis of inflammatory bowel disease. Front Immunol. 2022;13:829525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Zhang YL, Li ZJ, Gou HZ, Song XJ, Zhang L. The gut microbiota-bile acid axis: A potential therapeutic target for liver fibrosis. Front Cell Infect Microbiol. 2022;12:945368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Jiao N, Baker SS, Chapa-Rodriguez A, Liu W, Nugent CA, Tsompana M, et al. Suppressed hepatic bile acid signalling despite elevated production of primary and secondary bile acids in NAFLD. Gut. 2018;67:1881–1891. [DOI] [PubMed] [Google Scholar]
- 108. Puri P, Daita K, Joyce A, Mirshahi F, Santhekadur PK, Cazanave S, et al. The presence and severity of nonalcoholic steatohepatitis is associated with specific changes in circulating bile acids. Hepatology. 2018;67:534–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Krähenbühl S, Talos C, Lauterburg BH, Reichen J. Reduced antioxidative capacity in liver mitochondria from bile duct ligated rats. Hepatology. 1995;22:607–612. [DOI] [PubMed] [Google Scholar]
- 110. Krähenbühl S, Fischer S, Talos C, Reichen J. Ursodeoxycholate protects oxidative mitochondrial metabolism from bile acid toxicity: dose-response study in isolated rat liver mitochondria. Hepatology. 1994;20:1595–1601. [DOI] [PubMed] [Google Scholar]
- 111. Abrigo J, Olguín H, Tacchi F, Orozco-Aguilar J, Valero-Breton M, Soto J, et al. Cholic and deoxycholic acids induce mitochondrial dysfunction, impaired biogenesis and autophagic flux in skeletal muscle cells. Biol Res. 2023;56:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Li H, Shi J, Zhao L, Guan J, Liu F, Huo G, et al. Lactobacillus plantarum KLDS1.0344 and lactobacillus acidophilus KLDS1.0901 mixture prevents chronic alcoholic liver injury in mice by protecting the intestinal barrier and regulating gut microbiota and liver-related pathways. J Agric Food Chem. 2021;69:183–197. [DOI] [PubMed] [Google Scholar]
- 113. Yu JS, Youn GS, Choi J, Kim CH, Kim BY, Yang SJ, et al. Lactobacillus lactis and Pediococcus pentosaceus-driven reprogramming of gut microbiome and metabolome ameliorates the progression of non-alcoholic fatty liver disease. Clin Transl Med. 2021;11:e634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Zhou D, Pan Q, Xin FZ, Zhang RN, He CX, Chen GY, et al. Sodium butyrate attenuates high-fat diet-induced steatohepatitis in mice by improving gut microbiota and gastrointestinal barrier. World J Gastroenterol. 2017;23:60–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Fairfield B, Schnabl B. Gut dysbiosis as a driver in alcohol-induced liver injury. JHEP Rep. 2021;3:100220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Zhao T, Gu J, Zhang H, Wang Z, Zhang W, Zhao Y, et al. Sodium butyrate-modulated mitochondrial function in high-insulin induced HepG2 Cell dysfunction. Oxid Med Cell Longev. 2020;2020:1904609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Li W, Deng M, Gong J, Zhang X, Ge S, Zhao L. Sodium acetate inhibit TGF-β1-induced activation of hepatic stellate cells by restoring AMPK or c-Jun signaling. Front Nutr. 2021;8:729583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Wang X, Zhu M, Loor JJ, Jiang Q, Zhu Y, Li W, et al. Propionate alleviates fatty acid-induced mitochondrial dysfunction, oxidative stress, and apoptosis by upregulating PPARG coactivator 1 alpha in hepatocytes. J Dairy Sci. 2022;105:4581–4592. [DOI] [PubMed] [Google Scholar]
- 119. Deng M, Li X, Li W, Gong J, Zhang X, Ge S, et al. Short-chain fatty acids alleviate hepatocyte apoptosis induced by gut-derived protein-bound uremic toxins. Front Nutr. 2021;8:756730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Gao Z, Yin J, Zhang J, Ward RE, Martin RJ, Lefevre M, et al. Butyrate improves insulin sensitivity and increases energy expenditure in mice. Diabetes. 2009;58:1509–1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Lee TW, Liu HW, Lin YF, Lee TI, Kao YH, Chen YJ. Histone deacetylase inhibition improves metabolism and mitochondrial dynamics: A potential novel therapeutic strategy for sarcopenia coexisting with diabetes mellitus. Med Hypotheses. 2021;158:110724. [DOI] [PubMed] [Google Scholar]
- 122. Galmozzi A, Mitro N, Ferrari A, Gers E, Gilardi F, Godio C, et al. Inhibition of class I histone deacetylases unveils a mitochondrial signature and enhances oxidative metabolism in skeletal muscle and adipose tissue. Diabetes. 2013;62:732–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Eslick S, Williams EJ, Berthon BS, Wright T, Karihaloo C, Gately M, et al. Weight loss and short-chain fatty acids reduce systemic inflammation in monocytes and adipose tissue macrophages from obese subjects. Nutrients. 2022;14:765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. González-Bosch C, Boorman E, Zunszain PA, Mann GE. Short-chain fatty acids as modulators of redox signaling in health and disease. Redox Biol. 2021;47:102165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Syn WK, Teaberry V, Choi SS, Diehl AM. Similarities and differences in the pathogenesis of alcoholic and nonalcoholic steatohepatitis. Semin Liver Dis. 2009;29:200–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Zhu L, Baker SS, Gill C, Liu W, Alkhouri R, Baker RD, et al. Characterization of gut microbiomes in nonalcoholic steatohepatitis (NASH) patients: A connection between endogenous alcohol and NASH. Hepatology. 2013;57:601–609. [DOI] [PubMed] [Google Scholar]
- 127. Ruuskanen MO, Åberg F, Männistö V, Havulinna AS, Méric G, Liu Y, et al. Links between gut microbiome composition and fatty liver disease in a large population sample. Gut Microbes. 2021;13:1–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. de Medeiros IC, de Lima JG. Is nonalcoholic fatty liver disease an endogenous alcoholic fatty liver disease? - A mechanistic hypothesis. Med Hypotheses. 2015;85:148–152. [DOI] [PubMed] [Google Scholar]
- 129. Resta-Lenert S, Barrett KE. Enteroinvasive bacteria alter barrier and transport properties of human intestinal epithelium: Role of iNOS and COX-2. Gastroenterology. 2002;122:1070–1087. [DOI] [PubMed] [Google Scholar]
- 130. Abdelmegeed MA, Banerjee A, Jang S, Yoo SH, Yun JW, Gonzalez FJ, et al. CYP2E1 potentiates binge alcohol-induced gut leakiness, steatohepatitis, and apoptosis. Free Radic Biol Med. 2013;65:1238–1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Seitz HK, Bataller R, Cortez-Pinto H, Gao B, Gual A, Lackner C, et al. Alcoholic liver disease. Nat Rev Dis Primers. 2018;4:16. [DOI] [PubMed] [Google Scholar]
- 132. Yan T, Zhao Y. Acetaldehyde induces phosphorylation of dynamin-related protein 1 and mitochondrial dysfunction via elevating intracellular ROS and Ca(2+) levels. Redox Biol. 2020;28:101381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Zeng C, Chen M. Progress in nonalcoholic fatty liver disease: SIRT family regulates mitochondrial biogenesis. Biomolecules. 2022;12:1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Silva J, Spatz MH, Folk C, Chang A, Cadenas E, Liang J, et al. Dihydromyricetin improves mitochondrial outcomes in the liver of alcohol-fed mice via the AMPK/Sirt-1/PGC-1α signaling axis. Alcohol. 2021;91:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Fan H, Shen Y, Ren Y, Mou Q, Lin T, Zhu L, et al. Combined intake of blueberry juice and probiotics ameliorate mitochondrial dysfunction by activating SIRT1 in alcoholic fatty liver disease. Nutr Metab (Lond). 2021;18:50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Yang SJ, Choi JM, Kim L, Park SE, Rhee EJ, Lee WY, et al. Nicotinamide improves glucose metabolism and affects the hepatic NAD-sirtuin pathway in a rodent model of obesity and type 2 diabetes. J Nutr Biochem. 2014;25:66–72. [DOI] [PubMed] [Google Scholar]
- 137. Covarrubias AJ, Perrone R, Grozio A, Verdin E. NAD(+) metabolism and its roles in cellular processes during ageing. Nat Rev Mol Cell Biol. 2021;22:119–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Rajman L, Chwalek K, Sinclair DA. Therapeutic potential of NAD-boosting molecules: The in vivo evidence. Cell Metab. 2018;27:529–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Magnúsdóttir S, Ravcheev D, de Crécy-Lagard V, Thiele I. Systematic genome assessment of B-vitamin biosynthesis suggests co-operation among gut microbes. Front Genet. 2015;6:148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Ge H, Wei W, Tang L, Tian Y, Zhu Y, Luo Y, et al. CONSORT-Characteristics and metabolic phenotype of gut microbiota in NAFLD patients. Medicine (Baltimore). 2022;101:e29347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Liu LW, Xie Y, Li GQ, Zhang T, Sui YH, Zhao ZJ, et al. Gut microbiota-derived nicotinamide mononucleotide alleviates acute pancreatitis by activating pancreatic SIRT3 signalling. Br J Pharmacol. 2023;180:647–666. [DOI] [PubMed] [Google Scholar]
- 142. Feng S, Guo L, Wang H, Yang S, Liu H. Bacterial PncA improves diet-induced NAFLD in mice by enabling the transition from nicotinamide to nicotinic acid. Commun Biol. 2023;6:235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Lu X, Yang R, Chen Y, Chen D. NAD metabolic therapy in metabolic dysfunction-associated steatotic liver disease: Possible roles of gut microbiota. iScience. 2024;27:109174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Kim LJ, Chalmers TJ, Madawala R, Smith GC, Li C, Das A, et al. Host-microbiome interactions in nicotinamide mononucleotide (NMN) deamidation. FEBS Lett. 2023;597:2196–2220. [DOI] [PubMed] [Google Scholar]
- 145. Shats I, Williams JG, Liu J, Makarov MV, Wu X, Lih FB, et al. Bacteria boost mammalian host NAD metabolism by engaging the deamidated biosynthesis pathway. Cell Metab. 2020;31:564–579.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Shen C, Dou X, Ma Y, Ma W, Li S, Song Z. Nicotinamide protects hepatocytes against palmitate-induced lipotoxicity via SIRT1-dependent autophagy induction. Nutr Res. 2017;40:40–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Cantó C, Menzies KJ, Auwerx J. NAD(+) Metabolism and the control of energy homeostasis: A balancing act between mitochondria and the nucleus. Cell Metab. 2015;22:31–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Katsyuba E, Mottis A, Zietak M, De Franco F, van der Velpen V, Gariani K, et al. De novo NAD(+) synthesis enhances mitochondrial function and improves health. Nature. 2018;563:354–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Dall M, Hassing AS, Treebak JT. NAD(+) and NAFLD - caution, causality and careful optimism. J Physiol. 2022;600:1135–1154. [DOI] [PubMed] [Google Scholar]
- 150. Okabe K, Yaku K, Tobe K, Nakagawa T. Implications of altered NAD metabolism in metabolic disorders. J Biomed Sci. 2019;26:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Li DJ, Sun SJ, Fu JT, Ouyang SX, Zhao QJ, Su L, et al. NAD(+)-boosting therapy alleviates nonalcoholic fatty liver disease via stimulating a novel exerkine Fndc5/irisin. Theranostics. 2021;11:4381–4402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Lee S, Seok BG, Lee SJ, Chung SW. Inhibition of mitoNEET attenuates LPS-induced inflammation and oxidative stress. Cell Death Dis. 2022;13:127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Carpino G, Del Ben M, Pastori D, Carnevale R, Baratta F, Overi D, et al. Increased liver localization of lipopolysaccharides in human and experimental NAFLD. Hepatology. 2020;72:470–485. [DOI] [PubMed] [Google Scholar]
- 154. Maciejewska D, Łukomska A, Dec K, Skonieczna-Żydecka K, Gutowska I, Skórka-Majewicz M, et al. Diet-induced rat model of gradual development of non-alcoholic fatty liver disease (NAFLD) with lipopolysaccharides (LPS) secretion. Diagnostics (Basel). 2019;9:205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Hegazy MA, Mogawer SM, Alnaggar A, Ghoniem OA, Abdel Samie RM. Serum LPS and CD163 biomarkers confirming the role of gut dysbiosis in overweight patients with NASH. Diabetes Metab Syndr Obes. 2020;13:3861–3872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Wang Y, Zhang Y, Yang J, Li H, Wang J, Geng W. Lactobacillus plantarum MA2 ameliorates methionine and choline-deficient diet induced non-alcoholic fatty liver disease in rats by improving the intestinal microecology and mucosal barrier. Foods. 2021;10:3126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Li Y, Xu YJ, Tan CP, Liu Y. Sinapine improves LPS-induced oxidative stress in hepatocytes by down-regulating MCJ protein expression. Life Sci. 2022;306:120828. [DOI] [PubMed] [Google Scholar]
- 158. Shi W, An L, Zhang J, Li J. Periplaneta americana extract ameliorates lipopolysaccharide-induced liver injury by improving mitochondrial dysfunction via the AMPK/PGC-1α signaling pathway. Exp Ther Med. 2021;22:1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Liu Z, Guo J, Sun H, Huang Y, Zhao R, Yang X. α-Lipoic acid attenuates LPS-induced liver injury by improving mitochondrial function in association with GR mitochondrial DNA occupancy. Biochimie. 2015;116:52–60. [DOI] [PubMed] [Google Scholar]
- 160. Wang PF, Xie K, Cao YX, Zhang A. Hepatoprotective effect of mitochondria-targeted antioxidant mito-TEMPO against lipopolysaccharide-induced liver injury in mouse. Mediators Inflamm. 2022;2022:6394199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Hu W, Yan G, Ding Q, Cai J, Zhang Z, Zhao Z, et al. Update of indoles: Promising molecules for ameliorating metabolic diseases. Biomed Pharmacother. 2022;150:112957. [DOI] [PubMed] [Google Scholar]
- 162. Hubbard TD, Murray IA, Perdew GH. Indole and tryptophan metabolism: Endogenous and dietary routes to Ah receptor activation. Drug Metab Dispos. 2015;43:1522–1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Natividad JM, Agus A, Planchais J, Lamas B, Jarry AC, Martin R, et al. Impaired aryl hydrocarbon receptor ligand production by the gut microbiota is a key factor in metabolic syndrome. Cell Metab. 2018;28:737–749.e4. [DOI] [PubMed] [Google Scholar]
- 164. Hubbard TD, Murray IA, Bisson WH, Lahoti TS, Gowda K, Amin SG, et al. Adaptation of the human aryl hydrocarbon receptor to sense microbiota-derived indoles. Sci Rep. 2015;5:12689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Jin UH, Lee SO, Sridharan G, Lee K, Davidson LA, Jayaraman A, et al. Microbiome-derived tryptophan metabolites and their aryl hydrocarbon receptor-dependent agonist and antagonist activities. Mol Pharmacol. 2014;85:777–788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Sridharan GV, Choi K, Klemashevich C, Wu C, Prabakaran D, Pan LB, et al. Prediction and quantification of bioactive microbiota metabolites in the mouse gut. Nat Commun. 2014;5:5492. [DOI] [PubMed] [Google Scholar]
- 167. Krishnan S, Ding Y, Saedi N, Choi M, Sridharan GV, Sherr DH, et al. Gut Microbiota-derived tryptophan metabolites modulate inflammatory response in hepatocytes and macrophages. Cell Rep. 2018;23:1099–1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Stockinger B, Di Meglio P, Gialitakis M, Duarte JH. The aryl hydrocarbon receptor: Multitasking in the immune system. Annu Rev Immunol. 2014;32:403–432. [DOI] [PubMed] [Google Scholar]
- 169. Dong F, Hao F, Murray IA, Smith PB, Koo I, Tindall AM, et al. Intestinal microbiota-derived tryptophan metabolites are predictive of Ah receptor activity. Gut Microbes. 2020;12:1–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Min BH, Devi S, Kwon GH, Gupta H, Jeong JJ, Sharma SP, et al. Gut microbiota-derived indole compounds attenuate metabolic dysfunction-associated steatotic liver disease by improving fat metabolism and inflammation. Gut Microbes. 2024;16:2307568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Ji Y, Gao Y, Chen H, Yin Y, Zhang W. Indole-3-acetic acid alleviates nonalcoholic fatty liver disease in mice via attenuation of hepatic lipogenesis, and oxidative and inflammatory stress. Nutrients. 2019;11:2062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Kim D, Kim H, Kim K, Roh S. The protective effect of indole-3-acetic acid (IAA) on H(2)O(2)-damaged human dental pulp stem cells is mediated by the AKT pathway and involves increased expression of the transcription factor nuclear factor-erythroid 2-related factor 2 (Nrf2) and its downstream target heme oxygenase 1 (HO-1). Oxid Med Cell Longev. 2017;2017:8639485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Huang X, Qiu Y, Gao Y, Zhou R, Hu Q, He Z, et al. Gut microbiota mediate melatonin signalling in association with type 2 diabetes. Diabetologia. 2022;65:1627–1641. [DOI] [PubMed] [Google Scholar]
- 174. Fernández-Ortiz M, Sayed RKA, Fernández-Martínez J, Cionfrini A, Aranda-Martínez P, Escames G, et al. Melatonin/Nrf2/NLRP3 connection in mouse heart mitochondria during aging. Antioxidants (Basel). 2020;9:1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Zhang H, Yan A, Liu X, Ma Y, Zhao F, Wang M, et al. Melatonin ameliorates ochratoxin A induced liver inflammation, oxidative stress and mitophagy in mice involving in intestinal microbiota and restoring the intestinal barrier function. J Hazard Mater. 2021;407:124489. [DOI] [PubMed] [Google Scholar]
- 176. Barrea L, Annunziata G, Muscogiuri G, Di Somma C, Laudisio D, Maisto M, et al. Trimethylamine-N-oxide (TMAO) as novel potential biomarker of early predictors of metabolic syndrome. Nutrients. 2018;10:1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Sherriff JL, O’Sullivan TA, Properzi C, Oddo JL, Adams LA. Choline, Its potential role in nonalcoholic fatty liver disease, and the case for human and bacterial genes. Adv Nutr. 2016;7:5–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Yoo W, Zieba JK, Foegeding NJ, Torres TP, Shelton CD, Shealy NG, et al. High-fat diet-induced colonocyte dysfunction escalates microbiota-derived trimethylamine N-oxide. Science. 2021;373:813–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179. Tan X, Liu Y, Long J, Chen S, Liao G, Wu S, et al. Trimethylamine N-Oxide aggravates liver steatosis through modulation of bile acid metabolism and inhibition of farnesoid X receptor signaling in nonalcoholic fatty liver disease. Mol Nutr Food Res. 2019;63:e1900257. [DOI] [PubMed] [Google Scholar]
- 180. Hu Y, Zhao Y, Yuan L, Yang X. Protective effects of tartary buckwheat flavonoids on high TMAO diet-induced vascular dysfunction and liver injury in mice. Food Funct. 2015;6:3359–3372. [DOI] [PubMed] [Google Scholar]
- 181. Byndloss MX, Olsan EE, Rivera-Chávez F, Tiffany CR, Cevallos SA, Lokken KL, et al. Microbiota-activated PPAR-γ signaling inhibits dysbiotic Enterobacteriaceae expansion. Science. 2017;357:570–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182. Mills EL, Kelly B, Logan A, Costa A, Varma M, Bryant CE, et al. Succinate dehydrogenase supports metabolic repurposing of mitochondria to drive inflammatory macrophages. Cell. 2016;167:457–470.e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183. Wu P, Chen J, Chen J, Tao J, Wu S, Xu G, et al. Trimethylamine N-oxide promotes apoE(-/-) mice atherosclerosis by inducing vascular endothelial cell pyroptosis via the SDHB/ROS pathway. J Cell Physiol. 2020;235:6582–6591. [DOI] [PubMed] [Google Scholar]
- 184. Makrecka-Kuka M, Volska K, Antone U, Vilskersts R, Grinberga S, Bandere D, et al. Trimethylamine N-oxide impairs pyruvate and fatty acid oxidation in cardiac mitochondria. Toxicol Lett. 2017;267:32–38. [DOI] [PubMed] [Google Scholar]
- 185. Wang G, Jiao T, Xu Y, Li D, Si Q, Hao J, et al. Bifidobacterium adolescentis and Lactobacillus rhamnosus alleviate non-alcoholic fatty liver disease induced by a high-fat, high-cholesterol diet through modulation of different gut microbiota-dependent pathways. Food Funct. 2020;11:6115–6127. [DOI] [PubMed] [Google Scholar]
- 186. Zhao D, Shah NP. Synergistic application of black tea extracts and lactic acid bacteria in protecting human colonocytes against oxidative damage. J Agric Food Chem. 2016;64:2238–2246. [DOI] [PubMed] [Google Scholar]
- 187. Kim SJ, Choi SI, Jang M, Jeong YA, Kang CH, Kim GH. Combination of Limosilactobacillus fermentum MG4231 and MG4244 attenuates lipid accumulation in high-fat diet-fed obese mice. Benef Microbes. 2021;12:479–491. [DOI] [PubMed] [Google Scholar]
- 188. Huo Y, Lu X, Wang X, Wang X, Chen L, Guo H, et al. Bifidobacterium animalis subsp. lactis A6 alleviates obesity associated with promoting mitochondrial biogenesis and function of adipose tissue in mice. Molecules. 2020;25:1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189. Gu Y, Xiao X, Pan R, Zhang J, Zhao Y, Dong Y, et al. Lactobacillus plantarum dy-1 fermented barley extraction activates white adipocyte browning in high-fat diet-induced obese rats. J Food Biochem. 2021;45:e13680. [DOI] [PubMed] [Google Scholar]
- 190. Xiao X, Bai J, Li MS, Zhang JY, Sun XJ, Dong Y. Supplementation of fermented barley extracts with Lactobacillus Plantarum dy-1 inhibits obesity via a UCP1-dependent mechanism. Biomed Environ Sci. 2019;32:578–591. [DOI] [PubMed] [Google Scholar]
- 191. Gu Y, Bai J, Zhang J, Zhao Y, Pan R, Dong Y, et al. Transcriptomics reveals the anti-obesity mechanism of Lactobacillus plantarum fermented barley extract. Food Res Int. 2022;157:111285. [DOI] [PubMed] [Google Scholar]
- 192. Kobyliak N, Abenavoli L, Mykhalchyshyn G, Kononenko L, Boccuto L, Kyriienko D, et al. A multi-strain probiotic reduces the fatty liver index, cytokines and aminotransferase levels in NAFLD patients: Evidence from a randomized clinical trial. J Gastrointestin Liver Dis. 2018;27:41–49. [DOI] [PubMed] [Google Scholar]
- 193. Kobyliak N, Abenavoli L, Falalyeyeva T, Mykhalchyshyn G, Boccuto L, Kononenko L, et al. Beneficial effects of probiotic combination with omega-3 fatty acids in NAFLD: A randomized clinical study. Minerva Med. 2018;109:418–428. [DOI] [PubMed] [Google Scholar]
- 194. Mohamad Nor MH, Ayob N, Mokhtar NM, Raja Ali RA, Tan GC, Wong Z, et al. The effect of probiotics (MCP(®) BCMC(®) Strains) on hepatic steatosis, small intestinal mucosal immune function, and intestinal barrier in patients with non-alcoholic fatty liver disease. Nutrients. 2021;13:3192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195. Stachowska E, Portincasa P, Jamioł-Milc D, Maciejewska-Markiewicz D, Skonieczna-Żydecka K. The relationship between prebiotic supplementation and anthropometric and biochemical parameters in patients with NAFLD-A systematic review and meta-analysis of randomized controlled trials. Nutrients. 2020;12:3460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196. Li Z, Zhang H, Li Y, Chen H, Wang C, Wong V, et al. Phytotherapy using blueberry leaf polyphenols to alleviate non-alcoholic fatty liver disease through improving mitochondrial function and oxidative defense. Phytomedicine. 2020;69:153209. [DOI] [PubMed] [Google Scholar]
- 197. Rafiei H, Omidian K, Bandy B. Dietary polyphenols protect against oleic acid-induced steatosis in an in vitro model of NAFLD by modulating lipid metabolism and improving mitochondrial function. Nutrients. 2019;11:541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198. Badolati N, Masselli R, Sommella E, Sagliocchi S, Di Minno A, Salviati E, et al. The hepatoprotective effect of taurisolo, a nutraceutical enriched in resveratrol and polyphenols, involves activation of mitochondrial metabolism in mice liver. Antioxidants (Basel). 2020;9:410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199. Sun M, Gu Y, Glisan SL, Lambert JD. Dietary cocoa ameliorates non-alcoholic fatty liver disease and increases markers of antioxidant response and mitochondrial biogenesis in high fat-fed mice. J Nutr Biochem. 2021;92:108618. [DOI] [PubMed] [Google Scholar]
- 200. Zhang F, Zhang X, Gu Y, Wang M, Guo S, Liu J, et al. Hepatoprotection of lycii fructus polysaccharide against oxidative stress in hepatocytes and larval zebrafish. Oxid Med Cell Longev. 2021;2021:3923625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201. Zhang Z, Chen X, Cui B. Modulation of the fecal microbiome and metabolome by resistant dextrin ameliorates hepatic steatosis and mitochondrial abnormalities in mice. Food Funct. 2021;12:4504–4518. [DOI] [PubMed] [Google Scholar]
- 202. Kavyani M, Saleh-Ghadimi S, Dehghan P, Abbasalizad Farhangi M, Khoshbaten M. Co-supplementation of camelina oil and a prebiotic is more effective for in improving cardiometabolic risk factors and mental health in patients with NAFLD: A randomized clinical trial. Food Funct. 2021;12:8594–8604. [DOI] [PubMed] [Google Scholar]
- 203. Chambers ES, Viardot A, Psichas A, Morrison DJ, Murphy KG, Zac-Varghese SE, et al. Effects of targeted delivery of propionate to the human colon on appetite regulation, body weight maintenance and adiposity in overweight adults. Gut. 2015;64:1744–1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204. Chambers ES, Byrne CS, Rugyendo A, Morrison DJ, Preston T, Tedford C, et al. The effects of dietary supplementation with inulin and inulin-propionate ester on hepatic steatosis in adults with non-alcoholic fatty liver disease. Diabetes Obes Metab. 2019;21:372–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205. Brandt A, Jin CJ, Nolte K, Sellmann C, Engstler AJ, Bergheim I. Short-term intake of a fructose-, fat- and cholesterol-rich diet causes hepatic steatosis in mice: Effect of antibiotic treatment. Nutrients. 2017;9:1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206. Abdel-Razik A, Mousa N, Shabana W, Refaey M, Elzehery R, Elhelaly R, et al. Rifaximin in nonalcoholic fatty liver disease: Hit multiple targets with a single shot. Eur J Gastroenterol Hepatol. 2018;30:1237–1246. [DOI] [PubMed] [Google Scholar]
- 207. Jian J, Nie MT, Xiang B, Qian H, Yin C, Zhang X, et al. Rifaximin ameliorates non-alcoholic steatohepatitis in mice through regulating gut microbiome-related bile acids. Front Pharmacol. 2022;13:841132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208. Cobbold J, Atkinson S, Marchesi JR, Smith A, Wai SN, Stove J, et al. Rifaximin in non-alcoholic steatohepatitis: An open-label pilot study. Hepatol Res. 2018;48:69–77. [DOI] [PubMed] [Google Scholar]
- 209. Chong C, Orr D, Plank LD, Vatanen T, O’Sullivan JM, Murphy R. Randomised double-blind placebo-controlled trial of inulin with metronidazole in non-alcoholic fatty liver disease (NAFLD). Nutrients. 2020;12:937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210. Scott NA, Andrusaite A, Andersen P, Lawson M, Alcon-Giner C, Leclaire C, et al. Antibiotics induce sustained dysregulation of intestinal T cell immunity by perturbing macrophage homeostasis. Sci Transl Med. 2018;10:eaao4755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211. Delaune V, Orci LA, Lacotte S, Peloso A, Schrenzel J, Lazarevic V, et al. Fecal microbiota transplantation: A promising strategy in preventing the progression of non-alcoholic steatohepatitis and improving the anti-cancer immune response. Expert Opin Biol Ther. 2018;18:1061–1071. [DOI] [PubMed] [Google Scholar]
- 212. Mocanu V, Zhang Z, Deehan EC, Kao DH, Hotte N, Karmali S, et al. Fecal microbial transplantation and fiber supplementation in patients with severe obesity and metabolic syndrome: A randomized double-blind, placebo-controlled phase 2 trial. Nat Med. 2021;27:1272–1279. [DOI] [PubMed] [Google Scholar]
- 213. Huang W, Kong D. The intestinal microbiota as a therapeutic target in the treatment of NAFLD and ALD. Biomed Pharmacother. 2021;135:111235. [DOI] [PubMed] [Google Scholar]
- 214. Wang Y, Yang Z, Tang H, Sun X, Qu J, Lu S, et al. Faecal microbiota transplantation is better than probiotics for tissue regeneration of type 2 diabetes mellitus injuries in mice. Arch Physiol Biochem. 2024;130:333–341. [DOI] [PubMed] [Google Scholar]
- 215. Burz SD, Monnoye M, Philippe C, Farin W, Ratziu V, Strozzi F, et al. Fecal microbiota transplant from human to mice gives insights into the role of the gut microbiota in non-alcoholic fatty liver disease (NAFLD). Microorganisms. 2021;9:199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216. Xue L, Deng Z, Luo W, He X, Chen Y. Effect of fecal microbiota transplantation on non-alcoholic fatty liver disease: A randomized clinical trial. Front Cell Infect Microbiol. 2022;12:759306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217. Craven L, Rahman A, Nair Parvathy S, Beaton M, Silverman J, Qumosani K, et al. Allogenic fecal microbiota transplantation in patients with nonalcoholic fatty liver disease improves abnormal small intestinal permeability: A randomized control trial. Am J Gastroenterol. 2020;115:1055–1065. [DOI] [PubMed] [Google Scholar]
- 218. Ng SC, Xu Z, Mak J, Yang K, Liu Q, Zuo T, et al. Microbiota engraftment after faecal microbiota transplantation in obese subjects with type 2 diabetes: A 24-week, double-blind, randomised controlled trial. Gut. 2022;71:716–723. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.