Abstract
Excessive exposure to ultraviolet radiation (UVR) causes harmful effects on human skin. Pre‐exposure application of sunscreen can be protective, but not after damage already has occurred. There is a need for agents that can be applied post‐UVR exposure to repair the damage. We investigated a novel compound, NEO400, that appears to meet this medicinal need. NEO400 was created by conjugating linoleic acid to perillyl alcohol. UVR was repeatedly administered to the skin of mice over several weeks, where it caused the typical signs of UV damage, including scaling of the skin, DNA damage, and elevated levels of inflammatory cytokines. However, when NEO400 was applied immediately post‐UVR, it triggered the appearance of markers for dermal stem cell proliferation, and no signs of skin damage emerged. Furthermore, when NEO400 was applied to skin that already had incurred significant damage, it accelerated skin healing. When applied individually, linoleic acid and perillyl alcohol were ineffective, indicating that they had to be conjugated in order to exert therapeutic efficacy. None of these skin‐protective effects could be achieved with Aloe vera gel, a popular and widely used post‐exposure remedy. Our study suggests that NEO400 holds potential as a regenerative treatment for excessively UVR‐exposed skin.
Keywords: Aloe vera, linoleic acid, perillyl alcohol, skin damage, UV radiation
In the present work with mouse models, we demonstrate that a novel compound, NEO400, is able to profoundly protect skin against damage caused by UV radiation (UVR) when it is applied to skin post‐UVR exposure. In comparison, Aloe vera or linoleic acid are unable to achieve a similar level of protection. NEO400 was created by covalently conjugating linoleic acid to perillyl alcohol, a naturally occurring monoterpene. Our study introduces NEO400 as an effective skin protection remedy that can be applied to skin after UVR exposure already has occurred.

Abbreviations
- LA
linoleic acid
- NEO400
perillyl alcohol covalently linked to linoleic acid
- POH
perillyl alcohol
- UVR
ultraviolet radiation
INTRODUCTION
Moderate exposure of human skin to ultraviolet radiation (UVR) triggers certain health benefits, most notably pre‐vitamin D3 production. However, excessive exposure is linked to a variety of harmful effects, such as accelerated skin aging (photoaging) and the promotion of skin malignancies, including melanoma and nonmelanoma skin cancer. 1 , 2 From a clinical point of view, the visible acute response of skin to excessive UVR is erythema, an inflammatory reaction characterized by redness, warmth, and tenderness. It involves inflammatory cytokines such as interleukin 1 (IL‐1), IL‐3, IL‐6, tumor necrosis factor alpha (TNF‐α), and granulocyte/macrophage colony‐stimulating factor. Inflammation triggers a cascade of cytokines that modulate the immune system, including IL‐4, IL‐10, and prostaglandin E2 (PGE2), which in turn modulate the systemic immune responses through regulatory T‐cells. Nuclear factor kappa B (NF‐κB) activation induces the expression of cyclooxygenase‐2 (COX‐2), an inflammatory enzyme that converts arachidonic acid into prostaglandins. PGE2 leads to pain and edema as a result of local vasodilation from epidermal cells. 3 , 4
UVR exposure can damage the DNA through the formation of cyclobutane pyrimidine dimers (CPD) and 6‐4 photoproducts (6‐4 PP). Additional DNA damage can result as a consequence of the formation of reactive oxygen species (ROS), which can interact with several cellular components, leading to lipid peroxidation, protein carbonyl formation, and oxidative DNA damage. 2 , 3
Human skin possesses several protective mechanisms against UVR, which are designed to cope with the adverse effects and prevent further damage. The first line of defense is melanin, which dissipates UVR as heat. Another defense is presented by epidermal hyperplasia. At the molecular level, DNA repair mechanisms and activation of antioxidant pathways contribute to cytoprotection and cell survival, although in cases of excessive damage, pro‐apoptotic signaling dominates the response and leads to cell death. 2 , 3 Depending on the intensity of the UVR exposure and the extent of subsequent skin damage, it may become necessary to apply external interventions to support the natural physiological skin responses in order to strengthen recovery and return to homeostasis. This may involve the topical application of pharmacological compounds or natural substances. Among the topicals of choice are non‐steroidal anti‐inflammatory drugs to reduce pain and inflammation, although more commonly cooling and hydrating gels and creams are used to provide soothing comfort by helping the skin to lock in moisture.
Aloe vera gels, creams, and lotions are popular and widely used as remedies for sunburn and some other skin conditions. 5 , 6 , 7 However, while they contain moisturizing and anti‐inflammatory properties, 6 , 8 , 9 which provide rapid, short‐term relief to sunburned individuals, their impact on the actual skin healing process has not been clearly established. A 2014 review 7 of 18 clinical trials on the use of Aloe vera for burns, psoriasis, and other skin conditions found evidence that individuals with mild burns might heal faster, as compared to topical antibiotics. However, another study 10 with 20 sunburned participants found that applying Aloe vera cream twice a day for 3 weeks was not effective at healing such burns.
Beyond Aloe vera, variety of other natural compounds have been investigated for their potentially beneficial effects on the repair and healing of damaged skin. 11 , 12 For example, topical administration of d‐limonene revealed positive effects on wound healing in mice, which appeared mediated by its major metabolite, perillyl alcohol (POH). 13 POH is a natural monocyclic terpene derived from limonene and the mevalonate pathway in certain plants, such as citrus, peppermint, lavender and lilac oils, sage, cherries, and others. 14 Clinical studies in human cancer patients provided evidence that it might harbor anticancer activity. 15 Many terpenes have been shown to function as penetration enhancers (PEs), and POH in particular has displayed the ability to enhance the uptake and delivery of a number of functional drugs. 16 , 17 As natural compounds, terpenes are believed to possess greater safety profiles as compared to currently utilized PEs, which include surfactants, fatty acids/esters, and solvents. 17
Linoleic acid (LA) has a physiological role in maintaining the water permeability barrier of the skin as a constituent of acylglycosyl ceramides. Besides its structural role among the polyunsaturated fatty acids in cell membranes, LA gives rise to arachidonic acid, which is the major precursor of bioactive eicosanoids, which regulate a large number of physiological processes. LA deficiency, which presents as a scaly dermatitis and an impaired immune response, can be cured and prevented through the dietary intake of small amounts of LA or even simple topical application of LA to the affected area of skin. 18 , 19
Our group has created a novel molecule, termed NEO400, where POH was covalently conjugated to LA. We hypothesized that NEO400 would combine the most advantageous features of each partner component into a single entity. In the current study, we present evidence that this novel molecule is potently able to prevent and repair UVR‐induced skin damage, even when given post radiation, through a multi‐factorial mechanism involving DNA damage repair, inhibition of inflammatory cytokine production, and stimulation of dermal stem cell activity.
MATERIALS AND METHODS
Reagents
NEO400 was manufactured by Norac Pharma and kindly provided by NeOnc Technologies Inc. Immediately before use, it was suspended in glycerol: ethanol (90:10, vol/vol) to a final concentration of 10 mM (4.3 mg/mL) and applied to the skin of mice in a volume of 50 μL. POH and LA were purchased from Sigma‐Aldrich and were diluted with glycerol: ethanol solution (90:10, vol/vol) to a concentration of 13 and 7 mM, respectively. Aloe vera was purchased as a gel (100% Aloe Vera Gel, fragrance‐free, no color added, from Fruit of the Earth, Inc.), which was used straight from the tube following manufacturer's instructions to apply liberally to the sunburned or irritated skin. Sunscreen was commercially available SPF15 sunblock in the form of wipes for dogs (Well & Good, Petco), which was gently wiped onto the UVR‐exposed area.
Source of UVR
The source of UVR was a short‐wavelength UV lamp (VWR). The peak spectral output of this lamp was approximately 310 nm, with no energy detectable below 260 nm, approximately 0.6% between 260 and 280 nm (UV‐C), 72.7% between 280 and 320 nm (UV‐B), and 26.7% between 320 and 400 nm (UV‐A). A progressive UV exposure regimen was used, based on a protocol by Moloney and coworkers 20 with modifications by Oba and Edwards, 21 where radiation dosage of UV‐B per exposure was set at 54 mJ/cm2 for the first week, 72 mJ/cm2 for the second week, 90 mJ/cm2 for the third week, and 108 mJ/cm2 for the remaining weeks. The dosages were confirmed with a digital UVA/UVB meter (General Tools).
Animal model
All animal protocols were approved by the Institutional Animal Care and Use Committee (IACUC) of the University of Southern California (USC) and were conducted according to the NIH Guide for the Care and Use of Laboratory Animals. Female SKH1 Elite mice, a naturally hairless, immunocompetent mouse strain, was obtained from Charles River Laboratories. We decided to use female mice because male mice have a tendency to fight while in their cages; we wanted to keep extraneous skin damage to a minimum. For UVR exposure, the dosage per irradiation was set as described above and was delivered to the back of the animals. For post‐irradiation treatment, vehicle or test substances were applied to the same area of the back immediately following the UVR exposure. Each treatment schedule was applied to a group of three mice (n = 3). Photos of all animals were taken at different times to document the extent of skin damage and the healing process.
Quantifying skin damage
The extent of skin damage in response to the various treatment conditions was assessed by applying a semi‐quantitative skin damage score. 22 The range of this score was from 0 (no detectable changes in the appearance of the skin, as compared to untreated animals) to a maximum score of 4 (very severe skin damage). It was obtained by multiplying the severity of signs of injury (erythema, roughness, scaliness, denudation, erosion, ulceration, and scarring on a scale from 0 to 4) with the percent of the respectively affected skin area; sub‐areas from each animal were added to reach 100%.
Measuring cytokine levels
Levels of IL‐1β, IL‐6 and TNF‐α were measured in mouse plasma following UV exposure and treatment. Briefly, blood samples were taken from each mouse 4 h after the last cutaneous treatment. About 0.5 mL of blood was collected by cardiac puncture and placed into 0.4% sodium citrate, mixed well and then centrifuged at 1500 g for 15 min. Plasma was aliquoted in polypropylene tubes, frozen at −20°C and stored at −80°C before cytokine analyses. After thawing of all the serum samples, levels of IL‐1β, IL‐6, and TNF‐α were assayed simultaneously using DuoSet ELISA kits (R&D Systems) following manufacturer's instructions. Assays were performed in triplicate.
Measuring markers of DNA damage
Skin tissues were collected from the back of euthanized animals, fixed in formalin, and processed to detect the presence of cyclobutane pyrimidine dimers (CPD) and pyrimidine (6‐4) pyrimidone photoproducts (6‐4 PP), which represent two predominant types of UV‐induced DNA lesions. DNA was isolated, heat denatured, and absorbed onto a 96‐well DNA high‐binding plate, followed by probing with anti‐CPD or 6‐4 PP antibodies and detection with an HRP conjugated secondary antibody. The absorbance was determined and quantified against a pre‐determined standard run in parallel.
Analysis of stem cell activity
Markers of dermal stem cell activity were evaluated following 5 weeks of UVR exposure and different treatments. Skin tissue was harvested from euthanized mice at 24 h following the final exposure and treatment. Samples were embedded into OCT molds and immediately frozen. Sections were cut at 10 μm thickness and stained using standard techniques with antibodies to follistatin and integrin α6, which represent documented markers of skin stem cells. Antibodies were obtained from R&D Systems (Minneapolis, MN; catalog numbers: AF669‐SP and MAB13501‐SP, respectively). Slides were counter‐stained with hematoxylin.
Statistical analysis
All parametric data were analyzed using the Student t‐test to calculate the significance values. A probability value (p) <0.05 was considered statistically significant.
RESULTS
NEO400 prevents overt UV‐induced skin damage
NEO400 was generated by covalently conjugating POH to LA (see chemical structure in Figure 1). To investigate its potential protective effects against UVR‐induced skin damage, we used SKH1 Elite mice as a model. These immunocompetent mice are commonly used for this purpose, because their hairless status allows for convenient monitoring of skin effects. 23 For all treatment conditions, three mice per group (n = 3) were used. The backs of these mice were exposed to a 10‐min bout of UVR three times a week, for a total of 5 weeks (see outline of Schedule A in Figure 2A). Immediately following each irradiation, we topically applied NEO400 to the same skin area. Control mice received sunscreen, POH, LA, Aloe vera, or vehicle only treatment. Photos of mice were taken before any treatment and again at the completion of all treatments after 5 weeks. As presented in Figure 3A, repeated UVR exposure resulted in pronounced skin damage, characterized by erythema, scaly rash, and dry patches. In stark contrast, all animals that had received NEO400 remained clear of these skin lesions and presented with healthy‐looking, supple skin that closely resembled the normal skin of animals before any UVR exposure. In comparison, the skin of animals that had received sunscreen, Aloe vera, POH, or LA showed the same type of skin damage as UVR‐treated animals without any topical treatment. To quantify the extent of skin damage and protection, we applied a score that took into account the surface area and the severity of skin damage in each animal. As emphasized in Figure 3B, only NEO400 resulted in skin protection, and this effect was statistically highly significant p < 0.001. Together, these results indicated a protective effect of NEO400 that could not be mimicked by sunscreen, Aloe vera, or by the individual constituents of NEO400, POH, or LA.
FIGURE 1.
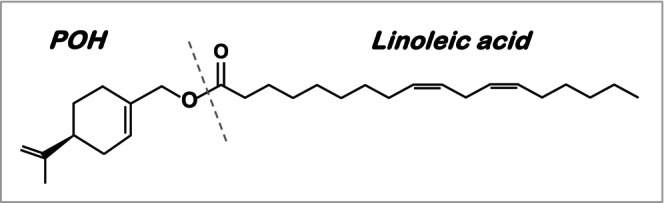
Chemical structure of NEO400. Perillyl alcohol was covalently conjugated to linoleic acid to create NEO400 (Synthesis, validation, and quality control of NEO400 was performed by Norac Pharma).
FIGURE 2.
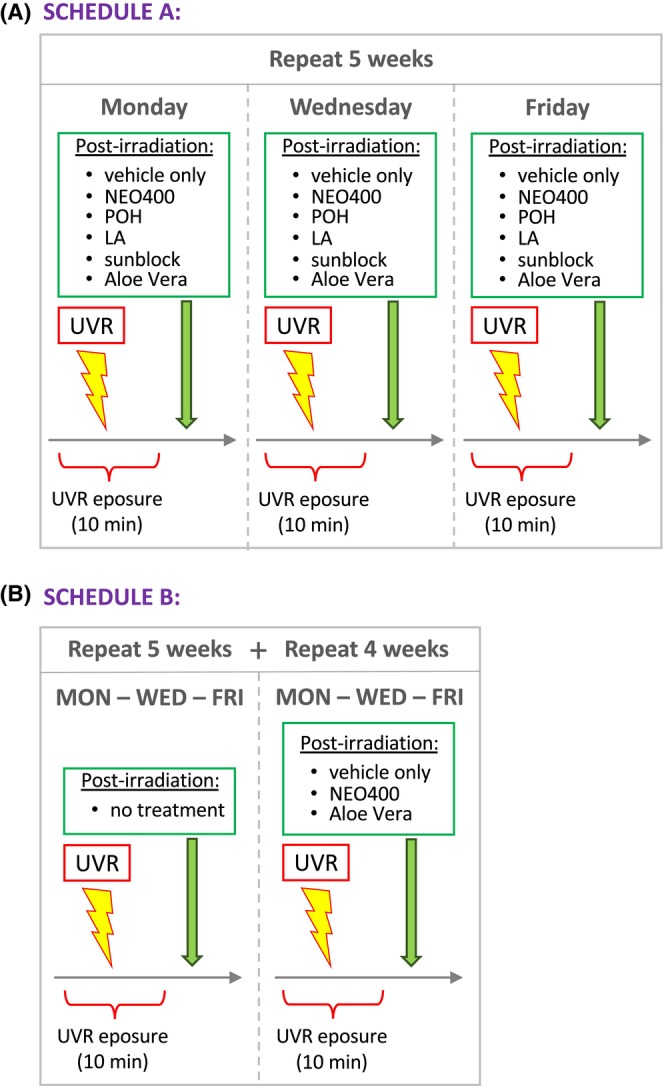
Outline of treatment schedules. In all cases, mice received 3× weekly treatments, similar to schedules previously established by other groups. 20 , 21 For Schedule A, treatment was performed for a total of five consecutive weeks. Exposure to UV light (of the back of the animal) was for 10 min in each session and was followed either by no additional treatment (or vehicle only) or by topical application of POH, LA, Aloe vera, sunblock, or NEO400. At the end of this 5‐week regimen, mice were evaluated and biological responses investigated. For Schedule B, mice received the same 3× per week UVR treatment as in Schedule A, but without any topical applications during the first five consecutive weeks. Thereafter, another 4 weeks of 3× per week UVR was added, but this time each UVR exposure was followed by topical treatment with NEO400, Aloe vera, or vehicle only. In both schedules, each individual treatment was applied to three mice (n = 3) in parallel.
FIGURE 3.

Macroscopic appearance of UVR‐exposed skin. (A) A total of 18 mice were separated into six treatment groups, where each UVR exposure was immediately followed by topical treatment over the course of 5 weeks (as per Schedule A). For each group of three mice each, only one representative animal is shown before the onset of any treatments. After completion of the 5‐week treatment, photos were again taken, and photos of all three mice in each group are shown. (B) The extent of skin damage in each mouse shown in part A was evaluated by applying a skin damage score from 0 to 4, where 0 indicated no damage and 4 indicated very severe damage (see Materials and Methods). Shown are the averages from each group of three mice. Except for NEO400, none of the other treatments achieved any significant reduction in the skin damage score as compared to UV + Vehicle, and skin damage in all of them was significantly worse as compared to the UV + NEO400 group. ***p < 0.001. (C) Three mice per treatment group received the 5‐week exposure to UVR without any post‐UVR treatment, which resulted in pronounced skin damage (exemplary photo “N/A") at 5 weeks. Thereafter, the mice were subjected to continued 3× per week UVR, but this time each exposure was immediately followed by topical application of NEO400 or Aloe Vera (as per Schedule B). Two and four weeks later, photos were taken, and representative outcomes are shown, along with enlargements of the irradiated skin area.
We next investigated the effects of NEO400 when applied to skin that already presented with obvious UVR‐induced damage. For this purpose, mice received the same 5‐week UVR exposure as outlined in Figure 2, but without any post‐exposure treatment. After 5 weeks, when there was clear skin damage with scaly rash and erythema (Figure 3C, left photo), we began to include NEO400 or Aloe vera into this treatment regimen. UVR exposure was continued for 4 more weeks, but during this time, the animals received NEO400 or Aloe vera immediately following each irradiation (three times per week; see Schedule B, outlined in Figure 2B). Photos of mice were taken 2 and 4 weeks later (7 and 9 weeks total UVR) and showed that, despite continued UV irradiation, the application of NEO400 resulted in healing of the macroscopic damage of the skin, whereas Aloe vera had no such beneficial effect (Figure 3C). These results indicated that NEO400 not only was able to prevent the appearance of UVR‐induced skin damage, but also exerted a healing effect on skin that already was severely damaged by UVR.
NEO400 prevents microscopic signs of UVR‐induced skin damage
To examine the effects of NEO400 in greater detail, skin sections were prepared from mice after 5 weeks of UVR exposure with or without NEO400 treatment (as per Schedule A in Figure 2A). Results from the microscopic analysis are shown in Figure 4 and were characterized as follows. The epidermis of UVR‐treated skin showed signs of atrophy and notable features such as, hypergranulosis, abnormal keratosis and dyskeratosis, hyperplasic epidermis, and abnormal structures with ductal differentiation (likely related to hair follicles). In comparison, skin from mice that also received NEO400 appeared normal, with flattened epidermis, and inflammation in the upper dermis was inconspicuous. In all, these microscopic indicators of skin health were consistent with the macroscopic appearance of skin shown above, and provided further evidence of the preventive and therapeutic potential of NEO400.
FIGURE 4.
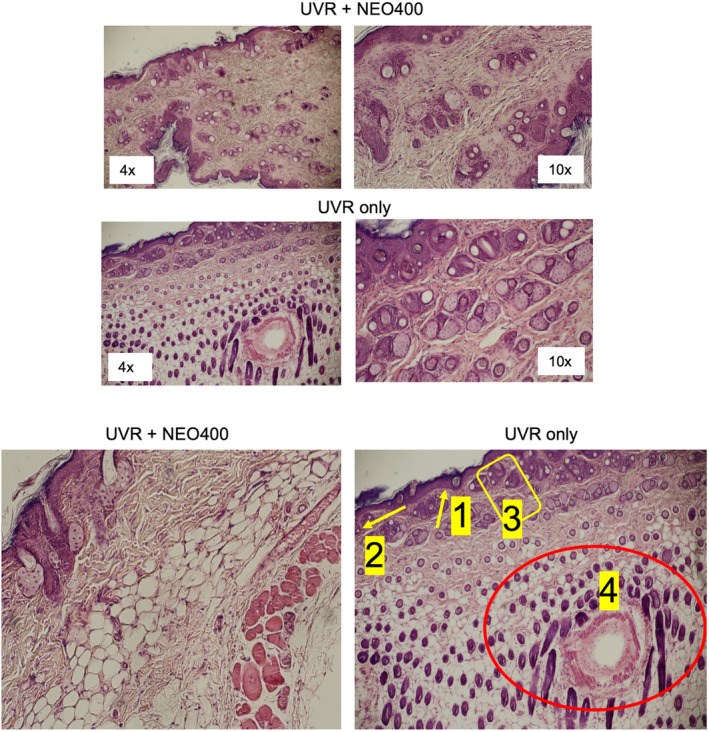
Microscopic appearance of UVR‐exposed skin. Skin sections were prepared from mice subjected to the 5‐week treatment cycle of UVR‐only, or UVR + NEO400 (Schedule A). Histological characteristics were examined under the microscope. As exemplified in these images, the epidermis of UVR‐only skin showed signs of: (1) hypergranulosis, (2) dyskeratosis, (3) hyperplasic epidermis, and (4) abnormal structures with ductal differentiation, which were not apparent in skin sections from mice that had received NEO400 immediately following UVR exposure.
NEO400 treatment normalizes cytokine levels after chronic UVR
Excessive UVR is well known to trigger an increase in inflammatory cytokines. We therefore investigated whether NEO400 would have an impact on serum levels of IL‐1β, IL‐6, and TNF‐α. Mice received UVR exposure with or without topical application of NEO400, Aloe vera, POH, LA, and POH mixed with LA. Control mice received vehicle only, or remained entirely untreated. The results are summarized in Figure 5 and reveal the following.
FIGURE 5.

Cytokine levels after UVR exposure. Blood from mice subjected to the 5‐week treatment cycle of UVR with or without immediately following topical treatments (Schedule A) was analyzed for the levels of IL‐1β, IL‐6, and TNF‐α. Shown are averages from each group of three mice. *p < 0.05; **p < 0.01; ***p < 0.001; n.s.: No statistical difference (p > 0.05).
As expected, UVR exposure resulted in increased serum levels of all three inflammatory cytokines, IL‐1β, IL‐6 and TNF‐α, and topical application of vehicle had no effect on this pronounced increase. In contrast, this increase was not apparent in serum collected from mice that had received NEO400 immediately following each UVR exposure. Mice exposed to UVR followed by topical application of Aloe vera, POH, LA, or POH combined with LA, showed an intermediate outcome, where serum levels of all three cytokines were higher than those after NEO400 treatment, but still lower than the levels reached with UVR alone (Figure 5).
Statistical analysis confirmed that levels of TNF‐α were significantly (p < 0.001) lower when mice were treated with NEO400 after UVR, as compared to UVR alone, or as compared to mice receiving Aloe vera after UVR (p < 0.01). Similarly, in the case of IL‐6 and IL‐1β, levels of these cytokines were also significantly (p < 0.05) lower when mice were treated with NEO400 after UVR, as compared to UVR alone. Although all other treatments reduced the average serum levels of IL‐6 and IL‐1β as well, the differences did not reach statistical significance. In all, the inclusion of NEO400 in this treatment regimen resulted in cytokine levels that were closest to those observed in non‐irradiated control mice. It is also noteworthy that the mixture of POH and LA in a combined application did not appear to exert stronger effects than the individual treatments with POH alone or LA alone, indicating that the mixture was unable to mimic the stronger effect of the conjugated fusion product, NEO400.
NEO400 maintains low levels of DNA damage markers in UVR‐exposed skin
We next investigated the extent of DNA damage in mouse skin after exposing mice to the treatment regimen outlined in Figure 2A. This was accomplished by determining the levels of cyclobutane pyrimidine dimers (CPD) and pyrimidine (6‐4) pyrimidone photoproducts (6‐4 PP), which represent two predominant types of UV‐induced DNA lesions. As shown in Figure 6, UVR caused a pronounced increase in the amount of both of these markers, as expected, and post‐irradiation treatment with vehicle‐only did not affect this increase. In contrast, when mice received NEO400 as the post‐irradiation treatment, CPD and 6‐4 PP levels were much lower (p < 0.001) and closer to the levels seen in control mice without any UVR exposure.
FIGURE 6.

Markers of DNA damage after UVR exposure. DNA was extracted from the skin of mice subjected to the 5‐week treatment cycle of UVR, with or without immediately following topical treatments (Schedule A), and DNA content of 6‐4 PP and CPD was determined. Shown are averages from each group of three mice. *p < 0.05; ***p < 0.001; n.s., no statistical difference (p > 0.05).
We also measured CPD and 6‐4 PP levels in UVR‐exposed mice that received Aloe vera, POH, or LA as the post‐irradiation treatment. Here, we noted an intermediate effect on both DNA damage markers, i.e., CPD and 6‐4 PP levels were lower than those in UVR‐only exposed mice. However, the protective effect of NEO400 clearly was the strongest: compared to post‐treatments with POH, LA, or Aloe vera, the greater difference made by NEO400 was highly significant (p < 0.001; Figure 6). Taken together, these data indicate that the protective effect of NEO400 on irradiated skin can also be observed at the level of DNA, where significantly less UVR‐induced damage was noted after application of NEO400.
NEO400 stimulates the expression of stem cell markers in UVR‐exposed skin
Following a 5‐week course of UVR exposure with and without additional topical applications of agents, as outlined in Figure 2A, we subjected mouse skin sections to immunohistochemical analysis for markers of skin stem cells. In particular, we probed the expression levels of follistatin and integrin α6, which are generally indicative of healthy, actively renewing skin. Expression levels of follistatin were found to be undetectable under all treatment conditions, including in skin from untreated control mice, but there was pronounced follistatin expression when NEO400 was applied post‐UVR (Figure 7). Similarly, expression levels of integrin α6 were barely detectable, but were strongly elevated in mouse skin that had received NEO400 post‐UVR. Together, these data suggest that the presence of NEO400 stimulated regenerative skin repair activity after UV irradiation.
FIGURE 7.

Detection of skin stem cell markers. Skin sections were prepared from mice subjected to the 5‐week treatment cycle of UVR, with or without immediately following topical treatments (Schedule A), and subjected to staining with antibodies to follistatin or integrin α6. Size bar: 100 μM. Enlargements for each image are shown as well.
DISCUSSION
The harmful effects of excessive UVR exposure on human skin are well established, but currently available post‐exposure interventions are suboptimal with regard to repairing and healing of the lesions. While sunscreens can be effective in preventing skin damage in the first place, they require conscientious application before sun exposure, along with mindful reapplications every 2 h. Not every person consistently meets these requirements, and as a consequence about one‐third of U.S. adults experience at least one sunburn each year, and children even more frequently. 24 , 25 Moreover, if UV rays do penetrate the sunscreen, they can still damage the skin and put the person at risk for the development of skin cancer. 26 , 27 , 28 Therefore, there is a clear need for agents that can be applied in conjunction with conventional sunscreens that will provide complete protection for the skin by preventing and/or repairing the damage caused by UVR, thereby preventing further detrimental sequelae, including melanoma and nonmelanoma skin cancer.
In this report, we characterized the novel compound NEO400 as an agent with desirable properties that appear to meet this important medical need. Our mouse models of UVR‐induced skin wounds presented with the typical features of erythema, elevated levels of inflammatory cytokines, scaling of the skin, and DNA damage. NEO400 was able to alleviate all of these skin damage indicators and return them closer to the levels observed in healthy, non‐damaged skin. Our data demonstrating that NEO400 prevents DNA damage that can occur with UVB radiation is especially important because both UVA and UVB have been attributed to cause DNA damage. Current sunscreens do not protect the dermal and subdermal cells of the skin once the UV rays have penetrated. Moreover, in our experiments, we have demonstrated that NEO400 can stimulate skin stem cells to repair the damaged skin.
The therapeutic effect of NEO400 was observed in two different types of models. In the first model, NEO400 was applied to the skin immediately following each irradiation session. In this case, it prevented the emergence of markers of skin damage, as there was no evidence of significant redness or skin scaling at any time. In the second model, NEO400 was not included during the first 5 weeks of repeated irradiations, but was only applied thereafter, at a time when obvious skin damage (notable scaling of the skin) already had occurred. In this case, despite continued irradiation, topical application of NEO400 resulted in returning the skin to its normal, healthy‐looking appearance. Combined, these observations indicate that NEO400 has both preventive and therapeutic activity.
It is noteworthy that Aloe vera had no noticeable benefit for skin healing in our models. Aloe vera creams and lotions are popular remedies for a variety of skin conditions, including irritations, minor wounds, burns from heat or radiation, psoriasis, pressure ulcers, genital herpes, acne vulgaris, frostbite, and others. 5 , 6 Multiple controlled trials have investigated their efficacy for the prevention and treatment of radiation‐induced side effects, for example during radiation therapy for cancer. In all, these results have not convincingly established major therapeutic benefit, 29 , 30 , 31 although there are indications that extensive pre‐ and post‐treatment with Aloe vera might reduce the likelihood of dermatitis secondary to cancer therapeutic ionizing radiation. 32 In our present UVR‐based study, we did not observe beneficial effects of Aloe vera treatment, consistent with results from a 2005 study with 20 sunburned participants that reported a lack of efficacy when compared to placebo. 10 In comparison, the benefit of topical NEO400 became readily apparent. It will be of interest to study NEO400 applications for skin damage induced by gamma radiation as well.
A preclinical study with mice exposed to graded doses of gamma radiation and treated with wound dressing gels containing concentrated Aloe vera extract investigated whether the timing of application would make a difference. The results revealed that the gel exerted some benefit, but not if applications were started 1 week after irradiation. 33 This latter result is noteworthy because in our present study, we find that NEO400 is also effective when it is applied to well‐established UVR‐induced skin lesions. In our Schedule B, mice were exposed to repeated UVR over the course of 5 weeks, which resulted in extensive skin damage. Only at this stage was NEO400 treatment initiated, along with further continued UVR exposure. Within 2 weeks, NEO400, but not Aloe vera, achieved a clear healing effect, indicating that it was able to not only prevent the emergence of UVR‐induced skin damage, but also accelerate the healing process of already established lesions.
An important consideration for using Aloe vera for therapeutic purposes is the fact that it is composed of dozens of potentially active constituents. 34 , 35 On one hand, this complexity might be beneficial in generating its multifactorial effects, including anti‐inflammatory, anti‐oxidant, vasoconstrictive, and other activities. On the other hand, it reduces the reliability and consistency of Aloe vera preparations, because plant composition is greatly affected by the plant's geographical location, climate, soil conditions, age, postharvest processing, and other variables. 36 Many investigators agree that acemannan, an acetylated glucosaminoglycan, represents the main active ingredient of the Aloe vera gel, 36 but others have found pectic substance (pectin, pectic acid, and arabinogalactan) as the key polysaccharide. 37 In addition to these surmised therapeutically active ingredients, it has been proposed that Aloe vera contains permeation enhancers that may support skin uptake of the other active biomolecules. 38 In all, the complex chemical nature and the variable presence of Aloe vera constituents might explain some of the discrepancies that have been reported from preclinical and clinical studies with this natural remedy. In comparison, NEO400 has the advantage of being a well‐defined, pure compound that could reliably be manufactured under stringent quality control measures.
Neither POH nor LA alone was able to achieve clear wound healing benefit. This was intriguing because both POH and LA are known to be able to penetrate the skin. We surmise that covalent conjugation of the two might result in altered physico‐ and physiochemical properties of the new molecule, leading to overall greater skin penetration, perhaps together with synergistic stimulation of the beneficial processes that participate in wound repair and skin healing, where NEO400 might provide favorable stimuli that are not readily achieved by its individual subunits. The veracity of this conjecture, however, is not yet supported by evidence and will have to await further studies. Similar considerations apply to Aloe vera, which also qualifies as a skin penetration enhancer, but, just like POH and LA, was unable to mimic the healing effect of NEO400 in our experimental conditions. Nonetheless, assuming that the positive outcomes presented in this current study can be further validated and expanded, NEO400 harbors the potential to become a new therapeutic for radiation‐induced skin damage.
CONCLUSION
The conjugate of POH and LA, as NEO400, is deemed to preserve the best features of its two parental molecules. It has shown benefit for minimizing skin damage and enhancement of skin repair in mouse models of UV radiation exposure. Importantly, NEO400 not only is able to provide preventative protection, but furthermore can repair skin damage that is already established. Together, these activities bode well for its further development as a therapeutic agent.
AUTHOR CONTRIBUTIONS
Stephen Swenson: methodology, investigation, formal analysis. Catalina Silva‐Hirschberg: methodology, investigation, formal analysis. Liliana Freeland: investigation. Kristen L. Chen: methodology, formal analysis. Nagore I. Marín‐Ramos: formal analysis. Axel H. Schönthal: methodology, formal analysis, visualization, manuscript draft. Thomas C. Chen: conceptualization, funding acquisition, project administration, supervision. All authors: review and editing.
CONFLICT OF INTEREST STATEMENT
Thomas C. Chen is founder and stakeholder of NeOnc Technologies, Inc. No conflict of interest was declared by the other authors.
ACKNOWLEDGMENT
We thank NeOnc Technologies for providing NEO400, which had been synthesized by Norac Pharma.
Swenson S, Silva‐Hirschberg C, Freeland L, et al. Therapeutic effect of NEO400, perillyl alcohol conjugated to linoleic acid, in a mouse model of UV‐induced skin damage. Photochem Photobiol. 2025;101:338‐349. doi: 10.1111/php.13998
Contributor Information
Axel H. Schönthal, Email: schontha@usc.edu.
Thomas C. Chen, Email: thomas.chen@med.usc.edu.
REFERENCES
- 1. Matsumura Y, Ananthaswamy HN. Toxic effects of ultraviolet radiation on the skin. Toxicol Appl Pharmacol. 2004;195:298‐308. [DOI] [PubMed] [Google Scholar]
- 2. Mohania D, Chandel S, Kumar P, et al. Ultraviolet radiations: skin defense‐damage mechanism. Adv Exp Med Biol. 2017;996:71‐87. [DOI] [PubMed] [Google Scholar]
- 3. Garmyn M, Young AR, Miller SA. Mechanisms of and variables affecting UVR photoadaptation in human skin. Photochem Photobiol Sci. 2018;17:1932‐1940. [DOI] [PubMed] [Google Scholar]
- 4. Sample A, He YY. Mechanisms and prevention of UV‐induced melanoma. Photodermatol Photoimmunol Photomed. 2018;34:13‐24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Liang J, Cui L, Li J, Guan S, Zhang K, Li J. Aloe vera: a medicinal plant used in skin wound healing. Tissue Eng Part B Rev. 2021;27:455‐474. [DOI] [PubMed] [Google Scholar]
- 6. Surjushe A, Vasani R, Saple DG. Aloe vera: a short review. Indian J Dermatol. 2008;53:163‐166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Grundmann O. Aloe Vera gel research review. Nat Med J. 2014;6. https://www.naturalmedicinejournal.com/journal/aloe‐vera‐gel‐research‐review [Google Scholar]
- 8. Byeon SW, Pelley RP, Ullrich SE, Waller TA, Bucana CD, Strickland FM. Aloe barbadensis extracts reduce the production of interleukin‐10 after exposure to ultraviolet radiation. J Invest Dermatol. 1998;110:811‐817. [DOI] [PubMed] [Google Scholar]
- 9. Reuter J, Jocher A, Stump J, Grossjohann B, Franke G, Schempp CM. Investigation of the anti‐inflammatory potential of Aloe vera gel (97.5%) in the ultraviolet erythema test. Skin Pharmacol Physiol. 2008;21:106‐110. [DOI] [PubMed] [Google Scholar]
- 10. Puvabanditsin P, Vongtongsri R. Efficacy of Aloe vera cream in prevention and treatment of sunburn and suntan. J Med Assoc Thai. 2005;88(Suppl 4):S173‐S176. [PubMed] [Google Scholar]
- 11. Criollo‐Mendoza MS, Contreras‐Angulo LA, Leyva‐Lopez N, Gutierrez‐Grijalva EP, Jimenez‐Ortega LA, Heredia JB. Wound healing properties of natural products: mechanisms of action. Molecules. 2023;28:598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Fernandes A, Rodrigues PM, Pintado M, Tavaria FK. A systematic review of natural products for skin applications: targeting inflammation, wound healing, and photo‐aging. Phytomedicine. 2023;115:154824. [DOI] [PubMed] [Google Scholar]
- 13. d'Alessio PA, Mirshahi M, Bisson JF, Bene MC. Skin repair properties of d‐Limonene and perillyl alcohol in murine models. Antiinflamm Antiallergy Agents Med Chem. 2014;13:29‐35. [DOI] [PubMed] [Google Scholar]
- 14. Crowell PL, Elson CE. Isoprenoids, health and disease. In: Wildman REC, ed. Neutraceuticals and Functional Foods. CRC Press; 2001:31‐54. [Google Scholar]
- 15. Chen TC, Fonseca CO, Schönthal AH. Preclinical development and clinical use of perillyl alcohol for chemoprevention and cancer therapy. Am J Cancer Res. 2015;5:1580‐1593. [PMC free article] [PubMed] [Google Scholar]
- 16. Chen TC, da Fonseca CO, Levin D, Schönthal AH. The Monoterpenoid perillyl alcohol: anticancer agent and medium to overcome biological barriers. Pharmaceutics. 2021;13:2167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sapra B, Jain S, Tiwary AK. Percutaneous permeation enhancement by terpenes: mechanistic view. AAPS J. 2008;10:120‐132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Cunnane SC, Anderson MJ. Pure linoleate deficiency in the rat: influence on growth, accumulation of n‐6 polyunsaturates, and [1–14C] linoleate oxidation. J Lipid Res. 1997;38:805‐812. [PubMed] [Google Scholar]
- 19. Whelan J, Fritsche K. Linoleic acid. Adv Nutr. 2013;4:311‐312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Moloney SJ, Edmonds SH, Giddens LD, Learn DB. The hairless mouse model of photoaging: evaluation of the relationship between dermal elastin, collagen, skin thickness and wrinkles. Photochem Photobiol. 1992;56:505‐511. [DOI] [PubMed] [Google Scholar]
- 21. Oba A, Edwards C. Relationships between changes in mechanical properties of the skin, wrinkling, and destruction of dermal collagen fiber bundles caused by photoaging. Skin Res Technol. 2006;12:283‐288. [DOI] [PubMed] [Google Scholar]
- 22. Bernatchez SF, Bichel J. The science of skin: measuring damage and assessing risk. Adv Wound Care. 2023;12:187‐204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Williams KA, Kolappaswamy K, Detolla LJ, Vucenik I. Protective effect of inositol hexaphosphate against UVB damage in HaCaT cells and skin carcinogenesis in SKH1 hairless mice. Comp Med. 2011;61:39‐44. [PMC free article] [PubMed] [Google Scholar]
- 24. Holman DM, Ding H, Berkowitz Z, Hartman AM, Perna FM. Sunburn prevalence among US adults, National Health Interview Survey 2005, 2010, and 2015. J Am Acad Dermatol. 2019;80:817‐820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Holman DM, Ragan KR, Julian AK, Perna FM. The context of sunburn among U.S. adults: common activities and sun protection behaviors. Am J Prev Med. 2021;60:e213‐e220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Iannacone MR, Hughes MC, Green AC. Effects of sunscreen on skin cancer and photoaging. Photodermatol Photoimmunol Photomed. 2014;30:55‐61. [DOI] [PubMed] [Google Scholar]
- 27. Nash JF, Tanner PR. Relevance of UV filter/sunscreen product photostability to human safety. Photodermatol Photoimmunol Photomed. 2014;30:88‐95. [DOI] [PubMed] [Google Scholar]
- 28. Boo YC. Emerging strategies to protect the skin from ultraviolet rays using plant‐derived materials. Antioxidants (Basel). 2020;9:637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Farrugia CE, Burke ES, Haley ME, Bedi KT, Gandhi MA. The use of Aloe vera in cancer radiation: an updated comprehensive review. Complement Ther Clin Pract. 2019;35:126‐130. [DOI] [PubMed] [Google Scholar]
- 30. Richardson J, Smith JE, McIntyre M, Thomas R, Pilkington K. Aloe vera for preventing radiation‐induced skin reactions: a systematic literature review. Clin Oncol. 2005;17:478‐484. [DOI] [PubMed] [Google Scholar]
- 31. Robijns J, Becherini C, Caini S, et al. Natural and miscellaneous agents for the prevention of acute radiation dermatitis: a systematic review and meta‐analysis. Support Care Cancer. 2023;31:195. [DOI] [PubMed] [Google Scholar]
- 32. Wang T, Liao J, Zheng L, Zhou Y, Jin Q, Wu Y. Aloe vera for prevention of radiation‐induced dermatitis: a systematic review and cumulative analysis of randomized controlled trials. Front Pharmacol. 2022;13:976698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Roberts DB, Travis EL. Acemannan‐containing wound dressing gel reduces radiation‐induced skin reactions in C3H mice. Int J Radiat Oncol Biol Phys. 1995;32:1047‐1052. [DOI] [PubMed] [Google Scholar]
- 34. Chelu M, Musuc AM, Popa M, Calderon Moreno J. Aloe vera‐based hydrogels for wound healing: properties and therapeutic effects. Gels. 2023;9:539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Enachi E, Boev M, Bahrim GE. Aloe vera plant – an important source of bioactive compounds with functional value. Innov Roman Food Biotechn. 2020;19:1‐20. [Google Scholar]
- 36. Minjares‐Fuentes R, Femenia A, Comas‐Serra F, Rodriguez‐Gonzalez VM. Compositional and structural features of the main bioactive polysaccharides present in the Aloe vera plant. J AOAC Int. 2018;101:1711‐1719. [DOI] [PubMed] [Google Scholar]
- 37. Hamman JH. Composition and applications of Aloe vera leaf gel. Molecules. 2008;13:1599‐1616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Cole L, Heard C. Skin permeation enhancement potential of Aloe vera and a proposed mechanism of action based upon size exclusion and pull effect. Int J Pharm. 2007;333:10‐16. [DOI] [PubMed] [Google Scholar]


