Abstract
Purpose
This systematic review and meta-analysis of clinical observational studies aims to clarify the correlation between the intake levels of fruits and vegetables and non-alcoholic fatty liver disease (NAFLD).
Materials and methods
PubMed, Embase, Web of Science, and the Cochrane Library were searched for studies on the association between vegetable or fruit intake with the risk of NAFLD from the foundation of each database up until September 2023. The relative risk (OR) and the 95% confidence interval (CI) were pooled for both the highest and lowest consumption levels of vegetables and fruits to explore their association with the incidence of NAFLD.
Results
The meta-analysis encompassed 11 studies with a total of 493,682 patients. A higher consumption of vegetables (OR = 0.78, 95% CI = 0.67–0.91) and fruits (OR = 0.88, 95% CI = 0.83–0.93) was found to have a negative correlation with the risk of NAFLD, denoting an inverse association. This correlation, however, varied among different ethnic groups and gender.
Conclusions
Our results indicate that increased consumption of vegetables and fruits is associated with a reduced likelihood of developing NAFLD.
Systematic review registration
https://www.crd.york.ac.uk/PROSPERO/#searchadvanced, identifier: CRD42023460430.
Keywords: vegetable, fruit, non-alcoholic fatty liver disease, diet, meta-analysis
1 Introduction
NAFLD, predominantly caused by metabolic syndrome, is closely associated with obesity, insulin resistance and hyperlipidemia (1). Over the past four decades, the incidence of NAFLD has steadily increased (2, 3), currently affecting 25% of the global adult population (3). The disease prevalence is about 30% in Asia (4), the US, and South America, 24% in Europe, and 13% in Africa (2, 5–7), making it the most widespread chronic liver disease worldwide (2). An estimated 20% of non-alcoholic steatohepatitis (NASH) patients progress to cirrhosis, and the risk of hepatocellular carcinoma in NASH patients surged 7.7·times between 2002 and 2016, from 2.1 to 16.2% (8). In the US, it is expected that NASH medical expenses per patient will jump from $3,636 to $6,968 between 2020 and 2039 (9). Similarly, in Japan, the annual healthcare cost for NASH ranged from 322,206 to 340,399 yen per patient between 2011 and 2017 (10), imposing a substantial economic burden. Therefore, attention must be focused on early disease detection in primary healthcare settings.
Obesity, over nutrition, a high-calorie diet, and a sedentary lifestyle contribute to the accumulation of liver fat (11, 12), and are crucial risk factors for NAFLD (13). The regulatory mechanism of NAFLD is connected with metabolism, heredity, intestinal microorganisms, and other factors (11, 14, 15). At present, the management of NAFLD is centered around reducing insulin resistance and limiting oxidative stress (13). Treatment strategies are founded on lifestyle management, such as modifying diet and increasing physical activity, with the intent of controlling weight and managing risk factors pertinent to metabolic syndrome (16, 17). Consequently, adhering to a balanced diet and maintaining a healthy lifestyle have become pivotal in treating and delaying the progression of this disease (18, 19).
Fruits and vegetables are plant-based foods, rich in dietary fiber, which can help maintain the balance of intestinal flora, reduce inflammation, and decrease fat accumulation in the liver. Moreover, fruits and vegetables are abundant in antioxidants that neutralize free radicals and diminish oxidative stress damage to the liver. The antioxidants (20) and anti-inflammatory compounds (21) in fruits and vegetables enhance insulin sensitivity, accelerate beta-oxidation, and inhibit new fat production (22). As a result, it has been hypothesized that an intake of fruits and vegetables correlates with a lower prevalence of NAFLD (23). However, the relationship between fruit and vegetable intake and NAFLD risk remains a subject of debate. Several observational studies suggest that higher dietary vegetable intake is associated with a lower NAFLD risk (24, 25). Yet, some research indicates that there is no such relationship (23, 26). A similar controversy exists in regard to the correlation between fruit intake and the risk of NAFLD (26–31). Even though the role of vegetable and fruit intake in NAFLD has drawn considerable public attention, no meta-analyses have demonstrated a correlation between vegetable and fruit consumption and NAFLD risk. Therefore, we undertook this meta-analysis to summarize the results of observational studies regarding the association between vegetable and fruit consumption and the risk of NAFLD.
2 Materials and methods
This systematic review and meta-analysis statement followed Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA), and the protocol was registered with PROSPERO (ID: CRD42023460430).
2.1 Study strategy
The researchers scoured Pubmed, Embase, Web of Science, and the Cochrane Library for studies on the correlation between vegetable or fruit consumption with the risk of NAFLD from the inception of these databases to September 2023. Key search terms included non-alcoholic Fatty Liver Disease, fruit*, vegetable*, among others. Detailed search strategies are presented in the Supplementary material. The Endnote software (X20 version) was utilized to eliminate duplicate documents fetched from each database, and the remaining potentially eligible documents were manually screened (Appendix 1).
2.2 Inclusion and exclusion criteria
Two researchers checked the titles and abstracts to select studies that met the inclusion and exclusion criteria. The full texts of these studies were examined to choose eligible studies. In instances where consensus on eligibility could not be reached, a third reviewer was engaged for discussion.
Inclusion criteria were as follows: (1) studies assessing the correlation between varying levels of vegetable and fruit consumption, and the risk of NAFLD; (2) studies that provide relative risk, odds ratio, hazard ratio, and their corresponding 95% confidence intervals; (3) observational studies such as cross-sectional studies, case-control studies, and cohort studies.
Papers were ineligible for inclusion using the following criteria: (1) duplicate papers; (2) irrelevance to the subject matter (irrelevant disease and observation indicators); (3) meta-analysis, reviews, letters, conference abstracts, case reports, guidelines, etc.; (4) animal experiments.
2.3 Data extraction
Two reviewers extracted basic information from the articles finally included. This information comprised the first author, publication year, country, study type, sample size, age of study population, sex ratio, follow-up time, disease diagnosis method, intake assessment method, model adjustment factors, and the relative risk RR (OR, HR) associated with the highest and lowest fruit and/or vegetable intake along with their corresponding 95% confidence intervals. Where possible, the maximally adjusted RR, OR, or HR ratio and 95% CI were extracted. Any disagreements during the review process, if any, were resolved by discussion or, if necessary, consultation with a third reviewer.
2.4 Quality assessment
The assessment of bias and quality of the included studies was performed independently by two reviewers, with discrepancies resolved by a third reviewer. The quality assessment adhered to our published protocols. The quality of case-control studies was evaluated using the Newcastle-Ottawa Scale (NOS) and categorized into high quality (score 7–9), medium quality (score 4–6), and low quality (score 0–3). The quality of cross-sectional studies was assessed using the AHRQ scale from the U.S. Agency for Healthcare Research and Quality, and these cross-sectional studies were assessed as low quality (score 0–3), medium quality (score 4–7), or high quality (score 8–11).
2.5 Statistical analysis
In this study, Stata (version 15.0) was utilized to gather and summarize the OR and its corresponding 95% confidence interval, and to develop a forest map. Heterogeneity was evaluated by the Q-test and I-square test. A random effects model was employed when I-squared was ≥50% and p < 0.1; under other circumstances, a fixed effects model was used. In the presence of high heterogeneity, we conducted subgroup (study type, continent, and intake assessment questionnaire) and stratified analyses to explore heterogeneity sources. Sensitivity analysis was performed by observing the results' stability after sequentially eliminating each article. The potential risk of publication bias was assessed by examining funnel plots. When dealing with ≧10 articles, publication bias was evaluated using Egger's test and Begg's test. If publication bias was present, further evaluation was conducted using the “trim-and-fill” method. A bilateral P < 0.05 indicates a notable distinction.
3 Results
3.1 Literature search and selection
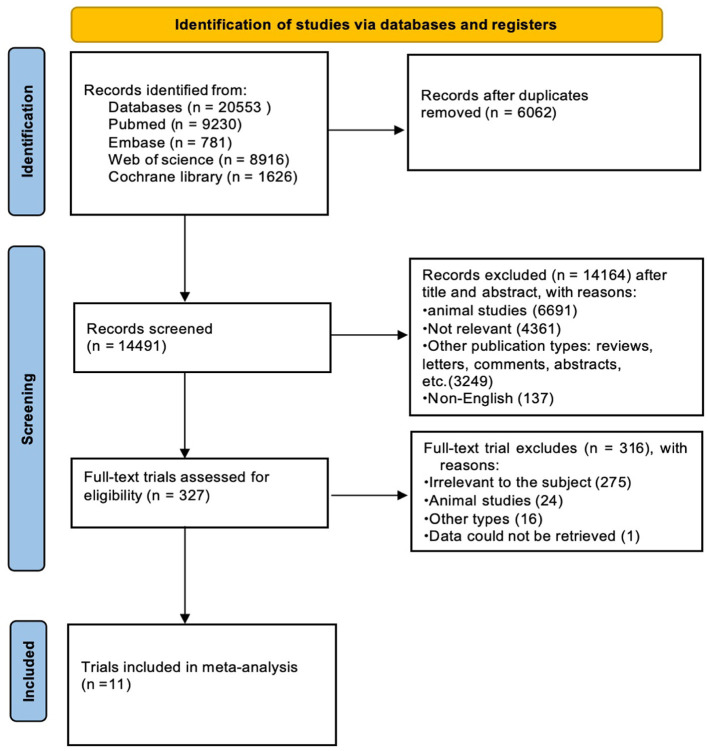
Papers related to the association between vegetable and/or fruit intake and the risk of NAFLD were searched from Pubmed (9,230 articles), Embase (781 articles), Web of Science (8,916 articles), and Cochrane Library (1,626 articles) from the inception of these databases until September 2023. After utilizing EndNote(X20) for automatic duplication removal, 14,491 related publications remained. Following a manual check for duplicates, 327 articles were left. These were then screened by their titles and abstracts according to inclusion and exclusion criteria. After a full-text review, only 11 papers were included. The literature screening process is illustrated in Figure 1.
Figure 1.
Flow diagram of study selection process.
3.2 Study characteristics
The 11 included studies comprised six cohort studies and five case-control studies. Of the chosen studies, eight were conducted in Asian countries, specifically China (four studies), Iran (two studies), South Korea (one study), and Japan (one study). Additionally, two studies were conducted in Europe (one in Italy and one in the United Kingdom), and one study was conducted in North America (the United States). There was a total of 493,682 participants, consisting of 221,779 males and 271,901 females, whose ages ranged from 18 to 79 years. All included studies were deemed to be of high quality considering their AHRQ and NOS scores (Tables 1, 2). In the included studies, three intake assessment questionnaires were used, including Food Frequency Questionnaire (FFQ) used in nine studies, BDHQ in one study, and Food Diary in one study. The attributes of the included studies are detailed in Table 3.
Table 1.
Quality assessment of six case-control studies.
| References | Selection | Comparability | Exposure | Overall score | |||||
|---|---|---|---|---|---|---|---|---|---|
| Is the case definition adequate? | Representativeness of the cases | Selection of controls | Definition of Controls | Comparability of cohorts on the basis of the design or analysis | Ascertainment of exposure | Same method of ascertainment for cases and controls | Non-response rate | ||
| Emamat et al. (24) | 1 | 1 | 1 | 1 | 2 | - | 1 | - | 7 |
| Giraldi et al. (32) | 1 | 1 | 1 | 1 | 2 | - | 1 | - | 7 |
| Noureddin et al. (29) | 1 | 1 | 1 | 1 | 2 | - | 1 | 1 | 8 |
| Tutunchi et al. (30) | 1 | 1 | 1 | 1 | 2 | - | 1 | 1 | 8 |
| Guo et al. (26) | 1 | 1 | 1 | 1 | 2 | - | 1 | 1 | 8 |
Table 2.
Quality assessment of six cross-sectional studies.
| Chan et al. (27) | Liu et al. (28) | Tajima et al. (23) | Kim and Shin (25) | Li et al. (33) | Du et al. (31) | |
|---|---|---|---|---|---|---|
| Define the source of information (survey, record review) | 1 | 1 | 1 | 1 | 1 | 1 |
| List inclusion and exclusion criteria for exposed and unexposed subjects (cases and controls) or refer to previous publications. | 1 | 1 | 1 | 1 | 1 | 1 |
| Indicate time period used for identifying patients. | 1 | 1 | 1 | 1 | 1 | 1 |
| Indicate whether or not subjects were consecutive if not population-based. | 1 | 1 | 1 | 1 | 1 | 1 |
| Indicate if evaluators of subjective components of study were masked to other aspects of the status of the participants. | 1 | 1 | 1 | 1 | 1 | 1 |
| Describe any assessments undertaken for quality assurance purposes (e.g., test/retest of primary outcome measurements). | 1 | 1 | 1 | 1 | 1 | 1 |
| Explain any patient exclusions from analysis. | 0 | 0 | 0 | 0 | 0 | 0 |
| Describe how confounding was assessed and/or controlled. | 1 | 1 | 1 | 1 | 1 | 1 |
| If applicable, explain how missing data were handled in the analysis. | 1 | 1 | 1 | 1 | 1 | 1 |
| Summarize patient response rates and completeness of data collection. | 1 | 1 | 1 | 1 | 1 | 1 |
| Clarify what follow-up, if any, was expected and the percentage of patients for which incomplete data or follow-up was obtained. | 1 | 1 | 1 | 1 | 1 | 1 |
| Total score | 10 | 10 | 10 | 10 | 10 | 10 |
Table 3.
Study characteristics of the association between fruit and vegetable intake levels and the incidence of NAFLD were evaluated.
| References | Country | Research type | Total number of participants | Baseline age (years) | Gender (male/female) | Follow-up period (years) | Methods of disease diagnosis | Quality of study |
|---|---|---|---|---|---|---|---|---|
| Chan et al. (27) | China | Cross-sectional study | 797 | 36.2–60.3 | 332/465 | / | Measurement of intrahepatic triglyceride content (IHTG) by 1H-MRS | Good |
| Liu et al. (28) | China | Cross-sectional study | 1,639 | 18.55 ± 1.48 | 880/759 | / | B-ultrasonic examination | Good |
| Tajima et al. (23) | Japan | Cross-sectional study | 2,444 | 40–69 | 977/1,467 | / | Abdominal ultrasonography | Good |
| Emamat et al. (24) | Iran | Case-control study | 999 | 43.26 ± 14.03 | 430/569 | / | Controlled attenuation parameter (CAP) score in Fibroscan exam | Good |
| Giraldi et al. (32) | Italy | Case-control study | 815 | 51.37 ± 16.67 | 509/304 | / | Presence of sonographic features of hepatic steatosis based on the presence of the bright liver pattern as recommended by the American Gastroenterology Association. | Good |
| Kim and Shin (25) | Korea | Cross-sectional study | 52,280 | 40–79 | 15,588/36,692 | 4.2 years | NAFLD was diagnosed based on FLI Participants with FLI ≥60 were defined as having NAFLD. | Good |
| Noureddin et al. (29) | America | Case-control study | 32,448 | 45–75 | 12,225/20,223 | / | NAFLD cases among eligible participants were identified using Medicare claims | Good |
| Li et al. (33) | China | Cross-sectional study | 26,891 | ≥18 | 12,727/14,164 | / | Abdominal ultrasonography | Good |
| Tutunchi et al. (30) | Iran | Case-control study | 210 | 30–60 | 90/120 | / | Abdominal ultrasonography | Good |
| Du et al. (31) | China | Cross-sectional study | 2,667 | 18–76 | 1,694/973 | / | Abdominal ultrasonography | Good |
| Guo et al. (26) | UK | Case-control study | 372,492 | 48.63–64.83 | 176,327/196,165 | / | / | Good |
| 465,15.5690ptReferences | Sources of intake assessment | Adjustment factors | Relationship between vegetables or fruits and NAFLD OR (LL, UL) | |||||
| Vegetables | Fruits | |||||||
| Chan et al. (27) | FFQ | Age, sex, BMI, smoke, drink, central obesity, triglyceride >1.7 mmol/l, reduced HDL-cholesterol, hypertension, impaired fasting glucose or diabetes, the PNPLA3 genotypes (CC vs. CG vs. GG genotypes), and Energy intake | 0.51 (0.3, 0.87)* | 0.50 (0.3, 0.84)* | ||||
| Liu et al. (28) | FFQ | Age, sex, BMI, economic income, smoking status, educational level, physical activity, family history of diabetes, stroke, and energy intake. | 0.81 (0.66, 1.04) | 0.84 (0.67, 1.07) | ||||
| Tajima et al. (23) | BDHQ | Age, lifestyle factors, and BMI | 0.83 (0.57, 1.21) | 0.73 (0.5, 1.07) | ||||
| Emamat et al. (24) | FFQ | Age, gender, BMI, energy intake, and physical activity | 0.36 (0.22, 0.56)* | / | ||||
| Giraldi et al. (32) | FFQ | Age, gender, total energy intake, diabetes status, smoking status, BMI, and physical activity. | 1.81 (0.68, 4.78) | 2.26 (0.97, 5.29) | ||||
| Kim and Shin (25) | FFQ | Age, education level, smoking status, alcohol consumption, physical activity, energy intake, and red and processed meat intake, BMI | 0.80 (0.69, 0.93)* | 0.83 (0.72, 0.95)* | ||||
| Noureddin et al. (29) | FFQ | BMI, alcohol intake, coffee intake, total soda intake, vigorous physical activity, and energy intake | 0.99 (0.88, 1.1) | 0.91 (0.81, 1.02) | ||||
| Li et al. (33) | FFQ | Age, sex, smoking status, drinking status, education level, occupation, household income, physical activity, family history of disease (including cardiovascular disease, hypertension, hyperlipidemia, and diabetes), hypertension, hyperlipidemia, and diabetes, total energy intake, “fruits and sweet” dietary pattern score, “healthy dietary pattern score, and “animal foods” dietary pattern score, vegetable intake and fruit intake, BMI | 0.81 (0.63, 1.05) | / | ||||
| Tutunchi et al. (30) | Food diary | Sex, education, physical activity, BMI, and WC, the relationships and effect sizes for the residual effects of this variable | 0.34 (0.16, 0.81)* | 0.54 (0.19, 1.56) | ||||
| Du et al. (31) | FFQ | Age, sex, educational attainment, BMI, WC, HC, BP, diabetes duration; family history, smoking, drinking, physical activity level, the consumption of bean products, salt, fish and sugary beverages, and biochemical index values (HbA1c, ALT, AST, and serum lipid levels). | 0.67 (0.51, 0.88)* | 1.15 (0.84, 1.59) | ||||
| Guo et al. (26) | FFQ | Age, sex, race, education level, Townsend Deprivation Index (quartiles), drinking status, smoking status, exercise, BMI, and diabetes. | 1.03 (0.93, 1.14) | 0.89 (0.81, 0.98)* | ||||
*Indicates that the result is significant.
3.3 Results of the meta-analyses
3.3.1 Vegetable intake
Eleven studies involving 493,682 participants reported the association between vegetable intake and NAFLD risk. A random-effects model was used for data analysis (I2 = 77.7%, p < 0.001). The results found that higher vegetable intake was linked to a reduced risk of NAFLD (OR = 0.78, 95% Cl: 0.67–0.91, p = 0.001; Figure 2A).
Figure 2.
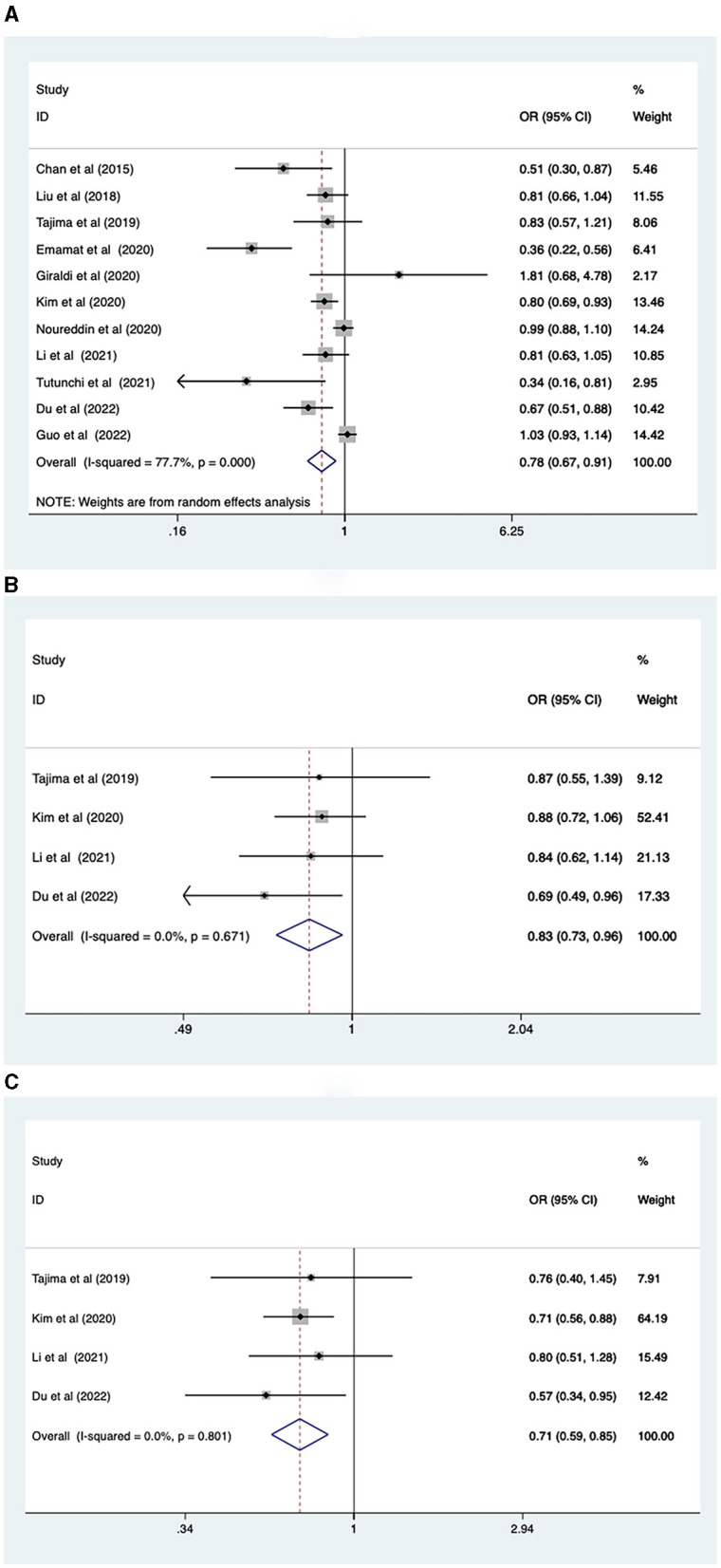
(A) Association between vegetable intake and risk of NAFLD; (B) NAFLD risk in men; (C) NAFLD risk in women.
Due to the greater heterogeneity in the analysis of total vegetable intake, subgroup analyses were conducted according to sex, study type, continent, and intake assessment questionnaire. The results revealed that increased levels of vegetable intake were associated with a reduced risk of NAFLD for both males (OR = 0.83, 95% CI: 0.73–0.96, p = 0.011), and females (OR = 0.71, 95% CI: 0.59–0.85, p < 0.001; Figures 2B, C). Regarding study types, higher levels of vegetable intake were connected with lower prevalence of NAFLD in cross-sectional studies (OR = 0.78, 95% CI: 0.70–0.86), p < 0.001. However, in case-control studies (OR = 0.79, 95% CI: 0.60–1.05, p = 0.107), no such association was observed (Supplementary Figure 1A). In terms of the geographic location of the study population, increased vegetable intake was inversely correlated with the risk of NAFLD in the Asian population (OR = 0.68, 95% CI: 0.58–0.82, p < 0.001). However, no correlation was found between vegetable intake and the risk of NAFLD in the European (OR = 1.10, 95%CI: 0.77–1.56, p = 0.6) and North American population (OR = 0.99, 95% CI: 0.89–1.11, p = 0.86; Supplementary Figure 1B). Regarding the intake assessment questionnaires, FFQ (OR = 0.80, 95% CI: 0.68–0.93, p = 0.005) and Food Diary (OR = 0.34, 95% CI: 0.15–0.77, p = 0.009) indicated that a higher vegetable intake was associated with decreased NAFLD risk. However, results from the BDHQ (OR = 0.83, 95% CI: 0.57–1.21, p = 0.332) showed the relationship between the two was not statistically significant (Supplementary Figure 1C).
When an adjustment factor was present in more than two studies, we conducted a single-factor heterogeneity analysis. Upon adjusting for age, gender, smoking and alcohol consumption status, physical activity, energy intake, BMI, economic income level, education level, and family history of diseases (hypertension, hyperlipidemia, cardiovascular disease, diabetes), we found that elevated levels of vegetable intake remained associated with a lower NAFLD risk (Supplementary Figures 2A–J). Yet, when we adjusted for hypertension, hyperlipidemia, diabetes, coffee intake, soft drinks, vegetable and fruit intake, and waist circumference, this association was not observed (Supplementary Figures 3A–G).
3.3.2 Fruit intake
Nine studies, involving 465,792 participants, reported the association between fruit intake and NAFLD risk. A fixed-effects model was used (I2 = 46.9%, p = 0.058). The analysis revealed that higher levels of fruit intake were associated with a lower risk of NAFLD (OR = 0.88, 95% CI: 0.83–0.93, p < 0.001; Figure 3A).
Figure 3.
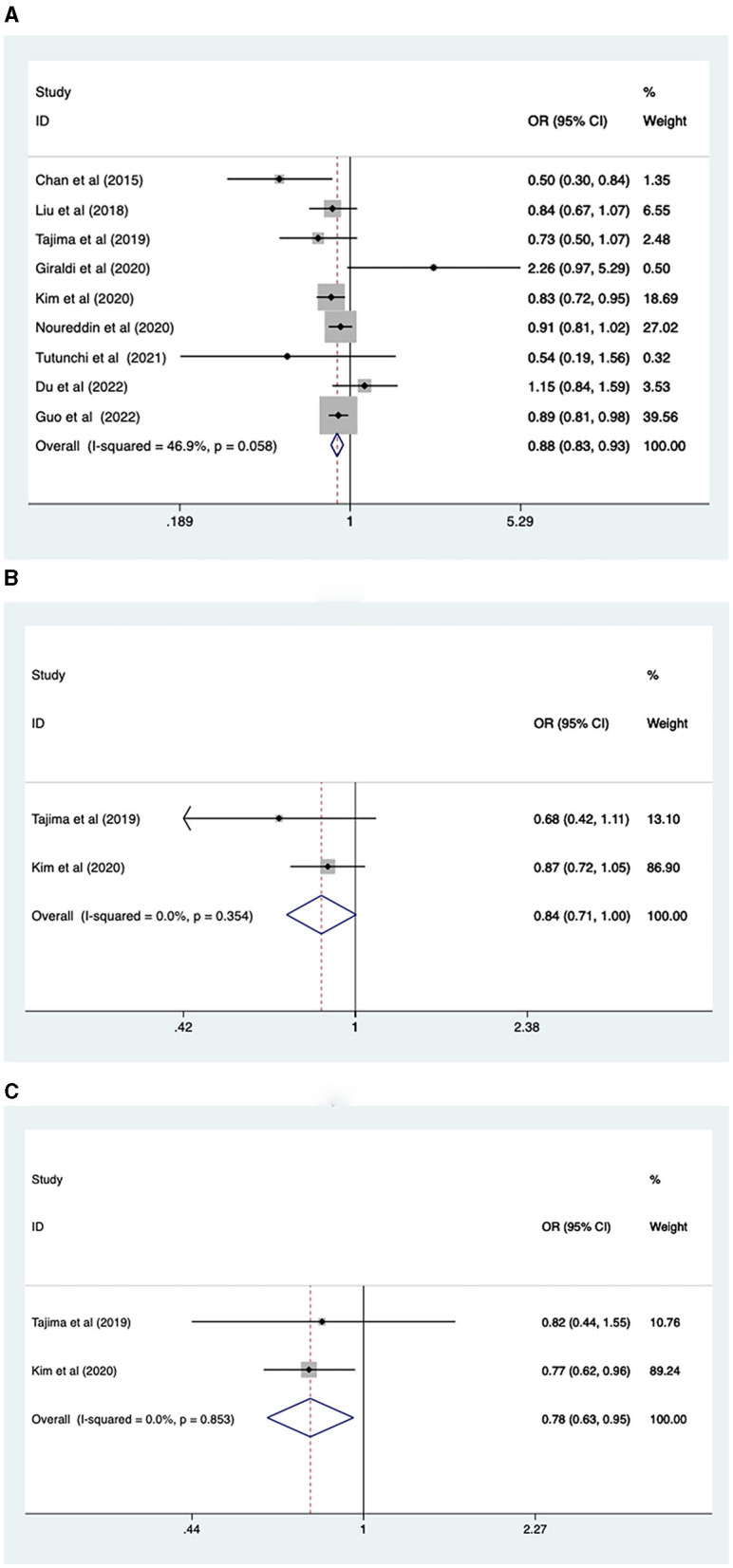
(A) Association between fruit intake and risk of NAFLD; (B) NAFLD risk in men; (C) NAFLD risk in women.
Due to the heterogeneity of total fruit intake results, we performed a subgroup analysis based on sex, study type, continent, and intake assessment questionnaire. The fruit intake level was found to be associated with the prevalence of NAFLD in females (OR = 0.78, 95% CI: 0.63–0.95, p = 0.016), but not in males (OR = 0.84, 95% CI: 0.7–1.00; Figures 3B, C). Cross-sectional (OR = 0.84, 95% CI: 0.75, 0.93, p = 0.001) and case-control (OR = 0.90, 95% CI: 0.84, 0.97, p = 0.006) studies showed that higher levels of fruit intake were associated with lower NAFLD risk (Supplementary Figure 4A). In Asian (OR = 0.83, 95% CI: 0.75, 0.92, p = 0.001) and European (OR = 0.90, 95% CI: 0.82, 0.99, p = 0.03) populations, higher levels of fruit intake were associated with a lower risk of NAFLD, while in the North American population (OR = 0.91, 95% CI: 0.81, 1.02, p = 0.109), fruit intake was not associated with lower risk of NAFLD (Supplementary Figure 4B). FFQ (or = 0.89, 95% CI: 0.83, 0.94, p < 0.001) showed that a higher level of fruit intake was related to a lower level of NAFLD risk, while those of BDHQ (or = 0.73, 95% CI: 0.50, 1.07, p = 0.105) and Food Diary (or = 0.54, 95% CI: 0.19, 1.55, p = 0.251) showed that fruit intake had no significant impact on the risk of NAFLD (Supplementary Figure 4C).
Following adjustment for variables such as alcohol consumption, BMI, education level, energy intake, and physical activity, we observed that a high intake of fruits was still significantly associated with a reduced risk of NAFLD (Supplementary Figures 5A–E). However, further adjustments for factors such as age, gender, smoking status, coffee consumption, soft drink intake, history of diabetes, family history of diseases (hypertension, hyperlipidemia, cardiovascular disease, and diabetes), and waist circumference did not reveal any substantial correlation between fruit consumption and a lower risk of NAFLD (Supplementary Figures 6A–H).
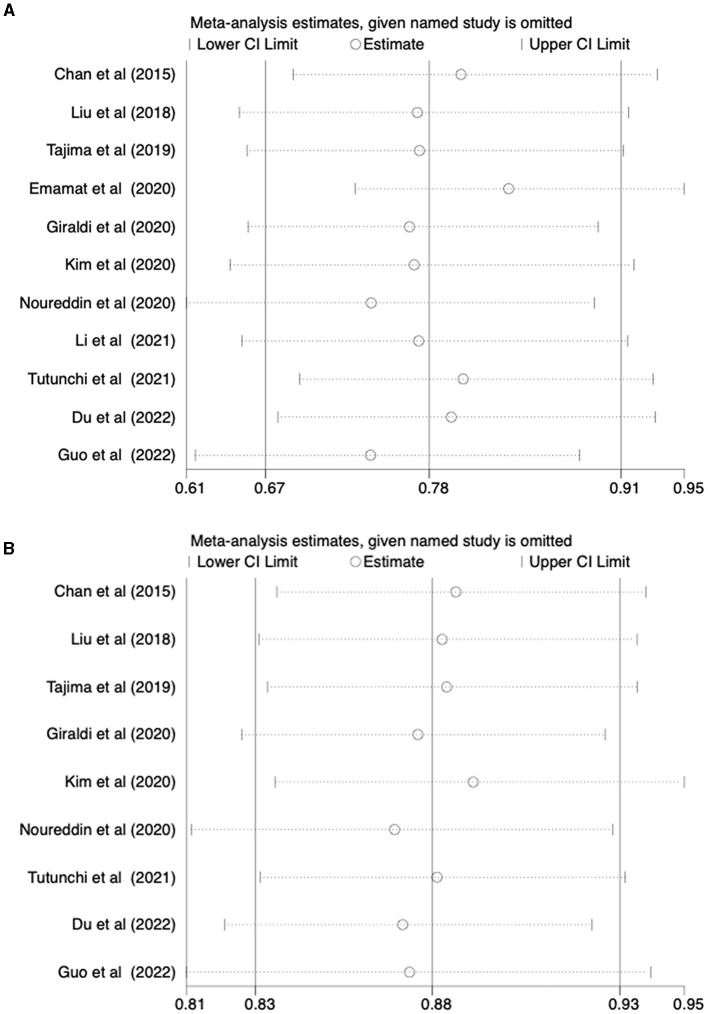
3.4 Sensitivity analyses and publication bias
A sensitivity analysis was conducted to evaluate the stability of the results regarding the intake of vegetables or fruits and the risk of NAFLD. After excluding each article one by one, the results remained stable (Figure 4). To further examine publication bias, Egger's (p = 0.022) and Begg's (p = 0.161) tests were conducted for the relationship between vegetable intake and the risk of NAFLD. The Egger's test showed evidence of publication bias (Figure 5A). Hence, the Trim and Fill method was employed to adjust for the asymmetry in the funnel plot. However, the result indicated that no trimming was necessary and the data remained unchanged. When using Duval's Trim and Fill method, no new studies were added, suggesting that publication bias did not impact the study results (Figure 5B). Additionally, we analyzed publication bias using Egger's (p = 0.822) and Begg's (p = 0.754) tests for the relationship between fruit intake and the risk of NAFLD, finding no evidence of publication bias (Figure 5C).
Figure 4.
(A) Outcome sensitivity analysis of vegetable intake and risk of NAFLD; (B) Outcome sensitivity analysis of fruit intake and risk of NAFLD.
Figure 5.
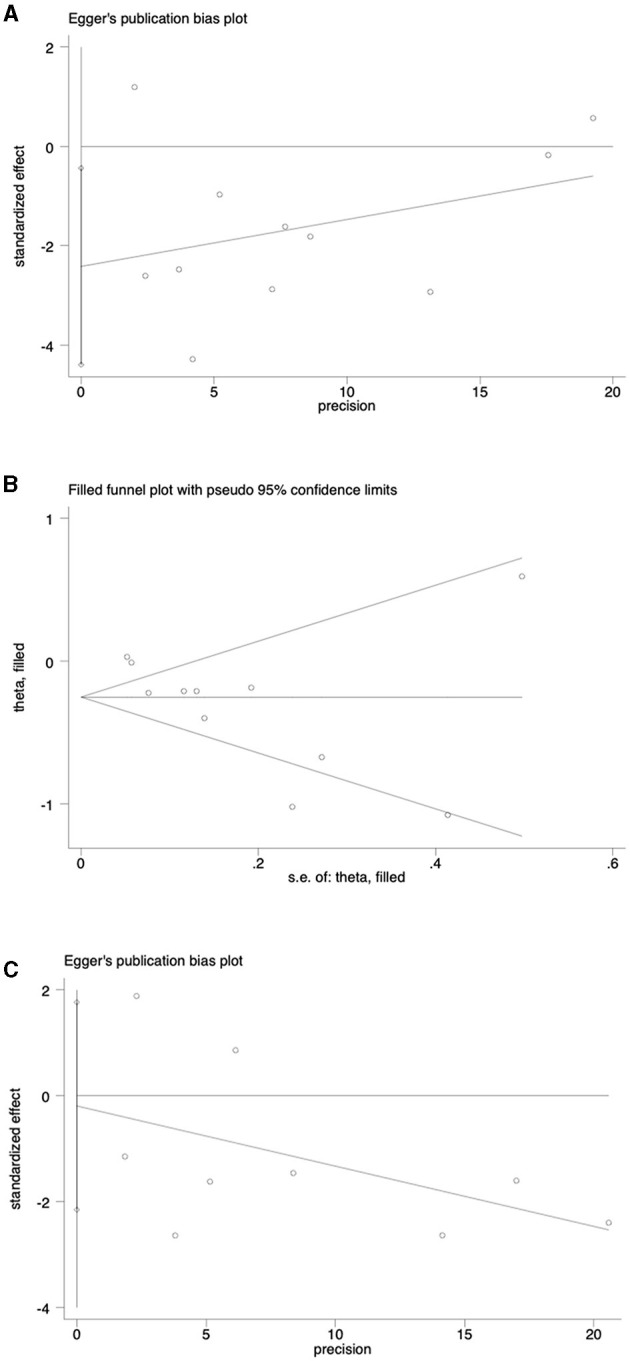
(A) Analysis of publication bias of outcomes associated with vegetable intake and risk of NAFLD; (B) Publication bias analysis was adjusted for the association between vegetable intake and NAFLD risk; (C) Analysis of publication bias for outcomes associated with fruit consumption and risk of NAFLD.
4 Discussion
This represents the inaugural meta-analysis study investigating the connection between the intake of vegetables and fruits and the risk of NAFLD. A total of 11 studies involving 493,682 participants were included in the analysis, and the results suggest a negative correlation between the consumption of vegetables and fruits and the risk of NAFLD.
Dietary fiber, which is abundant in fruits and vegetables, is a type of short-chain fatty acids (SCFAs) produced by the fermentation of intestinal microorganisms in the gastrointestinal tract. These SCFAs, like propionic acid and butyric acid, have numerous benefits, including maintaining the integrity of the intestinal barrier, reducing the inflammatory reaction in the liver, regulating appetite, and maintaining glucose balance at the systemic level. This is all helpful in maintaining the energy balance of the liver, improving insulin sensitivity, and regulating liver lipid metabolism (34, 35). Furthermore, dietary fiber also enhances satiety, thus promoting calorie restriction (36). Additionally, fruits and vegetables are rich in antioxidants, including vitamin C, vitamin E, beta-carotene, polyphenols, which neutralize free radicals and mitigate the detrimental effects of oxidative stress on the liver (37–41). It should also be noted that NAFLD is frequently accompanied by an inflammatory response. Therefore, inhibiting inflammation is crucial to alleviating NAFLD symptoms. Fruits and vegetables are rich in polyphenols and flavonoids, which possess anti-inflammatory properties and can reduce the severity of inflammation in the liver (41, 42).
It is observed that in some of the included studies, the relationship between intake levels of vegetables (23, 26, 28, 29, 32) and fruits (23, 28, 30–33) and the prevalence of NAFLD were not consistent with the conclusion in this meta-analysis. In particular, the proportion of related studies on fruit intake with inconsistent conclusions with this meta-analysis is relatively high. This discrepancy may be attributed to variations in disease diagnosis methods, study populations, adjustment factors, and dietary assessment methods.
The diagnosis of NAFLD differed across the included studies, and differences in the definition of fatty liver and the degree of medical diagnosis may have led to discrepancies in conclusions. Chan's study (27) measured intrahepatic triglyceride content (IHTG) using 1H-MRS within 8 weeks after the baseline visit of included participants, and the result showed that higher levels of vegetable and fruit intake were associated with a lower prevalence of NAFLD. Liu et al. (28) used B-ultrasound as the diagnostic basis, Giraldi et al. (32) used bright liver pattern and ultrasound features of liver steatosis as the diagnostic basis, and Noureddin et al. (29) identified eligible NAFLD patients through Medicare claims. Their results showed that vegetable and fruit intake levels were not associated with the prevalence of NAFLD. It is worth discussing that in the study with a large sample size conducted in South Korea by Kim and Shin (25), the incidence risk of NAFLD in the female population was related to vegetable and fruit intake, while in the male population, only fruit intake was found to be related. In the overall population, the incidence risk of NAFLD was associated with the intake of both vegetables and fruits. However, this conclusion may have significant bias because the diagnosis of NAFLD is based on the Fatty Liver Index (FLI), using a cutoff value of 60. However, in the study by Kim et al., 46% of the participants were of normal weight or underweight, and the accuracy of FLI in diagnosing NAFLD in lean NAFLD patients was low. This is because patients with low body mass index and NAFLD would not be able to reach the FLI limit for diagnosing NAFLD in the absence of increased GGT or triglycerides (included in the FLI calculation) (43). Furthermore, the accuracy of the critical value of 60 for diagnosing NAFLD is low in Asian populations, including in South Korea, because after the study by Kim et al., the ideal critical value for South Koreans was described to be equal to 29 (44–46).
Furthermore, results may vary between populations. Asians may have a stronger preference for leafy vegetables such as spinach and cabbage, while Europeans and Americans tend to consume vegetables like corn, squash, potatoes, onions, and broccoli, which contain higher levels of starch. This dietary difference could explain the diverse findings regarding the correlation between vegetable and fruit intake and NAFLD among participants in Asia, Europe, and America. Notably, there is limited literature from Europe and the Americas, necessitating cautious interpretation of these results and future confirmation through additional relevant studies. Additionally, we observed different results between males and females (23, 25, 31, 33), which may be attributed to notable differences in their dietary patterns (33). Studies have shown that females tend to increase their intake of fruits and vegetables more than males (47, 48), and there are sex-specific disparities in fatty acid oxidation and regulation of liver de novo lipogenesis (DNL), with males inhibiting DNL less rapidly than females, leading to a shift in cellular metabolism from fatty acid oxidation to esterification (49). Since gender-specific studies are relatively scarce, further clinical data are required to validate these conclusions.
Results may vary when adjusting factors in a study. After adjusting for social and economic status and other factors in Li et al.'s study (33), green leafy vegetables (GLV) were negatively correlated with NAFLD. However, further adjustment for BMI eliminated this negative correlation. We found that adjusting for BMI did not significantly alter the results in multiple studies, including Noureddin et al.'s study (29). Although GLV intake is negatively correlated with NAFLD in normal/overweight individuals, obesity-related metabolic complications such as hyperlipidemia and insulin resistance may significantly increase liver lipids, resulting in decreased insulin sensitivity. These complications cannot be regulated by lipid metabolism and GLV intake (50). Additionally, some reports show that obese individuals significantly underestimate their dietary intake in self-recording or interview evaluation (51), which may explain why some studies did not observe the relationship between GLV intake and NAFLD after adjusting for BMI. Liu et al. (28) investigated the relationship between dietary patterns and NAFLD in Chinese adolescents, finding no association between fruit and vegetable intake and the occurrence of NAFLD. Teenagers usually have excellent physiological functions and efficiently absorb nutrients from food. Meanwhile, teenagers are more prone to unhealthy eating patterns that could affect their BMI and contribute to differences in conclusions. Therefore, additional studies are required to confirm the reliability of this conclusion.
In studies examining the intake levels of total vegetables and total fruits (25) and their association with the prevalence of NAFLD, results show a negative correlation. However, research by Liu et al. (28) and Giraldi et al. (32) indicates no relationship between vegetable and fruit intake levels and the prevalence of NAFLD. Guo et al.'s study (26) found significant results for fruit intake but not for vegetable intake. These studies included vegetables and fruits within dietary patterns, which are typically composed of independent or interactive foods and complex nutrient combinations that affect human metabolism. Therefore, it is challenging to exclude the synergistic effects of nutritional foods on NAFLD. Furthermore, not all types of vegetables and fruits are associated with a reduced risk of chronic diseases, as they contain different components and bioactive phytochemicals (52). While vegetables are generally considered low-carbohydrate foods and those with high dietary fiber levels may reduce the risk of NAFLD (33), excessive intake of starchy vegetables might increase blood glucose and insulin resistance, which is detrimental to NAFLD patients (53). Similarly, fruits contain natural fructose, and high fructose intake increases hepatic de novo lipogenesis (DNL), reduces fatty acid β-oxidation (FAO), and leads to fatty acid deposition (54). Excessive fructose intake can also promote the development of NAFLD. Additionally, the type of fructose—natural fructose from fruits vs. industrial fructose—might lead to different research conclusions. In Tajima et al.'s study (23), fruit intake was negatively correlated with the fatty liver index in the elderly, whose dietary fructose mainly came from fruits (55). Despite inquiries about fruit intake in studies, younger individuals might mistakenly count industrial fructose and fruit juice consumption as fruit intake. This could mean that, among the younger population, the harmful effects of industrial fructose and soft drinks on NAFLD might outweigh the protective effects of fruits on NAFLD (55). These factors may explain the inconsistencies between the conclusions regarding fruit intake in the included studies and the results of this meta-analysis.
In addition, there are some factors that cannot be avoided in the included studies. For example, in Giraldi et al. (32), there may be large differences in samples and high variability, resulting in wide confidence intervals, and outcomes may be affected. In Li et al.'s study (33), fruit intake was recorded for both NAFLD patients and the control group. However, the results were not significant, possibly due to differences in the study population, adjustment factors, dietary assessment methods, or recall bias in participants' reporting of fruit intake, leading to the omission of ORs. This does not meet the inclusion criteria for studies on fruit intake and NAFLD prevalence in our research and consequently contributes to a degree of selection bias in this study. Furthermore, the assessment questionnaire itself has self-reporting bias and subjectivity, and different assessment methods have different and limited contents, such as the lack of eating methods of vegetables and fruits, the choice of types of vegetables and fruits, the combination of food and the time of eating, which may also lead to different outcomes.
5 Strengths and limitations
Our study has various strengths. Firstly, it is the first meta-analysis to investigate the association between vegetable and fruit intake and incidence of NAFLD, utilizing large sample sizes. Secondly, in most of the studies included in the meta-analysis, the incidence of major NAFLD risk factors was controlled. Finally, we conducted subgroup analysis and stratification analysis of confounding factors to explore the sources of heterogeneity in the association between vegetable and fruit intake and NAFLD events.
Our study has a few limitations. Firstly, the included studies in our analysis encompassed case-control studies. As these studies employed food assessment questionnaires to estimate dietary intake, we cannot entirely rule out the possibility of measurement errors resulting from under- or over-reporting of food group intake due to participants' subjective judgments or memory biases. This could introduce recall and selection bias. Secondly, even though most studies adjusted for potential risk factors of NAFLD, residual confounding is always a concern in all observational studies. Finally, ORs were pooled from the highest and lowest intake levels, but intake levels were not always consistent across studies. Because of limited data, we were unable to include all studies in the dose-response analysis.
6 Conclusion
In conclusion, this systematic review and meta-analysis provide evidence that higher fruit and vegetable intake is linked to a lower risk of NAFLD. However, given that the relationship between vegetable intake and NAFLD incidence varies across different populations (age, sex, and ethnicity), types of vegetables, and fruits, more high-quality prospective studies are desired to further elucidate this connection. Additionally, there are studies suggesting that excessive fruit intake may actually promote the development of NAFLD.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding authors.
Author contributions
RW: Data curation, Formal analysis, Investigation, Methodology, Software, Visualization, Writing – original draft. RY: Data curation, Formal analysis, Methodology, Software, Writing – review & editing. JJ: Investigation, Project administration, Software, Writing – review & editing. FL: Data curation, Investigation, Software, Writing – review & editing. HZ: Investigation, Software, Visualization, Writing – review & editing. ZC: Conceptualization, Methodology, Resources, Supervision, Writing – review & editing. HW: Project administration, Supervision, Validation, Writing – review & editing. SY: Conceptualization, Methodology, Project administration, Supervision, Validation, Writing – review & editing. JL: Conceptualization, Funding acquisition, Methodology, Project administration, Resources, Supervision, Validation, Writing – review & editing.
Funding Statement
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by the Fund Project: the National Natural Science Foundation of China (82174330); Scientific Research Fund of Shaanxi Provincial Science and Technology Department (2020ZDLSF05-15, 2022JQ-965, and No. 2022SF-338); Innovation Team of Science and Technology Department of Shaanxi Province (2022TD-55); Shaanxi Provincial Central Management Bureau team (2022-SLRH-LJ-002); Fund of Shaanxi Provincial Central Management Bureau (No. 2021-02-ZZ-004); and Scientific Research Foundation of Science and Technology Bureau of Xianyang City (L2022ZDYFSF007).
Abbreviations
NAFLD, non-alcoholic fatty liver disease; CI, confidence interval; OR, relative risk; NOS, Newcastle-Ottawa Scale; FAO, fatty acid beta-oxidation.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2024.1398184/full#supplementary-material
The complete retrieval formula.
Subgroup of the association between vegetable intake and the risk of NAFLD. (A) Research type; (B) Nation state; (C) Assessment questionnaire.
Stratified analysis of the association between vegetable intake and the risk of NAFLD (relevant). (A) age; (B) gender; (C) Smoking status; (D) Alcohol Consumption status; (E) physical exercise; (F) Energy intake; (G) BIM; (H) economic income; (I) educational level; (J) Family history of disease.
Stratified analysis of the association between vegetable intake and the risk of NAFLD (irrelevant). (A) High blood pressure, high cholesterol; (B) diabetes; (C) Coffee intake; (D) Soft drink intake; (E) Vegetable intake; (F) Fruit intake; (G) waistline.
Subgroup of the association between fruit consumption and the risk of NAFLD. (A) Study type heterogeneity; (B) National heterogeneity; (C) Heterogeneity of fruit intake was assessed.
Stratified analyses of the association between fruit consumption and the risk of NAFLD (relevant). (A) Alcohol Consumption status; (B) BMI; (C) educational level; (D) energy intake; (E) physical exercise.
Stratified analyses of the association between fruit consumption and the risk of NAFLD (irrelevant). (A) age; (B) gender; (C) smoking status; (D) Coffee intake; (E) Soft drink consumption; (F) diabetes; (G) Family history of disease; (H) waistline.
References
- 1.Loomba R, Sanyal AJ. The global NAFLD epidemic. Nat Rev Gastroenterol Hepatol. (2013) 10:686–90. 10.1038/nrgastro.2013.171 [DOI] [PubMed] [Google Scholar]
- 2.Huang DQ, El-Serag HB, Loomba R. Global epidemiology of NAFLD-related HCC: trends, predictions, risk factors and prevention. Nat Rev Gastroenterol Hepatol. (2021) 18:223–38. 10.1038/s41575-020-00381-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Estes C, Anstee QM, Arias-Loste MT, Bantel H, Bellentani S, Caballeria J, et al. Modeling NAFLD disease burden in China, France, Germany, Italy, Japan, Spain, United Kingdom, and United States for the period 2016-2030. J Hepatol. (2018) 69:896–904. 10.1016/j.jhep.2018.05.036 [DOI] [PubMed] [Google Scholar]
- 4.Li J, Zou B, Yeo YH, Feng Y, Xie X, Lee DH, et al. Prevalence, incidence, and outcome of non-alcoholic fatty liver disease in Asia, 1999-2019: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol. (2019) 4:389–98. 10.1016/S2468-1253(19)30039-1 [DOI] [PubMed] [Google Scholar]
- 5.Younossi ZM. Non-alcoholic fatty liver disease - a global public health perspective. J Hepatol. (2019) 70:531–44. 10.1016/j.jhep.2018.10.033 [DOI] [PubMed] [Google Scholar]
- 6.Vernon G, Baranova A, Younossi ZM. Systematic review: the epidemiology and natural history of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis in adults. Aliment Pharmacol Ther. (2011) 34:274–85. 10.1111/j.1365-2036.2011.04724.x [DOI] [PubMed] [Google Scholar]
- 7.Petersen KF, Dufour S, Feng J, Befroy D, Dziura J, Dalla Man C, et al. Increased prevalence of insulin resistance and nonalcoholic fatty liver disease in Asian-Indian Men. Proc Natl Acad Sci USA. (2006) 103:18273–7. 10.1073/pnas.0608537103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Younossi Z, Stepanova M, Ong JP, Jacobson IM, Bugianesi E, Duseja A, et al. Nonalcoholic steatohepatitis is the fastest growing cause of hepatocellular carcinoma in liver transplant candidates. Clin Gastroenterol Hepatol. (2019) 17:57. 10.1016/j.cgh.2018.05.057 [DOI] [PubMed] [Google Scholar]
- 9.Younossi ZM, Paik JM, Henry L, Yang J, Fernandes G, Stepanova M, et al. The growing economic and clinical burden of nonalcoholic steatohepatitis (NASH) in the United States. J Clin Exp Hepatol. (2023) 13:454–67. 10.1016/j.jceh.2022.12.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Terai S, Buchanan-Hughes A, Ng A, Lee IH, Hasegawa K. Comorbidities and healthcare costs and resource use of patients with nonalcoholic fatty liver disease (NAFLD) and nonalcoholic steatohepatitis (NASH) in the Japan medical data vision database. J Gastroenterol. (2021) 56:274–84. 10.1007/s00535-021-01759-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Buzzetti E, Pinzani M, Tsochatzis EA. The multiple-hit pathogenesis of non-alcoholic fatty liver disease (NAFLD). Metabolism. (2016) 65:1038–48. 10.1016/j.metabol.2015.12.012 [DOI] [PubMed] [Google Scholar]
- 12.Juanola O, Martínez-López S, Francés R, Gómez-Hurtado I. Non-alcoholic fatty liver disease: metabolic, genetic, epigenetic and environmental risk factors. Int J Environ Res Publ Health. (2021) 18:5227. 10.3390/ijerph18105227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Riazi K, Raman M, Taylor L, Swain MG, Shaheen AA. Dietary patterns and components in nonalcoholic fatty liver disease (NAFLD): what key messages can health care providers offer? Nutrients. (2019) 11:122878. 10.3390/nu11122878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brunner KT, Henneberg CJ, Wilechansky RM, Long MT. Nonalcoholic fatty liver disease and obesity treatment. Curr Obes Rep. (2019) 8:220–8. 10.1007/s13679-019-00345-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jelenik T, Kaul K, Séquaris G, Flögel U, Phielix E, Kotzka J, et al. Mechanisms of insulin resistance in primary and secondary nonalcoholic fatty liver. Diabetes. (2017) 66:2241–53. 10.2337/db16-1147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Barrea L, Verde L, Savastano S, Colao A, Muscogiuri G. Adherence to mediterranean diet: any association with NAFLD? Antioxidants. (2023) 12:71318. 10.3390/antiox12071318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Promrat K, Kleiner DE, Niemeier HM, Jackvony E, Kearns M, Wands JR, et al. Randomized controlled trial testing the effects of weight loss on nonalcoholic steatohepatitis. Hepatology. (2010) 51:121–9. 10.1002/hep.23276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Powell EE, Wong VW-S, Rinella M. Non-alcoholic fatty liver disease. Lancet. (2021) 397:2212–24. 10.1016/S0140-6736(20)32511-3 [DOI] [PubMed] [Google Scholar]
- 19.Alisi A, Cianfarani S, Manco M, Agostoni C, Nobili V. Non-alcoholic fatty liver disease and metabolic syndrome in adolescents: pathogenetic role of genetic background and intrauterine environment. Ann Med. (2012) 44:29–40. 10.3109/07853890.2010.547869 [DOI] [PubMed] [Google Scholar]
- 20.Carlsen MH, Halvorsen BL, Holte K, Bøhn SK, Dragland S, Sampson L, et al. The total antioxidant content of more than 3100 foods, beverages, spices, herbs and supplements used worldwide. Nutr J. (2010) 9:3. 10.1186/1475-2891-9-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Joseph SV, Edirisinghe I, Burton-Freeman BM. Fruit polyphenols: a review of anti-inflammatory effects in humans. Crit Rev Food Sci Nutr. (2016) 56:419–44. 10.1080/10408398.2013.767221 [DOI] [PubMed] [Google Scholar]
- 22.Salomone F, Godos J, Zelber-Sagi S. Natural antioxidants for non-alcoholic fatty liver disease: molecular targets and clinical perspectives. Liver Int. (2016) 36:12975. 10.1111/liv.12975 [DOI] [PubMed] [Google Scholar]
- 23.Tajima R, Kimura T, Enomoto A, Saito A, Kobayashi S, Masuda K, et al. No association between fruits or vegetables and non-alcoholic fatty liver disease in middle-aged men and women. Nutrition. (2019) 61:119–24. 10.1016/j.nut.2018.10.016 [DOI] [PubMed] [Google Scholar]
- 24.Emamat H, Farhadnejad H, Tangestani H, Saneei Totmaj A, Poustchi H, Hekmatdoost A. Association of allium vegetables intake and non-alcoholic fatty liver disease risk: a case-control study. Nutr Food Sci. (2020) 50:1075–83. 10.1108/NFS-11-2019-0334 [DOI] [Google Scholar]
- 25.Kim S-A, Shin S. Fruit and vegetable consumption and non-alcoholic fatty liver disease among Korean adults: a prospective cohort study. J Epidemiol Community Health. (2020) 74:1035–42. 10.1136/jech-2020-214568 [DOI] [PubMed] [Google Scholar]
- 26.Guo W, Ge X, Lu J, Xu X, Gao J, Wang Q, et al. Diet and risk of non-alcoholic fatty liver disease, cirrhosis, and liver cancer: a large prospective cohort study in UK Biobank. Nutrients. (2022) 14:245335. 10.3390/nu14245335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chan R, Wong VW-S, Chu WC-W, Wong GL-H, Li LS, Leung J, et al. Diet-quality scores and prevalence of nonalcoholic fatty liver disease: a population study using proton-magnetic resonance spectroscopy. PLoS ONE. (2015) 10:e0139310. 10.1371/journal.pone.0139310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu X, Peng Y, Chen S, Sun Q. An observational study on the association between major dietary patterns and non-alcoholic fatty liver disease in Chinese adolescents. Medicine. (2018) 97:e0576. 10.1097/MD.0000000000010576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Noureddin M, Zelber-Sagi S, Wilkens LR, Porcel J, Boushey CJ, Le Marchand L, et al. Diet associations with nonalcoholic fatty liver disease in an ethnically diverse population: the multiethnic cohort. Hepatology. (2020) 71:1940–52. 10.1002/hep.30967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tutunchi H, Saghafi-Asl M, Asghari-Jafarabadi M, Ostadrahimi A. Association between dietary patterns and non-alcoholic fatty liver disease: results from a case-control study. Arch Iran Med. (2021) 24:35–42. 10.34172/aim.2021.06 [DOI] [PubMed] [Google Scholar]
- 31.Du L-J, He Z-Y, Gu X, Hu X, Zhang X-X, Yang L-J, et al. Inverse association of fruit and vegetable consumption with nonalcoholic fatty liver disease in Chinese patients with type 2 diabetes mellitus. Nutrients. (2022) 14:214559. 10.3390/nu14214559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Giraldi L, Miele L, Aleksovska K, Manca F, Leoncini E, Biolato M, et al. Mediterranean diet and the prevention of non-alcoholic fatty liver disease: results from a case-control study. Eur Rev Med Pharmacol Sci. (2020) 24:7391–8. [DOI] [PubMed] [Google Scholar]
- 33.Li H, Wang X, Ye M, Zhang S, Zhang Q, Meng G, et al. Does a high intake of green leafy vegetables protect from NAFLD? Evidence from a large population study. Nutr Metab Cardiovasc Dis. (2021) 31:1691–701. 10.1016/j.numecd.2021.01.009 [DOI] [PubMed] [Google Scholar]
- 34.Zhang S, Zhao J, Xie F, He H, Johnston LJ Dai X, et al. Dietary fiber-derived short-chain fatty acids: a potential therapeutic target to alleviate obesity-related nonalcoholic fatty liver disease. Obes Rev. (2021) 22:e13316. 10.1111/obr.13316 [DOI] [PubMed] [Google Scholar]
- 35.Krawczyk M, Maciejewska D, Ryterska K, Czerwińka-Rogowska M, Jamioł-Milc D, Skonieczna-Żydecka K, et al. Gut permeability might be improved by dietary fiber in individuals with nonalcoholic fatty liver disease (NAFLD) undergoing weight reduction. Nutrients. (2018) 10:111793. 10.3390/nu10111793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.De Nucci S, Rinaldi R, Di Chito M, Donghia R, Giannuzzi V, Shahini E, et al. The replacement of only one portion of starchy carbohydrates with green leafy vegetables regresses mid and advanced stages of NAFLD: results from a prospective pilot study. Nutrients. (2023) 15:102289. 10.3390/nu15102289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wu H, Guo J-L, Yao J-J, Yu J-J, Xia R-Y, Huang W-Q, et al. Serum vitamin C levels and risk of non-alcoholic fatty liver disease: results from a cross-sectional study and Mendelian randomization analysis. Front Nutr. (2023) 10:1162031. 10.3389/fnut.2023.1162031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Scorletti E, Creasy KT, Vujkovic M, Vell M, Zandvakili I, Rader DJ, et al. Dietary vitamin E intake is associated with a reduced risk of developing digestive diseases and nonalcoholic fatty liver disease. Am J Gastroenterol. (2022) 117:927–30. 10.14309/ajg.0000000000001726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen Y, Wu M, Chen F, Wen X, Zhao L, Li G, et al. Potential role of inflammation in relation to dietary sodium and β-carotene with non-alcoholic fatty liver disease: a mediation analysis. Nutr Diabetes. (2022) 12:40. 10.1038/s41387-022-00218-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yang K, Chen J, Zhang T, Yuan X, Ge A, Wang S, et al. Efficacy and safety of dietary polyphenol supplementation in the treatment of non-alcoholic fatty liver disease: a systematic review and meta-analysis. Front Immunol. (2022) 13:949746. 10.3389/fimmu.2022.949746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Abenavoli L, Larussa T, Corea A, Procopio AC, Boccuto L, Dallio M, et al. Dietary polyphenols and non-alcoholic fatty liver disease. Nutrients. (2021) 13:20494. 10.3390/nu13020494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tan P, Jin L, Qin X, He B. Natural flavonoids: potential therapeutic strategies for non-alcoholic fatty liver disease. Front Pharmacol. (2022) 13:1005312. 10.3389/fphar.2022.1005312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zahrawi FM, Mehal WZ. Letter to the editor: concerns regarding the use of fatty liver index in studies of lean NAFLD. Hepatology. (2024) 79:E129. 10.1097/HEP.0000000000000754 [DOI] [PubMed] [Google Scholar]
- 44.Cho E-J, Jung G-C, Kwak M-S, Yang J-I, Yim J-Y, Yu S-J, et al. Fatty liver index for predicting nonalcoholic fatty liver disease in an asymptomatic Korean population. Diagnostics. (2021) 11:122233. 10.3390/diagnostics11122233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yang B-L, Wu W-C, Fang K-C, Wang Y-C, Huo T-I, Huang Y-H, et al. External validation of fatty liver index for identifying ultrasonographic fatty liver in a large-scale cross-sectional study in Taiwan. PLoS ONE. (2015) 10:e0120443. 10.1371/journal.pone.0120443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Takahashi S, Tanaka M, Higashiura Y, Mori K, Hanawa N, Ohnishi H, et al. Prediction and validation of nonalcoholic fatty liver disease by fatty liver index in a Japanese population. Endocr J. (2022) 69:463–71. 10.1507/endocrj.EJ21-0563 [DOI] [PubMed] [Google Scholar]
- 47.Cronin FJ, Krebs-Smith SM, Wyse BW, Light L. Characterizing food usage by demographic variables. J Am Diet Assoc. (1982) 81:661–73. 10.1016/S0002-8223(21)38912-X [DOI] [PubMed] [Google Scholar]
- 48.Stea TH, Nordheim O, Bere E, Stornes P, Eikemo TA. Fruit and vegetable consumption in Europe according to gender, educational attainment and regional affiliation-a cross-sectional study in 21 European countries. PLoS ONE. (2020) 15:e0232521. 10.1371/journal.pone.0232521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pramfalk C, Pavlides M, Banerjee R, McNeil CA, Neubauer S, Karpe F, et al. Sex-specific differences in hepatic fat oxidation and synthesis may explain the higher propensity for NAFLD in men. J Clin Endocrinol Metab. (2015) 100:4425–33. 10.1210/jc.2015-2649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Donnelly KL, Smith CI, Schwarzenberg SJ, Jessurun J, Boldt MD, Parks EJ. Sources of fatty acids stored in liver and secreted via lipoproteins in patients with nonalcoholic fatty liver disease. J Clin Invest. (2005) 115:1343–51. 10.1172/JCI200523621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Heitmann BL, Lissner L. Dietary underreporting by obese individuals–is it specific or non-specific? Br Med J. (1995) 311:986–9. 10.1136/bmj.311.7011.986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Boeing H, Bechthold A, Bub A, Ellinger S, Haller D, Kroke A, et al. Critical review: vegetables and fruit in the prevention of chronic diseases. Eur J Nutr. (2012) 51:637–63. 10.1007/s00394-012-0380-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Li X, Zhang T, Li H, Zhou Z, Li M, Zeng X, et al. Associations between intake of starchy and non-starchy vegetables and risk of hepatic steatosis and fibrosis. Hepatol Int. (2022) 16:846–57. 10.1007/s12072-022-10368-x [DOI] [PubMed] [Google Scholar]
- 54.Mao T, Sun Y, Xu X, He K. Overview and prospect of NAFLD: significant roles of nutrients and dietary patterns in its progression or prevention. Hepatol Commun. (2023) 7:234. 10.1097/HC9.0000000000000234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kanerva N, Sandboge S, Kaartinen NE, Männistö S, Eriksson JG. Higher fructose intake is inversely associated with risk of nonalcoholic fatty liver disease in older Finnish adults. Am J Clin Nutr. (2014) 100:1133–8. 10.3945/ajcn.114.086074 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The complete retrieval formula.
Subgroup of the association between vegetable intake and the risk of NAFLD. (A) Research type; (B) Nation state; (C) Assessment questionnaire.
Stratified analysis of the association between vegetable intake and the risk of NAFLD (relevant). (A) age; (B) gender; (C) Smoking status; (D) Alcohol Consumption status; (E) physical exercise; (F) Energy intake; (G) BIM; (H) economic income; (I) educational level; (J) Family history of disease.
Stratified analysis of the association between vegetable intake and the risk of NAFLD (irrelevant). (A) High blood pressure, high cholesterol; (B) diabetes; (C) Coffee intake; (D) Soft drink intake; (E) Vegetable intake; (F) Fruit intake; (G) waistline.
Subgroup of the association between fruit consumption and the risk of NAFLD. (A) Study type heterogeneity; (B) National heterogeneity; (C) Heterogeneity of fruit intake was assessed.
Stratified analyses of the association between fruit consumption and the risk of NAFLD (relevant). (A) Alcohol Consumption status; (B) BMI; (C) educational level; (D) energy intake; (E) physical exercise.
Stratified analyses of the association between fruit consumption and the risk of NAFLD (irrelevant). (A) age; (B) gender; (C) smoking status; (D) Coffee intake; (E) Soft drink consumption; (F) diabetes; (G) Family history of disease; (H) waistline.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding authors.