Abstract
Bloodstream infections (BSI) are one of the leading causes of morbidity and mortality in children and young adults receiving chemotherapy for malignancy or undergoing hematopoietic stem cell transplantation (HSCT). Antibiotic prophylaxis is commonly used to decrease the risk of BSI; however, antibiotics carry an inherent risk of complications. The aim of this manuscript is to review levofloxacin prophylaxis in pediatric oncology patients and HSCT recipients. We reviewed published literature on levofloxacin prophylaxis to prevent BSI in pediatric oncology patients and HSCT recipients. Nine manuscripts were identified. The use of levofloxacin is indicated in neutropenic children and young adults receiving intensive chemotherapy for leukemia or undergoing HSCT. These results support the efficacy of levofloxacin in pediatric patients with leukemia receiving intensive chemotherapy and should be considered in pediatric patients undergoing HSCT prior to engraftment.
Keywords: Blood stream infection, levofloxacin, pediatric bone marrow transplant, pediatric cancer, prophylaxis
Introduction
Bloodstream infections (BSI) are one of the leading causes of morbidity and mortality in pediatric oncology patients receiving intensive chemotherapy and undergoing hematopoietic stem cell transplantation (HSCT).1–3 Bacterial BSIs (BBSIs) have been reported in up to 66% of pediatric oncology and HSCT patients.4 Well-documented risk factors for BSIs in this patient population include the presence of a central venous catheter (CVC), high-dose chemotherapy, extended periods of neutropenia, graft-vs-host disease (GVHD), and mucosal barrier injury.5–8 Specifically, in adult HSCT recipients (age >18 years old), risk factors for BSIs include the use of unrelated graft sources, myeloablative conditioning regimens, acute GVHD, mucositis, transplant-associated thrombotic microangiopathy (TA-TMA), primary malignant disease, andsteroiduse.6,9 In multivariate analysis, pre-engraftmentBSIhasbeenassociatedwithengraftmentfailureandhig h-riskdiseasestatusatthetimeofHSCT.10
Prior large studies in adult oncology patients receiving myelosuppressive chemotherapy demonstrated levofloxacin prophylaxis diminished the incidence of fever, probable infection, and hospitalization and showed beneficial impacts on mortality.11,12 In comparison, studies evaluating trimethoprim-sulfamethoxazole, erythromycin, and amoxicillin-clavulanate prophylaxis show no clear overall benefit.13–15 In contrast, early studies using fluoroquinolone prophylaxis in pediatric acute leukemia patients reduced hospitalization rates, intensive care admissions, and bacteremia rates compared to historical controls.16,17
The use of prophylactic antibiotics to prevent bacterial infections has not been universally adopted for pediatric oncology and HSCT recipients. Previous clinical practice guidelines for antibacterial prophylaxis in pediatric cancer and HSCT patients only administered a weak strength of recommendation based on high-quality evidence to consider systematic antibacterial prophylaxis in children with acute myelogenous leukemia (AML) and relapsed acute lymphoblastic leukemia (ALL). For autologous HSCT (auto-HSCT) and allogeneic HSCT (allo-HSCT) patients, the guideline made weak recommendations against routine antibiotic prophylaxis in this patient cohort.18 This may be secondary to the negative consequences of routine antibiotic administration, including Clostridium difficile (C. difficile)associated diarrhea, development of bacterial resistance, and antibiotic-related toxicities.
Data gathered internally for quality improvement related to levofloxacin use by the Children’s Hospitals’ Solutions for Patient Safety (SPS) Hematology-Oncology central line-associated bloodstream infection (CLABSI) Improvement team suggests significant practice variation.19 In response, SPS Hematology-Oncology CLABSI leadership convened to conduct a literature review and synthesis to promote evidence-based standards. The objective of this manuscript is to provide a comprehensive summary of the current published data on levofloxacin prophylaxis in pediatric oncology patients and HSCT recipients.
Materials and methods
To conduct this review, a literature search using PubMed and Google Scholar was conducted on August 4, 2022, and updated on December 31, 2022, using the following search terms: “acute leukemia,” “pediatric,” “bone marrow transplant,” “hematopoietic stem cell transplantation,” “bacterial bloodstream infection,” “bone marrow transplant,” “fluoroquinolone,” “levofloxacin,” “prophylaxis.” No filters or publication time limits were applied to the search. Only studies that included children <18 years of age were included. This resulted in a total of 11 pediatric-focused studies. Because of this review’s specific focus, studies were excluded if levofloxacin prophylaxis was not explicitly studied; specifically, if the study focused only on other antibiotics and/or fluoroquinolone prophylaxis, these studies were excluded. All search results were imported to the EndNoteX9.0 reference manager, and all duplicates were removed.
Two reviewers screened each record; each report was retrieved, and no automation tools were used. The data obtained from each report included the number of patients enrolled in levofloxacin prophylaxis-focused studies, the age of the patient populations, and study outcomes, including bacterial BSI rate.
Results
Levofloxacin spectrum of antibacterial activity
Levofloxacin belongs to the fluoroquinolone family of antibiotics. Other fluoroquinolones in this class include ciprofloxacin, moxifloxacin, and gemifloxacin. Fluoroquinolones are highly active against Gram-positive and Gram-negative pathogens.20 Levofloxacin has increased activity against many respiratory pathogens, including Streptococcus pneumoniae, Mycoplasma pneumoniae, and Chlamydophila pneumoniae.20 Levofloxacin also has documented activity against some of the most common gram-positive organisms isolated from patients with hematologic malignancies, including coagulase-negative staphylococci, Staphylococcus aureus, and enterococci.21 The recommendations are not to use fluoroquinolones as first-line agents in children younger than 18, except when specific indications exist.
Associated toxicities with levofloxacin
Fluoroquinolones are generally well-tolerated. However, side effects and risks associated with this class of antibiotics can include transient arthralgias, tendinopathies, QTc interval prolongation, central nervous system toxicities, thrombocytopenia, hepatic dysfunction, renal dysfunction, and C. difficile disease.22 Additionally, although antibiotic prophylaxis can reduce the risk of serious infections in these immunocompromised patients, barriers to universal implementation have included the contribution toward breeding antibiotic resistance. Prior studies have analyzed stool samples with metagenomic sequencing from newly diagnosed pediatric patients being treated for ALL who received either levofloxacin or no antibacterial prophylaxis. The sequencing data showed there was an increase in the relative abundance of trimethoprim-sulfamethoxazole resistance genes (estimated mean fold change 5.9, 95% CI 3.6–9.6%, p < 0.0001), but this was not changed by levofloxacin prophylaxis (p = 0.46). However, the predominance of topoisomerase point mutations did increase over the course of induction chemotherapy in ALL patients who received levofloxacin prophylaxis (mean prevalence 10.4%, 95% CI 3.2–25.4) compared to baseline prior to the start of chemotherapy (mean prevalence 3.7%, 95% CI 0.2–22.5). No changes were observed in the gene expression of aminoglycoside, β-lactam, vancomycin, or multidrug resistance genes in the levofloxacin and no prophylaxis groups.23
Outcomes in HSCT patients who develop bloodstream infections
BSI alone is a significant independent predictor of treatment-related mortality (TRM). Poutsiaka et al. described increased TRM (HR 1.79, 95% CI 1.18–2.73, p = 0.007) after adjusting acute GVHD and allo-HSCT, with both predicting death three months after HSCT. In addition, they found that bacteremia with gram-negative rods (GNR) and vancomycin-resistant enterococcus (VRE) were significantly associated with increased mortality.24 Liu et al. confirmed the negative impact of BSI on 6-month survival post-HSCT and demonstrated that patients who developed BSI had an increased length of hospital stay (LOS).25 In a retrospective analysis, Dandoy et al. studied outcomes from 170 BSIs diagnosed in 100 (27%) of 374 pediatric patients undergoing HSCT.6 They showed that BSIs were associated with increased morbidity and mortality. Specifically, one-year non-relapse mortality (NRM) was significantly increased in patients with one (20/58, 34%) and more than one (17/30, 56%) BSI in the first year post-HSCT compared with those who did not develop BSI (27/194, 14%) (p = <0.0001). In addition, an increased risk of one-year NRM was noted in patients with at least one mucosal barrier injury laboratory-confirmed bloodstream infection (MBI-LCBI, OR 1.94, p = 0.018) and at least one secondary BSI (OR 2.87, p = 0.0023) but not in patients with CLABSI (OR 1.17, p = 0.68)6 Levinson et al. showed that in addition to increased NRM, patients who developed early BSI during the conditioning regimen and within ten days after HSCT (and prior to engraftment) had a two-fold increased risk of developing acute GVHD.26 These results demonstrate BSI is associated with significant harm to HSCT patients, increase the risk for adverse outcomes such as GVHD, prolong hospitalization, and increase hospital resource utilization.
Early studies in adult oncology and HSCT patients using levofloxacin prophylaxis
Early studies using fluoroquinolone, specifically levofloxacin, prophylaxis in patients with cancer focused on patients who became neutropenic after receiving chemotherapy for their underlying diagnosis. Levofloxacin prophylaxis decreased gram-positive and gram-negative bacteremia incidence and reduced infection-related mortality. Conversely, as expected, these studies also showed an increased incidence of bacteremia due to fluoroquinolone-resistant strains. Additionally, studies showed a survival advantage in patients receiving levofloxacin prophylaxis despite high resistance rates among bacterial isolates.27 Attempts to temper the emergence of resistant bacterial strains with prophylactic levofloxacin administration have included the use of a rotating schedule of various antibiotics.28
The use of levofloxacin prophylaxis in adult and pediatric cancer patients has largely been limited to retrospective cohort analyses until the first prospective, multicenter, double-blind, randomized, placebo-controlled trial was conducted in Italy from 2001 to 200330. In this study, 760 consecutive adult patients with acute leukemia, solid tumors, or lymphoma were randomized to receive daily oral levofloxacin or placebo. No patients undergoing HSCT were included in this study. Patients were risk stratified based on their underlying disease and expected duration of neutropenia. The primary study endpoint was fever occurrence requiring empirical antibacterial therapy during the period of neutropenia. In the study analysis, fever was evident for the period of neutropenia in 65% of patients who received levofloxacin prophylaxis compared to 85% of patients receiving placebo. More freedom from fever was observed in patients with acute leukemia, those with solid tumors, and those receiving treatment for lymphoma. Overall, the cohort who received levofloxacin had a lower incidence of bacteremias, microbiologically documented infections, and single-agent gram-negative bacteremias, especially due to Escherichia coli, compared to the placebo group. Medication compliance was good and similar in both the levofloxacin and placebo groups. The overall infection-related mortality rate was similar between the two groups, with 2% in the levofloxacin group and 4% in the placebo group (p = 0.36).29
Patients undergoing HSCT have more substantial hurdles as their expected risk period for bacterial infection is commonly more prolonged due to the intense myeloablative conditioning they receive and their neutrophil recovery, depending on the pace of their engraftment. For HSCT patients, prior retrospective studies have compared clinical and microbiological outcomes secondary to the impacts of changes to antibiotic prophylaxis practice. For example, in 2002, the Fred Hutchinson Cancer Research Center changed from ceftazidime to levofloxacin for antibacterial prophylaxis for adult HSCT recipients. The levofloxacin cohort (August 2002–2005) was compared to a group of historical controls who received ceftazidime for antibiotic prophylaxis from 2000 to 2002. This retrospective analysis demonstrated at day 100 from HSCT, patients receiving levofloxacin had increased rates of febrile episodes but had decreased rates of significant bacteremia compared to those receiving ceftazidime (19.2% vs. 29.6%, p = 0.02).30 While overall antibiotic therapy use did not differ between the levofloxacin and ceftazidime groups, the average acquisition costs for the levofloxacin group were lower than the ceftazidime group. Furthermore, in the levofloxacin group, there was no increase in rates of isolation of more resistant bacterial strains, the incidence of C. difficile infection, the incidence of infection at other body sites, and survival.
Initial prospective studies have examined fluoroquinolones (ciprofloxacin) specifically for bacterial infection prophylaxis in HSCT recipients. Still, these studies were performed several decades ago and were not randomized nor placebo-controlled.31,32 One of the first randomized, double-blinded, placebo-controlled single-center studies in HSCT recipients was conducted in Germany in 2007. However, an important difference is that patients in this study received either levofloxacin or placebo within seven days after absolute neutrophil recovery (ANC >0.5×109/L) was achieved following HSCT. The primary aim of this study was to evaluate the incidence of infections with proven or presumed bacterial origin after neutrophil recovery in patients receiving levofloxacin prophylaxis compared to patients receiving placebo. The study enrolled only 18 adult patients, and only 13 could be analyzed with respect to the primary aim, greatly limiting the power of the final analysis. This study demonstrated bacterial infections tended to be lower in patients receiving levofloxacin (20%) compared to placebo (50%), but this difference did not reach statistical significance.33
Levofloxacin prophylaxis in pediatric oncology and HSCT patients
Similar to the published literature in adult cancer and HSCT patients, early studies exclusively performed in pediatric patients were retrospective. There are few studies exclusively evaluating levofloxacin prophylaxis pediatric ALL population. Two international studies based in Saudi Arabia and Indonesia analyzed ciprofloxacin prophylaxis during different phases of ALL therapy and had conflicting results.16,34 In one of the larger, single-center prospective cohort studies of pediatric ALL, patients who received levofloxacin prophylaxis were compared to patients who received no prophylaxis or other prophylaxis during the induction phase of therapy on the total XVI study. Patients who received levofloxacin prophylaxis had decreased odds of febrile neutropenia, bacterial infection, and bloodstream infection by ≥70%. ALL patients who received levofloxacin prophylaxis had lower odds of C. difficile infection and broad-spectrum treatment antibiotic exposure by >95%.35 Additionally, a prospective study analyzing pediatric ALL patients who received either oral levofloxacin or moxifloxacin prophylaxis reported reduced bacteremia rates during induction therapy (10.9% in the prophylaxis group vs. 24.4% in the control group, p < 0.0001).36
Observational studies in pediatric ALL patients have shown no increased rates of neurotoxicity in patients receiving fluoroquinolone antibiotic prophylaxis throughout their therapy.37 Additionally, levofloxacin has been used as a step-down method of prophylaxis in pediatric neutropenic cancer patients and found to reduce intravenous antibiotic use at home and less IV antibiotic initiations within 24 h of a new healthcare encounter up to a week from discharge.38 Furthermore, levofloxacin prophylaxis has been found to be a cost-effective measure in pediatric acute myelogenous leukemia (AML) patients in reducing the frequency of intensive care unit (ICU) admissions and hospital costs.39
Exclusive analyses evaluating pediatric HSCT recipients and levofloxacin prophylaxis are rare. A single-center retrospective study based in Italy compared the outcomes of levofloxacin versus ciprofloxacin prophylaxis in allo-HSCT pediatric patients with hematologic malignancies.40 Levofloxacin prophylaxis correlated with reduced rates of bloodstream infections compared to the ciprofloxacin group (15% vs. 28.3%, p < 0.05) and rates of C. difficile infections (2.5% vs. 15%, p < 0.05). There was no difference in the number of febrile neutropenia days in the levofloxacin group (33.3%) compared to the ciprofloxacin group (36.7%, p = 0.74). Overall mortality at 30 days and 90 days after HSCT was not different between both prophylaxis groups.
The first multicenter, open-label, randomized trial in exclusively pediatric patients (ages 6 months to 21 years old) enrolled 200 patients with acute leukemia and 424 patients undergoing HSCT to receive levofloxacin prophylaxis or placebo between September 2011 and April 2016. Patients with acute leukemia were randomized to receive levofloxacin prophylaxis or placebo during two consecutive cycles of chemotherapy. HSCT recipients were randomized to receive levofloxacin prophylaxis or placebo during a single HSCT procedure. The primary outcome measure was the occurrence of bacteremia during the two chemotherapy cycles for acute leukemia patients or a single transplant procedure for HSCT procedures. The major findings from this study demonstrated a markedly reduced occurrence of bacteremia in acute leukemia patients in the levofloxacin prophylaxis group compared to the control group (21.9% vs. 43.4%, p = 0.001). This difference was not observed in the HSCT cohort, where the risk of bacteremia was similar between the levofloxacin prophylaxis group and the control groups (11.0% vs. 17.3%, p = 0.06). Secondary outcome measure analysis showed that all patients receiving levofloxacin prophylaxis had less fever and neutropenia (71.2% vs. 82.1%, p = 0.002). Additionally, there was no difference observed between the levofloxacin prophylaxis and placebo groups in risk of severe infection, invasive fungal disease, C. difficile-associated diarrhea, or musculoskeletal side effects.41
An observational study of 96 pediatric patients undergoing auto-HSCT compared patients who received levofloxacin prophylaxis to historical controls. Their main observations included a delay in time until the onset of the first fever in the levofloxacin cohort (median of 15 days) compared to historical controls (median of 11 days). Infectious complications were also higher in the historical controls compared to patients who received levofloxacin prophylaxis.42 Risk factors for breakthrough bacteremia on antibiotic prophylaxis with ciprofloxacin have included serotherapy with anti-thymocyte globulin and cord blood as stem cell sources.43
An international and multidisciplinary panel convened to publish a series of clinical practice guidelines for the use of antibacterial prophylaxis administration in pediatric cancer and HSCT patients.18 Based on this expert panel of recommendations, it was strongly recommended that systemic antibacterial prophylaxis for children should not be extended to patients whose therapy is not expected to result in severe neutropenia (ANC <0.5 × 109/L) for at least seven days. And if systemic antibacterial prophylaxis is planned, the preferred agent administered should be levofloxacin because of the recent data published on children and its microbiological spectrum of activity. We have summarized the key pertinent literature in Table 1.
Table 1.
Studies on levofloxacin prophylaxis in pediatric oncology patients or undergoing hematopoietic stem cell transplant.
| Study and Year | Study Design | Diagnosis | Age range | Number of patients enrolled | Main effects of levofloxacin prophylaxis |
|---|---|---|---|---|---|
|
| |||||
| Wolf J et al., 201735 | Single-institutional, observational cohort study | ALL | 0–12 years | 344 |
Bacteremia • Decreased rates of bloodstream infections in levofloxacin prophylaxis group OR 0.42 (95% CI 0.15–1.16, p = 0.09) Fever and neutropenia • No difference in rates of febrile neutropenia with levofloxacin prophylaxis OR 0.1.17 (95% CI 0.64–2.14, p = 0.60) Levofloxacin-associated toxicities • Reduced rates of C. difficile infections in levofloxacin prophylaxis group OR 0.04 (95% CI <0.01–0.36, p < 0.001) Antibiotic exposure • Antibiotic exposure and cumulative antibiotic exposure were significantly greater in patients receiving levofloxacin or other prophylaxis than in those receiving no prophylaxis (p < 0.001 for all comparisons) • Levofloxacin prophylaxis did reduce exposure to cefepime/ceftazidime, vancomycin, meropenem (p < 0.001) and aminoglycosides (p = 0.002) |
| Sulis et al., 201836 | Single-institution, prospective study | ALL | 1–21 years | 1,024 | Bacteremia • Lower rates of bacteremia in prophylaxis group treated on the DFFCI 11–001 protocol compared to the control group treated on the DFCI 05–001 protocol (10.9% vs 24.4%, p < 0.0001) Survival • No difference in rates of death during induction between prophylaxis group and control group (0.9% vs 2%) |
| Karol SE, et al., 202037 | Single-institutional, observational cohort study | ALL | 0–18 years | 598 |
Levofloxacin-associated toxicities • Fluoroquinolone prophylaxis during induction therapy for ALL did not increase the risk of peripheral neurotoxicity in children receiving vincristine during continuation - Any neuropathic pain (Grade 2+) HR 0.75, 95% CI 0.52–1.02 - Any neuropathy (Grade 2+) HR 0.92, 95% CI 0.39–1.82 - Any neuropathic pain or neuropathy (Grade 2+) HR 0.75, 95% CI 0.54–1.04 • High-grade neuropathic pain or neuropathy (Grade 3+) HR 1.06, 95% CI 0.51–2.22 |
| McCormick et al. 202039 | Retrospective cohort cost-effectiveness analysis study | AML | 0–21 years | 2,601 |
Bacteremia cost analysis • Prophylaxis cost $8,491 per bacteremia episode prevented compared with an average added hospital cost of $119,478 Survival cost analysis • Prophylaxis cost $220,457 per death avoided. In sensitivity analysis, at a wiIIingness-to-pay threshold of $100,000 per bacteremia episode avoided, prophylaxis remained cost-effective in 95% of simulations ICU† cost analysis • Prophylaxis cost $81,609 per ICU admission avoided, compared with an average added hospital cost of $94,181 |
| Servidio AG et al., 202140 | Single-institution, retrospective cohort study | HSCT recipients with hematologic malignancies | ≤13 years | 180 | Bacteremia • Reduced rates of bacteremia in levofloxacin group compared to the ciprofloxacin group (15% vs 28.3%, p < 0.05) Fever and neutropenia • Similar rates of fever and neutropenia in levofloxacin group compared to the ciprofloxacin group (33.3% vs 36.7%, p = 0.74) Levofloxacin-associated toxicities • Less C. difficile infections in levofloxacin group compared to the ciprofloxacin group (2.5% vs 15%, p < 0.05) Survival • Similar rates of 90-day overall mortality in levofloxacin group compared to ciprofloxacin group (8.3% vs 1%, p = 1.0) • Similar rates of 30-day overall mortality in levofloxacin group compared to ciprofloxacin group (1.7% vs 1.7%, p = 1.0) |
| Alexander et al., 201841 | Multicenter, open-label, randomized trial, patients | ALL!, AML@ and HSCT# recipients | 6 months–21 years | 624 |
Bacteremia • Acute leukemia: the likelihood of bacteremia was significantly lower in the levofloxacin prophylaxis group than in the control group (22% vs 43%; risk difference, 21.6%; 95% CI, 9%–34%, p = .001) • HSCT group: the risk of bacteremia was not significantly lower in the levofloxacin prophylaxis group (11% vs 17%; risk difference, 6%; 95% CI, 0.3%–13%; p = .06). Fever and neutropenia • Fever and neutropenia were less common in the levofloxacin group (71% vs 82%; risk difference, 11%; 95% CI, 4%–18%; p = .002). Levofloxacin-associated toxicities • No significant differences in C. difficile*-associated diarrhea (2% vs 5%; risk difference, 3%; 95% CI, −0.1% to 6%; p = .07) • No difference in musculoskeletal toxic effects at 2 months (11% vs 16%; risk difference, 5%; 95% CI, −2% to 11%; p = .15) or at 12 months (10% vs 14%; risk difference, 4%; 95% CI, −3% to 12%; p = .28) |
| Hafez et al., 201542 | Observational study, before-and-after study intervention analysis | Pediatric patients undergoing auto-HSCT | <18 years | 96 |
Infection • The incidence of infectious complications was higher in patients without levofloxacin (4/46) than those with levofloxacin (1/50) Fever and neutropenia • Median duration of febrile neutropenia lower in the historical control group compared to levofloxacin prophylaxis group (11 days vs 15 days, p ≤ 0.001) Antibiotic exposure • Median duration of empiric antibiotic use was lower in the levofloxacin group compared to the historical control cohort (10 days vs 14 days, p < 0.001) |
| Davis et al., 202244 | Single-institution, retrospective study with historical controls | AML, relapsed ALL | 6 months–21 years | 135 |
Bacteremia • 60% of patients in the pre-implementation group and 38% of patients in the postimplementation group developed CLABSI% • Reduction in gram negative rod bacteremia but observed a higher percentage of any number of levofloxacin non-susceptible GNR^ BSI events Levofloxacin-associated toxicities • The incidence of MDRO& and C. difficile-associated diarrhea was similar throughout both periods Survival • Death in the post-implementation period was significantly reduced |
| Gardner JC et al., 202245 | Single-institution, retrospective study | AML, auto- or allo-HSCT recipients | Less than 21 years | 60 |
Bacteremia • There was no difference found in the frequency of bacteremia between levofloxacin and clinician-directed prophylaxis (15.6% vs 10.4%, p = 0.49) Fever and neutropenia • No difference in incidence of febrile neutropenia in levofloxacin group vs clinician-directed prophylaxis group (62.5% vs 66.7%, p = 0.70) Levofloxacin-associated toxicities • No difference in rates of C. difficile infections in levofloxacin group vs clinician-directed prophylaxis group (12.5% vs 27.1%, p = 0.17) Survival • No differences in 30-day infection-related mortality between levofloxacin group and clinician-directed prophylaxis group (0% vs 2.1%, p = 1.0) Antibiotic exposure • Similar rates of antibiotic exposure days between both levofloxacin and clinician-directed prophylaxis groups (18.7 days vs 13.6 days, p = 0.31) |
| Margolis EB et al., 202146 | Prospective, single-center, cohort study | ALL | ≤18 years | 49 |
Bacteremia • No difference in bloodstream infections in no prophylaxis group vs levofloxacin group (11% vs 6%, p = 0.62) Fever and neutropenia • No difference in rates of febrile neutropenia in no prophylaxis group and levofloxacin prophylaxis group (67% vs 42%, p = 0.14) Levofloxacin-associated toxicities • Lower rates of C. difficile infection in levofloxacin group compared to no prophylaxis group (0% vs 17%, p = 0.04) • Increase in the prevalence of topoisomerase point mutations in the levofloxacin cohort (mean prevalence was 4% [95% CI 0.2–22.5] at baseline vs. 10% [3.2–25.4] after induction therapy) vs. those not receiving levofloxacin was 0% at baseline and 0% after induction therapy (p < 0.0001) Antibiotic exposure • Trend toward less antibiotic exposure days in the no prophylaxis group compared to levofloxacin group (31.7days vs 43.8days, p = 0.09) |
| Maser et al., 202047 | Literature review based cost-utility analysis | Relapsed ALL, AML | <21 years |
Cost analysis • Levofloxacin prophylaxis produced cost savings of $542.44 compared to no prophylaxis |
|
Abbreviations: ALL: Acute lymphoblastic leukemia
AML: Acute myelogenous leukemia
HSCT: Hematopoietic stem cell transplant
CLABSI: Central-line associated bloodstream infection
GNR: Gram-negative rod
MDRO: Multidrug resistant organism
C. difficile: Clostridium difficile
ICU: Intensive care unit.
Bacterial resistance with levofloxacin prophylaxis
One of the major concerns related to the use of antibiotics, specifically fluoroquinolone, prophylaxis is the emergence of multi-drug resistant organisms (MDRO) that are more difficult to treat. MDROs are defined as bacterial isolates that belong to one of the following categories: VRE, methicillin-resistant Staphylococcus aureus (MRSA), or multidrug-resistant gram-negative bacteria (MRGN), as described previously.48
The gut flora contains collections of antibiotic-resistance genes, collectively called the gastrointestinal resistome, which serve as a source of potential antibiotic resistance for bacteria.49 Margolis et al. evaluated the impact of levofloxacin prophylaxis on antibiotic resistance genes by comparing the gastrointestinal microbiome in fecal samples from pediatric ALL patients who received Levaquin prophylaxis (n = 31) and those who did not (n = 18). They found an increase in the prevalence of topoisomerase point mutations in the levofloxacin cohort (mean prevalence was 3.7% [95% CI 0.2–22.5] at baseline vs. 10.4% [3.2–25.4] after induction therapy) versus those not receiving levofloxacin was 0% at baseline and 0%after induction therapy (p < 0.0001). They did not find evidence of cross-class resistance to other antibiotics in the fluoroquinolone prophylaxis cohort.23
Bloodstream infections with VRE are emerging in pediatric and adult HSCT recipients.50 In a single-center report, the rate of VRE was substantially higher for adult patients than pediatric patients; and VREBSI resulted in inferior one-year OSpost-HSCT47. In addition, patients with VRE BSI have a significantly longer inpatient duration (attributable difference 2.1 days longer) and hospitalization costs.51 Enterococcus faecium has emerged as a leading cause of multiple-drug resistant enterococcal infection in the United States;52 as VRE is responsible for nearly 18% of all invasive enterococcal infections in North America, with an incidencenearlydoublinginrecentyears.52 Notably, E. faecium is intrinsically more antibiotic-resistant than E. faecalis, with more than half of its pathogenic isolates expressing resistance to vancomycin and ampicillin. As a result, treating infections caused by this species can be difficult.53 The primary mode of spread of VRE from patient-to-patient occurs through the hands of healthcare workers. Enterococci can persist for as long as 60 minutes after inoculation onto hands and last as long as four months on inanimate surfaces, where they can serve as a reservoir for ongoing transmission in the absence of regular econtamination.54,55 Antibiotic therapy leading to VREGI overgrowth may lead to unique pathogenesis and predisposition to gut translocation and bacteremia.56,57 Specifically, perturbation of normal commensal intestinal microbiota by antibiotics and domination by VRE was shown to precede VRE-BSI in allo-HSCT patients.57
MRSA produces virulent biofilms on invasive, foreign devices like endotracheal tubes and endovascular catheters.58,59 Biofilm facilitates MRSA survival and multiplication, prolonging the organism’s exposure to antibiotics as well as promoting the transfer of antibiotic resistance genes among strains.60 The use of antibiotics, particularly cephalosporins and fluoroquinolones, strongly correlates with MRSA colonization and infection. In 2007, Shaw etal. evaluated the frequency and outcome of patients who developed MRSA BSI over a 5-year period. The frequency of MRSA infections in autologous, MSD, and MUD transplants was 3, 6, and 9%, respectively. In 7% of the infections, MRSA was directly implicated inpatient mortality.61
Multi-drug resistant bacterial strains are defined by their resistance to three or more antibiotic classes: carbapenems (imipenem, meropenem); penicillin (piperacillin, ticarcillin, and piperacillin–tazobactam); cephalosporins (ceftazidime, cefepime); monobactams; aminoglycosides and fluoroquinolones. In the aforementioned 2014 European survey, the median reported rates of extended-spectrum beta-lactamase (ESBL)-producing gram-negative bacilli (GNB, 15–24%), aminoglycoside-resistant GNB (5–14%), and carbapenem-resistant P. aeruginosa (5–14%) were substantial.62 Consistent with the European survey, a recent study reported a 17.5% ESBL gram-negative colonization rate among HSCT patients in Germany, with only 2% of colonized patients developing bacteremia.63 In a 2015 report from MD Anderson Cancer Center,64 rates of stool colonization with multidrug-resistant Pseudomonas were 1.2% (12/794); however, seven (58%, 7/12) of the colonized patients went on to develop MDR Pseudomonas BSI. Differences in geography, infection control, and antibiotic stewardship likely contribute to the variable rates of these resistant pathogens.
Cost-effectiveness of levofloxacin prophylaxis in pediatric and HSCT patients
A published meta-analysis of healthcare-associated infections (HAIs) revealed that CLABSIs are associated with the highest cost of any HAI, averaging $45,814 perevent.65 A recent evaluation in pediatric HSCT and oncology patients with ambulatory BSIs demonstrated a $40,852 median hospital charge, with the room, pharmacy, and procedure charges accounting for more than 70% oftotalcharges.66 Finally, Wilson et al. utilized propensity scoring with matched cases while controlling for other covariates and defined the attributable cost of CLABSI to approximate $70,000 per BSI event in pediatric hematology oncology patients. In addition, patients with CLABSI had LOS that was 21.2 days longer than those without CLABSI (p < 0.0001).67
Additionally, a retrospective cohort analysis using data from the Pediatric Health Information System (PHIS) database evaluated the cost-effectiveness of levofloxacin prophylaxis compared to no prophylaxis in pediatric patients aged 0–21 years with AML during a single chemotherapy cycle. Their findings showed levofloxacin prophylaxis decreased the absolute risk of bacteremia by 17% and cost by $1464 compared to no prophylaxis—costing $8491 per bacteremia episode avoided. This is cost-effective, as an episode of bacteremia added an average of $119,478 to the encounter costs. Prophylaxis decreased absolute ICU admission risk by 2.1% costing $81,609 per ICU admission avoided. Finally, levofloxacin prophylaxis decreased absolute mortality risk by 0.7% and cost $220,457 per death avoided.39
Recommendations
These results support the effectiveness of levofloxacin in children and young adults with leukemia receiving intensive chemotherapy for treatment. In addition, patients undergoing HSCT may benefit from levofloxacin prophylaxis prior to engraftment. Further clinical trials are needed to determine the effectiveness of levofloxacin in reducing infection in other populations, including children with neuroblastoma or receiving therapy for sarcomas. A proposed algorithm outlining the recommended use of levofloxacin prophylaxis in pediatric oncology and HSCT patients is shown in Figure 1.
Figure 1.
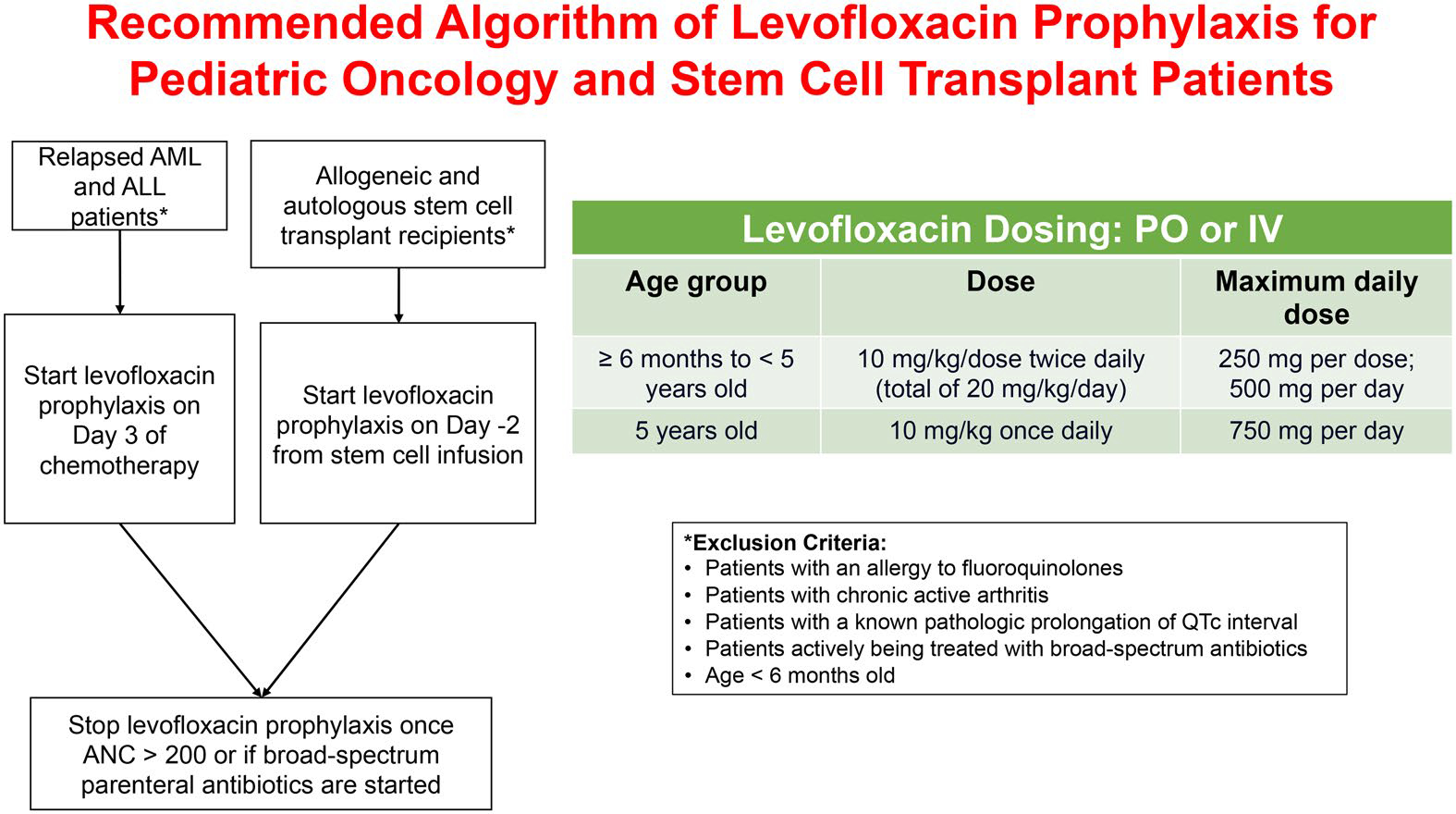
Proposed algorithm of levofloxacin prophylaxis for pediatric oncology patients and stem cell transplant patients.
The authors recommend levofloxacin prophylaxis for pediatric acute lymphoblastic leukemia, acute myeloid leukemia, and stem cell transplant recipients while neutropenic. Patients with underlying tendinopathy or cardiac arrhythmias should avoid levofloxacin prophylaxis. Clinicians should consider the risks and benefits of levofloxacin prophylaxis in patients with neuropathy.
While levofloxacin prophylaxis is associated with decreased infections, its use comes at a cost. The main concern over the use of prophylactic antibiotics is the emergence of antibiotic resistance. Quinolone use has been associated with multi-drug-resistant Staphylococcus aureus, multidrug-resistant Escherichia coli and Pseudomonas aeruginosa. Institutional infection prevention programs should be aware of system-wide prophylaxis use and monitor for the incidence and occurrence of multidrug-resistant organisms.
Funding
The author(s) reported there is no funding associated with the work featured in this article.
Footnotes
Disclosure statement
The authors declare there are no conflicts of interest to declare.
References
- 1.Dandoy CE, Kelley T, Gaur AH, et al. Outcomes after bloodstream infection in hospitalized pediatric hematology/oncology and stem cell transplant patients. Pediatr Blood Cancer. 2019;66(12):e27978. doi: 10.1002/pbc.27978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mattei D, Baretta V, Mazzariol A, et al. Characteristics and outcomes of bloodstream infections in a tertiary-care pediatric hematology-oncology unit: a 10-year study. J Clin Med. 2022;11(3):880. doi: 10.3390/jcm11030880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ustun C, Young JH, Papanicolaou GA, et al. Bacterial bloodstream infections (BSIs), particularly post-engraftment BSIs, are associated with increased mortality after allogeneic hematopoietic cell transplantation. Bone Marrow Transplant. 2019;54(8):1254–1265. doi: 10.1038/s41409-018-0401-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chang AK, Foca MD, Jin Z, et al. Bacterial bloodstream infections in pediatric allogeneic hematopoietic stem cell recipients before and after implementation of a central line-associated bloodstream infection protocol: a single-center experience. Am J Infect Control. 2016;44(12):1650–1655. doi: 10.1016/j.ajic.2016.04.229. [DOI] [PubMed] [Google Scholar]
- 5.Dandoy CE, Kim S, Chen M, et al. Incidence, risk factors, and outcomes of patients who develop mucosal barrier injury-laboratory confirmed bloodstream infections in the first 100 days after allogeneic hematopoietic stem cell transplant. JAMA Netw Open. 2020;3(1):e1918668. doi: 10.1001/jamanetworkopen.2019.18668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dandoy CE, Haslam D, Lane A, et al. Healthcare burden, risk factors, and outcomes of mucosal barrier injury laboratory-confirmed bloodstream infections after stem cell transplantation. Biol Blood Marrow Transplant. 2016;22(9):1671–1677. doi: 10.1016/j.bbmt.2016.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Freeman JT, Elinder-Camburn A, McClymont C, et al. Central line-associated bloodstream infections in adult hematology patients with febrile neutropenia: an evaluation of surveillance definitions using differential time to blood culture positivity. Infect Control Hosp Epidemiol. 2013;34(1):89–92. doi: 10.1086/668431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gudiol C, Garcia-Vidal C, Arnan M, et al. Etiology, clinical features and outcomes of pre-engraftment and post-engraftment bloodstream infection in hematopoietic SCT recipients. Bone Marrow Transplant. 2014;49(6):824–830. doi: 10.1038/bmt.2014.37. [DOI] [PubMed] [Google Scholar]
- 9.Mitchell AE, Derrington P, Turner P, Hunt LP, Oakhill A, Marks DI. Gram-negative bacteraemia (GNB) after 428 unrelated donor bone marrow transplants (UD-BMT): risk factors, prophylaxis, therapy and outcome. Bone Marrow Transplant. 2004;33(3):303–310. doi: 10.1038/sj.bmt.1704338. [DOI] [PubMed] [Google Scholar]
- 10.Kikuchi M, Akahoshi Y, Nakano H, et al. Risk factors for pre- and post-engraftment blood-stream infections after allogeneic hematopoietic stem cell transplantation. Transpl Infect Dis. 2015;17(1):56–65. doi: 10.1111/tid.12345. [DOI] [PubMed] [Google Scholar]
- 11.Cullen M, Steven N, Billingham L, et al. Antibacterial prophylaxis after chemotherapy for solid tumors and lymphomas. N Engl J Med. 2005;353(10):988–998. doi: 10.1056/NEJMoa0500781614ss8284. [DOI] [PubMed] [Google Scholar]
- 12.Gafter-Gvili A, Fraser A, Paul M, Leibovici L. Meta-analysis: antibiotic prophylaxis reduces mortality in neutropenic patients. Ann Intern Med. 2005;142(12 Pt 1):979–995. doi: 10.7326/0003-4819-142-12_part_1-200506210-00008. [DOI] [PubMed] [Google Scholar]
- 13.Castagnola E, Boni L, Giacchino M, et al. A multicenter, randomized, double blind placebo-controlled trial of amoxicillin/clavulanate for the prophylaxis of fever and infection in neutropenic children with cancer. Pediatr Infect Dis J. 2003;22(4):359–365. doi: 10.1097/01.inf.0000061014.97037.a8. [DOI] [PubMed] [Google Scholar]
- 14.van Eys J, Berry DM, Crist W, et al. Effect of trimethoprim/sulfamethoxazole prophylaxis on outcome of childhood lymphocytic leukemia. A pediatric oncology group study. Cancer. 1987;59(1):19–23. doi: 10.1002/1097-0142(19870101)59:1. [DOI] [PubMed] [Google Scholar]
- 15.Pizzo PA, Robichaud KJ, Edwards BK, Schumaker C, Kramer BS, Johnson A. Oral antibiotic prophylaxis in patients with cancer: a double-blind randomized placebo-controlled trial. J Pediatr. 1983;102(1):125–133. doi: 10.1016/s0022-3476(83)80310-2. [DOI] [PubMed] [Google Scholar]
- 16.Yousef AA, Fryer CJ, Chedid FD, Abbas AA, Felimban SK, Khattab TM. A pilot study of prophylactic ciprofloxacin during delayed intensification in children with acute lymphoblastic leukemia. Pediatr Blood Cancer. 2004;43(6):637–643. doi: 10.1002/pbc.20065. [DOI] [PubMed] [Google Scholar]
- 17.Kurt B, Flynn P, Shenep JL, et al. Prophylactic antibiotics reduce morbidity due to septicemia during intensive treatment for pediatric acute myeloid leukemia. Cancer. 2008;113(2):376–382. doi: 10.1002/cncr.23563. [DOI] [PubMed] [Google Scholar]
- 18.Lehrnbecher T, Fisher BT, Phillips B, et al. Guideline for antibacterial prophylaxis administration in pediatric cancer and hematopoietic stem cell transplantation. Clin Infect Dis. 2020;71(1):226–236. doi: 10.1093/cid/ciz1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Children’s Hospitals’ Solutions for Patient Safety. Results. January 26, 2023. https://www.solutionsforpatientsafety.org/.
- 20.Jackson MA, Schutze GE. The use of systemic and topical fluoroquinolones. Pediatrics. 2016;138(5):223–230. doi: 10.1542/peds.2016-2706. [DOI] [PubMed] [Google Scholar]
- 21.Rolston KV, Yadegarynia D, Kontoyiannis DP, Raad II, Ho DH. The spectrum of Gram-positive bloodstream infections in patients with hematologic malignancies, and the in vitro activity of various quinolones against Gram-positive bacteria isolated from cancer patients. Int J Infect Dis. 2006;10(3):223–230. doi: 10.1016/j.ijid.2005.05.007. [DOI] [PubMed] [Google Scholar]
- 22.Barnett ED, Lynfield R, Sawyer MH. Red Book: 2021–2024 Report of the Committee on Infectious Diseases. (32nd edition). 2021.
- 23.Margolis EB, Hakim H, Dallas RH, et al. Antibiotic prophylaxis and the gastrointestinal resistome in paediatric patients with acute lymphoblastic leukaemia: a cohort study with metagenomic sequencing analysis. Lancet Microbe. 2021;2(4):e159–e167. doi: 10.1016/s2666-5247(20)30202-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Poutsiaka DD, Price LL, Ucuzian A, Chan GW, Miller KB, Snydman DR. Blood stream infection after hematopoietic stem cell transplantation is associated with increased mortality. Bone Marrow Transplant. 2007;40(1):63–70. doi: 10.1038/sj.bmt.1705690. [DOI] [PubMed] [Google Scholar]
- 25.Liu CY, Lai YC, Huang LJ, et al. Impact of bloodstream infections on outcome and the influence of prophylactic oral antibiotic regimens in allogeneic hematopoietic SCT recipients. Bone Marrow Transplant. 2011;46(9):1231–1239. doi: 10.1038/bmt.2010.286. [DOI] [PubMed] [Google Scholar]
- 26.Levinson A, Pinkney K, Jin Z, et al. Acute gastrointestinal graft-vs-host disease is associated with increased enteric bacterial bloodstream infection density in pediatric allogeneic hematopoietic cell transplant recipients. Clin Infect Dis. 2015;61(3):350–357. doi: 10.1093/cid/civ285. [DOI] [PubMed] [Google Scholar]
- 27.Reuter S, Kern WV, Sigge A, et al. Impact of fluoroquinolone prophylaxis on reduced infection-related mortality among patients with neutropenia and hematologic malignancies. Clin Infect Dis. 2005;40(8):1087–1093. doi: 10.1086/428732. [DOI] [PubMed] [Google Scholar]
- 28.Craig M, Cumpston AD, Hobbs GR, Devetten MP, Sarwari AR, Ericson SG. The clinical impact of antibacterial prophylaxis and cycling antibiotics for febrile neutropenia in a hematological malignancy and transplantation unit. Bone Marrow Transplant. 2007;39(8):477–482. doi: 10.1038/sj.bmt.1705591. [DOI] [PubMed] [Google Scholar]
- 29.Bucaneve G, Micozzi A, Menichetti F, et al. Levofloxacin to prevent bacterial infection in patients with cancer and neutropenia. N Engl J Med. 2005;353(10):977–987. doi: 10.1056/NEJMoa044097. [DOI] [PubMed] [Google Scholar]
- 30.Guthrie KA, Yong M, Frieze D, Corey L, Fredricks DN. The impact of a change in antibacterial prophylaxis from ceftazidime to levofloxacin in allogeneic hematopoietic cell transplantation. Bone Marrow Transplant. 2010;45(4):675–681. doi: 10.1038/bmt.2009.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.de Witte T, Novakova I, Branolte J, Muytjens H, de Pauw B. Long-term oral ciprofloxacin for infection prophylaxis in allogeneic bone marrow transplantation. Pharm Weekbl Sci. 1987;9 Suppl:S48–S52. doi: 10.1007/BF02075260. [DOI] [PubMed] [Google Scholar]
- 32.De Pauw BE, Donnelly JP, De Witte T, Novakova IR, Schattenberg A. Options and limitations of long-term oral ciprofloxacin as antibacterial prophylaxis in allogeneic bone marrow transplant recipients. Bone Marrow Transplant. 1990;5(3):179–182. [PubMed] [Google Scholar]
- 33.Schmidt-Hieber M, Stroux A, Thiel E, Blau IW. Antibacterial prophylaxis with levofloxacin in patients after allogeneic stem cell transplantation and neutrophil reconstitution: results from a double-blinded, placebo-controlled phase III trial and overview of recent clinical practice. Leuk Lymphoma. 2010;51(1):157–160. doi: 10.3109/10428190903288480. [DOI] [PubMed] [Google Scholar]
- 34.Widjajanto PH, Sumadiono S, Cloos J, Purwanto I, Sutaryo S, Veerman AJ. Randomized double blind trial of ciprofloxacin prophylaxis during induction treatment in childhood acute lymphoblastic leukemia in the WK-ALL protocol in Indonesia. J Blood Med. 2013;4:1–9. doi: 10.2147/JBM.S33906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wolf J, Tang L, Flynn PM, et al. Levofloxacin prophylaxis during induction therapy for pediatric acute lymphoblastic leukemia. Clin Infect Dis. 2017;65(11):1790–1798. doi: 10.1093/cid/cix644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sulis ML, Blonquist TM, Stevenson KE, et al. Effectiveness of antibacterial prophylaxis during induction chemotherapy in children with acute lymphoblastic leukemia. Pediatr Blood Cancer. 2018;65(5):e26952. doi: 10.1002/pbc.26952. [DOI] [PubMed] [Google Scholar]
- 37.Karol SE, Sun Y, Tang L, et al. Fluoroquinolone prophylaxis does not increase risk of neuropathy in children with acute lymphoblastic leukemia. Cancer Med. 2020;9(18):6550–6555. doi: 10.1002/cam4.3249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Olson J, Mehra S, Hersh AL, et al. Oral step-down therapy with levofloxacin for febrile neutropenia in children with cancer. J Pediatric Infect Dis Soc. 2021;10(1):27–33. doi: 10.1093/jpids/piaa015. [DOI] [PubMed] [Google Scholar]
- 39.McCormick M, Friehling E, Kalpatthi R, Siripong N, Smith K. Cost-effectiveness of levofloxacin prophylaxis against bacterial infection in pediatric patients with acute myeloid leukemia. Pediatr Blood Cancer. 2020;67(10):e28469. doi: 10.1002/pbc.28469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Servidio AG, Simeone R, Zanon D, Barbi E, Maximova N. Levofloxacin versus ciprofloxacin-based prophylaxis during the pre-engraftment phase in allogeneic hematopoietic stem cell transplant pediatric recipients: a single-center retrospective matched analysis. Antibiotics (Basel). 2021;10(12):995–1004. doi: 10.3390/antibiotics10121523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Alexander S, Fisher BT, Gaur AH, et al. Effect of levofloxacin prophylaxis on bacteremia in children with acute leukemia or undergoing hematopoietic stem cell transplantation: a randomized clinical trial. JAMA. 2018;320(10):995–1004. doi: 10.1001/jama.2018.12512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hafez HA, Yousif D, Abbassi M, Elborai Y, Elhaddad A. Prophylactic levofloxacin in pediatric neutropenic patients during autologous hematopoietic stem cell transplantation. Clin Transplant. 2015;29(12):1112–1118. doi: 10.1111/ctr.12635. [DOI] [PubMed] [Google Scholar]
- 43.Choeyprasert W, Hongeng S, Anurathapan U, Pakakasama S. Bacteremia during neutropenic episodes in children undergoing hematopoietic stem cell transplantation with ciprofloxacin and penicillin prophylaxis. Int J Hematol. 2017;105(2):213–220. doi: 10.1007/s12185-016-2113-0. [DOI] [PubMed] [Google Scholar]
- 44.Davis A, Stevens AM, Brackett J, et al. Levofloxacin prophylaxis for pediatric leukemia patients: Longitudinal follow-up for impact on health care-associated infections. Pediatr Blood Cancer. 2022;69(7):e29525. doi: 10.1002/pbc.29525. [DOI] [PubMed] [Google Scholar]
- 45.Gardner JC, Courter JD, Dandoy CE, Davies SM, Teusink-Cross A. Safety and efficacy of prophylactic levofloxacin in pediatric and adult hematopoietic stem cell transplantation patients. Transplant Cell Ther. 2022;28(3):e1–167–e5. doi: 10.1016/j.jtct.2021.11.017. [DOI] [PubMed] [Google Scholar]
- 46.Ballen K, Woo Ahn K, Chen M, et al. Infection rates among acute leukemia patients receiving alternative donor hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2016;22(9):1636–1645. doi: 10.1016/j.bbmt.2016.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Maser B, Pelland-Marcotte MC, Alexander S, Sung L, Gupta S. Levofloxacin prophylaxis in hospitalized children with leukemia: a cost-utility analysis. Pediatr Blood Cancer. 2020;67(10):e28643. doi: 10.1002/pbc.28643. [DOI] [PubMed] [Google Scholar]
- 48.Mücke MM, Kessel J, Mücke VT, et al. The role of Enterococcus spp. and multidrug-resistant bacteria causing pyogenic liver abscesses. BMC Infect Dis. 2017;17(1):450. doi: 10.1186/s12879-017-2543-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Willmann M, Vehreschild M, Biehl LM, et al. Distinct impact of antibiotics on the gut microbiome and resistome: a longitudinal multicenter cohort study. BMC Biol. 2019;17(1):76. doi: 10.1186/s12915-019-0692-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vydra J, Shanley RM, George I, et al. Enterococcal bacteremia is associated with increased risk of mortality in recipients of allogeneic hematopoietic stem cell transplantation. Clin Infect Dis. 2012;55(6):764–770. doi: 10.1093/cid/cis550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Adams DJ, Eberly MD, Goudie A, Nylund CM. Rising vancomycin-resistant enterococcus infections in hospitalized children in the United States. Hosp Pediatr. 2016;6(7):404–411. doi: 10.1542/hpeds.2015-0196. [DOI] [PubMed] [Google Scholar]
- 52.Hidron AI, Edwards JR, Patel J, et al. NHSN annual update: antimicrobial-resistant pathogens associated with healthcare-associated infections: annual summary of data reported to the National Healthcare Safety Network at the Centers for Disease Control and Prevention, 2006–2007. Infect Control Hosp Epidemiol. 2008;29(11):996–1011. doi: 10.1086/591861. [DOI] [PubMed] [Google Scholar]
- 53.Arias CA, Murray BE. The rise of the Enterococcus: beyond vancomycin resistance. Nat Rev Microbiol. 2012;10(4):266–278. doi: 10.1038/nrmicro2761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Noskin GA, Stosor V, Cooper I, Peterson LR. Recovery of vancomycin-resistant enterococci on fingertips and environmental surfaces. Infect Control Hosp Epidemiol. 1995;16(10):577–581. doi: 10.1086/647011. [DOI] [PubMed] [Google Scholar]
- 55.Ray AJ, Hoyen CK, Taub TF, Eckstein EC, Donskey CJ. Nosocomial transmission of vancomycin-resistant enterococci from surfaces. JAMA. 2002;287(11):1400–1401. doi: 10.1001/jama.287.11.1400. [DOI] [PubMed] [Google Scholar]
- 56.Kamboj M, Blair R, Bell N, Sun J, Eagan J, Sepkowitz K. What is the source of bloodstream infection due to vancomycin-resistant enterococci in persons with mucosal barrier injury? Letter Research Support, N.I.H., Extramural. Infect Control Hosp Epidemiol. 2014;35(1):99–101. doi: 10.1086/674406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ubeda C, Taur Y, Jenq RR, et al. Vancomycin-resistant Enterococcus domination of intestinal microbiota is enabled by antibiotic treatment in mice and precedes bloodstream invasion in humans. J Clin Invest. 2010;120(12):4332–4341. doi: 10.1172/JCI43918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Archer NK, Mazaitis MJ, Costerton JW, Leid JG, Powers ME, Shirtliff ME. Staphylococcus aureus biofilms: properties, regulation, and roles in human disease. Virulence. 2011;2(5):445–459. doi: 10.4161/viru.2.5.17724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Foster TJ, Höök M. Surface protein adhesins of Staphylococcus aureus. Trends Microbiol. 1998;6(12):484–488. doi: 10.1016/s0966-842x(98)01400-0. [DOI] [PubMed] [Google Scholar]
- 60.Fridkin SK, Hageman JC, Morrison M, et al. Methicillin-resistant Staphylococcus aureus disease in three communities. N Engl J Med. 2005;352(14):1436–1444. doi: 10.1056/NEJMoa043252. [DOI] [PubMed] [Google Scholar]
- 61.Shaw BE, Boswell T, Byrne JL, Yates C, Russell NH. Clinical impact of MRSA in a stem cell transplant unit: analysis before, during and after an MRSA outbreak. Bone Marrow Transplant. 2007;39(10):623–629. doi: 10.1038/sj.bmt.1705654. [DOI] [PubMed] [Google Scholar]
- 62.Mikulska M, Viscoli C, Orasch C, et al. Aetiology and resistance in bacteraemias among adult and paediatric haematology and cancer patients. J Infect. 2014;68(4):321–331. doi: 10.1016/j.jinf.2013.12.006. [DOI] [PubMed] [Google Scholar]
- 63.Liss BJ, Vehreschild JJ, Cornely OA, et al. Intestinal colonisation and blood stream infections due to vancomycin-resistant enterococci (VRE) and extended-spectrum beta-lactamase-producing Enterobacteriaceae (ESBLE) in patients with haematological and oncological malignancies. Infection. 2012;40(6):613–619. doi: 10.1007/s15010-012-0269-y. [DOI] [PubMed] [Google Scholar]
- 64.Nesher L, Rolston KV, Shah DP, et al. Fecal colonization and infection with Pseudomonas aeruginosa in recipients of allogeneic hematopoietic stem cell transplantation. Transpl Infect Dis. 2015;17(1):33–38. doi: 10.1111/tid.12323. [DOI] [PubMed] [Google Scholar]
- 65.Zimlichman E, Henderson D, Tamir O, et al. Health care-associated infections: a meta-analysis of costs and financial impact on the US health care system. JAMA Intern Med. 2013;173(22):2039–2046. doi: 10.1001/jamainternmed.2013.9763. [DOI] [PubMed] [Google Scholar]
- 66.Wong Quiles CI, Gottsch S, Thakrar U, Fraile B, Billett AL. Health care institutional charges associated with ambulatory bloodstream infections in pediatric oncology and stem cell transplant patients. Pediatr Blood Cancer. 2016;64(2):324–329. doi: 10.1002/pbc.26194. [DOI] [PubMed] [Google Scholar]
- 67.Wilson MZ, Rafferty C, Deeter D, Comito MA, Hollenbeak CS. Attributable costs of central line-associated bloodstream infections in a pediatric hematology/oncology population. Am J Infect Control. 2014;42(11):1157–1160. doi: 10.1016/j.ajic.2014.07.025. [DOI] [PubMed] [Google Scholar]


