Abstract
Purpose of review
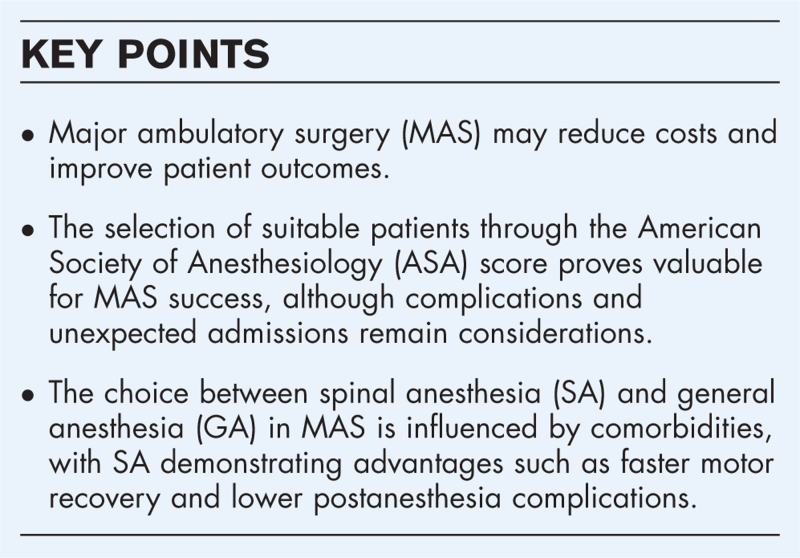
To assess current practice in the use of spinal anesthesia in major ambulatory surgery, highlighting its advantages over general anesthesia and identifying potential areas for improvement to facilitate a transition to a sustainable healthcare system.
Recent findings
Spinal anesthesia might be preferred in selected populations when compared to general anesthesia providing the highest standards of healthcare quality.
The use of local anesthetics with short half-life has proven to be efficient in achieving high anesthesia success rates. Spinal anesthesia does not increase perioperative complications; instead, it has shown a reduction in postoperative nausea and vomiting, an improvement in patient comfort, and a favorable economic impact when compared to general anesthesia.
Summary
Spinal anesthesia is an appropriate method for anesthesia in ambulatory patients, offering advantages over general anesthesia in selected populations.
The use of spinal anesthesia is expanding to meet surgical needs. Therefore, it is crucial to plan ahead and anticipate organizational failures in the ambulatory setting to maintain safety and efficiency during outpatient procedures and surgeries.
Keywords: ambulatory, in-day, spinal anesthesia, subarachnoid, surgery
INTRODUCTION
More than 230 million major surgical procedures are performed worldwide each year [1]. This dimension underscores the importance of strategically planning healthcare expenses budget, managing bed occupancy, using human resources efficiently and addressing morbidity and mortality. The overload in hospitalizations negatively impacts the quality of care, leading to increased fatigue of professionals and higher mortality rates [2].
In this context, major ambulatory surgery (MAS) might be the basis for decreasing costs while enhancing patient satisfaction [3]. MAS leads to improvement in mortality, when a suitable population is selected, and a lower readmission rate, positively impacting the bed occupancy [4▪].
MAS is defined as an intervention performed during a working day; including those that do not require hospital observation for >24 h. The number of major outpatient procedures is increasing progressively. In 2006 the percentage of outpatient procedures in the United States was around 65–70% [5], while in various European countries in 2021 the percentage of outpatient major surgeries was between 48% [6] and 52% [7].
There are multiple methods to perform anesthesia in outpatient surgery, with general anesthesia (GA) and spinal anesthesia (SA) being the most commonly used in the context of MAS. SA is an attractive approach due to its potential to decrease morbidity in certain populations, without detriment of anesthetic quality [8]. However, SA is not free of complications inherent to the procedure [9▪]. In addition, the time benefit between the start of the surgical procedure and discharge, as compared to GA, is under discussion [10,11].
Box 1.
no caption available
PATIENT SELECTION
How does the American Society of Anesthesiologists Physical Status score influence the outcome of major ambulatory surgery?
It could be hypothesized that the American Society of Anesthesiologists Physical Status (ASA PS) score might be useful to correctly select the population that will benefit the most from MAS. The use of the ASA PS in the specific context of MAS revealed a direct correlation between the number of complications and the ASA physical status [12], thereby endorsing the utilization of ASA PS grading for MAS.
Usually, outpatient procedures are performed in low-risk cases, excluding patients with ASA PS III or IV. However, there is evidence suggesting that outpatient procedures may be considered for ASA PS III patients, as long as their baseline pathology is stable and optimized when possible [13]. Furthermore, there was no significant difference in the rate of complications or unscheduled readmissions in ASA PS III patients when compared with ASA PS I or II patients [14].
However, in another study there is a significant relationship between ASA PS scores and unexpected admissions, with an odds ratio (OR) of 2.19 [95% confidence interval (CI) 1.10 to 4.34] for ASA PS III and an OR of 12.87 (95% CI 1.03 to 2.88) for ASA PS IV patients [15]. Almost half of the patients (46%) with a prolonged hospital stay were secondary to organizational and social issues. The second reason for the failure of the MAS circuit was related to surgical problems (38.6%). Only one in ten patients scheduled for MAS was not discharged appropriately due to anesthetic problems, such as a delay in the awakening time (6%) and urinary retention (3%). These results imply that for the proper functioning of a MAS unit it is crucial to have well organized logistics for both the procedural agenda and an adequate family support network for the patient in the immediate postoperative period.
Comorbidities influencing the choice of spinal anesthesia or general anesthesia
One of the advantages of SA over GA for major procedures is that by avoiding the use of systemic drugs, decompensation of stable pathologies can be prevented, thereby reducing perioperative morbidity [16].
Obesity is a metabolic disorder with an increasing incidence in the population, linked with a higher number of other comorbidities, and more consultations per patient [17]. Although obesity is sometimes considered a limiting condition for ambulatory surgery, a comprehensive evaluation should consider the patient's overall health status, and not only weight or body mass index (BMI) [18]. Airway manipulation in obese patients presents a challenge due to the tendency for airway collapse and the high incidence of difficult airway [19]. For this reason, SA may be an advantageous option over GA. It is necessary to highlight that obese patients have a greater number of technical failures of SA when compared to patients with normal weight. Nevertheless, the benefits of SA outweigh the associated risks [20].
One of the risks associated with GA is the needed ventilatory support, either with supplemental oxygen during sedation or by using airway devices such as laryngeal masks or endotracheal tubes. In populations with decreased functional reserve this may imply an increase in perioperative morbidity and mortality. In patients with chronic obstructive pulmonary disease (COPD) different types of anesthesia regimens were evaluated with SA leading to lower morbidity rates, such as pneumonia due to prolonged ventilation. Furthermore, there were no significant differences in mortality between [16]. A subgroup of patients with obstructive sleep apnea, demonstrate a greater susceptibility to the depressant effects of sedative medications in addition to having difficult airways. For this population avoidance of systemic sedatives and the use of SA may offer an advantage [18].
SPINAL ANESTHESIA, AN OLD PLAYER HAS JOINED THE GAME
The choice of anesthetic technique depends on several factors, such as the onset of action, the delay in motor recovery, postoperative nausea and vomiting (PONV), and postoperative pain among others. SA is offered as an alternative that presents a risk/benefit balance equivalent to GA, and in certain aspects, even superior. Having determined that SA offers advantages in certain outpatients for certain procedures, what are barriers to implementation and strategies for risk mitigation?
Preoperative issues
Practitioners might not be comfortable with SA for different reasons, e.g. the fear of adverse effects. In the case of SA, perhaps the biggest worry for patients and physicians are injuries in the central nervous system, despite their extreme rarity [9▪]. These concerns can be resolved with good interdisciplinary communication, outlining the benefits of this technique and its few adverse effects.
Another barrier for implementing SA is that the total time needed to achieve adequate anesthesia for the procedure is prolonged when you compare it to GA. An alternative to optimize total procedure times is the inclusion of a procedure room in which the spinal anesthetic can be performed, so that the patient enters the operating room already anesthetized. This incorporation has made it possible to reduce times and has also proved to be cost-effective [21,22].
Intraoperative issues
It is common to find patients who prefer GA rather than SA due to fear or unfamiliarity with the technique. “I want to be asleep. I don’t want to know anything” is a frequent remark. These comments can be addressed by dedicating time to dissipate doubts, calmly explaining the pros and cons of the procedure and the use of intraoperative sedation. If needed, different sedative schemes can be used [23], including propofol, midazolam or dexmedetomidine. There is also the possibility of using target-controlled titrated infusion, which reports excellent levels of intraoperative satisfaction, without increasing adverse events [24].
Functional recovery
Motor recovery time: Recovery of mobility is one of the requirements for discharge after a MAS procedure [25]. The delay in motor recovery depends directly on the medication used. In this regard, the use of ultra-short-acting drugs such as prilocaine or 2-chlorprocamine result in rapid recovery from motor block. Prilocaine is a safe and effective anesthetic [26▪]. Adverse effects such as a delay in diuresis are infrequent (1%) [27]. In a meta-analysis comparing low doses of bupivacaine and 2-chlorprocamine, the authors observed the superiority of the latter, showing significantly faster ambulation time and earlier discharge time (−84.6 min versus −88.0 min, P < 0.001), favoring the use of 2-chlorprocaine [28]. Therefore, the choice of the local anesthetic is key to optimize motor recovery.
Post dural puncture headache
Post dural puncture headache is a complication that can appear in up to 1% of punctures. Most of the time, it resolves spontaneously within a week [29], although sometimes it can be intense and disabling. Its frequency has been reduced with the introduction of atraumatic needles such as 25 gauge (G), 26 G and 27 G needles [30]. Patients aged between 12 and 19 years presented a higher risk of headache compared to those between 20 and 45 years (5% vs. 2%, respectively) [31]. These figures contrast with those found in a prospective study, which observed a 22–29% incidence of post puncture headache, with a 2.5-fold increased risk when a 25 G needle was used compared to a 27 G needle [32]. In addition to the needle gauge, other factors associated with post puncture headache were multiple attempts and the number of drops prior to administering medication.
Transient neurological symptoms
Transient neurological symptoms are defined as persistent sensory alterations after recovery of motor capacity, mainly described as pain radiating to the lower limbs, associated with a normal physical examination and imaging. The symptoms usually start in the first 24 h, and last for 5 days. The pathogenesis is unknown.
The appearance of transient neurological symptoms is more often observed with intrathecal lidocaine [33], with a similar incidence with mepivacaine and 2-chlorprocaine. Prilocaine or bupivacaine showed a low incidence of this syndrome [34].
Postanesthesia nausea and vomiting
Postoperative nausea and vomiting is a common adverse effect in GA, with an incidence of 30% up to 80% in at-risk populations [35]. In this regard, it has been shown that GA carries a higher risk of PONV compared to local or regional anesthesia [36]. Risk factors such as hypotension or the use of intrathecal morphine, have also been associated with increased PONV in patients receiving SA. However, in the context of MAS, the use of intrathecal opioids is limited due to the risk of respiratory depression, that could present many hours after the procedure, which requires monitoring of oxygenation up to 24 h [37]. Hypotensive episodes after administration of intrathecal anesthetics can be prevented with administration of vasoconstrictive agents after puncture. This has shown to decrease hypotensive episodes, with no increase in hypertensive episodes [38].
Urinary retention
Postoperative urinary retention (POUR) is a frequent complication of surgery. Its incidence varies in a wide range, from 5% to 70%, and is defined as the impossibility of generating spontaneous diuresis in the immediate postoperative period [39].
The risk factors associated with its occurrence are age, preexisting neurological diseases, prostatic pathology and type of anesthesia. In relation to the last point, it has been seen that general anesthesia has a lower risk compared to spinal anesthesia, and within this, local anesthetics with a longer half-life, such as bupivacaine, have an increased risk when compared to local anesthetics with a short half-life, such as prilocaine.
To prevent its occurrence, a restrictive use of fluids and avoidance of opioids, both intrathecal and systemic, is advocated [40]. It has been seen that the use of alpha-blockers prevents its appearance, so they could be indicated in patients at risk [41].
ECONOMIC IMPACT OF SPINAL ANESTHESIA
It has been shown that performing certain procedures on an outpatient basis is more cost-effective compared to the same procedure when hospitalization is required [42]. Yet, overall evidence about potential economic benefits for SA vs. GA remains inconsistent, likely given by the complexity of what to include in the definition of costs [43]. For example, a cost analysis for anterior cruciate ligament repair surgery, variables that made the procedure more expensive were, amongst others, preexisting comorbidity and receiving GA. Particularly, general anesthesia generated the greatest increase in costs [44]. When comparing GA and SA for lumbar laminectomy, the evidence showed shorter operating room time, shorter postoperative recovery time, a decrease in the postoperative pain perception and a reduction in total costs of 10% [45]. In contrast, evidence from short stay anorectal surgery favors SA given both, lower postoperative pain scores and lower rates of revision surgery [46]. Some data suggests, that patients at risk for higher subjective postoperative pain might easily be identified preoperatively [47]
CONCLUSION
In this review, we have evaluated the advantages of spinal anesthesia in major ambulatory surgeries. Spinal anesthesia for MAS is a safe, effective, and efficient technique in patients with an ASA status I-III when compared to GA. Despite SA in the ambulatory setting being a technique that is getting more attention, there are still challenging aspects limiting the increase of MAS pathways such as the adaptation of the institutions and the social context related to the patients. An appropriate patient selection and the highest anesthesia care standards during these procedures allow an expansion of outpatient scheduled surgery. Spinal anesthesia is an appropriate method for anesthesia in ambulatory patients, offering advantages over general anesthesia in selected populations.
Acknowledgements
Funding: Not applicable.
Ethics approval: Not applicable.
Consent to participate: Not applicable.
Consent for publication: Not applicable.
Availability of data and material: Not applicable.
Code availability: Not applicable.
Authors’ contributions: Ignacio Ledesma, Andrea Stieger, Markus Luedi, Carolina S. Romero wrote the article and approved the final version.
Compliance with ethical standards: This review article complies with ethical standards
Human and Animal Rights and Informed Consent: This article does not contain any studies with human or animal subjects performed by any of the authors.
Financial support and sponsorship
No funding was involved.
Conflicts of interest
There are no conflicts of interest.
REFERENCES AND RECOMMENDED READING
Papers of particular interest, published within the annual period of review, have been highlighted as:
▪ of special interest
▪▪ of outstanding interest
REFERENCES
- 1.Weiser TG, Regenbogen SE, Thompson KD, et al. An estimation of the global volume of surgery: a modelling strategy based on available data. Lancet 2008; 372:139–144. [DOI] [PubMed] [Google Scholar]
- 2.Bosque-Mercader L, Siciliani L. The association between bed occupancy rates and hospital quality in the English National Health Service. Eur J Health Econ 2023; 24:209–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ahmad NZ, Byrnes G, Naqvi SA. A meta-analysis of ambulatory versus inpatient laparoscopic cholecystectomy. Surg Endosc 2008; 22:1928–1934. [DOI] [PubMed] [Google Scholar]
- 4▪.Madsen HJ, Henderson WG, Dyas AR, et al. Inpatient versus outpatient surgery: a comparison of postoperative mortality and morbidity in elective operations. World J Surg 2023; 47:627–639. [DOI] [PubMed] [Google Scholar]; Recent study on morbidity and mortality in patients undergoing ambulatory vs. inpatient elective surgery.
- 5.Stanak M, Strohmaier C. Minimum volume standards in day surgery: a systematic review. BMC Health Serv Res 2020; 20:886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sistema Nacional de Salud (SNS) Ministerio de Salud de España. Intervenciones quirúgicas realizadas en hospitales del Sistema Nacional de Salud (SNS), frecuentación por 1.000 habitantes, porcentaje de intervenciones de Cirugía Mayor Ambulatoria (C.M.A.) sobre el total de intervenciones y días de espera para intervenciones no urgentes según comunidad autónoma. [Google Scholar]
- 7.Lefebvre-Hoang I, Yilmaz E. Etat de lieux des pratiques de chirurgie ambulatoire en. Dossiers DRESS 2019; 41:5–29. [Google Scholar]
- 8.del Río S, Fernández. Spinal anesthesia in ambulatory surgery? Are we near the optimal anesthetic technique for ambulatory surgical procedures? CIR MAY AMB 2013; 18:182–186. [Google Scholar]
- 9▪.Pozza DH, Tavares I, Cruz CD, Fonseca S. Spinal cord injury and complications related to neuraxial anaesthesia procedures: a systematic review. IJMS 2023; 24:4665. [DOI] [PMC free article] [PubMed] [Google Scholar]; Extensive review of complications of neuraxial anesthesia, which are infrequent but with serious consequences. Moreover, this technique is becoming increasingly relevant in the context of opioid-free anesthesia, so it is crucial to be aware of them.
- 10.Capdevila X, Aveline C, Delaunay L, et al. Factors determining the choice of spinal versus general anesthesia in patients undergoing ambulatory surgery: results of a multicenter observational study. Adv Ther 2020; 37:527–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chandler K, Jacob R, Kuntz Iv GE, et al. Operating room time comparison between spinal and general anesthesia in total knee arthroplasty: an institutional review. Orthop Rev. 2021;13. Available at: https://orthopedicreviews.openmedicalpublishing.org/article/28330-operating-room-time-comparison-between-spinal-and-general-anesthesia-in-total-knee-arthroplasty-an-institutional-review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Foley C, Kendall MC, Apruzzese P, De Oliveira GS. American Society of Anesthesiologists Physical Status Classification as a reliable predictor of postoperative medical complications and mortality following ambulatory surgery: an analysis of 2,089,830 ACS-NSQIP outpatient cases. BMC Surg 2021; 21:253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rajan N, Rosero EB, Joshi GP. Patient selection for adult ambulatory surgery: a narrative review. Anesth Analg 2021; 133:1415–1430. [DOI] [PubMed] [Google Scholar]
- 14.Ansell GL, Montgomery JE. Outcome of ASA III patients undergoing day case surgery. Br J Anaesth 2004; 92:71–74. [DOI] [PubMed] [Google Scholar]
- 15.Van Caelenberg E, De Regge M, Eeckloo K, Coppens M. Analysis of failed discharge after ambulatory surgery: unanticipated admission. Acta Chirurg Belg 2019; 119:139–145. [DOI] [PubMed] [Google Scholar]
- 16.Hausman MS, Jewell ES, Engoren M. Regional versus general anesthesia in surgical patients with chronic obstructive pulmonary disease: does avoiding general anesthesia reduce the risk of postoperative complications? Anesth Analg 2015; 120:1405–1412. [DOI] [PubMed] [Google Scholar]
- 17.Nightingale CE, Margarson MP, Shearer E, et al. Members of the Working Party. Peri-operative management of the obese surgical patient 2015: Association of Anaesthetists of Great Britain and Ireland Society for Obesity and Bariatric Anaesthesia. Anaesthesia 2015; 70:859–876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Servin F. Ambulatory anesthesia for the obese patient. Curr Opin Anaesthesiol 2006; 19:597–599. [DOI] [PubMed] [Google Scholar]
- 19.Langeron O, Birenbaum A, Le Saché F, Raux M. Airway management in obese patient. Minerva Anestesiol 2014; 80:382–392. [PubMed] [Google Scholar]
- 20.Ingrande J, Brodsky JB, Lemmens HJ. Regional anesthesia and obesity. Curr Opin Anaesthesiol 2009; 22:683–686. [DOI] [PubMed] [Google Scholar]
- 21.Torkki PM, Marjamaa RA, Torkki MI, et al. Use of anesthesia induction rooms can increase the number of urgent orthopedic cases completed within 7 h. Anesthesiology 2005; 103:401–405. [DOI] [PubMed] [Google Scholar]
- 22.Smith MP, Sandberg WS, Foss J, et al. High-throughput operating room system for joint arthroplasties durably outperforms routine processes. Anesthesiology 2008; 109:25–35. [DOI] [PubMed] [Google Scholar]
- 23.Xi C, Sun S, Pan C, et al. Different effects of propofol and dexmedetomidine sedation on electroencephalogram patterns: Wakefulness, moderate sedation, deep sedation and recovery. PLoS One 2018; 13:e0199120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hewson DW, Worcester F, Sprinks J, et al. Patient-maintained versus anaesthetist-controlled propofol sedation during elective primary lower-limb arthroplasty performed under spinal anaesthesia: a randomised controlled trial. Br J Anaesth 2022; 128:186–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.J Viñoles PA. Discharge criteria in ambulatory surgery. CIR MAY AMB 2013; 18:125–132. [Google Scholar]
- 26▪.Ambrosoli AL, Di Carlo S, Crespi A, et al. Safety and effectiveness of prilocaine for spinal anesthesia in day surgery setting: a retrospective study on a sample of 3291 patients. J Anesth Analg Crit Care 2023; 3:40. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study uses a large database to support the effectiveness of prilocaine at the anesthetic level and its low incidence of adverse effects.
- 27.Guntz E, Vasseur C, Ifrim D, et al. Intrathecal chloroprocaine or hyperbaric prilocaine for ambulatory knee surgery? A prospective randomized study. J Exp Orthop 2021; 8:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Saporito A, Ceppi M, Perren A, et al. Does spinal chloroprocaine pharmacokinetic profile actually translate into a clinical advantage in terms of clinical outcomes when compared to low-dose spinal bupivacaine? A systematic review and meta-analysis. J Clin Anesth 2019; 52:99–104. [DOI] [PubMed] [Google Scholar]
- 29.Turnbull DK, Shepherd DB. Postdural puncture headache: pathogenesis, prevention and treatment. Br J Anaesth 2003; 91:718–729. [DOI] [PubMed] [Google Scholar]
- 30.DelPizzo K, Luu T, Fields KG, et al. Risk of postdural puncture headache in adolescents and adults. Anesth Analg 2020; 131:273–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Aniceto L, Gonçalves L, Gonçalves L, et al. Incidence and severity of postdural puncture headache in nonobstetric patients undergoing subarachnoid block. Cureus 2023; 15:e47442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Weji BG, Obsa MS, Melese KG, Azeze GA. Incidence and risk factors of postdural puncture headache: prospective cohort study design. Perioper Med (Lond) 2020; 9:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stewart J, Gasanova I, Joshi GP. Spinal anesthesia for ambulatory surgery: current controversies and concerns. Curr Opin Anaesthesiol 2020; 33:746–752. [DOI] [PubMed] [Google Scholar]
- 34.Forget P, Borovac JA, Thackeray EM, Pace NL. Transient neurological symptoms (TNS) following spinal anaesthesia with lidocaine versus other local anaesthetics in adult surgical patients: a network meta-analysis. Cochrane Database Syst Rev 2019; 12:CD003006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gan TJ, Belani KG, Bergese S, et al. Fourth consensus guidelines for the management of postoperative nausea and vomiting. Anesth Analg 2020; 131:411–448. [DOI] [PubMed] [Google Scholar]
- 36.Rattenberry W, Hertling A, Erskine R. Spinal anaesthesia for ambulatory surgery. BJA Educ 2019; 19:321–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Borgeat A, Ekatodramis G, Schenker CA. Postoperative nausea and vomiting in regional anesthesia. Anesthesiology 2003; 98:530–547. [DOI] [PubMed] [Google Scholar]
- 38.Weinstein SM, Poultsides L, Baaklini LR, et al. Postoperative delirium in total knee and hip arthroplasty patients: a study of perioperative modifiable risk factors. Br J Anaesth 2018; 120:999–1008. [DOI] [PubMed] [Google Scholar]
- 39.Agrawal K, Majhi S, Garg R. Postoperative urinary retention: review of literature. WJA 2019; 8:1–12. [Google Scholar]
- 40.Baldini G, Bagry H, Aprikian A, Carli F. Postoperative urinary retention: anesthetic and perioperative considerations. Anesthesiology 2009; 110:1139–1157. [DOI] [PubMed] [Google Scholar]
- 41.Clancy C, Coffey JC, O’Riordain MG, Burke JP. A meta-analysis of the efficacy of prophylactic alpha-blockade for the prevention of urinary retention following primary unilateral inguinal hernia repair. Am J Surg 2018; 216:337–341. [DOI] [PubMed] [Google Scholar]
- 42.Recart A. Cirugía mayor ambulatoria. una nueva forma de entender la medicina quirúrgica. Rev Méd Clín Condes 2017; 28:682–690. [Google Scholar]
- 43.Ravi S, Krishna HM. Comparison of spinal anaesthesia with isobaric chloroprocaine and general anaesthesia for short duration ambulatory urological procedures. J Anaesthesiol Clin Pharmacol 2022; 38:91–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bokshan SL, Mehta S, DeFroda SF, Owens BD. What are the primary cost drivers of anterior cruciate ligament reconstruction in the United States? A cost-minimization analysis of 14,713 patients. Arthroscopy 2019; 35:1576–1581. [DOI] [PubMed] [Google Scholar]
- 45.Morris MT, Morris J, Wallace C, et al. An analysis of the cost-effectiveness of spinal versus general anesthesia for lumbar spine surgery in various hospital settings. Global Spine J 2019; 9:368–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Luedi MM, Kauf P, Evers T, et al. Impact of spinal versus general anesthesia on postoperative pain and long term recurrence after surgery for pilonidal disease. J Clin Anesth 2016; 33:236–242. [DOI] [PubMed] [Google Scholar]
- 47.Luedi MM, Schober P, Hammoud B, et al. Preoperative pressure pain threshold is associated with postoperative pain in short-stay anorectal surgery: a prospective observational study. Anesth Analg 2021; 132:656–662. [DOI] [PMC free article] [PubMed] [Google Scholar]