Abstract
Background:
Anal cancer is caused by human papillomavirus (HPV), particularly HPV-16, and is preceded by anal high-grade squamous intraepithelial lesions (HSIL). The incidence of anal cancer is highest among men who have sex with men (MSM) living with HIV (MSMLWH) and increases with age. However, most previous studies of anal HPV infection and anal HSIL were performed on men under 50 years of age, and relatively little is known about HSIL among older MSMLWH or MSM not living with HIV (MSM-Not-LWH).
Setting:
We enrolled MSM who were aged 50+ during 2018–2022 in San Francisco, California.
Methods:
129 MSMLWH and 109 MSM-not-LWH participated. All participants had anal HPV DNA testing (Atila Biosystems) and high-resolution anoscopy with biopsy of visible lesions.
Results:
Among MSMLWH, 47% had anal HSIL, 19% had HPV-16, and 51% had other oncogenic anal HPV types (excluding HPV-16). Among MSM-not-LWH, 37% had anal HSIL, 22% had HPV-16, and 34% had other oncogenic anal HPV types. Increasing age was not statistically associated with prevalent HSIL, HPV-16, or other oncogenic HPV infections in MSMLWH or MSM-not-LWH. HPV-16 (OR:45.1, 95% CI:15.8–129), other oncogenic HPV types (OR:5.95, 95% CI:2.74–12.9) were associated with increased odds of anal HSIL, adjusted for age, income, education, and HIV status.
Conclusion:
The prevalence of oncogenic anal HPV, anal HPV-16, and anal HSIL remain very high in older MSMLWH and MSM-not-LWH. With recent evidence showing that treating anal HSIL prevents anal cancer, MSM aged 50+ should be considered for anal cancer screening.
Keywords: HIV, aging, human papillomavirus, HSIL, high-grade squamous intraepithelial lesion, MSM
Introduction
Anal cancer is a rare cancer in the general population. In 2019 the incidence of anal cancer in the United States (US) was 2.1 per 100,000 people.1 Anal cancer is more common among older individuals; among people between the ages of 50 and 64 years the incidence was 5.0/100,000, and among people over age 65 years 7.4/100,000.1 Additionally, the incidence of anal cancer has been increasing; each year, between the years 2009 and 2019, there was a 1.7% increase in cases of anal cancer.1 The mortality rate from anal cancer has also been increasing and is highest among older individuals.1–3 There has also been a significant increase in the incidence of more advanced-stage anal cancer.2 Among white women older than 65, the incidence of anal cancer now exceeds the incidence of cervical cancer (8.6/100,000 versus 8.2/100,000, respectively).4
Although relatively rare in the general population, certain risk groups are at high risk for developing anal cancer. A recent meta-analysis ranked men who have sex with men living with HIV (MSMLWH) as the group with the highest incidence of anal cancer, 85.0/100,000.5 Age-specific summary statistics based primarily on the 1996–2015 US HIV Cancer Match Study show that MSMLWH aged 45–59 had an incidence of 99.7/100,000 PY, and for those ≥60 years of age, the incidence was 107.5/100,000 PY.5 The incidence rates of anal cancer in these high-risk groups are higher than the incidence rates of two other cancers with well-established screening recommendations in the general population6,7 – colon cancer among men (incidence 88.5/100,000 among men 50+ years)1, and cervical cancer among women (incidence 12.2/100,000 women 50+ years).1 More limited data are available on anal cancer incidence for MSM who are not living with HIV (MSM-not-LWH). A meta-analysis reported the pooled incidence of anal cancer for MSM-not-LWH of 5.5/100,000 based on only two cohort studies (from 1996 and 2008) and only 3 cases of anal cancer.8
More than 90% of anal cancers are caused by anal human papillomavirus (HPV) infections9, particularly HPV-16.10 Similar to cervical cancer, anal cancer is preceded by a precursor, anal high-grade squamous intraepithelial lesions (HSIL).11 Consistent with high rates of anal cancer, MSMLWH are also at the highest risk of anal HPV infections and anal HSIL.10,12,13 Although both age and HIV status have been demonstrated to be associated with anal cancer incidence, there have been few reports of anal HPV infection or anal HSIL, specifically among older MSMLWH12. It is unknown if the increase in incidence of anal cancer seen in older age groups results from persistent anal HPV infections acquired in youth that lead to cancer development over time or from newly acquired infections progressing to anal cancer more quickly.
A recent randomized controlled trial demonstrated that treating anal HSIL can prevent anal cancer among people living with HIV (PLWH).14 Based on these results, screening and treating anal HSIL will likely become recommended for PLWH. However, screening programs for anal HSIL have not yet become standard of care. Given the high risk of anal cancer among MSMLWH, including MSMLWH ≥50 years, it is critical to understand the prevalence of anal HPV infection and anal HSIL in these age groups.
Currently, there are few studies focusing on anal HPV infection or anal HSIL among older MSMLWH or older MSM-not-LWH12. Therefore, our study was performed to address this gap. In this report, we present baseline cross-sectional results from a three-year prospective cohort study addressing the prevalence and incidence of anal HPV infection and anal HSIL in older MSM living with and without HIV. The objectives of this study were to determine the type-specific prevalence of anal HPV infection and the prevalence of anal HSIL among MSMLWH and MSM-not-LWH ages 50 and older.
Methods
All methods and procedures were approved by the University of California, San Francisco (UCSF), Institutional Review Board, and were in accordance with the Helsinki Declaration of 1975, as revised in 2000.
The protocol and methods for our study, the Anal HPV, HIV and Aging (AHHA) Study, have been published elsewhere.15 Briefly, we recruited MSMLWH and MSM-not-LWH through a wide variety of methods16, including referrals from healthcare practitioners, flyers/posters/stickers posted in and around the San Francisco Bay Area, UCSF electronic medical records recruitment tools, advertisements in the newspaper, social media presence on Facebook, dating applications (apps), website updates, and direct recruitment by study outreach workers.
Participants were eligible for enrollment if they answered yes to the question, “Do you identify as a man or transperson who has sex with men?”. Subsequently, we asked a series of questions to help describe our participants including “What is your gender identity?” with the possible answer choices of (select all that apply): Transmale/Transman, Transfemale/Transwoman, Male, Gender Queer, Other, and Cis-female. Participants also responded to “What was your sex assigned at birth?” with the possible answer choices of male or female. Participants’ answers to the second two questions did not impact their eligibility for the study.
The other eligibility criteria were that participants must have been aged 50 or older, lived in the San Francisco Bay area, and had never been screened for anal cancer through high-resolution anoscopy (HRA) in the past (previous anal cytology was allowed). Outreach workers screened participants for eligibility by phone and scheduled potential participants for a visit at the UCSF Anal Neoplasia Center for Research and Education (ANCRE) clinic. Participants completed informed consent procedures and provided written informed consent. Participants self-reported HIV status. Participants who reported living with HIV had their status confirmed by review of medical charts and/or review of HIV medications. Participants reporting HIV negative status had a rapid test to confirm (OraQuick, OraSure Technologies Bethlehem, Pennsylvania).
Participants completed a behavioral questionnaire, and blood was collected to determine levels of CD4+ T-cells and HIV viral load. Participants next underwent collection of one anal swab for anal cytology and HPV DNA genotyping, followed by a digital anal rectal examination (DARE). Participants then underwent HRA with HRA-guided biopsy of visible lesions. If no lesion was identified on HRA, but HSIL was found on cytology, they were asked to return for a repeat HRA exam. DARE was performed as a standard of care procedure and no data were collected.
HPV genotyping was determined through Atila Biosystems Multiplex High-Risk HPV fluorescent detection (Atila BioSystems, Mountain View, CA) of 15 oncogenic HPV types (16, 18, 31, 33, 35, 39, 45, 51, 52, 53, 56, 58, 59, 66, 68). Anal histology samples were evaluated by a UCSF pathologist who was unaware of participants’ HIV status and HPV status. Results were classified as benign, low-grade squamous intraepithelial lesions (LSIL) and HSIL using Lower Anogenital Squamous Terminology Standardization Project for HPV-Associated Lesions (LAST) criteria,.21 If multiple lesions were found in a participant, the analysis used the highest-grade result.
Statistical Considerations
Anal HSIL was defined as a biopsy-confirmed diagnosis of HSIL or cancer (Table 1). Histology diagnoses of normal, atypia, and LSIL were classified as not having HSIL. Participants who did not have visible lesions on HRA were diagnosed as normal clinically and classified as not having HSIL.
Table 1:
Definitions and abbreviation of study outcomes
| Outcome | Abbreviation | Definition |
|---|---|---|
| Anal high-grade squamous intraepithelial lesion (HSIL) | HSIL | Biopsy-confirmed HSIL or cancer |
| Any oncogenic anal human papillomavirus (HPV) | Any Oncogenic HPV | Having any oncogenic anal HPV infection was defined as detection of at least one of the 15 oncogenic types (16, 18, 31, 33, 35, 39, 45, 51, 52, 53, 56, 58, 59, 66, 68) Including HPV-16 |
| Type-specific anal HPV | HPV-18, HPV-31, … HPV-68 | Detection of that type through HPV genotyping |
| Anal HPV infection (three-part mutually exclusive variable) | No HPV | Individuals with no detection of any HPV infection |
| HPV-16 | Individuals with detection of HPV-16 through HPV genotyping | |
| Other Oncogenic HPV (not HPV-16) | Individuals with other oncogenic anal HPV infection are defined as detection of at least one of the 14 oncogenic types (18, 31, 33, 35, 39, 45, 51, 52, 53, 56, 58, 59, 66, 68). Excluding individuals with HPV-16 |
Type-specific anal HPV infection was defined as a detection of that type through HPV genotyping; more than one HPV type could be identified in a sample. No detection of the specific type was classified as not having that HPV type. Having any oncogenic anal HPV infection was defined as detection of at least one of the 15 oncogenic HPV types evaluated (including HPV-16) (Table 1). Having HPV-16 was defined by detection of HPV-16. Having other oncogenic anal HPV infection was defined by detection of at least one of the 14 oncogenic HPV types evaluated, excluding HPV-16. HPV data for participants with insufficient material for evaluation and/or a negative internal control result (human beta-globin negative) were classified as missing.
In statistical analyses, a mutually exclusive three-part categorical variable for HPV was defined as negative test result for all oncogenic HPV types, positive test for an oncogenic HPV types excluding HPV-16, and positive for HPV-16 (Table 1). If a participant was positive for HPV-16 and other oncogenic HPV, they were categorized with the HPV-16 positive group.
Age was considered as a three-part categorical variable (50–59, 60–69, 70+) per protocol, and was also considered as a continuous variable, and as a two-part variable (<60, 60+) in regression analyses.
We summarized our participant characteristics stratified by HIV status using medians and interquartile range (IQR) for continuous variables and frequency and proportions for categorical variables. P-values comparing differences between MSMLSH and MSM-not-LWH were from chi-square tests for unordered categorical variables and ranked ANOVA for ordinal levels of continuous characteristics.
Prevalence was estimated as the mean and 95% confidence interval (CI) of the variable under consideration using generalized estimating equation (GEE models). In particular, we estimated type-specific prevalence of each anal HPV type and prevalence of any oncogenic HPV, other oncogenic HPV (excluding HPV-16), and anal HSIL (Table 1).
We hypothesized that both HPV-16 and other oncogenic HPV would increase the odds of anal HSIL. We assessed bivariable associates with anal HSIL via odds ratios (OR) and 95% CI in GEE models with a logit link for HPV-16 and other oncogenic HPV, age, HIV status, and sociodemographic factors known to be associated with HIV status. Effect modification was assessed with age*HIV status interaction term in bivariate analyses with the three-part age variable per protocol, and with age as a continuous variable or a two-part variable in exploratory analyses. Similarly, age*HPV (HPV-16/Other oncogenic/no HPV) interaction terms were also evaluated.
A multivariable model was created to estimate the association between anal HSIL and anal HPV infection, adjusted for combined HIV status and CD4+ level, and for sociodemographic factors (age, income, and education level) to control for potential confounding.
All analyses were conducted in SAS 9.4 (SAS Institute, Cary, NC).
Results
Participant characteristics related to HIV status.
All 238 participants responded ‘yes’ to the question: “Do you identify as a man or transperson who has sex with men?” Two hundred thirty participants also answered “male” to the question “What is your gender identity?” and all 230 also reported their sex assigned at birth as “male”. Of the eight remaining participants, three described their gender identity as “TransFemale/Transwoman”, one described it as “Other”, one declined to answer, and two responded ‘no’ to all gender identity questions. All 7 of these participants marked “male” to the sex assigned at birth question. One participant described her gender identity as “Cis-female”, and this individual left the sex assigned at birth field blank. As this eligibility criterion was based on the response to the initial question, this participant was considered eligible, and physician confirmed eligibility.
We enrolled 238 participants, 129 men who have sex with men living with HIV (MSMLWH) and 109 MSM not living with HIV (MSM-not-LWH). Because the majority of our participants identified as men, we grouped the participants living with HIV who identified as “Other” (n=1), declined (n=1), or did not respond (n=2) with the MSMLWH group and grouped the four participants not living with HIV who identified as “TransFemale/Transwoman” (n=3) or “Cis-female” (n=1) with MSM-not-LWH.
MSMLWH and MSM-not-LWH were similar in age, race/ethnicity, and history of smoking (Table 2). 54% of our participants were ages 50–59, 36% were 60–69, and 10% were 70+ years of age. 66% of men self-identified as non-Hispanic white, 10% as Black, 5% as Hispanic white, 7% as Asian, and 13% as multiracial (Table 2). However, there were some significant differences in demographic factors between MSMLWH and MSM-not-LWH. Compared with MSMLWH, MSM-not-LWH reported full-time employment more often (36% versus 15%, p<0.01), had higher annual household income—defined as making $84,000 or more annually (43% versus 27%, p<0.01), and more had at least a bachelor’s degree (71% versus 56%, p<0.01).
Table 2:
Participant Characteristics by Living with HIV (N=238).
| MSMLWH | MSM-not-LWH | ||||
|---|---|---|---|---|---|
| Characteristic* | n | (%) | n | (%) | p-value** |
| Total N | 129 | (54.2) | 109 | (45.8) | |
|
| |||||
| Age | 0.58 | ||||
| 50–59 | 69 | (53.5) | 60 | (55.0) | |
| 60–69 | 49 | (38.0) | 36 | (33.0) | |
| 70+ | 11 | (8.5) | 13 | (11.9) | |
|
| |||||
| Self-reported race/ethnicity | 0.24 | ||||
| Asian, American Indian, or Alaskan Native | 6 | (4.8) | 10 | (9.2) | |
| Black or African American | 15 | (11.9) | 8 | (7.3) | |
| Non-Hispanic White | 79 | (62.7) | 74 | (67.9) | |
| Hispanic White | 6 | (4.8) | 7 | (6.4) | |
| Other/Mixed | 20 | (15.9) | 10 | (9.2) | |
|
| |||||
| Employment Status | <.001 | ||||
| Employed full-time | 19 | (14.7) | 40 | (36.7) | |
| Employed part-time | 16 | (12.4) | 11 | (10.1) | |
| Retired | 42 | (32.6) | 32 | (29.4) | |
| Other*** | 52 | (40.3) | 26 | (23.9) | |
|
| |||||
| Income (annual) | 0.004 | ||||
| $0-$47,999 | 86 | (64.8) | 47 | (43.1) | |
| $48,000-$83,999 | 11 | (8.6) | 15 | (13.8) | |
| $84,000+ | 34 | (26.6) | 47 | (43.1) | |
|
| |||||
| Education | 0.011 | ||||
| Less than college | 8 | (6.3) | 12 | (11) | |
| Some college/technical school | 48 | (37.5) | 20 | (18.3) | |
| Bachelor’s degree | 35 | (27.3) | 35 | (32.1) | |
| Graduate or professional degree | 37 | (28.9) | 42 | (38.5) | |
|
| |||||
| Smoked at least 100 cigarettes, lifetime | 76 | (59.4) | 54 | (49.5) | 0.13 |
|
| |||||
| Undetectable HIV viral load | 118 | (94.4) | - | - | |
|
| |||||
| CD4+ level categories | |||||
| 0–199 | 7 | (5.7) | - | - | . |
| 200–499 | 33 | (26.8) | - | - | |
| 500+ | 83 | (67.5) | - | - | |
|
| |||||
| Ever had receptive anal intercourse | 125 | (97.7) | 100 | (92.6) | 0.067 |
|
| |||||
| Partners had receptive anal intercourse****, past 6 mo | 0.96 | ||||
| 0 | 73 | (57.5) | 62 | (57.4) | |
| 1 | 22 | (17.3) | 20 | (18.5) | |
| 2+ | 32 | (25.2) | 26 | (24.1) | |
|
| |||||
| Any oncogenic anal HPV | 90 | (70.9) | 61 | (57.0) | 0.027 |
|
| |||||
| Oncogenic anal HPV and HPV-16 | 0.058 | ||||
| No anal HPV | 37 | (28.7) | 46 | (42.2) | |
| Other HPV types (excluding HPV-16) | 66 | (51.2) | 37 | (33.9) | |
| HPV-16 | 24 | (18.6) | 24 | (22.0) | |
|
| |||||
| Biopsy Confirmed anal HSIL | 60 | (46.7) | 40 | (37.4) | 0.143 |
When totals do not sum to 238, participants were excluded because of missing data.
p-value for categorical variable from chi-square and from ranked ANOVA, for non-normally distributed continuous variables.
”Other” employment category included voluntary, unpaid employment, receiving government assistance (Medicare, Medicaid, Social Security), not employed, and decline; MSM, men who have sex with men; MSMLWH, men who have sex with men living with HIV; MSM-not-LWH, men who have sex with men not living with HIV; HSIL, high-grade squamous intraepithelial lesion; IQR, interquartile range
Most MSMLWH had undetectable HIV viral load at study enrollment; nonetheless, one-third had CD4+ T-cell counts below 500 cells/mm3 (Table 2). The median (IQR) CD4+ T-cell counts was 585 (400 – 795) cells/mm3. Essentially all participants reported having experienced receptive anal intercourse in their lifetime, and about 43% had a receptive partner in the past 6 months (with whom the participant was the receptive partner). MSM-not-LWH were more likely than MSMLWH to be HPV negative (42% versus 29%, p=0.06); however, the prevalence of HPV-16 was approximately equal in these groups (19% versus 22%, p=0.06) (Table 2).
MSMLWH had a higher prevalence of any oncogenic anal HPV infection compared with MSM-not-LWH (70.9% versus 57.0%, p=0.03) and had a higher mean number of HPV types detected concurrently (1.7 versus 1.4, p=0.05). The most common HPV types among MSMLWH were types: 53 (20.5%), 16 (18.9%), 68 (15.7%), 52 (15.7%), and 39 (13.4%). Among MSM-not-LWH, the most common HPV type were: 16 (22.5%), 51 (12.1%), 68 (11.2%), 52 (10.3%), and 39 (10.3%) (Figure 1).
Figure 1: Type-specific prevalence of anal HPV types for MSMLWH and MSM-not-LWH.
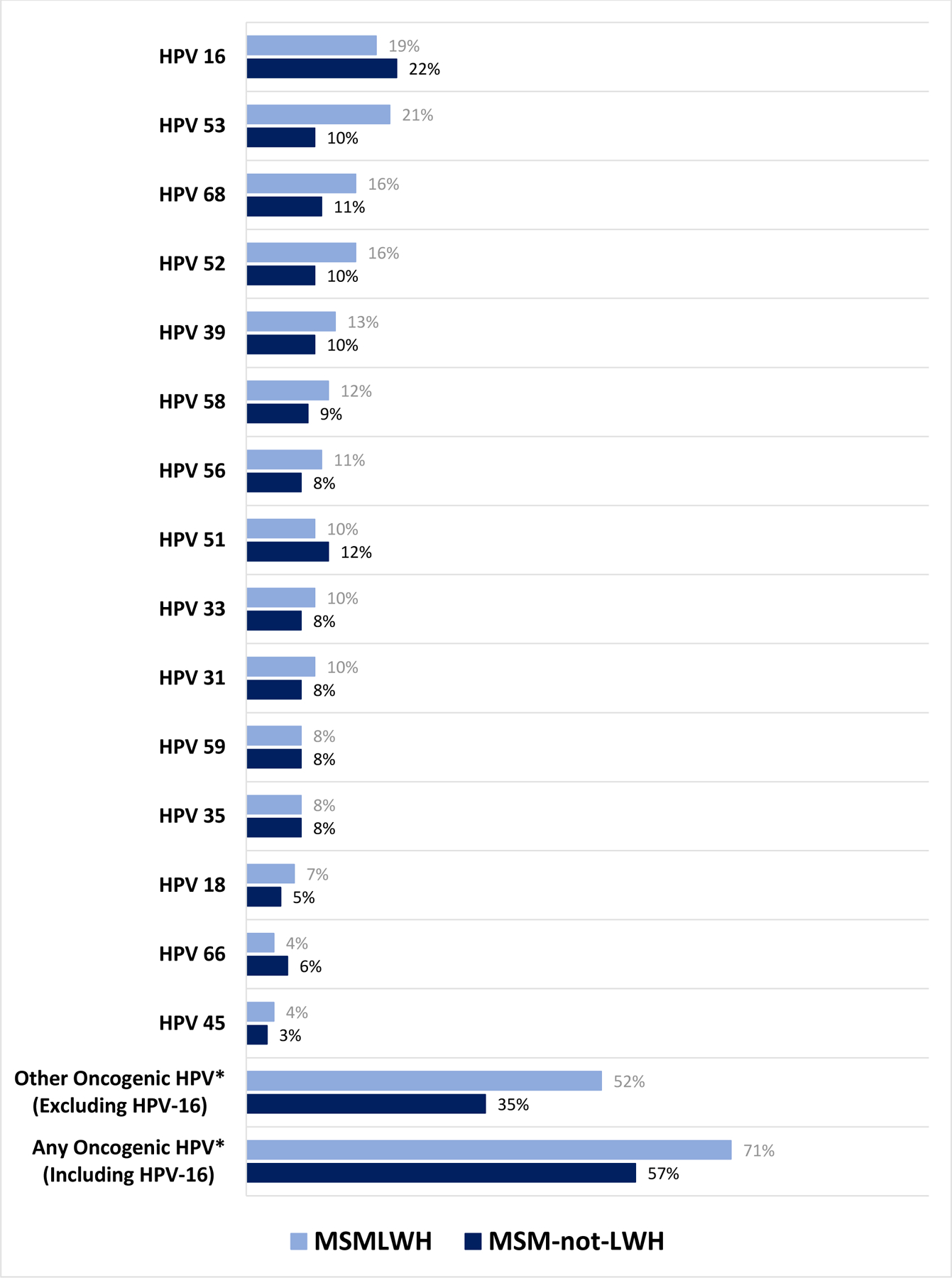
*Other Oncogenic HPV = One or more of the following HPV types: 18, 31, 33, 35, 39, 45, 51, 52, 53, 56, 58, 59, 66, 68
Three participants were omitted from further analyses because they were unable to complete the HRA and were missing the biopsy-confirmed HSIL results (1 MSMLWH and 2 MSM-not-LWH). An additional MSMLWH had missing HPV DNA results and is also omitted from further analyses.
Associations of anal HSIL with HIV, HPV, and age at enrollment.
The overall prevalence of biopsy-confirmed anal HSIL was 42.3% (51%−64%) in this population of older MSM, including one MSMLWH who had cancer (0.8%, 95% CI: 0.02%−4.3%) and was grouped with those having anal HSIL. While MSMLWH had a higher prevalence than MSM-not-LWH, 46.7% (38%−56%) versus 37.4% (29%−47%), respectively, this difference was not statistically significant (Table 2). Similarly, after accounting for both HIV and CD4+ level, HSIL prevalence did not differ statistically significantly between these groups (Table 3).
Table 3:
Associations of select variables with biopsy-confirmed anal HSIL (N=234*).
| HSIL Frequency | Unadjusted OR** | Adjusted OR*** | |||
|---|---|---|---|---|---|
| HSIL Risk Characteristic | HSIL cases/ row total | (%) | OR (95% CI) | aOR (95% CI) | P-value**** |
| All participants | 99/234 | (42.3) | |||
|
| |||||
| HIV Status and CD4+ Level | 0.21 | ||||
| No HIV (MSM-not-LWH) | 40/107 | (37.4) | Ref | Ref | |
| HIV and CD4:500+ | 36/82 | (43.9) | 1.31 (0.73–2.35) | 1.20 (0.57–2.50) | |
| HIV and CD4: <500 | 21/39 | (54.0) | 2.05 (0.98–4.27) | 2.50 (1.01–6.17) | |
| Missing CD4+ Level | 2/6 | (33.3) | NE* | ||
|
| |||||
| Anal HPV Infection | < 0.001 | ||||
| None | 11/83 | (13.3) | Ref | Ref | |
| Other Oncogenic HPV (not HPV-16) | 48/103 | (46.6) | 5.71 (2.72–12.0) | 5.95 (2.74–12.9) | |
| HPV-16 | 40/48 | (83.3) | 32.7 (12.2–88.0) | 45.1 (15.8–129) | |
|
| |||||
| Age | Ref 1.15 (0.69–1.94) |
Ref 1.23 (0.65–2.34) |
0.51 | ||
| <60 | 52/127 | (40.9) | |||
| 60+ | 47/107 | (44.0) | |||
|
| |||||
| Income | Ref 0.92 (0.53–1.59) NE |
Ref 0.71 (0.34–1.50) NE |
0.60 | ||
| $0-$59,999 | 54/126 | (43.0) | |||
| $60,000+ | 38/92 | (41.3) | |||
| Decline to answer/missing | 7/16 | (43.8) | |||
|
| |||||
| Education | Ref 1.43 (0.82–2.48) NE |
Ref 2.63 (1.26–5.50) NE |
0.015 | ||
| Less than graduate/ professional | 62/157 | (39.5) | |||
| Graduate/ professional+ | 37/76 | (48.7) | |||
| Missing/decline | 0/1 | (0.00) | |||
3 MSMLWH were excluded from analysis because they were missing biopsy-confirmed HSIL results and 1 MSM-not-LWH was excluded because of missing anal HPV DNA results
Unadjusted odds ratio (OR) and exact 95% confidence interval (CI)
Odds ratios are adjusted for age, income, education, and HIV status and current CD4+ level combined.
p-value from likelihood ratio statistic; Ref, reference; NE, not estimable
In our multivariable model, HPV-16 strongly increased the odds of prevalent anal HSIL (OR: 45.1, 95% CI: 15.8–129) as did other oncogenic HPV types (OR: 5.95, 95% CI: 2.74–12.9) when compared to not having any HPV infection (p<0.0001) (Table 3). The prevalence of anal HSIL, anal HPV-16, and other oncogenic HPV did not differ significantly by age group in either MSMLWH or MSM-not-LWH in bivariable analyses (Figure 2). In adjusted multivariable models of HSIL, age was evaluated as a categorical variable (50–59, 60–69, and 70+), per protocol, as a continuous variable and as a two-part variable (<60, 60+) to assess if age was an effect modifier of HIV status; it was not significant in any model evaluated. We similarly evaluated age as an effect modifier of the relationship between anal HPV infection and HSIL, and it was also non-significant in models. Interactions of age with HIV status and with HPV categories were not statistically significant in the model and are not presented in Table 3.
Figure 2: Prevalence of HSIL (A), HPV-16 (B), and other oncogenic HPV* (C), by age group, for MSMLWH and MSM-not-LWH.
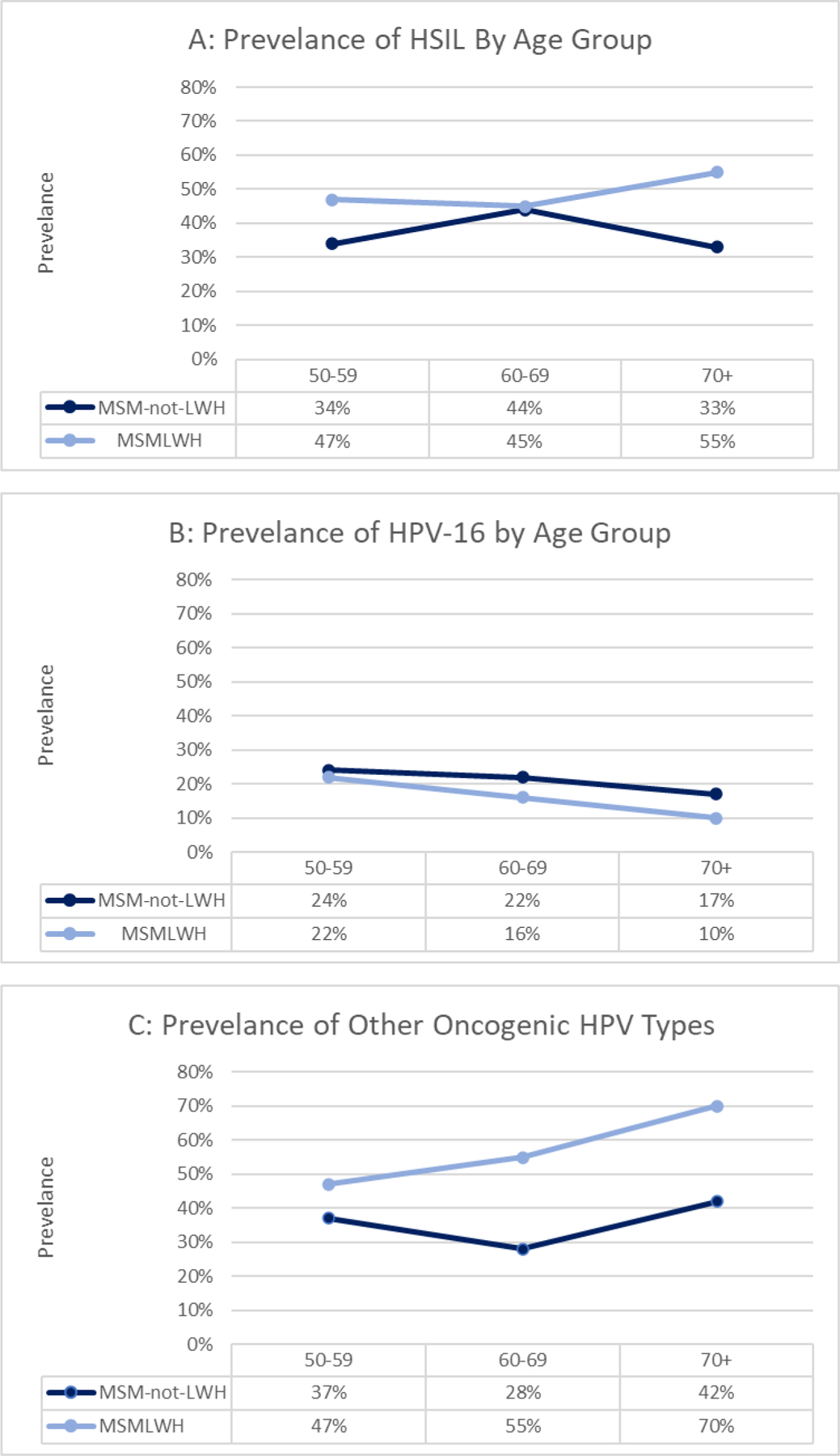
*Other Oncogenic HPV (excluding HPV-16) = One or more of the following HPV types: 18, 31, 33, 35, 39, 45, 51, 52, 53, 56, 58, 59, 66, 68; p-value for all comparisons non-significant; p-values generated using generalized estimating equation (GEE models) for each outcome as a function of age, HIV, and an age*HIV status interaction term.
Supplemental Figure A presents prevelances of anal HPV with HPV types grouped in three different categorizations: (1) HPV-16 plus other HPV types; (2) types included in the 9-valent vaccine (16, 18, 31, 33, 45, 52, 58) and types not included (35, 39, 51, 53, 56, 59, 66, 68); (3) and HPV types grouped by increasing detection in cervical cancers. Two percent of MSMLWH had only HPV-16 and 17% had HPV-16 plus at least one other oncogenic type. Among MSM-not-LWH, 6% had only HPV-16 and 17% had HPV-16 plus at least one other oncogenic type. 20% of MSMLWH and 18% of MSM-not-LWH had HPV types not included in the 9-valent HPV vaccine. Supplemental Table A presents the associations of anal HPV as categorized above. Participants with anal HPV types not included in the vaccine had an increased odds of anal HSIL (OR: 3.6, CI: 1.50–8.71).
Discussion
This is the first study that we are aware of that focused exclusively on evaluating anal HPV, anal HSIL, and HIV status among MSM ages 50 and over. We found the prevalence of anal HSIL, the precursor to anal cancer, to be high (37–47%) among both MSMLWH and MSM-not-LWH who are 50 years of age and older. A recent meta-analysis that included over 29,000 men of all ages worldwide found the pooled prevalence of anal HSIL among MSMLWH at 22.4% and 11.3% for MSM-not-LWH (the meta-analysis estimates are for all men, not solely those over 50 years).12 While the prevalence estimate that we report is considerably higher than these pooled estimates, they are within the ranges reported in studies from the United States, Canada, and Australia (31–55% 17–23 among MSMLWH and 20–42% 17–19 among MSM-not-LWH). Studies included in the meta-analysis varied by population demographics, geographic location, level of clinician experience in HRA, as well as by the protocol used to screen for or diagnose anal HSIL, which could account for much of the variability found across studies.
Nevertheless, our results are at the high end of the ranges reported by these studies and indicate that a large proportion of MSM who are ages 50 and older (both living with and without HIV) have anal HSIL. Although there was no significant difference in the prevalence of HSIL by age, even men in our oldest age group of 70 and older had a high prevalence of anal HSIL (MSMLWH, 55%, and MSM-not-LWH, 33%). Given the recent finding that treating anal HSIL can prevent anal cancer14, these results imply that older MSM are still very much at risk for anal cancer and should be included in screening protocols. Anal cancer screening guidelines are being revised based on the results of the Anal Cancer HSIL Outcomes Research (ANCHOR) Study14, and it is possible that screening for and treatment of anal HSIL will become standard of care among PLWH. Although our sample size in this study is limited, our data imply that screening recommendations in this population should not include an upper age limit, in contrast to cervical cancer screening recommendations, which currently end at 65 years for most women.24 Additionally, the high prevalence of HSIL among MSM-not-LWH suggest that we should also consider including them in anal cancer screening programs even though the data on anal cancer incidence in this group is scarce.
In previous studies of San Francisco Bay Area MSMLWH who were ≥18 years of age, the prevalence of oncogenic anal HPV was approximately 80%.20,25,26 While our current study had consistent results from the other San Francisco studies, the prevalence of oncogenic HPV was somewhat lower at 71% among MSMLWH (and 57% among MSM-not-LWH). This lower value could reflect the more limited number of HPV types included in our assay than in previous analyses. It could also reflect the older chronological age of our study population as many HPV infections present earlier in life may clear12 and no longer be present when the individual is tested for HPV in older ages. HPV detection among older participants may reflect the reactivation of latent infection, persistent infection, or new exposure to HPV. The cross-sectional nature of this analysis does not allow us to distinguish among these possibilities. Forty-three percent of MSMLWH and MSM-not-LWH had at least one partner with whom the participant was the anal receptive partner, and, therefore, new infections are a possibility.
Our results on anal HPV infection are also consistent with the recent meta-analysis described above that presented age-specific prevalence estimates of anal HPV infection from men recruited globally; their pooled estimate in their 55+ group was 67% for MSMLWH and 49% for MSM-not-LWH12. They also described a steady prevalence by age after the ages of 23–24, consistent with our findings of an elevated but consistent prevalence by age.
In our participants, HPV-16 prevalence showed a non-significant decreasing trend with age (see Figure 2). However, the most important risk factor for having prevalent anal HSIL was anal HPV-16 infection, followed by other oncogenic types (excluding HPV-16). Consistent with many previous studies of anal cancer and anal HSIL12,27,28, we found strong associations between anal HPV infection and anal HSIL among all participants as well as among MSMLWH and MSM-not-LWH when considered separately (data not shown). The inclusion of demographic variables in our models did not damper the association.
While the participants in our study likely did not benefit from primary prevention by HPV vaccination, it is important to note that 39, 51, 53, and 68 were also common in our population of older MSM, and these types are not included in any of the current HPV vaccines.29 Although these types are not often found in anal cancers10, this has not been well evaluated in older age groups. Approximately 18–20% of our population had anal HPV types that are not included in HPV vaccines. A recent analysis of HPV types from anal cancers in PLWH showed that some were associated with single infections with HPV 51 and 35, which are also not included in the nonavalent HPV vaccine.30 This is worth investigating further since the potential of the vaccine to prevent HPV-related cancers in PLWH may be slightly lower than in HIV-negative individuals.
Our study had several limitations. Because our target population is considered a hidden or hard-to-reach population, we enrolled a convenience sample of MSM or transpersons in and around San Francisco. We enrolled them through various methods, for example, social media and dating apps like Grindr, and referrals from HIV health care and/or service providers. As a result, our prevalence estimates may not be generalizable to all MSM or transpersons living in areas with demographics differing from San Francisco, with individuals who do not use social media or dating apps or do not utilize or need specialized health care or services. Also, the UCSF ANCRE clinic has been providing and promoting anal cancer screening for decades. One of the exclusion criteria was that individuals should not have been previously screened for anal cancer or anal HSIL, and many potential participants were not eligible because they had been screened in the past. Other locations with lower screening rates may have increased prevalence. Unfortunately, we did not have sufficient participants who did not identify as male to conduct separate analyses for individuals with different gender identities. These are important groups and future studies should focus on targeted outreach to these communities.
In summary, a large proportion of both older MSMLWH and MSM-not-LWH have the most important risk factors for anal cancer currently, anal HPV infection and anal HSIL. As the population of MSMLWH continues to age, public health professionals need to consider these older populations in their prevention strategies, as public health campaigns aimed at younger men may not reach or resonate with older MSM.20 In addition, the recent evidence that treatment of anal HSIL prevents anal cancer14, paired with the high prevalence of these risk factors in older MSM, warrant a recommendation that older MSM, regardless of HIV status, be considered for anal cancer screening.
Supplementary Material
Acknowledgments
The authors would like to thank the ANCRE Center staff for their efforts and the participants of the AHHA Study for their time and effort.
Source of Funding:
The study was funded by the National Institutes for Health, National Cancer Institute grant R01CA206477.
Footnotes
Prior presentation: Preliminary data was presented previously at the International Workshop on HIV & Aging 2022 (Virtual Meeting, October 13–14, 2022) and the International Papillomavirus Conference 2020 (Virtual Meeting, July 20–24, 2020).
Conflicts of Interest: The authors have no conflicts of interest to disclose.
References
- 1.National Cancer Institute. Surveillance, Epidemiology, and Ende Results Program. SEER*Explorer. Accessed July 25, 2022. https://seer.cancer.gov/statistics-network/explorer/application.html?site=34&data_type=1&graph_type=2&compareBy=sex&chk_sex_1=1&chk_sex_3=3&chk_sex_2=2&hdn_rate_type=1&race=1&age_range=1&stage=101&advopt_precision=1&advopt_show_ci=on&hdn_view=0&advopt_display=2
- 2.Deshmukh AA, Suk R, Shiels MS, et al. Recent Trends in Squamous Cell Carcinoma of the Anus Incidence and Mortality in the United States, 2001–2015. JNCI: Journal of the National Cancer Institute. 2020;112(8):829–838. doi: 10.1093/jnci/djz219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA: A Cancer Journal for Clinicians. 2021;71(3):209–249. doi: 10.3322/caac.21660 [DOI] [PubMed] [Google Scholar]
- 4.Deshmukh AA, Suk R, Shiels MS, et al. Incidence Trends and Burden of Human Papillomavirus-Associated Cancers Among Women in the United States, 2001–2017. J Natl Cancer Inst 2021;113(6):792–796. doi: 10.1093/jnci/djaa128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Clifford GM, Georges D, Shiels MS, et al. A meta-analysis of anal cancer incidence by risk group: Toward a unified anal cancer risk scale. International Journal of Cancer. 2021;148(1):38–47. doi: 10.1002/ijc.33185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.US Preventive Services Task Force, Davidson KW, Barry MJ, et al. Screening for Colorectal Cancer: US Preventive Services Task Force Recommendation Statement. JAMA 2021;325(19):1965–1977. doi: 10.1001/jama.2021.6238 [DOI] [PubMed] [Google Scholar]
- 7.US Preventive Services Task Force, Curry SJ, Krist AH, et al. Screening for Cervical Cancer: US Preventive Services Task Force Recommendation Statement. JAMA 2018;320(7):674–686. doi: 10.1001/jama.2018.10897 [DOI] [PubMed] [Google Scholar]
- 8.Machalek DA, Poynten M, Jin F, et al. Anal human papillomavirus infection and associated neoplastic lesions in men who have sex with men: a systematic review and meta-analysis. Lancet Oncol 2012;13(5):487–500. doi: 10.1016/S1470-2045(12)70080-3 [DOI] [PubMed] [Google Scholar]
- 9.Centers for Disease Control and Prevention (CDC). Cancers Associated with Human Papillomavirus, United States—2014–2018 | CDC Centers for Disease Control and Prevention (CDC); 2021. Accessed July 25, 2022. https://www.cdc.gov/cancer/uscs/about/data-briefs/no26-hpv-assoc-cancers-UnitedStates-2014-2018.htm [Google Scholar]
- 10.Lin C, Franceschi S, Clifford GM. Human papillomavirus types from infection to cancer in the anus, according to sex and HIV status: a systematic review and meta-analysis. Lancet Infect Dis 2018;18(2):198–206. doi: 10.1016/S1473-3099(17)30653-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Berry JM, Jay N, Cranston RD, et al. Progression of anal high-grade squamous intraepithelial lesions to invasive anal cancer among HIV-infected men who have sex with men. Int J Cancer. 2014;134(5):1147–1155. doi: 10.1002/ijc.28431 [DOI] [PubMed] [Google Scholar]
- 12.Wei F, Gaisa MM, D’Souza G, et al. Epidemiology of anal human papillomavirus infection and high-grade squamous intraepithelial lesions in 29 900 men according to HIV status, sexuality, and age: a collaborative pooled analysis of 64 studies. Lancet HIV 2021;8(9):e531–e543. doi: 10.1016/S2352-3018(21)00108-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Palefsky JM. Human papillomavirus-associated anal and cervical cancers in HIV-infected individuals: incidence and prevention in the antiretroviral therapy era. Curr Opin HIV AIDS 2017;12(1):26–30. doi: 10.1097/COH.0000000000000336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Palefsky JM, Lee JY, Jay N, et al. Treatment of Anal High-Grade Squamous Intraepithelial Lesions to Prevent Anal Cancer. N Engl J Med 2022;386(24):2273–2282. doi: 10.1056/NEJMoa2201048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hernandez AL, Weatherly CS, Gonzalez R, et al. Rationale and design of the Anal HPV, HIV and Aging (AHHA) study: Protocol for a prospective study of anal HPV infection and HSIL among men who have sex (MSM) or trans women living with and without HIV, ages 50 and older. Frontiers in Epidemiology. 2022;2. Accessed December 20, 2022. https://www.frontiersin.org/articles/10.3389/fepid.2022.992718 [DOI] [PMC free article] [PubMed]
- 16.Hernandez AL, Weatherly CS, Burrowes S, Jimenez J, Gonzalez R, Palefsky J. “The problem is that our culture is just so messed up about aging.” Recruiting older men who have sex with men (MSM) into clinical studies. BMC Medical Research Methodology. BMC Medical Research Methodology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lowe B, Goldstone SE, Rus S, et al. Detection of Human Papillomavirus in Anal Specimens Using the Hybrid Capture 2 Assay. Diagnostic Molecular Pathology. 2012;21(3):150. doi: 10.1097/PDM.0b013e318249fd6b [DOI] [PubMed] [Google Scholar]
- 18.Medina-Laabes DT, Suarez-Perez EL, Guiot HM, et al. Human Papillomavirus Correlates With Histologic Anal High-Grade Squamous Intraepithelial Lesions in Hispanics With HIV. J Low Genit Tract Dis 2018;22(4):320–325. doi: 10.1097/LGT.0000000000000416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Machalek DA, Poynten IM, Jin F, et al. A Composite Cytology-Histology Endpoint Allows a More Accurate Estimate of Anal High-Grade Squamous Intraepithelial Lesion Prevalence. Cancer Epidemiol Biomarkers Prev 2016;25(7):1134–1143. doi: 10.1158/1055-9965.EPI-15-1106 [DOI] [PubMed] [Google Scholar]
- 20.Hernandez AL, Efird JT, Holly EA, Berry JM, Jay N, Palefsky JM. Risk factors for anal human papillomavirus infection type 16 among HIV-positive men who have sex with men in San Francisco. J Acquir Immune Defic Syndr 2013;63(4):532–539. doi: 10.1097/QAI.0b013e3182968f87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gaisa MM, Sigel KM, Deshmukh AA, et al. Comparing Anal Cancer Screening Algorithms Using Cytology and Human Papillomavirus DNA Testing in 3 High-Risk Populations. J Infect Dis 2021;224(5):881–888. doi: 10.1093/infdis/jiaa801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.de Pokomandy A, Rouleau D, Ghattas G, et al. Prevalence, clearance, and incidence of anal human papillomavirus infection in HIV-infected men: the HIPVIRG cohort study. J Infect Dis 2009;199(7):965–973. doi: 10.1086/597207 [DOI] [PubMed] [Google Scholar]
- 23.Wilkin TJ, Chen H, Cespedes MS, et al. A Randomized, Placebo-Controlled Trial of the Quadrivalent Human Papillomavirus Vaccine in Human Immunodeficiency Virus-Infected Adults Aged 27 Years or Older: AIDS Clinical Trials Group Protocol A5298. Clin Infect Dis 2018;67(9):1339–1346. doi: 10.1093/cid/ciy274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Eun TJ, Perkins RB. Screening for Cervical Cancer. Medical Clinics of North America. 2020;104(6):1063–1078. doi: 10.1016/j.mcna.2020.08.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Palefsky JM, Holly EA, Ralston ML, Jay N. Prevalence and risk factors for human papillomavirus infection of the anal canal in human immunodeficiency virus (HIV)-positive and HIV-negative homosexual men. J Infect Dis 1998;177(2):361–367. doi: 10.1086/514194 [DOI] [PubMed] [Google Scholar]
- 26.Palefsky JM, Holly EA, Efirdc JT, et al. Anal intraepithelial neoplasia in the highly active antiretroviral therapy era among HIV-positive men who have sex with men. AIDS 2005;19(13):1407–1414. doi: 10.1097/01.aids.0000181012.62385.4a [DOI] [PubMed] [Google Scholar]
- 27.Kelly H, Chikandiwa A, Alemany Vilches L, Palefsky JM, de Sanjose S, Mayaud P. Association of antiretroviral therapy with anal high-risk human papillomavirus, anal intraepithelial neoplasia, and anal cancer in people living with HIV: a systematic review and meta-analysis. Lancet HIV 2020;7(4):e262–e278. doi: 10.1016/S2352-3018(19)30434-5 [DOI] [PubMed] [Google Scholar]
- 28.Palefsky JM, Lensing SY, Belzer M, et al. High Prevalence of Anal High-Grade Squamous Intraepithelial Lesions, and Prevention Through Human Papillomavirus Vaccination, in Young Men Who Have Sex With Men Living With Human Immunodeficiency Virus. Clin Infect Dis 2021;73(8):1388–1396. doi: 10.1093/cid/ciab434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.HPV Vaccination: What Everyone Should Know | CDC Published May 6, 2022. Accessed November 3, 2022. https://www.cdc.gov/vaccines/vpd/hpv/public/index.html
- 30.Chowdhury S, Darragh TM, Berry-Lawhorn JM, et al. HPV Type Distribution in Benign, High-Grade Squamous Intraepithelial Lesions and Squamous Cell Cancers of the Anus by HIV Status. Cancers (Basel) 2023;15(3):660. doi: 10.3390/cancers15030660 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


