Abstract
Background and Objectives
Cognitive decline rates in Alzheimer disease (AD) vary greatly. Disease-modifying treatments may alter cognitive decline trajectories, rendering their prediction increasingly relevant. We aimed to construct clinically applicable prediction models of cognitive decline in amyloid-positive patients with mild cognitive impairment (MCI) or mild dementia.
Methods
From the Amsterdam Dementia Cohort, we selected amyloid-positive participants with MCI or mild dementia and at least 2 longitudinal Mini-Mental State Examination (MMSE) measurements. Amyloid positivity was based on CSF AD biomarker concentrations or amyloid PET. We used linear mixed modeling to predict MMSE over time, describing trajectories using a cubic time curve and interactions between linear time and the baseline predictors age, sex, baseline MMSE, APOE ε4 dose, CSF β-amyloid (Aβ) 1–42 and pTau, and MRI total brain and hippocampal volume. Backward selection was used to reduce model complexity. These models can predict MMSE over follow-up or the time to an MMSE value. MCI and mild dementia were modeled separately. Internal 5-fold cross-validation was performed to calculate the explained variance (R2).
Results
In total, 961 participants were included (age 65 ± 7 years, 49% female), 310 had MCI (MMSE 26 ± 2) and 651 had mild dementia (MMSE 22 ± 4), with 4 ± 2 measurements over 2 (interquartile range 1–4) years. Cognitive decline rates increased over time for both MCI and mild dementia (model comparisons linear vs squared vs cubic time fit; p < 0.05 favoring a cubic fit). For MCI, backward selection retained age, sex, and CSF Aβ1–42 and pTau concentrations as time-varying effects altering the MMSE trajectory. For mild dementia, retained time-varying effects were Aβ1–42, age, APOE ε4, and baseline MMSE. R2 was 0.15 for the MCI model and 0.26 for mild dementia in internal cross-validation. A hypothetical patient with MCI, baseline MMSE 28, and CSF Aβ1–42 of 925 pg/mL was predicted to reach an MMSE of 20 after 6.0 years (95% CI 5.4–6.7) and after 8.6 years with a hypothetical treatment reducing decline by 30%.
Discussion
We constructed models for MCI and mild dementia that predict MMSE over time. These models could inform patients about their potential cognitive trajectory and the remaining uncertainty and aid in conversations about individualized potential treatment effects.
Introduction
Alzheimer disease (AD) is a progressive neurodegenerative disease with considerable variability in the rate of cognitive decline.1 The disease is highly prevalent, with roughly 100 million people estimated to be in the mild cognitive impairment (MCI) and dementia stages of the disease.2 From the MCI stage, it is estimated to take 4 years on average before people have progressed to dementia.3 New disease-modifying treatments targeting amyloid plaques slow disease progression in the MCI and mild dementia stages of AD.4-6 However, the clinical meaningfulness of these medications is debated.7 Two factors in this debate are the challenge of translating the identified 30% reduction in decline rates into outcomes relevant to patients and the complexity of assessing the impact of disease-modifying treatments on an individual's decline trajectory because of heterogeneity in progression.
Patients are highly interested in their expected disease course.8,9 To accommodate these needs, prediction models of individualized natural cognitive trajectories and the associated uncertainty are urgently needed. When these individualized natural course predictions are combined with intervention efficacy data, the putative intervention benefits can be personalized.
Predicting progression from MCI to dementia has received much attention in the literature.10 While the future risk of dementia can be predicted with reasonable precision using MRI and CSF biomarker information,10-12 this crude end point may not be the most meaningful to patients. In addition, patients with mild dementia do not benefit from these predictions while prognostic information is equally important to them. In a study on outcomes that matter to patients with AD and their caregivers, participants indicated cognitive decline to be among the most important factors.8 Earlier models predicting cognitive decline have been published.12-14 However, they are either limited to patients with MCI12 or the models have not been built for easy clinical use.13,14 Therefore, we aimed to construct clinically applicable prediction models of cognitive decline in amyloid-positive patients with MCI or mild dementia.
Methods
Design and Patients
In this longitudinal study, we included participants from the Amsterdam Dementia Cohort, which is a mixed memory clinic cohort of all patients with memory complaints presenting at Alzheimer Center Amsterdam. While the Amsterdam Dementia Cohort does not have exclusion criteria, elderly patients are often referred to the geriatric outpatient clinic and thus do not present themselves for inclusion in the cohort. Inclusion criteria for this study were a baseline diagnosis of MCI or mild dementia (clinical dementia rating of less than 2), amyloid positivity at baseline, and a baseline and at least 1 follow-up Mini-Mental State Examination (MMSE). Participants had their baseline visit between August 2002 and December 2022. This study followed the Transparent Report of a Multivariable Prediction Model for Individual Prognosis or Diagnosis reporting guideline.
At our memory clinic, a standardized 1-day diagnostic workup is performed, including medical history; neurologic, physical, and neuropsychological tests; MRI; and lumbar puncture.15 This includes measurements of height, weight, systolic and diastolic blood pressure, and information on depression with the Geriatric Depression Scale,16 education on the Verhage scale,17 and smoking history. Diagnosis of dementia due to AD and MCI was made in a multidisciplinary meeting.18 All diagnoses fulfilled the core clinical criteria, National Institute on Aging-Alzheimer's Association criteria.19,20 During annual follow-up, medical examination and neuropsychological tests were performed without blinding to information gathered at baseline.
We used the MMSE as the main cognitive outcome in this study.21 Those with MCI had a median follow-up of 3 (interquartile range [IQR] 2–5) years with on average 4 (SD 2) MMSE measurements per participant for a combined 1,315 measurements. The mild dementia group had a median of 2 (IQR 1–3) years of follow-up with an average of 3 (SD 2) MMSE measurements per participant for a combined 2,113 measurements. As an additional outcome to provide information on the decline in memory, we used the Dutch version of the Rey Auditory Verbal Learning Test (RAVLT) Immediate Recall score (total available: n = 2,855, MCI: n = 1,227, mild dementia: n = 1,628).22
Amyloid Positivity and CSF Measurements
Amyloid positivity was defined based on either AD biomarkers in CSF or on amyloid PET within 6 months after the baseline MMSE. β-Amyloid (Aβ) 1–42 and phosphorylated threonine 181 (pTau) information from CSF was available in 874 (91%) participants. Before 2018, sandwich ELISA was used (Innotest, Fujirebio, Gent, Belgium). Innotest Aβ values were drift corrected.23 From 2018 onward, CSF was analyzed using Elecsys (Roche, Rotkreuz, Switzerland). For the Innotest assays, a drift-corrected Aβ1–42 below 813 pg/mL was considered positive, and for Elecsys assays, a pTau/Aβ1–42 ratio of more than or equal to 0.020 was considered positive.24 For the prediction models, Innotest CSF values were bridged to Elecsys.25 In total, 860 participants were amyloid positive based on their CSF.
Amyloid PET imaging was performed for 309 (32%) participants using 3-Tesla Ingenuity TF PET/MRI, Ingenuity TF PET/CT, and Gemini TF PET/CT scanners (Philips Healthcare, Amsterdam, the Netherlands) with the 11C-Pittsburgh compound B, 18F-flutemetamol, and 18F-florbetaben compounds.26,27 Visual rating was performed according to company guidelines or for 11C-Pittsburgh compound B according to previously published methods and discriminated between positive scans (n = 297) and negative scans.28
Two hundred twenty-two (23%) participants had both CSF and PET measurements. Participants could be included when either their CSF or amyloid PET was positive. CSF and PET were concordant for 196 (20%) participants and discordant for 26 (3%) based on positive CSF and negative amyloid PET (n = 12) or vice versa (n = 14).
MRI Measurements
MRI was performed on site in 762 (79%) participants. Before 2008, 1-T and 1.5-T scanners were used (Magnetom Avanto, Impact, and Sonata, Siemens; Signa, GE Healthcare). From 2008 onward, 3-T scanners were used (Magnetom Siemens; Discovery MR750, Signa GE Medical Systems; Ingenuity TF PET/MR, Philips Medical Systems; and Titan, Toshiba Medical Systems). All scans were performed using a standardized protocol.29
Volumetric MRI measurements were the primary MRI biomarkers used in this study. Left and right hippocampal volume and whole brain volume were quantified using Freesurfer version 7.1 (available in 709 [74%] participants), visually checked, and scanner-related differences were adjusted for thorough harmonization using the ComBat procedure.30
Statistical Analyses
Baseline information was missing for some participants (eTable 1) on all predictors except for age, sex, and baseline MMSE and diagnosis. Missing information was imputed using multiple imputations by chained equations in 25 imputation data sets. Variables used in the identification of donors were selected based on a minimal correlation of 0.05 with the variable being imputed. Baseline diagnosis was used in all donor selections. Parameter estimates were pooled across the imputation sets. The distribution of imputed values and convergence were assessed visually.
We used linear mixed models to model MMSE over time, including a random slope and intercept per individual. Separate models were developed for MCI and mild dementia. First, the trajectory of MMSE over time (including baseline in the outcome) was described using only a cubic time curve. Subsequently, we used backward selection procedures to construct models predicting MMSE over follow-up using a cubic time curve and baseline measurements. The baseline measures could be included as predictors with a constant effect over time (no interaction with time) or as predictors with a time-varying effect (interaction of linear time × baseline predictor). In these models, baseline MMSE was included as a potential predictor. Backward selection was started from (1) a base model including age, sex, and baseline MMSE; (2) a biomarker model adding to the base model: CSF Aβ1–42 and pTau, MRI total brain and hippocampal volume, and APOE ε4; or (3) a full model adding a range of clinical variables and risk factors encompassing the Verhage score, Geriatric Depression Scale, systolic and diastolic blood pressure, body mass index (categorized as <25, 25–30, and >30 kg/m2), and smoking history. The various models represent variations in information availability in clinical settings. Variables that were selected (p < 0.10) in at least half of the 25 imputed sets were included in the final models pooled over all imputed sets. The time, age, and sex variables were preselected in all imputation sets.
We investigated the effect of the statistical method used by evaluating 2 additional modeling approaches without backward selection: no parameter penalization and ridge penalization. Both statistical methods were applied to the 3 models listed above. For ridge penalization,31 local shrinkage was applied in 4 groups: the cubic time curve, MMSE at baseline, other parameters without time-varying effects, and parameters with linear time-varying effects.
Predictive performance was assessed using internal 5-fold cross-validation for the no parameter penalization, backward selection, and ridge penalization models. Out-of-sample root mean squared error, median absolute deviation, and proportion of explained variance (R2) were calculated. Internal cross-validation of ridge penalization was performed using 5 imputed data sets because of computational limitations. For MCI and mild dementia, 1 statistical method and model (base, biomarker, full model) was selected to highlight based on the model performance and the number of parameters included in the models, favoring a more parsimonious model.
We visualized the predicted decline pattern in the MCI and mild dementia groups in one of the imputed data sets (arbitrarily the first) based on the estimated fixed and random effects, highlighting the 2nd, 16th, 50th, 84th, and 98th percentiles of predicted decline at different time points. To visualize the interindividual variation (error) and provide insight into the uncertainty surrounding individualized predictions, we plotted 1,000 samples from the random effect distribution around the predicted mean MMSE for a hypothetical patient with MCI and mild dementia. The hypothetical patients were based on the median predictor values in each group. The decline is also shown with hypothetical interventions that reduce the predicted mean MMSE decline by 10%, 30%, and 50%.
To simplify the interpretation of the predicted decline, we used the model to estimate the time to reach a threshold MMSE of 20 (indicating mild dementia) for MCI and 15 for moderate dementia.32 The time to the threshold MMSE was calculated for different baseline CSF Aβ1–42 and baseline MMSE measurements; other predictors were fixed at the median. In addition, we provide the time to threshold MMSE with a hypothetical intervention that reduced decline by 30%.
We performed external validation for all constructed models in data from Alzheimer's Disease Neuroimaging Initiative (ADNI).33 ADNI is a longitudinal self-referral scientific cohort of patients with cognitively unimpairment, MCI, and dementia aged 55–99 years.33 Important exclusion criteria were the presence of significant neurologic disease other than AD or prior psychiatric diagnoses interfering with the cognitive assessments. The cohort was launched in 2003 to test whether serial MRI, PET, other biological markers, and clinical and neuropsychological assessment can be combined to measure the progression of MCI and early AD. Baseline and follow-up measurements in ADNI included all information needed to validate the models. No visual MRI read information was available from ADNI. From ADNI, participants who met the inclusion criteria were included for this study (see eTable 2 for baseline characteristics). For the MCI sample, we selected those with “late” MCI.34 In total, 598 ADNI participants were included (389 MCI; 209 mild dementia), with 2 (IQR 1–4) years of follow-up and 4 (IQR 3–6) MMSE measurements on average. The mean age was 74 years (SD 8), and 41% were female.
The modeling and validation steps were also performed with RAVLT as the outcome (see eMethods for additional information). Exploratory analyses using visual MRI read biomarkers and excluding CSF as potential predictors are included in eMethods and eResults.
The highlighted models are available as a shiny app as a proof of concept for the implementation of prediction tools on predictmmse.com. Normally distributed variables are displayed with means and SD and skewed distributions with medians and IQRs. Model diagnostics were assessed graphically. All analyses were performed in R version 4.2.1,35 with the use of the “lme4,” “mgcv,” and “mice” packages.
Standard Protocol Approvals, Registrations, and Patient Consents
The study protocol of the Amsterdam Dementia Cohort was approved by the ethical review board of the VU University Medical Center (2016.061). Written informed consent was obtained from all patients for the use of their data for research purposes.
Data Availability
Data can be made available upon reasonable request.
Results
Within the Amsterdam Dementia Cohort, there were 1,789 amyloid-positive participants with MCI (n = 436) or mild dementia (n = 1,344) and a baseline MMSE measurement. Of those, 961 participants also had a follow-up MMSE measurement (Table 1), 310 of whom had MCI and 651 had mild dementia; 462 (48%) were female; over 90% were White, with an average age (SD) of 65 years (7). The mild dementia group without follow-up had a 1.7 (SE 0.2) point lower baseline MMSE and 93 (SE 12) pg/mL lower CSF Aβ1–42 concentration (eTable 3) than the group with follow-up.
Table 1.
Baseline Characteristics
| Total (n = 961) | Partition based on diagnosis | ||
| MCI (n = 310) | Mild dementia (n = 651) | ||
| Age at baseline, y, mean ± SD | 65 ± 7 | 66 ± 7 | 65 ± 7 |
| Female, n (%) | 461 (48.0) | 141 (45.5) | 320 (49.2) |
| MMSE at baseline, mean ± SD | 23.6 ± 3.9 | 26.5 ± 2.3 | 22.3 ± 3.8 |
| No. of MMSE measurements, mean ± SD | 3.6 ± 1.8 | 4.2 ± 2.1 | 3.2 ± 1.5 |
| Years between first and last MMSE, median (IQR) | 2.2 (1.2–3.6) | 3.1 (2.0–4.9) | 2.0 (1.1–3.1) |
| RAVLT Immediate Recall at baseline, mean ± SD | 25.3 ± 8.5 | 30.2 ± 7.3 | 22.9 ± 8.1 |
| Years of education,a mean ± SD | 12.2 ± 3.0 | 12.5 ± 3.1 | 12.0 ± 2.9 |
| Geriatric Depression Scale, median ± IQR | 2.8 ± 2.4 | 3.0 ± 2.5 | 2.7 ± 2.3 |
| Systolic blood pressure, mm Hg, mean ± SD | 147 ± 19 | 146 ± 19 | 148 ± 19 |
| Diastolic blood pressure, mm Hg, mean ± SD | 84 ± 10 | 84 ± 10 | 84 ± 10 |
| Body mass index, kg/m2, n (%) | |||
| Below 18.5 | 18 (2.0) | 3 (1.0) | 15 (2.5) |
| 18.5–25 | 522 (57.6) | 157 (53.2) | 365 (59.7) |
| 25–30 | 303 (33.4) | 118 (40.0) | 185 (30.3) |
| Over 30 | 63 (7.0) | 17 (5.8) | 46 (7.5) |
| Reported smoking status, n (%) | |||
| Never | 473 (49.7) | 146 (47.6) | 327 (50.8) |
| Stopped | 331 (34.8) | 108 (35.2) | 223 (34.6) |
| Current smoker | 147 (15.5) | 53 (17.3) | 94 (14.6) |
| CSF measures | |||
| β-Amyloid 1–42, pg/mL, mean ± SD | 757 ± 210 | 789 ± 217 | 742 ± 205 |
| Phosphorylated tau, pg/mL, median (IQR) | 32.2 (23.8–43.2) | 29.7 (22.9–41.0) | 33.5 (24.3–45.3) |
| MRI measures | |||
| Medial temporal atrophy score, mean ± SD | 1.1 ± 0.8 | 0.8 ± 0.7 | 1.3 ± 0.8 |
| Global atrophy score, mean ± SD | 0.9 ± 0.6 | 0.7 ± 0.6 | 1.0 ± 0.7 |
| Fazekas score, mean ± SD | 1.0 ± 0.8 | 1.0 ± 0.7 | 1.0 ± 0.8 |
| Lacunes visually present, n (%) | 152 (20.7) | 63 (25.0) | 89 (18.4) |
| Microbleeds visually present, n (%) | 62 (8.2) | 27 (10.5) | 35 (7.0) |
| Total brain volume, mL, mean ± SD | 1,078 ± 110 | 1,102 ± 113 | 1,066 ± 106 |
| Hippocampal volume, mL, mean ± SD | 6.8 ± 0.9 | 7.0 ± 0.9 | 6.6 ± 0.9 |
| APOE ε4 alleles, n (%) | |||
| 0 | 247 (26.4) | 67 (22.4) | 180 (28.3) |
| 1 | 447 (47.8) | 140 (46.8) | 307 (48.3) |
| 2 | 241 (25.8) | 92 (30.8) | 149 (23.4) |
Abbreviations: IQR = interquartile range; MMSE = Mini-Mental State Examination; RAVLT = Rey Auditory Verbal Learning Test.
Baseline characteristics shown before imputation.
Years of education were calculated from the Verhage score.
In both MCI and mild dementia, the yearly decline in MMSE increased during follow-up (model comparisons for linear vs squared, and squared vs cubic time fit; p < 0.05). In MCI, the average MMSE declined from 26.4 (95% CI 26.2–26.7) to 25.8 (25.5–26.1) after 18 months, to 24.2 (23.7–24.6) after 3 years, and to 21.0 (20.2–21.7) after 5 years. In mild dementia, the average MMSE declined from 22.4 (95% CI 22.0–22.7) to 19.8 (19.4–20.2), 15.3 (14.7–15.9), and 7.8 (6.8–8.9), respectively.
Internal cross-validation indicated that the models captured some of the variation in decline in MMSE in patients with MCI and mild dementia, albeit considerable uncertainty remained (Table 2). The backward selected models performed comparably with the models without penalization and had slightly lower performance than the ridge penalization model (eTables 4–6), but we highlight the backward selected models because they use fewer parameters. In the backward selected models, the biomarker model (model 2) performed slightly better than the basic model (model 1) and similar to the full model (model 3), albeit with fewer parameters. Thus, we focus on the biomarker model in the following sections. In the MCI group, the mean out-of-sample R2 and median absolute deviation in internal validation (Table 2) were 0.17 and 2.05, respectively, and in the mild dementia group, 0.26 and 2.83. This means that in half of the predictions made for patients with MCI, the observed MMSE deviated by less than 2 points from the predicted MMSE. Correspondingly, the deviation was less than approximately 3 points in mild dementia.
Table 2.
Predictive Performance of the MMSE Prediction Models in Internal Cross-Validation
| Model | No. of parameters (n; range over folds) | RMSE (range over folds) | MAD (range over folds) | R2 (range over folds) |
| Mild cognitive impairment | ||||
| Base model | ||||
| No penalization | 10 | 3.67 (3.36–3.97) | 2.17 (1.84–2.75) | 0.06 (−0.15 to 0.24) |
| Backward selection | 8 (7–8) | 3.62 (3.38–3.96) | 2.13 (1.85–2.50) | 0.09 (−0.15 to 0.23) |
| Ridge | 10 | 3.52 (3.20–3.93) | 2.09 (1.84–2.59) | 0.15 (−0.09 to 0.26) |
| Biomarker model | ||||
| No penalization | 18 | 3.48 (3.15–3.88) | 2.06 (1.77–2.50) | 0.16 (−0.04 to 0.30) |
| Backward selection | 13 (11–15) | 3.47 (3.13–3.85) | 2.05 (1.71–2.58) | 0.17 (−0.08 to 0.28) |
| Ridge | 18 | 3.39 (3.12–3.84) | 2.07 (1.84–2.54) | 0.21 (0.01 to 0.32) |
| Full model | ||||
| No penalization | 36 | 3.49 (3.12–3.88) | 2.14 (1.78–2.46) | 0.16 (−0.02 to 0.32) |
| Backward selection | 15 (13–19) | 3.51 (3.17–3.88) | 2.11 (1.86–2.58) | 0.14 (−0.10 to 0.30) |
| Ridge | 36 | 3.38 (3.08–3.86) | 2.07 (1.76–2.51) | 0.22 (0.02 to 0.34) |
| Mild dementia | ||||
| Base model | ||||
| No penalization | 10 | 4.77 (4.20–5.25) | 2.90 (2.80–3.01) | 0.22 (0.05 to 0.42) |
| Backward selection | 9 (8–9) | 4.75 (4.19–5.24) | 2.90 (2.80–3.10) | 0.22 (0.05 to 0.41) |
| Ridge | 10 | 4.48 (4.06–4.87) | 2.78 (2.73–2.87) | 0.31 (0.18 to 0.47) |
| Biomarker model | ||||
| No penalization | 20 | 4.65 (4.14–5.16) | 2.83 (2.56–3.02) | 0.26 (0.08 to 0.42) |
| Backward selection | 13 (12–15) | 4.65 (4.12–5.14) | 2.83 (2.55–2.96) | 0.26 (0.09 to 0.42) |
| Ridge | 20 | 4.44 (4.03–4.92) | 2.75 (2.62–2.86) | 0.32 (0.17 to 0.48) |
| Full model | ||||
| No penalization | 36 | 4.68 (4.17–5.14) | 2.78 (2.46–3.03) | 0.25 (0.09 to 0.44) |
| Backward selection | 19 (17–22) | 4.69 (4.20–5.14) | 2.84 (2.56–2.99) | 0.24 (0.09 to 0.43) |
| Ridge | 36 | 4.40 (3.97–4.86) | 2.70 (2.46–2.86) | 0.33 (0.18 to 0.50) |
Abbreviations: Aβ = β-amyloid; MAD = median absolute deviation; RMSE = root mean squared error.
The different prediction models were 5-fold internal cross-validation using all imputed data sets. Basic predictors included time, time2, time3, MMSE at baseline, age, sex, and the interaction of all these variables with a linear fit of time. Biomarker predictors also included APOE ε4 allele count, CSF Aβ1–42 and pTau, MRI normalized total brain and hippocampal volume, and the interaction of all these variables with a linear fit of time. The full model also included Geriatric Depression Scale, Verhage score, smoking history, body mass index, systolic and diastolic blood pressure, and their interactions with a linear fit of time. For the ridge prediction models, normalization of the variables was performed based on the mean and standard deviation of the variables in the imputed Amsterdam Dementia Cohort. For the ridge cross-validation, only 5 imputed data sets were used.
Different variables were retained in the MCI and mild dementia groups (Table 3, eTables 4, and 6). In MCI, baseline MMSE was retained as a time-constant effect and retained time-varying effects were age, sex, and CSF pTau and Aβ1–42. In mild dementia, retained time-constant effects were sex and CSF pTau and retained time-varying effects were age, baseline MMSE, APOE ε4, and CSF Aβ1–42. Volumetric MRI information was retained in none of the biomarker models.
Table 3.
Regression Coefficients of the Backward Selected Prediction Models of MMSE Over Time in MCI and Mild Dementia
| Variable names | MCI | Mild dementia |
| Coefficient (SE) | Coefficient (SE) | |
| Intercept | 12.9446 (2.4238) | −10.2450 (2.4825) |
| Years since baselinea | −1.6700 (1.1730) | −9.9597 (1.4078) |
| Years since baseline squareda | −0.2090 (0.0486) | −0.2196 (0.0582) |
| Years since baseline cubeda | 0.0141 (0.0091) | 0.0227 (0.0139) |
| Age at baseline, y | 0.0147 (0.0239) | 0.1020 (0.0274) |
| Sex, reference female | 0.4329 (0.3227) | −0.0811 (0.2718) |
| MMSE at baseline | 0.5283 (0.0534) | 1.0006 (0.0561) |
| No. of APOE ε4 alleles | — | 0.5419 (0.2842) |
| CSF pTau, pg/mL log transformed | −1.1162 (0.4153) | −1.0403 (0.2990) |
| CSF Aβ1–42, pg/mL | 0.0008 (0.0008) | 0.0019 (0.0010) |
| Interaction of years since baseline | ||
| Age at baseline, y | 0.0188 (0.0142) | 0.0376 (0.0156) |
| MMSE at baseline | — | 0.1299 (0.0345) |
| No. of APOE ε4 alleles | — | 0.4768 (0.1636) |
| CSF Aβ1–42, pg/mL | 0.0006 (0.0005) | 0.0009 (0.0006) |
| Sex, reference female | 0.2884 (0.1926) | — |
| CSF pTau, pg/mL log transformed | −0.3894 (0.2484) | — |
Abbreviations: Aβ = β-amyloid; MCI = mild cognitive impairment; MMSE = Mini-Mental State Examination.
In backward selection, the cubic time curve, age, and sex were forced into the model. The other variables were backward selected based on a p-value of <0.10 and were included in the final model if the variables were selected in at least half the imputed data set. Parameter estimates and standard error are based on pooling between the imputed data sets.
Centered by subtracting 2.3.
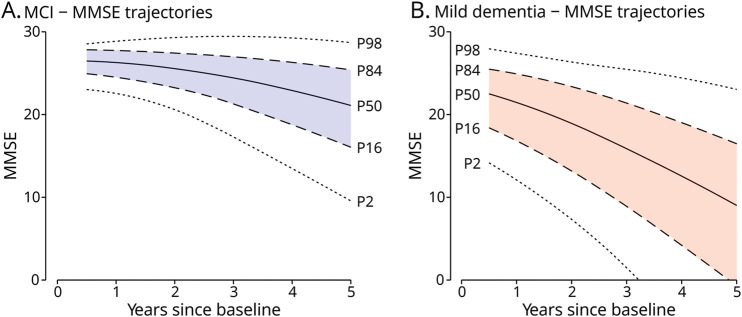
We visualized the heterogeneity in predicted decline in the study cohort (Figure 1) to allow patients to compare themselves with a representative “population.” The 84th (+1 SD from the mean) and 16th (−1 SD from the mean) percentiles of the predicted mean MMSE in the MCI group after 9 months were 28.0 and 24.7, respectively. After 5 years, the predicted mean MMSE measurements were 25.4 (84th percentile) and 16.2 (16th percentile). The 84th and 16th percentiles of the predicted mean MMSE in the mild dementia group after 9 months were 25.3 and 17.5, respectively, and 21.5 and 8.4 after 3 years. Against this population distribution, individualized predictions can be shown.
Figure 1. Distribution of Predicted MMSE Trajectories in the MCI and Mild Dementia Groups.
MMSE trajectories over time were estimated for all participants based on their patient characteristic and estimated random intercept and slope using the backward selected prediction models for mild cognitive impairment (A) and mild dementia (B) using the biomarker model. Arbitrarily, complete data from the first imputed data set were used. From all estimated MMSE trajectories, the predicted MMSE measurements at the 98th, 84th, 50th, 16th, and 2nd percentiles are plotted. Predicted MMSE measurements outside of the possible range (0–30) have not been plotted. Predicted MMSE is first displayed after 6 months. MCI = mild cognitive impairment, MMSE = Mini-Mental State Examination.
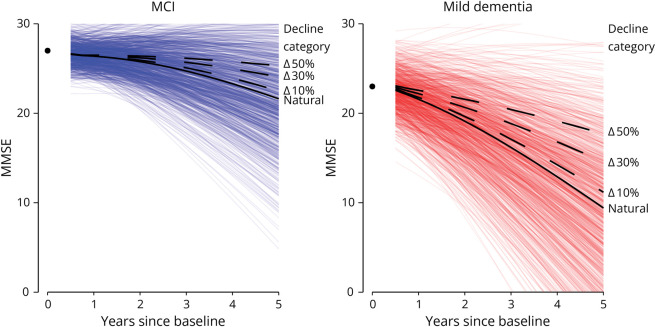
Next, we visualized the estimated trajectory with the unexplained interindividual variation (Figure 2). For the hypothetical patient with MCI with median predictor values, the predicted mean MMSE after 5 years was 21.0 (95% CI 20.9–23.0). By drawing 1,000 samples from the random-effect distribution, we can visualize the unexplained interindividual variation surrounding this predicted MMSE, showing 90% was between 30 and 13 MMSE points, indicating substantial variation for individuals with the same predictor values. As a next step, the MMSE predictions can also be made in the hypothetical situation where an intervention reduces decline by 30%. For this patient with MCI, a 30% reduction in decline would give a predicted mean MMSE after 5 years of 23.7. Within the interindividual variation surrounding the natural decline, this reduced decline places at the 69th percentile of the distribution. So, compared with the natural decline trajectory of 100 patients with MCI with the same predictor values, 69 are likely to have a lower MMSE after 5 years and 31 a higher MMSE. With a steeper predicted natural decline, the projected effect of interventions that reduce decline deviates more from the unexplained interindividual variation in MMSE (Figure 2).
Figure 2. Simulated Interindividual Variation Surrounding Predicted MMSE Trajectories.
Depicts predicted MMSE trajectories for hypothetical patients with MCI and mild dementia with median predictor values in each group. For the MCI group, the fixed parameter values were sex: male; age: 66 years; log CSF pTau: 3.415 ng/L; CSF Aβ1–42: 796.22; and baseline MMSE 27. For the mild dementia group, the fixed parameters values were sex: female; age: 66 years; APOE ε4 alleles: 2; log CSF pTau: 3.498 ng/L; CSF Aβ1–42: 758.8; and baseline MMSE 23. Predictions were made using the model based on the backward selection biomarker model. The black solid line is the predicted mean MMSE trajectory based on the median values, the “natural” trajectory. The colored lines surrounding the predicted MMSE depict 1,000 simulated random intercepts and effects, indicating interindividual variation in MMSE trajectories not explained by the available predictors. The dashed lines show expected MMSE trajectories with interventions that reduce decline by 10%, 30%, or 50%, respectively. The circle at year 0 indicated the baseline MMSE. Predicted MMSE is first presented after 6 months of follow-up. Aβ = β-amyloid; MCI = mild cognitive impairment; MMSE = Mini-Mental State Examination.
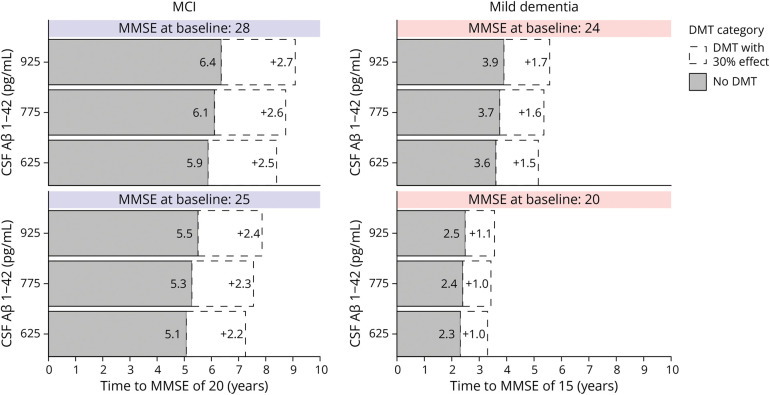
To make the results of the models more intuitive, we also visualized the models as a personalized predicted time to a certain MMSE value (Figure 3). The predicted mean time to reach an MMSE of 20 for a patient with MCI with a baseline MMSE of 28 and CSF Aβ1–42 of 925 pg/mL was 6.0 years (95% CI 5.4–6.7 years). For a patient with mild dementia with a baseline MMSE of 20 and CSF Aβ1–42 of 625 pg/mL, the predicted mean time to reach an MMSE of 15 was 2.3 years (95% CI 2.1–2.5). These estimates can also be used to evaluate potential time gains with inventions that reduce the rate of decline. With a hypothetical intervention that reduces decline by 30%, the time to threshold would be 8.6 years for the patient with MCI and 3.3 years for the patient with mild dementia.
Figure 3. Time to a Further Cognitive Stage.
Time to reach a threshold MMSE of 20 and 15 was calculated using the backward selected biomarker model. The median value of all selected predictors in the MCI and mild dementia groups was used, varying only CSF Aβ1–42 and MMSE at baseline. The CSF Aβ1–42 values approximately reflect the P25, P50, and P75 values in the overall cohort. For the MCI group, the fixed parameters values were sex: male; age: 66 years; and log CSF pTau: 3.415 ng/L. For the mild dementia group, the fixed parameter values were sex: female; age: 66 years; APOE ε4 alleles: 2; and log CSF pTau: 3.498 ng/L. The white areas indicate the expected additional time to reach the threshold MMSE with a hypothetical DMT that reduces decline by 30%. The statistical uncertainty surrounding the time estimates is left out for visual clarity and is given in the text. Aβ = β-amyloid; DMT = disease-modifying treatment; MCI = mild cognitive impairment; MMSE = Mini-Mental State Examination.
External validation of all prediction models in ADNI showed comparable performance between the model-building approaches (eTable 7). The mild dementia backward selected biomarker model (model 2) had an R2 of 0.20 and median absolute deviation of 2.19 in ADNI. The MCI model had an R2 of 0.21 and median absolute deviation of 1.97 (eTable 7).
In an additional set of analyses, we constructed prediction models for RAVLT Immediate Recall (eTables 8–10). Compared with the backward selected MMSE models, fewer variables were retained with time-varying effects. In internal cross-validation, RAVLT models in participants with MCI performed comparably with MMSE models with R2 ranging between 0.11 and 0.20 (eTable 11). Performance of the RAVLT models in mild dementia was slightly better than that of the MMSE models, with an R2 ranging between 0.32 and 0.34. This is also reflected in the external validation of the RAVLT models in ADNI, where the R2 of the mild dementia models clustered around 0.50 (eTable 12). The R2 of the MCI RAVLT models in ADNI ranged between 0.25 and 0.33.
Discussion
We constructed clinically applicable prediction models of cognitive decline measured by MMSE or RAVLT for patients with amyloid-positive MCI and mild dementia. Adding MRI and CSF biomarkers to base variables somewhat improved predictions, although the modest explained variance illustrates that making individualized predictions inherently comes with uncertainty. Our models can be used to predict the time to reach a certain level of MMSE or RAVLT. We incorporate these models in a calculator with visualization as a prototype tool to discuss prognosis, the uncertainty surrounding the predictions, and the impact of intervention strategies with patients.
The overall predictive performance of the models for both MCI and mild dementia indicates a substantial amount of variation in MMSE decline could already be explained by clinical variables age, sex, baseline MMSE, and time since baseline. Additional information on MRI volumetric and CSF Aβ1–42 and pTau biomarkers, representing etiologic disease characteristics,36 aided in the prediction of MMSE decline in our amyloid-positive sample. However, further increasing model complexity by adding other clinical and vascular risk factors did not improve predictive performance despite their known association with AD dementia.37,38 Potentially, tau PET information could improve predictive performance because of the association with AD-associated symptom severity,39 but we could not incorporate this because of a lack of data.
Compared with other studies that predicted MMSE decline, our models showed similar12 or even better13,14 predictive performance while requiring less12 or similar13,14 information. Two studies used MCI Biofinder patients13 or amyloid-positive ADNI patients12 to build prediction models for MMSE decline, after 2 and 4 years, based on demographic and plasma biomarker information13 or a wide variety of CSF, MRI volumetric, cognitive test, and vascular risk factor information.12 The “AD course map” model used cognitively normal patients or those with MCI clinical AD from ADNI and jointly modeled decline in cognition, PET hypometabolism and MRI cognitive thinning, and hippocampal deformation.14 One study predicted decline in functional impairment through the Clinical Dementia Rating Sum of Boxed Score and showed similarly modest predictive performance compared with the various MMSE studies.40 These former studies used different statistical techniques, namely linear regression,12,13 multivariate nonlinear mixed-effect models,14 gradient boosting,13,40 and different ways to weigh data.13 We investigated localized shrinkage with ridge regression and no penalization of linear mixed model coefficients as additional approaches, which both did not substantially alter the predictive performance compared with backward selection. Our finding that the statistical approach did not have a strong effect on the predictive performance follows the relative equivalence in performance from different statistical models in the literature. This implies 2 things. First, additional predictors are needed to capture the remaining unexplained variation. A possible avenue would be combining clinical predictors and polygenetic risk scores.41 Second, the use of novel statistical techniques does not result in large gain in performance. Thus, keeping models simple and the way in which the model can be used is more relevant than the small performance variation between techniques. The linear mixed model approach we used allows for prediction at any point in time within the data range (approximately 5 years in this study) and visualization of different sources of uncertainty in individualized prediction.
Patients with AD and their care partners want to know their future cognitive functioning.8 Our prediction models can be used to inform patients about their cognitive decline, but our results also indicate that providing a precise prognosis is challenging. Thus clinicians need to talk about the inherent uncertainty surrounding the predictions with their patients.42 Visualizations of the uncertainty can form the basis for meaningful doctor-patient conversations about the predicted cognitive decline.43
In the communication of prognostic information to patients, a link needs to be made between the answers models can provide and the questions patients and their care partners have such as “how long can I still drive a car” or “how long can I in my hobby.”8 The MMSE provides an indication of global cognition and does not answer these questions. However, no currently available cognitive test addresses all the questions patients have or takes into consideration the diversity in patients' living situations affecting the extent to which they can use their remaining cognitive function.44 In the future, we hope prediction models will become available directly predicting patient-reported outcomes such as quality of life and daily functioning. Such data are currently being collected,45 but long-term follow-up is needed to develop robust models. Until then, there is an important role for clinicians in translating the observed and predicted cognitive function scores into answers to patients' questions. We attempted to aid clinicians by translating the rate of decline into a clinically meaningful outcome by providing estimations of time to a certain MMSE level.
In both the analysis of the interindividual variation in decline and time to a certain MMSE level, we added hypothetical medication effects. By calculating the “additional” time to a certain MMSE level when slowing decline with hypothetical interventions, we provide an easier way to think about clinical meaningfulness than absolute changes in memory score. At the same time, these figures visualize that benefits be difficult to distinguish from variation in natural decline patterns. The applied hypothetical interventions extrapolate beyond the time frame of the amyloid-targeting therapy trial results.4-6 We assumed the effects would be stable over time and across disease stages or patient subgroups such as APOE subtype or sex. These assumptions could be inappropriate and long-term follow-up of patients undergoing treatment is essential to further refine such models in the future.
Strengths of this study include, first, the large, real-world population used to build the prediction models. We selected our sample to include patients who in theory could be eligible for the novel generation of disease-modifying treatments, i.e. patients with amyloid-positive MCI and mild dementia. In addition, participants were included from a tertiary memory clinic, a setting in which these interventions are likely to be implemented first. This makes our study highly relevant in helping shape the future patient journey.46 Second, we used straightforward statistical methods, improving the interpretability and acceptability of the final biomarker prediction models in a clinical setting. For clinical applicability, parsimonious and simple models are preferred over more complex statistical models.
There are some limitations that warrant discussion. First, we used MMSE as a measure of cognition because of the short time it takes to collect in the clinic and its widespread use. However, MMSE measurements show intraindividual variation in a cognitively normal population.47 Furthermore, MMSE measurements in our clinic are not always taken at the same time of day and patients with cognitive decline might score lower later in the day when they are more tired.48 Both factors increase unexplainable noise in the outcome, reducing predictability. As an alternative, we also modeled RAVLT as a second outcome. Contrary to what might have been expected, we did not find higher predictive accuracy for RALVT than for MMSE. Second, the models were built for use in memory clinics based on tertiary memory clinic data. Thus, generalizability to the general population could be limited. Although external validation in ADNI did not show diminished performance, indicating generalizability to an older population, MCI or dementia due to AD in the general population is likely to occur in individuals with more comorbidities than are present in either the development or validation cohort. Third, the selected mild dementia population had a slightly higher baseline MMSE score and Aβ1–42 than the average patient with amyloid-positive mild dementia in our cohort. However, as the rate of MMSE decline in mild dementia is modulated by these 2 predictors, the generalizability of the predictions should not be affected. Fourth, no information was available on the number of impaired cognitive domains from participants with MCI. This information may have improved predictions.49
We constructed clinically applicable models to predict MMSE and memory over time in patients with MCI or mild dementia due to AD. There is a need among patients and care partners for prognostic information on their cognitive trajectory. These models can provide such information, although our results also emphasize that the heterogeneity in cognitive trajectories can only be partially captured. The models come with an easy-to-use calculator allowing visualization of the predicted cognitive trajectories. Such a tool can form the basis for a meaningful discussion about patients' expected natural decline trajectory and how initiating hypothetical intervention strategies might alter this decline.
Glossary
- Aβ
β-amyloid
- AD
Alzheimer disease
- ADNI
Alzheimer's Disease Neuroimaging Initiative
- IQR
interquartile range
- MCI
mild cognitive impairment
- MMSE
Mini-Mental State Examination
- RAVLT
Rey Auditory Verbal Learning Test
Appendix. Authors
| Name | Location | Contribution |
| Pieter J. van der Veere, MD | Alzheimer Center and Department of Neurology, Department of Epidemiology and Biostatistics, Amsterdam Neuroscience, VU University Medical Center; and Amsterdam Neuroscience, Neurodegeneration, the Netherlands | Drafting/revision of the manuscript for content, including medical writing for content; study concept or design; analysis or interpretation of data |
| Jeroen Hoogland, MD | Department of Epidemiology and Biostatistics, Amsterdam Neuroscience, VU University Medical Center, the Netherlands | Drafting/revision of the manuscript for content, including medical writing for content; analysis or interpretation of data |
| Leonie N.C. Visser, PhD | Alzheimer Center and Department of Neurology, Department of Epidemiology and Biostatistics, Amsterdam Neuroscience, VU University Medical Center; and Amsterdam Neuroscience, Neurodegeneration, the Netherlands; Division of Clinical Geriatrics, Center for Alzheimer Research, Department of Neurobiology, Care Sciences and Society, Karolinska Institutet, Stockholm, Sweden; Medical Psychology, Amsterdam UMC Location AMC, University of Amsterdam; Amsterdam Public Health, Quality of Care, Personalized Medicine, the Netherlands | Drafting/revision of the manuscript for content, including medical writing for content |
| Argonde C. Van Harten, MD, PhD | Alzheimer Center and Department of Neurology, Amsterdam Neuroscience, VU University Medical Center; Amsterdam Neuroscience, Neurodegeneration, the Netherlands | Drafting/revision of the manuscript for content, including medical writing for content |
| Hanneke F. Rhodius-Meester, MD, PhD | Alzheimer Center and Department of Neurology, Amsterdam Neuroscience, VU University Medical Center; Amsterdam Neuroscience, Neurodegeneration; Internal Medicine, Geriatric Medicine Section, Amsterdam Cardiovascular Sciences Institute, Amsterdam UMC Location VUmc, the Netherlands | Drafting/revision of the manuscript for content, including medical writing for content |
| Sietske A.M. Sikkes, PhD | Alzheimer Center and Department of Neurology, Amsterdam Neuroscience, VU University Medical Center; Department of Clinical, Neuro and Developmental Psychology, Faculty of Movement and Behavioral Sciences, VU University, the Netherlands | Drafting/revision of the manuscript for content, including medical writing for content |
| Vikram Venkatraghavan, PhD | Alzheimer Center and Department of Neurology, Amsterdam Neuroscience, VU University Medical Center; Amsterdam Neuroscience, Neurodegeneration, the Netherlands | Drafting/revision of the manuscript for content, including medical writing for content; analysis or interpretation of data |
| Frederik Barkhof, MD, PhD, FRCR | Department of Radiology & Nuclear Medicine, Amsterdam UMC, Vrije Universiteit, the Netherlands; Queen Square Institute of Neurology and Centre for Medical Image Computing, University College London, London, United Kingdom | Drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data |
| Charlotte E. Teunissen, PhD | Amsterdam Neuroscience, Neurodegeneration; Neurochemistry Laboratory and Biobank, Department of Clinical Chemistry, Amsterdam Neuroscience, VU University Medical Center, the Netherlands | Drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data |
| Elsmarieke van de Giessen, MD, PhD | Amsterdam Neuroscience, Neurodegeneration; Department of Radiology & Nuclear Medicine, Amsterdam UMC, Vrije Universiteit, the Netherlands | Drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data |
| Johannes Berkhof, PhD | Department of Epidemiology and Biostatistics, Amsterdam Neuroscience, VU University Medical Center, the Netherlands | Drafting/revision of the manuscript for content, including medical writing for content; study concept or design; analysis or interpretation of data |
| Wiesje M. Van Der Flier, PhD | Alzheimer Center and Department of Neurology, Department of Epidemiology and Biostatistics, Amsterdam Neuroscience, VU University Medical Center; and Amsterdam Neuroscience, Neurodegeneration, the Netherlands | Drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data; study concept or design; analysis or interpretation of data |
Study Funding
Alzheimer Center Amsterdam and Neurochemistry Laboratory Amsterdam UMC have received unrestricted funding from Alzheimer Nederland, Stichting VUmc Fonds, Genootschap tot Steun Alzheimercentrum, Alzheimer Rally, and many others. Commercial partners in consortia or for contract research: Life-MI, Brain Research Center, AVID, Winterlight labs, Nutricia, ADx Neurosciences, Roche AG, Novartis-NL, Philips, Combinostics, Danone-Nutricia, Castor, Neurocast, FujiFilm-Toyama, Quanterix, Eli Lilly, AC-Immune, Axon Neurosciences, BioConnect, Bioorchestra, Brainstorm Therapeutics, Celgene, Cognition Therapeutics, EIP Pharma, Eisai, Fujirebio, Grifols, Instant Nano Biosensors, Merck, Novo Nordisk, PeopleBio, Siemens, Vivoryon. Grant funding: NWO, ZonMW, CVON, EU-JPND, EU-IMI, EU-IHI, Alzheimer Nederland, Hersenstichting, Health∼Holland Top Sector Life Sciences & Health, Stichting Dioraphte, Gieskes Strijbis Fonds, Edwin Bouw Fonds, Pasman stichting, stichting Equilibrio, European Commission (Marie Curie International Training Network), Innovative Medicines Initiatives 3 TR, EPND, National MS Society, Alzheimer Drug Discovery Foundation, Alzheimer Association, The Selfridges Group Foundation. Data collection and sharing for this project was funded by the Alzheimer's Disease Neuroimaging Initiative (ADNI) (NIH Grant U01 AG024904) and DOD ADNI (Department of Defense award number W81XWH-12-2-0012). ADNI is funded by the National Institute on Aging, the National Institute of Biomedical Imaging and Bioengineering, and through generous contributions from the following: AbbVie, Alzheimer's Association; Alzheimer's Drug Discovery Foundation; Araclon Biotech; BioClinica, Inc.; Biogen; Bristol-Myers Squibb Company; CereSpir, Inc.; Cogstate; Eisai Inc.; Elan Pharmaceuticals, Inc.; Eli Lilly and Company; EuroImmun; F. Hoffmann-La Roche Ltd and its affiliated company Genentech, Inc.; Fujirebio; GE Healthcare; IXICO Ltd.; Janssen Alzheimer Immunotherapy Research & Development, LLC.; Johnson & Johnson Pharmaceutical Research & Development LLC.; Lumosity; Lundbeck; Merck & Co., Inc.; Meso Scale Diagnostics, LLC.; NeuroRx Research; Neurotrack Technologies; Novartis Pharmaceuticals Corporation; Pfizer Inc.; Piramal Imaging; Servier; Takeda Pharmaceutical Company; and Transition Therapeutics. The Canadian Institutes of Health Research is providing funds to support ADNI clinical sites in Canada. Private sector contributions are facilitated by the Foundation for the NIH (fnih.org). The grantee organization is the Northern California Institute for Research and Education, and the study is coordinated by the Alzheimer's Therapeutic Research Institute at the University of Southern California. ADNI data are disseminated by the Laboratory for Neuro Imaging at the University of Southern California. This work has received support from a research grant from Eisai. This work used the Dutch national e-infrastructure with the support of the SURF Cooperative using grant no. EINF-2044.
Disclosure
F. Barkhof is part of the steering committee or is a Data Safety Monitoring Board member for Biogen, Merck, ATRI/ACTC, and Prothena, is a consultant for Roche, Celltrion, Rewind Therapeutics, Merck, IXICO, Jansen, and Combinostics; has research agreements with Merck, Biogen, GE Healthcare, and Roche; and is a cofounder and shareholder of Queen Square Analytics LTD. L.N.C. Visser has received research funding from ZonMW, Health Holland, Eisai, the Amsterdam Public Health research institute, and Alzheimer Nederland. E. van de Giessen has performed contract research for Heuron Inc., Roche, and 1st Biotherapeutics; and has a consultancy agreement with IXICO for the reading of PET scans. C.E. Teunissen has performed contract research for ADx Neurosciences, AC-Immune, Aribio, Axon Neurosciences, Beckman-Coulter, BioConnect, Bioorchestra, Brainstorm Therapeutics, Celgene, Cognition Therapeutics, EIP Pharma, Eisai, Eli Lilly, Fujirebio, Grifols, Instant Nano Biosensors, Merck, Novo Nordisk, Olink, PeopleBio, Quanterix, Roche, Siemens, Toyama, and Vivoryon; is an editor of Alzheimer's Research & Therapy, serves on the editorial boards of Medidact Neurologie/Springer and Neurology: Neuroimmunology & Neuroinflammation; and has had speaker contracts for Eli Lilly, Grifols, Novo Nordisk, Olink and Roche. S.A.M. Sikkes has been a consultant to Prothena Biosciences, Aribio, Cogstate, Biogen, Boehringer, and Toyama. W.M. van der Flier is a recipient of the IHI-Prominent, supported by the European Innovative Health Initiative Joint Undertaking (JU) under grant agreement No 101112145; has received grants from Nederlandse Organisatie voor Wetenschappelijk Onderzoek, Alzheimer Nederland, Cardiovascular Research Netherlands, Hersenstichting, Health Holland Topsector Life Sciences & Health, Stichting Dioraphte, Gieskes-Strijbis Fonds, Stichting Equilibrio, Edwin Bouw Fonds, Philips, Biogen, Novartis-NL, Life-MI, Avid Radiopharmaceuticals, Roche-BV, Fujifilm, Eisai, Combinostics, and ZonMW; has received speaker honoraria from Biogen, Danone, Eisai, WebMD Neurology (Medscape), Novo Nordisk, Springer Healthcare, and the European Brain Council; is a consultant to the Oxford Health Policy Forum, Roche, Biogen, and Eisai; has served on advisory boards for Biogen, Roche, and Eli Lilly; is a steering committee member for Partnerships for Action, Voices for Empowerment, and Think Brain Health; was an associate editor of Alzheimer's Research & Therapy in 2020 and 2021; and is an associate editor of Brain. The other authors report no relevant disclosures. Go to Neurology.org/N for full disclosures.
References
- 1.Livingston G, Huntley J, Sommerlad A, et al. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet. 2020;396(10248):413-446. doi: 10.1016/S0140-6736(20)30367-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gustavsson A, Norton N, Fast T, et al. Global estimates on the number of persons across the Alzheimer's disease continuum. Alzheimers Dement. 2023;19(2):658-670. doi: 10.1002/alz.12694 [DOI] [PubMed] [Google Scholar]
- 3.Vermunt L, Sikkes SAM, van den Hout A, et al. Duration of preclinical, prodromal, and dementia stages of Alzheimer's disease in relation to age, sex, and APOE genotype. Alzheimers Dement. 2019;15(7):888-898. doi: 10.1016/j.jalz.2019.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.van Dyck CH, Swanson CJ, Aisen P, et al. Lecanemab in early Alzheimer's disease. N Engl J Med. 2023;388(1):9-21. doi: 10.1056/NEJMoa2212948 [DOI] [PubMed] [Google Scholar]
- 5.Budd Haeberlein S, Aisen PS, Barkhof F, et al. Two randomized phase 3 studies of aducanumab in early Alzheimer's disease. J Prevent Alzheimers Dis. 2022;9(2):197-210. doi: 10.14283/jpad.2022.30 [DOI] [PubMed] [Google Scholar]
- 6.Sims JR, Zimmer JA, Evans CD, et al. Donanemab in early symptomatic Alzheimer disease: the TRAILBLAZER-ALZ 2 randomized clinical trial. JAMA. 2023;330(6):512-527. doi: 10.1001/jama.2023.13239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.The Lancet. Lecanemab for Alzheimer's disease: tempering hype and hope. Lancet. 2022;400(10367):1899. doi: 10.1016/S0140-6736(22)02480-1 [DOI] [PubMed] [Google Scholar]
- 8.Mank A, van Maurik IS, Bakker ED, et al. Identifying relevant outcomes in the progression of Alzheimer's disease; what do patients and care partners want to know about prognosis? Alzheimers Dement (NY). 2021;7(1):e12189. doi: 10.1002/trc2.12189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kunneman M, Smets EMA, Bouwman FH, et al. Clinicians' views on conversations and shared decision making in diagnostic testing for Alzheimer's disease: the ABIDE project. Alzheimers Dement (NY). 2017;3(3):305-313. doi: 10.1016/j.trci.2017.03.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen Y, Qian X, Zhang Y, et al. Prediction models for conversion from mild cognitive impairment to Alzheimer's disease: a systematic review and meta-analysis. Front Aging Neurosci. 2022;14:840386. doi: 10.3389/fnagi.2022.840386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.van Maurik IS, Vos SJ, Bos I, et al. Biomarker-based prognosis for people with mild cognitive impairment (ABIDE): a modelling study. Lancet Neurol. 2019;18(11):1034-1044. doi: 10.1016/S1474-4422(19)30283-2 [DOI] [PubMed] [Google Scholar]
- 12.Cullen NC, Leuzy A, Palmqvist S, et al. Individualized prognosis of cognitive decline and dementia in mild cognitive impairment based on plasma biomarker combinations. Nat Aging. 2021;1(1):114-123. doi: 10.1038/s43587-020-00003-5 [DOI] [PubMed] [Google Scholar]
- 13.Dansson HV, Stempfle L, Egilsdottir H, et al. Predicting progression and cognitive decline in amyloid-positive patients with Alzheimer's disease. Alzheimers Res Ther. 2021;13(1):151. doi: 10.1186/s13195-021-00886-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Koval I, Bône A, Louis M, et al. AD Course Map charts Alzheimer's disease progression. Sci Rep. 2021;11(1):8020. doi: 10.1038/s41598-021-87434-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.van der Flier WM, Scheltens P. Amsterdam dementia cohort: performing research to optimize care. J Alzheimers Dis. 2018;62(3):1091-1111. doi: 10.3233/JAD-170850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yesavage JA, Brink TL, Rose TL, et al. Development and validation of a geriatric depression screening scale: a preliminary report. J Psychiatr Res. 1982;17(1):37-49. doi: 10.1016/0022-3956(82)90033-4 [DOI] [PubMed] [Google Scholar]
- 17.Rijnen SJM, Meskal I, Emons WHM, et al. Evaluation of normative data of a widely used computerized neuropsychological battery: applicability and effects of sociodemographic variables in a Dutch sample. Assessment. 2020;27(2):373-383. doi: 10.1177/1073191117727346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van der Flier WM, Pijnenburg YA, Prins N, et al. Optimizing patient care and research: the Amsterdam Dementia Cohort. J Alzheimers Dis. 2014;41(1):313-327. doi: 10.3233/JAD-132306 [DOI] [PubMed] [Google Scholar]
- 19.Albert MS, DeKosky ST, Dickson D, et al. The diagnosis of mild cognitive impairment due to Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011;7(3):270-279. doi: 10.1016/j.jalz.2011.03.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McKhann GM, Knopman DS, Chertkow H, et al. The diagnosis of dementia due to Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011;7(3):263-269. doi: 10.1016/j.jalz.2011.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189-198. doi: 10.1016/0022-3956(75)90026-6 [DOI] [PubMed] [Google Scholar]
- 22.Rey A. L’examen Clinique en Psychologie. Presses Universitaries De France; 1958. [Google Scholar]
- 23.Tijms BM, Willemse EAJ, Zwan MD, et al. Unbiased approach to counteract upward drift in cerebrospinal fluid amyloid-β 1-42 analysis results. Clin Chem. 2018;64(3):576-585. doi: 10.1373/clinchem.2017.281055 [DOI] [PubMed] [Google Scholar]
- 24.van Harten AC, Wiste HJ, Weigand SD, et al. Detection of Alzheimer's disease amyloid beta 1-42, p-tau, and t-tau assays. Alzheimers Dement. 2022;18(4):635-644. doi: 10.1002/alz.12406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Willemse EAJ, van Maurik IS, Tijms BM, et al. Diagnostic performance of Elecsys immunoassays for cerebrospinal fluid Alzheimer's disease biomarkers in a nonacademic, multicenter memory clinic cohort: the ABIDE project. Alzheimers Dement (Amst). 2018;10(1):563-572. doi: 10.1016/j.dadm.2018.08.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.de Wilde A, van der Flier WM, Pelkmans W, et al. Association of amyloid positron emission tomography with changes in diagnosis and patient treatment in an unselected memory clinic cohort: the ABIDE project. JAMA Neurol. 2018;75(9):1062-1070. doi: 10.1001/jamaneurol.2018.1346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Konijnenberg E, Carter SF, Ten Kate M, et al. The EMIF-AD PreclinAD study: study design and baseline cohort overview. Alzheimers Res Ther. 2018;10(1):75. doi: 10.1186/s13195-018-0406-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zwan MD, Ossenkoppele R, Tolboom N, et al. Comparison of simplified parametric methods for visual interpretation of 11C-Pittsburgh compound-B PET images. J Nucl Med. 2014;55(8):1305-1307. doi: 10.2967/jnumed.114.139121 [DOI] [PubMed] [Google Scholar]
- 29.Rhodius-Meester HFM, Benedictus MR, Wattjes MP, et al. MRI visual ratings of brain atrophy and white matter hyperintensities across the spectrum of cognitive decline are differently affected by age and diagnosis. Front Aging Neurosci. 2017;9:117. doi: 10.3389/fnagi.2017.00117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fortin J-P, Cullen N, Sheline YI, et al. Harmonization of cortical thickness measurements across scanners and sites. Neuroimage. 2018;167:104-120. doi: 10.1016/j.neuroimage.2017.11.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.van de Wiel MA, Leday GG, Hoogland J, Heymans MW, van Zwet EW, Zwinderman AH. Think before you shrink: alternatives to default shrinkage methods can improve prediction accuracy, calibration and coverage. arXiv. 2023. doi: 10.48550/arXiv.2301.09890 [DOI] [Google Scholar]
- 32.Perneczky R, Wagenpfeil S, Komossa K, Grimmer T, Diehl J, Kurz A. Mapping scores onto stages: mini-mental state examination and clinical dementia rating. Am J Geriatr Psychiatry. 2006;14(2):139-144. doi: 10.1097/01.JGP.0000192478.82189.a8 [DOI] [PubMed] [Google Scholar]
- 33.Petersen RC, Aisen PS, Beckett LA, et al. Alzheimer's Disease Neuroimaging Initiative (ADNI): clinical characterization. Neurology. 2010;74(3):201-209. doi: 10.1212/WNL.0b013e3181cb3e25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Edmonds EC, McDonald CR, Marshall A, et al. Early versus late MCI: improved MCI staging using a neuropsychological approach. Alzheimers Dement. 2019;15(5):699-708. doi: 10.1016/j.jalz.2018.12.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.R: A Language and Environment for Statistical Computing. Version 4.2.1. R Foundation for Statistical Computing; 2022. R-project.org/. [Google Scholar]
- 36.Fehr J, Piccininni M, Kurth T, Konigorski S. Assessing the transportability of clinical prediction models for cognitive impairment using causal models. BMC Med Res Methodol. 2023;23(1):187. doi: 10.1186/s12874-023-02003-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang W, Chen Y, Chen N. Body mass index and trajectories of the cognition among Chinese middle and old-aged adults. BMC Geriatr. 2022;22(1):613. doi: 10.1186/s12877-022-03301-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Deschaintre Y, Richard F, Leys D, Pasquier F. Treatment of vascular risk factors is associated with slower decline in Alzheimer disease. Neurology. 2009;73(9):674-680. doi: 10.1212/WNL.0b013e3181b59bf3 [DOI] [PubMed] [Google Scholar]
- 39.Ossenkoppele R, Reimand J, Smith R, et al. Tau PET correlates with different Alzheimer's disease-related features compared to CSF and plasma p-tau biomarkers. EMBO Mol Med. 2021;13(8):e14398. doi: 10.15252/emmm.202114398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Devanarayan V, Ye Y, Charil A, et al. Predicting clinical progression trajectories of early Alzheimer's disease patients. Alzheimers Dement. 2024;20(3):1725-1738. doi: 10.1002/alz.13565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tomassen J, den Braber A, van der Lee SJ, et al. Amyloid-β and APOE genotype predict memory decline in cognitively unimpaired older individuals independently of Alzheimer's disease polygenic risk score. BMC Neurol. 2022;22(1):484. doi: 10.1186/s12883-022-02925-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Visser LNC, Pelt SAR, Kunneman M, et al. Communicating uncertainties when disclosing diagnostic test results for (Alzheimer's) dementia in the memory clinic: the ABIDE project. Health Expect. 2020;23(1):52-62. doi: 10.1111/hex.12964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.van Gils AM, Visser LNC, Hendriksen HMA, Georges J, van der Flier WM, Rhodius-Meester HFM. Development and design of a diagnostic report to support communication in dementia: Co-creation with patients and care partners. Alzheimers Dement (Amst). 2022;14(1):e12333. doi: 10.1002/dad2.12333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Apolinario D, Magaldi RM, Busse AL, Lopes LDC, Kasai JYT, Satomi E. Cognitive impairment and driving: a review of the literature. Dement Neuropsychol. 2009;3(4):283-290. doi: 10.1590/S1980-57642009DN30400004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dreves MAE, van Harten AC, Visser LNC, et al. Rationale and design of the ABOARD project (a personalized medicine approach for Alzheimer's disease). Alzheimers Dement (NY). 2023;9(2):e12401. doi: 10.1002/trc2.12401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.van der Flier WM, de Vugt ME, Smets EMA, Blom M, Teunissen CE. Towards a future where Alzheimer's disease pathology is stopped before the onset of dementia. Nat Aging. 2023;3(5):494-505. doi: 10.1038/s43587-023-00404-2 [DOI] [PubMed] [Google Scholar]
- 47.Feeney J, Savva GM, O'Regan C, King-Kallimanis B, Cronin H, Kenny RA. Measurement error, reliability, and minimum detectable change in the mini-mental state examination, Montreal cognitive assessment, and color trails test among community living middle-aged and older adults. J Alzheimers Dis. 2016;53(3):1107-1114. doi: 10.3233/JAD-160248 [DOI] [PubMed] [Google Scholar]
- 48.Mazzucco S, Li L, Tuna MA, et al. Time-of-day could affect cognitive screening performance in older patients with TIA and stroke. Cerebrovasc Dis. 2017;43(5-6):290-293. doi: 10.1159/000456673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kaduszkiewicz H, Eisele M, Wiese B, et al. Prognosis of mild cognitive impairment in general practice: results of the German AgeCoDe study. Ann Fam Med. 2014;12(2):158-165. doi: 10.1370/afm.1596 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data can be made available upon reasonable request.