Abstract
Competition is common in life, and intimate relationships are essential. Understanding how intimate relationships impact an individual’s competitive process is crucial. This study explored the impact of competitor gender on female competition using electroencephalography analysis. The results revealed that females exhibited a smaller median of the absolute value of reaction time difference (DRT) between their partners and their competitors when their partners were absent compared to when their partners were present. Additionally, females showed greater average amplitudes of N2 posterior contralateral component (N2pc) and Late Positive Potential (LPP), increased activation of the alpha frequency band, and enhanced theta frequency band functional connectivity between the central parietal lobe and occipital lobe. Furthermore, when competing with individuals of the same gender as opposed to individuals of the opposite gender, females exhibited greater average amplitudes of percentage of wins and N2pc. A significant negative correlation was noted between the DRT and the average wave amplitudes of N2pc and LPP. These findings suggest that females are more engaged in competitive tasks when partners are not present and have improved decision-making when competing with same-gender individuals. This study provides evidence for the influence of lovers on female competition, helping females adapt to social competition and promoting healthy relationships.
Keywords: EEG, competition, intimate relationships, brain functional connectivity
Introduction
In modern society, competition is a common phenomenon, and people will face challenges from competition in different fields and scenarios (Haran et al. 2024). Moreover, competition is a social behavior of human interaction established in social relationships and networks (Shaw et al. 2023). When friends, brothers, and sisters compete for the love of friends and parents, and employees compete for jobs, salary increases, the boss’ appreciation, and even the last piece of pizza, there will be competition. The theory of mind (ToM) refers to people’s cognition and understanding of their own and others’ mental states (Premack and Woodruff 1978). The ToM includes people’s understanding of psychological processes such as their own and others’ beliefs, intentions, desires, and emotions. In competition, individuals often employ the ToM to infer the intentions and goals of others, enabling them to better respond to competitors and make appropriate behavioral decisions (Prodan et al. 2023). In addition, the ToM can help people interpret other people’s behaviors and emotional reactions in the process of competition to better cope with the pressure and challenges of competition (Prodan and Visu-Petra 2022). In addition, the social relationships, states, and characteristics of competing parties may also have an impact on competition outcomes (Georgiev et al. 2013). Previous studies have shown that the psychological distance of intimate relationships can affect individuals’ decision-making preferences and risk assessment when making decisions, thus affecting competitive outcomes (Wong et al. 2023). Previous studies have shown that children use the ToM in competitive scenarios with close friends to predict their thoughts and achieve victory (Roberts et al. 2020). As one of the important social relationships among individuals, intimate relationships are closely related to individuals’ participation in social interaction, competition, and decision-making (Abiodun et al. 2020). People understand the mental state of others by observing and speculating about their behavior, words, and emotions. As one of the important social relationships of individuals, intimate relationships are influenced by the ToM and closely related to individuals’ participation in social interaction, competition, and decision-making (Abiodun et al. 2020).
There is no doubt that classic theories in the past were enlightening; however, previous theories mainly focused on males and some have questioned whether their results can represent the overall human race (Hodgson and Fischer 1979). Previous studies have shown that females generally have greater awareness of and influence on the process of interpersonal communication (Acitelli 1992). Females are often more enthusiastic and submissive than males, whereas males tend to be more dominant and indifferent than females (Suh et al. 2004). In addition, studies have shown that gender differences in peer relationships are related to different behavioral and emotional outcomes (Alarcón et al. 2020). For example, males tend to socialize and engage in competitive interactions within a larger group of peers, whereas females engage in longer, more intimate binary interactions (Alarcón et al. 2020). Thus, gender differences widely exist in the interaction activities of individuals in different relationships. The study of social competition processes and intimate relationships among males has yielded relatively clear findings, and studies of females, as equally important individuals, are also of great significance. A study by Zhang et al. (2021) showed that, influenced by the environment, women are more likely to exhibit activated brain activity in their right prefrontal region during acute stress competition, and competition is more intense. Previous studies have shown that people are more sensitive to interpersonal relationships in more intimate (such as romantic) relationships, and females generally exhibit greater interpersonal sensitivity than males (Jack et al. 2016). When individuals see faces of different genders in interpersonal relationships, male faces are more likely to trigger changes in brain activity than female faces (Zhang et al. 2016). Based on previous research, we hypothesize that in actual competition, competitors of different genders are more likely to stimulate different competitive impulses and coping strategies in female individuals than in male individuals, and their behavior and brain activity may differ significantly. In addition, previous studies have shown that the visual state of others can affect an individual’s attention to a specific target. When there is visual attention from others, the individual’s attention to the task will be disrupted to some extent (Capozzi et al. 2021). Therefore, we hypothesize that female individuals may also be more influenced by intimate relationships when facing competitive interaction scenarios, and the physical presence of their lovers and the gender of their competitors will both have an impact on female competitive behavior and brain activity.
Electroencephalography (EEG) can detect dynamic brain oscillations with high temporal resolution (i.e. milliseconds), can capture neural activities related to social interaction in real ecological environments, is inexpensive and easy to apply, and it has become one of the most commonly used techniques for exploring individual neural mechanisms and brain neural correlations (Helfrich and Knight 2019; Grootjans et al. 2024). Event-related potentials (ERPs) help researchers accurately capture the immediate response of the brain to specific stimuli by recording the electrophysiological signals of the brain during cognitive task execution (Helfrich and Knight 2019). Previous studies on ERP using EEG have shown that components such as N170, N2 posterior contralateral (N2pc), P3, Feedback Related Negativity (FRN), and Late Positive Potential (LPP) are influenced by competitive interactions (Lu et al. 2022; Yang et al. 2022; Yu et al. 2022; Dolci et al. 2023; Lin and Liang 2024). Among them, N2pc is considered to represent an individual’s attentional deployment in visual space, reflecting top–down attentional processes (Dolci et al. 2023). Research has indicated that when individuals have attentional perceptual differences toward target stimuli, there are differences in the average amplitudes of N2pc, with greater attentional focus resulting in faster and more pronounced N2pc responses (Forschack et al. 2023). Furthermore, LPP is a positively deflected component occurring around central-parietal electrode sites (Brothers et al. 2023). Studies have shown that individuals in competitive states exhibit significantly larger LPP amplitudes for negative outcomes compared with positive outcomes, indicating a close association between LPP and the processing of outcome–related social comparisons in the late stages of competition (Lin and Liang 2024).
In addition, to understand the brain activity of competition-related EEG from multiple perspectives, research has also been conducted in both the temporal and frequency domains. Research shows that brain activity in the theta band of the parietal lobe region is often reflected through the mirror neuron system to promote executive control and synchronous motor activity as part of a feedback system mediated by the success or failure of perception competition (Iacoboni et al. 2005; Clark 2013). Theta band oscillation transmits stimulus-specific information to the visual cortex under focused attention conditions and is related to successful working memory performance (Ekstrom et al. 2005). Brain activity in the theta frequency band reflects an individual’s ability to predict competitive task outcomes and behavioral adaptation. The alpha frequency band has been validated as a neural characteristic of individual attentional suppression toward target stimuli (Foxe and Snyder 2011). Researchers have found that individuals who win in competition exhibit greater alpha band energy and greater ability to monitor opponent activity than those who lose in competition (Putri et al. 2022). Functional connectivity analysis can help researchers understand the coordination and communication patterns between different functional regions of the brain and reveal the functional connectivity patterns of the brain during cognitive tasks (Alahmadi 2023). Recent meta-analysis reports suggest that social cognitive processes, such as inferring others’ intentions during competition, involve a balanced interaction of functional integration and segregation across several brain networks (Schurz et al. 2020). Xin et al. (2024) investigated the impact of group identification on individual brain functional connectivity during competition and observed significant functional connectivity in the individual’s frontal and right temporal regions. These findings suggest that group identification enhances an individual’s cognitive control and rational judgment during competitive tasks, promoting attention to others for predictive judgments of competitive behavior. Thus, individual brain functional connectivity reflects the state of the individual during tasks and is crucial for understanding brain activity. This study aimed to provide a comprehensive analysis and exploration of brain activity during competition from the perspectives of the temporal domain, the frequency domain, and the functional connectivity of individuals.
Based on previous research, we propose the following hypothesis: (i) we expect that competition among female individuals under different conditions will involve significant changes in the average amplitude of N2pc and late LPP components. When the lover is not present, females pay more attention to competitive tasks, and the average amplitude of N2pc and LPP in females will significantly increase. (ii) In terms of time frequency, when a lover is not present, the theta and alpha frequency bands closely related to attentional resource allocation will enhanced. (iii) During the competition process, the synchronization of connections between the theta and alpha frequency bands in the female brain will be enhanced. In this study, we used ERP, time–frequency analysis techniques, and brain functional connectivity techniques, combined with individual behavioral performance, to explore the companionship status of intimate relationships and the impact of an opponent’s gender on individual competition in females. We hope to gain a more comprehensive understanding of the cognitive processes, emotional responses, and brain activities of female individuals in competition, re-examine the impact of gender roles and social expectations on individual behavior, provide deeper insights and understanding for the study of intimate relationships and competitive behavior, promote gender equality and the development of gender education, and help females better realize their potential and cope with various challenges in life.
Materials and methods
Participants
The number of individuals required to participate in the study was calculated using G * power 3.1 (Faul et al. 2007); moreover, based on the analysis (effect size f = 0.4, α = 0.05, 1-β = 0.8, 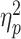 =0.14, analysis of variance: fixed effects, special effects, main effects, and interactions), the minimum required sample size was 52. The effect size f = 0.4 is the largest effect quantity (Meurs 2016). This study recruited a total of 83 college students (aged 18 to 25 yr, M = 19.7 yr, SD = 2.4), including 20 partners competing with the same gender, 21 partners competing with the opposite gender, 22 partners not involved in competition with the same gender, and 20 partners not involved in competition with the opposite gender. The 2 individuals who participate in the competition within the same group do not know each other. All individuals were right-handed with normal or corrected vision, normal color vision, no history of mental illness or brain disease, and a stable romantic status (duration of “love”: M = 9.21 mo, range from 2 to 36 mo). Considering that a female’s response to social stress may be influenced by the menstrual cycle (Kirschbaum et al. 1999; Duchesne and Pruessner 2013; Albert et al. 2015; Banis and Lorist 2017), none of the female participants included in this study were menstruating at the time of the study. After the experiment, a reward ranging from 10 to 20 yuan was provided based on performance. Based on the Declaration of Helsinki, the local school’s medical ethics committee approved this study. All individuals provided written informed consent.
=0.14, analysis of variance: fixed effects, special effects, main effects, and interactions), the minimum required sample size was 52. The effect size f = 0.4 is the largest effect quantity (Meurs 2016). This study recruited a total of 83 college students (aged 18 to 25 yr, M = 19.7 yr, SD = 2.4), including 20 partners competing with the same gender, 21 partners competing with the opposite gender, 22 partners not involved in competition with the same gender, and 20 partners not involved in competition with the opposite gender. The 2 individuals who participate in the competition within the same group do not know each other. All individuals were right-handed with normal or corrected vision, normal color vision, no history of mental illness or brain disease, and a stable romantic status (duration of “love”: M = 9.21 mo, range from 2 to 36 mo). Considering that a female’s response to social stress may be influenced by the menstrual cycle (Kirschbaum et al. 1999; Duchesne and Pruessner 2013; Albert et al. 2015; Banis and Lorist 2017), none of the female participants included in this study were menstruating at the time of the study. After the experiment, a reward ranging from 10 to 20 yuan was provided based on performance. Based on the Declaration of Helsinki, the local school’s medical ethics committee approved this study. All individuals provided written informed consent.
Tasks and procedures
The experiment consisted of a visual cue-target task (Cui et al. 2012; Cheng et al. 2015; Barraza et al. 2020). Individuals are required to respond to target stimuli by pressing buttons quickly. Unlike previous competition between humans and computers, this study selected unfamiliar individuals as competitors. The presence of real competitors plays a supervisory role for females, ensuring that individuals focus on completing experiments while creating real competitive scenarios that are more situationally valid. At the beginning of the experiment, individuals were asked to sit side by side in front of a computer screen 50 cm away (to avoid the influence of additional variables caused by location, under the conditions where a romantic companion was present, the seats were uniformly arranged with the female on the left and the competitor on the right, and the lover accompanied the female on the left. In the absence of a lover, the lover’s position was replaced by a stranger of the opposite gender to eliminate any effects solely attributed to gaze). To avoid confusion with physical stimuli or differences in results caused by environmental differences between conditions, individuals are asked to sit in fixed positions under different conditions while using the same sensory stimuli, and the stimuli are presented in the same order. The only difference is the participants’ intention to perform the task.
After the preliminary experimental preparation was completed, the individual first described the experimental requirements to the individual after taking a seat in the laboratory, and corresponding guidance was displayed on the screen. Before the formal experiment started, practice experiments were conducted, including 5 trials, and individuals became familiar with the competitive task process. At the beginning of each trial in the formal experiment, a 2,000 ms black screen was first displayed. The black screen was followed by a gray circle that appeared in the center of the screen for a duration of 600 ms to 1,500 ms followed by a green circle for target stimulation. The target stimulus remained on the screen until both parties responded. Afterward, 4 s of feedback were provided. During the experiment, individuals were required to press buttons as quickly as possible. Figure 1 shows the sequence of events during the task.
Fig. 1.

Experimental design.
In the competitive task, the original base score of both parties was 100 points. The winner was awarded 3 points to the total score, whereas 3 points were reduced from the loser’s score. After both parties pressed the button, the feedback screen displayed the winning or losing situation in the current competitive task, their respective bonus and bonus points, and their cumulative scores. After a black screen appeared for an interval of 2,000 ms, the next attempt was presented. The formal experiment consisted of 3 blocks, each containing 20 trials, for a total of 60 trials.
Data acquisition
The 64-channel EEG recording system of the Neuroscan Company records individual EEG signals. The 64-channel SynAmps 2 device records female EEG signals based on a connection to an amplifier through a head box. According to the 10–20 international EEG acquisition system, the EEG activity of individuals was collected using a 64-lead Ag/Agcl electrode cap, and data were continuously collected throughout the experiment. Both vertical and horizontal electromyography (VEOG and HEOG, respectively) signals were recorded during the experiment using bipolar recordings. VEOG electrodes were placed at the median position of 1 cm above and 1 cm below the left orbit, and HEOG electrodes were placed at the lateral canthus of the left and right eyes. The EEG signals were filtered online from 0.05 to 100 Hz with a sampling rate of 1,000 Hz. The impedance between all electrodes and the scalp was less than 5 K Ω.
EEG data were preprocessed using MATLAB R2018b with the EEGLAB toolbox (Makeig et al. 2004). The data preprocessing process included locating electrode points; deleting useless electrodes (HEOG, VEOG); re-referencing the whole-brain average; reducing sampling to 500 Hz; performing high-pass filtering and low-pass filtering with frequency limits of 1 and 45 Hz, respectively; segmenting with stimulus time points as zeros; and baseline correction with time points before the zero time point serving as baselines (ERP analysis selected the 200 ms before stimulus as the baseline, whereas time–frequency analysis, functional connectivity analysis, and source analysis selected the 2,000 ms before stimulus as the baseline). Independent component analysis removes interference artifacts caused by blinking head movements, etc., and manually removes poor-quality clips.
The segment of ERP analysis, traceability, and functional connectivity analysis data from −200 to 1,000 ms is selected, and the time range of the time–frequency analysis of EEG data segmentation is −2,000 to 1,000 ms, and the stimulus occurs as the zero point. In addition, the individual’s reaction time (RT; from the initial presentation of stimuli to the individual’s button response) was recorded using E-prime 3.0 and statistically analyzed using SPSS 23.0.
Data analysis
Behavior performance
Individual RTs and the number of winning experiments were recorded. To measure the closeness of individuals to complete tasks in the experiment and to avoid outliers in the data, the median DRT of the absolute value of the RT difference of each pair of individuals in all trials (hereafter referred to DRT) was calculated. The smaller the value is, the closer the subjects’ RT is.
 |
In addition, to quantify the performance of female individuals, we calculated the percentage of winning attempts, i.e. the percentage of wins (PWTs), among females (Cui et al. 2012; Cheng et al. 2015).
This study used 2-way analysis of variance to analyze DRT, with partner status and opponent gender as fixed variables and α set to 0.05. Moreover, 2-way analysis of variance was also conducted on PWT using partner status and opponent gender as fixed variables.
ERP and time–frequency data analysis
Based on previous research, this study quantified N2pc and LPP as average amplitudes in the range of 200 to 300 ms and 600 to 900 ms, respectively. The region of interest for N2pc is the parieto-occipital area (P3, PZ, P4, PO3, POZ, and PO4), whereas the central parietal area (CP3, CP1, CPZ, CP2, CP4, P3, P1, PZ, P2, and P4) is the region of interest for LPP (Brothers et al. 2023; Dolci et al. 2023). The presence of a romantic partner and the gender of the opponent are used as independent variables, and a 2-way analysis of variance is conducted. In the time–frequency analysis, with the help of the FieldTrip toolbox (a MATLAB toolbox), the EEG data were analyzed using multiwindow Fourier transformation (mtmconvol), and the window was smoothed using the Hanning window function to calculate the time–frequency distribution. Based on previous literature, this study selected FZ, CZ, CPZ, and PZ electrodes as electrodes of interest and conducted a 2-way analysis of variance on the theta frequency band (3 to 7 Hz) and alpha frequency band (8 to 12 Hz) power values from 0 to 1,000 ms (Peng et al. 2021).
Traceability and functional connectivity analysis
The phase locking value (PLV) is selected as a brain activity synchronization indicator for functional connectivity, and the PLV is calculated from individual EEG data (Lachaux et al. 1999). In short, this method calculates the brain phase difference between all electrode pairs in a time window and then evaluates the phase difference’s stability through all experiments (Burgess 2013). A current source density analysis was performed on the preprocessed data before calculating the PLV to avoid volumetric conduction effects. Then, the time–frequency matrix of all segmented stored phase information is obtained using a short-time Fourier transformation (window length, 400 ms; step, 10 Hz). Let φi and φj be the phase vectors of the signals between electrodes i and j at time window t and frequency f. By subtracting the phase values for each phase matrix, the phase difference between the 2 electrodes is obtained:
φij = φi · φj × (φj × the complex conjugate of φj)
For each time–frequency point, the consistency of the phase differences of all segments at that time–frequency point is calculated, and the PLV of each time–frequency point is obtained as follows:
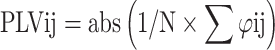 |
where N is the number of tests, and the PLV is calculated for each pair of electrodes (i, j). The PLV ranges from 0 to 1, where 0 indicates that the 2 signals are unsynchronized, and 1 indicates perfect synchronization. The −200 to 0 ms interval before stimulation was selected as the baseline for baseline correction. The analysis method adopted in this study involved a data-driven approach of fixed time windows and frequency bands of interest. Here, t-tests were conducted on each data point, and false discovery rate (FDR) correction was applied to reduce false positive results. Based on the previous literature and the spectrograms generated from this study, a time window of 0 to 1,000 ms was defined as the region of interest. The theta frequency band (3 to 7 Hz) and alpha frequency band (8 to 2 Hz) are defined as the frequency ranges of interest (Ekstrom et al. 2005; Mackenzie et al. 2024). The analysis was conducted using custom MATLAB code. The α level was set at 0.05 for all tests. The same sensory stimuli and stimuli presentation sequence were used to avoid physical stimulus confounds or context differences between conditions. The only difference was the intention with which the individuals performed the task.
To further determine the connected brain regions related to competition, a distributed source model using field trip minimum norm estimation was used to perform source localization analysis on the preprocessed scalp EEG. To solve the problem of multiple comparisons, a cluster-based permutation test method was adopted for correction, with a significance threshold of 0.05 set, and 1,000 permutation tests were conducted.
Correlation analysis
To understand the relationship between competitive behavior and the brain, this study performed Spearman’s bivariate correlation analysis (2-tailed) between behavior indicators that show significant differences, average amplitudes of ERP components, average power values in the time–frequency domain, and PLV. This analysis aimed to further explore the intrinsic correlations between cognitive neuromechanisms in female individuals during competition.
Results
Behavioral performance
The results revealed that when participants had lovers present (M = 61.04; SD = 23.91), their DRT was significantly greater than when their partners were absent [M = 47.27; SD = 22.62; F(1, 79) = 7.24, P = 0.009, 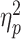 = 0.08; Fig. 2A]. When competing against same-gender opponents (M = 55.97; SD = 13.29), participants’ PWT was significantly greater than when competing against opposite-gender opponents [M = 45.54; SD = 15.07; F(1, 79) = 10.82, P = 0.002,
= 0.08; Fig. 2A]. When competing against same-gender opponents (M = 55.97; SD = 13.29), participants’ PWT was significantly greater than when competing against opposite-gender opponents [M = 45.54; SD = 15.07; F(1, 79) = 10.82, P = 0.002, 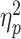 = 0.12; Fig. 2B].
= 0.12; Fig. 2B].
Fig. 2.
A) DRT of females participating in competitive tasks. B) PWT of female individuals in competitive tasks. The “box” depicts the median and the 25th and 75th quartiles, and the “whisker” shows the 5th and 95th percentiles. “* * *” indicates that the P-value is less than 0.001.
Furthermore, there were no significant main effects of opponent gender on DRT [F(1, 79) = 0.46, P = 0.501] or significant interactions between partner status and opponent gender [F(1, 79) = 0.52, P = 0.472]. Similarly, there were no significant main effects of partner status on PWT [F(1, 79) = 0.59, P = 0.445] or significant interactions between partner status and opponent gender [F(1, 79) = 0.01, P = 0.921].
ERP results
For the N2pc component, a significant main effect of partner status [F(1, 79) = 8.00, P = 0.006, 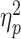 = 0.09] was observed. The average amplitude of the N2pc when the partner was absent (M = 8.22; SD = 8.82) was significantly greater than that when the partner was present (M = 2.45; SD = 10.06). There was also a significant main effect of opponent gender [F(1, 79) = 5.06, P = 0.02,
= 0.09] was observed. The average amplitude of the N2pc when the partner was absent (M = 8.22; SD = 8.82) was significantly greater than that when the partner was present (M = 2.45; SD = 10.06). There was also a significant main effect of opponent gender [F(1, 79) = 5.06, P = 0.02, 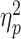 = 0.06]. The average amplitude of the N2pc was significantly greater for individuals competing against same-gender opponents (M = 7.67; SD = 9.70) than for those competing against opposite-gender opponents (M = 3.01; SD = 9.52). The interaction between partner status and opponent gender was marginally significant [F(1, 79) = 3.45, P = 0.067,
= 0.06]. The average amplitude of the N2pc was significantly greater for individuals competing against same-gender opponents (M = 7.67; SD = 9.70) than for those competing against opposite-gender opponents (M = 3.01; SD = 9.52). The interaction between partner status and opponent gender was marginally significant [F(1, 79) = 3.45, P = 0.067, 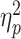 = 0.04]. Simple effect analysis revealed that when a partner was present, the amplitude of the N2pc induced by same-gender competition (M = 6.65; SD = 2.04) was significantly greater than that induced by opposite-gender competition (M = −1.56; SD = 1.98, P = 0.005). When competing against opposite-gender opponents, the amplitude of the N2pc was significantly greater when the partner was absent (M = 7.81; SD = 8.05) than when the partner was present (M = −1.56; SD = 8.64, P = 0.001; Fig. 3A).
= 0.04]. Simple effect analysis revealed that when a partner was present, the amplitude of the N2pc induced by same-gender competition (M = 6.65; SD = 2.04) was significantly greater than that induced by opposite-gender competition (M = −1.56; SD = 1.98, P = 0.005). When competing against opposite-gender opponents, the amplitude of the N2pc was significantly greater when the partner was absent (M = 7.81; SD = 8.05) than when the partner was present (M = −1.56; SD = 8.64, P = 0.001; Fig. 3A).
Fig. 3.
ERP results. A) N2pc component waveform and brain topography. B) LPP component waveform and brain topography.
For the LPP component, there was a significant main effect of partner status [F(1, 79) = 8.25, P = 0.005, 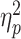 = 0.10]. The average amplitude of the LPP was significantly greater when a partner was absent (M = 8.51; SD = 4.63) than when a partner was present (M = 5.64; SD = 4.39; Fig. 3B). However, there was no significant main effect of opponent gender [F(1, 79) < 0.001, P = 0.986] and no significant interaction between partner status and opponent gender [F(1, 79) = 0.68, P = 0.411].
= 0.10]. The average amplitude of the LPP was significantly greater when a partner was absent (M = 8.51; SD = 4.63) than when a partner was present (M = 5.64; SD = 4.39; Fig. 3B). However, there was no significant main effect of opponent gender [F(1, 79) < 0.001, P = 0.986] and no significant interaction between partner status and opponent gender [F(1, 79) = 0.68, P = 0.411].
Results of the time–frequency analysis
The results of the 2-way analysis of variance revealed a significant main effect of partner status in the alpha frequency band [100 to 400 ms after stimulus onset; F(1, 79) = 5.62, P = 0.020, 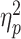 = 0.07]. Specifically, the power values in the alpha frequency band when the partner was absent (M = 202.34; SD = 146.97) were significantly greater than those when the partner was present (M = 142.33; SD = 71.22; Fig. 4). However, there was no significant main effect of opponent gender [F(1, 79) = 0.22, P = 0.644] and no significant interaction between partner status and opponent gender [F(1, 79) = 0.87, P = 0.355]. No significant differences in the theta frequency band were identified based on the results of 2-way analysis of variance.
= 0.07]. Specifically, the power values in the alpha frequency band when the partner was absent (M = 202.34; SD = 146.97) were significantly greater than those when the partner was present (M = 142.33; SD = 71.22; Fig. 4). However, there was no significant main effect of opponent gender [F(1, 79) = 0.22, P = 0.644] and no significant interaction between partner status and opponent gender [F(1, 79) = 0.87, P = 0.355]. No significant differences in the theta frequency band were identified based on the results of 2-way analysis of variance.
Fig. 4.

Topographic map of the 100 to 400 ms alpha frequency band and the large average time–frequency distribution.
EEG connection results during the simulation
The data-driven analysis results showed that in the theta frequency band (3 to 7 Hz) at 200 to 400 ms, a significant increase in the PLV was noted between the O10 and P8 electrodes in the brains of females when their partners were not present (M = 0.39; SD = 0.16) compared to when their partners were present (M = 0.27; SD = 0.10) [t(71.01) = −4.12, P < 0.001, Cohen’s d = −0.98]. Additionally, in the absence of partners (M = 0.38; SD = 0.14), a significantly greater PLV was noted between the O10 and CP6 electrodes in the female brains compared with that obtained when partners were present [M = 0.38; SD = 0.14; t(81) = −3.30, P = 0.001, Cohen’s d = −0.73; Fig. 5]. However, no significant differences were observed in the alpha frequency band or at other time intervals.
Fig. 5.
Distribution of functional connections in the theta frequency band from 200 to 400 ms for different lover companionship states.
Furthermore, further source analysis of the EEG signals revealed significant activation primarily in the central parietal lobe region. The brain regions showed greater activation when partners were not present than when partners were present, as shown in Fig. 6.
Fig. 6.
A visual state map of the average posterior brain traceability of female individuals competing in the presence of their partner (left) and absence of their partner (right) during a stimulation period of 200 to 400 ms. The red position indicates the presence of significantly activated brain regions.
Correlation analysis results
When females competed with the opposite gender while their partners were present, a significant negative correlation was observed between the average amplitude of the LPP and the DRT (r = −0.52, P = 0.015; Fig. 7A). On the other hand, when females competed with the opposite gender in the absence of their partners, a significant positive correlation was noted between the average amplitude of the N2pc and the DRT (r = 0.49, P = 0.029; Fig. 7B).
Fig. 7.

A) During the period of 600 to 900 ms, the average amplitude of LPP was significantly correlated with DRT. B) During the period of 200 to 300 ms, the average amplitude of the N2pc was significantly correlated with the DRT. C) During the period of 200 to 400 ms, the PLV of the theta frequency band CP6-O10 was significantly correlated with the PWT. D) During the period of 200 to 400 ms, the PLV of the theta frequency band CP6-O10 was significantly correlated with the DRT.
Furthermore, when females competed with the same gender while their partners were present, a significant negative correlation was noted between the PLV of the O10–CP6 electrodes in the theta frequency band (200 to 400 ms) and the PWT (r = −0.46, P = 0.042; Fig. 7C). When females competed with the same gender in the absence of their partners, a significant positive correlation was noted between the PLV of the O10–CP6 electrodes in the theta frequency band (200 to 400 ms) and the DRT (r = 0.51, P = 0.015; Fig. 7D).
Apart from these findings, no other significant correlations were observed.
Discussion
This study is based on the ToM and uses ERP, time–frequency analysis, and functional connectivity techniques to explore the synchronous activity of functional brain regions related to the influence of the companionship status of intimate relationships on competition. It has been found that intimate relationships affect females’ use of the ToM in competitive scenarios. Specifically, the close companionship of a lovers weakens the competitive state of females. In contrast, competition with the opposite gender occupies more of the attention resources that female individuals use for adjusting predictions. The research results are consistent with the research hypothesis.
Research has shown that compared to heterosexual individuals, female individuals have a greater PWT of competition when competing with same-gender individuals, which means that the difficulty of competition is lower (Morris et al. 2020). Moreover, compared to the situation where a lover is present, female individuals react faster when a lover is not present. Previous studies have shown that the visual state of others can affect an individual’s attention to a specific target location. When visual attention from others is present, the individual’s attention to the task will be affected, and the individual will be more inclined toward the position of the other person’s vision (Capozzi et al. 2021). In this study, it is also possible that the female individuals may feel nervous and unnatural under the attention of their partners due to their attention to their gaze. As a result, their attention to target stimuli may be disrupted when they complete competitive tasks with competitors. Compared to the condition where a partner is not present, the competitive state of female individuals decreases in the presence of a partner.
As mentioned earlier, the N2pc reflects both top–down attentional processes and the perceptual strength of target stimuli in the visual domain (Dolci et al. 2023; Forschack et al. 2023). In this study, the average amplitude of the N2pc in the female parietal occipital region was greater when partners were not present than when partners were present. This finding may be because when a lover is not present, female individuals have no interference from other people’s goals, or their emotional attention is not on the lover, which can lead to more focused attention being given to competitive stimuli. However, when partners are present, the gaze of the partner may introduce distractions and interfere with the processing of competitive stimuli, affecting higher-order evaluative processes (Capozzi et al. 2021; Kerzel and Huynh Cong 2022). Furthermore, in the present study, the average amplitude of the N2pc was greater when females competed with individuals of the same gender than when they competed with individuals of the opposite gender. Previous research has suggested that females tend to pay more attention to males in competitive contexts due to the adaptive advantage of monitoring potential threats related to physical harm, leading to more interference in target-focused attention (Pause et al. 2020). In the present study, the larger N2pc amplitude observed in female–male competition may be attributed to increased external attentional allocation during competition with the opposite gender, indicating more complex cognitive control processes among females (Zivony and Eimer 2022).
Additionally, the results of this study revealed that when partners were not present, females exhibited larger average amplitudes of the LPP in competitive tasks than when partners were present. Previous research has indicated that the LPP reflects the allocation of attentional resources based on motivation and the emotional salience of stimuli, with larger LPP amplitudes associated with increased motivation and more intense emotional stimuli (Hajcak and Foti 2020). When a lover is not present, females may pay more attention to competitive tasks and goals, which may generate more competitive motivation and result in greater LPP. In addition, combined with the ToM, when the lover is not around, females may be more focused on speculating on the intentions and behaviors of others in competitive tasks to better cope with competitors and make appropriate competitive behavior decisions in the subsequent process, resulting in a greater average amplitude of LPP (Prodan et al. 2023).
In terms of time–frequency analysis, the alpha frequency range in the central parietal region is associated with the excitatory and inhibitory processes of the cortical brain, reflecting the brain’s stability and balance (Klimesch 2012). Previous research has indicated that greater alpha power is often associated with a more relaxed state, which assists individuals in better focusing their attention (Wolf et al. 2015). In addition, the alpha band energy activity in the central parietal lobe may also be related to an individual’s alertness to task participation. Studies have shown that as alertness increases, the alpha band energy in the individual parietal lobe decreases (McDonnell et al. 2024). In this study, during the 100 to 400 ms period after the appearance of the target stimulus, the alpha frequency energy in the central parietal region of females was significantly greater when their partner was not present. This may also be because females are in a more relaxed state when their partner is not around, allowing them to concentrate and focus on competitive tasks. The difference in the alpha frequency range may reflect differences in cognitive processing strategies among females in competitive tasks, indicating that females are better able to adapt to the competitive environment and complete the competitive task when their partners are not present.
In addition, during the 200 to 400 ms period after the competition began, females showed greater theta frequency band functional connectivity in the central parietal occipital region of the brain when a lover was not present compared to when a lover was present. Recent studies have shown that functional connectivity in the theta frequency range among the frontal, central, and parietal regions tends to increase with the complexity of motor tasks and is associated with the flow of information processing in tasks (Van Hoornweder et al. 2022; Kodama et al. 2023). In addition, studies have shown that an increase in the theta frequency band functional connectivity strength in the parietal and occipital regions is associated with an increase in visual motor and cognitive needs (Studnicki and Ferris 2023). Considering the behavioral results of this study, females exhibited shorter DRT in competition when their partners were not present compared to when their partners were present, indicating a potentially more intense competitive task. In the absence of partners, females may face greater complexity in the competitive task and encounter more competition-related information, leading to increased functional connectivity in the theta frequency range. Moreover, combining the findings from the time–frequency analysis, when partners were not present, females were able to engage more attentively in the competitive task, make better predictions about the outcomes of the competition, and select appropriate behavioral strategies. This finding suggests that females could adapt their decision-making behavior in a timely manner to achieve success in competitive tasks (Cavanagh et al. 2010).
Notably, in this study, a significant positive correlation was noted between DRT and the amplitude of the N2pc, and a significant negative correlation was noted between DRT and the amplitude of the LPP. Given that the N2pc is a negative ERP component, it can be inferred that as DRT decreases, the average amplitudes of the N2pc and LPP increase. As mentioned earlier, the N2pc and LPP in this study reflect aspects such as attention and motivation in competitive tasks (Hajcak and Foti 2020; Forschack et al. 2023). When females engage in more intense competition with their opponents, their attention and motivation in the competition are also stronger, leading to larger N2pc and LPP amplitudes. Additionally, the present study revealed a significant negative correlation between DRT and the PLV. As mentioned earlier, stronger functional connectivity in the theta frequency range in the absence of partners indicates a more competitive state for females, and under such conditions, females perform better in the competitive task, resulting in shorter DRT compared to those of their opponents.
This study differs from previous human-machine competition or game experimental paradigms and instead simulates real competition scenarios between people, starting from a more socially valid environment, to explore the competitive behavior and brain activity changes of females affected by intimate relationships (Grootjans et al. 2024). However, this study also has several limitations. First, due to location limitations, the individuals investigated in this study were all from university campuses, and the group of lovers was also composed of college students. Therefore, the research results can only illustrate the social interactions and competition situations of university campuses. This result does not yet represent working couples, married couples, elderly partners, etc. Future research can expand the subject population to explore the impact of intimate relationships on competition in different age groups and scenarios. Notably, the experimental paradigm used in this study was a key–pressing race task. Therefore, the results are only applicable to competitive scenarios involving speed-based competitions. Caution should be exercised when generalizing these findings to other competitive contexts. Real-life competitive situations are diverse and varied. Future studies could consider incorporating a wider range of competitive task paradigms or employing alternative experimental techniques to increase ecological validity and make laboratory research more representative of real-life situations. This approach enhances the generalizability of laboratory findings and addresses the limitations of studying a single specific context. However, the research findings still have reference significance for exploring the behavior and brain activity status of female individuals in social competition. From a theoretical perspective, studying the impact of intimate relationships and the gender of competitors on female competition can help us gain a deeper understanding of the role of gender in social relationships. The traditional belief is that females place more emphasis on intimate relationships, whereas males place more emphasis on competition. However, the actual situation may be more complex. Studying the competitive behavior of females in intimate relationships can help us re-examine the impact of gender roles and social expectations on individual behavior, thereby promoting gender equality and the development of gender education. In practical terms, studying the competitive behavior of females in intimate relationships can help us better understand their performance and challenges in the workplace and society. When females enter the workplace, they face pressure to compete with males and may also face competition among females. Understanding the impact of intimate relationships and a competitor’s gender on female competitive behavior can help us implement more effective gender equality policies and leadership training, helping females better cope with challenges and realize their potential. I hope this study can provide inspiration for researchers in cognitive neuroscience, psychology, and other related fields.
Conclusion
In summary, this study, from the perspective of intimate relationships, demonstrates the impact of the presence of a lover and a competitor’s gender on female competition using EEG technology. The research findings revealed that when females competed with individuals of the same gender in the absence of their lover, they were able to focus more effectively on the competitive task, leading to greater success. However, this pattern was reversed when competing with unfamiliar individuals of the opposite gender. This study provides valuable insights into the impact of intimate relationships on the competitive behavior of females from the perspectives of cognitive neuroscience and behavior. It has important implications for fostering high–quality intimate relationships and promoting the development of healthy social competition.
Acknowledgements
Y.L. and S.J. designed the study; J.M. and S.J. conducted the experiments, collected data, and analyzed the data; M.X., J.G., J.J., S.J., and H.W. contributed to conceptualization, software, investigation, and writing—original draft; H.W. and S.J. wrote the paper.
Author contributions
Yingjie Liu (Funding acquisition, Methodology, Visualization, Writing—review & editing), Shuyu Jia (Data curation, Methodology, Software, Visualization, Writing—original draft, Writing—review & editing), Yujia Meng (Methodology), Miao Xing (Data curation), Jiaqi Guan (Data curation), Jinru Jiang (Data curation), and He Wang (Funding acquisition, Supervision, Writing—review & editing).
Funding
This research was supported by Hebei Province Education Science Planning-General Funded Project “The Moderation of Altruistic Punishment by Social Environmental Factors and Its Educational Implications” (2203198); The Key Research Project of North China University of Science and Technology in 2023 (ZD-RW-202319); National Education Science Planning-Youth Project of The Ministry of Education “The Influence of Social Moral Factors on Pain Empathy and Its Educational Implications” (EBA210396).
Conflict of interest statement: None declared.
Data availability
The data underlying this article will be shared on reasonable request to the corresponding author.
Contributor Information
Yingjie Liu, School of Public Health, North China University of Science and Technology, Hebei, China; School of Psychology and Mental Health, North China University of Science and Technology, Hebei, China.
Shuyu Jia, School of Psychology and Mental Health, North China University of Science and Technology, Hebei, China.
Yujia Meng, School of Psychology and Mental Health, North China University of Science and Technology, Hebei, China.
Miao Xing, School of Psychology and Mental Health, North China University of Science and Technology, Hebei, China.
Jiaqi Guan, School of Psychology and Mental Health, North China University of Science and Technology, Hebei, China.
Jinru Jiang, School of Psychology and Mental Health, North China University of Science and Technology, Hebei, China.
He Wang, School of Public Health, North China University of Science and Technology, Hebei, China; School of Psychology and Mental Health, North China University of Science and Technology, Hebei, China.
References
- Abiodun O, Sodeinde K, Jagun O, Ladele A, Adepoju A, Ohiaogu F, Adelowo O, Ojinni O, Adekeye J, Bankole O, et al. Influence of perception of family support and functioning on adolescent high-risk sexual behavior. Am J Trop Med Hyg. 2020:104(3):1153–1163. 10.4269/ajtmh.20-0732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acitelli LK. Gender differences in relationship awareness and marital satisfaction among young married couples. Personal Soc Psychol Bull. 1992:18(1):102–110. 10.1177/0146167292181015. [DOI] [Google Scholar]
- Alahmadi AAS. Functional connectivity of sub-cortical brain regions: disparities and similarities. Neuroreport. 2023:34(4):214–219. 10.1097/WNR.0000000000001881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alarcón G, Morgan JK, Allen NB, Sheeber L, Silk JS, Forbes EE. Adolescent gender differences in neural reactivity to a friend’s positive affect and real-world positive experiences in social contexts. Dev Cogn Neurosci. 2020:43:1–9. 10.1016/j.dcn.2020.100779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albert K, Pruessner J, Newhouse P. Estradiol levels modulate brain activity and negative responses to psychosocial stress across the menstrual cycle. Psychoneuroendocrinology. 2015:59:14–24. 10.1016/j.psyneuen.2015.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banis S, Lorist MM. The combined effects of menstrual cycle phase and acute stress on reward-related processing. Biol Psychol. 2017:125:130–145. 10.1016/j.biopsycho.2017.02.005. [DOI] [PubMed] [Google Scholar]
- Barraza P, Pérez A, Rodríguez E. Brain-to-brain coupling in the gamma-band as a marker of shared intentionality. Front Hum Neurosci. 2020:14:295. 10.3389/fnhum.2020.00295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brothers T, Morgan E, Yacovone A, Kuperberg G. Multiple predictions during language comprehension: friends, foes, or indifferent companions? Cognition. 2023:241:105602–105678. 10.1016/j.cognition.2023.105602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess AP. On the interpretation of synchronization in EEG hyperscanning studies: a cautionary note. Front Hum Neurosci. 2013:7:881–898. 10.3389/fnhum.2013.00881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capozzi F, Wahn B, Ristic J, Kingstone A. Prior attentional bias is modulated by social gaze. Atten Percept Psychophys. 2021:83(1):1–6. 10.3758/s13414-020-02194-w. [DOI] [PubMed] [Google Scholar]
- Cavanagh JF, Frank MJ, Klein TJ, Allen JJ. Frontal theta links prediction errors to behavioral adaptation in reinforcement learning. NeuroImage. 2010:49(4):3198–3209. 10.1016/j.neuroimage.2009.11.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng X, Li X, Hu Y. Synchronous brain activity during cooperative exchange depends on the gender of partner: a fNIRS-based hyperscanning study. Hum Brain Mapp. 2015:36(6):2039–2048. 10.1002/hbm.22754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark A. Whatever next? Predictive brains, situated agents, and the future of cognitive science. Behav Brain Sci. 2013:36(3):181–204. 10.1017/S0140525X12000477. [DOI] [PubMed] [Google Scholar]
- Cui X, Bryant DM, Reiss AL. NIRS-based hyperscanning reveals increased interpersonal coherence in superior frontal cortex during cooperation. NeuroImage. 2012:59(3):2430–2437. 10.1016/j.neuroimage.2011.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolci C, Boehler CN, Santandrea E, Dewulf A, Ben-Hamed S, Macaluso E, Chelazzi L, Rashal E. Integrated effects of top–down attention and statistical learning during visual search: an EEG study. Atten Percept Psychophys. 2023:85(6):1819–1833. 10.3758/s13414-023-02728-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duchesne A, Pruessner JC. Association between subjective and cortisol stress response depends on the menstrual cycle phase. Psychoneuroendocrinology. 2013:38(12):3155–3159. 10.1016/j.psyneuen.2013.08.009. [DOI] [PubMed] [Google Scholar]
- Ekstrom AD, Caplan JB, Ho E, Shattuck K, Fried I, Kahana MJ. Human hippocampal theta activity during virtual navigation. Hippocampus. 2005:15(7):881–889. 10.1002/hipo.20109. [DOI] [PubMed] [Google Scholar]
- Faul F, Erdfelder E, Lang AG, Buchner A. G*power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods. 2007:39(2):175–191. 10.3758/BF03193146. [DOI] [PubMed] [Google Scholar]
- Forschack N, Gundlach C, Hillyard S, Müller MM. Attentional capture is modulated by stimulus saliency in visual search as evidenced by event-related potentials and alpha oscillations. Atten Percept Psychophys. 2023:85(3):685–704. 10.3758/s13414-022-02629-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foxe JJ, Snyder AC. The role of alpha-band brain oscillations as a sensory suppression mechanism during selective attention. Front Psychol. 2011:2:154. 10.3389/fpsyg.2011.00154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgiev AV, Klimczuk AC, Traficonte DM, Maestripieri D. When violence pays: a cost-benefit analysis of aggressive behavior in animals and humans. Evol Psychol. 2013:11(3):678–699. 10.1177/147470491301100313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grootjans Y, Harrewijn A, Fornari L, Janssen T, de BruijnERA, van AtteveldtN, Franken IHA. Getting closer to social interactions using electroencephalography in developmental cognitive neuroscience. Dev Cogn Neurosci. 2024:67:101391. 10.1016/j.dcn.2024.101391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajcak G, Foti D. Significance?... significance! Empirical, methodological, and theoretical connections between the late positive potential and P300 as neural responses to stimulus significance: an integrative review. Psychophysiology. 2020:57(7):e13570–e13585. 10.1111/psyp.13570. [DOI] [PubMed] [Google Scholar]
- Haran U, Van Dijk D, Barina M, Krief M, Rosenzweig S. Winning isn’t everything: guilt proneness and competitive vs. non-competitive motivation. J Pers. 2024:92(2):457–479. 10.1111/jopy.12834. [DOI] [PubMed] [Google Scholar]
- Helfrich RF, Knight RT. Cognitive neurophysiology: event-related potentials. Handb Clin Neurol. 2019:160:543–558. 10.1016/B978-0-444-64032-1.00036-9. [DOI] [PubMed] [Google Scholar]
- Hodgson JW, Fischer JL. Sex differences in identity and intimacy development in college youth. J Youth Adolesc. 1979:8(1):37–50. 10.1007/BF02139138. [DOI] [PubMed] [Google Scholar]
- Iacoboni M, Molnar-Szakacs I, Gallese V, Buccino G, Mazziotta JC, Rizzolatti G. Grasping the intentions of others with one’s own mirror neuron system. PLoS Biol. 2005:3(3):0529–0535. 10.1371/journal.pbio.0030079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack C. Lambert, Christopher J. Hopwood. (2016). Sex differences in interpersonal sensitivities across acquaintances, friends, and romantic relationships, Personality and Individual Differences, 89, 162–165. [Google Scholar]
- Kerzel D, Huynh Cong S. Biased competition between targets and distractors reduces attentional suppression: evidence from the positivity posterior contralateral and distractor positivity. J Cogn Neurosci. 2022:34(9):1563–1575. 10.1162/jocn_a_01877. [DOI] [PubMed] [Google Scholar]
- Kirschbaum C, Kudielka BM, Gaab J, Schommer NC, Hellhammer DH. Impact of gender, menstrual cycle phase, and oral contraceptives on the activity of the hypothalamus-pituitary-adrenal axis. Psychosom Med. 1999:61(2):154–162. 10.1097/00006842-199903000-00006. [DOI] [PubMed] [Google Scholar]
- Klimesch W. α-band oscillations, attention, and controlled access to stored information. Trends Cogn Sci. 2012:16(12):606–617. 10.1016/j.tics.2012.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kodama T, Kojima T, Honda Y, Hosokawa T, Karashima A, Watanabe M. Contribution of default mode network to game and delayed-response task performance: power and connectivity analyses of theta oscillation in the monkey. Neurosci Lett. 2023:814:137465. 10.1016/j.neulet.2023.137465. [DOI] [PubMed] [Google Scholar]
- Lachaux JP, Rodriguez E, Martinerie J, Varela FJ. Measuring phase synchrony in brain signals. Hum Brain Mapp. 1999:8(4):194–208. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin H, Liang J. Competition influences outcome processing involving social comparison: an ERP study. Psychophysiology. 2024:61(4):e14477. 10.1111/psyp.14477. [DOI] [PubMed] [Google Scholar]
- Lu J, Li W, Xie Y, Huang Q, Li J. Prosociality moderates outcome evaluation in competition tasks. Sci Rep. 2022:12(1):11397. 10.1038/s41598-022-15570-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackenzie AK, Baker J, Daly RC, Howard CJ. Peak occipital alpha frequency mediates the relationship between sporting expertise and multiple object tracking performance. Brain Behav. 2024:14(2):e3434–e3447. 10.1002/brb3.3434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makeig S, Delorme A, Westerfield M, Jung TP, Townsend J, Courchesne E, Sejnowski TJ. Electroencephalographic brain dynamics following manually responded visual targets. PLoS Biol. 2004:2(6):e176–e192. 10.1371/journal.pbio.0020176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonnell AS, Crabtree KW, Cooper JM, Strayer DL. This is your brain on autopilot 2.0: the influence of practice on driver workload and engagement during on-road, partially automated driving. Hum Factors. 2024:66(8):2025–2040. 10.1177/00187208231201054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meurs J. The experimental design of postmortem studies: the effect size and statistical power. Forensic Sci Med Pathol. 2016:12(3):343–349. 10.1007/s12024-016-9793-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris JS, Link J, Martin JC, Carrier DR. Sexual dimorphism in human arm power and force: implications for sexual selection on fighting ability. J Exp Biol. 2020:223(Pt 2):jeb212365. [DOI] [PubMed] [Google Scholar]
- Pause BM, Storch D, Lübke KT. Chemosensory communication of aggression: women’s fine-tuned neural processing of male aggression signals. Philos Trans R Soc Lond Ser B Biol Sci. 2020:375(1800):20190270. 10.1098/rstb.2019.0270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng M, Wang X, Chen W, Chen T, Cai M, Sun X, Wang Y. Cooperate or aggress? An opponent’s tendency to cooperate modulates the neural dynamics of interpersonal cooperation. Neuropsychologia. 2021:162:108025–108034. 10.1016/j.neuropsychologia.2021.108025. [DOI] [PubMed] [Google Scholar]
- Premack D, Woodruff G. Does the chimpanzee have a theory of mind? Behav Brain Sci. 1978:1(4):515–526. 10.1017/S0140525X00076512. [DOI] [Google Scholar]
- Prodan N, Visu-Petra L. The art of telling the truth to deceive: a matter of intent. Studia UBB Psychol-Paed. 2022:67(1):87–98. 10.24193/subbpsyped.2022.1.05. [DOI] [Google Scholar]
- Prodan N, Ding XP, Szekely-Copîndean RD, Tănăsescu A, Visu-Petra L. Socio-cognitive correlates of primary school children’s deceptive behavior toward peers in competitive settings. Acta Psychol. 2023:240:104019–104033. 10.1016/j.actpsy.2023.104019. [DOI] [PubMed] [Google Scholar]
- Putri F, Susnoschi Luca I, Garcia Pedro JA, Ding H, Vučković A. Winners and losers in brain computer interface competitive gaming: directional connectivity analysis. J Neural Eng. 2022:19(4):046037. 10.1088/1741-2552/ac8451. [DOI] [PubMed] [Google Scholar]
- Roberts R, McCrory E, Bird G, Sharp M, Roberts L, Viding E. Thinking about others’ minds: mental state inference in boys with conduct problems and callous-unemotional traits. J Abnorm Child Psychol. 2020:48(10):1279–1290. 10.1007/s10802-020-00664-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schurz M, Maliske L, Kanske P. Cross-network interactions in social cognition: a review of findings on task related brain activation and connectivity. Cortex. 2020:130:142–157. 10.1016/j.cortex.2020.05.006. [DOI] [PubMed] [Google Scholar]
- Shaw DJ, Czekóová K, Mareček R, Havlice Špiláková B, Brázdil M. The interacting brain: dynamic functional connectivity among canonical brain networks dissociates cooperative from competitive social interactions. NeuroImage. 2023:269:119933–119944. 10.1016/j.neuroimage.2023.119933. [DOI] [PubMed] [Google Scholar]
- Studnicki A, Ferris DP. Parieto-occipital electrocortical dynamics during real-world table tennis. eNeuro. 2023:10(4):ENEURO.0463-22.2023. 10.1523/ENEURO.0463-22.2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suh EJ, Moskowitz DS, Fournier MA, Zuroff DC. Gender and relationships: influences on agentic and communal behaviors. Pers Relat. 2004:11(1):41–60. 10.1111/j.1475-6811.2004.00070.x. [DOI] [Google Scholar]
- Van Hoornweder S, Mora DAB, Depestele S, Frieske J, van DunK, Cuypers K, Verstraelen S, Meesen R. Age and interlimb coordination complexity modulate oscillatory spectral dynamics and large-scale functional connectivity. Neuroscience. 2022:496:1–15. 10.1016/j.neuroscience.2022.06.008. [DOI] [PubMed] [Google Scholar]
- Wolf S, Brölz E, Keune PM, Wesa B, Hautzinger M, Birbaumer N, Strehl U. Motor skill failure or flow-experience? Functional brain asymmetry and brain connectivity in elite and amateur table tennis players. Biol Psychol. 2015:105:95–105. 10.1016/j.biopsycho.2015.01.007. [DOI] [PubMed] [Google Scholar]
- Wong JCS, Yang JZ, Liu Z. It’s the thoughts that count: how psychological distance and affect heuristic influence support for aid response measures during the COVID-19 pandemic. Health Commun. 2023:38(12):2702–2710. 10.1080/10410236.2022.2109394. [DOI] [PubMed] [Google Scholar]
- Xin Q, Hao S, Xiaoqin W, Jiali P. Brain source localization and functional connectivity in group identity regulation of overbidding in contest. Neuroscience. 2024:541:101–117. 10.1016/j.neuroscience.2024.01.016. [DOI] [PubMed] [Google Scholar]
- Yang H, Duan Q, Peng M, Gu R, Sun X. Sex differences on the response to others’ gains and losses under cooperation and competition. Int J Psychophysiol. 2022:182:211–219. 10.1016/j.ijpsycho.2022.10.012. [DOI] [PubMed] [Google Scholar]
- Yu M, Lu J, Wang S. Forgiveness weakens counterempathy at the early and late stage of empathic responses to opponents’ expressions. Behav Neurosci. 2022:136(2):114–125. 10.1037/bne0000496. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Wei B, Zhao P, Zheng M, Zhang L. Gender differences in memory processing of female facial attractiveness: evidence from event-related potentials. Neurocase. 2016:22(3):317–323. 10.1080/13554794.2016.1151532. [DOI] [PubMed] [Google Scholar]
- Zhang R, Zhou X, Feng D, Yuan D, Li S, Lu C, Li X. Effects of acute psychosocial stress on interpersonal cooperation and competition in young women. Brain Cogn. 2021:151:105738–105748. 10.1016/j.bandc.2021.105738. [DOI] [PubMed] [Google Scholar]
- Zivony A, Eimer M. Categorization templates modulate selective attention. J Exp Psychol Hum Percept Perform. 2022:48(11):1294–1312. 10.1037/xhp0001058. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data underlying this article will be shared on reasonable request to the corresponding author.






