Abstract

In this paper, we quantify weak protein–protein interactions in solution using cross-interaction chromatography (CIC) and surface plasmon resonance (SPR) and demonstrate that they can be modulated by the addition of millimolar concentrations of free amino acids. With CIC, we determined the second osmotic virial cross-interaction coefficient (B23) as a proxy for the interaction strength between two different proteins. We perform SPR experiments to establish the binding affinity between the same proteins. With CIC, we show that the amino acids proline, glutamine, and arginine render the protein cross-interactions more repulsive or equivalently less attractive. Specifically, we measured B23 between lysozyme (Lys) and bovine serum albumin (BSA) and between Lys and protein isolates (whey and canola). We find that B23 increases when amino acids are added to the solution even at millimolar concentrations, corresponding to protein/ligand stoichiometric ratios as low as 1:1. With SPR, we show that the binding affinity between proteins can change by 1 order of magnitude when 10 mM glutamine is added. In the case of Lys and one whey protein isolate (WPI), it changes from the mM to the M range, thus by 3 orders of magnitude. Interestingly, this efficient modulation of the protein cross-interactions does not alter the protein’s secondary structure. The capacity of amino acids to modulate protein cross-interactions at mM concentrations is remarkable and may have an impact across fields in particular for specific applications in the food or pharmaceutical industries.
Introduction
Weak protein (cross-) interactions are ubiquitous in nature and pivotal to many cell functions. We define weak protein–protein interactions as cross-interactions whose standard free energy does not exceed 4 times the thermal energy (kBT). They are responsible for protein solubility but also for protein aggregation.1,2 Since decades, studies have been accumulating evidence that small molecules can act as osmolytes capable of screening these hydrophobic interactions and of rendering protein dispersions more stable.3−5 This screening mechanism by osmolytes preventing aggregation has been extensively observed in highly crowded environments using in vitro and in vivo studies6 and also in the nucleus of the cell.7−9 Yet, protein stability against aggregation is an important topic also beyond the context of cell biology, in fields where dilute solutions are prevalent. In the food industry, protein aggregation in dilute solutions plays a key role in a whole range of processes and phenomena. For instance, the precipitation of salivary proteins, including lysozyme and proline-rich proteins when interacting with certain food proteins, is assumed to be responsible for the perception of the astringent and dry mouthfeel sensation.10 Among those food proteins leading to an astringency sensation when interacting with salivary proteins, whey, and plant proteins are found to be majorly present in commercial protein isolates.11,12
Typically, the addition of salts at high concentrations to protein solutions enhances unfavorable interactions leading to a destabilization of the dispersions and to protein aggregation.2,13 Other molecules can destabilize protein solutions because they favor denaturation (e.g., urea).14,15 There has been intense research in the investigation of small molecules capable of stabilizing protein solutions. Trimethylamine N-oxide (TMAO) is considered the quintessential stabilizing molecule. It is believed that TMAO counteracts denaturing effects. Amino acids such as glycine and structurally equivalent ligands like betaine have been shown to be another class of small molecules that possess the ability to stabilize proteins in aqueous solutions.4,5,16 Arakawa and Timasheff state that amino acids alongside sugars and certain salts possess the ability to induce the preferential hydration of proteins and that this is a rather common property.4 However, this effect was shown in the molar concentration range of added amino acids.
In this paper, we show that amino acids have an effect on dilute protein solutions that is opposite to the typical effect of salts, i.e., that they reduce the net, unfavorable interactions between proteins. When studying dilute protein solutions, we used as a proxy for their stability two quantitative values, namely, the second osmotic virial cross-interaction coefficient (B23) and the equilibrium dissociation constant (KD).4,5,17−22 Pioneering theoretical works introduced the application of the Kirkwood–Buff theory to quantify in terms of B23 and KD the effect of osmolytes on protein interactions treated as perturbations of the chemical potential.21,23,24 We present here quantitative data showing that proline, glutamine, and arginine significantly change both values of B23 and KD of selected protein couples even at relatively low concentrations. For example, we show that the KD of lysozyme to α-lactalbumin increases by 1 order of magnitude when 10 mM glutamine is added to the solution.
Methods
Materials
Hen egg-white lysozyme (14.3 kDa, ≥95%, purchased from Roche) was used as the primary model protein and was stored at 4 °C. For the grafting of the self-interaction chromatography (SIC) column, a Tricorn 5/50 column (Cytiva, Column Volume of 1.178 mL) was manually grafted with lysozyme using as a resin TOYOPEARL-AF Formyl-650 M chromatography particles, sodium cyanoborohydride, potassium phosphate, and ethanolamine. The standard buffer used throughout the experiments was 50 mM sodium phosphate buffer at pH ≈ 6.9 consisting of monobasic and dibasic sodium phosphate in Milli-Q water. Note that we chose this buffer as our standard one since it mimics the salivary conditions based on the average salt composition of saliva.25,26 The commercial whey protein isolates were purchased from Agropur (Eden Prairie, MN, reference BiPRO α 9000 and BiPRO 9500) while the canola protein isolate was from Merit Functional Foods, Inc. (MA, Canada, reference Puratein HS). Before the experiments, the required proteins and protein isolates were weighed, typically at a protein content of 20 mg/mL, dissolved in the buffer solution, and vortexed several times to obtain a homogeneous solution. All amino acids were purchased from Thermo Scientific being l-arginine, l-glutamine, l-proline, l-serine, and l-glycine dissolved in the standard buffer.
Column Grafting
The experimental procedure for the custom-made column grafting with lysozyme for the SIC/cross-interaction chromatography (CIC) experiments in this study is a modified version of the protocol reported by Le Brun et al.27 First, 3 mL of TOYOPEARL AF-Formyl-650 M particles were washed 5–7 times with our standard 50 mM sodium phosphate buffer. The recovered particles were mixed with lysozyme at 10 mg/mL dissolved in buffer solution. Then, 90 mg of sodium cyanoborohydride was added serving as an activator to covalently bind the particles and proteins. This solution was placed on a shaker for 12–14 h overnight at room temperature. The next morning, the resin + lysozyme solution was washed with 200 mL of buffer using a membrane filter. A small sample was saved from the washing mixture to measure its lysozyme concentration indicating how much protein was washed away. Next, to cap the residual matrix reactive groups, 20 mL of buffer with 1 M ethanolamine (1.22 g) and 20 mg sodium cyanoborohydride were prepared. The recovered resin particles grafted with lysozyme were added and the solution was placed on the shaker for 4 h at room temperature. To remove any unbound material, the particles were washed again with 200 mL of buffer from which was kept again a small sample. As a last step, the recovered grafted resin particles were dissolved in buffer solution in a graduated cylinder and were let to settle. To verify the amount of lysozyme bound as grafted protein to the resin particles, absorbance measurements using the Nanodrop instrument were performed on the three kept small samples of the stock solution, the solution after the first wash and after the second wash. All experiments were performed on SIC columns with a grafted surface coverage of lysozyme of ≈45%. To pack the Tricorn 5/50 column the chromatography particles settled down in the graduated cylinder were prepared as a 58–60% slurry in buffer. The column was packed under pressure with the following flow rates: 0.75 mL/min for 15 min, then at 3 mL/min for 15 min, and again at 0.75 mL/min for 30 min. At the end of the column packing as well as at each start of a measurement series, a column performance test was performed with 50 μL of a 20 vol % acetone solution to evaluate the reference elution volume. The column was stored at 4 °C overnight and between experiment days.
SIC/CIC Experiments
To prepare a chromatography column for a CIC experiment, Lys is manually grafted on the column, as outlined in the previous section on Column Grafting, and for the optimized signal-to-noise ratio of the elution profile, we determined that the protein concentration should be in the range of ∼20 mg/mL which was injected in all CIC experiments shown in this work.
SIC/CIC experiments were conducted to probe the lysozyme-lysozyme or the interaction between lysozyme and different proteins (BSA, WPI BLG, WPI ALAC, and CPI NAP) in different solution environments (buffer alone or in the presence of an amino acid at different concentrations dissolved in buffer) from which the respective B22/B23 values were calculated. Before each measurement series, a column performance test was run with 20% Acetone in Milli-Q water. For each run of the experiments, 50 μL of lysozyme at 20 mg/mL was injected. Samples were injected after 10× column volume and with a constant flow rate of 0.75 mL/min at room temperature. The amino acids tested were proline, glycine, arginine, serine, and glutamine and their concentration was varied between 5 mM and 1.2 M (considering their respective solubility limit). For each amino acid, the concentration range was individually refined to be the lowest concentration possible (≥1 mM) to observe a change in the B22/B23 value. The upper range limit was set by the fact that toward the solubility limit of an amino acid, the buffer solution becomes turbid which very likely clogs and thus breaks the column. Therefore, to protect the grafted column the upper limit was chosen with caution in a range well below the solubility limit of the studied amino acid. In the case of glutamine, the least soluble amino acid tested here, given that the solubility limit is ∼280 mM in aqueous buffer solution, the measurement range never surpassed 100 mM.
Determination of B22/B23 by SIC/CIC
When quantifying the interaction of two proteins in solution, a common approach is to use the virial equation of state.5,20−22 In such equation, B23 is the second-order coefficient of the cross-term that depends on the concentration of protein 2 multiplied by the concentration of protein 3. This derivation uses statistical mechanics to link thermodynamics to the properties of molecules or proteins.21,23,24 The expansion of the osmotic pressure into its second virial coefficients allows for a measure of the nonideality of the solution and has been used to quantify intermolecular forces between molecules in dilute solutions. In other terms, B23 can be described by the cross-interaction energy or potential of mean force for two molecules as a function of separation distance and angular conformation.19−22,28 A negative/positive B23 is a signature for attractive/repulsive interactions, respectively, and when referenced to B23 = 0 that indicates an “ideal solution”. The self-interaction second osmotic virial coefficient (B22) is generally measured using traditional colloidal characterization techniques including static light scattering and sedimentation equilibrium analytical ultra-centrifugation (SE-AUC) and self-interaction chromatography (SIC).28−30 SIC has the unique capability to be extended to cross-interaction chromatography (CIC) quantifying the cross-interaction between two different proteins in terms of B23.
In SIC/CIC experiments, one evaluates the interactions between the injected protein in the mobile phase and the immobilized protein grafted on the column in terms of a measured retention volume. To experimentally determine B23 one will first compute the retention factor k′ from the measurement as follows
where V0 is the retention volume of noninteracting species which is calculated before each experiment with the column performance test using 20% acetone in Milli-Q water and Vr is the volume required to elute the injected protein in the mobile phase through the grafted column.
Then, B23 [mol ml g–2] can be computed as
where BHS is the
excluded volume or hard sphere contribution of the two interacting
proteins, ρs being the immobilization density, i.e.,
the number of covalently immobilized protein molecules per unit area
of the bare chromatography particles, and 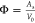 is the phase ratio, i.e., the total surface
available to the mobile phase protein. BHS is calculated as follows assuming a spherical shape
is the phase ratio, i.e., the total surface
available to the mobile phase protein. BHS is calculated as follows assuming a spherical shape
where r2,3 are the protein radii of the two proteins, NA refers to Avogadro’s number, and M2 is the averaged protein molecular weight of both proteins. Lysozyme has a molecular weight of ≈14,300 g/mol and a protein radius of (1.89 ± 0.03) nm.31 For BSA, the reported values for the radius and weight are 3.48 nm and 66463 g/mol, respectively.32
The assumption here is that we are only measuring two-body interactions, i.e., one injected free protein interacts with only one immobilized protein molecule at a time. This is valid since protein–protein (cross)-interactions are dominantly of short-range nature, meaning that they are dominant over a smaller distance than the diameter of the proteins involved. This constraint can be guaranteed by controlling the immobilized proteins grafted onto an effectively flat surface as the column. The last assumption is that the injected free proteins interact only with immobilized proteins grafted onto the column and not with each other. This can be verified by determining the variation for the calculated B22 value measured at a concentration of 5–30 mg/mL of the injected protein, here for lysozyme. For the Lys-Lys self-interaction, the obtained B22 value should remain constant. We determined this variation to be ∼0.2 × 10–4 mol mL g–2 for Lys-Lys and considered this variation in our error analysis.
In the main text and figures, we chose to report on the change of B23 (ΔB23) being the absolute difference between the second osmotic virial cross-coefficient value in the presence of the amino acid and the one in the standard buffer. Hereby, variations between sample runs and measurement days due to differences of column grafting and other instrument variations are eliminated.
SPR Experiments and Analysis
SPR experiments were conducted on a Biacore 8K (Cytiva) at room temperature. We used the commercial CM5 Sensor S chip (Biacore) which is already functionalized with a carboxylated dextran matrix. To immobilize lysozyme on the chip, the standard routine for amino coupling was applied. This routine consists of an activation step with EDC and NHS, then lysozyme is linked to the matrix at a concentration of ∼100 μg/mL in acetate buffer of pH 4.5. This optimal pH was evaluated by a pH scouting step beforehand. After the lysozyme was coupled to the chip, the surface was passivated by ethanolamine. To determine the binding affinity we set up multicycle kinetic studies of WPI in phosphate-buffered saline (PBS) first and then in the presence of amino acids. To enhance the SPR signal and reduce the so-called gradient effect inducing a significant refractive index mismatch, we had to increase the salt content. This is the reason why we switched to PBS as the standard buffer for our SPR experiments. Note that only the ionic strength is higher but the pH remains in the neutral range. The use of PBS improved the signal-to-noise ratio of the SPR signal but did not alter the obtained KD values. We determined that the optimized range for WPI BLG weakly and transiently interacting with the immobilized Lys onto the chip is to probe in the concentration range of 0.1–10 mg/mL of WPI BLG. We averaged over 10 experiments, each on >4 channels with different degrees of Lys covalently immobilized on two separate chips. We applied the same concentration range for HA and α-lac. KD yields a measure of the binding affinity of the interaction.18 The smaller the KD value, the higher the affinity between the two proteins. To determine KD from the SPR measurements, we used the Biacore Insight Software. From the measured SPR sensorgrams (which is SPR signal in Response Units (RU) vs time), we obtained the steady-state binding levels (Req) vs analyte concentration (C) first and then by a fitting applying the steady-state affinity model based on a 1:1 binding we determined KD
with Rmax being the analyte binding capacity of the functionalized surface and offset the response at zero analyte concentration.
CD Experiments and Analysis
All circular dichroism data were obtained on a Chirascan CD spectrometer (Applied Photophysics) using a quartz cuvette with a path length of 0.5 mm, allowing for a protein concentration of tens of μM. Lysozyme and WPI BLG were dissolved in our standard 50 mM sodium phosphate buffer at pH ∼ 6.9 to a final concentration of 25–50 μM and to this sample the amino acids were added. We monitored the influence of the added amino acid on the secondary shape at protein/ligand molar ratios of 1:2 and 1:20, which are similar to the CIC experimental conditions. The spectra were collected in the wavelength range of 190–280 nm with a scanning speed of 20 nm/min at room temperature. Each CD spectrum is an average of three repeated measurements and is corrected for buffer and added amino acid absorption. Note that we always added the amino acids to the protein injection sample and that we dissolved the respective amino acid in the standard elution buffer to eliminate a gradient effect. We analyzed and visualized the spectra by ourselves in Excel and Prism 9 (GraphPad).
Results and Discussion
Cross-Interactions between Lysozyme and Other Proteins are Repulsive or Attractive Depending on the Net Charge of the Proteins
To quantify the influence of small molecules on protein–protein cross-interactions, we applied cross-interaction chromatography (CIC), an extension of self-interaction chromatography (SIC) to quantify the cross-interaction between lysozyme (Lys) and a different protein of interest (refer to the Methods Section for detailed explanations).
As a proof-of-principle of CIC, as an extension of SIC, we quantified the interaction in terms of B23 between Lys and bovine serum albumin (BSA) in 50 mM sodium phosphate buffer at pH ∼ 6.9. In Figure 1A, we show a characteristic elution profile for Lys-BSA in comparison to one of the self-interacting Lys in the same buffer conditions. The measured retention volume for Lys-BSA is (1.98 ± 0.04) mL, which is eluted ∼0.2 mL later than the Lys-Lys peak retention volume. With the determined retention volume at the peak position of the elution profile, we calculated the corresponding B23 as explained in detail in the Methods Section. For Lys-BSA, we obtained a B23 value of (−1.6 ± 0.4) × 10–4 mol mL/g2 indicating that the interaction is slightly attractive, given that the obtained B23 value is negative. In contrast, the obtained data for the self-interacting lysozyme yielded a positive value for B22 (= 3 ± 0.2) 10–4 mol mL/g2. At the buffer condition tested at pH ∼ 6.9, Lys and BSA are oppositely charged. Thus, we expected a B23 value for Lys-BSA hinting at attractive interactions. This is in agreement with our obtained data and also confirmed by the literature.19,33 A brief remark on the peak shape of measured SIC/CIC elution profiles is that it contains several additional information that can be derived by a more elaborate peak deconvolution analysis. The peak width is a convolution of the molecular weight distribution with the interaction strength of the eluted protein with the grafted protein on the column. By visual inspection of the Lys-BSA peak shape to the one obtained for the Lys-Lys self-interaction (see Figure 1A), it can be stated that the Lys-BSA peak is broader. This can be expected since BSA possesses a higher molecular weight, larger hydrodynamic radius, and natural propensity to form dimers and trimers in buffer conditions containing salts. Lastly, note that we assume that the grafted Lys exhibits a random distribution. However, there still might be differences between bulk measurements and Lys grafted on the surface of a chromatography column. Overall, we have successfully managed to establish the CIC approach on the model system of the two globular proteins Lys-BSA and to quantify the cross-interaction strength in terms of B23.
Figure 1.
Characteristic elution profiles measured by the CIC approach monitoring the interactions of Lys-BSA (purple) in comparison to the self-interacting Lys-Lys (black) (A) and of Lys-WPI BLG (blue), Lys-WPI ALAC (green), and Lys-CPI NAP (orange) in comparison to the self-interacting Lys-Lys (dash-dotted black line) (B) in 50 mM sodium phosphate buffer at pH ∼ 6.9.
Next, we assessed the interaction strength of Lys with different commercial protein isolates. The protein isolates we tested are two types of whey protein isolates (WPIs), one enriched with β-lactoglobulin (BLG), referred to as WPI BLG, and the other one is enriched with α-lactalbumin (ALAC), referred to as WPI ALAC hereafter. In addition, a canola protein isolate rich in napin was tested and referred to as CPI NAP hereafter in our standard buffer condition. The isoelectric points have been found to be very close between the two WPIs, namely, to be at pH ∼ 4.7 for WPI BLG and at pH ∼ 5.0 for WPI ALAC (Supporting Information (SI) Figure 1A,B, respectively). From turbidity measurements, it can be concluded that WPI BLG interacts significantly stronger with lysozyme than WPI ALAC (SI Figure 2).
As shown in Figure 1B, the elution profiles corresponding to cross-interactions between Lys and the three different protein isolates studied are distinctly shifted in comparison to the peak position of the Lys-Lys self-interaction profile (black dotted line). These shifted peaks indicate that distinctly different interactions occur between the grafted Lys and the injected protein isolates. The obtained B23 values are (−1.0 ± 0.3) × 10–4 mol mL/g2 for Lys-WPI BLG and (−0.7 ± 0.4) × 10–4 mol mL/g2 for Lys-WPI ALAC. Both B23 values are negative revealing that the interactions between lysozyme and both WPIs are attractive. However, for the measured Lys-CPI NAP cross-interaction, we obtain a value of B23 = (4.6 ± 0.3) × 10–4 mol mL/g2. This indicates that the interaction between Lys and CPI NAP is repulsive. Note that CPI NAP, a protein extracted from canola seeds, is considered to be the plant-based structural analogue of lysozyme.34 They share similar molar mass, the secondary structure rich in helices, 4 disulfide bonds, and a high isoelectric point which was measured to be at pH ∼ 7.2 (see SI Figure 3). Thus, at the studied pH of ∼6.9, the two interacting proteins, Lys and CPI NAP, both possess a positive net charge which explains the observed repulsive nature of their interaction.
Presence of Proline at mM Concentrations Increases Repulsive Interactions between Lys and BSA
After having determined the B23 values for protein cross-interactions under standard buffer conditions, we monitored the influence of small molecules, namely, the addition of various amino acids to the buffer solution. In Figures 2 and 3, we report on the change of B23 (ΔB23) to focus on solely detecting the impact of the added amino acid on the protein cross-interactions and removing all other effects (e.g., of buffers and salts, see the Methods Section for more details). In Figure 2, we examined the influence of proline on the Lys-BSA interaction in terms of ΔB23. There is clearly a significant influence of proline that renders the cross-interaction between Lys and BSA more repulsive or equivalently less attractive (ΔB23 > 0, Figure 2). In particular, it should be recognized that a noticeable effect of proline is already observed at an addition of 10 mM, the lowest concentration probed. The injected protein concentration of BSA is 20 mg/mL corresponding to 0.3 mM which translates to a minimal stoichiometric protein: ligand ratio of 33.
Figure 2.
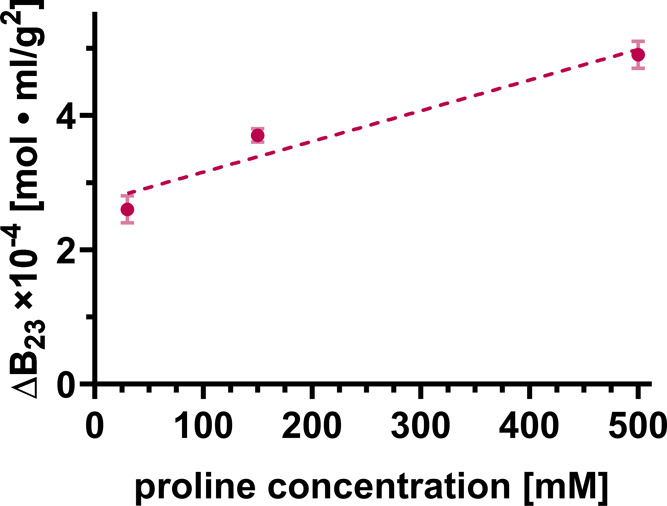
Change of B23 (ΔB23) for the interaction between lysozyme grafted to the column and BSA (at 20 mg/mL) in the presence of added proline dissolved in 50 mM sodium phosphate buffer at pH ∼ 6.9. The error bars reflect the measurement uncertainties (std of ΔB23).
Figure 3.

ΔB23 for the interaction between lysozyme, grafted to the column, and the two whey protein isolates, WPI BLG (circles) and WPI ALAC (squares), and the canola protein isolate, CPI NAP (triangles), is displayed for different amino acids (arginine (blue), glutamine (green), and proline (red)) at 1–100 mM dissolved in the protein injected and in the 50 mM sodium phosphate buffer at pH ∼ 6.9. The error bars reflect the measurement uncertainties (std of ΔB23).
Impact of Various Amino Acids on the Weakly Interacting Protein Cross-Interactions Requires Minimal Concentrations
As observed for the Lys-BSA interaction, the effect of amino acids rendering the protein cross-interaction more repulsive holds true for the cross-interactions between Lys and different protein isolates as shown in Figure 3. Furthermore, the three amino acids tested appear to yield the same influence on the two interacting proteins regardless of their respective charge and/or polarity. Note that proline is a nonpolar amino acid, glutamine is polar but not charged and arginine is a basic, positively charged amino acid. The mentioned effect manifests itself as an increasingly positive B23 value (ΔB23 > 0) with increasing amino acid concentration in comparison to the one obtained in the buffer condition. As a control experiment, we probed the influence of three established osmolytes which are not amino acids, namely, urea, betaine, and polyethylene glycol (PEG) 6k on the Lys-WPI BLG cross-interaction shown in SI Figure 4. For PEG 6k, we observed a destabilizing (ΔB23 < 0) effect. Over a large concentration range (10 to 300 mM), betaine and urea yielded no significant and a slightly positive/stabilizing effect, respectively.
Strikingly, we observe this influence at added amino acid concentrations as low as 1 mM for the two different WPIs interacting with Lys. This detectable positive shift of B23 to more positive values measured at concentrations as minimal as 1 mM corresponds to a stoichiometric protein/ligand ratio of ∼0.8 for WPI with a molecular weight slightly larger than Lys.
Addition of mM Concentrations of Amino Acids to Lys and WPI Individually or to Lys Interacting with WPI Does Not Affect the Secondary Structure of the Proteins
To verify if the presence of amino acids influences the secondary structure of the protein–protein cross-interactions, we performed Circular Dichroism (CD) experiments. The CD signal was monitored for the individual proteins as well as for mixtures interacting in the buffer first and then in the presence of amino acids at two different molar ratios as displayed in Figure 4 (refer to the Methods Section for details).
Figure 4.
Normalized CD curves for the individual proteins of lysozyme (A) and WPI BLG (B) at ∼25 μM and for the interacting Lys-WPI BLG sample (C) at ∼50 μM in buffer and in the presence of 1 mM proline and 10 mM proline and 10 mM glutamine. In (D), the calculated wavelength (λ) ratio between the CD signal measured at λ = 222 nm versus λ = 208 nm for the Lys and WPI BLG measured separately and together in buffer solutions (gray) and in the presence of 1 mM proline (light green) and 10 mM proline (dark green) and 10 mM glutamine (orange).
In Figure 4A–C, we compare, as a first visual inspection, the normalized CD curves for Lys (Figure 4A) and WPI BLG (Figure 4B) alone as well as for Lys-WPI BLG (Figure 4C) in the buffer solution with the ones measured in the presence of amino acids at different concentrations. Regardless of whether we added proline at a concentration of 1 or 10 mM or in the presence of 10 mM glutamine, the obtained curves still overlap with the respective curves of the protein tested in buffer solution (Figure 4A–C). This suggests that the secondary shape is unaffected by the presence of amino acids.
The calculated sum of the individual proteins versus the measured CD curves for proteins mixed together perfectly overlaps for both conditions, namely, in the absence and presence of added amino acids. As shown in Figure 4D, we calculated the wavelength ratio between the CD signal measured at 222 nm versus the one at 208 nm for the two proteins alone and measured together in buffer solutions and for the different amino acid conditions. Again, we see no significant change which supports our conclusion that the driving mechanism is not based on a molecular structural change of the weakly interacting proteins but is rather of an effective screening nature.
Dissociation Constant of Lys Interacting with Different Proteins Changes by Orders of Magnitude in the Presence of Millimolar Concentrations of Amino Acids
To gain a deeper insight into the effect of amino acids on protein–protein cross-interactions with respect to their binding affinity, SPR experiments were carried out evaluating the equilibrium dissociation constant KD. The interactions in the Lys-WPI BLG system are in the regime of weak, transient interactions. Accordingly, we expected the values for KD to be in the mM range, in contrast to antibody–antigen interactions which are tightly binding and possess KD values in the nM to pM range.35 Weak interactions with mM binding affinities are rarely studied by SPR since the time resolution for resolving their fast association and disassociation kinetics is at the limit of the commercially available SPR instruments.36 Here for this work, we optimized the concentration range of the injected protein to yield a reliable SPR response leading to a high degree of repeatability. Specifically, the data presented for the Lys-WPI BLG interaction was measured in over 30 individual measurements conducted on three different CM5 sensor chips and five different measurement days. A representative SPR sensorgram for Lys-WPI BLG in the concentration range of (0.1–10) mg/mL in PBS is shown in SI Figure 5A. From the sensorgram, the maximum constant SPR response of the injected WPI BLG is computed and plotted against its respective concentration as shown in Figure 5A. The fitting of this plot assuming a steady-state affinity model results in the characteristic KD value, a measure of the binding affinity, of the investigated protein cross-interaction. For the Lys-WPI BLG interaction in PBS, we obtained a characteristic average KD value of (2 ± 1) mM.
Figure 5.
(A) Representative steady-state affinity fitting for the SPR response of WPI BLG interacting with lysozyme in PBS yielding a KD value of (1.4 ± 0.2) mM which is indicated by the dashed line. (B) Steady-state affinity fitting for a representative SPR response for WPI BLG interacting with lysozyme in the presence of 10 mM arginine yielding a KD of (5 ± 1) M as indicated by the dashed line.
Once we demonstrated a reproducible KD for the Lys-WPI interaction, we probe the influence of proline, glycine, and arginine at 10 and 100 mM which was added to the WPI BLG injection as well as added to the running PBS buffer. A representative sensorgram for the Lys-WPI BLG interaction in the presence of arginine at 10 mM is shown in SI Figure 5B. The corresponding steady-state affinity fitting is displayed in Figure 5B and all KD values obtained from the fittings to all of the different conditions probed are summarized in Table S1. In SI Figure 6, we show that the added amino acids to the running buffer yield no measurable SPR signal which is more important than the PBS buffer alone when injected onto the SPR chip immobilized with Lys.
We observed a distinct influence on the KD being shifted from the mM to the M range in the presence of the amino acids. The large error is explained by the fact that the molar range pushes the experiments to the resolution limit of the instrument. Nevertheless, we consistently obtained a KD value for the Lys-WPI BLG interaction shifted by 3 orders of magnitude in the presence of any of the three amino acids tested.
The question arises if the observed influence of amino acids on the binding affinity of protein–protein cross-interactions holds beyond the weakly binding interaction regime yielding mM values for KD. As a first attempt, we performed SPR measurements to evaluate the binding affinities of Lys interacting with the protein α-lactalbumin (ALAC, molecular weight MW of ∼14.2 kDa) and with the glycosaminoglycan hyaluronic acid (HA hereafter, low weight of MW ∼ 100–500 kDa) in PBS buffer conditions and in the presence of 10 mM glutamine.
As shown in Table 1, we obtained binding affinities of (8.9 ± 0.4) × 10–05 and (1.4 ± 0.2) × 10–04 M for Lys-ALAC and Lys-HA in buffer solution, respectively. The presence of 10 mM glutamine shifts both binding affinities by 1 order of magnitude. This indicates that the effect of amino acids is not unique for the mM range.
Table 1. Averaged KD Values for the Lys-ALAC and Lys-HA Cross-Interactions in PBS Buffer and in the Presence of 10 mM Glutamine Measured by SPR Experiments.
| av. KD ± std [M] | ALACc | HA |
|---|---|---|
| in PBS | (8.9 ± 0.4) × 10–05 | (1.4 ± 0.2) × 10–04 |
| 10 mM Gln | (6.2 ± 0.3) × 10–04 | (2.7 ± 0.9) × 10–03 |
Conclusions
In this work, we quantified the effect of free amino acids on the interaction between lysozyme and a series of proteins in terms of changes in the second osmotic virial cross-interaction coefficient (B23) and in the equilibrium dissociation constant (KD). The protein combinations chosen were such that we had attractive (for Lys-BSA, Lys-WPI BLG, and Lys-WPI ALAC) or repulsive interaction (Lys-CPI NAP) in 50 mM phosphate buffer solutions at pH ∼ 6.9.
Regardless of the starting interaction regime for the specific protein system, we observed a significant influence of the added amino acid at millimolar concentrations on all tested protein systems. However, note that we are not in the concentration range where small molecules are known to act as crowders.6 In all cases, B23 became larger and more positive indicating a net change in interaction toward a more repulsive regime. The effects we found are not small, i.e., for Lys-BSA interacting in the presence of 500 mM added proline, we observed a value of B23 that is more than double compared to the initial one. In the case of lysozyme interacting with BSA or with any of the two whey protein isolates the initial B23 value is negative, indicating a net attractive interaction. For BSA already the addition of 10 mM of added proline changes the sign of the B23 value. For the whey protein isolates, 1 mM of added amino acid already shifts the sign; however, note that the initial B23 value is less negative. These data are significant since it shows that at protein to amino acid stoichiometric values as low as 0.8 we already observed a significant change in the interaction between proteins. A change in B23 implies a change in the chemical potential of the two proteins which can be mathematically treated as a perturbation of the chemical potentials.21,23,24 A direct consequence of such protein cross-interaction is an (observable) change of its corresponding equilibrium dissociation constant KD, a proxy for its binding affinity as well. We verified this statement by measuring changes in KD as a result of the addition of amino acids. The affinity measurements by SPR showed that 10 mM of added amino acid affects the binding constants expressed as a shift to weaker binding affinities. For the Lys-WPI BLG cross-interaction, we report on a shift of KD by 3 orders of magnitude from the weakly interacting millimolar regime. In short, we show that free amino acids can modulate weak protein–protein cross-interactions as was previously reported for strongly binding immunoglobulins in the presence of 5 mM histidine addition.37
We believe that the observed modulation of the protein cross-interaction by minimal concentrations of amino acids cannot be simply explained as a hydrotropic effect5−7,24 nor as crowding.6 In fact, we do not see any threshold in this behavior and we observe the effect at low stoichiometries of protein/ligand (e.g., ∼0.8) that can hardly be attributed to hydrotropic effects.
By means of CD measurements, we observed no changes in the secondary structure of the interacting proteins in the presence of amino acids. This implies that the modulating amino acids are only weakly interacting with the proteins. Also, that implies that the effect we have presented in this work does not depend on protein conformational changes, but it is the direct effect in the changes of the colloidal interaction between proteins. As we have shown in our recent work by complementary experimental approaches together with a predictive theoretical model,38 an amino acid can be present on the surface of a protein in a time-averaged fractional way, thus screening part of the protein interaction with other proteins (and with the solvent molecules) which leads to the change of its chemical potential. This small molecule effect already happens at low (e.g., millimolar) concentrations and can thus act as a powerful stabilizer for protein dispersions.
In this work, we confirm that this effect is rather broad and also applies to protein cross-interactions We investigated a range of protein systems, namely, of two different WPI and of one CPI, which go beyond the most reported model proteins (i.e., Lys and BSA). In all cases the observed effects are comparable. Consistent with our previous work and in particular with the proposed predictive theoretical model,38 the amino acid effects that we probed here are all similar in magnitude. This is because the root cause is a weak screening interaction of the amino acids with the proteins, which is not expected to change significantly across amino acids.
It is important to notice that the stabilization effects that we show here lead to large changes in the equilibrium dissociation constant. The mere presence of 10 mM glutamine in strongly binding systems such as Lys-HA (KD = 14 mM) and Lys-ALAC (KD = 0.9 μM) leads to a change in KD of close to 1 order of magnitude. This indicates that the effect of amino acids is not unique to weakly interacting protein systems. Furthermore, this highlights that free amino acids are potentially capable of modulating protein interactions across interaction regimes.
In the pharmaceutical field, amino acids such as arginine are employed as stabilizers in drug formulations without necessarily understanding the underlying mechanism. Here we have shown that the proteinogenic amino acids proline, arginine, and glutamine have a fundamental stabilizing effect on protein–protein cross-interactions. This effect leads to significant alterations in the equilibrium dissociation constant. We foresee implications of this work on the use of amino acids in the design and preparation of protein-based liquid formulations, in both food and pharmaceutical industries.
Furthermore, our results are relevant in the biological context. The actual concentrations of amino acids in living organisms are hard to determine accurately e.g., by measurements of the volume and dry mass. In the recently introduced theoretical model for cell size scaling laws by Rollin et al., the cellular density of impermeant species (that are for 99% metabolites) is found to be 120 mM, which is comparable to the external ionic density.39 They further state that metabolites are mainly composed of amino acids (73%), in particular of glutamine, glutamic and aspartic acid. This translates to approximate concentrations of the total concentration of free amino acids in mammalian cells being ∼88 mM, thus ≤100 mM. Therefore, our experiments match well with the actual physiological situation in living cells. The results presented in this paper apply to any form of protein cross-interaction, we have shown here the effect of amino acids only on weak interacting proteins. Future work will investigate the effect on stronger interacting protein systems.
Acknowledgments
The authors gratefully acknowledge support and insightful discussions with Dr. Christoph Hartmann (Nestlé Research) and Dr. Kelvin Lau (EPFL) and Dr. Quy Ong (EPFL).
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acs.jpcb.4c01086.
Measured ζ-potentials for the probed protein isolates (WPI BLG, WPI ALAC, and CPI NAP), turbidity measurements for the two whey protein isolates interacting with lysozyme as a function of molar ratio, representative SPR sensorgrams, CIC measurements of the effect of PEG, urea, and betaine on Lys-WPI BLG interactions, SPR sensorgrams probing amino acids on immobilized Lys, and averaged binding affinities for the Lys-WPI-BLG interaction in the presence of amino acids (PDF)
Author Contributions
The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript.
The research of P.M.W. was funded by Société des Produits Nestlé S.A.
The authors declare no competing financial interest.
This article published ASAP on July 12, 2024. The name indexing of author Juliana V. C. Silva has been updated and the corrected version reposted on July 25, 2024.
Supplementary Material
References
- Chandler D. Interfaces and the Driving Force of Hydrophobic Assembly. Nature 2005, 437 (7059), 640–647. 10.1038/nature04162. [DOI] [PubMed] [Google Scholar]
- Wang W.; Nema S.; Teagarden D. Protein Aggregation—Pathways and Influencing Factors. Int. J. Pharm. 2010, 390 (2), 89–99. 10.1016/j.ijpharm.2010.02.025. [DOI] [PubMed] [Google Scholar]
- Bruździak P.; Panuszko A.; Stangret J. Influence of Osmolytes on Protein and Water Structure: A Step To Understanding the Mechanism of Protein Stabilization. J. Phys. Chem. B 2013, 117 (39), 11502–11508. 10.1021/jp404780c. [DOI] [PubMed] [Google Scholar]
- Arakawa T.; Timasheff S. N. Preferential Interactions of Proteins with Solvent Components in Aqueous Amino Acid Solutions. Arch. Biochem. Biophys. 1983, 224 (1), 169–177. 10.1016/0003-9861(83)90201-1. [DOI] [PubMed] [Google Scholar]
- Timasheff S. N. Protein-Solvent Preferential Interactions, Protein Hydration, and the Modulation of Biochemical Reactions by Solvent Components. Proc. Natl. Acad. Sci. U.S.A. 2002, 99 (15), 9721–9726. 10.1073/pnas.122225399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou H.-X.; Rivas G.; Minton A. P. Macromolecular Crowding and Confinement: Biochemical, Biophysical, and Potential Physiological Consequences. Annu. Rev. Biophys. 2008, 37 (1), 375–397. 10.1146/annurev.biophys.37.032807.125817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel A.; Malinovska L.; Saha S.; Wang J.; Alberti S.; Krishnan Y.; Hyman A. A. ATP as a Biological Hydrotrope. Science 2017, 356 (6339), 753–756. 10.1126/science.aaf6846. [DOI] [PubMed] [Google Scholar]
- Rabbani G.; Choi I. Roles of Osmolytes in Protein Folding and Aggregation in Cells and Their Biotechnological Applications. Int. J. Biol. Macromol. 2018, 109, 483–491. 10.1016/j.ijbiomac.2017.12.100. [DOI] [PubMed] [Google Scholar]
- Burg M. B.; Ferraris J. D. Intracellular Organic Osmolytes: Function and Regulation. J. Biol. Chem. 2008, 283 (12), 7309–7313. 10.1074/jbc.R700042200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haslam E.; Lilley T. H.; Butler L. G. Natural Astringency in Foodstuffs—A Molecular Interpretation. Crit. Rev. Food Sci. Nutr. 1988, 27 (1), 1–40. 10.1080/10408398809527476. [DOI] [PubMed] [Google Scholar]
- Silletti E.; Vingerhoeds M. H.; Norde W.; Van Aken G. A. Complex Formation in Mixtures of Lysozyme-Stabilized Emulsions and Human Saliva. J. Colloid Interface Sci. 2007, 313 (2), 485–493. 10.1016/j.jcis.2007.05.030. [DOI] [PubMed] [Google Scholar]
- Assad-Bustillos M.; Cázares-Godoy A. C.; De Lavergne M. D.; Schmitt C.; Hartmann C.; Windhab E. Assessment of the Interactions between Pea and Salivary Proteins in Aqueous Dispersions. Innovative Food Sci. Emerging Technol. 2023, 84, 103290 10.1016/j.ifset.2023.103290. [DOI] [Google Scholar]
- Zhang Y.; Cremer P. S. The Inverse and Direct Hofmeister Series for Lysozyme. Proc. Natl. Acad. Sci. U.S.A. 2009, 106 (36), 15249–15253. 10.1073/pnas.0907616106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zetterholm S. G.; Verville G. A.; Boutwell L.; Boland C.; Prather J. C.; Bethea J.; Cauley J.; Warren K. E.; Smith S. A.; Magers D. H.; Hammer N. I. Noncovalent Interactions between Trimethylamine N -Oxide (TMAO), Urea, and Water. J. Phys. Chem. B 2018, 122 (38), 8805–8811. 10.1021/acs.jpcb.8b04388. [DOI] [PubMed] [Google Scholar]
- Ganguly P.; Polák J.; Van Der Vegt N. F. A.; Heyda J.; Shea J.-E. Protein Stability in TMAO and Mixed Urea–TMAO Solutions. J. Phys. Chem. B 2020, 124 (29), 6181–6197. 10.1021/acs.jpcb.0c04357. [DOI] [PubMed] [Google Scholar]
- Liao Y.-T.; Manson A. C.; DeLyser M. R.; Noid W. G.; Cremer P. S. Trimethylamine N -Oxide Stabilizes Proteins via a Distinct Mechanism Compared with Betaine and Glycine. Proc. Natl. Acad. Sci. U.S.A. 2017, 114 (10), 2479–2484. 10.1073/pnas.1614609114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asthagiri D.; Neal B. L.; Lenhoff A. M. Calculation of Short-Range Interactions between Proteins. Biophys. Chem. 1999, 78 (3), 219–231. 10.1016/S0301-4622(99)00028-9. [DOI] [PubMed] [Google Scholar]
- Ma W.; Yang L.; He L. Overview of the Detection Methods for Equilibrium Dissociation Constant KD of Drug-Receptor Interaction. J. Pharm. Anal. 2018, 8 (3), 147–152. 10.1016/j.jpha.2018.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tessier P. M.; Sandler S. I.; Lenhoff A. M. Direct Measurement of Protein Osmotic Second Virial Cross Coefficients by Cross-Interaction Chromatography. Protein Sci. 2004, 13 (5), 1379–1390. 10.1110/ps.03419204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon Y. U.; Curtis R. A.; Anderson C. O.; Blanch H. W.; Prausnitz J. M. Protein–Protein Interactions in Aqueous Ammonium Sulfate Solutions. Lysozyme and Bovine Serum Albumin (BSA). J. Solution Chem. 2000, 29 (8), 699–718. 10.1023/A:1005112927213. [DOI] [Google Scholar]
- Shimizu S. Estimating Hydration Changes upon Biomolecular Reactions from Osmotic Stress, High Pressure, and Preferential Hydration Experiments. Proc. Natl. Acad. Sci. U.S.A. 2004, 101 (5), 1195–1199. 10.1073/pnas.0305836101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu S.; Matubayasi N. Preferential Solvation: Dividing Surface vs Excess Numbers. J. Phys. Chem. B 2014, 118 (14), 3922–3930. 10.1021/jp410567c. [DOI] [PubMed] [Google Scholar]
- Shimizu S.; Boon C. L. The Kirkwood–Buff Theory and the Effect of Cosolvents on Biochemical Reactions. J. Chem. Phys. 2004, 121 (18), 9147–9155. 10.1063/1.1806402. [DOI] [PubMed] [Google Scholar]
- Pierce V.; Kang M.; Aburi M.; Weerasinghe S.; Smith P. E. Recent Applications of Kirkwood–Buff Theory to Biological Systems. Cell Biochem. Biophys. 2008, 50 (1), 1–22. 10.1007/s12013-007-9005-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ngamchuea K.; Chaisiwamongkhol K.; Batchelor-McAuley C.; Compton R. G. Chemical Analysis in Saliva and the Search for Salivary Biomarkers – a Tutorial Review. Analyst 2018, 143 (1), 81–99. 10.1039/C7AN01571B. [DOI] [PubMed] [Google Scholar]
- Nonaka T.; Wong D. T. W. Saliva Diagnostics. Annu. Rev. Anal. Chem. 2022, 15 (1), 107–121. 10.1146/annurev-anchem-061020-123959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Brun V.; Friess W.; Schultz-Fademrecht T.; Muehlau S.; Garidel P. Lysozyme-Lysozyme Self-Interactions as Assessed by the Osmotic Second Virial Coefficient: Impact for Physical Protein Stabilization. Biotechnol. J. 2009, 4 (9), 1305–1319. 10.1002/biot.200800274. [DOI] [PubMed] [Google Scholar]
- Tessier P. M.; Lenhoff A. M.; Sandler S. I. Rapid Measurement of Protein Osmotic Second Virial Coefficients by Self-Interaction Chromatography. Biophys. J. 2002, 82 (3), 1620–1631. 10.1016/S0006-3495(02)75513-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patro S. Y.; Przybycien T. M. Self-Interaction Chromatography: A Tool for the Study of Protein-Protein Interactions in Bioprocessing Environments. Biotechnol. Bioeng. 1996, 52 (2), 193–203. . [DOI] [PubMed] [Google Scholar]
- Behlke J.; Ristau O. Analysis of the Thermodynamic Non-Ideality of Proteins by Sedimentation Equilibrium Experiments. Biophys. Chem. 1999, 76 (1), 13–23. 10.1016/S0301-4622(98)00212-9. [DOI] [PubMed] [Google Scholar]
- Parmar A. S.; Muschol M. Hydration and Hydrodynamic Interactions of Lysozyme: Effects of Chaotropic versus Kosmotropic Ions. Biophys. J. 2009, 97 (2), 590–598. 10.1016/j.bpj.2009.04.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda S.; Nishinari K. Intermolecular Forces in Bovine Serum Albumin Solutions Exhibiting Solidlike Mechanical Behaviors. Biomacromolecules 2000, 1 (4), 757–763. 10.1021/bm005587o. [DOI] [PubMed] [Google Scholar]
- Wang G.; Wang S.; Wu H.; Yan C.; Liu Y. Interactions of Bovine Serum Albumin Molecules in an Aqueous Sodium Sulfate Solution Determined by an Osmotic Pressure Method. J. Solution Chem. 2018, 47 (3), 586–594. 10.1007/s10953-018-0738-8. [DOI] [Google Scholar]
- Perera S.; McIntosh T.; Wanasundara J. Structural Properties of Cruciferin and Napin of Brassica Napus (Canola) Show Distinct Responses to Changes in pH and Temperature. Plants 2016, 5 (3), 36 10.3390/plants5030036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuriyan J.; Konforti B.; Wemmer D.. The Molecules of Life: Physical and Chemical Principles; Garland Science, Taylor & Francis Group: New York, 2013. [Google Scholar]
- Hearty S.; Leonard P.; Ma H.; O’Kennedy R.. Measuring Antibody-Antigen Binding Kinetics Using Surface Plasmon Resonance. In Antibody Engineering; Nevoltris D.; Chames P., Eds.; Springer: New York, 2018; Vol. 1827, pp 421–455. [DOI] [PubMed] [Google Scholar]
- Le Brun V.; Friess W.; Bassarab S.; Mühlau S.; Garidel P. A Critical Evaluation of Self-Interaction Chromatography as a Predictive Tool for the Assessment of Protein–Protein Interactions in Protein Formulation Development: A Case Study of a Therapeutic Monoclonal Antibody. Eur. J. Pharm. Biopharm. 2010, 75 (1), 16–25. 10.1016/j.ejpb.2010.01.009. [DOI] [PubMed] [Google Scholar]
- Mao T.; Xu X.; Winkler P. M.; Siri C.; Poliukhina E.; Silva P. J.; Luo Z.; Ong Q.; Katz A.-A.; Stellacci F.. Amino Acids Stabilizing Effect on Protein and Colloidal Dispersions, arXiv:2404.11574. arXiv.org e-Print archive, April 17, 2024. https://doi.org/10.48550/arXiv.2404.11574. (accessed April 04, 23).
- Rollin R.; Joanny J.-F.; Sens P. Physical Basis of the Cell Size Scaling Laws. eLife 2023, 12, e82490 10.7554/eLife.82490. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.





