Abstract
Purpose
To analyze the copy number variation (CNV) in the X-linked genes BCORL1, POF1B, and USP9X in idiopathic diminished ovarian reserve (DOR).
Methods
This case-control study included 47 women, 26 with DOR and 21 in the control group. Age, weight, height, BMI, and FSH level were evaluated, as well as antral follicle count (AFC), oocyte retrieval after controlled ovarian stimulation, and metaphase II (MII) oocytes. The CNVs of BCORL1, USP9X, and POF1B genes were measured by quantitative real time PCR (qPCR) using two reference genes, the HPRT1 (X-linked) and MFN2 (autosomal). Protein–protein interaction network and functional enrichment analysis were performed using the STRING database.
Results
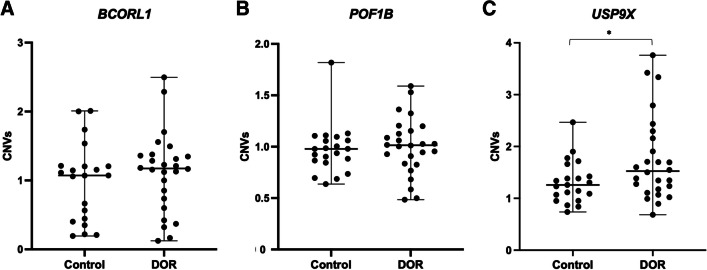
The mean age was 36.52 ± 4.75 in DOR women and 35.38 ± 4.14 in control. Anthropometric measures did not differ between the DOR and control groups. DOR women presented higher FSH (p = 0.0025) and lower AFC (p < .0001), oocyte retrieval after COS (p = 0.0004), and MII oocytes (p < .0001) when compared to the control group. BCORL1 and POF1B did not differ in copy number between DOR and control. However, DOR women had more copies of USP9X than the control group (p = 0.028).
Conclusion
The increase in the number of copies of the USP9X gene may lead to overexpression in idiopathic DOR and contribute to altered folliculogenesis and oocyte retrieval.
Supplementary information
The online version contains supplementary material available at 10.1007/s10815-024-03185-8.
Keywords: Ovarian failure, Copy number variation, Genomic instability; Aging; DOR
Introduction
The advance of age is inversely proportional to reproductive health, mainly after 37 years old in women, whose primary outcome is the reproductive senescence [1]. Diminished ovarian reserve (DOR) is a condition that may precede menopause whose ovaries lose their reproductive potential, impairing fertility [2, 3]. Women with DOR are usually poor responders during reproductive treatments and, if not age-related (< 40 years old), its diagnosis may be considered pathologic and may lead to premature ovarian insufficiency (POI) [4–7]. Increasingly prevalent in women of reproductive age [8–10], women with DOR usually present reduced pregnancy rates after assisted reproductive technologies (ARTs), mainly due to lower quality and quantity of matured oocytes after controlled ovarian stimulation, resulting in impaired fertilization, embryo quality, and implantation rate [11–13]. The reproductive outcomes observed may be due to the reduced number of euploid blastocysts in women with DOR [14], and as a result, they face all the physical and emotional challenges of ARTs with a low chance of achieving the goal of becoming pregnant [15].
The etiology of DOR is unclear, being a complex and multifactorial disease, with genetic and epigenetic components, environmental factors, autoimmune diseases, and medical treatment (iatrogenic exposure) [16–18]. Early aging and the cessation of ovarian activity before the age of 40 pose a challenge for reproductive medicine [5, 19–22]. Chromosomal abnormalities and altered gene regulation mainly in the X chromosome have been reported in POI and DOR, as X-linked genes play a key role in follicular dynamics and oogenesis, influencing the reproductive outcomes, as observed in Turner syndrome, fragile X syndrome, and early menopause [23]. Copy number variations (CNVs) are a class of structural genetic elements in the genome with variable frequency across populations [15, 24, 25]. Gain or loss of copies of DNA elements changes gene function and contributes to the pathogenesis of many diseases [26].
Array comparative genomic hybridization (array-CGH) has identified several CNVs affecting loci with a possible role in ovarian function and female fertility, as observed in Table 1. Several studies reported alterations in X-linked genes, such as EIF1AX, PCDH19, PCDH11X, POF1B, TGIF2LX, and SHOX and its relation with ovarian failure, disrupted folliculogenesis and oogenesis resulting in infertility [27–31]. In an end-to-end X chromosome analysis, Quilter et al. [28] observed differences in the number of copies between health and POI women. Besides corroborate genes already described in the literature as those related to ovarian failure, they presented new candidate genes, such as BCORL1 and USP9X. The USP9X is located in the short arm X chromosome at Xp11.4, a critical region for ovarian development, whereas POF1B and BCORL1 are located in the long arm of X chromosome, Xq21.1 and Xq26.1, respectively, being related to chromosome pairing and apoptosis (Fig. 1) [28, 32, 33].
Table 1.
Studies that identified CNVs with a possible role in ovarian function and female fertility using array comparative genomic hybridization (array-CGH)
| Reference | Resolution | N | Loci ou CNV | Candidate genes | Region |
|---|---|---|---|---|---|
| Sakka et al., [30] | CGH Microarray, 4 × 180 K | 3 POI; same family | Xp22.12 | EIF1AX | Tunisia |
| Bestetti et al., [34] | CGH Microarray 244 K/400 K | 67 (46,XX) POI | 37 ovary-related CNVs | BMP15, DIAPH2, CPEB1, BNC1, TP63, VLDLR | Italy |
| Yatsenko et al., [35] |
CGH Microarray 180 K |
111 POI | Xq22.1 and Xp22.23 | PCDH19, SHOX | Europe |
| Katari et al., [36] | aCGH 4 × 60 K | 46 POI | 16p12.3, Xq28, 3p22.2 | CETN2, HAUS7, MLH1 | USA |
| Jaillard et al., [23] | Oligonucleotide CGH Microarray 180 K | 60 OF | 19 CNVs (8 deletions and 11 duplications) | SYCE1, CLASP1, CENP-A, CDC16, RSPH1, KIF24, CSMD1, SEMA6D, KIAA1324 | France |
| Norling, et al., [37] | Customized 1 M Oligomarker Array-CGH Platform, 2.2 K | 26 POI | 2p16.2, 15q26.2, 3p21.31, 15q26.2, 12p13.33, 15q25.2, 19p13.3, Xp22.33, 17q21.2, 5q31.1, 2p11.2, 17q21.2, 2q34 | GDF9, DNAH6, TSPYL6, SMARCC1, CSPG5, ZFR2 | Sweden |
| Knauf, et al., [28] | Illumina 370 K BeadChip | 97 POI | Xq21.3 | PCDH11X, TGIF2LX | Netherland |
| McGuire et al., [38] | Illumina’s HumanCNV370-Duo DNA Analysis | 89 POI | 8q24.13, 10p15-p14, 10q26.3, 15q25.2, 18q21.32 | SYCE1, CPEB1 | America |
| Dudding et al. [39], | X Chromosome Tiling Path Array, 80 K | 50 POI | Xp22.33 and Xq13.3 | PRKX, ABCB7, ZDHHC15 | New Zeland |
| Ledig et al., [40] | Human Genome CGH Microarray Kit 105A, 21.7 K | 44 POI and 30 OD | 15 microdeletion; 29 microduplication | PLCB1, RB1CC1, MAP4K4;RBBP8; IMMP2L, FER1L6, MEIG1 | Germany |
| Quilter et al., [27] | X Chromosome Tiling Path Array, 1 M | 42 POI |
15 CNVs X chromosome |
XNPEP2, UTP14A, CENP1, PCDH19, VCX, STS, ZFX, BCORL1, USP9X, TSPAN7, POF1B, AIFM1 | United Kingdom |
| Aboura et al., [41] | Genomic DNA Array, 0.7 M | 99 POI | 1p21.1, 5p14.3, 5q13.2, 6p25.3, 14q32.33, 16p11.2, 17q12, Xq28 | DNAH5, NAIP, DUSP22, AKT1, NUPR1 | France |
CGH comparative genome hybridization, POI premature ovarian insufficiency, OD ovarian dysgenesis, OF ovarian failure
Fig. 1.
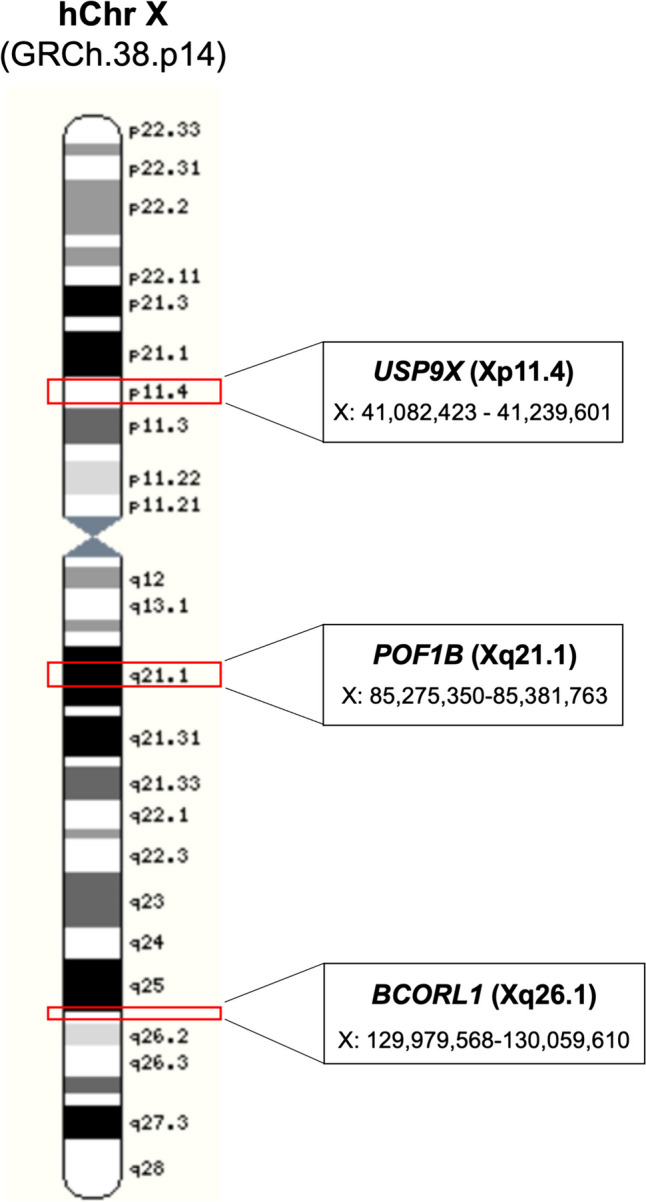
Ideogram of human X chromosome the spatial localization of the BCORL1, POF1B, and USP9X genes.
Modified from Ensemble (ensembl.org/index.html)
Despite clinical, biological, and hormonal tests, poor ovarian reserve is still difficult to diagnose, mainly because of its interpretative limitations [34]. Identification of molecular markers of ovarian reserve or insufficiency is important for understanding the menopausal process, reproductive lifespan, and other conditions associated with DOR. Loss or gain at the X chromosome predicted by CNVs alters gene expression that may promote ovarian failure and have predictive value as a biomarker for disease-association studies, both for early diagnosis and reproductive intervention in DOR women. We sought to evaluate the variations in the number of copies of the X-linked genes POF1B, BCORL1, and USP9X in women with DOR and their correlation with clinical features. We observed that women diagnosed with DOR had a higher number of copies of the USP9X gene than those with normal ovarian reserve [35–42].
Participants, material, and methods
Participants and ethics statement
This case–control study was approved by the Research Ethics Committee of the Ribeirão Preto Medical School (CAAE: 54777816.4.0000.5440) and ratified by the participating institutions. The participants were recruited in the Human Reproduction Division of the Department of Gynecology and Obstetrics of the Ribeirão Preto Medical School, University of São Paulo (FMRP-USP). The inclusion criteria were women aged between 18 and 40 years, regardless of race, ethnicity, and social status.
The DOR group (n = 26) was formed by women who had previously undergone at least one assisted fertilization treatment using conventional stimulation with a recovery of ≤ 3 oocytes/cycle and/or a low ovarian reserve profile (Poseidon 1, 2, 3, and 4) [43]. The low ovarian reserve was defined as early follicular phase antral follicle count (< 5). To confirm the absence of X-chromosome abnormalities, the 46,XX karyotype was required for women younger than 30 years. The control group (n = 21) was formed by women with normal ovarian reserve, considering ≥ 8 oocytes/cycle.
The exclusion criteria were tobacco smoking, patients with chromosome alterations, endocrine disease, autoimmune disorders, and syndromic POI, such as Turner and Fragile X syndrome, as well as women with a history of ovarian surgery and radio- or chemotherapy.
Clinical characterization
The clinical characterization of all volunteers was performed, including measurement of age, FSH, and antral follicle count (AFC). The serum concentration of FSH were evaluated using chemiluminescence assays (IMMULITE 2000 Immunoassay System, Siemens Healthcare Diagnostics, Los Angeles, CA, USA) and AFC by a Voluson S8 ultrasound (GE Healthcare Technologies Inc., USA).
Genomic DNA isolation
Genomic DNA was obtained from peripheral blood leukocytes using QIAamp DNA Micro kits (Qiagen, Germany), according to the manufacturer’s instructions. DNA concentration and integrity were determined using a spectrophotometer Nanodrop 2000c (ThermoFisher Scientific, MA, USA) and agarose gel 1.0% stained with GelRed™ (Uniscience Corp., USA), respectively. Samples with good quality and quantity were included in the CNV analysis.
Copy number variation analysis
The quantification of CNVs for the BCORL1, POF1B, and USP9X genes was performed using quantitative real time (qPCR). The variations in the copy number were normalized to single copy control genes MFN2 (autosomal, hChr 1p36.22) and HPRT1 (X-linked, hChr Xq26.2-q26.3). The quantification was carried out in triplicate using the detection system SYBR®Green Master Mix (Applied Biosystems, USA) in the instrument ViiA7 Real-Time PCR System (Applied Biosystems, Foster City, CA,). The qPCR reactions were analyzed in singleplex, in a final volume of 10 µl containing 2X SYBR®Green, 0.5 µM of each primer, and 50 ng of genomic DNA modified from Ottesen et al. [44]. The primer sequences are presented in Supplementary Table S1.
The quantification was analyzed using the comparative Cq (quantification cycle) method by calculating the variation in the copy number relative to the single copy gene ratio using 2−ΔΔCq [45]. The values from each target were normalized with the reference gene [ΔCq = Ct(target)/Ct(HPRT1 + MFN2/2)]. The ΔΔCq was calculated for each sample (x) relative to the reference normal female control (46,XX), to give an estimated copy number of each gene [2-(ΔCqx – ΔCqr) = 2−ΔΔCq]. A 46,XY male control was used to observe the relative differences between the quantification.
In silico analysis of protein–protein interaction
A protein–protein network of the USP9X was predicted using the STRING database (https://string-db.org, version 12.0). Network interactions were built with a score of 0.43 or more, selecting Homo sapiens as an organism and an initial input of one protein.
Statistical analysis
Quantitative variables are summarized as mean and standard deviation, and the difference in the CNVs between DOR and control groups was calculated using a t-test. Spearman’s correlation was used to correlate the quantitative variables. All results were analyzed using SAS® 9.0 software (SAS Institute Inc., North Carolina University, NC, USA), with a level of significance of 5% (p < 0.05).
Results
This case-control study included 26 women diagnosed with DOR and 21 in the control group. Age, height, weight, and BMI did not differ between DOR and control group (Table 2). FSH level was higher in DOR women (p = 0.0025). On the other hand, AFC (p < 0.001), oocytes retrieved after COS (p = 0.0004), and MII oocytes (p < 0.0001) were higher in the control group when compared to DOR women (Table 2).
Table 2.
Anthropometric, hormonal, and biological measures of DOR and control groups
| Variables | Control (n = 21) Mean ± SD |
DOR (n = 26) Mean ± SD |
p-value |
|---|---|---|---|
| Anthropometric measures | |||
| Age (years) | 35.38 ± 4.14 | 36.52 ± 4.75 | 0.395 |
| Height (m) | 1.64 ± 0.08 | 1.62 ± 0.05 | 0.3121 |
| Weight (Kg) | 68.18 ± 13.84 | 70.45 ± 13.29 | 0.5706 |
| BMI (Kg/m2) | 25.32 ± 4.41 | 26.74 ± 4.41 | 0.2760 |
| Hormonal parameters | |||
| FSH (IU/L) | 4.79 ± 1.94 | 7.43 ± 3.41 | 0.0025 |
| Patient history | |||
|
Previously pregnancies (Pregnancy/patients) |
13/12 | 10/9 | - |
|
Previously abortion (Abortion/patients) |
8/6 | 7/6 | - |
|
Previously delivery (Delivery/patients) |
5/5 | 3/3 | - |
|
Poor responder % (Patients/total) |
14.28% (3/21) | 80.76% (21/26) | |
| Current cycle | |||
| AFC (mean ± SD) | 15.57 ± 6.85 | 5.75 ± 2.62 | < .0001 |
| Oocyte retrieved (mean ± SD) | 7.74 ± 6.85 | 2.27 ± 2.27 | 0.0004 |
| MII oocytes (mean ± SD) | 6.18 ± 4.46 | 1.69 ± 1.89 | < .0001 |
DOR diminished ovarian reserve, AFC antral follicle count, MII oocyte metaphase II, FSH follicle stimulating hormone, BMI body mass index, SD standard deviation, in bold p < 0.005
Spearman’s coefficient showed a positive correlation between the variables age and FSH for both control (r2 = 0.48) and DOR (r2 = 0.38). A negative correlation was observed in the control group between age and AFC (r2 = − 0.38) and, in DOR women between FSH and AFC (r2 = − 0.53) (Fig. 3). The CNVs in the BCORL1 (p = 0.418) and POF1B (p = 0.965) genes (Fig. 2A and B) were similar between the DOR and control groups. However, DOR patients showed an increase in the number of copies of the USP9X gene (p = 0.028) when compared to the control group (Figs. 2C and 3).
Fig. 3.
Spearman’s correlation using the qualitative variables age, FSH, AFC, BCORL1, POF1B, and USP9X. Positive correlations are represented by values near to 1, whereas the negative correlations are represented by values near to − 1
Fig. 2.
Copy number variation (CNV) in the DOR and control women. A CNVs in the BCORL1 gene. B CNVs in the POF1B gene. C CNVs in the USP9X gene. *p < 0.05
The STRING protein–protein interaction (PPI) and functional network of the USP9X gene were built with a score of 0.43 or more. The strongest interaction was observed between USP9X and SMAD4 (score 0.971), followed by MCL1 (score 0.958), SNCA (score 0.941), and HUWE1 (score 0.908) (Fig. 4 and Table S2). However, this network had less interaction than expected, drawn from the human genome (PPI enrichment p-value, 0.128). The functional enrichment network presented the main biological process in which USP9X is involved (Table 3). According to Gene ontology (GO) [46, 47], the USP9X protein is involved in important biological processes, such as positive regulation of protein acetylation, catabolic process, and negative regulation of programmed cell death (Table 3).
Fig. 4.

USP9X protein interaction network. The thickest lines represent stronger interactions, and the thinnest lines represent lower interactions
Table 3.
The main functional enrichments in the USP9X protein network based on the lowest false discovery rate
| # Term ID | Term description | Count in gene set | False discovery rate |
|---|---|---|---|
| Biological process (GO) | |||
| GO:1901216 | Positive regulation of neuron death | 3 of 92 | 0.0405 |
| GO:1901983 | Regulation of protein acetylation | 3 of 94 | 0.0405 |
| GO:0042188 | Negative regulation of protein catabolic process | 3 of 112 | 0.0416 |
| GO:0009895 | Negative regulation of catabolic process | 4 of 329 | 0.0405 |
| GO:0043068 | Positive regulation of programmed cell death | 6 of 519 | 0.0022 |
| Cellular component (GO) | |||
| GO:0005829 | Cytosol | 10 of 5438 | 0.0436 |
| KEGG pathways | |||
| hsa04120 | Ubiquitin mediated proteolysis | 3 of 134 | 0.0175 |
*GO Gene ontology
Discussion
The X-linked genes are strongly associated with ovarian function and their misregulation has been linked to impaired ovarian reserve and premature failure. In our study, we found that the number of copies of the BCORL1 and POF1B genes was similar between the DOR and control groups; however, the USP9X showed an increased number of copies in women with DOR. The volunteers with DOR also presented higher FSH and lower AFC, oocyte retrieval, and MII oocyte rate when compared to women without DOR.
Women with DOR present regular menstrual cycles but a poor response when submitted to reproductive treatments, such as controlled ovarian stimulation (COS), when compared to healthy women of the same age [48]. The proper diagnosis of DOR prior to ART using molecular biomarkers may assist in the disease physiopathology knowledge, besides help to guide medical counseling, and consequently improve treatment outcomes in this population, which may represent nearly 30% of infertile women with familial history [23, 49]. The aging process affects ovarian resource through the increase of FSH levels during the early follicular phase, mainly due to a reduction in pituitary negative feedback [50, 51].
Genetic and epigenetic factors may be associated with pathologic DOR, especially in women with a familial history of premature menopause [15, 23]. Zhu et al. [15] showed the relationship between different genetic factors (e.g., mutations, variants, altered expression) and ovarian reserve and highlighted the relevance of genetic analysis in personalized medicine during the diagnosis of DOR. In our study, women with DOR had a higher number of copies of the USP9X gene compared to the control group. To our knowledge, this is the first study to describe a possible association of CNV in the USP9X gene in women with DOR. Moreover, there is no information in the literature on the possible consequences for USP9X copy number gain in ovary reserve.
Different from our study, Quilter and colleagues [28] observed a copy number loss in the USP9X gene in POI when compared to women without POI. The USP9X gene is in an important region for oogenesis that escapes the X chromosome inactivation (XCI). Mutations in the USP9X have been associated with the development of Turner Syndrome and neural disorders [28, 32, 52–54]. Moreover, deletion or loss of function of this gene increases chromosome segregation defects and chromosomal instability and may lead to primary amenorrhea in women [28, 55].
The STRING network showed that the USP9X protein has stronger correlations with SMAD4 (score 0.971) and MCL1 (score 0.958), both related to folliculogenesis and ovarian function. SMAD4 is a member of the SMAD family genes, and their proteins are intracellular mediators of the transforming growth factor β (TGF-β), essential for granulosa cell and oocyte communication [56]. On the other hand, the MCL1 is associated with follicle survival and ovarian reserve. Omari et al. [57] described a negative correlation between MCL1 gene and age; they demonstrated that MCL1 level in human germinal vesicles decreased with the advance of age.
In contrast to USP9X, the POF1B and BCORL1 showed no difference in the copy number between DOR and control women. Different from our data in DOR, the X-chromosome analysis in POI women showed CNV alterations in both genes, with an increased number of copies in the POF1B gene and a reduced number of copies in the BCORL1 [28]. POF1B is expressed in the ovary during embryonic development and escapes XCI [58, 59]. A point mutation in exon 10 of this gene may lead to loss of function, resulting in an abnormal apoptotic process during germ cell formation and a possible cause of POI [58]. BCORL1 is also related to apoptotic processes, and deletion in its region may lead to insufficient repression of apoptosis and the atresia of ovarian follicles [60].
Several studies have demonstrated the importance of X chromosome integrity and the correct dosage of X-linked genes for gene regulation, functionality, and its consequences for ovarian reserve and X-linked disorders [30, 36, 53, 61]. The association of CNVs in X-linked genes with DOR and other ovarian disorders could be helpful in understanding the genetic basis of these complex diseases, since genetic variants, such as age-, sex-, and ethnicity-related. The copy number gain of USP9X could be a characteristic of the Brazilian population, known for its high heterogeneity [62]. Brazil presents a large racial admixture, and the population covered by the service where we conducted this study is no different. Ferreira et al. [63] conducted a study in the same region of our study and showed the contribution of American Indian, African, and European populations.
There are some limitations that could be highlighted in our study. The average of FSH in women with DOR was not higher than 10 IU/L as observed for women with hypogonadism hypergonadotropic, although the AFC was compatible with DOR diagnosis. The reduced number of volunteers in both groups, the lack of sequencing of the USP9X, POF1B, and BCORL1 chromosomal regions, and the expression analysis of these genes to confirm the data also could be addressed as limitations. Nevertheless, the CNVs observed in women with idiopathic DOR in our study complement the previous findings of our group. Miranda–Furtado et al. [64] described an association between POI, XCI, and telomere length. In this study, women with idiopathic POI had a higher frequency of skewed XCI and shorter telomeres than women with regular menstrual cycles.
Numerical and structural chromosomal abnormalities, like loss or gain, in important genes related to ovarian function may be predictive of DOR. Our results reinforce the idea that CNVs are related to ovarian disorders and may be population specific. In conclusion, the increase in the number of copies of the USP9X gene may be related to overexpression in idiopathic DOR and may contribute to other X-linked alterations, such as skewed XCI, which often coincide with multiple epigenetic alterations.
Supplementary information
Below is the link to the electronic supplementary material.
Acknowledgements
The authors are extremely grateful to the subjects and their families for participating in this study. We also thank Cristiana C. Padovan Ribas for technical assistance in DNA extraction. We thank the members of the Human Reproduction Division at the Department of Gynecology and Obstetrics of the Ribeirao Preto Medical School, University of São Paulo, especially Océlia de Vasconcelos for blood collection, and Maria Albina Valladas Verceze and Tatiana Marina Vieira Giorgenon for measuring hormone concentrations; and Suleimy Mazin for performing the statistical analysis.
Author contribution
CLMF, FGOG, and RMR conceived the idea; FGOG and MRS recruited the patients and collected the samples; MRS, CGV, and LECMS performed the experimental analysis; CGV, CLMF, and RMR wrote the paper; all authors critically reviewed the manuscript and approved the final version.
Funding
This study was supported by Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES; Academic Excellence Program—PROEX and Graduate Support Program– PROAP), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Institutos Nacionais de Ciência e Tecnologia (INCT)-Saúde da Mulher, with the grant nº 465482/2014-7 and fellowship (CNPq-INCT: 176841/2022-9).
Data availability
No additional file is available for this study.
Declarations
Ethical approval
The study design was approved by the Research Ethics Committee of the University Hospital of the Ribeirao Preto Medical School, University of Sao Paulo (HC-FMRP-USP) under the protocol nº1.887.369 (CAAE: 54777816.4.0000.5440).
Consent to participate
All participants provided informed consent to participate in this study. These documents are with the corresponding author.
Consent for publication
All participants proved informed consent to publish these data.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Cristiana Libardi Miranda Furtado and Murilo Racy Soares have equal author’s contribution.
Contributor Information
Cristiana Libardi Miranda Furtado, Email: clibardim@gmail.com.
Rosana Maria dos Reis, Email: romareis@fmrp.usp.br.
References
- 1.Laopaiboon M, Lumbiganon P, Intarut N, Mori R, Ganchimeg T, Vogel JP, et al. Advanced maternal age and pregnancy outcomes: a multicountry assessment. BJOG. 2014;121(Suppl):49–56. 10.1111/1471-0528.12659 [DOI] [PubMed] [Google Scholar]
- 2.Cohen J, Chabbert-Buffet N, Darai E. Diminished ovarian reserve, premature ovarian failure, poor ovarian responder–a plea for universal definitions. J Assist Reprod Genet. 2015;32:1709–12. 10.1007/s10815-015-0595-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fritz R, Jindal S. Reproductive aging and elective fertility preservation. J Ovariam Res. 2018;11:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Park SU, Walsh L, Berkowitz KM. Mechanisms of ovarian aging. Reproduction. 2021;162:R19-33. 10.1530/REP-21-0022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pastore LM, Christianson MS, Stelling J, Kearns WG, Segars JH. Reproductive ovarian testing and the alphabet soup of diagnoses: DOR, POI, POF, POR, and FOR. J Assist Reprod Genet. 2018;35:17–23. 10.1007/s10815-017-1058-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Committee Opinion No. 589. Female age-related fertility decline. Fertil Steril. 2014;101:633–4. 10.1016/j.fertnstert.2013.12.032 [DOI] [PubMed] [Google Scholar]
- 7.Cakiroglu Y, Yuceturk A, Karaosmanoglu O, Kopuk S, Korun Z, Herlihy N, et al. Ovarian reserve parameters and IVF outcomes in 510 women with poor ovarian response (POR) treated with intraovarian injection of autologous platelet rich. Aging. 2022;14:2513–23 (Albany NY). 10.18632/aging.203972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Devine K, Mumford SL, Wu M, DeCherney AH, Hill MJ, Propst A. Diminished ovarian reserve in the United States assisted reproductive technology population: diagnostic trends among 181,536 cycles from the Society for Assisted Reproductive Technology Clinic Outcomes Reporting System. Fertil Steril [Internet]. 2015;104:612–619.e3. Available from: 10.1016/j.fertnstert.2015.05.017. (Elsevier Inc.). [DOI] [PMC free article] [PubMed]
- 9.ESHRE. Management of women with premature ovarian insufficiency. Hum Reprod. 2016;31:926–37. 10.1093/humrep/dew027 [DOI] [PubMed] [Google Scholar]
- 10.Hu S, Xu B, Jin L. Perinatal outcome in young patients with diminished ovarian reserve undergoing assisted reproductive technology. Fertil Steril. 2020;114:118-124.e1. 10.1016/j.fertnstert.2020.02.112 [DOI] [PubMed] [Google Scholar]
- 11.Levi AJ, Raynault MF, Bergh PA, Drews MR, Miller BT, Scott RT. Reproductive outcome in patients with diminished ovarian reserve. Fertil Steril. 2001;76:666–9 (Elsevier). 10.1016/S0015-0282(01)02017-9 [DOI] [PubMed] [Google Scholar]
- 12.The Practice Committee of the American Society for Reproductive Medicine. Aging and infertility in women. Fertil Steril. 2006;86:S248-52. 10.1016/j.fertnstert.2006.08.024 [DOI] [PubMed] [Google Scholar]
- 13.Zhu S, Jiang W, Liao X, Sun Y, Chen X, Zheng B. Effect of diminished ovarian reserve on the outcome of fresh embryo transfer in IVF/ICSI cycles among young women: a retrospective cohort study. BMC Women’s Health. 2024;24:1–10. 10.1186/s12905-024-03039-6. 10.1186/s12905-024-03039-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jaswa EG, McCulloch CE, Simbulan R, Ceders MI, Rosen MP. Diminished ovarian reserve is associated with reduced euploid rates via preimplantation genetic testing for aneuploidy independently from age: evidence for concomitant reduction in oocyte quality with quantity. Fertil Steril. 2021;115:966–73. 10.1016/j.fertnstert.2020.10.051 [DOI] [PubMed] [Google Scholar]
- 15.Zhu Q, Ma H, Wang J, Liang X. Understanding the mechanisms of diminished ovarian reserve: insights from genetic variants and regulatory factors. Reprod Sci [Internet]. Springer International Publishing; 2024. Available from: 10.1007/s43032-024-01467-1. [DOI] [PubMed]
- 16.Fusco F, Paciolla M, Chen E, Li X, Genesio R, Conti A, et al. Genetic and molecular analysis of a new unbalanced X;18 rearrangement: localization of the diminished ovarian reserve disease locus in the distal Xq POF1 region. Hum Reprod. 2011;26:3186–96. 10.1093/humrep/der266 [DOI] [PubMed] [Google Scholar]
- 17.Kaur M, Arora M. Diminished ovarian reserve, causes, assessment and management. Int J Infertil Fetal Med. 2013;4:45–55. 10.5005/jp-journals-10016-1060 [DOI] [Google Scholar]
- 18.Nesbit CB, Huang J, Singh B, Maher JY, Pastore LM, Segars J. New perspectives on the genetic causes of diminished ovarian reserve and opportunities for genetic screening: systematic review and meta-analysis. F&S Rev. 2020;1:1–15 (Elsevier). 10.1016/j.xfnr.2020.06.001 [DOI] [Google Scholar]
- 19.Narkwichean A, Maalouf W, Campbell B, Jayaprakasan K. Efficacy of dehydroepiandrosterone to improve ovarian response in women with diminished ovarian reserve: a meta-analysis. Reprod Biol Endocrinol. 2013;11:1–8. 10.1186/1477-7827-11-44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nikolaou D, Gilling-Smith C, Nikolaou D, Gilling-Smith C. Early ovarian ageing: are women with polycystic ovaries protected? Hum Reprod. 2004;19:217502179. 10.1093/humrep/deh419 [DOI] [PubMed] [Google Scholar]
- 21.ESHRE. Management of women with premature ovarian insufficiency. Guideline of the European Society of Human Reproduction and Embryology. EshreEu [Internet]. 2015. Available from: https://www.eshre.eu/Guidelines-and-Legal/Guidelines/Management-of-premature-ovarian-insufficiency.aspx. Accessed 15 Dec 2023.
- 22.de Boer E, den Tonkelaar I, te Velde E, Burger C, Klip H, van Leeuwen F, et al. A low number of retrieved oocytes at in vitro fertilization treatment is predictive of early menopause. Fertil Steril. 2022;77:978–85. 10.1016/S0015-0282(02)02972-2 [DOI] [PubMed] [Google Scholar]
- 23.Greene AD, Patounakis G, Segars JH. Genetic associations with diminished ovarian reserve: a systematic review of the literature. J Assist Reprod Genet. 2014;31:935–46. 10.1007/s10815-014-0257-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jaillard S, Akloul L, Beaumont M, Hamdi-Roze H, Dubourg C, Odent S, et al. Array-CGH diagnosis in ovarian failure: identification of new molecular actors for ovarian physiology. J Ovarian Res [Internet]. 2016;9:1–7. Available from: 10.1186/s13048-016-0272-5 [DOI] [PMC free article] [PubMed]
- 25.de Godoy VCSM, Bellucco FT, Colovati M, de Oliveira-Junior HR, Moysés-Oliveira M, Melaragno MI. Copy number variation (CNV) identification, interpretation, and database from Brazilian patients. Genet Mol Biol. 2020;43:1–7. 10.1590/1678-4685-gmb-2019-0218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang L, Shi J, Ouyang J, Zhang R, Tao Y, Yuan D, et al. X-CNV: genome-wide prediction of the pathogenicity of copy number variations. Genome Med. 2021;13:1–15. 10.1186/s13073-021-00945-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bione S, Rizzolio F, Sala C, Ricotti R, Goegan M, Manzini M, et al. Mutation analysis of two candidate genes for premature ovarian failure, DACH2 and POF1B. Hum Reprod. 2004;19:2759–66. 10.1093/humrep/deh502 [DOI] [PubMed] [Google Scholar]
- 28.Quilter CR, Karcanias AC, Bagga MR, Duncan S, Murray A, Conway GS, et al. Analysis of X chromosome genomic DNA sequence copy number variation associated with premature ovarian failure (POF). Hum Reprod. 2010;25:2139–50. 10.1093/humrep/deq158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Knauff E, Blauw H, Pearson P, Kok K, Wijmenga C, Veldink J, et al. Copy number variants on the X chromosome in women with primary ovarian insufficiency. Fertil Steril. 2011;95:1584–8. 10.1016/j.fertnstert.2011.01.018 [DOI] [PubMed] [Google Scholar]
- 30.Yatsenko SA, Rajkovic A. Genetics of human female infertility. Biol Reprod. 2019;101:549–66. 10.1093/biolre/ioz084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sakka R, Abdelhedi F, Sellami H, Pichon B, Lajmi Y, Mnif M, et al. An unusual familial Xp22.12 microduplication including EIF1AX: a novel candidate dosage-sensitive gene for premature ovarian insufficiency. Eur J Med Genet. 2022;65:1–9. [DOI] [PubMed]
- 32.Simpson JL. Genetic and phenotypic heterogeneity in ovarian failure: overview of selected candidate genes. Ann N Y Acad Sci. 2008;1135:146–54. 10.1196/annals.1429.019 [DOI] [PubMed] [Google Scholar]
- 33.Fortuño C, Labarta E. Genetics of primary ovarian insufficiency: a review. J Assist Reprod Genet. 2014;31:1573–85. 10.1007/s10815-014-0342-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ulrich N, Marsh EE. Ovarian reserve testing: a review of the options, their applications, and their limitations. Clin Obs Gynecol. 2019;62:228–37. 10.1097/GRF.0000000000000445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bestetti I, Castronovo C, Sironi A, Caslini C, Sala C, Rossetti R, et al. High-resolution array-CGH analysis on 46, XX patients affected by early onset primary ovarian insufficiency discloses new genes involved in ovarian function. Hum Reprod. 2019;34:574–83. 10.1093/humrep/dey389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yatsenko SA, Wood-Trageser M, Chu T, Jiang H, Rajkovic A. A high-resolution X chromosome copy-number variation map in fertile females and women with primary ovarian insufficiency. Genet Med. 2019;21:2275–84. 10.1038/s41436-019-0505-2 [DOI] [PubMed] [Google Scholar]
- 37.Katari S, Aarabi M, Kintigh A, Mann S, Yatsenko SA, Sanfilippo JS, et al. Chromosomal instability in women with primary ovarian insufficiency. Hum Reprod. 2018;33:531–8. 10.1093/humrep/dey012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Norling A, Hirschberg A, Rodriguez-Wallberg K, Iwarsson E, Wedell A, Barbaro M. Identification of a duplication within the GDF9 gene and novel candidate genes for primary ovarian insufficiency (POI) by a customized high-resolution array comparative genomic hybridization platform. Hum Reprod. 2014;29:1818–27. 10.1093/humrep/deu149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McGuire M, Bowden W, Engel N, Ahn H, Kovanci E, Rajkovic A. Genomic analysis using high-resolution single-nucleotide polymorphism arrays reveals novel microdeletions associated with premature ovarian failure. Fertil Steril. 2011;95:1595–600. 10.1016/j.fertnstert.2010.12.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dudding TE, Lawrence O, Winship I, Froyen G, Vandewalle J, Scott R, et al. Array comparative genomic hybridization for the detection of submicroscopic copy number variations of the X chromosome in women with premature ovarian failure. Hum Reprod. 2010;25:3159–60. 10.1093/humrep/deq284 [DOI] [PubMed] [Google Scholar]
- 41.Ledig S, Röpke A, Wieacker P. Copy number variants in premature ovarian failure and ovarian dysgenesis. Sex Dev. 2010;4:225–32. 10.1159/000314958 [DOI] [PubMed] [Google Scholar]
- 42.Aboura A, Dupas C, Tachdjian G, Portnoï M, Bourcigaux N, Dewailly D, et al. Array comparative genomic hybridization profiling analysis reveals deoxyribonucleic acid copy number variations associated with premature ovarian failure. J Clin Endocrinol Metab. 2009;94:4540–6. 10.1210/jc.2009-0186 [DOI] [PubMed] [Google Scholar]
- 43.Poseidon G. A new more detailed stratification of low responders to ovarian stimulation: from a poor ovarian response to a low prognosis concept. Fertil Steril. 2016;105:1452–3. 10.1016/j.fertnstert.2016.02.005 [DOI] [PubMed] [Google Scholar]
- 44.Ottesen A, Garn I, Aksglaede L, Juul A, Rajpert-De Meyts E. A simple screening method for detection of Klinefelter syndrome and other X-chromosome aneuploidies based on copy number of the androgen receptor gene. Mol Hum Reprod. 2007;13(10):745–50. 10.1093/molehr/gam053. 10.1093/molehr/gam053 [DOI] [PubMed] [Google Scholar]
- 45.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCT method. Methods. 2001;25:402–8. 10.1006/meth.2001.1262 [DOI] [PubMed] [Google Scholar]
- 46.Ashburner M, Ball C, Blake J, et al. Gene ontology: tool for the unification of biology. Nat Genet. 2000;25:25–9. 10.1038/75556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.The Gene Ontology Consortium. The Gene ontology knowledgebase in 2023. Genetics. 2023;224:1–14. 10.1093/genetics/iyad031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rasool S, Shah D. Fertility with early reduction of ovarian reserve: the last straw that breaks the Camel’s back. Fertil Res Pract. 2017;3:1–12. 10.1186/s40738-017-0041-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Moiseeva AV, Kudryavtseva VA, Nikolenko VN, Gevorgyan MM, Unanyan AL, Bakhmet AA, et al. Genetic determination of the ovarian reserve: a literature review. J Ovarian Res [Internet]. 2021;14:1–9. Available from: 10.1186/s13048-021-00850-9. (BioMed Central). [DOI] [PMC free article] [PubMed]
- 50.Broekmans FJ, Scheffer GJ, Bancsi LFJMM, Dorland M, Blankenstein MA, Te Velde ER. Ovarian reserve tests in infertility practice and normal fertile women. Maturitas. 1998;30:205–14. [DOI] [PubMed]
- 51.Orlowski M, Sarao M. Physiology, follicle stimulating hormone. In: Treasure Island F, editor. StatPearls [Internet]. 2023. Available from: https://www.ncbi.nlm.nih.gov/books/NBK535442/. Accessed 10 Mar 2024. [PubMed]
- 52.Trolle C, Nielsen MM, Skakkebæk A, Lamy P, Vang S, Hedegaard J, et al. Widespread DNA hypomethylation and differential gene expression in Turner syndrome. Sci Rep. 2016;6:1–14 (Nature Publishing Group). 10.1038/srep34220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jolly LA, Parnell E, Gardner AE, Corbett MA, Pérez-Jurado LA, Shaw M, et al. Missense variant contribution to USP9X-female syndrome. npj Genomic Med. 2020;5:1–11. 10.1038/s41525-020-00162-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Viuff M, Gravholt CH. Turner syndrome and fertility. Ann Endocrinol (Paris) [Internet]. 2022;83:244–9. Available from: 10.1016/j.ando.2022.06.001. (The Authors). [DOI] [PubMed]
- 55.Skowyra A, Allan LA, Saurin AT, Clarke PR. USP9X limits mitotic checkpoint complex turnover to strengthen the spindle assembly checkpoint and guard against chromosomal instability. Cell. 2018;23:852–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kaune H, Peyrache E, Williams SA. Oocyte-derived Smad4 is not required for development of the oocyte or the preimplantation embryo. Theriogenology. 2015;83:897–903. 10.1016/j.theriogenology.2014.11.024 [DOI] [PubMed] [Google Scholar]
- 57.Omari S, Waters M, Naranian T, Kim K, Perumalsamy AL, Chi M, et al. MCL-1 is a key regulator of the ovarian reserve. Cell Death Dis. 2015;6:1–12 (Nature Publishing Group). 10.1038/cddis.2015.95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lacombe A, Lee H, Zahed L, Choucair M, Muller JM, Nelson SF, et al. Disruption of POF1B binding to nonmuscle actin filaments is associated with premature ovarian failure. Am J Hum Genet. 2006;79:113–9. 10.1086/505406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pelosi E, Forabosco A, Schlessinger D. Genetics of the ovarian reserve. Front Genet. 2015;6:1–20. 10.3389/fgene.2015.00308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Beke A, Piko H, Haltrich I, Csomor J, Matolcsy A, Fekete G, et al. Molecular cytogenetic analysis of Xq critical regions in premature ovarian failure. Mol Cytogenet. 2013;6:1–8. 10.1186/1755-8166-6-62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bauters M, Weuts A, Vandewalle J, Nevelsteen J, Marynen P, Van Esch H, et al. Detection and validation of copy number variation in X-linked mental retardation. Cytogenet Genome Res. 2008;123:44–53. 10.1159/000184691 [DOI] [PubMed] [Google Scholar]
- 62.Parra F, Amado R, Lambertucci J, Rocha J, Antunes C, Pena SD. Color and genomic ancestry in Brazilians. Proc Natl Acad Sci U S A. 2003;7:177–82. 10.1073/pnas.0126614100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ferreira L, Mendes-Junior C, Wiezel C, Luizon M, Simões AL. Genomic ancestry of a sample population from the state of São Paulo. Brazil Am J Hum Biol. 2006;18:702–5. 10.1002/ajhb.20474 [DOI] [PubMed] [Google Scholar]
- 64.Miranda-Furtado CL, Luchiari HR, ChielliPedroso DC, Kogure GS, Caetano LC, Santana BA, et al. Skewed X-chromosome inactivation and shorter telomeres associate with idiopathic premature ovarian insufficiency. Fertil Steril. 2018;110:476-485.e1. 10.1016/j.fertnstert.2018.04.017 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No additional file is available for this study.