Abstract
Peripheral nerve injury (PNI) represents a serious clinical and public health problem due to its high incurrence and poor spontaneous recovery. Compared to autograft, which is still the best current practice for long-gap peripheral nerve defects in clinics, the use of polymer-based biodegradable nerve guidance conduits (NGCs) has been gaining momentum as an alternative to guide the repair of severe PNI without the need of secondary surgery and donor nerve tissue. However, simple hollow cylindrical tubes can barely outperform autograft in terms of the regenerative efficiency especially in critical sized PNI. With the rapid development of tissue engineering technology and materials science, various functionalized NGCs have emerged to enhance nerve regeneration over the past decades. From the aspect of scaffold design considerations, with a specific focus on biodegradable polymers, this review aims to summarize the recent advances in NGCs by addressing the onerous demands of biomaterial selections, structural designs, and manufacturing techniques that contributes to the biocompatibility, degradation rate, mechanical properties, drug encapsulation and release efficiency, immunomodulation, angiogenesis, and the overall nerve regeneration potential of NGCs. In addition, several commercially available NGCs along with their regulation pathways and clinical applications are compared and discussed. Lastly, we discuss the current challenges and future directions attempting to provide inspiration for the future design of ideal NGCs that can completely cure long-gap peripheral nerve defects.
Keywords: peripheral nerve regeneration, nerve guidance conduits, tissue engineering, scaffold design
1. Introduction
The peripheral nerve system consists of numerous nerve branches outside of the central nerve system (CNS; brain and spinal cord (SpC)) and builds up the entire body to transmit signals from and to the CNS. Trauma, tumor, and invasive surgical procedures can all cause peripheral nerve injury (PNI), where neuronal loss and axonal degradation ultimately result in the formation of gaps between two ends of peripheral nerves and bring burden to patients such as pain, weakness of sensation, uncontrollable muscle stretching and movement, and paralysis [1]. Annually there are over 360 000 people suffering from PNI in the U.S. and millions of cases globally [2]. Peripheral nerve is one of the tissues that are capable of self-repairing after injury unlike CNS or avascular cartilage [3–5]. The two commonly used classification systems of PNI are the Seddon classification and the Sunderland classification. From mild to severe, PNI is classified into neurapraxia, axonotmesis, and neurotmesis according to the Seddon classification [6], or first degree to fifth degree in terms of the Sunderland classification [7, 8]. Neurapraxia (first degree) refers to a major conducting blockage with some degree of myelin injury or ischemia but no axon loss and it can be excellently recovered in weeks to months [9, 10]. Axonotmesis (second, third, and fourth degree) involves the loss of axonal continuity, but the surrounding connective tissues such as the endoneurium, perineurium, and epineurium remain fully intact or only partially disrupted [9, 11]. This type of PNI has poor self-recovery capacity and surgery is generally required. Neurotmesis (fifth degree) stands for the most severe PNI where the entire nerve trunk including both the nerve fibers and the surrounding connective tissues is disrupted, and spontaneous recovery is nearly impossible [11].
After severe PNI, the nerve fibers at the distal end of injured site undergo Wallerian degeneration, which is a process predominated by actions of macrophages that infiltrate through the leaky blood-nerve barrier [12, 13]. As illustrated in figure 1, the degenerated fibers and axon fragments are rapidly cleared from the broken-down distal myeline sheath. Meanwhile, Schwann cells (SCs) proliferate, dedifferentiate, and align with the external basal lamina to form a highly oriented structure called the ‘bands of Büngner’, which guide the axonal sprouts regenerate parallelly along the tubular structure of nerve fibers from the proximal end to the distal target end [14, 15]. The regenerated axonal sprouts undergo myelination and eventually recover target functions. However, if disorganized axonal sprouts are formed or the oriented axonal sprouts cannot cross the whole defect area, the target functions fail to recover if no further interventions are applied.
Figure 1.
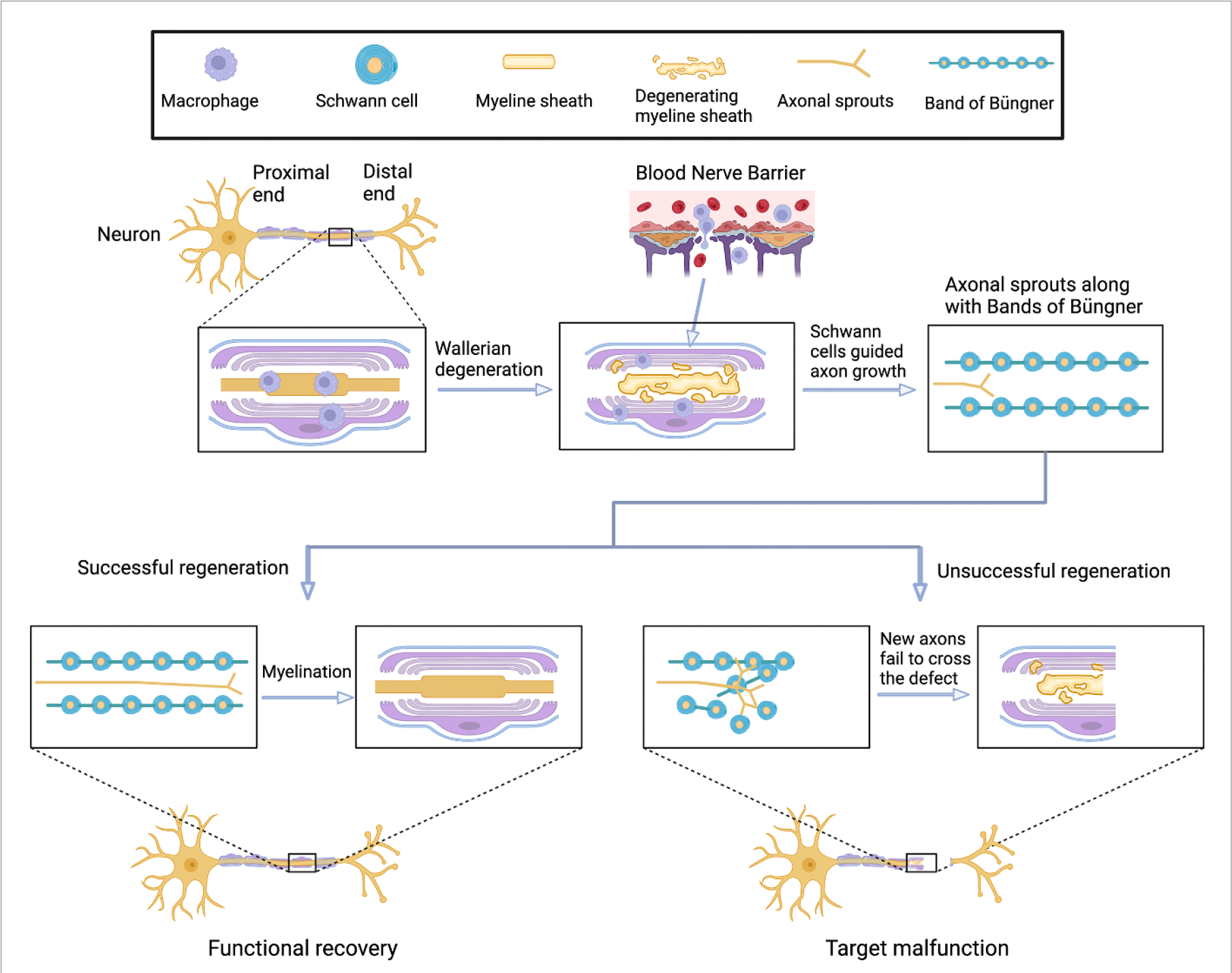
Illustration of peripheral nerve degeneration and regeneration after injury. Peripheral nerves possess self-repairing ability due to actions of immune cells and SCs. Axons regrow in the defect area with the guide of ‘bands of Büngner’, which was formed by highly ordered SCs. In the scenario while the ‘bands of Büngner’ failed to form, new axons cannot cross the whole defect area and functional recovery is unsuccessful. NGCs are therefore required to better guide the regrowth of axons from the proximal end to the distal end of the never tissue. Created with BioRender.com.
Therefore, in clinics, the length of nerve defect is a key indicator for selection of treatment strategies. For short nerve gaps (<5 mm), current practice involves tension-free suturing of the proximal and distal stumps in the injury site, so called neurorrhaphy [16]. The ‘gold standard’ for larger PNI is still autograft, which is associated with a series of drawbacks such as the need for multiple surgeries, limited donor availability, dimension/structure/property mismatch between the donor and defect areas, loss of function at the donor site, and potential for neuroma [17, 18]. Polymer-based nerve guidance conduits (NGCs) provide a promising alternative for repairing large sized nerve gaps by bridging the proximal and distal ends of nerve defects in a manner that not only guides the aligned growth of axonal sprouts but also prevents the ingrowth of undesired cells such as fibroblasts. Nevertheless, simple hollow cylindrical conduit often failed to completely regenerate long-gap PNI due to lack of systematic physicochemical, biological, and topological cues [19].
With the rapid progress of tissue engineering techniques, advanced NGCs such as those manufactured with novel biomaterials, designed in biomimetic complexes, equipped with photothermal/electrical/magnetic stimulating abilities, nanofunctionalized, and bio-functionalized have been developed to enhance the cure of large peripheral nerve defects. In this review, we aim to summarize these recent advancements of NGCs along with their commercialization and clinical applications from the aspects of selections of biomaterials, structural designs, and manufacturing routes.
2. Scaffold requirements
Tissue regeneration is associated with four continuous and overlapping stages including hemostasis, inflammation, repair, and remodeling [20, 21]. For peripheral nerve regeneration (PNR) guided by tissue engineering scaffolds, NGCs interact closely with various types of cells including SCs, fibroblasts, and immune cells (mainly mast cells and macrophages) to regulate all these processes and ultimately enhance nerve regeneration. Hence, functional NGCs should satisfy certain minimal requirements such as good biocompatibility, suitable biodegradability and mechanical properties, and other functionalities to provide sufficient regenerative potential and avoid treatment failure.
2.1. Anatomy of peripheral nervous system
Understanding the anatomical structure of peripheral nervous system is considered a prerequisite to design functional NGCs for enhanced nerve regeneration. Figure 2 illustrates the cross-sectional anatomy of a peripheral nerve. The entire nerve trunk is separated into multilayered microstructure by three types of connective tissues including endoneurium, perineurium, and epineurium, which are all mainly composed of collagen fibers [22]. In a nerve trunk, the myelinated or unmyelinated Schwann cell-axon unit is surrounded by the endoneurium layer, where the electrical isolation of every individual axon by the endoneurium maximizes the accuracy of signal transmissions between CNS and peripheral target tissues [23]. A number of these endoneurium covered axon units are then grouped together to form separate bundles (called fascicles) and are covered by the perineurium, which are condensed into a perineurial sheath to majorly resist external forces [1]. Individual fascicles are connected continuously by internal epineurium while the external epineurium stands for the outmost protective and connective layer that encircles all fascicles, and internal adipose tissues and blood vessels [24, 25].
Figure 2.
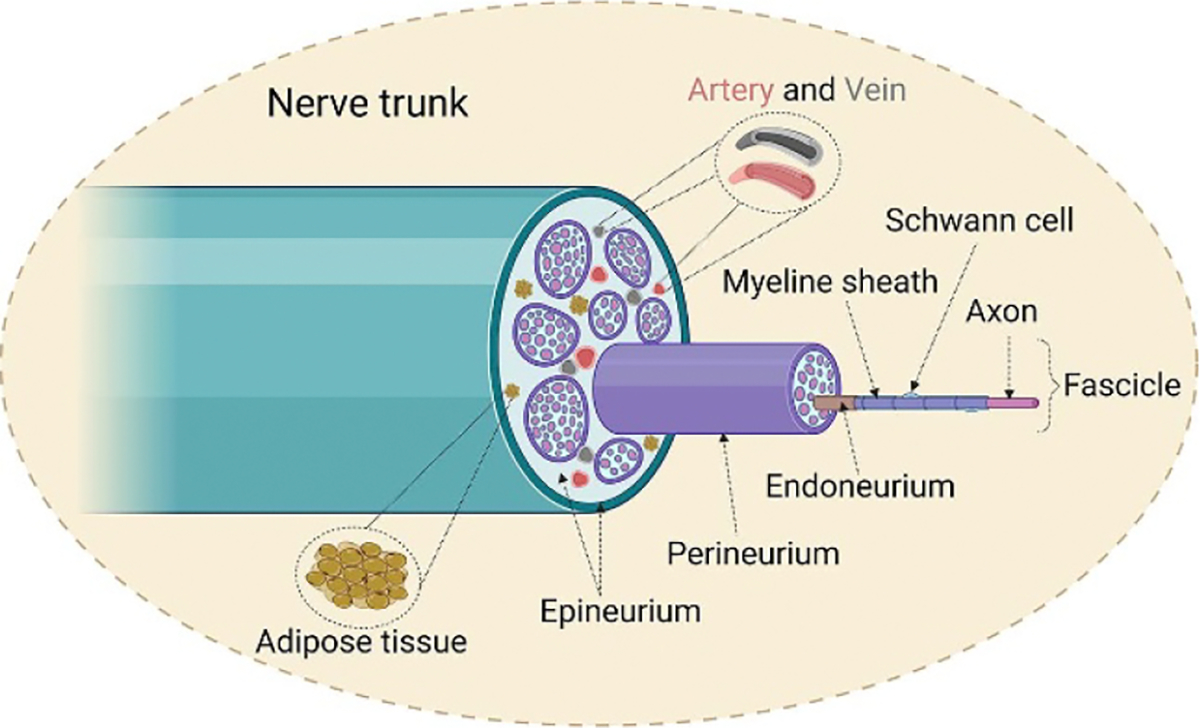
Illustration of cross-sectional anatomy of a peripheral nerve trunk. The entire nerve trunk mainly consists of three different layers of connective tissues and signal-transmission fascicles, which were formed by bundles of myelinated or unmyelinated axons. Created with BioRender.com.
2.2. Scaffold requirements
2.2.1. Biocompatibility
One of the minimal requirements of NGCs is their good biocompatibility, where obvious toxicity should be avoided. According to international standard ISO 10993–5, three categories of test can be performed to evaluate the in vitro toxicity of biomedical devices including NGCs: extract test, direct contact test, and indirect contact test. Extractions of NGCs can be obtained by soaking the NGCs or thin films of the corresponding biomaterials in extraction solutions such as PBS, physiological saline solution, or culture medium [26, 27]. The the ratio of the standard surface area or mass of the samples to the volume of the extraction solutions can be found in ISO 10993–12 depending upon the shape, thickness, and porosity of the tested devices. For instance, for irregularly shaped porous devices, like NGCs, this ratio is 0.1 g ml−1 (for every 0.1 g NGCs, 1 ml of extraction solution is required to soak the NGCs). It is noted that if the cell viability of the material extract is lower than 70% compared to the blank group, which is the group of liquid extraction solution containing no tested material, the material is considered toxic, and the half-maximal inhibitory concentration (IC50), the concentration of the extractions reflecting inhibition of cell viability by half, must be established. In addition, ideal NGCs should support cell attachment, proliferation, and migration to eventually guide the oriented growth of axonal sprouts along with the ‘bands of Büngner’ generated within the NGCs. These are generally conducted by direct contact test, which requires seeding cells directly on the NGCs or material equivalent of NGCs such as flat porous films or scaffolds made of the same materials used to engineer NGCs. Lastly, NGCs should not elicit adverse immunological responses such as local allergic reactions [28]. We propose several strategies to mitigate this issue: (1) using biocompatible materials that would generally not cause severe allergic responses; (2) adapting surface modification techniques to improve the biocompatibility of NGCs; (3) administering anti-inflammatory drugs post-implantation to suppress immune response; (4) engineering NGCs with biodegradable materials to prevent host tissues from long-term exposure of foreign implants.
2.2.2. Biodegradability
The first generation of artificial peripheral NGCs were made of non-degradable silicone [29]. Unfortunately, peripheral nerve compression syndrome is often observed in clinics after applying this material, resulting from the consistent pressure on nerves from such a non-resorbable NGC with the regeneration of nerve tissue [30]. The use of biodegradable NGCs is thus preferred to not only avoid compression syndrome but also eliminate the necessity of secondary surgery for removal of the implant after recovery. For biodegradable NGCs, the rate of degradation is one of the essential features determining treatment effectiveness. Generally, the degradation profile of NGCs should accommodate the rate of nerve regeneration: the NGC should be fully or largely resorbed upon the complete regeneration of nerve tissue [31, 32]. Moreover, since the absorption of degradation fluid is often associated with the swelling of surrounding tissues, burst degradation should be avoided to abolish the potential of local inflammation caused by degradation-induced tissue swelling [33]. However, if the degradation rate of NGCs is too slow in comparison to the nerve regeneration rate, it may still result in compression syndrome.
The rate of axonal elongation proceeds approximately 1 mm per day across different vertebrate species, but the delay before regenerated axons advance and their critical sizes do differ [34, 35], resulting in different axonal regeneration and function recovery time across species. For example, for a 10 mm sciatic nerve injury in rat (critical size = ~ 15 mm), axonal elongation (axonal phase) starts around the third week after injury (after the fluid, matrix, and cellular phases) and the new axons can across the whole gap in 4 weeks within a silicone tube [36]. While the complete motor and sensory functional recovery in hand of humans (critical size = ~ 40 mm) after brachial plexus injuries could take 9–12 months or as much as 800 d, with the maximal regeneration rate being ~1 mm per day [37, 38]. In addition, motor nerves generally exhibited poor recovery than sensory nerves and motor recovery takes longer than sensory recovery [37]. For example, studies have shown that patients with cubital tunnel syndrome (a disease with both sensory and motor nerve malfunctions on the elbow) who received surgical intervention within 10 months after showing symptoms had both good sensory and motor functional recovery, while recovery of motor function was incomplete in those who operated 10 or more months after the onset of symptoms [39]. The presence of scar tissue also hampers and delays the regeneration of nerves [40]. Overall, the ideal degradation rate of NGCs would need to be customized depending upon its application in a certain scenario. And thus, those with tunable biodegradation rate would be of advantage for a wider application of PNR.
2.2.3. Mechanical properties
Overall, NGCs should possess certain strength and stiffness to withstand pressures from surrounding tissues and also have flexibility to maintain continuity during daily activities, which may cause collapse or kinking of NGCs. The contrast of mechanical properties, especially elastic modulus, between the nerve implant and the surrounding nerve tissues at the defect area should be minimized to avoid tension, which will ultimately cause failure of regeneration and catastrophically hamper functional recovery [41]. Mismatched moduli between biomaterials and host tissue will also cause chronic inflammation [42]. However, the mechanical properties of peripheral nerves change with the variations of species, surrounding microenvironment, locations, cellular constituents, and age [43]. For example, the ultimate tensile strengths and Young’s moduli of acellularized and fresh native rat sciatic nerve range from 1 MPa–6 MPa and 0.6 MPa–14 MPa, respectively [44, 45]. The ultimate tensile strength, Young’s modulus, and strain at break of porcine tibial nerves are 0.87 ± 0.29 MPa, 7.43 ± 1.69 MPa, and 16% [46]. In contrast, the ultimate tensile strength and Young’s modulus of human tibial nerve is 3.91 ± 0.92 MPa, and 9.5 ± 2.84 MPa [47]. Moreover, the elastic moduli of sciatic nerves in living mice were found to be significantly higher in young mice (~391 Pa) compared to that of juvenile (~131 Pa) and adult mice (~227 Pa) [43]. In another case, the Young’s moduli of human digital collateral nerves were found lower in thumb (~39.71 MPa) than in other fingers (58.15–73.04 MPa) [48]. Therefore, it is important to develop biomaterials with a wide-range, fine-tunable mechanical properties for repairing different peripheral nerves in different patients.
More importantly, mechanical properties have been increasingly recognized as major parameters regulating cellular responses as they alter the crosstalk between cells and biomaterials [49, 50]. Gu et al [51] reported that the substrate stiffness (elastic modulus) of polyacrylamide gels is a key parameter influencing cell adhesion, viability, proliferation, migration, and neurotrophic actions of SCs, where gels with a moderate modulus (7.45 kPa) showed optimal performance. Neurite extension of PC12 cells was found to increase with the increasing of elasticity (decreasing of stiffness) of polyethylene glycol (PEG) hydrogels [52]. Neuronal differentiation was enhanced of neural stem cells cultured on methacrylamide chitosan (MAC) with an elastic modulus less than 1 kPa whereas oligodendryocyte differentiation was upregulated of those cultured on stiffer MAC hydrogels (>7 kPa) [53]. However, unlike hydrogels, Wang et al [42] synthesized biodegradable waterborne polyurethane (BWPU) NGCs with different mechanical properties and found that BWPU with higher elastic modulus (3.890 ± 0.052 MPa in dry state and 0.478 ± 0.030 MPa in hydrated state) exhibited outstanding in vivo sciatic nerve regeneration capability compared to the group with lower modulus (1.384 ± 0.012 MPa in dry state and 0.224 ± 0.004 MPa in hydrated state). This is probably because the mechanisms of cellular response against different levels of mechanical stimulation (e.g. kPa and MPa) are different.
2.2.4. Bio-functionalities
Ideal NGCs should not only provide physical guidance but also possess bio-functionality to promote nerve regeneration. A common strategy for improving the PNR potential of NGCs involves providing biological cues, such as loading growth factors like nerve growth factor (NGF) and brain-derived neurotropic factor (BDNF) into NGCs [54–56]. However, the half-life and stability of these biological molecules are typically low, both during the loading process and upon release in tissue environment. This characteristic makes it challenging for them to sustainably contribute to the regeneration of nerve tissue over an extended period. In recent years, epigenetic regulation of nerve regeneration has become an popular topic, providing new therapeutic opportunities to improve neural repair by orchestrating the transcription processes of nerve regeneration-associated genes (RAG) [57, 58]. This process involves the acetylation and methylation of histone proteins, as well as the methylation of DNA and microRNAs, which ultimately influence the transcription of nerve RAG without modifying the genes themselves [57]. For example, folic acid has been reported to regulate axonal regeneration of rodent central nervous system through DNA methylation [59]. Our group previously confirmed that biodegradable citrate-based NGCs loaded with folic acid could promote PNR partially by enhancing the global DNA methylation of SCs [60]. Recent findings indicated that such a beneficial trait of enhanced axonal regeneration and accompanying molecular alterations of DNA methylation triggered by folic acid in F0 generation could be inherited transgenerationally even beyond the F3 generation [61], demonstrating the effectiveness of epigenetic regulation in regenerating nerve tissues triggered by biomolecules like folic acid. However, following nerve injury, there are typically numerous genes affected. Navigating to those that contribute the most to nerve regeneration and precisely controlling their transcription process represents one of the significant obstacles in applying epigenetic regulation for PNR.
The close interactions between vascular and neural systems underscore the important role of angiogenesis in PNR [62]. In fact, NGCs supplemented with vascular endothelial growth factors A (VEGF-A) has demonstrated to induce intraneural angiogenesis and enhance axonal regeneration [63]. Vascularized NGCs have been shown to stimulate revascularization and enhance nerve regeneration by providing a favorable nutritional microenvironment that not only accelerates axonal regeneration but also minimizes fibroblast infiltration [64, 65]. A recently developed NGC combined with VEGF-A overexpressing SCs exhibited efficient sciatic nerve repair and the authors proposed that the underlying molecular mechanism behind the angiogenesis-triggered nerve regeneration might be related to elevated activation of the VEGFR2/ERK signalling pathway [66]. However, sustained administration of VEGF did not enhance new blood vessel formation on autograft, nor did it improve nerve functional recovery in the long term (16 weeks) [67]. It is believed that providing a stable blood supply may play a more important role than the administration of VEGF alone to facilitate intraneural angiogenesis as PNR is a dynamic process that thrives on nutritive blood supply [65]. Overall, NGCs with angiogenetic potential is desired for accelerated PNR. But angiogenesis is a complex process, inappropriate localization and concentration of vascularization may on the contrary cause adverse effects to PNR.
PNI triggers a cascade of inflammatory responses, playing a crucial role in facilitating tissue regeneration and remodeling. The complexity of this process is further heightened by subsequent surgical procedure and the implantation of NGCs, involving alterations in local immune cells and immunomodulatory factors [68, 69]. Therefore, leveraging immunomodulation mediated by NGCs emerges as another effective strategy to enhance PNR. In the initial stages of tissue regeneration, inflammation proves advantageous by rapidly eliminating debris and wastes produced by PNI. Nevertheless, in later stages, an overreacting immune response drives scarring and fibrosis, ultimately leading to failure of nerve regeneration and functional recovery [70]. Hence, at this juncture, immunosuppression becomes important to better modulate the overall regeneration process. Mokarram et al [71] reported that local delivery of either Interferon-gamma (IFN-γ) or Interleukin-4 (IL-4) from NGCs could modulate the phenotype of macrophage within the polymeric scaffolds and thus promote PNR by polarizing macrophages toward pro-inflammatory (M1) or anti-inflammatory (M2) phenotypes, respectively. They found that the initial polarization of macrophages to M2 phenotype resulted in increased SC infiltration and accelerated axonal regeneration in a 15 mm rat sciatic nerve defect model. In another study, Sun et al [72] synthesized lithium-magnesium-silicone bioceramics-containing NGCs and demonstrated that the scaffolds promoted macrophage polarization toward M2 phenotype which subsequently facilitated the migration and differentiation of SCs and ultimately enhanced PNR and motor functional recovery in a rat sciatic nerve defect model. We postulate that biomaterials displaying intrinsic immunomodulatory effects, including chitosan [73], citrate [74], folate [75], and Flammulina velutipes [76] hold considerable promise as either standalone biomaterials or loaded biomolecules augmenting PNR during the engineering of NGCs.
2.2.5. Other properties
Porosity is another important material property that needs to be considered for NGCs. Ideally, the wall of NGCs should be porous to allow for cell attachment and nutrient and waste transportations. But the pore size should be limited to isolate the infiltration of scar-forming cells such as fibroblasts, which hampers nerve regeneration [40]. It was believed that if the wall pore size is less than 5 μm, cells and tissues are unable to proliferate while if it is larger than 30 μm, entry of tissues becomes excessive [77]. To verify this, Meek and Den Dunnen [77] investigated the nerve regeneration ability of porous Neurolac®, an FDA-approved bioresorbable NGC made of poly(DL-lactic-co-caprolactone) (PLCL). They specifically selected pore sizes ranging from 10 to 20 μm. However, they concluded that these porous Neurolac® NGCs demonstrated no beneficial effect compared to previous findings obtained from non-porous NGCs. A possible reason for this negative outcome might be that this work did not directly compare the nerve regeneration abilities of porous and nonporous NGCs, making it somewhat unfair to juxtapose their in vivo data with previously published results. In a comparison study, highly permeable collagen NGCs were found to significantly promote PNR compared to non-permeable silicone NGCs [78]. Overall, we believe that ideal NGCs should possess a certain degree of porosity. In addition to porosity, functional NGCs with specific properties have increasingly emerged as effective strategy to enhance PNR by providing external neural stimulation, such as electrical [79, 80], magnetic [81, 82], piezoelectric [83, 84], photoacoustic [85], and ultrasound [86, 87] stimulations, or enabling controlled release of drugs and growth factors with the aid of external magnetic field [88, 89], ultrasound stimulation [90, 91], and photothermal effect [92, 93].
Table 1 summarizes the major findings, polymer compositions, microstructures, fabrication techniques, degradation rate and mechanical properties, bio-functionalities, animal models, defect lengths, and in vivo observation time of representative studies aimed for PNR. We attempt to provide a first-sight overview to those looking for developing advanced biodegradable conduits as they pick biomaterials, design microstructures, and select manufacturing routes to tune the properties discussed in this section and ultimately achieve an outstanding overall performance of NGCs for accelerated PNR.
Table 1.
Summary of recent biodegradable polymeric scaffolds for peripheral nerve regeneration.
| Material classifications | Materials | Structural characteristics | Fabrication techniques | Biodegradation rate; mechanical properties | Bio-functionalities | Major results | Animal model; defect gap; longest observation time | References |
|---|---|---|---|---|---|---|---|---|
| Tissue-derived extracellular matrixes (ECMs) and polysaccharides | Adipose tissue-derived decellularized ECM hydrogel, and chitin | Chitin NGC, filled with decellularized ECM hydrogel. | Lyophilization, decellularization, in vitro cell laden of ADSCs. | N/A | Scaffolds were made of bioactive ECM and further loaded with rat adipose tissue derived mesenchymal stem cells (ADSCs). | Co-culture of the filler material loaded-ADSCs with SCs increased the proliferation of the latter. Chitin NGCs loaded with ADSCs-laden decellularized ECM hydrogels promoted motor functional recovery. | Rat sciatic nerve; 10 mm; 12 weeks. | [94] |
| Cell-derived ECMs, polysaccharides, and proteins | MSC-derived ECM or SCs-derived ECM, Chitosan, and silk fibroin | ECM-modified, porous hollow chitosan NGC, filled with silk fibroin. | Solvent casting, lyophilization, decellularization | N/A | Cell derived ECM was the major source of bio-functionalities. | BMSCs-derived, or SCs-derived ECMs modified NGCs showed outperformed in vivo PNR in terms of histological and functional assessments compared to pure chitosan-silk fibroin NGCs. | Rat sciatic nerve; 10 mm; 12 weeks. | [95, 96] |
| Tissue-derived ECMs | Decellularized nerve ECM hydrogel | Multiple microchannels | Unidirectional lyophilization | Scaffold maintained microchannel structures 4 weeks post-implantation. | Decellularized nerve matrix hydrogel from porcine sciatic nerve was the major source of bio-functionalities. NGF was also loaded into the scaffolds. | The stiffness and protein composition of the ECM influence cell morphology of SCs which ultimately play important roles in regulating phenotype of SCs. Scaffolds loaded with NGF demonstrated the best in vivo nerve regeneration. | Rat sciatic nerve; 15 mm; 12 weeks. | [97] |
| Natural polymers | Collagen | Porous single hollow tube with average pore size of 215 μm | Self-assembly, lyophilization, and chemical crosslinking with formaldehyde | N/A | The biomaterials used were the major source of bio-functionality without further modification. | NGCs showed high permeability to macromolecules as large as bovine serum albumin (68 kDa); compared to silicone NGCs, collagen NGCs demonstrated enhanced axonal regeneration, myelination, and vascularization in vivo. | Rat sciatic nerve; 5 or 10 mm; 8 weeks. | [78, 98] |
| Natural polymers | Collagen | Mineralized collagen NGC filled with pure collagen fibers | Self-assembly, mineralization, chemical crosslinking with EDC, and lyophilization; films were wrapped into scaffolds | 60% and 80% weight loss within 28 d in vitro of mineralized and unmineralized materials; ultimate tensile strength and Young’s Modulus were 0.75 ± 0.17 GPa, 15.04 ± 2.04 GPa in dry condition and 0.03 ± 0.01 GPa, 0.11 ± 0.03 GPa in wet for 30 min. | The biomaterials used were the major source of bio-functionality without further modification. | NGCs containing mineralized collagen demonstrated higher mechanical strength, prolonged degradation, and superior in vitro and in vivo potential for PNR. | Rat sciatic nerve; 10 mm; 12 weeks. | [99] |
| Natural polymers | Gelatin | Solid hollow tubes with a rough outer surface and a smooth inner lumen | Dip-coating, chemical crosslinking with genipin | Degradation of NGCs became obvious 6 weeks post-operation; mechanical properties N/A. | The biomaterials used were the major source of bio-functionality without further modification. | Restored muscle function within 4 week; numerous nerve fibers, mostly unmyelinated, regenerated 6 weeks post implantation. However, the dense scar tissue formation at the outer area of the newly formed nerve tissue might be a concern. | Rat sciatic nerve; 10 mm; 8 weeks. | [100] |
| Natural polymers, synthetic polymers | Gelatin methacrylate (GelMA) and polyethylene glycol diacrylate (PEGDA) | Four different designs: a single channel hollow tube, a tube with multiple microchannels, a branched, and a biomimetic branched human facial NGC | Rapid continuous 3D printing | Degradation rate N/A; Young’s moduli ranging from 0.3–4.5 MPa in wet for overnight as determined via unconfined compression testing. | The biomaterials used were the major source of bio-functionality without further modification. | With a single printing platform using a single material composition, the technique is capable of rapidly printing (within 10 min) customized NGCs with various shapes including a complex life-size branched NGC mimicking the human facial zygomatic branches, the buccal branches, the marginal mandibular branch, and the cervical branch. | Mouse sciatic nerve; 4 mm; 10 weeks. | [101] |
| Natural polymers | Silk fibroin (SF) | Hollow tubes with either aligned or random distributed SF fibers. | Electrospinning to make SF films with aligned or non-aligned fibers, then rolling the films into hollow tubes | N/A | NGCs were loaded with glial cell lien-derived neurotrophic factor (GDNF) and nerve growth factor (NGF). | The aligned SF fibers promoted the outgrowth rate and augmented the length of axons along the SF fiber direction of both dorsal root ganglions (DRG) sensory neurons and spinal cord (SpC) motor neurons, in comparison to the group with randomly oriented SF fibers. The loaded GDNF and NGF were sustainedly released from the SF NGC in vitro over 4 weeks. | No in vivo study performed. | [54] |
| Natural polymers | Cellulose, soybean protein isolate (SPI) | Cellulose/SPI film-based conduit (CSFC) or sponge-based conduit (CSSC) | CSFC was made by solvent casting, while CSSC was made by lyophilization | N/A | The biomaterials used were the major source of bio-functionality without further modification. | Both scaffolds were capable of repairing a 10 mm rat sciatic nerve gap in months while CSSC showed a higher repairing potential due to higher radial permeability. | Rat sciatic nerve; 10 mm; 3 months. | [102] |
| Natural polymers | Chitosan | Porous single hollow tube | Dip-coating, lyophilization | The suture retention forces dropped from ~2 to 1.04 N after in vitro degradation for 8 weeks in lysozyme, inferring that these NGCs might be able to retain structural integrity for at least 8 weeks. | NGCs were loaded with BMSCs-derived SCs or sciatic nerve-derived SCs. | Chitosan scaffolds seeded with BMSCs-derived SCs bridged a critical-sized 12 mm rat sciatic nerve gap in three months. The overall repairing ability is approaching to that of autografts. | Rat sciatic nerve; 12 mm; 3 months. | [103] |
| Natural polymers, synthetic polymers | Chitosan, carboxymethyl chitosan (CMC), polyaniline (PANI) | Double layered: chitosan hollow tube, filled with DHF-loaded, PANI modified CMC hydrogel | Polycondensation to synthesize CMC-PANI, chemical crosslinking with DCC to load DHF, electrodeposition to prepare chitosan NGCs | ~30% and ~20% weight loss within 8 weeks in vitro with or without DHF-loaded NGCs; the ultimate tensile strength and Young’s Modulus of DHF-loaded NGC were 0.94 ± 0.07 and 3.61 ± 0.24 MPa, respectively. | DHF, which is a small molecule with similar functions to BDNF but much longer biological half-life, was loaded into scaffolds. | The DHF-loaded double-layered CMC-based nerve guidance hydrogel achieved in vivo never repair ability to that of autografts in a 10 mm rat sciatic nerve defect model. | Rat sciatic nerve; 10 mm; 12 weeks. | [104] |
| Synthetic polymers, Natural polymers | Poly(lactic-co-glycolic acid) (PLGA) and collagen | PLGA (50:50) conduit filled with collagen gel, which was imbedded with rat dental pulp cells | Commercially available tube; self-assembly to make cell-laden collagen gels | NGCs were completely resorbed 2 months post-operation; mechanical properties N/A. | NGCs were loaded with dental pulp cells. | Cell-laden NGCs successfully bridged a 7 mm gap in the bilateral buccal branches of rat facial nerve and the tubes were resorbed in vivo in 2 months. | Rat bilateral buccal branches of facial nerve; 7 mm; 2 months. | [105] |
| Synthetic polymers | Poly(ε-caprolactone) (PCL) | Porous hollow tube with aligned fibers coated with a concentration gradient of NGF | Electrospinning | Biodegradation rate N/A; the ultimate tensile strengths, Young’s moduli, and strain at break were approximately 6 MPa, 20 MPa, and 5%, respectively. | NGCs were coated with a concentration gradient of NGF. | NGCs were confirmed to enhance and attract the directional neurite growth of dorsal root ganglion (DRG) neurons toward the direction containing higher concentration of NGF; NGCs were capable of repairing a 15 mm rat sciatic nerve defect within 12 weeks and the performances are comparable to those of autografts. | Rat sciatic nerve; 15 mm; 12 weeks. | [55] |
| Synthetic polymers | poly(lactide-ε-caprolactone) (PLCL) |
Three designs: a single channel NGC, an NGC with hundreds of microchannels, and a one immobilized with substance P (SP) on the latter design. | Ring-opening polymerization to synthesize prepolymers, electrospinning or micropattern rolling to fabricate NGCs, chemical crosslinking with CDI to immobilize SP. | Molecular weight drops over time in vivo: ~84 kDa, 39 kDa, and 19 kDa after 4, 8, 12 weeks of sub-q implantation, respectively; microchannel constructs collapsed 12 weeks post-implantation; The ultimate tensile strength, Young’s modulus, and strain at break of the NGCs still maintained ~2.5 MPa, ~10 MPa, and ~50% 12 weeks post implantation. | Topographical cues; NGCs were immobilized with substance P, a stem-cell recruitment factor. | NGCs with hundreds of microchannels showed increased recruitment capability of host stem cells, and therefore achieved better nerve functional recovery in comparison to single channel NGCs in vivo. SP-immobilization on the multichannel NGCs further promoted nerve regeneration. |
Rat sciatic nerve; 10 mm; 12 weeks. | [106] |
| Synthetic polymers | PGS | Cuboidal bars | Polycondensation to make prepolymers, thermal crosslinking and melt-processed to make bars. | The dimension retained unchanged 7 d post-implantation. The lengths and widths decreased by 20% and 15% 21 d post-implantation, respectively. By 35 d post-implantation, these reductions increased to 32% and 50%, respectively. | The main objective of this study was to evaluate the in vitro and in vivo response of PGS for neural reconstruction application. Therefore, no further bio-functionalities were applied. | Compared to commercial PLGA (50:50), PGS films showed similar or superior cellular response of SCs. PGS bars demonstrated favourable in vivo responses in comparison of PLGA when implanted underneath rat sciatic nerve on the underlying muscle bed, showing less inflammation, fibrosis, and tissue swelling. | Rat sciatic nerve; no gap; 60 d. | [107] |
| Synthetic polymers | Crosslinked urethane doped POC polyesters (CUPE) | Porous hollow tubes with single channel or multiple channels; porous hollow tube loaded with folic acid | Polycondensation to make prepolymers; dip-coating, thermal crosslinking, and particular leaching to make porous NGCs. | CUPE has tunable biodegradation rate (few weeks to more than a year) [108]. The ultimate tensile strengths, Young’s moduli, and strains at break ranging from ~1–3 MPa, ~0.6–1.4 MPa, and ~318%–122%, respectively [109]. | Folic acid was loaded into NGCs to epigenetically regulate neural regeneration [60]. | Numbers of Channels showed no significant influence of the mechanical properties of CUPE NGCs. CUPE scaffolds loaded with folic acid demonstrated functions regulating migration, neurotrophic release, and accelerated nerve repair. | Rat sciatic nerve; 22 mm; 12 weeks [60]. Rat sciatic nerve; 10 mm; 8 weeks [109]. | [60, 109] |
| Synthetic polymers, natural polymers | PEGDA and GelMA | Solid hollow tubes loaded with living platelets | Rapid continuous 3D printing | ~1% weight loss in vitro within 20 h in collagenase; dynamic mechanical analysis testing revealed that the NGCs could regain the printed structure after removing the force (⩽0.3 N). | Live platelets were mixed with bio-ink and then 3D printed into NGCs. | Live platelets loaded in the 3D printed conduits sustainedly released growth factors for a long period (>500 h). Platelets-loaded conduits showed significantly promoted nerve regeneration in vivo. |
Rat sciatic nerve; 10 mm; 12 weeks | [110] |
| Synthetic polymers, natural polymers | Polyurethane acrylate (PUA), gelatin | Gelatin coated PUA films with nanoscale groove pattern arrays (350-nm width) | UV-assisted capillary force lithography | N/A | Topographical cues; PUA material was coated with 0.1% gelatin and modified by oxygen plasma treatment to enhance cell adhesion. | Surface modified nanoscale ridge/groove pattern arrays alone can rapidly and effectively induce the differentiation of human embryonic stem cell (hESCs) into a neuronal lineage without the use of any biological factors. | No in vivo study performed. | [111] |
| Synthetic polymers | Polyurethane (PU), PCL, and PEG | Single channel NGC filled with a porous inner tubular scaffold | Emulsion polymerization, Lyophilization | NGCs completely degraded 12 weeks post-implantation; the tensile moduli of the two formulations were 3.89 MPa and 1.38 MPa in dry condition, and 0.48 MPa and 0.22 MPa in wet (soaked in PBS for overnight). | The biomaterials used were the major source of bio-functionality without further modification. | By varying the PEG concentration, two groups of biodegradable waterborne PU NGCs with different moduli were synthesized. The group with higher elastic modulus (3.89 MPa in dry condition) displayed superior nerve repair that is similar to that of autograft in vivo. | Rat sciatic nerve; 10 mm; 12 weeks | [42] |
3. Selection of biomaterials
The choice of biomaterials is an important first step in designing functional NGCs as it largely determines the above-mentioned material properties. As discussed in section 2.2.2, biodegradable materials are preferred over non-degradable materials, in this section, we focus on the discussion of biodegradable NGCs made of both natural and synthetic polymers.
3.1. Natural polymers
The main natural polymers used for NGCs include most of the organic compounds found in extracellular matrixes (ECMs) and their derivatives. Natural polymers generally have good biocompatibility, non-toxicity of their biodegradation products, low immunogenicity, and excellent biomimetic properties [112, 113]. But they often exhibit inferior mechanical properties, low processability, and less consistency due to the batch-to-batch variations of animal sources [114]. The use of some natural polymers such as collagen and hyaluronic acid are also associated with high cost [5].
3.1.1. ECM and derivatives
ECM is a complex network composed of many species including proteins, proteoglycans, and polysaccharides [115]. ECMs derived from cells or decellularized tissue ECMs contain abundant morphological and biological cues for PNR as they provide a biomimetic local microenvironment, which is suitable for the attachment, proliferation, and migration of SCs and can modulate the differentiation of neural stem cells by binding or regulating growth factors [95, 116]. For example, Xu et al found that the phenotype of SCs can be regulated by adjusting the stiffness and protein composition of the ECM via influencing cell morphology of SCs [117]. ECM proteins including laminin, fibronectin, and type IV collagen have also been revealed to promote the adhesion of SCs and influence the biological behaviors of SCs in the process of remyelination after PNI [118]. Decellularized ECMs from adipose tissue or nerve tissue have been verified to promote PNR [119, 120]. Notably, decellularized nerve ECM such as Avance® (Axogen, USA), which is a processed human nerve allograft, has already been approved by the FDA for the surgical repair of peripheral nerve gaps (further discussed in section 6).
However, decellularized tissue ECMs sometimes exhibit several drawbacks such as immune rejection, pathogen transfer, and low mechanical properties. On the one hand, incomplete removal of cellular materials from tissue ECMs may retain pathogen or components causing inflammatory response, which may ultimately result in failure of repairing [121]. On the other hand, many bioactive factors favoring nerve regeneration could be removed from the harsh decellularization process, during which it is also inevitable to damage the structural integrity of the 3D hierarchical microstructures, resulting in inferior mechanical strength [97, 122, 123]. As alternatives, cell derived ECMs [95, 96] and hydrogels [97, 124] derived from decellularized tissue ECMs have drawn considerable attention lately. Gu et al [95, 96] demonstrated that the peripheral nerve regenerative outcomes of chitosan-silk fibroin scaffolds in repairing a 10 mm rat sciatic nerve gap could be significantly improved by adding a ECM layer directly derived from either SCs or bone marrow mesenchymal stem cells (BMSCs). Additionally, hydrogels derived from decellularized nerve matrix have been used for PNR because they not only preserve the high bioactivity and ECM-mimicking nanofibrous structure, but also provide high tunability for further modifications such as growth factors and cell loadings [97, 124]. Gong et al [125] filled ECM-mimicking hydrogels into a 3D printed gelatin methacryloyl (GelMAs) nerve conduit and successfully promoted PNI due to the morphological and biochemical cues provided by the ECM-mimicking hydrogels, which supported the outgrowth of neuron. Hydrogels derived from porcine decellularized nerve matrix have been shown to repair a 15 mm sciatic nerve gap in rat by promoting the activation of M2 macrophages and enhancing myelination [124].
3.1.2. Proteins
The most commonly used protein materials for PNI include collagen, gelatin, and silk fibroin. As the main structural protein in the body, collagen is abundant in many tissues such as bone, cartilage, tendon, skin, as well as the connective tissues in nerve trunk including endoneurium, perineurium, and epineurium. As a natural biopolymer extracted from various animal tissues such as bovine tendons, rat tails, porcine skin, and jellyfish, collagen-based materials demonstrated excellent biocompatibility and tunable biodegradability [126–128]. Kemp et al [78] examined the peripheral nerve regenerative potential of collagen NGCs in comparison to non-permeable silicone ones and found that the collagen tubes significantly enhanced the axonal regeneration, myelination, and vascularization in both a 5- and a10 mm-gap in rat sciatic nerves. However, as a biopolymer, collagen NGCs usually exhibit inferior mechanical strength and are prone to be absorbed in vivo even sooner than nerve tissue regenerates, failing to support and guide PNR over time. On one hand, these characteristics make collagen hydrogels or fibers suitable to be used as a filler material in NGCs to facilitate the ingrowth of cells and therefore accelerate the regeneration of axons [129–131]. On the other hand, one can tune the mechanical property and degradability of collagen by incorporating inorganic minerals such as apatite and silicon into collagen as mineralized collagen often offers enhanced mechanical properties, superior biocompatibility, and decreased biodegradation rate [132–134]. Duan et al [99] recently constructed a biphasic NGC with a mineralized collagen layer serving as the outer tube while pure collagen fibers act as a filler (MC@Col). Compared to pure collagen conduit, the MC@Col group demonstrated significantly enhanced mechanical properties, prolonged degradation, and more importantly, promoted the attachment and alignment of SCs and facilitated in vivo PNR in rats. Notably, there are several collagen-based commercialized conduits such as NeuraGen® Nerve Guide by Integra LifeSciences Co., NeuroMatrix® Conduit and Neuroflex® Conduit by Collagen Matrix, INC. etc., which are discussed in section 6.
As a hydrolyzed and denatured form of collagen, gelatin is popularly used in tissue engineering because it possesses structural fragments of collagen that is able to activate cell functions and ECM production [135]. In an early attempt made by Chen et al [100], solid hollow tubular genipin crosslinked gelatin NGCs were fabricated with a rough outer surface and a smooth inner lumen, which maintained structural integrity in vivo for 6 weeks and repaired and partially recovered muscle functions in a 10 mm rat sciatic nerve gap in rat within as short as only 4 weeks. However, most of the regenerated axons were unmyelinated, and the formation of a dense scar tissue at the outer area of the regenerated nerve might be a concern as it might act as a barrier preventing the myelination and maturation of nerves. As a result, gelatin has been recently more frequently combined with many other biomaterials such as various synthetic polyesters [8, 136–138] and natural biopolymers like silk fibroin [139, 140], chitosan [141, 142], alginate [143], etc. aiming to combine the advantages provided by gelatin and other components. In addition, the amine groups and hydroxyl groups on the surface of gelatin are highly robust, allowing them to covalently bond with the carboxyl groups in methacrylate (such as methacryloyl and methacrylic anhydrite). Such a reaction produces a high-promising photocrosslinkable material, gelatin methacrylate (GelMA), as the carbon-carbon double bonds in methacrylate can be further photoinitiated to rapidly cure the polymer through free radical polymerization. As a result, GelMA has been frequently used for biomedical applications such as to create in situ photocrosslinkable hydrogels or serve as a promising bioink for biofabrication in tissue engineering to manufacture NGCs with rather complicated designs [101, 144–147].
Silk fibroin (SF) is mainly produced by silkworms and spiders. As one can picture the strength of spider webs seen in daily life, silk fibroin is a natural protein with outstanding mechanical properties. As a result, it has been popularly used in load-bearing scenarios such as bioresorbable bone fixation devices [148, 149], load-bearing scaffolds for dermal tissue regeneration [150] and bone regeneration [151]. In addition, due to its excellent biocompatibility, superb flexural strength, and good elasticity, numerous of SF-based NGCs have been developed and encouraging outcomes have been achieved [54, 152–154]. For example, Madduri et al [54] manufactured SF-based NGCs with either randomly oriented or aligned fibers on the lumen surface, which is further loaded with glial cell line-derived neurotrophic factor (GDNF) and NGF. The loaded factors were sustainedly released from the SF NGCs over 4 weeks in vitro, regulating PNR over a relatively long duration. Moreover, compared to the non-aligned group, the alignment of the SF fibers has been shown to promote the outgrowth velocity and augment the length of axons regenerated along the fiber orientation of both dorsal root ganglions (DRG) sensory neurons and SpC motor neurons. In a more recent attempt, enzymatically crosslinked SF-based NGCs have been used as a platform to compare and optimize the loading methods (crosslinking or adsorption) of GDNF and NGF to the NGCs, attempting to achieve a more controllable release of such factors for favored PNR [152]. It has been shown that the group bearing GDNF loaded by adsorption method exhibited the best overall performance in terms of the bioactivity and release profile of the neurotrophic factors and the in vivo nerve regeneration capability in a 10 mm rat sciatic nerve gap [152]. Similar to other proteins, it is also a common strategy to combine SF with other materials to tune the overall performance of SF-based NGCs. For example, it has been discovered that by introduction of hyaluronic acid into SF, the composite conduits exhibited superior hydrophilicity, flexibility and stability and ultimately increased cytocompatibility and well supported the proliferation and migration of embryonic stem cells [155].
3.1.3. Polysaccharides
Polysaccharides are carbohydrate-based polymers composed of sugar molecules in its molecular structure [156, 157]. Polysaccharides used for the synthesis of NGCs mainly include cellulose and derivatives, alginate, chitosan/chitin and derivatives, and hyaluronic acid. In this section, we focus on the discussions of cellulose and chitosan/chitin as they are the two most abundant natural polymers on earth and have been widely used in tissue engineering.
As the most abundant natural polymer, bacterial- and plant-derived cellulose has drawn considerable attention due to its low cost, excellent biocompatibility, high water retention, and unique mechanical properties. A recent computational analysis indicated that the fibril-fibril sliding in aligned cellulose networks offers the materials excellent plasticity, while the non-covalently bonded bundled cellulose network provides the material stress-dependent elasticity, stiffening and plasticity beyond the yielding point [158]. However, cellulose is not considered biodegradable in human due to lack of appropriate enzymes to break the β-1,4-glucose linkages in the molecule [159, 160]. But its derivatives such as methylcellulose and carboxymethyl cellulose are excellent biodegradable polymers that has been popularly applied in drug delivery and tissue engineering [161–163]. Moreover, cellulose is frequently combined with another biodegradable natural polymer, soy protein isolate (SPI) to engineer NGCs for PNR as the latter possesses great film/sponge-forming performance [102, 164–166]. It has been reported that both of the cellulose/SPI film-based conduit (CSFC) and sponge-based conduit (CSSC) demonstrated sufficient capability to repair a 10 mm rat sciatic nerve gap [102]. Compared to CSFC, CSSC showed a higher repairing efficiency as the latter had a much higher porosity and permeability, which is favorable for cell attachment and nutrient/wastage transportations [102].
Chitin is the second most abundant natural polymer which is only behind the plant-derived cellulose on earth. It is highly concentrated in the shells of crabs, shrimps, and other crustaceans [167]. Chitin is considered the precursor of chitosan as the latter is derived from the former through a process termed deacetylation, when the amount of acetyl function groups in the repeating units of chitin is reduced [168]. Therefore, chitin and chitosan share many desirable biological properties including (1) broad-spectrum activity against bacteria, yeast, and fungi; (2) antitumor and immunomodulatory effects; (3) enhance blood coagulation and promote wound healing [169]. However, chitin is barely soluble in many solvents, making it difficult to manipulate. In contrast, chitosan is fully soluble in mild acidic environments and thus can be easily manufactured into various biomaterials such as dental implants [170], skin regeneration scaffolds [171], wound dressing hydrogels [172], and of course NGCs [103, 104, 173]. It has been evidenced that chitosan NGCs loaded with BMSCs-derived SCs obtained approachable outcomes compared to autografts and could bridge a critical-sized 12 mm rat sciatic nerve gap [103]. Recently, encouraging in vivo results which are overall comparable to those achieved by autografts have been reported using a precursor SCs-derived ECM modified chitosan-SF based composite scaffold [174]. Four weeks after the implantation, such a scaffold exhibited apparent elongation of axons and significant improvement in behavioral tests, which are similar to those of autografts when bridging an 8 mm gap in the upper brachial plexus (a proximal nerve defect) in rat. In addition, carboxymethyl chitosan (CMC), which is a water-soluble chitosan derivative, has been shown promising in promoting PNR. For example, conductive polyaniline modified CMC hydrogel conduit were loaded with 7,8-dihydroxcyflavone (DHF), a nature molecule mimics the function of BDNF, to promote PNR [104].
3.2. Synthetic polymers
Compared to natural polymers, synthetic polymers possess outstanding advantages such as good batch-to-batch consistency, well-tunable and controllable mechanical properties and biodegradation rate, ease of processing, manufacturing, and biofunctionalization. But they usually have lower biocompatibility. The fast degradation of some synthetic polymers also raises concerns about their cytotoxicity and immunogenicity.
3.2.1. Polyesters
Aliphatic polyesters (i.e. polyesters without benzene ring within their structure in contrast to aromatic polymers) are the most widely used biodegradable synthetic polymers as the ester bond in such a structure is susceptible to hydrolysis in vivo. Some of the examples include thermoplastic polylactic acid (PLA), polyglycolic acid (PGA), poly(ε-caprolactone) (PCL), and their copolymers poly(lactic-co-glycolic acid) (PLGA) and poly(lactide-ε-caprolactone) (PLCL), and thermosetting poly(glycerol sebacate) PGS and poly(diol citrate).
PLA/PGA/PLGA standard for some of the most well-known polyesters for biomedical applications. They are synthesized through a polycondensation reaction (lactic acid, glycolic acid, or both) or a ring opening polymerization (lactide, glycolide, or both). Due to the existence of chiral carbon in lactic acid, three forms of PLA exist: PLLA, PDLA, and PDLLA, among which PLLA exhibits higher crystallinity and chemical stability, making it more resistant to hydrolysis and thus a slower biodegradation rate but a higher mechanical property [175]. By adding D-isomers into the polymerization reaction of PLLA, the resulted polymer, known as PDLLA, cannot pack as tightly as PLLA and therefore resulting in a lower mechanical strength but a higher degradation rate [176]. Therefore, PLA-based polymers have been widely used in nerve regeneration as they provide highly tunable mechanical properties and degradation rate to satisfy the materials requirements as previously discussed in section 2. For example, a NGC made of PLA non-woven fabric was used to successfully repair a 7 mm buccal branch facial nerve defect in rat within 13 weeks [177]. PGA has a similar structure to PLA, but the latter contains a methyl group on its repeating unit, making it more hydrophobic and more resistant to mechanical deformation than PGA. However, when PGA on its own degrades, it goes through a ‘bulk degradation’ and loses a large deal of its mechanical strength, making it less frequently used as a neat scaffolding component [178]. Instead, PLGA copolymers have been widely used thanks to their tunable properties as a higher ratio of lactic acid compartment in PLGA leads to higher mechanical strength but longer degradation time. PLGA (50:50) NGCs loaded with dental pulp cells successfully bridged a 7 mm gap in the bilateral buccal branches of rat facia nerve and the tubes were resorbed in vivo in 2 months [105]. In general, PLGA has a degradation rate ranging from a few weeks up to 24 months (PLLA) depending on the ratio of lactide and glycolide, and molecular weight of the copolymer [179, 180]. One of the adverse effects of a PLGA/PLA/PGA scaffold is the inflammation reaction caused by its degradation products, which are lactic acid or/and glycolic acid that make the surrounding area more acidic. In a study using PLGA scaffolding to promote growth of SCs, it was found that after 4 weeks, the pH of the liquid solution from the degradation process had a pH as low as 3.41 [181].
PCL/PLCL represents another species of important thermoplastic polyester. The 5 methylene groups on the repeating unit of PCL make this polymer degrade slower (2–3 years) compared to PLGA-based biomaterials [182, 183]. Therefore, PCL is more suitable for applications where longer regenerative process is required. One of the reasons that makes PCL a good polymer is the ease at which it can be combined with other materials to tune its properties. For example, it can be combined with magnesium phosphate to increase its degradation rate [184], blended with bioactive glass [185] or bioceramics [186] to increase its mechanical properties, copolymerized with lactic acid to form PLCL [187], or simply blended with PLA to form PCL/PLA composites [188]. It was reported that the addition of PLA improved the mechanical properties of PCL and led to a better printability of PCL/PLA composites [188]. Recently developed PLCL-based NGCs have shown encouraging results when repairing 8–12 mm rat sciatic nerve gaps [138, 189, 190]. Moreover, PCL is one of the ideal materials for preparing nanofibers using electrospinning technique [191], which is one of the key techniques used to manufacture NGCs that will be discussed in section 5. For example, Zhu et al [55] managed to regenerate nerve tissues across a 15 mm rat sciatic nerve defect using an NGC with highly aligned electrospun PCL nanofibers coated with a concentration gradient of NGF.
Differing from the above-mentioned linear polyesters, PGS and poly(diol citrate) are biodegradable thermosetting polyesters as they are derived from monomers with multifunctionality (glycerol or citrate) [192, 193]. As thermosetting polyesters, the mechanical properties and degradation rate can be easily tuned by adjusting the thermal crosslinking conditions and the monomer ratio of acid and alcohol. Moreover, the unreacted side functional groups in these branched polymers allow for many convenient modifications such as fluorophore amino acid doping for in vitro and in vivo imaging [27, 194], urethane doping for augmented elasticity [108, 109], peptide or protein coating for enhanced cell attachment [195]. Importantly, PGS and poly(diol citrate) on their own are excellent elastomers with good tensile strength and large elongation ratio thanks to the strong intermolecular force provided by the hydrogen bonding of hydroxyl groups and thermal crosslinking, making them extremely suitable to be used in soft tissue regeneration including nerve. An early comparative study verified that the in vitro nerve regenerative effects of PGS NGC were similar to or superior to that of PLGA one, and demonstrated a promoted in vivo response with less inflammation, fibrosis, and surrounding tissue swelling [107]. This is probably because the degradation of PGS demonstrates little water uptake and the resulted degradation products are less acidic compared to PLGA, as the former degrades following a process called surface erosion (with much less bulky degradation in comparison to PLGA) and presents a linear loss in mass with minimal loss in mechanical strength [196]. We found similar degradation properties in poly(diol citrate)-based materials such as poly(octamethylene citrate) (POC) and urethane-doped crosslinked POC polyesters (CUPE) [108, 197]. Folic acid-loaded CUPE NGCs have been shown to guide the directional migration of SCs and repaired a 10 mm rat sciatic nerve gap possibly due to the epigenetic regulation of folic acid-triggered DNA methylation [60]. Our group has long been working on citrate-based biomaterials for tissue regeneration, drug delivery, and bioimaging over the last two decades. Citric acid is an intermediate in the Krebs cycle that participates in metabolism processes. It also possesses excellent antioxidant, antibacterial and intrinsic immunomodulatory effect [198, 199]. We believe that POC- or CUPE-based elastomers could be promising biomaterials in combination with other biochemical cues or physical stimulations to manufacture NGCs for enhanced PNR.
3.2.2. Polyethers
Polyethers are generally not biodegradable in vivo as the ether bond is relatively stable even at extreme pH values. However, polyether-based materials such as PEG and polypropylene glycol are frequently applied in biomedical engineering mainly due to their high hydrophilicity and high-water retention provided by the ether bond as it forms hydrogen bonding to water. PEG-based hydrogels have been proven to be promising in regulating nerve regeneration [200–202]. The elastic moduli or stiffnesses of hydrogels are generally in the range of kPa, which are key parameters regulating cellular responses of SCs and neurons as discussed in section 2.2.3. PEG-based hydrogels have a stiffness that is very easy to customize with just a small tweak of PEG concentration. For example, Gunn et al [52] fabricated PEG-based hydrogels with an elastic modulus ranging from tens of kPa to hundreds of kPa by only adjusting the PEG-diacrylate concentration from 50 to 200 mg ml−1 when preparing the hydrogels. And such an increase of modulus demonstrated a decrease of neurite extension of PC12 cells [52]. Besides hydrogels, it is a common strategy to incorporate PEG into other polymers to increase the hydrophilicity and biocompatibility of the material. In a study conducted by Serra et al [203], 5% w/w of PEG incorporation into PLA not only improved the processability for 3D printing of the PLA/PEG polyblend, but also increased the surface roughness, wettability and degradation time of the polymer. In addition, PEG derivatives such as PEG diacrylate (PEGDA) is an excellent bioink when mixed with GelMA for 3D printing of advanced and customized NGCs [101, 110, 147].
3.2.3. Polyurethanes (PU)
PU are not biodegradable. But the urethane bond is a good source of hydrogen bonding to surrounding molecules, increasing the mechanical strength by building strong intermolecular forces. The mechanical properties of PU can be easily modified by adjusting the ratio of polyol to isocyanate. Higher ratios of polyol lead to a softer PU polymer, and vice versa, because polyol governs the softer sections of PU as it generally contains a longer carbon chain lengths while isocyanate on the other had has shorter sections with greater crystallinity [204]. Moreover, the hard segment aggregation in PU can act as ‘pseudo-cross-links’ to make the materials behave as an excellent elastomer [205]. Ester-based polyurethanes combine the good biodegradability of polyesters with the good mechanical properties generated by polyurethanes and have been widely used in tissue engineering especially for soft regeneration where elasticity is required. As a result, many polyester-polyurethane-based materials have been developed for nerve regeneration [42, 60, 109, 206, 207].
Overall, selection of biomaterials is a complicated process as there are many specifications that need to be considered. Great efforts have been made to make composite NGCs aiming to combine the advantages provided by the various components and many examples such as the combination of GelMA and PEGDA, cellulose and SPI, PLGA and collagen have already been provided above. In a recent study, PCL-PEG-PU-based NGCs have been developed attempting to build a biodegradable waterborne polymer as PCL is a aliphatic polyester with good biodegradability, PEG is a hydrophilic component with good biocompatibility, and polyurethane offers great mechanical strength and elasticity [42]. But it was found that by slightly changing the ratio of PEG in the composite polymer, NGCs demonstrated distinct properties and nerve regeneration ability. As a result, the strategy of combining different polymers also brings up new challenges as the overall performance of the composite material such as mechanical properties, processability, biodegradation rate, cellular responses, and in vivo nerve regenerative potential, is largely dependent on the ratios of each component. How to balance the requirement for each property and find the optimized compositional combination is a painstaking work.
4. Structural designs
4.1. Single channel
NGCs with a single channel non-porous tubular structure represent the most straightforward and simplest design (figure 3(A)). The design of the tubular structure acts as an interface between the nerve and surrounding tissue, blocking scar cells while allowing the entry of essentials (e.g. oxygen and nutrients). By taking advantages of the good elasticity and biocompatibility of polydimethylsiloxane (PDMS), the first generation of conduits for PNR is a cylindrical silicone tube which simply provided a physical guidance and mechanical support when bridging a 6 mm rat sciatic nerve gap [29, 36]. As people realized the importance of scaffolding permeability in tissue regeneration for nutrient/wastage transportation, porous hollow tubes were developed (figure 3(B)) and better regenerative ability has been achieved [102, 208]. NGCs made of electrospun fibers are good examples of porous design as the fibrous polymer network offers a highly porous structure that mimics the architecture of ECMs [209, 210]. Moreover, the fiber orientation during electrospinning can be conveniently manipulated to align the longitudinal direction of NGCs, aiming to provide a topographical cue for alignment of SCs and thus guide the axonal growth. For example, Frost et al [211] made a double-layered NGC with aligned electrospun fibers along the inside lumen to guide the axonal regeneration while randomly oriented fibers at the outside to provide a stronger mechanical support.
Figure 3.
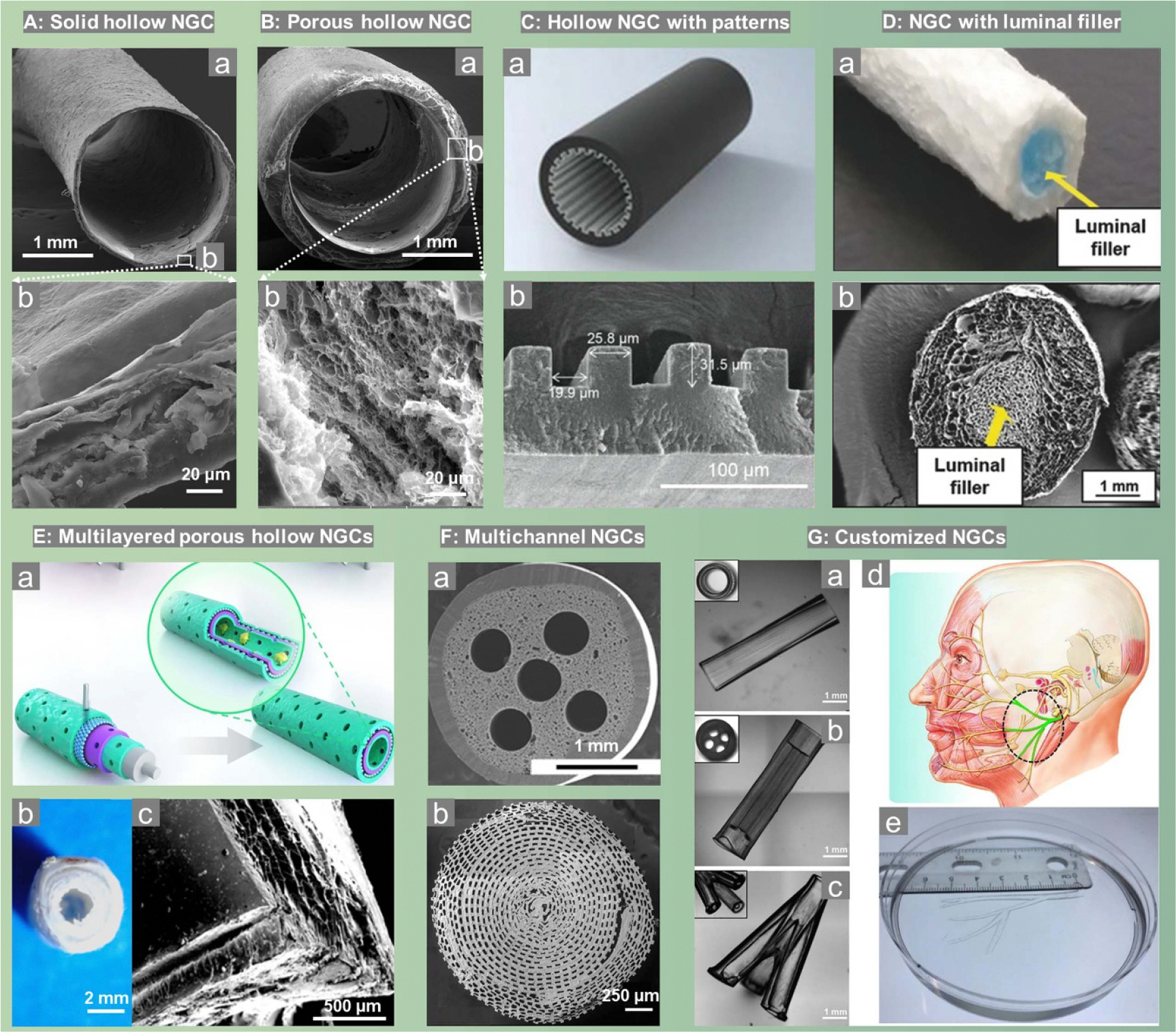
Different scaffold designs of NGCs. (A) and (B), single channel solid and porous hollow NGCs. Reproduced from [102]. © IOP Publishing Ltd All rights reserved. (C), single channel hollow NGCs with inner pattern (grooves). [212] John Wiley & Sons. © 2023 Wiley-VCH GmbH (D), single channel porous NGC with luminal filler. [213] John Wiley & Sons. © 2017 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim. (E) multilayered porous single channel hollow NGCs. Reproduced from [214]. CC BY 4.0. Reprinted from [8], Copyright (2020), with permission from Elsevier. (F): multichannel NGCs with several (F-a). [109] John Wiley & Sons. © 2013 Wiley Periodicals, Inc. or hundreds of (F-b) Reproduced from [106]. CC BY 4.0 microchannels. (G): 3D printed customized NGCs with (a) single channel, (b) multichannel, (c) bifurcated, and (d), (e), human life-size NGC mimicking the human facial nerve system. Reprinted from [101], Copyright (2018), with permission from Elsevier.
Aligning with the design of aligned electrospun fibers, NGCs with aligned inner surface patterns have been developed. As shown in figure 3(C), a conductive conduit with a micropatterned surface of 20 μm width grooves have been recently fabricated and such a patterned surface has been approved to promote the elongation of SCs [212]. In combination with an external electrical stimulation, this patterned NGC exhibited a much greater effectiveness promoting neural growth and bridging rat sciatic nerve gap [212]. Similarly, Hu et al [215] directly modified Morpho butterfly wing, which exhibits parallel nano-ridge structure with numerous micrometer-sized grooves, with reduced graphene oxide (rGO) and BDNF encapsulated GelMA hydrogel. Such a topographical cue in combination with the biochemical cues and electric stimulation overall exhibited great performance in repairing 10 mm rat sciatic nerve defect.
In native nerve trunk, axons grow in fascicles. As a result, researchers tried to design NGCs containing filler materials, either hydrogel or oriented fibers to mimic the nerve fascicle structure and provide a more cell-friendly microenvironment compared to hollow NGCs. Figure 3(D) shows a typical photo and SEM image of such a design [213]. The out conduit made of crosslinked collagen not only provided an optimized mechanical support, but also allowed high wall permeability to mitigate the risk of neuroma formation. While the inner hyaluronic acid-based luminal filler with aligned pores offered a neuro-conductive environment for better nerve regeneration, making the biphasic NGC capable of promoting the regrowth of axons across a 10 mm sciatic nerve gap in rat [213].
Multilayered hollow NGCs (figure 3(E)) are another type of structural design that aligns with the strategy of using two or more biomaterials in one system aiming to combine the advantages provided by each component. But the development of multilayered NGCs does not require to make copolymers or polyblends or do any other chemical reactions such as chemical grafting. Instead, it is a convenient approach to build NGCs with good overall performance. For example, figure 3(E–a) illustrates the schematical design of a porous multilayered NGC: the inner-most and the outer-most layers are consisted of a polydopamine (PDA) and arginylglycylaspartic acid (RGD) mixed layer to facilitate cell adhesion, while the inside two layers are constituted by a mix of PCL and single-layered or multilayered graphene to grant electrical conductivity, biodegradability, and mechanical strength [214]. Such a design promoted axonal growth and remyelination after PNI when repairing a 10 mm rat sciatic nerve gap. Figure 3(E-b and E-c) display the typical digital photo and SEM images of multilayered NGCs, where a triple-layered conduit using PCL and gelatin was fabricated by firstly 3D printing of an inner PCL layer, followed by a dip-coating of gelatin hydrogels, and lastly electrospinning of an outer PCL nanofibrous layer [8].
4.2. Multichannel
Underlying the design of multichannel NGCs is that they seem to better resemble the structure of the multiple basal lamina tubes (fascicles) in native nerve trunks as illustrated in figure 2. As a result, they may limit the unwanted axons dispersion compared to that when regenerating across single hollow tubes, which results in inappropriate target reinnervation [216]. Indeed, NGCs with multiple microchannels (figure 3(F)) have been shown to favor PNR in many studies [97, 101, 106, 109, 217]. Rao et al [97] found that ECM-based scaffolds with longitudinally aligned microchannels at desirable channel size (20–50 μm) further enhanced axonal growth, SC migration and fasciculation, and PNR. A recent report by Park and coauthors [106] demonstrated that NGCs with hundreds of microchannels showed promoted stem cell recruitment capability and therefore achieved better functional recovery in comparison to single channel NGCs as evaluated with a 10 mm rat sciatic nerve defect model (figure 3(F–b)).
Studies have also been conducted to investigate the influence of channel number on the property and axonal regeneration potential of NGCs [109, 216]. Tran et al [109] managed to fabricate multichannel NGCs with various channel numbers (from 1 to 5 channels) made of biodegradable CUPE elastomers. Interestingly, it was found that the channel numbers showed no significant influence on the mechanical properties of the CUPE NGCs. Collagen NGCs with 1, 2, 4, and 7 sub-millimeter diameter channels were fabricated and the authors found that 4-channel collagen NGC is a favorable structure for PNR [216].
4.3. Customized
Many of the nerve tissues such as facial trigeminal nerve are bifurcated, making researchers develop NGCs with bifurcated or customized structure to better mimic the nature. The rapid advancement of 3D printing technique makes bifurcated and customized NGCs possible as this technology can conveniently engineer NGCs with complex structure at the aid of computer programs. Johnson et al [218] fabricated a customized conduit containing bifurcating sensory and motor never pathway directly from 3D scanned patient anatomies. This customized conduit achieved successful regeneration of complex nerve injuries across a bifurcated 10 mm rat sciatic nerve and enhanced functional return of the regenerated nerve tissue [218]. As depicted in figure 3(G), customized NGCs with (a) single channel, (b) multichannel, (c) bifurcated, and (d), (e), human life-size NGC mimicking the human facial nerve system have been rapidly and conveniently engineered using a single 3D printing system with the same material combination [101]. Therefore, engineering NGCs with customized shapes, sizes, and microstructural characteristics might be a good future direction. But how to broaden the selection of biomaterials suitable for this technique is a challenging process.
5. Fabrication techniques
5.1. Solvent casting and Dip-coating
Solvent casting and dip-coating represent two of the simplest and economic ways to fabricate hollow NGCs as these methods do not require any expensive instrument except a simple mold system (a mandrel to control the inner diameter while an outer mold to control the wall thickness of NGCs) figures 4(A) and (B) depicts the schematic illustrations of solvent casting [219] and dip-coating [220] methods, respectively. The mold materials are usually some of the inexpensive and slippery materials such as silicone and polytetrafluoroethylene (PTFE) to facilitate demolding. When small particles such as water-soluble salts and sugar particles are mixed with water insoluble polymer solutions, a porous hollow tube then can be easily made by dip-coating of the mixture, followed by particular leaching.
Figure 4.

Different Fabrication techniques of NGCs. (A), Solvent casting. Reprinted from [219], Copyright (2021), with permission from © 2021 The Authors. Publishing services by Elsevier B.V. on behalf of KeAi Communications Co. Ltd (B), Dip-coating. Reproduced from [220]. CC BY 3.0. Copyright © 2010 Shanfeng Wang and Lei Cai. (C), Unidirectional lyophilization. Reprinted from [219], Copyright (2021), with permission from © 2021 The Authors. Publishing services by Elsevier B.V. on behalf of KeAi Communications Co. Ltd (D), Electrospinning. Reprinted with permission from [221]. Copyright (2020) American Chemical Society. (E), 3D printing. Reprinted from [101], Copyright (2018), with permission from Elsevier. (F). Biomimetic. Reproduced from [174]. © The Author(s). Published by IOP Publishing Ltd CC BY 4.0. (G). Wrapping/rolling. Reproduced from [106]. CC BY 4.0
5.2. Lyophilization
The principle of lyophilization is sublimation, which is a process that solid-state solvents directly removed from materials in gas-state under vacuum, leaving behind a porous 3D scaffold. And the pore size and distribution within the scaffold are simply a replicate of those of solid-state solvents. Therefore, one can tune the pore size, porosity, and pore orientation distributed within the scaffolds by controlling the nucleation and crystal growth process of solvents. For example, by applying a unidirectional freezing (figure 4(C)), a chitosan-based NGC with aligned microchannel porosity is obtained, which showed promising functional recovery in combination with other biochemical cues when repairing a 15 mm critical-size rat sciatic nerve defect [219].
5.3. Electrospinning
Electrospinning is a versatile technique able to produce nanofibers from polymer solutions or melts. The arrangement of electrospun fibers is highly tunable and can be made to simulate the hierarchical architectures of ECMs [222], or aligned with any predefined orientations [223]. Since many studies have revealed the importance of aligned topographical cue to guide the migration of SCs and thus guide the axonal outgrowth and ultimately help the bridging of nerve gaps [55, 106, 189, 223], electrospinning standards for one of the most widely used technique to fabricate NGCs with aligned fibrous inner morphologies. However, aligned electrospun fibers alone usually exhibit inferior mechanical strength. It is a common strategy to add an outer layer with randomly oriented fibers to provide a better mechanical support. Figure 4(D) illustrates a workflow using electrospinning technology to make a double-layered polymeric mat with a random and an aligned fibrous layer. Interestingly, a self-forming multichannel NGC can be constructed spontaneously after implantation thanks to the use of a shape memory polymer, poly(lactide-co-trimethylene carbonate).
5.4. 3D printing
3D printing, or additive manufacturing, is the most versatile technology to fabricate NGCs especially those with complex microstructure and customized size and shape. Scaffolds produced by 3D printing also have significantly higher reproductivity as all the process are precisely controlled by computer programs [224]. For example, four distinct designs (figure 3(G)) were manufactured with a single 3D printing platform using a single bioink (GelMA and PEGDA) [101]. The printing platform is depicted in figure 4(E) a digital micromirror device chip which is composed of about four million micromirrors is affiliated to a continuous 3D printer to facilitate the production of customized 3D scaffolds with the aid of either computer-aided design models, or computed tomography scans, or magnetic resonance imaging scans [101]. That means this technology is able to rapidly replicate customized conduits as soon as the 3D anatomical scanning from the patient is available. A drawback of this technique is it rules out many biomaterials with good properties as not all available biomaterials are of good printability.
5.5. Biomimetic
Biomimetic approaches are particularly useful when obtaining cell derived ECM layers. Figure 4(F) shows an example of building an ECM layer atop silk-chitosan composite materials [174]. Briefly, skin-derived precursor SCs were either co-cultured with bundles of silk fibers or injected into chitosan conduits to obtain sufficient ECM secretion, followed by decellularization to get ECM modified fiber bundles or conduits. Then the ECM coated bundles of silk fibers were cut into desired size and inserted into the ECM coated conduits to obtain the final composite scaffolds which are ready for subsequent evaluations. As discussed in section 3.1.3, such a scaffold achieved outstanding overall in vivo performance that is comparable to autografts.
5.6. Wrapping/rolling
Wrapping/rolling is a simple but effective way to fabricate NGCs with various structures. Generally, a thin sheet or mat needs to be premade, which is then wrapped/rolled on its own or against a mandrel. For example, single channel SF nerve conduits with either randomly oriented or aligned SF fibers along the longitudinal direction of the conduit were made by rolling SF sheets on a Teflon-coated steel mandrel [54]. In another study, PLCL thin film with many micrometer-sized grooves was made by bathing the polymer solution into methanal to precipitate PLCL atop a patterned PDMS mold from chloroform. Then the patterned PLCL sheet was rolled against a stainless steel microneedle with a small amount of residual solvent acted as adhesive, forming a homogenous conduit with hundreds of microchannels (figure 4(G)) [106]. A drawback of this method is that the materials used must have certain strength and flexibility to allow for repeatedly rolling and if no appropriate action such as suturing or crosslinking is taken, the wrapped conduit may unwrap on its own after implantation.
6. Commercialized products
It is always one of the ultimate goals for researchers to translate developed technologies from bench to bedside. The global PNI market size was estimated at 1.54 billion in 2023 and is expected to grow at a compound annual growth rate of 7.7% by 2030 [225]. In this section, we attempt to summarize the clearance pathways and classify current commercially available NGCs aimed to repair peripheral nerve gaps in terms of their microstructures and selection of biomaterials. So that to probably provide some guide to those looking to develop new commercialized NGCs.
Table 2 summarizes the materials composition, structural characteristics, storage recommendation, acceptable gap length, along with their regulation pathways of FDA-approved products for peripheral nerve repair. Overall, commercialized NGCs are made of natural biopolymers, synthetic polymers, or a combination of the two. Almost all products need to be stored in a dry environment. NGCs made of type I collagen, polyglycolic acid, and porcine small intestine submucosa need to be stored at room temperature; those made of PLCL need to be stored at an environment lower than room temperature; and as the only commercial NGC made of decellularized human nerve allografts, Avance® needs to be stored in freezer. In addition, most commercial NGCs are indicated for use where gap closure can be achieved by flexion of the extremity; several NGCs are indicated where there is no substantial loss of nerve tissue; the remaining NGCs are intended indicated for use within their acceptable gap lengths. For example, Neurolac® accepts peripheral nerve discontinuity up to 20 mm [226], and Avance® accepts peripheral nerve discontinuity up to 70 mm [227]. Below we classify the commercial products in terms of their microstructures.
Table 2.
FDA-approved commercially available NGCs. Data collected from company websites and FDA database searched with a product code of JXI [228].
| Product | Materials | Structures | Storage conditions | Acceptable gap | Company | FDA clearance or 510 K number | Decision date |
|---|---|---|---|---|---|---|---|
| NeuraGen® Nerve Guide | Type I collagen | Absorbable, semi-permeable, smooth, inner membrane. Absorbable porous outer layer. | Store at room temperature. Avoid excessive heat or humidity. Do not refrigerate. | Gap closure achieved by flexion of the extremity. | Integra LifeSciences Co. | K011168 | 6/22/2001 |
| Neurawrap® Nerve Protector | Type I collagen | Absorbable, semi-permeable inner membrane. Absorbable porous outerlLayer. longitudinal slit. | Store at room temperature. Avoid excessive heat or humidity. Do not refrigerate. | No substantial loss of nerve tissue. | Integra LifeSciences Co. | K041620 | 7/16/2004 |
| NeuraGen® 3D Nerve Guide Matrix | Type I bovine collagen, glycosam inoglycan | Resorbable, semi-permeable, porous membrane. Resorbable, longitudinally aligned porous matrix. | 10 °C–30 °C. | Gap closure achieved by flexion of the extremity. | Integra LifeSciences Co. | K163457 | 1/6/2017 |
| NeuroMatrix® Conduit | Type I collagen | Resorbable, semi-permeable, collagen tubular matrix. | N/A | Gap closure achieved by flexion of the extremity. | Collagen Matrix, Inc. | K012814 | 9/21/2001 |
| NeuroMend® Wrap | Type I collagen | Resorbable, semi-permeable, self-curl structure with a longitudinal slit. | N/A | Gap closure achieved by flexion of the extremity. | Collagen Matrix, Inc. | K060952 | 7/14/2006 |
| Neuroflex® Conduit | Type I Collagen | Flexible, resorbable, semi-permeable, collagen tubular matrix. | 15 °C–30 °C Avoid excessive heat or humidity. | Gap closure achieved by flexion of the extremity. | Collagen Matrix, Inc. | K131541 | 4/3/2014 |
| Neurolac® Nerve Guide | poly (DL-lactide-ε-caprolactone) | Bioresorbable, high transparency enable, synthetic tubular structure. | −18 °C–8 °C Store in a dark, dry place. | Up to 20 mm. | Polyganics BV | K050573 | 5/4/2005 |
| Neurolac® TW Nerve Guide | poly (DL-lactide-ε-caprolactone) | Bioresorbable, high transparency enable, synthetic thin-wall tubular structure. | −18 °C–8 °C Store in a dark, dry place. | Up to 20 mm. | Polyganics BV | K112267 | 10/20/2011 |
| Neurotube® Nerve Conduit | polyglycolic acid | Absorbable woven polyglycolic acid mesh tube. | 20 °C–30 °C. | More than or equal to 8 mm, but less than or equal to 30 mm. | Synovis MCA | K983007 | 3/22/1999 |
| Nerbridge® Nerve Regeneration Guidance Conduit | Polyglycolic acid, collagen | Flexible, resorbable, semi-permeable tubular membrane matrix. Inner porous collagen scaffold. | 1 °C–30 °C Avoid excessive heat or humidity. | Gap closure achieved by flexion of the extremity. | TOYOBO Co. | K152967 | 6/22/2016 |
| Reaxon® Direct | Chitosan | Flexible, resorbable, chitosan transparent tube. | N/A | Gap closure achieved by flexion of the extremity. | Medovent GmbH | K180222 | 4/24/2018 |
| AxoGuard nerve Protector® | Porcine small intestine submucosa | Bioabsorbable, porous extracellular matrix. | Stored in a clean, dry location at room temperature. | Gap closure achieved by flexion of the extremity. | Cook Biotech Inc. | K132660 | 01/10/2014 |
| Avance® nerve graft | Human nerve allograft | Decellularized and cleansed extracellular matrix. | −20–− 40 °C Storage is limited to 6 months. | Less than or equal to 70 mm. | AxoGen Co. | Cleared under FDA 21 CFP Part 1271 regulations | 5/4/2015 |
| AxoGuard nerve connector® | Porcine small intestine submucosa | Flexible, resorbable, porous, semi-translucent tube. | Stored in a clean, dry location at room temperature. | Gap closure achieved by flexion of the extremity. | Cook Biotech Inc. | K162741 | 10/31/2016 |
| Axoguard HA+ Nerve Protector® | Porcine small intestinal submucosa, sodium hyaluronate, sodium alginate | Remodelling extracellular matrix base-layer, double-side resorbable hyaluronate-alginate gel coating. | Stored in a clean, dry location at room temperature. | No gap allowed. | AxoGen Co. | K223640 K231708 |
04/07/2023 10/12/2023 |
| NeuroShield™ | Chitosan | Bioabsorbable, porous transparent membrane. | N/A | Gap closure achieved by flexion of the extremity. | Monarch Bioimplants GmbH | K190246 | 5/31/2019 |
| SaluBridge™ Nerve Cuff | Polyvinyl alcohol, saline. | Flexible tubular sheath. | N/A | Gap closure achieved by flexion of the extremity. | SaluMedica, L.L.C. | K002098 | 11/24/2000 |
| SaluTunnel™ Nerve Protector | Polyvinyl alcohol, saline. | Flexible tubular sheath with longitudinal slit. | N/A | No substantial loss of nerve tissue. | SaluMedica, L.L.C. | K100382 | 08/05/2010 |
6.1. Single channel hollow tubular structure
Hollow tubular NGCs provide a protective environment for the repairing of peripheral nerves after injury. It stands for the most fundamental structure, and thus most commercialized products follow this structural design. Common materials used in producing the hollow tubular NGCs are type I collagen, PLCL, PGA, chitosan, and porcine small intestine submucosa. Several examples of FDA-approved commercial hollow tubular NGCs are NeuraGen® Nerve Guide, NeuroMatrix® Conduit, Neuroflex® Conduit, Neurolac® Nerve Guide, Neurolac® TW Nerve Guide, Neurotube® Nerve Conduit, Reaxon® Direct, AxoGuard nerve connector®, and SaIuBridge™ Nerve Cuff.
6.2. Single channel tubular structure with fillers
As discussed in section 4.1, filler materials are inserted into the hollow portion of hollow tubular NGCs to guide SCs and aid in axonal regeneration. For example, NeuraGen® 3D Neural Guidance Matrix has a hollow section filled with glycosaminoglycan [3], and Nerbridge® Nerve Regeneration Guidance Conduit has a hollow section filled with collagen [10]. The materials used in the outer layers of the filled tubular NGCs are essentially the same as those used in hollow tubular NGCs, which means that the storage conditions of the filled tubular structures are similar to those of hollow tubular NGCs.
6.3. Wrapped structures
Wrapped NGCs are available for a more convenient surgery process as they can be applied in clinic without the process of inserting the defects end into a conduit. Instead, a longitudinal slit is added to the conduit so that it can be opened and placed easily over the injured nerve. Since the wrapped NGCs are a sheet-like structure, additional sutures are required to secure the longitudinal seams when installing the wrapped NGCs. Aside from the longitudinal slit, the materials and structures of several wrapped NGCs are identical to their hollow tubular version, which results in similar storage conditions. Some wrapped NGCs also add a coating layer to their surface. For example, Axoguard HA+ Nerve Protector® adds a short-term resorbable hyaluronate-alginate gel coating to enhance nerve glide and reduce soft tissue attachment [229]. Several other example of commercially approved wrapped NGCs are Neurawrap® Nerve Protector, NeuroMend® Wrap, AxoGuard nerve Protector®, NeuroShield™, and SaluTunnel™ Nerve Protector.
6.4. Biomimetic structure
Biomimetic NGCs are quite different compared to the first three structures. The grafted NGCs are decellularized human nerve tissue derived and cleansed ECM. It is an allograph with high biomimetic structure and therefore showed one of the most powerful abilities to repair up to 70 mm peripheral nerve gap, serving as a good alternative of autografts. However, as a decellularized tissue ECM, it has a relatively short shelf life (six months when stored between −20 °C to −40 °C. Additionally, the Avance® nerve graft received non-510 clearance from the FDA in 2015 (under the FDA 21 CFP Part 1271 regulations).
7. Current challenges, future perspectives, and conclusions
Although great efforts have been attempted by many researchers and encouraging results have been reported in many studies, it is still a big challenge nowadays to design artificial NGCs that can outperform or perform comparably to autografts especially when repairing long peripheral nerve gaps. For example, many of the studies discussed here used an 8–10 mm rat sciatic nerve defect as animal model with only a few demonstrated acceptable performances when bridging a 12–15 mm gaps. In addition, most of the current animal studies are done on rats or rabbits, which are not suitable models particularly when aiming at repairing human critical peripheral nerve defects (5–30 cm) due to its small size (⩽3 cm) and species-specific neurobiological regenerative profile [35]. It is necessary to conduct more in vivo studies with longer gaps in large animals before the developed NGCs can be transferred from bench to bedside.
Given the complex structure of the peripheral nervous system, designing and creating NGCs with ideal structures that closely resemble the anatomy of native nerve tissues remains challenging. For example, while NGCs with surface grooves have shown favorable results in guiding the migration and proliferation of SCs, the optimal depths and widths of these grooves are still debated. Additionally, the swelling of NGCs made from different biomaterials, each with varying swelling ratios, along with the infiltration of cells and tissues post-implantation, will undoubtedly alter the groove dimensions, further complicating their design. This issue also arises when selecting the channel diameter of multichannel scaffolds. Therefore, when designing multichannel NGCs or NGCs with surface patterns, we suggest selecting biomaterials with a low swelling ratio or accounting for swelling when calculating the size of surface patterns or inner channel diameters.
The selection of biomaterials remains another challenge. For instance, ECM-based materials demonstrated close resemblance of native nerve tissues. However, one of the current major limitations of ECM involves the use of Matrigel, an ill-defined mixture of ECM proteins and GFs derived from Engelbreth-Holm-Swarm mouse tumor, which may cause concerns of pathologies transfer [230]. The recently developed endometrial extracellular matrixbased hydrogels provide an alternative to overcome this issue [230]. Such obtained hydrogels supported the growth of mouse and human endometrial organoids that are comparable to Matrigel. We feel that this could be a future direction to develop ECM-based hydrogels for PNR which is to directly derive ECM from a native healthy nerve tissue without the use of Matrigel to decrease the chance of pathologies transfer.
In addition, many current NGCs lack sufficient bio-functionalities to achieve nerve regeneration potential comparable to that of autografts. Increasing evidence has highlighted the promising potential of extracellular vesicles (EVs) including microvesicles and exosomes that secreted by various types of cells in promoting tissue regeneration [231, 232]. These EVs carry intracellular cargo such as lipids, proteins, and genetic nucleic acids, which facilitate intercellular communication and thus play important roles in regenerative medicine by orchestrating the cell recruitment, differentiation, and immunomodulation processes [233]. Compared to cell-based therapies, EVs not only hold similar therapeutic functions to those of their parent cells, but also possess lower immunogenicity likely due to their less abundant transmembrane proteins [232, 234]. Particularly, EVs derived from SCs or mesenchymal stem cells (MSCs) have been demonstrated to improve nerve functional recovery by increasing the formation of blood vessels and axonal sprouts [235, 236]. Nevertheless, a major hurdle in EVs-based therapies for PNR is the limited production and off-target release of these vesicles. We believe this could be possibly tackled by applying EVs-based therapies in combination with advanced NGCs, or in other words by applying NGCs with capability to deliver EVs locally and sustainedly in a controlled manner. A recent study indicated that F127-polycitrate-polyethyleneimine (FE) hydrogel could be used as an excellent carrier to deliver EVs to the nerve lesion site for up to 56 d, owing to the good electrostatic interaction between FE hydrogel and EVs [237]. In another attempt, local production and controlled release of EVs were realized by encapsulating SCs into a superparamagnetic hydrogel, which could manipulate the location and concentration of EVs secreted from SCs by adjusting the external rotation magnetic field [238]. The optimized design ultimately resulted in accelerated nerve regeneration and functional recovery in rats by simultaneously promoting axon growth, angiogenesis, and inflammatory regulation. It has been demonstrated that some other physical signals like mechanical [239, 240], electrical [241], and acoustic [242] stimulations could boost the secretion of EVs. In addition, environmental factors like hypoxia [243] and acidity [244], or the incorporation of chemical molecules like iron [245], could also promote the secretion of EVs. Designing NGCs with capability to controlled release of EVs by manipulating external stimulation or regulating the local microenvironment could be a promising future direction for leveraging EVs-based therapy to enhance PNR.
Overall, the expectation of implanting NGCs for long gap PNR has transcended the mere provision of mechanical support for guiding axonal regrowth. It now necessitates the ability to regenerate or even to innervate nerve tissues involving regenerative medicine, which aims to restore tissue and organ function without the use of permanent implants by applying various biodegradable proregenerative biomaterials [246]. The biofunctionalization of NGCs emerges as an effective strategy to enhance the regenerative potential of biomaterials. This involves incorporating biological cues to regulate the overall PNR process, which is a multifaceted procedure encompassing the proliferation and migration of SCs, the recruitment of macrophages, axon growth and myelination, vascular regeneration, and inflammation control. As discussed previously, selecting biomaterials with intrinsic biofunctions such as epigenetic regulation, angiogenetic response, and immunomodulatory effect might be an effective step to fulfill the bio-functionality of advanced NGCs. The selection of biomaterials poses another challenge as a single component is typically insufficient to satisfy all the material requirements discussed in section 2. From this aspect of view, materials with multiple functions should be given higher priority. For instance, folic acid has been recognized as an effective molecule to play epigenetic regulation for enhanced nerve regeneration through DNA methylation [59–61]. It has also been demonstrated to influence the immunomodulatory process by suppressing inflammation, which is a potential mechanism to promote PNR [247–249]. Similarly, citric acid as an intermediate in the Krebs circle has been revealed to fuel the metabolism of MSCs and facilitate bone regeneration through ‘metabonegenic’ regulation [27, 246, 250]. Citric acid also possesses excellent antioxidant, antibacterial, and immunomodulatory effects [198, 199]. It is noted that both folic acid and citric acid are able to increase the local acidity, which is beneficial to the secretion of EVs from cells that may eventually facilitate PNR. An alternative way in regarding to selections of biomaterial might be combining multiple components to develop composite materials. However, this brings up new complications. For example, even one of the most commonly applied copolymers, PLGA, increasing of the degradation rate is always associated with a sacrifice of mechanical strength. Balancing each material property and identifying the optimal combination that confers excellent overall PNR ability is a meticulous process. This task is further complicated by the fact that different patients may exhibit distinct reactions to the same materials system. Designing customized materials with the aid of artificial intelligence (AI) could possibly tackle this issue [251–253].
Although current commercially available NGCs have demonstrated some encouraging outcomes, many were approved via the 510 K pathway, which requires devices to be as safe and effective as, or substantially equivalent (SE) to, a previously marked device. This makes it challenging to significantly improve the nerve regeneration potential of the newly marketed devices, as incorporating additional bio-functionalities is difficult to receive FDA clearance. Besides, no commercial products currently offer customized NGCs tailored to the specific location, shape, and size of the defect in patients. As discussed above, future devices may address this issue with advancements in AI. Additionally, the potential to repair nerve defects longer than 30 mm remains poor [254]. We believe that the further addition of bio-functionalities is essential to revolutionize commercial NGCs in hope of repairing long peripheral nerve gaps in clinics. However, this will be a long process, as adding more complexity to the design of commercial NGCs requires substantial evidence to justify the safety and efficacy of the device for FDA approval.
In conclusion, by presenting the anatomy of peripheral nerve tissue and understanding the nerve regeneration mechanisms, we discussed several material requirements of NGCs for PNR. With a focus on the discussion of various types of biodegradable polymers, including both natural and synthetic ones, we then compared some of the commonly used structural designs and fabrication techniques of NGCs. Lastly, we did a survey and comparison of commercially available NGCs for nerve repair.
Acknowledgments
This work was partially supported by Nationals Institute of Health Grants (R01NS123433, and R01HL158204).
Footnotes
Conflict of interest
Dr Jian Yang and The Pennsylvania State University have a financial interest in Acuitive Technologies, Inc. and Aleo BME, Inc. These interests have been reviewed by the University’s Institutional and Individual Conflict of Interest Committees and are currently being managed by the University.
Data availability statement
No new data were created or analysed in this study.
References
- [1].Lee SK and Wolfe SW 2000. Peripheral nerve injury and repair J. Am. Acad. Orthop. Surg 8 243–52 [DOI] [PubMed] [Google Scholar]
- [2].Patel NP, Lyon KA and Huang JH 2018. An update–tissue engineered nerve grafts for the repair of peripheral nerve injuries Neural Regen. Res 13 764–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Lin Y-C and Marra KG 2012. Injectable systems and implantable conduits for peripheral nerve repair Biomed. Mater 7 024102. [DOI] [PubMed] [Google Scholar]
- [4].Huebner EA and Strittmatter SM 2009. Axon regeneration in the peripheral and central nervous systems Cell Biology of the Axon (Springer; ) pp 305–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Yu L, Cavelier S, Hannon B and Wei M 2023. Recent development in multizonal scaffolds for osteochondral regeneration Bioact. Mater 25 122–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Seddon H 1975. Surgical disorders of the peripheral nerves pp xiii,336–xiii
- [7].Sunderland S 1968. Nerves and Nerve Injuries (Edinburgh, E& S Livingstone Ltd; ) [Google Scholar]
- [8].Liu S, Sun L, Zhang H, Hu Q, Wang Y and Ramalingam M 2021. High-resolution combinatorial 3D printing of gelatin-based biomimetic triple-layered conduits for nerve tissue engineering Int. J. Biol. Macromol. 166 1280–91 [DOI] [PubMed] [Google Scholar]
- [9].Robinson LR 2000. Traumatic injury to peripheral nerves Muscle Nerve 23 863–73 [DOI] [PubMed] [Google Scholar]
- [10].Qian Y, Lin H, Yan Z, Shi J and Fan C 2021. Functional nanomaterials in peripheral nerve regeneration: scaffold design, chemical principles and microenvironmental remodeling Mater. Today 51 165–87 [Google Scholar]
- [11].Kaya Y and Sarikcioglu L 2015 Sir Herbert Seddon (1903–1977) and his classification scheme for peripheral nerve injury Child’s Nerv. Syst 31 177–80 [DOI] [PubMed] [Google Scholar]
- [12].Brück W 1997. The role of macrophages in Wallerian degeneration Brain Pathol. 7 741–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Liu P, Peng J, Han G-H, Ding X, Wei S, Gao G, Huang K, Chang F and Wang Y 2019. Role of macrophages in peripheral nerve injury and repair Neural Regen. Res 14 1335–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Panzer KV, Burrell JC, Helm KV, Purvis EM, Zhang Q, Le AD, O’Donnell JC and Cullen DK 2020. Tissue engineered bands of büngner for accelerated motor and sensory axonal outgrowth Front. Bioeng. Biotechnol 8 580654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Arslantunali D, Dursun T, Yucel D, Hasirci N and Hasirci V 2014. Peripheral nerve conduits: technology update Med. Devices 7 405–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Hu Y, Wu Y, Gou Z, Tao J, Zhang J, Liu Q, Kang T, Jiang S, Huang S and He J 2016. 3D-engineering of cellularized conduits for peripheral nerve regeneration Sci. Rep. 6 1–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Bellamkonda RV 2006. Peripheral nerve regeneration: an opinion on channels, scaffolds and anisotropy Biomaterials 27 3515–8 [DOI] [PubMed] [Google Scholar]
- [18].Kim Y-T, Haftel VK, Kumar S and Bellamkonda RV 2008. The role of aligned polymer fiber-based constructs in the bridging of long peripheral nerve gaps Biomaterials 29 3117–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Marquardt LM and Sakiyama-Elbert SE 2013. Engineering peripheral nerve repair Curr. Opin. Biotechnol. 24 887–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Xiong Y, Mi B-B, Lin Z, Hu Y-Q, Yu L, Zha K-K, Panayi AC, Yu T, Chen L and Liu Z-P 2022. The role of the immune microenvironment in bone, cartilage, and soft tissue regeneration: from mechanism to therapeutic opportunity Mil. Med. Res 9 65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Rodrigues M, Kosaric N, Bonham CA and Gurtner GC 2019. Wound healing: a cellular perspective Physiol. Rev. 99 665–706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Kaplan B and Levenberg S 2022. The role of biomaterials in peripheral nerve and spinal cord injury: a review Int. J. Mol. Sci. 23 1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Hirakawa H, Okajima S, Nagaoka T, Kubo T, Takamatsu T and Oyamada M 2004. Regional differences in blood–nerve barrier function and tight-junction protein expression within the rat dorsal root ganglion Neuroreport 15 405–8 [DOI] [PubMed] [Google Scholar]
- [24].Flores AJ, Lavemia C and Owens PW 2000. Anatomy and physiology of peripheral nerve injury and repair Am. J. Orthop 29 167–73 (available at: http://europepmc.org/abstract/MED/10746467) [PubMed] [Google Scholar]
- [25].Ilfeld BM, Preciado J and Trescot AM 2016. Novel cryoneurolysis device for the treatment of sensory and motor peripheral nerves Expert Rev. Med. Devices 13 713–25 [DOI] [PubMed] [Google Scholar]
- [26].Jung O, Smeets R, Hartjen P, Schnettler R, Feyerabend F, Klein M, Wegner N, Walther F, Stangier D and Henningsen A 2019. Improved in vitro test procedure for full assessment of the cytocompatibility of degradable magnesium based on ISO 10993–5/-12 Int. J. Mol. Sci. 20 255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Ma C, Tian X, Kim JP, Xie D, Ao X, Shan D, Lin Q, Hudock MR, Bai X and Yang J 2018. Citrate-based materials fuel human stem cells by metabonegenic regulation Proc. Natl Acad. Sci 115 E11741–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].De Ruiter G C, Malessy MJ, Yaszemski MJ, Windebank AJ and Spinner RJ 2009. Designing ideal conduits for peripheral nerve repair Neurosurg. Focus 26 E5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Lundborg G, Gelberman RH, Longo FM, Powell HC and Varon S 1982. In vivo regeneration of cut nerves encased in silicone tubes: growth across a six-millimeter gap J. Neuropathol. Exp. Neurol. 41 412–22 [DOI] [PubMed] [Google Scholar]
- [30].Schlosshauer B, Dreesmann L, Schaller H-E and Sinis N 2006. Synthetic nerve guide implants in humans: a comprehensive survey Neurosurgery 59 740–8 [DOI] [PubMed] [Google Scholar]
- [31].Nectow AR, Marra KG and Kaplan DL 2012. Biomaterials for the development of peripheral nerve guidance conduits Tissue Eng. B 18 40–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Harley B, Spilker M, Wu J, Asano K, Hsu H-P, Spector M and Yannas I 2004. Optimal degradation rate for collagen chambers used for regeneration of peripheral nerves over long gaps Cells Tissues Organs 176 153–65 [DOI] [PubMed] [Google Scholar]
- [33].Muheremu A and Ao Q 2015. Past, present, and future of nerve conduits in the treatment of peripheral nerve injury Biomed. Res. Int. 2015 237507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Gutmann E, Guttmann L, Medawar P and Young J 1942. The rate of regeneration of nerve J. Exp. Biol 19 14–44 [Google Scholar]
- [35].Kaplan HM, Mishra P and Kohn J 2015. The overwhelming use of rat models in nerve regeneration research may compromise designs of nerve guidance conduits for humans J. Mater. Sci., Mater. Med 26 1–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Williams LR, Longo FM, Powell HC, Lundborg G and Varon S 1983. Spatial-temporal progress of peripheral nerve regeneration within a silicone chamber: parameters for a bioassay J. Comp. Neurol. 218 460–70 [DOI] [PubMed] [Google Scholar]
- [37].Höke A 2011. A (heat) shock to the system promotes peripheral nerve regeneration J. Clin. Invest. 121 4231–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Gordon T, Eva P and Borschel GH 2015. Delayed peripheral nerve repair: methods, including surgical ‘cross-bridging’to promote nerve regeneration Neural Regen. Res 10 1540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Ma CHE, Omura T, Cobos EJ, Latrémolière A, Ghasemlou N, Brenner GJ, Van Veen E, Barrett L, Sawada T and Gao F 2011. Accelerating axonal growth promotes motor recovery after peripheral nerve injury in mice J. Clin. Invest. 121 4332–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Zheng F, Li R, He Q, Koral K, Tao J, Fan L, Xiang R, Ma J, Wang N and Yin Y 2020. The electrostimulation and scar inhibition effect of chitosan/oxidized hydroxyethyl cellulose/reduced graphene oxide/asiaticoside liposome based hydrogel on peripheral nerve regeneration in vitro Mater. Sci. Eng. C 109 110560. [DOI] [PubMed] [Google Scholar]
- [41].Howarth HM, Alaziz T, Nicolds B, O’Connor S and Shah SB 2019. Redistribution of nerve strain enables end-to-end repair under tension without inhibiting nerve regeneration Neural Regen. Res 14 1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Wang Y, Liang R, Lin J, Chen J, Zhang Q, Li J, Wang M, Hui X, Tan H and Fu Q 2021. Biodegradable polyurethane nerve guide conduits with different moduli influence axon regeneration in transected peripheral nerve injury J. Mater. Chem. B 9 7979–90 [DOI] [PubMed] [Google Scholar]
- [43].Rosso G and Guck J 2019. Mechanical changes of peripheral nerve tissue microenvironment and their structural basis during development APL Bioeng. 3 036107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Dinis T, Elia R, Vidal G, Dermigny Q, Denoeud C, Kaplan D, Egles C and Marin F 2015. 3D multi-channel bi-functionalized silk electrospun conduits for peripheral nerve regeneration J. Mech. Behav. Biomed. Mater 41 43–55 [DOI] [PubMed] [Google Scholar]
- [45].Borschel GH, Kia KF, Kuzon WM Jr and Dennis RG 2003. Mechanical properties of acellular peripheral nerve J. Surg. Res. 114 133–9 [DOI] [PubMed] [Google Scholar]
- [46].Zilic L, Garner PE, Yu T, Roman S, Haycock JW and Wilshaw SP 2015. An anatomical study of porcine peripheral nerve and its potential use in nerve tissue engineering J. Anat 227 302–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Kerns J, Piponov H, Helder C, Amirouche F, Solitro G and Gonzalez M 2019. Mechanical properties of the human tibial and peroneal nerves following stretch with histological correlations Anat. Rec. 302 2030–9 [DOI] [PubMed] [Google Scholar]
- [48].Botero SS, Honecker S, Jmal H, Bahlouli N, Liverneaux PA and Facca S 2018. The biomechanical properties of 44 human digital collateral nerves from fresh frozen cadavers J. Cell. Immunotherapy 4 38–40 [Google Scholar]
- [49].Janmey PA and McCulloch CA 2007. Cell mechanics: integrating cell responses to mechanical stimuli Annu. Rev. Biomed. Eng 9 1–34 [DOI] [PubMed] [Google Scholar]
- [50].Schoen I, Pruitt BL and Vogel V 2013. The Yin-Yang of rigidity sensing: how forces and mechanical properties regulate the cellular response to materials Annu. Rev. Mater. Res 43 589–618 [Google Scholar]
- [51].Gu Y, Ji Y, Zhao Y, Liu Y, Ding F, Gu X and Yang Y 2012. The influence of substrate stiffness on the behavior and functions of Schwann cells in culture Biomaterials 33 6672–81 [DOI] [PubMed] [Google Scholar]
- [52].Gunn JW, Turner SD and Mann BK 2005. Adhesive and mechanical properties of hydrogels influence neurite extension J. Biomed. Mater. Res. A 72 91–97 [DOI] [PubMed] [Google Scholar]
- [53].Leipzig ND and Shoichet MS 2009. The effect of substrate stiffness on adult neural stem cell behavior Biomaterials 30 6867–78 [DOI] [PubMed] [Google Scholar]
- [54].Madduri S, Papaloizos M and Gander B 2010. Trophically and topographically functionalized silk fibroin nerve conduits for guided peripheral nerve regeneration Biomaterials 31 2323–34 [DOI] [PubMed] [Google Scholar]
- [55].Zhu L, Jia S, Liu T, Yan L, Huang D, Wang Z, Chen S, Zhang Z, Zeng W and Zhang Y 2020. Aligned PCL fiber conduits immobilized with nerve growth factor gradients enhance and direct sciatic nerve regeneration Adv. Funct. Mater. 30 2002610 [Google Scholar]
- [56].Vögelin E, Baker J, Gates J, Dixit V, Constantinescu MA and Jones N 2006. Effects of local continuous release of brain derived neurotrophic factor (BDNF) on peripheral nerve regeneration in a rat model Exp. Neurol. 199 348–53 [DOI] [PubMed] [Google Scholar]
- [57].Shin JE and Cho Y 2017. Epigenetic regulation of axon regeneration after neural injury Mol. Cells 40 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Oh YM, Mahar M, Ewan EE, Leahy KM, Zhao G and Cavalli V 2018. Epigenetic regulator UHRF1 inactivates REST and growth suppressor gene expression via DNA methylation to promote axon regeneration Proc. Natl Acad. Sci 115 E12417–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Iskandar BJ, Rizk E, Meier B, Hariharan N, Bottiglieri T, Finnell RH, Jarrard DF, Banerjee RV, Skene JP and Nelson A 2010. Folate regulation of axonal regeneration in the rodent central nervous system through DNA methylation J. Clin. Invest. 120 1603–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Kim GB, Chen Y, Kang W, Guo J, Payne R, Li H, Wei Q, Baker J, Dong C and Zhang S 2018. The critical chemical and mechanical regulation of folic acid on neural engineering Biomaterials 178 504–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Madrid A, Alisch RS, Rizk E, Papale LA, Hogan KJ and Iskandar BJ 2023. Transgenerational epigenetic inheritance of axonal regeneration after spinal cord injury Environ. Epigenet 9 dvad002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Saio S, Konishi K, Hohjoh H, Tamura Y, Masutani T, Iddamalgoda A, Ichihashi M, Hasegawa H and Mizutani K-I 2021. Extracellular environment-controlled angiogenesis, and potential application for peripheral nerve regeneration Int. J. Mol. Sci. 22 11169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Hobson MI, Green CJ and Terenghi G 2000. VEGF enhances intraneural angiogenesis and improves nerve regeneration after axotomy J. Anat. 197 591–605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Donzelli R, Capone C, Sgulò FG, Mariniello G and Maiuri F 2016. Vascularized nerve grafts: an experimental study Neurol. Res 38 669–77 [DOI] [PubMed] [Google Scholar]
- [65].Saffari TM, Bedar M, Hundepool CA, Bishop AT and Shin AY 2020. The role of vascularization in nerve regeneration of nerve graft Neural Regen. Res 15 1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Wu P, Tong Z, Luo L, Zhao Y, Chen F, Li Y, Huselstein C, Ye Q, Ye Q and Chen Y 2021. Comprehensive strategy of conduit guidance combined with VEGF producing Schwann cells accelerates peripheral nerve repair Bioact. Mater 6 3515–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Lee J-Y, Giusti G, Friedrich PF, Bishop AT and Shin AY 2016. Effect of vascular endothelial growth factor administration on nerve regeneration after autologous nerve grafting J. Reconstr. Microsurg 32 183–8 [DOI] [PubMed] [Google Scholar]
- [68].Li X, Guan Y, Li C, Zhang T, Meng F, Zhang J, Li J, Chen S, Wang Q and Wang Y 2022. Immunomodulatory effects of mesenchymal stem cells in peripheral nerve injury Stem Cell Res. Ther 13 1–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Zhang B, Su Y, Zhou J, Zheng Y and Zhu D 2021. Toward a better regeneration through implant-mediated immunomodulation: harnessing the immune responses Adv. Sci 8 2100446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Julier Z, Park AJ, Briquez PS and Martino MM 2017. Promoting tissue regeneration by modulating the immune system Acta Biomater. 53 13–28 [DOI] [PubMed] [Google Scholar]
- [71].Mokarram N, Merchant A, Mukhatyar V, Patel G and Bellamkonda RV 2012. Effect of modulating macrophage phenotype on peripheral nerve repair Biomaterials 33 8793–801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Sun Y, Zhang H, Zhang Y, Liu Z, He D, Xu W, Li S, Zhang C and Zhang Z 2023. Li–Mg–Si bioceramics provide a dynamic immuno-modulatory and repair-supportive microenvironment for peripheral nerve regeneration Bioact. Mater 28 227–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Moran HB, Turley JL, Andersson M and Lavelle EC 2018. Immunomodulatory properties of chitosan polymers Biomaterials 184 1–9 [DOI] [PubMed] [Google Scholar]
- [74].Williams NC and O’Neill LA 2018. A role for the Krebs cycle intermediate citrate in metabolic reprogramming in innate immunity and inflammation Front. Immunol. 9 141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Fernández-Villa D, Aguilar MR and Rojo L 2019. Folic acid antagonists: antimicrobial and immunomodulating mechanisms and applications Int. J. Mol. Sci. 20 4996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Chen F, Wu M, Wu P, Xiao A, Ke M, Huselstein C, Cai L, Tong Z and Chen Y 2021. Natural flammulina velutipes-based nerve guidance conduit as a potential biomaterial for peripheral nerve regeneration: in vitro and in vivo studies ACS Biomater. Sci. Eng 7 3821–34 [DOI] [PubMed] [Google Scholar]
- [77].Meek MF and Den Dunnen W F 2009. Porosity of the wall of a Neurolac® nerve conduit hampers nerve regeneration Microsurgery 29 473–8 [DOI] [PubMed] [Google Scholar]
- [78].Kemp SW, Syed S, Walsh SK, Zochodne DW and Midha R 2009 Collagen nerve conduits promote enhanced axonal regeneration, schwann cell association, and neovascularization compared to silicone conduits Tissue Eng. A 15 1975–88 [DOI] [PubMed] [Google Scholar]
- [79].Choe G, Han UG, Ye S, Kang S, Yoo J, Cho YS and Jung Y 2023. Effect of electrical stimulation on nerve-guided facial nerve regeneration ACS Biomater. Sci. Eng 9 3512–21 [DOI] [PubMed] [Google Scholar]
- [80].Nguyen HT, Sapp S, Wei C, Chow JK, Nguyen A, Coursen J, Luebben S, Chang E, Ross R and Schmidt CE 2014. Electric field stimulation through a biodegradable polypyrrole-co-polycaprolactone substrate enhances neural cell growth J. Biomed. Mater. Res. A 102 2554–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Stölting MN, Arnold AS, Haralampieva D, Handschin C, Sulser T and Eberli D 2016. Magnetic stimulation supports muscle and nerve regeneration after trauma in mice Muscle Nerve 53 598–607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Chen J, Zhou X-J and Sun R-B 2020. Effect of the combination of high-frequency repetitive magnetic stimulation and neurotropin on injured sciatic nerve regeneration in rats Neural Regen. Res 15 145–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Ma Y, Wang H, Wang Q, Cao X and Gao H 2023. Piezoelectric conduit combined with multi-channel conductive scaffold for peripheral nerve regeneration Chem. Eng. J. 452 139424 [Google Scholar]
- [84].Qian Y, Xu Y, Yan Z, Jin Y, Chen X, Yuan W-E and Fan C 2021. Boron nitride nanosheets functionalized channel scaffold favors microenvironment rebalance cocktail therapy for piezocatalytic neuronal repair Nano Energy 83 105779 [Google Scholar]
- [85].Zheng N, Fitzpatrick V, Cheng R, Shi L, Kaplan DL and Yang C 2022. Photoacoustic carbon nanotubes embedded silk scaffolds for neural stimulation and regeneration Acs Nano 16 2292–305 [DOI] [PubMed] [Google Scholar]
- [86].Park SC, Oh SH, Seo TB, Namgung U, Kim JM and Lee JH 2010. Ultrasound-stimulated peripheral nerve regeneration within asymmetrically porous PLGA/Pluronic F127 nerve guide conduit J. Biomed. Mater. Res. B 94 359–66 [DOI] [PubMed] [Google Scholar]
- [87].Kim JR, Oh SH, Kwon GB, Namgung U, Song KS, Jeon BH and Lee JH 2013. Acceleration of peripheral nerve regeneration through asymmetrically porous nerve guide conduit applied with biological/physical stimulation Tissue Eng. A 19 2674–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Huang W-C, Lin C-C, Chiu T-W and Chen S-Y 2022. 3D gradient and linearly aligned magnetic microcapsules in nerve guidance conduits with remotely spatiotemporally controlled release to enhance peripheral nerve repair ACS Appl. Mater. Interfaces 14 46188–200 [DOI] [PubMed] [Google Scholar]
- [89].Giannaccini M, Calatayud MP, Poggetti A, Corbianco S, Novelli M, Paoli M, Battistini P, Castagna M, Dente L and Parchi P 2017. Magnetic nanoparticles for efficient delivery of growth factors: stimulation of peripheral nerve regeneration Adv. Healthcare Mater 6 1601429. [DOI] [PubMed] [Google Scholar]
- [90].Zhang H, Wang H, Wen B, Lu L, Zhao Y and Chai R 2023. Ultrasound-responsive composited conductive silk conduits for peripheral nerve regeneration Small Struct. 4 2300045 [Google Scholar]
- [91].Zhu Y, Jin Z, Wang J, Chen S, Hu Y, Ren L, Wang Y, Song Q, Tian X and Xie F 2020. Ultrasound-guided platelet-rich plasma injection and multimodality ultrasound examination of peripheral nerve crush injury npj Regen. Med 5 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Donsante A, Xue J, Poth KM, Hardcastle NS, Diniz B, O’Connor DM, Xia Y and Boulis NM 2020. Controlling the release of neurotrophin-3 and chondroitinase ABC enhances the efficacy of nerve guidance conduits Adv. Healthcare Mater 9 2000200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Jiang L, Wu X, Wang Y, Liu C, Wu Y, Wang J, Xu N, He Z, Wang S and Zhang H 2023. Photothermal controlled-release immunomodulatory nanoplatform for restoring nerve structure and mechanical nociception in infectious diabetic ulcers Adv. Sci 10 2300339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Li Y, Chen Z, Zhou J, Guan Y, Xing J, Niu Z, Zhang B, Zeng Q, Pei X and Wang Y 2023. Combining chitin biological conduits with injectable adipose tissue-derived decellularised matrix hydrogels loaded with adipose-derived mesenchymal stem cells for the repair of peripheral nerve defects in rats Colloids Surf. A 658 130743 [Google Scholar]
- [95].Gu Y, Li Z, Huang J, Wang H, Gu X and Gu J 2017. Application of marrow mesenchymal stem cell-derived extracellular matrix in peripheral nerve tissue engineering J. Tissue Eng. Regen. Med. 11 2250–60 [DOI] [PubMed] [Google Scholar]
- [96].Gu Y, Zhu J, Xue C, Li Z, Ding F, Yang Y and Gu X 2014. Chitosan/silk fibroin-based, Schwann cell-derived extracellular matrix-modified scaffolds for bridging rat sciatic nerve gaps Biomaterials 35 2253–63 [DOI] [PubMed] [Google Scholar]
- [97].Rao Z, Lin T, Qiu S, Zhou J, Liu S, Chen S, Wang T, Liu X, Zhu Q and Bai Y 2021. Decellularized nerve matrix hydrogel scaffolds with longitudinally oriented and size-tunable microchannels for peripheral nerve regeneration Mater. Sci. Eng. C 120 111791. [DOI] [PubMed] [Google Scholar]
- [98].Li S-T, Archibald SJ, Krarup C and Madison RD 1992. Peripheral nerve repair with collagen conduits Clin. Mater 9 195–200 [DOI] [PubMed] [Google Scholar]
- [99].Duan G, Li C, Yan X, Yang S, Wang S, Sun X, Zhao L, Song T, Pan Y and Wang X 2023. Construction of a mineralized collagen nerve conduit for peripheral nerve injury repair Regen. Biomater 10 rbac089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Chen Y-S, Chang J-Y, Cheng C-Y, Tsai F-J, Yao C-H and Liu B-S 2005. An in vivo evaluation of a biodegradable genipin-cross-linked gelatin peripheral nerve guide conduit material Biomaterials 26 3911–8 [DOI] [PubMed] [Google Scholar]
- [101].Zhu W, Tringale KR, Woller SA, You S, Johnson S, Shen H, Schimelman J, Whitney M, Steinauer J and Xu W 2018. Rapid continuous 3D printing of customizable peripheral nerve guidance conduits Mater. Today 21 951–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Gan L, Zhao L, Zhao Y, Li K, Tong Z, Yi L, Wang X, Li Y, Tian W and He X 2016. Cellulose/soy protein composite-based nerve guidance conduits with designed microstructure for peripheral nerve regeneration J. Neural Eng 13 056019. [DOI] [PubMed] [Google Scholar]
- [103].Ao Q, Fung C-K, Tsui AY-P, Cai S, Zuo H-C, Chan Y-S and Shum DK-Y 2011. The regeneration of transected sciatic nerves of adult rats using chitosan nerve conduits seeded with bone marrow stromal cell-derived Schwann cells Biomaterials 32 787–96 [DOI] [PubMed] [Google Scholar]
- [104].Deng P, Chen F, Zhang H, Chen Y and Zhou J 2022. Multifunctional double-layer composite hydrogel conduit based on chitosan for peripheral nerve repairing Adv. Healthcare Mater 11 2200115. [DOI] [PubMed] [Google Scholar]
- [105].Sasaki R, Aoki S, Yamato M, Uchiyama H, Wada K, Ogiuchi H, Okano T and Ando T 2011. PLGA artificial nerve conduits with dental pulp cells promote facial nerve regeneration J. Tissue Eng. Regen. Med 5 823–30 [DOI] [PubMed] [Google Scholar]
- [106].Park D, Kim D, Park SJ, Choi JH, Seo Y, Kim D-H, Lee S-H, Hyun JK, Yoo J and Jung Y 2022. Micropattern-based nerve guidance conduit with hundreds of microchannels and stem cell recruitment for nerve regeneration npj Regen. Med 7 62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Sundback CA, Shyu JY, Wang Y, Faquin WC, Langer RS, Vacanti JP and Hadlock TA 2005. Biocompatibility analysis of poly (glycerol sebacate) as a nerve guide material Biomaterials 26 5454–64 [DOI] [PubMed] [Google Scholar]
- [108].Dey J, Xu H, Shen J, Thevenot P, Gondi SR, Nguyen KT, Sumerlin BS, Tang L and Yang J 2008. Development of biodegradable crosslinked urethane-doped polyester elastomers Biomaterials 29 4637–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Tran RT, Choy WM, Cao H, Qattan I, Chiao JC, Ip WY, Yeung KWK and Yang J 2014. Fabrication and characterization of biomimetic multichanneled crosslinked-urethane-doped polyester tissue engineered nerve guides J. Biomed. Mater. Res. A 102 2793–804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Tao J, Liu H, Wu W, Zhang J, Liu S, Zhang J, Huang Y, Xu X, He H and Yang S 2020. 3D-printed nerve conduits with live platelets for effective peripheral nerve repair Adv. Funct. Mater. 30 2004272 [Google Scholar]
- [111].Lee MR, Kwon KW, Jung H, Kim HN, Suh KY, Kim K and Kim K-S 2010. Direct differentiation of human embryonic stem cells into selective neurons on nanoscale ridge/groove pattern arrays Biomaterials 31 4360–6 [DOI] [PubMed] [Google Scholar]
- [112].Kaur M, Mehta A and Gupta R 2018. Biomedical applications of synthetic and natural biodegradable polymers Green Sustain. Adv. Mater 2 281–310 [Google Scholar]
- [113].Prajapati SK, Jain A, Jain A and Jain S 2019. Biodegradable polymers and constructs: a novel approach in drug delivery Eur. Polym. J 120 109191 [Google Scholar]
- [114].Moon SH, Hwang HJ, Jeon HR, Park SJ, Bae IS and Yang YJ 2023. Photocrosslinkable natural polymers in tissue engineering Front. Bioeng. Biotechnol 11 1127757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Gonzalez-Perez F, Udina E and Navarro X 2013. Extracellular matrix components in peripheral nerve regeneration Int. Revi. Neurobiol 108 257–75 [DOI] [PubMed] [Google Scholar]
- [116].Yang C-Y, Huang W-Y, Chen L-H, Liang N-W, Wang H-C, Lu J, Wang X and Wang T-W 2021. Neural tissue engineering: the influence of scaffold surface topography and extracellular matrix microenvironment J. Mater. Chem. B 9 567–84 [DOI] [PubMed] [Google Scholar]
- [117].Xu Z, Orkwis JA, DeVine BM and Harris GM 2020. Extracellular matrix cues modulate Schwann cell morphology, proliferation, and protein expression J. Tissue Eng. Regen. Med 14 229–42 [DOI] [PubMed] [Google Scholar]
- [118].Yu P, Zhang G, Hou B, Song E, Wen J, Ba Y, Zhu D, Wang G and Qin F 2023. Effects of ECM proteins (laminin, fibronectin, and type IV collagen) on the biological behavior of Schwann cells and their roles in the process of remyelination after peripheral nerve injury Front. Bioeng. Biotechnol 11 1133718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Lin G, Albersen M, Harraz AM, Fandel TM, Garcia M, McGrath MH, Konety BR, Lue TF and Lin C-S 2011. Cavernous nerve repair with allogenic adipose matrix and autologous adipose-derived stem cells Urology 77 1509.e1–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Choi J, Kim JH, Jang JW, Kim HJ, Choi SH and Kwon SW 2018. Decellularized sciatic nerve matrix as a biodegradable conduit for peripheral nerve regeneration Neural Regen. Res. 13 1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Philips C, Cornelissen M and Carriel V 2018. Evaluation methods as quality control in the generation of decellularized peripheral nerve allografts J. Neural Eng 15 021003. [DOI] [PubMed] [Google Scholar]
- [122].Boriani F, Fazio N, Fotia C, Savarino L, Nicoli Aldini N, Martini L, Zini N, Bernardini M and Baldini N 2017. A novel technique for decellularization of allogenic nerves and in vivo study of their use for peripheral nerve reconstruction J. Biomed. Mater. Res. A 105 2228–40 [DOI] [PubMed] [Google Scholar]
- [123].Kasper M, Deister C, Beck F and Schmidt CE 2020. Bench-to-bedside lessons learned: commercialization of an acellular nerve graft Adv. Healthcare Mater 9 2000174. [DOI] [PubMed] [Google Scholar]
- [124].Lin T, Liu S, Chen S, Qiu S, Rao Z, Liu J, Zhu S, Yan L, Mao H and Zhu Q 2018. Hydrogel derived from porcine decellularized nerve tissue as a promising biomaterial for repairing peripheral nerve defects Acta Biomater. 73 326–38 [DOI] [PubMed] [Google Scholar]
- [125].Gong H, Fei H, Xu Q, Gou M and Chen HH 2020. 3D-engineered GelMA conduit filled with ECM promotes regeneration of peripheral nerve J. Biomed. Mater. Res. A 108 805–13 [DOI] [PubMed] [Google Scholar]
- [126].Yu L, Rowe DW, Perera IP, Zhang J, Suib SL, Xin X and Wei M 2020. Intrafibrillar mineralized collagen-hydroxyapatite-based scaffolds for bone regeneration ACS Appl. Mater. Interfaces 12 18235–49 [DOI] [PubMed] [Google Scholar]
- [127].Schmidt M, Dornelles R, Mello R, Kubota E, Mazutti M, Kempka A and Demiate I 2016. Collagen extraction process Int. Food Res. J 23 913 [Google Scholar]
- [128].Yu L, Martin IJ, Kasi RM and Wei M 2018. Enhanced intrafibrillar mineralization of collagen fibrils induced by brushlike polymers ACS Appl. Mater. Interfaces 10 28440–9 [DOI] [PubMed] [Google Scholar]
- [129].Chen Y-S, Hsieh C-L, Tsai C-C, Chen T-H, Cheng W-C, Hu C-L and Yao C-H 2000. Peripheral nerve regeneration using silicone rubber chambers filled with collagen, laminin and fibronectin Biomaterials 21 1541–7 [DOI] [PubMed] [Google Scholar]
- [130].Lee J-Y, Giusti G, Friedrich PF, Archibald SJ, Kemnitzer JE, Patel J, Desai N, Bishop AT and Shin AY 2012. The effect of collagen nerve conduits filled with collagen-glycosaminoglycan matrix on peripheral motor nerve regeneration in a rat model JBJS 94 2084–91 [DOI] [PubMed] [Google Scholar]
- [131].Fujimaki H, Matsumine H, Osaki H, Ueta Y, Kamei W, Shimizu M, Hashimoto K, Fujii K, Kazama T and Matsumoto T 2019. Dedifferentiated fat cells in polyglycolic acid-collagen nerve conduits promote rat facial nerve regeneration Regen. Ther 11 240–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [132].Yu L and Wei M 2021. Biomineralization of collagen-based materials for hard tissue repair Int. J. Mol. Sci. 22 944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133].Oosterlaken BM, Vena MP and de With G 2021. In vitro mineralization of collagen Adv. Mater. 33 2004418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [134].Hu C, Yu L and Wei M 2017. Biomimetic intrafibrillar silicification of collagen fibrils through a one-step collagen self-assembly/silicification approach RSC Adv 7 34624–32 [Google Scholar]
- [135].Nichol JW, Koshy ST, Bae H, Hwang CM, Yamanlar S and Khademhosseini A 2010. Cell-laden microengineered gelatin methacrylate hydrogels Biomaterials 31 5536–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [136].Mohammadi M, Ramazani Saadatabadi A, Mashayekhan S and Sanaei R 2020. Conductive multichannel PCL/gelatin conduit with tunable mechanical and structural properties for peripheral nerve regeneration J. Appl. Polym. Sci. 137 49219 [Google Scholar]
- [137].Pozzobon LG, Sperling LE, Teixeira CE, Malysz T and Pranke P 2021. Development of a conduit of PLGA-gelatin aligned nanofibers produced by electrospinning for peripheral nerve regeneration Chem. Biol. Interact. 348 109621. [DOI] [PubMed] [Google Scholar]
- [138].Lee HS, Jeon EY, Nam JJ, Park JH, Choi IC, Kim SH, Chung JJ, Lee K, Park JW and Jung Y 2022. Development of a regenerative porous PLCL nerve guidance conduit with swellable hydrogel-based microgrooved surface pattern via 3D printing Acta Biomater. 141 219–32 [DOI] [PubMed] [Google Scholar]
- [139].Wang H, Wan H, Wang Q, Ma Y, Su G, Cao X and Gao H 2023. Engineered multifunctional silk fibroin/gelatin hydrogel conduit loaded with miR-29a@ ZIF-8 nanoparticles for peripheral nerve regeneration Smart Mater. Med 4 480–92 [Google Scholar]
- [140].Lin C-C, Chang J-J, Yung M-C, Huang W-C and Chen S-Y 2020. Spontaneously micropatterned silk/gelatin scaffolds with topographical, biological, and electrical stimuli for neuronal regulation ACS Biomater. Sci. Eng. 6 1144–53 [DOI] [PubMed] [Google Scholar]
- [141].Mohseni M and Ramazani Saadatabadi A 2021. Highly conductive self-electrical stimuli core-shell conduit based on PVDF-chitosan–gelatin filled with in-situ gellan gum as a possible candidate for nerve regeneration: a rheological, electrical, and structural study Appl. Nanosci 11 2199–213 [Google Scholar]
- [142].Vishnoi T, Singh A, Teotia AK and Kumar A 2019. Chitosan-gelatin-polypyrrole cryogel matrix for stem cell differentiation into neural lineage and sciatic nerve regeneration in peripheral nerve injury model ACS Biomater. Sci. Eng 5 3007–21 [DOI] [PubMed] [Google Scholar]
- [143].Sun D 2023. Sacrificial gelatin of PAM-Alginate-BC hydrogel tube with tunable diameter as nerve conduit J. Biomater. Sci. Polym. Ed. 34 1398–407 [DOI] [PubMed] [Google Scholar]
- [144].Chen S, Wang Y, Lai J, Tan S and Wang M 2023. Structure and properties of gelatin methacryloyl (GelMA) synthesized in different reaction systems Biomacromolecules 24 2928–41 [DOI] [PubMed] [Google Scholar]
- [145].Ye W et al. 2020. 3D printing of gelatin methacrylate-based nerve guidance conduits with multiple channels Mater. Des. 192 108757 [Google Scholar]
- [146].Gao S, Tang Y, Sun W, Liu Z, Zhao T, Li X, Wang T, Liao G, Xu T and Zheng G 2023. 3D-bioprinted GelMA nerve guidance conduits promoted peripheral nerve regeneration by inducing trans-differentiation of MSCs into SCLCs via PIEZO1/YAP axis Mater. Today Adv. 17 100325 [Google Scholar]
- [147].Liu J, Zhang B, Li L, Yin J and Fu J 2021. Additive-lathe 3D bioprinting of bilayered nerve conduits incorporated with supportive cells Bioact. Mater 6 219–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [148].Heimbach B, Yu L and Wei M 2020. In vitro behavior of silk fibroin-based composite resorbable bone fixation devices Materialia 9 100587 [Google Scholar]
- [149].Perrone GS, Leisk GG, Lo TJ, Moreau JE, Haas DS, Papenburg BJ, Golden EB, Partlow BP, Fox SE and Ibrahim AM 2014. The use of silk-based devices for fracture fixation Nat. Commun. 5 3385. [DOI] [PubMed] [Google Scholar]
- [150].Han F, Liu S, Liu X, Pei Y, Bai S, Zhao H, Lu Q, Ma F, Kaplan D and Zhu H 2014. Woven silk fabric-reinforced silk nanofibrous scaffolds for regenerating load-bearing soft tissues Acta Biomater. 10 921–30 [DOI] [PubMed] [Google Scholar]
- [151].Murchio S, Benedetti M, Berto A, Agostinacchio F, Zappini G and Maniglio D 2022. Hybrid Ti6Al4V/silk fibroin composite for load-bearing implants: a hierarchical multifunctional cellular scaffold Materials 15 6156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [152].Carvalho CR, Chang W, Silva-Correia J, Reis RL, Oliveira JM and Kohn J 2021. Engineering silk fibroin-based nerve conduit with neurotrophic factors for proximal protection after peripheral nerve injury Adv. Healthcare Mater 10 2000753. [DOI] [PubMed] [Google Scholar]
- [153].Wang C, Jia Y, Yang W, Zhang C, Zhang K and Chai Y 2018. Silk fibroin enhances peripheral nerve regeneration by improving vascularization within nerve conduits J. Biomed. Mater. Res. A 106 2070–7 [DOI] [PubMed] [Google Scholar]
- [154].Yang Y, Ding F, Wu J, Hu W, Liu W, Liu J and Gu X 2007. Development and evaluation of silk fibroin-based nerve grafts used for peripheral nerve regeneration Biomaterials 28 5526–35 [DOI] [PubMed] [Google Scholar]
- [155].Hu Z, Das SK, Yan S, You R, Li X, Luo Z, Li M, Zhang Q and Kaplan DL 2020. Stability and biodegradation of silk fibroin/hyaluronic acid nerve conduits Composites B 200 108222 [Google Scholar]
- [156].Su L, Feng Y, Wei K, Xu X, Liu R and Chen G 2021. Carbohydrate-based macromolecular biomaterials Chem. Rev. 121 10950–1029 [DOI] [PubMed] [Google Scholar]
- [157].Maji B 2019. Functional Polysaccharides for Biomedical Applications (Elsevier; ) pp 1–31 [Google Scholar]
- [158].Zhang Y, Yu J, Wang X, Durachko DM, Zhang S and Cosgrove DJ 2021. Molecular insights into the complex mechanics of plant epidermal cell walls Science 372 706–11 [DOI] [PubMed] [Google Scholar]
- [159].Hickey RJ and Pelling AE 2019. Cellulose biomaterials for tissue engineering Front. Bioeng. Biotechnol. 7 45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [160].Wang B, Lv X, Chen S, Li Z, Sun X, Feng C, Wang H and Xu Y 2016. In vitro biodegradability of bacterial cellulose by cellulase in simulated body fluid and compatibility in vivo Cellulose 23 3187–98 [Google Scholar]
- [161].Niemczyk-Soczynska B, Gradys A, Kolbuk D, Krzton-Maziopa A and Sajkiewicz P 2019. Crosslinking kinetics of methylcellulose aqueous solution and its potential as a scaffold for tissue engineering Polymers 11 1772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [162].Bonetti L, De Nardo L and Farè S 2021. Thermo-responsive methylcellulose hydrogels: from design to applications as smart biomaterials Tissue Eng. B 27 486–513 [DOI] [PubMed] [Google Scholar]
- [163].Stalling SS, Akintoye SO and Nicoll SB 2009. Development of photocrosslinked methylcellulose hydrogels for soft tissue reconstruction Acta Biomater. 5 1911–8 [DOI] [PubMed] [Google Scholar]
- [164].Luo L, Gan L, Liu Y, Tian W, Tong Z, Wang X, Huselstein C and Chen Y 2015. Construction of nerve guide conduits from cellulose/soy protein composite membranes combined with Schwann cells and pyrroloquinoline quinone for the repair of peripheral nerve defect Biochem. Biophys. Res. Commun. 457 507–13 [DOI] [PubMed] [Google Scholar]
- [165].Zhao Y, Zhang Q, Zhao L, Gan L, Yi L, Zhao Y, Xue J, Luo L, Du Q and Geng R 2017. Enhanced peripheral nerve regeneration by a high surface area to volume ratio of nerve conduits fabricated from hydroxyethyl cellulose/soy protein composite sponges ACS Omega 2 7471–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [166].Dong Q, Ai J, Xiao A, Wu P, Wu M, Liu X, Huselstein C, Cai L, Feng X and Chen Y 2023. Nerve defect treatment with a capping hydroxyethyl cellulose/soy protein isolate sponge conduit for painful neuroma prevention ACS Omega 8 30850–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [167].Gupta B and Edwards J 2019. Advanced Textiles for Wound Care (Elsevier; ) pp 55–104 [Google Scholar]
- [168].Kean T and Thanou M 2011. Chitin and chitosan: sources, production and medical applications Renewable Resources for Functional Polymers and Biomaterials: Polysaccharides, Proteins and Polyesters (RSC Publishing; ) pp 292–318 [Google Scholar]
- [169].Kou SG, Peters L and Mucalo M 2022. Chitosan: a review of molecular structure, bioactivities and interactions with the human body and micro-organisms Carbohydrate Polym 282 119132. [DOI] [PubMed] [Google Scholar]
- [170].Fakhri E, Eslami H, Maroufi P, Pakdel F, Taghizadeh S, Ganbarov K, Yousefi M, Tanomand A, Yousefi B and Mahmoudi S 2020. Chitosan biomaterials application in dentistry Int. J. Biol. Macromol. 162 956–74 [DOI] [PubMed] [Google Scholar]
- [171].Madni A, Kousar R, Naeem N and Wahid F 2021. Recent advancements in applications of chitosan-based biomaterials for skin tissue engineering J. Bioresources Bioprod. 6 11–25 [Google Scholar]
- [172].Lu J, Fan X, Hu J, Li J, Rong J, Wang W, Chen Y, Liu W, Chen J and Chen Y 2023. Construction and function of robust and moist bilayer chitosan-based hydrogel wound dressing Mater. Des. 226 111604 [Google Scholar]
- [173].Jiang Z, Zhang Y, Wang Y, Wang S, Chang J, Liu W and Han B 2023. Multichannel nerve conduit based on chitosan derivates for peripheral nerve regeneration and Schwann cell survival Carbohydrate Polym. 301 120327. [DOI] [PubMed] [Google Scholar]
- [174].Song L, Guo Q, Guo J, Xu X, Xu K, Li Y, Yang T, Gu X, Cao R and Cui S 2022. Brachial plexus bridging with specific extracellular matrix-modified chitosan/silk scaffold: a new expand of tissue engineered nerve graft J. Neural Eng 19 026010. [DOI] [PubMed] [Google Scholar]
- [175].Lee HI, Heo Y, Baek S-W, Kim D-S, Song DH and Han DK 2021. Multifunctional biodegradable vascular PLLA scaffold with improved x-ray opacity, anti-inflammation, and re-endothelization Polymers 13 1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [176].Annunziata M, Nastri L, Cecoro G and Guida L 2017. The use of poly-d, l-lactic acid (PDLLA) devices for bone augmentation techniques: a systematic review Molecules 22 2214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [177].Matsumine H, Sasaki R, Yamato M, Okano T and Sakurai H 2014. A polylactic acid non-woven nerve conduit for facial nerve regeneration in rats J. Tissue Eng. Regen. Med 8 454–62 [DOI] [PubMed] [Google Scholar]
- [178].Budak K, Sogut O and Aydemir Sezer U 2020. A review on synthesis and biomedical applications of polyglycolic acid J. Polym. Res 27 1–19 [Google Scholar]
- [179].Mir M, Ahmed N and Ur Rehman A 2017. Recent applications of PLGA based nanostructures in drug delivery Colloids Surf. B 159 217–31 [DOI] [PubMed] [Google Scholar]
- [180].Stefaniak K and Masek A 2021. Green copolymers based on poly (lactic acid)—short review Materials 14 5254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [181].Lu P, Wang G, Qian T, Cai X, Zhang P, Li M, Shen Y, Xue C and Wang H 2021. The balanced microenvironment regulated by the degradants of appropriate PLGA scaffolds and chitosan conduit promotes peripheral nerve regeneration Mater. Today Bio 12 100158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [182].Terzopoulou Z, Zamboulis A, Koumentakou I, Michailidou G, Noordam MJ and Bikiaris DN 2022. Biocompatible synthetic polymers for tissue engineering purposes Biomacromolecules 23 1841–63 [DOI] [PubMed] [Google Scholar]
- [183].Middleton JC and Tipton AJ 2000. Synthetic biodegradable polymers as orthopedic devices Biomaterials 21 2335–46 [DOI] [PubMed] [Google Scholar]
- [184].Wu F, Liu C, O’Neill B, Wei J and Ngothai Y 2012. Fabrication and properties of porous scaffold of magnesium phosphate/polycaprolactone biocomposite for bone tissue engineering Appl. Surf. Sci 258 7589–95 [Google Scholar]
- [185].Liverani L, Lacina J, Roether JA, Boccardi E, Killian MS, Schmuki P, Schubert DW and Boccaccini AR 2018. Incorporation of bioactive glass nanoparticles in electrospun PCL/chitosan fibers by using benign solvents Bioact. Mater 3 55–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [186].Shor L, Güçeri S, Wen X, Gandhi M and Sun W 2007. Fabrication of three-dimensional polycaprolactone/hydroxyapatite tissue scaffolds and osteoblast-scaffold interactions in vitro Biomaterials 28 5291–7 [DOI] [PubMed] [Google Scholar]
- [187].Jeong SI, Kim SH, Kim YH, Jung Y, Kwon JH, Kim B-S and Lee YM 2004. Manufacture of elastic biodegradable PLCL scaffolds for mechano-active vascular tissue engineering J. Biomater. Sci. Polym. Ed. 15 645–60 [DOI] [PubMed] [Google Scholar]
- [188].Haq RHA, Rahman MNA, Ariffin AMT, Hassan MF, Yunos MZ and Adzila S 2017. Characterization and mechanical analysis of PCL/PLA composites for FDM feedstock filament IOP Conf. Ser.: Mater. Sci. Eng 226 012038 [Google Scholar]
- [189].Zhang D, Li Z, Shi H, Yao Y, Du W, Lu P, Liang K, Hong L and Gao C 2022. Micropatterns and peptide gradient on the inner surface of a guidance conduit synergistically promotes nerve regeneration in vivo Bioact. Mater 9 134–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [190].Zhang D, Yang W, Wang C, Zheng H, Liu Z, Chen Z and Gao C 2020. Methylcobalamin-loaded PLCL conduits facilitate the peripheral nerve regeneration Macromol. Biosci 20 1900382. [DOI] [PubMed] [Google Scholar]
- [191].Kanungo I, Fathima NN, Rao JR and Nair BU 2013. Influence of PCL on the material properties of collagen based biocomposites and in vitro evaluation of drug release Mater. Sci. Eng. C 33 4651–9 [DOI] [PubMed] [Google Scholar]
- [192].Wang Y, Ameer GA, Sheppard BJ and Langer R 2002. A tough biodegradable elastomer Nat. Biotechnol. 20 602–6 [DOI] [PubMed] [Google Scholar]
- [193].Yang J, Webb AR and Ameer GA 2004. Novel citric acid-based biodegradable elastomers for tissue engineering Adv. Mater 16 511–6 [Google Scholar]
- [194].Yang J, Zhang Y, Gautam S, Liu L, Dey J, Chen W, Mason RP, Serrano CA, Schug KA and Tang L 2009. Development of aliphatic biodegradable photoluminescent polymers Proc. Natl Acad. Sci 106 10086–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [195].Abrbekoh FN, Valizadeh N, Hassani A, Ghale H, Mahboob SA, Rahbarghazi R, Khoshfetrat AB and Madipour M 2022. Combination of polyglycerol sebacate coated with collagen for vascular engineering J. Cardiovascular Thoracic Res 14 172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [196].Loh XJ, Karim AA and Owh C 2015. Poly (glycerol sebacate) biomaterial: synthesis and biomedical applications J. Mater. Chem. B 3 7641–52 [DOI] [PubMed] [Google Scholar]
- [197].Yang J, Webb AR, Pickerill SJ, Hageman G and Ameer GA 2006. Synthesis and evaluation of poly (diol citrate) biodegradable elastomers Biomaterials 27 1889–98 [DOI] [PubMed] [Google Scholar]
- [198].Wu K, Fu M, Zhao Y, Gerhard E, Li Y, Yang J and Guo J 2023. Anti-oxidant anti-inflammatory and antibacterial tannin-crosslinked citrate-based mussel-inspired bioadhesives facilitate scarless wound healing Bioact. Mater 20 93–110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [199].Guo J, Wang W, Hu J, Xie D, Gerhard E, Nisic M, Shan D, Qian G, Zheng S and Yang J 2016. Synthesis and characterization of anti-bacterial and anti-fungal citrate-based mussel-inspired bioadhesives Biomaterials 85 204–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [200].Berkovitch Y, Cohen T, Peled E, Schmidhammer R, Florian H, Teuschl AH, Wolbank S, Yelin D, Redl H and Seliktar D 2018. Hydrogel composition and laser micropatterning to regulate sciatic nerve regeneration J. Tissue Eng. Regen. Med 12 1049–61 [DOI] [PubMed] [Google Scholar]
- [201].Kong X-B, Tang Q-Y, Chen X-Y, Tu Y, Sun S-Z and Sun Z-L 2017. Polyethylene glycol as a promising synthetic material for repair of spinal cord injury Neural Regen. Res 12 1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [202].Berkovitch Y, Yelin D and Seliktar D 2015. Photo-patterning PEG-based hydrogels for neuronal engineering Eur. Polym. J 72 473–83 [Google Scholar]
- [203].Serra T, Ortiz-Hernandez M, Engel E, Planell JA and Navarro M 2014. Relevance of PEG in PLA-based blends for tissue engineering 3D-printed scaffolds Mater. Sci. Eng. C 38 55–62 [DOI] [PubMed] [Google Scholar]
- [204].Das A and Mahanwar P 2020. A brief discussion on advances in polyurethane applications Adv. Ind. Eng. Polym. Res 3 93–101 [Google Scholar]
- [205].Oprea S 2010. The effect of chain extenders structure on properties of new polyurethane elastomers Polym. Bull. 65 753–66 [Google Scholar]
- [206].Chiono V, Sartori S, Rechichi A, Tonda-Turo C, Vozzi G, Vozzi F, D’Acunto M, Salvadori C, Dini F and Barsotti G 2011. Poly (ester urethane) guides for peripheral nerve regeneration Macromol. Biosci 11 245–56 [DOI] [PubMed] [Google Scholar]
- [207].Wu Y, Wang L, Guo B, Shao Y and Ma PX 2016. Electroactive biodegradable polyurethane significantly enhanced Schwann cells myelin gene expression and neurotrophin secretion for peripheral nerve tissue engineering Biomaterials 87 18–31 [DOI] [PubMed] [Google Scholar]
- [208].Zhang S, Wang J, Zheng Z, Yan J, Zhang L, Li Y, Zhang J, Li G, Wang X and Kaplan D 2021. Porous nerve guidance conduits reinforced with braided composite structures of silk/magnesium filaments for peripheral nerve repair Acta Biomater. 134 116–30 [DOI] [PubMed] [Google Scholar]
- [209].Magaz A, Faroni A, Gough JE, Reid AJ, Li X and Blaker JJ 2018. Bioactive silk-based nerve guidance conduits for augmenting peripheral nerve repair Adv. Healthcare Mater 7 1800308. [DOI] [PubMed] [Google Scholar]
- [210].Vijayavenkataraman S 2020. Nerve guide conduits for peripheral nerve injury repair: a review on design, materials and fabrication methods Acta Biomater. 106 54–69 [DOI] [PubMed] [Google Scholar]
- [211].Frost HK, Andersson T, Johansson S, Englund-Johansson U, Ekström P, Dahlin LB and Johansson F 2018. Electrospun nerve guide conduits have the potential to bridge peripheral nerve injuries in vivo Sci. Rep. 8 16716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [212].Lu S, Chen W, Wang J, Guo Z, Xiao L, Wei L, Yu J, Yuan Y, Chen W and Bian M 2023. Polydopamine-decorated PLCL conduit to induce synergetic effect of electrical stimulation and topological morphology for peripheral nerve regeneration Small Methods 7 2200883. [DOI] [PubMed] [Google Scholar]
- [213].Ryan AJ, Lackington WA, Hibbitts AJ, Matheson A, Alekseeva T, Stejskalova A, Roche P and O’Brien FJ 2017. A physicochemically optimized and neuroconductive biphasic nerve guidance conduit for peripheral nerve repair Adv. Healthcare Mater 6 1700954. [DOI] [PubMed] [Google Scholar]
- [214].Qian Y, Zhao X, Han Q, Chen W, Li H and Yuan W 2018. An integrated multi-layer 3D-fabrication of PDA/RGD coated graphene loaded PCL nanoscaffold for peripheral nerve restoration Nat. Commun. 9 323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [215].Hu Y, Chen Z, Wang H, Guo J, Cai J, Chen X, Wei H, Qi J, Wang Q and Liu H 2022. Conductive nerve guidance conduits based on Morpho butterfly wings for peripheral nerve repair ACS Nano 16 1868–79 [DOI] [PubMed] [Google Scholar]
- [216].Yao L, de Ruiter G C, Wang H, Knight AM, Spinner RJ, Yaszemski MJ, Windebank AJ and Pandit A 2010. Controlling dispersion of axonal regeneration using a multichannel collagen nerve conduit Biomaterials 31 5789–97 [DOI] [PubMed] [Google Scholar]
- [217].Shahriari D, Shibayama M, Lynam DA, Wolf KJ, Kubota G, Koffler JY, Tuszynski MH, Campana WM and Sakamoto JS 2017. Peripheral nerve growth within a hydrogel microchannel scaffold supported by a kink-resistant conduit J. Biomed. Mater. Res. A 105 3392–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [218].Johnson BN, Lancaster KZ, Zhen G, He J, Gupta MK, Kong YL, Engel EA, Krick KD, Ju A and Meng F 2015. 3D printed anatomical nerve regeneration pathways Adv. Funct. Mater. 25 6205–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [219].Manoukian OS, Rudraiah S, Arul MR, Bartley JM, Baker JT, Yu X and Kumbar SG 2021. Biopolymer-nanotube nerve guidance conduit drug delivery for peripheral nerve regeneration: in vivo structural and functional assessment Bioact. Mater 6 2881–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [220].Wang S and Cai L 2010. Polymers for fabricating nerve conduits Int. J. Polym. Sci 2010 1–20 [Google Scholar]
- [221].Wang J, Xiong H, Zhu T, Liu Y, Pan H, Fan C, Zhao X and Lu WW 2020. Bioinspired multichannel nerve guidance conduit based on shape memory nanofibers for potential application in peripheral nerve repair ACS Nano 14 12579–95 [DOI] [PubMed] [Google Scholar]
- [222].Fu L, Yang Z, Gao C, Li H, Yuan Z, Wang F, Sui X, Liu S and Guo Q 2020. Advances and prospects in biomimetic multilayered scaffolds for articular cartilage regeneration Regen. Biomater 7 527–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [223].Quan Q, Meng H-Y, Chang B, Liu G-B, Cheng X-Q, Tang H, Wang Y, Peng J, Zhao Q and Lu S-B 2019. Aligned fibers enhance nerve guide conduits when bridging peripheral nerve defects focused on early repair stage Neural Regen. Res 14 903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [224].Pati F 2016. Gantelius J and Svahn H A 2016 3D bioprinting of tissue/organ models Angew. Chem., Int. Ed. 55 4650–65 [DOI] [PubMed] [Google Scholar]
- [225].2023 Peripheral Nerve Injury Market Size, Share & Trends Analysis Report By Product (Nerve Conduit, Nerve Protector), By Surgery (Direct Nerve Repair, Nerve Grafting), By Application, By Region, And Segment Forecasts, 2024–2030. Grand View Research; ) [Google Scholar]
- [226].Regenity Neurolac (available at: https://regenity.com/solution/nerve-repair/)
- [227].Axogen Avance Nerve Graft (available at: https://ir.axogeninc.com/press-releases/detail/737/axogen-inc-announces-clearance-from-fda-to-proceed-with)
- [228].FDA FDA database product code JXI (available at: www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfPMN/pmn.cfm)
- [229].Axogen Axoguard HA+ nerve protector (available at: www.axogeninc.com/products/axoguard-ha-nerve-protector/)
- [230].Jamaluddin MFB, Ghosh A, Ingle A, Mohammed R, Ali A, Bahrami M, Kaiko G, Gibb Z, Filipe EC and Cox TR 2022. Bovine and human endometrium-derived hydrogels support organoid culture from healthy and cancerous tissues Proc. Natl Acad. Sci 119 e2208040119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [231].Nederveen JP, Warnier G, Di Carlo A, Nilsson MI and Tarnopolsky MA 2021. Extracellular vesicles and exosomes: insights from exercise science Front. Physiol 11 604274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [232].Murphy DE, de Jong O G, Brouwer M, Wood MJ, Lavieu G, Schiffelers RM and Vader P 2019. Extracellular vesicle-based therapeutics: natural versus engineered targeting and trafficking Exp. Mol. Med 51 1–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [233].Gurung S, Perocheau D, Touramanidou L and Baruteau J 2021. The exosome journey: from biogenesis to uptake and intracellular signalling Cell Commun. Signaling 19 1–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [234].Ong S-G and Wu JC 2015. Exosomes as potential alternatives to stem cell therapy in mediating cardiac regeneration (American Heart Association; ) pp 7–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [235].Zhang W, Fang X-X, Li Q-C, Pi W and Han N 2023. Reduced graphene oxide-embedded nerve conduits loaded with bone marrow mesenchymal stem cell-derived extracellular vesicles promote peripheral nerve regeneration Neural Regen. Res 18 200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [236].Yu M, Gu G, Cong M, Du M, Wang W, Shen M, Zhang Q, Shi H, Gu X and Ding F 2021. Repair of peripheral nerve defects by nerve grafts incorporated with extracellular vesicles from skin-derived precursor Schwann cells Acta Biomater. 134 190–203 [DOI] [PubMed] [Google Scholar]
- [237].Wang C, Wang M, Xia K, Wang J, Cheng F, Shi K, Ying L, Yu C, Xu H and Xiao S 2021. A bioactive injectable self-healing anti-inflammatory hydrogel with ultralong extracellular vesicles release synergistically enhances motor functional recovery of spinal cord injury Bioact. Mater 6 2523–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [238].Xia B, Gao X, Qian J, Li S, Yu B, Hao Y, Wei B, Ma T, Wu H and Yang S 2023. A novel superparamagnetic multifunctional nerve scaffold: a remote actuation strategy to boost in situ extracellular vesicles production for enhanced peripheral nerve repair Adv. Mater. 36 2305374. [DOI] [PubMed] [Google Scholar]
- [239].Xia B, Gao J, Li S, Huang L, Zhu L, Ma T, Zhao L, Yang Y, Luo K and Shi X 2020. Mechanical stimulation of Schwann cells promote peripheral nerve regeneration via extracellular vesicle-mediated transfer of microRNA 23b-3p Theranostics 10 8974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [240].Hao R, Hu S, Zhang H, Chen X, Yu Z, Ren J, Guo H and Yang H 2023. Mechanical stimulation on a microfluidic device to highly enhance small extracellular vesicle secretion of mesenchymal stem cells Mater. Today Bio 18 100527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [241].Fukuta T, Nishikawa A and Kogure K 2020. Low level electricity increases the secretion of extracellular vesicles from cultured cells Biochem. Biophys. Rep 21 100713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [242].Ambattu LA, Ramesan S, Dekiwadia C, Hanssen E, Li H and Yeo LY 2020. High frequency acoustic cell stimulation promotes exosome generation regulated by a calcium-dependent mechanism Commun. Biol 3 553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [243].Patton MC, Zubair H, Khan MA, Singh S and Singh AP 2020. Hypoxia alters the release and size distribution of extracellular vesicles in pancreatic cancer cells to support their adaptive survival J. Cell. Biochem. 121 828–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [244].Nakase I, Ueno N, Matsuzawa M, Noguchi K, Hirano M, Omura M, Takenaka T, Sugiyama A, Bailey Kobayashi N and Hashimoto T 2021. Environmental pH stress influences cellular secretion and uptake of extracellular vesicles FEBS Open Bio 11 753–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [245].Yanatori I, Richardson DR, Dhekne HS, Toyokuni S and Kishi F 2021. CD63 is regulated by iron via the IRE-IRP system and is important for ferritin secretion by extracellular vesicles Blood J. Am. Soc. Hematol 138 1490–503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [246].Wang H, Huddleston S, Yang J and Ameer GA 2023. Enabling proregenerative medical devices via citrate-based biomaterials: transitioning from inert to regenerative biomaterials Adv. Mater. 36 2306326. [DOI] [PubMed] [Google Scholar]
- [247].Huang C, Li Z, Qu W and Guo W 2022. Astaxanthin-folic acid combined treatment potentiates neuronal regeneration and functional recovery after brachial plexus avulsion and reimplantation Front. Neurosci. 16 923750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [248].Iskandar BJ, Nelson A, Resnick D, Pate Skene J, Gao P, Johnson C, Cook TD and Hariharan N 2004. Folic acid supplementation enhances repair of the adult central nervous system Ann. Neurol. 56 221–7 [DOI] [PubMed] [Google Scholar]
- [249].Kolb AF and Petrie L 2013. Folate deficiency enhances the inflammatory response of macrophages Mol. Immunol. 54 164–72 [DOI] [PubMed] [Google Scholar]
- [250].Ma C, Kuzma ML, Bai X and Yang J 2019. Biomaterial-based metabolic regulation in regenerative engineering Adv. Sci 6 1900819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [251].Zhu Z, Ng DWH, Park HS and McAlpine MC 2021. 3D-printed multifunctional materials enabled by artificial-intelligence-assisted fabrication technologies Nat. Rev. Mater 6 27–47 [Google Scholar]
- [252].Wan J, Li X, Dai H-N, Kusiak A, Martinez-Garcia M and Li D 2020. Artificial-intelligence-driven customized manufacturing factory: key technologies, applications, and challenges Proc. IEEE 109 377–98 [Google Scholar]
- [253].Krishna DV and Sankar MR 2023. Engineered approach coupled with machine learning in biofabrication of patient-specific nerve guide conduits-review Bioprinting 30 e00264 [Google Scholar]
- [254].Parker BJ, Rhodes DI, O’Brien CM, Rodda AE and Cameron NR 2021. Nerve guidance conduit development for primary treatment of peripheral nerve transection injuries: a commercial perspective Acta Biomater. 135 64–86 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No new data were created or analysed in this study.


