Abstract
Background:
The Sequential Organ Failure Assessment (SOFA) score monitors organ failure and defines sepsis but may not fully capture factors influencing sepsis mortality. Socioeconomic and demographic impacts on sepsis outcomes have been highlighted recently.
Objective:
To evaluate the prognostic value of SOFA scores against demographic and social health determinants for predicting sepsis mortality in critically ill patients, and to assess if a combined model increases predictive accuracy.
Methods:
The study utilized retrospective data from the MIMIC-IV database and prospective external validation from the Penn State Health cohort. A Random Forest model incorporating SOFA scores, demographic/social data, and the Charlson Comorbidity Index was trained and validated.
Findings:
In the MIMIC-IV dataset of 32,970 sepsis patients, 6,824 (20.7%) died within 30 days. A model including demographic, socioeconomic, and comorbidity data with SOFA scores improved predictive accuracy beyond SOFA scores alone. Day 2 SOFA, age, weight, and comorbidities were significant predictors. External validation showed consistent performance, highlighting the importance of delta SOFA between days 1 and 3.
Conclusion:
Adding patient-specific demographic and socioeconomic information to clinical metrics significantly improves sepsis mortality prediction. This suggests a more comprehensive, multidimensional prognostic approach is needed for accurate sepsis outcome predictions.
Keywords: Sepsis, machine learning, Sequential Organ Failure Assessment score, MIMIC database, mortality
INTRODUCTION
The Sepsis-related Organ Failure Assessment (SOFA) score, initially devised by Vincent et al. [1], was designed to understand the progression and impact of organ dysfunction in sepsis, complementing other severity scores. Later known as the ‘Sequential’ Organ Failure Assessment score, it was incorporated into the diagnostic criteria for sepsis (Sepsis-3) in 2016 [2]. The SOFA score provides an objective measure of sequential organ dysfunction or recovery during a patient’s hospitalization, contrasting with other scoring systems like the APACHE II score [3]. It relies heavily on laboratory data, which may not always reflect the current clinical condition of critically ill patients. Additionally, among the six SOFA domains, four hinge on laboratory results, potentially delaying the identification of critical changes in a patient’s condition. Although other severity-of-illness scores exist, no scoring system exclusively predicts mortality in sepsis [4, 5]. Recognizing its limitations, a review is currently underway to update and improve the SOFA score for more accurate, sepsis-specific applications [6].
Recent literature suggests that socioeconomic status and demographic background may play a more definitive role in the outcomes of sepsis than previously recognized [7–10]. In fact, artificial intelligence has facilitated data-driven approaches to uncovering existing healthcare disparities [11]. These social determinants of health (SDoH) may provide immediate, specific insight into patient risk without the need for expensive tests. Thus, our study sought to assess the predictive power of SOFA scores relative to demographic and socioeconomic factors for sepsis prognosis. We hypothesized that a combined approach using SOFA scores measured on the days following sepsis onset, together with social health determinants, could offer a more accurate prediction of mortality.
METHODS
We accessed anonymized patient data from the Medical Information Mart for Intensive Care (MIMIC)-IV database (version 2.2, Jan 6 2023), which includes critical care data from Beth Deaconess Medical Center [12]. This resource was chosen for its comprehensive data and patient mortality information. Using existing code [13], we calculated SOFA scores for patients fitting Sepsis-3 criteria [2].
From the onset of suspected infection, we tracked the highest daily SOFA score and its change between each day of critical illness. Additionally, we computed daily changes in SOFA (delta SOFA) and cumulative daily SOFA scores (sum SOFA). This method ensured data completeness, with no need for imputation except when patients were discharged from the ICU. We also gathered demographic and sociological data, specifically gender, age, weight, height, insurance status (Medicare, Medicaid, private insurance), marital status and race as well as the Charlson Comorbidity Index (CCI, [14]), focusing on 30-day mortality as the primary outcome.
Using Python’s scikit-learn library (version 1.3.2)[15], we trained a Random Forest model, evaluating it through 5-fold cross-validation. We tested six model types, including (1) models that were trained on 3-day and 8-day retrospective data, (2) models with and without daily SOFA organ component measures, and (3) models with and without patient socioeconomic, demographic and comorbidity data. Metrics such as feature importance, PPV, NPV, sensitivity, specificity, and AUROC were assessed during each iteration.
We then externally validated our best predictive model with real-world, observational data from critically ill patients having sepsis and forming part of a prospective research cohort at Penn State Health (PSH) from Aug 2020 – Feb 2024. Eligible participants were adults over 18 years, identified within 48 hours of critical illness onset. Sepsis was defined according to Sepsis-3 criteria, and critical illness required continuous intravenous vasopressors or ongoing respiratory support. Patients with active hematologic malignancies or those on immune-altering therapies were excluded.
Given that the validation data set included SOFA scores measured every other day, the original model was re-tuned to only use odd-numbered days. We also compared feature importance between a streamlined dataset for a Random Forest model with both the MIMIC and PSH data. Our analysis code is available on Github, and the study adhered to TRIPOD guidelines for predictive model reporting [16].
RESULTS
Of 299712 unique patients included in the MIMIC-IV database, 32970 had sepsis. Among these, 57.8% were male and the mean age was 66.7 ± 16 years. Supplemental Table 1 details the SDoH factors included in the study. A total of 6824 patients (20.7%) died within 30 days of the sepsis onset.
For each of the six scenarios described above, 25 different machine learning models were trained. Figure 1A describes five metrics pertaining to the model having the highest comparative AUROC (75%, with 95% CI 73 – 77%). This model demonstrated 77% sensitivity (95% CI 75 – 77%), 74% accuracy (95% CI 72 – 76%) and a precision of 43% (95% CI 39 – 47%). The most effective model combined demographic, socioeconomic, and comorbidity data with total SOFA score and SOFA organ component measures over an 8-day period post-sepsis. However, adding just demographic, socioeconomic, and comorbidity data to the total SOFA score (i.e., without individual organ dysfunction measures) significantly enhanced 30-day mortality prediction as compared with total SOFA score alone. This improvement was evident in analyses conducted at both 3 and 8 days after sepsis onset.
Figure 1.

Comparative Metrics and Feature Importance for 30-Day Mortality Prediction Based on Parameters derived from Medical Information Mart for Intensive Care (MIMIC)-IV dataset.
(A) This spider plot illustrates the efficacy of different predictive models over a span of 3 and 8 days following a sepsis diagnosis. ‘SOFA’ represents models utilizing exclusively SOFA-related parameters, including total SOFA score, its daily change (delta), cumulative sum, and AUROC values. ‘Dem’ extends the ‘SOFA’ model by incorporating demographic and socioeconomic factors such as insurance status, marital status, ethnicity, age, gender, body weight, height, and the Charlson comorbidity index. ‘All’ encompasses all ‘Dem’ variables plus daily, organ-specific scores (e.g., day-to-day SOFA respiratory scores). Metrics of model performance include positive predictive value (PPV), negative predictive value (NPV), and the receiver operating characteristic (ROC) curve area. (B) This figure displays the relative feature importance of various parameters in predicting 30-day mortality following sepsis. The x-axis is segmented by square brackets indicating an 8-day timeline post-sepsis onset, with day 1 at the bracket’s left end progressing to day 8 at the right. The parameters include daily SOFA component scores for respiration, coagulation, liver function, cardiovascular stability, central nervous system (CNS) activity, and renal performance. ‘Delta SOFA’ quantifies the day-over-day variation in SOFA scores, while ‘sum SOFA’ aggregates SOFA scores over two consecutive days. ‘Total AUC’ represents the cumulative SOFA score up to the current day. ‘SOFA max’ and ‘SOFA max day’ denote the peak SOFA score recorded for a patient and the specific day it was registered. Conversely, ‘SOFA min’ and ‘SOFA min day’ indicate the lowest SOFA score and its corresponding day. ‘SOFA range’ is the time span between the highest and lowest SOFA scores, providing a measure of fluctuation in organ function. ‘SOFA long stay’ tallies the total days a patient’s SOFA scores are monitored within the ICU, reflecting the duration of critical care received.
With respect to feature importance, SOFA organ component scores measured on day 2 were most predictive of 30-day mortality (Figure 1B). Amongst organ systems affected by sepsis, cardiovascular dysfunction, renal dysfunction, and central nervous system dysfunction were most predictive of 30-day mortality. Equally important was the relative feature importance of age, weight, height, marital status and CCI as compared with organ-specific or overall SOFA measures (Figure 1B).
Figure 2A illustrates the results of external validation using prospective data from 105 septic, critically ill patients undergoing care at PSH. In this data set, 52.4% of patients were male, with a mean age was 66.5 ± 15 years. The microbial sources of sepsis in this cohort are enumerated in Supplementary Table 2. Nineteen patients (18.1%) died within 30 days. When individual metrics were compared between discovery and validation cohorts, there was a noted increase in sensitivity and negative predictive value, and to a minor degree AUROC, at the expense of a lower specificity and positive predictive value. The negative predictive value of the model derived from MIMIC data appeared to decrease when using data derived from every other day of critical illness. Otherwise, the model’s performance remained consistent.
Figure 2.
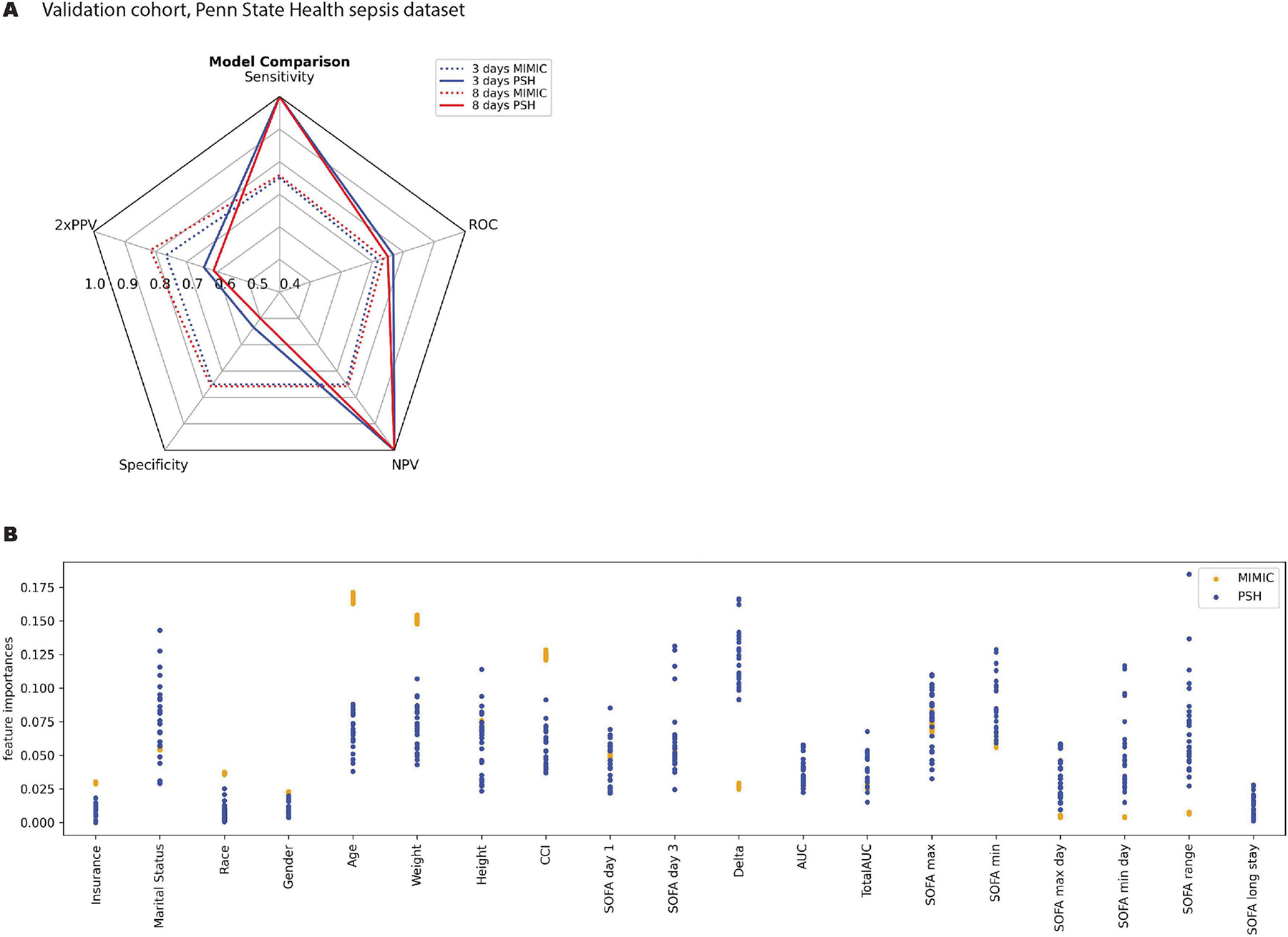
External Validation of 30-Day Mortality Prediction Model, Utilizing Data from Septic Patients Treated at Penn State Health
(A) This spider plot compares the performance of the predictive model trained on the MIMIC dataset on a validation subset of MIMIC (‘MIMIC’) versus the external validation data obtained from Penn State Electronic Medical Record (‘PSH’). All abbreviations are defined in the legend for Figure 1. (B) This figure compares the relative feature importance in predicting 30-day mortality following sepsis for MIMIC (blue) and PSH (orange). We extracted patient characteristics and clinical variables from the first three days of illness onset alone. To minimize the risk of model overfitting, we streamlined the number of features following the initial discovery phase.
Interestingly, in the streamlined model, age, weight, height and CCI continued to comprise the most important features (Figure 2B). The importance of marital status was variable as compared with results derived from the MIMIC model, while ‘delta SOFA’ between days 1 – 3 of critical illness proved to be far better correlated with mortality in the PSH data set.
DISCUSSION
Historically, a higher SOFA score, signaling severe organ failure, has been closely linked with poorer clinical outcomes [17, 18]. However, our analysis proposes a paradigm shift. Our pivotal finding is the pronounced impact of patient-specific and social risk factors on 30-day mortality, overshadowing the predictive relevance of organ dysfunction severity [10].
Our data indicate that, by day 2 of sepsis, certain organ dysfunction measures can predict mortality with some degree of reliability. This finding is consistent with previous literature that indicates enhanced prognostic value of SOFA score when measured later in a patient’s hospitalization [19, 20]. Yet, it is the integration of a patient’s age, weight, height, marital status and comorbidity profile (as encapsulated by the Charlson Comorbidity Index) that significantly amplifies prognostic precision as compared with the SOFA score alone or any of its individual organ components. By Day 8, models enriched with these variables not only sustained but also enhanced the predictive accuracy of mortality, with model performance metrics surpassing those based solely on clinical measures.
The inclusion of individual organ dysfunction parameters only modestly improved the model’s performance, reinforcing the premise that, while clinical measures of organ dysfunction are not to be overlooked, they are evidently less predictive of patient outcomes compared to SDoH. This is a critical observation, as it underscores the limitations of current clinical-only prognostic models and highlights the potential for improved risk stratification through the incorporation of other, readily available, patient-specific factors.
We externally validated the best model generated from publicly available data on a subset of patients receiving health care at our institution. We found that, while the predictive model remained stable, delta SOFA between days 1 and 3 of critical illness was a far stronger predictor of mortality in our patient population. The prognostic utility of delta SOFA has been previously reported [21], although it is not currently in widespread clinical use.
Past efforts to improve on the SOFA score have produced mixed results [22–24]. However, our research supports these endeavors, suggesting that an enhanced SOFA score incorporating SDoH could sharpen the predictive accuracy for mortality in sepsis patients. This augmented model may be particularly beneficial for patients who, due to cognitive impairments caused by their illness, are unable to provide a detailed medical history. Given the large regional variability in socioeconomic status and ethnic diversity, it may also be necessary to adjust the model to reflect this local variability. Methods such as federated learning can be utilized for this purpose [25].
Although our study offers valuable insights, it is important to acknowledge its limitations. A significant constraint was that our validation dataset recorded SOFA scores every 48 hours instead of every 24 hours. This less frequent recording could have affected the accuracy of validation, given that peak SOFA scores usually manifest within the first 24 hours of sepsis onset. Furthermore, our exclusive use of the Sepsis-3 diagnostic criteria might not encompass the full range of clinical presentations and outcomes. This is particularly relevant since other frameworks, like Sepsis-2 [26], are still widely used in clinical practice. Finally, as the validation cohort was small, with minimal numbers for many subgroups, one must be cautious in interpreting the data in a general manner; rather, it should be approached as a preliminary glance at the model performance. Further external validation, using diverse populations, a community healthcare setting and temporal separation of development/validation datasets are underway to address this limitation.
Our study’s findings have implications beyond the immediate scope of sepsis and can be applied to various models across different medical domains [27, 28]. Integrating SDoH and demographic data with clinical scoring systems can enhance predictive accuracy and provide a more holistic understanding of patient outcomes. This approach is particularly relevant in chronic diseases such as diabetes and heart failure, where socioeconomic factors profoundly impact disease progression and mortality. Moreover, incorporating SDoH into predictive models for infectious diseases like COVID-19 can improve resource allocation and patient management in pandemics, highlighting the broader applicability of our findings across diverse medical fields.
In conclusion, our investigation underscores the imperative to revisit the prognostic frameworks for sepsis. It is evident that a multidimensional approach, encompassing both clinical and non-clinical factors, is crucial for a more accurate prediction of outcomes. Our work contributes to the growing body of evidence that supports the integration of broader patient information, extending beyond the confines of physiological and laboratory measures, into prognostic models for sepsis.
Supplementary Material
Integrating SOFA with socio-demographic data improves sepsis mortality prediction
Delta SOFA between days 1–3 is a critical predictor of 30-day sepsis mortality
External validation shows consistent model performance in different patient cohorts
Funding
Funding was provided by the National Institute of General Medical Sciences R35GM150695 (ASB), endowment funds from the Department of Anesthesiology at Penn State Health (ES), and start-up funds from the Department of Public Health Sciences (VA).
List of abbreviations
- AUROC
area under the receiver operating characteristic curve
- CCI
Charlson comorbidity index
- ICU
intensive care unit
- MIMIC
Medical Information Mart for Intensive Care
- NPV
negative predictive value
- PPV
positive predictive value
- PSH
Penn State Health
- SOFA
sequential (or sepsis-associated) organ failure assessment
Footnotes
Competing interests
The authors declare that they have no competing interests.
Ethics approval and consent to participate
De-identified and publicly-available human data was accessed via Physionet [29] and utilized in accordance with PhysioNet Credentialed Health Data Use Agreement 1.5.0. Prospective validation data was collected in accordance with the Penn State Human Subjects Protection Office (IRB study# 15328, approved 7/27/2020).
Availability of data and materials
The datasets analyzed during the current study are available in the MIMIC-IV version 2.2 database repository, available at https://physionet.org/content/mimiciv/2.2. Validation data from PSH will be made available from the investigator on reasonable request. The code used to analyze MIMIC-IV data is publicly available and can be accessed here: https://github.com/rasman/SOFA.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Vincent JL, Moreno R, Takala J, Willatts S, De Mendonca A, Bruining H, Reinhart CK, Suter PM, Thijs LG: The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med 1996, 22(7):707–710. [DOI] [PubMed] [Google Scholar]
- 2.Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, Bellomo R, Bernard GR, Chiche JD, Coopersmith CM et al. : The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016, 315(8):801–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Knaus WA, Draper EA, Wagner DP, Zimmerman JE: APACHE II: a severity of disease classification system. Crit Care Med 1985, 13(10):818–829. [PubMed] [Google Scholar]
- 4.Minne L, Abu-Hanna A, de Jonge E: Evaluation of SOFA-based models for predicting mortality in the ICU: A systematic review. Crit Care 2008, 12(6):R161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moreno RP, Metnitz PGH, Almeida E, Jordan B, Bauer P, Campos RA, Iapichino G, Edbrooke D, Capuzzo M, Le Gall JR et al. : SAPS 3 - From evaluation of the patient to evaluation of the intensive care unit. Part 2: Development of a prognostic model for hospital mortality at ICU admission. Intensive Care Medicine 2005, 31(10):1345–1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moreno R, Singer M, Rhodes A: Why the Sequential Organ Failure Assessment score needs updating? Crit Care Sci 2024, 36:e20240296en. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Minejima E, Wong-Beringer A: Impact of Socioeconomic Status and Race on Sepsis Epidemiology and Outcomes. J Appl Lab Med 2021, 6(1):194–209. [DOI] [PubMed] [Google Scholar]
- 8.Galiatsatos P, Brigham EP, Pietri J, Littleton K, Hwang S, Grant MC, Hansel NN, Chen ES: The effect of community socioeconomic status on sepsis-attributable mortality. J Crit Care 2018, 46:129–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Galiatsatos P, Follin A, Alghanim F, Sherry M, Sylvester C, Daniel Y, Chanmugam A, Townsend J, Saria S, Kind AJ et al. : The Association Between Neighborhood Socioeconomic Disadvantage and Readmissions for Patients Hospitalized With Sepsis. Critical Care Medicine 2020, 48(6):808–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Galiatsatos P, Kachalia A, Belcher HME, Hughes MT, Kahn J, Rushton CH, Suarez JI, Biddison LD, Golden SH: Health equity and distributive justice considerations in critical care resource allocation. The Lancet Respiratory Medicine 2020, 8(8):758–760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen IY, Szolovits P, Ghassemi M: Can AI Help Reduce Disparities in General Medical and Mental Health Care? AMA J Ethics 2019, 21(2):E167–179. [DOI] [PubMed] [Google Scholar]
- 12.Johnson AE, Stone DJ, Celi LA, Pollard TJ: The MIMIC Code Repository: enabling reproducibility in critical care research. J Am Med Inform Assoc 2018, 25(1):32–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Johnson A, Pollard T, Blundell J, a-chahin, Gow B, erinhong, Schubert M, Dang K, Paris N, shu98 et al. : MIT-LCP/mimic-code: MIMIC Code v2.4.0. In., v2.4.0 edn: Zenodo; 2023. [Google Scholar]
- 14.Charlson ME, Pompei P, Ales KL, MacKenzie CR: A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987, 40(5):373–383. [DOI] [PubMed] [Google Scholar]
- 15.Pedregosa F, Varoquaux G, Gramfort A, Vincent M, Bertrand T, Grisel O, Blondel M, Prettenhofer P, Weiss R, Dubourg V et al. : Scikit-learn: Machine Learning in Python. Journal of Machine Learning Research 2011, 12:2825–2830. [Google Scholar]
- 16.Patzer RE, Kaji AH, Fong Y: TRIPOD Reporting Guidelines for Diagnostic and Prognostic Studies. JAMA Surg 2021, 156(7):675–676. [DOI] [PubMed] [Google Scholar]
- 17.Raith EP, Udy AA, Bailey M, McGloughlin S, MacIsaac C, Bellomo R, Pilcher DV, Australian, New Zealand Intensive Care Society Centre for O, Resource E: Prognostic Accuracy of the SOFA Score, SIRS Criteria, and qSOFA Score for In-Hospital Mortality Among Adults With Suspected Infection Admitted to the Intensive Care Unit. JAMA 2017, 317(3):290–300. [DOI] [PubMed] [Google Scholar]
- 18.Falcao ALE, Barros AGA, Bezerra AAM, Ferreira NL, Logato CM, Silva FP, do Monte A, Tonella RM, de Figueiredo LC, Moreno R et al. : The prognostic accuracy evaluation of SAPS 3, SOFA and APACHE II scores for mortality prediction in the surgical ICU: an external validation study and decision-making analysis. Ann Intensive Care 2019, 9(1):18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moreno R, Vincent JL, Matos R, Mendonca A, Cantraine F, Thijs L, Takala J, Sprung C, Antonelli M, Bruining H et al. : The use of maximum SOFA score to quantify organ dysfunction/failure in intensive care. Results of a prospective, multicentre study. Working Group on Sepsis related Problems of the ESICM. Intensive Care Med 1999, 25(7):686–696. [DOI] [PubMed] [Google Scholar]
- 20.Vincent JL, de Mendonca A, Cantraine F, Moreno R, Takala J, Suter PM, Sprung CL, Colardyn F, Blecher S: Use of the SOFA score to assess the incidence of organ dysfunction/failure in intensive care units: Results of a multicenter, prospective study. Critical Care Medicine 1998, 26(11):1793–1800. [DOI] [PubMed] [Google Scholar]
- 21.de Grooth HJ, Geenen IL, Girbes AR, Vincent JL, Parienti JJ, Oudemans-van Straaten HM: SOFA and mortality endpoints in randomized controlled trials: a systematic review and meta-regression analysis. Crit Care 2017, 21(1):38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Arakawa M, Levy JH, Fujimori K, Kondo K, Iba T: A new SOFA score calculation to improve the predictive performance for mortality in sepsis-associated disseminated intravascular coagulopathy patients. J Crit Care 2021, 64:108–113. [DOI] [PubMed] [Google Scholar]
- 23.Lee HJ, Ko BS, Ryoo SM, Han E, Suh GJ, Choi SH, Chung SP, Lim TH, Kim WY, Kwon WY et al. : Modified cardiovascular SOFA score in sepsis: development and internal and external validation. BMC Med 2022, 20(1):263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu Y, Gao K, Deng H, Ling T, Lin J, Yu X, Bo X, Zhou J, Gao L, Wang P et al. : A time-incorporated SOFA score-based machine learning model for predicting mortality in critically ill patients: A multicenter, real-world study. Int J Med Inform 2022, 163:104776. [DOI] [PubMed] [Google Scholar]
- 25.Sheller MJ, Edwards B, Reina GA, Martin J, Pati S, Kotrotsou A, Milchenko M, Xu W, Marcus D, Colen RR et al. : Federated learning in medicine: facilitating multi-institutional collaborations without sharing patient data. Scientific Reports 2020, 10(1):12598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Levy MM, Fink MP, Marshall JC, Abraham E, Angus D, Cook D, Cohen J, Opal SM, Vincent JL, Ramsay G et al. : 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Crit Care Med 2003, 31(4):1250–1256. [DOI] [PubMed] [Google Scholar]
- 27.Pappas G, Queen S, Hadden W, Fisher G: The Increasing Disparity in Mortality between Socioeconomic Groups in the United States, 1960 and 1986. New England Journal of Medicine 1993, 329(2):103–109. [DOI] [PubMed] [Google Scholar]
- 28.Stringhini S, Carmeli C, Jokela M, Avendaño M, Muennig P, Guida F, Ricceri F, d’Errico A, Barros H, Bochud M et al. : Socioeconomic status and the 25 × 25 risk factors as determinants of premature mortality: a multicohort study and meta-analysis of 1·7 million men and women. Lancet 2017, 389(10075):1229–1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Goldberger AL, Amaral LA, Glass L, Hausdorff JM, Ivanov PC, Mark RG, Mietus JE, Moody GB, Peng CK, Stanley HE: PhysioBank, PhysioToolkit, and PhysioNet: components of a new research resource for complex physiologic signals. Circulation 2000, 101(23):E215–220. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


