Abstract
Background & Aims:
Treatment with immune checkpoint inhibitors (ICIs) for hepatocellular carcinoma (HCC) prior to liver transplantation (LT) has been reported; however, ICIs may elevate the risk of allograft rejection and impact other clinical outcomes. This study aims to summarize the impact of ICI use on post-LT outcomes.
Methods:
In this individual patient data meta-analysis, we searched databases to identify HCC cases treated with ICIs before LT, detailing allograft rejection, HCC recurrence, and overall survival. We performed Cox regression analysis to identify risk factors for allograft rejection.
Results:
Among 91 eligible patients, with a median (IQR) follow-up of 690.0 (654.5) days, there were 24 (26.4%) allograft rejections, 9 (9.9%) HCC recurrences, and 9 (9.9%) deaths. Age (adjusted hazard ratio [aHR] per 10 years 0.72, 95% CI 0.53–0.99, p = 0.044) and ICI washout time (aHR per 1 week 0.92, 95% CI 0.86–0.99, p = 0.022) were associated with allograft rejection. The median (IQR) washout period for patients with ≤20% probability of allograft rejection was 94 (196) days. Overall survival did not differ between cases with and without allograft rejection (log-rank test, p = 0.2). Individuals with HCC recurrence had fewer median (IQR) ICI cycles than those without recurrence (4.0 [1.8] vs. 8.0 [9.0]; p = 0.025). The proportion of patients within Milan post-ICI was lower for those with recurrence vs. without (16.7% vs. 65.3%, p = 0.032).
Conclusion:
Patients have acceptable post-LT outcomes after ICI therapy. Age and ICI washout length relate to the allograft rejection risk, and a 3-month washout may reduce it to that of patients without ICI exposure. Number of ICI cycles and tumor burden may affect recurrence risk. Large prospective studies are necessary to confirm these associations.
Keywords: Immune Checkpoint Inhibitors, Hepatocellular Carcinoma, Liver Neoplasms, Liver Transplantation, Graft Rejection, Recurrence
Graphical Abstract

Age and ICI washout length relate to the allograft rejection risk, and a 3-month washout may reduce it to that of patients without ICI exposure
The data underscore the need for large-scale multicenter studies to provide robust evidence supporting the efficacy and safety of ICI for HCC in LT candidates
Introduction
Primary liver cancer is the sixth most diagnosed cancer and the third leading cause of death due to cancer, of which hepatocellular carcinoma (HCC) comprises 75 to 85% of cases.1 Liver transplantation (LT) is a preferred treatment for patients with HCC given its curative nature, particularly for those with liver dysfunction or multifocal disease.2 While LT is one of the best treatment options for patients with early-stage HCC, most patients are diagnosed at intermediate or advanced stages, making them unsuitable candidates for LT at initial presentation. However, an increasing proportion of patients with HCC may be eligible for LT with successful local control of tumor using locoregional treatments (LRTs) or systemic therapy.3–5
In the last decade, immune checkpoint inhibitors (ICIs), including anti-programmed cell death 1 (PD-1), anti-programmed cell death ligand 1 (PD-L1), and antibodies to cytotoxic T lymphocyte antigen-4, have revolutionized the treatment landscape of many cancers, including HCC.6–8 Several phase III studies reported a higher overall survival of checkpoint inhibitors (in combination with other compounds) compared to tyrosine kinase inhibitors making this option the first-line therapy for advanced HCC. Most recently, Emerald-1, a double-blinded, placebo-controlled phase III randomized clinical trial, reported that the combination of durvalumab, bevacizumab, and transarterial chemoembolization (TACE) is superior to TACE alone for objective responses and progression-free survival (hazard ratio [HR] 0.77, 95% CI 0.61–0.98) for TACE-eligible unresectable HCC. These data suggest that ICI in combination with LRT may represent a more robust treatment regimen for intermediate-stage HCC compared to TACE alone.9 Small clinical trials reported promising efficacy of ICI treatment in early-stage resectable HCC.10–12
While the indications for ICI treatment are expected to increase for a broader group of patients with HCC, there exists a potential concern regarding the use of ICIs before LT as it may increase the risk of allograft rejection, which in some cases can lead to death. The interval period between the last dose of ICI and the time of LT (ICI washout period), ICI cycles, and other factors like tumor burden can potentially impact the likelihood of LT rejection or HCC recurrence.13,14 Several small studies and case series have attempted to demonstrate ICIs as a possible treatment strategy for downstaging.13,15 However, there is insufficient safety data regarding the use of ICIs prior to LT given the limited sample size of each report. Therefore, we conducted a systematic review and individual patient data (IPD) meta-analysis to summarize our current understanding of the impact of using ICIs prior to LT on post-transplant outcomes, including allograft rejection and HCC recurrence.
Materials and methods
Study design
This systematic review with IPD meta-analysis was performed based on preferred reporting items for a systematic review and meta-analysis of IPD16 and an outline for assessment and synthesis of case reports and case series in systematic review projects. 17 Currently, most of the evidence regarding using ICIs in HCC cases prior to LT is derived from non-comparative case reports and case series. Therefore, we gathered and pooled all related case reports and case series based on available guidelines for this purpose. We registered the protocol of our study on the International Prospective Register of Systematic Reviews (PROSPERO)– CRD42023494951.
Data source and search strategy
We searched PubMed, Web of Science (all editions of core collections and ProQuest ™ Dissertations & Theses Citation), and Scopus until September 09, 2023 without language limitation (Table S1). For each database, we developed a comprehensive search strategy using different keywords for three main concepts including HCC, LT, and ICIs (Supplementary Appendix 1).
To minimize possible publication bias, we searched for meeting abstracts in both Web of Science and Scopus databases. Furthermore, we manually searched for available meeting abstracts published between 2020 and 2024 in eight congresses, including Digestive Disease Week and the main congresses of EASL, AASLD, APASL, ASCO, ESMO, AACR, and EACR.
We also examined the references of included studies and related reviews to find any additional relevant studies. We set automatic alerts in PubMed and Scopus to identify newly published studies during the analysis and writing of our manuscript. We performed a final search on January 13, 2024.
Eligibility criteria and outcome
We included any study types in which ICIs had been used in patients with HCC prior to LT. We evaluated outcomes including the occurrence of allograft rejection, the result of rejection treatment (fully recovered, graft loss, death), overall survival status (alive or deceased), the incidence of HCC recurrence, and pathology findings on explant. We included studies with no time, language, and location limitations. Studies reporting non-HCC liver cancer, use of ICIs only after LT, and use of ICIs without LT were excluded. Studies without providing IPD on allograft rejection status were excluded. A full list of inclusion and exclusion criteria can be seen in Table S2.
Study screening and selection
All identified studies were imported to EndNote software version 21. Two authors (MSR-Z and YHY) independently screened titles and abstracts of studies at the first step and reviewed full texts at the next step for possible inclusion in this project. In the case of overlapping patient cases, we included the most recent one or the publication with the more complete data. Any disagreements were resolved by mutual consensus between MSR-Z and YHY or by consultation with a third author (JDY).
Data extraction and collection of individual patient data
We extracted the following data from included studies: age, gender, liver disease etiology, Barcelona Clinic Liver Cancer (BCLC) stage and alpha-fetoprotein (AFP) before commencing and after the completion of immunotherapy, details of the ICI regimens including dosage, duration, and cycle number, interventions other than ICIs before LT, pathology findings on explant (number of viable tumors, largest diameters of viable tumors, tumor differentiation, and microvascular invasion), and maximum follow-up duration. Post-LT outcomes of interest included allograft rejection, rejection treatment outcome, HCC recurrence, or death.
Allograft rejection was classified as acute cellular, acute antibody-mediated, or chronic rejection. Data related to the severity of rejection (mild, moderate, severe) was extracted based on the rejection activity index (RAI). The RAI is scored according to the criteria defined by the International Banff schema for liver allograft rejection, where scores of 4–5, 6–7, and 8–9 are considered mild, moderate, and severe, respectively.18 We contacted the authors of the included publications to capture missing data.
Quality assessment and critical appraisal
We used criteria developed by Murad et al.17 for assessing case reports and case series. For cohort studies, we employed the Newcastle–Ottawa Scale.19 In both the scales and scoring systems, we made necessary modifications to their questions to align them with our project. There was one open-label clinical trial, and we used the same criteria to assess case series. Full methodology for performing quality assessment and critical appraisal can be seen in Supplementary Appendix 2.
Ethical considerations
In this study, we analyzed de-identified cases of previously published studies and included more data only if needed under the original permission of the Institutional Review Board of participating centers. The Institutional Review Board of Cedars-Sinai Medical Center approved this study (STUDY00003175).
Statistical analysis
For descriptive statistics, we presented categorical variables with frequency (percentages) and continuous variables with median (IQR). The chi-squared (or Fisher’s exact) and Mann-Whitney tests were used to assess the association between demographic, clinical, and therapeutic variables including the ICI regimen types, cycles, and washout time and our outcomes of interest. For the evaluation of the effect of ICI on tumor burden (AFP, BCLC), we utilized the Wilcoxon and Chi-square tests, or alternatively, the Fisher’s exact test when appropriate. We employed Kendall’s tau-b correlation and Kruskal-Wallis test to examine the effect of ICI cycles on tumor size and number reported on the explants' pathology findings. Additionally, we employed Kendall’s tau-b correlation test to analyze the correlation between ICI cycles and washout period with the RETREAT score.20
We utilized univariable and multivariable Cox proportional hazards regression models to determine the HR and 95% CI of different factors associated with allograft rejection. We also used a Cox proportional hazards regression model to assess the effect of allograft rejection on overall survival. A p value <0.05 was considered statistically significant in all analyses. We used R software version 4.3.3 for all analyses.
Results
Study selection
Our initial literature search yielded 2,248 records. Following the removal of duplicates, title and abstract screening, and exclusion of irrelevant full texts, 22 studies met eligibility criteria. Additionally, manual searches of conference abstracts21–24 and backward citation checking in reviews25,26 found six additional studies. In our updated search on January 13, 2024, we identified five more related studies.27–31 Hence, we targeted 33 studies, encompassing 127 cases, for pooled analysis. Contact with all study authors led to IPD of 24 studies (71 cases).15,18,22,24–44 Six studies (20 cases)13,21,45–48 did not provide IPD but had sufficient data in their main paper including ICI regimen, allograft rejection status, and follow-up time for inclusion. Therefore, the systematic review included 30 studies,13,15,18,21,22,24–48 comprising 91 cases (Fig, 1, Table S1, and Supplementary Appendix 3).
Fig. 1. PRISMA flowchart diagram.
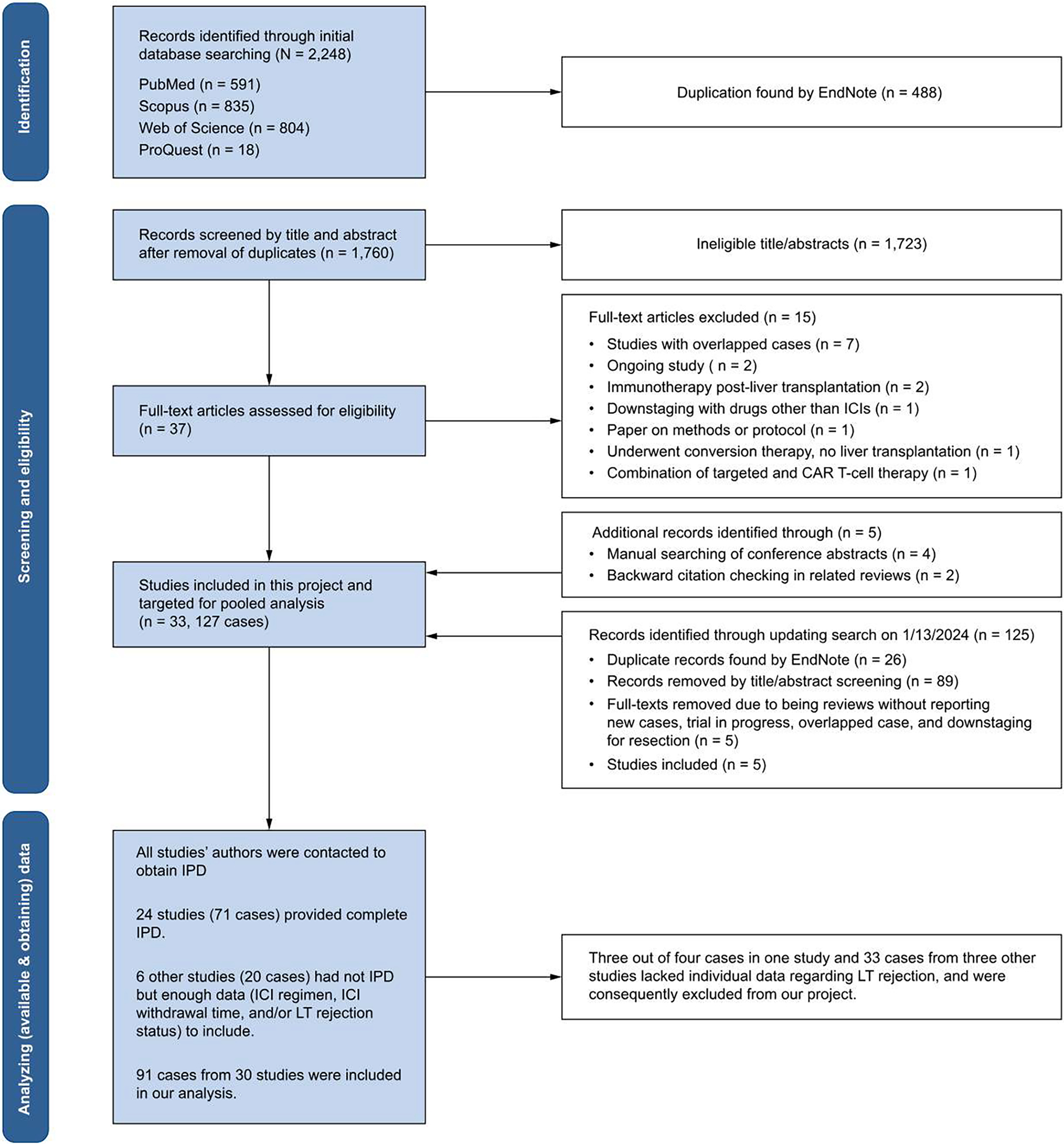
CAR, chimeric antigen receptor; ICIs, immune checkpoint inhibitors; LT, liver transplant.
Study critical appraisal
Of 27 case reports/case series and one clinical trial, three studies were categorized as good-quality, 19 studies as fair-quality, and five studies as low-quality. Among these 27 studies, we were able to obtain missing data for 21 studies. Considering the new data, the results for critical appraisal for these 21 studies changed to 15 as good-quality and six as fair-quality. For six remaining studies we could not obtain full IPD, four were fair- and two were low-quality. We also included three retrospective cohort studies. One of them was categorized as fair-quality and the other two as low-quality studies. After obtaining IPD, two were regarded as good and one as fair-quality. Missing data, short follow-up time, and using other interventions in addition to ICI were the main reasons for the low-quality in all evaluated studies. Full results for the evaluation of the quality of the included studies have been provided in Supplementary Appendix 4.
Baseline characteristics of included cases
Most of the included cases were from USA (n = 42, [46.2%]) and China (n = 34, [37.4%]) (Table S3). Median age (IQR) of the included patients was 61 (13.2) years and 81.2% of cases were male. The most common liver disease etiology was viral hepatitis (77.5%). Among cases with available data, most were beyond Milan criteria (64 cases, 81.0%) and classified as BCLC B (35 cases, 45.5%) before starting ICI treatment. Twenty cases (26.0%) were in BCLC A, and twenty-two (28.6%) were in BCLC C+D. A total of 9 (12.3%) had AFP levels exceeding 1,000 ng/ml before ICI treatment, while all but two cases had AFP levels less than 1,000 ng/ml after the completion of ICI treatment. There was no significant difference in the frequency of allograft rejection or HCC recurrence based on AFP levels or pre-treatment BCLC staging (Table 1).
Table 1.
Demographic and clinical characteristics.
| Variable1 | Total population median (IQR)/frequency (%) | Allograft rejection status |
p value | FICC recurrence status5 |
p value | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Yes (24 [26.4]) | No (67 [73.6]) | Yes (9 [9.9]) | No (82 [90.1]) | |||||||||||
|
| ||||||||||||||
| Age, year | 61 (13.2) | 54 (19) | 62 (10) | 0.023 | 53.5 (10.5) | 61.5 (12.2) | 0.112 | |||||||
| Male gender | 65 (81.2) | 19 (82.6) | 46 (80.7) | 1.000 | 6 (75.0) | 59 (81.9) | 0.640 | |||||||
| HCC etiology | ||||||||||||||
| HBV | 38 (47.5) | 11 (47.8) | 27 (47.4) | 0.318 | 7 (87.5) | 31 (43.1) | 0.107 | |||||||
| HCV | 24 (30.0) | 10 (43.5) | 14 (24.6) | 0 (0.0) | 24 (33.3) | |||||||||
| MASLD | 6 (7.5) | 1 (4.3) | 5 (8.8) | 0 (0.0) | 6 (8.3) | |||||||||
| MetALD/Alcohol | 6 (7.5) | 1 (4.3) | 5 (8.8) | 1 (12.5) | 5 (6.9) | |||||||||
| Other3 | 6 (7.5) | 0 (0.0) | 6 (10.5) | 0 (0.0) | 6 (8.3) | |||||||||
| AFP before ICI, ng/ml | 20.1 (189.5) | 56.9 (245.8) | 12.7 (171) | 0.189 | 20.5 (130.6) | 20.1 (190.1) | 0.944 | |||||||
| AFP before ICI (>1,000) | 9 (12.3) | 4 (17.4) | 5 (10.0) | 0.450 | 1 (16.7) | 8 (11.9) | 0.560 | |||||||
| AFP after ICI, ng/ml | 4.14 (4.3) | 5.2 (13.4) | 4.0 (4.7) | 0.176 | 5.3 (19.4) | 4.1 (4.4) | 0.350 | |||||||
| AFP after ICI (>1,000) | 2 (2.7) | 1 (4.3) | 1 (2.0) | 0.534 | 1 (16.7) | 1 (1.5) | 0.159 | |||||||
| BCLC pre-ICI | ||||||||||||||
| A | 20 (26.0) | 5 (23.8) | 15 (26.8) | 0.850 | 0 (0.0) | 20 (29.0) | 0.186 | |||||||
| B | 35 (45.5) | 9 (42.9) | 26 (46.4) | 4 (50.0) | 31 (44.9) | |||||||||
| C & D | 22 (28.6) | 7 (33.3) | 15 (26.8) | 4 (50.0) | 18 (26.1) | |||||||||
| Milan criteria before ICI (within) | 15 (19.0) | 5 (22.7) | 10 (17.5) | 0.750 | 0 (0.0) | 15 (22.1) | 0.341 | |||||||
| Milan criteria post-ICI (within) | 33 (60.0) | 14 (66.7) | 19 (55.9) | 0.610 | 1 (16.7) | 32 (65.3) | 0.032 | |||||||
| ICI regimen type | ||||||||||||||
| Nivolumab2 | 45 (49.5) | 13 (54.2) | 32 (47.8) | 0.308 | 3 (37.5) | 42 (57.5) | 0.060 | |||||||
| Pembrolizumab | 21 (23.1) | 6 (25.0) | 15 (22.4) | 2 (25.0) | 9 (12.3) | |||||||||
| Atezolizumab (+bevacizumab) | 14 (15.4) | 1 (4.2) | 13 (19.4) | 0 (0.0) | 14 (19.2) | |||||||||
| Other2 | 11 (12.1) | 4 (16.7) | 7 (10.4) | 3 (37.5) | 8 (11.0) | |||||||||
| ICI class (anti-PD-14) | 76 (83.5) | 23 (95.8) | 53 (79.1) | 0.105 | 8 (100) | 58 (79.5) | 0.340 | |||||||
| ICI cycles | 8 (9) | 7 (16.5) | 8 (8.2) | 0.626 | 4.0 (1.8) | 8.0 (9.5) | 0.025 | |||||||
| ICI washout period, day | 42 (71) | 22 (18.0) | 43.0 (86.5) | <0.001 | 23.5 (20.8) | 42 (88) | 0.205 | |||||||
| Any interventions than ICI | 87 (96.7) | 23 (100) | 64 (95.5) | 0.567 | 8 (100) | 69 (95.8) | 1.000 | |||||||
| TKIs | 44 (48.9) | 12 (52.2) | 32 (47.8) | 0.901 | 7 (87.5) | 27 (37.5) | 0.009 | |||||||
| Surgery | 21 (23.3) | 5 (21.7) | 16 (23.9) | 1.000 | 3 (37.5) | 18 (25.0) | 0.426 | |||||||
| Systemic chemotherapy | 3 (3.3) | 1 (4.3) | 2 (3.0) | 1.000 | 0 (0.0) | 3 (4.2) | 1.000 | |||||||
| Locoregional treatment | 70 (77.8) | 23 (100) | 47 (70.1) | 0.007 | 6 (75.0) | 64 (88.9) | 0.261 | |||||||
|
| ||||||||||||||
| Explant pathology findings | ||||||||||||||
| Number of viable tumors | 0.278 | 0.089 | ||||||||||||
| 0 | 24 (33.8) | 6 (28.6) | 18 (36.0) | 0 (0.0) | 24 (36.9) | |||||||||
| 1 | 18 (25.4) | 8 (38.1) | 10 (20.0) | 1 (16.7) | 17 (26.2) | |||||||||
| >1 | 29 (40.8) | 7 (33.3) | 22 (44.0) | 5 (83.3) | 24 (36.9) | |||||||||
| Largest tumor size, mm | 15 (41) | 15 (61.0) | 14 (32.6) | 0.301 | 46.5 (41.5) | 9 (35.5) | 0.017 | |||||||
| Tumor differentiation | ||||||||||||||
| No viable tumor | 24 (33.8) | 7 (33.3) | 17 (34.0) | 0 (0.0) | 24 (36.9) | |||||||||
| Well or very well | 12 (16.9) | 2 (9.5) | 10 (20.0) | 0.632 | 0 (0.0) | 12 (18.5) | 0.056 | |||||||
| Moderate | 23 (32.4) | 7 (33.3) | 16 (32.0) | 4 (66.7) | 19 (29.2) | |||||||||
| Poor | 12 (16.9) | 5 (23.8) | 7 (14.0) | 2 (33.3) | 10 (15.4) | |||||||||
| Microvascular invasion (Yes) | 15 (22.1) | 5 (23.8) | 10 (21.3) | 1.000 | 2 (33.3) | 13 (21.0) | 0.607 | |||||||
| Allograft rejection Status (Yes) | 24 (26.4) | - | - | - | 2 (25.0) | 22 (30.1) | 1.000 | |||||||
AFP, alpha-fetoprotein; BCLC, Barcelona Clinic Liver Cancer; HCC, hepatocellular carcinoma; ICI, immune checkpoint inhibitor; LT, liver transplantation; MASLD, metabolic dysfunction-associated steatotic liver disease; MetALD, metabolic alcohol related liver disease; PD-1, programmed cell death protein 1; PD-L1, programmed death ligand 1; TKIs, tyrosine kinase inhibitors.
Number (%) of missing data for each variable: Rejection: 0 (0), Recurrence: 10 (11), Age: 11 (12.2), Gender: 11 (12.1), HCC etiology: 11 (12.1), ICI regimen: 0 (0), ICI class: 0 (0), ICI cycle: 12 (13.2), ICI washout period: 0 (0), AFP before ICI: 18 (19.8), AFP after ICI: 18 (19.8), BCLC before ICI: 14 (15.4), Any other Interventions than ICI: 1 (1.1), tyrosine kinase inhibitors: 1 (1.1), surgery: 1 (1.1), systemic chemotherapy: 1 (1.1), locoregional therapy: 1 (1.1), Milan criteria before ICI: 12 (13.2), Milan criteria post-ICI: 36 (39.6), Number of viable tumors: 20 (22.0), Largest diameter of tumor: 22 (24.2), Tumor differentiation: 20 (22.0), and Microvascular invasion: 23 (25.3).
Three cases that used other immune checkpoint inhibitors in addition to nivolumab (nivolumab-toripalimab-sintilimab-tislelizumab (1), and nivolumab-ipilimumab (2)) were categorized as nivolumab.
Other immune checkpoint inhibitors include durvalumab (n = 1), camrelizumab (n = 4), and sintilimab (n = 6).
This category includes 74 cases with anti-PD-1 alone and two cases with PD-1 and anti-CTLA-4 (nivolumab-ipilimumab).
One clinical trial with a total of 10 patients reported one case of HCC recurrence. As individual patient data could not be obtained, characteristics of these 10 patients were not included in the table.
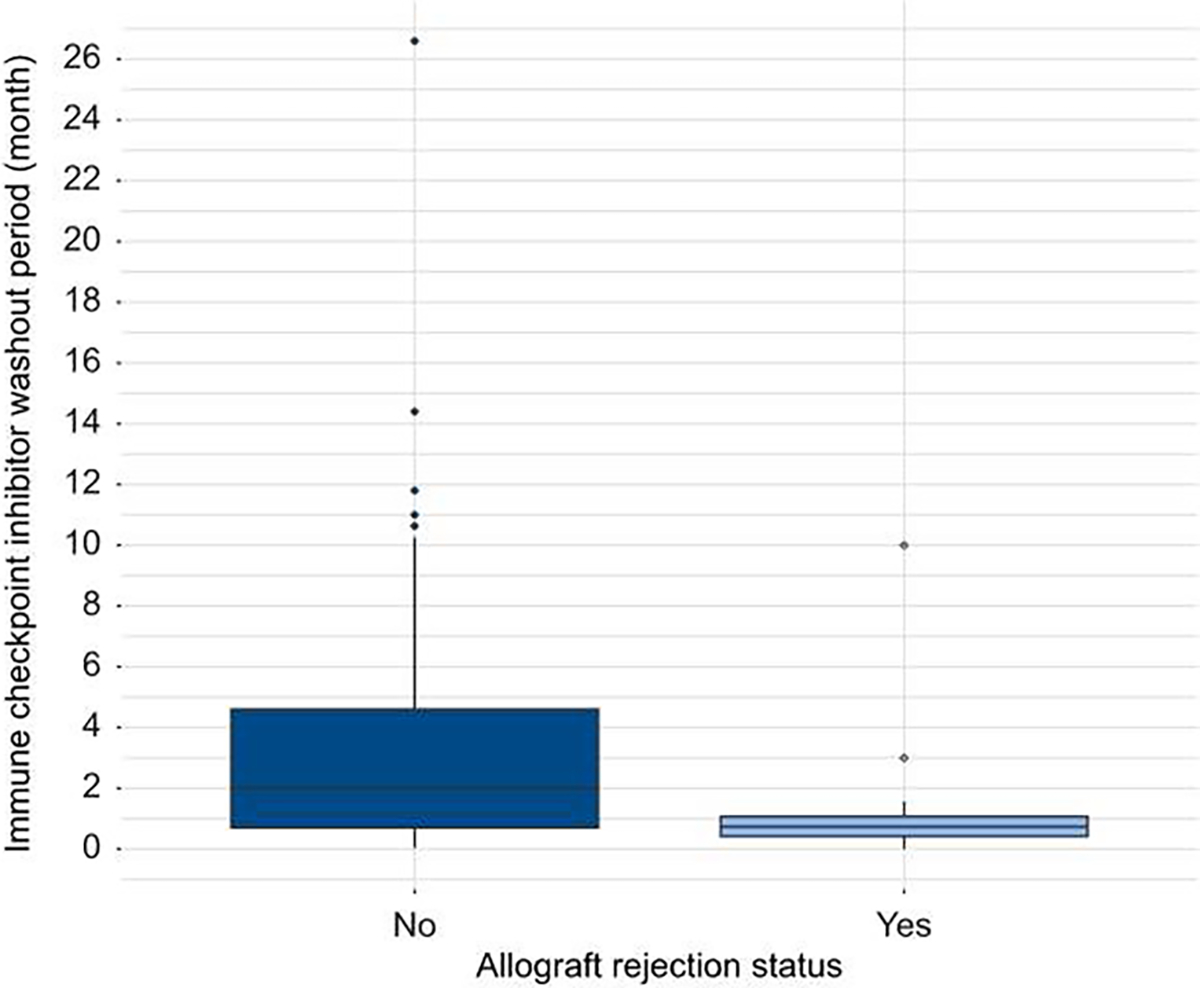
Nivolumab was used in 49.5% of cases alone or in combination with other ICIs. Other common ICIs were pembrolizumab (23.1%) and atezolizumab together with bevacizumab (15.4%). The median (IQR) ICI cycles (number) and ICI washout period (days) were 8 (9) and 42 (71), respectively. ICI washout period (days) was shorter for patients who had rejection vs. those without rejection (22 vs. 43 days, p <0.001) (Fig. 2) Complete characteristics of cases including tumor burden features have been provided in Table 1 and Table S4).
Fig. 2. Comparison of immune checkpoint inhibitor washout period in patients with and without allograft rejection.
Evaluation of the effect of ICIs on the HCC burden
After completing ICI treatment, eight cases (11.1%) showed no viable tumor, 33 cases (45.8%) were BCLC 0-A, 25 cases (34.7%) were BCLC B, and six cases (8.3%) were BCLC C+D. Data on Milan criteria status post-IC! treatment were available for 55 cases, with 60.0% meeting the Milan criteria. The median (IQR) AFP levels significantly decreased from 20.1 (189.5) before ICI to 4.14 (4.3) after ICI (p <0.0001). Based on the pathology findings of the explant, 24 (33.8%) cases showed no viable tumor, 12 (16.9 %) were well or very well differentiated, and 53 (77.9%) had no microvascular invasion. Using Kendall’s tau-b correlation, we found that the number of ICI cycles was inversely associated with the largest diameter of the tumor on explant (τ = −0.28, p = 0.0014). Additionally, we found that the median (IQR) ICI cycles were significantly different among patients with no viable tumor (10.0 [9.5]), one viable tumor (6.5 [13.8]), and more than one viable tumor (7 [7]) (p = 0.044).
Description of post-LT outcomes
During a median (IQR) of maximum follow-up was 690.0 (654.5) days, nine (9.9%) patients died (Table S4). There were 24 (26.4%) cases of allograft rejection, comprising 23 cellular rejections and one antibody-mediated rejection (Tables 2 and S4). The median (IQR) time to rejection for these 24 cases was 10.0 (30.8) days. Of 17 cases with available data regarding the severity of rejection, nine were classified as mild, four as moderate, and four as severe.
Table 2.
Characteristics of patients with liver allograft rejection.
| Age, year, gender, (reference) | HCC etiology | ICI regimen, cycles, & washout period, day | BCLC staging pre-ICI | Other intervention before LT | Allograft rejection time, day | Rejection treatment regimen | Rejection treatment outcome | Tumor burden on explant | Overall status, last follow-up time, day |
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| 64, M, 41 | HCV | Nivolumab, 23, 16 | C | TKI, LRT | 9 | Steroid, TG | Resolved | NV | Alive, 480 |
| 54, M, 30 | MASLD | Atezolizumab, 2, 47 | B | Bevacizumab, LRT | 89 | Steroid | Resolved | BM | Alive, 278 |
| 65, M, 38 | HCV | Nivolumab, 10, 10 | A | Surgery, LRT | 14 | TG, Steroid, PP, IVIG | Resolved | NV | Alive, 1,186 |
| 68, M, 24 | HCV | Nivolumab, 6, 11 | A | LRT | 10 | TG, IVIG, RTX | Resolved | NV | Alive, 1,131 |
| NA, 13 | NA | Pembrolizumab, NA, 30 | NA | NA | 13 | Steroid, PP | Resolved | NA | Alive, 13 |
| 68, M, 44 | HCV | Nivolumab, 3, 300 | B | TKI, LRT | 990 | Steroid | Resolved | WM | Alive, 1,170 |
| 29, M,43 | HBV | Nivolumab, 25, 22 | B | LRT | 131 | Steroid | Resolved | WM | Alive, 1,637 |
| 53, M,43,1 | HCV | Nivolumab, 25, 2 | B | LRT | 169 | No treatment | Resolved | NV | Alive, 1,365 |
| 56, F, 43 | HCV | Nivolumab, 7, 23 | D | LRT | 84 | Steroid | Resolved | BM | Alive, 367 |
| 30, M, 15 | HBV | Nivolumab, 25, 22 | B | LRT | 108 | Increase TAC | Resolved | NV | Alive, 480 |
| 66, M,18,2 | MetALD | Sintilimab, 4, 26 | C | TKI, LRT, Surgery | 4 | IVIG, Steroid, increase ARM | Resolved | BM | Alive, 892 |
| 55, M, 18 | HBV | Pembrolizumab, 2, 24 | C | TKI, LRT | 22 | IVIG, Steroid, increase ARM | Resolved | BM | Alive, 944 |
| 52, M, 18 | HBV | Pembrolizumab, 1, 29 | A | TKI, LRT | 15 | IVIG, Steroid, increase ARM | Resolved | WM | Alive, 1,012 |
| 41, M,18 | HBV | Sintilimab, 10, 21 | C | TKI, LRT | 9 | IVIG, Steroid, increase ARM | Resolved | BM | Alive, 912 |
| 50, M, 18 | HBV | Pembrolizumab, 4, 17 | C | LRT | 8 | IVIG, Steroid, increase ARM | Resolved | BM | Alive, 577 |
| 38, M, 18 | HBV | Sintilimab, 8, 14 | C | TKI, LRT | 4 | IVIG, Steroid, increase ARM | Resolved | BM | Alive, 1,003 |
| 43, M,18 | HBV | Camrelizumab, 5, 90 | B | TKI, LRT, Surgery | 7 | IVIG, Steroid, increase ARM | Resolved | BM | Alive, 899 |
| 51, M, 18 | HBV | Pembrolizumab, 3, 20 | B | TKI, LRT | 4 | Steroid, increase ARM | Resolved | BM | Alive, 1,054 |
| 48, F,18,3 | HBV | Pembrolizumab, 3, 7 | B | LRT | 7 | IVIG, Steroid, increase ARM | Resolved | BM | Dead, 173 |
| 57, M, 36 | HCV | Nivolumab, 4, 42 | B | LRT | 10 | Steroid | Resolved | NA | Dead, 355 |
| 61, F, 38 | HCV | Nivolumab, 19, 35 | A | TKI, LRT | 12 | TG, Steroid, PP, IVIG | Re-transplant | WM | Alive, 1,916 |
| 67, F, 24 | HCV | Nivolumab, 21, 40 | A | LRT | 6 | TG, IVIG, RTX, PP | Re-transplant | WM | Alive, 1,947 |
| 37, M, 45 | HBV | Nivolumab, 8, 0 | NA | TKI, LRT, Surgery, Systemic chemotherapy | 1 | NA | Dead | NA | Dead, 1 |
| 65, M, 47 | HCV | Nivolumab, 52, 8 | NA | TKI, LRT, Surgery | 6 | Steroid, TG | Dead | NV | Dead, 10 |
ARM, anti-rejection medication; BCLC, Barcelona Clinic Liver Cancer; BM, viable tumor beyond Milan criteria; HCC, hepatocellular carcinoma; ICI, immune checkpoint inhibitor; IVIG, intravenous immunoglobulin; LRT. locoregional therapy; LT, liver transplantation; MASLD, metabolic dysfunction-associated steatotic liver disease; MetALD, metabolic alcohol related liver disease; NA, not available; NV, non-viable tumor; PP, plasmapheresis; RTX, rituximab; TAC, tacrolimus; TG, thymoglobulin; TKI, tyrosine kinase inhibitor; WM, viable tumor within Milan criteria.
This case exhibited features of antibody-mediated rejection based on biopsy results but was not clinically diagnosed as such. Remarkably, it fully resolved without treatment. The remaining 23 cases in this table were classified as cellular rejection.
This case had HCC recurrence 245 days after LT.
This case had HCC recurrence 43 days after LT.
Twenty of 24 cases resolved with treatment including thymoglobulin, plasmapheresis, intravenous immunoglobulin, rituximab, increasing anti-rejection medication such as tacrolimus, and steroid, Four cases resulted in graft loss. In two cases, patients were retransplanted 8 and 22 days following rejection. The first one showed no rejection during follow-up. The latter had mild acute cellular rejection 9 days after retransplantation, which was successfully managed with steroids. Both retransplanted cases showed no graft loss after more than 5 years of follow-up. For the other two rejections, the treatment was unsuccessful and led to death. Nivolumab was used in both cases resulting in death, with washout periods of 0 and 8 days. Further, among 20 patients with resolved rejection, two deaths occurred 173 and 355 days after LT for reasons not related to rejection.
Nine cases (9.9%) experienced HCC recurrence (Tables 3 and S4). One case of recurrence was reported in a small clinical trial,21 among 10 patients. As individual patient data could not be obtained, these 10 patients were not included in the subsequent analysis. The median (IQR) time to HCC recurrence was 7.8 (15.1) months. The ICI regimen in these recurrence cases included nivolumab (n = 3), sintilimab (n = 2), pembrolizumab (n = 2), and camrelizumab (n = 1). Except for one case with an ICI cycle number of 32, all other cases had six ICI cycles or less. Based on the explant pathology findings, five cases had viable tumor exceeding Milan criteria, one had viable tumor within Milan criteria, and data were not available for two cases.
Table 3.
Characteristics of patients with HCC recurrence following liver transplantation.*
| Age, year, gender, (reference) | HCC etiology | ICI regimen, cycles, washout period, day | AFP, ng/ml |
BCLC staging pre-ICI | Other intervention | Milan criteria status post-ICI | Tumor burden on explant | HCC recurrence time, day | Overall status, last follow-up time, day | |
|---|---|---|---|---|---|---|---|---|---|---|
| Pre-ICI | Post-ICI | |||||||||
|
| ||||||||||
| 66, M,18,1 | MetALD | Sintilimab, 4, 26 | 28.1 | 28.1 | C | TKI, LRT, Surgery | BM | BM | 245 | Alive, 892 |
| 48, F,18 | HBV | Pembrolizumab, 4, 167 | 180.8 | 6.5 | C | TKI, LRT, Surgery | BM | BM | 108 | Alive, 985 |
| 48, F,18,1 | HBV | Pembrolizumab, 3, 7 | 38,700 | 16,182.3 | B | LRT | BM | BM | 43 | Dead, 173 |
| 63, M, 18 | HBV | Nivolumab, 6, 28 | 12.8 | 4.2 | C | TKI, LRT | BM | BM | 221 | Dead, 996 |
| 37, M, 18 | HBV | Nivolumab, 4, 60 | 11.7 | 3.0 | B | TKI, LRT | BM | BM | 703 | Alive, 813 |
| 57, M, 46 | HBV | Camrelizumab, 2, 18 | NA | NA | C | TKI | NA | NA | NA | Alive, 344 |
| 50, M, 46 | HBV | Sintilimab, 1, 21 | NA | NA | B | TKI | NA | NA | NA | Alive, 88 |
| 57, M, 43 | HBV | Nivolumab, 32, 3 | 1.9 | 1.9 | B | TKI, LRT, Surgery | WM | WM | 747 | Alive, 1,803 |
AFP, alpha-fetoprotein; BCLC, Barcelona Clinic Liver Cancer; BM, viable tumor, beyond Milan criteria; HCC, hepatocellular carcinoma; ICI, immune checkpoint inhibitor; LRT, locoregional therapy; LT, liver transplantation; MetALD, metabolic alcohol related liver disease; NA, not available; TKI, tyrosine kinase inhibitor.
While there were a total of nine HCC recurrences, individual patient data could not be obtained in one case, so this was not included in the table.
These cases experienced an allograft rejection, which was resolved with treatment.
Factors affecting post-LT outcomes
Allograft rejection
In multivariable analysis, age (per 10-year interval: adjusted HR 0.72, 95% CI 0.53–0.99, p = 0.044) and ICI washout (per week: adjusted HR 0.92, 95% CI 0.86–0.99, p = 0.022) were significantly associated with allograft rejection (Table 4). The same results were observed with sensitivity analysis excluding two studies (11 cases) of low-quality (Table S5).
Table 4.
Univariable and multivariable Cox regression models for determining factors impacting on allograft rejection.*
| Univariable model |
Multivariable model |
|||
|---|---|---|---|---|
| Variable | HR, 95% CI | p value | HR, 95% CI | p value |
|
| ||||
| Age (10-year interval) | 0.755 (0.580, 0.984) | 0.037 | 0.724 (0.530, 0.991) | 0.044 |
| Male gender (Ref: female) | 1.083 (0.368, 3.183) | 0.885 | - | |
| ICI washout period (weeks) | 0.921 (0.855, 0.991) | 0.028 | 0.923 (0.862, 0.989) | 0.022 |
| ICI cycles | 1.006 (0.965, 1.049) | 0.767 | - | |
| Anti-PD-L1 ICI subtype (Ref: anti-PD-1) | 0.196 (0.026, 1.458) | 0.112 | - | |
| No viral HCC etiology (Ref: viral) | 0.291 (0.068, 1.241) | 0.095 | - | |
| AFP >1,000, pre-ICI (Ref: <1,000) | 1.646 (0.559, 4.848) | 0.366 | - | |
| AFP >1,000, post-ICI (Ref: <1,000) | 1.884 (0.252, 14.098) | 0.537 | - | |
| BCLC B-D, pre-ICI (Ref: A) | 1.137 (0.416, 3.108) | 0.802 | - | |
| BCLC B-D post-ICI, (Ref: A, 0, and no viable tumor) | 1.591 (0.687, 3.687) | 0.278 | - | |
| Using other interventions than ICI | ||||
| Tyrosine kinase inhibitors | 1.284 (0.566, 2.911) | 0.549 | ||
| Surgery | 0.939 (0.347, 2.538) | 0.901 | ||
| Systemic chemotherapy | 1.558 (0.209, 11.61) | 0.665 | ||
| Locoregional treatment | 2.84* 108 (0, inf) | 0.997 | ||
| Viable tumor on explant pathology (Ref: no viable tumor) | 1.285 (0.499, 3.33) | 0.599 | - | |
AFP, alpha-fetoprotein; BCLC, Barcelona Clinic Liver Cancer; HR, hazard ratio; ICI, immune checkpoint inhibitor; LT, liver transplantation; PD-1, programmed cell death protein 1; PD-L1, programmed death ligand 1; BCLC, Barcelona clinic liver cancer.
Statistically significant variables in the univariable model were included in the multivariable model.
We evaluated the association between allograft rejection and overall survival. Of nine decedents, two were related to allograft rejection. On Kaplan-Meier curve analysis (Fig. 3), we found no statistically significant difference in survival between cases with and without allograft rejection (HR 2.16, 95% CI 0.58–8.1, p = 0.2, log-rank test). The median overall survival was not reached for both groups. The cumulative overall survival probability (95% CI) at 5 years following LT for cases with allograft rejection was 83% (69%, 100%). For cases without rejection, the survival probabilities (95% CI) at 4 years following LT were 88% (76%, 100%). The last death in groups with and without rejection occurred at 11.8 and 33.2 months following LT.
Fig. 3. Kaplan-Meier curve comparing overall survival between cases with and without liver transplant allograft rejection.
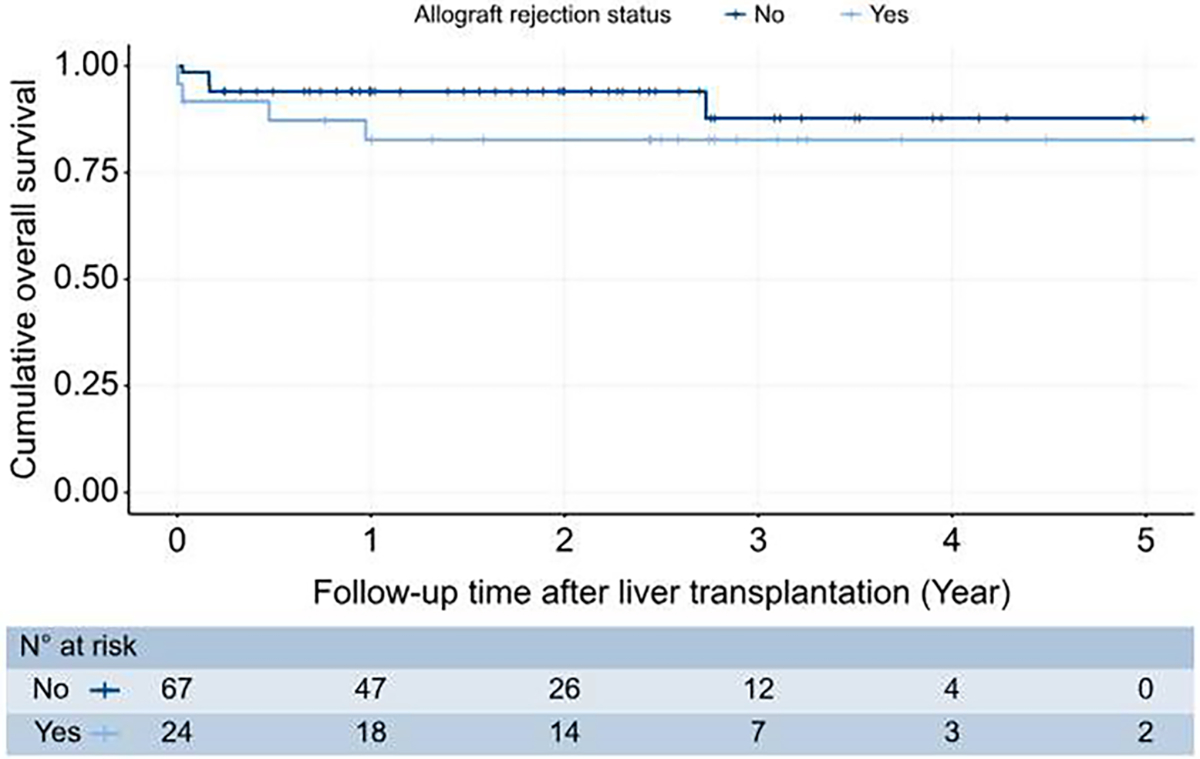
The Kaplan-Meier curve showed no difference between cases with and without allograft rejection regarding overall survival (HR 2.158, 95% CI 0.575–8.098, p = 0.2, log-rank test). HR, hazard ratio.
Determining the safest ICI washout period to avoid allograft rejection
Using a multivariable Cox regression model adjusted for age and ICI washout period, we predicted the probability of having LT rejection for each case. We then selected cases that had a probability of LT rejection ≤20% or 30%. The median (IQR) washout time for patients with less than 20% and 30% probability of rejection were 94 (196) and 72 (136) days, respectively (Fig. 4).
Fig. 4. Immune checkpoint inhibitor washout period, patient age, and probability of allograft rejection based on a Cox regression model adjusted for age and Immune checkpoint inhibitor washout period.
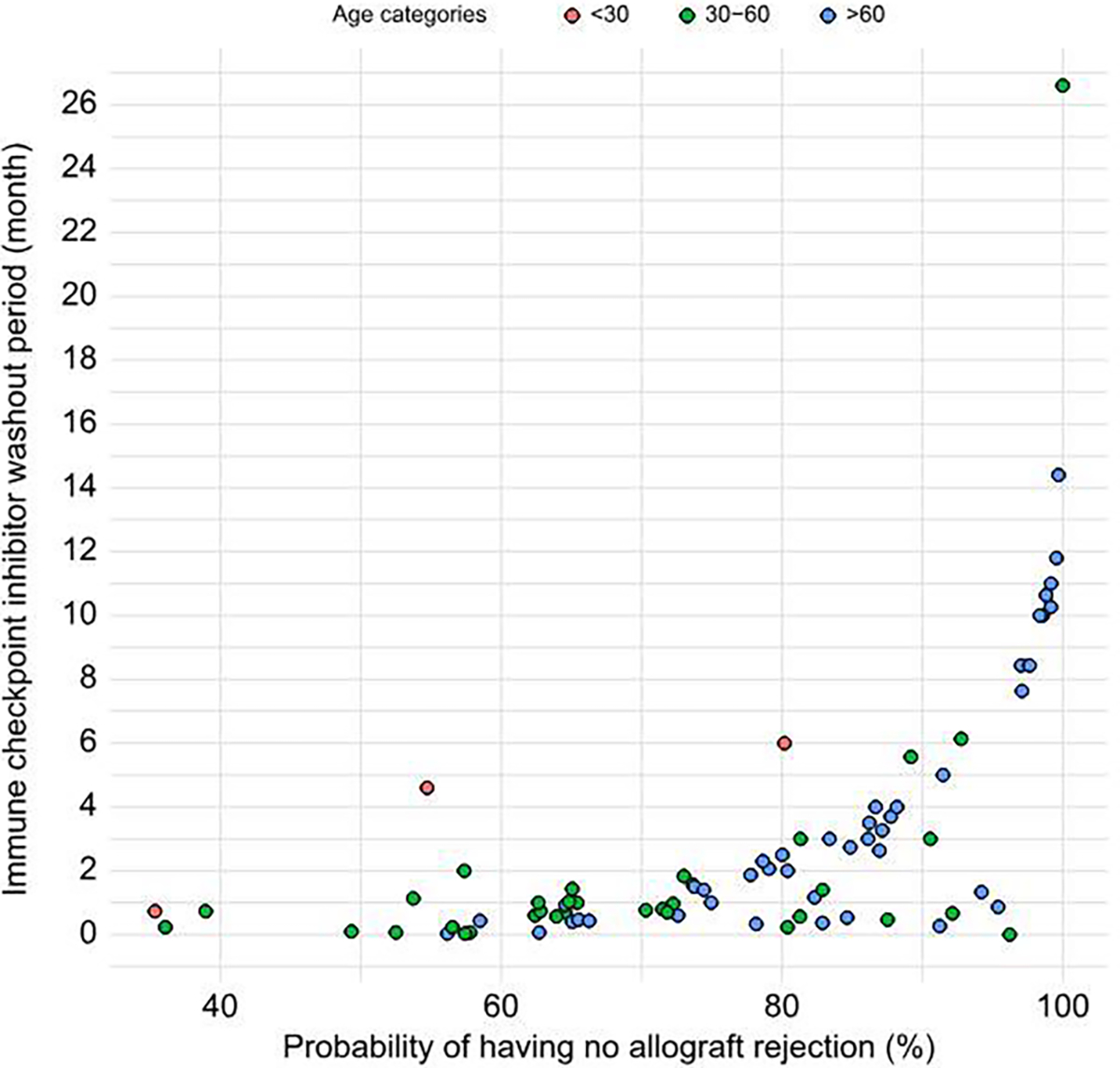
The median (IQR) washout period(in days) for patients with less than 20% and 30% probability of allograft rejection was 94 (196) and 72 (136), respectively.
HCC recurrence
Individuals with HCC recurrence had fewer median (IQR) ICI cycles than those without recurrence (4.0 [1.8]) vs. 8.0 [9.5], respectively; p = 0.025) (Table 1). Due to the high amount of missing data on AFP at the time LT, we utilized AFP at the time of ICI completion and were able to calculate the RETREAT score20 for 66 cases. We found a significant inverse correlation for both ICI cycles (Kendall’s τ = −0.21, p = 0.025) and ICI washout period (Kendall’s τ = −0.3, p = 0.001) with RETREAT score. A total of 55 cases had data on Milan criteria status at the completion of ICI treatment, of whom six had HCC recurrence. While there was one HCC recurrence among 33 cases within Milan criteria post-ICI completion, there were five cases of recurrence among the 22 cases beyond Milan criteria (p = 0.032). For the other two cases with HCC recurrence, Milan criteria status at the completion of ICI was not available. Among tumor-related factors, larger tumor size on explant was associated with HCC recurrence (p = 0.017). Given the limited number of observed events of HCC recurrence in our study, we opted against conducting Cox regression analysis.
Discussion
In this systematic review and IPD meta-analysis, we described post-LT outcomes of 91 patients with HCC who used ICIs prior to LT. During a median follow-up of 1.9 years after LT, there were 24 allograft rejections (mostly mild in severity), nine HCC recurrences, and nine deaths. Patienťs age and ICI washout period had a significant inverse association with allograft rejection, with each 10-year increase in a patienťs age and 1-week increase in ICI washout period decreasing the risk of allograft rejection by 28% (1, 47%) and 8% (1, 14%), respectively. An ICI washout period of more than 94 days may decrease the risk of allograft rejection to 20% or less. We also reported that more than 83% of allograft rejection could be resolved with medical management, and overall survival among patients with allograft rejection was comparable to those without rejection. However, considering the short follow-up time, the effect of allograft rejection on overall survival needs more investigation.
ICIs have been utilized in the pre-transplant setting, as included in the current project, and also in the neoadjuvant setting before resection.49,50 Although the majority of included cases in our project did not meet the Milan criteria before starting ICI treatment, the intention for ICI treatment (e.g. downstaging, bridging to LT) could not be ascertained in the current study. Nevertheless, our results lay out the rationale for conducting a future study that aims to evaluate the efficacy and safety of pre-LT ICI treatment for downstaging or bridging to LT, particularly considering recent data suggesting higher progression-free survival with combination therapy than TACE alone. Such studies will be critical to determine the appropriate candidates who would benefit from downstaging treatment with ICI vs. locoregional treatment alone for successful listing and receiving LT.
ICIs can enhance cell-mediated immunity and improve antigen recognition, leading to an increased antitumor response. However, this potential benefit from their anti-cancer activity can also lead to various adverse events related to immune activation, including allograft rejection,41 which is one of the main problems with using ICIs pre-LT. Allograft rejection has been documented following the use of ICIs after organ transplantations in retrospective or systematic reviews of such studies.51–54 In the context of LT, the cells infiltrating the allograft express PD-1, while cholangiocytes, hepatocytes, as well as cells along the sinusoids in the liver graft, express PD-L1. The PD-1/PD-L1 interaction works as a negative regulator of immune response. The use of ICIs in this setting will block this interaction, affecting the balance between pathogenic and regulatory T cells, and ultimately, it may increase the effect and infiltration of T cells on the liver graft predisposing it to rejection.55–57
More prospective studies can shed light on the rate of rejection based on the different ICIs used. Published literature regarding allograft rejection following ICI use before LT is currently restricted to case reports/series and some very low sample size studies. When adjusting the ICI washout period, one should specifically consider both the half-life of different ICIs (usually more than 4 weeks) and also the time period that these ICIs can occupy their targets, which may be even longer.58 For example, the serum half-life of anti-PD-1 inhibitors like pembrolizumab and nivolumab is reported to be up to about 25 days.14 However, the average occupancy of more than 70% of PD-1 molecules expressed by T cells can persist sustainably for up to 2 months after infusion, and this occurs independently of dosage.59 Furthermore, by multiple infusions, this occupancy can be more than 50% even after 200 days.60 Adjusting the interval between the last ICI dose and LT presents another challenge in practice. Anticipating donor availability is often difficult, affecting ICI washout timing. However, it is important to note that this interval can influence allograft rejection risk. In the current project, considering all ICI regimens and classes together, the ICI washout period proved to be a significant factor affecting allograft rejection. We had a limited sample size to do subgroup analysis with adequate power and, therefore, could not calculate this time for each ICI class and regimen. Future projects should also investigate the impact of subtype of ICIs on post-LT allograft rejection.
In line with our results, previous descriptive evaluations have proposed that younger age might be a risk factor for allograft rejection in the setting of using ICI pre-LT.3 It is also consistent with literature that LT patients of older age encounter less acute rejection than those of younger age.61 While patients’ age is not a modifiable risk factor for allograft rejection, a longer ICI washout period and stronger immunosuppression regimen should be considered after LT for younger patients. Allograft rejection can occur even with high-dose immunosuppression regimens, and incorporating at least one drug from classes other than corticosteroids may yield better outcomes.62 In our project, steroids were the mainstay of treatment for allograft rejection. Thymoglobulin, plasmapheresis, intravenous immunoglobulin, rituximab were among the other frequent strategies for rejection treatment, respectively. Among the 24 allograft rejections we identified, there were only two deaths following allograft rejection, with ICI washout periods of O and 8 days. Additionally, there were two cases of rejection that required retransplantation. In these cases, the ICI washout periods were 35 and 40 days. Management of LT rejection seems to be appropriate, as our analysis showed that LT rejection did not have a significant effect on the overall survival status of patients.
We noted that the number of ICI cycles was significantly lower among cases with HCC recurrence compared to those without recurrence. Additionally, achieving tumor burden within Milan criteria after completion of ICI appears to be a useful method for lowering the risk of HCC recurrence, as we observed that most HCC recurrences occurred among patients beyond Milan criteria after ICI completion. AFP greater than 1,000 ng/ml is a well-established risk factor for post-LT HCC recurrence. In our study, there were only two cases with AFP levels greater than 1,000 ng/ml after ICI completion. One case with an AFP level of 16,182 ng/ml experienced HCC recurrence at 43 days and death at 173 days post-LT, supporting the current AASLD practice guideline to avoid LT in patients with AFP greater than 1,000 ng/ml63. These results should be interpreted with caution given the short follow-up time.
There are several limitations in our study. Most cases were from case reports/series or cohort studies with very low sample size and without a control group instead of systematic analysis of a prospective study with an intention-to-treat analysis. There is a risk of potential publication biases with a higher likelihood of reporting positive outcomes (exceptional responders) and under-reporting of negative outcomes. We also expect potential residual confounding despite limited multivariable analysis. Most reported cases were not biopsy-proven rejections and it is unclear if it is directly related to ICI use. Additionally, the follow-up time of the cases in the main studies was not extensive, and therefore we did not observe a high number of events, particularly regarding HCC recurrence and mortality, which limited us from performing further statistical analysis for these outcomes. In most cases, we evaluated patients who have used anti-PD-1 as a single agent in their ICI regimen, which is not standard-of-care with a limited representation of current standard-of-care ICI (atezolizumab and bevacizumab or durvalumab and tremelimumab). There was heterogeneity in study design including intention of treatment (e.g. downstaging, bridging to LT), timing of ICI therapy in relation to LT, and use of concurrent or prior locoregional therapy; therefore, prospective studies would better define how to implement ICIs as downstaging or bridging therapy to LT. As most patients received additional pre-transplant therapy, the relative contribution of ICI therapy vs. other treatments to post-LT outcomes remains unknown, although locoregional therapies would not impact the risk of our primary outcome (i.e., graft rejection). Finally, several relevant variables were missing in a large proportion of patients, including post-LT immunosuppression regimen and tumor burden at the completion of ICI treatment.
While our findings underscore the significance of age and ICI washout period in allograft rejection and suggest a potential impact of ICI cycles and tumor burden upon completion of ICI on HCC recurrence, these results, along with numerous other considerations, warrant validation and incorporation into future studies. It is essential to determine which patients, based on specific characteristics like AFP level, BCLC classification, and HCC etiology, benefit most from ICIs in the pre-LT phase. We need to establish the optimal timing for initiating ICIs and their integration with other treatments like LRTs for downstaging. Fortunately, many clinical trials and prospective studies investigating the use of ICIs as a downstaging tool are currently underway.60,64,65 Another important challenge in using ICIs in the setting of pre-LT involves the immunosuppression approach used after LT, and the ideal immunosuppression regimen based on the ICI regimen characteristics (types, cycles, washout period) should be investigated in future studies.
In conclusion, our study showed that post-LT outcomes appear to be acceptable in patients who receive ICI prior to LT. ICI washout period may be a modifiable factor to minimize the risk of allograft rejection, and maintaining a minimum 3-month washout period appears reasonable. The data underscore the need for large-scale multicenter studies to provide robust evidence supporting the efficacy and safety of ICIs for patients with HCC, particularly in the context of downstaging or bridging to LT.
Supplementary Material
Highlights.
Acceptable risk of allograft rejection, cancer recurrence, and mortality among patients with HCC who receive ICIs prior to LT.
Increased age and ICI washout period were associated with reduced chance of allograft rejection.
A median ICI washout period of 94 days or more was associated with a risk of allograft rejection ≤20%.
Over 80% of allograft rejection was resolved with medical management and overall survival was unaffected.
An increased number of ICI cycles and within Milan criteria post-ICI completion were associated with a lower risk of HCC recurrence.
Impact and implications.
This systematic review and individual patient data meta-analysis of 91 patients with hepatocellular carcinoma and immune checkpoint inhibitor use prior to liver transplantation suggest acceptable overall post-transplant outcomes. Older age and longer immune checkpoint inhibitor washout period have a significant inverse association with the risk of allograft rejection. A 3-month washout may reduce it to that of patients without immune checkpoint inhibitor exposure. Additionally, a higher number of immune checkpoint inhibitor cycles and tumor burden within Milan criteria at the completion of immunotherapy may predict a decreased risk of hepatocellular carcinoma recurrence, but this observation requires further validation in larger prospective studies.
Acknowledgment
We would like to express our gratitude to Lai Wei from Huazhong University of Science and Technology in China, and Hyejee Ohm from the University of Alberta in Edmonton, Alberta, Canada. They generously shared the individual participant data of their published cases with us.
Financial support
Dr. Yang’s research is supported by NCI KOS CA259534. Dr. Singal’s research is supported by NCI R01. Dr. Tran’s research is supported by NIMHD K23 (K23MD017217).
Abbreviations
- AFP
alpha-fetoprotein
- BCLC
Barcelona Clinic Liver Cancer
- HR
hazard ratio
- ICI
immune checkpoint inhibitor
- IPD
individual patient data
- LRT
locoregional treatments
- LT
liver transplantation
- PD-1
programmed cell death 1
- PD-L1
programmed cell death ligand 1
- RAI
rejection activity index
Footnotes
Conflict of interest
Ju Dong Yang provides a consulting service for AstraZeneca, Eisai, Exact Sciences, Exelixis, Fujifilm Medical Sciences, and Gilead Sciences. Neehar Parikh has served as a consultant or advisor for Genentech, Fujifilm Medical, Eisai, Exelixis, Merck, Exact Sciences, Freenome, and Gilead. Amit Singal has served as a consultant or on advisory boards for Genentech, AstraZeneca, Bayer, Eisai, Exelixis, Merck, Elevar, Boston Scientific, Sirtex, HistoSonics, Fujifilm Medical Sciences, Exact Sciences, Glycotest, Abbott, Roche, Freenome, and GRAIL. Neil Mehta has served as a consultant or advisor for Exelixis, Fujifilm Medical, Genentech, Eisai, Exelixis, Exact Sciences, and Merck. Sherrie Bhoori serves as an advisor or lecturer for Roche, AstraZeneca, Boston Scientific, Terumo. Beau B. Toskich serves as an advisor for Genentech, Eisai, and Astra Zeneca. Robyn D. Gartrell’s laboratory receives funding from Hyundai Hope on Wheels Hope Scholar Award, Swim Across America, Rally Foundation, StacheStrong and Musella Foundation. Bruno Sangro reports consulting or advisory fees from AstraZeneca, Bayer, Boston Scientific, Bristol-Myers Squibb, Eisai, lncyte, IPSEN, Roche, Sirtex Medical, and Terumo; reports being an invited speaker for AstraZeneca, Bristol-Myers Squibb, Eisai, lncyte, IPSEN, Roche, and Sirtex Medical; research funding (to institution) from Bristol-Myers Squibb and Sirtex Medical. Tarek Hassanein serves as an advisory committee member or review panelist for AbbVie, Cymabay, Gilead, HepQuant, Madrigal, Mallinckrodt; has received grant and research support from AbbVie, Amgen, Biolinq, Bristol-Myers Squibb, Astra Zeneca, Boehringerlngelheim, Bristol-Myers Squibb, COUR, DURECT Corporation, Escient, Galectin, Gilead, Grifols, HepQuant, Intercept, Janssen, Merck, Mirum, NeuroBo, Novartis, Novo Nordisk, Pfizer, Regeneron, Salix Pharmaceuticals, Sonic lncytes. Takeda, Terns Pharmaceuticals, Valeant; and also involved in speaking engagements and teaching for for AbbVie, Gilead, Intercept, Mallinckrodt, Salix Pharmaceuticals. Davendra Sohal has served on the speakers Bureau for Astra Zeneca since January 2024, lncyte since January 2021, and Seagen since January 2023; and has received consulting fees or honoraria from Astra Zeneca (ended Jan 2024), Replimune (ended Jan 2024), Cancer Commons (ended Jun 2023), TransThera (ended Jun 2022), Totus Medicines (ended Jul 2023), Valar Labs (ended Dec 2022), Aadi (ended Jun 2023), Elevar, Regeneron; and has received research funding from Aadi, Ability Pharma, Amgen, Apexigen, Astellas, Astra Zeneca, Bexion, Bristol-Myers Squibb, FibroGen, Genentech, Hengrui, Merck, Mirati, NextCure, PanCAN, Regeneron, Roche, Triumvira. Nguyen H Tran has served as an advisor for Astrazeneca, Genentech, Helsinn and TEMPUS. She is a recipient of the K23MD017217–01A1. Parissa Tabrizian serves as an advisor for Bayer. Astrazeneca, boston scientific. -honorarium. Mehmet Akce has been involved in research projects with Bristol-Myers Squibb-Ono Pharmaceutical (Inst), Xencor (Inst), Merck Sharp & Dohme (Inst), Eisai (Inst), GSK (Inst), Bayer (Inst), Relay (Inst), ProDa BioTech (Inst), Exelixis (lnst),and AstraZeneca (Inst) and also has consulting or advisory roles for Eisai, Ipsen, Exelixis, GSK, QED, lsofol, Curio Science, AstraZeneca, Genentech, lncyte, and Taiho. Other authors declare no conflict of interest.
Please refer to the accompanying ICMJE disclosure forms for further details.
Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jhep.2024.06.042.
Data availability statement
Data analyzed during the study are available from the corresponding author by request.
Code for International Prospective Register of Systematic Reviews (PROSPERO): CRD42023494951.
References
- [1].Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2021;71:209–249. [DOI] [PubMed] [Google Scholar]
- [2].Lucey MR, Furuya KN. Foley DP. Liver transplantation. New Engl J Med 2023;389:1888–1900. [DOI] [PubMed] [Google Scholar]
- [3].Matevish L, Patel MS, Vagefi PA. Downstaging techniques for hepatocellular carcinoma in candidates awaiting liver transplantation. Surg Clin North America 2023;104(1):145–162. [DOI] [PubMed] [Google Scholar]
- [4].Tabrizian P, Holzner ML, Mehta N, et al. Ten-year outcomes of liver transplant and downstaging for hepatocellular carcinoma. JAMA Surg 2022;157:779–788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Yao FY, Ferrell L, Bass NM, et al. Liver transplantation for hepatocellular carcinoma: comparison of the proposed UCSF criteria with the Milan criteria and the Pittsburgh modified TNM criteria. Liver Transpl 2002;8:765–774. [DOI] [PubMed] [Google Scholar]
- [6].Pardall DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer 2012;12:252–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Sharma P, Allison JP. The future of immune checkpoint therapy. Science 2015;348:56–61. [DOI] [PubMed] [Google Scholar]
- [8].Faivre S, Rimassa L, Finn RS. Molecular therapies for HCC: looking outside the box. J Hepatol 2020;72:342–352. [DOI] [PubMed] [Google Scholar]
- [9].Lencioni R, Kudo M, Erinjeri J, et al. EMERALD-1: a phase 3, randomized, placebo-controlled study of transarterial chemoembolization combined with durvalumab with or without bevacizumab in participants with unresectable hepatocellular carcinoma eligible for embolization. Am Soc Clin Oneal 2024. https://meetings.asco.org/abstracts-presentations/229367. [Google Scholar]
- [10].Kaseb AO, Hasanov E, Cao HST, et al. Perioperative nivolumab monotherapy versus nivolumab plus ipilimumab in resectable hepatocellular carcinoma: a randomised, open-label, phase 2 trial. Lancet Gastroenterol Hepatol 2022:7:208–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Marron TU, Fiel Ml, Hamon P. et al. Neoadjuvant cemiplimab for resectable hepatocellular carcinoma: a single-arm, open-label, phase 2 trial. Lancet Gastroenterol Hepatol 2022;7:219–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Shi Y-H, Ji Y, Liu W-R, et al. A phase Ib/II, open-label study evaluating the efficacy and safety of Toripalimab injection (JS001) or combination with Lenvatinib as a neoadjuvant therapy for patients with resectable hepatocellular carcinoma (HCC). Cancer Res 2021;81. 486–486. [Google Scholar]
- [13].Kuo FC, Chen CY, Lin NC, et al. Optimizing the safe washout period for liver transplantation following immune checkpoint inhibitors with atezolizumab, nivolumab, or pembrolizumab. Transpl Proc 2023;55:878---883. [DOI] [PubMed] [Google Scholar]
- [14].Tran NH, Muñoz S, Thompson S, et al. Hepatocellular carcinoma downstaging for liver transplantation in the era of systemic combined therapy with anti-VEGF/TKI and immunotherapy. Hepatology 2022;76:1203–1218. [DOI] [PubMed] [Google Scholar]
- [15].Tabrizian P, Florman SS, Schwartz ME. PD-1 inhibitor as bridge therapy to liver transplantation? Am J Transplant 2021;21:1979–1980. [DOI] [PubMed] [Google Scholar]
- [16].Stewart LA, Clarke M, Rovers M, et al. Preferred reporting items for systematic review and meta-analyses of individual participant data: the PRISMA-IPD statement. Jama 2015;313:1657–1665. [DOI] [PubMed] [Google Scholar]
- [17].Murad MH, Sultan S, Haffar S, et al. Methodological quality and synthesis of case series and case reports. BMJ Evid Based Med 2018;23:60–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Wang T, Chen Z, Liu Y, et al. Neoadjuvant programmed cell death 1 inhibitor before liver transplantation for HCC is not associated with increased graft loss. Liver Transpl 2023;29:598–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Lo CK, Mertz D, Loeb M. Newcastle-Ottawa Scale: comparing reviewers' to authors' assessments. BMC Med Res Methodol 2014;14:45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Mehta N, Heimbach J, Harnois DM, et al. Validation of a risk estimation of tumor recurrence after transplant (RETREAT) score for hepatocellular carcinoma recurrence after liver transplant. JAMA Oneal 2017;3:493–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Lv Z, Xia Q, Feng H. Neoadjuvant PD-1 blockade with Pembrolizumab combined with Lenvatinib therapy in patients with hepatocellular carcinoma beyond Milan criteria before liver transplantation (PLENTY): a single-site pilot randomized controlled trial. J Hepatol 2023;78:S454. [Google Scholar]
- [22].Liu M, Lizaola-Mayo B, Jayasekera C, et al. Downstaging hepatocellular carcinoma with checkpoint inhibitor therapy safely improves access to curative liver transplantation: a case series. J Hepatol 2023:78:S590–S591. [DOI] [PubMed] [Google Scholar]
- [23].Xing H, Li L, Zhang QB, et al. The effect of pre-transplant immunotherapy on the prognosis of transplant receipents with hepatocellular carcinoma. Hepatology 2022;76:S534–S535. [Google Scholar]
- [24].Tl Hassanein, Alqassim N, Diaz-Moreno J, et al. Complementing locoregional therapies with immune checkpoint inhibitors in advanced HCC improves disease-free survival and decreases drop- out from the liver transplant list. Hepatology 2023;78:S1823–S1825. [Google Scholar]
- [25].Solina GA, Ferreira RPCPC, D'Albuquerque LAC, et al. Atezolizumab plus bevacizumab as a bridge for liver transplant in hepatocellular carcinoma. Braz J Transplant 2023;26. [Google Scholar]
- [26].Liou H, Mody K, Boyle AW, et al. Neoadjuvant radiation lobectomy and immunotherapy for angioinvasive HCC resulting in complete pathologic response. Hepatology 2021;74:525–527. [DOI] [PubMed] [Google Scholar]
- [27].Zhao Y, Chen D, Yang B, et al. Successful liver transplantation with ctDNA clearance after PD-1 inhibitor plus FOLFOX-HAIC treatment in HCC: a case report. Oneal Lett 2024;27:51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Lucas AP, Lewis AR, Kasi PM, et al. Abscopal downstaging of intermediate stage hepatocellular via combination cryoablation and immunotherapy with complete pathologic response. Radial Case Rep 2024;19:910–914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].De Simone P, Ghinolfi D, Palladino S, et al. First-in-human liver transplantation from a centenarian deceased donor after brain death. Am J Transpl 2023;24(2):304–307. [DOI] [PubMed] [Google Scholar]
- [30].Bhoori S, Dosi M, Bellia V, et al. Tracing anti-cancer immunity in patients undergoing liver transplantation for HCC after downstaging with immunotherapy. Dig Liver Dis 2023;55. S217–S217. [Google Scholar]
- [31].Ohm H, Khwaja R, Karachiwala H. lmmunotherapy before liver transplant in unresectable hepatocellular carcinoma: a case report. J Gastrointest Oneal 2023;14:2644–2649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Giudicelli H, Roux C, Mansel A, et al. Successful advanced hepatocellular carcinoma downstaging with atezolizumab-Bevacizumab and radioembolization before liver transplantation. Clin Res Hepatol Gastroenterol 2023;47:102167. [DOI] [PubMed] [Google Scholar]
- [33].Chouik Y, Erard D, Demian H, et al. Case Report: successful liver transplantation after achieving complete clinical remission of advanced HCC with Atezolizumab plus Bevacizumab combination therapy. Front lmmunol 2023;14:1205997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Rudolph M, Shah SA, Quillin R, et al. Immune checkpoint inhibitors in liver transplant: a case series. J Gastrointest Oneal 2023;14:1141–1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Schmiderer A, Zoller H, Niederreiter M, et al. Liver transplantation after successful downstaging of a locally advanced hepatocellular carcinoma with systemic therapy. Dig Dis 2023;41:641–644. [DOI] [PubMed] [Google Scholar]
- [36].Tow CY, Castrodad-Rodríguez CA, Panarelli N, et al. Finding nivo: a case report of 2 forms of nivolumab-induced liver injury in an allograft liver in the immediate post-transplant period. Transpl Proc 2022;54:2794–2796. [DOI] [PubMed] [Google Scholar]
- [37].Abdelrahim M, Esmail A, Umoru G, et al. lmmunotherapy as a neoadjuvant therapy for a patient with hepatocellular carcinoma in the pretransplant setting: a case report. Curr Oneal 2022;29:4267–4273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Dave S, Yang K, Schnickel GT, et al. The impact of treatment of hepatocellular carcinoma with immune checkpoint inhibitors on pre- and post-liver transplant outcomes. Transplantation 2022;106:e308–e309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Kang E, Martinez M, Moisander-Joyce H, et al. Stable liver graft post anti-PD1 therapy as a bridge to transplantation in an adolescent with hepatocellular carcinoma. Pediatr Transpl 2022;26:e14209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Kang S, Magliocca J, Sellers M, et al. Successful liver transplantation of recurrent fibrolamellar carcinoma following clinical and pathologic complete response to triple immunochemotherapy: a case report. Oneal Res Treat 2022;45:430–437. [DOI] [PubMed] [Google Scholar]
- [41].Aby ES, Lake JR. Immune checkpoint inhibitor therapy before liver transplantation - case and literature review. Transplant Direct 2022;8:E1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Sogbe M, López-Guerra D, Blanco-Fernández G, et al. Durvalumab as a successful downstaging therapy for liver transplantation in hepatocellular carcinoma: the importance of a washout period. Transplantation 2021;105:e398–e400. [DOI] [PubMed] [Google Scholar]
- [43].Simoes CC, Thung SN, Fiel Ml, et al. Morphology of tumor and nontumor tissue in liver resection specimens for hepatocellular carcinoma following nivolumab therapy. Mod Pathol 2021;34:823–833. [DOI] [PubMed] [Google Scholar]
- [44].Peterson J, Stanek S, Kalman R, et al. Nivolumab as a bridge to liver transplantation in advanced hepatocellular carcinoma. Am J Gastroenterol 2021;116. S1159–S1159. [Google Scholar]
- [45].Yin J, Wen M, Cheng J, et al. A patient with failed liver transplantation after the use of PD-1 blockade combined with lenvaxen. Front Med (Lausanne) 2022;9:712466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Duan B, Li W, Cao J, et al. Immune checkpoint inhibitors combined with TKls as a bridge therapy for advanced HCC before liver transplantation. Chin J Hepatobiliary Surg 2022;28:28–32. [Google Scholar]
- [47].Nordness MF, Hamel S, Godfrey CM, et al. Fatal hepatic necrosis after nivolumab as a bridge to liver transplant for HCC: are checkpoint inhibitors safe for the pretransplant patient? Am J Transplant 2020;20:87S–883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Schwacha-Eipper B, Minciuna I, Banz V, et al. lmmunotherapy as a downstaging therapy for liver transplantation. Hepatology 2020;72:1488–1490. [DOI] [PubMed] [Google Scholar]
- [49].Chao J, Zhu Q, Chen D, et al. Case report: transarterial chemoembolization in combination with tislelizumab downstages unresectable hepatocellular carcinoma followed by radical salvage resection. Front Oncol 2021;11 :667555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Xin H, Zhang C, Ding Z, et al. TACE plus PD-1 inhibitor (Camrelizumab) treatment for bridging to tumor resection in HCC: case reports. Clin Res Hepatol Gastroenterol 2022;46:101777. [DOI] [PubMed] [Google Scholar]
- [51].Abdel-Wahab N, Safa H, Abudayyeh A, et al. Checkpoint inhibitor therapy for cancer in solid organ transplantation recipients: an institutional experience and a systematic review of the literature. J lmmunother Cancer 2019;7:106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Gassmarm D, Weiler S, Mertens JC, et al. Liver allograft failure after nivolumab treatment-A case report with systematic literature research. Transpl Direct 2018;4:e376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Fisher J, Zeitouni N, Fan W, et al. Immune checkpoint inhibitor therapy in solid organ transplant recipients: a patient-centered systematic review. J Am Acad Dermatol 2020;82:1490–1500. [DOI] [PubMed] [Google Scholar]
- [54].Kayali S, Pasta A, Plaz Torres MC, et al. Immune checkpoint inhibitors in malignancies after liver transplantation: a systematic review and pooled analysis. Liver Int 2023;43:8--17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Qiao ZY, Zhang ZJ, Lv ZC, et al. Neoadjuvant programmed cell death 1 (PD-1) inhibitor treatment in patients with hepatocellular carcinoma before liver transplant: a cohort study and literature review. Front lmmunol 2021;12:653437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Shi XL, Mancham S, Hansen BE, et al. Counter-regulation of rejection activity against human liver grafts by donor PD-L1 and recipient PD-1 interaction. J Hepatol 2016;64:1274–1282. [DOI] [PubMed] [Google Scholar]
- [57].Tanaka K, Albin MJ, Yuan X, et al. PDL1 is required for peripheral transplantation tolerance and protection from chronic allograft rejection. J lmmunol 2007;179:5204–5210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Gao Q, Anwar IJ, Abraham N, et al. Liver transplantation for hepatocellular carcinoma after downstaging or bridging therapy with immune checkpoint inhibitors. Cancers (Basel) 2021;13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Brahmer JR, Drake CG, Wollner I, et al. Phase I study of single-agent anti-programmed death-1 (MDX-1106) in refractory solid tumors: safety, clinical activity, pharmacodynamics, and immunologic correlates. J Clin Oncol 2010;28:3167–3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Alghamdi S, AI-Hamoudi W. Hepatocellular carcinoma: the role of immunotherapy and transplantation in the era of transplant oncology. Cancers (Basel) 2023;15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].De Simone P, Battistella S, Lai Q, et al. lmmunosuppression for older liver transplant recipients. Transpl Rev (Orlando) 2024;38:100817. [DOI] [PubMed] [Google Scholar]
- [62].Tabrizian P, Yu A, Debnath N, et al. lmmunotherapy and liver transplantation: the future or the failure? Surg Clin North Am 2024;104:163–182. [DOI] [PubMed] [Google Scholar]
- [63].Singal AG, Llovet JM, Yarchoan M, et al. AASLD Practice Guidance on prevention, diagnosis, and treatment of hepatocellular carcinoma. Hepatology 2023;78:1922–1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Tabrizian P, Zeitlhoefler M, Hassan AT, et al. lmmunotherapy for transplantation of hepatocellular carcinoma: the next frontier in adjunctive therapy. Curr Opin Organ Transpl 2023;29(2):144–154. [DOI] [PubMed] [Google Scholar]
- [65].Gorji L, Brown ZJ, Pawlik TM. Advances and considerations in the use of immunotherapies for primary hepato-biliary malignancies. Surg Oncol 2023;52:102031. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data analyzed during the study are available from the corresponding author by request.
Code for International Prospective Register of Systematic Reviews (PROSPERO): CRD42023494951.


