Abstract
Tumor necrosis has been reported to represent an independent prognostic factor in colorectal cancer, but its evaluation methods have not been described in sufficient detail to introduce tumor necrosis evaluation into clinical use. To study the potential of tumor necrosis as a prognostic indicator in colorectal cancer, criteria for 3 methods for its evaluation were defined: the average percentage method (tumor necrosis percentage of the whole tumor), the hotspot method (tumor necrosis percentage in a single hotspot), and the linear method (the diameter of the single largest necrotic focus). Cox regression models were used to calculate cancer-specific mortality hazard ratios (HRs) for tumor necrosis categories in 2 colorectal cancer cohorts with more than 1800 cases. For reproducibility assessment, 30 cases were evaluated by 9 investigators, and Spearman’s rank correlation coefficients and Cohen’s kappa coefficients were calculated. We found that all 3 methods predicted colorectal cancer-specific survival independent of other prognostic parameters, including disease stage, lymphovascular invasion, and tumor budding. The greatest multivariable HRs were observed for the average percentage method (cohort 1: HR for ≥ 40% vs. <3% 3.03, 95% CI, 1.93-4.78; cohort 2: HR for ≥ 40% vs. < 3% 2.97; 95% CI, 1.63-5.40). All 3 methods had high reproducibility, with the linear method showing the highest mean Spearman’s correlation coefficient (0.91) and Cohen’s kappa (0.70). In conclusion, detailed criteria for tumor necrosis evaluation were established. All 3 methods showed good reproducibility and predictive ability. The findings pave the way for the use of tumor necrosis as a prognostic factor in colorectal cancer.
Key Words: Tumor necrosis, prognosis, colorectal cancer, reproducibility, histopathology
Colorectal cancer (CRC) is one of the most common causes of cancer deaths worldwide and the incidence is continuously increasing.1 Predictive and prognostic factors for CRC include the tumor-node-metastasis (TNM) classification, mismatch repair (MMR) status, lymphatic or vascular invasion, perineural invasion, BRAF or RAS mutations, grade, and tumor budding.2,3 To refine the prognostic classification, additional parameters have been investigated, and tumor necrosis has proven to be an independent prognostic factor in several studies.4–6 These studies have mostly visually estimated tumor necrosis percentage and then categorized it based on certain cutoff values.5 However, the optimal evaluation method is not clear, and the specifics of the evaluation methods have not been described in sufficient detail to introduce tumor necrosis evaluation into clinical use.
Tumor budding is an example of a histopathologic parameter that has been successfully adapted into clinical decision-making, based on its strong prognostic value and reproducible criteria for its evaluation, established by the International Tumor Budding Consensus Conference (ITBCC).7 Tumor necrosis could also be evaluated from hematoxylin and eosin-stained samples and it does not require any complex staining or analysis methods. However, more detailed criteria are required to firmly establish it as a useful prognostic parameter. In this study, we aimed to address this by thoroughly describing 3 methods for tumor necrosis evaluation and comparing their prognostic value in more than 1800 CRC cases and reproducibility among 9 pathologists and researchers.
MATERIALS AND METHODS
Patients
Two cohorts were analyzed, cohort 1 from Central Finland Central Hospital (N=1343) and cohort 2 from Oulu University Hospital (N=1011). Cohort 1 has been retrospectively collected and includes patients from 2000 to 2015.8 Cohort 2 has been prospectively collected since 2006. It was previously described from 2006 to 20149,10 and has now been extended until 2020. Both cohorts consist of CRC patients who have undergone tumor resection and from whom adequate tumor samples have been available. Patients who received preoperative radiotherapy or chemoradiotherapy were excluded from the analysis (cohort 1, N=243; cohort 2, N=235), and after their exclusion, there were 1100 patients for cohort 1 and 776 patients for cohort 2. Patients who died within 30 days after surgery were further excluded from survival analysis (cohort 1, N=37; cohort 2, N=5). Colorectal cancer-specific survival was used as the study endpoint, and it was defined as the time from operation to colorectal cancer-related death or the end of follow-up.
In survival analysis, the follow-up was limited to 10 years, considering that most colorectal cancer deaths occur within that time. The median follow-up time for censored cases was 10 years (IQR 7.3 to 10) for cohort 1 and 5.6 years (IQR 3.7 to 9.3) for cohort 2. The total number of deaths in cohort 1 was 531, of which 296 were cancer deaths. Cohort 2 had a total of 244 deaths, of which 135 were cancer deaths.
Histopathologic Analyses
Tumor resection specimens were fixed using 10% formalin, embedded in paraffin, and hematoxylin and eosin (H&E) stained. Basic tumor parameters such as TNM stage, grade, and invasion status have been previously collected from tumor samples in both cohorts.8,11,12 TNM stage was determined by the Union for International Cancer Control/The American Joint Committee on Cancer (UICC/AJCC) criteria. The grade was assessed using the WHO criteria. MMR status and BRAF V600E mutation status were analyzed using immunohistochemistry.8,11,12 For cohort 2, neuroendocrine differentiation was determined using immunohistochemistry. Tissue microarrays were stained for chromogranin A (clone LK2H10, code MA5-13096, ER1, 1:400; Thermo Fisher, Waltham, MA) and synaptophysin (clone MRQ-40, code 336R-95, ER2, 1:20, Cell Marque, Rocklin, CA) with Leica Bond RX automated stainer and BOND Polymer Refine Detection kit (Leica DS9800). For antigen retrieval BOND Epitope Retrieval Solution 2 (Leica AR9640, 30min, 100°C) was used. The expression levels of chromogranin A (CHGA) and synaptophysin (SYP) were assessed as the percentage of positive tumor cells. These percentages were then categorized into 3 groups: negative (0% positive tumor cells for both CHGA and SYP), low (1% to 9% positive tumor cells for either CHGA or SYP), high (≥10% positive tumor cells for either CHGA or SYP).
Tumor necrosis was identified as an area that had nuclear shrinkage, fragmentation, and disappearance, frequently associated with eosinophilia and neutrophil infiltration. A detailed description of the criteria used in its evaluation with example images is included in the Tumor necrosis evaluation manual (Supplementary file 1, Supplemental Digital Content 1, http://links.lww.com/PAS/B909). Tumor necrosis percentage was estimated in cohort 1 cases for a previous study,4 but after defining the new criteria, all cases were re-evaluated for this study. Necrosis evaluations were done blinded to the study endpoint. Three necrosis estimation methods were used in both cohorts (Fig. 1). First, in the “average percentage method,” the percentage of tumor necrosis relative to the tumor epithelial area in all available tumor slides was visually evaluated. Second, in the “hotspot method,” a circle with a radius of 1 mm (corresponding to an average field-of-view using a 10x objective magnification in a microscope) was placed in a necrotic hotspot, where the necrotic area would cover the largest possible proportion of circle. The percentage of the necrotic area relative to the total circle area was then visually evaluated. Third, in the “linear method,” the maximum length of a single necrotic region was measured. These evaluations were conducted on digital microscope slides scanned with either Hamamatsu (NanoZoomer S60 or NanoZoomer-XR) or Leica Aperio AT2 slide scanner. An average of 3 digital slides (range, 1 to 18) per case were available for cohort 2, while one digital slide per case, including the deepest tumor invasion, was available for cohort 1. Thirty consecutive cases from cohort 2 were used to investigate the potential influence of slide selection on the results of cohort 1. In these cases, Spearman’s correlation coefficients between tumor necrosis evaluation from a single slide with the deepest tumor invasion and tumor necrosis evaluation using multiple slides were 0.94 for the average percentage method, 0.88 for the hotspot method, and 0.86 for the linear method, indicating high consistency between the results based on analysis of single tumor slide with the deepest invasion vs. multiple slides.
FIGURE 1.
Three evaluation methods for tumor necrosis in colorectal cancer. (A) Overview of a tumor sample. (B) Evaluation of tumor necrosis using the average percentage method. (C) Evaluation of tumor necrosis using the hotspot method. (D) Evaluation of tumor necrosis using the linear method. Scale bars correspond to 2.5 mm.
Statistical Analyses
Statistical analyses were performed with IBM SPSS Statistics for Windows (version 26.0; IBM Corp.). P value of <0.05 was considered statistically significant.
Tumor necrosis measurements were classified into 3 categories, following the example of the Immunoscore®13 and the ITBCC classification for tumor budding.7 The cutoff values were determined based on the previous study,14 as well as the shape of the receiver-operating-characteristics (ROC) curves (endpoint: cancer death), where the lowest and highest segments were more concave than the middle segment. The 3 categories for each estimation method were <3%, 3% to 39.9% and ≥40% for the average percentage method, <4%, 4% to 79.9% and ≥80% for the hotspot method, and ≤500 µm, 501 to 3500 µm and >3500 µm for the linear method. Tumor characteristics were cross-tabulated with the 3-category tumor necrosis measurements. The Chi-square test was used to determine the statistical significance.
Colorectal cancer-specific survival was assessed using the Kaplan-Meier method and Cox regression models. The proportional hazards assumption for the Cox regression model was checked using time-dependent variables. Covariates included in the multivariable models were age (<65, 65-75, >75), sex (female, male), stage (I-II, III, IV), tumor budding (grade 1, 2, 3), lymphovascular invasion (no, yes), grade (low-grade, high-grade), year of operation (cohort 1: 2000 to 2005, 2006 to 2010, 2011 to 2015; cohort 2: 2006 to 2010, 2011 to 2015, 2016 to 2020), tumor location (proximal colon, distal colon, rectum), BRAF status (wild-type, mutant), and MMR status (proficient, deficient). Cases with missing data (BRAF status: 1 patient in cohort 1 and 7 patients in cohort 2) were excluded from the multivariable survival models. For subgroup analysis, 2-category necrosis variables were used: average percentage method, <40% and ≥40%; hotspot method <80% and ≥80%; linear method ≤3500 µm and >3500 µm.
The reproducibility of tumor necrosis evaluation was examined by using Spearman’s correlation coefficients and Cohen’s kappa coefficients. In addition to the 3-category variables, the 2-category variables were included in Cohen’s kappa analysis.
RESULTS
After excluding patients who received neoadjuvant treatment, 1100 patients were analyzed in cohort 1 and 776 patients in cohort 2. The 3 necrosis evaluation methods mainly showed similar associations with tumor and patient characteristics, including high stage, MMR proficient status, BRAF wild-type status, nonmucinous/nonsignet ring cell histology, and high tumor grade (all P<0.001) (Table 1; and Table S1, Supplemental Digital Content 2, http://links.lww.com/PAS/B910, Table S2, Supplemental Digital Content 3, http://links.lww.com/PAS/B911). Age, sex, neuroendocrine differentiation, and lymphatic or venous invasion did not have a statistically significant association with any necrosis estimation method. The hotspot method (Table S1, Supplemental Digital Content 2, http://links.lww.com/PAS/B910) and the average percentage method (Table 1) both showed association with distal tumor location in cohorts 1 and 2, while the linear method was not significantly associated with tumor location (P=0.238) in cohort 2 (Table S2, Supplemental Digital Content 3, http://links.lww.com/PAS/B911).
TABLE 1.
Patient and Tumor Characteristics according to Tumor Necrosis Percentage (average necrosis percentage method) in Cohorts 1 and 2
| Cohort 1, N=1100 | Cohort 2, N=776 | |||||||
|---|---|---|---|---|---|---|---|---|
| Tumor necrosis percentage, N (%) | Tumor necrosis percentage, N (%) | |||||||
| Variable | Total, N (%) | <3% | 3-39.9% | ≥40% | Total, N (%) | <3% | 3-39.9% | ≥40% |
| Sex | ||||||||
| Male | 557 (51%) | 122 (22%) | 399 (72%) | 36 (6.5%) | 412 (53%) | 116 (28%) | 271 (66%) | 25 (6.1%) |
| Female | 543 (49%) | 131 (24%) | 376 (69%) | 36 (6.6%) | 364 (47%) | 119 (33%) | 216 (59%) | 29 (8.0%) |
| P | 0.662 | 0.166 | ||||||
| Age | ||||||||
| <65 | 290 (26%) | 59 (20%) | 212 (73%) | 19 (6.6%) | 233 (30%) | 66 (28%) | 154 (66%) | 13 (5.6%) |
| 65-75 | 381 (35%) | 82 (22%) | 272 (71%) | 27 (7.1%) | 285 (37%) | 88 (31%) | 174 (61%) | 23 (8.1%) |
| >75 | 429 (39%) | 112 (26%) | 291 (68%) | 26 (6.1%) | 258 (33%) | 81 (31%) | 159 (62%) | 18 (7.0%) |
| P | 0.389 | 0.704 | ||||||
| Tumor location | ||||||||
| Proximal colon | 536 (49%) | 151 (28%) | 354 (66%) | 31 (5.8%) | 323 (42%) | 110 (34%) | 190 (59%) | 23 (7.1%) |
| Distal colon | 404 (37%) | 62 (15%) | 306 (76%) | 36 (8.9%) | 205 (26%) | 41 (20%) | 148 (72%) | 16 (7.8%) |
| Rectum | 160 (15%) | 40 (25%) | 115 (72%) | 5 (3.1%) | 248 (32%) | 84 (34%) | 149 (60%) | 15 (6.0%) |
| P | <0.001 | 0.007 | ||||||
| Stage | ||||||||
| I | 184 (17%) | 62 (34%) | 120 (65%) | 2 (1.1%) | 187 (24%) | 89 (48%) | 97 (52%) | 1 (0.5%) |
| II | 408 (37%) | 83 (20%) | 293 (72%) | 32 (7.8%) | 253 (33%) | 71 (28%) | 157 (62%) | 25 (9.9%) |
| III | 355 (32%) | 77 (22%) | 258 (73%) | 20 (5.6%) | 251 (32%) | 64 (25%) | 170 (68%) | 17 (6.8%) |
| IV | 153 (14%) | 31 (20%) | 104 (68%) | 18 (12%) | 85 (11%) | 11 (13%) | 63 (74%) | 11 (13%) |
| P | <0.001 | <0.001 | ||||||
| Histologic subtype | ||||||||
| Adenocarcinoma | 995 (90%) | 201 (20%) | 725 (73%) | 69 (6.9%) | 700 (90%) | 188 (27%) | 458 (65%) | 54 (7.7%) |
| Mucinous carcinoma | 77 (7.0%) | 39 (51%) | 36 (47%) | 2 (2.6%) | 61 (7.9%) | 39 (64%) | 22 (36%) | 0 (0%) |
| Signet ring cell carcinoma | 28 (2.5%) | 13 (46%) | 14 (50%) | 1 (3.6%) | 15 (1.9%) | 8 (53%) | 7 (47%) | 0 (0%) |
| P | <0.001 | <0.001 | ||||||
| Neuroendocrine differentiation* | ||||||||
| 0% | — | — | — | — | 560 (72%) | 162 (29%) | 358 (64%) | 40 (7.1%) |
| 1–9% | — | — | — | — | 157 (20%) | 47 (30%) | 98 (62%) | 12 (7.6%) |
| ≥10% | — | — | — | — | 43 (5.5%) | 15 (35%) | 26 (60%) | 2 (4.7%) |
| Missing data | 16 (2.1%) | |||||||
| P | 0.905 | |||||||
| WHO grade | ||||||||
| Low-grade | 903 (82%) | 188 (21%) | 662 (73%) | 53 (5.9%) | 665 (86%) | 197 (30%) | 429 (64%) | 40 (6.0%) |
| High-grade | 197 (18%) | 65 (33%) | 113 (57%) | 19 (19%) | 111 (14%) | 38 (34%) | 59 (53%) | 14 (13%) |
| P | <0.001 | 0.011 | ||||||
| Lymphovascular invasion | ||||||||
| No | 858 (78%) | 202 (24%) | 596 (69%) | 60 (7.0%) | 429 (55%) | 148 (34%) | 258 (60%) | 23 (5.4%) |
| Yes | 242 (22%) | 51 (21%) | 179 (74%) | 12 (5.0%) | 347 (45%) | 87 (25%) | 229 (66%) | 31 (8.9%) |
| P | 0.327 | 0.006 | ||||||
| Mismatch repair status | ||||||||
| MMR proficient | 931 (85%) | 188 (20%) | 684 (73%) | 59 (6.3%) | 652 (84%) | 170 (26%) | 439 (67%) | 43 (6.6%) |
| MMR deficient | 169 (15%) | 65 (38%) | 91 (54%) | 13 (7.7%) | 124 (16%) | 65 (52%) | 48 (39%) | 11 (8.9%) |
| P | <0.001 | <0.001 | ||||||
| BRAF status | ||||||||
| Wild-type | 916 (83%) | 187 (20%) | 668 (73%) | 61 (6.7%) | 662 (85%) | 174 (26%) | 445 (67%) | 43 (6.5%) |
| Mutant | 182 (17%) | 65 (36%) | 106 (58%) | 11 (6.0%) | 107 (14%) | 55 (51%) | 41 (38%) | 11 (10%) |
| Missing data | 2 (0.2%) | 7 (0.9%) | ||||||
| P | <0.001 | <0.001 | ||||||
Neuroendocrine differentiation was determined for cohort 2 using synaptophysin and chromogranin A immunohistochemistry.
— indicates missing data for neuroendocrine differentiation in cohort 1.
MMR indicates mismatch repair.
In ROC analysis, the average percentage and hotspot methods had a slightly higher area under the curve (AUC) in predicting patients with colorectal cancer-specific mortality than the linear method, but the difference was within the limits of 95% CIs (Figure S1, Supplemental Digital Content 4, http://links.lww.com/PAS/B912). As categorical variables, all 3 methods reached statistical significance in Kaplan-Meier analysis (Fig. 2), univariable Cox regression models, and multivariable Cox regression models (Table 2; and Table S3, Supplemental Digital Content 5, http://links.lww.com/PAS/B913; Table S4, Supplemental Digital Content 6, http://links.lww.com/PAS/B914; and Table S5, Supplemental Digital Content 7, http://links.lww.com/PAS/B915). The survival substantially declined in the category with the highest necrosis (≥40%, ≥80%, or >3500 µm) compared to the 2 necrosis categories with the least amount of tumor necrosis (Fig. 2). The greatest hazard ratios were observed in both univariable and multivariable models for the average necrosis percentage ≥40% vs. <3% (univariable cohort 1: HR 2.41; 95% CI, 1.55-3.74 and cohort 2: HR 4.85; 95% CI, 2.73-8.16; multivariable cohort 1: HR 3.03; 95% CI, 1.93-4.78, cohort 2: HR 2.97; 95% CI, 1.63-5.40) (Table 2, and Table S3, Supplemental Digital Content 5, http://links.lww.com/PAS/B913).
FIGURE 2.
Tumor necrosis evaluation methods and survival. Kaplan-Meier cancer-specific survival curves for the 3 tumor necrosis evaluation methods in cohort 1 (A-C) and cohort 2 (D-F).
TABLE 2.
Cox Proportional Hazards Regression Models for the Associations between Tumor Necrosis Evaluation Methods and Colorectal Cancer-specific Survival in Cohorts 1 and 2
| Cohort 1, N=1063 | Cohort 2, N=771 | |||||||
|---|---|---|---|---|---|---|---|---|
| Variable | No. of cases | No. of events | Univariable HR (95% CI) | Multivariable HR (95% CI) | No. of cases | No. of events | Univariable HR (95% CI) | Multivariable HR (95% CI) |
| Average percentage method | ||||||||
| <3% | 244 | 55 | 1 (referent) | 1 (referent) | 233 | 25 | 1 (referent) | 1 (referent) |
| 3-39.9% | 751 | 210 | 1.26 (0.94-1.70) | 1.47 (1.07-2.00) | 484 | 88 | 1.75 (1.12-2.73) | 1.11 (0.71-1.74) |
| ≥40% | 68 | 31 | 2.41 (1.55-3.74) | 3.03 (1.93-4.78) | 54 | 22 | 4.85 (2.73-8.16) | 2.97 (1.63-5.40) |
| P trend | <0.001 | <0.001 | <0.001 | 0.003 | ||||
| Hotspot method | ||||||||
| <4% | 175 | 38 | 1 (referent) | 1 (referent) | 131 | 15 | 1 (referent) | 1 (referent) |
| 4-79.9% | 812 | 224 | 1.28 (0.91-1.80) | 1.39 (0.97-2.00) | 529 | 81 | 1.34 (0.77-2.32) | 0.84 (0.47-1.51) |
| ≥80% | 76 | 34 | 2.51 (1.58-3.98) | 2.76 (1.72-4.24) | 111 | 39 | 3.80 (2.09-6.90) | 1.82 (0.96-3.44) |
| P trend | <0.001 | <0.001 | <0.001 | 0.004 | ||||
| Linear method | ||||||||
| ≤500 μm | 287 | 71 | 1 (referent) | 1 (referent) | 129 | 17 | 1 (referent) | 1 (referent) |
| 501-3500 μm | 693 | 193 | 1.15 (0.88-1.51) | 1.36 (1.02-1.80) | 505 | 81 | 1.27 (0.75-2.15) | 1.24 (0.72-2.13) |
| >3500 μm | 83 | 32 | 1.69 (1.11-2.57) | 2.06 (1.34-3.17) | 137 | 37 | 2.50 (1.40-4.44) | 1.96 (1.06-3.62) |
| P trend | 0.029 | 0.001 | 0.001 | 0.016 | ||||
Cox proportional hazards regression model was adjusted for age (<65, 65-75, >75), sex (female, male), stage (I-II, III, IV), tumor budding (grades 1, 2, 3), lymphovascular invasion (no, yes), grade (low-grade, high-grade), year of operation (cohort 1: 2000-2005, 2006-2010, 2011-2015; Cohort 2: 2006-2010, 2011-2015, 2016-2020), tumor location (proximal colon, distal colon, rectum), BRAF status (wild-type, mutant) and mismatch repair status (proficient, deficient). Cases with missing data (BRAF status: 1 patient in cohort 1 and 7 patients in cohort 2) were excluded from the multivariable survival models. CI indicates confidence interval; HR, hazards ratio.
Considering that most tumors were MMR proficient (cohort 1, 85%; cohort 2, 84%), we conducted additional sensitivity analysis limited to MMR proficient cases. Kaplan-Meier analyses showed statistically significant survival associations for the average percentage method and hotspot method in both cohorts (Figure S2, Supplemental Digital Content 8, http://links.lww.com/PAS/B916). In multivariable Cox regression models, all 3 methods showed statistically significant association with survival, and the average percentage method (≥40% vs. <3%) showed the greatest HRs (cohort 1: HR 2.60; 95% CI, 1.58-4.29; cohort 2: HR 3.42; 95% CI, 1.78-6.55) (Table S6, Supplemental Digital Content 9, http://links.lww.com/PAS/B915).
Additional subgroup analyses of the prognostic value of tumor necrosis are presented as forest plots (Figure S3, Supplemental Digital Content 10, http://links.lww.com/PAS/B917). In these analyses, tumor necrosis predicted mortality in a wide range of patient subgroups defined by clinical, pathologic, and molecular characteristics. Although tumor necrosis appeared to show stronger prognostic significance in females (linear method) and lower T stages (hotspot method and linear method) in cohort 1 and patients with low-grade tumors (average percentage method and hotspot method) in cohort 2, no consistent findings of a statistically significant difference according to any clinicopathologic feature were observed across both cohorts.
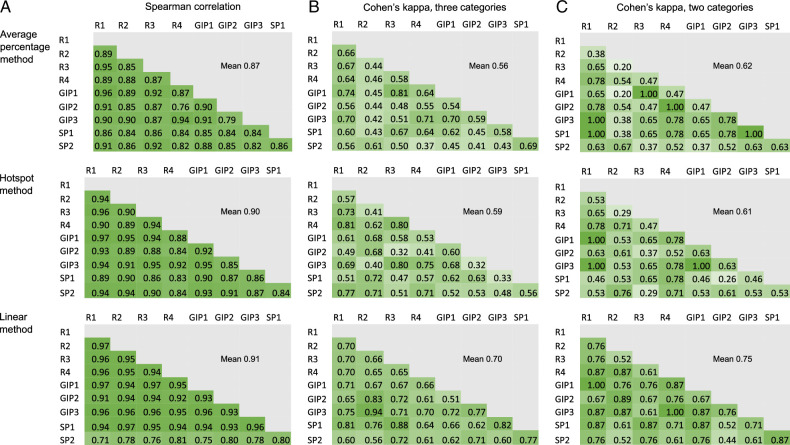
The reproducibility of the 3 necrosis estimation methods was tested (Fig. 3). Tumor necrosis was assessed from 30 tumor samples by 3 gastrointestinal pathologists, 2 specializing pathologists, and 4 researchers. The mean Spearman’s correlation coefficient for the average necrosis percentage was 0.87, for the hotspot method 0.90, and the linear measure 0.91, indicating excellent reproducibility and minimal difference between the estimation methods. However, when categorized into 3-category variables or 2-category variables, the linear method reached slightly higher mean Cohen’s kappa than the other methods (3-category: average necrosis percentage 0.56, hotspot method 0.59, linear method 0.70; 2-category: mean necrosis percentage 0.62, hotspot method 0.61, linear method 0.75) (Fig. 3). These results suggest that the linear method had slightly higher accuracy in measuring cases near category cut-points than the other 2 methods.
FIGURE 3.
Reproducibility analysis for the 3 tumor necrosis estimation methods. The reproducibility was measured with Spearman rank correlation analysis (A) and Cohen’s kappa (B–C).GIP, gastrointestinal pathologist; R, researcher; SP, specializing pathologist.
DISCUSSION
Our objective was to define an accurate and reproducible tumor necrosis evaluation method to enable its routine application as a prognostic parameter in CRC. Two large cohorts consisting of more than 1800 patients were analyzed with 3 necrosis estimation methods (average necrosis percentage, necrosis percentage in a single hotspot, and the largest diameter of a single necrotic focus). All 3 methods showed prognostic value independent of other tumor and patient characteristics, as well as reasonably high reproducibility.
Several previous colorectal cancer studies5,6,15,16 have evaluated tumor necrosis as the percentage of the necrotic area of the total tumor area, classified as none, ≤10%, 10% to 30%, and ≥30%. Richards et al.5 tested the reproducibility of this evaluation between 2 researchers, the interobserver intraclass correlation coefficient was 0.86. This evaluation method has also been used for upper urinary tract cell carcinoma17 and lung cancer.15 In non-small cell lung cancer, reproducibility kappa was 0.8 between 2 researchers.15 In some studies, necrosis percentage was also classified into 2 categories, such as absent/present18 or little (<10%)/abundant (≥10%) necrosis.19,20 A more subjective 3-grade assessment (rare areas of necrosis, frequent small areas of necrosis and broad areas of necrosis) has also been applied.21 Previous studies have used different cutoff values and various numbers of categories, which makes it difficult to compare different studies with each other.
We hypothesized that the hotspot method and the linear method could have better reproducibility than estimating the total necrosis percentage, as the measurements based on a single hotspot may be easier to reproduce than evaluations based on the whole tumor. However, all 3 methods reached reasonably high reproducibility, with the linear method showing the highest kappa coefficients, when evaluated as a categorical variable. Nevertheless, the average percentage method seemed to slightly outperform the linear method in predicting survival. This may be related to the linear measure being affected by the shape of the necrotic area (high values for long but thin foci). Based on our results, all 3 evaluation methods for tumor necrosis appear to show high reproducibility and prognostic value, and it is difficult to recommend any of the 3 methods over the other 2.
Tumor necrosis is associated with poor differentiation, advanced stage, and more aggressive tumor behavior.4,14,22 The factors contributing to the adverse prognostic effect of tumor necrosis are not clear, but some hypotheses can be formed. Necrosis is a form of unregulated cell death where the cell membranes rupture, releasing the contents of the cell and eliciting an inflammatory response.23,24 Large amounts of necrosis are associated with systemic inflammation and weaker local inflammatory response.5,25 Tumor necrosis has been related to elevated serum levels of proinflammatory cytokines and tumor aggression.4,26,27 Consequently, the presence of necrosis may influence the tumor microenvironment by the release of damage-associated molecular patterns (DAMPs) and proinflammatory cytokines, thus leading to more aggressive cancer.6,23 Furthermore, necrosis may be a sign or sequel of activated proliferation and anti-apoptotic pathways in tumors which are hallmarks of cancer progression.28 Necrosis has been hypothesized to be associated with insufficient blood flow to tumors and hypoxia but there are studies for and against this.14,26,29 The formation of tumor necrosis is likely dependent on many factors which could explain the adverse effects related to abundant necrosis.14,29
Other promising prognostic markers in CRC include tumor budding,7 Immunoscore®,13 modified Glasgow prognostic score (mGPS),30, and tumor stroma ratio,31 among others. Of these prognostic markers, tumor budding is widely used in clinical practice.3 In addition to its use in identifying high-risk stage II CRC patients for the consideration of adjuvant therapy, tumor budding has proven a useful marker in identifying high-risk pT1 tumors after endoscopic or transanal tumor removal.3 In our study, tumor necrosis had prognostic value independent of tumor budding, disease stage, and tumor molecular features including MMR status and BRAF mutation status. Similar to tumor budding, it is easy to evaluate tumor necrosis from H&E-stained samples making it an accessible prognostic marker. Our study defines criteria for tumor necrosis evaluation that can be used to assess whether stage II CRC patients with necrotic tumors would benefit from adjuvant treatment or if tumor necrosis could guide adjuvant treatment options for stage III patients.
The limitations of our study must be considered. First, when sampling tumors, pathologists may avoid overtly necrotic regions, as these are not useful for evaluating tumor morphology or conducting molecular analyses. This may have reduced the average necrosis percentage observed in the tumors. Therefore, it should be studied whether systematic sampling of the most necrotic regions could influence the survival results. Second, the analyses of cohort 1 were based on a single tumor section with the deepest invasion. However, a high correlation was observed between tumor necrosis measurements from a single tumor section with the deepest invasion and measurements from multiple tumor sections. Third, although the study included 2 large cohorts, the necrosis evaluation was based on a retrospective analysis of the tumor samples. A prospective study could assess whether tumor necrosis evaluation could identify patients who would benefit from standard adjuvant treatments or anti-angiogenesis treatments such as anti-VEGF antibody therapies. Fourth, the relevance of tumor necrosis in patients with neoadjuvant-treated rectal cancer is unclear, and it has not been investigated whether tumor necrosis evaluation could be done from preoperative biopsies. Fifth, although the cohorts were large, the number of MMR-deficient cases was relatively low, making the survival estimates less accurate in that patient subgroup. Sixth, these analyses focus mainly on the prognostic relevance of tumor necrosis in resected tumors, while largely excluding primarily metastatic cases that are treated with systemic chemotherapy only.
CONCLUSIONS
Tumor necrosis can be reproducibly evaluated on H&E-stained sections, which makes it an accessible prognostic factor. All 3 estimation methods predicted patient survival independently of other prognostic parameters and had reasonably high reproducibility. The findings pave the way for the use of tumor necrosis as a prognostic factor in colorectal cancer.
Supplementary Material
ACKNOWLEDGMENTS
The study benefited from samples/data from Northern Finland Biobank Borealis (Oulu, Finland) and Central Finland Biobank (Jyväskylä, Finland). Part of the work was carried out with the support of Biocenter Oulu, the Transgenic and Tissue Phenotyping Core Facility, endowed by the University of Oulu, Finland, and Biocenter Finland.
Footnotes
The research was done by the Declaration of Helsinki. Cohort 1 analyses were approved by the Regional Medical Research Ethics Committee of the Wellbeing Services county of Central Finland (Dnro 13U/2011, 1/2016, 8/2020, and 2/2023), the Finnish Medicines Agency (Fimea, FIMEA/2023/001573), and the Central Finland Biobank (BB23-0172). Cohort 2 studies were conducted under permission from the Regional Medical Research Ethics Committee of the Wellbeing Services county of North Ostrobothnia (25/2002, 42/2005, 122/2009, 37/2020), Fimea (FIMEA/2022/001941), and Biobank Borealis (BB-2017_1012). Participants gave written informed consent for the study in cohort 2. For cohort 1, the need to obtain informed consent from the study patients was waived (Dnro FIMEA/2023/001573).
The datasets generated and analyzed during this study are not publicly available. The sharing of data will require approval from relevant ethics committees and biobanks. Further information, including the procedures to obtain and access data from Finnish biobanks, are described at https://finbb.fi/en/fingenious-service.
M.K., M.J.M., and J.P.V. were involved in conceptualization. M.K., P.S., H.E., H.K., V.V.T., V.K.Ä., M.A., A.T., and J.P.V. were involved in data curation. M.K., J.-P.M., M.J.M., and J.P.V. were involved in funding acquisition. M.K., P.S., H.E., H.K., V.V.T., V.K.Ä., M.A., J.R., S.M., T.R., J.S., T.T.M., O.L., E.-V.W., O.H., T.T.S., J.B., J.-P.M., A.T., M.J.M., and J.P.V. were involved in investigation. M.J.M. and J.P.V. were involved in the methodology. M.K. and J.P.V. were involved in the formal analysis. J.-P.M., M.J.M., and J.P.V. were involved in resources. M.J.M. and J.P.V. were involved in supervision. M.K., H.K., V.V.T., V.K.Ä., V.-M.P., J.K., K.S., M.J.M. and J.P.V. were involved in validation. M.K. and J.P.V. were involved in visualization. M.K. and J.P.V. were involved in writing—the original draft. All authors were involved in writing—review and editing.
Conflicts of Interest and Source of Funding: This study was funded by Cancer Foundation Finland (59-5619 to J.P.V.), Finnish Medical Foundation (6021 to J.P.V.; 6259 to M.K.), Oulu Medical Research Foundation (to M.K.), Sigrid Jusélius Foundation (230229 to J.P.V.), and Finnish State Research Funding (to M.J.M. and J.P.V.). T.T. Seppälä reports consultation fees from Amgen Finland, Tillots Pharma, and Nouscom, being a co-owner and CEO of Healthfund Finland Ltd, and a position in the Clinical Advisory Board and a minor shareholder of LS Cancer Diag Ltd. For the remaining authors, none were declared.
Supplemental Digital Content is available for this article. Direct URL citations are provided in the HTML and PDF versions of this article on the journal's website, www.ajsp.com.
Contributor Information
Meeri Kastinen, Email: meeri.kastinen@student.oulu.fi.
Päivi Sirniö, Email: paivi.sirnio@oulu.fi.
Hanna Elomaa, Email: elomaa.hannak@gmail.com.
Ville K. Äijälä, Email: ville.aijala@student.oulu.fi.
Henna Karjalainen, Email: henna.karjalainen@student.oulu.fi.
Vilja V. Tapiainen, Email: vilja.tapiainen@student.oulu.fi.
Vesa-Matti Pohjanen, Email: vesa-matti.pohjanen@oulu.fi.
Janette Kemppainen, Email: janette.savela@student.oulu.fi.
Katja Sliashynskaya, Email: katja.sliashynskaya@pohde.fi.
Maarit Ahtiainen, Email: maarit.ahtiainen@hyvaks.fi.
Jukka Rintala, Email: jukka.rintala@pohde.fi.
Sanna Meriläinen, Email: sanna.merilainen@ppshp.fi.
Tero Rautio, Email: tero.rautio@pohde.fi.
Juha Saarnio, Email: juha.saarnio@oulu.fi.
Taneli T. Mattila, Email: taneli.mattila@pohde.fi.
Outi Lindgren, Email: outi.lindgren@pohde.fi.
Erkki-Ville Wirta, Email: erkki-ville.wirta@fimnet.fi.
Olli Helminen, Email: olli.helminen@oulu.fi.
Toni T. Seppälä, Email: toni.seppala@tuni.fi.
Jan Böhm, Email: jan.bohm@hyvaks.fi.
Jukka-Pekka Mecklin, Email: jukka-pekka.mecklin@hyvaks.fi.
Anne Tuomisto, Email: anne.tuomisto@oulu.fi.
Markus J. Mäkinen, Email: markus.makinen@oulu.fi.
Juha P. Väyrynen, Email: juha.vayrynen@oulu.fi.
REFERENCES
- 1.Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–249. [DOI] [PubMed] [Google Scholar]
- 2.Nagtegaal ID, Odze RD, Klimstra D, et al. The 2019 WHO classification of tumours of the digestive system. Histopathology. 2020;76:182–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Argilés G, Tabernero J, Labianca R, et al. Localised colon cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2020;31:1291–1305. [DOI] [PubMed] [Google Scholar]
- 4.Kastinen M, Sirniö P, Elomaa H, et al. Immunological and prognostic significance of tumour necrosis in colorectal cancer. Br J Cancer. 2023;128:2218–2226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Richards CH, Roxburgh CSD, Anderson JH, et al. Prognostic value of tumour necrosis and host inflammatory responses in colorectal cancer. Br J Surg. 2012;99:287–294. [DOI] [PubMed] [Google Scholar]
- 6.Pollheimer MJ, Kornprat P, Lindtner RA, et al. Tumor necrosis is a new promising prognostic factor in colorectal cancer. Hum Pathol. 2010;41:1749–1757. [DOI] [PubMed] [Google Scholar]
- 7.Lugli A, Kirsch R, Ajioka Y, et al. Recommendations for reporting tumor budding in colorectal cancer based on the International Tumor Budding Consensus Conference (ITBCC) 2016. Mod Pathol. 2017;30:1299–1311. [DOI] [PubMed] [Google Scholar]
- 8.Elomaa H, Ahtiainen M, Väyrynen SA, et al. Prognostic significance of spatial and density analysis of T lymphocytes in colorectal cancer. Br J Cancer. 2022;127:514–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Väyrynen JP, Väyrynen SA, Sirniö P, et al. Platelet count, aspirin use, and characteristics of host inflammatory responses in colorectal cancer. J Transl Med. 2019;17:199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Väyrynen JP, Tuomisto A, Väyrynen SA, et al. Preoperative anemia in colorectal cancer: relationships with tumor characteristics, systemic inflammation, and survival. Sci Rep. 2018;8:1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sajanti S, Sirniö P, Väyrynen JP, et al. VE1 immunohistochemistry accurately detects BRAF V600E mutations in colorectal carcinoma and can be utilized in the detection of poorly differentiated colorectal serrated adenocarcinoma. Virchows Arch. 2014;464:637–643. [DOI] [PubMed] [Google Scholar]
- 12.Seppälä TT, Böhm JP, Friman M, et al. Combination of microsatellite instability and BRAF mutation status for subtyping colorectal cancer. Br J Cancer. 2015;112:1966–1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pagès F, Mlecnik B, Marliot F, et al. International validation of the consensus Immunoscore for the classification of colon cancer: a prognostic and accuracy study. Lancet. 2018;391:2128–2139. [DOI] [PubMed] [Google Scholar]
- 14.Väyrynen SA, Väyrynen JP, Klintrup K, et al. Clinical impact and network of determinants of tumour necrosis in colorectal cancer. Br J Cancer. 2016;114:1334–1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Swinson DEB, Jones JL, Richardson D, et al. Tumour necrosis is an independent prognostic marker in non-small cell lung cancer: correlation with biological variables. Lung Cancer. 2002;37:235–240. [DOI] [PubMed] [Google Scholar]
- 16.Ye H, Wang Y, Yao S, et al. Necrosis score as a prognostic factor in stage I–III colorectal cancer: a retrospective multicenter study. Discov Oncol. 2023;14:61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Langner C, Hutterer G, Chromecki T, et al. Tumor necrosis as prognostic indicator in transitional cell carcinoma of the upper urinary tract. J Urol. 2006;176:910–913. [DOI] [PubMed] [Google Scholar]
- 18.Kuo FY, Eng HL, Li WF, et al. Tumor necrosis is an indicator of poor prognosis among hepatoma patients undergoing resection. J Surg Res. 2023;283:1091–1099. [DOI] [PubMed] [Google Scholar]
- 19.Gao JF, Arbman G, Wadhra TI, et al. Relationships of tumor inflammatory infiltration and necrosis with microsatellite instability in colorectal cancers. World J Gastroenterol. 2005;11:2179–2183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Park JH, van Wyk H, Roxburgh CSD, et al. Tumour invasiveness, the local and systemic environment and the basis of staging systems in colorectal cancer. Br J Cancer. 2017;116:1444–1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Paarnio K, Väyrynen JP, Väyrynen SA, et al. TLR2 and TLR4 in colorectal cancer: relationship to tumor necrosis and markers of systemic inflammation. Neoplasma. 2022;69:1418–1424. [DOI] [PubMed] [Google Scholar]
- 22.Schneider NI, Langner C. Prognostic stratification of colorectal cancer patients: current perspectives. Cancer Manag Res. 2014;6:291–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Karsch-Bluman A, Feiglin A, Arbib E, et al. Tissue necrosis and its role in cancer progression. Oncogene. 2019;38:1920–1935. [DOI] [PubMed] [Google Scholar]
- 24.Yee PP, Li W. Tumor necrosis: a synergistic consequence of metabolic stress and inflammation. Bioessays. 2021;43:e2100029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sirniö P, Väyrynen JP, Mutt SJ, et al. Systemic inflammation is associated with circulating cell death released keratin 18 fragments in colorectal cancer. Oncoimmunology. 2020;9:1783046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guthrie GJK, Roxburgh CSD, Horgan PG, et al. Does interleukin-6 link explain the link between tumour necrosis, local and systemic inflammatory responses and outcome in patients with colorectal cancer? Cancer Treat Rev. 2013;39:89–96. [DOI] [PubMed] [Google Scholar]
- 27.Pennel KAF, Quinn JA, Nixon C, et al. CXCL8 expression is associated with advanced stage, right sidedness, and distinct histological features of colorectal cancer. J Pathol Clin Res. 2022;8:509–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. [DOI] [PubMed] [Google Scholar]
- 29.Caruso RA, Branca G, Fedele F, et al. Mechanisms of coagulative necrosis in malignant epithelial tumors (Review). Oncol Lett. 2014;8:1397–1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McMillan DC. The systemic inflammation-based Glasgow Prognostic Score: a decade of experience in patients with cancer. Cancer Treat Rev. 2013;39:534–540. [DOI] [PubMed] [Google Scholar]
- 31.Sullivan L, Pacheco RR, Kmeid M, et al. Tumor stroma ratio and its significance in locally advanced colorectal cancer. Curr Oncol. 2022;29:3232–3241. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.