Abstract
Background and purpose:
Treatment of malignancies with chemotherapy and surgery is often associated with disease recurrence and metastasis. Immunotherapy improves cancer treatment by creating an active response against tumor antigens. Various cancer cells express a large amount of glucose-regulated protein 78 (GRP78) protein on their surface. Stimulating the immune system against this antigen can expose cancer cells to the immune system. Herein, we investigated the effectiveness of a cGRP78-based vaccine against different cancer cells.
Experimental approach:
BALB/c mice were immunized with the cGRP78. The humoral immune response against different cancer cells was assessed by Cell-ELISA. The cellular immunity response was determined by splenocyte proliferation assay with different cancer antigens. The effect of vaccination on metastasis was investigated in vaccinated mice by injecting melanoma cancer cells into the tail of mice.
Findings/Results:
These results indicated that the cGRP78 has acceptable antigenicity and stimulates the immune system to produce antibodies. After three injections, the amount of produced antibody was significantly different from the control group. Compared to the other three cell types, Hela and HepG2 showed the highest reaction to the serum of vaccinated mice. Cellular immunity against the B16F10 cell line had the best results compared to other cells. The metastasis results showed that after 30 days, the growth of B16F10 melanoma cancer cells was not noticeable in the lung tissue of vaccinated mice.
Conclusion and implications:
Considering the resistance of vaccinated mice to metastasis, this vaccine offers a promising prospect for cancer treatment by inhibiting the spread of cancer cells.
Keywords: cGRP78 vaccine, Metastasis, Melanoma, Immunotherapy.
INTRODUCTION
The initial stages of malignancy treatment include chemotherapy, surgery, and radiotherapy. However, in most cases, there will be the possibility of disease recurrence and metastasis after these steps. One of the new research fields that creates an active response against tumor antigens is immunotherapy against cancer (1). Immunotherapy activates the immune system against cancer surface antigens, and T cells play a special role in this mechanism (2).
On the other hand, cancer cells can escape from the immune system by hiding their antigens from T cells. Therefore, one of the most important goals of immunotherapy in cancer treatment is to create tumor-specific T cells (3). One of the new trials in the prevention and treatment of malignancies that uses immunotherapy mechanisms is the use of surface antigens of cancer cells to develop cancer vaccines (4). The main goal of anti-tumor vaccines is to induce an appropriate and long-lasting immune response against tumors, which is able to stop the recurrence of the tumor. An effective vaccine that can better treat cancer cells should be able to rouse cellular and humoral immune responses against cancer cells (1,5,6). The production of heat shock protein (HSP) housekeeping proteins, which are produced in the cells of all living organisms, increases in some pathological conditions such as hypoxia or heat shock (7). The high rate of proliferation in cancer cells continuously leads to a lack of nutrients and oxygen (hypoxia) in these cells. As a result, members of the Hsp70 families increase their expression in these cells, particularly the extracellular and membrane-bound cells, and migrate there more than they normally do (7). In the following, antigen-presenting cells are activated against these antigens and play their role in cancer immunotherapy.
Glucose-regulated protein 78 (GRP78) is an active chaperone in the endoplasmic reticulum and a member of the HSP70 family. The most important function of GRP78 is in regulating intracellular transport of proteins, receptor-dependent endocytosis, and apoptosis. Another activity of GRP78 is targeting misfolded proteins for proteasomal degradation and regulating calcium homeostasis in cells (8,9). Many articles have reported high expression of GRP78 on the surface of cancer cells (10,11,12,13,14). In cancer cells and stressed cells, the high expression of GRP78 in the endoplasmic reticulum causes the migration of this protein to the cell surface, which rarely happens in normal cells. The amount of GRP78 on the cell surface is directly related to the growth rate, malignancy, anti-apoptotic activity, drug resistance, and metastasis of cancer cells (9,15,16).
Various cancer cells, such as breast cancer, melanoma, hepatoma, osteosarcoma, and pancreatic cancer, express a large amount of GRP78 protein on their surface (17,18). In the study by Cai et al, the role of GRP78 in the chemotherapy process of breast cancer was confirmed using in vivo breast cancer xenografts, which makes this antigen an attractive candidate for use in immunotherapy and vaccination (10). Moreover, there is a lot of evidence showing that GRP78 is closely related to the progression and poor prognosis of lung cancer. Therefore, this protein has a significant role in lung cancer therapy and can potentially be considered a suitable candidate for lung cancer vaccination (11,19). On the other hand, several studies have revealed that GRP78 may play an important role in the development and progression of hepatocellular carcinoma. In melanoma cancer cells, increased surface expression of GRP78 is associated with drug resistance and malignant progression (14). It has been found that overexpression of cell surface GRP78 boosts the proliferation, growth, and migration of cancer cells, and the suppression of this receptor has reduced metastasis to the lung and increased the survival of the tested mice (11,19,20). These findings indicate cell surface GRP78 as an encouraging cancer cell-specific biomarker and a beneficial target for the treatment and imaging of tumor cells (17,18).
Many studies show that antibodies that bind to the N-terminal domain of GRP78 increase the growth and metastasis of tumor cells, but antibodies against the C-terminal domain of this protein inhibit the growth and metastasis of cancer cells (21,22). This shows the importance of choosing cGRP78 as a suitable target for removing cancer cells. Producing antibodies against this protein or using it as a vaccine against cancer cells can open new horizons in cancer treatment.
In previous studies, our investigations using bioinformatics and laboratory data showed that the production of the C-terminal domain of the GRP78 protein maintained its original structure (21). This truncated recombinant protein can be a suitable candidate as an anticancer vaccine because it causes the immune system to respond against the C-terminal protein GRP78, which scientists have shown will lead to the destruction of cancer cells. Unlike most cancer-specific antigen vaccines, a cGRP78-based vaccine can act against many cancer cells, and depending on the level of surface expression of GRP78 on cancer cells, it can eliminate or reduce the growth and metastasis of cancer cells.
This study aimed to investigate the reaction of a cGRP78-based vaccine on various cancer cells, such as hepatoma, lung, breast, melanoma, and cervical cancer, and to evaluate its effect on preventing the metastasis of melanoma cancer cells (B16F10) in a mouse model.
MATERIALS AND METHODS
Cell lines and mice
Cancer cell lines including 4T1, B16F10, HepG2, A549, Hela, and normal cell line including HUVEC were purchased from the cell bank of the Pasteur Institute of Iran and cultured in DMEM and RPMI culture mediums enriched with 10% fetal bovine serum (FBS; DNAbiotech Co., Iran) in a humidified incubator under 5% CO2 at 37 °C depending on the cell types (23). The cells were passaged at the appropriate time and frozen for later use.
The mice used in this study were 4-week-old BALB/c males purchased from Royan Laboratory Animal Center of Isfahan, kept in standard environmental and nutritional conditions. All the steps of working with mice have been done according to the Animal Welfare Guidelines, and the proposal of this research was approved by the Institutional Ethical Committee and Research Advisory Committee of Isfahan University of Medical Sciences in Iran (Ethical No. 1398.849).
Preparation of cGRP78
Cloning of cGRP78 recombinant protein has been performed in our previous study (21). The gene for this recombinant protein (690 bp) was cloned in the pET22b vector. In the following, its purification was carried out with the help of the histidine tag and by the nickel-nitrilotriacetic acid (Ni-NTA) column. The expression of this recombinant protein was done at 30 °C for 16 h, and its purification was performed by a native purification protocol (Invitrogen). Sodium dodecyl-sulfate polyacrylamide gel electrophoresis (SDS-PAGE) was used to evaluate the purity of the recombinant protein, and a western blot with an anti-GRP78 antibody (Abcam Co., UK) was used to confirm it. SDS-PAGE and western blot were performed according to the procedure mentioned in the previous article (24).
Mouse immunization with cGRP78
To investigate the response of the humoral and cellular immune systems of mice vaccinated with the recombinant protein cGRP78 against different cancer cells, 4-week-old BALB/c mice (n = 16) were randomly divided into two groups. The mice of the test group were injected with 0.1 mL of 500 μg/mL cGRP78 recombinant protein (50 pg/mice) in three boosters with a ten-day interval, complete and incomplete Freund's adjuvant as a vaccine. Also, phosphate-buffered saline (PBS; DNAbiotech Co., Iran) was injected in three boosters with complete and incomplete Freund's adjuvant in the control group. The injection was performed peritoneally in the left and right flanks of the mice. Before each injection, blood was taken from two mice from the test and control groups via a capillary tube of the left eye to track the antibodies produced in the mice's bodies against cGRP78 using the enzyme-linked immunosorbent assay (ELISA) technique. After finishing the vaccination of mice, 4 mice from each group were used to collect blood and isolate serum to perform the humoral immunity test against cancer cells. Moreover, 4 mice were sacrificed and their spleens were used to perform the cellular immune response test against cancer cells.
Evaluation of the immunized mice serum response to different cancer cells
Antibodies against cGRP78 in the sera of immunized mice were assayed by ELISA. Firstly, the cGRP78 protein was prepared with a final concentration of 50 μg/mL in the coating buffer (carbonate bicarbonate buffer, pH 9.4). Then, 100 μL of it was added to each well in 96-well plates (5 μg of protein in each well). The plate was incubated at 37 °C for 16 h. After washing with PBST (PBS buffer with 0.1% between 20), 100 μL of blocking buffer (5% skim milk; Merck, Germany) in PBS buffer) was poured into each well to block the wells. Serial dilutions were prepared from the sera of immunized and control mice (1:100, 1:200, 1:500, and 1:1000) and exposed to cGRP78 for 1 h with triplicate repetitions. After washing, horseradish peroxidase (HRP) conjugated goat anti-mouse IgG (Sigma-Aldrich, USA) with a dilution of 1:50,000 was added in the wells, and 3, 3', 5,5' tetramethylbenzidine (TMB; DNAbiotech Co., Iran) was used to track the bound antibodies. At the end of the reaction, after stopping the reaction with H2SO4 (0.16 M; Merck, Germany), absorbance was measured at 450 nm with the ELISA Reader (Bio-Rad, model 680, California, USA).
After confirming the production of specific antibodies against cGRP78 recombinant protein in immunized mice, the serum of these mice was collected, and the reaction of the serum antibodies with different cancer cells was evaluated by the cell-ELISA method. Each 96-well plate was cultured with 30,000 different cancer cells, including 4T1, HepG2, A549, Hela, and HUVEC cells as a negative control, and B16F10 cells as a positive control, for 24 h. B16F10 cells have shown high surface expression of GRP78 in different references, so it is suitable as a positive control (25). Paraformaldehyde (Merck, Germany) with a concentration of 4% was added to each well and incubated for 15 min at room temperature. After collecting the medium and washing wells, a 5% skim milk (Merck, Germany) solution was used for blocking overnight at 4 °C. After washing three times, serum obtained from immunized and control mice was used with two different dilutions (1/500 and 1/1000) with three repetitions on different cancer cells, and the control cells were incubated for 1 h at 37 °C in a shaker incubator. After washing three times, 100 μL of goat anti-mouse HRP-conjugated antibody was added to the wells and incubated for another hour in a shaker incubator at 37 °C. TMB was used to evaluate the number of antibodies attached to the surface of the cells, and after stopping the reaction, the results were estimated by an ELISA reader at 450 nm.
Splenocyte proliferation response with different cancer cell antigen
The growth-stimulating potency of cancer cell antigens on cGRP78-immunized mouse spleen lymphocytes was evaluated by the MTT test. The isolated splenocytes of the vaccinated and control groups were cultured at a density of 1×105 cells per well in a 96-well plate with 20 μg/mL of 4T1, HepG2, A549, Hela, HUVEC (negative control), and B16F10 (positive control) cells antigens cultured in complete medium (26). Cancer cell antigens were prepared by freeze-thawing method (27). After 72 h, MTT solution (DNAbiotech, Iran) was added to the wells and incubated for 4 h. Then, the medium on the cells was removed, and dimethyl sulfoxide (DMSO; DNAbiotech, Iran) was used to dissolve the formazan crystals. The amount of color produced was evaluated at a wavelength of 570 nm with an ELISA reader. All experiments were performed in triplicates.
In vivo metastatic tumor treatment
According to the results of humoral and cellular immunity tests on different cancer cells, the melanoma cancer cell B16F10 was selected for an in-vivo metastasis test. Four-week-old BALB/c mice were randomly divided into test and control groups (8 mice in each group). Then, 5×104 B16F10 cells were injected into all mice through the tail vein. Immediately after the injection of cancer cells, the mice in the test group were vaccinated with the cGRP78 protein in three boosters with an interval of ten days. The control group was injected with PBS instead of cGRP78 protein. The first injection of vaccine and PBS (in the control group) was done with complete Freund's adjuvant, and the subsequent injections were done with incomplete Freund's adjuvant. After one month, all mice were sacrificed according to the Animal Welfare Guidel ines (28), and their spleen, lung, and liver were examined for black cancerous nodules of B16F10 (29).
Statistical analysis
All experiments were performed three times. Data obtained from different experiments were analyzed using a one-way ANOVA followed by Tukey post-test. Also, a significant difference between the two groups was found by Student's t-tests. All statistical analysis was performed using SPSS software (version 26.0; SPSS Inc.) and GraphPad Prism 9. P-values ≤ 0.05 were considered statistically significant.
RESULTS
Expression and purification of the cGRP78 protein
The expression of the cGRP78 protein was investigated under different conditions, and the optimal expression was obtained 16 h after induction with 0.5 mM IPTG at 30 °C. Purification of the recombinant protein was performed under native conditions using a Ni-NTA column. Native purification condition allows the resulting protein to retain its structure and do not require refolding. The purified 28-kDa band in SDS-PAGE indicates that the cGRP78 protein was obtained with appropriate purity. The western blot technique was used to confirm the obtained protein (Fig. 1A and B).
Fig. 1.
The SDS-PAGE and western blotting of cGRP78 protein. (A) Expression and purification of cGRP78 recombinant protein. Line 1: soluble fraction from protein expression in E. coli before purification; lines 2 and 3: recombinant protein cGRP78 after purification by Ni-NTA; line 4: PageRuler™ plus Prestained Protein Ladder. (B) Western blotting of cGRP78. Line 1: purified protein; line 2: Prestained Protein Ladder. SDS-PAGE, Sodium dodecyl-sulfate polyacrylamide gel electrophoresis; GRP, glucose-regulated protein 78; Ni-NTA, nickel-nitrilotriacetic acid.
Humoral immunity reaction with different cancer cells
S p ecific antibodies against recombinant cGRP78 were induced in BALB/c mice after v a ccination. As shown in Fig. 2, after three injections, the amount of antibody produced (mean of OD ~ 1.13) is significantly different from the control group (comparison of means with a t-test, P-value 0.001).
Fig. 2.

Anti-cGRP78 IgG titer after mouse immunization. OD represents the amount of produced antibody. **P < 0.01 and ***P < 0.001 indicate significant differences in comparison with the control group. GRP, glucose-regulated protein 78; OD, optical density.
In the next step, the reaction of the immunized mouse serum and the control serum after the third injection was analyzed on different cancer cells coated on the ELISA plate. Its results are shown in Fig. 3. In this test, two human cancer cells (A549 and Hela) and three mouse cancer cells (4T1, B16F10, and HepG2) were used. Examining the obtained results showed that, despite the use of human cells and the possibility of a non-specific mouse antibody reaction with these cells, the serum antibodies acted specifically and identified the cGRP78 antigen. The results of this test indicated that Hela (mean of OD ~ 0.72) and HepG2 (mean of OD ~ 0.68) cells had the highest reaction with antibodies obtained from vaccinated mice (Fig. 3). Comparison between three groups with One-way ANOVA, P-value 0.01.
Fig. 3.
Humoral immune status of immune mice against different types of cancer cells. Two human cancer cells (A549 and Hela) and three mouse cancer cells (4T1, B16F10, and HepG2) were used to examine the humoral immunity in vaccinated mice. Hela and HepG2 cells reacted the most with the serum of vaccinated mice. *P < 0.05 and **P < 0.01 indicate significant differences compared to the respective control group. OD, Optical density.
Cellular immune response and splenocyte proliferation induced by cGRP78
To evaluate the induction of cell-mediated immunity after injection of the cGRP78 vaccine, the proliferation ability of splenocytes exposed to different cancer cell antigens was investigated. Splenocytes obtained from vaccinated mice and the control group were isolated and cultured. The proliferation of splenocytes in the presence and absence of five cancer cell antigens was measured after 72 h using the MTT assay. Also, as a control group, antigens of a normal cell (HUVEC) were used instead of cancer cell antigens. The results shown in Fig. 4 confirm that the B16F10 antigens more than other cancer cell antigens increased the proliferation of splenocytes (mean of OD ~ 0.9) in vaccinated mice compared to its respective control group. Also, the comparison of the cellular immune status of vaccinated mice in the “vaccinated + antigen group” (vaccinated mouse splenocyte along with cancer antigens) and the “vaccinated -antigen group” (vaccinated mouse splenocyte in the absence of cancer antigens) showed that the proliferation of splenocytes of immunized mice in the presence of antigen was significantly higher than when there was no antigen in the environment.
Fig. 4.
Cellular immune status of vaccinated mice against different types of cancer cells in the “vaccinated + antigen group” (vaccinated mouse splenocyte along with cancer antigens) and “vaccinated -antigen group” (vaccinated mouse splenocyte in the absence of cancer antigens). The B16F10 cells' antigens more than other cancer cell antigens increased the proliferation of splenocytes in vaccinated mice. *P < 0.05, **P < 0.01, ***P < 0.001 indicates significant differences compared to the respective control and ###P < 0.001 indicates the differences between vaccinated groups. OD, Optical density.
In vivo tumor treatment experiments
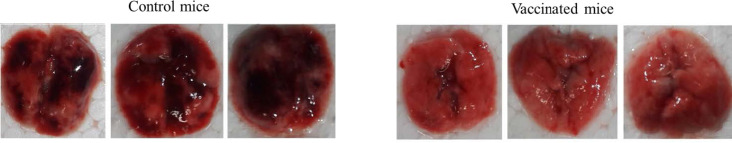
Considering that in the cellular immunity test, B16F10 cells stimulated cellular immunity more than other cancer cell lines, to evaluate the capability of the cGRP78 vaccine in the prevention of B16F10 metastasis, cancer cells were injected into the blood of cGRP78-vaccinated and control mice. The lung, liver, and spleen tissues of mice in both groups were examined for cancer nodules. The results showed that after 30 days, the growth of cancer cells was not noticeable in the lung, liver, and spleen tissues of cGRP78-vaccinated mice, but the mice in the control group contained black cancerous nodules resulting from the growth of B16F10 cells. The results of comparing the lungs of vaccinated mice and the control group are shown in Fig. 5.
Fig. 5.
Investigating lung metastasis of melanoma cells in vaccinated mice. The black nodules in the lungs of control group mice indicate the metastasis of cancer cells in the lungs.
DISCUSSION
GRP78, as a member of the HSP70 family, prevents the aggregation of unfolded proteins by binding to hydrophobic residues in the ER. Interestingly, due to the GRP78 overexpression in cancer tissues and its emergence on the surface of cancer cells, this antigen is a potential target for cancer immunotherapy (30).
In a wide range of cancers such as lung (10,20), hepatocellular (12), breast (11,19,31), cervical (13), bladder (22), melanoma (14), gastrointestinal tract (2,32), and ovary (21), overexpression of GRP78 induces resistance to numerous drug agents. Therefore, it is possible to increase the sensitivity of tumors to drug treatment through immunotherapy and by inhibiting GRP78. As a result, this method can limit the growth rate of tumors.
The production of antibodies against different epitopes of GRP78 has been done by different researchers, and the results have been reported in the literature. In a study conducted by Misra et al. antibodies were raised against the GRP78 carboxyl domain-induced apoptosis in prostate and melanoma cells (33). In another study, performed on colon and breast cancer cells, the use of the high-affinity monoclonal antib ody Mab-159 (KD = 1.7 nmol/L) against surface GRP78 inhibited tumor cell proliferation (34).
In our previous investigation, the recombinant C-terminal domain of GRP78 protein was evaluated by bioinformatics tools and produced in E. coli. Experimental evaluations of this antigen by circular dichroism and ELISA methods revealed that the two-and three-dimensional structures of both recombinant and native proteins are very similar to each other. For this reason, this truncated recombinant protein can be an interesting candidate for vaccination against cancer (21).
The humoral immunity capacity of immunized mice against various cancer antigens was tested using cell ELISA after vaccination with the cGRP78 protein. The results indicate that the cGRP78 protein has acceptable antigenicity and stimulates the immune system to produce antibodies.
Vaccination with this recombinant antigen stimulates humoral immunity against cancer cell antigens, and the serum antibodies identified A549, Hela, 4T1, B16F10, and HepG2 cell lines. It should be noted that Hela and HepG2 cells showed the highest reaction with the serum of vaccinated mice, which may be due to the high expression of GRP78 at the level of these two cell lines (19,35).
The stimulatory effect of cancer antigens on the proliferation ability of splenocytes from immunized mice was investigated after vaccination by cGRP78. This antigen significantly increased the proliferative excitability in splenocytes of the vaccinated mice compared to non-vaccinated mice. This shows the stimulating effect of the cGRP78 vaccine on the proliferation of immune cells (36). According to the results, it can be concluded that the cellular immune response reacts more to the B16F10 cancer cell line and the probability of removing this cell is higher by the cellular immune system.
Considering that the role of cellular immunity in cancer immunotherapy is more important than humoral immunity, and on the other hand, the obtained results show high cellular immunity against melanoma cells, B16F10 (a melanoma cell line) was used for in vivo testing and further investigation.
In a study by Zhang et al. four-weak B16F10 neoantigens were used as vaccine targets. These antigens fused to the diphtheria toxin, and this recombinant vaccine elicited anti-tumor CD8+ T cell responses. “DTT-neo Ag” vaccine inhibited tumor growth at a rate of 88% and 100% in the preventive and therapeutic models, respectively (37). In another study, an mRNA vaccine developed was able to significantly and specifically protect mice against B16F10 melanoma tumor progression. This mRNA vaccine elicited a cellular immune response characterized by the production of interferon-gamma and the induction of cytotoxic T lymphocytes (38). In our study, the proliferation of splenocytes in the presence of cancer cell antigens showed that the recombinant GRP78 vaccine is also able to stimulate cellular immunity. Moreover, the results of the metastasis of B16-F10 melanoma cells in our investigation showed that vaccinated mice with cGRP78 were more resistant to melanoma metastasis, and the immune systems of these animal models were more effective in inhibiting the spread of cancer cells. Among the limitations of this project, we can mention the lack of appropriate tumorigenesis in mice and the ethical problems caused by working on laboratory animals. By using tissue engineering and designing in vitro tests suitable for tumorigenesis, these problems can be solved to some extent.
CONCLUSION
The results of our studies on vaccination by cGRP78 can open a new approach to the clinical management of melanoma cancer patients. This in vivo investigation clarified that cGRP78 could be an interesting target for additional research in melanoma cancer therapy. Moreover, considering the acceptable results of this vaccine in inhibiting melanoma metastasis in a mouse model, which is caused by the effective stimulation of cellular immunity, this vaccine can be used for other cancer cell lines that have good results in stimulating cellular immunity (such as 4T1 and Hela). However, it is necessary to perform more detailed tests in vivo on other cell lines used in this research.
Conflict of interest statement
All authors declared no conflict of interest in this study.
Authors' contributions
H. Bakherad carried out most of the experi m ents including making the constructs, contributed to the design and the supervision of the study, analyzed the data and prepared the figures; H. Zare wrote the manuscript and conducted the statistical analysis; A. Nasr Esfahani carried out vaccine preparation, administration, and results; H. Aghamollaei supervised the pathologic experiments; S.L. Mousavi Gargari provided scientific comments and suggestions; M. Aliomrani provided laboratory animals. W. Ebrahimizadeh modified the text and corrected the manuscript. The finalized article has been approved by all authors.
Acknowledgments
This research was funded by the Vice-Chancellery of Research of Isfahan University of Medical Sciences Through Grant No. 198261.
REFERENCES
- 1.Gheybi E, Salmanian AH, Fooladi AAI, Salimian J, Hosseini HM, Halabian R, et al. Immunogenicity of chimeric MUC1-HER2 vaccine against breast cancer in mice. Iran J Basic Med Sci. 2018;21(1):26–32. doi: 10.22038/IJBMS.2017.25686.6335. DOI: 10.22038/IJBMS.2017.25686.6335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liu J, Fu M, Wang M, Wan D, Wei Y, Wei X. Cancer vaccines as promising immuno-therapeutics: platforms and current progress. J Hematol Oncol. 2022;15(1):1–26. doi: 10.1186/s13045-022-01247-x. 28. DOI: 10.1186/s13045-022-01247-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abbott M, Ustoyev Y. Cancer and the immune system: the history and background of immunotherapy. Semin Oncol Nurs. 2019;35(5):1–5. doi: 10.1016/j.soncn.2019.08.002. 150923. DOI: 10.1016/j.soncn.2019.08.002. [DOI] [PubMed] [Google Scholar]
- 4.Josephs DH, Bax HJ, Karagiannis SN. Tumour-associated macrophage polarisation and re-education with immunotherapy. Front Biosci (Elite Ed) 2015;7(2):293–308. doi: 10.2741/E735. DOI: 10.2741/E735. [DOI] [PubMed] [Google Scholar]
- 5.Soliman H. Developing an effective breast cancer vaccine. Cancer Control. 2010;17(3):183–190. doi: 10.1177/107327481001700307. DOI: 10.1177/107327481001700307. [DOI] [PubMed] [Google Scholar]
- 6.Lakshminarayanan V, Thompson P, Wolfert MA, Buskas T, Bradley JM, Pathangey LB, et al. Immune recognition of tumor-associated mucin MUC1 is achieved by a fully synthetic aberrantly glycosylated MUC1 tripartite vaccine. Proc Natl Acad Sci USA. 2012;109(1):261–266. doi: 10.1073/pnas.1115166109. DOI: 10.1073/pnas.1115166109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mahmood K, Jadoon S, Mahmood Q, Irshad M, Hussain J. Synergistic effects of toxic elements on heat shock proteins. Biomed Res Int. 2014;2014(564136):1–18. doi: 10.1155/2014/564136. DOI: 10.1155/2014/564136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aghamollaei H, Ghanei M, Rasaee MJ, Latifi AM, Bakherad H, Fasihi-Ramandi M, et al. Isolation and characterization of a novel nanobody for detection of GRP78 expressing cancer cells. Biotechnol Appl Biochem. 2021;68(2):239–246. doi: 10.1002/bab.1916. DOI: 10.1002/bab.1916. [DOI] [PubMed] [Google Scholar]
- 9.Farshbaf M, Khosroushahi AY, Mojarad-Jabali S, Zarebkohan A, Valizadeh H, Walker PR. Cell surface GRP78: an emerging imaging marker and therapeutic target for cancer. J Control Release. 2020;328:932–941. doi: 10.1016/j.jconrel.2020.10.055. DOI: 10.1016/j.jconrel.2020.10.055. [DOI] [PubMed] [Google Scholar]
- 10.Cai Y, Zheng Y, Gu J, Wang S, Wang N, Yang B, et al. Betulinic acid chemosensitizes breast cancer by triggering ER stress-mediated apoptosis by directly targeting GRP78. Cell Death Dis. 2018;9(6):1–16. doi: 10.1038/s41419-018-0669-8. 636. DOI: 10.1038/s41419-018-0669-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xia S, Duan W, Liu W, Zhang X, Wang Q. GRP78 in lung cancer. J Transl Med. 2021;19(1):1–14. doi: 10.1186/s12967-021-02786-6. 118. DOI: 10.1186/s12967-021-02786-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Luo C, Xiong H, Chen L, Liu X, Zou S, Guan J, et al. GRP78 Promotes hepatocellular carcinoma proliferation by increasing FAT10 expression through the NF-κB pathway. Exp Cell Res. 2018;365(1):1–11. doi: 10.1016/j.yexcr.2018.02.007. DOI: 10.1016/j.yexcr.2018.02.007. [DOI] [PubMed] [Google Scholar]
- 13.Qiu J, Zhou S, Cheng W, Luo C. LINC00294 induced by GRP78 promotes cervical cancer development by promoting cell cycle transition. Oncol Lett. 2020;20(5):1–7. doi: 10.3892/ol.2020.12125. 262. DOI: 10.3892/ol.2020.12125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gonzalez-Gronow M, Gopal U, Austin RC, Pizzo SV. Glucose-regulated protein (GRP78) is an important cell surface receptor for viral invasion, cancers, and neurological disorders. IUBMB life. 2021;73(6):843–854. doi: 10.1002/iub.2502. DOI: 10.1002/iub.2502. [DOI] [PubMed] [Google Scholar]
- 15.Gonzalez-Gronow M, Pizzo SV. Physiological roles of the autoantibodies to the 78-kilodalton glucose-regulated protein (GRP78) in cancer and autoimmune diseases. Biomedicines. 2022;10(6):1–13. doi: 10.3390/biomedicines10061222. 1222. DOI: 10.33 90/biomedicines 10061222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee CH, Tsai HY, Chen CL, Chen JL, Lu CC, Fang YP, et al. Isoliquiritigenin inhibits gastric cancer sternness, modulates tumor microenvironment, and suppresses tumor growth through glucose-regulated protein 78 downregulation. Biomedicines. 2022;10(6):1–18. doi: 10.3390/biomedicines10061350. 1350. DOI: 10.33 90/biomedicines 10061350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Albakova Z, Mangasarova Y, Albakov A, Gorenkova L. HSP70 and HSP90 in cancer: cytosolic, endoplasmic reticulum and mitochondrial chaperones of tumorigenesis. Front Oncol. 2022;12(829520):1–14. doi: 10.3389/fonc.2022.829520. DOI: 10.3389/fonc.2022.829520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gifford JB, Hill R. GRP78 influences chemoresistance and prognosis in cancer. Curr Drug Targets. 2018;19(6):701–708. doi: 10.2174/1389450118666170615100918. DOI: 10.2174/1389450118666170615100918. [DOI] [PubMed] [Google Scholar]
- 19.Du T, Li H, Fan Y, Yuan L, Guo X, Zhu Q, et al. The deubiquitylase OTUD3 stabilizes GRP78 and promotes lung tumorigenesis. Nat Commun. 2019;10(1):1–15. doi: 10.1038/s41467-019-10824-7. 2914. DOI: 10.1038/s41467-019-10824-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yao X, Liu H, Zhang X, Zhang L, Li X, Wang C, et al. Cell surface GRP78 accelerated breast cancer cell proliferation and migration by activating STAT3. PloS One. 2015;10(5):1–17. doi: 10.1371/journal.pone.0125634. e0125634. DOI: 10.1371/journal.pone.0125634. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 21.Aghamollaei H, Mousavi Gargari SL, Ghanei M, Rasaee MJ, Amani J, Bakherad H, et al. Structure prediction, expression, and antigenicity of c-terminal of GRP78. Biotechnol Appl Biochem. 2017;64(1):117–125. doi: 10.1002/bab.1455. DOI: 10.1002/bab.1455. [DOI] [PubMed] [Google Scholar]
- 22.Hernandez I, Cohen M. Linking cell-surface GRP78 to cancer: from basic research to clinical value of GRP78 antibodies. Cancer Lett. 2022;524:1–14. doi: 10.1016/j.canlet.2021.10.004. DOI: 10.1016/j.canlet.2021.10.004. [DOI] [PubMed] [Google Scholar]
- 23.Eghbali-Feriz S, Taleghani A, Al-Najjar H, Emami SA, Rahimi H, Asili J, et al. Anti-melanogenesis and anti-tyrosinase properties of Pistacia atlantica subsp. mutica extracts on B16F10 murine melanoma cells. Res Pharm Sci. 2018;13(6):533–545. doi: 10.4103/1735-5362.245965. DOI: 10.4103/1735-5362.245965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bakherad H, Setayesh N, Gargari SLM, Ebrahimizadeh W, Mavandadnejad F, Faghfuri E, et al. Expression of recombinant G-CSF receptor domains and their inhibitory role on G-CSF function. Res Pharm Sci. 2020;15(4):381–389. doi: 10.4103/1735-5362.293516. DOI: 10.4103/1735-5362.293516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.de Ridder GG, Gonzalez-Gronow M, Ray R, Pizzo SV. Autoantibodies against cell-surface GRP78 promote tumor growth in a murine model of melanoma. Melanoma Res. 2011;21(1):35–43. doi: 10.1097/CMR.0b013e3283426805. DOI: 10.1097/CMR.0b013e3283426805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Göbel TW, Schneider K, Schaerer B, Mejri I, Puehler F, Weigend S, et al. IL-18 stimulates the proliferation and IFN-γ release of CD4+ T cells in the chicken: conservation of a Th1-like system in a nonmammalian species. J Immunol. 2003;171(4):1809–1815. doi: 10.4049/jimmunol.171.4.1809. DOI: 10.4049/jimmunol.171.4.1809. [DOI] [PubMed] [Google Scholar]
- 27.Herr W, Ranieri E, Olson W, Zarour H, Gesualdo L, Storkus WJ. Mature dendritic cells pulsed with freeze-thaw cell lysates define an effective in vitro vaccine designed to elicit EBV-specific CD4+ and CD8+ T lymphocyte responses. Blood. 2000;96(5):1857–1864. DOI: 10.1182/blood.V96.5.1857. [PubMed] [Google Scholar]
- 28.Ahmadi-Noorbakhsh S, Mirabzadeh Ardakani E, Sadighi J, Aldavood SJ, Farajli Abbasi M, Farzad-Mohajeri S, et al. Guideline for the care and use of laboratory animals in Iran. Lab Anim (NY) 2021;50(11):303–305. doi: 10.1038/s41684-021-00871-3. DOI: 10.1038/s41684-021-00871-3. [DOI] [PubMed] [Google Scholar]
- 29.Cheng WF, Chang MC, Sun WZ, Jen YW, Liao CW, Chen YY, et al. Fusion protein vaccines targeting two tumor antigens generate synergistic anti-tumor effects. PloS One. 2013;8(9):1–8. doi: 10.1371/journal.pone.0071216. e71216. DOI: 10.1371/journal.pone.0071216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ciocca DR, Cayado-Gutierrez N, Maccioni M, Cuello-Carrion FD. Heat shock proteins (HSPs) based anti-cancer vaccines. Curr Mol Med. 2012;12(9):1183–1197. doi: 10.2174/156652412803306684. DOI: 10.2174/156652412803306684. [DOI] [PubMed] [Google Scholar]
- 31.Parvizpour S, Razmara J, Pourseif MM, Omidi Y. In silico design of a triple-negative breast cancer vaccine by targeting cancer testis antigens. BioImpacts. 2019;9(1):45–56. doi: 10.15171/bi.2019.06. DOI: 10.15171/bi.2019.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Benedetti R, Dell'Aversana C, Giorgio C, Astorri R, Altucci L. Breast cancer vaccines: new insights. Front Endocrinol. 2017;8(270):1–7. doi: 10.3389/fendo.2017.00270. DOI: 10.3389/fendo.2017.00270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Misra UK, Mowery Y, Kaczowka S, Pizzo SV. Ligation of cancer cell surface GRP78 with antibodies directed against its COOH-terminal domain up-regulates p53 activity and promotes apoptosis. Mol Cancer Ther. 2009;8(5):1350–1362. doi: 10.1158/1535-7163.MCT-08-0990. DOI: 10.1158/1535-7163.MCT-08-0990. [DOI] [PubMed] [Google Scholar]
- 34.Liu R, Li X, Gao W, Zhou Y, Wey S, Mitra SK, et al. Monoclonal antibody against cell surface GRP78 as a novel agent in suppressing PI3K/AKT signaling, tumor growth, and metastasis. Clin Cancer Res. 2013;19(24):6802–6811. doi: 10.1158/1078-0432.CCR-13-1106. DOI: 10.1158/1078-0432.CCR-13-1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Haraguchi-Suzuki K, Kawabata-Iwakawa R, Suzuki T, Suto T, Takazawa T, Saito S. Local anesthetic lidocaine-inducible gene, growth differentiation factor-15 suppresses the growth of cancer cell lines. Sci Rep. 2022;12(1):1–14. doi: 10.1038/s41598-022-18572-3. 14520. DOI: 10.1038/s41598-022-18572-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rai R, Kennedy AL, Isingizwe ZR, Javadian P, Benbrook DM. Similarities and differences of Hsp70, hsc70, Grp78 and mortalin as cancer biomarkers and drug targets. Cells. 2021;10(11):1–19. doi: 10.3390/cells10112996. 2996. DOI: 10.3390/cells10112996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang Y, Lin Z, Wan Y, Cai H, Deng L, Li R. The Immunogenicity and anti-tumor efficacy of a rationally designed neoantigen vaccine for B16F10 mouse melanoma. Front Immunol. 2019;10(2472):1–16. doi: 10.3389/fimmu.2019.02472. DOI: 10.3389/fimmu.2019.02472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mockey M, Bourseau E, Chandrashekhar V, Chaudhuri A, Lafosse S, Le Cam E, et al. mRNA-based cancer vaccine: prevention of B16 melanoma progression and metastasis by systemic injection of MART1 mRNA histidylated lipopolyplexes. Cancer Gene Ther. 2007;14(9):802–814. doi: 10.1038/sj.cgt.7701072. DOI: 10.1038/sj.cgt.7701072. [DOI] [PubMed] [Google Scholar]