Key Points
Question
Is the novel self-management intervention for pain called Skills to Manage Pain (STOMP) efficacious in reducing chronic pain in people with HIV?
Findings
In this randomized clinical trial of 278 people with HIV and chronic pain, STOMP was associated with significantly reduced pain immediately after the intervention (Brief Pain Inventory total score mean difference, −1.25 points) with sustained improvement compared to enhanced usual care at 3 months (Brief Pain Inventory total score mean difference, −0.62 points).
Meaning
The results demonstrated STOMP as an efficacious chronic pain intervention for people with HIV with potential for widespread implementation.
Abstract
Importance
Chronic pain is a common condition for which efficacious interventions tailored to highly affected populations are urgently needed. People with HIV have a high prevalence of chronic pain and share phenotypic similarities with other highly affected populations.
Objective
To evaluate the efficacy of a behavioral pain self-management intervention called Skills to Manage Pain (STOMP) compared to enhanced usual care (EUC).
Design, Setting, and Participants
This randomized clinical trial included adults with HIV who experienced at least moderate chronic pain for 3 months or more. The study was set at the University of Alabama at Birmingham and the University of North Carolina–Chapel Hill large medical centers from August 2019 to September 2022.
Intervention
STOMP combined 1-on-1 skill-building sessions delivered by staff interventionists with group sessions co-led by peer interventionists. The EUC control group received the STOMP manual without any 1-on-1 or group instructional sessions.
Main Outcomes and Measures
The primary outcome was pain severity and the impact of pain on function, measured by the Brief Pain Inventory (BPI) summary score. The primary a priori hypothesis was that STOMP would be associated with a decreased BPI in people with HIV compared to EUC.
Results
Among 407 individuals screened, 278 were randomized to STOMP intervention (n = 139) or EUC control group (n = 139). Among the 278 people with HIV who were randomized, the mean (SD) age was 53.5 (10.0) years; 126 (45.0%) identified as female, 146 (53.0%) identified as male, 6 (2.0%) identified as transgender female. Of the 6 possible 1-on-1 sessions, participants attended a mean (SD) of 2.9 (2.5) sessions. Of the 6 possible group sessions, participants attended a mean (SD) of 2.4 (2.1) sessions. Immediately after the intervention compared to EUC, STOMP was associated with a statistically significant mean difference for the primary outcome, BPI total score: −1.25 points (95% CI, −1.71 to −0.78 points; P < .001). Three months after the intervention, the mean difference in BPI total score remained statistically significant, favoring the STOMP intervention −0.62 points (95% CI, −1.09 to −0.14 points; P = .01).
Conclusion and Relevance
The findings of this randomized clinical trial support the efficaciousness of STOMP as an intervention for chronic pain in people with HIV. Future research will include implementation studies and work to understand the optimal delivery of the intervention.
Trial Registration
ClinicalTrials.gov Identifier: NCT03692611
This randomized clinical trial assessed the efficacy of a behavioral pain self-management program compared with enhanced usual care in patients with HIV and chronic pain.
Introduction
Chronic pain is a common condition for which effective interventions tailored to highly affected populations are urgently needed.1 Chronic pain is defined as pain that persists for at least 3 months and is considered a chronic disease associated with disability and high health care utilization.2,3 It is a prevalent comorbidity in many groups with biopsychosocial complexity, including veterans, older adults, and people with serious illnesses, such as cancer and HIV.4,5,6,7
The US Department of Health and Human Services’ National Pain Strategy promotes pain self-management as key to achieving widespread pain relief and emphasizes developing pain self-management interventions in highly affected populations.8 Self-management generally is defined as the ability to manage the symptoms, treatments, consequences, and lifestyle changes for individuals living with a chronic condition.9 Self-management interventions have been widely adopted for other chronic diseases, including diabetes and hypertension.10,11 Pain self-management interventions can leverage nonclinicians and peers to address core self-management behaviors, including problem-solving, clinical decision-making, collaborating with clinicians, and taking action toward goals.12,13,14 Pain self-management has been studied for various pain syndromes with positive results, including low back pain and fibromyalgia.15,16
This study focused on people with HIV, who share similarities to other highly affected populations. Chronic pain prevalence in people with HIV is reported to be between 30% and 85% and is often attributed to musculoskeletal origins with multisite pain, or pain in at least 3 anatomical locations, being a common phenotype.4,17 People with HIV can also experience pain related to disease-modifying treatments (eg, antiretroviral therapy) or common medical comorbidities (eg, vascular disease).18 Chronic pain is especially important to address in this population given high rates of mental health and substance use comorbidities, making prescribing opioid pain medication risky.19,20
We developed a novel pain self-management intervention called Skills to Manage Pain (STOMP).21 STOMP combines 1-on-1 skill-building sessions delivered by staff interventionists with group sessions co-led by peer interventionists. Before the trial began, we hypothesized that people with HIV randomized to the STOMP intervention would have decreased average pain severity and improved function immediately after and 3 months after the intervention in comparison to the enhanced usual care (EUC) control group.
Methods
Study Design and Participants
Participants in this randomized clinical trial were recruited from clinics at the University of Alabama at Birmingham and the University of North Carolina–Chapel Hill. The study was approved by the University of Alabama at Birmingham and the University of North Carolina–Chapel Hill institutional review boards with oversight provided by a data and safety monitoring board that met 8 times between April 2020 and April 2023 composed of clinicians and researchers with expertise in pain, statistical methods, and clinical trials. The trial protocol is published elsewhere and appears in Supplement 1.22 We followed the Consolidated Standards of Reporting Trials (CONSORT) reporting guideline.23
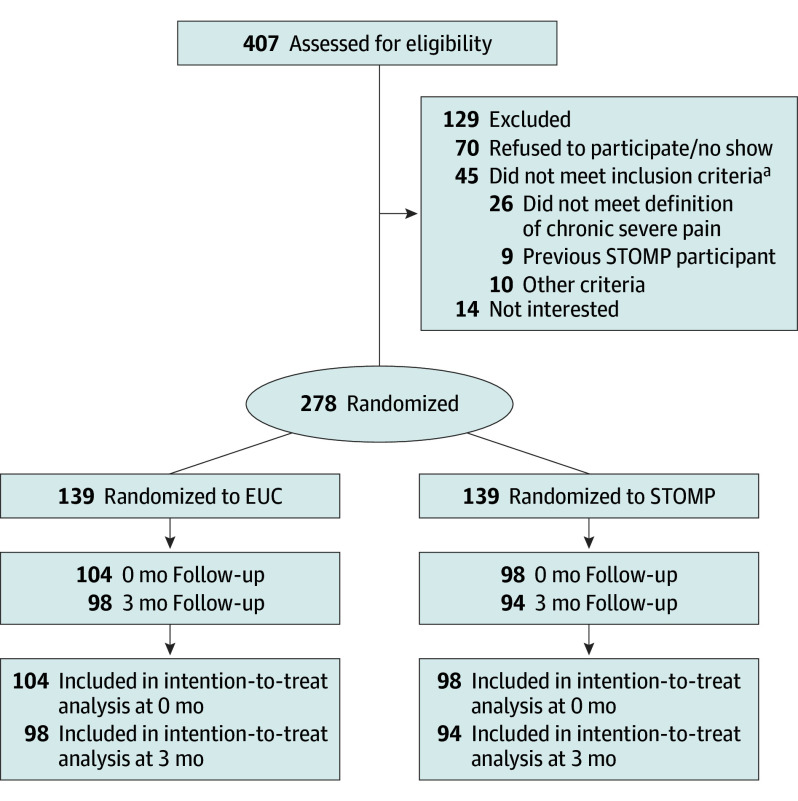
Eligible participants were people with HIV, 18 years or older, spoke English, reported at least moderate chronic pain for 3 months or more on the Brief Chronic Pain Screening Questionnaire,24 rated pain as moderately severe and impairing pleasure and enjoyment (average of all 3 items on the Pain, Enjoyment of Life and General Activity [PEG] scale was at least 4 points),25 and able to attend the group sessions with no plans for surgery during the study period. Participants who were unable to attend group sessions, previously participated in the STOMP pilot study, or did not have access to a phone were excluded from the study (Figure 1). Eligible individuals were enrolled from August 2019 to September 2022.
Figure 1. CONSORT Diagram.
Of 407 people assessed for eligibility, 278 were randomized to either the pain self-management intervention, known as Skills to Manage Pain (STOMP), or enhanced usual care (EUC). The results were assessed immediately after the intervention (0-mo time point) and 3 months after the intervention (3-mo time point).
aParticipants with HIV who were 18 years or older, spoke English, reported at least moderate chronic pain for 3 months or more impairing enjoyment of life, and were able to attend the group sessions without plans for surgery during the study period.
Randomization to Study Groups
After written informed consent was obtained, participants were randomly assigned using a 1:1 ratio to the STOMP or EUC groups. The study statistician (D.M.L.) used SAS statistical software, version 9.4 (SAS Institute), to generate the randomization scheme stratified by long-term opioid therapy at baseline (taking prescribed opioids for at least 3 months) and chronic multisite pain (pain in at least 3 locations or pain all over). Principal investigators and outcome assessors were blinded to allocation to intervention or control groups. All participants had access to all available clinical services at their study sites. Group allocations were divided into 7 blocks with each randomizing 20 participants to the intervention and 20 to EUC.
Among 407 individuals screened, 280 (32%) met eligibility and provided written informed consent, 278 were randomized to STOMP (n = 139) or EUC (n = 139), and 2 withdrew prior to randomization. Overall, 139 were recruited from the University of North Carolina–Chapel Hill, and 139 were recruited from the University of Alabama at Birmingham. All randomized participants completed baseline assessments, and 202 (73%) completed assessments immediately after the intervention (104 [74.8%] from the STOMP group vs 98 [70.5%] from the EUC group). At 3 months after the intervention, 172 (61.9%) completed assessments. Three participants randomized to STOMP were found to be ineligible postrandomization due to having participated in the pilot; they were included in data collection but did not participate in any intervention sessions. Consistent with other studies, they were recorded as having zero sessions in the analysis.26,27 No participants withdrew from the study.
STOMP Intervention Group
The novel behavioral intervention for pain called STOMP and its development are described elsewhere, and the manual appears in Supplement 2.21,28 STOMP, a pain self-management intervention based on social cognitive theory, includes alternating group and 1-on-1 sessions delivered throughout 12 weeks.22
One-on-one sessions were led by staff interventionists who were social workers and health educators trained to deliver the STOMP intervention. All participants received an initial 1-on-1 pain education session and then were asked to choose 5 of 8 remaining sessions based on topics most interesting to them. These topics were physical activity, weight loss, relaxation, sleep, building self-worth, talking with friends and family about pain, and taking opioids.
The group sessions were co-led by peer and staff interventionists. Peers were people with HIV with chronic pain with good communication and pain self-management skills who shared tips and strategies from 1-on-1 sessions and prior life experience. The first intervention session was a group in which all study participants and peers signed a confidentiality agreement. There were 6 one-hour group sessions for each 12-week intervention block.
Staff and peer interventionists attended training up-front. Both groups received training from the principal investigator (J.S.M.) and the study psychologist (W.D.) on chronic pain, pain self-management, and the intervention manual (Supplement 2). Further peer training included mock individual and group sessions and receiving parts of the intervention from staff interventionists. Staff followed a robust reminder protocol to avoid missed sessions. Peers were compensated $500 for the initial training session and an additional $1500 per 12-week block.
Fidelity to the intervention was assessed by blinded assessors (W.D. and L.B.) using the Yale Adherence and Competence Scale,29 a system for rating adherence and competence in delivering behavioral treatments. A session was considered to be conducted with fidelity if it scored a minimum of 4 out of 8 possible points in both the frequency and extensiveness and the skill level categories in each domain (eg, general aspects of sessions, such as rapport-building and empathy, and then covering content for individual sessions). Once an interventionist completed 4 of 5 consecutive sessions with fidelity, they were certified as having the competence necessary to conduct the intervention. All of the sampled intervention sessions were completed with fidelity. Periodic supervision calls with staff and peer interventionists provided ongoing opportunities for training.29
Beginning in March 2020, COVID-19–related modifications were made to the trial protocol, including pivoting all intervention procedures to a remote format (Supplement 1). At the University of Alabama at Birmingham, only the first of the 7 groups completed study procedures in person. University of North Carolina–Chapel Hill enrollment started after March 2020, thus intervention delivery occurred entirely by audio-only technology. At both sites, audio-video technology was briefly trialed but due to internet connectivity issues, it was used inconsistently by only 4 participants. All other intervention sessions were conducted via audio only after March 2020.
EUC Control Group
Recipients of EUC received the STOMP manual (Supplement 2) and a brief staff-led overview. The study did not interfere with usual care.
Outcomes and Follow-Up
Study measures were collected initially in person and then via phone beginning in March 2020 due to the COVID-19 pandemic lockdowns. Questions were read to the participants by study staff and responses were entered into REDCap.30 The choice of these measures was informed by the Initiative on Methods, Measurement, and Pain Assessment in Clinical Trials recommendation on clinically important outcomes in pain clinical trials.31 Pain measures included the Brief Pain Inventory (BPI) and PEG scale, with the BPI total score as the primary outcome.30 BPI is a 12-item questionnaire that can be reported as a summary score (BPI-total). The BPI has 2 subscales: pain severity (BPI–pain severity) and pain-related functional interference (BPI–functional interference).32 BPI items are on a scale from 0 to 10; a higher score indicates more pain severity or functional interference. The PEG scale asks 3 questions derived from the BPI about pain severity and the impact of pain on enjoyment of life and general activities.25 The BPI subscales and PEG scale results were secondary pain outcomes.
Sociodemographic information was collected at enrollment, including basic information about pain location and characteristics. Race and ethnicity data were self-reported and included as variables based on known racial disparities in HIV treatment, chronic pain care, and recruitment in clinical trials of patients with chronic pain. Before the trial began, we hypothesized that STOMP would improve self-efficacy and mood and reduce reliance on less optimal coping strategies such as pain distress (often referred to as pain catastrophizing or negative pain appraisal).33 Therefore, we measured both pain self-efficacy (Pain Self-Efficacy Questionnaire [PSEQ]),34 mood (Patient Health Questionnaire depression scale [PHQ-8]),35 and pain catastrophizing (Pain Catastrophizing Scale [PCS]),36 although the study was not powered to detect differences in these outcomes.
Statistical Power
The desired sample size was 280 participants for 85% power, 1-unit minimum detectable difference, and 25% attrition rate. The Initiative on Methods, Measurement, and Pain Assessment in Clinical Trials guidelines suggest that a change of 1 point in BPI total score is the minimum clinically significant difference,37 and the mean (SD) difference found in the pilot trial was 1 (2.4) points.21 The sample needed to be divisible by 20 because 20 participants progressed through the study simultaneously.
Adverse Events
Participants self-reported adverse events (AEs) as part of routine outcome assessments. When a participant reported an AE, they were asked if it was related to the study. Events reported by participants as related to the study were clinically adjudicated by the site principal investigator. All AEs were reported to the site institutional review board and discussed at data safety and monitoring board meetings.
Statistical Analyses
The statistical analysis plan is available in Supplement 3. The primary objective was to evaluate the efficacy of the STOMP intervention for pain severity and functional interference immediately after the intervention (ie, 3 to 4 months after completing baseline study measures). All analyses used an intention-to-treat approach using SAS.32 statistical software (SAS Institute). Linear mixed-effects models with fixed effects for treatment assignment, study visit (baseline, immediately after the intervention, and 3 months after the intervention), and their interaction were included with random effects for participants and 10-person training group. The effect of STOMP accounting for any baseline differences was estimated by predicted between-group differences (ie, least-squares mean difference) at each postintervention time point. All modeling assumptions were examined using residual analyses. No adjustments were made to P values or CIs for multiple comparisons of the primary outcome.
For missing data, prespecified multiple imputation using chained equations was used to account for missing follow-up outcomes.37 Results from all sensitivity analyses (eMethods in Supplement 4) were similar to the results from the primary analysis. We also conducted a responder analysis to assess participants who achieved at least 30% improvement in BPI total score, the conventional clinically meaningful difference in BPI score,38 from baseline to immediately after the intervention using generalized linear mixed models with similar fixed and random effects as in the primary analysis. The statistical significance threshold was P < .05 with 2-sided testing.
Results
Among the 278 people with HIV who were randomized, the mean (SD) age was 53.5 (10.0) years; 126 (45.0%) identified as female, 146 (53.0%) identified as male, 6 (2.0%) identified as transgender female; 7 (3.0%) identified as American Indian/Alaska Native or other race, 225 (81.0%) identified as Black or African American, and 46 (17.0%) identified as White. Sociodemographic characteristics, pain characteristics, long-term opioid use, and primary and secondary outcome scores were similar between the 2 groups at baseline (Table 1). At the time of enrollment, 216 (78.0%) had multisite pain, 65 (23.4%) self-reported long-term opioid use, and average pain rating as moderate to severe (mean [SD] BPI-total, 6.4 [1.1] points). The most common pain locations were 210 people (75.5%) with lower back pain, 150 (54.0%) with knee pain, and 146 (52.5%) with numbness or tingling in hands and feet.
Table 1. Baseline Characteristics for Total Sample and by Study Arm.
| Variable | No. (%) | ||
|---|---|---|---|
| All participants (N = 278) | EUC (n = 139) | STOMP (n = 139) | |
| Age, mean (SD), y | 53.5 (10.0) | 53.3 (10.4) | 53.7 (9.6) |
| Gender | |||
| Female | 126 (45) | 62 (45) | 64 (46) |
| Male | 146 (53) | 74 (53) | 72 (52) |
| Transgender female | 6 (2) | 3 (2) | 3 (2) |
| Race | |||
| Black or African American | 225 (81) | 118 (85) | 107 (77) |
| White | 46 (17) | 19 (14) | 27 (19) |
| Other or American Indian/Alaska Native | 7 (3) | 2 (1) | 5 (4) |
| Site | |||
| University of Alabama at Birmingham | 139 (50) | 72 (52) | 67 (48) |
| University of North Carolina–Chapel Hill | 139 (50) | 67 (48) | 72 (52) |
| Ethnicity | |||
| Hispanic | 3 (1) | 1 (1) | 2 (1) |
| Non-Hispanic | 275 (99) | 138 (99) | 137 (99) |
| Detectable viral load, >200 copies/mL | |||
| No | 236 (94.4) | 116 (93.5) | 120 (95.2) |
| Yes | 14 (5.6) | 8 (6.5) | 6 (4.8) |
| Are you currently taking any anti-HIV medications? | |||
| No | 6 (2) | 2 (1) | 4 (3) |
| Yes | 271 (98) | 136 (99) | 135 (97) |
| BPI total score, mean (SD)a | 6.4 (1.7) | 6.4 (1.7) | 6.3 (1.7) |
| BPI pain severity subscore, mean (SD)a | 6.5 (1.8) | 6.6 (1.8) | 6.4 (1.8) |
| BPI functional interference subscore, mean (SD)a | 6.4 (2.2) | 6.4 (2.3) | 6.5 (2.2) |
| Have you been taking an opioid or narcotic for pain for 3 mo or more? | |||
| No | 213 (76.6) | 107 (77.0) | 106 (76.3) |
| Yes | 65 (23.4) | 32 (23.0) | 33 (23.7) |
| Multisite pain (>3 sites of pain) | |||
| No | 62 (22) | 30 (22) | 32 (23) |
| Yes | 216 (78) | 109 (78) | 107 (77) |
| Pain locations | |||
| Numbness or tingling in hands and/or feet | 146 (52.5) | 71 (51.1) | 75 (54.0) |
| Headache | 82 (29.5) | 43 (30.9) | 39 (28.1) |
| Abdominal | 61 (21.9) | 30 (21.6) | 31 (22.3) |
| Lower back | 210 (75.5) | 101 (72.7) | 109 (78.4) |
| Hip | 131 (47.1) | 67 (48.2) | 64 (46.0) |
| Shoulder | 121 (43.5) | 57 (41.0) | 64 (46.0) |
| Knee | 150 (54.0) | 75 (54.0) | 75 (54.0) |
| Pain everywhere in your body | 56 (20.1) | 28 (20.1) | 28 (20.1) |
| Other | 135 (48.6) | 70 (50.4) | 65 (46.8) |
| Scores of secondary outcome measures | |||
| PEG scalea | 7.5 (1.6) | 7.5 (1.7) | 7.4 (1.6) |
| PHQ-8b | 9.1 (5.8) | 9.1 (6.1) | 9.1 (5.6) |
| PCSc | 39.8 (13.7) | 39.9 (14.0) | 39.8 (13.5) |
| PTSD screeningd | 1.4 (1.6) | 1.6 (1.6) | 1.3 (1.5) |
| PSEQe | 33.6 (14.5) | 32.5 (15.4) | 34.6 (13.4) |
| AUDIT-Cf | |||
| No risk | 259 (93.2) | 127 (91.4) | 132 (95.0) |
| Low risk | 17 (6.1) | 11 (7.9) | 6 (4.3) |
| Medium risk | 2 (0.7) | 1 (0.7) | 1 (0.7) |
| Cocaine use history | |||
| None | 128 (46.0) | 58 (42.4) | 69 (49.6) |
| Past use | 144 (51.8) | 78 (56.1) | 66 (47.5) |
| Current use | 6 (2.2) | 2 (1.4) | 4 (2.9) |
Abbreviations: AUDIT-C, Alcohol Use Disorders Identification Test–Concise; BPI, Brief Pain Inventory; EUC, enhanced usual care; PCS, Pain Catastrophizing Scale; PEG, Pain, Enjoyment of Life and General Activity; PHQ-8, Patient Health Questionnaire depression scale; PSEQ, Pain Self-Efficacy Questionnaire; PTSD, posttraumatic stress disorder; STOMP, Skills to Manage Pain.
Scores ranged from 0 to 10 points. BPI measured pain severity and functional interference. The PEG scale measured pain severity and enjoyment of activities.
Scale measured depressive symptoms and ranged from 0 to 24 points.
Scale measured pain catastrophizing symptoms and ranged from 0 to 52 points.
Scale measured PTSD symptoms and ranged from 0 to 4 points.
Scale measured self-efficacy and ranged from 0 to 60 points.
Scale measured risk for alcohol use disorder. For male respondents, high risk was indicated by a score greater than 5. For female respondents, high risk was indicated by a score greater than 4.
Information about intervention adherence can be found in eTable 1 in Supplement 4. Of the 6 possible 1-on-1 sessions, participants attended a mean (SD) of 2.9 (2.5) sessions. Of the 6 possible group sessions, participants attended a mean (SD) of 2.4 (2.1) sessions.
Primary Outcome
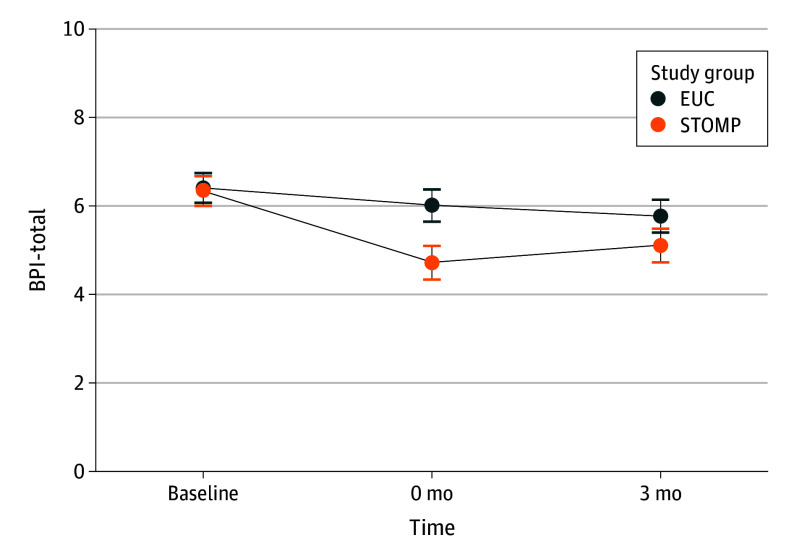
Immediately after the intervention, STOMP was associated with a statistically significant mean difference for the primary outcome, BPI-total compared to EUC: −1.25 (95% CI, −1.71 to −0.78; P < .001). Three months after the intervention, the mean difference in BPI-total remained statistically significant, favoring the STOMP intervention: −0.62 (95% CI, −1.09 to −0.14; P = .01) (Figure 2)
Figure 2. Brief Pain Inventory Total Scores (BPI-Total) Throughout Trial.
The BPI-total values were measured from before the intervention (baseline) to immediately after the intervention (0 mo) to 3 months after the intervention (3 mo) for both the Skills to Manage Pain (STOMP) intervention group and the enhanced usual care (EUC) control group.
Secondary Outcomes
Differences were also observed in the secondary pain outcomes immediately after the intervention favoring the STOMP intervention: BPI–pain severity: −1.10 (95% CI, −1.60 to −0.61; P < .001); BPI–functional interference: −1.52 (95% CI, −2.12 to −0.91; P < .001); PEG: −1.34 (95% CI, −1.86 to −0.82; P < .001); PHQ-8: −2.27 (95% CI, −3.39 to −1.14; P < .001); PSEQ: 4.10 (95% CI, 0.74 to 7.45; P = .02); and PCS: −4.22 (95% CI, −7.18 to −1.27; P = .005) (Table 2; eFigure in Supplement 4).
Table 2. Least-Squares Means of Primary and Secondary Outcome Measures and Between-Group Differences.
| Outcome | Time point | Least-squares mean (SD) | Between-group mean difference (95% CI) | P value | ||
|---|---|---|---|---|---|---|
| All participants | EUC | STOMP | ||||
| BPI total scorea | Baseline | 6.37 (0.13) | NA | NA | NA | NA |
| Immediately postintervention | NA | 5.99 (0.19) | 4.74 (0.19) | −1.25 (−1.71 to −0.78) | <.001 | |
| 3-mo Follow-up | NA | 5.74 (0.19) | 5.13 (0.19) | −0.62 (−1.09 to −0.14) | .01 | |
| BPI pain severity subscorea | Baseline | 6.48 (0.14) | NA | NA | NA | NA |
| Immediately postintervention | NA | 6.23 (0.19) | 5.13 (0.2) | −1.10 (−1.60 to −0.61) | <.001 | |
| 3-mo Follow-up | NA | 6.11 (0.2) | 5.25 (0.2) | −0.86 (−1.37 to −0.36) | .001 | |
| BPI–functional interference subscorea | Baseline | 6.43 (0.15) | NA | NA | NA | NA |
| Immediately postintervention | NA | 5.93 (0.23) | 4.41 (0.23) | −1.52 (−2.12 to −0.91) | <.0001 | |
| 3-mo Follow-up | NA | 5.57 (0.23) | 4.92 (0.24) | −0.65 (−1.27 to −0.03) | .04 | |
| PEG scalea | Baseline | 7.46 (0.14) | NA | NA | NA | NA |
| Immediately postintervention | NA | 6.94 (0.2) | 5.61 (0.21) | −1.34 (−1.86 to −0.82) | <.001 | |
| 3-mo Follow-up | NA | 6.78 (0.21) | 5.68 (0.21) | −1.10 (−1.63 to −0.57) | <.001 | |
| PSEQb | Baseline | 33.56 (0.87) | NA | NA | NA | NA |
| Immediately postintervention | NA | 36.44 (1.28) | 40.53 (1.31) | 4.10 (0.74 to 7.45) | .02 | |
| 3-mo Follow-up | NA | 35.91 (1.31) | 40.73 (1.33) | 4.82 (1.38 to 8.26) | .01 | |
| PHQ-8c | Baseline | 9.1 (0.35) | NA | NA | NA | NA |
| Immediately postintervention | NA | 8.66 (0.47) | 6.39 (0.48) | −2.27 (−3.39 to −1.14) | <.001 | |
| 3-mo Follow-up | NA | 7.69 (0.48) | 7.25 (0.48) | −0.44 (−1.59 to 0.71) | .45 | |
| PCSd | Baseline | 39.84 (1.18) | NA | NA | NA | NA |
| Immediately postintervention | NA | 38.57 (1.44) | 34.35 (1.46) | −4.22 (−7.18 to −1.27) | .005 | |
| 3-mo Follow-up | NA | 35.99 (1.46) | 34.24 (1.48) | −1.76 (−4.77 to 1.26) | .25 | |
Abbreviations: BPI, Brief Pain Inventory; EUC, enhanced usual care; NA, not applicable; PCS, Pain Catastrophizing Scale; PEG, Pain, Enjoyment of Life and General Activity; PHQ-8, Patient Health Questionnaire depression scale; PSEQ, Pain Self-Efficacy Questionnaire; STOMP, Skills to Manage Pain.
Scores ranged from 0 to 10 points. BPI measured pain severity and functional interference. The PEG scale measured pain severity and enjoyment of activities.
Scale measured self-efficacy and ranged from 0 to 60 points.
Scale measured depressive symptoms and ranged from 0 to 24 points.
Scale measured pain catastrophizing symptoms and ranged from 0 to 52 points.
At 3 months between-group differences persisted for the following secondary outcomes: BPI-pain severity: −0.86 (95% CI, −1.37 to −0.36; P = .001); BPI–functional interference: −0.65 (95% CI, −1.27 to −0.03; P = .04); PEG: −1.10 (95% CI, −1.63 to −0.57; P < .001); and PSEQ: 4.82 (95% CI, 1.38 to 8.26; P = .01). Between-group differences at 3 months were no longer significant for pain catastrophizing (PCS) or mood (PHQ-8). (Table 2).
In the responder analysis, STOMP had a significantly higher response rate (>30% improvement in BPI total score) compared to EUC (21 of 104 [37%] vs 21 of 98 [20%]; P = .01) immediately after the intervention with a sustained BPI improvement at 3 months (Table 3). The EUC group demonstrated improved pain scores during the study period.
Table 3. Proportion of Participants Who Reported Improvement in BPI Total Score by Study Arm and Between-Group Differences.
| >30% Reduction in BPI-total | No./total No. (%) | Between-group mean difference (95% CI) | |
|---|---|---|---|
| EUC | STOMP | ||
| Baseline | 0/139 (0) | 0/139 (0) | NA |
| Immediately postintervention | 21/104 (20) | 36/98 (37) | 2.36 (1.15-4.86) |
| 3-mo Follow-up | 21/98 (21) | 50/94 (53) | 1.72 (0.82-3.60) |
Abbreviations: BPI-total, Brief Pain Inventory total score; EUC, enhanced usual care; NA, not applicable; STOMP, Skills to Manage Pain.
Adverse Events
A total of 187 AEs were reported during the study in both the intervention and EUC groups (eTable 2 in Supplement 4). The majority of AEs were grouped under the categories of illness (eg, urinary tract or respiratory infection, COVID-19 infection, heart failure), injury (eg, car crash, fall), or surgery (eg, joint replacement, biopsy, amputation). Two severe AEs occurred in the EUC group requiring hospitalization Participants deemed these events related to the study (fall during the enrollment visit and surgery); however, the severe AEs were later adjudicated by the site principal investigator as unrelated to the study. All other AEs were deemed unrelated to the study.
Discussion
Among people with HIV and chronic pain, those who received the STOMP intervention demonstrated meaningful improvements in average pain ratings compared to EUC in this randomized clinical trial. Immediately after the intervention, the mean difference between the STOMP and EUC groups for all pain outcomes was greater than 1 point on a scale of 0 to 10, the metric of clinical significance in pain clinical trials.37 There were sustained but attenuated statistically significant differences at 3 months for pain outcomes. This suggests STOMP may be similarly efficacious as pharmacologic treatments for chronic pain but given its nature as a behavioral intervention may also be safer.39,40,41 Pain self-efficacy improved immediately after the intervention and 3 months after the intervention, indicating the intervention worked as designed. STOMP has the potential to improve the lives of people with HIV with chronic pain.
Although other pain self-management studies have included peers, these interventions did not produce clinically significant results.42,43,44 STOMP is the first peer-involved pain self-management intervention to our knowledge that produced a clinically meaningful result. This milestone could be explained by several possible reasons. First, peers participated in extensive training and received compensation; lack of peer compensation has been problematic for peer retention in other pain self-management studies.42,43 Second, peer-led sessions may be especially important due to a host of biopsychosocial challenges (eg, stigma, substance use, and racism) faced by people with HIV, leading to worse chronic pain outcomes and social isolation.45
STOMP focused on pain self-management as an essential behavioral approach, but it is not the only behavioral approach. HIV-PASS is a behavioral intervention for people with HIV who have both chronic pain and depression.46 Although STOMP did not specifically focus on individuals with mental health comorbidities, baseline PHQ-8 scores (mean [SD], 9.1 [5.8] points) were consistent with mild-to-moderate depressive symptoms. HIV-PASS was found to decrease the BPI functional interference score by an average of 1.3 points immediately after the intervention. However, it is a high-intensity intervention with up-front in-person meetings with the patient, primary care physician, and a behavioral health specialist,46,47 which affects its implementation potential. Also, no changes were seen in HIV-PASS secondary outcomes, and changes in pain did not persist beyond the immediate postintervention intervention period. Regardless, multiple behavioral interventions are absolutely essential to the field and should be available for use and adaptation.
On average, STOMP demonstrated patient-reported benefits, despite most participants attending fewer than half of the group and 1-on-1 sessions, with 34 participants (24%) attending no sessions. Although adherence was low and small differences were attenuated over time, we hypothesized that the combination of 1-on-1 and group sessions plus our intensive intervention session reminder protocol was sufficient for short-term behavioral change. For long-term behavioral maintenance, different approaches may be needed.48 We speculated that lower-than-anticipated adherence may have resulted from study procedures during the COVID-19 pandemic when maintaining and addressing medical needs in people with HIV was particularly challenging.49 Nonetheless, given the findings, future research should aim to identify the optimal and minimally effective number of STOMP sessions and the specific combination regimen of individual and group sessions.
Strengths and Limitations
The primary strength of the STOMP trial was that it was a randomized clinical trial of chronic pain in a large group of people with HIV.46,50,51 Participants were recruited from 2 different sites with few restrictions on enrollment (ie, all pain types were considered for enrollment) and no limitations on age, enhancing generalizability. Finally, the intervention was delivered with high fidelity, most likely due to close adherence to the study protocol, use of the STOMP manual, and rigorous training of peer and staff interventionists.
The primary limitation of the STOMP trial was that its patient population was very specific—people with HIV and chronic pain—which may have decreased the generalizability of the findings to patients with other conditions. In addition, we reported immediate and 3-month postintervention results, which is a short follow-up period and a common criticism of chronic pain trials.52,53 The study sample was also less than half women (45%), likely because HIV is more common in men; therefore, the results may not generalize easily to other chronic pain populations in which women are more prevalent.54 STOMP delivery precluded participants from being blinded, although study staff and investigators remained blinded until study completion.
Conclusions
The findings of this randomized clinical trial demonstrated that STOMP may be an efficacious chronic pain intervention for people with HIV. The STOMP intervention also has the potential to be tailored to other highly affected groups including cancer survivors, older adults, or veterans who frequently experience chronic multisite pain. Implementation studies and research on the optimal delivery of STOMP will be helpful for future use of this efficacious intervention for chronic pain.
Trial Protocol
STOMP Manual
Statistical Analysis Plan
eMethods. Multiple Imputation
eTable 1. STOMP Intervention Adherence
eTable 2. Adverse Events (AEs)
eFigure. Secondary Outcomes from Baseline to Immediately Postintervention (0 Months) and 3 Months
Data Sharing Statement
References
- 1.Institute of Medicine Committee on Advancing Pain Research, Care, and Education . Relieving Pain in America: A Blueprint for Transforming Prevention, Care, Education, and Research. National Academies Press;2011. [PubMed] [Google Scholar]
- 2.Treede RD, Rief W, Barke A, et al. Chronic pain as a symptom or a disease: the IASP Classification of Chronic Pain for the International Classification of Diseases (ICD-11). Pain. 2019;160(1):19-27. doi: 10.1097/j.pain.0000000000001384 [DOI] [PubMed] [Google Scholar]
- 3.Cohen SP, Vase L, Hooten WM. Chronic pain: an update on burden, best practices, and new advances. Lancet. 2021;397(10289):2082-2097. doi: 10.1016/S0140-6736(21)00393-7 [DOI] [PubMed] [Google Scholar]
- 4.Madden VJ, Parker R, Goodin BR. Chronic pain in people with HIV: a common comorbidity and threat to quality of life. Pain Manag. 2020;10(4):253-260. doi: 10.2217/pmt-2020-0004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Miaskowski C, Blyth F, Nicosia F, et al. A biopsychosocial model of chronic pain for older adults. Pain Med. 2019. [DOI] [PubMed] [Google Scholar]
- 6.Baria AM, Pangarkar S, Abrams G, Miaskowski C. Adaption of the biopsychosocial model of chronic noncancer pain in veterans. Pain Med. 2019;20(1):14-27. doi: 10.1093/pm/pny058 [DOI] [PubMed] [Google Scholar]
- 7.Glare P, Aubrey K, Gulati A, Lee YC, Moryl N, Overton S. Pharmacologic management of persistent pain in cancer survivors. Drugs. 2022;82(3):275-291. doi: 10.1007/s40265-022-01675-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.National Institute of Health; Interagency Pain Research Coordinating Committee . Federal Pain Research Strategy. Accessed on December 1, 2023. https://www.iprcc.nih.gov/sites/default/files/documents/FPRS_Research_Recommendations_Final_508C.pdf
- 9.Dwarswaard J, Bakker EJ, van Staa A, Boeije HR. Self-management support from the perspective of patients with a chronic condition: a thematic synthesis of qualitative studies. Health Expect. 2016;19(2):194-208. doi: 10.1111/hex.12346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li R, Liang N, Bu F, Hesketh T. The effectiveness of self-management of hypertension in adults using mobile health: systematic review and meta-analysis. JMIR Mhealth Uhealth. 2020;8(3):e17776. doi: 10.2196/17776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shrivastava SR, Shrivastava PS, Ramasamy J. Role of self-care in management of diabetes mellitus. J Diabetes Metab Disord. 2013;12(1):14. doi: 10.1186/2251-6581-12-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Darnall BD, Roy A, Chen AL, et al. Comparison of a single-session pain management skills intervention with a single-session health education intervention and 8 sessions of cognitive behavioral therapy in adults with chronic low back pain: a randomized clinical trial. JAMA Netw Open. 2021;4(8):e2113401-e2113401. doi: 10.1001/jamanetworkopen.2021.13401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Azizoddin DR, Adam R, Kessler D, et al. Leveraging mobile health technology and research methodology to optimize patient education and self-management support for advanced cancer pain. Support Care Cancer. 2021;29(10):5741-5751. doi: 10.1007/s00520-021-06146-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Matthias MS, Daggy J, Adams J, et al. Evaluation of a peer coach-led intervention to improve pain symptoms (ECLIPSE): rationale, study design, methods, and sample characteristics. Contemp Clin Trials. 2019;81:71-79. doi: 10.1016/j.cct.2019.04.002 [DOI] [PubMed] [Google Scholar]
- 15.Geraghty AWA, Maund E, Newell D, et al. Self-management for chronic widespread pain including fibromyalgia: a systematic review and meta-analysis. PLoS One. 2021;16(7):e0254642. doi: 10.1371/journal.pone.0254642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sandal LF, Bach K, Øverås CK, et al. Effectiveness of app-delivered, tailored self-management support for adults with lower back pain-related disability: a selfBACK randomized clinical trial. JAMA Intern Med. 2021;181(10):1288-1296. doi: 10.1001/jamainternmed.2021.4097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Merlin JS, Westfall AO, Heath SL, et al. Brief report: IL-1β levels are associated with chronic multisite pain in people living with HIV. J Acquir Immune Defic Syndr. 2017;75(4):e99-e103. doi: 10.1097/QAI.0000000000001377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jiao JM, So E, Jebakumar J, George MC, Simpson DM, Robinson-Papp J. Chronic pain disorders in HIV primary care: clinical characteristics and association with healthcare utilization. Pain. 2016;157(4):931-937. doi: 10.1097/j.pain.0000000000000462 [DOI] [PubMed] [Google Scholar]
- 19.Nkhoma K, Norton C, Sabin C, Winston A, Merlin J, Harding R. Self-management interventions for pain and physical symptoms among people living with HIV: a systematic review of the evidence. J Acquir Immune Defic Syndr. 2018;79(2):206-225. doi: 10.1097/QAI.0000000000001785 [DOI] [PubMed] [Google Scholar]
- 20.Merlin JS, Long D, Becker WC, et al. Brief report: the association of chronic pain and long-term opioid therapy with HIV treatment outcomes. J Acquir Immune Defic Syndr. 2018;79(1):77-82. doi: 10.1097/QAI.0000000000001741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Merlin JS, Westfall AO, Long D, et al. A randomized pilot trial of a novel behavioral intervention for chronic pain tailored to individuals with HIV. AIDS Behav. 2018;22(8):2733-2742. doi: 10.1007/s10461-018-2028-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jones KF, Bair MJ, Orris SM, et al. Evaluation of the efficacy and mechanisms of a novel intervention for chronic pain tailored to people with HIV: The STOMP protocol. Contemp Clin Trials. 2023;129:107163. doi: 10.1016/j.cct.2023.107163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Merkow RP, Kaji AH, Itani KMF. The CONSORT Framework. JAMA Surg. 2021;156(9):877-878. doi: 10.1001/jamasurg.2021.0549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Merlin JS, Walcott MM, Herbey I, et al. Qualitative investigation of a Brief Chronic Pain Screening tool in HIV-infected patients. AIDS Patient Care STDS. 2014;28(4):176-182. doi: 10.1089/apc.2014.0006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Krebs EE, Lorenz KA, Bair MJ, et al. Development and initial validation of the PEG, a three-item scale assessing pain intensity and interference. J Gen Intern Med. 2009;24(6):733-738. doi: 10.1007/s11606-009-0981-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ferrand RA, McHugh G, Rehman AM, et al. ; BREATHE Trial Group . Effect of once-weekly azithromycin vs placebo in children with HIV-associated chronic lung disease: the BREATHE randomized clinical trial. JAMA Netw Open. 2020;3(12):e2028484. doi: 10.1001/jamanetworkopen.2020.28484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rehman AM, Ferrand R, Allen E, Simms V, McHugh G, Weiss HA. Exclusion of enrolled participants in randomised controlled trials: what to do with ineligible participants. BMJ Open. 2020;10(12):e039546. doi: 10.1136/bmjopen-2020-039546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Merlin JS, Young SR, Johnson MO, et al. Intervention mapping to develop a social cognitive theory-based intervention for chronic pain tailored to individuals with HIV. Contemp Clin Trials Commun. 2018;10:9-16. doi: 10.1016/j.conctc.2018.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Carroll KM, Nich C, Sifry RL, et al. A general system for evaluating therapist adherence and competence in psychotherapy research in the addictions. Drug Alcohol Depend. 2000;57(3):225-238. doi: 10.1016/S0376-8716(99)00049-6 [DOI] [PubMed] [Google Scholar]
- 30.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377-381. doi: 10.1016/j.jbi.2008.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Smith SM, Dworkin RH, Turk DC, et al. Interpretation of chronic pain clinical trial outcomes: IMMPACT recommended considerations. Pain. 2020;161(11):2446-2461. doi: 10.1097/j.pain.0000000000001952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Atkinson TM, Mendoza TR, Sit L, et al. The Brief Pain Inventory and its “pain at its worst in the last 24 hours” item: clinical trial endpoint considerations. Pain Med. 2010;11(3):337-346. doi: 10.1111/j.1526-4637.2009.00774.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Crombez G, De Paepe AL, Veirman E, Eccleston C, Verleysen G, Van Ryckeghem DML. Let’s talk about pain catastrophizing measures: an item content analysis. PeerJ. 2020;8:e8643. doi: 10.7717/peerj.8643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nicholas MK. The pain self-efficacy questionnaire: taking pain into account. Eur J Pain. 2007;11(2):153-163. doi: 10.1016/j.ejpain.2005.12.008 [DOI] [PubMed] [Google Scholar]
- 35.Kroenke K, Strine TW, Spitzer RL, Williams JB, Berry JT, Mokdad AH. The PHQ-8 as a measure of current depression in the general population. J Affect Disord. 2009;114(1-3):163-173. doi: 10.1016/j.jad.2008.06.026 [DOI] [PubMed] [Google Scholar]
- 36.Sullivan MJ, Bishop SR, Pivik J. The Pain Catastrophizing Scale: development and validation. Psychol Assess. 1995;7(4):524. doi: 10.1037/1040-3590.7.4.524 [DOI] [Google Scholar]
- 37.Li P, Stuart EA, Allison DB. Multiple imputation: a flexible tool for handling missing data. JAMA. 2015;314(18):1966-1967. doi: 10.1001/jama.2015.15281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dworkin RH, Turk DC, Farrar JT, et al. ; IMMPACT . Core outcome measures for chronic pain clinical trials: IMMPACT recommendations. Pain. 2005;113(1-2):9-19. doi: 10.1016/j.pain.2004.09.012 [DOI] [PubMed] [Google Scholar]
- 39.Arnold LM, Lu Y, Crofford LJ, et al. A double-blind, multicenter trial comparing duloxetine with placebo in the treatment of fibromyalgia patients with or without major depressive disorder. Arthritis Rheum. 2004;50(9):2974-2984. doi: 10.1002/art.20485 [DOI] [PubMed] [Google Scholar]
- 40.Jones K, Wechsler S, Zulewski D, Wood L. Pharmacological and nonpharmacological management of chemotherapy-induced peripheral neuropathy: a scoping review of randomized controlled trials. J Palliat Med. 2022;25(6):964-995. doi: 10.1089/jpm.2021.0512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Krebs EE, Gravely A, Nugent S, et al. Effect of opioid vs nonopioid medications on pain-related function in patients with chronic back pain or hip or knee osteoarthritis pain: the SPACE randomized clinical trial. JAMA. 2018;319(9):872-882. doi: 10.1001/jama.2018.0899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Matthias MS, Bair MJ, Ofner S, et al. Peer support for self-management of chronic pain: the evaluation of a peer coach-led intervention to improve pain symptoms (ECLIPSE) trial. J Gen Intern Med. 2020;35(12):3525-3533. doi: 10.1007/s11606-020-06007-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Matthias MS, Hirsh AT, Ofner S, Daggy J. Exploring the relationships among social support, patient activation, and pain-related outcomes. Pain Med. 2022;23(4):676-685. doi: 10.1093/pm/pnab306 [DOI] [PubMed] [Google Scholar]
- 44.Crotty M, Prendergast J, Battersby MW, et al. Self-management and peer support among people with arthritis on a hospital joint replacement waiting list: a randomised controlled trial. Osteoarthritis Cartilage. 2009;17(11):1428-1433. doi: 10.1016/j.joca.2009.05.010 [DOI] [PubMed] [Google Scholar]
- 45.Merlin JS, Young SR, Johnson MO, et al. Using patient perspectives to inform the development of a behavioral intervention for chronic pain in patients with HIV: a qualitative study. Pain Med. 2017;18(5):879-888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Uebelacker LA, Pinkston MM, Busch AM, et al. HIV-PASS (Pain and Sadness Support): randomized controlled trial of a behavioral health intervention for interference due to pain in people living with HIV, chronic pain, and depression. Psychosom Med. 2023;85(3):250-259. doi: 10.1097/PSY.0000000000001172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Uebelacker LA, Weisberg RB, Herman DS, et al. Pilot randomized trial of collaborative behavioral treatment for chronic pain and depression in persons living with HIV/AIDS. AIDS Behav. 2016;20(8):1675-1681. doi: 10.1007/s10461-016-1397-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kwasnicka D, Dombrowski SU, White M, Sniehotta F. Theoretical explanations for maintenance of behaviour change: a systematic review of behaviour theories. Health Psychol Rev. 2016;10(3):277-296. doi: 10.1080/17437199.2016.1151372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jiang H, Zhou Y, Tang W. Maintaining HIV care during the COVID-19 pandemic. Lancet HIV. 2020;7(5):e308-e309. doi: 10.1016/S2352-3018(20)30105-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Scott W, Arkuter C, Kioskli K, et al. Psychosocial factors associated with persistent pain in people with HIV: a systematic review with meta-analysis. Pain. 2018;159(12):2461-2476. doi: 10.1097/j.pain.0000000000001369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nkhoma K, Norton C, Sabin C, Winston A, Merlin J, Harding R. Self-management Interventions for Pain and Physical Symptoms Among People Living With HIV: A Systematic Review of the Evidence. J Acquir Immune Defic Syndr. 2018;79(2):206-225. doi: 10.1097/QAI.0000000000001785 [DOI] [PubMed] [Google Scholar]
- 52.Garcia LM, Birckhead BJ, Krishnamurthy P, et al. Three-month follow-up results of a double-blind, randomized placebo-controlled trial of 8-week self-administered at-home behavioral skills-based virtual reality (VR) for chronic low back pain. J Pain. 2022;23(5):822-840. doi: 10.1016/j.jpain.2021.12.002 [DOI] [PubMed] [Google Scholar]
- 53.Hemkens LG. How routinely collected data for randomized trials provide long-term randomized real-world evidence. JAMA Netw Open. 2018;1(8):e186014. doi: 10.1001/jamanetworkopen.2018.6014 [DOI] [PubMed] [Google Scholar]
- 54.Povshedna T, Swann SA, Levy SLA, et al. Global prevalence of chronic pain in women with HIV: a systematic review and meta-analysis. Open Forum Infect Dis. 2023;10(8):ofad350. doi: 10.1093/ofid/ofad350 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
STOMP Manual
Statistical Analysis Plan
eMethods. Multiple Imputation
eTable 1. STOMP Intervention Adherence
eTable 2. Adverse Events (AEs)
eFigure. Secondary Outcomes from Baseline to Immediately Postintervention (0 Months) and 3 Months
Data Sharing Statement