Abstract
Introduction
Africa exhibits a considerably high prevalence of the hepatitis B virus among pregnant women. Furthermore, there is a discernible lack of a well-established surveillance system to adequately monitor and comprehend the epidemiology of the hepatitis B virus, particularly among pregnant women. The eradication efforts of the virus in Africa have been impeded by the significant disease burden in the region, and there is a lack of evidence regarding the pooled prevalence of the hepatitis B virus in Africa. Consequently, this systematic review and meta-analysis aims to determine the prevalence of hepatitis B virus infection among pregnant women in Africa.
Methods
We conducted a systematic literature search using reputable databases such as PubMed, Advanced Google Scholar, Scopus, and the Cochrane Library. The search spanned from July 2013 to July 2023 and included all relevant articles published within this period. To identify potentially eligible articles, we conducted a comprehensive manual review of the reference lists of the identified studies. Our review encompassed articles from the African Journal Online. The analysis focused on observational studies published in peer-reviewed journals that reported the prevalence of hepatitis B surface antigen-positive testing among pregnant women. We utilized the Newcastle-Ottawa critical appraisal checklist to assess the methodological quality of each paper. Finally, a meta-analysis was conducted using a random-effects model.
Results
Out of the 774 studies identified, 31 studies involving 33,967 pregnant women were selected for the meta-analysis. According to the random-effects model, the combined prevalence of hepatitis B virus among pregnant women was 6.77% [95% CI: 5.72, 7.83]. The I2 statistic was calculated to be 95.57% (p = 0.00), indicating significant heterogeneity among the studies. The high I2 value of 95.57% suggests a substantial degree of heterogeneity. A subgroup meta-analysis revealed that factors such as time-dependent bias, sample size dependence, or individual variation among study participants contributed to this heterogeneity (p-difference < 0.05).
Conclusion
According to the findings of this study, the pooled prevalence of hepatitis B infection among pregnant women in Africa was found to be intermediate-high. It is recommended that policymakers implement hepatitis B virus immunization programs targeting pregnant women and their new-born babies at higher risk of exposure.
1. Introduction
Hepatitis is a medical condition characterized by liver inflammation [1–3]. Various virus families are known to cause liver damage [1, 4, 5]. Several medically necessary viruses pose a significant risk to the well-being of millions of people worldwide [3, 4, 6]. Hepatitis B virus (HBV) is a substantial global public health issue [7–9] and belongs to the hepadnavirus family, a group of deoxyribonucleic acid (DNA) viruses [2]. It primarily affects the liver and can lead to both short-term and long-term health problems [8, 9], resulting in significant levels of illness and death [7, 10–14]. World Health Organization (WHO) estimates that 254 million people will be living with chronic hepatitis B infection in 2022 [15]. It is estimated that over 1 million individuals die each year due to chronic liver disease caused by this virus [10, 12].
The hepatitis B virus is a significant public health concern and is particularly widespread among pregnant women in sub-Saharan Africa [16, 17]. It is estimated that approximately 65 million individuals in Africa are infected with HBV, with a mortality rate of 25% [8]. Among pregnant women, the prevalence of HBV infection in different countries of the African region was reported as 7.5% in Sudan [18], 9.3% in Kenya [19], 3.2% in Eritrea [20], and 3.1% in Rwanda [21]. The prevalence of HBV among pregnant women in sub-Saharan Africa ranges from 9–20% [8], posing a persistent and significant public health challenge, particularly in this region. The infection is associated with severe complications, including cirrhosis and liver cancer [8, 16, 17, 22], can be transmitted prenatally, through unprotected sexual contact, intravenous drug use, contaminated blood, and blood products, contaminated injections during medical procedures, and injection drug use and unsafe medical practices [10, 23].
Hepatitis B virus infection demonstrates a noteworthy inclination for vertical and horizontal transmission, posing potential risks to mothers and infants [16, 17, 24]. Consequently, adverse birth outcomes, including stillbirth, fetal loss, neonatal death, premature birth, and low birth weight, may ensue [23]. The risk of acquiring chronic HBV infection varies with age. Around 90% of infections occur during the perinatal period [11]. If an infant’s mother is positive for both hepatitis B surface antigen (HBsAg)and hepatitis B e antigen (HBeAg), there is a 90% likelihood of the infant developing chronic infection by age 6 [12]. For children above six years old, the chances of developing chronic hepatitis B virus are 30–50% and 5–10% [25]. Pregnant women who test positive for both HBsAg and HBeAg have a 70–90% chance of transmitting the infection to their new-born infants. If they test positive for only HBsAg, the chances decrease to 10–40% [8].
In order to prevent up to 96% of transmission, pregnant women should be routinely evaluated for HBsAg, and new-borns whose mothers test positive should receive the HBV vaccine at delivery [8, 26]. The majority of pregnant women, however, do not receive routine screening during antenatal care (ANC) [27]. For both the mother and the new-born, an HBV-complicated pregnancy causes challenges in the management of drugs or medication administration [14]. Despite a potent vaccination, HBV infection remains one of the significant public health issues, primarily in developing nations [28].
The global hepatitis strategy of the WHO, which is endorsed by all member states, aims to reduce new hepatitis infections by 90%, decrease deaths by 65%, and provide treatment for 80% of those living with these illnesses between 2016 and 2030 [29]. Given the scope and severity of the issue, prevention and control of viral hepatitis require a high level of attention [3]. Therefore, controlling the HBV among pregnant women may help break the chain of transmission [7]. However, global progress toward the elimination goals has been slow, particularly in Africa, where the burden of HBV is high [27, 30] and with also a poor uptake of HBV vaccination and an incomplete HBV three-dose vaccine coverage, HBsAg prevalence in the WHO Africa region in remains high [31].
Furthermore, there is a significant deficiency in an efficient surveillance system for monitoring and understanding the epidemiology of HBV among pregnant women [32]. Despite the existence of several studies investigating the prevalence of HBV and its related factors in Africa, the outcomes of this study will provide valuable insights for the development of appropriate strategies to mitigate HBV infection among pregnant women. Currently, there is a lack of comprehensive studies with a specific focus on pregnant women in Africa. This systematic review and meta-analysis aims to determine the pooled prevalence of hepatitis B virus infection among pregnant women. This study’s findings can inform the development of surveillance strategies, advocate for early screening during pregnancy, and facilitate the formulation of effective preventive measures. Hence, the objective of this meta-analysis is to determine the prevalence of hepatitis B virus infection among pregnant women in Africa.
2. Methods
2.1. Protocol for the study and reporting
The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) standards for the literature search approach, study selection, data extraction, and outcome reporting were followed when conducting this systematic review and meta-analysis (SRMA). The Newcastle-Ottawa scale (NOS) review guideline was modified to create eligibility criteria based on the Condition, Context, and Population (COCOPO) principle [33]. Zotero (version 6.0) reference management software and Rayyan software tools were used to download, organize, review, and cite related articles in systematic and meta-analysis literature reviews [34]. The protocol has been identified as CRD42023442426 and registered in the PROSPERO database.
2.2. Variables and measures
These systematic reviews and meta-analyses encompassed all observational research studies conducted in Africa that examined the prevalence of HBV infection among pregnant women. This includes cohort, case-control, and cross-sectional studies that assessed the presence of HBV through the detection of positive hepatitis B surface antigen (HBsAg) using rapid diagnostic tests (RDTs), enzyme-linked immunosorbent assay(ELISA), or a combination of both.
2.3. Inclusion and exclusion criteria
We included a comprehensive range of observational studies, such as cross-sectional, case-control, prospective, and retrospective cohorts, that reported their findings in peer-reviewed journals. Moreover, we considered studies eligible for inclusion if they were conducted in Africa, focused on screening pregnant women for HBV, and published in English. Furthermore, our study exclusively incorporates research that adheres to rigorous methodological standards determined by the NOS [33]. We excluded studies, including case reports and case series, from our analysis. For studies published in multiple reports, we excluded duplicates and those that did not involve pregnant women. Additionally, we did not consider studies published in questionable, scholarly, open-access (predatory) journals, following the guidelines provided by Ross-White and colleagues [35].
2.4. Study design and search strategy
We conducted an extensive literature search to locate previous systematic reviews, meta-analysis studies, and protocols relevant to the topic of interest. This search used the PROSPERO database and the abstracts of effects (DARE) reviews to ensure no similar study had been registered and published. Various biomedical databases, including PubMed, Advanced Google Scholar, Scopus, and the Cochrane Library, were utilized to search for eligible articles published between July 18, 2013, and July 18, 2023. We expressly limited our search to studies published in English. To identify potentially eligible articles, we thoroughly reviewed the reference lists of the identified studies, which also included articles from the African Journal Online (AJO). To refine our search strategy, we combined medical subject headings (MeSH terms), keywords, and Boolean operators (AND and OR). Additionally, we conducted a secondary search using all identified keywords and index terms. Finally, we examined the reference lists of identified papers and articles to identify any further relevant studies. The database search was performed using terms such as "Prevalence," "Hepatitis B Virus," "Infection," "Pregnant Women," and "Africa" (S1 File).
2.5. Study selection and screening
The study selection process was conducted in two stages, following strict inclusion and exclusion criteria. Initially, two authors (YML and AAA) independently evaluated the titles and abstracts of the articles. Subsequently, the same two authors (YML and AAA) individually obtained and scrutinized the complete papers of the eligible articles. To ensure consensus, all three authors (SBK, NG, and RAA) agreed upon the selected articles at each stage of the screening process. In case of any disagreements, a third author (NG) was consulted to resolve them.
2.6. Data extraction
The authors’ pre-tested technique was used to extract the data from the included studies. The first author’s last name, the publication year, the study location, the study design, the study year, the HBV-specific antigen reported, the screening method, the number of pregnant women who were screened for HBV (HBsAg), the number of pregnant women who were screened but tested positive for HBV (HBsAg+), how many HBV-infected women tested positive for HBeAg, as well as the risk variables that have been publicly disclosed were all retrieved by two authors (YML and AAA). For further information, the authors of the included studies were contacted when needed. Three additional writers (NG, SBK, and RAA) randomly chose and cross-checked the extracted data.
2.7. Measures for data quality control
The methodological quality of each publication in cross-sectional and cohort studies was assessed using the NOS critical evaluation checklist [33]. This measure was also used to determine the likelihood of biased study results. The NOS employs a star rating methodology to assess the risk of bias in three areas: study group selection, group comparability, and result determination. The NOS awards a maximum of ten stars for each study. Based on the NOS classification for quality evaluation in cross-sectional studies, articles were categorized as unsatisfactory (0–4 stars), satisfactory (5–6 stars), good (7–8 stars), or very good (9–10 stars) [36]. Conversely, in cohort studies, acceptable quality included good (1–2 stars in comparability, 3–4 stars in selection, and 2–3 stars in result or exposure domains), fair (1–2 stars in comparison, 2–3 stars in outcome, and two stars in selection domains), and poor (0–1 star in selection, 0 stars in comparability, or 0–1 star in outcome or exposure domains) [33]. Only studies that scored at least seven out of ten were included in our review study after being evaluated against these criteria. Two authors (Y.M.L. and A.A.A.) independently assessed the quality of each study, and any discrepancies were resolved through discussion with a third independent reviewer (NG) (S2 File).
Where: *(asterisks) correspond to ratings assigned for each item according to the Newcastle Ottawa Quality Assessment Scale; HbcAb: hepatitis B core antibody; IGM: Immunoglobulin M; HBV: hepatitis B virus; HDV: hepatitis D virus; ANC: antenatal care; ELISA: Enzyme-linked immunosorbent assay; PCR: polymerase chain reaction; HBsAg: hepatitis B surface antigen; HBs: anti-Hepatitis B surface; HBe: anti-Hepatitis B envelope; HBc: anti-Hepatitis B core; HBeAg: hepatitis B envelope antigen; HBcAg: hepatitis B core antigen.
2.8. Statistical analysis
The statistical software package Stata (version 16.0, Stata Corp. LP based in College Station, United States of America) was used to conduct all analyses. A random effects meta-analysis model was used. This model was based on the DerSimonian, and Laird approaches and was employed to combine the prevalence of hepatitis B virus in pregnant women in Africa. Statistical significance was determined using a p-value and a 95% confidence interval. The random effect model was used for analyses with statistical heterogeneity, while the fixed effect model was used for analyses without heterogeneity. Statistical heterogeneity was assessed using the I-squared (I2) statistic test [37]. We performed a meta-regression and subgroup analysis to explore possible causes of heterogeneity. To evaluate publication bias, we visually examined the asymmetry of the funnel plot and conducted Egger’s test [38]. The p-value was < 0.05, indicating statistical evidence for publication bias. Additionally, a counter-enhanced funnel plot was employed to differentiate between asymmetry caused by publication bias and asymmetry caused by other factors. A sensitivity analysis was performed to assess the impact of a single study on the overall estimate.
3. Results
3.1. Search results
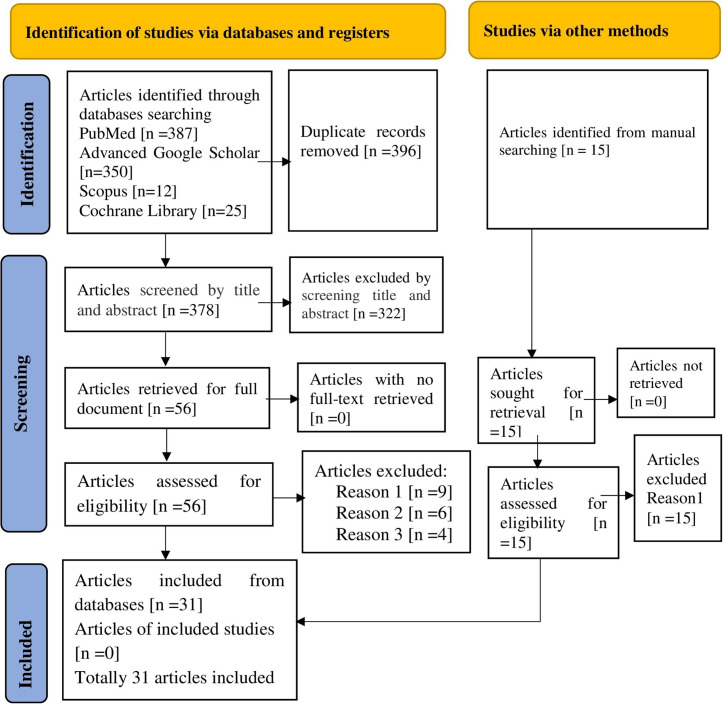
Initially, a total of 774 studies were retrieved from electronic databases. After removing 396 duplicate items, 378 articles remained for review based on their title and abstracts. Subsequently, 322 research papers were excluded. Four of the remaining 56 full-text papers were excluded as they did not report the outcome of interest, six were excluded due to the unavailability of full-text articles, and nine were excluded due to low methodological quality. Finally, 31 studies that met the inclusion criteria were selected and invited to participate in the study (Fig 1).
Fig 1. A PRISMA flow diagram showing the screening and inclusion of the studies.
Reason 1: Poor methodological quality of data. Reason 2: The full textile article is unavailable. Reason 3: Not reporting the outcome of interest.
3.2. Study characteristics
The analysis incorporated studies published between 2013 and 2023. A comprehensive total of 31 articles were utilized to determine the overall prevalence of hepatitis B virus infection among pregnant women in Africa. The sample size ranged from 124 to 12138; twenty-seven out of the thirty-one studies (87.1%) were facility-based cross-sectional studies, three (9.68%) were retrospective medical record reviews, and one (3.23%) was a prospective cohort study.
The methodological quality score was more significant than or equal to seven in all twenty-one studies. Out of 31 articles, 10 (32.26%) were conducted in Ethiopia, seven (22.58%) in Nigeria, two (6.45%) in Ghana, two (6.45%) in South Sudan, two (6.45%) in Sudan, and one (3.23%) each from Uganda, Burkina Faso, Chad, Somalia, South Africa, Egypt, Cameroon and Gambia (Table 1).
Table 1. Characteristics of studies included in the review from 2013 to 2023 (N = 33,967).
| Author, Year of Publication | Country | Study Population | Study Type | Sample Size | Pregnant women tested positive | Prevalence [95%CI] | Quality |
|---|---|---|---|---|---|---|---|
| Mortada EL-Shabrawi et al., 2014 [39] | Egypt | Pregnant Women | Facility-based cross-sectional survey | 2,000 | 35 | 1.8% [1.7–10.2] | 7 |
| Frambo et al., 2014 [40] | Cameroon | Pregnant Women | Facility-based cross-sectional survey | 176 | 17 | 9.7% [5.7–15] | 7 |
| Mohammed Hammad Abuelgasim and Mohammed Basheer Koko Baraka, 2015 [18] | Sudan | Pregnant Women | Facility-based cross-sectional survey | 160 | 12 | 7.5%[5.4–9.8] | 7 |
| Dahie and Heyle, 2017 [8] | Somalia | Pregnant Women | Facility-based cross-sectional survey | 364 | 15 | 4.1%[3.3–6.4] | 7 |
| Stephen Kirbak et al., 2017 [41] | South Sudan | Pregnant Women | Facility-based cross-sectional survey | 280 | 31 | 11%[8.1–12.8] | 8 |
| Atilola G et al., 2018 [42] | Nigeria | Pregnant Women | Facility-based cross-sectional survey | 353 | 37 | 10.5% [7.5–14.2] | 7 |
| Gasim et al., 2019 [43] | Sudan | Pregnant Women | Facility-based cross-sectional survey | 900 | 162 | 18%[15.3–19.7] | 8 |
| Bittaye et al., 2019 [44] | Gambia | Pregnant Women | Facility-based cross-sectional survey | 426 | 39 | 9.2[6.9–13.2] | 9 |
| Peter Asaga et al., 2019 [45] | Nigeria | Pregnant Women | Facility-based cross-sectional survey | 200 | 39 | 19.5[14.2–21.5] | 8 |
| Magaji et al., 2020 [46] | Nigeria | Pregnant Women | Facility-based cross-sectional survey | 3238 | 241 | 7.4[6.6–8.4] | 8 |
| Kwadzokpui et al., 2020 [47] | Ghana | Pregnant Women | Facility-based cross-sectional survey | 213 | 7 | 3.3[2.3–3.9] | 8 |
| Bancha et al., 2020 [16] | Ethiopia | Pregnant Women | Facility-based cross-sectional survey | 675 | 49 | 7.3%[5–9] | 9 |
| Dortey BA et al., 2020 [48] | Ghana | Pregnant Women | Facility-based cross-sectional survey | 221 | 17 | 7.7%[6.7,8.7] | 8 |
| Michael Pou and Dube, 2021 [49] | South Sudan | Pregnant Women | Facility-based cross-sectional survey | 234 | 16 | 6.8% [3.8–10.3] | 9 |
| Tadiwos et al., 2021 [13] | Ethiopia | Pregnant Women | Facility-based cross-sectional survey | 479 | 44 | 9.2%[4.2–14.2] | 8 |
| Atalay et al., 2021 [50] | Ethiopia | Pregnant Women | Facility-based cross-sectional survey | 215 | 11 | 5.1%[1.1–12.2] | 8 |
| Iliyasu et al., 2022 [51] | Nigeria | Pregnant Women | Facility-based cross-sectional survey | 394 | 46 | 11.7% [6.1–17.1] | 7 |
| Kassaw et al., 2022 [52] | Ethiopia | Pregnant Women | Facility-based cross-sectional survey | 381 | 25 | 6.6%[4.2–8.9] | 8 |
| Atwine et al., 2022 [25] | Uganda | Pregnant Women | Facility-based cross-sectional survey | 341 | 7 | 2.1%[0.5–3.5] | 8 |
| Argaw et al., 2022 [9] | Ethiopia | Pregnant Women | Facility-based cross-sectional survey | 338 | 11 | 3.3%[1.5–5] | 7 |
| Amaike C et al., 2022 [27] | Nigeria | Pregnant Women | Retrospective chart review | 706 | 82 | 11.6%[6.5–18.1] | 7 |
| Afolabi A et al., 2022 [53] | Nigeria | Pregnant Women | Retrospective chart review | 4300 | 110 | 2.6%[1.8–3.2] | 7 |
| Gebretsadik., et al., 2022 [54] | Ethiopia | Pregnant Women | Facility-based cross-sectional survey | 124 | 10 | 8.1%[9.8–13.8] | 7 |
| Joseph Davey et al., 2022 [55] | South Africa | Pregnant Women | Retrospective chart review | 1194 | 8 | 6.7%[3.4–13.2] | 8 |
| Ukpe et al., 2023 [56] | Nigeria | Pregnant Women | Facility-based cross-sectional survey | 291 | 23 | 7.9%[4.8–11.2] | 8 |
| Umer et al., 2023 [12] | Ethiopia | Pregnant Women | Facility-based cross-sectional survey | 300 | 24 | 8%[5.3–11] | 7 |
| Kampe et al., 2023 [11] | Ethiopia | Pregnant Women | Facility-based cross-sectional survey | 368 | 21 | 5.7%[3.7–8.6] | 7 |
| Ouoba et al., 2023 [57] | Burkina Faso | Pregnant Women | Facility-based cross-sectional survey | 1622 | 106 | 6.5%[5.4–7.8] | 10 |
| Israel E et al., 2023 [23] | Ethiopia | Pregnant Women | Facility-based cross-sectional survey | 484 | 8 | 1.7%[1.5–4.1] | 9 |
| Debsikreo N et al., 2023 [7] | Chad | Pregnant Women | Facility-based cross-sectional survey | 458 | 33 | 7.2%[5–10] | 7 |
| Tesfu et al., 2023 [14] | Ethiopia | Pregnant Women | prospective cohort | 12138 | 369 | 3.1%[1.8–4.9] | 8 |
| Total | 33,967 | 1701 | 7.5%[5.1–10.6] | 7.8 | |||
Where; HBV: hepatitis B Virus; N: Sample Size
3.3. Pooled prevalence of hepatitis B virus among pregnant women in Africa
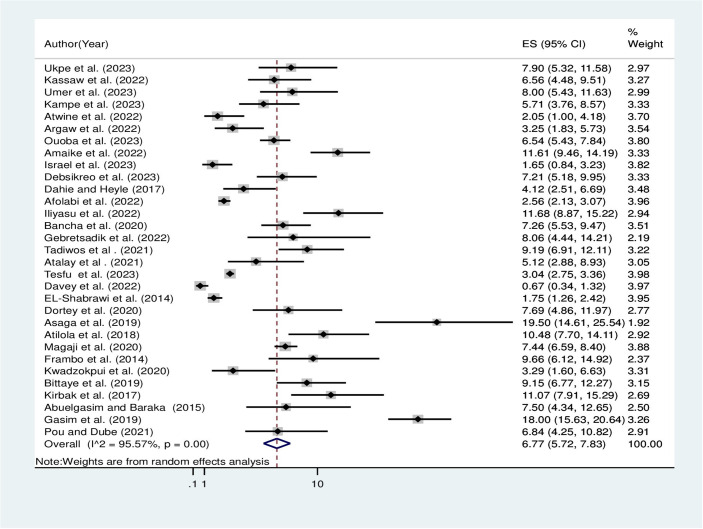
The overall pooled prevalence of hepatitis B virus among pregnant women in Africa using the fixed effect model was 3.16% [95% CI: 2.98, 3.35]. When using the fixed effect model, the pooled effect size of the hepatitis B virus among pregnant women showed significant heterogeneity among the included studies (I2) of 95.57% (P = 0.00). As a result, the final prevalence was determined using a random-effect model to account for the observed heterogeneity. The pooled prevalence of hepatitis B virus among pregnant women in Africa using the random effect model was 6.77% [95% CI: 5.72, 7.83] (Fig 2).
Fig 2. Overall pooled prevalence of hepatitis B virus among pregnant women in Africa, 2023.
3.4. Subgroup analysis by country and region
Subgroup analysis revealed that the aggregated estimate of HBV infection among pregnant women was assessed based on country and region. Consequently, we identified variations in the prevalence of HBV infection among the countries reviewed. The specific prevalence rates of HBV were 9.73%, 5.44%, 2.05%, 6.54%, 7.21%, 4.12%, 0.67%, 1.75%, 4.68%, 9.66%, 9.15%, 8.69%, and 15.12% in Nigeria, Ethiopia, Uganda, Burkina Faso, Chad, Somalia, South Africa, Egypt, Ghana, Cameroon, Gambia, South Sudan, and Sudan, respectively. Additionally, regional disparities in the prevalence of HBV infection were also observed in this review. The detailed prevalence rates of HBV were 8.58%, 4.94%, 7.21%, 0.67%, and 9% in the West, East, Central, Southern, and Northern regions of Africa, respectively (Table 2).
Table 2. Subgroup analysis of the pooled prevalence of hepatitis B virus among pregnant women in Africa, 2023.
| Variables | Characteristics | NS | Pooled prevalence 95%CI | I2 | P-value |
|---|---|---|---|---|---|
| Country | Nigeria | 7 | 9.73[6.32,13.14] | 97.08% | 0.00 |
| Ethiopia | 10 | 5.44[3.95,6.93] | 87.37% | 0.00 | |
| Uganda | 1 | 2.05[1,4.18] | - | - | |
| Burkina Faso | 1 | 6.54[5.43,7.84] | - | - | |
| Chad | 1 | 7.21[5.18,9.95] | - | - | |
| Somalia | 1 | 4.12[2.51,6.69] | - | - | |
| South Africa | 1 | 0.67[0.34,1.32] | - | - | |
| Egypt | 1 | 1.75[1.26,2.42] | - | - | |
| Ghana | 2 | 4.68[2.71,6.66] | - | - | |
| Cameroon | 1 | 9.66[6.12,14.92] | - | - | |
| Gambia | 1 | 9.15[6.77,12.27] | - | - | |
| South Sudan | 2 | 8.69[6.26,11.11] | - | - | |
| Sudan | 2 | 15.12[12.98,17.26] | - | - | |
| Region | West | 12 | 8.58[6.33,10.83] | 95.57% | 0.00 |
| East | 12 | 4.94[3.71,6.17] | 85.22% | 0.00 | |
| Central | 1 | 7.21[5.18,9.95] | - | - | |
| Southern | 1 | 0.67[0.34,1.32] | - | - | |
| Northern | 5 | 9[1.8,16.21] | 97.83 | 0.00 |
Where: NS: is the number of studies; CI: confidence Interval, and I2: I–Squared.
Meta-regression analysis revealed that the observed heterogeneity could be accounted for by two factors: publication year (P = 0.002) and sample size (P = 0.004) (Table 3). It is widely recognized that meta-analyses focusing on prevalence often exhibit substantial variability. Consequently, genuine heterogeneity in prevalence estimates is expected to arise due to variations in the timing and location of the conducted studies. These factors should be interpreted cautiously as they may not be discriminatory [58]. I2 estimates can sometimes be unreliable due to inadequate power and precision. Factors contributing to high heterogeneity include time-dependent bias, dependence on sample size, study location, regional differences, mean/median sample size, HBV screening methods, methodological quality, and individual variation among study participants [58, 59].
Table 3. Meta-regression analysis of factors influencing between-study heterogeneity for the pooled prevalence of HBV infection among pregnant women in Africa, 2023.
| Source of heterogeneity | Coef | Std. Err | T | P>|t| | [95% Conf. Interval] |
|---|---|---|---|---|---|
| Year of publication | .5583528 | .1608809 | 3.47 | 0.002 | .2293144,.8873912 |
| Sample size | .94912222 | .3054847 | 3.11 | 0.004 | .3243358,1.573909 |
3.5. Publication bias
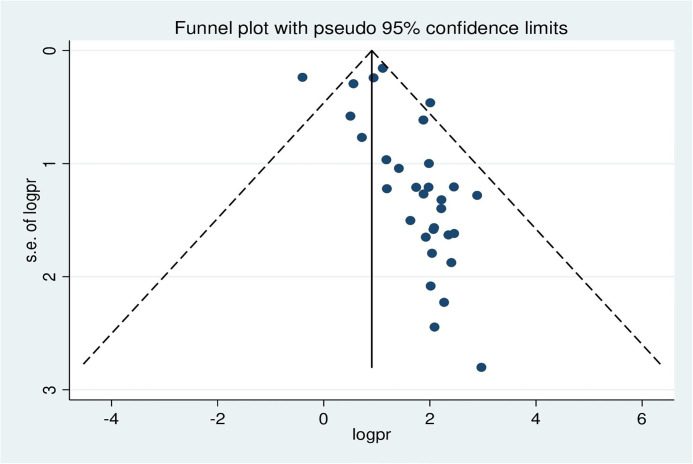
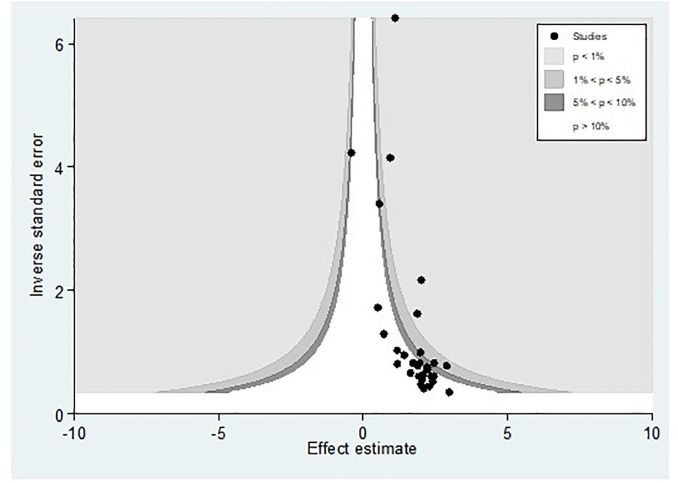
Publication bias was assessed using a funnel plot and Egger’s regression test. The funnel plot depicted evidence of asymmetry (Fig 3).
Fig 3. A funnel plot illustrates publication bias regarding the prevalence of hepatitis B virus among pregnant women in Africa, 2023.
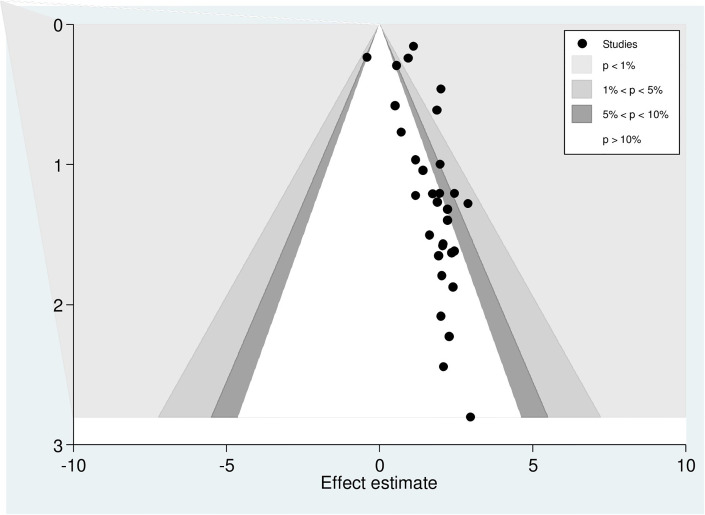
Egger’s regression test was statistically significant, with a P-value of 0.00. However, it is essential to note that the funnel plot and Egger’s test primarily assess the risk of slight study bias, and smaller studies tend to have more significant variance. While an asymmetrical plot could indicate publication bias, other factors could explain funnel plot asymmetry. To further investigate this, we conducted a contour-enhanced funnel plot to differentiate between asymmetry caused by publication bias and asymmetry caused by other factors. The contour-enhanced funnel plot suggests that the "missing" studies are expected to be located in areas of high statistical significance (shaded areas), while most available studies are not statistically significant. This indicates that the observed asymmetry may not be solely due to publication bias based on statistical significance. Therefore, it is probable that the asymmetry can be attributed to several other factors, including but not limited to study size, study effect, study design, study location, regional variances, HBV screening methods, methodological quality, and individual variations among study participants (Fig 4).
Fig 4. Counter-enhanced funnel plots for publication bias for the prevalence of hepatitis B virus among pregnant women in Africa, 2023.
Similar findings were observed when conducting a metric (inverse) counter-enhanced funnel plot (Fig 5).
Fig 5. Metric inverse counter-enhanced funnel plots of publication bias for the prevalence of hepatitis B virus among pregnant women in Africa, 2023.
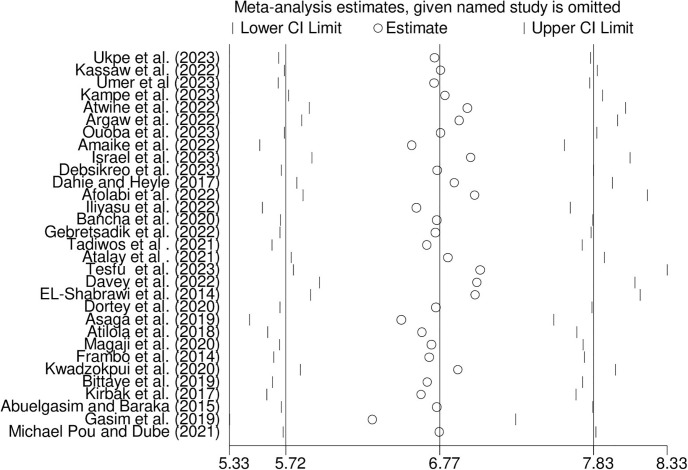
3.6. Sensitivity analysis
We performed a sensitivity analysis to assess how each study affected the overall summary estimate of the meta-analysis. No studies were outside the confidence bounds in the sensitivity analysis, suggesting that all studies had a nearly equal influence on the pooled prevalence (Fig 6).
Fig 6. Sensitivity analysis for the prevalence of hepatitis B virus among pregnant women in Africa, 2023.
4. Discussion
Among the various viruses that cause hepatitis, HBV is responsible for both acute and chronic infections. It is the most prevalent and severe liver infection globally, leading to significant morbidity and mortality [8, 9, 60]. However, there is limited documentation regarding the national prevalence of hepatitis B infection among pregnant women. The concern surrounding HBV infection in pregnant women stems from the potential transmission of the virus to their new-borns during delivery. By identifying the hepatitis status of pregnant mothers, the risk of virus transmission can be mitigated, thereby reducing the likelihood of chronic hepatitis development in infants.
To contribute to the expanding body of evidence on HBV in Africa, we have conducted a systematic review and meta-analysis to investigate the prevalence of HBV among pregnant women. Our analysis encompasses studies published between July 2013 and July 2023. In this systematic review and meta-analysis, 31 studies indicated a relatively high prevalence rate of 6.77%. According to the classification of HBV endemicity based on HBsAg prevalence–low (<2%), lower-intermediate (2–4.99%), higher-intermediate (5–7.99%), and high (>8%) [32, 61], our findings suggest a higher-intermediate endemicity of HBV infection among pregnant women in Africa. The reported range of HBV infection among pregnant women in Africa varies from 9% to 20% [8]. These results similar with a meta-analysis conducted in Nigeria (6.49%) [32], Ethiopia (5.78%) [60] and (7.4%) [62], a cross-sectional study conducted in Khartoum, Sudan(7.5%) [18], a retrospective review of pregnant women in Ghana (6%) [17], Wolaita Sodo in southern Ethiopia (7.5%) [63], Deder Hospital in Eastern Ethiopia (6.9%) [64], and Addis Ababa, Ethiopia (6%) [65]. However, our findings are lower than the prevalence rates reported in a meta-analysis study in Nigeria (9.5%) [22], Kenya (9.3%) [19], Cameroon (11.2%) [66], a cross-sectional study in Nigeria (10.9%) [67], Northwest Ethiopia (8%) [5], and Tigray in Ethiopia (11.6%) [68]. On the other hand, our meta-analysis study reveals higher prevalence compared to a systematic review and meta-analysis conducted in Ethiopia (4.75%) [69], Bangladesh (4%) [28], Ghana (4.6%) [10], Ambo town in central Ethiopia (4.99%) [70], Arba Minch South Ethiopia (4.3%) [71], Eritrea (3.2%) [20], Iranian pregnant women (1.2%) [72] and a cross-sectional study conducted in Eretria(3.2%) [20], (3.1%) in Rwanda [21] and (1.3%) Cairo, Egypt [2].
In the subgroup analysis, there was a significant variation in the prevalence of HBV infection among the subnational regions of Africa. According to the findings of this analysis, the northern region showed the highest prevalence of HBV at 9%, while the eastern region had the lowest prevalence at 4.94%. This discrepancy can be attributed to variations in the number of studies included in the analysis, as the northern region of Africa was limited to only five.
The explanation for the overall variations in the prevalence of HBV infection among pregnant women can be attributed to a combination of factors, including sociodemographic characteristics, cultural and environmental differences, behavioural risk factors for HBV infection, methodological variances, and natural disparities associated with different geographical situations. Factors such as awareness levels, sample size, study participants, study design, and infection prevention practices within different communities across countries and regions also contribute to the observed differences.
The findings of this study provide essential data for assessing the effectiveness of existing prevention and control strategies in Africa. Moreover, this study serves as a valuable resource for developing and implementing efficient public health management strategies, with the ultimate goal of achieving elimination by 2030. Prevention of mother-to-child transmission (PMTCT) of HBV infection is a crucial component of initiatives aimed at advancing the elimination of viral hepatitis. According to existing guidelines, it is advised to conduct maternal screening, administer antiviral therapy during the third trimester of high-risk pregnancies, implement universal and timely HBV birth dose vaccination, and administer post-exposure prophylaxis with hepatitis B immunoglobulin to selected neonates [73–76].
5. Limitation of the study
Significant variation was observed among the included studies, which may affect the reliability of the combined estimate. A thorough analysis was conducted to examine the potential sources of this variation. The findings indicated that differences in screening methods, study settings, and countries could have influenced the observed variation. The high levels of variation pose challenges for similar meta-analytic studies on HBV in Africa. Additionally, there was variability in the reporting quality of the studies included. However, it is vital to acknowledge the possibility of underestimating or overestimating the outcome variable. Obtaining accurate data on the prevalence of HBV infection among pregnant women is crucial for effectively managing the vertical transmission of the disease. A search was conducted in four databases, and only studies published in English were included. This may have resulted in the exclusion of relevant articles and specific reports on the prevalence of HBV infection among pregnant women in Africa.
6. Conclusion
The pooled prevalence of hepatitis B infection among pregnant women in Africa was found to be intermediate-high.
7. Recommendation
Therefore, governmental and non-governmental organizations must augment their endeavours towards integrating reproductive health services, specifically HBV screening, as standard elements of ANC clinics in Africa. Moreover, it is crucial to deliver comprehensive health education and treatment when a positive HBV status is identified in order to avert HBV transmission from mother to child. Consequently, policymakers in the maternal health sector are strongly advised to enforce HBV immunization programs tailored specifically for pregnant women and their new-born infants who have been exposed to the virus. Furthermore, researchers should undertake analogous studies on related factors.
Supporting information
(DOCX)
(DOCX)
(DOCX)
Acknowledgments
We want to thank all authors of the articles that were included.
Data Availability
All relevant data are included in the paper and its Supporting information files.
Funding Statement
The author(s) received no specific funding for this work.
References
- 1.Prevention & Control of Viral Hepatitis Infection: A Strategy for Global Action © World Health Organization 2011.
- 2.Abdulkadhim Sayah M, Khaled Younis Albahadly W, Subhi Farhan S, Qasem S, Majeed Al-Tamimi S, Al-Shalah SAJ, et al. Investigate the Presence of HBV Surface Antigen in Pregnant Women, Cairo City in Egypt. Arch Razi Inst [Internet]. 2022. Oct [cited 2023 Jul 22];77(5). Available from: doi: 10.22092/ARI.2022.359511.2438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shiferaw F, Letebo M, Bane A. Chronic viral hepatitis: policy, regulation, and strategies for its control and elimination in Ethiopia. BMC Public Health. 2016. Dec;16(1):769. doi: 10.1186/s12889-016-3459-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bedaso A, Duko B, Fedlu R. Knowledge on HBV vaccine and vaccination status among health care workers of Hawassa University Comprehensive Specialized Hospital, Hawassa, southern Ethiopia: a cross sectional study. BMC Res Notes. 2018. Dec;11(1):912. doi: 10.1186/s13104-018-4023-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Asemahagn MA. Epidemiology of hepatitis B and C virus infections among patients who booked for surgical procedures at Felegehiwot referral hospital, Northwest Ethiopia. Chemin I, editor. PLOS ONE. 2020. Jun 17;15(6):e0234822. doi: 10.1371/journal.pone.0234822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Haile K, Timerga A, Mose A, Mekonnen Z. Hepatitis B vaccination status and associated factors among students of medicine and health sciences in Wolkite University, Southwest Ethiopia: A cross-sectional study. Wang J, editor. PLOS ONE. 2021. Sep 21;16(9):e0257621. doi: 10.1371/journal.pone.0257621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Debsikreo N, Mankréo BL, Ouangkake M, Jotham M, Ndiaye AJS, Leye N, et al. Prevalence of hepatitis B virus and associated factors among pregnant women in the health facilities, N’djamena, Chad [Internet]. In Review; 2023 Feb [cited 2023 Jul 11]. https://www.researchsquare.com/article/rs-2522118/v1
- 8.Dahie HA, Heyle AA. Prevalence of Hepatitis B and Its Associated Factors Among Pregnant Women in Mogadishu, Somalia. Arch Bus Res [Internet]. 2017. Nov 30 [cited 2023 Jul 11];5(11). Available from: http://www.scholarpublishing.org/index.php/ABR/article/view/3876 [Google Scholar]
- 9.Argaw B, Kedir S, Mustefa A, Yesse M, Hussen L, Abdella B, et al. Sero-Prevalence, Infectivity, and Associated Risk Factors of Hepatitis B Virus Among Pregnant Women Attending Antenatal Care in Sankura Primary Hospital, Silte Zone, Southern Ethiopia, 2021. Open Microbiol J. 2022. Jul 20;16(1):e187428582206030. [Google Scholar]
- 10.Shelter Agbeko Bobie Stephen Henry Afakorzi, Manortey Stephen. Prevalence of Hepatitis B infections and associated risk factors among pregnant women at Hawa Memorial Saviour Hospital in the Abuakwa North Municipality, Ghana. World J Adv Res Rev. 2022. Oct 30;16(1):904–14. [Google Scholar]
- 11.Kampe A, Kannaiyan Abbai M, Tilahun D, Daka D, Aliyo A, Dedecha W, et al. Seroprevalence of Hepatitis B Virus Infection and Associated Factors Among Pregnant Women Attending Antenatal Care At Public Hospitals in Borena Zone, Southern Ethiopia. Health Serv Res Manag Epidemiol. 2023. Jan;10:233339282311619. doi: 10.1177/23333928231161946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Umer A, Teklemariam Z, Ayele F, Mengesha MM. Prevalence of hepatitis B infection and its associated factors among pregnant mothers attending antenatal care at public hospitals at Hararghe, Eastern Ethiopia. Front Glob Womens Health. 2023. Apr 27;4:1056488. doi: 10.3389/fgwh.2023.1056488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tadiwos MB, Kanno GG, Areba AS, Kabthymer RH, Abate ZG, Aregu MB. Sero-Prevalence of Hepatitis B Virus Infection and Associated Factors Among Pregnant Women Attending Antenatal Care Services in Gedeo Zone, Southern Ethiopia. J Prim Care Community Health. 2021. Jan;12:215013272199362. doi: 10.1177/2150132721993628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tesfu MA, Habtemariam TT, Belay NB. Risk factors associated with Hepatitis B virus infection among pregnant women attending public hospitals in Addis Ababa, Ethiopia. Khudyakov YE, editor. PLOS ONE. 2023. Apr 26;18(4):e0284646. doi: 10.1371/journal.pone.0284646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Easterbrook PJ, Luhmann N, Bajis S, Min MS, Newman M, Lesi O, et al. WHO 2024 hepatitis B guidelines: an opportunity to transform care. Lancet Gastroenterol Hepatol. 2024. Jun;9(6):493–5. doi: 10.1016/S2468-1253(24)00089-X [DOI] [PubMed] [Google Scholar]
- 16.Bancha B, Kinfe AA, Chanko KP, Workie SB, Tadese T. Prevalence of hepatitis B viruses and associated factors among pregnant women attending antenatal clinics in public hospitals of Wolaita Zone, South Ethiopia. Ciccozzi M, editor. PLOS ONE. 2020. May 7;15(5):e0232653. doi: 10.1371/journal.pone.0232653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Antuamwine BB, Herchel ED, Bawa EM. Comparative prevalence of hepatitis B virus infection among pregnant women accessing free maternal care in a tertiary hospital in Ghana. Chemin I, editor. PLOS ONE. 2022. Mar 4;17(3):e0263651. doi: 10.1371/journal.pone.0263651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mohammed Hammad Abuelgasim Mohammed Basheer Koko Baraka. Prevalence of Hepatitis B Infection among Pregnant Women at Khartoum Teaching Hospital, Sudan. J US-China Med Sci [Internet]. 2015. Jun 28 [cited 2024 Apr 21];12(2). Available from: http://www.davidpublisher.org/index.php/Home/Article/index?id=17199.html [Google Scholar]
- 19.Gatheru Z, Murila F, Mbuthia J, Okoth F, Kanyingi F, Mugo F, et al. Factors Associated with Hepatitis B Surface Antigen Seroprevalence amongst Pregnant Women in Kenya. Open J Obstet Gynecol. 2018;08(05):456–67. [Google Scholar]
- 20.Fessehaye N. Prevalence of Hepatitis B Virus Infection and Associated Seromarkers among Pregnant Women in Eritrea. 2018;10. [Google Scholar]
- 21.Muvunyi CM, Habtu M, Nyamusi MM, Imunya JMM. Factors Associated with Hepatitis B Surface Antigen Seropositivity among Pregnant Women in Kigali, Rwanda: A Cross Sectional Study. J Community Public Health Nurs [Internet]. 2017. [cited 2024 Apr 24];03(04). Available from: https://www.omicsonline.org/open-access/factors-associated-with-hepatitis-b-surface-antigen-seropositivity-among-pregnant-women-in-kigali-rwanda-a-cross-sectional-study-2471-9846-1000192-92961.html [Google Scholar]
- 22.Ajuwon BI, Yujuico I, Roper K, Richardson A, Sheel M, Lidbury BA. Hepatitis B virus infection in Nigeria: a systematic review and meta-analysis of data published between 2010 and 2019. BMC Infect Dis. 2021. Dec;21(1):1120. doi: 10.1186/s12879-021-06800-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Israel E, Hizkel I, Geta T, Feleke T, Samuel B, Markos D. Triple sexually transmitted infections among pregnant woman in the context of Elimination of mother to child transmission in Southern Ethiopia: Reports from a survey of questionnaires and laboratory studies. Front Glob Womens Health. 2023. Jun 19;4:1190170. doi: 10.3389/fgwh.2023.1190170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moghaddasifar I, Lankarani B. K, Moosazadeh M, Afshari M, Malary M. Prevalence of Hepatitis B Virus infection among Pregnant Women in Iran: A Systematic Review and Meta-analysis. Iran J Cancer Prev [Internet]. 2016. Oct 4 [cited 2023 Jul 21];In Press(In Press). Available from: https://brieflands.com/articles/ijcm-3703.html [Google Scholar]
- 25.Atwine B, Suleiman MA, Odongo AO, Manenga E. Knowledge and Risk Factors of Hepatitis B Disease Among Women Attending Antenatal Care at Arua Regional Referral Hospital, Arua, Uganda [Internet]. In Review; 2022 Sep [cited 2023 Jul 11]. https://www.researchsquare.com/article/rs-2102123/v1
- 26.Alemu AA, Zeleke LB, Aynalem BY, Kassa GM. Hepatitis B Virus Infection and Its Determinants among Pregnant Women in Ethiopia: A Systematic Review and Meta-Analysis. Infect Dis Obstet Gynecol. 2020. Jun 11;2020:1–11. doi: 10.1155/2020/9418475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Amaike C,Harry LU,Afolaranmi T,Odiari AO,Adesuyi A,Ocheke A. Seroprevalence of Hepatitis B and C virus infections among pregnant women attending antenatal care in a secondary health facility in Northern Nigeria,2022. [Google Scholar]
- 28.Banik S, Datta A, Ghosh A, Ghosh KY, Debi H. The prevalence of hepatitis B virus infection in Bangladesh: a systematic review and meta-analysis. Epidemiol Infect. 2022;150:e47. doi: 10.1017/S0950268822000061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bhadoria AS, Khwairakpam G, Grover GS, Pathak VK, Pandey P, Gupta R. Viral Hepatitis as a Public Health Concern: A Narrative Review About the Current Scenario and the Way Forward. Cureus [Internet]. 2022. Feb 4 [cited 2024 Apr 18]; Available from: https://www.cureus.com/articles/78246-viral-hepatitis-as-a-public-health-concern-a-narrative-review-about-the-current-scenario-and-the-way-forward [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Smith S, Harmanci H, Hutin Y, Hess S, Bulterys M, Peck R, et al. Global progress on the elimination of viral hepatitis as a major public health threat: An analysis of WHO Member State responses 2017. JHEP Rep. 2019. Aug;1(2):81–9. doi: 10.1016/j.jhepr.2019.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sonderup MW, Spearman CW. Global Disparities in Hepatitis B Elimination—A Focus on Africa. Viruses. 2022. Jan 3;14(1):82. doi: 10.3390/v14010082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Olakunde BO, Adeyinka DA, Olakunde OA, Uthman OA, Bada FO, Nartey YA, et al. A systematic review and meta-analysis of the prevalence of hepatitis B virus infection among pregnant women in Nigeria. Sonderup MW, editor. PLOS ONE. 2021. Oct 29;16(10):e0259218. doi: 10.1371/journal.pone.0259218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bae JM. A suggestion for quality assessment in systematic reviews of observational studies in nutritional epidemiology. Epidemiol Health. 2016. Apr 26;38:e2016014. doi: 10.4178/epih.e2016014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rayyan—AI Powered Tool for Systematic Literature Reviews, https://www.rayyan.ai/.
- 35.Ross-White A, Godfrey CM, Sears KA, Wilson R. Predatory publications in evidence syntheses. J Med Libr Assoc [Internet]. 2019. Jan 4 [cited 2024 Apr 19];107(1). Available from: http://jmla.pitt.edu/ojs/jmla/article/view/491 doi: 10.5195/jmla.2019.491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Newcastle—Ottawa Quality Assessment Scale Adapted For Cross Sectional Studies. This Scale Has Been Adapted From The Newcastle-Ottawa Quality Assessment Scale For Cohort Studies To Provide Quality Assessment Of Cross Sectional Studies.
- 37.Rücker G, Schwarzer G, Carpenter JR, Schumacher M. Undue reliance on I2 in assessing heterogeneity may mislead. BMC Med Res Methodol. 2008. Dec;8(1):79. doi: 10.1186/1471-2288-8-79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Egger M, D Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997. Sep 13;315(7109):629–34. doi: 10.1136/bmj.315.7109.629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mortada EL-Shabrawi, Mohamed Farouk Mohamed, Mona Salah El Din Hamdi, Mohamed Ehab, Shaimaa Shaaban Khamiss and Hanaa El-Karaksy,Prevalence of Hepatitis B Virus Infection among Egyptian Pregnant Women—A Single Center Study,2014.
- 40.Frambo AAB, Atashili J, Fon PN, Ndumbe PM. Prevalence of HBsAg and knowledge about hepatitis B in pregnancy in the Buea Health District, Cameroon: a cross-sectional study. 2014; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stephen Kirbak AL, Ng’ang’a Z, Omolo J, Idris H, Usman A, Mbabazi WB. Sero-prevalence for Hepatitis B virus among pregnant women attending antenatal clinic in Juba Teaching Hospital, Republic of South Sudan. Pan Afr Med J [Internet]. 2017. [cited 2024 Apr 21];26. Available from: http://www.panafrican-med-journal.com/content/article/26/72/full/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Atilola Glory, Tomisin Obadara, Randle Mayowa, Isaac Komolafe O., Odutolu Gbenga, Olomu Josephine, Laide Adenuga;Epidemiology of HBV in Pregnant Women, South West Nigeria, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gasim RAE, Eltayeb N, Khidir IE. Hepatitis B Virus Infection in Pregnant Women, in Al Fashir Town, North Darfur State, Sudan. Open J Med Microbiol. 2019;09(01):28–36. [Google Scholar]
- 44.Bittaye M, Idoko P, Ekele BA, Obed SA, Nyan O. Hepatitis B virus sero-prevalence amongst pregnant women in the Gambia. BMC Infect Dis. 2019. Dec;19(1):259. doi: 10.1186/s12879-019-3883-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Peter Asaga M, Adamu Chipago S, Philomena Ehi A. High Prevalence of Hepatitis B Virus Infection among Pregnant Women Attending Antenatal Care in Central Nigeria. J Infect Dis Epidemiol [Internet]. 2019. Mar 31 [cited 2024 Apr 21];5(1). Available from: https://www.clinmedjournals.org/articles/jide/journal-of-infectious-diseases-and-epidemiology-jide-5-068.php?jid=jide [Google Scholar]
- 46.Magaji FA, Okolo MO, Hassan Z, Shambe IH, Pam VC, Ocheke AN, et al. Prevalence of hepatitis B virus infection among pregnant women in Jos, Nigeria. Ann Afr Med. 2020;19(3):176–81. doi: 10.4103/aam.aam_20_19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kwadzokpui PK, Akorsu EE, Abaka-Yawson A, Quarshie SS, Amankwah SA, Tawiah PA. Prevalence and Knowledge of Hepatitis B Virus Infection among Pregnant Women in the Ningo-Prampram District, Ghana. Int J Hepatol. 2020. Apr 28;2020:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dortey BA, Anaba EA, Lassey AT, Damale NKR, Maya ET. Seroprevalence of Hepatitis B virus infection and associated factors among pregnant women at Korle-Bu Teaching Hospital, Ghana,2020. Chemin I, editor. PLOS ONE. 2020. Apr 22;15(4):e0232208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Michael Pou M, Dube J. Seroprevalence and associated risk factors for Hepatitis B Virus infections among apparently healthy pregnant mothers attending Anc in Rubkona primary health care center in Rubkona County, Unity State, South Sudan. Arch Hepat Res. 2021. Mar 5;004–13. [Google Scholar]
- 50.Atalay AA, Abebe RK, Dadhi AE, Bededa WK. Seroprevalence of hepatitis B virus among pregnant women attending Antenatal care in Dilla University Referral Hospital Gedio Zone, Ethiopia; health facility based cross-sectional study. Spradley FT, editor. PLOS ONE. 2021. Mar 25;16(3):e0249216. doi: 10.1371/journal.pone.0249216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Iliyasu MY, Sahal MR, Inusa T, Ismail S, Umar RD, Tahir H, et al. Prevalence and Determinants of Hepatitis B Virus Antigenemia among Pregnant Women Attending Antenatal Clinics in Some Hospitals in Bauchi Metropolis. J Adv Microbiol. 2022. Dec 12;65–73. [Google Scholar]
- 52.Kassaw B, Abera N, Legesse T, Workineh A, Ambaw G. Sero-prevalence and associated factors of hepatitis B virus among pregnant women in Hawassa city public hospitals, Southern Ethiopia: Cross-sectional study design. SAGE Open Med. 2022. Jan;10:205031212211407. doi: 10.1177/20503121221140778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.As A. Afolabi A, Adekanle A, Atolagbe J, Afolabi O, Oshineye A, Folami O. Prevalence of Hepatitis B Among Pregnant Women At LAUTECH Teaching Hospital, Osogbo: A 5-Year Study: ORIGINAL ARTICLE. WNJMS [Internet]. 2022. Dec. 14 [cited 2023 Jul. 26];5(2 (Jul-Dec). Available from: https://wnjms.com.ng/journal/index.php/wnjms/article/view/103. [Google Scholar]
- 54.Gebretsadik D, Assefa M, Fenta GM, Daba C, Ali A, Tekele SG. High Seroprevalence of Hepatitis B and C Virus Infections among Pregnant Women Attending Antenatal Clinic in Borumeda General Hospital, Northeast Ethiopia. Masaki N, editor. BioMed Res Int. 2022. Aug 28;2022:1–11. doi: 10.1155/2022/1395238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Joseph Davey D, Hsiao N yuan, Wendy Spearman C, Sonderup M, Hu NC, Mashele N, et al. Low prevalence of hepatitis B virus infection in HIV-uninfected pregnant women in Cape Town, South Africa: implications for oral pre-exposure prophylaxis roll out. BMC Infect Dis. 2022. Sep 1;22(1):719. doi: 10.1186/s12879-022-07697-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ukpe MT, Abasiattai AM, Utuk NM, Umoh AV, Ibanga GJ. Prevalence of hepatitis B virus infection among antenatal attendees in a university teaching hospital in Southern Nigeria. Adesh Univ J Med Sci Res. 2023. Jun 21;5:9–16. [Google Scholar]
- 57.Ouoba S, Ko K, Lingani M, Nagashima S, Guingané AN, Bunthen E, et al. Intermediate hepatitis B virus infection prevalence among 1622 pregnant women in rural Burkina Faso and implications for mother-to-child transmission. Sci Rep. 2023. Apr 14;13(1):6115. doi: 10.1038/s41598-023-32766-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Thorlund K, Imberger G, Johnston BC, Walsh M, Awad T, Thabane L, et al. Evolution of Heterogeneity (I2) Estimates and Their 95% Confidence Intervals in Large Meta-Analyses. Emmert-Streib F, editor. PLoS ONE. 2012. Jul 25;7(7):e39471. doi: 10.1371/journal.pone.0039471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nussbaumer-Streit B, Mayr V, Dobrescu A, Chapman A, Persad E, Klerings I, et al. The Effectiveness of Quarantine alone or in Combination with Other Public Health Measures to Control Coronavirus Disease 2019: a Rapid Review. Cochrane Database Syst Rev [Internet]. 2020. Mar 27 [cited 2024 Jan 17]; Available from: http://doi.wiley.com/10.1002/14651858.CD202001 [Google Scholar]
- 60.Asgedom YS, Kassie GA, Woldegeorgis BZ, Meskele Koyira M, Kebede TM. Seroprevalence of hepatitis B virus infection and factors associated among pregnant women in Ethiopia: A systematic review and meta-analysis. Womens Health. 2024. Jan;20:17455057241235881. doi: 10.1177/17455057241235881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.World Health Organization. Global policy report on the prevention and control of viral hepatitis in WHO Member States [Internet]. Geneva: World Health Organization; 2013. [cited 2023 Jul 21]. 212 p. https://apps.who.int/iris/handle/10665/85397 [Google Scholar]
- 62.Belyhun Y, Maier M, Mulu A, Diro E, Liebert UG. Hepatitis viruses in Ethiopia: a systematic review and meta-analysis. BMC Infect Dis. 2016. Dec;16(1):761. doi: 10.1186/s12879-016-2090-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tadesse M, Tafesse G, Hajare ST, Chauhan NM. Assessment of prevalence of Hepatitis B virus and its associated factors among pregnant women from Wolaita Sodo, Ethiopia. J Clin Virol Plus. 2022. Jun;2(2):100069. [Google Scholar]
- 64.Umare A, Seyoum B, Gobena T, Haile Mariyam T. Hepatitis B Virus Infections and Associated Factors among Pregnant Women Attending Antenatal Care Clinic at Deder Hospital, Eastern Ethiopia. Chemin IA, editor. PLOS ONE. 2016. Nov 29;11(11):e0166936. doi: 10.1371/journal.pone.0166936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Desalegn Z, Wassie L, Beyene HB, Mihret A, Ebstie YA. Hepatitis B and human immunodeficiency virus co-infection among pregnant women in resource-limited high endemic setting, Addis Ababa, Ethiopia: implications for prevention and control measures. Eur J Med Res. 2016. Dec;21(1):16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bigna JJ, Amougou MA, Asangbeh SL, Kenne AM, Noumegni SRN, Ngo-Malabo ET, et al. Seroprevalence of hepatitis B virus infection in Cameroon: a systematic review and meta-analysis. BMJ Open. 2017. Jun;7(6):e015298. doi: 10.1136/bmjopen-2016-015298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Talla C, Itanyi IU, Tsuyuki K, Stadnick N, Ogidi AG, Olakunde BO, et al. Hepatitis B infection and risk factors among pregnant women and their male partners in the Baby Shower Programme in Nigeria: a cross-sectional study. Trop Med Int Health. 2021. Mar;26(3):316–26. doi: 10.1111/tmi.13531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kiros KG, Goyteom MH, Tesfamichael YA, Mekonen HH, Gebru TH, Gebrehiwot TG, et al. Seroprevalence of Hepatitis B Virus Infection, Mother-To-Child Transmission, and Associated Risk Factors Among Delivering Mothers in Tigray Region, Northern Ethiopia: a Cross-Sectional Study. Infect Dis Ther. 2020. Dec;9(4):901–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kebede KM, Abateneh DD, Belay AS. Hepatitis B virus infection among pregnant women in Ethiopia: a systematic review and Meta-analysis of prevalence studies. BMC Infect Dis. 2018. Dec;18(1):322. doi: 10.1186/s12879-018-3234-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wakjira M, Darega J, Oljira H, Tura MR. Prevalence of hepatitis B virus and its associated factors among pregnant women attending antenatal care in Ambo town, Central Ethiopia: A cross-sectional study. Clin Epidemiol Glob Health. 2022. May;15:101054. [Google Scholar]
- 71.Yohanes T, Zerdo Z, Chufamo N. Seroprevalence and Predictors of Hepatitis B Virus Infection among Pregnant Women Attending Routine Antenatal Care in Arba Minch Hospital, South Ethiopia. Hepat Res Treat. 2016. Jan 24;2016:1–7. doi: 10.1155/2016/9290163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Badfar G, Shohani M, Nasirkandy MP, Mansouri A, Abangah G, Rahmati S, et al. Epidemiology of hepatitis B in pregnant Iranian women: a systematic review and meta-analysis. Arch Virol. 2018. Feb;163(2):319–30. doi: 10.1007/s00705-017-3551-6 [DOI] [PubMed] [Google Scholar]
- 73.Matthews PC, Ocama P, Wang S, El-Sayed M, Turkova A, Ford D, et al. Enhancing interventions for prevention of mother-to-child- transmission of hepatitis B virus. JHEP Rep. 2023. Aug;5(8):100777. doi: 10.1016/j.jhepr.2023.100777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Prevention of Mother-To-Child Transmission of Hepatitis B Virus: Guidelines on Antiviral Prophylaxis in Pregnancy © World Health Organization July 2020. [PubMed]
- 75.Wang M, Bian Q, Zhu Y, Pang Q, Chang L, Li R, et al. Real-world study of tenofovir disoproxil fumarate to prevent hepatitis B transmission in mothers with high viral load. Aliment Pharmacol Ther. 2019. Jan;49(2):211–7. doi: 10.1111/apt.15064 [DOI] [PubMed] [Google Scholar]
- 76.Operationalizing elimination of mother-to-child transmission of Hepatitis B virus in the Western Pacific Region © World Health Organization 2021.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
(DOCX)
(DOCX)
Data Availability Statement
All relevant data are included in the paper and its Supporting information files.