Abstract
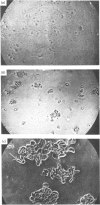
The present paper describes the purification and function of a haemagglutinin from the amoebocyte lysate of the horseshoe crab Carcinoscorpius rotundicauda. The purified protein consisted of a single subunit of Mr 24 000 and agglutinated human blood-group-A+ erythrocytes. Its haemagglutinin activity was inhibited by purified lysate, coagulogen, but not by sugars. The haemagglutinin differed immunologically and in activity from the sialic-acid-binding lectin carcinoscorpin present in the haemolymph. It caused aggregation of forma-fixed amoebocytes, and on the basis of this observation its role in cell-cell adhesion is proposed. This new haemagglutinin promotes cell-cell aggregation in amoebocytes in a manner that shares some similarities with thrombospondin-mediated platelet aggregation in vertebrates [Jaffe, Leuang, Nachman, Levin & Moseher (1981) Nature (London) 295, 246-248].
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bishayee S., Dorai D. T. Isolation and characterisation of a sialic acid-binding lectin (carcinoscorpin) from Indian horseshoe crab Carcinoscorpius rotunda cauda. Biochim Biophys Acta. 1980 May 29;623(1):89–97. doi: 10.1016/0005-2795(80)90011-2. [DOI] [PubMed] [Google Scholar]
- Cladaras C., Cottam G. L. Dietary alteration of translatable mRNA sequences coding for rat liver pyruvate kinase. J Biol Chem. 1980 Dec 10;255(23):11499–11503. [PubMed] [Google Scholar]
- Dorai D. T., Srimal S., Mohan S., Bachhawat B. K., Balganesh T. S. Recognition of 2-keto-3-deoxyoctonate in bacterial cells and lipopolysaccharides by the sialic acid binding lectin from the horseshoe crab Carcinoscorpius rotunda cauda. Biochem Biophys Res Commun. 1982 Jan 15;104(1):141–147. doi: 10.1016/0006-291x(82)91951-9. [DOI] [PubMed] [Google Scholar]
- Gartner T. K., Gerrard J. M., White J. G., Williams D. C. Fibrinogen is the receptor for the endogenous lectin of human platelets. Nature. 1981 Feb 19;289(5799):688–690. doi: 10.1038/289688a0. [DOI] [PubMed] [Google Scholar]
- Gartner T. K., Phillips D. R., Williams D. C. Expression of thrombin-enhanced platelet lectin activity is controlled by secretion. FEBS Lett. 1980 May 5;113(2):196–199. doi: 10.1016/0014-5793(80)80590-4. [DOI] [PubMed] [Google Scholar]
- Jaffe E. A., Leung L. L., Nachman R. L., Levin R. I., Mosher D. F. Thrombospondin is the endogenous lectin of human platelets. Nature. 1982 Jan 21;295(5846):246–248. doi: 10.1038/295246a0. [DOI] [PubMed] [Google Scholar]
- Jorgensen J. H., Smith R. F. Preparation, sensitivity, and specificity of Limulus lysate for endotoxin assay. Appl Microbiol. 1973 Jul;26(1):43–48. doi: 10.1128/am.26.1.43-48.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- March S. C., Parikh I., Cuatrecasas P. A simplified method for cyanogen bromide activation of agarose for affinity chromatography. Anal Biochem. 1974 Jul;60(1):149–152. doi: 10.1016/0003-2697(74)90139-0. [DOI] [PubMed] [Google Scholar]
- Marchialonis J. J., Edelman G. M. Isolation and characterization of a hemagglutinin from Limulus polyphemus. J Mol Biol. 1968 Mar 14;32(2):453–465. doi: 10.1016/0022-2836(68)90022-3. [DOI] [PubMed] [Google Scholar]
- Shishikura F., Sekiguchi K. Agglutinins in the horseshoe crab hemolymph: purification of a potent agglutinin of horse erythrocytes from the hemolymph of Tachypleus tridentatus, the Japanese horseshoe crab. J Biochem. 1983 Jun;93(6):1539–1546. doi: 10.1093/oxfordjournals.jbchem.a134292. [DOI] [PubMed] [Google Scholar]
- Yeaton R. W. Invertebrate lectins: II. Diversity of specificity, biological synthesis and function in recognition. Dev Comp Immunol. 1981 Fall;5(4):535–545. doi: 10.1016/s0145-305x(81)80028-6. [DOI] [PubMed] [Google Scholar]