Abstract
As a complementary and alternative therapy, acupuncture is widely used in the prevention and treatment of various diseases. However, the understanding of the mechanism of acupuncture effects is still limited due to the lack of systematic biological validation. Notably, proteomics technologies in the field of acupuncture are rapidly evolving, and these advances are greatly contributing to the research of acupuncture. In this study, we review the progress of proteomics research in analyzing the molecular mechanisms of acupuncture for neurological disorders, pain, circulatory disorders, digestive disorders, and other diseases, with an in-depth discussion around acupoint prescription and acupuncture manipulation modalities. The study found that proteomics has great potential in understanding the mechanisms of acupuncture. This study will help explore the mechanisms of acupuncture from a proteomic perspective and provide information to support future clinical decisions.
Keywords: Proteomics, Acupuncture, Electroacupuncture, Acupoints
1. Genral introduction of acupuncture with its holistic therapeutic theoreis
Acupuncture belongs to the category of complementary and alternative medicine, which originated in China more than 3000 years ago [1]. It began to spreadg to the America and Europe since the 16th century and since then it has attracted worldwide attention [2]. According to the traditional theories of meridians and acupoints in Classic Ancient Chinese Medical bookes (e.g., HuangDi NeiJing, also known as The Yellow Emperor's Canon of Internal Medicine) [3], acupuncture therapies achieves the effects of treating various diseases by balancing the body's energy [4]. The Chinese medical theories believes that the whole body communicates and interacts between the internal organs and external body surface through meridians, along which are composed of many scattered functional areas so called acupoints [5,6]. Acupuncture needles of 25–50 mm in length and 0.25–0.45 mm in diameter are usually used to pierce the acupoints and produce soreness, numbness, and heaviness [6,7]. With the development of modern science and technologies, electrical stimulation has been extended to the traditional manual needling operation. Therefore, electroacupuncture (EA), which is widely used as a stimulation strategy, was also included in the scope of this review. Studies have shown that acupuncture has a high safety profile with few side effects [7].
The use of acupuncture for the treatment of various diseases has gained global recognition [8]. Clinical studies have shown that acupuncture has clear advantages especially in the treatment of pain [[9], [10], [11]], neurological disorders [12,13] and digestive disorders [14,15]. However, the mechanisms of effects associated with acupuncture are not fully understood, and further efficacy evaluations and validations by modern systems biology are still with challenges. Therefore, exploring biological mechanisms of acupuncture is a key issue that needs to be addressed. Acupuncture has been shown to regulate multiple biological processes and pathways from a “holistic view” with multi-level, multi-target, dynamic regulation [16]. In the last two decades, proteomics has been widely used to explore the mechanisms of acupuncture, owing to the advantages of high sensitivity, large scale, high throughput, and its similarity to acupuncture in terms of holistic and dynamic changes. Proteomics provides a powerful technical platform to explore the biological mechanisms of holistic regulation of acupuncture [17,18].
In this review, we will fist introduce the proteomics technique, then to briefly summarize the current applications and new outcomes of this technique in the acupuncture studies. Finally, we will discuss future research directions of acupuncture to raise caution that systematic and holistic detections and investigations should be focus for better exploring mechanisms of acupuncture therapies. This may conform more to the concept of the Chinese medicine theories from a “holistic view” with multi-level, multi-target, dynamic regulations.
2. The general development and category of the systematic proteomics technologies
Proteomics refers to the systematic study of the identity, variable abundance, distribution, modification, interaction, structure, and function of a large profile of proteins and their involvement in disease [19]. Since proteins are directly involved in the entire process of physiology, proteomics enables dynamic monitoring of changes in protein expression to clarify the underlying mechanisms of disease and further identify specific biomarkers as well as potential therapeutic targets [[20], [21], [22]].
Sample collection and preparation is a great starting point for obtaining valid information in the process of proteomics analysis [23]. In general, samples used for proteomics analysis can be divided into two categories: tissues (stomach, lungs, kidneys, brain, etc.) and biological fluids (plasma, serum, saliva, urine, tear fluid, cerebrospinal fluid, etc.) [24,25]. The more accepted methods of sample collection and preservation of tissues were the fresh-frozen method and formalin-fixed paraffin-embedded method [26]. There were also protocols and best practice tutorials for biological fluid sample collection [27,28]. However, there are still many challenges in the sample collection process. In the case of plasma, for example, differences in centrifugation methods, freezing temperatures, and time of blood collection can all have an impact on the results [29]. The complexity of the sample collection process should be given adequate attention.
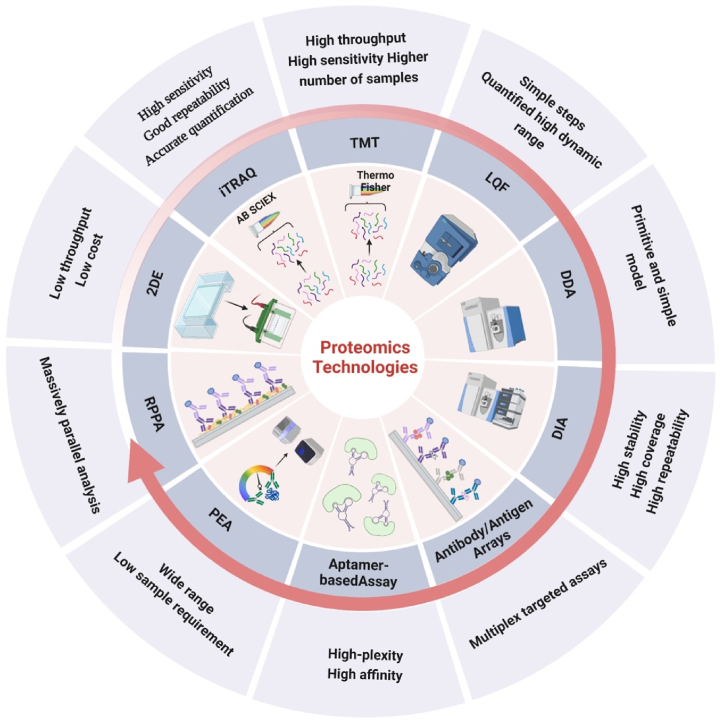
The development of proteomics depends to some extent on protein isolation techniques and the ability to identify and analyze proteins [30]. Currently, the most commonly used proteomics techniques include 2D electrophoresis (2-DE), mass spectrometry (MS), antibody/antigen arrays, etc. In the early discovery phase, protein screening is usually performed using low throughput methods such as 2-DE. This technique is still used today due to its robustness and low cost. The liquid chromatography-tandem mass spectrometry (LC-MS) has been increasingly chosen as the current core separation and identification for the proteomics research. Compared with 2-DE, LC-MS can be substantially more efficient, with advantages such as higher sensitivity and a larger detection range with lower injection samples [31]. With the advancement of technologies, various aspects of MS have been further optimized, especially the development of quantification techniques (e.g. isobaric tags for relative and absolute quantitation [iTRAQ], tandem mass tag [TMT], Label-free quantitation [LQF]), as well as the mass spectrometry scanning modes (data-dependent acquisition [DDA], data-independent acquisition [DIA]), etc. (Fig. 1) [32]. To meet clinical needs for easier detection and operation as well as faster imaging and recognization, many high-throughput and high-complexity protein chips technologies relying on the affinity reagents have emerged, such as antibody/antigen arrays, aptamer-based assays, proximity extension assay (PEA), reverse phase protein arrays (RPPA), etc [[33], [34], [35], [36]]. In addition, due to the high complexity of the human proteome, fractionation must be performed regardless of the analytical method used.
Fig. 1.
Overview of the main application features of proteomics technologies. The figure lists the current mainstream and innovative proteomics technologies. It also briefly describes the characteristics of each type of proteomics technology.
3. Application of proteomics in acupuncture research
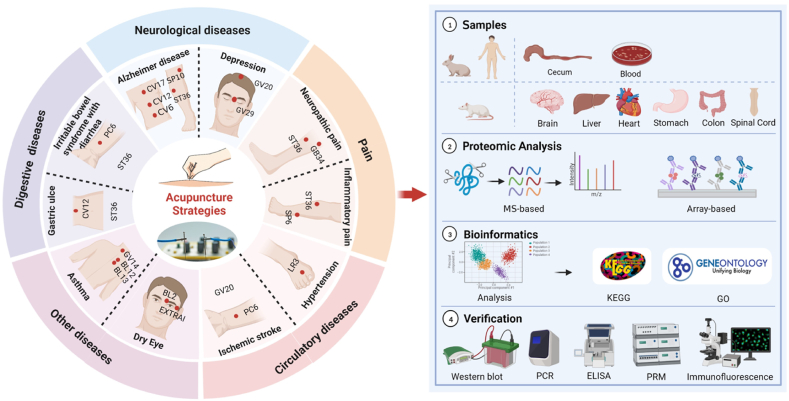
Recently, proteomics technologies have been actively applied to the field of acupuncture to identify differentially expressed proteins (DEPs), to screen and discover biomarkers, and to further analyze the biological functions of DEPs that modulated by acupuncture therapies. These findings have helped clearify the efficacy and elucidate the mechanisms of acupuncture regulations. Fig. 2 depicts the general scheme of proteomics techniques that were acchieved in fields of acupuncture treatment. Proteomics techniques have been applied mainly in studies where the intervention modality is acupuncture and electro-acupuncture (EA), and a few studies have investigated the mechanism of action of acupuncture tonic and diaphoretic techniques. Body fluid (e.g., blood, tears), and tissue organs (e.g., brain tissue, spinal cord tissue, heart tissue, lung tissue, liver tissue, stomach tissue, etc.) from humans or animals after acupuncture interventions were used for proteomics studies. LC-MS and iTRAQ were the most commonly used proteomics techniques for acupuncture, followed by 2-DE and MALDI-TOF-MS, only several studies chose label-free and antibody array. For the bioinformatics annotation after the quantification, the Gene ontology (GO) and the Kyoto Encyclopedia of Genes and Genomes (KEGG) was the mostly used to assess the molecular function, cellular composition, and biological processes, as well as to determine the interaction between proteins and biological pathways. For the post experimental pathway validations, Western blot (WB), ELISA were further used to make the results more reliable. In addition, a few studies have explored protein-protein interaction networks in depth.
Fig. 2.
General research scheme of proteomics techniques acchieved in acupuncture studies. The inner ring (white) in the diagram on the left lists the main modalities of the acupuncture stimulation strategy (acupuncture and electroacupuncture). The outer ring is five color blocks corresponding to neurological, pain, circulatory, digestive, and other disorders. The middle circle is a common prescription of acupoints for major diseases. The figure on the right is a schematic diagram of the sample sources, proteomic methods, bioinformatics analysis, and validation of proteomics studies in acupuncture research.
The overall effects of acupuncture include non-specific physiological effects and pathology-specific effects. Several studies have shown that acupuncture altered protein expression in humans and rats in healthy states produces [37,38]. It was suggested that the non-specific physiological changes of acupuncture were achieved through multiple biological pathways involving DEPs. However, more studies have explored the specific effects of acupuncture in disease states. Examples include acupuncture for neurological disorders, pain, circulatory disorders, and digestive disorders.
3.1. Proteomics studies in acupuncture against neurological disorders
Alzheimer's disease (AD) is a neurodegenerative disease that seriously endangers human brain memory and cognition [39], and imposes a huge economic burden on patients' families and society [40]. At present, there are no effective drugs that can curb the development of AD in clinical practice, only help with symptomatic relief [41]. Recent studies reported that acupuncture can help improve memory and cognitive impairment [42,43]. Table 1 summarizes the recent proteomics studies that provided scientific evidence for the acupuncture therapies for neurological disorders. SAMP8 mice have been widely used in studies of acupuncture for AD because of their markedly reduced learning and memory [44]. The biological effect produced by acupuncture of AD was related to alterations in protein expression. Hippocampus and amygdala were the main samples used for proteomic analysis. The composition of the acupuncture prescription has been key to the efficacy of acupuncture treatment. CV17, CV12, CV6, SP10, and ST36 were the commonly used acupuncture prescriptions, and BL23 and GV20 were often co-applied. The frequency of acupuncture is related to the specific disease and the animal model taken. Most studies chose to treat six times per week for a total of four weeks.
Table 1.
Application of proteomics in acupuncture for neurological disorders.
| Diseases | Therapy | Acupoints | Frequencies | Samples | Methods | DEPs | Functions and related pathways | Ref |
|---|---|---|---|---|---|---|---|---|
| AD | Acupuncture | CV17 CV12 CV6 SP10 ST36 |
3.5 min, 6/week, 4 weeks |
SAMP8 mice; hippocampus | iTRAQ Labeling; LC-MS/MS; |
605 DEPs (286 ↑, 319 ↓) | 1. Acupuncture improved the learning, memory ability and regulates. 2. Synaptic and mitochondrial functions were enriched for regulating pathways. |
[32] |
| Acupuncture | CV17 CV12 CV6 SP10 ST36 |
3.5 min, 6/week, 4 weeks |
SAMP8 mice; hippocampus | iTRAQ Labeling; LC-MS/MS; Immunofluorescence validation |
605 DEPs (286 ↑, 319 ↓) | 1. Acupuncture improved learning and memory ability. 2. Enrichment analysis showed that most of the proteins affected by needling were neuronal protrusions, the cytoskeleton, and involved biological processes such as intermediate filaments, keratin fibres, myelin sheaths, postsynaptic densities, and nerve fibre projections. |
[33] | |
| Acupuncture | CV17 CV12 CV6 SP10 ST36 |
3.5 min, 6/week, 4 weeks |
SAMP8 mice; hippocampus | iTRAQ Labeling; HPLC-MS/MS; Immunofluorescence and WB validation |
158 DEPs | 1. Acupuncture improved learning memory in the hippocampus. 2. Functional analysis showed that acupuncture could increase cytoskeleton-related proteins and “small G proteins” simultaneously. |
[34] | |
| Acupuncture | CV17 CV 12 CV6 SP10 ST36 |
3.5 min, 6/week, 4 weeks |
SAMP8 mice; hippocampal lipid rafts | HPLC-MS/MS | 39DEPs | 1. Acupuncture improved the cognitive ability. 2.3 cellular signaling pathways were involved: G protein-coupled receptor signaling, enzyme-linked receptor signaling, and ion channel-mediated signaling. |
[35] | |
| Acupuncture | CV17 CV12 CV6 SP10 ST36 |
3.5 min, 6/week, 4 weeks |
SAMP8 mice; hippocampus | iTRAQ Labeling; LC-MS/MS; |
299 DEPs | 1. Acupuncture significantly improved learning memory, and neuronal mitochondria were the key target. 2. KEGG results showed that oxidative phosphorylation, Parkinson's disease and protein processing in the endoplasmic reticulum are key pathways. |
[38] | |
| Acupuncture | BL23 GV20 SP10 BL17 |
11 min, 6/week, 8 weeks |
SAMP8 mice; hippocampus | 2-DE; MALDI-TOF-MS; WB validation |
13DEPs (9 ↑, 4 ↓) | 1.Acupuncture modulated the expression of a variety of structural and functional proteins in hippocampal mitochondria for the treatment. 2. NFL, ATP-β, TBB2A and NDUS1 were validated. |
[39] | |
| Acupuncture | GV20 BL23 BL17 SP10 |
11 min, 6/week, 8 weeks |
SAMP8 mice; amygdala | 2-DE; MALDI-TOF-MS |
9DEPs (6 ↑, 3 ↓) | 1.Acupuncture could improve mitochondrial energy metabolism, oxidative stress and reduce the production of Aβ to achieve the potential therapeutic effect. | [41] | |
| EA | GV20 BL23 GV16 |
20 min, 6/week, 16 weeks |
APP/PS1 transgenic mice; Cerebral cortex and hippocampus |
LQF; WB validation |
319DEPs (149 ↑, 170 ↓) | 1. EA prevented the decline of learning memory ability and the formation of Aβ age spots in the brain in a young rat model of AD. 2. KEGG analysis showed that the differential proteins were involved in alcohol addiction and the Apelin signaling pathway, among others. |
[42] | |
| VD | Acupuncture | ST36 GV20 |
20 min, 6/week, 2 weeks |
Wistar rats; hippocampus | iTRAQ labeling; LC -MS/MS; WB validation |
31DEPs | 1. Acupuncture could improve cognitive function by reducing the production of reactive oxygen species and increasing the survival of neuronal cells. 2. GO analysis showed that most of the DEPs were related to oxidative stress, apoptosis and synaptic function. |
[50] |
| PD | EA | GB34 | 20 min, 7/week, 2 weeks |
C57BL/6 mice; SN |
2-DE; MALDI-TOF MS; WB validation |
22 DEPs (21 ↑, 1 ↓) | 1. EA could protect DA neuron injury in mouse PD model. 2. CypA and NSF were selected as representative proteins for verification |
[61] |
| EA | GB34 GB39 |
20 min, 1/day, 12 days |
C57BL/6 mice; striatum | 2-DE; MALDI-TOF; WB validation |
13DEPs (12 ↑, 1 ↓) | 1. EA protected the cells of DA neurons. 2. HAGH was validated. |
[62] | |
| EA | GV14 GV20 |
10 min, 6/week, 4 weeks |
SD rats; the motor cortex | iTRAQ labeling; HPLC-MS; WB validation |
95 DEPs (60 ↑, 35 ↓) | 1. EA improved PD by attenuating dyskinesia in 6-OHDA rats. 2. KEGG pathway analysis showed that EA regulates AMPK signaling and adhesion pathways in impaired motor cortex. |
[63] | |
| Depression | EA | GV20 GV29 |
30 min, 7/week, 2 weeks |
Wistar rats; PFC |
iTRAQ labeling; HPLC-MS; WB validation |
52DEPs | 1. EA alleviated depression-like aphasic symptoms and anxiety behaviors, accompanied by improved synaptic morphology and an increase in PFC neurons. 2. KEGG pathway analysis identified seven pathways, including dopaminergic signaling, that contribute to the modulatory effects of EA. |
[55] |
| EA | GV20 GV29 |
30 min, 7/week, 1 weeks |
Wistar rats; Hippocampus |
iTRAQ; HPLC-MS/MS |
274DEPs (145 ↑, 129 ↓) | 1. EA improved antidepressant performance in depressed rats by protecting synaptic and mitochondrial functions in the hippocampus. 2. KEGG analysis revealed 5 important pathways including NAFLD, oxidative phosphorylation, Parkinson's disease, Alzheimer's disease, and Huntington's disease. |
[57] | |
| EA | GV20 GV29 |
20 min, 7/week, 4 weeks |
SPF rats; hippocampus | iTRAQ labeling; LC-MS/MS |
27DEPs (14↑, 13 ↓) | 1. EA improved depressive-like symptoms by regulating differential proteins. 2.27 proteins related to emotional disorders were successfully identified by iTRAQ. |
[56] | |
| Insomnia | Acupuncture | GV20 GV14 GV9 |
15 min, 1/day, 7 days |
SD rats; hypothalamic | iTRAQ labeling; LC-MS/MS; |
45 DEPs (28 ↑, 17 ↓) | 1. Acupuncture could effectively improve sleep by regulating protein expression processes. 2. Enrichment of GO and KEGG pathways showed that prolamin, NMDA receptor synaptic nuclear signaling and neuronal migration factor, transmembrane protein 41B and microtubule-associated protein 1B were closely associated with neuromodulation or insomnia treatment. |
[64] |
| Epilepsy | Acupuncture | HT8 | 6 min, 1/day, 3 days |
C57BL/6 mice; hippocampus | 2-DE; MALDI-TOF-MS; WB validation |
11DEPs | 1. Acupuncture altered the protein expression profile of the hippocampus, favoring neuronal survival in kainic acid-treated mice. 2. PURA and PP5 were selected for validation. |
[65] |
| Maternal separation | Acupuncture | HT8 | 1min, 7/week, 1 weeks |
SD rats; hypothalamic | 2-DE; MALDI-TOF-MS |
27DEPs (9 ↑, 5 ↓) | 1. Acupuncture could induce severe stressful conditions in early life. | [66] |
AD, Alzheimer disease; VD, Vascular dementia; PD, Parkinson's disease; DEPs, differentially expressed proteins; WB, Western blot; iTRAQ, isobaric tags for relative and absolute quantitation; LC–MS/MS, liquid chromatography with tandem mass spectrometry detection; HPLC, high performance liquid chromatography; 2DE, 2D electrophoresis; MALDI-TOF-MS, matrix-assisted laser desorption/ionization time of flight mass spectrometry; MSMS, tandem mass spectrometry; LQF,Label-free quantitation; GO, Gene ontology; KEGG, Kyoto Encyclopedia of Genes and Genomes; SD,Sprague-Dawley; SPF, specific pathogen free; PFC, Prefrontal cortex; SN, the substantia nigra; EA, electroacupuncture.
Several experiments reported that learning and memory abilities can be improved by acupuncture in animal models. Proteomic analysis showed that acupuncture substantially upregulated the expression of neuronal protrusion and cytoskeleton-related proteins, which underlie the structure and function of synaptic plasticity [45]. Another study achieved similar results and showed that DEPs were also involved in biological processes such as intermediate filaments, keratin fibers, myelin sheaths, postsynaptic density, and nerve fiber protrusions [46]. Further exploration of the neuronal cytoskeleton revealed that acupuncture at these points increased both cytoskeleton-associated proteins and small G proteins [47]. Nie et al. [48] reported that acupuncture recruited more kinases, ion channel proteins, and transmembrane signaling receptors, including G protein-coupled receptor-mediated pathways, which plays a key role in AD [49].
In addition, another key target for acupuncture in the treatment of AD was the neuronal mitochondria. Mitochondrial dysfunction was closely associated with the core pathological features of AD [50]. Acupuncture intervention in SAMP8 mice significantly upregulated the expression of mitochondria-related proteins [51]. Liang et al. [52] found that DEPs were involved in the regulation of mitochondrial function as well as structure after proteomic analysis. One of them, NFL, was one of the biomarkers of neurodegeneration and was closely associated with cognitive dysfunction [53]. Some researchers also found that the function of the differential protein involves oxidative stress and β-amyloid (Aβ) production in addition to mitochondrial energy metabolism [54]. Apart from SAMP8 mice, APP/PS1 double transgenic mice is another frequently used species for the AD study which expressing mouse/human amyloid precursor protein (Mo/HuAPP695swe) and mutant human progerin 1 (PS1-DE9). Zhang et al. [55] chosed the APP/PS1 juvenile mice to explore the potential mechanisms of acupuncture in the treatment of AD from a preventive perspective. This was known as “prevention before illness” in Chinese medicine theory. This study confirmed that EA was effective in preventing learning and memory deficits in APP/PS1 juvenile mice in adulthood and significantly reduced the formation of cortical and hippocampal Aβ age spots in AD model mice in adulthood. DEPs in the study such as epoxide hydrolase 4 (EH4), neurolysin, histone-H 3, GNB 5, Aβ, calsyntenin-3, myoglobin, metallothionein-1, and neurogranin have also been reported to be directly or indirectly associated with AD or Aβ [[56], [57], [58], [59], [60], [61]].
Vascular dementia (VD) is the second most common cognitive disorder after AD [62]. GV20 and ST36 have cerebral blood flow and anti-inflammatory effects in the ischemic area of the node [63]. Acupuncture ST36 and GV20 improved cognitive function in rats [64]. Proteomic analysis showed that S100B and SOD1 were differential proteins and the results were verified by Western blot to be reliable. Levels of S100B and SOD1 were considered to be markers of efficacy in subcortical vascular dementia [65,66]. The above studies suggest that proteomics is a useful tool to investigate the mechanisms underlying the effects of acupuncture prescription for AD. Through a variety of proteomic assays, it has been confirmed that acupuncture can modulate specific proteins and signaling pathways in multiple regions of the brain to improve cognitive levels in AD and VD.
Next, Parkinson's disease (PD) is also one of the predominant diseases treated with acupuncture [67]. The major behavioral impairment in PD was caused by a massive loss of dopaminergic (DA) substantia nigra (SN) neurons and depletion of striatal dopamine. GB34 was widely used to treat movement disorders because it protected SN and striatal DA neurons [68,69]. EA stimulation of GB34 protected nigrostriatal DA neurons in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) mice [70]. Nine pinprick-specific proteins were found to be involved in cell death regulation, inflammation, or injury repair [71]. Proteomic analysis of the striatum of MPTP mice suggested that differential proteins may be involved in cellular metabolism. The protein HAGH was validated for reliable results. In addition, EA significantly improved spontaneous ground plane locomotion and stick-turning performance in a 6-hydroxydopamine (6-OHDA) unilateral injection-induced PD rat model [72]. EA-altered DEPs were involved in increasing autophagy, mRNA processing, and ATP binding as well as maintaining neurotransmitter homeostasis. In summary, EA facilitated the survival of DA neurons and attenuated toxicity such as oxidative stress, thus exerting a neuroprotective effect on different brain regions of PD mice.
Apart from the AD and PD, depression is another common neurological disorder [73]. EA has also been shown to be effective in the treatment of depression. In Chinese medicine, GV 20 and GV 29 are both part of the governor meridian, which reaches up to the medulla oblongata, so these two points are more effective in treating mental disorders. Therefore, these two acupoints are frequently used in combination for the treatment of depression [74,75]. Studies using samples from the prefrontal cortex have found that EA can alleviate symptoms of pleasure deficit, increase the number of PFC neurons and modulate dopaminergic signaling pathways to treat depression [76]. Analysis of the KEGG pathway revealed seven significantly enriched pathways in which the dopaminergic synapse, cocaine addiction, amphetamine addiction, and alcoholism pathways share the same four upregulated proteins. They were all important components of the dopaminergic synaptic signaling pathway. GUO et al. [77] also demonstrated that dopamine may be involved in the antidepressant-like effects of EA. In another study [78], EA treatment was also found to have an antidepressant effect by protecting mitochondrial function in the synapse and hippocampus. Further research is needed in the future to investigate the specific mechanism of action of EA in depression and whether the differential proteins identified are associated with EA or depression.
Moreover, acupuncture can also treat neurological disorders such as insomnia, epilepsy and maternal separation. Studies have shown that acupuncture treats neurological disorders by improving neuronal function, increasing neuronal survival, and promoting neurodevelopment [[79], [80], [81]].
3.2. Application of proteomics in acupuncture analgesia
In addition to the ability of acupuncture to treat neurological disorders, the effectiveness of acupuncture for analgesia has also been demonstrated [82,83]. The diseases in which proteomics techniques have been applied to study acupuncture analgesia include neuropathic pain (NP), inflammatory pain, and chronic pain (Table 2). Different types of pain involve various sites and mechanisms. Current samples applied for proteomic analysis include various regions of brain tissue, spinal cord, blood, etc.
Table 2.
Application of proteomics in acupuncture analgesia.
| Diseases | Therapy | Acupoints | Frequencies | Samples | Methods | DEPs | Functions and related pathways | Ref |
|---|---|---|---|---|---|---|---|---|
| NP | EA | ST36 GB34 |
30 min, 1/day, 12 days |
Wistar rats; hippocampal | 2-DE; MALDI-TOF MS; PCR and WB validation |
19 DEPs (11 ↑, 8 ↓) | 1. EA can reverse the reduction of thermal pain thresholds in the affected foot and plantar in the CCI model and had an analgesic effect on neuropathic pain. 2. Functional analysis revealed that DEPs are involved in cysteine metabolism, valine, leucine and isoleucine degradation and MAPK signaling. |
[72] |
| EA | ST36 GB34 |
30 min, 1/day, 12 days |
Wistar rats; hypothalamus | 2-DE; MALDI-TOF MS; PCR and WB validation |
17 DEPs | 1. EA was able to improve the affected foot and plantar thermal pain thresholds in the CCI model. 2. Functional analysis showed that changes in the expression of several proteins involved in REDOX enzyme activity, REDOX, protein binding, and glycolysis/gluconeogenesis/glucose metabolism may be involved. |
[71] | |
| EA | ST36 | 30 min, 7/week, 1 weeks |
SD rats; hypothalamus | 2-DE; MALDI-TOF MS; |
36 DEPs | 1. EA had a relieving effect on mechanical pain. 2. DEPs were involved in inflammation, enzyme metabolism and signal transduction. |
[73] | |
| EA | GB30 GB34 |
30 min, 7/week, 3 weeks |
SD rats; hippocampal | TMT Labeling; LC-MS/MS; ELISA and WB validation |
53 DEPs (17 ↑, 36 ↓) | 1.EA improved neuropathic pain by eliminating mechanical pain sensitivity and memory deficits. 2. KEGG pathway analysis showed that DEPs were mainly enriched in drug metabolism, cytochrome P450 and isobiomass metabolism through cytochrome P450 pathway. |
[74] | |
| KOA | EA | ST36 GB34 |
30 min, 7/week, 2 weeks |
SD rats; synovial | DIA-MS; WB validation |
222 DEPs (144 ↑, 78 ↓) | 1. EA could alleviate inflammatory pain behaviors and cartilage damage. 2. GO analysis suggested that cytokines secreted by macrophages may be involved in the regulation of KOA by EA. |
[75] |
| DPN | EA | ST36 BL23 |
30 min, 4/week, 4 weeks |
SD rats; The L4-5 spinal |
TMT Labeling; LC-MS/MS; |
97 DEPs (14 ↑, 83 ↓) | 1. EA could improve DPN by regulating mechanical pain threshold and fasting blood glucose level in rats. 2. KEGG pathway enrichment analysis suggested that oxidative phosphorylation was a major factor involved in the effects of EA therapy on DPN. |
[76] |
| BCP | EA | ST36 BL60 |
30 min, 7/week, 1 weeks |
SD rats; the L4–L6 DRGs | PEX100 protein microarray (antibody array); WB validation |
176 DEPs | 1. EA alleviated mechanical pain behavior and to some extent bone destruction in BCP rats. 2. KEGG pathway analysis showed that ErbB signaling pathway, PI3K-Akt signaling pathway and MAPK signaling pathway were closely related to BCP or cancer. |
[77] |
| pain aversion | EA | ST36 SP6 |
30 min, 7/week, 2 weeks |
SD rats; The amygdala |
iTRAQ labeling; LC-MS/MS; WB validation |
11 DEPs | 1. EA could increase the claw threshold of inflammatory pain, confirming the possible mechanism of EA in the central nervous system of pain aversion. 2. Glyceraldehyde-3-phosphate dehydrogenase, glutamate transporter-1 and P21-activated kinase 6 were selected for validation. |
[78] |
| Inflammatory pain | EA | ST36 SP6 |
20 min, 1/day, 2 days |
SD rats; The lumbar spinal cords |
2-DE; MS/MS; |
8 DEPs (4 ↑, 4 ↓) | 1. EA stimulation attenuates CFA-induced inflammatory hyperalgesia. 2.8 loci were differentially phosphorylated proteins. |
[79] |
| Chronic Myofascial Pain | Acupuncture | 6 local twitch responses | 20 min, 1/week, 4 weeks |
SD rats; the gray matter | TMT Labeling; LC-MS/MS; PRM Validation |
107 DEPs (47 ↑, 60 ↓) | 1. Dry needling ameliorated chronic myofascial pain by modulating nociceptive mechanical thresholds in the left hind paw in an active myofascial trigger point rat model. 2. GO and KEGG enrichment results showed that dry needling can significantly modulate tightly connected pathways. |
[80] |
| Chronic Pain | EA | LI10 LI11 |
30 min, 4/week, 3 weeks |
SD rats; PFC and hippocampus |
iTRAQ; LC-MS/MS; Immunofluorescence and WB validation |
8 DEPs(PFC); 14 DEPs(hippocampus) |
1. EA could alleviate chronic pain syndrome, inhibit the cognitive dysfunction caused by chronic pain and restore normal cellular structure. 2. The MARKS in the PFC and PAK2 and ACAT1 in the hippocampus were further validated. |
[81] |
| SCI | EA | GV6 GV9 T7 T11 |
20 min, 4/week, 3 weeks |
SD rats; The spinal cord |
2-DE; MALDI-TOF MS; Immunofluorescence and WB validation |
15 DEPs | 1.EA could improve neuronal survival and thus contribute to SCI recovery. 2. ANXA5 and CRMP2 were further validated. |
[82] |
| Migraine | Acupuncture | DU20 DU24 GB13 GB8 GB20 |
30 min, 3/week, 4 weeks |
Human; Blood |
DIA; HPLC-MS/MS |
29 DEPs | 1.Acupuncture could be effective in relieving migraines. 2. KEGG analysis showed differences in riboflavin metabolism and glycolytic pathways after acupuncture. This suggested that acupuncture modulation of migraine was associated with changes in energy metabolism. |
[83] |
NP, neuropathic pain; KOA, knee osteoarthritis; DPN, Diabetic Painful Neuropathy; BCP, Bone Cancer Pain; SCI, Spinal cord injury; WB, Western blot; TMT, tandem mass tag; DIA,indicates data-independent acquisition; PCR, polymerase chain reaction; ELISA, Enzyme-linked Immunosorbent Assay; PRM, Parallel reaction monitoring; DRGs, Dorsal root ganglions; CFA, Complete Freund's adjuvant.
In terms of the frequency of acupuncture, the frequency of treatment varied for the same animal models chosen. Each acupuncture treatment lasts 20–30 min. The treatment course is usually higher in SD rats than in Wistar rats. In terms of acupoint selection, ST36 and GB34 are reported to be the key acupoints for analgesia in Chinese medicine, the ability of which by traditional needling stimulation have been reported by recent laboratory studies [84,85]. EA interventions with ST36 and GB34 were reported to reverse the reduction in thermal pain thresholds in the affected foot and plantar surface of chronic constrictive injury (CCI) rats [86,87]. Proteomic analysis of the hippocampus and hypothalamus using a 2-DE combined with MALDI-TOF MS strategy showed that EA analgesia may be achieved through the regulation of multiple protein expression as well as signaling pathways, including inflammation, enzyme metabolism and signal transduction. Similar results were obtained in a study by Sung et al. [88] Notably, Gong et al. [89] chose GB34 combined with GB30 by EA to intervene in the hippocampus of NP mice, reporting that hippocampal TMEM126A plays an important anti-inflammatory role in EA for neuropathic pain. Furthermore, pain caused by other diseases, such as osteoarthritis of the knee (KOA), diabetic painful neuropathy (DPN), and bone cancer pain (BCP), could also be treated with EA on the acupoints of ST36 and GB34 [[90], [91], [92]]. These studies revealed by different proteomic analysis techniques that EA could alleviate inflammatory pain behavior and cartilage damage, could inhibit cytokines secreted by macrophages in KOA, could regulate oxidative phosphorylation in DPN disease, and could alleviate pain and bone destruction in BCP disease. Among them, Wang et al. [93] found that the potential mechanism of EA treatment for BCP by PEX100 protein microarray may be related to mTOR phosphorylation and mTOR pathway.
ST36 and SP6 are another common pairs of acupoint prescriptions for analgesia, especially for inflammatory pain. Several studies have demonstrated that EA interventions on ST36 and SP6 reduced thermal nociceptive hypersensitivity in rats in a complete Freund's adjuvant (CFA)-induced inflammatory pain model [94,95]. On the one hand, a study performed and validated proteomic analysis of the amygdala, showing that the expression of GAPDH, GLT-1, and PAK6 was closely associated with EA intervention in this disease. On the other hand, the spinal cord samples was collected for phosphoproteomic analysis, and a total of eight differentially phosphorylated proteins were identified (Table 2). These proteins are involved in processes such as cellular signaling, protein transport, and transcription. In summary, both central and peripheral mechanisms of acupuncture for inflammatory pain have been initially confirmed. Valuable protein biomarkers for acupuncture analgesia were provided.
Chronic pain is widespread across the globe. Several studies have shown that acupuncture helps to modulate the mechanical threshold of nociception and alleviate chronic pain syndromes [96,97]. Li et al. used acupuncture to intervene in the gray matter of chronic fascial pain mice for proteomic analysis and validated Actin α3, Calsequestrin-1, and microalbumin α by parallel reaction monitoring (PRM). Studies have confirmed the dominant role of the tight junction pathway in the management of chronic pain with acupuncture. Another study showed that in addition to relieving chronic pain, acupuncture can inhibit chronic pain-induced cognitive dysfunction and restore normal cellular structure. Proteomic analysis of the PFC and hippocampus revealed that marcks, pak2, and acat1 may be potential targets for acupuncture treatment. This indicates that the central mechanism of acupuncture to improve pain involves multiple brain regions, further demonstrating that acupuncture is a holistic, multi-targeted treatment modality.
In addition, Li et al. [98] investigated the effect of EA on spinal cord injury (SCI) and demonstrated that EA is useful for neuronal survival. Proteomic analysis identified ANXA 5 and CRMP 2 as key pinprick-specific proteins. In another study, Liu et al. [99] selected human blood as a sample to investigate the mechanism of acupuncture intervention in migraine. The study demonstrated that acupuncture can relieve migraine by regulating energy metabolic pathways.
3.3. Application of proteomics in acupuncture for circulatory diseases
This section evaluates the proteomic changes associated with acupuncture in both human and animal models of hypertension and ischemic stroke (IS) to investigate the potential mechanisms through which acupuncture can alleviate circulatory disorders. Table 3 provided comprehensive details regarding the specific acupuncture methods utilized, the acupoints selected, the samples, and the proteomics methodologies employed in this section.
Table 3.
Application of proteomics in acupuncture for circulatory diseases.
| Diseases | Therapy | Acupoints | Frequencies | Samples | Methods | DEPs | Functions and related pathways | Ref |
|---|---|---|---|---|---|---|---|---|
| Hypertension | acupuncture | LR3 | 5 min, 7/week, 1 weeks |
SD rats; The medulla |
2-DE; MALDI-TOF MS; qRT-PCR, WB and ELISA validation |
23 DEPs | 1. Acupuncture could significantly reduce systolic blood pressure in rats, but not enough to reduce blood pressure to normal level. 2. Reduced oxidative stress may be a potential mechanism for acupuncture in the treatment of hypertension. |
[89] |
| EA; TRFM; TRDM |
LR3 | 20 min, 7/week, 2 weeks |
SHR and WKY rats; hypothalamus | Label-free; LC-MS; PRM-MS validation |
117 (EA/M), 61 (TRFM/M) and 86 (TRDM/M) DEPs | 1. TRDM, TRFM and EA procedures reduced blood pressure measurements, with TRDM being the most effective of the techniques used. 2.12(RF/M),11(RD/M),15(EA/M) pathways were significantly different in terms of protein enrichment, respectively. |
[86] | |
| TRFM; TRDM |
LR3 | 20 min, 7/week, 2 weeks |
SHR and WKY rats; the parietal cortex | Label-free; LC-MS |
72 DEPs in TRFM/M 1043 DEPs in TRDM/M |
1. Acupuncture had a blood pressure regulating effect, and the TRDM group had a better antihypertensive effect than the TRFM group. 2. TRDM improved oxidative phosphorylation, regulates vasoconstriction and smooth muscle proliferation, improves lipid metabolism and reduces glucose metabolism, improves inflammation and endothelial function, and reduces sympathetic excitability. |
[87] | |
| RF; RD; EA |
LR3 | 20 min, 7/week, 2 weeks |
SHR and WKY rats; Cerebellum |
Label-free; LC-MS; PRM-MS validation |
96,133 and 216 DEPs | 1.RD, RF, and EA at LR3 lowered blood pressure, with RD being the most effective. 2. KEGG pathway enrichment indicated that there were significant differences in protein enrichment in 12 (RF/M), 11 (RD/M) and 15 (EA/M) pathways respectively. |
[88] | |
| IS | EA | GV20 GV24 |
30 min, 7/week, 2 weeks |
SPF rats; hippocampal | TMT labeling; LC -MS/MS; WB validation |
218DEPs (168 ↑, 50 ↓) | 1.EA significantly alleviated neurological deficits, spatial learning and memory deficits, and cerebral infarction in cerebral ischemic stroke rats. 2. GO enrichment analysis showed that DEPs were associated with brain and neurodevelopment. |
[92] |
| EA | MS6 BL10 GB20 LI4 PC6 B40 SP6 ST36 |
30 min, 1/day, 10 days |
Human; Blood |
2-DE; MALDI-TOF/TOF-MS; WB validation |
7 DEPs (6 ↑, 1 ↓) | 1.EA could improve the muscle strength of the upper and lower limbs to treat acute IS. 2.7 DEPs were found. SerpinG1, coagulation proteins and C3 were validated. |
[93] | |
| Acupuncture | GV20 2 points beside GV20 |
30 min, 6/week, 2 weeks |
SD rats; the brain | SDS-PAGE; LC-MS/MS; |
414 DEPs | 1. Acupuncture improved neurological function scores and motor dysfunction in a middle cerebral artery occlusion model rat. 2. DEPs were mainly involved in cellular signal transduction, protein transport, and exerted their biological functions through various synaptic and metabolic pathways. |
[99] | |
| I/R injury of the heart | EA | PC6 | 30 min, 1/week, 1 weeks |
SD rats; heart tissue | 2-DE; Antibody and WB validation |
26DEPs | 1. EA reduced I/R injury in rat heart. 2. The expression of myocardial interleukin-1 B-converting enzyme (ICE) was gradually decreased by EA treatment, while the expression of glycogen synthase-3 a (GSK-3) was increased in EA. |
[102] |
IS, ischemic stroke; I/R, Ischemia-reperfusion; WB, Western blot; SDS-PAGE, sodium dodecyl sulphate-polyacrylamide gel electrophoresis; qRT-PCR, Quantitative Reverse Transcription-polymerase Chain Reaction; SHR, spontaneously hypertensive rats; WKY, Wistar-Kyoto; TRFM, twirling reinforcing manipulation; TRDM, twirling reducing manipulation; RF,twirling reinforcing manipulation group; RD,twirling reducing manipulation group.
Acupuncture has been shown to be an effective treatment of hypertension [100]. Previous research has established that hypertension pathogenesis is linked to the central nervous system's malfunctioning brain regions [101]. Proteomics studies have confirmed the effectiveness of acupuncture in treating hypertension [[102], [103], [104], [105]]. Four studies have used acupuncture to intervene on LR3 in spontaneously hypertensive rats (SHRs) and found more than 100,000 DEPs in various brain regions, including the medulla oblongata, hypothalamus, parietal cortex, and cerebellum (refer to Table 3). The majority of DEPs underwent validation through parallel reaction monitoring (PRM), and the results were found to be generally consistent with the experimental trends observed. Early studies have found that acupuncture on LR3 relieves hypertension compared to non-acupoints [105]. Proteomics studies have shown that needling LR3 can alter the protein expression profile of SHRs, and the regulatory mechanisms involve multiple biological processes in multiple brain regions. Out of these findings, the neoplastic neurotrophic factor (NENF) has been suggested as a possible target for hypertension treatment [102]. Mechanisms frequently detected by acupuncture antihypertensive proteomics primarily include improved oxidative phosphorylation and regulation of vasoconstriction and smooth muscle proliferation [103,104].
Different methods of acupuncture manipulation were one of the influencing factors that produced the aforementioned differential expression of proteins and biological functions. The Twirling reducing manipulation (TRDM) and the Twirling reinforcing manipulation (TRFM) are both acupuncture manipulation methods that achieve augmentation and decrementation by manually twisting the needle. Interestingly, these studies compared also the impact ofthe TRDM with the TRFM on managing hypertension, reported that TRDM was the most effective in lowering the blood pressure [[102], [103], [104]]. In the future, it would be worthwhile to explore the hypotensive effects of specific acupuncture operations on various acupoints. A further selection of other brain regions for proteomic analysis will help to systematically reveal the potential mechanisms of action of different acupuncture manipulations.
IS is a circulatory disease characterized by high mortality and self-injury rates [106]. Acupuncture can significantly improve the motor dysfunction and cognitive level caused by stroke [107]. Recently, researchers have been actively investigating reliable biomarkers for IS, which can help identify effective targets and mechanisms for acupuncture treatment of IS. Notably, differential expression of various proteins, including Pak4, Akt3, Efnb2, SerpinG1, complement component I, C3, C4B, and beta-2-glycoprotein I, were observed after acupuncture treatment for IS [108,109]. In particular, Pak4, Akt3, and Efnb2 are considered to be important neuroprotective factors [[110], [111], [112]]. Complement components and beta-2-glycoprotein I also play an important role in the pathogenesis of IS [113,114]. Thus, it was hypothesized that the neuroprotective effects of acupuncture for IS may be mediated through mechanisms such as complementary activation, inhibition of apoptosis, and anti-thrombosis. Other reports also showed that acupuncture increased the expression levels of Cdc42 and GFAP in rats with middle cerebral artery obstruction [115]. GFAP can be used to identify stroke subtypes, which can be extremely helpful for acupuncture precision treatment [116,117]. Additionally, acupuncture can play a cardioprotective role against I/R injury by regulating cytochrome P450 and glycogen synthase kinase-3 a [118].
3.4. Application of proteomics in acupuncture for digestive system diseases
According to Chinese medicine principle, therapeutic effects may differ when using different combinations with acupoints. Therefore, identifying a scientific and reasonable effective combination of acupoints is crucial for improving acupuncture efficacy. There are some commonly used acupoint combinations for treating digestive system diseases by acupuncture [119,120]. For example, ST36-CV12 and ST36-PC6 have been applied to ameliorate gastrointestinal disorders (Table 4) [[121], [122], [123], [124], [125]].
Table 4.
Application of proteomics in acupuncture for digestive system diseases.
| Diseases | Therapy | Acupoints | Frequencies | Samples | Methods | DEPs | Findings | Ref |
|---|---|---|---|---|---|---|---|---|
| Stress gastric ulcer | Acupuncture | CV12 ST36 |
10 min, 7/week, 1 weeks |
Wistar rats; Stomach tissues |
SDS-PAGE; nano-LC/MS | 14 DEPs | 1.The combination of CV12 and ST36 was superior to single acupoints and other acupoints combinations for the prevention of stress ulcers. 2. DEPs species included enzymes, backbone proteins, transport proteins and heat shock proteins. |
[105] |
| gastric ulcer | EA | CV12 ST36 |
20 min, 7/week, 1 weeks |
SD rats; Stomach tissues |
TMT labeling; HPLC-MS; ELISA; WB validation |
133 DEPs (55 ↑, 78 ↓) | 1. Stimulation of CV12 and ST36 by EA improved gastric ulcers. 2. KEGG results showed that the gastric acid secretion pathway was significantly regulated after acupuncture. |
[106] |
| CAG | acupuncture | RN12 PC6 ST36 |
20 min, 3/week, 20 weeks |
Human; peripheral blood | iTRAQ Labeling; HPLC-MS/MS; ELISA validation |
66 DEPs | 1. Acupuncture could improve the histopathological changes of gastric mucosa in CAG patients. 2. Bioinformatics analysis showed that actin binding proteins (ABPs) and Notch signaling pathway related proteins were closely related to the occurrence and development of CAG. |
[107] |
| IBS-D | Acupuncture | ST36 PC6 CV4 |
18 min, 6/week, 4 weeks |
SD rats; colon | LQF; LC-MS/MS; WB validation |
56 DEPs (37 ↑, 19 ↓) | 1. Acupuncture could alleviate IBS-D. 2. IBS-D was related to processes such as energy metabolism and muscle excitation/contraction, and acupuncture could reverse the impaired normal energy metabolism. |
[108] |
| Enteritis | EA | ST36 | 30min, 2/week, 3weeks |
New Zealand white rabbits; The PVS on the cecum |
TMT; LC-MS/MS; WB validation |
110 DEPs (65 ↑, 45 ↓) | 1. EA could relieve bleeding, ulcers, adhesions and thickening of the intestinal wall of enteritis and regulate the inflammatory process. 2. Enrichment analysis revealed possible involvement in the treatment of enterocolitis through processes such as promotion of inflammatory cell proliferation, antigen expression and cell adhesion. |
[110] |
| T2DM with NAFLD | acupuncture | BL13 BL20 BL23 LI4 ST36 SP6 LR3 |
20 min, 6/week, 2 weeks |
db/db and db/m mice; liver tissues |
TMT/iTRAQ labelling; HPLC-MS/MS; ELISA; PRM validation |
122 DEPs (73 ↑, 49 ↓) | 1. Acupuncture could reduce the risk of NAFLD by reducing lipid storage in liver cells, inhibiting de novo fat synthesis, and promoting fatty acid oxidation. 2. DEPs of KEGG pathway are mainly involved in PPAR signaling pathway, fatty acid biosynthesis, fatty acid metabolism, fatty acid elongation, fat digestion and absorption. |
[109] |
CAG, chronic atrophic gastritis; IBS-D, Irritable bowel syndrome with diarrhea; T2DM, Type 2 diabetes mellitus; NAFLD, non-alcoholic fatty liver disease; PVS,primo vascular system; DA,attenuates dopaminergic; WB, Western blot.
The acupuncture treatment with acupoint combination of ST36-CV12 on gastric ulcer rats model displayed 14 relevant DEPs, mainly including enzymes, motor (skeletal) proteins, transporter proteins, and heat shock proteins [121]. The same combination of acupoints using EA on a gastric ulcer rats model evaluated proteomic changes and found 133 DEPs in another study by the same research team, which may be due to different proteomic techniques used [122]. These studies suggested that acupuncture improves gastric ulcer symptoms by regulating abnormal gastric acid secretion, stimulating the immune system, positively regulating apoptosis, and promoting the ability to repair gastric mucosal damage. Another report also assessed proteomic changes in the peripheral blood serum of patients with acupuncture intervention in chronic atrophic gastritis (CAG) [123]. In addition to ST36-PC6, RN12 was added to the selection of acupoints. Bioinformatic analysis speculated that acupuncture achieved the treatment of CAG by modulating actin-binding proteins and gap-signaling pathway-related proteins. In conclusion, the above studies indicated that interfering with different combinations of acupoints can lead to different protein expression and pathways. The proteomic study of acupuncture with multiple acupoint combinations deserves further in-depth investigation.
In irritable bowel syndrome (IBS-D), proteomic analysis has shown an association with biological processes such as energy metabolism and muscle excitation/contraction [124]. Acupuncture reversed the impairment of normal energy metabolism and used Atp5a1 and Bpnt1 as key targets. Moreover, TMT-based proteomic analysis was utilized to identify changes in protein expression in rabbit models of enteritis treated by EA, revealing that most of the identified DEPs were related to inflammatory and immune processes [126]. Among them, CD40, CD45, HLA-DRA1, LAMP1, JAGN1 and FGL1 were alleviated or reversed by EA treatment, showing that EA has significant anti-inflammatory effects.
In a study of acupuncture for the treatment of type 2 diabetes mellitus (T2DM) combined with nonalcoholic fatty liver disease (NAFLD), liver tissue from mice was analyzed using proteomics [125]. KEGG analysis revealed that DEPs were mainly enriched in the PPAR signaling pathway, fatty acid biosynthesis, fatty acid metabolism, fatty acid elongation, fat digestion, and absorption. Acupuncture was found to reduce the risk of hepatocyte steatosis by inhibiting lipid resynthesis, promoting fatty acid oxidation, and regulating the expression of proteins related to glucose and lipid metabolism. These findings may provide new targets and pathways for acupuncture to treat digestive disorders. In addition the frequency of acupuncture for digestive disorders is generally low compared to other systemic disorders. Each treatment typically lasts 10–30 min. The overall course of treatment is 1–4 weeks. Except for CAG, of course, which is related to the fact that the samples are human blood and the characteristics of the disease.
3.5. Application of proteomics in acupuncture for other diseases
In addition to the diseases mentioned above, proteomics has been applied in the field of acupuncture for treating respiratory diseases, ophthalmic diseases, immune system diseases, gynecological diseases, etc. Acupoints GV14, BL12, and BL13 are frequently used in acupuncture treatment for Asthma, which is a chronic inflammatory disease of the respiratory tract that has a wide impact worldwide [127]. A proteomic study showed that acupuncture reduced the expression of SLC3A2, ATP1A3, S100A8, RAGE, and S100A11 and increased the expression of CC10, ANXA5, sRAGE, and Cyp A in lung tissues and serum of an Obamacin-induced mouse asthma model [[128], [129], [130]]. These results suggested that acupuncture could treat asthma by regulating processes such as iron sagging, oxidative stress, immune regulation, gene expression, and protein synthesis.
In ophthalmic diseases, acupuncture can improve ocular surface disease index scores and symptoms in patients with dry eye disease (DED) by intervening at common acupuncture points such as BL2,EXTRAI and stimulating lacrimal gland secretion. In a proteomic study on the effect of acupuncture on tear secretion in rabbits, six proteins were upregulated while five were downregulated [131]. Of these, Annexin A1 and S100A9 were considered as potential therapeutic targets for DED [132]. Another study showed a twofold increase in tear protein counts of secreted proteins and a significant decrease in cytosolic proteins after acupuncture [133]. Tong L et al. [134] found similar results.
Gynecological disorders, such as ovarian hyperstimulation syndrome (OHSS), can also be effectively treated by acupuncture. A proteomics study reported that the EA therapy could reduce oocyte numbers and preserve the vascular barrier against OHSS by triggering a CD200-mediated anti-inflammatory response, and could reverse the down-regulation of CD200 in the ovaries of the OHSS rat model [135]. Overall, this study provided a scientific basis for the potential targets of acupuncture for OHSS.
4. Proteomic characterization of acupuncture mechanisms
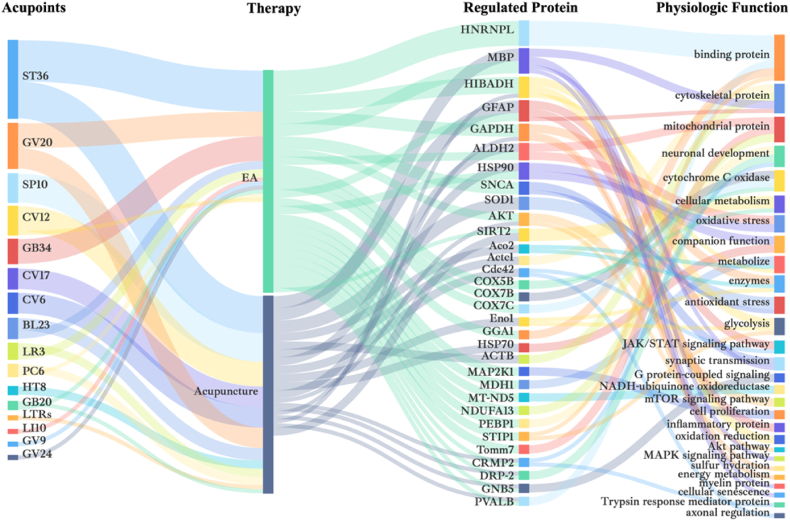
In summary, all proteomics studies in the field of acupuncture have provided a large number of proteins that may be involved in the mechanism of disease amelioration by acupuncture. Most acupuncture regulation of proteins may be specific to each disease type or sample tissue collected. However, a deeper comparison of the above proteomics studies applied to acupuncture reveals a small amount of overlapping protein regulation (Fig. 3). Interestingly, some of the proteins were regulated in multiple diseases as well as in different modalities of acupuncture intervention. Overall, the higher frequencies in the selection of acupuncture points were ST36,SP10,CV12,GV20 and GB34.
Fig. 3.
Proteomic characterization of acupuncture mechanisms. The diagram summarizes the overlapping acupoints, therapy, regulated proteins, and phsiologic functions.
Based on the physiological functions of the proteins, the overlapping proteins (in order of overlap frequency) can be broadly categorized as binding proteins, cytoskeletal proteins, mitochondrial proteins, neuronal development-related proteins, cytochrome C oxidase, cellular metabolism, oxidative stress, companion function, metabolism, enzymes, and antioxidative stress-related proteins. These regulated proteins are mainly involved in inflammation, apoptosis, neuronal damage repair, mitochondrial function, signaling, energy metabolism, and other processes. The major pathways involved include glycolysis, JAK/STAT signaling pathway, mTOR signaling pathway, and MAPK signaling pathway.
Of these, HNRNPL, MBP and HIBADH were all present in pain [136,137]. HIBADH is strongly correlated with oxidoreductase activity and redox [138,139]. And the process of acupuncture analgesia involved ameliorating oxidative damage to the cytoskeleton, as well as energy exchange [140]. Acupuncture reduced the expression of HNRNPL, regulated the stability of coupling proteins, and affected neuronal activity, resulting in analgesic effects. MBP not only can relieve pain by participating in the regulation of MAPK signaling pathway, but also widely found in neurological diseases, circulatory diseases [17]. MBP is associated with synapse-associated myelin formation, and its expression has been linked to neuronal cell injury. Acupuncture was able to regulate MBP levels, improve neurodegenerative lesions, and protect ischemic brain tissue. In conclusion, acupuncture is able to ameliorate diseases by regulating multiple proteins and participating in multiple pathways. It is of great interest to apply proteomics techniques to explore the mechanism of action of acupuncture.
5. Concluding remarks
Acupuncture is an integrative medical therapy, which has great potential in the prevention and treatment of various diseases. Proteomics-based approaches and bioinformatics annotation can help identify potential biomarkers and provide biological intepretations of potential mechanisms for acupuncture treatments. According to published proteomic studies to date, acupuncture could modulate a large number of key proteins in multiple systems and organs. However, the specific mechanisms by which acupuncture interacts with these different systems have not been clearly elucidated. First, in terms of sample selection, acupuncture has not been well studied on body fluids, involving only blood and tears. The process protocol for sample collection was also not kept up to date. Sample selection is directly related to the needs of clinical research. Only by expanding the space for sample selection and standardizing the process of sample collection can we cover the scope of acupuncture in treating more diseases. Second, the use of proteomics technology was generally less advanced in the field of acupuncture. Only one study used antibody arrays. The quality of bioinformatics analyses that follow proteomics was variable due to the long span of years. The lack of a validation process in some studies reduced the credibility of the results. In addition, most of the existing studies were based on disease states, and there were fewer studies applying proteomics technology to explore the specificity of acupuncture points in physiological states. There were insufficient comparative studies on the frequency of acupuncture, manipulation, and prescription of different acupoints.
Therefore, in the future, consideration should be given to expanding the selection of diseases and conducting systematic studies by combining metabolomic, transcriptomic, and other multi-omics techniques in order to elucidate the scientific basis for the efficacy of acupuncture. Further in-depth exploration from a broader and more systematic perspective using advanced proteomics technologies that are more targeted, sensitive and reproducible. To strengthen the research on acupuncture techniques and acupuncture parameters with a view to applying proteomics technology to standardize the therapeutic criteria for acupuncture treatment of different diseases. In addition to acupuncture and EA, other stimulation strategies, such as cupping and acupuncture piercing, have also been studied by applying proteological techniques [[141], [142], [143]]. In conclusion, proteomics-based acupuncture studies are expected to complement new discoveries in acupuncture modernization and thus deepen the understanding of the mechanisms of acupuncture action. And ultimately improve the patient's disease state at the individual level.
Funding
This study was supported by the National Natural Science Foundation of China (grant number. 81874502); National Key Research and Development Program (grant number. 2018YFC1706000); Jilin Province Science and Technology Development Project (grant number. YDZJ202301ZYTS165).
Data availability statement
This research was not applicable. No data was used for the research described in the article.
CRediT authorship contribution statement
Zhen Zhong: Conceptualization, Visualization, Writing – original draft. Meng-Meng Sun: Project administration. Min He: Project administration. Hai-Peng Huang: Supervision. Guan-Yu Hu: Supervision. Shi-Qi Ma: Investigation, Formal analysis. Hai-Zhu Zheng: Formal analysis, Investigation. Meng-Yuan Li: Data curation, Methodology. Lin Yao: Data curation, Methodology. De-Yu Cong: Funding acquisition, Writing – review & editing. Hong-Feng Wang: Funding acquisition, Writing – review & editing.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
Author would like to thank BioRender for providing a great plateform to create beautiful schematic diagrams for scientific publications.
Contributor Information
De-Yu Cong, Email: congdeyu666@sina.com.
Hong-Feng Wang, Email: wanghf@ccucm.edu.cn.
Abbreviations
- 2DE
2D electrophoresis
- DEPs
differentially expressed proteins
- DIA
indicates data-independent acquisition
- EA
electroacupuncture
- ELISA
Enzyme-linked Immunosorbent Assay
- GO
Gene ontology
- HPLC
high performance liquid chromatography
- iTRAQ
isobaric tags for relative and absolute quantitation
- KEGG
Kyoto Encyclopedia of Genes and Genomes
- LC–MS/MS
liquid chromatography with tandem mass spectrometry detection
- LQF
Label-free quantitation
- MALDI-TOF-MS
matrix-assisted laser desorption/ionization time of flight mass spectrometry; MSMS, tandem mass spectrometry
- PCR
polymerase chain reaction
- PRM
Parallel reaction monitoring
- SDS-PAGE
sodium dodecyl sulphate-polyacrylamide gel electrophoresis
- TMT
tandem mass tag
- TRDM
twirling reducing manipulation
- TRFM
twirling reinforcing manipulation
References
- 1.Han J.-S., Ho Y.-S. Global trends and performances of acupuncture research. Neurosci. Biobehav. Rev. 2011;35:680–687. doi: 10.1016/j.neubiorev.2010.08.006. [DOI] [PubMed] [Google Scholar]
- 2.Zhuang Y., Xing J., Li J., Zeng B.-Y., Liang F. International Review of Neurobiology. Elsevier; 2013. History of acupuncture research; pp. 1–23. [DOI] [PubMed] [Google Scholar]
- 3.Lehmann H. Acupuncture in ancient China: how important was it really? Journal of Integrative Medicine. 2013;11:45–53. doi: 10.3736/jintegrmed2013008. [DOI] [PubMed] [Google Scholar]
- 4.Chen H., Yang M., Ning Z., Lam W.L., Zhao Y.K., Yeung W.F., Ng B.F.-L., Ziea E.T.-C., Lao L. A Guideline for randomized controlled trials of acupuncture. Am. J. Chin. Med. 2019;47:1–18. doi: 10.1142/S0192415X19500010. [DOI] [PubMed] [Google Scholar]
- 5.Longhurst J.C. Defining meridians: a modern basis of understanding. Journal of Acupuncture and Meridian Studies. 2010;3:67–74. doi: 10.1016/S2005-2901(10)60014-3. [DOI] [PubMed] [Google Scholar]
- 6.Kaptchuk T.J. Acupuncture: theory, efficacy, and practice. Ann. Intern. Med. 2002;136:374–383. doi: 10.7326/0003-4819-136-5-200203050-00010. [DOI] [PubMed] [Google Scholar]
- 7.Witt C.M., Pach D., Brinkhaus B., Wruck K., Tag B., Mank S., Willich S.N. Safety of acupuncture: results of a prospective observational study with 229,230 patients and introduction of a medical information and consent form. Forsch Komplementmed. 2009;16:91–97. doi: 10.1159/000209315. [DOI] [PubMed] [Google Scholar]
- 8.Allen J., Mak S.S., Begashaw M., Larkin J., Miake-Lye I., Beroes-Severin J., Olson J., Shekelle P.G. Use of acupuncture for adult health conditions, 2013 to 2021: a systematic review. JAMA Netw. Open. 2022;5 doi: 10.1001/jamanetworkopen.2022.43665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Epstein A.S., Liou K.T., Romero S.A.D., Baser R.E., Wong G., Xiao H., Mo Z., Walker D., MacLeod J., Li Q., Barton-Burke M., Deng G.E., Panageas K.S., Farrar J.T., Mao J.J. Acupuncture vs massage for pain in patients living with advanced cancer: the IMPACT randomized clinical trial. JAMA Netw. Open. 2023;6 doi: 10.1001/jamanetworkopen.2023.42482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mawla I., Ichesco E., Zöllner H.J., Edden R.A.E., Chenevert T., Buchtel H., Bretz M.D., Sloan H., Kaplan C.M., Harte S.E., Mashour G.A., Clauw D.J., Napadow V., Harris R.E. Greater somatosensory afference with acupuncture increases primary somatosensory connectivity and alleviates fibromyalgia pain via insular γ-aminobutyric acid: a randomized neuroimaging trial. Arthritis Rheumatol. 2021;73:1318–1328. doi: 10.1002/art.41620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mao J.J., Liou K.T., Baser R.E., Bao T., Panageas K.S., Romero S.A.D., Li Q.S., Gallagher R.M., Kantoff P.W. Effectiveness of electroacupuncture or auricular acupuncture vs usual care for chronic musculoskeletal pain among cancer survivors: the PEACE randomized clinical trial. JAMA Oncol. 2021;7:720–727. doi: 10.1001/jamaoncol.2021.0310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ben-Arye E., Hausner D., Samuels N., Gamus D., Lavie O., Tadmor T., Gressel O., Agbarya A., Attias S., David A., Schiff E. Impact of acupuncture and integrative therapies on chemotherapy-induced peripheral neuropathy: a multicentered, randomized controlled trial. Cancer. 2022;128:3641–3652. doi: 10.1002/cncr.34422. [DOI] [PubMed] [Google Scholar]
- 13.Friedemann T., Kark E., Cao N., Klaßen M., Meyer-Hamme G., Greten J.H., Rostock M., Buhlmann E., Zhao A., Schröder S. Acupuncture improves chemotherapy-induced neuropathy explored by neurophysiological and clinical outcomes - the randomized, controlled, cross-over ACUCIN trial. Phytomedicine. 2022;104 doi: 10.1016/j.phymed.2022.154294. [DOI] [PubMed] [Google Scholar]
- 14.Wu X.-K., Gao J.-S., Ma H.-L., Wang Y., Zhang B., Liu Z.-L., Li J., Cong J., Qin H.-C., Yang X.-M., Wu Q., Chen X.-Y., Lu Z.-L., Feng Y.-H., Qi X., Wang Y.-X., Yu L., Cui Y.-M., An C.-M., Zhou L.-L., Hu Y.-H., Li L., Cao Y.-J., Yan Y., Liu L., Liu Y.-X., Liu Z.-S., Painter R.C., Ng E.H.Y., Liu J.-P., Mol B.W.J., Wang C.C. Acupuncture and doxylamine-pyridoxine for nausea and vomiting in pregnancy : a randomized, controlled, 2 × 2 factorial trial. Ann. Intern. Med. 2023;176:922–933. doi: 10.7326/M22-2974. [DOI] [PubMed] [Google Scholar]
- 15.Qi L.-Y., Yang J.-W., Yan S.-Y., Tu J.-F., She Y.-F., Li Y., Chi L.-L., Wu B.-Q., Liu C.-Z. Acupuncture for the treatment of diarrhea-predominant irritable bowel syndrome: a pilot randomized clinical trial. JAMA Netw. Open. 2022;5 doi: 10.1001/jamanetworkopen.2022.48817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang A., Sun H., Wang P., Han Y., Wang X. Future perspectives of personalized medicine in traditional Chinese medicine: a systems biology approach. Compl. Ther. Med. 2012;20:93–99. doi: 10.1016/j.ctim.2011.10.007. [DOI] [PubMed] [Google Scholar]
- 17.Lai X., Wang J., Nabar N.R., Pan S., Tang C., Huang Y., Hao M., Yang Z., Ma C., Zhang J., Chew H., He Z., Yang J., Su B., Zhang J., Liang J., Sneed K.B., Zhou S.-F. Proteomic response to acupuncture treatment in spontaneously hypertensive rats. PLoS One. 2012;7 doi: 10.1371/journal.pone.0044216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cressey D. Acupuncture for mice. Nature. 2010;465:538. doi: 10.1038/465538a. [DOI] [PubMed] [Google Scholar]
- 19.Gershon D. Proteomics technologies: probing the proteome. Nature. 2003;424:581–587. doi: 10.1038/424581a. [DOI] [PubMed] [Google Scholar]
- 20.Wilkins M.R., Sanchez J.C., Gooley A.A., Appel R.D., Humphery-Smith I., Hochstrasser D.F., Williams K.L. Progress with proteome projects: why all proteins expressed by a genome should be identified and how to do it. Biotechnol. Genet. Eng. Rev. 1996;13:19–50. doi: 10.1080/02648725.1996.10647923. [DOI] [PubMed] [Google Scholar]
- 21.Suhre K., McCarthy M.I., Schwenk J.M. Genetics meets proteomics: perspectives for large population-based studies. Nat. Rev. Genet. 2021;22:19–37. doi: 10.1038/s41576-020-0268-2. [DOI] [PubMed] [Google Scholar]
- 22.Thongboonkerd V., LaBaer J., Domont G.B. Recent advances of proteomics applied to human diseases. J. Proteome Res. 2014;13:4493–4496. doi: 10.1021/pr501038g. [DOI] [PubMed] [Google Scholar]
- 23.Mischak H., Critselis E., Hanash S., Gallagher W.M., Vlahou A., Ioannidis J.P.A. Epidemiologic design and analysis for proteomic studies: a primer on -omic technologies. Am. J. Epidemiol. 2015;181:635–647. doi: 10.1093/aje/kwu462. [DOI] [PubMed] [Google Scholar]
- 24.Gillette M.A., Carr S.A. Quantitative analysis of peptides and proteins in biomedicine by targeted mass spectrometry. Nat. Methods. 2013;10:28–34. doi: 10.1038/nmeth.2309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dayon L., Cominetti O., Affolter M. Proteomics of human biological fluids for biomarker discoveries: technical advances and recent applications. Expert Rev. Proteomics. 2022;19:131–151. doi: 10.1080/14789450.2022.2070477. [DOI] [PubMed] [Google Scholar]
- 26.Dapic I., Baljeu-Neuman L., Uwugiaren N., Kers J., Goodlett D.R., Corthals G.L. Proteome analysis of tissues by mass spectrometry. Mass Spectrom. Rev. 2019;38:403–441. doi: 10.1002/mas.21598. [DOI] [PubMed] [Google Scholar]
- 27.Lygirou V., Makridakis M., Vlahou A. Biological sample collection for clinical proteomics: existing SOPs. Methods Mol. Biol. 2015;1243:3–27. doi: 10.1007/978-1-4939-1872-0_1. [DOI] [PubMed] [Google Scholar]
- 28.Nakayasu E.S., Gritsenko M., Piehowski P.D., Gao Y., Orton D.J., Schepmoes A.A., Fillmore T.L., Frohnert B.I., Rewers M., Krischer J.P., Ansong C., Suchy-Dicey A.M., Evans-Molina C., Qian W.-J., Webb-Robertson B.-J.M., Metz T.O. Tutorial: best practices and considerations for mass-spectrometry-based protein biomarker discovery and validation. Nat. Protoc. 2021;16:3737–3760. doi: 10.1038/s41596-021-00566-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Geyer P.E., Voytik E., Treit P.V., Doll S., Kleinhempel A., Niu L., Müller J.B., Buchholtz M.-L., Bader J.M., Teupser D., Holdt L.M., Mann M. Plasma Proteome Profiling to detect and avoid sample-related biases in biomarker studies. EMBO Mol. Med. 2019;11 doi: 10.15252/emmm.201910427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thongboonkerd V. Proteomics., Forum Nutr. 2007;60:80–90. doi: 10.1159/000107076. [DOI] [PubMed] [Google Scholar]
- 31.Macklin A., Khan S., Kislinger T. Recent advances in mass spectrometry based clinical proteomics: applications to cancer research. Clin. Proteonomics. 2020;17:17. doi: 10.1186/s12014-020-09283-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Geyer P.E., Holdt L.M., Teupser D., Mann M. Revisiting biomarker discovery by plasma proteomics. Mol. Syst. Biol. 2017;13:942. doi: 10.15252/msb.20156297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kopf E., Zharhary D. Antibody arrays--an emerging tool in cancer proteomics. Int. J. Biochem. Cell Biol. 2007;39:1305–1317. doi: 10.1016/j.biocel.2007.04.029. [DOI] [PubMed] [Google Scholar]
- 34.Syu G.-D., Dunn J., Zhu H. Developments and applications of functional protein microarrays. Mol. Cell. Proteomics. 2020;19:916–927. doi: 10.1074/mcp.R120.001936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Huang J., Chen X., Fu X., Li Z., Huang Y., Liang C. Advances in aptamer-based biomarker discovery. Front. Cell Dev. Biol. 2021;9 doi: 10.3389/fcell.2021.659760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lundberg M., Eriksson A., Tran B., Assarsson E., Fredriksson S. Homogeneous antibody-based proximity extension assays provide sensitive and specific detection of low-abundant proteins in human blood. Nucleic Acids Res. 2011;39:e102. doi: 10.1093/nar/gkr424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sun H., Zhang A., Yan G., Zhang Y., Meng X., Liu L., Xie N., Cheng W., Wang X. Acupuncture targeting and regulating multiple signaling pathways related to Zusanli acupoint using iTRAQ-based quantitative proteomic analysis. Acupuncture and Related Therapies. 2014;2:51–56. doi: 10.1016/j.arthe.2014.03.002. [DOI] [Google Scholar]
- 38.Xu Y.-D., Wang Y., Park G.-H., Yin L.-M., Ran J., Liu Y.-Y., Yang Y.-Q. Non-specific physiological background effects of acupuncture revealed by proteomic analysis in normal rats. BMC Compl. Alternative Med. 2014;14:375. doi: 10.1186/1472-6882-14-375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Braak H., Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 1991;82:239–259. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- 40.Jack C.R.J. Advances in Alzheimer's disease research over the past two decades. Lancet Neurol. 2022;21:866–869. doi: 10.1016/S1474-4422(22)00298-8. [DOI] [PubMed] [Google Scholar]
- 41.Klyucherev T.O., Olszewski P., Shalimova A.A., Chubarev V.N., Tarasov V.V., Attwood M.M., Syvänen S., Schiöth H.B. Advances in the development of new biomarkers for Alzheimer's disease. Transl. Neurodegener. 2022;11:25. doi: 10.1186/s40035-022-00296-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yu C.-C., Du Y.-J., Wang S.-Q., Liu L.-B., Shen F., Wang L., Lin Y.-F., Kong L.-H. Experimental evidence of the benefits of acupuncture for alzheimer's disease: an updated review. Front. Neurosci. 2020;14 doi: 10.3389/fnins.2020.549772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Su X.-T., Wang L.-Q., Li J.-L., Zhang N., Wang L., Shi G.-X., Yang J.-W., Liu C.-Z. Acupuncture therapy for cognitive impairment: a delphi expert consensus survey. Front. Aging Neurosci. 2020;12 doi: 10.3389/fnagi.2020.596081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liu B., Liu J., Shi J.-S. SAMP8 mice as a model of age-related cognition decline with underlying mechanisms in alzheimer's disease. J Alzheimers Dis. 2020;75:385–395. doi: 10.3233/JAD-200063. [DOI] [PubMed] [Google Scholar]
- 45.Zhang X.-Z., Liu T., Jia Y.-J., Han J.-X., Nie K. Effects of sanjiao acupuncture on learning and memory function and related genes in Alzheimer's disease model mice. J. Chin. Med. 2019:1056–1061. doi: 10.13288/j.11-2166/r.2019.12.014. [DOI] [Google Scholar]
- 46.Liu T., Zhang X.-Z., Han J.-X., Nie K. Using bioinformatics tools to explore cellular mechanisms of “Triple Energizer Acupuncture Method” in treating senile dementia. Acupunct. Res. 2019:424–429. doi: 10.13702/j.1000-0607.180412. [DOI] [PubMed] [Google Scholar]
- 47.Liu T., He J., Zhang X.-Z., Jia Y.-J., Han J.-X., Nie K. Effect of sanjiao acupuncture on cytoskeleton recombination related proteins in hippocampal neurons of senescence accelerated mouse. Chinese Journal of Integrated Chinese and Western Medicine. 2019:1463–1468. [Google Scholar]
- 48.Nie K., Zhang X.-Z., Zhao L., Jia Y.-J., Han J.-X. [Effect of acupuncture on transmembrane signal pathway in AD mice: an analysis based on lipid-raft proteomics] Zhongguo Zhong Xi Yi Jie He Za Zhi. 2014;34:991–996. [PubMed] [Google Scholar]
- 49.Huang Y., Rafael Guimarães T., Todd N., Ferguson C., Weiss K.M., Stauffer F.R., McDermott B., Hurtle B.T., Saito T., Saido T.C., MacDonald M.L., Homanics G.E., Thathiah A. G protein-biased GPR3 signaling ameliorates amyloid pathology in a preclinical Alzheimer's disease mouse model. Proc. Natl. Acad. Sci. U.S.A. 2022;119 doi: 10.1073/pnas.2204828119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ashleigh T., Swerdlow R.H., Beal M.F. The role of mitochondrial dysfunction in Alzheimer's disease pathogenesis. Alzheimers Dement. 2023;19:333–342. doi: 10.1002/alz.12683. [DOI] [PubMed] [Google Scholar]
- 51.Liu T., Zhang X.-Z., Jia Y.-J., Han J.-X., Nie K., Using bioinformatics tools to analyse " Yi Qi Tiao Xue Fu Ben Pei Yuan Key targets of acupuncture in the treatment of dementia in aging. Chinese Journal of Integrated Chinese and Western Medicine. 2018:1474–1478. [Google Scholar]
- 52.Liang M., Li G., Zhu H., Gong Q., Dong K., Long C., Li Y., Sayrash J. Effect of acupuncture on hippocampal mitochondrial proteome expression in SAMP8 mouse model with Alzheimer disease. J. Acupunct. Tuina. Sci. 2018;16:67–79. doi: 10.1007/s11726-018-1026-2. [DOI] [Google Scholar]
- 53.Dhiman K., Gupta V.B., Villemagne V.L., Eratne D., Graham P.L., Fowler C., Bourgeat P., Li Q.-X., Collins S., Bush A.I., Rowe C.C., Masters C.L., Ames D., Hone E., Blennow K., Zetterberg H., Martins R.N. Cerebrospinal fluid neurofilament light concentration predicts brain atrophy and cognition in Alzheimer's disease. Alzheimers Dement (Amst). 2020;12 doi: 10.1002/dad2.12005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Liang M., Zhu H., Dong K., Li G.-C. Effects of bushen huoxue acupuncture method on proteomics expression in amygdaloid tissue of SAMP8. Chin. J. Inf. Tradit. Chin. Med. 2018:58–63. [Google Scholar]
- 55.Zhang S.-J., Sun N.-N., Gao J.-F. [Protective function of electroacupuncture on young mouse model of Alzheimer's disease and proteomic study] Zhongguo Zhen Jiu. 2021;41:295–302. doi: 10.13703/j.0255-2930.20200203-k0002. [DOI] [PubMed] [Google Scholar]
- 56.Ogawa O., Zhu X., Lee H.-G., Raina A., Obrenovich M.E., Bowser R., Ghanbari H.A., Castellani R.J., Perry G., Smith M.A. Ectopic localization of phosphorylated histone H3 in Alzheimer's disease: a mitotic catastrophe? Acta Neuropathol. 2003;105:524–528. doi: 10.1007/s00401-003-0684-3. [DOI] [PubMed] [Google Scholar]
- 57.Chowdhury U.N., Islam M.B., Ahmad S., Moni M.A. Network-based identification of genetic factors in ageing, lifestyle and type 2 diabetes that influence to the progression of Alzheimer's disease. Inform. Med. Unlocked. 2020;19 doi: 10.1016/j.imu.2020.100309. [DOI] [Google Scholar]
- 58.Kodani S.D., Morisseau C. Role of epoxy-fatty acids and epoxide hydrolases in the pathology of neuro-inflammation. Biochimie. 2019;159:59–65. doi: 10.1016/j.biochi.2019.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Uchida Y., Gomi F. The role of calsyntenin-3 in dystrophic neurite formation in Alzheimer's disease brain. Geriatr. Gerontol. Int. 2016;16(Suppl 1):43–50. doi: 10.1111/ggi.12737. [DOI] [PubMed] [Google Scholar]
- 60.Comes G., Manso Y., Escrig A., Fernandez-Gayol O., Sanchis P., Molinero A., Giralt M., Carrasco J., Hidalgo J. Influence of transgenic metallothionein-1 on gliosis, CA1 neuronal loss, and brain metal levels of the Tg2576 mouse model of alzheimer's disease. Int. J. Mol. Sci. 2017;18 doi: 10.3390/ijms18020251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Manso Y., Comes G., López-Ramos J.C., Belfiore M., Molinero A., Giralt M., Carrasco J., Adlard P.A., Bush A.I., Delgado-García J.M., Hidalgo J. Overexpression of metallothionein-1 modulates the phenotype of the Tg2576 mouse model of alzheimer's disease. J Alzheimers Dis. 2016;51:81–95. doi: 10.3233/JAD-151025. [DOI] [PubMed] [Google Scholar]
- 62.Farooq M.U., Min J., Goshgarian C., Gorelick P.B. Pharmacotherapy for vascular cognitive impairment. CNS Drugs. 2017;31:759–776. doi: 10.1007/s40263-017-0459-3. [DOI] [PubMed] [Google Scholar]
- 63.Liu S., Wang Z., Su Y., Qi L., Yang W., Fu M., Jing X., Wang Y., Ma Q. A neuroanatomical basis for electroacupuncture to drive the vagal-adrenal axis. Nature. 2021;598:641–645. doi: 10.1038/s41586-021-04001-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yang J., Wang X., Zhang M., Xiao L., Zhu W., Ji C., Liu C. Acupuncture as a multifunctional neuroprotective therapy ameliorates cognitive impairment in a rat model of vascular dementia: a quantitative iTRAQ proteomics study. CNS Neurosci. Ther. 2018;24:1264–1274. doi: 10.1111/cns.13063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Levada O.A., Traïlin A.V. [Serum level of S100B as a marker of progression of vascular mild cognitive impairment into subcortical vascular dementia and therapy effectiveness] Lik Sprava. 2012:53–59. [PubMed] [Google Scholar]
- 66.Datta A., Qian J., Chong R., Kalaria R.N., Francis P., Lai M.K.P., Chen C.P., Sze S.K. Novel pathophysiological markers are revealed by iTRAQ-based quantitative clinical proteomics approach in vascular dementia. J. Proteonomics. 2014;99:54–67. doi: 10.1016/j.jprot.2014.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rajendran P.R., Thompson R.E., Reich S.G. The use of alternative therapies by patients with Parkinson's disease. Neurology. 2001;57:790–794. doi: 10.1212/wnl.57.5.790. [DOI] [PubMed] [Google Scholar]
- 68.Kang J.M., Park H.J., Choi Y.G., Choe I.H., Park J.H., Kim Y.S., Lim S. Acupuncture inhibits microglial activation and inflammatory events in the MPTP-induced mouse model. Brain Res. 2007;1131:211–219. doi: 10.1016/j.brainres.2006.10.089. [DOI] [PubMed] [Google Scholar]
- 69.Park H.-J., Lim S., Joo W.-S., Yin C.-S., Lee H.-S., Lee H.-J., Seo J.C., Leem K., Son Y.-S., Kim Y.-J., Kim C.-J., Kim Y.-S., Chung J.-H. Acupuncture prevents 6-hydroxydopamine-induced neuronal death in the nigrostriatal dopaminergic system in the rat Parkinson's disease model. Exp. Neurol. 2003;180:93–98. doi: 10.1016/s0014-4886(02)00031-6. [DOI] [PubMed] [Google Scholar]
- 70.Jeon S., Kim Y.J., Kim S.-T., Moon W., Chae Y., Kang M., Chung M.-Y., Lee H., Hong M.-S., Chung J.-H., Joh T.H., Lee H., Park H.-J. Proteomic analysis of the neuroprotective mechanisms of acupuncture treatment in a Parkinson's disease mouse model. Proteomics. 2008;8:4822–4832. doi: 10.1002/pmic.200700955. [DOI] [PubMed] [Google Scholar]
- 71.Kim S.-T., Moon W., Chae Y., Kim Y.J., Lee H., Park H.-J. The effect of electroaucpuncture for 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced proteomic changes in the mouse striatum. J. Physiol. Sci. 2010;60:27–34. doi: 10.1007/s12576-009-0061-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Li M., Li L., Wang K., Su W., Jia J., Wang X. The effect of electroacupuncture on proteomic changes in the motor cortex of 6-OHDA Parkinsonian rats. Brain Res. 2017;1673:52–63. doi: 10.1016/j.brainres.2017.07.027. [DOI] [PubMed] [Google Scholar]
- 73.Rickards H. Depression in neurological disorders: an update. Curr. Opin. Psychiatr. 2006;19:294–298. doi: 10.1097/01.yco.0000218601.17722.5b. [DOI] [PubMed] [Google Scholar]
- 74.Jiang H., Zhang X., Wang Y., Zhang H., Li J., Yang X., Zhao B., Zhang C., Yu M., Xu M., Yu Q., Liang X., Li X., Shi P., Bao T. Mechanisms underlying the antidepressant response of acupuncture via PKA/CREB signaling pathway. Neural Plast. 2017;2017 doi: 10.1155/2017/4135164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Duan D., Yang X., Ya T., Chen L. Hippocampal gene expression in a rat model of depression after electroacupuncture at the Baihui and Yintang acupoints. Neural Regen Res. 2014;9:76–83. doi: 10.4103/1673-5374.125333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhang J., Zhang J., Zhang Z., Zheng Y., Zhong Z., Yao Z., Cai X., Lao L., Huang Y., Qu S. Dopaminergic signaling in prefrontal cortex contributes to the antidepressant effect of electroacupuncture: an iTRAQ-based proteomics analysis in a rat model of CUMS. Anat. Rec. 2021;304:2454–2469. doi: 10.1002/ar.24732. [DOI] [PubMed] [Google Scholar]
- 77.Guo Z., Tu Y., Guo T.-W., Wu Y.-C., Yang X.-Q., Sun L., Yang X.-J., Zhang W.-Y., Wang Y., Zhang X.-H. Electroacupuncture pretreatment exhibits anti-depressive effects by regulating hippocampal proteomics in rats with chronic restraint stress. Neural Regen Res. 2015;10:1298–1304. doi: 10.4103/1673-5374.162764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zhang J., Zhang Z., Zhang J., Zhong Z., Yao Z., Qu S., Huang Y. Electroacupuncture improves antidepressant effects in CUMS rats by protecting hippocampal synapse and mitochondrion: an ultrastructural and iTRAQ proteomic study. Evid Based Complement Alternat Med. 2019;2019 doi: 10.1155/2019/3424698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Xu Y., Li X., Man D., Su X., A G. iTRAQ-based proteomics analysis on insomnia rats treated with Mongolian medical warm acupuncture. Biosci. Rep. 2020;40 doi: 10.1042/BSR20191517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bae C.-H., Kim D.-S., Jun Y.L., Kwon S., Park H.-J., Hahm D.-H., Lee H., Kim S.-T. Proteomic analysis of the effect of acupuncture on the suppression of kainic Acid-induced neuronal destruction in mouse hippocampus. Evid Based Complement Alternat Med. 2013;2013 doi: 10.1155/2013/436315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kim H.-J., Park H.J., Hong M.S., Song J.Y., Park H.-K., Jo D.J., Park S.W., HwanYun D., Park H.-K., Yang J.-S., Ban J.Y., Chung J.-H. Effect by acupuncture on hypothalamic expression of maternally separated rats: proteomic approach. Neurol. Res. 2010;32(Suppl 1):69–73. doi: 10.1179/016164109X12537002794129. [DOI] [PubMed] [Google Scholar]
- 82.Cao J., Tu Y., Orr S.P., Lang C., Park J., Vangel M., Chen L., Gollub R., Kong J. Analgesic effects evoked by real and imagined acupuncture: a neuroimaging study. Cerebr. Cortex. 2019;29:3220–3231. doi: 10.1093/cercor/bhy190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Qiao L., Guo M., Qian J., Xu B., Gu C., Yang Y. Research advances on acupuncture analgesia. Am. J. Chin. Med. 2020;48:245–258. doi: 10.1142/S0192415X20500135. [DOI] [PubMed] [Google Scholar]
- 84.Chen Y., Zhou Y., Li X.-C., Ma X., Mi W.-L., Chu Y.-X., Wang Y.-Q., Mao-Ying Q.-L. Neuronal GRK2 regulates microglial activation and contributes to electroacupuncture analgesia on inflammatory pain in mice. Biol. Res. 2022;55:5. doi: 10.1186/s40659-022-00374-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Jiang M., Chen X., Zhang L., Liu W., Yu X., Wang Z., Zheng M. Electroacupuncture suppresses glucose metabolism and GLUT-3 expression in medial prefrontal cortical in rats with neuropathic pain. Biol. Res. 2021;54:24. doi: 10.1186/s40659-021-00348-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Gao Y., Chen S., Xu Q., Yu K., Wang J., Qiao L., Meng F., Liu J. Proteomic analysis of differential proteins related to anti-nociceptive effect of electroacupuncture in the hypothalamus following neuropathic pain in rats. Neurochem. Res. 2013;38:1467–1478. doi: 10.1007/s11064-013-1047-7. [DOI] [PubMed] [Google Scholar]
- 87.Gao Y.-H., Chen S.-P., Wang J.-Y., Qiao L.-N., Meng F.-Y., Xu Q.-L., Liu J.-L. Differential proteomics analysis of the analgesic effect of electroacupuncture intervention in the hippocampus following neuropathic pain in rats. BMC Compl. Alternative Med. 2012;12:241. doi: 10.1186/1472-6882-12-241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Sung H.J., Kim Y.S., Kim I.S., Jang S.-W., Kim Y.R., Na D.S., Han K.H., Hwang B.G., Park D.S., Ko J. Proteomic analysis of differential protein expression in neuropathic pain and electroacupuncture treatment models. Proteomics. 2004;4:2805–2813. doi: 10.1002/pmic.200300821. [DOI] [PubMed] [Google Scholar]
- 89.Gong D., Yu X., Jiang M., Li C., Wang Z. Differential proteomic analysis of the Hippocampus in rats with neuropathic pain to investigate the use of electroacupuncture in relieving mechanical allodynia and cognitive decline. Neural Plast. 2021;2021:1–10. doi: 10.1155/2021/5597163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Chen W., Zhang X.-N., Su Y.-S., Wang X.-Y., Li H.-C., Liu Y.-H., Wan H.-Y., Qu Z.-Y., Jing X.-H., He W. Electroacupuncture activated local sympathetic noradrenergic signaling to relieve synovitis and referred pain behaviors in knee osteoarthritis rats. Front. Mol. Neurosci. 2023;16 doi: 10.3389/fnmol.2023.1069965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Yu X., Chen X., Liu W., Jiang M., Wang Z., Tao J. Proteomics analysis of the spinal dorsal horn in diabetic painful neuropathy rats with electroacupuncture treatment. Front. Endocrinol. 2021;12 doi: 10.3389/fendo.2021.608183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wang W., Zhou Y., Cai Y., Wang S., Shao F., Du J., Fang J., Liu J., Shao X., Liu B., Fang J., Liang Y. Phosphoproteomic profiling of rat's dorsal root ganglia reveals mTOR as a potential target in bone cancer pain and electro-acupuncture’s analgesia. Front. Pharmacol. 2021;12 doi: 10.3389/fphar.2021.593043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wang W., Zhou Y., Cai Y., Wang S., Shao F., Du J., Fang J., Liu J., Shao X., Liu B., Fang J., Liang Y. Phosphoproteomic profiling of rat's dorsal root ganglia reveals mTOR as a potential target in bone cancer pain and electro-acupuncture’s analgesia. Front. Pharmacol. 2021;12 doi: 10.3389/fphar.2021.593043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wu Y., Jiang Y., Shao X., He X., Shen Z., Shi Y., Wang C., Fang J. Proteomics analysis of the amygdala in rats with CFA-induced pain aversion with electro-acupuncture stimulation. J. Pain Res. 2019;12:3067–3078. doi: 10.2147/JPR.S211826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Lee S.-H., Kim S.-Y., Kim J.-H., Jung H.-Y., Moon J.-H., Bae K.-H., Choi B.-T. Phosphoproteomic analysis of electroacupuncture analgesia in an inflammatory pain rat model. Mol. Med. Rep. 2012;6:157–162. doi: 10.3892/mmr.2012.879. [DOI] [PubMed] [Google Scholar]
- 96.Li L., Huang Q., Barbero M., Liu L., Nguyen T., Xu A., Ji L. Proteins and signaling pathways response to dry needling combined with static stretching treatment for chronic myofascial pain in a RAT model: an explorative proteomic study. Int. J. Mol. Sci. 2019;20 doi: 10.3390/ijms20030564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Zhang J.-F., Williams J.P., Shi W.-R., Qian X.-Y., Zhao Q.-N., Lu G.-F., An J.-X. Potential molecular mechanisms of electroacupuncture with spatial learning and memory impairment induced by chronic pain on a rat model. Pain Physician. 2022;25:E271–E283. [PubMed] [Google Scholar]
- 98.Li W.-J., Pan S.-Q., Zeng Y.-S., Su B.-G., Li S.-M., Ding Y., Li Y., Ruan J.-W. Identification of acupuncture-specific proteins in the process of electro-acupuncture after spinal cord injury. Neurosci. Res. 2010;67:307–316. doi: 10.1016/j.neures.2010.04.012. [DOI] [PubMed] [Google Scholar]
- 99.Liu L., Li W., Wang L., Gong P., Lyu T., Liu D., Zhang Y., Guo Y., Liu X., Tang M., Hu H., Liu C., Li B. Proteomic and metabolomic profiling of acupuncture for migraine reveals a correlative link via energy metabolism. Front. Neurosci. 2022;16 doi: 10.3389/fnins.2022.1013328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Fan H., Yang J.-W., Wang L.-Q., Huang J., Lin L.-L., Wang Y., Zhang N., Liu C.-Z. The hypotensive role of acupuncture in hypertension: clinical study and mechanistic study. Front. Aging Neurosci. 2020;12:138. doi: 10.3389/fnagi.2020.00138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kelly D.M., Rothwell P.M. Blood pressure and the brain: the neurology of hypertension. Practical Neurol. 2020;20:100–108. doi: 10.1136/practneurol-2019-002269. [DOI] [PubMed] [Google Scholar]
- 102.Liang J., Wu J., Zhang X., Hao X., Zeng T., Sun J., Ji Z., Park K., Li K., Liu Q. Proteomics analysis of the hypothalamus in spontaneously hypertensive rats treated with twirling reinforcing manipulation, twirling reducing manipulation or electroacupuncture. Exp. Ther. Med. 2021;21:381. doi: 10.3892/etm.2021.9812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Wu J., Zeng T., Liang J., Zhang X., Xie Q., Lv T., Wong P.Y., Ji Z., Liu Q. Effects of different acupuncture manipulations on protein expression in the parietal cortex of spontaneously hypertensive rats. Journal of Traditional Chinese Medical Sciences. 2021;8:257–264. doi: 10.1016/j.jtcms.2021.07.009. [DOI] [Google Scholar]
- 104.Zhang X., Hao X., Liang J., Wu J., Xue Y., Wu X., Wei P., Wang Y., Xiao M., Zhao J., Liu Q. Proteomics analysis of the cerebellum in spontaneously hypertensive rats treated with twirling reinforcing manipulation, twirling reducing manipulation or electroacupuncture. Review. 2020 doi: 10.21203/rs.3.rs-101204/v1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Lai X., Wang J., Nabar N.R., Pan S., Tang C., Huang Y., Hao M., Yang Z., Ma C., Zhang J., Chew H., He Z., Yang J., Su B., Zhang J., Liang J., Sneed K.B., Zhou S.-F. Proteomic response to acupuncture treatment in spontaneously hypertensive rats. PLoS One. 2012;7 doi: 10.1371/journal.pone.0044216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Virani S.S., Alonso A., Aparicio H.J., Benjamin E.J., Bittencourt M.S., Callaway C.W., Carson A.P., Chamberlain A.M., Cheng S., Delling F.N., Elkind M.S.V., Evenson K.R., Ferguson J.F., Gupta D.K., Khan S.S., Kissela B.M., Knutson K.L., Lee C.D., Lewis T.T., Liu J., Loop M.S., Lutsey P.L., Ma J., Mackey J., Martin S.S., Matchar D.B., Mussolino M.E., Navaneethan S.D., Perak A.M., Roth G.A., Samad Z., Satou G.M., Schroeder E.B., Shah S.H., Shay C.M., Stokes A., VanWagner L.B., Wang N.-Y., Tsao C.W. Heart disease and stroke statistics-2021 update: a report from the American heart association. Circulation. 2021;143:e254–e743. doi: 10.1161/CIR.0000000000000950. [DOI] [PubMed] [Google Scholar]
- 107.Kuang X., Fan W., Hu J., Wu L., Yi W., Lu L., Xu N. Acupuncture for post-stroke cognitive impairment: a systematic review and meta-analysis. Acupunct. Med. 2021;39:577–588. doi: 10.1177/09645284211009542. [DOI] [PubMed] [Google Scholar]
- 108.Su K., Hao W., Lv Z., Wu M., Li J., Hu Y., Zhang Z., Gao J., Feng X. Electroacupuncture of Baihui and Shenting ameliorates cognitive deficits via Pten/Akt pathway in a rat cerebral ischemia injury model. Front. Neurol. 2022;13 doi: 10.3389/fneur.2022.855362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Pan S., Zhan X., Su X., Guo L., Lv L., Su B. Proteomic analysis of serum proteins in acute ischemic stroke patients treated with acupuncture. Exp. Biol. Med. 2011;236:325–333. doi: 10.1258/ebm.2011.010041. [DOI] [PubMed] [Google Scholar]
- 110.Xiao J., Cai T., Fang Y., Liu R., Flores J.J., Wang W., Gao L., Liu Y., Lu Q., Tang L., Zhang J.H., Lu H., Tang J. Activation of GPR40 attenuates neuroinflammation and improves neurological function via PAK4/CREB/KDM6B pathway in an experimental GMH rat model. J. Neuroinflammation. 2021;18:160. doi: 10.1186/s12974-021-02209-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Zhang T., Shi Z., Wang Y., Wang L., Zhang B., Chen G., Wan Q., Chen L. Akt3 deletion in mice impairs spatial cognition and hippocampal CA1 long long-term potentiation through downregulation of mTOR. Acta Physiol. 2019;225 doi: 10.1111/apha.13167. [DOI] [PubMed] [Google Scholar]
- 112.Zeng X., Hunt A., Jin S.C., Duran D., Gaillard J., Kahle K.T. EphrinB2-EphB4-RASA1 signaling in human cerebrovascular development and disease. Trends Mol. Med. 2019;25:265–286. doi: 10.1016/j.molmed.2019.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Ma Y., Liu Y., Zhang Z., Yang G.-Y. Significance of complement system in ischemic stroke: a comprehensive review. Aging Dis. 2019;10:429–462. doi: 10.14336/AD.2019.0119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Grossi C., Artusi C., Meroni P., Borghi M.O., Neglia L., Lonati P.A., Oggioni M., Tedesco F., De Simoni M.-G., Fumagalli S. β2 glycoprotein I participates in phagocytosis of apoptotic neurons and in vascular injury in experimental brain stroke. J. Cerebr. Blood Flow Metabol. 2021;41:2038–2053. doi: 10.1177/0271678X20984551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Wu X., Ni J., An H., Gao Y., Li M., Huang Z., Xu J. Effect of cluster needling at scalp acupoints on differential protein expression in rat brain tissue after acute focal cerebral ischemia. Journal of Traditional Chinese Medical Sciences. 2020;7:316–324. doi: 10.1016/j.jtcms.2020.07.010. [DOI] [Google Scholar]
- 116.Dagonnier M., Donnan G.A., Davis S.M., Dewey H.M., Howells D.W. Acute stroke biomarkers: are we there yet? Front. Neurol. 2021;12 doi: 10.3389/fneur.2021.619721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Luger S., Jæger H.S., Dixon J., Bohmann F.O., Schaefer J., Richieri S.P., Larsen K., Hov M.R., Bache K.G., Foerch C. Diagnostic accuracy of glial fibrillary acidic protein and ubiquitin carboxy-terminal hydrolase-L1 serum concentrations for differentiating acute intracerebral hemorrhage from ischemic stroke. Neurocritical Care. 2020;33:39–48. doi: 10.1007/s12028-020-00931-5. [DOI] [PubMed] [Google Scholar]
- 118.Tsou M.-T., Ho J.-Y., Lin C.-H., Chiu J.-H. Proteomic analysis finds different myocardial protective mechanisms for median nerve stimulation by electroacupuncture and by local somatothermal stimulation. Int. J. Mol. Med. 2004;14:553–563. [PubMed] [Google Scholar]
- 119.Cheong K.B., Zhang J., Huang Y. The effectiveness of acupuncture in postoperative gastroparesis syndrome--a systematic review and meta-analysis. Compl. Ther. Med. 2014;22:767–786. doi: 10.1016/j.ctim.2014.05.002. [DOI] [PubMed] [Google Scholar]
- 120.Takahashi T. Acupuncture for functional gastrointestinal disorders. J. Gastroenterol. 2006;41:408–417. doi: 10.1007/s00535-006-1773-6. [DOI] [PubMed] [Google Scholar]
- 121.Wang Z.-H., Shan C.-X., Zhou D., Yan X.-K., Wang F.-C. Comparison of effects of acupuncture with different points qon prevention of stress gastric ulcer and screening of differentially expressed proteins. J. Jilin Univ. (Earth Sci. Ed.) 2013:441–447+420. [Google Scholar]
- 122.Wang L.-Y., Deng W.-Y., Zhou D., Wang F.-C. Effects of electroacupuncture on the expression of serum somatostatin and gastrin in gastric ulcer model rats. Tokugawa National Medicine. 2021:2543–2547. [Google Scholar]
- 123.Li F., Yang B., Liu Y., Tang T., Wang C., Li M., Lv S., Qi Q., Liu H., Shi Z., Wu H., Wang X. Acupuncture regulates serum differentially expressed proteins in patients with chronic atrophic gastritis: a quantitative iTRAQ proteomics study. Evid Based Complement Alternat Med. 2021;2021 doi: 10.1155/2021/9962224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Liu J., Peng R., Tan Q., Li B., Chen J., Liu G., Wang Y., Li C., Li J., Wang H. Proteomic analysis of rat colonic mucosa following acupuncture treatment for irritable bowel syndrome with diarrhea. PLoS One. 2022;17 doi: 10.1371/journal.pone.0273853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Wang G., Li M., Yu S., Guan M., Ma S., Zhong Z., Guo Y., Leng X., Huang H. Tandem mass tag-based proteomics analysis of type 2 diabetes mellitus with non-alcoholic fatty liver disease in mice treated with acupuncture. Biosci. Rep. 2022;42 doi: 10.1042/BSR20212248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Nan S., Wan J., Lei Q., Wang X., Ma N., Yin R., Zhu J., Ding M., Ding Y. The involvement of the primo vascular system in local enteritis and its modification by electroacupuncture. Front. Immunol. 2022;13 doi: 10.3389/fimmu.2022.1072996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Reddel H.K., Bacharier L.B., Bateman E.D., Brightling C.E., Brusselle G.G., Buhl R., Cruz A.A., Duijts L., Drazen J.M., FitzGerald J.M., Fleming L.J., Inoue H., Ko F.W., Krishnan J.A., Levy M.L., Lin J., Mortimer K., Pitrez P.M., Sheikh A., Yorgancioglu A.A., Boulet L.-P. Global Initiative for Asthma Strategy 2021: executive summary and rationale for key changes. Eur. Respir. J. 2022;59 doi: 10.1183/13993003.02730-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Tang W., Dong M., Teng F., Cui J., Zhu X., Wang W., Wuniqiemu T., Qin J., Yi L., Wang S., Dong J., Wei Y. TMT-based quantitative proteomics reveals suppression of SLC3A2 and ATP1A3 expression contributes to the inhibitory role of acupuncture on airway inflammation in an OVA-induced mouse asthma model. Biomed. Pharmacother. 2021;134 doi: 10.1016/j.biopha.2020.111001. [DOI] [PubMed] [Google Scholar]
- 129.Xu Y.-D., Cui J.-M., Wang Y., Yin L.-M., Gao C.-K., Liu X.-Y., Wei Y., Liu Y.-Y., Jiang Y.-L., Shan C.-X., Yang Y.-Q. Proteomic analysis reveals the deregulation of inflammation-related proteins in acupuncture-treated rats with asthma onset. Evid Based Complement Alternat Med. 2012;2012 doi: 10.1155/2012/850512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Wang Y., Cui J., Ma S., Liu Y., Yin L., Yang Y. Proteomics analysis of component in serum with anti-asthma activity derived from rats treated by acupuncture. Journal of Acupuncture and Tuina Science. 2009;7:326–331. [Google Scholar]
- 131.Qiu X., Gong L., Sun X., Guo J., Chodara A.M. Efficacy of acupuncture and identification of tear protein expression changes using iTRAQ quantitative proteomics in rabbits. Curr. Eye Res. 2011;36:886–894. doi: 10.3109/02713683.2011.601843. [DOI] [PubMed] [Google Scholar]
- 132.Kannan R., Das S., Shetty R., Zhou L., Ghosh A., Deshpande V. Tear proteomics in dry eye disease. Indian J. Ophthalmol. 2023;71:1203–1214. doi: 10.4103/IJO.IJO_2851_22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Liu Q., Liu J., Ren C., Cai W., Wei Q., Song Y., Yu J. Proteomic analysis of tears following acupuncture treatment for menopausal dry eye disease by two-dimensional nano-liquid chromatography coupled with tandem mass spectrometry. Int. J. Nanomed. 2017;12:1663–1671. doi: 10.2147/IJN.S126968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Tong L., Zhou L., Koh S.K., Gao Y., Htoon H.M., Hou A., Deng L., Wei Q.-P., Lim P. Changes in tear proteome after acupuncture treatment in dry eye. Clin. Ophthalmol. 2021;15:4585–4590. doi: 10.2147/OPTH.S334942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Chen L., Huang X., Wang L., Wang C., Tang X., Gu M., Jing J., Ma R., Ge X., Yao B. Electroacupuncture reduces oocyte number and maintains vascular barrier against ovarian hyperstimulation syndrome by regulating CD200. Front. Cell Dev. Biol. 2021;9 doi: 10.3389/fcell.2021.648578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Gao Y., Chen S., Xu Q., Yu K., Wang J., Qiao L., Meng F., Liu J. Proteomic analysis of differential proteins related to anti-nociceptive effect of electroacupuncture in the hypothalamus following neuropathic pain in rats. Neurochem. Res. 2013;38:1467–1478. doi: 10.1007/s11064-013-1047-7. [DOI] [PubMed] [Google Scholar]
- 137.Gao Y.-H., Chen S.-P., Wang J.-Y., Qiao L.-N., Meng F.-Y., Xu Q.-L., Liu J.-L. Differential proteomics analysis of the analgesic effect of electroacupuncture intervention in the hippocampus following neuropathic pain in rats. BMC Compl. Alternative Med. 2012;12:241. doi: 10.1186/1472-6882-12-241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Hajszan T., MacLusky N.J., Leranth C. Short-term treatment with the antidepressant fluoxetine triggers pyramidal dendritic spine synapse formation in rat hippocampus. Eur. J. Neurosci. 2005;21:1299–1303. doi: 10.1111/j.1460-9568.2005.03968.x. [DOI] [PubMed] [Google Scholar]
- 139.Zhou L., Huang K.-X., Kecojevic A., Welsh A.M., Koliatsos V.E. Evidence that serotonin reuptake modulators increase the density of serotonin innervation in the forebrain. J. Neurochem. 2006;96:396–406. doi: 10.1111/j.1471-4159.2005.03562.x. [DOI] [PubMed] [Google Scholar]
- 140.Goldman N., Chen M., Fujita T., Xu Q., Peng W., Liu W., Jensen T.K., Pei Y., Wang F., Han X., Chen J.-F., Schnermann J., Takano T., Bekar L., Tieu K., Nedergaard M. Adenosine A1 receptors mediate local anti-nociceptive effects of acupuncture. Nat. Neurosci. 2010;13:883–888. doi: 10.1038/nn.2562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Hu J., Weng L., Zhang C., Zhao S.-M., Ge K.-W., DI K., Cao M., Wang H.-S., Zhao L.-G., Liu L.-Y. [Components of drugs in acupoint sticking therapy and its mechanism of intervention on bronchial asthma based on UPLC-Q-TOF-MS combined with network pharmacology and experimental verification] Zhongguo Zhongyao Zazhi. 2022;47:1359–1369. doi: 10.19540/j.cnki.cjcmm.20210903.401. [DOI] [PubMed] [Google Scholar]
- 142.Liu Z., Chen C., Li X., Zhao C., Li Z., Liang W., Lin Y. Is cupping blister harmful?-A proteomical analysis of blister fluid induced by cupping therapy and scald. Compl. Ther. Med. 2018;36:25–29. doi: 10.1016/j.ctim.2017.11.002. [DOI] [PubMed] [Google Scholar]
- 143.Patel S., Ling J., Kim S.J., Schey K.L., Rose K., Kuchtey R.W. Proteomic analysis of macular fluid associated with advanced glaucomatous excavation. JAMA Ophthalmol. 2016;134:108–110. doi: 10.1001/jamaophthalmol.2015.4105. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This research was not applicable. No data was used for the research described in the article.