Abstract
Atherosclerosis (AS) is a chronic inflammatory disease resulting from dysregulated lipid metabolism, constituting the pathophysiological foundation of cardiovascular and cerebrovascular diseases. AS has a high incidence rate and mortality rate worldwide. As such, traditional Chinese medicine (TCM) has been widely used recently due to its stable therapeutic effect and high safety. Ganoderma lucidum polysaccharides (GLP) are the main active ingredients of Ganoderma lucidum, a Chinese herbal medicine. Research has also shown that GLP has anti-inflammatory and antioxidant properties, regulates gut microbiota, improves blood glucose and lipid levels, and inhibits obesity. Most of the current research on GLP anti-AS is focused on animal models. Thus, its clinical application remains to be discovered. In this review, we combine relevant research results and start with the pathogenesis and risk factors of GLP on AS, proving that GLP can prevent and treat AS, providing a scientific basis and reference for the future prevention and treatment of AS with GLP.
Keywords: Ganoderma lucidum polysaccharide, Atherosclerosis, Intestinal flora, Obesity, Research progress
1. Introduction
Atherosclerosis (AS) is a chronic and progressive vascular disease induced by various factors, such as an imbalance of lipid metabolism [1]. The pathogenesis of AS is complex, mainly involving inflammation, oxidative stress, and gut microbiota dysbiosis. Additionally, epidemiological investigations have revealed that hyperlipidemia, hyperglycemia, obesity, and other important risk factors related to AS [2]. Consequently, AS is a significant cause of increased cardiovascular disease morbidity and mortality worldwide. Therefore, studying the pathogenesis of AS and the effective prevention of its risk factors is substantial.
With the development of modern medicine, significant progress has been made in the prevention and treatment of AS. Currently, there are two kinds of AS treatment measures. One is percutaneous transluminal coronary angioplasty (PTCA). However, it is prone to postoperative restenosis and bleeding symptoms [3]. The second is oral statins, which can reduce lipid deposition and effectively control the release of inflammatory factors, improve endothelial function, and improve the anti-AS efficacy [4]. However, statins are prone to causing muscle pain and injury symptoms, leading to adverse reactions such as liver and kidney damage and elevated blood glucose levels [5]. Thus, we urgently need an anti-AS drug with few side effects and high efficacy. Ganoderma lucidum, also known as Reishi, is the dried fruiting body of either Ganoderma lucidum (Leyss. ex Fr.) Karst or Ganoderma sinense, in which both fungi belong to the family Polyporaceae. It is also known for its dual use in both medicine and food. Moreover, it has the efficacy of strengthening the body, nourishing and strengthening, prolonging life, and anti-aging. Recently, Ganoderma lucidum has been applied in the treatment of AS, and its main active ingredient Ganoderma lucidum polysaccharides (GLP), have been found to play an essential role in the anti-AS process [6]. Notably, modern medical research has shown that GLP plays a vital role in regulating lipid metabolism and has various pharmacological effects such as protecting the liver and nervous system, regulating immunity, anticancer, antioxidant, and controlling blood lipids and blood glucose [7].
This review primarily focuses on the treatment and prevention of AS by GLP, reviewing the direct and indirect protective effects of GLP on AS. This review aims to lay a foundation and provide direction for future research on GLP in the context of AS.
2. Ganoderma lucidum polysaccharide
Ganoderma lucidum polysaccharides (GLP), classified as homopolysaccharides and heteropolysaccharides, are polymeric macromolecular carbohydrates consisting of not less than 10 monosaccharides linked by glycosidic bonds. Homopolysaccharides consist of a single glucose or galactose. In contrast, heteropolysaccharides consist of multiple monosaccharides in different proportions [8]. More than 200 polysaccharides have been isolated from Ganoderma lucidum substrates, Ganoderma lucidum spores and mycelium, mainly including β-D-glucan, α-D-glucan, α-D-mannan, Ganoderma lucidum proteoglycans, Ganoderma lucidum degradation polysaccharides, and Ganoderma lucidum wall-breaking spore polysaccharides [9]. Studies have also demonstrated that the pharmacological effects and biological activities of GLP are affected by the chemical structure, such as antioxidant activity when there is β-D-Glc p in the side chain and immunomodulatory activity when there is α-L-Fuc p in the side chain. Additionally, the magnitude of the antioxidant and immunomodulatory activities are affected by the monosaccharides; the greater the number of monosaccharides, the more significant the activity. Furthermore, the composition of the monosaccharides is regulated by the enzymes of the Ganoderma lucidum during the growth period, which plays an essential role in the GLP anti-AS effect [10].
3. The main mechanisms of GLP in the treatment of AS
3.1. GLP is an antioxidant
Studies have shown that plasma levels of oxidized low-density lipoprotein (ox-LDL) are positively correlated with the development of AS [11,12]. Moreover, it was found that plasma levels of reactive oxygen species (ROS), represented by H2O2 and ox-LDL, were significantly elevated in the plasma of mice modeling AS. It was also found that the superoxide produced by excessive ROS could lead to vascular dysfunction and accelerate the development of AS [13].
Wihastuti et al. showed that different doses of Ganoderma lucidum polysaccharide peptide (PSP) significantly reduced H2O2 and ox-LDL levels in T2DM rats compared to the high-fat diet (HFD) control group, suggesting that PSP can inhibit AS through antioxidant effects [14]. Wu and Zhao et al. also identified that GLP could reduce serum malondialdehyde (MDA) levels and oxidative stress indicators such as aortic ROS and down-regulate the expression of NADPH oxidase, which is closely related to ROS generation [15,16]. Thus, the research results indicate that GLP can exert antioxidant effects to treat AS by reducing H2O2, ox-LDL, and ROS levels.
3.2. GLP repairs damaged endothelial cells
Repair of endothelial dysfunction is also an essential mechanism for AS treatment by GLP. Endothelial dysfunction is considered the initiating factor and central aspect of AS. When cholesterol metabolism is disturbed, LDL-C is deposited in the vasculature, and ox-LDL induces macrophage adhesion to endothelial cells (EC), forming early atheromatous plaques [17]. Notably, endothelial progenitor cells (EPC) play a positive role in endothelial repair after injury, promoting the restoration of endothelial function and inhibiting plaque formation in AS progression [18]. The primary mechanism by which GLP repairs endothelial cells is by increasing the number of EPCs.
Wihastuti et al. showed that PSP could increase the number of EPCs and improve endothelial dysfunction in rats. Likewise, they found that EPCs can also increase anti-inflammatory cytokines such as IL-10, inhibit the inflammatory response, and slow the AS process [19]. Circulating endothelial cells (CEC) are considered the only specific indicator in vivo that directly responds to vascular endothelial cell damage, and a higher number represents more severe EC damage [20]. As such, Sargowo et al. screened 34 stable angina and 37 high-risk patients according to the ESC guidelines for stable coronary artery disease and the Framingham risk score, with no control group, and administered PSP 750 mg/day in 3 divided doses for 90 consecutive days. The results showed that the CEC values of both groups were significantly reduced following PSP administration, suggesting that PSP can dramatically improve endothelial dysfunction [21]. Interestingly, the values of EPC were considerably reduced in both groups following PSP administration, which is likely due to the reduced degree of EC injury when patients undergo PSP administration. High turnover is no longer required to maintain cellular homeostasis and, therefore, does not induce an increase in EPC either. Furthermore, a study on the number of EPCs in patients with myocardial infarction found that their EPC values returned to baseline levels within 60 days [22]. Therefore, whether the number of EPCs is negatively correlated with the development of AS remains in question. Thus, GLP's mechanism of action on EPCs still needs to be investigated with large sample sizes and more in-depth studies.
3.3. GLP is anti-inflammatory
Studies have shown that macrophages can be polarized into M1 and M2 types, release pro-inflammatory and anti-inflammatory factors, respectively, and transform into each other under certain conditions [23]. During the inflammatory progression of AS, macrophages are mainly polarized into M1 types, which can release pro-inflammatory factors such as tumor necrosis factor-alpha (TNF-α) and interleukin 6 (IL-6). Likewise, they can produce ROS, nitric oxide synthase, and other substances to promote the development of AS [23]. The Notch signaling pathway also plays a vital role in the development of the cell, including four receptors (Notch1-Notch4) and five ligands (delta-like, DLL1, DLL3, DLL4, jagged-1, and jagged-2) [24]. Moreover, recent investigations have shown that DLL ligands and multiple Notch signaling expressions are upregulated in AS plaques [25]. Furthermore, one study reported that DLL4 binding to the Notch1 receptor promotes macrophage polarization toward a pro-inflammatory M1 phenotype, generating high levels of ROS and accelerating the progression of AS plaques [26].
Li et al. found that the GLP-treated group's MDA level and ROS fluorescence intensity were significantly lower than those of the control group. Additionally, the expression of Notch1 signaling and DLL4 in the treated group was also significantly reduced, suggesting that GLP can inhibit macrophage polarization toward the M1 phenotype and slow down the progression of AS by regulating the expression of Notch1 signaling and DLL4 signaling [27]. Thus, this suggests that GLP can treat AS by exerting anti-inflammatory effects by regulating Notch1 and DLL4 signaling, thereby inhibiting the polarization of macrophages to the M1 phenotype. However, it has also been shown that Ganoderma lucidum polysaccharide peptide (Gl-PS) can improve the immune response and exert anti-tumor immunity by promoting the polarization of macrophages to the M1 type [28]. Therefore, GLP can inhibit macrophage transformation to M1 type to play an anti-inflammatory role and promote macrophage transformation to M1 to play an immunomodulatory role. Furthermore, studies have shown that at the beginning of the inflammatory response, the proportion of M1-type macrophages increases, which is conducive to the clearance of pathogenic microorganisms. With the progression of the inflammatory response, the proportion of M1-type macrophages decreases, and the conversion of macrophage M1-type to M2 promotes the repair of inflammatory injury [29,30]. Hence, we postulate that GLP, with its unique advantages in TCM encompassing multiple targets, stages, and pathways, may modulate macrophage polarization towards beneficial phenotypes across various phases of inflammatory response. Thus, elucidating the mechanisms by which GLP exerts its anti-inflammatory effects during macrophage phenotype transition holds pivotal implications for future therapeutic strategies targeting AS.
3.4. GLP regulates intestinal flora
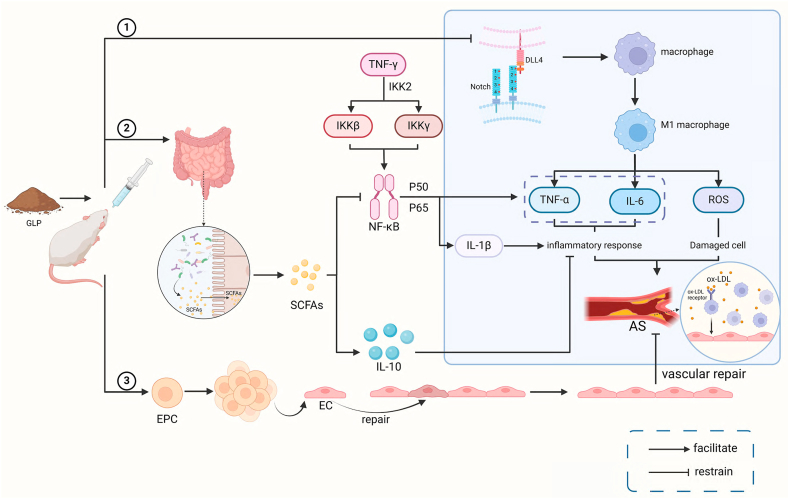
Many lines of evidence suggest a significant correlation between dysbiosis of the gut flora and AS. Additionally, evidence suggests that dysbiosis of the gut flora causes systemic chronic inflammation, foam cell accumulation, endothelial dysfunction, and lipid accumulation, which in turn leads to AS [[31], [32], [33]]. Short-chain fatty acids (SCFAs) are metabolites of the bacteria in the gut and are essential in maintaining human intestinal homeostasis [34]. Notably, butyrate is one of the representatives of SCFAs. It has been reported that SCFAs can increase the expression of the anti-inflammatory factor IL-10, inhibit the production of pro-inflammatory cytokines, and play an anti-inflammatory role [35]. Recently, Chen et al. found that GLPs could increase the amount of butyrate and valerate and exert the anti-inflammatory effects of SCFAs [36]. Recent investigations have also demonstrated that oral butyrate can inhibit nuclear factor κB (NF-κB) transport, reduce pro-inflammatory cytokine expression, decrease monocyte-EC adhesion, impede foam cell formation, promote reverse cholesterol transport, and interfere with the AS process [37]. In summary, we hypothesize that GLP can treat AS by modulating gut microbiota dysbiosis by increasing certain SCFAs' levels. Although we have not found any experiments in which GLP regulates the intestinal flora to directly inhibit AS, many studies have shown that GLP can control AS-related risk factors to treat AS. The main mechanism of GLP in treating AS is shown in Fig. 1.
Fig. 1.
The main mechanism of GLP treating AS.
Fig. 1 ① GLP inhibits the binding of Notch1 and DLL4, thereby inhibiting macrophage polarization towards the M1 type and reducing TNF-α. The release of IL-6 and ROS inhibits inflammation and cellular damage and slows down the progression of atherosclerosis; ② We indicate that GLP can regulate gut microbiota, increase the number of SCFAs and the expression of anti-inflammatory factor IL-10, and inhibit NF-κ Transport B, reduce TNF-α、IL-6, IL-1β pro-inflammatory cytokine expression, thereby treating AS; ③ GLP increases the number of EPCs, repairs damaged endothelial cells, improves endothelial dysfunction, and inhibits AS plaque formation. Created with BioRender.com.
4. The primary mechanism of GLP in preventing AS
4.1. GLP inhibits obesity
Obesity is a metabolic syndrome caused by an imbalance in energy intake or consumption. Research has shown that the pathogenesis of obesity and AS is related to inflammatory responses, and many inflammatory mediators, such as macrophages and T cells, play essential roles in the progression of obesity and AS. Thus, we hypothesize that inhibiting obesity can be used to prevent AS [38].
It has been suggested that the sterol-regulatory element binding proteins (SREBP) pathway can promote the production and release of adipogenic genes such as fatty acid synthase (FAS) and acetyl coenzyme A carboxylase (ACC) to accelerate adipogenesis via the up-regulation of SREBP-1c and peroxisome proliferator-activated receptor (PPAR) expression [39]. It was found that the expression level of the SREBP-1c/FAS pathway was significantly elevated in obese mice compared to normal mice [40]. Moreover, recent studies have shown that PSP can reduce adipogenesis by inhibiting the expression of SREBP1c, FAS, and ACC [41]. Sporoderm-broken spore powder of Ganoderma lucidum (SSPL) contains all the active components of Ganoderma lucidum and has a relatively high GLP content [42]. Zhong et al. experimentally found that compared with the control group of mice on a HFD, mice in the SSPL-treated group showed a significantly slower rate of weight gain and significantly lower levels of SREBP-1c, PPARγ, FAS, and TG in the liver [43]. These findings suggest that GLP-rich SSPL can slow down the process of obesity by inhibiting adipogenesis by regulating the SREBP pathway. Although GLP is the most abundant, SSPL contains Ganoderma lucidum triterpenoic acid and other active ingredients. Thus, more in-depth and accurate experiments are needed to prove that GLP improves the symptoms of obesity by inhibiting the SREBP pathway. A research group from Fudan University extracted a proteoglycan from Ganoderma lucidum entity and named it FYGL (Fudan-Yueyang Ganoderma lucidum). As such, it was found that FYGL can accelerate the degradation and metabolism of triglycerides by activating the AMPKα signaling pathway, thus preventing fat accumulation and inhibiting the progression of obesity [44]. However, this idea still needs to be further investigated and explored.
Studies have shown that changes in gut flora composition are strongly associated with obesity, additionally, Goodman found that an increase in the proportion of thick-walled phylum/Anthrobacterium promotes the progression of obesity [45,46]. To investigate whether GLP inhibits obesity by regulating the intestinal flora, Chang et al. conducted experiments using water extract of Ganoderma lucidum mycelium (WEGL) as a therapeutic agent at different doses in a model of HFD (HFD) mice [47]. The results showed that WEGL could slow down the rate of weight gain, inhibit the accumulation of fat in HFD model mice, and restore the ratio of the thick-walled phylum of Ganoderma lucidum to the anaplasmosis phylum of HFD model mice to the same level as that of normal mice. However, there was no significant difference in the gut microbiota of the mice treated with 2 % WEGL from that of the HFD model mice, likely related to the insufficient dosage of 2 % WEGL. Chang et al. also isolated the active ingredient from WEGL for experiments and found that high molecular weight polysaccharides (>300 kDa) had a significant anti-obesity effect on HFD-fed mice. This finding suggests that WEGL may exert its anti-obesity effect through its high molecular weight polysaccharides by regulating intestinal flora and intestinal barrier function and reducing the ratio of thick-walled phylum to anamorphic phylum. It was also found that GLP reduced the number of pro-obesity gut flora, such as Barnesella and Vibrio pseudobutyricum, in the intestinal tracts of C57BL/6J mice while increasing the abundance of anti-obesity gut flora, such as Eckermannia and acid-producing anaplasmodiales [48]. G protein-coupled receptors (GPRs) are SCFA receptors widely present in intestinal epithelial cells, regulating the immune response of intestinal epithelial cells or neutrophils through G protein-coupled receptor 41 (GPR41) or G protein-coupled receptor 43 (GPR43), which can inhibit obesity [49]. Moreover, Wang et al. found Ganoderma lucidum spore powder polysaccharide (BSGLP) may activate GPR43 expression in adipose tissue by regulating the intestinal flora and increasing the level of SCFAs, thus regulating metabolism and suppressing obesity [42]. The decrease in the ratio of Bacteroides thickeniensis/Bacteroides anomalies, the increase in the abundance of anti-obesity flora in the intestine, the reduction in the abundance of pro-obesity flora, and the up-regulation of the levels of GPR43 and SCFAs are all results of the experiment of Wang et al. which also summarizes the mechanism by which GLP may regulate the intestinal flora to inhibit obesity. Thus, this shows that compared with statin, GLP can inhibit obesity, which is a very promising advantage. However, the objects of related research are mostly active ingredients such as WEGL and BSGLP. These ingredients' complex composition and unknown polysaccharide purity may have affected the experimental results. Furthermore, the related research on regulating intestinal flora by high-purity GLP for the treatment of obesity is still unclear, and the complex mechanism of its regulation of intestinal flora is still the target of future research.
In summary, GLP mainly inhibits obesity by inhibiting fat generation and accumulation and regulating intestinal microbiota homeostasis.
4.2. GLP inhibits hyperlipidemia
Clinical manifestations of hyperlipidemia include elevated serum cholesterol (TC), triglycerides (TG), and low-density lipoprotein cholesterol (LDL-C) [50]. Research has found [51,52] when blood lipid levels are high, LDL-C will be converted into ox-LDL, forming a marker for early AS through pro-inflammatory reactions known as fat streaks. In addition, blood lipid levels are positively correlated with the progression of AS plaques [53]. Thus, prevention of AS can be achieved by inhibiting hyperlipidemia.
Wu et al. [54] using HFD rats as a model, found that GLP could reduce plasma TC, TG, and LDL-C levels and increase HDL-C levels. They also found that the levels of antioxidant enzymes superoxide dismutase (SOD) and glutathione peroxidase (GPx) were significantly increased [55]. The results suggest that GLP has a lipid-lowering effect. However, how it exerts its antioxidant effect to reduce the lipid level needs further investigation. NF-e2-related factor 2 (Nrf2) is a crucial regulator of tissue oxidative stress. It can bind to the antioxidant response element (ARE) in the promoter region of antioxidant genes, thus exerting its full antioxidant potential [56]. Recent studies have indicated that activation of the Nrf2/HO-1 signaling pathway can exert antioxidant effects [57]. To investigate whether GLP can reduce blood lipids by regulating the Nrf2/HO-1 signaling pathway, Li et al. established a mouse T2DM model for experiments. The results showed that a high dose of GLP (400 mg/kg) significantly reduced the concentrations of TC, TG, and LDL-C in the blood of mice, up-regulated the expression of the antioxidant enzymes SOD and GSH-Px, and significantly increased the protein expression levels of Nrf2 and HO-1. In contrast, a low dose of GLP (100 mg/kg) had no significant modulatory effect on the Nrf2/HO-1 signaling pathway [58]. Therefore, this suggests that high-purity GLP can activate the Nrf2/HO-1 pathway and inhibit the oxidative stress response, thus reducing the blood lipid level.
Hyperlipidemia is closely associated with related inflammatory factors such as interleukin 1β (IL-1β), TNF-α, IL-6, and monocyte chemotactic protein 1 (MCP-1) [59]. Recently, it has been shown that the Toll-like receptor 4 (TLR4)/NF-κB signaling pathway plays a crucial role in the regulation of inflammatory responses, and TLR4 activates the NF-κB nuclear translocation to produce a variety of inflammatory cytokines, such as TNF-α, IL-1β, and IL-6 [60]. The myeloid differentiation factor (Myd88) is a signaling factor in the TLR signaling pathway. Its structural domain can accelerate the activation and translocation of NF-κB, inducing the synthesis and release of inflammatory cytokines [61]. Recently, Wang et al. showed that GLP could inhibit the expression of NF-κB, TLR4, and MyD88. Furthermore, it reduces the release of inflammatory factors TNF-α, IL-1β, IL-6, and IL-10 to lower lipid levels [62].
The transport of cholesterol from peripheral tissues to the liver and its discharge into the bile as bile acids (BA) or free cholesterol is known as reverse cholesterol transport (RCT); by which cholesterol in the blood can be transported from peripheral tissues to the liver for recirculation or excretion, lowering the body's blood lipid level [63]. Liver X receptor α (LXRα), as an essential cholesterol metabolism receptor, regulates the critical transporter proteins ATP-binding cassette transporter G1 (A1ABCG1) and ATP-binding cassette transporter A1 (A1ABCA1) involved in the RCT process [64]. Notably, Zheng and Huang found that enhanced LXRα ABCA1, and ABCG1 expression levels contribute to BA synthesis and fecal excretion, promoting cholesterol dissolution and excretion from the body [65]. Recently, Wang et al. also established a mouse model of hyperlipidemia and showed that GLP could promote RCT and regulate lipid metabolism by activating the LXRα-ABCA1/ABCG1 pathway and enhancing the expression levels of LXRα and ABCA1 and ABCG1. Furthermore, they found that GLP reduced blood TC, TG, and LDL-C concentrations in mice [62].
Thus, GLP can inhibit hyperlipidemia by antioxidizing, inhibiting inflammatory responses, and promoting reverse cholesterol transport.
4.3. GLP inhibits hyperglycemia
Research has shown that high blood glucose levels can accelerate the development of AS in Apo E −/- mice, and elevated blood sugar levels can increase carotid intima-media thickness [66]. Therefore, we hypothesize that preventing AS can also be achieved by inhibiting hyperglycemia.
Some studies have pointed out that elevated blood glucose is associated with absolute or relative insufficiency of insulin secretion, which is mainly secreted by pancreatic β-cells [67]. Streptozotocin (STZ) is a valuable experimental drug that causes damage to pancreatic β-cells [68]. To investigate the mechanism of blood glucose regulation by GLP, Li et al. used STZ rats as a model. As such, they found that GLP can improve the symptoms of insufficient insulin secretion by repairing the damaged pancreatic β-cells or increasing their number to control blood glucose [69]. Zhu et al. also found that a synthetic GLP (PSG-1) could significantly improve the shrinkage of pancreatic islets in diabetic rats by hematoxylin and eosin staining of pancreatic islet tissues. Additionally, they noted that GLP could suppress the expression of pro-apoptotic protein Bcl-2 associated X protein (Bax) in pancreatic cells and promote the expression of anti-apoptotic protein Bax. Simultaneously it could inhibit the expression of the anti-apoptotic protein Bcl-2 associated with X protein in pancreatic cells [70]. Therefore, GLP mainly improves relative or absolute insulin deficiency symptoms by protecting pancreatic cells, thereby regulating blood glucose.
Gupta et al. showed that down-regulation of pancreatic duodenal homology box protein 1 (Pdx-1) expression in the pancreas could negatively affect pancreatic β-cell function and survival. In contrast, peroxisome proliferator-activated receptor γ (PPARγ) upregulated approximately 40 % of Pdx-1 expression in mouse β-cells [71]. Furthermore, Yu et al. found that FYGL, a proteoglycan extracted from Ganoderma lucidum entities, could promote PPARγ expression in the pancreas of T2D rats and upregulate Pdx-1 expression to increase the number of pancreatic β-cells. Thus, FYGL was able to restore pancreatic islet function and lower blood glucose [72].
Hepatic gluconeogenesis (HGP) is essential for maintaining systemic glucose homeostasis, and studies have shown that an excessive increase in HGP significantly elevates blood glucose [73]. The main pathways of HGP are glycogenolysis (breakdown of glucose polymer glycogen) and gluconeogenesis (synthesis of glucose from 3-carbon precursors). Additionally, hepatic glycogen phosphorylase (GP), fructose 1,6-bisphosphatase (FBPase), phosphoenolpyruvate carboxykinase (PEPCK), and glucose-6-phosphatase (G6Pase) are the key enzymes that promote the process of gluconeogenesis and glycogenolysis [74]. Thus, inhibiting the expression of the above vital enzymes has been regarded as essential to inhibiting HGP and lowering blood glucose. Xiao et al. also identified that GLP could lower fasting blood glucose levels in mice and significantly reduce the mRNA expression levels of GP, G6Pase, FBPase, and PEPCK [75]. Moreover, the FAM3C family (family with sequence similarity 3, FAM3C) consisting of FAM3A- D has been reported to be associated with the regulation of glucose metabolism, and the FAM3C -heat shock factor 1 (HSF1)-calmodulin (CaM) signaling pathway can overexpress FAM3C and activate the HSF1- CaM- AKT signaling pathway, inhibit the expression of gluconeogenic genes, and significantly improve hyperglycemia in mice [76,77]. Furthermore, PAN et al. used T2DM mice as a model for their study. The results showed that the GLP group's fasting blood glucose level was reduced, the expression levels of FAM3C, HSF1, CaM and p - AKT/AKT in the liver of mice in the 400 mg/kg GLP group were significantly increased, while the expression levels of FAM3C, HSF1, CaM and p - AKT/AKT in the 100 mg/kg GLP group were either slightly increased or did not have any significant changes [78]. This suggests that GLP may inhibit the expression of gluconeogenic genes by activating the FAM3C-HSF1-CaM signaling pathway. In doing so, it reduces blood glucose concentrations. The above experimental results indicate that GLP inhibits HGP and decreases blood glucose levels.
Recently, it has also been shown that GLP can restore the total abundance of intestinal flora, reduce the abundance of harmful bacteria such as rumenococci and bacilli, and increase the abundance of beneficial bacteria such as Brucella abortus and Dehalobacterium. In doing so, it can regulate the homeostasis of the intestinal flora, attenuate the symptoms of insulin resistance, and reduce blood glucose in rats with T2DM [36].
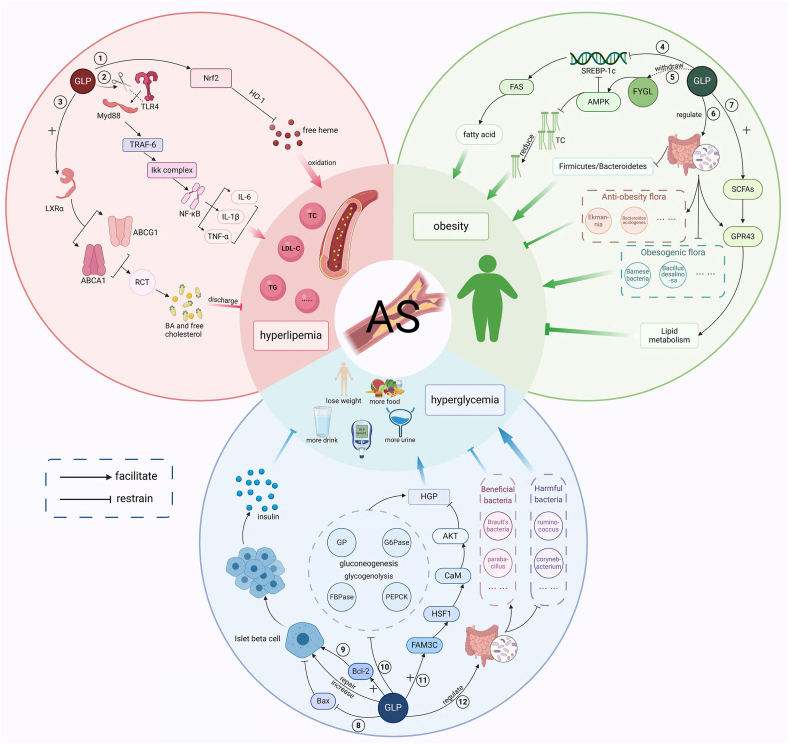
Thus, GLP can inhibit hyperglycemia by protecting or increasing the number of pancreatic cells, inhibiting hepatic glucose production, and regulating intestinal flora. Fig. 2 shows the primary mechanism of GLP in preventing AS.
Fig. 2.
The primary mechanism of GLP in preventing AS.
Fig. 2 ① GLP activates the Nrf2/HO-1 pathway, inhibits the oxidative stress response of free heme, thereby reducing blood lipid levels; ②GLP inhibits the TLR4/MyD88/NF-κB signaling pathway by reducing the binding of MyD88 to TLR4 and consequently decreases the expression of TNF-α, IL-1β, and IL-6, thereby lowering blood lipid levels; ③ GLP activates the LXR α-ABCA1/ABCG1 pathway to promote RCT, thereby promoting the synthesis of BA and the excretion of free cholesterol out of the body, resulting in reduced blood lipid levels; ④ GLP inhibits the expression of SREBP1c and FAS, reduces fatty acid production, thus inhibiting obesity; ⑤ The extracted Ganoderma lucidum protein polysaccharide FYGL activates the AMPK signaling pathway, inhibiting the expression of SREBP-1c/FAS pathway on the one hand, and reducing fat accumulation and inhibiting obesity on the other hand; ⑥ GLP regulates gut microbiota, reduces the proportion of Firmicutes/Bacteroidetes and the abundance of obesity promoting bacteria such as Bacillus and Deinococcus, increases the abundance of anti-obesity bacteria such as Ekmann and Acidophilus, and inhibits obesity; ⑦ GLP increases SCFAs levels, activates GPR43 expression in adipose tissue, regulates lipid metabolism, and inhibits obesity; ⑧ - ⑨ GLP inhibits the expression of pro apoptotic protein Bax, promotes the expression of anti-apoptotic protein Bcl-2, repairs and increases pancreatic islets β cells; The number of cells promotes insulin production, thereby reducing blood glucose; ⑩ GLP inhibits the expression of GP, G6Pase, FBPase, and PEPCK, thereby inhibiting gluconeogenesis and glycolysis, inhibiting HGP and lowering blood glucose; ⑪ GLP's overexpression of FAM3C activates the HSF1 CaM AKT signaling pathway, inhibits gluconeogenesis, and improves blood glucose levels; ⑫ GLP regulates the homeostasis of gut microbiota, reduces the abundance of harmful bacteria such as Ruminococcus and Corynebacterium, increases the abundance of beneficial bacteria such as Brucella and Parabacteria, and lowers blood glucose. Created with BioRender.com.
5. Summary and outlook
In recent years, GLP has made good progress in preventing and treating AS, but most of the research has been focused on preclinical studies and still lacks high-quality and large-sample clinical data. There are still some deficiencies in the current relevant preclinical studies, among which purity is the primary issue. Some experiments did not mention the specific purity of GLP in the study. Others used BSGLP, PSG-1, and FYGL, which are substances with lower purity and may affect the experimental results. The extraction process of high-purity GLP is cumbersome and expensive; therefore, in the future, we need to explore a purity standard that can guarantee therapeutic efficacy and reduce the cost. In addition, because there are many species of Ganoderma lucidum, even if the same species, the composition of the polysaccharides obtained using different extraction methods at different developmental stages is not the same. The structure of the GLPs applied in various experiments is distant and lacks a certain degree of comparability. Thus, the obtained experimental results may be somewhat controversial. Secondly, studies have shown that TCM can better utilize its therapeutic advantages in the early or subclinical stage of the disease [79]. Whether GLP has a better therapeutic effect for the early stage of AS is also an essential step in better utilizing the role of TCM in the future. In addition, the different ways of GLP administration in animal experiments may also affect the results; the experimental administration methods are mainly intraperitoneal injection, gavage, and oral administration. Oral administration has the disadvantage of uncontrollable dosage, the operation of gavage is more complicated, and it is easy to cause damage to the animal's organism. The intraperitoneal injection administration also has the problems of poor accuracy and slow onset of effect; whether there is a more reasonable way of administration and dosage form is also the next step to be explored. Finally, most of the existing studies lasted for 4–12 weeks, but the lipid-lowering efficacy of statins was better than that of GLP during this period [80]. This may be related to the slow onset of action and long duration of treatment of TCM, and extending the dosage period may be an essential means to improve the efficacy of GLP.
Based on many high-quality studies and reviews in recent years, we believe that GLP can be used as a promising active ingredient of TCM for preventing and treating AS. GLP's prevention and treatment of AS mainly focuses on regulating the pathogenesis and controlling the risk factors. In terms of pathogenesis, GLP mainly treats AS by improving endothelial dysfunction and inhibiting inflammation and oxidative reactions; moreover, studies have shown that butyrate, one of the representatives of SCFAs, can inhibit AS, and GLP can increase the amount of butyrate by regulating intestinal flora. However, relevant experiments about GLP regulating intestinal flora to inhibit AS have yet to be found. As such, research in this direction can be carried out in the next step. In terms of risk factors, high blood lipids, high blood glucose, and obesity seriously affect the progression of AS, and GLP can inhibit AS by lowering blood lipids and blood glucose levels; moreover, AS is closely related to obesity, and many inflammatory mediators and pathways play an essential role in the progression of both [38]. GLP can also regulate the intestinal flora to inhibit obesity, and the intestinal flora plays a critical role in the development of AS [81]. In this regard, it is worth thinking about suppressing AS by treating obesity through GLP. Ganoderma lucidum is considered an elixir of immortality and is famous for its anti-aging effects. Notably, GLP is anti-aging by scavenging oxygen free radicals through antioxidation and regulating aging genes [82]. Aging plays a vital role in AS, and an increase in the number of senescent cells and senescence-associated secretory phenotype (SASP) accelerates the AS process [83]. Autophagy plays a cellular scavenging role in maintaining cellular tissue homeostasis through lysosomal degradation and recycling of damaged organelles and misfolded proteins [84]. Studies have confirmed that autophagy can be anti-aging by inhibiting oxidative stress and significantly reducing ROS levels [85]. In addition, mitochondrial dysfunction is a major cause of cellular senescence and is associated with the overproduction of ROS [86]. Recently, Guo et al. found that Ganoderma atrum polysaccharide (PSG) can reduce ROS levels by activating autophagy, thus inhibiting mitochondrial dysfunction in anti-aging [87]. In agreement, this review introduces novel concepts regarding the anti-aging mechanism of GLP, offering a fresh perspective on its potential for treating AS. In the future, it is worth considering and investigating the therapeutic role of GLP in treating AS by activating autophagy and restoring mitochondrial dysfunction.
This review summarizes the common mechanisms of AS prevention and treatment by GLP and presents the main progress and shortcomings of the current relevant basic research. We suppose the conformational relationship of GLP can be further elucidated. In that case, the related mechanism of GLP anti-AS can be better explored, and the relevant clinical trials can be increased. As such, GLP is expected to become a novel therapeutic drug for treating AS.
Data availability statement
This manuscript is a review article and is not applicable to the Data availability statement situations.
Funding
This research was supported and funded by the National Natural Science Foundation of China Youth Fund (81303028),the Shandong Natural Science Foundation (ZR2022MH208),the Jinan Science and Technology Development Plan (202019027, 202134007),the Shandong Province Traditional Chinese Medicine Technology Project (2019-0107, 2019-0094, Z-2022060, Z-2023020),the Shandong Geriatrics Society Science and Technology Public Relations Project (LKJGG2021Y041, LKJGG2021W116).
CRediT authorship contribution statement
YiZheng Ma: Writing – original draft, Visualization. JingBo Han: Writing – original draft, Visualization. KangFeng Wang: Writing – review & editing, Visualization. Huan Han: Writing – original draft. YiBin Hu: Writing – original draft. He Li: Writing – review & editing, Writing – original draft. ShengXian Wu: Writing – review & editing. LiJuan Zhang: Writing – review & editing, Funding acquisition.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Contributor Information
He Li, Email: helishx2021@hotmail.com.
ShengXian Wu, Email: wushx@sina.com.
LiJuan Zhang, Email: zhanglijuansdutcm@163.com.
References
- 1.Jebari-Benslaiman S., Galicia-García U., Larrea-Sebal A., Olaetxea J.R., Alloza I., Vandenbroeck K., Benito-Vicente A., Martín C. Pathophysiology of atherosclerosis. Int. J. Mol. Sci. 2022;23:3346. doi: 10.3390/ijms23063346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Björkegren J.L.M., Lusis A.J. Atherosclerosis: recent developments. Cell. 2022;185:1630–1645. doi: 10.1016/j.cell.2022.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hartzler G.O. PTCA in evolution: why is it so popular? Cleve. Clin. J. Med. 1990;57:121–124. doi: 10.3949/ccjm.57.2.121. [DOI] [PubMed] [Google Scholar]
- 4.Jia J., Zhang L., Wang L., Ji C., Xia R., Yang Y. A systematic review and meta-analysis on the efficacy of statins in the treatment of atherosclerosis. Ann. Palliat. Med. 2021;10:6793–6803. doi: 10.21037/apm-21-1243. [DOI] [PubMed] [Google Scholar]
- 5.Bellosta S., Corsini A. Statin drug interactions and related adverse reactions: an update. Expet Opin. Drug Saf. 2018;17:25–37. doi: 10.1080/14740338.2018.1394455. [DOI] [PubMed] [Google Scholar]
- 6.Oh K.K., Adnan M., Cho D.H. A network pharmacology analysis on drug-like compounds from Ganoderma lucidum for alleviation of atherosclerosis. J. Food Biochem. 2021;45 doi: 10.1111/jfbc.13906. [DOI] [PubMed] [Google Scholar]
- 7.Cör Andrejč D., Knez Ž., Knez Marevci M. Antioxidant, antibacterial, antitumor, antifungal, antiviral, anti-inflammatory, and nevro-protective activity of Ganoderma lucidum: an overview. Front. Pharmacol. 2022;13 doi: 10.3389/fphar.2022.934982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jiang Y., Chang Y., Liu Y., Zhang M., Luo H., Hao C., Zeng P., Sun Y., Wang H., Zhang L. Overview of Ganoderma sinense polysaccharide-an adjunctive drug used during concurrent Chemo/Radiation therapy for cancer treatment in China. Biomed. Pharmacother. 2017;96:865–870. doi: 10.1016/j.biopha.2017.09.060. [DOI] [PubMed] [Google Scholar]
- 9.Liu W., Wang H., Pang X., Yao W., Gao X. Characterization and antioxidant activity of two low-molecular-weight polysaccharides purified from the fruiting bodies of Ganoderma lucidum. Int. J. Biol. Macromol. 2010;46:451–457. doi: 10.1016/j.ijbiomac.2010.02.006. [DOI] [PubMed] [Google Scholar]
- 10.Lu J., He R., Sun P., Zhang F., Linhardt R.J., Zhang A. Molecular mechanisms of bioactive polysaccharides from Ganoderma lucidum (Lingzhi), a review. Int. J. Biol. Macromol. 2020;150:765–774. doi: 10.1016/j.ijbiomac.2020.02.035. [DOI] [PubMed] [Google Scholar]
- 11.Qin S. LDL and HDL oxidative Modification and atherosclerosis. Adv. Exp. Med. Biol. 2020;1276:157–169. doi: 10.1007/978-981-15-6082-8_10. [DOI] [PubMed] [Google Scholar]
- 12.Zampetaki A., Dudek K., Mayr M. Oxidative stress in atherosclerosis: the role of microRNAs in arterial remodeling. Free Radic. Biol. Med. 2013;64:69–77. doi: 10.1016/j.freeradbiomed.2013.06.025. [DOI] [PubMed] [Google Scholar]
- 13.Karunakaran D., Geoffrion M., Wei L., Gan W., Richards L., Shangari P., DeKemp E.M., Beanlands R.A., Perisic L., Maegdefessel L., Hedin U., Sad S., Guo L., Kolodgie F.D., Virmani R., Ruddy T., Rayner K.J. Targeting macrophage necroptosis for therapeutic and diagnostic interventions in atherosclerosis. Sci. Adv. 2016;2 doi: 10.1126/sciadv.1600224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wihastuti T.A., Amiruddin R., Cesa F.Y., Alkaf A.I., Setiawan M., Heriansyah T. Decreasing angiogenesis vasa vasorum through Lp-PLA2 and H2O2 inhibition by PSP from Ganoderma lucidum in atherosclerosis: in vivo diabetes mellitus type 2. J. Basic Clin. Physiol. Pharmacol. 2020;30 doi: 10.1515/jbcpp-2019-0349. [DOI] [PubMed] [Google Scholar]
- 15.Wu F., Meng G., Chang S., Xu J. The anti - atherosclerotic effect of Ganoderma lucidum Polysaccharides via down - regulation of vascular NADPH oxidases expression in atherosclerosis rats. Chin. Pharmacol. Bull. 2012;28:944–947. [Google Scholar]
- 16.Zhao H.B., Lin S.Q., Liu J.H., Lin Z.B. Polysaccharide extract isolated from ganoderma lucidum protects rat cerebral cortical neurons from hypoxia/reoxygenation injury. J. Pharmacol. Sci. 2004;95:294–298. doi: 10.1254/jphs.sc0040011. [DOI] [PubMed] [Google Scholar]
- 17.Borén J., Chapman M.J., Krauss R.M., Packard C.J., Bentzon J.F., Binder C.J., Daemen M.J., Demer L.L., Hegele R.A., Nicholls S.J., Nordestgaard B.G., Watts G.F., Bruckert E., Fazio S., Ference B.A., Graham I., Horton J.D., Landmesser U., Laufs U., Masana L., Pasterkamp G., Raal F.J., Ray K.K., Schunkert H., Taskinen M.R., van de Sluis B., Wiklund O., Tokgozoglu L., Catapano A.L., Ginsberg H.N. Low-density lipoproteins cause atherosclerotic cardiovascular disease: pathophysiological, genetic, and therapeutic insights: a consensus statement from the European Atherosclerosis Society Consensus Panel. Eur. Heart J. 2020;41:2313–2330. doi: 10.1093/eurheartj/ehz962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hirsch E.Z., Chisolm G.M., 3rd, White H.M. Reendothelialization and maintenance of endothelial integrity in longitudinal denuded tracks in the thoracic aorta of rats. Atherosclerosis. 1983;46:287–307. doi: 10.1016/0021-9150(83)90179-x. [DOI] [PubMed] [Google Scholar]
- 19.Wihastuti T.A., Heriansyah T. The inhibitory effects of polysaccharide peptides (PsP) of Ganoderma lucidum against atherosclerosis in rats with dyslipidemia. Heart Int. 2017;12:e1–e7. doi: 10.5301/heartint.5000234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mody P., Joshi P.H., Khera A., Ayers C.R., Rohatgi A. Beyond coronary Calcification, family history, and C-reactive protein: cholesterol Efflux capacity and cardiovascular risk Prediction. J. Am. Coll. Cardiol. 2016;67:2480–2487. doi: 10.1016/j.jacc.2016.03.538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sargowo D., Ovianti N., Susilowati E., Ubaidillah N., Widya Nugraha A., Vitriyaturrida, Siwi Proboretno K., Failasufi M., Ramadhan F., Wulandari H., Waranugraha Y., Hayuning Putri D. The role of polysaccharide peptide of Ganoderma lucidum as a potent antioxidant against atherosclerosis in high risk and stable angina patients. Indian Heart J. 2018;70:608–614. doi: 10.1016/j.ihj.2017.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Alessio A.M., Beltrame M.P., Nascimento M.C., Vicente C.P., de Godoy J.A., Silva J.C., Bittar L.F., Lorand-Metze I., de Paula E.V., Annichino-Bizzacchi J.M. Circulating progenitor and mature endothelial cells in deep vein thrombosis. Int. J. Med. Sci. 2013;10:1746–1754. doi: 10.7150/ijms.6887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lin P., Ji H.H., Li Y.J., Guo S.D. Macrophage Plasticity and atherosclerosis therapy. Front. Mol. Biosci. 2021;8 doi: 10.3389/fmolb.2021.679797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vanderbeck A., Maillard I. Notch signaling at the crossroads of innate and adaptive immunity. J. Leukoc. Biol. 2021;109:535–548. doi: 10.1002/jlb.1ri0520-138r. [DOI] [PubMed] [Google Scholar]
- 25.Chen W., Liu Y., Chen J., Ma Y., Song Y., Cen Y., You M., Yang G. The Notch signaling pathway regulates macrophage polarization in liver diseases. Int Immunopharmacol. 2021;99 doi: 10.1016/j.intimp.2021.107938. [DOI] [PubMed] [Google Scholar]
- 26.Fukuda D., Aikawa E., Swirski F.K., Novobrantseva T.I., Kotelianski V., Gorgun C.Z., Chudnovskiy A., Yamazaki H., Croce K., Weissleder R., Aster J.C., Hotamisligil G.S., Yagita H., Aikawa M. Notch ligand delta-like 4 blockade attenuates atherosclerosis and metabolic disorders. Proc. Natl. Acad. Sci. U.S.A. 2012;109:E1868–E1877. doi: 10.1073/pnas.1116889109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li Y., Tang J., Gao H., Xu Y., Han Y., Shang H., Lu Y., Qin C. Ganoderma lucidum triterpenoids and polysaccharides attenuate atherosclerotic plaque in high-fat diet rabbits. Nutr. Metabol. Cardiovasc. Dis. 2021;31:1929–1938. doi: 10.1016/j.numecd.2021.03.023. [DOI] [PubMed] [Google Scholar]
- 28.Sun L.X., Lin Z.B., Lu J., Li W.D., Niu Y.D., Sun Y., Hu C.Y., Zhang G.Q., Duan X.S. The improvement of M1 polarization in macrophages by glycopeptide derived from Ganoderma lucidum. Immunol. Res. 2017;65:658–665. doi: 10.1007/s12026-017-8893-3. [DOI] [PubMed] [Google Scholar]
- 29.Hsieh J.Y., Smith T.D., Meli V.S., Tran T.N., Botvinick E.L., Liu W.F. Differential regulation of macrophage inflammatory activation by fibrin and fibrinogen. Acta Biomater. 2017;47:14–24. doi: 10.1016/j.actbio.2016.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Murray P.J. Macrophage polarization. Annu. Rev. Physiol. 2017;79:541–566. doi: 10.1146/annurev-physiol-022516-034339. [DOI] [PubMed] [Google Scholar]
- 31.Hao H., Li Z., Qiao S.Y., Qi Y., Xu X.Y., Si J.Y., Liu Y.H., Chang L., Shi Y.F., Xu B., Wei Z.H., Kang L.N. Empagliflozin ameliorates atherosclerosis via regulating the intestinal flora. Atherosclerosis. 2023;371:32–40. doi: 10.1016/j.atherosclerosis.2023.03.011. [DOI] [PubMed] [Google Scholar]
- 32.Liu Y., Dou C., Wei G., Zhang L., Xiong W., Wen L., Xiang C., Chen C., Zhang T., Altamirano A., Chen Y., Zhang T.E., Yan Z. Usnea improves high-fat diet- and vitamin D3-induced atherosclerosis in rats by remodeling intestinal flora homeostasis. Front. Pharmacol. 2022;13 doi: 10.3389/fphar.2022.1064872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lv Z., Shan X., Tu Q., Wang J., Chen J., Yang Y. Ginkgolide B treatment regulated intestinal flora to improve high-fat diet induced atherosclerosis in ApoE(-/-) mice. Biomed. Pharmacother. 2021;134 doi: 10.1016/j.biopha.2020.111100. [DOI] [PubMed] [Google Scholar]
- 34.Morrison D.J., Preston T. Formation of short chain fatty acids by the gut microbiota and their impact on human metabolism. Gut Microb. 2016;7:189–200. doi: 10.1080/19490976.2015.1134082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Smith P.M., Howitt M.R., Panikov N., Michaud M., Gallini C.A., Bohlooly Y.M., Glickman J.N., Garrett W.S. The microbial metabolites, short-chain fatty acids, regulate colonic Treg cell homeostasis. Science. 2013;341:569–573. doi: 10.1126/science.1241165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen M., Xiao D., Liu W., Song Y., Zou B., Li L., Li P., Cai Y., Liu D., Liao Q., Xie Z. Intake of Ganoderma lucidum polysaccharides reverses the disturbed gut microbiota and metabolism in type 2 diabetic rats. Int. J. Biol. Macromol. 2020;155:890–902. doi: 10.1016/j.ijbiomac.2019.11.047. [DOI] [PubMed] [Google Scholar]
- 37.Du Y., Li X., Su C., Xi M., Zhang X., Jiang Z., Wang L., Hong B. Butyrate protects against high-fat diet-induced atherosclerosis via up-regulating ABCA1 expression in apolipoprotein E-deficiency mice. Br. J. Pharmacol. 2020;177:1754–1772. doi: 10.1111/bph.14933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rocha V.Z., Libby P. Obesity, inflammation, and atherosclerosis. Nat. Rev. Cardiol. 2009;6:399–409. doi: 10.1038/nrcardio.2009.55. [DOI] [PubMed] [Google Scholar]
- 39.Li W., Li Y., Wang Q., Yang Y. Crude extracts from Lycium barbarum suppress SREBP-1c expression and prevent diet-induced fatty liver through AMPK activation. BioMed Res. Int. 2014;2014 doi: 10.1155/2014/196198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fang K., Wu F., Chen G., Dong H., Li J., Zhao Y., Xu L., Zou X., Lu F. Diosgenin ameliorates palmitic acid-induced lipid accumulation via AMPK/ACC/CPT-1A and SREBP-1c/FAS signaling pathways in LO2 cells. BMC Compl. Alternative Med. 2019;19:255. doi: 10.1186/s12906-019-2671-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhong D., Xie Z., Huang B., Zhu S., Wang G., Zhou H., Lin S., Lin Z., Yang B. Ganoderma lucidum polysaccharide peptide Alleviates Hepatoteatosis via modulating bile acid metabolism Dependent on FXR-SHP/FGF. Cell. Physiol. Biochem. 2018;49:1163–1179. doi: 10.1159/000493297. [DOI] [PubMed] [Google Scholar]
- 42.Sang T., Guo C., Guo D., Wu J., Wang Y., Wang Y., Chen J., Chen C., Wu K., Na K., Li K., Fang L., Guo C., Wang X. Suppression of obesity and inflammation by polysaccharide from sporoderm-broken spore of Ganoderma lucidum via gut microbiota regulation. Carbohydr. Polym. 2021;256 doi: 10.1016/j.carbpol.2020.117594. [DOI] [PubMed] [Google Scholar]
- 43.Zhong B., Li F.L., Zhao J.Y., Fu Y., Peng C. Sporoderm-broken spore powder of Ganoderma lucidum ameliorate obesity and inflammation process in high-fat diet-induced obese mice. Food Nutr. Res. 2022;66 doi: 10.29219/fnr.v66.8745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang Y., Yu F., Zheng X., Li J., Zhang Z., Zhang Q., Chen J., He Y., Yang H., Zhou P. Balancing adipocyte production and lipid metabolism to treat obesity-induced diabetes with a novel proteoglycan from Ganoderma lucidum. Lipids Health Dis. 2023;22:120. doi: 10.1186/s12944-023-01880-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Turnbaugh P.J., Ley R.E., Mahowald M.A., Magrini V., Mardis E.R., Gordon J.I. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature. 2006;444:1027–1031. doi: 10.1038/nature05414. [DOI] [PubMed] [Google Scholar]
- 46.Goodman A.L., Kallstrom G., Faith J.J., Reyes A., Moore A., Dantas G., Gordon J.I. Extensive personal human gut microbiota culture collections characterized and manipulated in gnotobiotic mice. Proc. Natl. Acad. Sci. U.S.A. 2011;108:6252–6257. doi: 10.1073/pnas.1102938108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chang C.J., Lin C.S., Lu C.C., Martel J., Ko Y.F., Ojcius D.M., Tseng S.F., Wu T.R., Chen Y.Y., Young J.D., Lai H.C. Ganoderma lucidum reduces obesity in mice by modulating the composition of the gut microbiota. Nat. Commun. 2015;6:7489. doi: 10.1038/ncomms8489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Khan I., Huang G.X., Li X.A., Leong W., Xia W.R., Hsiao W.L.W. Mushroom polysaccharides from Ganoderma lucidum and Poria cocos reveal prebiotic functions. J. Funct.Foods. 2018;41:191–201. doi: 10.1016/j.jff.2017.12.046. [DOI] [Google Scholar]
- 49.Kim M.H., Kang S.G., Park J.H., Yanagisawa M., Kim C.H. Short-chain fatty acids activate GPR41 and GPR43 on intestinal epithelial cells to promote inflammatory responses in mice. Gastroenterology. 2013;145:396–406. doi: 10.1053/j.gastro.2013.04.056. e1-10. [DOI] [PubMed] [Google Scholar]
- 50.Yang L., Li Z., Song Y., Liu Y., Zhao H., Liu Y., Zhang T., Yuan Y., Cai X., Wang S., Wang P., Gao S., Li L., Li Y., Yu C. Study on urine metabolic profiling and pathogenesis of hyperlipidemia. Clin. Chim. Acta. 2019;495:365–373. doi: 10.1016/j.cca.2019.05.001. [DOI] [PubMed] [Google Scholar]
- 51.Ou H.C., Chou W.C., Hung C.H., Chu P.M., Hsieh P.L., Chan S.H., Tsai K.L. Galectin-3 aggravates ox-LDL-induced endothelial dysfunction through LOX-1 mediated signaling pathway. Environ. Toxicol. 2019;34:825–835. doi: 10.1002/tox.22750. [DOI] [PubMed] [Google Scholar]
- 52.Bentzon J.F., Otsuka F., Virmani R., Falk E. Mechanisms of plaque formation and rupture. Circ. Res. 2014;114:1852–1866. doi: 10.1161/circresaha.114.302721. [DOI] [PubMed] [Google Scholar]
- 53.Liu Y., Zhu Y., Jia W., Sun D., Zhao L., Zhang C., Wang C., Chen G., Fu S., Bo Y., Xing Y. Association between lipid profiles and presence of carotid plaque. Sci. Rep. 2019;9 doi: 10.1038/s41598-019-54285-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wu S. Hypolipidaemic and anti-lipidperoxidant activities of Ganoderma lucidum polysaccharide. Int. J. Biol. Macromol. 2018;118:2001–2005. doi: 10.1016/j.ijbiomac.2018.07.082. [DOI] [PubMed] [Google Scholar]
- 55.Riccio A.V., Costa B.K., Alonso M.A., Affonso F.J., França D.S., Nichi M., Belli C.B., McLean A.K., Boakari Y.L., Fernandes C.B. Comparative Assessment of oxidative and antioxidant Parameters in Mule and Horse Neonates during their First month of extrauterine adaptation. Animals (Basel) 2023;13 doi: 10.3390/ani13243878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lee J.M., Li J., Johnson D.A., Stein T.D., Kraft A.D., Calkins M.J., Jakel R.J., Johnson J.A. Nrf2, a multi-organ protector? Faseb. J. 2005;19:1061–1066. doi: 10.1096/fj.04-2591hyp. [DOI] [PubMed] [Google Scholar]
- 57.Xu D., Xu M., Jeong S., Qian Y., Wu H., Xia Q., Kong X. The role of Nrf2 in liver disease: novel molecular mechanisms and therapeutic approaches. Front. Pharmacol. 2018;9:1428. doi: 10.3389/fphar.2018.01428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Li H.N., Zhao L.L., Zhou D.Y., Chen D.Q. Ganoderma lucidum polysaccharides ameliorates hepatic steatosis and oxidative stress in db/db mice via targeting nuclear factor E2 (Erythroid-Derived 2)-related factor-2/heme oxygenase-1 (HO-1) pathway. Med Sci Monit. 2020;26 doi: 10.12659/msm.921905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Devaki M., Nirupama R., Yajurvedi H.N. Chronic stress-induced oxidative damage and hyperlipidemia are accompanied by atherosclerotic development in rats. Stress. 2013;16:233–243. doi: 10.3109/10253890.2012.719052. [DOI] [PubMed] [Google Scholar]
- 60.Nie Y., Luo F., Wang L., Yang T., Shi L., Li X., Shen J., Xu W., Guo T., Lin Q. Anti-hyperlipidemic effect of rice bran polysaccharide and its potential mechanism in high-fat diet mice. Food Funct. 2017;8:4028–4041. doi: 10.1039/c7fo00654c. [DOI] [PubMed] [Google Scholar]
- 61.Zeng X., Zhang X., Wei D. Toonaciliatin K attenuates the lung injury induced by lung infection of H1N1 influenza virus by regulating the NF-κB/MyD88/TLR-7 pathway in mice. Arch. Med. Sci. 2020;16:1387–1393. doi: 10.5114/aoms.2019.86220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wang W., Zhang Y., Wang Z., Zhang J., Jia L. Ganoderma lucidum polysaccharides improve lipid metabolism against high-fat diet-induced dyslipidemia. J. Ethnopharmacol. 2023;309 doi: 10.1016/j.jep.2023.116321. [DOI] [PubMed] [Google Scholar]
- 63.Mangum L.C., Hou X., Borazjani A., Lee J.H., Ross M.K., Crow J.A. Silencing carboxylesterase 1 in human THP-1 macrophages perturbs genes regulated by PPARγ/RXR and RAR/RXR: down-regulation of CYP27A1-LXRα signaling. Biochem. J. 2018;475:621–642. doi: 10.1042/bcj20180008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ignatova I.D., Schulman I.G. Liver X receptors and atherosclerosis: it is not all cholesterol. Arterioscler. Thromb. Vasc. Biol. 2014;34:242–243. doi: 10.1161/atvbaha.113.302987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Huang F., Zheng X., Ma X., Jiang R., Zhou W., Zhou S., Zhang Y., Lei S., Wang S., Kuang J., Han X., Wei M., You Y., Li M., Li Y., Liang D., Liu J., Chen T., Yan C., Wei R., Rajani C., Shen C., Xie G., Bian Z., Li H., Zhao A., Jia W. Theabrownin from Pu-erh tea attenuates hypercholesterolemia via modulation of gut microbiota and bile acid metabolism. Nat. Commun. 2019;10:4971. doi: 10.1038/s41467-019-12896-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Veerman K.J., Venegas-Pino D.E., Shi Y., Khan M.I., Gerstein H.C., Werstuck G.H. Hyperglycaemia is associated with impaired vasa vasorum neovascularization and accelerated atherosclerosis in apolipoprotein-E deficient mice. Atherosclerosis. 2013;227:250–258. doi: 10.1016/j.atherosclerosis.2013.01.018. [DOI] [PubMed] [Google Scholar]
- 67.Stumvoll M., Goldstein B.J., van Haeften T.W. Type 2 diabetes: principles of pathogenesis and therapy. Lancet. 2005;365:1333–1346. doi: 10.1016/s0140-6736(05)61032-x. [DOI] [PubMed] [Google Scholar]
- 68.Verma N., Amresh G., Sahu P.K., Mishra N., Singh A.P., Rao Ch V. Antihyperglycemic activity, antihyperlipedemic activity, haematological effects and histopathological analysis of Sapindus mukorossi Gaerten fruits in streptozotocin induced diabetic rats. Asian Pac. J. Tropical Med. 2012;5:518–522. doi: 10.1016/s1995-7645(12)60091-1. [DOI] [PubMed] [Google Scholar]
- 69.Li F., Zhang Y., Zhong Z. Antihyperglycemic effect of ganoderma lucidum polysaccharides on streptozotocin-induced diabetic mice. Int. J. Mol. Sci. 2011;12:6135–6145. doi: 10.3390/ijms12096135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhu K., Nie S., Li C., Lin S., Xing M., Li W., Gong D., Xie M. A newly identified polysaccharide from Ganoderma atrum attenuates hyperglycemia and hyperlipidemia. Int. J. Biol. Macromol. 2013;57:142–150. doi: 10.1016/j.ijbiomac.2013.03.009. [DOI] [PubMed] [Google Scholar]
- 71.Gupta D., Jetton T.L., Mortensen R.M., Duan S.Z., Peshavaria M., Leahy J.L. In vivo and in vitro studies of a functional peroxisome proliferator-activated receptor gamma response element in the mouse pdx-1 promoter. J. Biol. Chem. 2008;283:32462–32470. doi: 10.1074/jbc.M801813200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yu F., Teng Y., Li J., Yang S., Zhang Z., He Y., Yang H., Ding C.F., Zhou P. Effects of a ganoderma lucidum proteoglycan on type 2 diabetic rats and the recovery of rat pancreatic islets. ACS Omega. 2023;8:17304–17316. doi: 10.1021/acsomega.3c02200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lewis G.F., Carpentier A.C., Pereira S., Hahn M., Giacca A. Direct and indirect control of hepatic glucose production by insulin. Cell Metabol. 2021;33:709–720. doi: 10.1016/j.cmet.2021.03.007. [DOI] [PubMed] [Google Scholar]
- 74.McCormack J.G., Westergaard N., Kristiansen M., Brand C.L., Lau J. Pharmacological approaches to inhibit endogenous glucose production as a means of anti-diabetic therapy. Curr. Pharmaceut. Des. 2001;7:1451–1474. doi: 10.2174/1381612013397393. [DOI] [PubMed] [Google Scholar]
- 75.Xiao C., Wu Q.P., Cai W., Tan J.B., Yang X.B., Zhang J.M. Hypoglycemic effects of Ganoderma lucidum polysaccharides in type 2 diabetic mice. Arch Pharm. Res. (Seoul) 2012;35:1793–1801. doi: 10.1007/s12272-012-1012-z. [DOI] [PubMed] [Google Scholar]
- 76.Shen L., Ao L., Xu H., Shi J., You D., Yu X., Xu W., Sun J., Wang F. Poor short-term glycemic control in patients with type 2 diabetes impairs the intestinal mucosal barrier: a prospective, single-center, observational study. BMC Endocr. Disord. 2019;19:29. doi: 10.1186/s12902-019-0354-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Chen Z., Wang J., Yang W., Chen J., Meng Y., Feng B., Chi Y., Geng B., Zhou Y., Cui Q., Yang J. FAM3C activates HSF1 to suppress hepatic gluconeogenesis and attenuate hyperglycemia of type 1 diabetic mice. Oncotarget. 2017;8:106038–106049. doi: 10.18632/oncotarget.22524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Pan R., Lou J., Wei L. Significant effects of Ganoderma lucidum polysaccharide on lipid metabolism in diabetes may be associated with the activation of the FAM3C-HSF1-CAM signaling pathway. Exp. Ther. Med. 2021;22:820. doi: 10.3892/etm.2021.10252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zhang D.Y., Cheng Y.B., Guo Q.H., Shan X.L., Wei F.F., Lu F., Sheng C.S., Huang Q.F., Yang C.H., Li Y., Wang J.G. Treatment of masked hypertension with a Chinese herbal formula: a randomized, placebo-controlled trial. Circulation. 2020;142:1821–1830. doi: 10.1161/circulationaha.120.046685. [DOI] [PubMed] [Google Scholar]
- 80.Yang Y., Xie J., Zhou J., Yu C., Lin W., Liu D., Wang X. Effect of ganoderma lucidum polysaccharide on TLR4/NF-kappa B signaling pathway in ApoE/atherosclerotic mice. Chinese Journal of Basic Medicine in Traditional Chinese Medicine. 2019;25:56–59+67. doi: 10.19945/j.cnki.issn.1006-3250.2019.01.020. [DOI] [Google Scholar]
- 81.Xue H., Chen X., Yu C., Deng Y., Zhang Y., Chen S., Chen X., Chen K., Yang Y., Ling W. Gut microbially produced indole-3-propionic acid inhibits atherosclerosis by promoting reverse cholesterol transport and its deficiency is causally related to atherosclerotic cardiovascular disease. Circ. Res. 2022;131:404–420. doi: 10.1161/circresaha.122.321253. [DOI] [PubMed] [Google Scholar]
- 82.Wang J., Cao B., Zhao H., Feng J. Emerging roles of ganoderma lucidum in anti-aging. Aging Dis. 2017;8:691–707. doi: 10.14336/ad.2017.0410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Liu D., Liu J., Zhang D., Yang W. Advances in relationship between cell senescence and atherosclerosis. Zhejiang Da Xue Xue Bao Yi Xue Ban. 2022;51:95–101. doi: 10.3724/zdxbyxb-2021-0270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zhang H., Simon A.K. Polyamines reverse immune senescence via the translational control of autophagy. Autophagy. 2020;16:181–182. doi: 10.1080/15548627.2019.1687967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ornatowski W., Lu Q., Yegambaram M., Garcia A.E., Zemskov E.A., Maltepe E., Fineman J.R., Wang T., Black S.M. Complex interplay between autophagy and oxidative stress in the development of pulmonary disease. Redox Biol. 2020;36 doi: 10.1016/j.redox.2020.101679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hong S.H., Cha H.J., Hwang-Bo H., Kim M.Y., Kim S.Y., Ji S.Y., Cheong J., Park C., Lee H., Kim G.Y., Moon S.K., Yun S.J., Chang Y.C., Kim W.J., Choi Y.H. Anti-proliferative and pro-apoptotic effects of licochalcone A through ROS-mediated cell cycle arrest and apoptosis in human bladder cancer cells. Int. J. Mol. Sci. 2019;20 doi: 10.3390/ijms20153820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Guo M., Zhang K., Zhang D., Zhou Y., Liu L., Wu Y., Zhou X., Nie S. Ganoderma atrum polysaccharide relieves mitochondrial dysfunction to alleviate hydrogen peroxide-induced senescence via activating autophagy. Journal of Future Foods. 2022;2:241–252. doi: 10.1016/j.jfutfo.2022.06.005. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This manuscript is a review article and is not applicable to the Data availability statement situations.