Abstract
Background:
Spinal cord injury (SCI) is a severe condition that often leads to persistent damage of nerve cells and motor dysfunction. Autophagy is an intracellular system that regulates the recycling and degradation of proteins and lipids, primarily through lysosomal-dependent organelle degradation. Numerous publications have highlighted the involvement of autophagy in the secondary injury of SCI. Therefore, gaining a comprehensive understanding of autophagy research is crucial for designing effective therapies for SCI.
Methods:
Dates were obtained from Web of Science, including articles and article reviews published from its inception to October 2023. VOSviewer, Citespace, and SCImago were used to visualized analysis. Bibliometric analysis was conducted using the Web of Science data, focusing on various categories such as publications, authors, journals, countries, organizations, and keywords. This analysis was aimed to summarize the knowledge map of autophagy and SCI.
Results:
From 2009 to 2023, the number of annual publications in this field exhibited wave-like growth, with the highest number of publications recorded in 2020 (44 publications). Our analysis identified Mei Xifan as the most prolific author, while Kanno H emerged as the most influential author based on co-citations. Neuroscience Letters was found to have published the largest number of papers in this field. China was the most productive country, contributing 232 publications, and Wenzhou Medical University was the most active organization, publishing 39 papers.
Conclusion:
We demonstrated a comprehensive overview of the relationship between autophagy and SCI utilizing bibliometric tools. This article could help to enhance the understanding of the field about autophagy and SCI, foster collaboration among researchers and organizations, and identify potential therapeutic targets for treatment.
Keywords: autophagy, bibliometric, mTOR, spinal cord injury
1. Introduction
Spinal cord injury (SCI) is a debilitating condition that results in permanent motor dysfunction and sensory disturbance. Currently, approximately 20 million cases has been discovered globally. The incidence of SCI continues to rise in both developed and non-developed countries. This trend is expected to persist in the future.[1] SCI leads to the loss of autonomic control over bowel, urinary bladder, and sexual function,[2] significantly impacting patients’ daily lives. The pathological process of SCI involves both primary mechanical injury and secondary injury, with the latter being the primary cause of disability. Secondary injury encompasses oxidative stress reactions, such as the production of oxyradicals and lipid peroxidation aggregation,[3] as well as spinal cord edema, vessel remodeling, ischemia, blood-spinal cord barrier damage,[4] bleeding, neuroinflammation,[5] ion disturbance,[6] and glutamate excitatory toxicity.[7] These factors contribute to damage to afferent neurons and motor nerves, resulting in sensory and motor disturbances. Current treatments for SCI include drug therapy, surgery, and rehabilitation, but none of these approaches can achieve a complete cure. It may be attributed to weak regeneration of spinal cord nerve[8,9] and excessive apoptosis and autophagy due to the accumulation of pro-inflammatory factors.[10] numerous studies have explored apoptosis. Thus, growing attention is now being paid to autophagy as a potential treatment avenue.
Autophagy is a cellular process where damaged organelles are degraded through lysosome-dependent pathways in eukaryotic cells under conditions of nutritional deficiency or oxidative stress. It plays a crucial role in maintaining internal environmental stability.[11] Autophagy is also involved in various cellular activities, including quality control, early development, and cell differentiation.[12] It has both beneficial and harmful effects: it enables the utilization of damaged organelles and proteins but excessive induction can lead to autophagic cell death, which impairs cells and differs from apoptosis-induced cell death.[13] Autophagy is generally considered as a mechanism for promoting cell survival.[14] However, autophagic cell death, which is non-apoptotic programmed cell death, occurs in dying cells and is mediated by autophagy rather than induced by it.[13] Apoptotic cell death depends on the activation of caspases[15] and clearance of cell debris by surrounding cells, while autophagy produces autophagosomes that are eliminated by lysosomes independently of caspase activation.[16] Autophagic cell death can damage brain neurons in cases of cerebral trauma and cerebral infarction.[17] Activation of the protein kinase B (AKT)/rapamycin (mTOR) pathway to attenuate autophagy has been shown to promote motor function recovery.[18] However, relevant publications have demonstrated that autophagy protects nerve cells from death following SCI.[19,20] Thus, proper regulation of autophagy is crucial for motor function recovery and nerve cell regeneration at different stages after SCI. Different treatments can be used to achieve the appropriate degree of autophagy based on the varying levels observed during different periods after SCI.
Currently, the death of nerve cells in SCI has been known to occur through multiple pathways, including necroptosis, apoptosis, pyroptosis, and autophagy. While autophagy has received less attention, it still holds promise as a target for SCI treatment. Necroptosis is a passive and uncontrolled form of cell death, apoptosis has been extensively studied without significant effective treatments, and our understanding of pyroptosis in SCI remains limited. With the important role of autophagy discovered in SCI, it represents a hopeful therapeutic target.
Bibliometric analysis is an effective and widely utilized tool for collecting vast amounts of information on publications within a specific field.[21] This strategy allows for the visualization and analysis of selected papers, including summarizing the number of publications in a given field, assessing the publication landscape of relevant journals, identifying key organizations and authors, and aiding researchers in understanding the current hotspots and trends. Additionally, it broadens research perspectives, assists in identifying reputable journals, and facilitates collaboration opportunities. Furthermore, bibliometric analysis provides valuable insights into regulating autophagy for SCI treatment.
In this article, we have compiled critical information from screened publications on autophagy in SCI. It included annual publications, authors, co-cited authors, journals, countries, organizations, references, and keywords. We presented a network of related papers aiming to provide a clear map of autophagy in SCI. Our objective was to advance research progress in autophagy and SCI through this comprehensive analysis.
2. Methods
2.1. Date source and search strategy
Web of Science (WOS) is a comprehensive database that covers a wide range of publications from various fields,[22] encompassing over 12,000 publications.[23] With its extensive time coverage compared to other databases such as Scopus and PubMed, WOS is widely recognized as suitable for bibliometric analysis.[24] We conducted a search in the Science Citation Index Expanded and Social Science Citation Index within the time span of 1985 to 2023. The search formula used was Topic = (“autophagy”) AND Topic = (“spinal cord injury”). We restricted the search to English-language papers and included only articles and review articles. We screened the articles based on titles, abstracts, and full texts, excluding any irrelevant papers. The publication dates were exported as plain text file” and record content contained “Full Record and Cited References.”
2.2. Date analysis
For bibliometric analysis, VOSviewer and Citespace software were employed using the exported data. Additionally, GraphPad Prism and SCImago were utilized to analyze the geographic distribution of countries and visualize the proportion of country-wise publications and organization-wise publications. VOSviewer, a Java-based software, aided in generating visualization maps with clusters, time, and density, enabling the construction of maps for authors, coauthors, journals, countries, organizations, and keywords. Citespace, another Java-based software created by Chaomei Chen,[25] was used to generate lists of burst keywords and link strength over time.
2.3. Review method
The literature search was conducted in WOS, resulting in the selection of 643 publications. After excluding duplicates and irrelevant articles, we were left with 621 publications consisting solely of articles and review articles. Ultimately, 282 papers that were closely relevant to autophagy and SCI remained for bibliometric analysis. These 282 papers were chosen after careful elimination of duplications and publications not directly related to autophagy and SCI. The flowchart illustrating the process of literature screening is presented in Figure 1.
Figure 1.
Publication screening flowchart.
3. Results
3.1. Publication summary
A total of 282 publications relating to autophagy and SCI were identified from 2009 to 2023, comprising 249 articles and 33 reviews. The annual trend of publications is depicted in Figure 2, with the first article being published in 2009. The lowest number of publications occurred in 2010 (1 publication), while the highest number was recorded in 2020 (44 publications). In recent years, there has been a fluctuating increase in the annual number of publications.
Figure 2.
Annual output of autophagy in SCI. SCI = spinal cord injury.
3.2. Authors
A total of 1527 authors were mentioned across the 282 publications. Based on individual publication counts, the top 10 authors were selected, and their respective number of papers, institutions, locations, total citations, average citations, and total link strength are displayed in Table 1. Mei, Xifan is the most prolific author with 18 papers, followed by Xiao, Jian (17 papers), Xu, Huazi (12 papers), Gao, Kai (10 papers), and Zhang, Hongyu (10 papers). Additionally, 108 authors contributed 3 or more papers and were considered for author collaboration analysis (Fig. 3A).
Table 1.
The top 10 authors in the field of autophagy and spinal cord injury.
| Rank | Author | Count | Institution | Location | Total citations | Average citations | Total link strength |
|---|---|---|---|---|---|---|---|
| 1 | Mei, Xifan | 18 | Jinzhou Med Univ | China | 573 | 31.83 | 23 |
| 2 | Xiao, Jian | 17 | Wenzhou Med Univ | China | 498 | 29.29 | 31 |
| 3 | Xu, Huazi | 12 | Wenzhou Med Univ | China | 412 | 34.33 | 24 |
| 4 | Gao, Kai | 10 | Xi An Jiao Tong Univ,Jining 1 Peoples Hosp | China | 392 | 39.20 | 9 |
| 5 | Zhang, Hongyu | 10 | Wenzhou Med Univ | China | 203 | 20.30 | 18 |
| 6 | Wang, Xiangyang | 9 | Wenzhou Med Univ | China | 156 | 17.33 | 21 |
| 7 | Zhou, Kailiang | 9 | Wenzhou Med Univ | China | 227 | 25.22 | 21 |
| 8 | Chen, Jian | 8 | Three Gorges Cent Hosp Chongqing | China | 195 | 24.37 | 8 |
| 9 | Yuan, Yajiang | 8 | Jinzhou Med Univ | China | 216 | 27.00 | 12 |
| 10 | Kanno, Haruo | 7 | Tohoku Med & Pharmaceut Univ | Japan | 557 | 79.57 | 7 |
Figure 3.
The visualization of authors (A) and co-cited authors (B) on research of autophagy in SCI. SCI = spinal cord injury.
3.3. Co-cited authors
A total of 8470 authors were co-cited in connection with the 282 articles, with 59 authors being co-cited at least 20 times (Fig. 3B). The most co-cited authors were Kanno, H (194 co-citations), Mizushima, N (123 co-citations), and Basso, DM (94 co-citations). Table 2 lists the top 10 co-cited authors, who accrued at least 65 citations.
Table 2.
Top 10 co-cited author.
| Rank | Co-cited author | Total citations | Institution | Location |
|---|---|---|---|---|
| 1 | Kanno, H | 194 | Tohoku Med & Pharmaceut Univ | Japan |
| 2 | Mizushima, N | 123 | Univ Tokyo, Grad Sch Med, Dept Biochem & Mol Biol | Japan |
| 3 | Basso, DM | 94 | Ohio State Univ | USA |
| 4 | Chen, HC | 84 | Jilin Univ | China |
| 5 | Wang, ZY | 78 | Shandong Agr Univ | China |
| 6 | Zhou, KL | 78 | Wenzhou Med Univ | China |
| 7 | Klionsky, DJ | 77 | Univ Michigan | USA |
| 8 | Levine, B | 72 | Univ Texas Southwestern Med Ctr Dallas | USA |
| 9 | Liu, S | 67 | Virginia Commonwealth Univ | USA |
| 10 | Tang, PF | 67 | Department of Orthopedic Medicine | China |
3.4. Journals
The 282 articles were published in a total of 136 journals. Table 3 presents the top 12 journals based on publication count, including their respective number of publications, citations, impact factor (IF) for 2022, and Journal Citation Reports division for 2022. Among these top journals, 2 were classified as Q1 in the Journal Citation Reports, 7 as Q2, and 3 as Q3. Neuroscience Letters published the most relevant publications on autophagy and SCI, with a total of 16 articles. Moreover, Oxidative Medicine and Cellular Longevity had the highest IF among these top 12 journals. For journal relation analysis based on clusters and times, journals that published at least 2 papers were included (Fig. 4A and B).
Table 3.
The top 12 productive journals correlated with autophagy and spinal cord injury.
| Rank | Journal | Count | Percentage | Total citations | IF (2022) | JCR division (2022) |
|---|---|---|---|---|---|---|
| 1 | Neuroscience Letters | 16 | 5.67% | 244 | 2.5 | Q3 |
| 2 | Molecular Neurobiology | 14 | 4.96% | 690 | 5.1 | Q2 |
| 3 | Neural Regeneration Research | 12 | 4.26% | 231 | 6.1 | Q2 |
| 4 | Neurochemical Research | 10 | 3.55% | 191 | 4.4 | Q2 |
| 5 | Oxidative Medicine and Cellular Longevity | 7 | 2.48% | 162 | 7.3 | Q2 |
| 6 | Spine | 6 | 2.13% | 252 | 3.0 | Q3 |
| 7 | Molecular Medicine Reports | 6 | 2.13% | 122 | 3.4 | Q3 |
| 8 | Frontiers in Pharmacology | 6 | 2.13% | 77 | 5.6 | Q1 |
| 9 | Journal of Neurotrauma | 5 | 1.77% | 211 | 4.2 | Q2 |
| 10 | Journal of Cellular and Molecular Medicine | 5 | 1.77% | 193 | 5.3 | Q2 |
| 11 | Spinal Cord | 5 | 1.77% | 74 | 2.2 | Q3 |
| 12 | Biomedicine & Pharmacotherapy | 5 | 1.77% | 25 | 7.5 | Q1 |
JCR = Journal Citation Reports.
Figure 4.
The visualization of journals (A, B) and co-cited journals (C) on research of autophagy in SCI. SCI = spinal cord injury.
3.5. Co-cited journals
A total of 1806 co-cited journals were associated with the 282 articles, with 164 journals being co-cited at least 20 times (Fig. 4C). Autophagy was the most co-cited journal (551 co-citations), followed by Journal of Neurotrauma (468 co-citations) and Molecular Neurobiology (318 co-citations). Of the top 10 co-cited journals, 6 were classified as Q1, 3 as Q2, and one as Q3. Additionally, 70% (7/10) of these top co-cited journals had an IF over 5.
3.6. Country
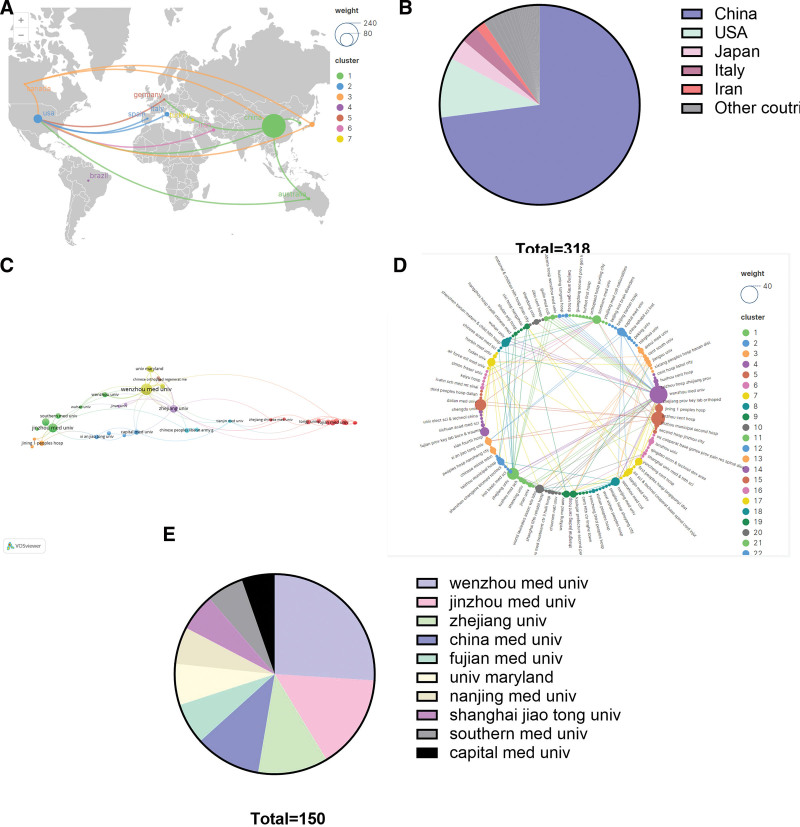
The 282 publications involved contributions from 24 countries. Table 4 presents the top 10 countries, with China leading in the number of publications (232 publications), followed by the USA (31 publications) and Japan (11 publications). Japan possessed the highest average citation count, followed by the USA and Italy. The international collaboration between countries, involving collaborations with at least 2 other countries, is illustrated in Figure 5A, while Figure 5B displays the proportion of each country’s publications.
Table 4.
The most co-cited journals associated with autophagy and spinal cord injury.
| Rank | Co-cited journal | Total citations | IF (2022) | JCR division (2022) |
|---|---|---|---|---|
| 1 | Autophagy | 551 | 13.3 | Q1 |
| 2 | J Neurotraum | 468 | 4.2 | Q2 |
| 3 | Mol Neurobiol | 318 | 5.1 | Q2 |
| 4 | J Neurosci | 300 | 5.3 | Q1 |
| 5 | Nature | 269 | 64.8 | Q1 |
| 6 | Neurobiol Dis | 255 | 6.1 | Q1 |
| 7 | Cell | 244 | 64.5 | Q1 |
| 8 | Exp Neurol | 240 | 5.3 | Q1 |
| 9 | PLOS One | 227 | 3.7 | Q2 |
| 10 | Neuroscience | 212 | 3.3 | Q3 |
JCR = Journal Citation Reports.
Figure 5.
The visualization of country (A and B) and organization (C–E) on research of autophagy in SCI. SCI = spinal cord injury.
3.7. Organization
A total of 318 organizations were associated with the 282 publications. Table 5 lists the top 10 productive organizations, all of which are high schools, with 90% (9/10) located in China. Wenzhou Medical University ranked first in productivity with 39 publications, followed by Jinzhou Medical University with 23 publications and Zhejiang University with 17 publications. Figure 5C shows organizational collaborations based on 38 organizations collaborating at least 3 times, Figure 5E illustrates the proportion of the top 10 organizations, and Figure 5D displays a radial analysis of the collaborations between all organizations related to the 282 papers.
Table 5.
The top 10 productive countries in the field of autophagy and spinal cord injury.
| Rank | Country | Documents | Percentage | Total citations | Average citations |
|---|---|---|---|---|---|
| 1 | China | 232 | 82.27% | 5159 | 22.24 |
| 2 | USA | 31 | 10.99% | 1703 | 54.94 |
| 3 | Japan | 11 | 3.90% | 694 | 63.09 |
| 4 | Italy | 9 | 3.19% | 316 | 35.11 |
| 5 | Iran | 5 | 1.77% | 77 | 15.40 |
| 6 | Australia | 4 | 1.42% | 129 | 32.25 |
| 7 | South Korea | 3 | 1.06% | 97 | 32.33 |
| 8 | Turkey | 3 | 1.06% | 46 | 15.33 |
| 9 | Brazil | 2 | 0.71% | 19 | 9.50 |
| 10 | Canada | 2 | 0.71% | 63 | 31.50 |
3.8. Papers
Among the 282 publications, 38 publications received over 50 citations. Table 6 summarizes the top 10 cited publications, with the most cited paper being “Health benefits of anthocyanins and molecular mechanisms: Update from the recent decade” by Li, Daotong (273 citations). Furthermore, 14 papers reached 100 citations.
Table 6.
The top 10 productive organizations published literature related to autophagy and spinal cord injury.
| Rank | Organization | Country | Documents | Total citations | Average citations |
|---|---|---|---|---|---|
| 1 | Wenzhou Med Univ | China | 39 | 1022 | 26.21 |
| 2 | Jinzhou Med Univ | China | 23 | 604 | 26.26 |
| 3 | Zhejiang Univ | China | 17 | 456 | 26.82 |
| 4 | China Med Univ | China | 16 | 320 | 20.00 |
| 5 | Fujian Med Univ | China | 10 | 208 | 20.80 |
| 6 | Univ Maryland | USA | 10 | 886 | 88.60 |
| 7 | Nanjing Med Univ | China | 9 | 250 | 27.78 |
| 8 | Shanghai Jiao Tong Univ | China | 9 | 160 | 17.78 |
| 9 | Southern Med Univ | China | 9 | 164 | 18.22 |
| 10 | Capital Med Univ | China | 8 | 88 | 11.00 |
3.9. Co-cited reference
A total of 10,837 co-cited references were related to the 282 papers. Figure 6 includes 38 publications with at least 20 co-citations. Table 7 showcases the top 10 co-cited papers, with the paper by Tang, Peifu in 2014 receiving the highest number of co-citations (66 co-citations), followed by Kanno, Haruoaruo in 2009 (63 co-citations) and Kanno, Haruo in 2011 (59 co-citations).
Table 7.
Top 10 most citied paper.
| Rank | Paper | Citations | Journal | Author | Institution | Location |
|---|---|---|---|---|---|---|
| 1 | Health benefits of anthocyanins and molecular mechanisms: Update from recent decade | 273 | Critical Reviews in Food Science and Nutrition | Li, Daotong | China Agr Univ | China |
| 2 | Systemic bisperoxovanadium activates Akt/mTOR, reduces autophagy, and enhances recovery following cervical spinal cord injury | 176 | PLOS One | Walker, Chandler L | Indiana Univ Sch Med | USA |
| 3 | Disrupted autophagy after spinal cord injury is associated with ER stress and neuronal cell death | 159 | Cell Death & Disease | Liu, S | Univ Maryland | USA |
| 4 | Rapamycin promotes autophagy and reduces neural tissue damage and locomotor impairment after spinal cord injury in mice | 153 | Journal of Neurotrauma | Sekiguchi, Akira | Tohoku Univ | Japan |
| 5 | Resveratrol protects against spinal cord injury by activating autophagy and inhibiting apoptosis mediated by the SIRT1/AMPK signaling pathway | 141 | Neuroscience | Zhao, Haosen | Jinzhou Med Univ | China |
| 6 | Autophagy reduces neuronal damage and promotes locomotor recovery via inhibition of apoptosis after spinal cord injury in rats | 139 | Molecular Neurobiology | Tang, Peifu | Chinese Peoples Liberat Army Gen Hosp | China |
| 7 | Reactive astrocytes undergo M1 microglia/macrophages-induced necroptosis in spinal cord injury | 139 | Molecular Neurodegeneration | Fan, Hong | Fourth Mil Med Univ | China |
| 8 | Function and mechanisms of autophagy in brain and spinal cord trauma | 136 | Antioxidants & Redox Signaling | Lipinski, Marta M. | Univ Maryland, Sch Med | USA |
| 9 | Regulation of autophagy and ubiquitinated protein accumulation by bFGF promotes functional recovery and neural protection in a rat model of spinal cord injury | 127 | Molecular Neurobiology | Zhang, Hong-Yu | Wenzhou Med Coll | China |
| 10 | Autophagy induction stabilizes microtubules and promotes axon regeneration after spinal cord injury | 124 | Proceedings of the National Academy of Sciences of the United States of America | He, Miao | Chinese Acad Sci | China |
FGF = fibroblast growth factor.
Figure 6.
The visualization of co-cited reference.
3.10. Keywords
A total of 1170 keywords were identified through co-occurrence analysis, with 59 keywords appearing at least 10 times and 27 keywords appearing at least 20 times. The most frequently occurring keyword was autophagy (n = 204), followed by SCI (n = 165), apoptosis (n = 130), inflammation (n = 62), and cell death (n = 60). The visual analysis, based on the 27 keywords appearing at least 20 times, is presented in Figure 7. These keywords were divided into 4 clusters (Fig. 7A). The different clusters represent diverse categories: Cluster 1 (red) focused on beclin-1, cell death, disease, mechanisms, neurons, neuroprotection, rapamycin, and up-regulation; Cluster 2 (green) encompassed apoptosis, autophagy, expression, oxidative stress, rats, SCI, and spinal-cord-injury; Cluster 3 (blue) included activation, cells, inflammation, pathway, protects, recovery, and regeneration; Cluster 4 (yellow) consisted of damage, functional recovery, inhibition, and model. Figure 7B and C depicts correlation analyses based on time and density. Burst keywords display the frequency of keywords in specific time periods, providing information about research hotspots from the past to the present and indicating current focal points as well as potential future breakthroughs. The most relevant burst keywords are listed in Figure 7D.
Figure 7.
The visualization of keywords (A, B, C) and keywords burst (D).
4. Discussion
From a bibliometric perspective, this study provided a systematic analysis of autophagy and SCI, yielding visual and comprehensive results. These findings not only reflected the overall research trends in the field but also uncovered current hotspots and cutting-edge developments.
4.1. General information
Regarding annual publications, the first publication in this field was published in 2009, and there has been a fluctuating increase in annual publications since then. The highest number of publications occurred in 2020, indicating a growing interest in the relationship between autophagy and SCI. Over the past 14 years, there have been 5 years with more than 30 publications. As the understanding of autophagy mechanisms, such as mTOR[26] and microRNA,[27] deepens, we can expect further research in this area to be published.
Among the most productive authors in this field, Mei Xifan, Xiao Jian, Xu Huazi, Gao Kai, and Zhang Hongyu have each contributed at least 10 papers. Notably, Xiao Jian, Xu Huazi, and Gao Kai are affiliated with Wenzhou Medical University, suggesting that scholars from this institution are particularly interested in autophagy in SCI. Mei Xifan, from Jinzhou Medical University, has published the highest number of papers (18) and focuses on the regulation of the silent information regulator sirtuin 1/AMP-activated protein kinase (AMPK) signaling pathway and autophagy. Kanno H (194 co-citations) and Mizushima N (123 co-citations) are the most frequently co-cited authors in this field, both hailing from Japan, indicating the high quality of Japanese scholars’ contributions. It is worth noting that Kanno H ranks among the top authors. Additionally, top 2, top 3, and top 9 co-cited paper all were published by him, demonstrating his significant achievements and authority in the field of autophagy and SCI (Table 8). Additionally, Akira Sekiguchi (57 co-citations, rank 5) and Kanno Haruo, both affiliated with Tohoku University School of Medicine, have collaborated multiple times on autophagy and SCI research. In China, Tang Peifu, working at the Chinese People’s Liberation Army General Hospital, has made substantial contributions to autophagy and SCI research. Tang Peifu ranks among the top ten coauthors, has the sixth most cited papers, and is involved in the most co-cited papers, focusing on hydrogel, fracture, apoptosis, SCI, and autophagy. Similarly, Zhang Hongyu, employed at Wenzhou Medical University, has made significant contributions to this field, ranking fifth among authors, ninth among cited papers, and tenth among co-cited papers. Zhang Hongyu research primarily focuses on autophagy, SCI, and endoplasmic reticulum stress.
Table 8.
Top 10 most co-cited papers.
| Rank | Title | Author | Citation | Institution | Location |
|---|---|---|---|---|---|
| 1 | Autophagy reduces neuronal damage and promotes locomotor recovery via inhibition of apoptosis after spinal cord injury in rats | Tang, Peifu | 66 | Chinese Peoples Liberat Army Gen Hosp | China |
| 2 | Spinal cord injury induces upregulation of Beclin 1 and promotes autophagic cell death | Kanno, Haruo | 63 | Tohoku University School of Medicine | Japan |
| 3 | Induction of autophagy and autophagic cell death in damaged neural tissue after acute spinal cord injury in mice | Kanno, Haruo | 59 | Tohoku University School of Medicine | Japan |
| 4 | A sensitive and reliable locomotor rating scale for open field testing in rats |
D M Basso | 57 | Department of Cell Biology | USA |
| 5 | Rapamycin promotes autophagy and reduces neural tissue damage and locomotor impairment after spinal cord injury in mice | Akira Sekiguchi | 57 | Tohoku University School of Medicine | Japan |
| 6 | Function and mechanisms of autophagy in brain and spinal cord trauma | Marta M Lipinski | 50 | University of Maryland School of Medicine | USA |
| 7 | Disrupted autophagy after spinal cord injury is associated with ER stress and neuronal cell death | S Liu | 50 | University of Maryland School of Medicine | USA |
| 8 | Autophagy is activated in injured neurons and inhibited by methylprednisolone after experimental spinal cord injury | Hsien-Chih Chen | 44 | Chang Gung Memorial Hospital | China |
| 9 | The role of autophagy in spinal cord injury | Haruo Kanno | 43 | Tohoku University School of Medicine | Japan |
| 10 | Regulation of autophagy and ubiquitinated protein accumulation by bFGF promotes functional recovery and neural protection in a rat model of spinal cord injury | Hong-Yu Zhang | 41 | Wenzhou Medical College | China |
FGF = fibroblast growth factor.
Neuroscience Letters has published the highest number of relevant articles, while Molecular Neurobiology has the second highest number of publications and the highest total citation count, indicating its prominence in the field. Oxidative Medicine and Cellular Longevity had the highest IF(7.3) in 2022, suggesting its relative authority and reliability. Journal of Neurotrauma emerged as the second most co-cited journal and ninth in terms of publication volume, emphasizing its focus on nervous system trauma. Autophagy, a highly co-cited journal, explores autophagy mechanisms in various diseases. Furthermore, one of the earliest papers in this field was published in Autophagy in 2009.[28] Nature and Cell, as renowned journals, have published exceptionally creative and impactful papers, making them frequently cited by scholars to support their theories and studies. In terms of country representation, Chinese researchers have published the majority of papers (82.27%), with China also receiving the highest total citations. However, the average number of citations per paper from China is the lowest among the top 3 productive countries (China, USA, and Japan), indicating that the quality of papers from China does not always reach the highest international standards. Conversely, Japan has the highest average number of citations, followed by the USA, suggesting the high quality of their publications related to autophagy and SCI.
With half of the most productive authors affiliated with Wenzhou Medical University, this institution has published the most papers on autophagy in SCI research. Although Chinese organizations account for a high proportion of productivity, the University of Maryland, the only American institution in the top 10, received nearly 3 times the average number of citations compared to Nanjing Medical University (the highest-cited Chinese organization).
The most cited paper in this field was “Health benefits of anthocyanins and molecular mechanisms: Update from the recent decade,”[29] which offered insights into the molecular mechanisms of autophagy. Therefore, researchers preferred to cite this paper to explain the role of autophagy in SCI. Additionally, studies have shown that anthocyanins have neuroprotective effects[30] and multifaceted effects,[31] contributing to motor functional recovery in SCI. The most co-cited paper, “Autophagy Reduces Neuronal Damage and Promotes Locomotor Recovery via Inhibition of Apoptosis After SCI in Rats,” highlights the importance of inducing autophagy early to protect nerve cells and promote motor recovery through the inhibition of apoptosis.[32]
4.2. The hotspots and topical issues
Keywords are often considered condensed versions of full articles, and frequently appearing keywords are thought to represent hotspots in specific fields. Burst keywords connect keywords with time to reveal relevant hotspots in specific years. Based on the keywords and burst keywords, this section will discuss mesenchymal stem cells (MSCs), inflammatory response, and therapeutic targets.
4.2.1. MSCs
MSCs, adherent fibroblast-like cells, were first discovered in human bone marrow in the 1960s.[33] MSCs also discovered in various human tissues, including the liver,[34] lungs,[35] peripheral blood,[36] periosteum,[37] salivary glands,[38] adipose tissue,[39] synovial membrane,[40] umbilical cord blood,[41] blood vessel walls,[42] Wharton jelly,[43] dental pulp,[44] placental tissue,[45] tendon,[46] skeletal muscle,[47] menstrual blood,[48] and amniotic fluid.[49] What sets them apart from other stem cells is their ability to regulate immunity.[50] The mechanisms behind the immunomodulation of MSCs include migrating to sites of inflammation or injury, changing the type of CD4+ T helper cell, inducing tolerogenic dendritic cells, regulating macrophage polarization and promoting their repair function, producing immunosuppressive soluble factors, and inducing immune tolerance.[51] The inflammatory microenvironment plays a vital role in the immunoregulatory effects of MSCs and induces autophagy.[52,53] Inflammatory factors such as immune interferon-γ and tumor necrosis factor-α primarily contribute to the autophagy of MSCs during inflammation.[52] MSCs can inhibit inflammation through exosomes,[54] promote axon regeneration and neuron survival by secreting brain-derived neurotrophic factor and β-nerve growth factor,[55,56] and repair blood vessels by releasing angiogenic factors such as vascular endothelial growth factor, platelet-derived growth factor, and fibroblast growth factor.[57] Inhibition of autophagy by knocking down Beclin 1 can significantly enhance the therapeutic effects on experimental autoimmune encephalomyelitis.[58] Exosomes secreted by MSCs transport miRNA and immunosuppressive proteins to targeted cells, providing a treatment option without aging problems and rejection reactions.[59,60] MSCs promote fusion of autophagosomes with lysosomes, and through excretion of exosomes, they can regulate autophagy via the AMPK/mTOR and AKT/mTOR pathways after SCI.[61,62] The multiple functions of exosomes, including accelerating axonal regeneration and angiogenesis, modulating the inflammatory microenvironment and immune response, suppressing apoptosis, and maintaining the integrity of the blood-spinal cord barrier,[63,64] have been revealed. Clinically, MSCs have been used to treat liver diseases,[65] diabetes mellitus,[66] and peripheral nerve injuries.[67] Relevant research has discovered that TGF-β helps MSCs protect nerve regeneration.[68] With more special functions of MSCs being discovered, the effectiveness of MSCs in treating SCI has been proven, and the question of how to improve their efficacy further remains to be addressed.
4.2.2. Inflammatory response
Inflammation, a defensive response, aims to eliminate pathogens, damaged tissues, and cancer.[69] However, excessive inflammation can damage tissue, and necrosis can perpetuate inflammation, leading to its persistence.[70] Additionally, the inflammatory response is critical to the secondary injury of SCI.[71] When a lesion occurs after SCI, immune cells pass through the blood-spinal cord barrier and migrate to the injury site to eliminate damaged tissue through phagocytosis and the release of anti-inflammatory factors. The polarization of macrophages and microglia plays an important role in the mechanism of SCI. Pro-inflammatory M1 macrophages appear early to phagocytose damaged tissue and promote the recruitment of leukocytes from peripheral blood. Conversely, anti-inflammatory M2 macrophages repair damaged tissues in the later stage.[72,73] Therefore, fine-tuned alteration of macrophage polarization can alleviate inflammation and promote tissue repair.[74]
Recent research has shown that autophagy in macrophages can limit the development of inflammation, and the regulation of Beclin 1 can control the production of inflammatory factors.[75] Mitophagy, a physiological metabolic process, eliminates damaged or dysfunctional mitochondria. Dysfunctional mitochondria can exacerbate inflammation, and proper mitophagy can balance inflammation through innate immunity responses, regardless of endogenous or exogenous sources of inflammation.[76] In addition, reactive oxygen species (ROS) produced by mitochondria and damage-associated molecular patterns (DAMPs) can induce inflammatory responses by promoting inflammasome formation. Mitophagy can prevent excessive inflammatory responses induced by ROS and DAMPs.[77] Hence, enhancing mitophagy can alleviate inflammation caused by ROS and DAMPs. Targeting mitophagy is also a promising direction for treating SCI.
AMPK is an important regulator of cellular energy and metabolism. Activation of the AMPK pathway induces autophagy, restricts inflammation metabolism,[78] and attenuates pain induced by inflammation by inhibiting NF-κB activation and IL-1β expression.[79] Selective induction of AMPK may be a reliable target for inflammation after SCI.
Inflammation after SCI is closely linked to autophagy. Liu discovered that Tripartite motif-containing 14 upregulates IL-12 and IL-23 expression to promote inflammation through crosstalk with lysine-specific demethylase 4D, inhibiting autophagic degradation epigenetically.[80] Conversely, vascular endothelial growth factor,[81] metformin,[82] rapamycin,[83] and curcumin[84] activate autophagy to suppress inflammation. Therefore, identifying proper targets for autophagy could regulate the extent of inflammation, minimizing damage after SCI. The timing of inflammation after SCI presents a practical opportunity to modulate autophagy to alleviate inflammatory damage.
4.2.3. Therapeutic targets
Autophagy plays a significant role in secondary injury and tissue repair, and targeted medicine can be more effective with fewer side effects. Therefore, it is necessary to identify proper targets for the treatment of SCI.
Rapamycin, an antifungal metabolite, has been found to inhibit immunity and proliferation in mammalian cells.[85] It promotes the expression of microtubule-associated protein 1 light chain 3 and Beclin1 at the site of injury, inducing autophagy and increasing the number of neurons and astrocytes with light chain 3 in the spinal cord.[32] The mammalian target of mTOR signaling pathway, an evolutionarily conserved serine/threonine kinase,[86] regulates processes such as death, survival, metabolism, proliferation, and growth.[87] This pathway, located in downstream effector of the phosphatidylinositol-3 kinase/AKT pathway, consists of 2 parts: mechanistic target of rapamycin complex 1 and mTORC2. The mechanistic target of rapamycin complex 1 pathway, which is sensitive to rapamycin, includes mTOR, regulatory-associated protein of mTOR, mammalian lethal with SEC13 protein 8, 40-kDa proline-rich Akt substrate, and DEP domain-containing mTOR-interacting protein.[88] It controls the process of autophagy through various intracellular and extracellular cues, including stress, energy status, oxygen, amino acids, and growth factors.[89] On the other hand, mTORC2, which is insensitive to rapamycin, is composed of mTOR, rapamycin-insensitive companion of mammalian target of rapamycin, mammalian lethal with SEC13 protein 8, DEP domain-containing mTOR-interacting protein, mammalian stress-activated protein kinase-interacting protein, and Protor1/2.[90,91] It regulates cell survival, proliferation, and cytoskeleton remodeling.[92] Autophagy is activated by AMPK, which senses cellular energy levels and balances cell metabolism. However, mTOR inhibits autophagy. Under conditions of glucose deprivation, AMPK activates autophagy by phosphorylating Ulk1.[93] Phosphorylated Ulk1 is a key regulator of autophagy. Both AMPK and mTOR can regulate the phosphorylation of Ulk1 to control autophagy. Regulating autophagy has the potential to improve motor function, as dysregulation of autophagy is one of the causes of severe outcomes in SCI. Therefore, inhibiting or activating autophagy signaling pathways such as mTOR and AMPK can enhance functional recovery. Several studies have provided evidence for this theory, such as the discovery of the regulation of the AKT/mTOR/signal transducer and activator of transcription 3 pathway by adenosine triphosphate[94] and the role of the transient receptor potential melastatin 7 channel in reducing blood-spinal cord barrier damage through the mTOR/Jumonji domain-containing protein-3 axis after SCI.[95] In clinical practice, rapamycin analogues like deforolimus, everolimus, and temsirolimus are used to treat various cancers.[96] With further research, more treatments for SCI will likely be discovered in the future.
Beclin 1, the first mammalian autophagy-related gene, was discovered by the Levine group in 1999. It is involved in multiple biological processes, including development, lifespan, and diseases. Beclin 1 is the mammalian homologue of yeast Atg6/vps30,[97] although the amino acid sequences of Beclin 1 and Atg6/vps30 differ substantially (39.1% sequence similarity and 24.4% sequence identity). The Beclin 1 complex, located downstream of the mTOR signaling pathway, initiates the formation of autophagosomes and is regulated by the mTOR pathway to control autophagy.[98] The tumor suppressing function of Beclin 1 has been well established.[99] During autophagy, Beclin 1 is phosphorylated by Ulk1 and serves as a scaffold for the phosphatidylinositol-3 kinase complex, promoting the localization of autophagy-related proteins to the autophagic vacuole.[100] Bcl-2, an antiapoptotic gene, interacts with Beclin 1 to regulate the balance between autophagy and apoptosis by inhibiting autophagy.[101] The Beclin 1-Bcl-2 complex maintains this balance. Autophagy, induced by Beclin 1, is inhibited by the expression of Bcl-2. Nutrient starvation, a potent physiological inducer of autophagy, promotes the dissociation between Beclin 1 and its repressors.[102] Moreover, cell death is weakly associated with autophagy when only Bcl-2 is expressed, while it is strongly linked to autophagy when Beclin 1 is activated alone. Many viruses have evolved mechanisms to inhibit autophagy, such as binding to Beclin-1, which inhibits autophagosome formation and impairs autophagy.[103] Understanding how viruses inhibit Beclin 1 could provide insights into regulating autophagy in SCI treatment. Up-regulation of Beclin 1 and promotion of autophagic cell death have been observed after SCI. The expression of Beclin 1 is initially up-regulated at 4 hours, peaks at 3 days, and remains at a high level at 21 days.[104] Therefore, inhibitors of autophagy may be potential and practical targets for treating SCI. Furthermore, Beclin 1 also mediates autophagy against apoptosis following SCI, playing an important role in the neuroprotection of the spinal cord.[105]
Axon regeneration after SCI is challenging due to the presence of extracellular inhibitory factors, limited axon growth capacity, and a lack of neurotrophic factors.[106] Promoting axon regeneration requires activating growth programs and alleviating growth inhibitory pathways.[10] In vitro studies have shown that inducing autophagy can reduce scar formation and promote axon regeneration at the lesion site after SCI by degrading the superior cervical ganglia protein 10, thereby stabilizing microtubules.[107, 108] The cytoskeleton, composed of microtubules and actin, plays a crucial role in axon regeneration. Research has indicated that dysregulated cytoskeleton dynamics can result in a growth-incompetent structure, but pharmacological modulation can reverse this effect.[109] The mechanism of axon regeneration is complex, and potential treatment targets include phosphatase and tensin homolog/mTOR,[110] the mitogen-activated protein kinase pathway,[111] and signal transducer and activator of transcription 3.[112] Recently, researchers discovered that ROS-Scavenging Hydrogels, a new material designed to remove ROS could promote nerve regeneration and improve the microenvironment to restore motor function after SCI.[113] As technology and materials continue to develop, more effective substances for promoting axon regeneration are likely to emerge.
5. Conclusion
In conclusion, this study utilized bibliometric tools to provide an overview of authors, influential journals, productive countries/organizations, popular papers, and burst keywords related to autophagy and SCI. By exploring this map, readers can better understand the relationship between autophagy and SCI, promote international collaboration among relevant organizations or countries, and guide scholars in selecting cooperative partners to accelerate the process of research in autophagy and SCI. With concerted efforts, future research will uncover potential and effective targets for treatment.
Acknowledgments
Thanks to Shanghai HaoSheng Biotechnology Co., Ltd. for providing language editing services.
Author contributions
Conceptualization: Zhenyu Zhang.
Visualization: Yangjun Xu, Wei He, Zhongwei He, Jinxiang Shang.
Writing – original draft: Fei Wang, Zhenyu Zhang.
Writing – review & editing: Songou Zhang, Xiang Wang.
Abbreviations:
- AKT
- activation of the protein kinase B
- AMPK
- AMP-activated protein kinase
- DAMPs
- damage-associated molecular patterns
- IF
- impact factor
- MSCs
- mesenchymal stem cells
- ROS
- reactive oxygen species
- SCI
- spinal cord injury
- WOS
- Web of Science
This study was supported by the Zhejiang Provincial Department of Health Project (2022KY1313 to Xujun Hu and 2022KY1288 to Jinxiang Shang), Shaoxing Science and Technology Plan Project (2023A14009) to Wei He and Shaoxing People’s Hospital Youth Project (2023YB01) to Fei Wang.
The authors have no conflicts of interest to disclose.
The datasets generated during and/or analyzed during the current study are publicly available.
How to cite this article: Wang F, Zhang S, Xu Y, He W, Wang X, He Z, Shang J, Zhenyu Z. Mapping the landscape: A bibliometric perspective on autophagy in spinal cord injury. Medicine 2024;103:29(e38954).
References
- [1].GBD 2016 Traumatic Brain Injury and Spinal Cord Injury Collaborators. Global, regional, and national burden of traumatic brain injury and spinal cord injury, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019;18:56–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Saadoun S, Papadopoulos MC. Targeted perfusion therapy in spinal cord trauma. Neurotherapeutics. 2020;17:511–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Li F, Wang H, Chen H, et al. Mechanism of ferroptosis and its role in spinal cord injury. Front Neurol. 2022;13:926780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Park CS, Lee JY, Choi HY, Ju BG, Youn I, Yune TY. Protocatechuic acid improves functional recovery after spinal cord injury by attenuating blood-spinal cord barrier disruption and hemorrhage in rats. Neurochem Int. 2019;124:181–92. [DOI] [PubMed] [Google Scholar]
- [5].Lin S, Mei X. Role of NLRP3 inflammasomes in neuroinflammation diseases. Eur Neurol. 2021;83:576–80. [DOI] [PubMed] [Google Scholar]
- [6].Liu WM, Wu JY, Li FC, Chen Q-X. Ion channel blockers and spinal cord injury. J Neurosci Res. 2011;89:791–801. [DOI] [PubMed] [Google Scholar]
- [7].Liu D, Thangnipon W, McAdoo DJ. Excitatory amino acids rise to toxic levels upon impact injury to the rat spinal cord. Brain Res. 1991;547:344–8. [DOI] [PubMed] [Google Scholar]
- [8].Yang Y, Pang M, Chen Y-Y, et al. Human umbilical cord mesenchymal stem cells to treat spinal cord injury in the early chronic phase: study protocol for a prospective, multicenter, randomized, placebo-controlled, single-blinded clinical trial. Neural Regen Res. 2020;15:1532–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Nagoshi N, Tsuji O, Nakamura M, Okano H. Cell therapy for spinal cord injury using induced pluripotent stem cells. Regen Ther. 2019;11:75–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].He X, Li Y, Deng B, et al. The PI3K/AKT signalling pathway in inflammation, cell death and glial scar formation after traumatic spinal cord injury: mechanisms and therapeutic opportunities. Cell Prolif. 2022;55:e13275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Levine B, Kroemer G. Autophagy in the pathogenesis of disease. Cell. 2008;132:27–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Ichimiya T, Yamakawa T, Hirano T, et al. Autophagy and autophagy-related diseases: a review. Int J Mol Sci . 2020;21:8974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Kroemer G, Levine B. Autophagic cell death: the story of a misnomer. Nat Rev Mol Cell Biol. 2008;9:1004–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Yu L, Strandberg L, Lenardo MJ. The selectivity of autophagy and its role in cell death and survival. Autophagy. 2008;4:567–73. [DOI] [PubMed] [Google Scholar]
- [15].Li J, Yuan J. Caspases in apoptosis and beyond. Oncogene. 2008;27:6194–206. [DOI] [PubMed] [Google Scholar]
- [16].Lockshin RA, Zakeri Z. Apoptosis, autophagy, and more. Int J Biochem Cell Biol. 2004;36:2405–19. [DOI] [PubMed] [Google Scholar]
- [17].Larsen KE, Sulzer D. Autophagy in neurons a review. Histol Histopathol. 2002;17:897. [DOI] [PubMed] [Google Scholar]
- [18].Walker CL, Walker MJ, Liu N-K, et al. Systemic bisperoxovanadium activates Akt/mTOR, reduces autophagy, and enhances recovery following cervical spinal cord injury. PLoS One. 2012;7:e30012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Erlich S, Alexandrovich A, Shohami E, Pinkas-Kramarski R. Rapamycin is a neuroprotective treatment for traumatic brain injury. Neurobiol Dis. 2007;26:86–93. [DOI] [PubMed] [Google Scholar]
- [20].Sekiguchi A, Kanno H, Ozawa H, Yamaya S, Itoi E. Rapamycin promotes autophagy and reduces neural tissue damage and locomotor impairment after spinal cord injury in mice. J Neurotrauma. 2012;29:946–56. [DOI] [PubMed] [Google Scholar]
- [21].Donthu N, Kumar S, Mukherjee D, Pandey N, Lim WM. How to conduct a bibliometric analysis: an overview and guidelines. J Bus Res. 2021;133:285–96. [Google Scholar]
- [22].Merigó JM, Yang J-B. A bibliometric analysis of operations research and management science. Omega. 2017;73:37–48. [Google Scholar]
- [23].Wu H, Li Y, Tong L, Wang Y, Sun Z. Worldwide research tendency and hotspots on hip fracture: a 20-year bibliometric analysis. Arch Osteoporos. 2021;16:1–14. [DOI] [PubMed] [Google Scholar]
- [24].Chadegani AA, Salehi H, Yunus MM, et al. A comparison between two main academic literature collections: web of Science and Scopus databases. arXiv preprint arXiv:13050377. 2013;9:18. [Google Scholar]
- [25].Chen C. CiteSpace II: detecting and visualizing emerging trends and transient patterns in scientific literature. J Am Soc Inf Sci Technol. 2006;57:359–77. [Google Scholar]
- [26].Jung CH, Ro S-H, Cao J, Otto NM, Kim D-H. mTOR regulation of autophagy. FEBS Lett. 2010;584:1287–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Frankel LB, Lund AH. MicroRNA regulation of autophagy. Carcinogenesis. 2012;33:2018–25. [DOI] [PubMed] [Google Scholar]
- [28].Kanno H, Ozawa H, Sekiguchi A, Itoi E. The role of autophagy in spinal cord injury. Autophagy. 2009;5:390–2. [DOI] [PubMed] [Google Scholar]
- [29].Li DT, Wang PP, Luo YH, Zhao MY, Chen F. Health benefits of anthocyanins and molecular mechanisms: update from recent decade. Review. Crit Rev Food Sci Nutr. 2017;57:1729–41. [DOI] [PubMed] [Google Scholar]
- [30].Kim KT, Nam TK, Park YS, Kim Y-B, Park S-W. Neuroprotective effect of anthocyanin on experimental traumatic spinal cord injury. J Korean Neurosurg Soc. 2011;49:205–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Wang J, Ma C, Rong W, et al. Bog bilberry anthocyanin extract improves motor functional recovery by multifaceted effects in spinal cord injury. Neurochem Res. 2012;37:2814–25. [DOI] [PubMed] [Google Scholar]
- [32].Tang PF, Hou HP, Zhang LC, et al. Autophagy reduces neuronal damage and promotes locomotor recovery via inhibition of apoptosis after spinal cord injury in rats. Mol Neurobiol. 2014;49:276–87. [DOI] [PubMed] [Google Scholar]
- [33].Castro-Malaspina H, Gay RE, Resnick G, et al. Characterization of human bone marrow fibroblast colony-forming cells (CFU-F) and their progeny. Blood. 1980;56:289–301. [PubMed] [Google Scholar]
- [34].Campagnoli C, Roberts IA, Kumar S, Bennett PR, Bellantuono I, Fisk NM. Identification of mesenchymal stem/progenitor cells in human first-trimester fetal blood, liver, and bone marrow. Blood. 2001;98:2396–402. [DOI] [PubMed] [Google Scholar]
- [35].Rolandsson Enes S, Andersson Sjöland A, Skog I, et al. MSC from fetal and adult lungs possess lung-specific properties compared to bone marrow-derived MSC. Sci Rep. 2016;6:29160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Villaron EM, Almeida J, López-Holgado N, et al. Mesenchymal stem cells are present in peripheral blood and can engraft after allogeneic hematopoietic stem cell transplantation. Haematologica. 2004;89:1421–7. [PubMed] [Google Scholar]
- [37].Fukumoto T, Sperling J, Sanyal A, et al. Combined effects of insulin-like growth factor-1 and transforming growth factor-β1 on periosteal mesenchymal cells during chondrogenesis in vitro. Osteoarthritis Cartilage. 2003;11:55–64. [DOI] [PubMed] [Google Scholar]
- [38].Rotter N, Oder J, Schlenke P, et al. Isolation and characterization of adult stem cells from human salivary glands. Stem Cells Dev. 2008;17:509–18. [DOI] [PubMed] [Google Scholar]
- [39].Zuk PA, Zhu M, Mizuno H, et al. Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng. 2001;7:211–28. [DOI] [PubMed] [Google Scholar]
- [40].De Bari C, Dell’Accio F, Tylzanowski P, et al. Multipotent mesenchymal stem cells from adult human synovial membrane. Arthritis Rheum. 2001;44:1928–42. [DOI] [PubMed] [Google Scholar]
- [41].Erices A, Conget P, Minguell JJ. Mesenchymal progenitor cells in human umbilical cord blood. Br J Haematol. 2000;109:235–42. [DOI] [PubMed] [Google Scholar]
- [42].Murray IR, West CC, Hardy WR, et al. Natural history of mesenchymal stem cells, from vessel walls to culture vessels. Cell Mol Life Sci. 2014;71:1353–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Watson N, Divers R, Kedar R, et al. Discarded Wharton jelly of the human umbilical cord: a viable source for mesenchymal stromal cells. Cytotherapy. 2015;17:18–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Pierdomenico L, Bonsi L, Calvitti M, et al. Multipotent mesenchymal stem cells with immunosuppressive activity can be easily isolated from dental pulp. Transplantation. 2005;80:836–42. [DOI] [PubMed] [Google Scholar]
- [45].Vellasamy S, Sandrasaigaran P, Vidyadaran S, George E, Ramasamy R. Isolation and characterisation of mesenchymal stem cells derived from human placenta tissue. World J Stem Cells. 2012;4:53–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Costa-Almeida R, Calejo I, Gomes ME. Mesenchymal stem cells empowering tendon regenerative therapies. Int J Mol Sci . 2019;20:3002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Lemos DR, Eisner C, Hopkins CI, Rossi FMV. Skeletal muscle-resident MSCs and bone formation. Bone. 2015;80:19–23. [DOI] [PubMed] [Google Scholar]
- [48].Bozorgmehr M, Gurung S, Darzi S, et al. Endometrial and menstrual blood mesenchymal stem/stromal cells: biological properties and clinical application. Front Cell Dev Biol. 2020;8:497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].int Anker PS, Scherjon SA, Kleijburg-Van der Keur C, et al. Amniotic fluid as a novel source of mesenchymal stem cells for therapeutic transplantation. Blood. 2003;102:1548–9. [DOI] [PubMed] [Google Scholar]
- [50].Najar M, Raicevic G, Fayyad-Kazan H, Bron D, Toungouz M, Lagneaux L. Mesenchymal stromal cells and immunomodulation: a gathering of regulatory immune cells. Cytotherapy. 2016;18:160–71. [DOI] [PubMed] [Google Scholar]
- [51].English K. Mechanisms of mesenchymal stromal cell immunomodulation. Immunol Cell Biol. 2013;91:19–26. [DOI] [PubMed] [Google Scholar]
- [52].Dang S, Yu Z-m, Zhang C-y, et al. Autophagy promotes apoptosis of mesenchymal stem cells under inflammatory microenvironment. Stem Cell Res Ther. 2015;6:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Dunbar H, Weiss DJ, Rolandsson Enes S, Laffey JG, English K. The inflammatory lung microenvironment; a key mediator in MSC licensing. Cells. 2021;10:2982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Zhang S, Teo KYW, Chuah SJ, Lai RC, Lim SK, Toh WS. MSC exosomes alleviate temporomandibular joint osteoarthritis by attenuating inflammation and restoring matrix homeostasis. Biomaterials. 2019;200:35–47. [DOI] [PubMed] [Google Scholar]
- [55].Luzuriaga J, Polo Y, Pastor-Alonso O, et al. Advances and perspectives in dental pulp stem cell based neuroregeneration therapies. Int J Mol Sci . 2021;22:3546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Luo Y, Xu T, Liu W, et al. Exosomes derived from GIT1-overexpressing bone marrow mesenchymal stem cells promote traumatic spinal cord injury recovery in a rat model. Int J Neurosci. 2021;131:170–82. [DOI] [PubMed] [Google Scholar]
- [57].Menezes K, Rosa BG, Freitas C, et al. Human mesenchymal stromal/stem cells recruit resident pericytes and induce blood vessels maturation to repair experimental spinal cord injury in rats. Sci Rep. 2020;10:19604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Dang S, Xu H, Xu C, et al. Autophagy regulates the therapeutic potential of mesenchymal stem cells in experimental autoimmune encephalomyelitis. Autophagy. 2014;10:1301–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Kordelas L, Rebmann V, Ludwig A, et al. MSC-derived exosomes: a novel tool to treat therapy-refractory graft-versus-host disease. Leukemia. 2014;28:970–3. [DOI] [PubMed] [Google Scholar]
- [60].Yaghoubi Y, Movassaghpour A, Zamani M, Talebi M, Mehdizadeh A, Yousefi M. Human umbilical cord mesenchymal stem cells derived-exosomes in diseases treatment. Life Sci. 2019;233:116733. [DOI] [PubMed] [Google Scholar]
- [61].Gu J, Jin ZS, Wang CM, et al. Bone marrow mesenchymal stem cell-derived exosomes improves spinal cord function after injury in rats by activating autophagy. Drug Des Dev Ther. 2020;14:1621–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Chen Y, Tian Z, He L, et al. Exosomes derived from miR-26a-modified MSCs promote axonal regeneration via the PTEN/AKT/mTOR pathway following spinal cord injury. Stem cell Res Ther. 2021;12:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Ren Z, Qi Y, Sun S, Tao Y, Shi R. Mesenchymal stem cell-derived exosomes: hope for spinal cord injury repair. Stem Cells Dev. 2020;29:1467–78. [DOI] [PubMed] [Google Scholar]
- [64].Harrell CR, Jovicic N, Djonov V, Arsenijevic N, Volarevic V. Mesenchymal stem cell-derived exosomes and other extracellular vesicles as new remedies in the therapy of inflammatory diseases. Cells. 2019;8:1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Lou G, Chen Z, Zheng M, Liu Y. Mesenchymal stem cell-derived exosomes as a new therapeutic strategy for liver diseases. Exp Mol Med. 2017;49:e346–e346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Zang L, Hao H, Liu J, Li Y, Han W, Mu Y. Mesenchymal stem cell therapy in type 2 diabetes mellitus. Diabetol Metab Syndr. 2017;9:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Dong R, Liu Y, Yang Y, Wang H, Xu Y, Zhang Z. MSC-derived exosomes-based therapy for peripheral nerve injury: a novel therapeutic strategy. Biomed Res Int. 2019;2019:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Luo H, Zhang Y, Zhang Z, Jin Y. The protection of MSCs from apoptosis in nerve regeneration by TGFβ1 through reducing inflammation and promoting VEGF-dependent angiogenesis. Biomaterials. 2012;33:4277–87. [DOI] [PubMed] [Google Scholar]
- [69].Medzhitov R. Origin and physiological roles of inflammation. Nature. 2008;454:428–35. [DOI] [PubMed] [Google Scholar]
- [70].Nathan C, Ding A. Nonresolving inflammation. Cell. 2010;140:871–82. [DOI] [PubMed] [Google Scholar]
- [71].Hellenbrand DJ, Quinn CM, Piper ZJ, Morehouse CN, Fixel JA, Hanna AS. Inflammation after spinal cord injury: a review of the critical timeline of signaling cues and cellular infiltration. J Neuroinflamm. 2021;18:1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Yunna C, Mengru H, Lei W, Weidong C. Macrophage M1/M2 polarization. Eur J Pharmacol. 2020;877:173090. [DOI] [PubMed] [Google Scholar]
- [73].Kong X, Gao J. Macrophage polarization: a key event in the secondary phase of acute spinal cord injury. J Cell Mol Med. 2017;21:941–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Liu YC, Zou XB, Chai YF, Yao Y-M. Macrophage polarization in inflammatory diseases. Int J Biol Sci. 2014;10:520–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Xu J, Kong L, Oliver BA, et al. Constitutively active autophagy in macrophages dampens inflammation through metabolic and post-transcriptional regulation of cytokine production. Cell Rep. 2023;42:112708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Deretic V. Autophagy in inflammation, infection, and immunometabolism. Immunity. 2021;54:437–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Zhao Y, Huang S, Liu J, et al. Mitophagy contributes to the pathogenesis of inflammatory diseases. Inflammation. 2018;41:1590–600. [DOI] [PubMed] [Google Scholar]
- [78].O’Neill LA, Hardie DG. Metabolism of inflammation limited by AMPK and pseudo-starvation. Nature. 2013;493:346–55. [DOI] [PubMed] [Google Scholar]
- [79].Xiang H-C, Lin L-X, Hu X-F, et al. AMPK activation attenuates inflammatory pain through inhibiting NF-κB activation and IL-1β expression. J Neuroinflamm. 2019;16:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Liu D, Zhao Z, She Y, et al. TRIM14 inhibits OPTN-mediated autophagic degradation of KDM4D to epigenetically regulate inflammation. Proc Natl Acad Sci USA. 2022;119:e2113454119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Wang H, Wang Y, Li D, et al. VEGF inhibits the inflammation in spinal cord injury through activation of autophagy. Biochem Biophys Res Commun. 2015;464:453–8. [DOI] [PubMed] [Google Scholar]
- [82].Wang C, Liu C, Gao K, et al. Metformin preconditioning provide neuroprotection through enhancement of autophagy and suppression of inflammation and apoptosis after spinal cord injury. Biochem Biophys Res Commun. 2016;477:534–40. [DOI] [PubMed] [Google Scholar]
- [83].Chen H-C, Fong T-H, Hsu P-W, Chiu W-T. Multifaceted effects of rapamycin on functional recovery after spinal cord injury in rats through autophagy promotion, anti-inflammation, and neuroprotection. J Surg Res. 2013;179:e203–10. [DOI] [PubMed] [Google Scholar]
- [84].Li W, Yao S, Li H, Meng Z, Sun X. Curcumin promotes functional recovery and inhibits neuronal apoptosis after spinal cord injury through the modulation of autophagy. J Spinal Cord Med. 2021;44:37–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Li J, Kim SG, Blenis J. Rapamycin: one drug, many effects. Cell Metab. 2014;19:373–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Kim YC, Guan KL. mTOR: a pharmacologic target for autophagy regulation. J Clin Invest. 2015;125:25–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Laplante M, Sabatini DM. mTOR signaling at a glance. J Cell Sci. 2009;122:3589–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Dibble CC, Cantley LC. Regulation of mTORC1 by PI3K signaling. Trends Cell Biol. 2015;25:545–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Laplante M, Sabatini DM. mTOR signaling in growth control and disease. Cell. 2012;149:274–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Zinzalla V, Stracka D, Oppliger W, Hall MN. Activation of mTORC2 by association with the ribosome. Cell. 2011;144:757–68. [DOI] [PubMed] [Google Scholar]
- [91].Fu W, Hall MN. Regulation of mTORC2 signaling. Genes. 2020;11:1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Dai H, Thomson AW. The “other” mTOR complex: new insights into mTORC2 immunobiology and their implications. Am J Transplant. 2019;19:1614–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Kim J, Kundu M, Viollet B, Guan K-L. AMPK and mTOR regulate autophagy through direct phosphorylation of Ulk1. Nat Cell Biol. 2011;13:132–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Sun ZG, Hu LY, Wen YM, et al. Adenosine triphosphate promotes locomotor recovery after spinal cord injury by activating mammalian target of rapamycin pathway in rats. Neural Regen Res. 2013;8:101–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Park CS, Lee JY, Seo KJ, Kim IY, Ju BG, Yune TY. TRPM7 mediates BSCB disruption after spinal cord injury by regulating the mTOR/JMJD3 axis in rats. Mol Neurobiol. 2023;61:662–77. [DOI] [PubMed] [Google Scholar]
- [96].Pópulo H, Lopes JM, Soares P. The mTOR signalling pathway in human cancer. Int J Mol Sci . 2012;13:1886–918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Aita VM, Liang XH, Murty V, et al. Cloning and genomic organization of beclin 1, a candidate tumor suppressor gene on chromosome 17q21. Genomics. 1999;59:59–65. [DOI] [PubMed] [Google Scholar]
- [98].Pattingre S, Espert L, Biard-Piechaczyk M, Codogno P. Regulation of macroautophagy by mTOR and Beclin 1 complexes. Biochimie. 2008;90:313–23. [DOI] [PubMed] [Google Scholar]
- [99].Wijshake T, Zou Z, Chen B, et al. Tumor-suppressor function of Beclin 1 in breast cancer cells requires E-cadherin. Proc Natl Acad Sci USA. 2021;118:e2020478118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Zachari M, Ganley Ian G. The mammalian ULK1 complex and autophagy initiation. Essays Biochem. 2017;61:585–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Pattingre S, Tassa A, Qu X, et al. Bcl-2 antiapoptotic proteins inhibit Beclin 1-dependent autophagy. Cell. 2005;122:927–39. [DOI] [PubMed] [Google Scholar]
- [102].Levine B, Sinha SC, Kroemer G. Bcl-2 family members: dual regulators of apoptosis and autophagy. Autophagy. 2008;4:600–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Münz C. Beclin-1 targeting for viral immune escape. Viruses. 2011;3:1166–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Kanno H, Ozawa H, Sekiguchi A, Itoi E. Spinal cord injury induces upregulation of Beclin 1 and promotes autophagic cell death. Neurobiol Dis. 2009;33:143–8. [DOI] [PubMed] [Google Scholar]
- [105].Wang Z-Y, Lin J-H, Muharram A, Liu W-G. Beclin-1-mediated autophagy protects spinal cord neurons against mechanical injury-induced apoptosis. Apoptosis. 2014;19:933–45. [DOI] [PubMed] [Google Scholar]
- [106].Liu K, Tedeschi A, Park KK, He Z. Neuronal intrinsic mechanisms of axon regeneration. Annu Rev Neurosci. 2011;34:131–52. [DOI] [PubMed] [Google Scholar]
- [107].Giger RJ, Hollis ER, Tuszynski MH. Guidance molecules in axon regeneration. Cold Spring Harbor Perspect Biol. 2010;2:a001867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].He M, Ding Y, Chu C, Tang J, Xiao Q, Luo Z-G. Autophagy induction stabilizes microtubules and promotes axon regeneration after spinal cord injury. Proc Natl Acad Sci USA. 2016;113:11324–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Blanquie O, Bradke F. Cytoskeleton dynamics in axon regeneration. Curr Opin Neurobiol. 2018;51:60–9. [DOI] [PubMed] [Google Scholar]
- [110].Park KK, Liu K, Hu Y, Kanter JL, He Z. PTEN/mTOR and axon regeneration. Exp Neurol. 2010;223:45–50. [DOI] [PubMed] [Google Scholar]
- [111].Hammarlund M, Nix P, Hauth L, Jorgensen EM, Bastiani M. Axon regeneration requires a conserved MAP kinase pathway. Science. 2009;323:802–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Mehta ST, Luo X, Park KK, Bixby JL, Lemmon VP. Hyperactivated Stat3 boosts axon regeneration in the CNS. Exp Neurol. 2016;280:115–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Liu D, Lu G, Shi B, et al. ROS-scavenging hydrogels synergize with neural stem cells to enhance spinal cord injury repair via regulating microenvironment and facilitating nerve regeneration. Adv Healthcare Mater. 2023;12:e2300123. [DOI] [PubMed] [Google Scholar]