Abstract
Globally over 2 million women are diagnosed with breast cancer each year despite major advances in detection and treatment of the disease. Breast cancer is comprised of several distinct subtypes and understanding the heterogeneity of the disease has become crucial for treatment planning. Therapeutic strategies span from a hormone therapy-based focus for women with estrogen receptor positive breast cancer to targeting human epidermal growth factor (HER2) by small molecules, antibody-drug-conjugates (ADC) and monoclonal antibodies in those with HER2 overexpression. Other novel treatment strategies for select subgroups of patients include the cyclin-dependent kinase 4/6 (CDK4/6) inhibitors for women with estrogen receptor positive tumors, the poly ADP ribose polymerase (PARP) inhibitors for those with BRCA mutations, and phosphoinositide 3-kinase (PI3K) inhibitors for women with tumors harboring phophatidylinositol-4,5-bisphosphate 3 kinase catalytic subunit alpha (PIK3CA) mutations. In contrast, the treatment for women with triple negative breast cancer has until recently been solely limited to chemotherapy. The profound impact of immunotherapy on cancer treatment in general has created much hope for its potential in breast cancer. This review will focus on the current advances and the research of immunotherapy in breast cancer, particularly on immune checkpoint inhibitors, adoptive cell transfer and cancer vaccines.
Relevant targets in different breast cancer subtypes and current standard of care
Breast cancer imposes a major health and economic challenge despite many major advances in detection and treatment. In 2018, 2.1 million women, globally, were diagnosed with breast cancer, leading to 62,700 deaths. In 2020, in the United States 279,100 individuals are expected to be affected by breast cancer.1 Breast cancer exhibits a lot of inter-patient heterogeneity. As cancer treatment has evolved towards personalized medicine, understanding the heterogeneity of breast cancer has become crucial for treatment planning. Based on transcriptomic analysis, breast cancer has been classified into luminal A, luminal B, human epidermal growth factor (HER2) overexpressing, basal-like and normal-like tumors.2 Immunohistochemically, breast cancer is classified based on the presence or absence of hormone receptors (estrogen receptor (ER) and progesterone receptor (PR)) and the HER2.3 Further subtyping of breast cancer malignancies using transcriptomic assays such as MammaPrint, Oncotype DX and PAM50 and others are now routinely employed to guide adjuvant therapy. Women with hormone receptor positive disease are foremost treated with hormonal therapy directed towards blocking estrogen receptor signaling or suppression of estrogen production. Genomic assessment in addition to ER and PR expression allows identification of a high-risk subgroup who will be best served with adjuvant chemotherapy followed by hormonal therapy. All patients with early stage breast cancer that express HER2 should receive anti-HER2 targeting biologics in combination with chemotherapy as neoadjuvant therapy, followed by extended adjuvant therapy with HER2 targeting small molecules such as neratinib and or antibody drug conjugates, ado-trastuzumab emtansine (ADC) for high-risk patients. For women with metastatic disease, multiple tissue-based approaches are used to define therapeutic targets. Universal testing includes assessment of ER, PR and HER2 expression either by immunohistochemistry or dual probe in situ hybridization as well as genomic assays determining phophatidylinositol-4,5-bisphosphate 3 kinase catalytic subunit alpha (PIK3CA) mutations as pre-requisite for hormonal therapy in combination with the phosphoinositide 3-kinase (PI3K) inhibitor, alpelisib.4 The ability to exploit homologous recombination deficiencies therapeutically with poly ADP ribose polymerase (PARP) inhibitors has led to routine testing for BRCA and other homologous recombination DNA repair deficient (HRD) mutations in the tumor or in the germline.5,6 Other genomic targets including ER gene mutations serve as eligibility criteria for clinical trials testing novel anti-estrogens.7, 8, 9 Tumors not expressing ER, PR or HER2 are referred to as triple negative breast cancer (TNBC). Patients with TNBC have increased risk of developing metastatic disease and when metastatic, overall survival (OS) is poorer (Table 1). With the absence of clearly defined therapeutic targets, such as BRCA mutations, the treatment of TNBC has remained a therapeutic challenge. For early-stage TNBC, cytotoxic chemotherapy remains the only choice of therapy. As expected by the profound impact that immunotherapy has had on cancer therapy in general, there has been a broad focus on immunotherapy in breast cancer. Over the last year, for women with metastatic TNBC, immune checkpoint inhibitors in combination with chemotherapy have been approved for tumors that express PD-L1 (Table 1). This review will focus on the current role of immunotherapy, particularly immune checkpoint inhibitors, adoptive cell transfer and cancer vaccines in breast cancer.
Table 1.
Comparing chemotherapy and immune checkpoint clinical trials in advanced breast cancer.
| Trial ID | Regimen | Phase | Subtype | N | Median PFS, months | Median OS, months | ORR (%) | References | ||
|---|---|---|---|---|---|---|---|---|---|---|
| NCT00938652 | Gemcitabine + Carboplatin: | III | TNBC | O'Shaughnessy et al, 2014 | ||||||
| Intention to treat | 30 | |||||||||
| (-) Iniparib | 258 | 4.1 | 11.1 | - | ||||||
| (+) Iniparib | 261 | 5.1 | 12.2 | - | ||||||
| 1st line therapy | ||||||||||
| (-) Iniparib | 149 | 4.6 | 13.9 | - | ||||||
| (+) Iniparib | 148 | 5.6 | 12.3 | - | ||||||
| 2nd/3rd line therapy | ||||||||||
| (-) Iniparib | 109 | 2.9 | 8.1 | - | ||||||
| (+) Iniparib | 113 | 4.2 | 12.1 | - | ||||||
| TNT | 1st line Carboplatin versus Docetaxel: | III | TNBC | Tutt et al, 2018 | ||||||
| NCT00532727 | Carboplatin | 188 | 3.1 | 12.4 | 31 | |||||
| Docetaxel | 188 | 4.4 | 12.3 | 36 | ||||||
| ASCENT | Sacituzumab versus TPC: | III | TNBC | Bardia et al, 2020 | ||||||
| NCT02574455 | Sacituzumab | 235 | 5.6 | 12.1 | 35 | |||||
| TPC | 233 | 1.7 | 6.7 | 5 | ||||||
| IMpassion130 | Nab-Paclitaxel: | III | TNBC | Schmid et al, 2018 | ||||||
| NCT02425891 | (-) atezolizumab | 449 | 5.6 | 17.6 | 45.9 | |||||
| PD-L1 - positive | 183 | 5.5 | 17.9 | 42.6 | ||||||
| PD-L1 - negative | 266 | 5.6 | - | |||||||
| (+) atezolizumab | 450 | 7.4 | 21.3 | 56.0 | ||||||
| PD-L1 - positive | 185 | 8.5 | 25.4 | 58.9 | ||||||
| PD-L1 - negative | 265 | 5.6 | - | |||||||
| IMpassion131 | Paclitaxel: | III | TNBC | Miles et al, 2020 | ||||||
| NCT03125902 | (-) atezolizumab | 220 | 5.6 | 22.8 | 47 | |||||
| PD-L1 - positive | 101 | 6.0 | 28.3 | 55 | ||||||
| (+) atezolizumab | 431 | 5.7 | 19.2 | 54 | ||||||
| PD-L1 - positive | 191 | 5.7 | 22.1 | 63 | ||||||
| KEYNOTE-012 | Pembrolizumab: | Ib | TNBC | 32 | 1.9 | 11.2 | 18.5 | Nanda et al, 2016 | ||
| NCT01848834 | PD-L1+ | |||||||||
| KEYNOTE-086 | Pembrolizumab: | II | TNBC | 170 | 2.0 | 9.0 | 5.3 | Adams et al, 2019 | ||
| NCT02447003 | PD-L1 - positive | 105 | 2.0 | 8.8 | 5.7 | |||||
| PD-L1 - negative | 64 | 1.9 | 9.7 | 4.7 | ||||||
| KEYNOTE-119 | Pembrolizumab versus TPC: | III | TNBC | Cortes et al, 2019 | ||||||
| NCT02555657 | Pembrolizumab | |||||||||
| PD-L1, CPS>= 10 | 96 | 2.1 | 12.7 | - | ||||||
| PD-L1, CPS>= 1 | 203 | 2.1 | 10.7 | - | ||||||
| Total | 312 | 2.1 | 9.9 | - | ||||||
| TPC | ||||||||||
| PD-L1, CPS>= 10 | 98 | 3.4 | 11.6 | - | ||||||
| PD-L1, CPS>= 1 | 202 | 3.1 | 10.2 | - | ||||||
| Total | 310 | 3.3 | 10.8 | - | ||||||
| KEYNOTE-150 | Pembrolizumab + eribulin | Ib/II | TNBC | 149 | Tolaney et al, 2020 | |||||
| NCT02513472 | PD-L1 positive | |||||||||
| No prior therapy | 29 | 6.1 | 21.0 | 34.5 | ||||||
| 1-2 prior therapies | 45 | 4.1 | 14.0 | 24.4 | ||||||
| PD-L1 negative | ||||||||||
| No prior therapy | 31 | 3.5 | 15.2 | 16.1 | ||||||
| 1-2 prior therapies | 44 | 3.9 | 15.5 | 18.2 | ||||||
| KEYNOTE-355 | TPC: | III | TNBC | Cortes et al, 2020 | ||||||
| NCT02819518 | (-) pembrolizumab | 281 | 5.6 | - | - | |||||
| PD-L1 - positive (CPS >=10) | 103 | 5.6 | - | - | ||||||
| (+) pembrolizumab | 566 | 7.5 | - | - | ||||||
| PD-L1 - positive (CPS >=10) | 220 | 9.7 | - | - | ||||||
| JAVELIN | Avelumab: | I | All | |||||||
| NCT01772004 | All | 168 | 1.4 | 8.1 | 3.0 | Dirix et al, 2018 | ||||
| PD-L1 - positive | 12 | 1.4 | 11.3 | 16.7 | ||||||
| PD-L1 - negative | 124 | 1.4 | 6.8 | 1.6 | ||||||
| TNBC | 58 | 1.4 | 9.2 | 5.2 | ||||||
| PD-L1 - positive | 9 | - | - | 22.2 | ||||||
| PD-L1 - negative | 39 | - | - | 2.6 | ||||||
| TONIC | Nivolumab: | II | TNBC | 66 | - | - | 20 | Voorwerk et al, 2019 | ||
| NCT02499367 | Without induction | 12 | - | - | 17 | |||||
| Irradiation (3 × 8 Gy) | 12 | - | - | 8 | ||||||
| Cyclophosphamide | 12 | - | - | 8 | ||||||
| Cisplatin | 13 | - | - | 23 | ||||||
| Doxorubicin | 17 | - | - | 35 | ||||||
Abbreviation: CPS, combined positive score; N, number; PD-L1, programmed cell death-ligand 1; PFS, progression free survival; ORR, overall response rate; OS, overall survival; TNBC, triple negative breast cancer; TPC, docetaxel, cisplatin, and irinotecan.
Immunotherapy: broad concepts
The clinical development of immune-oncology has been grouped into immune checkpoint inhibitors, vaccines and cell-based therapies. The premise that cancer cells engage different biological mechanisms to evade immune suppression has led to major breakthroughs in many cancers, including breast cancer. Currently approved immune checkpoint inhibitors include multiple monoclonal antibodies targeting immune checkpoint receptors and their ligands, including programmed cell death-1/programmed cell death-ligand 1 (PD-1/PD-L1) and cytotoxic T-lymphocyte-associated antigen 4 (CTLA-4). However, unlike in tumors like melanoma, benefits for women with breast cancer are more modest and so far, appear to be more limited to TNBC. CTLA-4 inhibitors have not been established in breast cancer. Hence, there is much interest in further defining the optimal setting for immunotherapy in breast cancer and the development of predictive biomarkers for response to anti-PD-1/PD-L1, anti-CTLA-4 inhibitors and other immune strategies. Much interest is directed towards the composition of specific tumor immune cell infiltrates such as tumor infiltrating lymphocytes (TILs), myeloid derived suppressor cells (MDSCs), tumor-associated macrophages (TAMs), natural killer (NKs) cells and dendric cells (DCs). In addition to the impact of PD-1 and PD-L1 expression in tumors and TILs, emerging data further points to interfering inflammatory, genetic and epigenetic signatures, as well as host factors such tumor location, gender, obesity and the microbiome. In most diseases, immune checkpoint inhibitors have shown to have synergistic interactions with other anti-cancer modalities and thus it is not surprising that immune checkpoint inhibitor combinations with other cytotoxic, biologic, and epigenetic therapies, as well as other strategies to overcome resistance are the focus of the more than 500 trials currently evaluating immune checkpoint inhibitors in breast cancer worldwide. Unlike immune checkpoint inhibitors, the development of vaccines and cell-based therapy strategies usually require an accessible target, ideally one that is also restricted to the tumor. While many vaccines and chimeric antigen receptor T cells (CAR-T) approaches have been focused on breast cancer or have included patients with breast cancer, to date immune therapy beyond PD-L1/PD-1 inhibitors remains experimental for patients with breast cancer.
Immune checkpoint inhibitors
Immune checkpoint inhibitors in metastatic breast cancer
Immune checkpoint inhibitors used in cancer therapy include monoclonal antibodies against CTLA-4, PD-1 or PD-L1, the latter of which are now approved in many hematological malignancies and solid tumors including ipilimumab and tremelimumab (anti-CTLA-4 antibodies); cemiplimab, nivolumab and pembrolizumab (anti-PD-1 antibodies); and atezolizumab, avelumab, and durvalumab (anti-PD-L1 antibodies), and many more are currently under development (Fig. 1A). Checkpoint inhibitors have only recently been approved for metastatic breast cancer, and specifically only for TNBC and tumors with high microsatellite instability (MSI-H). Pembrolizumab received approval in MSI-H tumors irrespective of cancer type and location.10 MSI-H is linked to DNA mismatch repair and is typically associated with high tumor mutation burden (TMB). Microsatellite instability is more frequently reported in cancers associated with Lynch disease such as endometrial, colon, ovarian and stomach cancer, yet is quite rarely seen in patients with breast cancer. With a prevalence of MSI-H in endometrial cancer reported as high as 17-31%, it is less than 2% in breast cancer, thus not routinely tested.11 Immune checkpoint inhibitors have initially been evaluated in various subtypes of breast cancer. The available data from completed clinical trials suggest that patients with ER positive (ER+) breast tumors derive minimal benefits from treatment with immune checkpoint inhibitors. The phase Ib trial, KEYNOTE-028, explored the safety of pembrolizumab in patients with ER+, HER2-, PD-L1 positive advanced breast cancer.12 An overall response rate (ORR) of 12% was observed in the 25 patients recruited for the study. Several trials evaluated immune checkpoint inhibitors as single agents in TNBC. A phase Ib, non-randomized, clinical trial (KEYNOTE-012) enrolled 27 patients with advanced, PD-L1 positive TNBC and found an ORR of 18.5%.13 Later a larger single arm Phase II study, KEYNOTE-086, enrolling 170 patients with previously treated metastatic TNBC showed an ORR of 5.7% with a median progression free survival (PFS) of 2 months, albeit 62% of the recruited patients had PD-L1 expression.14 In KEYNOTE-119, patients with metastatic TNBC were randomized to receive either pembrolizumab or a chemotherapeutic drug as monotherapy15 and even after stratification for PD-L1 expression and prior exposure to neoadjuvant or adjuvant chemotherapy, pembrolizumab did not result in any significant benefit. In KEYNOTE-150, 167 patients with metastatic TNBC received a combination of pembrolizumab and eribulin mesylate for a PFS of 4.1 months. In the TONIC trial, patients with metastatic TNBC were randomized into cohorts to receive nivolumab without induction, or with induction with irradiation, cyclophosphamide, cisplatin or doxorubicin.16 The cohort receiving cisplatin showed an ORR of 23%, while those receiving doxorubicin showed an ORR of 35%. The JAVELIN trial allowed enrollment of 168 patients with different breast cancer subtypes (HER2+: 15.5%, ER+: 42.9%, and TNBC: 34.5%) to be treated with escalating doses of avelumab.17 The ORR across all groups was 3%, whereas patients with TNBC showed ORR of 5.2%, and patients with HER2 positive disease showed ORR of 2.8%. A higher response was noted in patients with PD-L1 positive TNBC (22.2% versus 2.6%), but only 12 of 168 patients had PD-L1 positive tumors.
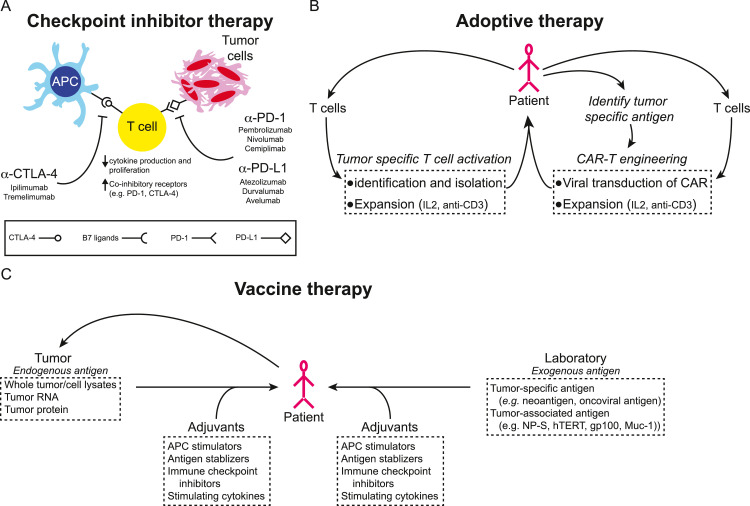
Fig. 1.
Adaptive immune therapies. (A) T cell activity is in part regulated through immune checkpoint cell surface receptors and ligands expressed in the microenvironment. Tumor cells and the tumor microenvironment down-regulate T cell cytokine production and proliferation by activating these checkpoints resulting in an exhausted T cell phenotype. Inhibiting antibodies that recognize and interfere with checkpoint receptor/ligand interactions (e.g. αPD-1, αPD-L1, and αCTLA-4) have been developed to promote T cell cytotoxic activity and an anti-tumor immune response. (B) Adoptive T cell therapies are derived from patient or donor T cells. T cells may be engineered through viral transduction to recognize tumor specific antigens (CAR-T cells) or by culturing T cells with tumor specific antigen and isolating activated T cells. Tumor directed T cells are then expanded and re-introduced to the patient to mount an adoptive therapy response. (C) Therapeutic cancer vaccines are generally either derived from endogenous antigens prepared from the patient's tumor or from exogenous antigens known to be tumor specific or associated with cancer or specific types of cancer. These antigens, together with adjuvants to boost immune response, are introduced to the patient to stimulate an effector and memory T cell response.
Taken together, these trials suggest that immune checkpoint inhibitors may predominantly have a role in TNBC. A closer look at subset analyses further points towards limited benefits beyond first-line therapy, e.g., the response rate of atezolizumab in combination with nab-paclitaxel in the phase Ib trial was 67% for untreated patients versus 29% for patients treated with two or more prior therapies. Hence, most phase III trials studying combinations of immune checkpoint inhibitors with chemotherapy limit enrollment to previously untreated metastatic TNBC patients. Immune checkpoint inhibitors in combination with chemotherapy are now approved in previously untreated, locally recurrent unresectable or metastatic TNBC with PD-L1 overexpression based on the IMpassion130 and the KEYNOTE-355 phase III trials. The IMpassion130 trial randomized 902 patients with previously untreated metastatic TNBC to receive either a combination of atezolizumab and nab-paclitaxel, or placebo and nab-paclitaxel.18,19 Patients were stratified based on the presence or absence of PD-L1 expression. The PFS in patients with PD-L1 negative tumors remained at 5.6 months for nab-paclitaxel with or without atezolizumab, whereas the median PFS was 7.5 months for the combination versus 5.0 month in the chemotherapy alone arm in the 40% of patients with PD-L1 overexpressing tumors. The second interim analysis, as well as the final data, showed clinically significant benefit in OS with the combination therapy only in patients with PD-L1 positive TNBC (25 months versus 18 months). The overall survival for patients with PD-L1 negative tumors was 20 months in both arms.20 The role of atezolizumab in TNBC was further evaluated in IMpassion131, a very similar trial, recently reported at the 2020 ESMO meeting. In a 2:1 randomization, 651 patients with untreated metastatic TNBC with 45% PD-L1 positive tumor were enrolled, evaluating atezolizumab (A) in combination with paclitaxel (P) (instead of nab-paclitaxel, nP) given weekly.21 Survival outcomes suggested that both trials enrolled similar populations (OS: IMpassion130: nP+A=21 months, nP=19 months; and IMpassion131: P+A= 19 months, P=23 months). In contrast to IMpassion130, there were no benefits seen with the addition of atezolizumab to paclitaxel, regardless of PD-L1 expression. Albeit not statistically different, this trial showed a trend towards worse OS in the atezolizumab arm (P+A= 22 months, P=28 months). These findings raise concerns in both patients and physicians. A third large randomized trial, KEYNOTE-355, evaluated the benefits of the PD-1 inhibitor, pembrolizumab in 847 patients with previously untreated locally recurrent inoperable or metastatic TNBC. Patients received either a taxane (nab paclitaxel or paclitaxel) or gemcitabine and carboplatin weekly, with or without pembrolizumab, and stratified by PD-L1 expression (using the CPS scores: CPS≥1: 75% or CPS ≥10: 38%) and type of chemotherapy. Unlike in the IMpassion trials which uses an immune cell score (immune cells in tumor cells of >1%, PD-L1 Ventana SP-142), KEYNOTE-355 uses combined positive score (CPS). CPS scores are calculated by the number of PD-L1-positive cells (tumor cells, lymphocytes, and macrophages by IHC Dako 22c3), divided by the number of viable tumor cells multiplied by 100. Similarly, to the IMpassion trials, pembrolizumab added no benefits to the overall intention-to-treat (ITT) group (PFS 7.5 versus 5.6 months, p=NS). Pre-planned stratification suggested that benefits were limited to patients with high PD-L1 (CPS>10) only (PFS 9.5 versus 5.6 months, P=0.0012). Low expression of PD-L1 (CPS>1) was not sufficient to convey benefits to the addition of pembrolizumab.
KEYNOTE-355, IMpassion130 and IMpassion131 have common findings. Unlike for other diseases, testing of PD-L1 expression is relevant in TNBC and patients with tumors with absent or low PD-L1 expression should be spared the potential physical and financial tolls associated with immunotherapy outside of a clinical trial. Comparing the control arms of IMpassion130 and IMpassion131 reaffirms the findings from multiple studies, that nab-paclitaxel does not convey clear benefits over paclitaxel in TNBC. However, overall survival even in the non-immunotherapy control arms appears longer than previously reported in other trials22 (Table 1) which may be in part explained by the 30% of the patients who had de novo metastatic disease. Paclitaxel in combination with atezolizumab offered no benefit to those with PD-L1-positive TNBC. One could be inclined to implicate the required use of steroids when using paclitaxel and atezolizumab. A retrospective study in metastatic lung cancer has shown decreased efficacy in patients receiving corticosteroids during their treatment compared to patients who were not treated with corticosteroids at the time of immunotherapy initiation. For breast cancer, this remains confounded, as the benefits of pembrolizumab were upheld in the paclitaxel and gemcitabine/carboplatin arms in the KEYNOTE-355 metastatic trial,23 where steroids are commonly used, as well as in the early-stage breast cancer trial, KEYNOTE-522.24 These findings suggest that when using atezolizumab, nab-paclitaxel should be the taxane of choice and avoidance of steroids wherever possible seems prudent. Unlike in other diseases where immune checkpoint inhibitors may replace chemotherapy, immune checkpoint inhibitors alone or in combination with CTLA-4 antibodies have not been studied in larger cohorts. Smaller single-arm trials showed modest response to immune checkpoint inhibitors when administered alone.
Immune checkpoint inhibitors in early-stage breast cancer
Several large randomized trials are currently ongoing to study the impact on immune checkpoint inhibitors on pathological response rate in patients with early stage TNBC (Table 2). KEYNOTE-173, a phase Ib trial tested the efficacy of pembrolizumab in combination with standard of care neoadjuvant chemotherapy in 60 TNBC patients.25 Recruited patients were divided into six cohorts to receive six different regimens of pembrolizumab and paclitaxel, with or without carboplatin. A pathologic complete response (pCR) rate of 60% was observed across all cohorts. Higher PD-L1 expression and greater density of TILs in the tumor microenvironment correlated with higher pCR rate. The GeparNuevo trial, also conducted in the neoadjuvant setting, tested the addition of durvalumab to nab-paclitaxel.26 The trial recruited 174 patients with TNBC, stratified based on stromal TIL density. Adding durvalumab increased the pCR from 44% to 53%, but this increase was not statistically significant. As part of ISPY-2, pembrolizumab was added to standard of care neoadjuvant paclitaxel. Pembrolizumab increased the pCR rate significantly. In patients with TNBC, the pCR rate was increased from 20% to 60%, and in patients with HER2-positive cancer, the pCR rate changed from 16% to 46%.27 The largest reported clinical trial to date is KEYNOTE-522,24 where 1,174 patients with stage II or III TNBC received pembrolizumab in addition to carboplatin plus paclitaxel followed by doxorubicin or epirubicin plus cyclophosphamide in a 2:1 ratio. The study's pre-planned co-primary endpoints included both pCR and event-free survival. First endpoint results of this study which recruited many young women (median age 49, 56% premenopausal, 51% node-positive) have been reported. Unlike for patients with metastatic TNBC, significant benefits from pembrolizumab were observed in all groups of patients with early-stage breast cancer, irrespective of PD-L1 expression. However, it should be noted that more than 80% of the patients had PD-L1 positive disease, which is much higher than observed in patients with metastatic disease (~40-45%). The pCR rate was higher for pembrolizumab with chemotherapy then the chemotherapy alone arm (64.8% versus 51.2%, P=0.00055). Overall, patients with tumors that showed higher PD-L1 expression fared better with or without pembrolizumab compared to those with lower PD-L1 expression: the pCR rates for the PD-L1 CPS < 1 group (45.3% versus 30.3%), PD-L1 CPS ≥ 1 (68.9% versus 54.9%), CPS ≥ 10 (77.9% versus 59.8%), and CPS ≥ 20 (81.7% versus 62.5%). Patients with more advanced stage disease were noted to receive the greatest absolute benefit from the addition of pembrolizumab to standard of care chemotherapy. At the first planned interim analysis, the event free survival endpoint was not statistically different. While an increase in pCR has been associated with better outcomes, several studies have shown that an increased pCR rate induced by carboplatin or other agents did not necessarily translate to superior DFS and OS.
Table 2.
Immune checkpoint neo-adjuvant clinical trials in breast cancer.
| Trial ID | Regimen | Phase | Subtype | N | PathCR, % | References | ||
|---|---|---|---|---|---|---|---|---|
| GeparNuevo | nab-paclitaxel followed by EC: | II | TNBC | 174 | Loibl et al, 2019 | |||
| NCT02685059 | (-) durvalumab | 44 | ||||||
| (+) duvalumab | 53 | |||||||
| IMpassion031 | nab-paclitaxel and AC: | III | TNBC | 333 | Mittendorf et al, 2020 | |||
| (-) atezolizumab | 168 | 41.1 | ||||||
| PD-L1 - positive | 75 | 49.3 | ||||||
| PD-L1 - negative | 93 | 34.4 | ||||||
| (+) atezolizumab | 165 | 57.6 | ||||||
| PD-L1 - positive | 77 | 68.8 | ||||||
| PD-L1 - negative | 88 | 47.7 | ||||||
| ISPY2 | paclitaxel followed by AC: | II | TNBC | 29 | Nanda et al, 2020 | |||
| NCT01042379 | (-) pembrolizumab | 20 | ||||||
| (+) pembrolizumab | 60 | |||||||
| KEYNOTE-173 | pembrolizumab + taxane followed by AC: | I/II | TNBC | 60 | Schmid et al, 2020 | |||
| NCT02622074 | (-) carboplatin | 10 | 22 | |||||
| (+) carboplatin | 50 | 33 | ||||||
| KEYNOTE-522 | paclitaxel + carboplatin followed by EC: | III | TNBC | 602 | Schmid et al, 2020 | |||
| NCT03036488 | (-) pembrolizumab | 201 | 51.2 | |||||
| PD-L1 - positive | 164 | 54.9 | ||||||
| PD-L1 - negative | 33 | 30.3 | ||||||
| (+) pembrolizumab | 401 | 64.8 | ||||||
| PD-L1 - positive | 334 | 68.9 | ||||||
| PD-L1 - negative | 64 | 45.3 | ||||||
Abbreviation: AC, dexorubicin and cyclophosphamide; EC, epirubicin and cyclophosphamide; N, number; PathCR, pathologic complete response.
A somewhat smaller trial has recently reported the impact of atezolizumab on pCR in women with early stage TNBC. In IMpassion031, 165 of 333 patients received atezolizumab in addition to chemotherapy and 168 were randomized to placebo with chemotherapy.28 Similar to the findings with pembrolizumab in the KEYNOTE-522 study, atezolizumab plus chemotherapy was associated with a higher pCR rate: 58% versus 41%, P=0.0044. And again, patients with high PD-L1 expressing tumors showed a further increase in the pCR rate, in both the atezolizumab and the control arms: 69% versus 49%. The addition of immune therapy was generally well tolerated and did not add untoward toxicities outside what is already well established for immune checkpoint inhibitors. Thus, much emphasis has been placed on defining better prognostic factors and predictors of response and turning immune silent into immune responsive tumors.
Predictive markers of immune response
Tumor immune infiltrates
Many contributing immune modulators are currently being studied. The role of TILs density and its association are emerging as a prognostic factor and possibly as a predictor of response. Among the various breast cancer subtypes, a high influx of TILs is most prevalent in TNBC but is also observed in HER2-positive tumors29. Based on gene expression profiles, TNBC has been further classified into six subtypes including basal-like 1, basal-like 2, immunomodulatory, mesenchymal, mesenchymal stem-like and luminal androgen receptor subtype.30 Among these different subtypes of TNBC, the immunomodulatory subtype, which comprises about 20% of TNBC, has the highest density of TILs, highest expression of CTLA-4, PD-L1 and PD-1 and has been considered to be the most likely subtype to respond to immune checkpoint blockade.30 Studies suggest that the presence of stromal lymphocytes have a more robust correlation with PFS and OS than intra-tumoral lymphocytes.31, 32, 33 The composition of TILs is heterogeneous with up to 75% T cell infiltrates.34 While the presence of CD8+ T cells in TILs has been positively correlated with better treatment outcome35, other studies further suggest an important role for the ratio of CD8+ T cells to Regulatory T (Treg) cells in the TIL population.36 Many ongoing studies highlight the importance of other immune cells including CD4+ T cells, Treg cells and NK cells. While there is still much unknown about the composition of TILs and the exact role of individual contributors overall, the relevance of the immune infiltrate in TNBC cancer is unequivocally established.
In tumors containing more than 50% lymphocytes in their environment, patients are thought to have a better prognosis.37 Such tumors tend to respond well to neoadjuvant chemotherapy.38,39 TILs have been shown to predict better PFS in several early-stage breast cancer trials.40,41 Yet this association does not universally translate to better OS. This may be in part explained by the heterogeneity even in the immunomodulatory subtype of TNBC, which may require further stratification.42 Although TILs are emerging as robust prognostic and predictive markers of therapy response, they are not yet routinely tested and are not clinically accepted as outcome markers in patient care.
PD-L1 and PD-1 expression in the tumor immune environment
Further analysis of the two IMpassion trials has suggested that while immune infiltrates are associated with better prognosis, TILs per se may not independently predict response to immune checkpoint inhibitors in patients with metastatic tumors. Emens et al. 43 reported that PD-L1 positive immune infiltrates were associated with better PFS and OS in patients receiving atezolizumab, while TIL rich tumors without PD-L1 expression did not show benefit from atezolizumab. Similar findings were reported by Brockhoff et al. in patients with early stage TNBC.44 The group analyzed the relevance of PD-1/PD-L1 scores and TIL expression in 103 TNBC patients and suggested a better PFS and OS in tumors and immune infiltrate with high PD-1 expression.
Multiple large, randomized studies clearly suggest that high PD-L1 expression is a pre-requisite for response to immunotherapy in metastatic breast cancer, however the relevance of PD-L1 in early-stage breast cancer, as a predictive marker, is more complicated. As reported in the KEYNOTE-522 trial, CPS >1 PD-L1 expression was found in more than 80% of the patients enrolled in the study and while all patients appeared to benefit from the addition of pembrolizumab, PD-L1 expression conferred a better response to both the immune checkpoint inhibitor and chemotherapy alone arm suggesting a strong role for the immune environment in the therapy response in early stage TNBC.42
Several studies have evaluated the type of assay for PD-L1 staining and location of its expression on tumor cells versus surrounding immune infiltrates,45,46 as well as PD-1 expression in tumors.47 So far for breast cancer, the scoring of PD-L1 expression in tumors seems to bear a stronger correlation with response.41,48, 49, 50, 51 Much emphasis has been placed on the role of HRD mutations in breast cancer and their role in therapy response. In many centers, women are now routinely tested for BRCA mutations either by germline assays or in the tumors. Several studies have shown a higher pCR rate when adding carboplatin to the regimen suggesting that BRCA mutations may confer a better response to chemotherapy particularly to platinum-based therapies. Emerging data further suggests a possibility to replace chemotherapy with PARP inhibitors. Thus, many recent studies are focused on integrating PARP inhibitors with or instead of chemotherapy into immune checkpoint inhibitors. So far, the role of BRCA and other HRD mutations as a predictor of immune checkpoint inhibitors has not yet been established in clinical studies. In fact, a subset analysis of BRCA mutations from the IMpassion 131 trial concluded that even in HRD associated tumors, PD-L1 expression remains the sole predictor for immunotherapy response.43
Immune silence and immune exhaustion in ER+ tumors
Understanding the host and tumor microenvironment factors that determine an immune silent or immune-unresponsive tumor environment continues to be the focus of many preclinical and clinical investigations. Factors implicated in poor response to immune checkpoint inhibitors include absence of PD-L1 expression, low TIL number, TMB 52, 53, 54, 55, 56, 57, 58, 59, 60, 61, 62, 63, unfavorable host microbiome55,61, female gender and the presence of liver metastasis .64 Patients with ER+ tumors often meet many or all of these conditions. Furthermore, tumor antigens in ER+ tumors may not be sufficient to trigger an immune response. Additionally, continued tumor antigen exposure may prompt anergy and exhaustion of the adaptive immune response leading to low levels of partially exhausted CTL with poor response to single agent immune checkpoint inhibitors.65 High levels of forkhead box P3 (Foxp3) expressing T cells may further contribute to poor outcomes in breast cancer. Several preclinical studies suggest that epigenetic modulators may increase PD-L1 expression and the number of CD8+ T cells, while decreasing the number of regulatory T cells (Foxp3+ Tregs).66, 67, 68 The mechanistic rationale for combinations of epigenetic modulators to prime cells to immunotherapy and the exploitation of PARP inhibitors induced generation of neoantigens has led to multiple clinical trials combining immune checkpoint inhibitors with histone deacetylase (HDAC) inhibitors and PARP inhibitors.
Epigenetic immune priming
Several groups explored HDAC inhibitors to boost response to immune checkpoint inhibitors in both estrogen receptor-negative (ER−) and ER+ breast cancer. In preclinical models in lung and kidney cancer and other diseases, epigenetic priming by HDAC inhibition led to PD-L1 upregulation on tumor cells.68 In ER− cell lines, PD-L1 upregulation was associated with increased human leukocyte antigen (HLA)-DR tumor cell expression, CD4+ FoxP3+ CTLA-4high Treg down-regulation, increased T cell tumor infiltration, prompting clinical studies with HDACi to potentiate immune checkpoint inhibitor blockade in TNBC and other cancers. The outcomes of these trials are eagerly awaited. A trial performed by our group solidified the findings of other immunotherapy trials in ER+ breast cancers. A trial planned to enroll 87 patients was halted early for lack of efficacy in ER+ patients. We found that most tumors were devoid of PD-L1 expression and had relatively low TIL levels (<2%), yet nonetheless had activated Tregs. Treatment with an HDAC inhibitor led to depletion of Tregs (FoxP3+/CTLA4+ CD4+). The analysis of preplanned Tregs and exhausted T-cell signatures found that high dual expression of CTLA4+/PD-1+ in CD8+ T cells, was found to correlate with prolonged PFS. This signature of T cell exhaustion could also be detected in peripheral blood cells. These findings are now further explored in a randomized trial with preselection of patients with partially exhausted T cells.
PARP inhibitors and immune checkpoint inhibitors
PARP are a superfamily of enzymes involved in poly ADP-ribosylation (PARylation) and are therapeutically explored in tumors with deficiencies in DNA damage signaling. Currently four PARP inhibitors, olaparib, talazoparib, rucaparib and niraparib are approved in patients with somatic or germline BRCA mutations. These agents show clinical efficacy in breast, ovarian, pancreatic and prostate cancer with a BRCA mutation or homologous recombination repair (HRR) deficiencies. In breast cancer, PARP inhibitors are effective but the duration of response is relatively short.6,69 Hence there has been much emphasis placed on developing rational combination therapies with PARP inhibitors, including immune checkpoint inhibitors. BRCA mutations are thought to generate neoantigens by accumulation of mutations70,71 and recruitment of TILs to the tumor microenvironment.72 A trial combining niraparib with pembrolizumab conducted by our group showed promising activity in 62 patients with ovarian cancer and 5 patients with TNBC73 despite progression on prior platinum. Expanding on this study, the TOPACIO/Keynote162 trial evaluated the efficacy of pembrolizumab in combination with niraparib in TNBC patients and observed durable responses in 21% of the patients, irrespective of their BRCA1/2 or PD-L1 status, however in those with BRCA mutations, ORR was 47%.
There are several ongoing clinical trials comparing olaparib (NCT04191135) and other PARP inhibitors with immune checkpoint inhibitors.
PARP inhibitors have been integrated into neoadjuvant therapy with immune checkpoint inhibitors and chemotherapy. Durvalumab, olaparib and paclitaxel was tested in the ISPY2 trial and compared it to paclitaxel treatment alone. All patients received doxorubicin and cyclophosphamide afterwards. Patients having HER2 negative breast cancer were primarily recruited for the study. While increasing the pCR rate in all subtypes of breast cancer, those with TNBC achieved a 47% pCR rate compared to 27% in the paclitaxel control group. Many studies are currently underway to determine the role of PARP inhibitors and immune checkpoint inhibitors. In the absence of randomized trials with careful determination of platinum-resistance, it has yet to be determined what the optimal setting will be, and whether PARP inhibitors will be able to replace platinum-based therapies or have a role in platinum-therapy resistance.
Other targets of interest
Much research is further devoted to studying the roles of host factors such as the microbiome, obesity or other inflammation markers such as IL6, erthrocyte sedimentation rate and C reactive protein (Fig. 2). Like in other diseases, several research groups have initiated clinical trials to target tumor associated macrophages, NK cells and other components of the immune system either with antibodies including CD3 targeted bispecific, oncolytic viruses, vaccines or CAR-T. Among the most advanced targets are 41BB, BCMA, CD19, CD20, CD47, CSF1R, HER2, IDO, LAG3, MUC1, NY-ESO, STAT3, STING, WT1, HPV. TIGIT, TIM3. So far, no clear target has emerged as clearly promising in breast cancer.
Fig. 2.
Predictive and prognostic biomarkers for TNBC. Approved TNBC biomarkers include PD-L1 tumor positivity and microsatellite instability, which represent a relatively small percentage of TNBC tumors. As such, the search for predictive and/or prognostic biomarkers for the treatment of TNBC continues to be a robust area of research. These include a myriad number of clinical, tumor, microenvironment, and peripheral blood features.
Abbreviation: BRCAmt, BRCA mutation; cDC, classical dendritic cell; HER2, human epidermal growth factor 2; IFN, interferon; LDH, lactate dehyolrogenase; MDSC, myeloid derived suppressor cell; peCTLs, partially exhausted cytotoxic T lymphocytes; TAM, tumor associated macrophage; TCR, T cell receptor; TMB, tumor mutation burden; TME, tumor microenviroment; Tregs, regulatory T cells.
Adverse effects of immune checkpoint inhibitors
Immune related adverse effects may be directed against any organ and are mostly unpredictable. Albeit rare, severe and irreversible endocrine failure including the pituitary, thyroid and pancreas have been routinely reported with all immune checkpoint inhibitors. Similarly, mild to fatal organ toxicity such as hepatitis, pneumonitis, pancreatitis, colitis, skin rash, as well as the potential for cardiac and bone marrow failure and neurological toxicities should be discussed with every patient prior to initiating immunotherapy (reviewed in details by Martins et al.74 and FDA package insert of individual immune checkpoint inhibitors). Adverse effects of immune checkpoint inhibitors were not potentiated by chemotherapy or other combinations as randomized studies in both metastatic disease and early-stage breast cancer have shown, and it does not appear that the adverse effects in breast cancer are different than in other diseases. The management of toxicities includes interruptions or discontinuation of immune checkpoint inhibitors, aggressive supportive care and steroid use (oral to high dose intravenous) as first response. Other modalities such as tumor necrosis factor (TNF) alpha, IL6 and inosine-5'-monophosphate dehydrogenase (IMPDH) inhibitors have shown some promise but are not yet clearly established.
Adoptive cell transfer
Tumor associated antigens can be derived from genetically mutated or epigenetically modified proteins or from proteins that are over-expressed in tumor cells but are also expressed by normal cells of the body (Fig. 1B). Additionally, some antigens referred to as neoantigens or tumor specific antigens are restricted to tumor cells.71,75,76 There is a high correlation between the total mutational burden in tumor cells and the repertoire of neoantigens being expressed. Neoantigens can generate both CD4+ and CD8+ T cell responses. Emerging immune therapies that specifically target cancer cells such as adoptive cell transfer and cancer vaccines target tumor neoantigens.
Adoptive cell transfer is the process by which lymphocytes are isolated from the patient, cultured and expanded ex vivo and then reinfused in the lymphodepleted patient. Non-myeloablative lymphodepletion using chemotherapeutic drugs such as cyclophosphamide and fludarabine is frequently employed to further reduce exhausted T cells and Treg cells that contribute to an immunosuppressive environment. Ongoing studies suggest that lymphodepletion in combination with autologous hematopoietic stem cell rescue may lead to a more persistent response.77 One of the major strategies for adoptive cell transfer include TIL therapy. After surgical resection, tumor sections are cultured in vitro in the presence of high doses of IL2 to selectively grow and expand T cells. The tumor cells are analyzed by whole-exome and transcriptome sequencing and compared with normal cells to identify neoantigens specific to the patient.78 Immature dendric cells are either transfected with genes that would express the corresponding neoantigen or are pulsed with synthetic neoantigenic peptides. TILs, cocultured in the presence of dendritic cells, expressing all the identified neoantigens are further selected based on a positive T cell response such as secretion of IFN gamma. During reinfusion, the patients are also treated with IL2. Three ongoing clinical trials test the efficacy and safety of TIL therapy in combination with pembrolizumab (NCT01174121) or as monotherapy (NCT04111510 and NCT01462903) in patients with refractory metastatic breast cancer.
Another adoptive cell transfer strategy is the use of CAR-T cells, with the goal to recognize a specific neoantigen expressed on the surface of tumor cells in an major histocompatibility complex (MHC)-independent manner. Autologous T cells are harvested from the peripheral blood of the patient and are genetically modified to express a CAR against a tumor neoantigen. The cells are then expanded in vitro in the presence of cytokines and reinfused in the patient. CAR-T cells are modular and have an extracellular target recognition domain covalently joined through a transmembrane hinge domain to intracellular signaling domains, often with up to three intracellular domains, responsible for activation, co-stimulation and inhibition of T cells.79 To increase the efficacy of CAR-T cells, their design has been further modified to release proinflammatory cytokines at the target tissue. Such modified cells, also known as TRUCK T cells (T cells redirected for antigen unrestricted cytokine killing) can release cytokines in a constitutive or inducible manner in the target tissue.80 CAR-T cells have shown considerable promise and approval for the treatment of several malignancies. Broader applications and improvement in their design, feasibility and ease of manufacturing are under active investigation. However, their use in breast cancer has been challenged by the limited number of known breast tumor neoantigens that can be targeted and is sufficiently restricted. MUC1 is a transmembrane protein that is expressed several folds higher and is aberrantly glycosylated in breast cancer cells and is associated with an aggressive cancer phenotype.81 Ongoing preclinical studies and clinical trials are investigating the efficacy of anti-MUC1 CAR-T cells. In the ongoing phase I/II trial NCT02587689, the safety and adverse side effects of MUC1 directed CAR-T cells are being tested in patients with invasive TNBC with MUC1 positive tumors. In another ongoing phase I clinical trial NCT04020575, patients with MUC1 positive breast cancer are being treated with huMNC2-CAR44, a CAR-T cell specific for a cleaved form of MUC1. Patients will be divided into four cohorts – dose escalation will be tested in cohort 1 while dose expansion will be tested in cohorts 2-4. Patients with luminal subtype disease will be recruited in cohort 2, patients with HER2 positive disease and TNBC will be recruited in cohort 3 and 4, respectively. The ongoing phase I clinical trial NCT04025216 is a dose escalation study designed to identify the appropriate dosage for using CART-TnMUC1 in patients with TNBC. Patients will be grouped into three cohorts and will receive CART-TnMUC1 as a monotherapy, or in combination with cyclophosphamide or fludarabine. Epithelial cell adhesion molecule (EpCAM) is another transmembrane glycoprotein that is overexpressed in various cancers including breast.82 In the clinical trial NCT02915445, CAR-T cells recognizing EpCAM is being evaluated in patients with recurrent breast cancer with distant metastasis. Patients were lymphodepleted using cyclophosphamide before treatment with the CAR-T cells. HER2 directed CAR-T cell therapy has shown considerable promise in preclinical studies. In the ongoing phase I clinical trial NCT03740256, the safety and efficacy of HER2 specific CAR-T cells in combination with CAdVEC, an oncolytic adenovirus, is being tested in patients with HER2 positive breast cancer. Preclinical research suggests that there might be other promising targets for CAR-T therapy in patients with breast cancer such as folate receptor alpha, TEM8, ROR1, NK cell activating receptor ligands.83, 84, 85 While HER2 is a well-validated target for small molecular and monoclonal antibodies, the HER2 CAR-T approaches have been associated with excessive toxicity. Other targets of interest in breast cancer and in Phase II trials are CAR-T cells directed against CEA, mesothelin, CD133, CD70, CD44 variant domain 6 and multiple 4th generation CAR-T cells targeting HER2, GD2, and CD44v6. None of the CAR-T cell trials are currently in phase III testing for breast cancer.
Cancer vaccines
Therapeutic cancer vaccines are usually designed to generate an anti-tumor immune response to enhance current therapies or to induce immune surveillance to prevent recurrence. Cancer vaccines have been found to elicit both CD8+ and CD4+ T cell responses in the patient (Fig. 1C). Multiple preclinical and clinical studies have demonstrated that cancer vaccines can successfully induce immunological memory.86 The ideal target for an anti-cancer vaccine would be a restricted neoantigen or, in the absence of well-validated neoantigen target in breast cancer, a tumor associated antigen. Vaccine strategies are currently explored using autologous or allogeneic tumor cells, DNA, RNA, proteins or short antigenic peptide epitopes. To elucidate a robust anti-tumor immune response, autologous tumor cells are typically further combined with a strong adjuvant or cytokines or alternatively genetically modified in vitro to express granulocyte macrophage colony stimulating factor (GM-CSF) before being reinfused in the patient. Preclinical studies and early phase clinical trials have shown accumulation of dendritic cells, macrophages and eosinophils at the injection sites when autologous tumor cell vaccines are combined with GM-CSF and many labs use Bacillus Calmette-Guerin (BCG) as a substitute adjuvant to GM-CSF. Several autologous tumor cell and dendritic cell combinations have shown some promising activity in preclinical studies and in some early phase clinical trials87,88 leading to much interest in the field. Given the relevance of HER2 as a therapeutic target, a considerable focus has been placed on using HER2 as a target in vaccine strategies. Over 75 studies have evaluated patients with stage II or III breast cancer with low, intermediate and high HER2 expression.
One of the notable HER2 vaccine trials involved nelipepimut-S (NeuVax, E75,), a HLA A2/A3-restricted HER-2/neu (HER2) peptide, combined with GM-CSF with the promise that the vaccine will raise a T cell response to the HER2 receptor and reduce the risk of recurrence. Nelipepimut-S combined with GM-CSF was initially studied in 195 breast cancer patients with lymph node-positive and high-risk lymph node-negative patients with HER2 (immunohistochemistry 1-3+) expressing tumors in a Phase I/II trial. The vaccinated group showed a 24-month landmark analysis disease-free survival (DFS) of 94.3% versus 86.8% (P=0.08). It was noted that 65% of patients received suboptimal vaccine doses. It should be noted that this trial did not include trastuzumab.89
The encouraging Phase II results prompted a multi-center, randomized, double-blind phase III trial with 758 patients with similar characteristics (NCT01479244).90 Though nelipepimut-S (NeuVax) was found to be well-tolerated, the phase III trial showed no significant improvement in DFS between the two groups. Estimated 3-year DFS rates were 77.1% in the vaccine group versus 77.5% in the placebo group and the trial was stopped for futility.
NeuVax has been further studied in a phase II trial randomizing 275 early-stage breast cancer patients with low and intermediate HER2 expression (1+/2+) receiving GM-CSF with or without NeuVax, however in this trial the patients all received trastuzumab.91 Early reports from this trial suggest a clinically meaningful difference in median DFS in favor of the NeuVax plus trastuzumab arm with hazard ratios (HRs) of 0.67 and a 34.9% reduction in the relative risk of recurrence without evidence of additional cardiotoxicity. The final results await peer review.
In an alternative way to target HER2, a randomized phase II trial (NCT00524277) enrolled 456 node-positive, as well as high risk node negative, patients to receive HER2 derived peptides, such as GP2 and AE37. HLA-A2 positive patients received the HER2/Neu Peptide GP2 and GM-CSF vaccine versus GM-CSF alone, whereas the HLA-A2 negative patients received the modified HER2/Neu Peptide AE37 + GM-CSF vaccine versus GM-CSF alone. In the primary analysis, there was no benefit to the 5-year overall DFS between the groups.92 Subset analysis suggested that patients with HER2 low-expressing tumors and advanced stage may benefit from the E37 vaccine (P=0.039; HR=0.375; 95%CI: 0.142, 0.988). The GP2 vaccine arm showed no differences in DFS, with the exception of the HER2 positive patients, with a trend toward improved DFS (P=0.052). Further studies may be needed to obtain approval for this strategy.92 Other vaccines involve oncolytic viruses such as Newcastle disease virus (NDV), influenza virus and avian influenza virus with the goal to prompt anti-tumor immune response through multiple signaling mechanisms including stimulation of cytotoxic T cells and delayed type hypersensitivity responses, production of cytokines and interferons, thereby contributing to an inflammatory tumor microenvironment.
In a clinical trial designed by Ahlert et al., 63 patients with primary breast cancer and 27 patients with pretreated metastatic breast cancer were treated with NDV infected autologous tumor cell vaccine.93 Patients who received 1.5 × 106 tumor cells with 33% viability showed a benefit in OS (P=0.026) and a trend towards DFS improvement (P=0.089). However, these findings have not been further supported in larger trials.
Several clinical trials including NCT02348320 have begun to evaluate personalized polyepitope DNA vaccines. Although some initial trials present potential, more studies are needed to establish the use of DNA vaccines as monotherapy or in combination, while many strategies offer promise, to date there are no approved vaccine strategies for breast cancer. Taken together, despite much focus and interest, cancer vaccines in breast cancer have not reached integration into standard of care treatment.
Conclusion and future directions
In addition to the therapeutic strategies discussed above, other novel therapies including Toll-like receptor (TLR) agonists, CD40 agonist, CD47 inhibitors are also being actively explored in preclinical studies and early phase clinical trials. These three therapies focus more on targeting the innate immune response. However, the innate and the adaptive immune systems work in close coordination and not independently, thus integration of such strategies with checkpoint inhibitors are likely needed. Immune checkpoint inhibitors so far have shown the most promise in the clinic, yet often require combination with other therapies, particularly in breast cancer. The approval of immune checkpoint inhibitors in TNBC is a major advance, yet the limited benefits to patients with high PD-L1 expressing tumors points to the strong need of further research and development in this field. In order to improve immune strategies in breast cancer, therapeutic approaches will need to consider the important role played by the tumor microenvironment in facilitating the immune responses as well as several defined and emerging confounding host factors.
Declaration of Competing Interest
The authors declare that they have no conflict of interests.
References
- 1.Siegel R.L., Miller K.D., Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70:7–30. doi: 10.3322/caac.21590. [DOI] [PubMed] [Google Scholar]
- 2.Perou C.M., Sørlie, T., Eisen M.B., et al. Molecular portraits of human breast tumours. Nature. 2000;406:747–752. doi: 10.1038/35021093. [DOI] [PubMed] [Google Scholar]
- 3.Cancer Genome Atlas Network Comprehensive molecular portraits of human breast tumours. Nature. 2012;490:61–70. doi: 10.1038/nature11412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Markham A. Alpelisib: first global approval. Drugs. 2019;79:1249–1253. doi: 10.1007/s40265-019-01161-6. [DOI] [PubMed] [Google Scholar]
- 5.Litton J.K., Hurvitz S.A., Mina L.A., et al. Talazoparib versus chemotherapy in patients with germline BRCA1/2-mutated HER2-negative advanced breast cancer: final overall survival results from the EMBRACA trial. Ann Oncol. 2020;31:1526–1535. doi: 10.1016/j.annonc.2020.08.2098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Robson M.E., Tung N., Conte P., et al. OlympiAD final overall survival and tolerability results: Olaparib versus chemotherapy treatment of physician’s choice in patients with a germline BRCA mutation and HER2-negative metastatic breast cancer. Ann Oncol. 2019;30:558–566. doi: 10.1093/annonc/mdz012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carlson B. Oncotype DX test offers guidance for women debating chemotherapy. Biotechnol Healthc. 2006;3:12–14. [PMC free article] [PubMed] [Google Scholar]
- 8.Parker J.S., Mullins M., Cheang M.C., et al. Supervised risk predictor of breast cancer based on intrinsic subtypes. J Clin Oncol. 2009;27:1160–1167. doi: 10.1200/jco.2008.18.1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wallden B., Storhoff J., Nielsen T., et al. Development and verification of the PAM50-based Prosigna breast cancer gene signature assay. BMC Med Genomics. 2015;8:54. doi: 10.1186/s12920-015-0129-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lemery S., Keegan P., Pazdur R. First FDA approval agnostic of cancer site - when a biomarker defines the indication. N Engl J Med. 2017;377:1409–1412. doi: 10.1056/NEJMp1709968. [DOI] [PubMed] [Google Scholar]
- 11.Zhao P., Li L., Jiang X., et al. Mismatch repair deficiency/microsatellite instability-high as a predictor for anti-PD-1/PD-L1 immunotherapy efficacy. J Hematol Oncol. 2019;12:54. doi: 10.1186/s13045-019-0738-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rugo H.S., Delord J.P., Im S.A., et al. Safety and antitumor activity of pembrolizumab in patients with estrogen receptor-positive/human epidermal growth factor receptor 2-negative advanced breast cancer. Clin Cancer Res. 2018;24:2804–2811. doi: 10.1158/1078-0432.Ccr-17-3452. [DOI] [PubMed] [Google Scholar]
- 13.Nanda R., Chow L.Q., Dees E.C., et al. Pembrolizumab in patients with advanced triple-negative breast cancer: phase Ib KEYNOTE-012 study. J Clin Oncol. 2016;34:2460–2467. doi: 10.1200/jco.2015.64.8931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Adams S., Schmid P., Rugo H.S., et al. Pembrolizumab monotherapy for previously treated metastatic triple-negative breast cancer: cohort A of the phase II KEYNOTE-086 study. Ann Oncol. 2019;30:397–404. doi: 10.1093/annonc/mdy517. [DOI] [PubMed] [Google Scholar]
- 15.Cortés J., Lipatov O., Im S.A., et al. LBA21 - KEYNOTE-119: Phase III study of pembrolizumab (pembro) versus single-agent chemotherapy (chemo) for metastatic triple negative breast cancer (mTNBC) Ann Oncol. 2019;30:v859–v860. doi: 10.1093/annonc/mdz394.010. [DOI] [Google Scholar]
- 16.Voorwerk L., Slagter M., Horlings H.M., et al. Immune induction strategies in metastatic triple-negative breast cancer to enhance the sensitivity to PD-1 blockade: the TONIC trial. Nat Med. 2019;25:920–928. doi: 10.1038/s41591-019-0432-4. [DOI] [PubMed] [Google Scholar]
- 17.Dirix L.Y., Takacs I., Jerusalem G., et al. Avelumab, an anti-PD-L1 antibody, in patients with locally advanced or metastatic breast cancer: a phase 1b JAVELIN Solid Tumor study. Breast Cancer Res Treat. 2018;167:671–686. doi: 10.1007/s10549-017-4537-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schmid P., Rugo H.S., Adams S., et al. Atezolizumab plus nab-paclitaxel as first-line treatment for unresectable, locally advanced or metastatic triple-negative breast cancer (IMpassion130): updated efficacy results from a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2020;21:44–59. doi: 10.1016/s1470-2045(19)30689-8. [DOI] [PubMed] [Google Scholar]
- 19.Schmid P., Adams S., Rugo H.S., et al. Atezolizumab and nab-paclitaxel in advanced triple-negative breast cancer. N Engl J Med. 2018;379:2108–2121. doi: 10.1056/NEJMoa1809615. [DOI] [PubMed] [Google Scholar]
- 20.L. Emens, Paper presented at the ESMO virtual congress 2020, virtual, Sep 2020.
- 21.Miles D., Andre J.F., Gligorov S., et al. 317TiP - IMpassion131: Phase III study comparing 1L atezolizumab with paclitaxel vs placebo with paclitaxel in treatment-naive patients with inoperable locally advanced or metastatic triple negative breast cancer (mTNBC) Ann Oncol. 2017;28:v105. doi: 10.1093/annonc/mdx365.080. [DOI] [Google Scholar]
- 22.Dent R.A., Mainwaring P.N., Tan T.J.Y., et al. Survival in triple-negative breast cancer (TNBC): Evidence from the SEER database 2010-2011. J Clin Oncol. 2015;33:e12075. doi: 10.1200/jco.2015.33.15_suppl.e12075. e12075-e12075. [DOI] [Google Scholar]
- 23.Cortes J., Cescon D.W., Rugo H.S., et al. KEYNOTE-355: Randomized, double-blind, phase III study of pembrolizumab + chemotherapy versus placebo + chemotherapy for previously untreated locally recurrent inoperable or metastatic triple-negative breast cancer. J Clin Oncol. 2020;38:1000. doi: 10.1200/JCO.2020.38.15_suppl.1000. 1000-1000. [DOI] [PubMed] [Google Scholar]
- 24.Schmid P., Cortes J., Pusztai L., et al. Pembrolizumab for early triple-negative breast cancer. N Engl J Med. 2020;382:810–821. doi: 10.1056/NEJMoa1910549. [DOI] [PubMed] [Google Scholar]
- 25.Schmid P., Salgado R., Park Y.H., et al. Pembrolizumab plus chemotherapy as neoadjuvant treatment of high-risk, early-stage triple-negative breast cancer: results from the phase 1b open-label, multicohort KEYNOTE-173 study. Ann Oncol. 2020;31:569–581. doi: 10.1016/j.annonc.2020.01.072. [DOI] [PubMed] [Google Scholar]
- 26.Loibl S., Untch M., Burchardi N., et al. A randomised phase II study investigating durvalumab in addition to an anthracycline taxane-based neoadjuvant therapy in early triple-negative breast cancer: clinical results and biomarker analysis of GeparNuevo study. Ann Oncol. 2019;30:1279–1288. doi: 10.1093/annonc/mdz158. [DOI] [PubMed] [Google Scholar]
- 27.Nanda R., Liu M.C., Yau C., et al. Effect of pembrolizumab plus neoadjuvant chemotherapy on pathologic complete response in women with early-stage breast cancer: an analysis of the ongoing phase 2 adaptively randomized I-SPY2 trial. JAMA Oncol. 2020;6:676–684. doi: 10.1001/jamaoncol.2019.6650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mittendorf E.A., Zhang H., Barrios C.H., et al. Neoadjuvant atezolizumab in combination with sequential nab-paclitaxel and anthracycline-based chemotherapy versus placebo and chemotherapy in patients with early-stage triple-negative breast cancer (IMpassion031): a randomised, double-blind, phase 3 trial. Lancet. 2020;396:1090–1100. doi: 10.1016/s0140-6736(20)31953-x. [DOI] [PubMed] [Google Scholar]
- 29.Cimino-Mathews A., Thompson E., Taube J.M., et al. PD-L1 (B7-H1) expression and the immune tumor microenvironment in primary and metastatic breast carcinomas. Hum Pathol. 2016;47:52–63. doi: 10.1016/j.humpath.2015.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lehmann B.D., Jovanović B., Chen X., et al. Refinement of triple-negative breast cancer molecular subtypes: implications for neoadjuvant chemotherapy selection. PLoS One. 2016;11:e0157368. doi: 10.1371/journal.pone.0157368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mahmoud S.M., Paish E.C., Powe D.G., et al. Tumor-infiltrating CD8+ lymphocytes predict clinical outcome in breast cancer. J Clin Oncol. 2011;29:1949–1955. doi: 10.1200/jco.2010.30.5037. [DOI] [PubMed] [Google Scholar]
- 32.Mora A.Della, Bastianelli L., Pistelli M., et al. C23 - Stromal peritumoral and intratumoral infiltrating lymphocytes: how immunity influences prognosis in triple negative breast cancer. Ann Oncol. 2017;28:vi31–vi32. doi: 10.1093/annonc/mdx424.022. [DOI] [Google Scholar]
- 33.Khoury T., Nagrale V., Opyrchal M., et al. Prognostic significance of stromal versus intratumoral infiltrating lymphocytes in different subtypes of breast cancer treated with cytotoxic neoadjuvant chemotherapy. Appl Immunohistochem Mol Morphol. 2018;26:523–532. doi: 10.1097/pai.0000000000000466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zgura A., Galesa L., Bratila E., et al. Maedica (Bucur) 2018;13:317–320. doi: 10.26574/maedica.2018.13.4.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu S., Lachapelle J., Leung S., et al. CD8+ lymphocyte infiltration is an independent favorable prognostic indicator in basal-like breast cancer. Breast Cancer Res. 2012;14:R48. doi: 10.1186/bcr3148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Waldman A.D., Fritz J.M., Lenardo M.J. A guide to cancer immunotherapy: from T cell basic science to clinical practice. Nat Rev Immunol. 2020;20:651–668. doi: 10.1038/s41577-020-0306-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.V. M. T. D. Jong, paper presented at the ESMO Virtual Congress 2020, Virtual, Sep 2020.
- 38.Garcia-Martinez E., Gil G.L., Benito A.C., et al. Tumor-infiltrating immune cell profiles and their change after neoadjuvant chemotherapy predict response and prognosis of breast cancer. Breast Cancer Res. 2014;16:488. doi: 10.1186/s13058-014-0488-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stanton S.E., Adams S., Disis M.L. Variation in the incidence and magnitude of tumor-infiltrating lymphocytes in breast cancer subtypes: a systematic review. JAMA Oncol. 2016;2:1354–1360. doi: 10.1001/jamaoncol.2016.1061. [DOI] [PubMed] [Google Scholar]
- 40.Denkert C., von Minckwitz G., Darb-Esfahani S., et al. Tumour-infiltrating lymphocytes and prognosis in different subtypes of breast cancer: a pooled analysis of 3771 patients treated with neoadjuvant therapy. Lancet Oncol. 2018;19:40–50. doi: 10.1016/s1470-2045(17)30904-x. [DOI] [PubMed] [Google Scholar]
- 41.Adams S., Gray R.J., Demaria S., et al. Prognostic value of tumor-infiltrating lymphocytes in triple-negative breast cancers from two phase III randomized adjuvant breast cancer trials: ECOG 2197 and ECOG 1199. J Clin Oncol. 2014;32:2959–2966. doi: 10.1200/jco.2013.55.0491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang D.Y., Jiang Z., Ben-David Y., et al. Molecular stratification within triple-negative breast cancer subtypes. Sci Rep. 2019;9:19107. doi: 10.1038/s41598-019-55710-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.L. Emens, paper presented at the San Antonio Breast Cancer Symposium, San Antonio, Texas, 2018.
- 44.Brockhoff G., Seitz S., Weber F., et al. The presence of PD-1 positive tumor infiltrating lymphocytes in triple negative breast cancers is associated with a favorable outcome of disease. Oncotarget. 2018;9:6201–6212. doi: 10.18632/oncotarget.23717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Guo H., Ding Q., Gong Y., et al. Comparison of three scoring methods using the FDA-approved 22C3 immunohistochemistry assay to evaluate PD-L1 expression in breast cancer and their association with clinicopathologic factors. Breast Cancer Res. 2020;22:69. doi: 10.1186/s13058-020-01303-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Davis A.A., Patel V.G. The role of PD-L1 expression as a predictive biomarker: an analysis of all US food and drug administration (FDA) approvals of immune checkpoint inhibitors. J Immunother Cancer. 2019;7:278. doi: 10.1186/s40425-019-0768-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Khunger M., Hernandez A.V., Pasupuleti V., et al. Programmed cell death 1 (PD-1) ligand (PD-L1) expression in solid tumors as a predictive biomarker of benefit from PD-1/PD-L1 axis inhibitors: a systematic review and meta-analysis. JCO Precision Oncol. 2017;1:1–15. doi: 10.1200/po.16.00030. [DOI] [PubMed] [Google Scholar]
- 48.Ibrahim E.M., Al-Foheidi M.E., Al-Mansour M.M., et al. The prognostic value of tumor-infiltrating lymphocytes in triple-negative breast cancer: a meta-analysis. Breast Cancer Res Treat. 2014;148:467–476. doi: 10.1007/s10549-014-3185-2. [DOI] [PubMed] [Google Scholar]
- 49.Salgado R., Denkert C., Campbell C., et al. Tumor-infiltrating lymphocytes and associations with pathological complete response and event-free survival in HER2-positive early-stage breast cancer treated with lapatinib and trastuzumab: a secondary analysis of the NeoALTTO trial. JAMA Oncol. 2015;1:448–454. doi: 10.1001/jamaoncol.2015.0830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ignatiadis M., Van den Eynden G., Roberto S., et al. Tumor-infiltrating lymphocytes in patients receiving trastuzumab/pertuzumab-based chemotherapy: a TRYPHAENA substudy. J Natl Cancer Inst. 2019;111:69–77. doi: 10.1093/jnci/djy076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tsao M.S., Kerr K.M., Kockx M., et al. PD-L1 immunohistochemistry comparability study in real-life clinical samples: results of blueprint phase 2 project. J Thorac Oncol. 2018;13:1302–1311. doi: 10.1016/j.jtho.2018.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rizvi N.A., Hellmann M.D., Snyder A., et al. Cancer immunology. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science. 2015;348:124–128. doi: 10.1126/science.aaa1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.McGranahan N., Furness A.J., Rosenthal R., et al. Clonal neoantigens elicit T cell immunoreactivity and sensitivity to immune checkpoint blockade. Science. 2016;351:1463–1469. doi: 10.1126/science.aaf1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Snyder A., Makarov V., Merghoub T., et al. Genetic basis for clinical response to CTLA-4 blockade in melanoma. N Engl J Med. 2014;371:2189–2199. doi: 10.1056/NEJMoa1406498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Vetizou M., Pitt J.M., Daillère R., et al. Anticancer immunotherapy by CTLA-4 blockade relies on the gut microbiota. Science. 2015;350:1079–1084. doi: 10.1126/science.aad1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Blank C.U., Haanen J.B., Ribas A., et al. CANCER Immunology. The “cancer immunogram”. Science. 2016;352:658–660. doi: 10.1126/science.aaf2834. [DOI] [PubMed] [Google Scholar]
- 57.Chen D.S., Mellman I. Elements of cancer immunity and the cancer-immune set point. Nature. 2017;541:321–330. doi: 10.1038/nature21349. [DOI] [PubMed] [Google Scholar]
- 58.Pitt J.M., Vétizou M., Daillère R., et al. Resistance mechanisms to immune-checkpoint blockade in cancer: tumor-intrinsic and -extrinsic factors. Immunity. 2016;44:1255–1269. doi: 10.1016/j.immuni.2016.06.001. [DOI] [PubMed] [Google Scholar]
- 59.Bellmunt J., de Wit R., Vaughn D.J., et al. Pembrolizumab as second-line therapy for advanced urothelial carcinoma. N Engl J Med. 2017;376:1015–1026. doi: 10.1056/NEJMoa1613683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Herbst R.S., Soria J.C., Kowanetz M., et al. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature. 2014;515:563–567. doi: 10.1038/nature14011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sivan A., Corrales L., Hubert N., et al. Commensal bifidobacterium promotes antitumor immunity and facilitates anti-PD-L1 efficacy. Science. 2015;350:1084–1089. doi: 10.1126/science.aac4255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ribas A., Hamid O., Daud A., et al. Association of pembrolizumab with tumor response and survival among patients with advanced melanoma. JAMA. 2016;315:1600–1609. doi: 10.1001/jama.2016.4059. [DOI] [PubMed] [Google Scholar]
- 63.Goldinger S.M., Tsai K.K., Tumeh P., et al. Correlation between metastatic site and response to anti-Programmed Death-1 (PD-1) agents in melanoma. J Clin Oncol. 2016;34:9549. doi: 10.1200/JCO.2016.34.15_suppl.9549. 9549-9549. [DOI] [Google Scholar]
- 64.Tumeh P.C., Hellmann M.D., Hamid O., et al. Liver metastasis and treatment outcome with Anti-PD-1 monoclonal antibody in patients with melanoma and NSCLC. Cancer Immunol Res. 2017;5:417–424. doi: 10.1158/2326-6066.Cir-16-0325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Loo K., Tsai K.K., Mahuron K., et al. Partially exhausted tumor-infiltrating lymphocytes predict response to combination immunotherapy. JCI Insight. 2017;2 doi: 10.1172/jci.insight.93433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Terranova-Barberio M., Thomas S., Munster P.N. Epigenetic modifiers in immunotherapy: a focus on checkpoint inhibitors. Immunotherapy. 2016;8:705–719. doi: 10.2217/imt-2016-0014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Shen L., Ciesielski M., Ramakrishnan S., et al. Class I histone deacetylase inhibitor entinostat suppresses regulatory T cells and enhances immunotherapies in renal and prostate cancer models. PLoS One. 2012;7:e30815. doi: 10.1371/journal.pone.0030815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Terranova-Barberio M., Thomas S., Ali N., et al. HDAC inhibition potentiates immunotherapy in triple negative breast cancer. Oncotarget. 2017;8:114156–114172. doi: 10.18632/oncotarget.23169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Litton J.K., Hurvitz S.A., Mina L.A., et al. Talazoparib versus chemotherapy in patients with germline BRCA1/2-mutated HER2-negative advanced breast cancer: final overall survival results from the EMBRACA trial. Ann Oncol. 2020;31:1526–1535. doi: 10.1016/j.annonc.2020.08.2098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Segal N.H., Parsons D.W., Peggs, K.S., et al. Epitope landscape in breast and colorectal cancer. Cancer Res. 2008;68:889–892. doi: 10.1158/0008-5472.Can-07-3095. [DOI] [PubMed] [Google Scholar]
- 71.Schumacher T.N., Schreiber R.D. Neoantigens in cancer immunotherapy. Science. 2015;348:69–74. doi: 10.1126/science.aaa4971. [DOI] [PubMed] [Google Scholar]
- 72.Wang Z., Sun K., Xiao Y., et al. Niraparib activates interferon signaling and potentiates anti-PD-1 antibody efficacy in tumor models. Sci Rep. 2019;9:1853. doi: 10.1038/s41598-019-38534-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Konstantinopoulos P.A., Waggoner S., Vidal G.A., et al. Single-arm phases 1 and 2 trial of niraparib in combination with pembrolizumab in patients with recurrent platinum-resistant ovarian carcinoma. JAMA Oncol. 2019;5:1141–1149. doi: 10.1001/jamaoncol.2019.1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Martins F., Sofiya L., Sykiotis G.P., et al. Adverse effects of immune-checkpoint inhibitors: epidemiology, management and surveillance. Nat Rev Clin Oncol. 2019;16:563–580. doi: 10.1038/s41571-019-0218-0. [DOI] [PubMed] [Google Scholar]
- 75.Jiang T., Shi T., Zhang H., et al. Tumor neoantigens: from basic research to clinical applications. J Hematol Oncol. 2019;12:93. doi: 10.1186/s13045-019-0787-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kawakami Y., Rosenberg S.A. Human tumor antigens recognized by T-cells. Immunol Res. 1997;16:313–339. doi: 10.1007/bf02786397. [DOI] [PubMed] [Google Scholar]
- 77.Muranski P., Boni A., Wrzesinski C., et al. Increased intensity lymphodepletion and adoptive immunotherapy–how far can we go? Nat Clin Pract Oncol. 2006;3:668–681. doi: 10.1038/ncponc0666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Deniger D.C., Pasetto A., Robbins P.F., et al. T-cell responses to tp53 “hotspot” mutations and unique neoantigens expressed by human ovarian cancers. Clin Cancer Res. 2018;24:5562–5573. doi: 10.1158/1078-0432.Ccr-18-0573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Roselli E., Frieling J.S., Thorner K., et al. CAR-T engineering: optimizing signal transduction and effector mechanisms. BioDrugs. 2019;33:647–659. doi: 10.1007/s40259-019-00384-z. [DOI] [PubMed] [Google Scholar]
- 80.Chmielewski M., Abken H. TRUCKs: the fourth generation of CARs. Expert Opin Biol Ther. 2015;15:1145–1154. doi: 10.1517/14712598.2015.1046430. [DOI] [PubMed] [Google Scholar]
- 81.Rakha E.A., Boyce R.W., Abd El-Rehim D., et al. Expression of mucins (MUC1, MUC2, MUC3, MUC4, MUC5AC and MUC6) and their prognostic significance in human breast cancer. Mod Pathol. 2005;18:1295–1304. doi: 10.1038/modpathol.3800445. [DOI] [PubMed] [Google Scholar]
- 82.Sadeghi S., Hojati Z., Tabatabaeian H. Cooverexpression of EpCAM and c-myc genes in malignant breast tumours. J Genet. 2017;96:109–118. doi: 10.1007/s12041-017-0748-0. [DOI] [PubMed] [Google Scholar]
- 83.Wallstabe L., Göttlich C., Nelke L.C., et al. ROR1-CAR T cells are effective against lung and breast cancer in advanced microphysiologic 3D tumor models. JCI Insight. 2019;4 doi: 10.1172/jci.insight.126345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Byrd T.T., Fousek K., Pignata A., et al. TEM8/ANTXR1-Specific CAR T Cells as a Targeted Therapy for Triple-Negative Breast Cancer. Cancer Res. 2018;78:489–500. doi: 10.1158/0008-5472.Can-16-1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Han Y., Xie W., Song D.G., et al. Control of triple-negative breast cancer using ex vivo self-enriched, costimulated NKG2D CAR T cells. J Hematol Oncol. 2018;11:92. doi: 10.1186/s13045-018-0635-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Emens L.A. Cancer vaccines: on the threshold of success. Expert Opin Emerg Drugs. 2008;13:295–308. doi: 10.1517/14728214.13.2.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lin M., Liang S., Jiang F., et al. 2003-2013, a valuable study: autologous tumor lysate-pulsed dendritic cell immunotherapy with cytokine-induced killer cells improves survival in stage IV breast cancer. Immunol Lett. 2017;183:37–43. doi: 10.1016/j.imlet.2017.01.014. [DOI] [PubMed] [Google Scholar]
- 88.Tomasicchio M., Semple L., Esmail A., et al. An autologous dendritic cell vaccine polarizes a Th-1 response which is tumoricidal to patient-derived breast cancer cells. Cancer Immunol Immunother. 2019;68:71–83. doi: 10.1007/s00262-018-2238-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Mittendorf E.A., Clifton G.T., Holmes J.P., et al. Clinical trial results of the HER-2/neu (E75) vaccine to prevent breast cancer recurrence in high-risk patients: from us military cancer institute clinical trials group study I-01 and I-02. Cancer. 2012;118:2594–2602. doi: 10.1002/cncr.26574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Mittendorf E.A., Lu B., Melisko M., et al. Efficacy and safety analysis of nelipepimut-s vaccine to prevent breast cancer recurrence: a randomized, multicenter, phase III clinical trial. Clin Cancer Res. 2019;25:4248–4254. doi: 10.1158/1078-0432.Ccr-18-2867. [DOI] [PubMed] [Google Scholar]
- 91.Clifton G.T., Hale D., Vreeland T.J., et al. Results of a randomized phase IIb trial of nelipepimut-S + trastuzumab versus trastuzumab to prevent recurrences in patients with high-risk HER2 low-expressing breast cancer. Clin Cancer Res. 2020;26:2515–2523. doi: 10.1158/1078-0432.Ccr-19-2741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Brown T.A., Mittendorf E.A., Hale D.F. Prospective, randomized, single-blinded, multi-center phase II trial of two HER2 peptide vaccines, GP2 and AE37, in breast cancer patients to prevent recurrence. Breast Cancer Res Treat. 2020;181:391–401. doi: 10.1007/s10549-020-05638-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ahlert T., Sauerbrei W., Bastert G., et al. Tumor-cell number and viability as quality and efficacy parameters of autologous virus-modified cancer vaccines in patients with breast or ovarian cancer. J Clin Oncol. 1997;15:1354–1366. doi: 10.1200/jco.1997.15.4.1354. [DOI] [PubMed] [Google Scholar]