Abstract
Adenoid cystic carcinoma (ACC) is an uncommon tumor that usually appears in the major salivary glands of the head and neck region, including the minor glands in the oral cavity, sinonasal tract, and other sites. ACC of the head and neck may have a low-grade histological appearance. This malignant tumor has unusual clinical characteristics such as occasional regional lymph node metastases and a prolonged yet continuously advancing clinical course. Additionally, it is an invasive tumor with perineural invasion, difficult-to-clear margins, metastasis, and localized recurrence. The cribriform and tubular proliferation of basaloid cells, which mostly display a myoepithelial cellular phenotype, are ACC's distinct histologic characteristics. The degree of genetic alterations and aneuploidy observed in tumor genomes are linked to the severity of histologic grade, which correlates with clinical prognosis. The three predominant cell types (PCTs) i.e., conventional ACC (C-ACC), myoepithelial-predominant ACC (M-ACC), and epithelial-predominant ACC (E-ACC)—and their respective applications will be reviewed. The function of extracellular matrix (ECM) components such as laminin, type IV collagen, fibronectin, and tenascin are also emphasized. An attempt has been made to explore the recent molecular diversity, regulatory pathways prevalent in PCT, ECM with its genetic changes, and translational utility with targeted therapies for ACC.
Keywords: Adenoid cystic carcinoma, Extracellular matrix, Myoepithelial, Laminin
Introduction
As a rare tumor form, Adenoid cystic carcinoma (ACC) accounts for only less than 1% of all malignant head and neck tumors and less than 10% of all salivary gland neoplasms with a predilection for major salivary glands.1 More than one-third of cases occur in minor glands in the oral cavity, sinonasal tract, or rarely other sites.2 It is characterized by perineural invasion and slow growth dynamics compared with other carcinomas and tends to have a low incidence of spread to local and regional lymph nodes.3 Nonetheless, local and distant recurrences are quite common after resection of the primary tumor. This high recurrence rate likely reflects the known tendency for perineural invasion with occult extension beyond surgical margins and a tendency for hematogenous dissemination at the early stages of tumor development.4 The most common sites for metastatic disease are the lungs followed by bone and liver. Late relapses (>5 years postoperatively) are well documented as are reports of rapid tumor progression after an extended period of indolent disease.5 The clinical outcome in ACC is correlated to histologic grade, which is correlated to the degree of aneuploidy and genetic alterations present in the tumor genomes. Histogenesis of ACC is uncertain; the disease may originate from stem cells that have multidirectional differentiation. It is composed of epithelial (luminal) and myoepithelial (abluminal) cells arranged in heterogeneous morphological growth patterns, including tubular, cribriform, and solid structures. The extracellular matrix (ECM) comprises a heterogeneous and versatile network of macromolecules synthesized and secreted by embryonic cells. In the ACC sequence, ECM is formed by a variety of components including collagen, elastin, fibronectin, laminins, glycosaminoglycans, proteoglycans, and other glycoproteins, mainly secreted by epithelial cells, myoepithelial cells, cancer-associated fibroblasts, immune cells, and endothelial cells.6 The significance of ECM was initially proposed by Shintani et al, in 1997 that the basement membrane, a component of the ECM, acts as a barrier that is compromised during cell invasion. They proposed that tenascin, a protein found in the ECM, could play a role in facilitating the detachment of cancer cells, thereby enhancing the invasive capabilities of ACC.7 Recently, Arnolt et al, in 2020 investigated the expression of 28 ECM components in ACC, mucoepidermoid carcinoma, and salivary duct carcinoma and gave an insight into profiling of the ECM composition specific to SGC.8 Currently, a 3-tier grading scheme based on the percentage of solid components is widely used for ACC. Grade 1 is tubular and cribriform growth with no solid component; Grade 2 is cribriform or <30% solid component; and Grade 3 is ≥ 30% solid component.9 Studies have shown that the solid structure ACC type has a worse prognosis compared with that of the other histological subtypes.10 Interestingly, according to the histological trend of different SGCs, the ECM was also found to play an equal role in high-grade transformation. ACC development requires the participation of cellular components and the tumor microenvironment (TME) for its progression. Therefore, ECM plays a crucial role in the TME by providing a supportive environment for tumor cell survival. Specific ECM elements have been shown to promote infiltration of cancer cells and metastases. While recent transcriptomic research has identified ECM signatures that predict patient survival in various cancer types, the ECM makeup of SGC remains largely unexplored. Understanding the typical histomorphological and genetic characteristics of ACC is essential for profiling the ECM specific to this cancer type. Knowledge of both epithelial and ECM components will be vital for effectively targeting the TME in the future.11 This review provides a comprehensive summary of the current understanding regarding the association of predominant cell types (PCT) and ECM with histopathology, immunohistochemistry, ECM characteristics, recent molecular diversity, regulatory pathways including genetic alterations, and the translational potential with targeted therapies for ACC. According to predominant cell type it will be divided into myoepithelial-predominant ACC (M-ACC), epithelial-predominant ACC (E-ACC), and conventional ACC (C-ACC) to determine the prognosis in each type.10 The function of ECM components such as laminin, type IV collagen, fibronectin, and tenascin are also emphasized.
M-ACC
Myoepithelial cells reside between luminal epithelial cells and the stromal compartment, allowing them to interact with all other cell types in the microenvironment. The solid component of ACC is usually formed predominantly by the proliferation of the epithelium (Fig. 1A). Nonetheless, as shown in the literature, excessive proliferation of the myoepithelium has also been observed to form solid structures in regular practice12 which can be identified by the presence of antigens such as CK14, p63, S100 protein, α-smooth muscle antigen, p53 and CD10.13 Myoepithelial cells potentially serve a role in curbing the aggressive biological tendencies of solid ACCs. The reason behind this phenomenon is that these cells can function as tumor suppressors by enhancing the proliferation of ECM rather than breaking it down. This is attributed to the copious secretion of ECM by myoepithelial cells and their comparatively low expression levels of matrix-degrading enzymes like urokinase and matrix metalloproteinases (MMP).14 To comprehend these tumors and explore the potential involvement of normal myoepithelial host cells in cancer, researchers generated immortal cell lines and transplantable xenografts from diverse human myoepithelial tumors in the salivary gland and breast. The cell lines maintain a normal myoepithelial phenotype, while the xenografts consistently accumulate a substantial extracellular matrix.15 Additional in-depth analyses encompassing ultrastructural, immunocytochemical, molecular, and biochemical aspects, elucidate that myoepithelial cells exhibit a propensity for secreting minimal amounts of matrix-degrading proteinases. Conversely, they demonstrate elevated levels of maspin and other anti-invasive proteinase inhibitors which accumulate within the myoepithelial matrix. In normal breast ducts and ductal carcinoma in situ, myoepithelial cells selectively express maspin and specific proteinase inhibitors. While myoepithelial proliferated neoplasms in the breast and salivary glands may display various grades and behaviors, their tendency towards a benign or low-grade nature is attributed to the mentioned inherent characteristics. This supports the hypothesis that host myoepithelial cells play a crucial role in regulating the transition from in situ to invasive carcinoma, acting as a significant defense against cancer invasion. Moreover, myoepithelial cells showcase the ability to induce epithelial morphogenesis, particularly spheroid formation i. e rossete like arrangement of cell in spherical arrangement which effectively impede tumor-cell invasion in vitro.15,16 This may offer insight into the improved prognosis observed in M-ACC. The molecular network encompasses the prominently up-regulated gene DLX4, DLX6, SOX11, OTX1 (orthodenticle homeobox 1 transcription factor) as well as KRT16. Also, abnormalities in p53 coupled with HER-2/neu overexpression or the loss of pRb expression play a significant role in the dedifferentiation process in low-grade ACC which is expressed in the cribriform and tubular areas of ACC.17 SOX11 inhibitor has been demonstrated to yield improved prognoses in breast carcinomas, clinical trials could be explored for its potential application in ACC as well (Fig. 2).18 Identifying the PCT component becomes crucial for targeted interventions, offering insights into overall survival rates and facilitating tailored treatment approaches.
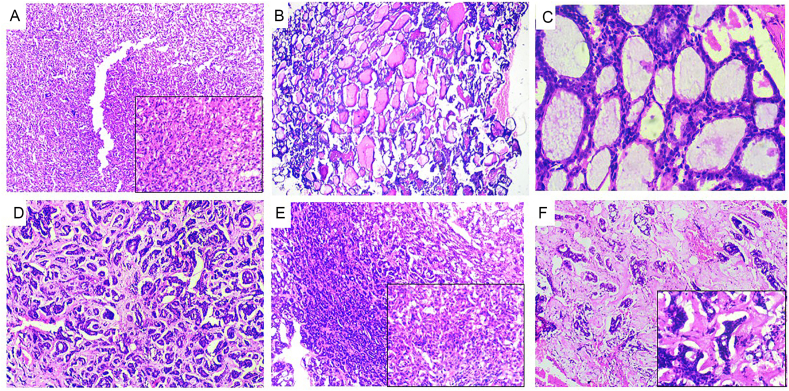
Fig. 1.
Figure (A) 10X depicting predominance of myoepithelial cells residing between luminal epithelial cells and the stromal compartment. Inset (40X) showing angulated, deeply basophilic, eccentrically placed nuclei of myoepithelial cell (B) under 4X depicts cribriform structures of low cuboidal epithelial cells with eosinophilic coagulum in center (C) 40X depicts epithelial low cuboidal, hypechromatic, polyhedral epithelial cells with eosinophilic coagulum in center. (D) Multiple areas of small tubular structures lined by angulated hyperchromatic myoepithelial cells with extracellular matrix (E) Showing predominance of epithelial cells with reduction or absence of myoepithelial cells with a transition of solid compartment to hyalinized stroma is seen. (F) Depicts abundantly hyalinized extracellular matrices with few solid tubular structures.
Fig. 2.
Flowchart outlining the predominant cell type of ACC along with its associated immunohistochemical features, molecular signaling pathways, and targeted therapeutic interventions.
E-ACC
E-ACC is characterized by either solid or cribriform (Fig. 1B, C) and tubular structures, (Fig. 1D) showing reduction or absence of myoepithelial cells with predominance of epithelial cells. (Fig. 1E) This can be identified by positive expression for cytokeratins AE1/3, CK34βE12, CK5/6, CK7, CK14, and CK18, indicating the presence of these cytokeratins in ACC of oral cavity.19 Conversely, CK18 and CK20 consistently showed negative results while CK7+/CK20- pattern aligns with ACC typically found in major salivary glands.19 Compared to M-ACC and C-ACC, E-ACC is associated with a poorer prognosis, possibly attributed to the role of specific genes (DLX1, keratin 16, MYB, SOX11, EN1, C-KIT, COX-2, BCL-2 and STATH) in development and differentiation. These genes contribute to cancer cell adaptation to their environment, enhancing survival through increased response to cell-to-cell defense mechanisms and resistance to therapy.14 The homeobox gene family has pluripotent stem cell-defining genes like homeotic (Hox) genes, which is assumed to confer cancer stem cell characteristics to ACC.14,20 In E-ACC, up-regulation of homeobox DLX family members, specifically DLX6 and DLX5, was observed, mirroring an experimental pattern seen in breast carcinoma cell lines which can be attributed to poor prognosis.21 Human-ACC 2A (HACC-2A) cell line demonstrated abundant expression of epithelial markers such as EGFR, Pan-cytokeratin, and E-cadherin, consistent with their epithelial phenotype. The conclusion drawn was that heightened expression contributes to increased tumorigenicity.22 Bcl-2 is a downstream target of MYB, which was highly expressed in HACC-2A cell line and it is typically overexpressed in ACC with fusion of MYB-NFIB gene, but not in other salivary gland tumors.23 The fusion of the MYB oncogene with the transcription factor NFIB, resulting from the translocation t (6:9) (q22-23; p23-24), increases the expression of the protein MYB, which is involved in the growth of tumors and is linked to a more aggressive clinical course. It is a crucial and early oncogenic event in classical ACC and high grade transformation.24,25 Hence, gene fusions play a role in the initial stages of tumorigenesis, serving as diagnostic and prognostic markers for various diseases. Potential mechanisms and treatment targets for ACC include the genes NOTCH1,26 VEGF, FGFR1,27 MMP,28 EN1, DLX6, and OTX1.14 NOTCH1 mutations are indicative of a poor-prognosis of disease, marked by a solid histological phenotype and have a high propensity for liver and bone spread.26 The enzyme γ-secretase is responsible for NOTCH cleavage and is targeted by gamma-secretase inhibitors. Ferrarotto et al. conducted a phase II trial using AL101, a selective gamma-secretase inhibitor, in patients with advanced ACC carrying activating NOTCH mutations, achieving a Disease Control Rate of 68.7%, despite notable gastrointestinal toxicity and asthenia.26 Additionally, brontictuzumab, a monoclonal antibody targeting NOTCH, showed promising results in a phase I trial with a Disease Control Rate of 25% in patients with various refractory solid tumors overexpressing NOTCH.29 In approximately 80% of ACC cases, VEGFR is overexpressed, leading to numerous clinical trials investigating anti-angiogenic drugs. Dovitinib, axitinib, sunitinib, regorafenib, sorafenib, nintedanib, and lenvatinib primarily target VEGFR and PDGFR and have undergone phase II trials in patients with advanced chemo-refractory ACC, and progression-free survival durations ranged from 4.3 to 17.5 months (Fig. 2).30
C-ACC
C-ACC manifests with differing proportions of three distinct growth patterns known as cribriform, tubular, and solid, without specific cellular preferences. The conventional histologic subtype is characterized by a solid component exceeding 30%, the presence of tumor necrosis, and a Ki-67 index of ≥30%.10 The cribriform subtype, the most common, features islands of basaloid cells surrounded by cyst-like spaces of variable sizes, creating a pattern resembling “Swiss cheese.”4 The tubular pattern displays a comparable cytologic appearance, with tumor cells arranged in nests surrounded by varying amounts of eosinophilic, often hyalinized stroma. In contrast, the solid histologic subtype is characterized by aggregates of basaloid cells, lacking tubules or pseudocystic formations, and predominantly composed of epithelial or myoepithelial cells, as previously described.31 Immunohistochemistry is frequently essential to definitively distinguish it from M-ACC and E-ACC. As such, the balanced presence of these cells determines the morphology of C-ACC. Biomarkers, such as c-KIT, vascular endothelial growth factor receptor (VEGFR),27 Ki-67, and p53, have been linked to biologic aggressiveness and poor prognosis.4,32 As per Xuan et al.'s study, a comparison of three PCTs revealed that C-ACC exhibits a more favorable prognosis compared to the other two. E-ACC was associated with more aggressive pathologic characteristics, including a higher frequency of high-grade transformation and a predominant solid component. The rate of lymph node metastasis for C-ACC is low, estimated to be approximately 10%.33 As per recent literature, the overexpression of MYB can occur through various mechanisms, including the relocation of NFIB enhancer elements to positions upstream or downstream of MYB. The primary genomic alterations include a t(6;9) chromosomal translocation or, less frequently, a t(8;9) translocation. These events lead to fusions involving the oncogenes MYB or MYBL1 and the transcription factor gene NFIB.34 Activations of MYB/MYBL1 rearrangements, whether through gene fusion or other mechanisms, are detected in more than 80% of ACCs and represent potential therapeutic targets.24,35 Also the co-occurrence of MYC and MYB overexpression correlates with a more advanced clinical stage and shorter disease-free survival.36 In C-ACC, the loss of heterozygosity at either the p53 or RB gene was observed more frequently in the solid components compared to the cribriform and tubular areas within the same tumor mass. This observation has led to speculation about a potential progression from the cribriform and tubular types of ACC to the solid type.17 Additionally, reports have indicated gains in the 4q12 region due to the overexpression of C-KIT, a gene encoding a Type III tyrosine kinase receptor. C-KIT has emerged as a primary target in clinical trials, with a relevant group of tyrosine kinase inhibitors being studied. A phase II study investigating the combination of imatinib and cisplatin showed that 11% of evaluated patients achieved partial response after induction chemotherapy. These findings suggest that tyrosine kinase inhibitors alone may not be adequate for patients with ACC.37
In conclusion, our review and AJCC staging results specifically highlight a significant difference between E-ACC and C-ACC, suggesting that E-ACC tends to be at a more advanced disease stage. Collectively, these outcomes may elucidate the comparatively poorer prognosis for E-ACC patients compared to M-ACC and C-ACC.38
ECM in ACC progression
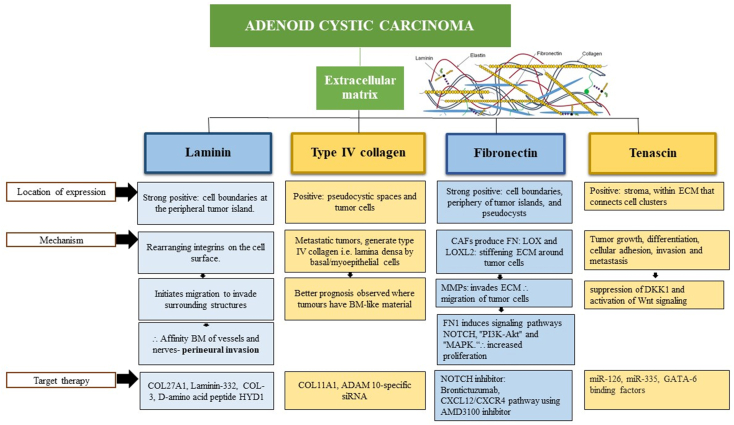
The relationships between ECM characteristics and prognosis can be independent or merely correlational to the PCT as per our hypothesis. In the ECM, basement membranes (BM) act as a significant impediment to the dissemination of malignant tumor cells (Fig. 1F). The ECM has heightened chemotactic response which promotes invasion and growth and gives cells the tendency to invade nerves and endothelial cells.30 Cheng et al. identified ECM within the pseudocystic space and surrounding tumor nests in ACC, demonstrating that cells produce specific ECM to aid infiltration through tissues abundant in BM.39 Certain mutations act as “driver mutations,” guiding carcinogenesis, suggesting targeted treatment possibilities. Systemic therapies traditionally involve polychemotherapy, but recent translational research supports targeted therapy, especially in ECM cell in ACC. Several studies have put forth the notion that purified laminin, type IV collagen, fibronectin, and others, are produced to facilitate invasion32 and recent targeted therapies are emphasized. A detailed discussion on this topic will follow.
Laminin
Laminin, a glycoprotein present in the stroma of ACC serves a key structural role by functioning as a primary component of the basal lamina and significantly influences the interaction between epithelial and endothelial cells. Its diverse biological activity encompasses the regulation of growth, differentiation, morphology, and migration in both normal and neoplastic cells.40 Shintani et al. observed a consistent strong positive expression of laminin and type IV collagen along cell boundaries at the periphery of tumor islands in all cases. Additionally, the immunostaining was evident along the lining of pseudocysts.7 Upon activation in tumorigenesis, these proteins facilitate cell proliferation, differentiation, and metastasis.41 As per recent reports in 2023, certain instances of ACC exhibited increased expression in the BM, indicating that cells utilize laminin for traversal and migration to invade surrounding structures.42 This process involves rearranging integrins on the cell surface, providing an explanation for the affinity of tumors like ACC and polymorphous adenocarcinoma for the BM of vessels and nerves.43 Recent genomic pathway revealed, upregulation of collagen 27A1 (COL27A1) specifically in ACC, with notably lower expression levels detected in other SGC's. The elevated expression of COL27A1 RNA and protein in ACC, suggests the potential for COL27A1 to serve as a promising target for further exploration.8 Additionally, Laminin-332, a large multidomain molecule of laminin family is involved in cell adhesion and matrix assembly also plays a crucial role in the development of various cancers, making it a promising target for cancer therapeutics. Alterations in the expression of laminin-332 have been reported at the transcriptional, translational, and posttranslational levels in carcinogenesis.44 Potential inhibitors of laminin-332, e.g. COL-3, a chemically modified tetracycline known to inhibit the expression of the laminin γ2 chain gene in melanoma are identified.45 Furthermore, specific peptides can disrupt signal transduction induced by laminin-332 and inhibit laminin-332-induced cell migration. The synthetic d-amino acid peptide HYD1 (KIKMVISWKG) demonstrates reversible inhibition of cytoskeleton-dependent tumor cell migration on laminin-332,46, while peptides containing the tripeptide motif KLP, which is homologous to laminin-332, have been demonstrated to inhibit the growth of peritoneal tumors (Fig. 3).47 This suggests potential utility in ACC and warrants further investigation.
Fig. 3.
Flowchart outlining immunohistochemical features, molecular signaling pathways, and targeted therapeutic interventions of extracellular matrix comprising laminin, type IV collagen, fibronectin, and tenascin.
Type IV collagen
The collagen subfamily is made up of 28 different forms of collagen that range in both form and function. During carcinogenesis, collagen undergoes modifications to influence the TME, promoting malignant cellular proliferation.48 Histologically, the cribriform type is identified by numerous cystic structures formed by relatively small polygonal and spindle-shaped cells. These structures, known as pseudocysts, contain extracellular matrices within their lumina. Antibody staining to type IV collagen reveals positive results in the pseudocystic spaces. It is noteworthy that the diverse biochemical elements of BM can be identified in neoplastic cells, and past research has associated such upregulation of ADAM 10 and COL11A1 gene expression with aberrant tumor behavior.49 Proteomic studies of breast cancers revealed that in less metastatic tumors, only stromal cells produce laminin and collagen IV; whereas, in highly metastatic tumors, both stromal cells and tumor cells generate these BM proteins.50 Ultrastructural studies showed the pseudocystic spaces of ACCs was lined by an uninterrupted BM consisting of lamina lucida and lamina densa which were characterized by their extraordinary thicknesses proving that this lamina densa was produced by the tumor cells with basal/myoepithelial characteristics.51 The passive behavior of ACCs may also reflect the tumor's degree of differentiation, which attempts to mimic normal tissue and exude BM-like material.52 This may explain the better prognosis observed where the tumours have BM-like material. In the realm of targeted therapy, recent findings highlight the crucial involvement of ADAM 10 in cell migration, tumor progression, and metastasis through the proteolytic shedding of cell surface proteins. Studies have unveiled that ADAM 10 has ability to cleave collagen type IV in the basement membrane, underscoring its relevance in tumor metastasis.53 Notably, the downregulation of ADAM 10 using ADAM 10-specific siRNA led to a significant growth inhibition of ACC cells. This discovery positions ADAM 10 as a promising and novel therapeutic target for treating ACC. According to recent reports in 2022 by Arolt et al., their findings denote a novel mechanism of extracellular matrix production in carcinomas, holding potential significance for the future. Their research elucidates the expression pattern of COL11A1 across different carcinoma types, offering insights that may aid in the classification of tumors for potential COL11A1-related therapies in the future (Fig. 3).8
Fibronectin (FN)
FN consist of two subunits that are covalently linked through disulfide bonds at their C-termini.54 Cell adhesion plays a crucial role in maintaining tissue integrity and represents a fundamental mechanism underlying the invasion and metastasis of cancer. Immunohistochemical analysis provided evidence of strong positive staining for FN and laminin along cell boundaries, both within and at the periphery of tumor islands and pseudocysts, at the margins of epithelial cell cords, and extensively between tumor cells.55 It is involved in the stages of morphogenesis, repair, and remodeling.56 The FN matrix contributes positively to the advancement of tumors and undergoes significant activation in the vicinity of the vascular network surrounding the tumor.57 Cancer-associated fibroblasts (CAFs) generate FN, and subsequently, these cells arrange them in ECM. CAFs facilitate the invasion of ACC by establishing an invasive path through a mechanism distinct from the secretion of soluble factors. Additionally, the involvement of FN in early angiogenesis was stated, as it contributes to the formation of neovasculature that supplies nutrients to the growing neoplasm.58 CAFs produce lysyl oxidase (LOX) and LOX-like protein 2 (LOXL2), which facilitate the cross-linking of fibrillar type I collagen. This process leads to the stiffening of the matrix, generating mechanotactic cues that actively support the invasion of neoplastic cells.59 They facilitate invasion through the induction of epithelial–mesenchymal transition (EMT) and contribute to invasion by either inducing the expression of MMPs in cancer cells or expressing MMPs themselves.60 FN causes activation of ECM-tumor cell adhesion, which is due to significant upregulation of signaling pathways such as NOTCH, PI3K-Akt and MAPK36 signaling pathway which stimulates growth, invasion, and cell proliferation, through the modulation of MMPs and the initiation of EMT.36 According Zhang et al., in 2023 stated the inhibition of MMPs restrained CAF and ACC invasion, highlighting the importance of ECM degradation in cancer invasion. Moreover, CAFs expressed CXCL12, and interference with the CXCL12/CXCR4 pathway using AMD3100 inhibited ACC invasion facilitated by CAFs.33 Furthermore, the application of brontictuzumab, a monoclonal antibody inhibiting the NOTCH1 pathway, has shown efficacy in ACC patients. A phase I study revealed its effectiveness in numerous cancer patients, among them those with ACC displaying partial responses and three cases of sustained stable disease were observed in ACC individuals with evidence of NOTCH1 pathway activation (Fig. 3).26
Tenascin (TN)
TN plays an important role in embryonic development, wound healing, and tumor formation.61 TN is typically found in the stroma, particularly with connective tissue that connects cell clusters. According to the staining pattern, it is implicated in the induction and progression of SGC.62 In vitro results reported by Shrestha et al. suggest that the enhanced expression of TN in neoplastic lesions may have potential implications in tumor growth, differentiation, cellular adhesion, invasion and metastasis. This effect was achieved through a mechanism that entailed the downregulation of Dickkopf-1 (DKK1) and the upregulation of Wnt signaling.63 This would partly explain its prominence in the undifferentiated tumors that we found, as in solid ACC. Contrary to that, TN staining was not present in more differentiated tumors, as tubular ACC. Amongst the targeted therapies, miR-126 and miR-335 as well as GATA-6 binding factors and corticoids downregulate mRNA levels of tenascin-C. In light of these results blocking inflammation by corticoids could be useful as anti-cancer treatment to block tenascin-C expression. A fully humanized antibody F16 that specifically recognizes the domain A1 of tenascin-C (generated by alternative splicing) has been developed by the company Philogen as a cytokine/chemokine combination therapy (Fig. 3).64
Therefore, ECM constituents according to previous reviews suggest that LN and type IV collagen were always present around morphologically well-differentiated duct-like structures in all tumors studied. BM interruption could not be seen in the malignant tumors, on the contrary BM production was evident, which is probably related to invasion. FN was present in the stroma of all tumors, but TN was mostly observed in less differentiated and higher degree of malignancy tumors, being more intense in the solid subtype of ACC.
Conclusion
The prevalent clinical classification of SGC relies significantly on the histologic features of the primary lesion, serving as a key reference for most pathologists. This review provides prognostic information and facilitates the identification of distinct subtypes of ACC characterized by dominant myoepithelial, epithelial cell, and stroma types. While molecular diagnosis is increasingly adopted in the classification of salivary gland tumors, a precise molecular classification based on the PCT, ECM, and associated targeted therapies is expected to be an adjunct. In the foreseeable future, this strategy shows potential for enhancing prognostic predictions, with the potential to impact therapeutic responses.
Disclosure of competing interest
The authors have none to declare.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.mjafi.2024.05.012.
Appendix A. Supplementary data
The following is the supplementary data to this article:
References
- 1.Ei-Naggar A.K. International Agency; 2017. WHO Classification of Head and Neck Tumours. [Google Scholar]
- 2.Khan A.J., DiGiovanna M.P., Ross D.A., et al. Adenoid cystic carcinoma: a retrospective clinical review. Intl Journal of Cancer. 2001 Jun 20;96(3):149–158. doi: 10.1002/ijc.1013. [DOI] [PubMed] [Google Scholar]
- 3.Li N., Xu L., Zhao H., El-Naggar A.K., Sturgis E.M. A comparison of the demographics, clinical features, and survival of patients with adenoid cystic carcinoma of major and minor salivary glands versus less common sites within the Surveillance, Epidemiology, and End Results registry. Cancer. 2012 Aug 15;118(16):3945–3953. doi: 10.1002/cncr.26740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Azumi N., Battifora H. The cellular composition of adenoid cystic carcinoma. An immunohistochemical study. Cancer. 1987 Oct 1;60(7):1589–1598. doi: 10.1002/1097-0142(19871001)60:7<1589::aid-cncr2820600729>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 5.Spiro R.H., Huvos A.G., Strong E.W. Adenoid cystic carcinoma of salivary origin. A clinicopathologic study of 242 cases. Am J Surg. 1974 Oct;128(4):512–520. doi: 10.1016/0002-9610(74)90265-7. [DOI] [PubMed] [Google Scholar]
- 6.Mariano F.V., Noronha A.L.F., Gondak R.O., et al. Carcinoma ex pleomorphic adenoma in a Brazilian population: clinico-pathological analysis of 38 cases. Int J Oral Maxillofac Surg. 2013 Jun;42(6):685–692. doi: 10.1016/j.ijom.2013.02.012. [DOI] [PubMed] [Google Scholar]
- 7.Shintani S., Alcalde R.E., Matsumura T., Terakado N. Extracellular matrices expression in invasion area of adenoid cystic carcinoma of salivary glands. Cancer Lett. 1997 Jun;116(1):9–14. doi: 10.1016/s0304-3835(97)04730-7. [DOI] [PubMed] [Google Scholar]
- 8.Arolt C., Meyer M., Hoffmann F., et al. Expression profiling of extracellular matrix genes reveals global and entity-specific characteristics in adenoid cystic, mucoepidermoid and salivary duct carcinomas. Cancers. 2020 Aug 31;12(9):2466. doi: 10.3390/cancers12092466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Szanto P.A., Luna M.A., Tortoledo M.E., White R.A. Histologic grading of adenoid cystic carcinoma of the salivary glands. Cancer. 1984 Sep 15;54(6):1062–1069. doi: 10.1002/1097-0142(19840915)54:6<1062::aid-cncr2820540622>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 10.Xuan L., Yuan J., Zhang H., Zhang Y., Liu H. Dominant cell type analysis predicts head and neck adenoid cystic carcinoma outcomes. Ann Diagn Pathol. 2022 Feb;56 doi: 10.1016/j.anndiagpath.2021.151867. [DOI] [PubMed] [Google Scholar]
- 11.Gerarduzzi C., Hartmann U., Leask A., Drobetsky E. The matrix revolution: matricellular proteins and restructuring of the cancer microenvironment. Cancer Res. 2020 Jul 1;80(13):2705–2717. doi: 10.1158/0008-5472.CAN-18-2098. [DOI] [PubMed] [Google Scholar]
- 12.Bell D., Roberts D., Kies M., Rao P., Weber R.S., El-Naggar A.K. Cell type-dependent biomarker expression in adenoid cystic carcinoma: biologic and therapeutic implications. Cancer. 2010 Dec 15;116(24):5749–5756. doi: 10.1002/cncr.25541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Terada T. Ductal adenoma of the breast: immunohistochemistry of two cases. Pathol Int. 2008 Dec;58(12):801–805. doi: 10.1111/j.1440-1827.2008.02315.x. [DOI] [PubMed] [Google Scholar]
- 14.Bell D., Bell A.H., Bondaruk J., Hanna E.Y., Weber R.S. In-depth characterization of the salivary adenoid cystic carcinoma transcriptome with emphasis on dominant cell type. Cancer. 2016 May 15;122(10):1513–1522. doi: 10.1002/cncr.29959. [DOI] [PubMed] [Google Scholar]
- 15.Sternlicht M.D., Barsky S.H. The myoepithelial defense: a host defense against cancer. Med Hypotheses. 1997 Jan;48(1):37–46. doi: 10.1016/s0306-9877(97)90022-0. [DOI] [PubMed] [Google Scholar]
- 16.Bani D., Riva A., Bigazzi M., Bani Sacchi T. Differentiation of breast cancer cells in vitro is promoted by the concurrent influence of myoepithelial cells and relaxin. Br J Cancer. 1994 Nov;70(5):900–904. doi: 10.1038/bjc.1994.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nagao T., Sugano I., Ishida Y., et al. Hybrid carcinomas of the salivary glands: report of nine cases with a clinicopathologic, immunohistochemical, and p53 gene alteration analysis. Mod Pathol. 2002 Jul;15(7):724–733. doi: 10.1097/01.MP.0000018977.18942.FD. [DOI] [PubMed] [Google Scholar]
- 18.Shepherd J.H., Uray I.P., Mazumdar A., et al. The SOX11 transcription factor is a critical regulator of basal-like breast cancer growth, invasion, and basal-like gene expression. Oncotarget. 2016 Mar 15;7(11):13106–13121. doi: 10.18632/oncotarget.7437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Terada T. Adenoid cystic carcinoma of the oral cavity: immunohistochemical study of four cases. Int J Clin Exp Pathol. 2013;6(5):932–938. [PMC free article] [PubMed] [Google Scholar]
- 20.Gao J., Ma K., Zhang L., Li T., Zhao B., Jiang Y. Paired related homeobox 1 attenuates autophagy via acetyl-CoA carboxylase 1-regulated fatty acid metabolism in salivary adenoid cystic carcinoma. FEBS Open Bio. 2022 May;12(5):1006–1016. doi: 10.1002/2211-5463.13367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Morini M., Astigiano S., Gitton Y., et al. Mutually exclusive expression of DLX2 and DLX5/6 is associated with the metastatic potential of the human breast cancer cell line MDA-MB-231. BMC Cancer. 2010 Dec;10(1):649. doi: 10.1186/1471-2407-10-649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Warner K.A., Oklejas A.E., Pearson A.T., et al. UM-HACC-2A: MYB-NFIB fusion-positive human adenoid cystic carcinoma cell line. Oral Oncol. 2018 Dec;87:21–28. doi: 10.1016/j.oraloncology.2018.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Persson M., Andrén Y., Mark J., Horlings H.M., Persson F., Stenman G. Recurrent fusion of MYB and NFIB transcription factor genes in carcinomas of the breast and head and neck. Proc Natl Acad Sci U S A. 2009 Nov 3;106(44):18740–18744. doi: 10.1073/pnas.0909114106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brill L.B., Kanner W.A., Fehr A., et al. Analysis of MYB expression and MYB-NFIB gene fusions in adenoid cystic carcinoma and other salivary neoplasms. Mod Pathol. 2011 Sep;24(9):1169–1176. doi: 10.1038/modpathol.2011.86. [DOI] [PubMed] [Google Scholar]
- 25.Mitani Y., Rao P.H., Futreal P.A., et al. Novel chromosomal rearrangements and break points at the t(6;9) in salivary adenoid cystic carcinoma: association with MYB-NFIB chimeric fusion, MYB expression, and clinical outcome. Clin Cancer Res. 2011 Nov 15;17(22):7003–7014. doi: 10.1158/1078-0432.CCR-11-1870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ferrarotto R., Mitani Y., Diao L., et al. Activating NOTCH1 mutations define a distinct subgroup of patients with adenoid cystic carcinoma who have poor prognosis, propensity to bone and liver metastasis, and potential responsiveness to Notch1 inhibitors. J Clin Orthod. 2017 Jan 20;35(3):352–360. doi: 10.1200/JCO.2016.67.5264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Andreasen S., Kiss K., Melchior L.C., Laco J. The ETV6-RET gene fusion is found in ETV6-rearranged low-grade sinonasal adenocarcinoma without NTRK3 involvement. Am J Surg Pathol. 2018 Jul;42(7):985–988. doi: 10.1097/PAS.0000000000001069. [DOI] [PubMed] [Google Scholar]
- 28.Hämetoja H., Mäkitie A., Bäck L., et al. Matrix metalloproteinase-7, -8, -9, -15, and -25 in minor salivary gland adenoid cystic carcinoma. Pathol Res Pract. 2021 Jan;217 doi: 10.1016/j.prp.2020.153293. [DOI] [PubMed] [Google Scholar]
- 29.Ferrarotto R., Eckhardt G., Patnaik A., et al. A phase I dose-escalation and dose-expansion study of brontictuzumab in subjects with selected solid tumors. Ann Oncol. 2018 Jul;29(7):1561–1568. doi: 10.1093/annonc/mdy171. [DOI] [PubMed] [Google Scholar]
- 30.Tchekmedyian V., Sherman E.J., Dunn L., et al. Phase II study of lenvatinib in patients with progressive, recurrent or metastatic adenoid cystic carcinoma. J Clin Orthod. 2019 Jun 20;37(18):1529–1537. doi: 10.1200/JCO.18.01859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Andreadis D., Epivatianos A., Poulopoulos A., et al. Detection of C-KIT (CD117) molecule in benign and malignant salivary gland tumours. Oral Oncol. 2006 Jan;42(1):56–64. doi: 10.1016/j.oraloncology.2005.06.014. [DOI] [PubMed] [Google Scholar]
- 32.Vila L., Liu H., Al-Quran S.Z., Coco D.P., Dong H.J., Liu C. Identification of c-kit gene mutations in primary adenoid cystic carcinoma of the salivary gland. Mod Pathol. 2009 Oct;22(10):1296–1302. doi: 10.1038/modpathol.2009.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang C.Y., Xia R.H., Han J., et al. Adenoid cystic carcinoma of the head and neck: clinicopathologic analysis of 218 cases in a Chinese population. Oral Surgery, Oral Medicine, Oral Pathology and Oral Radiology. 2013 Mar;115(3):368–375. doi: 10.1016/j.oooo.2012.11.018. [DOI] [PubMed] [Google Scholar]
- 34.Brayer K.J., Frerich C.A., Kang H., Ness S.A. Recurrent fusions in MYB and MYBL1 define a common, transcription factor-driven oncogenic pathway in salivary gland adenoid cystic carcinoma. Cancer Discov. 2016 Feb;6(2):176–187. doi: 10.1158/2159-8290.CD-15-0859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xiao H.D., Bifulco C.B. Oral, Head and Neck Oncology and Reconstructive Surgery [Internet] Elsevier; 2018. Cellular and molecular Pathology; pp. 57–78.https://linkinghub.elsevier.com/retrieve/pii/B9780323265683000026 [cited 2024 Apr 17] [Google Scholar]
- 36.da Silva F.J., Carvalho de Azevedo J., Ralph A.C.L., Pinheiro J. de JV., Freitas V.M., Calcagno D.Q. Salivary glands adenoid cystic carcinoma: a molecular profile update and potential implications. Front Oncol. 2023;13 doi: 10.3389/fonc.2023.1191218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Feeney L., Hapuarachi B., Adderley H., et al. Clinical disease course and survival outcomes following disease recurrence in adenoid cystic carcinoma with and without NOTCH signaling pathway activation. Oral Oncol. 2022 Oct;133 doi: 10.1016/j.oraloncology.2022.106028. [DOI] [PubMed] [Google Scholar]
- 38.Amin M.B., Edge S.B., editors. AJCC Cancer Staging Manual. 8th ed. Springer; New York: 2017. [Google Scholar]
- 39.Cheng J., Saku T., Okabe H., Furthmayr H. Basement membranes in adenoid cystic carcinoma. An immunohistochemical study. Cancer. 1992 Jun 1;69(11):2631–2640. doi: 10.1002/1097-0142(19920601)69:11<2631::aid-cncr2820691103>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 40.Tashiro Y., Yonezawa S., Kim Y.S., Sato E. Immunohistochemical study of mucin carbohydrates and core proteins in human ovarian tumors. Hum Pathol. 1994 Apr;25(4):364–372. doi: 10.1016/0046-8177(94)90144-9. [DOI] [PubMed] [Google Scholar]
- 41.Berndt A., Gaßler N., Franz M. Invasion-associated reorganization of laminin 332 in oral squamous cell carcinomas: the role of the laminin γ2 chain in tumor biology, diagnosis, and therapy. Cancers. 2022 Oct 7;14(19):4903. doi: 10.3390/cancers14194903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Scarini J.F., De Lima-Souza R.A., Lavareze L., et al. Heterogeneity and versatility of the extracellular matrix during the transition from pleomorphic adenoma to carcinoma ex pleomorphic adenoma: cumulative findings from basic research and new insights. Front Oral Health. 2023 Apr 17;4 doi: 10.3389/froh.2023.942604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Caselitz J., Schmitt P., Seifert G., Wustrow J., Schuppan D. Basal membrane associated substances in human salivary glands and salivary gland tumours. Pathol Res Pract. 1988 Aug;183(4):386–394. doi: 10.1016/S0344-0338(88)80084-0. [DOI] [PubMed] [Google Scholar]
- 44.Sathyanarayana U.G., Toyooka S., Padar A., et al. Epigenetic inactivation of laminin-5-encoding genes in lung cancers. Clin Cancer Res. 2003 Jul;9(7):2665–2672. [PubMed] [Google Scholar]
- 45.Seftor R.E.B., Seftor E.A., Kirschmann D.A., Hendrix M.J.C. Targeting the tumor microenvironment with chemically modified tetracyclines: inhibition of laminin 5 gamma2 chain promigratory fragments and vasculogenic mimicry. Mol Cancer Therapeut. 2002 Nov;1(13):1173–1179. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.