Abstract
Radiation induced pneumonitis is a common side effect of thoracic radiotherapy. We report a case of a patient diagnosed with symptomatic radiation pneumonitis and a serious contra-indication for corticosteroids. For that reason, the patient was treated with nintedanib instead. After several weeks of treatment her symptoms and chest CT improved significantly. This case shows that nintedanib might be an effective treatment of radiation pneumonitis if corticosteroids are contra-indicated.
Keywords: Radiation pneumonitis, Nintedanib, NSCLC, Case report
1. Introduction
Symptomatic pneumonitis is a potentially serious complication of radiation therapy. It most commonly occurs after treatment of locally advanced non-small cell lung cancer (NSCLC) with chemoradiotherapy (between 10 and 25 %) but may also occur after radiotherapy for other indications. Radiation pneumonitis (RP) is characterized by inflammation and damage to the lung tissue, leading to fatigue, coughing, shortness of breath, hypoxemia, fever and chest pain. If symptomatic, treatment with corticosteroids, supplemental oxygen or even mechanical ventilation in severe cases may be necessary [1].
In this case report we present a case of a 72-year old patient who had been treated with chemoradiation for stage IIIB adenocarcinoma of the lung subsequently developing RP grade 2 according to the Common Terminology Criteria of Adverse Events (CTCAE v5, National Cancer Institute). The patient suffered from severe glaucoma which is a contraindication for corticosteroid treatment and therefore was treated with nintedanib. Nintedanib is used in the treatment of idiopathic pulmonary fibrosis (IPF) but it has been proposed as a drug to prevent the development of RP in a recent clinical trial [2]. To the best of our knowledge, this case report is the first report of treatment of RP with nintedanib only and provides further insight into the development and treatment of RP.
2. Case report
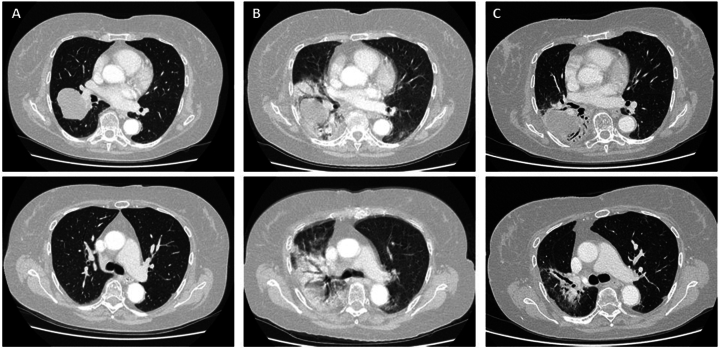
In December 2021, a 72-year old patient was diagnosed with cT3N2M0 stage IIIB NSCLC of the right lung. Her medical history consisted of atrial fibrillation, hypertension and severe glaucoma with a remaining vision of 20 % and 40 % in respectively the left and right eye. The patient was treated with 4 cycles cisplatin-pemetrexed and concurrent radiotherapy in 30 fractions of 2Gy. The chemoradiotherapy ended in March 2022 and the patient was subsequently started on adjuvant Durvalumab. In August 2022, the patient developed severe dyspnea and fatigue and was unable to perform her daily activities. A CT scan was made which showed a severe consolidation in the right lung (Fig. 1B) compared to the pre-treatment scan (Fig. 1A).
Fig. 1.
Representative images of chest CT showing the development and recovery of radiation pneumonitis
Description: Text CT images of the chest A) in April, just after the start of durvalumab and after completion of chemoradiotherapy with no signs of radiation pneumonitis and B) in August 2022, showing severe radiation pneumonitis with breathing artefacts due to severe dyspnea, and C) in November 2022, showing partial recovery of the consolidations.
Additional tests showed a diffusion capacity of the lung for carbon monoxide (DLCO) of 51 % (91 % before treatment) and an arterial pO2 of only 7.6 kPa (reference values 11–14 kPa) with normal pH and pCO2 levels. There were no signs of bacterial or viral infection. The clinical symptoms and the unilateral, typical abnormalities on the CT images were most suspicious for radiation pneumonitis. Since the patient suffered from severe glaucoma and already had impaired vision it was decided not to start a long-lasting gradually weaning course of corticosteroids, since this may worsen the existing glaucoma [3]. Instead, the patient started with 150mg nintedanib twice daily. At a return visit two weeks later the patient was able to walk the stairs, go about her daily activities and was less tired. The CT scan showed improvement of consolidations (Fig. 1C) and her symptoms improved further over the following weeks. The treatment was stopped after 10 weeks. At the time of writing there are no signs of dyspnea and no signs of progressive disease. Durvalumab remained discontinued to prevent a relapse of the pneumonitis.
3. Discussion
Nintedanib is a tyrosine-kinase inhibitor approved for the treatment of IPF after the INBUILD study [4] and the access was later expanded to treat other progressive interstitial lung diseases (ILD). The working mechanism of nintedanib is not yet fully understood but the drug is known to inhibit the activity of the Platelet Derived Growth Factor (PDGF), Fibroblast Growth Factor (FGF) and the Vascular Endothelial Growth Factor (VEGF). The development of radiation pneumonitis has been mostly attributed to double stranded DNA-breaks, the formation of reactive oxygen species (ROS), tumor necrosis factor-α (TNF-α), interleukin-6 and the influx of immune cells leading to an ongoing inflammatory cascade. This ongoing status results in local remodeling and the influx of fibroblasts, myofibroblasts, increased extracellular matrix and collagen and vascular changes [5]. Treatment with nintedanib most likely targets this remodeling process. The inhibition PDFG leads to reduced proliferation, expansion, migration and survival of myofibroblasts. FGF is associated with collagen synthesis and stimulation of fibroblasts and is strongly upregulated in IPF animal-models, inhibiting FGF will therefore most likely contribute to reduced remodeling in the lung. VEGF is a pro-angiogenic receptor that has also been shown to further stimulate PDGF. Therefore, inhibition could further reduce the effects of PDGF [6]. Another contributing factor might be the reduction of inflammation induced edema, as is also seen after anti-VEGF (Bevacizumab) for brain edema due to radiation necrosis after stereotactic radiotherapy of brain metastases [7]. The possible relevance of inhibiting PDGF and VEGF can be supported by two mouse models. In the first model, PDGF was blocked in mice who were treated with 20 Gy thorax irradiation and this resulted in a reduction of radiation induced lung injury [8]. The second study created an immunotherapy-like pneumonitis model where VEGF-inhibition led to less alveolar leakage and mitigation of pneumonitis [9]. However, the exact mechanism in the reduction of fibrosis and RP through VEGF remains incompletely understood [6].
There is limited research on the use of nintedanib for the treatment of RP. A mouse study by De Ruysscher et al. [10] found that nintedanib could reduce inflammation, alveolar debris and resolve edema and vasculitis in thoracic irradiated animals, showing its potential to mitigate radiation injury. Furthermore, as discussed previously, a clinical trial has been performed by Dy et al. [2]. This was a phase 2 randomized placebo controlled trial in which the use of nintedanib was studied as prophylaxis for RP. However, the trial had to be stopped prematurely due to low accrual and was further complicated by the change in the standard of care with the introduction of Durvalumab as adjuvant therapy. A total of eight patients were included and none of the patients who received nintedanib (n = 5) developed pneumonitis, while two patients in the control group did (n = 3). However, the sample size was too small to show a significant difference. Very recently, another clinical trial investigated the use of prednisone taper combined with nintedanib for the treatment of RP compared with prednisone taper only. The study compared 18 patients with ≥G2 radiation pneumonitis who received prednisone plus nintedanib with 12 patients with RP treated with prednisone plus placebo. The nintedanib group had less pulmonary exacerbations of RP at one year compared to the control group (p = 0.037) [11], which further supports the potential of using nintedanib for the prevention or treatment of RP.
4. Conclusion
This case demonstrates that patients with a contra indication for systemic corticosteroids can be treated for RP with nintedanib only. More studies are needed to determine the safety and efficacy of this treatment in this specific patient category.
Ethics statement
Review and/or approval by an ethics committee was not needed for this study because it is a retrospective case-report. The patient has signed an informed consent form agreeing to share the case anonymously.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data availability statement
Since this is a case report of a single patient, there is no additional data available.
CRediT authorship contribution statement
M.E. Kuipers: Writing – original draft, Visualization, Investigation. K.C.J. Van Doorn-Wink: Writing – review & editing. P.E. Postmus: Writing – review & editing, Investigation.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Hanania A.N., Mainwaring W., Ghebre Y.T., Hanania N.A., Ludwig M. Radiation-induced lung injury: assessment and management. Chest. 2019;156(1):150–162. doi: 10.1016/j.chest.2019.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dy G.K., Prasad D., Kumar P., Attwood K., Adjei A.A. A phase 2 randomized, double-blind, placebo-controlled study evaluating nintedanib versus placebo as prophylaxis against radiation pneumonitis in patients with unresectable NSCLC undergoing chemoradiation therapy. J. Thorac. Oncol. 2021;16(3):e19–e20. doi: 10.1016/j.jtho.2020.11.019. [DOI] [PubMed] [Google Scholar]
- 3.Roberti G., Oddone F., Agnifili L., et al. Steroid-induced glaucoma: epidemiology, pathophysiology, and clinical management. Surv. Ophthalmol. 2020;65(4):458–472. doi: 10.1016/j.survophthal.2020.01.002. [DOI] [PubMed] [Google Scholar]
- 4.Flaherty K.R., Wells A.U., Cottin V., et al. Nintedanib in progressive fibrosing interstitial lung diseases. N. Engl. J. Med. 2019;381(18):1718–1727. doi: 10.1056/nejmoa1908681. [DOI] [PubMed] [Google Scholar]
- 5.Cytlak U.M., Dyer D.P., Honeychurch J., Williams K.J., Travis M.A., Illidge T.M. Immunomodulation by radiotherapy in tumour control and normal tissue toxicity. Nat. Rev. Immunol. 2022;22(2):124–138. doi: 10.1038/s41577-021-00568-1. [DOI] [PubMed] [Google Scholar]
- 6.Wollin L., Wex E., Pautsch A., et al. Mode of action of nintedanib in the treatment of idiopathic pulmonary fibrosis. Eur. Respir. J. 2015;45(5):1434–1445. doi: 10.1183/09031936.00174914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Khan M., Zhao Z., Arooj S., Liao G. Bevacizumab for radiation necrosis following radiotherapy of brain metastatic disease: a systematic review & meta-analysis. BMC Cancer. 2021;21(1):167. doi: 10.1186/s12885-021-07889-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dadrich M., Nicolay N.H., Flechsig P., Bickelhaupt S., Hoeltgen L., Roeder F., et al. Combined inhibition of TGFβ and PDGF signaling attenuates radiation-induced pulmonary fibrosis. OncoImmunology. 2016;5(5) doi: 10.1080/2162402X.2015.1123366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Iwai T., Sugimoto M., Patel H., Yorozu K., Kurasawa M., Kondoh O. Anti-VEGF antibody protects against alveolar exudate leakage caused by vascular hyperpermeability, resulting in mitigation of pneumonitis induced by immunotherapy. Mol. Cancer Therapeut. 2021;20(12):2519–2526. doi: 10.1158/1535-7163.MCT-21-0031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.De Ruysscher D., Granton P.V., Lieuwes N.G., van Hoof S., Wollin L., Weynand B., et al. Nintedanib reduces radiation-induced microscopic lung fibrosis but this cannot be monitored by CT imaging: a preclinical study with a high precision image-guided irradiator. Radiother. Oncol. 2017;124(3):482–487. doi: 10.1016/j.radonc.2017.07.014. [DOI] [PubMed] [Google Scholar]
- 11.Rimner A., Moore Z.R., Lobaugh S., et al. Randomized, phase II, placebo-controlled trial of nintedanib for the treatment of radiation pneumonitis. Int. J. Radiat. Oncol. Biol. Phys. 2023 Mar;7 doi: 10.1016/j.ijrobp.2023.02.030. S0360-3016(23)00177-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Since this is a case report of a single patient, there is no additional data available.