Abstract
Aim: This study evaluated associations between CYP3A4*22 and variants in other pharmacogenes (CYP3A5, SULT2A1, ABCB1, ABCG2, ERCC1) and the risk for palbociclib-associated toxicities.
Materials & methods: Two hundred cancer patients who received standard-of-care palbociclib were genotyped and associations with toxicity were evaluated retrospectively.
Results: No significant associations were found for CYP3A4*22, CYP3A5*3, ABCB1_rs1045642, ABCG2_rs2231142, ERCC1_rs3212986 and ERCC1_rs11615. Homozygous variant carriers of SULT2A1_rs182420 had higher incidence of dose modifications due to palbociclib toxicity (odds ratio [OR]: 4.334, 95% CI: 1.057–17.767, p = 0.042). ABCG2_rs2231137 variant carriers had borderline higher incidence of grade 3–4 neutropenia (OR: 4.14, 95% CI: 0.99–17.37, p = 0.052).
Conclusion: Once validated, SULT2A1 and ABCG2 variants may be useful to individualize palbociclib dosing to minimize toxicities and improve treatment outcomes.
Keywords: : Breast cancer, CDK4/6 inhibitors, palbociclib, pharmacogenetics, toxicity
Plain language summary
Article highlights.
Palbociclib treatment is limited by dose-limiting toxicities, in particular neutropenia. Biomarkers to predict toxicity and individualize dosing are lacking.
In patients receiving standard-of-care therapy with palbociclib (n = 200), associations between variants in relevant pharmacogenes (i.e., CYP3A4, CYP3A5, SULT2A1, ABCB1, ABCG2, ERCC1) and palbociclib toxicity end points (i.e., treatment modification due to toxicity, any grade toxicity, grade 3–4 neutropenia) were investigated.
CYP3A4*22, and other candidate pharmacogenes including CYP3A5*3, ABCG2_rs2231142, ERCC1_rs3212986 and ERCC1_rs11615, were not associated with any of the toxicity end points (p > 0.05).
Multivariable analyses for SULT2A1_rs182420 revealed that homozygous variant carriers (C/C) had a greater risk for toxicities that required treatment modifications (odds ratio [OR]: 4.33, 95% CI: 1.06–17.77, p = 0.042) compared with SULT2A1 heterozygous and wildtype patients.
ABCG2_rs2231137 was borderline associated with an increased risk for grade 3–4 neutropenia (OR: 4.137, 95% CI: 0.99–17.37), p = 0.052.
Upon independent validation, SULT2A1_rs182420 and ABCG2_2231137 may predict palbociclib toxicity and could be useful to guide palbociclib dosing to minimize toxicities and improve therapeutic benefit.
1. Background
The introduction of the first cyclin-dependent kinases 4 and 6 (CDK4/6) inhibitor palbociclib in 2015 was practice changing, as it improved both progression-free and overall survival significantly in patients with metastatic breast cancer [1,2]. US FDA approval of other CDK4/6 inhibitors ribociclib (2017) and abemaciclib (2017) followed rapidly and CDK4/6 inhibitors are currently the front-line agents in the treatment of hormone receptor (HR)-positive, human epidermal growth factor receptor 2 (HER2)-negative (HR+/HER2-) advanced or metastatic breast cancer [3]. Globally, palbociclib remains the most frequently prescribed CDK4/6 inhibitor [4–8], and its use is expected to increase even further as clinical studies showed outcome benefits in other cancer types such as head and neck squamous cell carcinoma [9] and soft tissue sarcoma [10].
Palbociclib is administered at a fixed daily dose of 125 mg once daily for 21 days followed by 7 days off. This one-size-fits-all dosing approach results in large inter-individual variability in pharmacokinetics and unpredictable toxicity due to reported exposure–response relationships for safety, in particular neutropenia [11–13]. Adverse drug reactions (ADRs), such as grade 3–4 neutropenia that occurs in ∼60% of patients [1], often result in dose reductions (36%), treatment interruptions (71%), or even permanent discontinuation of palbociclib (8%) [14]. ADRs not only endanger patient safety and quality of life, but also can require treatment discontinuation that adversely impacts the efficacy of CDK4/6 inhibitor therapy, and waste healthcare resources [15,16].
Thus far, no safety biomarker is used clinically to predict the risk for palbociclib-induced toxicities. Palbociclib is metabolized primarily by the polymorphic enzymes, cytochrome P450 family 3 subfamily A member 4 (CYP3A4) and sulfotransferase family 1A member 1 (SULT2A1). It is well known that CYP3A4 inhibitors (e.g., itraconazole, erythromycin) cause clinically relevant pharmacokinetic interactions with palbociclib and its concomitant use with strong CYP3A4 inhibitors should therefore be avoided [13]. The CYP3A4 gene is also known to have clinically relevant single nucleotide polymorphisms (SNPs), of which CYP3A4*22 (rs35599367, 15389C>T) is the most common variant that leads to decreased protein expression and activity in individuals who are heterozygous carriers, which corresponds to ∼15% of White people [17–19]. Consequently, CYP3A4*22 is expected to be associated with increased palbociclib systemic concentrations and ADR risk, similar to reported associations for pazopanib, docetaxel, paclitaxel, and vemurafenib [20–23]. A previous pharmacogenetic study reported ABCB1_rs1128503 and ERCC1_rs11615 as potential risk factors for grade 3–4 neutropenia in non-Asian patients treated with palbociclib (p < 0.1) [24]; however, no variants of the main metabolizing enzymes CYP3A4 or SULT2A1 were included. Peruzzi et al. did show a significant univariate association between CYP3A4*22 and the risk for early dose-limiting toxicities in a combined cohort including all three CDK4/6 inhibitors [7], but associations with palbociclib specifically and SULT2A1 variants were not evaluated. Therefore, the objective of this retrospective genetic association study was to investigate the association of CYP3A4*22 and other SNPs in relevant pharmacogenes with palbociclib toxicity in patients with cancer.
2. Materials & methods
2.1. Study design
This retrospective pharmacogenetic association study was conducted in a pooled cohort of patients with breast cancer who received palbociclib treatment at Roswell Park Comprehensive Cancer Center (Buffalo, NY) or University of Michigan Rogel Cancer Center (Ann Arbor, MI), all of whom had banked germline DNA samples. Samples for patients treated at Roswell Park were banked at the Data Bank and Biorepository Shared Resource, while samples for patients treated at University of Michigan Center were collected and analyzed within the Michigan Genomics Initiative (MGI), a genetic data repository encompassing >84,000 Michigan Medicine patients [25]. Clinical data from both Roswell Park and MGI subjects were abstracted from electronic health records including demographics (e.g., age, gender, race), baseline measurements (e.g., body weight, height, blood cell counts), treatment data (e.g., palbociclib dose, start/stop dates, concurrent medications) and toxicity outcomes (e.g., incidence of grade 3–4 toxicities, dose reductions due to toxicities) by study personnel blinded to genotype data. Toxicities were retrospectively graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events (NCI CTCAE) v5.0. Patient information and genetic data were de-identified by honest brokers prior to analysis. This retrospective study was approved by the institutional review boards of both Roswell Park (protocol no. BDR-122719) and the University of Michigan (protocol no. HUM00161844).
2.2. Selection of SNPs
Common functional variants with a risk allele frequency of at least 4% in White patients were selected in genes encoding enzymes (CYP3A4/5, SULT2A1) and drug transporters (P-gp, BCRP) that are involved in the metabolism and disposition of palbociclib. The common reduced-activity variant CYP3A5*3 (risk allele frequency: 82–95% in White people) may impact palbociclib exposure [26] and response because of considerable overlap in substrate specificity of CYP3A4 and CYP3A5 [27]. In addition, reduced-activity variants in ABCB1 and ABCG2 genes were selected because palbociclib is a substrate of the polymorphic drug efflux transporters P-glycoprotein (P-gp, encoded by ABCB1) and Breast Cancer Resistance Protein (BCRP, encoded by ABCG2). Instead of the previously reported ABCB1_rs1128503 [24], we evaluated the ABCB1 SNP rs1045642 which is in strong linkage disequilibrium with rs1128503 [28]. SULT2A1_rs2637125 has been associated with decreased SULT2A1 enzyme expression [29]. A previous pharmacogenetic study reported ABCB1_rs1128503 and ERCC1_rs11615 as potential risk factors for grade 3–4 neutropenia in non-Asian patients treated with palbociclib (p < 0.1) [24]; however, no variants of the main metabolizing enzymes CYP3A4 or SULT2A1 were included. In addition, pharmacodynamic SNPs in ERCC1 were selected because of the previously reported associations with palbociclib toxicity [24]. Details of the SNPs included in this analysis are shown in Table 1.
Table 1.
Investigated single nucleotide polymorphisms.
| Gene | SNP | Normal function allele | Reduced function/risk allele | Nucleotide variation | Annotation | Frequency reduced function/risk allele (%)a | WT | HTZ | HMZ | HWE p-value |
|---|---|---|---|---|---|---|---|---|---|---|
| CYP3A4 | rs35599367 | G | A | 15389C>T | *22 | 4 | 187 | 13 | 0 | 0.40 |
| CYP3A5 | rs776746 | T | C | 6986A>G | *3 | 89 | 2 | 38 | 158 | 0.80 |
| ABCB1 | rs1045642 | G | A | 3435C>T | 50 | 67 | 92 | 41 | <0.05b | |
| ABCG2 | rs2231142 | G | T | 421C>A | 10 | 155 | 41 | 4 | 0.083 | |
| rs2231137 | C | T | 34G>A | 6 | 183 | 17 | 0 | 0.13 | ||
| SULT2A1 | rs2637125 | G | A | 10660T>G | 13 | 152 | 37 | 7 | <0.05 | |
| rs182420 | T | C | 23 | 122 | 61 | 13 | 0.16 | |||
| rs2547238 | G | C | 15 | 100 | 82 | 14 | <0.05 | |||
| ERCC1 | rs3212986 | C | A | 8092C>A | 25 | 116 | 65 | 15 | 0.17 | |
| rs11615 | G | A | 354T>C | 59 | 35 | 97 | 64 | 0.51 |
Allele frequency in the global population as reported in the NCBI Allele Frequency Aggregator (ALFA) project.
HWE p-value = 0.18 in White patients only and therefore retained in this analysis.
ALFA: Allele Frequency Aggregator www.ncbi.nlm.nih.gov/snp/docs/gsr/alfa/ (Feb 16); HMZ: Homozygous; HTZ: Heterozygous variant; HWE: Hardy–Weinberg equilibrium; SNP: Single nucleotide polymorphism; WT: Wildtype.
2.3. Genotyping
At the Roswell Park Genomics Shared Resource, high-quality genomic DNA samples from Roswell Park patients were processed for array analysis using the Infinium Global Diversity Array with Enhanced PGx (GDA, Illumina, San Diego, CA), as per manufacturer’s protocol. Briefly, 200 ng (20 ng/μl) of high-quality genomic DNA was amplified, fragmented, precipitated and resuspended in Illumina’s custom hybridization buffer. DNA was then hybridized to HumanCytoSNP-12 BeadChips (Illumina). Subsequently, the BeadChips were scanned using the Illumina iScan Reader after which the image data were transferred to Illumina GenomeStudio software for data processing, validation of assay controls, and report generation. For genetic analysis, the Illumina PGx Analysis – Microarray App within Illumina Connected Analytics was used to convert raw intensity files from the Illumina iScan reader to pharmacogenetic results. Germline DNA samples from MGI participants were analyzed by customized Illumina Infinium CoreExome-24 bead arrays, as previously described [25]. Genotype calling for the Illumina arrays was performed utilizing Illumina GenomeStudio software. Quality control procedures at Roswell Park and the University of Michigan ensured that only samples with a call rate of at least 99% were included.
2.4. Statistical analysis
Utilizing Chi-square tests, the distribution of genotypes was tested for Hardy–Weinberg Equilibrium (HWE) at α = 0.05. SNPs that were not in HWE, underwent retesting in White patients only and were retained in the analysis if they were in HWE in this subset of patients. Three dichotomized end points for toxicity were studied: the primary end point was treatment modification due to toxicity (i.e., dose reduction or treatment discontinuation), whereas any grade 3–4 toxicity and grade 3–4 neutropenia were secondary end points. All variants were initially evaluated using the additive genetic model (logistic regression analysis). For those associations with p < 0.05, dominant or recessive models were tested using the Fisher’s exact test. Based on the genotype distribution of the variants listed in Table 1, the most appropriate genetic model was selected (i.e., dominant or recessive) [30]. Our primary hypothesis was that CYP3A4*22 carriers (i.e., heterozygous plus homozygous variant carriers) had an increased risk for the primary end point treatment modifications due to toxicity. Univariable genetic associations with p-values < 0.05 were tested in a multivariable model. Multivariable logistic regression analyses were performed with any of the toxicity end points as dependent variable and genotype (standard α = 0.05) plus any significant confounders as independent variables (p < 0.2 in univariable Fisher’s exact or logistic regression analysis for confounders). Potential confounding variables that were evaluated included age at palbociclib initiation, gender, race, body weight, height, body surface area, body mass index, treatment site, cancer type, breast cancer stage, baseline white blood cell counts, baseline absolute neutrophil counts, baseline platelet counts and concurrent use of hormonal therapy, potent CYP3A4-modulating drugs or proton pump inhibitors. Statistical analyses were performed using SAS software, Version 9.4 (SAS Institute Inc., Cary, NC). Considering the a priori defined single primary analyses and exploratory nature of all secondary analyses, no correction for multiple testing was applied.
3. Results
3.1. Patients
A total of 200 patients who initiated treatment with palbociclib between 2015 and 2019 were included in this retrospective study. Demographic and baseline data are shown in Table 2. Most patients were White (90.5%), female (91.5%) and received treatment for HR+/HER2- breast cancer (85.5%), started at the recommended dose of 125 mg once daily (94.5%), with concurrent hormonal therapy with an aromatase inhibitor (61.5%). The majority of patients did not concomitantly use interacting drugs such as proton pump inhibitors (69.5%) or potent CYP3A4 modulating agents (97.0%).
Table 2.
Demographics.
| Parameter | Value |
|---|---|
| Number of patients | 200 |
| Study site, n (%) – Roswell Park Comprehensive Cancer Center – University of Michigan Rogel Cancer Center |
93 (46.5) 107 (53.5) |
| Age (years) at initiation of palbociclib, median (range) | 61.0 (32.8–86.0) |
| Ethnic composition, n (%) – White – Black – Asian – Other – Not reported |
181 (90.5) 9 (4.5) 7 (3.5) 2 (1.0) 1 (0.5) |
| Sex, n (%) – Female – Male |
183 (91.5) 17 (8.5) |
| Height (m), median (range) | 1.63 (1.47–1.85) |
| Body weight (kg), median (range) | 75.3 (38.9–146.6) |
| Body surface area (m2), median (range) | 1.8 (1.3–2.7) |
| Body mass index (kg/m2), median (range) | 28.0 (15.4–56.0) |
| Cancer – Breast – Soft tissue sarcoma – Prostate – Squamous cell carcinoma – Other |
182 (91.0) 5 (2.5) 2 (1.0) 2 (1.0) 9 (4.5) |
| HR+/HER2- status, n (%) – Yes – Other – Not reported/determined |
171 (85.5) 8 (4.0) 21 (10.5) |
| Palbociclib starting dose (QD), n (%) – 125 mg – 100 mg – 75 mg – Not reported |
189 (94.5) 8 (4.0) 1 (0.5) 2 (1.0) |
| Concurrent hormonal therapy, n (%) – Aromatase inhibitor (letrozole, anastrozole, exemestane) – Fulvestrant – Anastrozole/letrozole + fulvestrant – No hormonal therapy |
123 (61.5) 52 (26.0) 5 (2.5) 20 (10) |
| Concurrent use of proton pump inhibitors, n (%) – Yes – No – Not reported |
58 (29.0) 139 (69.5) 3 (1.5) |
| Concurrent use of potent CYP3A4 modulating drugs (e.g., ketoconazole, ritonavir, carbamazepine), n (%) – Inducer – Inhibitor – No – Not reported |
2 (1.0) 1 (0.5) 194 (97.0) 3 (1.5) |
| Baseline measurements | |
| Hemoglobin (g/dl) | 12.7 (8.4–18.3) |
| White blood cell count (×109/l) | 6.0 (2.0–18.9) |
| Absolute neutrophil count (×109/l) | 3.8 (0.9–225.0) |
| Platelet count (×109/l) | 243.0 (3.7–594.0) |
HER: Human epidermal growth factor receptor 2.
3.2. Genetic associations with palbociclib toxicity
Of the 200 evaluable patients, 76 (38%) experienced toxicities that required modification of palbociclib therapy (dose reduction or discontinuation). Grade 3–4 toxicities, which were evaluable for 197 patients, occurred in 114 patients (58%), including grade 3–4 neutropenia which was reported in 92 patients (47%). Three variants (i.e., ABCB1_rs1045642, SULT2A1_rs2637125, SULT2A1_rs2547238) did not follow the expected HWE distribution. ABCB1_rs1045642 was in HWE in the subset of White patients and was therefore retained in the analysis whereas the other two were removed (Table 1).
Table 3 summarizes the pharmacogenetic associations with palbociclib toxicity. For the main SNP of interest, CYP3A4*22, no significant associations with any of the palbociclib toxicity end points were observed. Significant univariable associations were observed for ABCG2_rs2231137 and SULT2A1_rs182420. For ABCG2_rs2231137, heterozygous T allele carriers were more likely than wildtype (C/C) patients to develop grade 3–4 neutropenia (odds ratio [OR]: 2.999, 95% confidence interval, CI: 1.014–8.864, p = 0.047). This association, however, did not retain significance in the multivariable analysis (p = 0.052). For SULT2A1_rs182420, homozygous variant carriers (C/C) had a greater risk for toxicities that required treatment modifications (OR: 5.909, 95% CI: 1.571–22.231, p = 0.009) and this association remained significant in the multivariable model (OR: 4.334, 95% CI: 1.057–17.767, p = 0.042, Table 3 & Figure 1). The other tested variants (i.e., CYP3A5*3, ABCB1_rs1045642, ABCG2_rs2231142, ERCC1_rs3212986 and ERCC1_rs11615 were not associated with any of the toxicity end points (all p > 0.05). A sensitivity analysis in a more homogeneous subset of patients consisting of only White, female patients with breast cancer (n = 161) generated results that were not meaningfully different. Also in this population, SULT2A1_rs182420 was significantly associated with treatment modifications due to toxicity (OR: 4.457, 95% CI: 1.118–17.766, p = 0.034).
Table 3.
Associations of genetic variants with palbociclib-induced toxicity.
| Gene | SNP | Group comparison | Treatment modification due to toxicity | Any grade 3–4 toxicity | Grade 3–4 neutropenia | |||
|---|---|---|---|---|---|---|---|---|
| OR (95% CI) | p-value | OR (95% CI) | p-value | OR (95% CI) | p-value | |||
| Univariate analyses | ||||||||
| CYP3A4 | rs35599367 | *1/*22 (n = 13) vs. *1/*1 (n = 187) | 2.00 (0.64–6.18) | 0.23 | 1.02 (0.31–3.34) | 0.97 | 1.65 (0.50–5.38) | 0.41 |
| CYP3A5 | rs776746 | *3/*3 (n = 158) vs. *1/*3 (n = 38) vs. *1/*1 (n = 2) | *1/*3 vs. *1/*1: >999.999 (<0.001–>999.999) *3/*3 vs. *1/*1: >999.999 (<0.001–>999.999) |
0.97 | *1/*3 vs. *1/*1: 2.36 (0.14–41.27) *3/*3 vs. *1/*1: 1.23 (0.075–20.00) |
0.25 | *1/*3 vs. *1/*1: 1.18 (0.068–20.26) *3/*3 vs. *1/*1: 0.81 (0.050–13.25) |
0.60 |
| ABCB1 | rs1045642 | A/A (n = 41) vs. G/A (n = 92) vs. G/G (n = 67) | G/A vs. G/G: 0.82 (0.43–1.56) A/A vs. G/G: 0.72 (0.32–1.62) |
0.70 | G/A vs. G/G: 0.91 (0.48–1.73) A/A vs. G/G: 0.82 (0.37–1.81) |
0.88 | G/A vs. G/G: 0.98 (0.52–1.85) A/A vs. G/G:1.17 (0.53–2.57) |
0.89 |
| ABCG2 | rs2231142 | T/T (n = 4) vs. G/T (n = 41) vs. G/G (n = 155) | G/T vs. G/G: 0.94 (0.46–1.92) T/T vs. G/G: 1.63 (0.22–11.86) |
0.87 | G/T vs. G/G: 1.52 (0.74–3.13) T/T vs. G/G: 0.79 (0.11–5.74) |
0.50 | G/T vs. G/G: 1.43 (0.72–2.86) T/T vs. G/G: 1.24 (0.17–9.00) |
0.59 |
| rs2231137 | C/T (n = 17) vs. C/C (n = 183) | 2.53 (0.92–6.97) | 0.072 | 2.54 (0.80–8.10) | 0.11 | 2.999 (1.014–8.864) | 0.047 | |
| SULT2A1 | rs182420 | C/C (n = 13) vs. T/C (n = 61) vs. T/T (n = 122) | T/C vs. T/T: 0.806 (0.422–1.541) C/C vs. T/T: 5.507 (1.440–21.057) |
0.026 | T/C vs. T/T: 0.75 (0.40–1.39) C/C vs. T/T: 1.52 (0.44–5.22) |
0.46 | T/C vs. T/T: 0.56 (0.29–1.05) C/C vs. T/T: 2.21 (0.65–7.58) |
0.054 |
| C/C (n = 13) vs. T/C + T/T (n = 183) | 5.909 (1.571–22.231) | 0.009 | – | – | – | – | ||
| ERCC1 | rs3212986 | A/A (n = 15) vs. C/A (n = 65) vs. C/C (n = 116) | C/A vs. C/C 0.75 (0.40–1.42) A/A vs. C/C: 1.29 (0.44–3.78) |
0.55 | C/A vs. C/C: 0.62 (0.33–1.16) A/A vs. C/C: 0.51 (0.17–1.51) |
0.21 | C/A vs. C/C: 0.71 (0.38–1.31) A/A vs. C/C: 0.64 (0.22–1.93) |
0.46 |
| rs11615 | A/A (n = 35) vs. G/A (n = 97) vs. G/G (n = 64) | G/A vs. G/G: 0.83 (0.43–1.58) A/A vs. G/G: 1.10 (0.48–2.53) |
0.73 | G/A vs. G/G: 1.23 (0.64–2.35) A/A vs. G/G: 0.82 (0.36–1.88) |
0.56 | G/A vs. G/G: 1.32 (0.70–2.51) A/A vs. G/G: 0.72 (0.31–1.68) |
0.30 | |
| Multivariable analyses | ||||||||
| ABCG2 | rs2231137 | C/T (n = 17) vs. C/C (n = 183)a | – | – | – | – | 4.14 (0.99–17.37) | 0.052 |
| SULT2A1 | rs182420 | C/C (n = 13) vs. T/C + T/T (n = 183)b | 4.334 (1.057–17.767) | 0.042 | – | – | – | – |
Significant associations (p < 0.05) are shown in bold.
Covariates included: gender, institute, BMI, height, baseline white blood cell, absolute neutrophil and platelet counts.
Covariates included: gender, ECOG status, breast cancer (yes/no), HR-HER status, institute, BMI, baseline white blood cell count.
CI: Confidence interval; OR: Odds ratio; SNP: Single nucleotide polymorphism; VAR: Variant allele; WT: Wild type allele.
Figure 1.
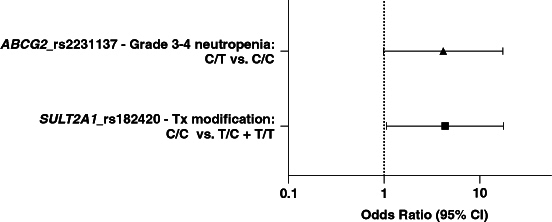
Multivariable associations between pharmacogenetic variants and palbociclib toxicity. SULT2A1_rs182420 homozygous (C/C) variant patients were at increased risk for toxicity-related treatment modifications (OR: 4.33, 95% CI: 1.06–17.77, p = 0.042). Albeit not statistically significant, ABCG2_rs2231137 heterozygous (C/T) variant carriers were more likely to develop grade 3–4 neutropenia (OR: 4.14, 95% CI: 0.99–17.37, p = 0.052).
CI: Confidence interval; Tx: Treatment.
4. Discussion
This real-world study investigated associations between pharmacogenetic variants and the incidence of palbociclib toxicities. For our main SNP of interest, CYP3A4*22, no significant associations with any of the toxicity end points were observed. The number of CYP3A4*22 variant carriers was quite small in our cohort (n = 13), hence the effects of this SNP on palbociclib toxicity should be analyzed in a larger patient cohort.
To our knowledge, this is the first study analyzing pharmacogenetic associations of the main metabolizing enzymes CYP3A4/5 or SULT2A1 with toxicity from palbociclib treatment specifically. No association was found for CYP3A4/5 variants. For CYP3A5*3, there is a case report of a patient with the CYP3A5*1/*3 genotype that had low palbociclib plasma concentrations [26], but the current literature provides insufficient evidence of an actual genetic association with palbociclib pharmacokinetics or toxicity. Homozygous variant carriers of SULT2A1_rs182420 (C/C genotype) were more likely to require treatment modifications (i.e., dose reduction or treatment discontinuation) compared with heterozygous (T/C) and wild type (T/T) patients. A plausible explanation for this association is impaired SULT2A1-mediated sulfonation of palbociclib, as others have reported lower SULT2A1-mediated conversion of dehydroepiandrosterone to dehydroepiandrosterone sulfate in carriers of the variant C allele [31,32]. Carriers of the ABCG2_rs2231137 variant T genotype had a greater risk for grade 3–4 neutropenia, though this association did not retain significance in the multivariable analysis (p = 0.052). This association, if real, may be caused by increased palbociclib exposure due to reduced BCRP expression, and palbociclib efflux, in variant carriers [33]. The associations involving SULT2A1_rs182420 and ABCG2_rs2231137 need to be tested in independent cohorts of palbociclib-treated patients to confirm their clinical validity and should be explored in cohorts with measured palbociclib systemic exposure for mechanistic validation.
Previously, Iwata et al. reported that ABCB1_rs1128503 (C/C vs. T/T) and ERCC1_rs11615 (A/A vs. G/G) were potential risk factors for grade 3–4 neutropenia on cycle 1 day 15 in non-Asians (p < 0.1), though these associations were not statistically significant in their analysis [24]. Our analysis of ABCB1_rs1045642, which is in strong linkage disequilibrium with rs1128503 [28], and ERCC1_11615 was not able to replicate these associations with any of the palbociclib toxicity end points.
In addition to SNPs in pharmacodynamic genes (e.g., ERCC1) that were included in the pharmacogenetic study by Iwata and colleagues [24], the present study evaluated SNPs in CYP3A and SULT2A1 genes that play a major role in palbociclib pharmacokinetics and were therefore expected to influence the pharmacological effect. Another strength of this study was the selection of clinically relevant/treatment affecting end points, i.e., dose reductions, treatment modifications and discontinuation due to toxicity. In contrast, early grade 3–4 neutropenia was the only toxicity end point in the study by Iwata et al. [24].
One of the limitations of this retrospective study was the lack of available pharmacokinetic samples, which precluded us from testing the hypothesis that the associations involving SULT2A1_rs182420 and ABCG2_2231137 were the result of increased systemic exposure to palbociclib due to impaired drug metabolism and efflux, respectively. To investigate genetic associations with both palbociclib pharmacokinetics and toxicity, a prospective study (BDR-122719) is ongoing in which DNA, plasma samples at steady-state, and toxicity data are collected from Roswell Park patients receiving standard-of-care treatment with palbociclib. This prospective cohort will also be utilized to clinically validate the genetic associations with toxicity that were found in this retrospective study. Demonstration of clinical validation is needed before the clinical utility of genotype-guided dosing can be evaluated in prospective clinical studies [34]. Future studies should also test genetic associations for the other CDK4/6 inhibitors ribociclib and abemaciclib to determine if the significant associations observed in the current study are specific to palbociclib. In the event of shared germline indicators of toxicity risk, dose reduction for abemaciclib and/or ribociclib should also be considered in SULT2A1_rs182420 and ABCG2_2231137 carriers [35]. However, if pharmacogenetic associations are CDK4/6 inhibitor-specific, variant carriers could be switched to another CDK4/6 inhibitor. For example, the lack of a significant association with ABCB1_rs1045642 in the present palbociclib analysis, while this SNP was associated with early dose-limiting toxicities and dose reductions in the combined CDK4/6 inhibitor study [7], suggests that for carriers of this variant palbociclib may be the preferred CDK4/6 inhibitor. Other limitations include the low number of CYP3A4*22 variant carriers, retrospective toxicity data collection that could have led to patient misclassification, and heterogeneity of the patient population (e.g., palbociclib dose and cancer type), all of which limits the ability to detect true associations.
5. Conclusion
In this retrospective study, SULT2A1_rs182420 was a significant predictor for palbociclib toxicity leading to treatment modifications including dose reductions and treatment discontinuation. This association will need to be confirmed in independent cohorts, ideally with collection of pharmacokinetic end points. Confirmation of these associations could lead to individualized, genotype-guided dosing of palbociclib to minimize toxicities and improve drug efficacy.
Acknowledgments
The authors acknowledge the MGI participants, Precision Health at the University of Michigan, the University of Michigan Medical School Central Biorepository and the University of Michigan Advanced Genomics Core for providing data and specimen storage, management, processing and distribution services and the Center for Statistical Genetics in the Department of Biostatistics at the School of Public Health for genotype data curation, imputation and management in support of the research reported in this publication.
Funding Statement
This work was supported by National Cancer Institute (NCI) grant P30CA016056 involving the use of Roswell Park Comprehensive Cancer Center’s Genomics, Biomedical Research Informatics, Data Bank and Biorepository, Biostatistics and Statistical Genomics Shared Resources.
Author contributions
AKL Goey and DL Hertz contributed to the conceptual design and provided supervision. AKL Goey wrote the original draft. AKL Goey, M Hwang, B Paulson, S Ozair and TL O'Connor collected clinical data. S Ozair, TL O'Connor and S Gandhi enrolled patients and supported clinical interpretation of the results. C Wang curated the data. C Wang, D Mhandire and KM Attwood performed statistical analysis. All authors critically read, revised, and approved the final manuscript.
Financial disclosure
This work was supported by National Cancer Institute (NCI) grant P30CA016056 involving the use of Roswell Park Comprehensive Cancer Center’s Genomics, Biomedical Research Informatics, Data Bank and Biorepository, Biostatistics and Statistical Genomics Shared Resources. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Competing interests disclosure
The authors have no competing interests or relevant affiliations with any organization or entity with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Writing disclosure
No writing assistance was utilized in the production of this manuscript.
Ethical conduct of research
The authors state that they have obtained appropriate approval from the institutional review boards at Roswell Park Comprehensive Cancer Center (protocol no. BDR-122719) and at the University of Michigan (protocol no. HUM00161844). In addition, informed consent has been obtained from the participants involved.
References
- 1.Finn RS, Martin M, Rugo HS, et al. Palbociclib and letrozole in advanced breast cancer. N Engl J Med. 2016;375(20):1925–1936. doi: 10.1056/NEJMoa1607303 [DOI] [PubMed] [Google Scholar]
- 2.Turner NC, Slamon DJ, Ro J, et al. Overall survival with palbociclib and fulvestrant in advanced breast cancer. N Engl J Med. 2018;379(20):1926–1936. doi: 10.1056/NEJMoa1810527 [DOI] [PubMed] [Google Scholar]
- 3.NCCN clinical practice guidelines in oncology – breast cancer (version 2.2024). https://www.nccn.org/professionals/physician_gls/pdf/breast.pdf. [DOI] [PubMed]
- 4.Husinka L, Koerner PH, Miller RT, Trombatt W. Review of cyclin-dependent kinase 4/6 inhibitors in the treatment of advanced or metastatic breast cancer. J Drug Assess. 2020;10(1):27–34. doi: 10.1080/21556660.2020.1857103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Marineau A, St-Pierre C, Lessard-Hurtubise R, David ME, Adam JP, Chabot I. Cyclin-dependent kinase 4/6 inhibitor treatment use in women treated for advanced breast cancer: integrating ASCO/NCODA patient-centered standards in a community pharmacy. J Oncol Pharm Pract. 2023;29(5):1144–1153. doi: 10.1177/10781552221102884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Luyendijk M, Blommestein H, Uyl-De Groot C, Siesling S, Jager A. Regulatory approval, reimbursement, and clinical use of cyclin-dependent kinase 4/6 inhibitors in metastatic breast cancer in the Netherlands. JAMA Netw Open. 2023;6(2):e2256170. doi: 10.1001/jamanetworkopen.2022.56170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Peruzzi E, Gerratana L, Montico M, et al. Association of ADME gene polymorphisms on toxicity to CDK4/6 inhibitors in patients with HR+/HER2- metastatic breast cancer. Biomed Pharmacother. 2023;167:115479. doi: 10.1016/j.biopha.2023.115479 [DOI] [PubMed] [Google Scholar]
- 8.Low JL, Lim E, Bharwani L, et al. Real-world outcomes from use of CDK4/6 inhibitors in the management of advanced/metastatic breast cancer in Asia. Ther Adv Med Oncol. 2022;14:17588359221139678. doi: 10.1177/17588359221139678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Adkins D, Ley J, Neupane P, et al. Palbociclib and cetuximab in platinum-resistant and in cetuximab-resistant human papillomavirus-unrelated head and neck cancer: a multicentre, multigroup, Phase II trial. Lancet Oncol. 2019;20(9):1295–1305. doi: 10.1016/S1470-2045(19)30405-X [DOI] [PubMed] [Google Scholar]
- 10.Dickson MA, Schwartz GK, Keohan ML, et al. Progression-free survival among patients with well-differentiated or dedifferentiated liposarcoma treated with CDK4 inhibitor palbociclib: a Phase II clinical trial. JAMA Oncol. 2016;2(7):937–940. doi: 10.1001/jamaoncol.2016.0264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Groenland SL, Martinez-Chavez A, Van Dongen MGJ, et al. Clinical pharmacokinetics and pharmacodynamics of the cyclin-dependent kinase 4 and 6 inhibitors palbociclib, ribociclib, and abemaciclib. Clin Pharmacokinet. 2020;59(12):1501–1520. doi: 10.1007/s40262-020-00930-x [DOI] [PubMed] [Google Scholar]
- 12.Sun W, O'dwyer PJ, Finn RS, et al. Characterization of neutropenia in advanced cancer patients following palbociclib treatment using a population pharmacokinetic–pharmacodynamic modeling and simulation approach. J Clin Pharmacol. 2017;57(9):1159–1173. doi: 10.1002/jcph.902 [DOI] [PubMed] [Google Scholar]
- 13.Clinical pharmacology and biopharmaceutics review palbociclib. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2015/207103Orig1s000ClinPharmR.pdf.
- 14.Dieras V, Rugo HS, Schnell P, et al. Long-term pooled safety analysis of palbociclib in combination with endocrine therapy for HR+/HER2- advanced breast cancer. J Natl Cancer Inst. 2019;111(4):419–430. doi: 10.1093/jnci/djy109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li N, Du EX, Chu L, et al. Real-world palbociclib dosing patterns and implications for drug costs in the treatment of HR+/HER2- metastatic breast cancer. Expert Opin Pharmacother. 2017;18(12):1167–1178. doi: 10.1080/14656566.2017.1351947 [DOI] [PubMed] [Google Scholar]
- 16.Hershman DL, Shao T, Kushi LH, et al. Early discontinuation and non-adherence to adjuvant hormonal therapy are associated with increased mortality in women with breast cancer. Breast Cancer Res Treat. 2011;126(2):529–537. doi: 10.1007/s10549-010-1132-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Okubo M, Murayama N, Shimizu M, Shimada T, Guengerich FP, Yamazaki H. CYP3A4 intron 6 C>T polymorphism (CYP3A4*22) is associated with reduced CYP3A4 protein level and function in human liver microsomes. J Toxicol Sci. 2013;38(3):349–354. doi: 10.2131/jts.38.349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Werk AN, Cascorbi I. Functional gene variants of CYP3A4. Clin Pharmacol Ther. 2014;96(3):340–348. doi: 10.1038/clpt.2014.129 [DOI] [PubMed] [Google Scholar]
- 19.Wang D, Guo Y, Wrighton SA, Cooke GE, Sadee W. Intronic polymorphism in CYP3A4 affects hepatic expression and response to statin drugs. Pharmacogenomics J. 2011;11(4):274–286. doi: 10.1038/tpj.2010.28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goey AK, With M, Agema BC, et al. Effects of pharmacogenetic variants on vemurafenib-related toxicities in patients with melanoma. Pharmacogenomics. 2019;20(18):1283–1290. doi: 10.2217/pgs-2019-0101 [DOI] [PubMed] [Google Scholar]
- 21.Bins S, Huitema ADR, Laven P, et al. Impact of CYP3A4*22 on pazopanib pharmacokinetics in cancer patients. Clin Pharmacokinet. 2019;58(5):651–658. doi: 10.1007/s40262-018-0719-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sim S, Bergh J, Hellstrom M, Hatschek T, Xie H. Pharmacogenetic impact of docetaxel on neoadjuvant treatment of breast cancer patients. Pharmacogenomics. 2018;19(16):1259–1268. doi: 10.2217/pgs-2018-0080 [DOI] [PubMed] [Google Scholar]
- 23.De Graan AJ, Elens L, Sprowl JA, et al. CYP3A4*22 genotype and systemic exposure affect paclitaxel-induced neurotoxicity. Clin Cancer Res. 2013;19(12):3316–3324. doi: 10.1158/1078-0432.CCR-12-3786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Iwata H, Umeyama Y, Liu Y, et al. Evaluation of the association of polymorphisms with palbociclib-induced neutropenia: pharmacogenetic analysis of PALOMA-2/-3. Oncologist. 2021;26(7):e1143–e1155. doi: 10.1002/onco.13811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fritsche LG, Gruber SB, Wu Z, et al. Association of polygenic risk scores for multiple cancers in a phenome-wide study: results from The Michigan Genomics Initiative. Am J Hum Genet. 2018;102(6):1048–1061. doi: 10.1016/j.ajhg.2018.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Roncato R, Gerratana L, Palmero L, et al. An integrated pharmacological counselling approach to guide decision-making in the treatment with CDK4/6 inhibitors for metastatic breast cancer. Front Pharmacol. 2022;13:897951. doi: 10.3389/fphar.2022.897951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu YT, Hao HP, Liu CX, Wang GJ, Xie HG. Drugs as CYP3A probes, inducers, and inhibitors. Drug Metab Rev. 2007;39(4):699–721. doi: 10.1080/03602530701690374 [DOI] [PubMed] [Google Scholar]
- 28.Hodges LM, Markova SM, Chinn LW, et al. Very important pharmacogene summary: ABCB1 (MDR1, P-glycoprotein). Pharmacogenet Genom. 2011;21(3):152–161. doi: 10.1097/FPC.0b013e3283385a1c [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhai G, Teumer A, Stolk L, et al. Eight common genetic variants associated with serum DHEAS levels suggest a key role in ageing mechanisms. PLoS Genet. 2011;7(4):e1002025. doi: 10.1371/journal.pgen.1002025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lewis CM. Genetic association studies: design, analysis and interpretation. Brief Bioinform. 2002;3(2):146–153. doi: 10.1093/bib/3.2.146 [DOI] [PubMed] [Google Scholar]
- 31.Goodarzi MO, Antoine HJ, Azziz R. Genes for enzymes regulating dehydroepiandrosterone sulfonation are associated with levels of dehydroepiandrosterone sulfate in polycystic ovary syndrome. J Clin Endocrinol Metab. 2007;92(7):2659–2664. doi: 10.1210/jc.2006-2600 [DOI] [PubMed] [Google Scholar]
- 32.Garcia-Anguita A, Ortega L, Garces C. Relationship between polymorphisms in the sulfotransferase SULT2A1 gene and dehydroepiandrosterone sulfate concentration in children. Exp Biol Med (Maywood). 2013;238(2):163–166. doi: 10.1177/1535370212473698 [DOI] [PubMed] [Google Scholar]
- 33.Poonkuzhali B, Lamba J, Strom S, et al. Association of breast cancer resistance protein/ABCG2 phenotypes and novel promoter and intron 1 single nucleotide polymorphisms. Drug Metab Dispos. 2008;36(4):780–795. doi: 10.1124/dmd.107.018366 [DOI] [PubMed] [Google Scholar]
- 34.Hertz DL, Arwood MJ, Stocco G, Singh S, Karnes JH, Ramsey LB. Planning and conducting a Pharmacogenetics Association Study. Clin Pharmacol Ther. 2021;110(3):688–701. doi: 10.1002/cpt.2270 [DOI] [PubMed] [Google Scholar]
- 35.Hertz DL, Mcshane LM, Hayes DF. Defining Clinical utility of germline indicators of toxicity risk: a perspective. J Clin Oncol. 2022;40(16):1721–1731. doi: 10.1200/JCO.21.02209 [DOI] [PMC free article] [PubMed] [Google Scholar]


