Abstract
Background
Damage from insect herbivores can elicit a wide range of plant responses, including reduced or compensatory growth, altered volatile profiles, or increased production of defence compounds. Specifically, herbivory can alter floral development as plants reallocate resources towards defence and regrowth functions. For pollinator-dependent species, floral quantity and quality are critical for attracting floral visitors; thus, herbivore-induced developmental effects that alter either floral abundance or attractiveness may have critical implications for plant reproductive success. Based on past work on resource trade-offs, we hypothesize that herbivore damage-induced effects are stronger in structural floral traits that require significant resource investment (e.g. flower quantity), as plants reallocate resources towards defence and regrowth, and weaker in secondary floral traits that require less structural investment (e.g. nectar rewards).
Methods
In this study, we simulated early-season herbivore mechanical damage in the domesticated jack-o-lantern pumpkin Cucurbita pepo ssp. pepo and measured a diverse suite of floral traits over a 60-d greenhouse experiment.
Key Results
We found that mechanical damage delayed the onset of male anthesis and reduced the total quantity of flowers produced. Additionally, permutational multivariate analysis of variance (PERMANOVA) indicated that mechanical damage significantly impacts overall floral volatile profile, though not output of sesquiterpenoids, a class of compounds known to recruit specialized cucumber beetle herbivores and squash bee pollinators.
Conclusions
We show that C. pepo spp. pepo reduces investment in male flower production following mechanical damage, and that floral volatiles do exhibit shifts in production, indicative of damage-induced trait plasticity. Such reductions in male flower production could reduce the relative attractiveness of damaged plants to foraging pollinators in this globally relevant cultivated species.
Keywords: Cucurbita pepo ssp. pepo, Cucurbitaceae, simulated herbivory, energetic trade-offs, greenhouse experiment, floral traits, phenology
Graphical Abstract
Graphical Abstract.
INTRODUCTION
Herbivory is a critical force driving plant population dynamics (Maron and Crone, 2006), species interactions (Grunseich et al., 2020) and plant investment in reproductive and vegetative growth (Lemoine et al., 2017). For example, herbivores are estimated to remove 13–16 % of crop plant biomass prior to harvest and similarly reduce substantial amounts of biomass in natural ecosystems (Culliney, 2014; Jia et al., 2018). While this direct loss of biomass is significant, herbivores can also impact plants through induced changes in defence and reproductive functions (Schiestl et al., 2014). Interestingly, trade-offs may arise when damaged plants reallocate resources and upregulate defences, thereby reducing available resources for reproductive floral functions or altering floral traits due to coupled biochemical pathways (Herms and Matteson, 1992; Lemoine et al., 2017). Given that 88 % of global plant species depend on animal pollination (Ollerton and Winfree, 2011), it is essential to discern the precise effects of herbivore damage on floral traits. Despite this need, the impacts of herbivore damage on floral cues are far less understood than impacts on vegetative biomass, particularly for animal-pollinated species.
Past work with wild plants has shown that herbivore damage can alter floral quantity and phenology, as plants reallocate limited resources to defence and growth functions over reproduction (Schiestl et al., 2014). For example, in response to herbivory, plants may increase or decrease floral abundance, changing the number of inflorescences within a given area (Schiestl et al., 2014; Jacobsen and Raguso, 2018). Plasticity in floral abundance is considered an adaptive trait, allowing for enhanced plant reproduction in more favourable environmental conditions that support the resources needed to complete floral development (Sawicki et al., 2015). Beyond biotic and abiotic impacts on floral abundance, herbivory-induced loss of developing buds may also lead to a delayed initiation of anthesis, a shift in floral phenology, and a change in the total number of flowers produced (Strauss, 1997; Avila-Sakar et al., 2003). For crop species, these floral phenological shifts can be particularly critical as they impact the development of harvestable fruits, especially when wild pollinators with distinct phenologies contribute significantly to pollination (Russo et al., 2013).
In particular, herbivory can induce changes in floral traits that pollinators use to assess floral quality and nutritional rewards (Chittka and Raine, 2006; Farré-Armengol et al., 2013). Because pollinators use diverse visual, olfactory and gustatory cues to determine how frequently and for how long they will interact with a flower (Goulson, 1999; Junker and Parachnowitsch, 2015), herbivore-induced changes in floral resource investment could result in reduced pollination services on damaged plants (Kevan and Baker, 1983; Strauss, 1997). Floral abundance, floral nutritional quality, floral size, the amount of nectar and pollen produced per flower, nectar sugar concentration, pollen nutrient composition and floral volatile organic compound (VOC) composition are all known to influence plant–pollinator interactions (Strauss, 1997). Nectar provides crucial carbohydrates, attracting pollinator visitation and fuelling foraging bouts; thus, changes in either the abundance or concentration of nectar resources could potentially alter pollinator visitation and plant fitness (Roldán-Serrano and Guerra-Sanz, 2005). Similarly, bees forage for pollen to provision their offspring, and pollen is essential for larval development (Roulston and Cane, 2002); thus, changes in the availability or abundance of pollen could alter pollinator efforts (Vaudo et al., 2016). Specialized wild bees that provision nests with a limited set of pollen types may be particularly sensitive to floral VOCs that are used to orient towards specific host plant patches (Farré-Armengol et al., 2013). Therefore, a change in the amount or composition of VOC blend could alter foraging decisions (Kessler et al., 2011). Given that changes in any one of these pollinator cues could alter plant fitness, it is particularly important to understand the impact of herbivory on animal-pollinated crop species, which make up ~75 % of global crop production (Klein et al., 2007).
Plants in the genus Cucurbita represent a pollinator-dependent crop native to the Americas that are both culturally and economically important and have served as a model system to examine herbivore–pollinator interactions. Domestication of Cucurbita crops began >10 000 years ago in central Mexico with farmers selecting for plants with larger, less bitter fruit, inadvertently selecting against strong defence production of bitter cucurbitacin compounds (Smith, 1997). Today, nutrient-rich Cucurbita crops contribute significantly to foodways across the world and generate over 10 million USD in global production revenue (FAOSTAT, 2020). Further, wild and cultivated Cucurbita species are primarily monoecious, and are host to specialist insect herbivores and bee pollinators that have coevolved alongside the diversification of plant defences and floral traits (Shapiro and Mauck, 2018). Across the range of Cucurbita production in North America, the wild hoary squash bee, Xenoglossa (Peponapis) pruinosa is a main contributor of pollination services, even when managed bees are utilized (Petersen et al., 2014). Further, there is evidence that these specialist bees are sensitive to floral cues, including floral density and floral volatiles (Andrews et al., 2007; McGrady et al., 2020). Female squash bees provision nests only with pollen from plants in the Cucurbita genus, therefore, changes in male floral resources are particularly important in their foraging decisions (Hurd et al., 1971). While studies of plant–insect interactions within Cucurbita have revealed an array of inducible defences elicited by herbivores (Andrews et al., 2007; Brzozowski et al., 2019; Thompson et al., 2024), less is known about the effect of herbivore damage on floral traits, especially in male flowers, in this culturally and economically valuable domesticated species.
In this study, we investigate whether simulated early-season foliar herbivore mechanical damage in the most cultivated Cucurbita species in the USA, C. pepo ssp. pepo (USDA-NASS, 2021), alters investment in plant reproduction, especially considering male flower production and nutritional quality cues used by bee pollinators. Given the diversity of herbivore- and pollinator-induced responses within the genus Cucurbita (Theis et al., 2014), we closely designed our experiment to complement past work on the wild C. pepo ssp. texana (Theis et al., 2009) and evaluate whether mechanical damage that simulates chewing herbivore damage induces consistent floral effects in an untested and economically valuable cultivated variety, C. pepo ssp. pepo. Male flowers are first to bloom by several days in both cultivated C. pepo spp. pepo and wild C. pepo spp. texana flowers (Loy, 2004; Theis et al., 2009); therefore effects of resource reallocation due to mechanical damage are most likely to influence development of male flowers. We hypothesize that early-season mechanical damage-induced effects in C. pepo ssp. pepo are stronger for structural floral traits that require significant resource investments and weaker for secondary floral traits that require less structural investment due to reallocation of resources towards defence functions following mechanical damage. Specifically, we predict that mechanical damage will reduce plant investment in flower quantity as observed in C. pepo ssp. texana (Theis et al., 2009) due to the energetic cost of flower production. In addition to predicting fewer flowers in C. pepo ssp. pepo, we expect that the date of first flower will be delayed relative to undamaged plants as plants reallocate resources from reproductive to defence functions in response to mechanical damage. Finally, we predict that the amount and quality of both flower pollen and nectar, as well as the production of floral VOCs, will be less affected in cultivated C. pepo ssp. pepo plants due to their lower structural investment (Nepi et al., 2001; Roddy et al., 2021). By determining the effects of simulated herbivory on multiple pollinator-relevant floral traits, we quantify potential mechanisms mediating herbivore–pollinator interactions that could impact the production of a critical global crop.
MATERIALS AND METHODS
Plant cultivation
We selected a single cultivar (as per Theis et al., 2009) and planted two seeds of Cucurbita pepo ssp. pepo of the variety ‘Racer Plus (F1)’ (Johnny’s Selected Seeds, Fairfield, ME) into each of 100 3 × 3 × 2.5-cm pots filled with organic potting soil (Kellogg’s Garden Products, Carson, CA) on 28 February 2021. We selected this variety based on resistance to powdery mildew and a semi-bush growth habit, making it more amenable to greenhouse experiments, both common traits in top-selling varieties of C. pepo seeds (Weng and Sun, 2011). Further, this variety is commonly planted within our growing region between April and September, coinciding with the time of this experiment (personal communication with farmers). We germinated potted seeds at 21.6 °C under 242 μmol light (16:8, light:dark cycle) in a growth chamber (CMP050 Conviron). Every 3 d we watered seeds ad libitum and we applied half a teaspoon (~5 g) of controlled release fertilizer pellets to pots 8 d after planting to the soil surface (Osmocote Flower & Vegetable, NPK 14-14-14) (Theis et al., 2014). Eighteen days after planting, over 80 % of pots contained emerged plants, and at this time we thinned plants to achieve one plant per pot (as in Theis et al., 2009).
On 1April we transplanted 84 plants into round pots measuring 30.5 cm in diameter at the rim, 24.13 cm in diameter at the base, and 29.5 cm tall filled with 18.9 L of a local organic soil blend made from compost, rice hulls and mineral sand (Thunder Garden, Geogrowers, Austin, TX, USA) and relocated them to a plastic-enclosed hoop-house at the Brackenridge Field Laboratory (Austin, TX, USA). The hoop-house protected potted plants from herbivore damage and precluded any pollinator visits (as in Edge et al., 2012). This enclosure experienced ambient light regimes and maintained a mean temperature of 24.8 °C (s.d. = 5.01) and relative humidity of 79.14 % (s.d. = 19.35 %) over the course of the experiment by means of an evaporative air conditioner, measured by a Drop D3 data-logger (KestrelMeasurements, USA). At the time of transplanting, all plants had a minimum of two true leaves. We watered the plants via drip irrigation (1.58 L delivered at 0730 h daily) and added 5 g of fertilizer every 2 weeks starting on the day of transplanting to ensure plants were neither water- nor nutrient-limited. We enclosed plots with a trellis system to support and contain plant vines, consisting of 2-inch square wire fencing 4 feet high.
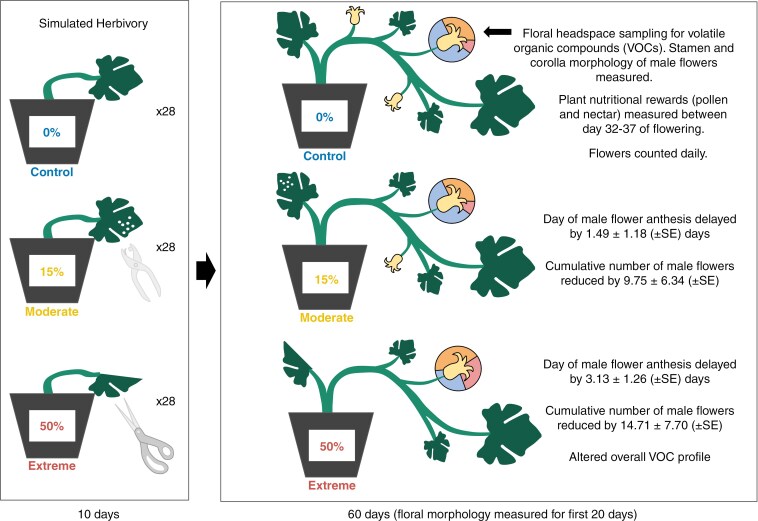
Simulated herbivory through mechanical damage treatments
To facilitate comparisons between the domesticated pumpkin, C. pepo spp. pepo, and its wild relative, C. pepo spp. texana, we followed the simulated herbivory treatment protocols of Theis et al. (2009). We chose a simulated herbivory approach via mechanical damage over herbivory via live herbivores to (1) examine the specific effect of tissue removal on floral traits and (2) reduce variation in the herbivore treatment, which can be influenced by tissue quality for live herbivores (Barman et al., 2023). Further, in the absence of studies examining oral secretion effects of the primary Cucurbita herbivores (the striped cucumber beetle, Acalymma vitattum, and squash bug, Anasa tristis; see discussions in Grunseich et al., 2020; Marmolejo et al., 2021; review Kallure et al., 2022), our mechanical damage approach allows a general test of resource trade-off effects, independent of herbivore species-specific defence cascades, which have been documented in other systems (e.g. Moreira et al., 2015). We implemented a randomized complete block design, whereby we grouped plants into 28 blocks so that plants within a block had the same number of true leaves (across-blocks range = 0–9, mean ± s.e. = 5 ± 0.21) and randomly assigned one plant per block to each of the three mechanical damage treatments (as in Hladun and Adler, 2009, Supplementary Data Fig. S1). Treatments consisted of 15 % leaf area removal (hereafter ‘moderate’ mechanical damage) by hole punch, 50 % leaf area removal (hereafter ‘extreme’ mechanical damage) by scissors, or control treatment under which leaves were held with the same grasp and for the same amount of time as used in damage treatments but did not experience leaf removal (as per Quesada et al., 1995; Theis et al., 2009) to control for plant hormonal responses to mechanical stimulation separate from mechanical damage (Waterman et al., 2019). Moderate and extreme mechanical damage levels reflect field-realistic levels of damage incurred by A. vitattum, previously observed in common garden experiments on C. pepo ssp. pepo (Hoffmann et al., 1996; Brzozowski et al., 2016). Over the course of a 10-d treatment window (2–11 April), we applied the assigned mechanical damage treatments to each expanding leaf whose axillary flower bud measured 8–15 mm long, using bleach-sterilized instruments and by taking care to avoid damaging leaf mid-veins, where many herbivores avoid feeding (Supplementary Data Fig. S2) (Avila-Sakar et al., 2003; Malishev and Sanson, 2015). We confirmed the level of leaf removal with the LeafByte app (Getman-Pickering et al., 2020). Our 10-d treatment window coincided with the pre-floral vegetative growth stage during which, under field conditions, palatable young plant tissues are at greatest risk of herbivory (Tallamy and Krischik, 1989; Haber et al., 2021). We also counted the number of true leaves and floral buds formed over the course of the 10-d treatment window to measure impacts of mechanical damage on plant vegetative growth (Supplementary Data Table S1).
Plant responses to mechanical damage
Floral phenology and morphology.
To assess the effects of mechanical damage on floral phenology, we surveyed plants daily, starting on the date of the first flower observed, 12 April 2021 (Supplementary Data Table S1). Over the 60-d flowering window, we counted and sexed each flower and recorded the date of the first anthesis for each plant (as in Theis et al., 2009). For the first 30 days, we also recorded morphological measurements for each flower. During this time all flowers produced were male. Measurements were chosen based on relevance to pollinator foraging decisions among species and cultivars in the Cucurbita genus (Strauss, 1997). Specifically, we measured stamen length, anther length, corolla angle, corolla width, corolla length and corolla size (corolla length times width) (as per Pélabon et al., 2013) (Supplementary Data Fig. S3).
Floral nutritional rewards.
To assess the effects of mechanical damage on plant investment in nutritional rewards, we collected nectar and pollen from all male flowers occurring on a given plant. We made these collections between 0730 and 0930 h over the course of 6 d (days 32–37 of the flowering window) to control for the well-documented temporal variation in nectar volume (Nepi et al., 2001). We chose to sample nectar and pollen during a period when most plants were producing flowers daily. We planned collections blocked by the date of first flower to control for variation in plant development speed; plants that did not produce a flower within this window (16.67 % of plants) were sampled on day 37 (as in Barber et al., 2013). We used a 10-μL micropipette to draw nectar from the nectaries of each flower and to measure nectar volume (Chan et al., 2021). We measured total sugar concentration from 10-μL drops of nectar with a Brix refractometer (RZ, Guangdong, China). If a clear reading was not possible with the first drop, we used a second drop (Hladun and Adler, 2009).
We harvested whole anthers from the plants into 2-mL centrifuge tubes, taking care to minimize any pollen shed while collecting. In the laboratory, we trimmed the stamen away from the base of the anther, weighed whole anthers on a carat scale (precision 0.1 mg), and then vortexed the tubes for 10 s to facilitate pollen shed in the tube. We then used forceps to scrape pollen from the anthers into the tube, weighed the shed pollen (as in Harth et al., 2016), and then stored it at −80 °C (Vaudo et al., 2020). Pollen samples were then uncapped, covered with a fine mesh screen cover, and placed in a drying cabinet at 36 °C for 24 h to dehydrate (Vaudo et al., 2016). We recorded the dry weights of each sample (precision 0.01 mg) as our measure of pollen quantity, given that a significant relationship exists between pollen volume and dry weight (Roulston et al., 2000; ln(volume) = (ln(dry weight) + 12.5)/0.95) and pollen load volume is a likely mechanism by which pollinators assess quality (Neff, 2008). Further, pollen dry weight has been used as a response variable in past herbivory studies (Theis et al., 2009).
Floral volatile cues.
To assess the effects of mechanical foliar damage on floral VOCs, we conducted dynamic headspace sampling (as per Theis et al., 2009). For each plant, we sampled the headspace of one male flower from 0600 to 1200 h during the sixth week of flowering. A vacuum pumped air through a filter loaded with 45 mg Hayesep-Q 80/100 into polyethylene bags enclosing the flowers at a rate of 250 mL min−1 (Marmolejo et al., 2021). This cleaned incoming air and minimized volatile contaminants from the shared airspace among treatments for a given flower. Floral volatiles were trapped by pulling air out of the sample bags at a rate of 200 mL min−1 across another Hayesep-Q-loaded filter (Theis et al., 2009; Marmolejo et al., 2021) (Supplementary Data Fig. S4). Volatiles collected in the filter traps were stored at room temperature and wrapped in aluminium foil to avoid degradation from light until elution.
Volatiles were eluted with 150 µL dichloromethane under a constant stream of N gas into gas chromatography–mass spectrometry (GC–MS) vials containing a narrow insert to avoid evaporation (as per Helms et al., 2019; Marmolejo et al., 2021). A volume of 2.9 µL of an anisole standard was added as an internal standard to all elutions. All headspace samples were stored at −80 °C in 2-mL clear vials with Teflon-lined caps (Maia et al., 2019). Volatiles were identified by GC–MS at the Penn State Center for Chemical Ecology (University Park, PA). A volume of 1 µL of a sample was injected in splitless mode onto a non-polar column (Agilent HP-5ms, 30 m length, 0.25 mm internal diameter, 0.25 µm phase thickness) installed in an Agilent 7890B/5977B single quadrupole GC/MS using helium carrier gas at 0.7 mL min−1. Initial temperature was held at 40 °C for 2 min and then increased at a rate of 10 °C min−1 until reaching 300 °C, at which point the maximum temperature was held for 4 min. Compounds were identified by utilizing the Agilent MassHunter Unknowns Analysis Program and searching the NIST database to identify components of sample spectra. To quantify the compounds, single representative ions were chosen for each analyte, and the peak area of each compound at their respective quantitative ion was converted to an approximated total ion count (TIC) response using a TIC response factor. This was then divided by the equivalent reconstructed TIC peak area of the internal standard and multiplied by the mass of the internal standard added to each sample.
Statistical analysis
To examine if simulated herbivory through mechanical damage influenced plant investment in initial reproductive flower formation, we constructed two separate generalized linear mixed models of leaf count and bud count on the tenth and last days of the mechanical damage treatments using treatment as a fixed effect, and accounting for block as a random intercept with a Poisson distribution, using the glmer function in the ‘lme4’ package (Bates et al., 2015). We then evaluated the ratio of Pearson’s χ2 to residual degrees of freedom to assess for overdispersion of the model and characterize parameter significance with the likelihood ratio statistic extracted with the Anova function in the ‘car’ package (Fox et al., 2021).
To examine if the effect of the mechanical damage treatments impacted plant investment in reproductive flower formation over the flower window, we used generalized mixed models to test for the main effect of treatment on the Poisson responses of first flowering day as well as cumulative flower counts, accounting for block as a random intercept. We followed the approach of Theis et al. (2009) to examine the effect of mechanical damage on cumulative flowers produced in each 5-d interval within the 60-d flowering window by constructing a model of cumulative male flower count as a factor of assigned treatment, accounting for block as a random intercept, and using a Poisson distribution with the glmer function. For intervals in which female flowers were present, we also modelled the ratio of male to female flowers as a factor of assigned treatment, accounted for block as a random intercept, and used a Poisson distribution with the glmer function. To account for repeated comparisons of flower count data over time, we calculated adjusted P-values with the Bonferroni correction with the p.adjst function and n = 12. Finally, given delayed and low female flower production, we modelled whether a plant produced any female flowers (Y, N) as a factor of treatment, accounting for block as a random intercept with a binomial distribution using the glmer function. For all flower models, we checked for overdispersion by assessing the ratio of Pearson’s χ2 to residual degrees of freedom and model significance with the likelihood ratio statistic extracted with the Anova function, as above.
We then analysed the impact of mechanical damage on floral traits separately for each flower sex, given well-established differences in flower phenology by sex, and the distinct cues and rewards each flower sex offers pollinators (Theis et al., 2014). Specifically, for the six floral morphological traits measured (stamen length, anther length, corolla angle, corolla width, corolla length and corolla size), we utilized a linear model and a MANOVA test to examine the fixed effect of treatment while controlling for random effects of block using the manova function and assessing parameter significance with a Wilks’ λ statistical test.
Next, we constructed linear mixed models to assess whether mechanical damage influenced flower nutritional rewards. Specifically, we modelled the responses of nectar volume, sugar concentration and pollen production (dry mass), as a function of treatment, accounting for plant block as a random factor with a Gaussian distribution using the lmer function in the ‘lme4’ package. We used the log transformation on nectar volume and pollen dry mass to meet assumption of normality of the linear model.
To examine floral volatile output, we first used linear mixed effect models to test whether VOC output, measured as nanograms emitted per 6-h sampling period, varied significantly by mechanical damage treatment. VOC output responses were log-transformed to meet model assumptions of normality. We modelled total emissions as well as emissions of four specific compounds [dimethoxybenzene, linalool, (E)-4,8-dimethyl-1,3,7-nonatriene (DMNT), methyl salicylate] and two classes of compounds (aromatic and sesquiterpenoid compounds) relevant to pollinating insects as a function of treatment, accounting for block as a random intercept and with a Poisson distribution, using the lmer function. To account for these seven tests of VOC emission components we calculated adjusted P-values with the Bonferroni correction with the p.adjst function. We also assessed the effect of mechanical damage treatment on similarity/dissimilarity of all VOCs emitted by permutational multivariate analysis of variance (PERMANOVA) using the adonis2 function with a Bray–Curtis dissimilarity index in the ‘vegan’ package, followed by a pairwise PERMANOVA to compare treatment groups with the permanova_pairwise function in the ‘ecole’ package (Fan et al., 2019; Smith, 2021; Werrie et al., 2021). We conducted all analyses above using the R statistical software program (v. 4.1.1).
RESULTS
Plant development
At the end of the 10-d treatment window, leaf treatment damage level did not influence the number of true leaves formed (χ2(1) = 0.67, P = 0.714). Across treatments, plants had formed a mean of 9.56 ± 0.26 (± s.e.) true leaves by the end of the 10-d treatment (range = 3–17 leaves) (Supplementary Data Fig. S5). Similarly, the number of floral buds formed by the end of the 10-d treatment window was unaffected by mechanical damage treatment (χ2(1) = 0.89, P = 0.641) (Supplementary Data Fig. S5). Overall, a mean of 10.00 ± 0.26 (± s.e.) floral buds had formed per plant by the tenth day of treatments (range = 1–20 floral buds).
Flower phenology and abundance
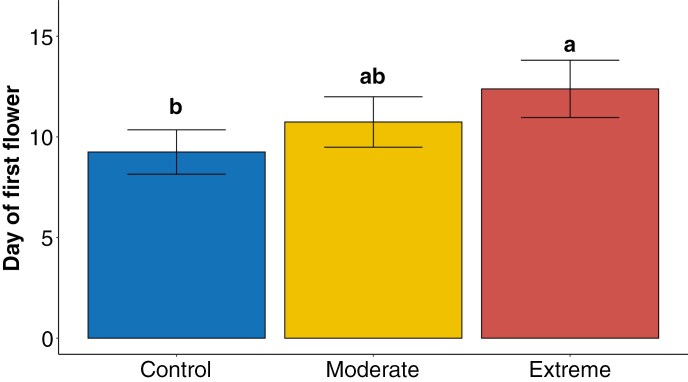
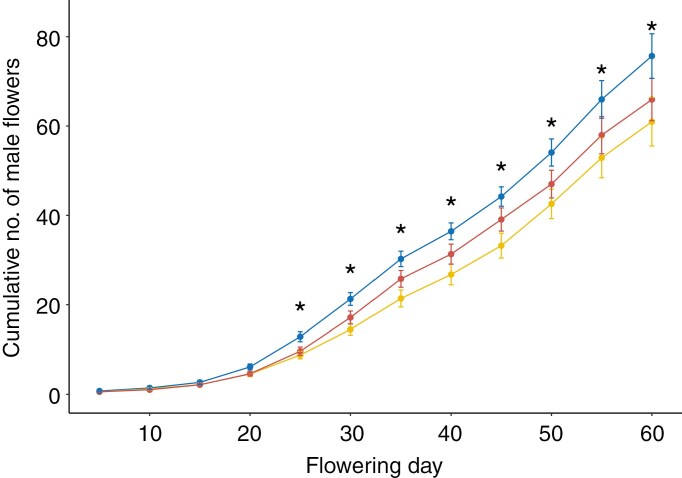
Mechanical damage treatment significantly impacted the day of first male flower (χ2(2) = 10.45, P = 0.005) (Fig. 1, Supplementary Data Table S2). Across treatments, mean day of first male flower was day 10.75 ± 6.59 (± s.e.). Specifically, 15 % moderate mechanical damage delayed the date of the first male flower by a mean of 1.49 ± 1.18 (± s.e.) days and 50 % extreme mechanical damage plants delayed the date of the first male flower by 3.13 ± 1.26 (± s.e.) days compared with undamaged control plants. By the end of the 60-d flowering window, both 15 and 50 % mechanical damage significantly reduced the cumulative number of male flowers produced by 9.75 ± 6.34 (± s.e.) and by 14.71 ± 7.70 (± s.e.) respectively, as compared with undamaged plants (z = 2.74, P = 0.017; z = 2.87, P = 0.011 respectively) (Fig. 2, Supplementary Data Tables S3 and S4). Over 3 weeks passed between the date of the first male flower and the date of the first female flower on any given plant, irrespective of treatment, with the first female flower produced on day 29 and mean first day of female flower production falling on day 47.88 ± 8.66 (± s.e.) across all treatments (Supplementary Data Fig. S6). The first day of female flower was not influenced by mechanical damage treatment (χ2(2) = 0.99, P = 0.609). After the onset of female flower anthesis, daily female flower production remained low with a mean of 0.02 ± 0.01 (± s.e.) female flowers per plant per day, leading to a cumulative total of 2.02 ± 0.23 (± s.e.) flowers produced per plant. In contrast, daily male flower production was 1.14 ± 0.11 (± s.e.) flowers per plant per day, leading to a total of 97.79 ± 70.01 (± s.e.) flowers per plant. Less than half the plants produced female flowers at all (N = 34), but whether a plant produced female flowers was not dependent on mechanical damage treatment (χ2(2) = 0.035, P = 0.982).
Fig. 1.
Mean day of first male flower produced by C. pepo ssp. pepo plants subjected to control (0 %), moderate (15 %) and extreme (50 %) levels of mechanical damage by leaf area removal in simulated herbivory treatments. Error bars represent standard error of the mean. Letters above error bars indicate significant differences between treatments (α = 0.05).
Fig. 2.
Cumulative number of male flowers produced by C. pepo ssp. pepo plants subjected to control (blue, 0 %), moderate (yellow, 15 %) and extreme (red, 50 %) levels of leaf area removal mechanical damage in simulated herbivory treatments. Error bars represent standard error of the mean of each 5-d interval. Asterisks above error bars indicate significant differences between treatments tested at each interval (α = 0.05).
Flower morphology, nutritional rewards and chemical cues
Mechanical damage treatment did not influence male floral morphology with respect to stamen, anther and corolla length, nor did it impact corolla angle and width (Wilks’ λ = 0.99, F12,2926 = 0.75, P = 0.418). Because female flowers were not present until later in the experiment, with a mean of only 0.76 ± 0.13 (± s.e.) per plant, we were unable to assess effects of mechanical damage on female flower morphology, nutritional rewards, or volatiles.
Neither nectar volume nor sugar concentration in male flowers was affected by mechanical damage treatment (χ2(2) = 0.072, P = 0.964 and χ2(2) = 0.37, P = 0.831, respectively). Across treatment levels, mean nectar volume was 27.65 ± 2.18 mL (± s.e.) per flower and sugar concentration was 34.01 ± 0.41 (± s.e.) % (Supplementary Data Fig. S7a, b). Similarly, pollen production assessed as dry pollen mass was not impacted by mechanical damage treatment (χ2(2) = 2.08, P = 0.354), averaging 41.63 ± 2.97 (± s.e.) mg per anther across treatment levels (Supplementary Data Fig. S7c).
Mechanical damage treatments did not affect the total volume of fragrance produced in male flowers (χ2(2) = 3.91, P = 0.991), nor production of any individual compound of interest previously shown to be linked to herbivore or pollinator interactions [1,4-dimethoxybenzene (χ2(2) = 1.79, P = 1.000), DMNT (χ2(2) = 3.89, P = 0.999), linalool (χ2(2) = 2.20, P = 1.000), methyl salicylate (χ2(2) = 1.35, P = 1.000)]. We found six of the top ten compounds found in C. pepo ssp. texana in our samples, while four of the compounds were absent, namely 6-methyl-5-hepten-2-one, p-anisaldehyde, 1,2,4-trimethoxybenzene and geranyl acetone. We also did not observe an effect of mechanical damage on the pollinator-relevant volatile classes of sesquiterpenes (χ2(2) = 4.11, P = 0.899) or aromatic compounds (χ2(2) = 4.54, P = 0.724). While mean emissions of several compounds were highest in extreme mechanical damage-treated flowers, these differences were not significant when controlling for block as a random effect (Supplementary Data Table S5). However, PERMANOVA revealed that the overall VOC profile was significantly impacted by mechanical damage treatment (F = 2.38, P = 0.034), with significant differences existing between control and extreme mechanical damage treatments (F = 3.86, P = 0.042) (Supplementary Data Table S6).
DISCUSSION
In this study, we found that simulated herbivory through mechanical damage delayed flower phenology and reduced overall flower count for an ecologically and economically valuable species. Specifically, we found that both 15 and 50 % leaf removal damage altered floral traits in the jack-o-lantern pumpkin, C. pepo ssp. pepo. At 50 % extreme mechanical damage, the day of first male flower was delayed by a mean of 3.13 d, overall male flower output was reduced by a mean of 14.71 flowers per plant by the end of the study period, and the VOC profile was distinct from control plants. In contrast, we did not find that mechanical damage influenced individual component floral VOC levels, nectar, or pollen production in male flowers. Female flower production was significantly delayed from the time of male anthesis, precluding our ability to examine effects of mechanical damage on most female floral traits. However, we found no evidence to suggest that mechanical damage had an effect on female floral phenology. Our results resonate with past work documenting delayed flowering onset in response to simulated and insect-mediated herbivory (Freeman et al., 2003; Theis et al., 2009; Brody and Irwin, 2012; Lemoine et al., 2017), but further quantify the differential impacts of simulated herbivory on a suite of reproductive traits; this study is also among the first to examine these patterns for a globally cultivated crop species.
Our study found that simulated herbivory through mechanical damage delays the onset of male flowering with eventual impacts on overall male flower production. We found no differences in the number of true leaves or floral buds formed at the end of the 10-d treatment window, but we found a pronounced delay in male flowering among the 50 % mechanical damage treatment plants, with lower overall flower counts, by the end of the 60-d flowering window. Though similar in direction to past work on the wild subspecies, we documented a 1- to 3-d delay for the 50 % mechanical damage treatment in C. pepo ssp. pepo, while Theis and colleagues documented a 7- to 11-d delay for the same treatment in C. pepo ssp. texana, though they similarly found no effects of herbivory on female flowering (Theis et al., 2009). Removal of plant tissue can reduce the availability of nutrients to support floral development as plants reallocate resources towards defence and regrowth and simultaneously experience reduced photosynthetic capacity with the direct loss of foliar tissue (Mattson, 1980). That we observed similar but dampened effects of mechanical damage on flower phenology in C. pepo ssp. pepo relative to the wild cultivar suggests a lower level of resource reallocation in this variety of cultivated C. pepo ssp. pepo.
In addition to delayed male flower onset, we documented an effect of simulated herbivory through mechanical damage on the cumulative number of male flowers produced across the 60-d flowering period. Similar results were found in studies of cultivated butternut squash (Cucurbita moschata), summer squash (C. pepo ssp. ovifera) (Woodson and Fargo, 1991; Hladun and Adler, 2009) and white turnip (Brassica rapa) (Schiestl et al., 2014), as well as the wildflower Ipomopsis aggregata (Brody and Irwin, 2012), using a mixture of insect-mediated herbivory (Woodson and Fargo, 1991; Hladun and Adler, 2009; Schiestl et al., 2014) and simulated herbivory approaches (Brody and Irwin, 2012). In these studies, insect-mediated herbivory reduced male flower production, resulted in overall lower fruit and seed weights (Hladun and Adler, 2009) and reduced perfect flower production and bee visitation (Schiestl et al., 2014), and simulated herbivory reduced total flowers (Brody and Irwin, 2012) but increased inflorescences per flower (Brody and Irwin, 2012). Yet in the wild Texas gourd (C. pepo ssp. texana), plants subjected to the same levels of simulated herbivory rebounded from an initial reduction in male flower production, so that by the end of a 40-d flowering window there were no differences in cumulative male flowers produced (Theis et al., 2009). Rebounds in male flower production in wild taxa may be due to the increased evolutionary pressure for combined male and female fitness, whereas in cultivated species breeding programmes may select for traits that favour conservation of female over male flower production. Recent work found that generalist bees were more likely to visit patches of a commercial pumpkin field with higher male floral density and that fruit yield was positively related to pollinator visitation rate (McGrady et al., 2020). Thus, our results suggest that C. pepo spp. pepo plants that sustain extreme herbivore damage in the vegetative phase could experience reduced pollinator visitation and subsequent fitness.
In our study, neither floral morphology nor the nutritional rewards of pollen and nectar were impacted by mechanical damage treatment, mirroring findings among Cucurbita species (e.g. Theis et al., 2009, 2014) and Brassica nigra (Bruinsma et al., 2014). Male nectar production, nectar concentration and pollen production in both the wild Texas gourd (C. pepo ssp. texana) and cultivated butternut squash (C. moschata) show similar immutability to herbivory as found in our study of the jack-o-lantern pumpkin (Hladun and Adler, 2009; Theis et al., 2009). These findings contrast with research on the wildflower I. aggregata, where mechanical damage decreased nectar production and reduced nectar sugar concentration (Irwin and Brody, 2011). Across Cucurbita plant species, reductions in pollen production within a given flower have only been observed when mechanical damage occurred after male meiosis within a given flower or along the same running vine branch of the plant (Quesada et al., 1995; Avila-Sakar et al., 2003). We collected pollen samples several weeks after mechanical damage treatments from flowers that were not yet buds at the time of mechanical damage treatments. Taken with our results, these findings indicate that pollen reduction may not be a typical outcome of early-season Cucurbita herbivore-damage-induced tissue loss, when plants can slow floral development rather than reduce floral quality (Strauss, 1997). Past work indicates that while nectar volume and concentration are particularly high in Cucurbita species, even modest reductions in both volume and concentration have been found to reduce generalist bee visitation rates and subsequent seed-set (Roldán-Serrano and Guerra-Sanz, 2005).
Simulated herbivore damage did not influence floral display morphology in our study, nor in past studies of the Texas gourd (Theis et al., 2009). Nor did insect-mediated herbivory alter floral morphology in the more distantly related member of the Cucurbitaceae family, Cucumis sativus (Barber et al., 2015). However, studies of Brassica species show that both plant species and herbivore identity influence whether insect-mediated herbivory induces changes in this trait (Schiestl et al., 2014; Rusman et al., 2019). When insect-mediated herbivory altered the floral display morphology of B. nigra, the authors implicated energetic trade-offs between bud development and leaf growth and the coupling of biochemical pathways synthesizing foliar and floral volatiles in this change (Rusman et al., 2019). Several studies document variation in foliar and root volatile responses to insect-mediated herbivory in the Cucurbitaceae family depending on herbivore species and tissue type consumed (Brzozowski et al., 2019; Grunseich et al., 2020; Thompson et al., 2024), yet such variation in herbivore damage has yet to be explored in floral responses.
We found that extreme 50 % mechanical damage levels significantly altered the overall composition but not the quantity of the floral VOC profile as compared with control plants. Past work indicates that impacts of insect-mediated herbivory on volatiles are highly dependent on the specific herbivore and plant species, where decreases, increases or no changes have been documented in the constituent compounds within a floral VOC blend or the total volume emitted (Lucas-Barbosa et al., 2011). In cases where floral blends were impacted by herbivory, the mechanism proposed in Jacobsen and Raguso (2018) states that floral VOC production will either decline due to energetic resource trade-offs between defensive and reproductive chemical synthesis or increase if defensive and reproductive biochemical pathways are correlated. As the biosynthesis of Cucurbita floral VOC production remains unstudied (Barman et al., 2023), we can hypothesize but not evaluate whether trade-offs or coupled biosynthetic pathways led to altered floral VOC composition without affecting production volume. A previous study of the wild gourd C. pepo ssp. texana showed an increase in terpenoids and aromatic components with simulated herbivore damage treatments carried out in a shared greenhouse (Theis et al., 2009). A main component class of Cucurbita floral volatiles, sesquiterpenoids, are known to attract both beneficial specialist squash bee pollinators and detrimental specialist cucumber beetle herbivores (Barman et al., 2023), and varieties that produce more of this compound attract more cucumber beetles (Theis et al., 2014). In our study, we did not find significant increases in any specific chemical compound or chemical class of compounds in response to mechanical damage. Yet C. pepo spp. pepo produced sesquiterpenoids at levels lower than other cultivated pumpkin species, including C. maxima and C. moschata, irrespective of damage treatment (Theis et al., 2014), suggesting that these lower volatile production levels might not be as sensitive to the impacts of simulated herbivory.
The observed significant delay in female flowering coupled with sparse total female flower production limited our assessment of female floral traits; however, we do note that the 3-week delay between male and female anthesis was twice (Chan and Raine, 2021) or four (Stapleton et al., 2000) times as long as reported from a field trial studying C. pepo ssp. pepo. The interval between male and female anthesis did not vary based on mechanical damage treatment and instead may be due to the lack of male pollen removal in greenhouse settings. Richardson and Stephenson (1989) found that in the absence of pollen removal the staminate phase of the protandrous Campanula rapunculoides was extended 4-fold. In our study, only one flower per plant had pollen removed for analysis, and this removal occurred ~1 week before the first female flowers bloomed. Delayed female anthesis, especially in the presence of limited pollen removal as observed in our study, could be a strategy to avoid sexual interference between male and female functions in a species with short-lived blooms such as C. pepo ssp. pepo (Barrett, 2002). Theis et al. (2014) also acknowledged that many of the Cucurbita taxa they tested in a greenhouse setting did not produce female flowers and therefore they similarly only reported herbivory effects on male flowers. A future experiment testing the effect of extensive mechanical pollen removal on female anthesis could clarify the cause of low female floral abundance for Cucurbita taxa in greenhouse settings.
Conclusions
Overall, we show that simulated herbivory in the early vegetative growth phase has substantial impacts on C. pepo spp. pepo floral phenology and abundance. In cucurbits, specialized herbivores often continue to feed on foliar, root and floral tissues throughout plant development, not just for the 10-d period of damage in our study, and thus there may be stronger reductions in flower production over the plant life cycle as energetic trade-offs accumulate (DeVeaux and Shultz, 1985; Haber et al., 2021). Further, the alterations to flowering phenology and floral abundance we documented in response to simulated herbivory underscore the potential indirect effects of foliar damage on floral resource availability. For example, decreases in floral abundance and delays in phenology could result in direct changes to the overlap between the flowering window and pollinator foraging, potentially altering whether pollination levels are sufficient for plant reproduction (Bailes et al., 2015). Beyond potential pollinator–plant phenological mismatches, herbivory-induced shifts in flowering may be exacerbated by changes in climate that also alter overall plant phenology (e.g. Elzinga et al., 2007) especially for crops, given that farmers frequently adjust planting dates in response to temperature or precipitation regimes (Fatima et al., 2020). In agricultural systems across the globe, farmers of pollinator-dependent crops are navigating management of both pest-control and pollination services (Lichtenberg et al., 2017). Thus, increased knowledge of herbivory impacts on floral traits, especially for pollinator-dependent crops, could enhance grower ability to target herbivores that are most likely to impact floral output, pollinator attraction, and fruit yields.
SUPPLEMENTARY DATA
Supplementary data are available at Annals of Botany online and consist of the following. Figure S1: experimental randomized complete block design of the 84 experimental plants (brown circles) arranged into 28 blocks (pink circle) of three plants that run the width of one of four greenhouse tables. Figure 2: axial floral buds (e.g. circled in blue) within the 8–15 mm range displayed in the centre with smaller buds not yet ready for herbivory treatment in lower left central and central cluster. Figure S3: morphometric measurements taken on each male flower produced 1–30 d after mechanical damage treatments. Figure S4: volatile sampling pump set-up. Figure S5: mean count of (a) true leaves and (b) floral buds produced by C. pepo ssp. pepo plants subjected to control (blue, 0 %), moderate (yellow, 15 %) and extreme (red, 50 %) levels of leaf area removal (simulated herbivory through mechanical damage treatments) on the tenth and last day of herbivory treatments. Error bars represent standard error of the mean. Figure S6: mean day of (a) first female flower and (b) difference between mean male and female flowers produced by C. pepo ssp. pepo plants subjected to control (blue, 0 %), moderate (yellow, 15 %) and extreme (red, 50 %) levels of leaf area removal (simulated herbivory treatments). Figure S7: mean nectar (a) sugar concentration and (b) sugar volume, and (c) pollen dry mass of male flowers produced on the 32nd to 37th days of flowering by C. pepo ssp. pepo plants subjected to control (blue, 0 %), moderate (yellow, 15 %) and extreme (red, 50 %) levels of leaf area removal (simulated herbivory treatments). Table S1: dataset characteristics from greenhouse simulated herbivory experiment study. Table S2: results of Tukey’s honest significant difference test of the effect of simulated herbivory through mechanical damage treatment level on the day of first male flower given in log scale. Table S3: analysis of deviance results of generalized linear mixed models of the effect of the three simulated herbivory treatments through mechanical damage (d.f. = 2) on cumulative male flower counts, and the ratio of cumulative female to male flower counts in 5-d increments. Ratio of female to male flower models is reported after the first female flower observed. Table S4: multiple comparisons of mean cumulative male flowers produced by simulated herbivory through mechanical damage treatment in 5-d intervals. Table S5: average emission rates of male flowers ± standard error in nanograms per flower per hour for 0 % (control), 15 % (moderate) and 50 % (extreme) mechanical damage. Table S6: pairwise PERMANOVA comparisons of VOCs between simulated herbivory through mechanical damage treatment levels using the Bray–Curtis dissimilarity index and Bonferroni adjustment for multiple comparisons of P-values.
ACKNOWLEDGEMENTS
The authors thank Larry Gilbert, Robert Plowes and Thomas Juenger for hosting this experiment at the UT Brackenridge Field Laboratory; Jason Bonnette, Colin Morrison and Jason Lawson for their assistance in setting up the experiment; and Kristen Brochu and Avehi Singh for guidance in greenhouse cultivation of cucurbits. Special thanks to Anjel Helms and Morgan Thompson for their expert advice in volatile collection methods, and Jared Gregory Ali, Nate McCartney and the PennState Center for Chemical Ecology for analysing volatile samples.
Contributor Information
Hannah L Gray, Department of Integrative Biology, University of Texas at Austin, Austin, TX 78712, USA.
Nicholas A Ivers, Department of Integrative Biology, University of Texas at Austin, Austin, TX 78712, USA.
Leeah I Richardson, Department of Integrative Biology, University of Texas at Austin, Austin, TX 78712, USA.
Margarita M López-Uribe, Department of Entomology, Pennsylvania State University, University Park, PA 16802, USA.
Shalene Jha, Department of Integrative Biology, University of Texas at Austin, Austin, TX 78712, USA; Lady Bird Johnson Wildflower Center, University of Texas, Austin, TX 78739, USA.
FUNDING
H.L.G. was supported by the United States Department of Agriculture (USDA) Postdoctoral Fellowship Program (Project No. 2020-67034-31758). M.M.L.-U. was supported by the USDA National Institute of Food and Agriculture and Hatch Appropriations (Project PEN04716 Accession No. 1020527) and the National Science Foundation CAREER Award (DEB-2046474).
AUTHOR CONTRIBUTIONS
H.L.G. designed the greenhouse experiment with substantial input by S.J. and M.M.L.-U. H.L.G., N.A.I. and L.I.R. conducted the experiment and H.L.G. analysed the data. H.L.G. wrote the initial manuscript draft and all authors contributed to revisions. Funding was acquired by H.L.G., S.J. and M.M.L.-U. to support this research.
DATA AVAILABILITY
All data associated with this manuscript can be found at ofs.org under DOI 10.17605/OSF.IO/WZ7MJ upon publication.
LITERATURE CITED
- Andrews ES, Theis N, Adler LS. 2007. Pollinator and herbivore attraction to Cucurbita floral volatiles. Journal of Chemical Ecology 33: 1682–1691. [DOI] [PubMed] [Google Scholar]
- Avila‐Sakar G, Simmers SM, Stephenson AG. 2003. The interrelationships among leaf damage, anther development, and pollen production in Cucurbita pepo ssp. texana (Cucurbitaceae). International Journal of Plant Sciences 164: 395–404. [Google Scholar]
- Bailes EJ, Ollerton J, Pattrick JG, Glover BJ. 2015. How can an understanding of plant–pollinator interactions contribute to global food security? Current Opinion in Plant Biology 26: 72–79. [DOI] [PubMed] [Google Scholar]
- Barber NA, Kiers ET, Theis N, Hazzard RV, Adler LS. 2013. Linking agricultural practices, mycorrhizal fungi, and traits mediating plant–insect interactions. Ecological Applications 23: 1519–1530. [DOI] [PubMed] [Google Scholar]
- Barber NA, Milano NJ, Kiers ET, et al. 2015. Root herbivory indirectly affects above- and below-ground community members and directly reduces plant performance. Journal of Ecology 103: 1509–1518. [Google Scholar]
- Barman M, Tenhaken R, Dötterl S. 2023. A review on floral scents and pigments in cucurbits: their biosynthesis and role in flower visitor interactions. Scientia Horticulturae 322: 112402. [Google Scholar]
- Barrett S. 2002. Sexual interference of the floral kind. Heredity 88: 154–159. [DOI] [PubMed] [Google Scholar]
- Bates D, Mächler M, Bolker B, Walker S. 2015. Fitting linear mixed-effects models using lme4. Journal of Statistical Software 67. https://doi.org/ 10.48550/arXiv.1406.5823. [DOI] [Google Scholar]
- Brody AK, Irwin RE. 2012. When resources don’t rescue: flowering phenology and species interactions affect compensation to herbivory in Ipomopsis aggregata. Oikos 121: 1424–1434. [Google Scholar]
- Bruinsma M, Lucas-Barbosa D, ten Broeke CJM, et al. 2014. Folivory affects composition of nectar, floral odor and modifies pollinator behavior. Journal of Chemical Ecology 40: 39–49. [DOI] [PubMed] [Google Scholar]
- Brzozowski LJ, Leckie BM, Gardner J, Hoffmann MP, Mazourek M. 2016. Cucurbita pepo subspecies delineates striped cucumber beetle (Acalymma vittatum) preference. Horticulture Research 3: 16028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brzozowski LJ, Mazourek M, Agrawal AA. 2019. Mechanisms of resistance to insect herbivores in isolated breeding lineages of Cucurbita pepo. Journal of Chemical Ecology 45: 313–325. [DOI] [PubMed] [Google Scholar]
- Chan DSW, Raine NE. 2021. Phenological synchrony between the hoary squash bee (Eucera pruinosa) and cultivated acorn squash (Cucurbita pepo) flowering is imperfect at a northern site. Current Research in Insect Science 1: 100022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chittka L, Raine N. 2006. Recognition of flowers by pollinators. Current Opinion in Plant Biology 4: 428–435. [DOI] [PubMed] [Google Scholar]
- Culliney TW. 2014. Crop losses to arthropods. In: Pimentel D, Peshin R. .eds. Integrated pest management: pesticide problems, Vol. 3. Dordrecht: Springer, 201–225. [Google Scholar]
- DeVeaux JS, Shultz EB. 1985. Development of buffalo gourd (Cucurbita foetidissima) as a semiarid land starch and oil crop. Economic Botany 39: 454–472. [Google Scholar]
- Edge AA, van Nest BN, Johnson JN, et al. 2012. Diel nectar secretion rhythm in squash (Cucurbita pepo) and its relation with pollinator activity. Apidologie 43: 1–16. [Google Scholar]
- Elzinga JA, Atlan A, Biere A, Gigord L, Weis AE, Bernasconi G. 2007. Time after time: flowering phenology and biotic interactions. Trends in Ecology & Evolution 22: 432–439. [DOI] [PubMed] [Google Scholar]
- Fan J, Zhang W, Zhang D, Wang G, Cao F. 2019. Flowering stage and daytime affect scent emission of Malus ioensis ‘Prairie Rose’. Molecules 24: 2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FAOSTAT. 2020. Food and Agriculture Organization of the United Nations Statistical Database. Rome: FAO. http://faostat.fao.org. Accessed 28 June 2022. [Google Scholar]
- Farré-Armengol G, Filella I, Llusia J, Peñuelas J. 2013. Floral volatile organic compounds: between attraction and deterrence of visitors under global change. Perspectives in Plant Ecology, Evolution and Systematics 15: 56–67. [Google Scholar]
- Fatima Z, Ahmed M, Hussain M, et al. 2020. The fingerprints of climate warming on cereal crops phenology and adaptation options. Scientific Reports 10: 18013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox J, Weisberg S, Adler D, et al. 2021. car: Companion to Applied Regression. http://socserv.socsci.mcmaster.ca/jfox/ (25 March 2023, date last accessed).
- Freeman RS, Brody AK, Neefus CD. 2003. Flowering phenology and compensation for herbivory in Ipomopsis aggregata. Oecologia 136: 394–401. [DOI] [PubMed] [Google Scholar]
- Getman‐Pickering ZL, Campbell A, Aflitto N, Grele A, Davis JK, Ugine TA. 2020. LeafByte: a mobile application that measures leaf area and herbivory quickly and accurately. Methods in Ecology and Evolution 11: 215–221. [Google Scholar]
- Goulson D. 1999. Foraging strategies of insects for gathering nectar and pollen, and implications for plant ecology and evolution. Perspectives in Plant Ecology, Evolution and Systematics 2: 185–209. [Google Scholar]
- Grunseich J, Thompson M, Hay A, et al. 2020. Risky roots and careful herbivores: sustained herbivory by a root-feeding herbivore attenuates indirect plant defenses. Functional Ecology 34: 1779–1789. [Google Scholar]
- Haber AI, Wallingford AK, Grettenberger IM, Ramirez Bonilla JP, Vinchesi-Vahl AC, Weber DC. 2021. Striped cucumber beetle and western striped cucumber beetle (Coleoptera: Chrysomelidae). Journal of Integrated Pest Management 12: 1–1. doi: https://doi.org/ 10.1093/jipm/pmaa026. [DOI] [Google Scholar]
- Harth JE, Winsor JA, Weakland DR, Nowak KJ, Ferrari MJ, Stephenson AG. 2016. Effects of virus infection on pollen production and pollen performance: implications for the spread of resistance alleles. American Journal of Botany 103: 577–583. [DOI] [PubMed] [Google Scholar]
- Helms A, Ray S, Matulis N, et al. 2019. Chemical cues linked to risk: cues from below-ground natural enemies enhance plant defences and influence herbivore behaviour and performance. Functional Ecology 33: 798–808. [Google Scholar]
- Herms DA, Mattson WJ. 1992. The dilemma of plants: to grow or defend. Quarterly Review of Biology 67: 283–335. [Google Scholar]
- Hladun KR, Adler LS. 2009. Influence of leaf herbivory, root herbivory, and pollination on plant performance in Cucurbita moschata. Ecological Entomology 34: 144–152. [Google Scholar]
- Hoffmann MP, Robinson RW, Kyle MM, Kirkwyland JJ. 1996. Defoliation and infestation of Cucurbita pepo genotypes by diabroticite beetles. HortScience 31: 439–442. [Google Scholar]
- Hurd PD, Linsley EG, Whitaker TW. 1971. Squash and gourd bees (Peponapis, Xenoglossa) and the origin of the cultivated Cucurbita. Evolution 25: 218–234. [DOI] [PubMed] [Google Scholar]
- Irwin RE, Brody AK. 2011. Additive effects of herbivory, nectar robbing and seed predation on male and female fitness estimates of the host plant Ipomopsis aggregata. Oecologia 166: 681–692. [DOI] [PubMed] [Google Scholar]
- Jacobsen DJ, Raguso RA. 2018. Lingering effects of herbivory and plant defenses on pollinators. Current Biology 28: R1164–R1169. [DOI] [PubMed] [Google Scholar]
- Jia S, Wang X, Yuan Z, et al. 2018. Global signal of top-down control of terrestrial plant communities by herbivores. Proceedings of the National Academy of the Sciences of the USA 115: 6237–6242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Junker RR, Parachnowitsch AL. 2015. Working towards a holistic view on flower traits – how floral scents mediate plant-animal interactions in concert with other floral characters. Journal of the Indian Institute of Science 95: 43–67. [Google Scholar]
- Kallure GS, Kumari A, Shinde BA, Giri AP. 2022. Characterized constituents of insect herbivore oral secretions and their influence on the regulation of plant defenses. Phytochemistry 193: 113008. [DOI] [PubMed] [Google Scholar]
- Kessler A, Halitschke R, Poveda K. 2011. Herbivory-mediated pollinator limitation: negative impacts of induced volatiles on plant-pollinator interactions. Ecology 92: 1769–1780. [DOI] [PubMed] [Google Scholar]
- Kevan PG, Baker HG. 1983. Insects as flower visitors and pollinators. Annual Review of Entomology 28: 407–453. [Google Scholar]
- Klein AM, Vaissière BE, Cane JH, et al. 2007. Importance of pollinators in changing landscapes for world crops. Proceedings Biological Sciences 274: 303–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemoine N, Doublet D, Salminen J, Burkepile D, Parker J. 2017. Responses of plant phenology, growth, defense, and reproduction to interactive effects of warming and insect herbivory. Ecology 98: 1817–1828. [DOI] [PubMed] [Google Scholar]
- Lichtenberg EM, Kennedy CM, Kremen C, et al. 2017. A global synthesis of the effects of diversified farming systems on arthropod diversity within fields and across agricultural landscapes. Global Change Biology 23: 4946–4957. [DOI] [PubMed] [Google Scholar]
- Loy JB. 2004. Morpho-physiological aspects of productivity and quality in squash and pumpkins (Cucurbita spp.). Critical Reviews in Plant Sciences 23: 337–363. [Google Scholar]
- Lucas-Barbosa D, van Loon JJA, Dicke M. 2011. The effects of herbivore-induced plant volatiles on interactions between plants and flower-visiting insects. Phytochemistry 72: 1647–1654. [DOI] [PubMed] [Google Scholar]
- Maia ACD, Grimm C, Schubert M, et al. 2019. Novel floral scent compounds from night-blooming Araceae pollinated by cyclocephaline scarabs (Melolonthidae, Cyclocephalini). Journal of Chemical Ecology 45: 204–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malishev M, Sanson GD. 2015. Leaf mechanics and herbivory defence: how tough tissue along the leaf body deters growing insect herbivores. Austral Ecology 40: 300–308. [Google Scholar]
- Marmolejo LO, Thompson MN, Helms AM. 2021. Defense suppression through interplant communication depends on the attacking herbivore species. Journal of Chemical Ecology 47: 1049–1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maron J, Crone E. 2006. Herbivory: effects on plant abundance, distribution and population growth. Proceedings of the Royal Society B: Biological Sciences 273: 2575–2584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson WJ. 1980. Herbivory in relation to plant nitrogen content. Annual Review of Ecology and Systematics 11: 119–161. [Google Scholar]
- McGrady CM, Troyer R, Fleischer SJ, Strange J. 2020. Wild bee visitation rates exceed pollination thresholds in commercial Cucurbita agroecosystems. Journal of Economic Entomology 113: 562–574. [DOI] [PubMed] [Google Scholar]
- Moreira X, Abdala-Roberts L, Hernández-Cumplido J, Cuny MAC, Glauser G, Benrey B. 2015. Specificity of induced defenses, growth, and reproduction in lima bean (Phaseolus lunatus) in response to multispecies herbivory. American Journal of Botany 102: 1300–1308. [DOI] [PubMed] [Google Scholar]
- Neff JL. 2008. Components of nest provisioning behavior in solitary bees (Hymenoptera: Apoidea). Apidologie 39: 30–45. [Google Scholar]
- Nepi M, Guarnieri M, Pacini E. 2001. Nectar secretion, reabsorption, and sugar composition in male and female flowers of Cucurbita pepo. International Journal of Plant Sciences 162: 353–358. [Google Scholar]
- Ollerton J, Winfree R, Tarrant S. 2011. How many flowering plants are pollinated by animals? Oikos 120: 321–326. [Google Scholar]
- Pélabon C, Osler NC, Diekmann M, Graae BJ. 2013. Decoupled phenotypic variation between floral and vegetative traits: distinguishing between developmental and environmental correlations. Annals of Botany 111: 935–944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen JD, Reiners S, Nault BA. 2014. Pollination services provided by bees in pumpkin fields supplemented with either Apis mellifera or Bombus impatiens or not supplemented. PLoS One 8: e69819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quesada M, Bollman K, Stephenson AG. 1995. Leaf damage decreases pollen production and hinders pollen performance in Cucurbita texana. Ecology 76: 437–443. [Google Scholar]
- Richardson TE, Stephenson AG. 1989. Pollen removal and pollen deposition affect the duration of the staminate and pistillate phases in Campanula rapunculoides. American Journal of Botany 76: 532–538. [Google Scholar]
- Roddy A, Martínez-Perez C, Teixido A, et al. 2021. Towards the flower economics spectrum. New Phytologist 299: 665–672. [DOI] [PubMed] [Google Scholar]
- Roldán-Serrano AS, Guerra-Sanz JM. 2005. Reward attractions of zucchini flowers (Cucurbita pepo L.) to bumblebees (Bombus terrestris L.). European Journal of Horticultural Science 70: 23–28. [Google Scholar]
- Roulston TH, Cane JH, Buchmann SL. 2000. What governs protein content of pollen: pollinator preferences, pollen-pistil interactions, or phylogeny? Ecological Monographs 70: 617–643. [Google Scholar]
- Roulston TH, Cane J. 2002. The effect of pollen protein concentration on body size in the sweat bee Lasioglossum zephyrum (Hymenoptera: Apiformes). Evolutionary Ecology 16: 49–65. [Google Scholar]
- Rusman Q, Lucas-Barbosa D, Poelman EH, Dicke M. 2019. Ecology of plastic flowers. Trends in Plant Science 24: 725–740. [DOI] [PubMed] [Google Scholar]
- Russo L, Debarros N, Yang S, Shea K, Mortensen D. 2013. Supporting crop pollinators with floral resources: network-based phenological matching. Ecology and Evolution 3: 3125–3140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawicki M, Barka A, Clément C, Vaillant-Gaveau N, Jacquard C. 2015. Cross-talk between environmental stresses and plant metabolism during reproductive organ abscission. Journal of Experimental Biology 66: 1707–1719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiestl FP, Kirk H, Bigler L, Cozzolino S, Desurmont GA. 2014. Herbivory and floral signaling: phenotypic plasticity and tradeoffs between reproduction and indirect defense. New Phytologist 203: 257–266. [DOI] [PubMed] [Google Scholar]
- Shapiro LR, Mauck KE. 2018. Chemically-mediated interactions among cucurbits, insects and microbes. In: Tabata J. ed. Chemical ecology of insects. Boca Raton: CRC Press, 2017, 55–90. [Google Scholar]
- Smith B. 1997. The initial domestication of Cucurbita pepo in the Americas 10,000 years ago. Science 276: 932–934. [Google Scholar]
- Smith RJ. 2021. Ecole: School of Ecology Package v. 0.9-2021. https://github.com/phytomosaic/ecole (2 January 2023, date last accessed). [Google Scholar]
- Stapleton SC, Wien HC, Morse RA. 2000. Flowering and fruit set of pumpkin cultivars under field conditions. HortScience 35: 1074–1077. [Google Scholar]
- Strauss SY. 1997. Floral characters link herbivores, pollinators, and plant fitness. Ecology 78: 1640–1645. [Google Scholar]
- Tallamy D, Krischik V. 1989. Variation and function of cucurbitacins in Cucurbita: an examination of current hypotheses. American Naturalist 133: 766–786. [Google Scholar]
- Theis N, Barber NA, Gillespie SD, Hazzard RV, Adler LS. 2014. Attracting mutualists and antagonists: plant trait variation explains the distribution of specialist floral herbivores and pollinators on crops and wild gourds. American Journal of Botany 101: 1314–1322. [DOI] [PubMed] [Google Scholar]
- Theis N, Kesler K, Adler LS. 2009. Leaf herbivory increases floral fragrance in male but not female Cucurbita pepo subsp. texana (Cucurbitaceae) flowers. American Journal of Botany 96: 897–903. [DOI] [PubMed] [Google Scholar]
- Thompson MN, Arriaga J, Bradford BJ, Kurian R, Strozier G, Helms AM. 2024. Belowground insect herbivory induces systemic volatile emissions that strengthen neighbouring plant resistance aboveground. Plant, Cell & Environment 47: 714–725. [DOI] [PubMed] [Google Scholar]
- USDA-NASS. 2021. Vegetable Crops 2020. https://downloads.usda.library.cornell.edu/usda-esmis/files/02870v86p/j6731x86f/9306tr664/vegean21.pdf (6 May 2023, date last accessed).
- Vaudo AD, Patch H, Mortensen D, Tooker J, Grozinger C. 2016. Macronutrient ratios in pollen shape bumble bee (Bombus impatiens) foraging strategies and floral preferences. Proceedings of the National Academy of Sciences of the USA 113: E4035–E4042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaudo AD, Tooker JF, Patch HM, et al. 2020. Pollen protein: lipid macronutrient ratios may guide broad patterns of bee species floral preferences. Insects 11: 132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waterman JM, Cazzonelli CI, Hartley SE, Johnson SN. 2019. Simulated herbivory: the key to disentangling plant defence responses. Trends in Ecology & Evolution 34: 447–458. [DOI] [PubMed] [Google Scholar]
- Weng Y, Sun ZY. 2011. Major cucurbit crops. In: Wang Y-H, Behera T, Kole C. eds. Genetics, genomics, and breeding of cucurbits. Boca Raton: CRC Press, 1–15. [Google Scholar]
- Werrie P, Burgeon C, Le Goff G, Hance T, Fauconnier M. 2021. Biopesticide trunk injection into apple trees: a proof of concept for the systemic movement of mint and cinnamon essential oils. Frontiers in Plant Science 12: 650132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodson DW, Fargo SW. 1991. Interactions of temperature and squash bug density (Hemiptera: Coreidae) on growth of seedling squash. Journal of Economic Entomology 84: 886–890. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data associated with this manuscript can be found at ofs.org under DOI 10.17605/OSF.IO/WZ7MJ upon publication.