Abstract
The total synthesis of the Ganoderma meroterpenoid ganoapplanin, an inhibitor of T-type voltage-gated calcium channels, is reported. Our synthetic approach is based on the convergent coupling of a readily available aromatic polyketide scaffold with a bicyclic terpenoid fragment. The three contiguous stereocenters of the terpenoid fragment, two of which are quaternary, were constructed by a diastereoselective, titanium-mediated iodolactonization. For the fusion of the two fragments and to simultaneously install the crucial biaryl bond, we devised a highly effective two-component coupling strategy. This event involves an intramolecular 6-exo-trig radical addition of a quinone monoacetal followed by an intermolecular aldol reaction. A strategic late-stage oxidation sequence allowed the selective installation of the remaining oxygen functionalities and the introduction of the characteristic spiro bisacetal structure of ganoapplanin.
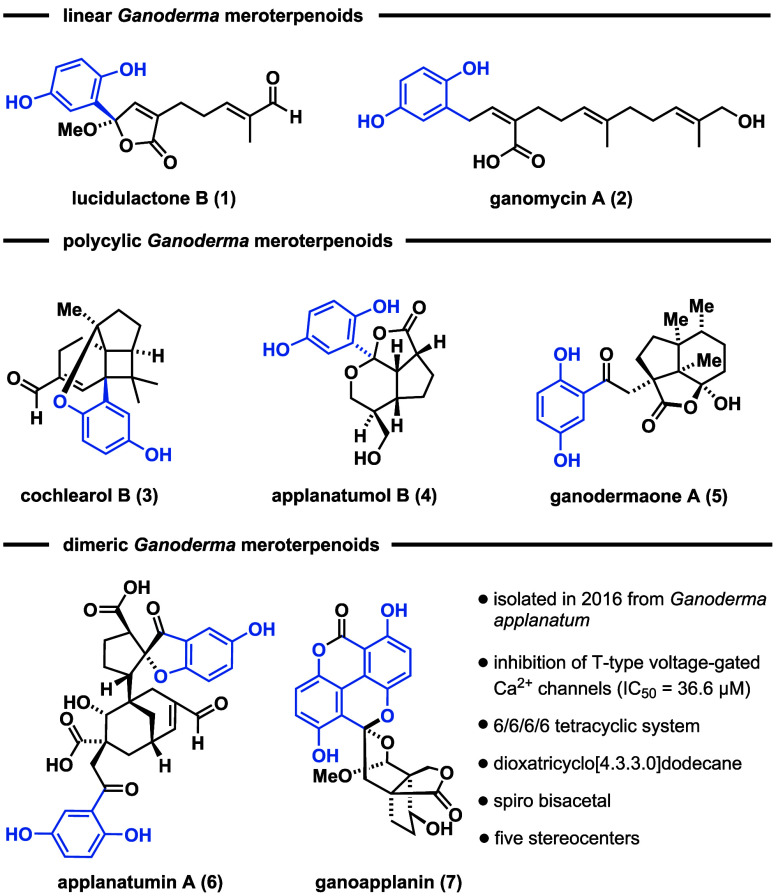
Ganoderma meroterpenoids (GMs) are a class of structurally diverse natural products, comprising more than hundred members, isolated from fungi of the genus Ganoderma.1 Structurally, these secondary metabolites share a common hydroquinone motif, connected to a farnesyl- or geranyl-derived terpenoid scaffold. According to the known structural modifications, three subclasses have been identified: linear, polycyclic, and dimeric GMs (Figure 1). Linear GMs consist of a hydroquinone connected to either a C10 (e.g., lucidulactone B (1)2) or a C15 terpenoid chain (e.g., ganomycin A (2)3). Conversely, cochlearol B (3),4 applanatumol B (4),5 and ganodermaone A (5)6 contain a hydroquinone motif, connected to a polycyclic ring system. The subclass of dimeric GMs, as exemplified by applanatumin A (6)7 and ganoapplanin (7),8 consists of two hydroquinone units attached to a terpenoid backbone, further increasing the level of structural complexity. Several GMs exhibit notable bioactivity profiles including antioxidant, antifibrotic, and antimicrobial activities.1
Figure 1.
Selected structures of Ganoderma meroterpenoids and their classification.
Altogether, these features render GMs appealing targets for total synthesis.9,10 While polycyclic GMs, such as cochlearol B (3)11−14 and applanatumol B (4),15,16 have been synthesized numerous times, there are only few reports on the synthesis of dimeric GMs.17,18 Notably, the synthesis of ganoapplanin (7) has not been achieved so far.
Ganoapplanin (7), isolated by Qiu in racemic form from the fungus Ganoderma applanatum in 2016, stands out from a structural perspective.8 It features five contiguous stereocenters, two of which are quaternary, and an unprecedented spiro bisacetal skeleton, constructed from a 6/6/6/6 tetracyclic system, including a tetra-ortho substituted biaryl motif (highlighted in blue) and a dioxatricyclo[4.3.3.0]dodecane scaffold.
In addition to its structural complexity, racemic 7 inhibits T-type voltage-gated calcium channels (IC50 = 36.6 μM), showing potential as a lead compound for the development of novel therapeutics against neurodegenerative diseases (e.g., epilepsy and Parkinson’s disease).19,20
In recent years, our group has developed synthetic methods to access polysubstituted arenes21 and heteroarenes22 to facilitate the total syntheses of natural products structurally related to the GMs.23,24 However, the distinctive substitution pattern of ganoapplanin (7), particularly the highly congested central region, connecting the terpenoid scaffold with the tetra-ortho substituted biaryl motif, proved incompatible with those protocols. We therefore drew inspiration from the proposed biosynthesis of 7 (Scheme 1A),8 which involves the nucleophilic addition of phenol 8 to lingzhilactone (9) followed by bisacetalization and diazotation to yield 10. A subsequent intramolecular Gomberg–Bachmann cyclization (Pschorr cyclization) via I and II then forges the C3–C3a biaryl bond. Suspecting that the bisacetal scaffold of 10 would be unstable and difficult to access by synthetic methods, we evaluated alternative strategies that would allow its late-stage installation. Finally, the proposed aryl radical I guided us toward a convergent strategy involving a radical addition/aldol reaction cascade. Herein, we present the realization of this strategy, resulting in the first total synthesis of ganoapplanin (7).
Scheme 1. (A) Proposed Biosynthesis of Ganoapplanin (7) and (B) Retrosynthetic Disconnections.

In our retrosynthesis (Scheme 1B), we decided to install both the lactone moiety of ganoapplanin (7) and the C4a oxygen functionality of chromene 11, required for spiro bisacetal formation, via late-stage oxidation (vide infra).25 Further simplification of 12 provided dearomatized acetal 13, which contains the retron for an intramolecular 1,4-addition/aldol reaction between quinone monoacetal 14 and aldehyde 15. This strategy was inspired by the seminal work of Utimoto,26 a related recent report by Inoue27 and Li,28 which was envisioned to install the crucial C3–C3a biaryl and C1–C2 bonds in a single operation. Aldehyde 15 was envisioned to be accessible via a titanium(IV)-mediated iodolactonization of alkene 16,29,30 which can be dissected to aldehyde 17 vinyl iodide 18.
The southern fragment 15(31) was synthesized in five steps as shown in Scheme 2A. The sequence started with a one-pot Nozaki–Hiyama–Kishi (NHK) reaction between aldehyde 17(32,33) and vinyl iodide 18(34,35) to form the corresponding secondary alcohol, which was protected in situ to give TBS ether 16. Treatment of 16 with Ti(Ot-Bu)4, CuO and excess iodine, conditions previously reported by Taguchi,29,30 furnished bicyclic lactone 19 as a single diastereomer in up to 61% yield on decagram scale. The excellent diastereoselectivity has previously been attributed to a chairlike transition state and stereoelectronic effects favoring an orthogonal alignment of the alkene and the allylic substituent (OTBS) for the 5-exo-trig cyclization of lll to lV. Conversion to the aldehyde was achieved by removing the methylester by employing Krapcho conditions (LiCl, H2O, DMSO, 150 °C), followed by allylation of the lactone and subsequent ozonolysis to yield aldehyde 15.
Scheme 2. (A) Synthesis of Aldehyde 14 and a (B) Radical 1,4-Addition/Aldol Sequence.

Having achieved the synthesis of aldehyde 15, we turned our attention to the construction of the aromatic fragment 14 (Scheme 2B). For this purpose, phenol 22(36,37) was initially protected as its methoxymethyl (MOM) ether with concomitant ester formation. The ester group was reduced by treatment with DIBAL-H to afford benzylic alcohol 23. Exposure of 23 to a one-pot mesylation/bromination protocol yielded benzyl bromide 24, which was then subsequently treated with excess 1,4-hydroquinone in the presence of potassium carbonate to furnish benzyl ether 25.
Eventually, oxidative dearomatization using phenyliodine(III) diacetate (PIDA) gave quinone monoacetal 14 in 82% yield.38 With access to the aromatic and terpenoid fragments 14 and 15, the stage was set for their fusion. Initially, we investigated the intramolecular 1,4-addition of 14. We found that metalation of 14 via metal/halogen exchange employing either t-BuLi or Turbo Grignard (i-PrMgCl•LiCl) only led to decomposition. However, treating 14 with azobis(isobutyronitrile) (AIBN) and tributyltin hydride (n-Bu3SnH) at elevated temperatures successfully initiated the intended 1,4-addition. Unfortunately, efforts to achieve the subsequent aldol reaction by deprotonation with either LDA, KHMDS, or LHMDS at −78 °C followed by the addition of aldehyde 15 failed to yield the desired aldol-product (see the Supporting Information for details). We then focused on a one-pot procedure to realize the radical 1,4-addition/aldol reaction cascade. Eventually, we discovered that radical initiation with triethyl borane and oxygen39 in the presence of tributyltin hydride induced the intramolecular radical 1,4-addition40 of 14 and also promoted the intermolecular aldol reaction with aldehyde 15 to provide 13 as an inconsequential mixture of diastereomers in 81% yield.26,27,41 Triethyl borane plays a dual role in this process: (1) radical initiation to enable the 6-exo-trig cyclization of aryl radical V and (2) formation of boron enolate V to promote the aldol reaction with 15. This sequence facilitated the convergent fusion of both fragments in high yields, establishing the crucial C3–C3a bond and the C1–C2 bond in a single step. It is important to note that the presence of both reagents, triethyl borane and tributyltin hydride, was critical for the reaction as the desired reactivity was not observed in the absence of either reagent. To the best of our knowledge, the substrate combination employed in this step is unprecedented in the chemical literature rendering it a unique transformation. Noteworthy, a stepwise process involving isolation of the 1,4-addition product and subsequent generation of boron enolate V in the presence of either dibutylboron triflate (n-Bu2BOTf) or dicyclohexylboron triflate (c-Hex2BOTf) were ineffective to afford 13 (see the Supporting Information for details). Surprisingly, attempts to perform the sequence in the presence of the C4a oxygen functionality failed and only the 1,4-addition product was formed (see the Supporting Information for details). Similar issues have been observed in previous attempts to install the C3–C3a bond via transition metal-catalyzed cross-coupling reactions. Consequently, we aimed for a late-stage oxidation to install the missing C4a phenol.
To form the biaryl motif, we then proceeded with the aromatization of 13 (Scheme 3). To this end, secondary alcohol 13 was first oxidized with Dess–Martin periodinane (DMP) to the ketone, followed by treatment with p-toluenesulfonic acid (p-TsOH) to give 26 in around 54% yield, although this process proved to be unreliable and difficult to reproduce. We found that exposure of the ketone to 1,8-diazabicyclo[5.4.0]undec-7-ene (DBU) leads to the formation of an inconsequential mixture of diastereomers, which upon treatment with p-TsOH undergoes smooth aromatization. Contrary to our initial expectations, the 1H NMR spectra of the 1,3-diketones obtained after DMP oxidation, as well as DBU treatment did not indicate any enol form. While this step clearly facilitates the acid-catalyzed aromatization, the exact role of DBU, presumably only epimerization, remains uncertain.
Scheme 3. Late-Stage Oxidations and Completion of the Synthesis of Ganoapplanin.

With synthetic access to phenol 26, we then proceeded with the endgame of the synthesis. Debenzylation of 26 to alcohol 27 was affected by treatment with Pearlma’s catalyst under 50 bar hydrogen pressure. Oxidation of the resulting neopentylic alcohol 27 to the corresponding aldehyde was found to require protection of the phenol to prevent significant decomposition. Chemoselective acetylation of the phenol was achieved by treatment with acetic anhydride and triethylamine. Exposure to DMP yielded aldehyde 28, which was converted to phenol 12 through sequential deprotections with bromotrimethylsilane (TMSBr) and hydrogen fluoride pyridine, respectively. We then attempted to install the missing oxygen functionality via selective C4a oxidation. After several failed attempts, we found that oxidation of 12 with phenyliodine bis(trifluoroacetate) (PIFA) in an aqueous mixture of acetone and acetonitrile led to smooth oxidation to give quinone 29. The presence of an electron-rich aromatic system proved to be crucial for this oxidation, as no C4a oxidation could be achieved in the presence of the lactone at C7a. The conversion of 29 to its hydroquinone VII was accomplished using sodium dithionite, resulting in the formation of an unstable intermediate, presumably hemiacetal VIII. Immediate treatment with p-TsOH and trimethyl orthoformate in methanol formed spiro bisacetal 11 as a single diastereomer. We believe that the excellent diastereoselectivity is a result of anomeric stabilization, as observed in the single crystal X-ray structure of ganoapplanin (7) (see the Supporting Information for details). With access to the complete carbon scaffold of ganoapplanin (7), the final steps required a seemingly straightforward benzylic oxidation. Given the scarcity of reports on benzylic oxidations in the presence of unprotected phenols, we decided to convert phenol 11 into its acetyl ester 30. Initial attempts to realize the oxidation of the benzylic position using standard conditions such as Jones reagent, 2,3-dichloro-5,6-dicyano-1,4-benzoquinone (DDQ) and MeOH or DDQ and tert-butyl hydroperoxide (TBHP) resulted in decomposition. However, employing a copper(l) mediated oxidation protocol in the presence of TBHP42 allowed for the formation of lactone 31 in 47% yield. Subsequent deacetylation was then affected by treatment with potassium carbonate in methanol to give ganoapplanin (7).
The choice of the employed protecting group proved to be crucial, as earlier attempts using benzyl protecting groups on the phenols failed to give 7 due to unsuccessful deprotections. The spectroscopic data for synthetic 7 were in full agreement with those reported in the literature.8 In summary, we have accomplished the first total synthesis of the dimeric Ganoderma meroterpenoid ganoapplanin (7). The bicyclic terpenoid fragment 15 was constructed employing a diastereoselective, titanium(IV)-mediated iodolactonization. For the fusion with arene component 14 we developed a highly efficient two-component radical 1,4-addition/aldol reaction sequence. Two late-stage oxidations facilitated the installation of the characteristic spiro bisacetal and lactone moieties of ganoapplanin (7). We expect that the efficiency of the developed key sequence will facilitate the synthesis of structurally related natural products.
Acknowledgments
This research was funded in whole or in part by the Austrian Science Fund (FWF) [10.55776/P33894] and the Tyrolean Science Fund TWF (F.33842/7-2021 to N.M.). For open access purposes, the author has applied a CC BY public copyright license to any author accepted manuscript version arising from this submission. We acknowledge the European Research Council under the European Union’s Horizon 2020 research and innovation program (grant agreement no. 101000060), the Austrian Academy of Sciences (OeAW), and the Center for Molecular Biosciences CMBI. Dr. Ondřej Kováč is grateful to the Experientia Foundation for financial support. We are grateful to Dr. Tobias Pinkert (University of Innsbruck) and Dr. Franz-Lucas Haut (FU Berlin) for assistance in the preparation of this manuscript and Prof. Christoph Kreutz (University of Innsbruck) and Prof. Thomas Müller (University of Innsbruck) for NMR and HRMS studies.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/jacs.4c08291.
Experimental procedures and characterization data for all new compounds as well as optimization tables (PDF)
Author Contributions
§ N.M. and O.K. contributed equally.
The authors declare no competing financial interest.
Supplementary Material
References
- Peng X.; Qiu M. Meroterpenoids from Ganoderma Species: A Review of Last Five Years. Nat. Prod. Bioprospect. 2018, 8 (3), 137–149. 10.1007/s13659-018-0164-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X.-F.; Yan Y.-M.; Wang X.-L.; Ma X.-J.; Fu X.-Y.; Cheng Y.-X. Two New Compounds from Ganoderma Lucidum. Journal of Asian Natural Products Research 2015, 17 (4), 329–332. 10.1080/10286020.2014.960858. [DOI] [PubMed] [Google Scholar]
- Mothana R. A. A.; Jansen R.; Jülich W.-D.; Lindequist U. Ganomycins A and B, New Antimicrobial Farnesyl Hydroquinones from the Basidiomycete Ganoderma Pfeifferi. J. Nat. Prod. 2000, 63 (3), 416–418. 10.1021/np990381y. [DOI] [PubMed] [Google Scholar]
- Dou M.; Di L.; Zhou L.-L.; Yan Y.-M.; Wang X.-L.; Zhou F.-J.; Yang Z.-L.; Li R.-T.; Hou F.-F.; Cheng Y.-X. Cochlearols A and B, Polycyclic Meroterpenoids from the Fungus Ganoderma Cochlear That Have Renoprotective Activities. Org. Lett. 2014, 16 (23), 6064–6067. 10.1021/ol502806j. [DOI] [PubMed] [Google Scholar]
- Luo Q.; Di L.; Yang X.-H.; Cheng Y.-X. Applanatumols A and B, Meroterpenoids with Unprecedented Skeletons from Ganoderma Applanatum. RSC Adv. 2016, 6 (51), 45963–45967. 10.1039/C6RA05148K. [DOI] [Google Scholar]
- Zhang J.-J.; Qin F.-Y.; Meng X.-H.; Yan Y.-M.; Cheng Y.-X. Renoprotective Ganodermaones A and B with Rearranged Meroterpenoid Carbon Skelotons from Ganoderma Fungi. Bioorganic Chemistry 2020, 100, 103930. 10.1016/j.bioorg.2020.103930. [DOI] [PubMed] [Google Scholar]
- Luo Q.; Di L.; Dai W.-F.; Lu Q.; Yan Y.-M.; Yang Z.-L.; Li R.-T.; Cheng Y.-X. Applanatumin A, a New Dimeric Meroterpenoid from Ganoderma Applanatum That Displays Potent Antifibrotic Activity. Org. Lett. 2015, 17 (5), 1110–1113. 10.1021/ol503610b. [DOI] [PubMed] [Google Scholar]
- Li L.; Li H.; Peng X.-R.; Hou B.; Yu M.-Y.; Dong J.-R.; Li X.-N.; Zhou L.; Yang J.; Qiu M.-H. (±)-Ganoapplanin, a Pair of Polycyclic Meroterpenoid Enantiomers from Ganoderma Applanatum. Org. Lett. 2016, 18 (23), 6078–6081. 10.1021/acs.orglett.6b03064. [DOI] [PubMed] [Google Scholar]
- Kawamoto Y.; Ito H. Total Synthesis of Ganoderma Meroterpenoids – Progresses since 2014. Asian J. Org. Chem. 2024, 13, e202300633 10.1002/ajoc.202300633. [DOI] [Google Scholar]
- Šimek M.; Bártová K.; Issad S.; Hájek M.; Císařová I.; Jahn U. Unified Total Synthesis of Diverse Meroterpenoids from Ganoderma Applanatum. Org. Lett. 2022, 24 (25), 4552–4556. 10.1021/acs.orglett.2c01633. [DOI] [PubMed] [Google Scholar]
- Mashiko T.; Shingai Y.; Sakai J.; Kamo S.; Adachi S.; Matsuzawa A.; Sugita K. Total Synthesis of Cochlearol B via Intramolecular [2 + 2] Photocycloaddition. Angew. Chem. Int. Ed 2021, 60 (46), 24484–24487. 10.1002/anie.202110556. [DOI] [PubMed] [Google Scholar]
- Mashiko T.; Shingai Y.; Sakai J.; Adachi S.; Matsuzawa A.; Kamo S.; Sugita K. Enantioselective Total Syntheses of (+)-Ganocin A and (−)-Cochlearol B. Org. Lett. 2023, 25 (46), 8382–8386. 10.1021/acs.orglett.3c03572. [DOI] [PubMed] [Google Scholar]
- Richardson A. D.; Vogel T. R.; Traficante E. F.; Glover K. J.; Schindler C. S. Total Synthesis of (+)-Cochlearol B by an Approach Based on a Catellani Reaction and Visible-Light-Enabled [2 + 2] Cycloaddition. Angew. Chem. Int. Ed 2022, 61 (31), e202201213 10.1002/anie.202201213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Q.; Ma X.; Qiao J.; He W.; Jiang M.; Shao H.; Zhao Y. Total Synthesis of Ganoderma Meroterpenoids Cochlearol B and Its Congeners Driven by Structural Similarity and Biological Homology. Chem. Eur. J. 2024, 30 (17), e202400084 10.1002/chem.202400084. [DOI] [PubMed] [Google Scholar]
- Liu Y.-L.; Deng T.; Sui H.-L.; Zhou M.; Qin H.-B. Total Synthesis of (±)-Applanatumol B. Tetrahedron Lett. 2023, 124, 154584. 10.1016/j.tetlet.2023.154584. [DOI] [Google Scholar]
- Uchida K.; Kawamoto Y.; Kobayashi T.; Ito H. Total Synthesis of Applanatumol B. Org. Lett. 2019, 21 (16), 6199–6201. 10.1021/acs.orglett.9b01901. [DOI] [PubMed] [Google Scholar]
- Zhang W.; Xiao D.; Wang B. A Concise Total Synthesis of Cochlearoid B. Org. Biomol. Chem. 2018, 16 (18), 3358–3361. 10.1039/C8OB00615F. [DOI] [PubMed] [Google Scholar]
- Shao H.; Gao X.; Wang Z.; Gao Z.; Zhao Y. Divergent Biomimetic Total Syntheses of Ganocins A–C, Ganocochlearins C and D, and Cochlearol T. Angew. Chem. Int. Ed 2020, 59 (19), 7419–7424. 10.1002/anie.202000677. [DOI] [PubMed] [Google Scholar]
- Zaichick S. V.; McGrath K. M.; Caraveo G. The Role of Ca2+ Signaling in Parkinson’s Disease. Disease Models & Mechanisms 2017, 10 (5), 519–535. 10.1242/dmm.028738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajakulendran S.; Hanna M. G. The Role of Calcium Channels in Epilepsy. Cold Spring Harb Perspect Med. 2016, 6 (1), a022723. 10.1101/cshperspect.a022723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unzner T. A.; Grossmann A. S.; Magauer T. Rapid Access to Orthogonally Functionalized Naphthalenes: Application to the Total Synthesis of the Anticancer Agent Chartarin. Angew. Chem. Int. Ed 2016, 55 (33), 9763–9767. 10.1002/anie.201605071. [DOI] [PubMed] [Google Scholar]
- Feierfeil J.; Magauer T. De Novo Synthesis of Benzannelated Heterocycles. Chem. Eur. J. 2018, 24 (6), 1455–1458. 10.1002/chem.201705662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamarija I.; Marsh B. J.; Magauer T. Ring Expansion of 1-Indanones to 2-Halo-1-Naphthols as an Entry Point to Gilvocarcin Natural Products. Org. Lett. 2021, 23 (23), 9221–9226. 10.1021/acs.orglett.1c03530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Röder L.; Wurst K.; Magauer T. Synthesis of the Tetracyclic Spiro-Naphthoquinone Chartspiroton. Org. Lett. 2024, 26, 3065. 10.1021/acs.orglett.4c00695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- While a free C4a position was found to be key for the fragment coupling, removal of the lactone function was critical to achieve selective C4a oxidation.
- Nozaki K.; Oshima K.; Utimoto K. Facile Routes to Boron Enolates. Et3B-Mediated Reformatsky Type Reaction and Three Components Coupling Reaction of Alkyl Iodides, Methyl Vinyl Ketone, and Carbonyl Compounds. Tetrahedron Lett. 1988, 29 (9), 1041–1044. 10.1016/0040-4039(88)85330-9. [DOI] [Google Scholar]
- Nagatomo M.; Kamimura D.; Matsui Y.; Masuda K.; Inoue M. Et 3 B-Mediated Two- and Three-Component Coupling Reactions via Radical Decarbonylation of α-Alkoxyacyl Tellurides: Single-Step Construction of Densely Oxygenated Carboskeletons. Chem. Sci. 2015, 6 (5), 2765–2769. 10.1039/C5SC00457H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Z.; Zhang X.; Guo Z.; Chen Y.; Mu T.; Li A. Total Synthesis of Aplysiasecosterol A. J. Am. Chem. Soc. 2018, 140 (29), 9211–9218. 10.1021/jacs.8b05070. [DOI] [PubMed] [Google Scholar]
- Kitagawa O.; Inoue T.; Taguchi T. Diastereoselective Iodocarbocyclization of 4-Pentenylmalonate Derivatives: Application to Cyclosarkomycin Synthesis. Tetrahedron Lett. 1994, 35 (7), 1059–1062. 10.1016/S0040-4039(00)79965-5. [DOI] [Google Scholar]
- Inoue T.; Kitagawa O.; Oda Y.; Taguchi T. Diastereoselective Iodocarbocyclization Reaction of 2- or 3-Oxy-4-Pentenylmalonate Derivatives. J. Org. Chem. 1996, 61 (23), 8256–8263. 10.1021/jo961076+. [DOI] [PubMed] [Google Scholar]
- Magauer T.; Rode A.; Wurst K. A General Entry to Ganoderma Meroterpenoids: Synthesis of Lingzhiol via Photoredox Catalysis. ChemRxiv May 2022, 10.26434/chemrxiv-2022-svqft. [DOI] [Google Scholar]
- Morrill C.; Péter Á.; Amalina I.; Pye E.; Crisenza G. E. M.; Kaltsoyannis N.; Procter D. J. Diastereoselective Radical 1,4-Ester Migration: Radical Cyclizations of Acyclic Esters with SmI2. J. Am. Chem. Soc. 2022, 144 (30), 13946–13952. 10.1021/jacs.2c05972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohara K.; Trowbridge A.; Smith M. A.; Gaunt M. J. Thiol-Mediated α-Amino Radical Formation via Visible-Light-Activated Ion-Pair Charge-Transfer Complexes. J. Am. Chem. Soc. 2021, 143 (46), 19268–19274. 10.1021/jacs.1c09445. [DOI] [PubMed] [Google Scholar]
- Wollnitzke P.; Essig S.; Gölz J. P.; Von Schwarzenberg K.; Menche D. Total Synthesis of Ajudazol A by a Modular Oxazole Diversification Strategy. Org. Lett. 2020, 22 (16), 6344–6348. 10.1021/acs.orglett.0c02188. [DOI] [PubMed] [Google Scholar]
- Riaz M. T.; Pohorilets I.; Hernandez J. J.; Rios J.; Totah N. I. Preparation of 2-(Trimethylsilyl)Methyl-2-Propen-1-Ol Derivatives by Cobalt Catalyzed Sp2-Sp3 Coupling. Tetrahedron Lett. 2018, 59 (29), 2809–2812. 10.1016/j.tetlet.2018.06.018. [DOI] [Google Scholar]
- Huang C.; Xiong J.; Guan H.-D.; Wang C.-H.; Lei X.; Hu J.-F. Discovery, Synthesis, Biological Evaluation and Molecular Docking Study of (R)-5-Methylmellein and Its Analogs as Selective Monoamine Oxidase A Inhibitors. Bioorg. Med. Chem. 2019, 27 (10), 2027–2040. 10.1016/j.bmc.2019.03.060. [DOI] [PubMed] [Google Scholar]
- Kita Y.; Yata T.; Nishimoto Y.; Chiba K.; Yasuda M. Selective Oxymetalation of Terminal Alkynes via 6- Endo Cyclization: Mechanistic Investigation and Application to the Efficient Synthesis of 4-Substituted Isocoumarins. Chem. Sci. 2018, 9 (28), 6041–6052. 10.1039/C8SC01537F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imbos R.; Minnaard A. J.; Feringa B. L. A Highly Enantioselective Intramolecular Heck Reaction with a Monodentate Ligand. J. Am. Chem. Soc. 2002, 124 (2), 184–185. 10.1021/ja017200a. [DOI] [PubMed] [Google Scholar]
- Curran D. P.; McFadden T. R. Understanding Initiation with Triethylboron and Oxygen: The Differences between Low-Oxygen and High-Oxygen Regimes. J. Am. Chem. Soc. 2016, 138 (24), 7741–7752. 10.1021/jacs.6b04014. [DOI] [PubMed] [Google Scholar]
- Villar F.; Equey O.; Renaud P. Desymmetrization of 1,4-Dien-3-Ols and Related Compounds via Ueno–Stork Radical Cyclizations. Org. Lett. 2000, 2 (8), 1061–1064. 10.1021/ol005613v. [DOI] [PubMed] [Google Scholar]
- Nozaki K.; Oshima K.; Utimoto K. Trialkylborane as an Initiator and Terminator of Free Radical Reactions. Facile Routes to Boron Enolates via α-Carbonyl Radicals and Aldol Reaction of Boron Enolates. Bull. Chem. Soc. Jpn. 1991, 64 (2), 403–409. 10.1246/bcsj.64.403. [DOI] [Google Scholar]
- Tanaka H.; Oisaki K.; Kanai M. Ligand-Free, Copper-Catalyzed Aerobic Benzylic Sp3 C–H Oxygenation. Synlett 2017, 28 (13), 1576–1580. 10.1055/s-0036-1588969. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.