Abstract
CONTEXT:
Diabetic eyes suffer from variety of complications including macular edema. Cataract surgery is the most commonly done procedure throughout the world and majority would be diabetics. As pseudophakic-cystoid macular edema (CME) is a known complication following cataract surgery, our study concentrated on finding the role of prophylactic topical nonsteroidal anti-inflammatory drugs (NSAIDs) on change in total macular volume (TMV) postcataract surgery in diabetic eyes.
AIMS:
To evaluate the role of NSAIDs on change in TMV postcataract surgery in diabetic eyes.
SETTINGS AND DESIGN:
Retrospective comparative study.
SUBJECTS AND METHODS:
Data were collected from the medical records department of our institute constituting diabetics undergoing cataract surgery from June-2021 to February-2022. Eighty diabetic eyes were divided into two groups: one group were given topical nepafenac drops and another who were not given. Demographic details, diabetic retinopathy stage, preoperative optical coherence tomography (OCT), and postoperative day (POD) 7, day 28, and 3 months OCT were collected. Statistical analysis was done to compare the change in TMV between both the groups.
STATISTICAL ANALYSIS USED:
Student’s t-test and Chi-squared/Fisher’s exact test were employed to find statistically significant differences between the two groups using SPSS-22.0 software.
RESULTS:
In our study, the mean age in the group with nepafenac was 60.93 ± 5.86 years and 31 (77.5%) had moderate nonproliferative diabetic retinopathy (NPDR), and in the group without nepafenac, the mean age was 58.53 ± 7.41 years and 30 (75%) had moderate NPDR. Majority of the individuals in the study group were known diabetic for 2–5 years. Change in TMV at POD 3 months among two groups was not statistically significant; P = 0.758 (P < 0.05-significant).
CONCLUSIONS:
Our study concluded that topical-NSAIDs played no role in postoperative period following cataract surgery with respect to change in TMV in diabetic eyes. Thus, prophylactic usage of topical-NSAIDs can be a burden on patient as it has no role in prevention of pseudophakic-CME in those with the duration of diabetes mellitus <5 years and with mild-to-moderate NPDR.
Keywords: Nonsteroidal anti-inflammatory drugs, pseudophakic cystoid macular edema, total macular volume
Introduction
Cataract constitutes predominant causative factor of visual-impairment among diabetic eyes, as incidence and progression of cataract increase in diabetes mellitus (DM).[1] Diabetic eyes are known to have various complications including macular edema. Pseudophakic-cystoid macular edema (CME) is one of the most predominant causative factors for visual impairment in the postoperative period of cataract surgery.[2] Accumulation of fluid in outer plexiform layer and inner nuclear layer of retina with the formation of tiny cyst like cavities is a major cause.[3]
Pseudophakic-CME is usually a common etiology in causing vision loss following cataract surgery.[4] This incidence further increases in the setting of co-morbidities like diabetic retinopathy(DR). As cataract surgery is most commonly performed and surgery being one of the most common causes of pseudophakic CME, it is quintessential to search preventive measure to decrease the incidence of pseudophakic CME. Nonsteroidal anti-inflammatory drugs (NSAIDs) primarily act by blocking cyclooxygenase enzymes responsible for prostaglandin synthesis, thus keeping check on intraocular inflammation. However, previous studies have still not come into uniform opinion on prophylactic usage of NSAID eyedrops alone or in combination with steroid eyedrops for the prevention of pseudophakic CME postcataract extraction.[5]
Our study assesses the role of topical nepafenac on change in total macular volume (TMV) in diabetic eyes undergoing small incision cataract surgery (SICS) with posterior chamber intraocular lens implantation.
Subjects and Methods
Ethics committee clearance
Not applicable as it was a retrospective study.
Source of data
Medical records department of our institute.
Study design
A retrospective comparative study.
Study period
September 2021 to February 2022.
Inclusion criteria
Age >18 years
Visually significant cataract in eyes of DR patients having clear media.
Exclusion criteria
Patients with a history of pan retinal laser photocoagulation
Patients with a history of any prior intraocular surgery to the eye which has undergone cataract surgery
Patients with central macular thickness >260 µ before cataract surgery
Patients with severe nonproliferative DR (NPDR), very severe NPDR, and the proliferative diabetic retinopathy
Duration of DM more than 5 years.
Methodology
Demographic data, history, clinical examination, and details of investigations were collected. Detailed ocular examination including uncorrected visual acuity, anterior segment evaluation and grading of cataract was done.
Posterior segment examination was done using Indirect Ophthalmoscope and 20D lens.
Grading of DR according to Early Treatment Diabetic Retinopathy Study(ETDRS) was done. Optical coherence tomography(OCT) with macular cube was done and TMV values were collected.
The patients were divided into two groups: Group A (diabetic eye with nepafenac 0.1% eyedrops) who had received topical nepafenac drops with conventional antibiotic steroid eyedrops postcataract surgery and Group B (diabetic eyes without nepafenac 0.1% eyedrops) who had only conventional antibiotic steroid eyedrops postcataract surgery with 40 patients in each group who had underwent cataract surgery.
TMV values of preoperative, postoperative day (POD) 7, POD 28, and POD 3 months on OCT were collected in both the groups. Then, statistical analysis was done to look for significance in change in TMV postcataract surgery in between the groups.
Sample size estimation
Based on the previous study done by Entezari et al.,[1] following values were taken:
Standard deviation 1 (sd1) of case group = 17
Standard devotion 2 (sd2) of control group = 32
Then sample size was calculated using the below formula:

n = sample size in one group
Zα/2 = critical value of the normal distribution at α of 0.05, i.e., 1.96
Zβ = critical value of the normal distribution at β. For β =20% (power of 80%)
=0.84
SD2 = Combined variance = [(sd1)2+ (sd2)2]/2=
Where sd1 = SD in Case group = 17
Where sd2 = SD in Control group = 32
On substituting, total sample size rounded off to each group (n) = 40
Sample size for Group A = 40
Sample size for Group B = 40.
Statistical analysis
The data collected were analyzed statistically with descriptive statistics that include mean, percentage, and SDs wherever applicable. Suitable nonparametric and parametric tests were used. Student’s t-test was applied to assess if statistically significant difference existed among means of the two independent groups. Chi-squared/Fisher’s exact test was employed to find significance of study parameters between two groups. Statistical software (SPSS 22.0; IBM corp, Chicago, United States of America) was used for statistical analysis. Microsoft Excel and Word were used to generate graphs and tables.
Results
The study included total 80 eyes with 40 each in the two groups. The mean age group in Group A was 60.93 ± 5.86 years and in Group B was 58.43 ± 7.41. Of these, majority were male, 28 (70%) in Group A and 23 (57.5%) in Group B. Systemic hypertension accounted for the maximum number among associated co-morbidities in both the groups. It amounted to 7 eyes(17.5%) and 10eyes (25%) in Group A and B, respectively [Table 1]. Majority of the individuals in the study group were known diabetic for 2–5 years in both the groups amounting to 22 (55%) and 27 (67.5%) in Group A and Group B, respectively [Table 2]. Grading of DR according to ETDRS showed moderate NPDR being maximum in both the groups amounting to 31 (77.5%) and 30 (75%) in Group A and Group B, respectively. Change in TMV from baseline (pre-operative) was calculated. It amounted to 0.04 ± 0.16 µm3, 0.16± 0.19 µm3 and 0.32 ± 0.27 µm3 on POD 7, POD 28 and POD 3 months respectively in Group A. In Group B the values were 0.01 ± 0.3 µm3, 0.14 ± 0.21 µm3, and 0.3 ± 0.3 µm3 on POD 7, POD 28 and POD 3 months respectively in Group B. P value was calculated for change in TMV from baseline between both groups on POD 7, POD 28, and POD 3 months. The values were found to be 0.5784, 0.6584, and 0.7548 respectively (P < 0.05 was taken as significant). No statistically significant difference existed between the two groups [Table 3, Figures 1 and 2].
Table 1.
Systemic illness frequency distribution table
| Systemic illness | Diabetic eyes with nepafenac (n=40), n (%) | Diabetic eyes without nepafenac (n=40), n (%) | Total (n=80), n (%) | P | ||||
|---|---|---|---|---|---|---|---|---|
| DM | 40 (100) | 40 (100) | 80 (100) | 1.000 | ||||
| Hypertension | 7 (17.5) | 10 (25) | 17 (21.3) | 0.292 | ||||
| Ischemic heart disease | 6 (15) | 3 (7.5) | 9 (11.3) | 0.481 | ||||
| Retroviral disease | 2 (5) | 2 (5) | 4 (5) | 1.000 |
DM: Diabetes mellitus
Table 2.
Duration of diabetic mellitus frequency distribution table
| Duration of DM (years) | Diabetic eyes with nepafenac, n (%) | Diabetic eyes without nepafenac, n (%) | Total, n (%) | |||
|---|---|---|---|---|---|---|
| Up to–2 | 9 (22.5) | 17 (42.5) | 26 (32.5) | |||
| 2–5 | 22 (55) | 27 (67.5) | 49 (61.3) | |||
| >5 | 15 (37.5) | 8 (20) | 23 (28.8) | |||
| Total | 40 (100) | 40 (100) | 80 (100) | |||
| Mean±SD | 4.83±2.53 | 3.96±2.9 | 4.39±2.74 |
SD: Standard deviation, DM: Diabetes mellitus
Table 3.
Change in total macular volume
| TMV | Diabetic eyes with nepafenac | Diabetic eyes without nepafenac | Total | P | ||||
|---|---|---|---|---|---|---|---|---|
| Results | ||||||||
| Preoperative | 9.63±0.21 | 9.52±0.28 | 9.57±0.25 | 0.036 | ||||
| POD 7 | 9.67±0.22 | 9.53±0.31 | 9.6±0.27 | 0.016 | ||||
| POD 28 | 9.8±0.27 | 9.66±0.26 | 9.73±0.27 | 0.023 | ||||
| POD 3 months | 9.96±0.37 | 9.82±0.32 | 9.89±0.35 | 0.072 | ||||
| Change from baseline | ||||||||
| POD 7 | 0.04±0.16 | 0.01±0.3 | 0.02±0.24 | 0.5784 | ||||
| POD 28 | 0.16±0.19 | 0.14±0.21 | 0.15±0.2 | 0.6584 | ||||
| POD 3 months | 0.32±0.27 | 0.3±0.3 | 0.31±0.28 | 0.7548 |
TMV: Total macular volume, POD: Postoperative day
Figure 1.
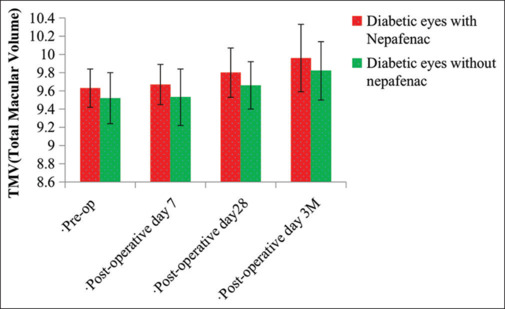
Comparative assessment of total macular volume preoperative, postoperative day 7, postoperative day 28, and postoperative 3 months. TMV: Total macular volume
Figure 2.
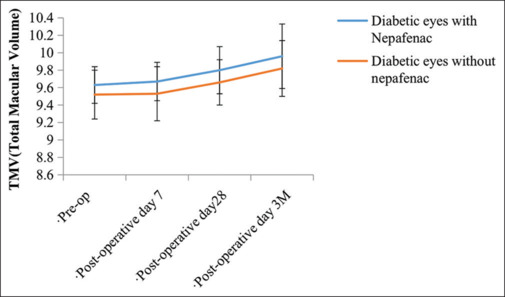
Graphical representation showing comparative change in total macular volume between two groups at preoperative, postoperative day 7, postoperative day 28, and postoperative 3 months. TMV: Total macular volume
Discussion
Cataract surgery constitutes most common cause for pseudophakic CME ranging from 1% to 30%.[6] Intraocular inflammation is the major culprit in the development of the macular edema following intraocular surgery. Pro-inflammatory prostaglandins play a pivotal role in leakage of fluid from the perifoveal capillaries into the layers of retina resulting in increased macular volume.
The management of pseudophakic CME is based on the pathogenesis. In inflammation cascade, cell membrane lipids will be converted to arachidonic acid by phospholipase A2, and prostaglandins will then be formed due to cyclooxygenases (COX). Corticosteroids decrease inflammation by keeping a check on phospholipase A2, and NSAIDs block COX. Two predominant COX isoforms are COX-1 and COX-2, and the latter is being major expressed in retina.[7]
Nepafenac, a prodrug that is transformed into the active drug, amfenac, via intraocular hydrolases, has been shown to have superior posterior segment activity and corneal penetration in rabbit models.[8] As we all know the role of topical nepafenac eyedrops in the well-developed diabetic macular edema, the same has been tried as a prophylactic measure in preventing pseudophakic CME.
A randomized controlled trial was done by Entezari et al. on 108 diabetic eyes who underwent phacoemulsification and intraocular lens (IOL) implantation. Among these 54 eyes had received conventional postoperative care with steroid drop, while other group’s 54 eyes were given preoperative diclofenac drops 4 times a day with a steroid drop. Further continued till 6 weeks following the surgery. The study showed that there exists a useful effect of topical diclofenac in the prevention of macular thickness increase postcataract procedure in diabetic patients.[1]
Randomized controlled trial was conducted by Medić et al. on 55 NPDR eyes who were undergoing cataract surgery. Then they were randomized to receive pre-operative Diclofenac eyedrops (n = 27) or placebo (n = 28). The study showed that patients who were treated preoperatively with diclofenac drops had significantly minimal intraocular concentrations of interleukin-12 and slight more of central foveal thickness, showing that addition of preoperative and postoperative treatment using topical NSAIDs decreases the incidence of postoperative macular edema in patients with DR.[9]
Randomized double masked single-center clinical trial was done by Miyake et al. on 59 patients who had undergone phacoemulsification with the foldable IOL implantation. Thirty patients had received nepafenac (0.1%) eyedrops and 29 patients fluorometholone for 5 weeks following surgery. The study postulated that nepafenac was more effective as compared to fluorometholone in the prevention of angiographic CME and blood aqueous barrier breakage. The results also showed that nepafenac played a role in faster visual recovery.[10]
Randomized control study was conducted by Shilpy and Patel on 140 patients who were made to receive prednisolone 1% (Group 1) versus Nepafenac + prednisolone 1% (Group 2) following Phacoemulsification. Examination was done on 1st day, 1-week and 1 month. Visual acuity, slit lamp evaluation for looking inflammatory findings in anterior segment and CMEwere done. Study postulated that addition of Nepafenac 0.1% with prednisolone 1% significantly decreases intraocular inflammation after phacoemulsification in postoperative period.[11]
Randomized controlled trial conducted was by Campa et al. on 144 patients who underwent cataract extraction with implantation of IOL. All the patients were stratified into three groups receiving bromfenac/nepafenac with dexamethasone or dexamethasone alone postoperatively. The study showed that coadministration of nepafenac/bromfenac plus steroids postcataract surgery can lower the incidence of pseudophakic CME as compared with the steroid monotherapy.[12]
Retrospective study was done by Sengupta et al. on records of patients with the clinical and OCT based diagnosis of acute Pseudophakic CME. Records were retrospectively reviewed for best-corrected visual acuity and OCT-based parameters at the time of presentation with PCME. The study included 69 eyes of the 69 patients. The study concluded that patients who received both topical prednisolone plus topical nepafenac had eventually lead to resolution of pseudophakic CME in 50% of eyes at 6 weeks.[13]
Conclusions
As our study showed that there is no role of prophylactic topical NSAIDs in the postoperative periods following cataract surgery in diabetic eyes with mean duration of DM <5 years in preventing the rise of macular volume, patients can be subjected to less burden with the minimal eyedrops and thus increasing patients compliance. Thus, patients with DM <5 years with mild-to-moderate NPDR undergoing SICS with PCIOL implantation do not need prophylactic NSAIDs.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Entezari M, Ramezani A, Nikkhah H, Yaseri M. The effect of topical sodium diclofenac on macular thickness in diabetic eyes after phacoemulsification: A randomized controlled trial. Int Ophthalmol. 2017;37:13–8. doi: 10.1007/s10792-016-0209-4. [DOI] [PubMed] [Google Scholar]
- 2.Stock RA, Galvan DK, Godoy R, Bonamigo EL. Comparison of macular thickness by optical coherence tomography measurements after uneventful phacoemulsification using ketorolac tromethamine, nepafenac, versus a control group, preoperatively and postoperatively. Clin Ophthalmol. 2018;12:607–11. doi: 10.2147/OPTH.S157738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Salmon JF. Kanski’s Clinical Ophthalmology – A Systematic Approach. 9th. China: Elsevier; 2020. Acquired macular disorders; p. 606. [Google Scholar]
- 4.Flach AJ. The incidence, pathogenesis and treatment of cystoid macular edema following cataract surgery. Trans Am Ophthalmol Soc. 1998;96:557–634. [PMC free article] [PubMed] [Google Scholar]
- 5.Kessel L, Tendal B, Jørgensen KJ, Erngaard D, Flesner P, Andresen JL, et al. Post-cataract prevention of inflammation and macular edema by steroid and nonsteroidal anti-inflammatory eye drops: A systematic review. Ophthalmology. 2014;121:1915–24. doi: 10.1016/j.ophtha.2014.04.035. [DOI] [PubMed] [Google Scholar]
- 6.McCannel AC, Berrocal AM, Holder GE, Kim SJ, Leonard BC, Rosen RB, et al., editors. Vol. 12. Sanfrancisco, CA: AAO in Collaboration with European Board of Ophthalmology, Subcommittee; 2019. Basic and Clinical Science Course. Retina and Vitreous; p. 157. [Google Scholar]
- 7.Jiramongkolchai K, Lalezary M, Kim SJ. Influence of previous vitrectomy on incidence of macular oedema after cataract surgery in diabetic eyes. Br J Ophthalmol. 2011;95:524–9. doi: 10.1136/bjo.2010.182477. [DOI] [PubMed] [Google Scholar]
- 8.Gamache DA, Graff G, Brady MT, Spellman JM, Yanni JM. Nepafenac, a unique nonsteroidal prodrug with potential utility in the treatment of trauma-induced ocular inflammation: I. Assessment of anti-inflammatory efficacy. Inflammation. 2000;24:357–70. doi: 10.1023/a:1007049015148. [DOI] [PubMed] [Google Scholar]
- 9.Medić A, Jukić T, Matas A, Vukojević K, Sapunar A, Znaor L. Effect of preoperative topical diclofenac on intraocular interleukin-12 concentration and macular edema after cataract surgery in patients with diabetic retinopathy: A randomized controlled trial. Croat Med J. 2017;58:49–55. doi: 10.3325/cmj.2017.58.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Miyake K, Ota I, Miyake G, Numaga J. Nepafenac 0.1% versus fluorometholone 0.1% for preventing cystoid macular edema after cataract surgery. J Cataract Refract Surg. 2011;37:1581–8. doi: 10.1016/j.jcrs.2011.03.052. [DOI] [PubMed] [Google Scholar]
- 11.Shilpy N, Patel DB. Comparison of nepafenac plus steroid versus steroid alone for control of ocular inflammation after phacoemulsification. International Journal of Contemporary Medical Research. 2019;6:A1–A3. [Google Scholar]
- 12.Campa C, Salsini G, Perri P. Comparison of the efficacy of dexamethasone, nepafenac, and bromfenac for preventing pseudophakic cystoid macular edema: An open-label, prospective, randomized controlled trial. Curr Eye Res. 2018;43:362–7. doi: 10.1080/02713683.2017.1396615. [DOI] [PubMed] [Google Scholar]
- 13.Sengupta S, Vasavada D, Pan U, Sindal M. Factors predicting response of pseudophakic cystoid macular edema to topical steroids and nepafenac. Indian J Ophthalmol. 2018;66:827–30. doi: 10.4103/ijo.IJO_735_17. [DOI] [PMC free article] [PubMed] [Google Scholar]


