Abstract
Myogenesis is a highly orchestrated process whereby muscle precursor cells, myoblasts, develop into muscle fibers to form skeletal muscle during embryogenesis and regenerate adult muscle. Here, we studied the RNA-binding protein FUS (fused in sarcoma), which has been implicated in muscular and neuromuscular pathologies but is poorly characterized in myogenesis. Given that FUS levels declined in human and mouse models of skeletal myogenesis, and that silencing FUS enhanced myogenesis, we hypothesized that FUS might be a repressor of myogenic differentiation. Interestingly, overexpression of FUS delayed myogenesis, accompanied by slower production of muscle differentiation markers. To identify the mechanisms through which FUS inhibits myogenesis, we uncovered RNA targets of FUS by ribonucleoprotein immunoprecipitation (RIP) followed by RNA-sequencing (RNA-seq) analysis. Stringent selection of the bound transcripts uncovered Tnnt1 mRNA, encoding troponin T1 (TNNT1), as a major effector of FUS influence on myogenesis. We found that in myoblasts, FUS retained Tnnt1 mRNA in the nucleus, preventing TNNT1 expression; however, reduction of FUS during myogenesis or by silencing FUS released Tnnt1 mRNA for export to the cytoplasm, enabling TNNT1 translation and promoting myogenesis. We propose that FUS inhibits myogenesis by suppressing TNNT1 expression through a mechanism of nuclear Tnnt1 mRNA retention.
Keywords: Myogenesis, TNNT1, ribonucleoprotein complex, translation
Introduction
Skeletal muscle is an organ with essential functions in mobility, physical performance, and whole-body metabolism.1 Skeletal muscle is comprised of bundles of muscle fibers, which are multinucleated syncytia capable of individual contraction. Myogenesis is the process through which muscle precursor cells form muscle fibers during embryonic development and regenerate muscle in the adult to preserve mass and function. Myogenesis starting from muscle precursor cells includes many steps. A quiescent muscle stem cell (a satellite cell) is first activated to become a myoblast, the myoblast then proliferates and becomes committed to undergo fusion, myoblasts subsequently fuse to become a myotube, and myotubes then mature through further fusion to form a muscle fiber.2 Impaired myogenesis underlies a number of muscle diseases such as sarcopenia, muscular dystrophies, and myopathies.3–7
To coordinate this complex set of events, the expression and function of myogenic regulatory factors are tightly regulated. Myogenin (MYOG), an essential, muscle-specific transcription factor for the proper differentiation of myogenic progenitors, is expressed primarily during early hours after the induction of differentiation from myoblasts to myocytes; the myocyte-specific enhancer factor 2C (MEF2C), another transcription regulatory protein, is induced early but remains elevated; and myosin heavy chain (MYH), a motor protein in muscle filaments, is expressed at late stages of myogenesis when myocytes fuse to form myotubes.2 For proper temporal regulation of myogenesis, in addition to the transcriptional myogenic program, a host of posttranscriptional regulatory noncoding RNAs, primarily microRNAs (known as myomiRs),8 and circular and linear noncoding RNAs,9–12 contribute to this regulatory process. Finally, a large number of RNA-binding proteins (RBPs) robustly regulate gene expression programs in muscle at the posttranscriptional level, ensuring the precise and timely expression of muscle differentiation factors as myogenesis progresses.13,14 Examples of RBPs involved in myogenesis include muscle blind-like (MBNL) protein family members (MBNL1, MBNL2, and MBNL3), HuR, and AUF1.14
In a recent study identifying circSamd4 as a circRNA that contributes to the transcriptional increase of MYH mRNA levels during myogenesis,12 we found the RBP FUS (fused in sarcoma) as a strong interacting partner of circSamd4. Although FUS did not influence circSamd4 activity and we did not pursue this observation at that time, the discovery that FUS levels declined during myogenic progression was intriguing. Thus, we decided to investigate the possibility that FUS might influence myogenesis. Expressed across many tissues, FUS is a multifunctional DNA- and RNA-binding protein involved in transcription, splicing, mRNA stability, and translation.15,16 Through gain- and loss-of-function experiments in mouse C2C12 myoblasts we found that FUS inhibits myogenesis, while affinity binding assays further identified Tnnt1 mRNA as an effector of FUS actions on myogenesis. Consistent with the notion that FUS associated with and retained Tnnt1 mRNA in the nucleus, reducing FUS levels during myogenesis or by RNA silencing permitted Tnnt1 mRNA levels to increase in the cytoplasm. This elevation in the cytoplasmic levels of Tnnt1 mRNA in turn promoted TNNT1 translation. The encoded protein, troponin T1 (TNNT1), is a subunit of the troponin complex that binds tropomyosin and anchors thin filaments in striated muscle, participating in muscle contraction.17 In sum, we present evidence that FUS represses TNNT1 production in proliferating myoblasts, and that declining FUS levels with advancing myogenesis allows TNNT1 to be expressed to support the muscle contraction program.
Results
FUS expression levels decline during myogenesis
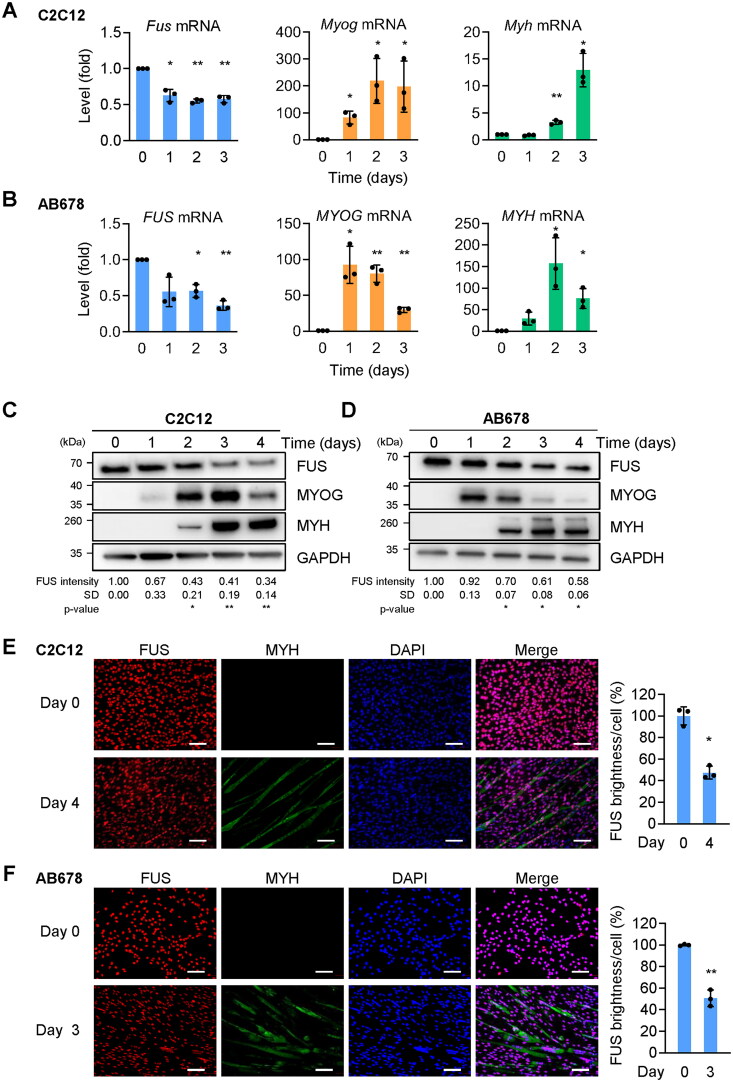
To evaluate the possible function of FUS in myogenesis, we first studied FUS expression in mouse and human myoblast models of myogenic differentiation. Myoblasts were cultured to high density in growth medium (GM) containing 20% fetal bovine serum (FBS) for C2C12 and 10% FBS for AB678 cells, and then the medium was replaced by differentiation medium (DM) containing 2% horse serum. The levels of Fus mRNA (mouse) and FUS mRNA (human) were quantified by reverse transcription (RT) followed by quantitative (q) polymerase chain reaction (PCR) analysis in mouse C2C12 and human AB678 myoblast cultures, respectively, over the course of 4 days. These measurements were carried out alongside measurements for the mRNAs encoding myogenic markers—Myog and Myh mRNAs in C2C12 cells, MYOG and MYH mRNAs in AB678 cells.18 We observed declining abundance of Fus mRNA in C2C12 cells and FUS mRNA in AB678 cells (Figure 1A and B), while the mRNAs encoding myogenic marker proteins showed increased expression levels at different levels, as previously documented, with Myog (MYOG) mRNA increasing first, and Myh (MYH) mRNA increasing later.2 The levels of the encoded proteins, FUS, MYOG, and MYH (Figure 1C and D), generally followed the patterns of the mRNAs. In this paradigm, FUS levels declined moderately over time, as shown and quantified (Figure 1C and D). Of note, MYOG levels increased earlier in AB678 cells relative to C2C12 cells, as myogenesis generally progressed somewhat slower in C2C12 cells; similarly, MYH levels increased slightly later in C2C12 as myogenesis reached completion—by day 4 in C2C12 cells and day 3 in AB678 cells. The reduction in FUS expression levels (red signal) was confirmed by immunofluorescence microscopy at the late stages of myogenesis, day 4 for C2C12 cells (Figure 1E) and day 3 for AB678 cells (Figure 1F), when myoblasts were actively undergoing fusion and myotubes were visible, as confirmed by evaluating the presence of MYH (green) (Figure 1E and F); DAPI (blue) was used to visualize nuclei. Quantification of FUS brightness per cell (Materials and Methods) indicated significant reductions to less than 50% compared to day 0. Together, these results indicate that in mouse and human myogenic differentiation, FUS expression levels declined with myogenesis.
Figure 1.
Reduced FUS levels during myogenic differentiation. (A) Proliferating mouse C2C12 myoblasts were placed in differentiation medium for the times indicated, and the levels of Myog and Myh mRNAs (encoding myogenic proteins MYOG and MYH, respectively), as well as Fus mRNA (encoding FUS), were measured by RT-qPCR analysis. Data were normalized to 18s rRNA levels. (B) Proliferating human AB678 myoblasts were placed in differentiation medium for the times indicated, and the levels of MYOG and MYH mRNAs, encoding myogenic proteins MYOG and MYH, as well as FUS mRNA (encoding FUS) were measured by RT-qPCR analysis. Data were normalized to 18S rRNA levels. (C, D) The levels of the proteins encoded by the mRNAs in (A) and (B) were assessed by Western blot analysis, as shown in (C) and (D), respectively. GAPDH was included as a loading control. The levels of FUS were quantified by densitometric quantification of the FUS signals using ImageJ, and normalized to GAPDH signals. (E, F) Detection of FUS by immunofluorescence microscopy in C2C12 cultures and days 0 and 4 (E) and AB678 cultures at days 0 and 3 (F). MYH signals were included to monitor myotube formation and DAPI was used to stain nuclei. FUS brightness per cell was calculated from the images at day 4 (E) and day 3 (F). Data in (A–F) represent the means ± SD of a minimum of three independent experiments. Statistical significance (*P < 0.05; **P < 0.01) was assessed with Student’s t test. Scale bars, 100 μm.
Myogenesis is promoted by silencing FUS, reduced by overexpressing FUS
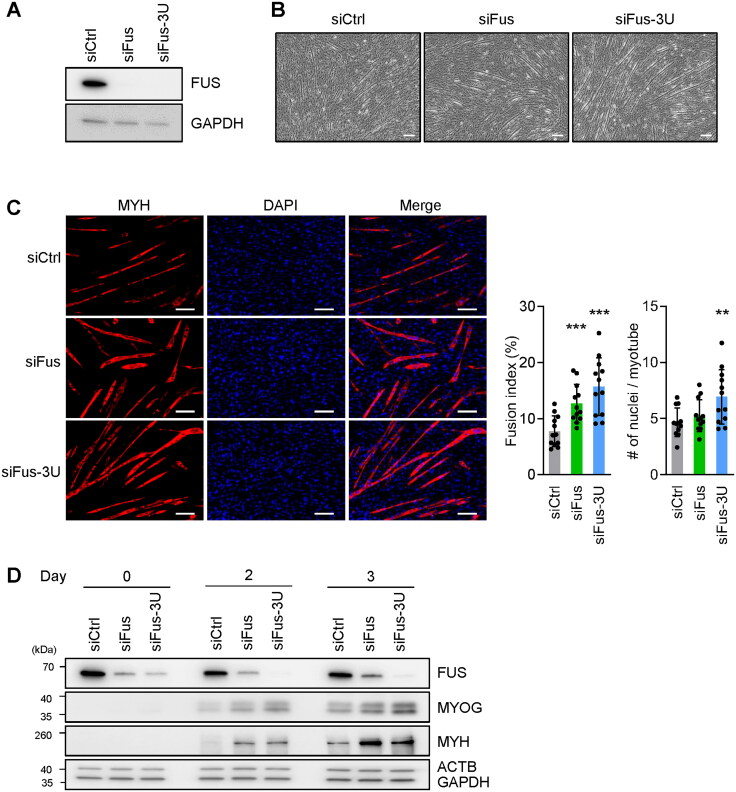
To investigate the possible involvement of FUS in myogenesis, we sought to modulate FUS expression levels and examine if there are any changes in myogenesis. FUS production was reduced in C2C12 myoblasts using siRNAs directed at Fus mRNA; siFus was directed at the coding region and siFus-3U at the 3′ untranslated region (3′UTR) of Fus mRNA. Proliferating C2C12 myoblasts were transfected with the siRNAs (siFus, siFus-3U, and control siCtrl) and 48 h later FUS silencing was confirmed by Western blot analysis (Figure 2A). The three transfection groups were induced to differentiate 24 h after the siRNA transfections and evaluated 3 days into the myogenic program. Interestingly, by day 3, while the siCtrl C2C12 population showed a few clear myotubes, C2C12 populations transfected with siFus or siFus-3U already had developed myotubes that were larger (longer and thicker) and more abundant in number (Figure 2B). To compare and quantify the extent of differentiation, immunofluorescence microscopy was used to visualize MYH (red) and nuclei (DAPI, blue) (Figure 2C). The fusion index (the ratio of the number of nuclei in myotubes relative to total nuclei) and the number of nuclei per myotube were calculated from multiple immunofluorescence images as a measure of myotube formation. As shown, FUS silencing significantly enhanced the formation of myotubes, increasing both the fusion index and the number of nuclei per myotubes, consistent with the microscopy results (Figure 2C). In addition, after silencing FUS, the expression of myogenic marker proteins MYOG and MYH, detected by Western blot analysis, revealed more robust expression levels early in myogenesis (Figure 2D), supporting the notion that silencing FUS expression promoted myogenic differentiation. The increased expression of MYH expression after silencing FUS was also confirmed in AB678 myoblast undergoing differentiation (Supplementary material, Figure S1). Together, these results indicate that reducing FUS expression promoted myogenesis.
Figure 2.
FUS silencing promotes myotube formation. (A–C) Proliferating C2C12 cells were transfected with siRNAs directed at Fus mRNA [either the coding region (siFus), or the 3′UTR (siFus-3U)], and then they were induced to differentiate and were studied 3 days later. Analyses included monitoring the levels of FUS (A), the levels of myotube formation by phase-contrast microscopy (B), and the levels of MYH-positive myotubes by fluorescence microscopy (using DAPI to stain nuclei) (C, left). Fusion indices and number of nuclei per myotube were also quantified (C, right). (D) Cells were processed as explained for (A–C), but the levels of the proteins shown were assessed by Western blot analysis at days 0, 2, and 3 into differentiation. ACTB and GAPDH were included as internal controls. Data in (C) represent the means ± SD and from three separate fields per experiment. Statistical significance (**P < 0.01; ***P < 0.001) was assessed with Student’s t test. Scale bars, 100 μm.
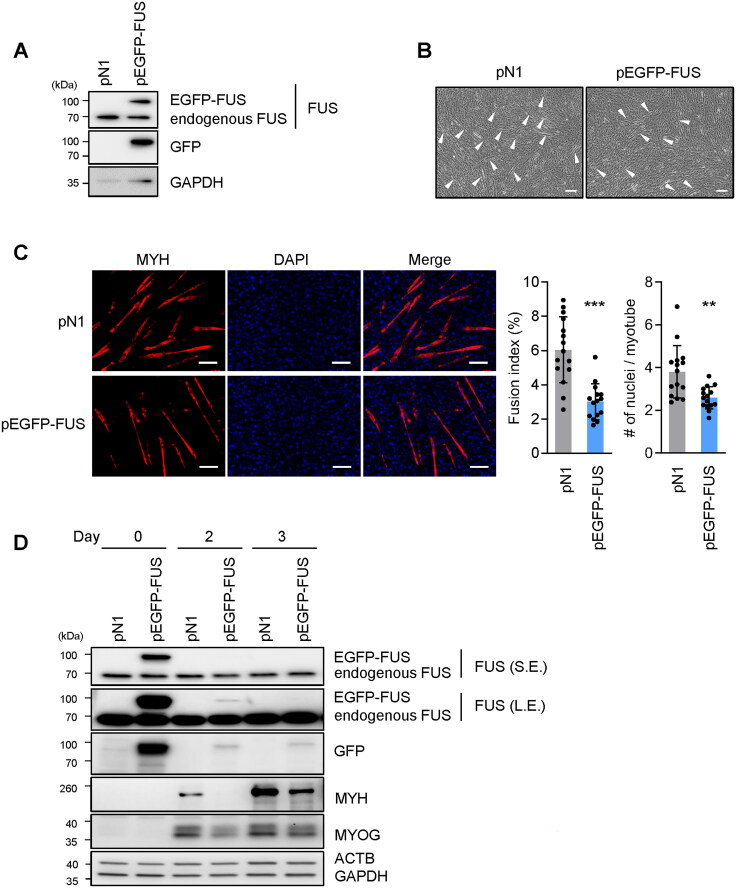
To test whether overexpressing FUS might have the opposite effect on myogenesis, we used a plasmid expressing EGFP-tagged human FUS (pEGFP-FUS), alongside a control plasmid (pN1). Proliferating C2C12 cells were transfected with the plasmids and harvested 24 h later; overexpression of the fusion protein was confirmed by Western blot analysis, which revealed that the fusion protein was larger than FUS and was expressed at approximately the same level as the endogenous protein (Figure 3A). After overexpressing FUS, myotube formation was reduced, as determined by inspection of phase-contrast micrographs (Figure 3B). This effect was confirmed by immunofluorescence microscopy; by 3 days into myogenesis, staining for MYH revealed that overexpressing FUS caused myotubes to be smaller, with significantly reduced fusion indices and fewer nuclei per myotube (Figure 3C). In addition, Western blot analysis revealed that overexpressing FUS decreased the levels of MYH and MYOG by 2 or 3 days into myogenesis (Figure 3D). It was interesting to note that EGFP-FUS could not be stably overexpressed, as the chimeric protein EGFP-FUS appeared to be labile and was degraded by day 1 after plasmid transfection; this degradation was independent of differentiation, as it was observed also in myoblasts cultured in proliferating conditions (not shown). These results suggest that overexpressing FUS, even transiently, during early stages of myogenesis, had a persistent effect in repressing C2C12 myogenic differentiation.
Figure 3.
FUS overexpression impairs myotube formation. (A–C) Proliferating C2C12 cells were transfected with either a control plasmid expressing only EGFP pEGFP-N1 (pN1) or a plasmid to overexpress FUS fused to EGFP (pEGFP-FUS); 24 h later, C2C12 cells were induced to differentiate and studied on day 3 of myogenesis. Analyses included monitoring the levels of endogenous FUS and ectopically overexpressed FUS (EGFP-FUS) (A), monitoring myotube formation by phase-contrast microscopy (B), and evaluating the levels of MYH-positive myotubes by fluorescence microscopy using DAPI to stain nuclei (C, left). Fusion indices and number of nuclei per myotube were also quantified (C, right). (D) Cells were processed as explained for (A–C), but the levels of the proteins shown were assessed by Western blot analysis at days 0, 2, and 3 of myogenesis. S.E., short exposure; L.E., long exposure. ACTB and GAPDH were included as internal controls. In (B), arrowheads indicate myotubes. Data in (C) represent the means ± SD and from three separate fields per experiment. Statistical significance (**P < 0.01; ***P < 0.001) was assessed with Student’s t test. Scale bars, 100 μm.
Identification of myogenesis-associated FUS targets
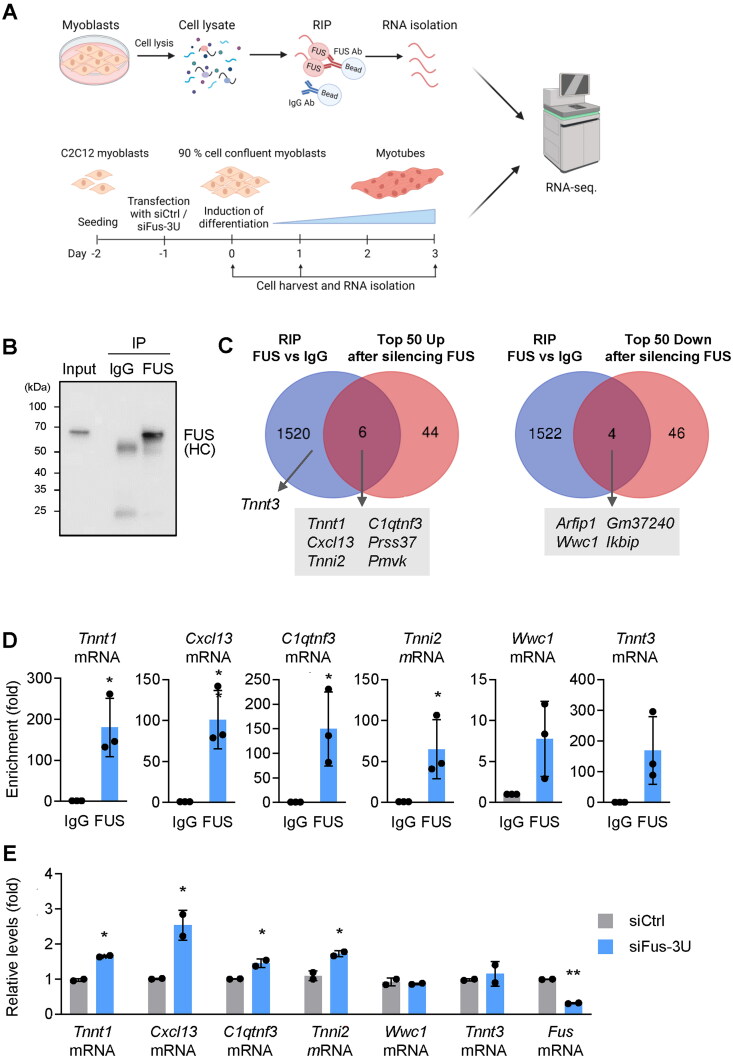
To begin to understand the mechanisms by which FUS suppressed myogenesis, we investigated how FUS regulated gene expression during myogenesis using a two-pronged approach. First, we identified RNAs that were direct targets of FUS by preparing lysates from proliferating C2C12 cells and employing an anti-FUS antibody to carry out ribonucleoprotein immunoprecipitation (RIP), isolating the bound RNA, and performing RNA-sequencing (RNA-seq) analysis of the bound transcripts (Figure 4A, top) (GSE267277); in control reactions, we used pre-immune IgG. As shown in Figure 4B, FUS was efficiently immunoprecipitated. Quality control experiments included detection of Fus mRNA and Gria1 mRNA, two known targets of FUS,19,20 as being enriched in the FUS RIP samples (Supplementary material, Figure S2A); the levels of Gapdh mRNA (not a target of FUS), measured in the same RIP samples, served to normalize for differences in sample input (Supplementary material, Figure S2A).
Figure 4.
Screening of new targets of FUS by RNA-seq analysis. (A) Workflow of sample preparation for total RNA-seq analysis; created using BioRender. Lysates were prepared from proliferating C2C12 cells and an anti-FUS antibody was used to perform RIP analysis; after isolating the RNA bound to the anti-FUS beads and the IgG beads, RNA-seq analysis was carried out (top). Proliferating C2C12 cells were transfected with siFus-3U or control siCtrl; 24 h later, myoblasts were induced to differentiate and total RNA was collected on days 0, 1, and 3 for RNA-seq-mediated identification of mRNAs differentially abundant (bottom). RNA-seq data are deposited in GSE267276. (B) Quality control of the RIP analysis in C2C12 cells was confirmed by Western blot analysis to detect FUS in each immunoprecipitated sample; (HC), heavy IgG chain. (C) Venn diagrams of the transcriptomes identified by RIP-seq (top arm, panel A) and by total RNA-seq analysis after FUS silencing (bottom arm, panel A). Those RNAs showing enrichments > 4-fold and padj < 0.05 with anti-FUS antibody (relative to IgG) were considered as FUS-bound RNAs. “Top 50 Up” and “Top 50 Down” refer to RNAs showing significantly different levels at day 0 in siFus-3U relative to siCtrl (padj < 0.05). (D, E) Validation of select mRNAs identified at the intersection of being altered in abundance when FUS was silenced and being associated with FUS. Enrichments (D) were validated by RIP followed by RT-qPCR analysis (normalized to Gapdh mRNA, not a target of FUS). Total mRNA levels (E) were measured in proliferating C2C12 cells (Gapdh mRNA was used for normalization of RT-qPCR results). Data in (D, E) represent the means ± SD from three and two independent replicates, respectively. Statistical significance (*P < 0.05; **p < 0.01; ***P < 0.001) was assessed with Student’s t test. In (B), the image is representative from three independent experiments.
Second, we silenced FUS using siFus-3U (siCtrl in control populations) and identified RNAs that were differentially abundant during myogenesis at days 0, 1, and 3 by performing RNA-seq analysis (Figure 4A, bottom). RT-qPCR analysis at these time points was used to evaluate the efficiency of Fus mRNA silencing and the corresponding changes in the levels of myogenesis markers Myog and Myh mRNAs as differentiation progressed (Supplementary material, Figure S2B). The quality of the biological replicates of the RNA-seq analysis is shown on the PCA plots (Supplementary material, Figure S3A). Further insight into the role of FUS in myogenesis was gained by performing Gene Set Enrichment Analysis (GSEA) of the Gene Ontology (GO) dataset of the transcriptomes identified by RNA-seq analysis. As shown, silencing FUS activated multiple GO pathways relevant to muscle development at the three time points (Supplementary material, Figure S3B). The loss-of-function analysis on day 0 (before placing the myoblasts in differentiation medium), revealed that FUS silencing promoted transcriptomic programs consistent with skeletal muscle tissue development, striated muscle contraction, and muscle structure development (enrichment score > 0.5) (Supplementary material, Figure S3C).
Although in the two analyses described in Figure 4A we found some long noncoding (lnc)RNAs, we focused on mRNAs, as lncRNAs are not generally well conserved between mouse and human, and in this paradigm, both human and mouse myogenesis responded similarly to perturbations of FUS levels. In the FUS RIP followed by RNA-seq analysis, RNAs bound to FUS were identified by comparing to the levels of each RNA associated with control IgG, using cut-offs of > 4-fold enrichment and padj < 0.05 (Supplementary material, Table S1); by these criteria, 1,526 mRNAs were enriched in the FUS RIP (Figure 4C). The RNA-seq analysis after FUS silencing for 24 h (day −1 to day 0) also had stringent cut-offs for both increased and decreased mRNAs (fold > 1.49 for the top 50 mRNAs elevated, fold < 0.66 for the top 50 mRNAs reduced; padj < 0.05 for both) (Supplementary material, Table S2). Those transcripts showing significant enrichment in FUS RIP and differential abundance after FUS silencing are indicated at the intersections of the Venn diagrams. FUS-bound mRNAs showing increased abundance after silencing FUS for 24 h (collected at day 0 in Figure 4A) were Tnnt1, Cxcl13, Tnni2, C1qtnf3, Prss37, and Pmvk mRNAs (Supplementary material, Figure S4). We also identified several mRNAs that both were targets of FUS by RIP and declined when FUS was silenced (Arfip1, Wwc1, Gm37240, Ikbip mRNAs; Supplementary material, Figure S4), suggesting that FUS might promote their expression before the onset of myogenesis; we decided not to pursue these targets at this time, as the effects of FUS on their expression levels may be indirect.
To validate these mRNAs, we first performed RIP analysis by conducting FUS IP and IgG IP in proliferating C2C12 myoblasts, followed by quantification of individual mRNAs—Tnnt1, Cxcl13, C1qtnf3, Tnni2, Wwc1, and Tnnt3 mRNAs—in the IP material by RT-qPCR analysis. These results were normalized to the levels of Gapdh mRNA in the same samples, as Gapdh mRNA is not a target of FUS. The data, represented as enrichment of the mRNAs in FUS IP vs IgG IP, confirmed that these mRNAs were significantly more abundant in the FUS IP samples (Figure 4D). We included Tnnt3 mRNA in this validation set, as several mRNAs encoding proteins in the troponin family were found to be targets of FUS, even though Tnnt3 mRNA levels were not changed when FUS was silenced (Figure 4C).
Further validation was carried out by individually measuring by RT-qPCR analysis the levels of the FUS target mRNAs examined in Figure 4C 24 h after silencing FUS (day 0 in Figure 4A). As shown, the levels of Tnnt1, Cxcl13, C1qtnf3, and Tnni2 mRNAs in C2C12 cells increased by silencing FUS before the start of differentiation, while the levels of Tnnt3 and Wwc1 mRNAs did not (Figure 4E). Inclusion of Fus mRNA in this analysis confirmed the efficiency of FUS silencing.
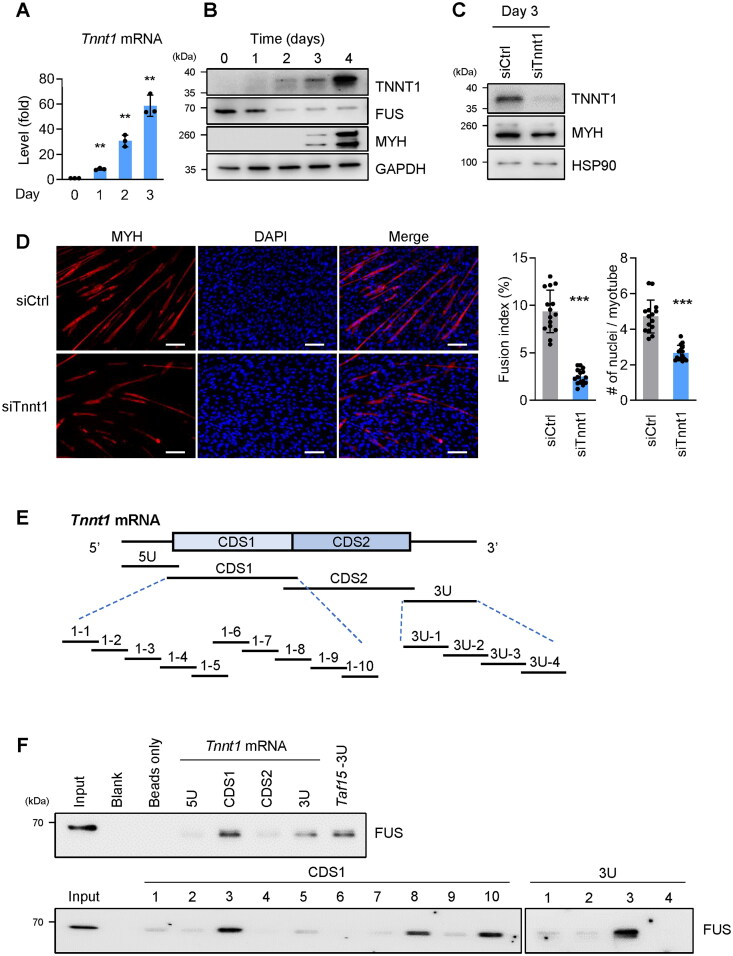
We surveyed the impact of these targets on myogenesis by individually silencing them and studying the impact of reducing these mRNAs on myotube formation. C2C12 cells were transfected with siCtrl or with siTnnt1, siTnnt3, siC1qtnf3 or siTnni2, and myotube formation on day 3 was evaluated by phase-contrast microscopy (Supplementary material, Figure S5). Compared with the control population, the reduction in myotube formation was particularly pronounced after silencing Tnnt1 mRNA. Before focusing on this interaction further, RIP analysis comparing FUS IP vs IgG IP in human AB678 myoblasts similarly revealed a strong enrichment in TNNT1 mRNA associated with FUS (Supplementary material, Figure S6A and B). Troponin T binds the troponin complex, which also comprises the calcium binding subunit (troponin C) and the inhibitory subunit (troponin I), to tropomyosin for muscle contraction.17 Unlike cardiac muscle troponin T2 (TNNT2), TNNT1 and TNNT3 are expressed in skeletal muscle.17 Reduced levels of TNNT1 or mutation in the TNNT1 gene reduces muscle function and is associated with myopathies.21,22 In light of these results, we focused on the impact of the FUS-Tnnt1 mRNA complex during myogenesis.
FUS abundance correlates inversely with TNNT1 expression levels during myogenesis
In C2C12 myoblasts undergoing myogenic differentiation, the expression levels of Tnnt1 mRNA and TNNT1 protein (evaluated by RT-qPCR and Western blot analyses, respectively) revealed robust, time-dependent increases in the abundance of both (Figure 5A and B); conversely, the levels of FUS markedly declined, and the levels of MYH strongly increased with the onset of myogenesis. We then tested the effect of silencing TNNT1 by transfecting siTnnt1, triggering differentiation for 72 h, and measuring the levels of TNNT1, the myogenic marker MYH, and loading control HSP90, by Western blot analysis (Figure 5C). Lowering TNNT1 levels was found to suppress myotube formation, as assessed by phase contrast microscopy (Supplementary material, Figure S5), as well as by immunofluorescence microscopy to detect myotubes by tracking MYH signals (red) (Figure 5D); myotube formation was greatly diminished by 72 h into myogenesis, as evaluated by visual inspection and by calculating fusion indices and numbers of nuclei per myotube (Figure 5D). The inverse pattern of expression and function of FUS and TNNT1 during myogenesis prompted us to analyze more closely a possible role for FUS in negatively controlling TNNT1 expression in this paradigm.
Figure 5.
Lowering TNNT1 expression levels suppresses myogenesis. (A, B) In C2C12 myoblasts undergoing myogenesis, Tnnt1 mRNA expression levels were quantified by RT-qPCR analysis, normalized to 18s rRNA levels (A), and the levels of TNNT1, along with the levels of FUS, MYH, and loading control GAPDH, were assessed by Western blot analysis (B). (C, D) TNNT1 expression was silenced in C2C12 myoblasts by transfection of siTnnt1 (siCtrl in control transfections); 24 h later, cells were induced to differentiate and 3 days after that the levels of TNNT1 (and controls MYH and HSP90) were measured by Western blot analysis (C) and the formation of myotubes was assessed by detecting MYH (and DAPI-stained nuclei) using fluorescence microscopy, followed by quantification of fusion indices and number of nuclei per myotube (D). (E) Schematic of biotinylated RNAs prepared spanning the mouse Tnnt1 mRNA for biotin pulldown analysis. The biotinylated RNAs synthesized spanned the 5′UTR (5U), the coding region (CDS1, CDS2) and the 3′UTR (3U). From CDS1 and 3U, smaller biotinylated RNAs corresponding to segments of the larger RNAs were prepared for analysis. (F) Biotin pulldown analysis using the biotinylated RNAs shown in (E) and C2C12 cell lysates; after the incubation, the presence of FUS-RNA complexes was detected by using streptavidin beads to bring down the complexes, followed by detection of FUS by Western blot analysis. Data in (A, D) represent the means ± SD of three or more independent experiments. Significance (**P < 0.01, ***P < 0.001) was assessed with Student’s t test in all panels. Scale bars, 100 µm.
To identify the segment of Tnnt1 mRNA that associated with FUS, we prepared biotinylated segments spanning the Tnnt1 mRNA and tested their ability to pull down FUS. First, long biotinylated RNAs spanning the 5′UTR (Tnnt1-5U), coding region (Tnnt1-CDS1, Tnnt1-CDS2), and 3′UTR (Tnnt1-3U) of Tnnt1 mRNA were transcribed in vitro as shown in the schematic (Figure 5E); biotinylated Taf15-3U was also synthesized to serve as a positive control for interaction with FUS.23 After incubating C2C12 lysates with these biotinylated RNAs, FUS bound most robustly Tnnt1-CDS1 and Tnnt1-3U (Figure 5F, top). Further subdivision of Tnnt1-CDS1 and Tnnt1-3U (which spanned 400 nt and 200 nt, respectively) was performed to identify more precisely the location(s) of interaction with FUS. Smaller biotinylated RNA probes tiling Tnnt1 mRNA CDS1 and 3U (Figure 5E, bottom) were prepared and biotin pulldown was performed again. The additional results indicated that FUS bound CDS1 RNA segments 1–3, 1–8, and 1–10, as well as 3U region 3U-3 (Figure 5F, bottom). The interaction of FUS with these regions of TNNT1 mRNA was confirmed in AB678 cells (Supplementary material, Figure S6C and D). Similar to C2C12 cells, FUS in AB678 cells was found to bind both the TNNT1 mRNA coding region (CDS2) and 3′UTR (3U) that were prepared using the human TNNT1 mRNA as template. In sum, these results detailed the inverse correlation between TNNT1 levels and FUS levels throughout myogenesis and confirmed the physical binding of FUS to the mRNA encoding TNNT1.
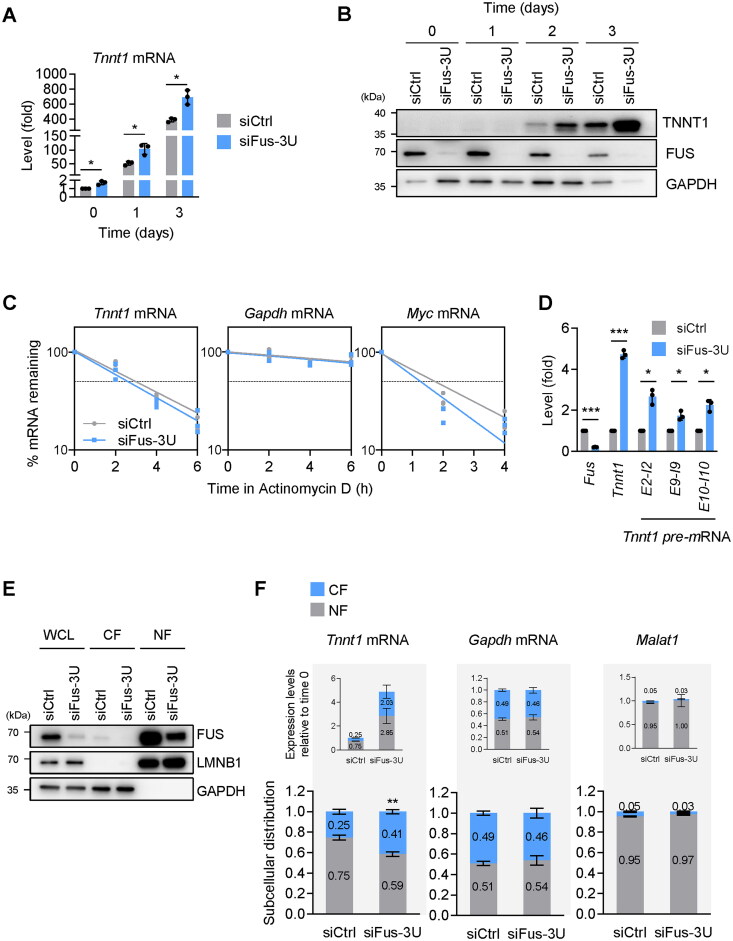
Silencing FUS promotes TNNT1 expression
To establish at the molecular level how FUS regulated TNNT1 production, C2C12 cells transfected with siCtrl and siFus-3U were induced to undergo myogenesis, and the levels of Tnnt1 mRNA and TNNT1 were analyzed at different time points by RT-qPCR and Western blot analyses, respectively. As shown, Tnnt1 mRNA levels were significantly higher after FUS silencing, and TNNT1 protein levels displayed the same pattern of increased expression (Figure 6A and B). Similar results were seen after silencing FUS in AB678 cells: the levels of TNNT1 mRNA and TNNT1 were elevated after triggering myogenesis (Supplementary material, Figure S6E and F).
Figure 6.
Silencing FUS promotes TNNT1 expression. (A, B) FUS was silenced in C2C12 myoblasts by transfecting siFus-3U (or siCtrl in control transfections); 24 h later, they were placed in differentiation medium and the levels of Tnnt1 mRNA (and 18S rRNA for normalization) were quantified by RT-qPCR analysis (A), and the levels of TNNT1 protein (and controls FUS and GAPDH) by Western blot analysis, at the times shown after the start of differentiation. (C) In C2C12 cells transfected as explained in (A), the relative stability of Tnnt1 mRNA (as well as stable control Gapdh mRNA and labile control Myc mRNA) in each transfection group was measured by incubating cells with 2.5 μg/mL of actinomycin D to block de novo transcription, collecting total RNA at the time shown, and measuring the levels of mRNAs (normalized to 18s rRNA levels) at each time after adding actinomycin D to evaluate their decay rates. (D) Proliferating C2C12 cells were transfected with siFus-3U (or siCtrl in control transfections); 48 h later, the relative levels of mature Tnnt1 mRNA as well as nascent Tnnt1 pre-mRNAs were measured by RT-qPCR analysis using specific primers. 18s rRNA was used as internal control for normalization of RT-qPCR data. (E) C2C12 cells were processed as described in (D) and whole-cell lysates (WCL), cytoplasmic fraction (CF), and nuclear fraction (NF) were prepared. Western blot analysis was used to ascertain the subcellular localization of FUS in each transfection group and each fraction; the nuclear protein Lamin B1 (LMNB1) and the cytoplasmic protein GAPDH were included as controls. (F) RNA was prepared from the CF and NF in the cells described in (E) and the levels of Tnnt1 mRNA and controls Gapdh mRNA and Malat1 (a nuclear lncRNA) were quantified by RT-qPCR analysis. The data were represented in two ways: as the subcellular distribution of RNAs in NF vs CF in both transfection groups (each equal to 1.0, bottom graphs) and as the expression levels of RNAs in NF and CF in both transfection groups (each relative to siCtrl, top graphs). Data in (A, C, D, F) represent the means ± SD of three or more individual experiments; other data are representative from three independent experiments. Significance (*P < 0.05, **P < 0.01, ***P < 0.001) was assessed with Student’s t test.
We postulated that FUS might lower the stability of Tnnt1 mRNA, as reported for other RBPs that associated with mRNA 3′UTRs.24–27 To test this possibility, proliferating C2C12 cells were transfected with siCtrl or siFus-3U; 48 h later, they were treated with actinomycin D, a drug that blocks RNA polymerase II and hence suppresses de novo transcription of mRNAs and permits the quantification of mRNA half-lives. RNA was harvested 0, 2, 4, and 6 h later and RT-qPCR analysis was performed to measure the levels of RNAs remaining at the different time points; this experiment revealed that silencing FUS did not affect the relative stability of Gapdh mRNA (a control stable transcript encoding the housekeeping protein GAPDH), and it slightly lowered the stability of Myc mRNA, a labile mRNA included here as a control to test the efficiency of RNA pol II inhibition (Figure 6C). However, the relative half-life of Tnnt1 mRNA, contrary to expectation, did not increase by FUS silencing, as both transfection groups exhibited Tnnt1 mRNA half-lives of ∼3 h. Therefore, these results suggested that the rise in Tnnt1 mRNA after silencing was not due to increased Tnnt1 mRNA stability. The RNA-seq analysis identified a number of isoforms expressed from the Tnnt1 gene; as shown (Supplementary material, Figure S7A), all the variants overall increased by days 2 and 3 after silencing FUS. The four most abundant Tnnt1 mRNA variants showed comparable expression patterns (Supplementary material, Figure S7B), supporting the notion that no particular splicing variant accounted for the accumulation of Tnnt1 mRNA, and that increased transcription was a major driver of the increase in Tnnt1 mRNA levels after silencing FUS.
To more directly establish if FUS silencing affected the rate of transcription of the Tnnt1 gene, we quantified the levels of the Tnnt1 mRNA precursor Tnnt1 pre-mRNA, a surrogate measure of nascent transcription, by RT-qPCR analysis using primer pairs spanning intron-exon junctions. As shown in Figure 6D, 48 h after silencing FUS in C2C12 cells, the levels of Tnnt1 pre-mRNA were higher in the siFus-3U cells than in control cells. Taken together, we concluded that FUS silencing increased both the transcription of Tnnt1 mRNA and the levels of TNNT1 protein.
FUS inhibits TNNT1 mRNA export and translation
We then focused on the impact of the interaction between FUS and Tnnt1 mRNA on TNNT1 expression. We first studied whether Tnnt1 mRNA export from the nucleus to the cytoplasm might be influenced by FUS. We silenced FUS in proliferating C2C12 cells and quantified the relative levels of Tnnt1 mRNA in the nucleus and the cytoplasm. C2C12 cells were fractionated into the cytoplasmic fraction (CF) and the nuclear fraction (NF), and the quality of the fractionation was assessed by monitoring the levels of the nuclear protein lamin B1 (LMNB1) and the cytoplasmic protein GAPDH (Figure 6E). We then measured by RT-qPCR analysis the levels of Tnnt1 mRNA in each fraction, along with the levels of the control transcripts Gapdh mRNA and the nuclear lncRNA Malat1 (Figure 6F). We plotted the levels of the transcripts as a fraction of the total (a value of 1) both independently of the effect of FUS silencing (bottom graphs), and relative to the levels after FUS silencing (top graphs). As shown, Malat1 was confirmed to be in the NF and did not change after FUS silencing, while Gapdh mRNA was found in CF and NF and remained similarly unchanged by FUS silencing. Strikingly, however, Tnnt1 mRNA levels, as a fraction of the total in the cell, increased in the cytoplasm after FUS silencing (from 0.25 to 0.41, bottom graphs); in addition, Tnnt1 mRNA overall increased in the cell after silencing FUS, further elevating the levels in the cytoplasm by ∼8-fold (0.25 vs 2.03, top graphs). These results suggested that FUS suppressed both Tnnt1 mRNA transcription and Tnnt1 mRNA export to the cytoplasm, as lowering FUS increased both Tnnt1 mRNA transcription and Tnnt1 mRNA export to the cytoplasm.
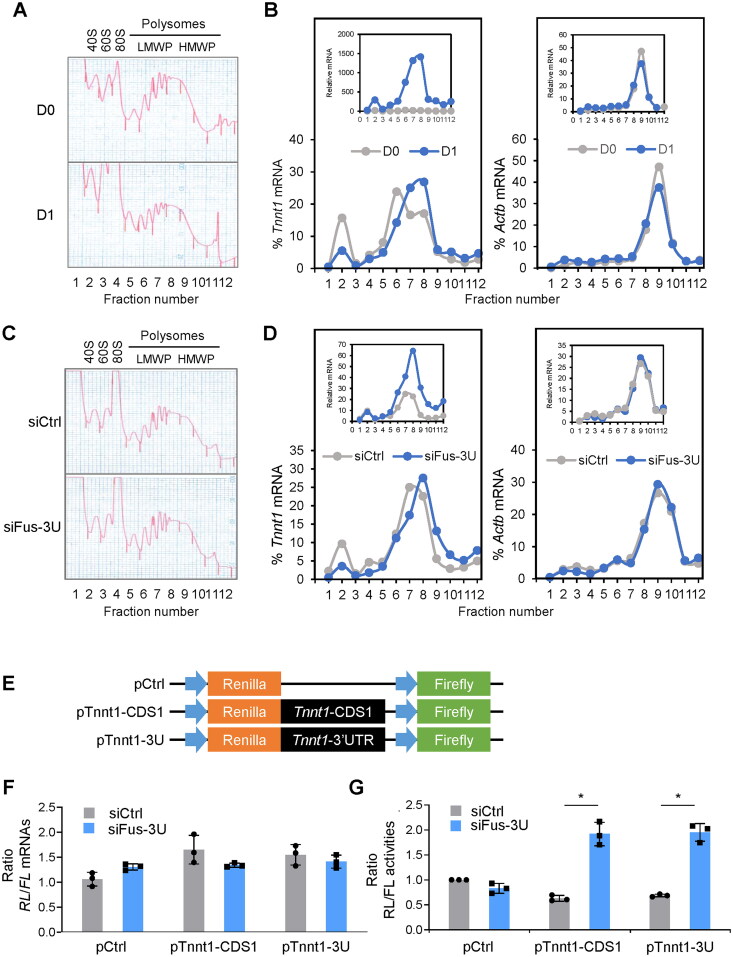
Second, given the relative rise in cytoplasmic Tnnt1 mRNA levels after reducing FUS levels (Figure 6F), and a gradual accumulation of cytoplasmic Tnnt1 mRNA during myogenesis (Supplementary material, Figure S8), we investigated if there was a concomitant change in translational efficiency after inducing myogenesis in C2C12 cells. We fractionated the cytoplasm of C2C12 myoblasts in proliferating conditions at day 0 (D0) and 24 h into differentiation (D1) by centrifugation through sucrose gradients (10–50% sucrose) to assess the association of Tnnt1 mRNA with the translation machinery. Polysome traces revealed that relative to D0, the ribosome subunits (40S, 60S) and monosomes (80S), in fractions ∼2–4, increased in D1, while the polysome fractions, including low- and high-molecular-weight (LMWP and HMWP, respectively, in fractions ∼5–8 and ∼9–12) decreased in D1 (Figure 7A). This decline was mirrored by a slight reduction in the relative distribution of Actb mRNA, encoding the housekeeping protein ACTB (β-Actin), across the fractions. Actb mRNA was measured in each fraction by RT-qPCR analysis and each value per fraction was calculated as a percentage of the total Actb mRNA in the entire gradient (set at 100%) (Materials and Methods); as shown, Actb mRNA levels peaked at fraction ∼9, suggesting that this mRNA was highly translated; in keeping with the overall modest reduction in polysomes at D1, this peak was slightly less pronounced in the D1 sample (Figure 7B, right). This reduction was observed both after considering Actb mRNA as a percentage of the total gradient in D0 and D1 (bottom graph) and after considering any changes in total Actb mRNA in D1 relative to D0 (top graph). In contrast, Tnnt1 mRNA peaked at a heavier fraction of the gradient in D1 (fraction 8) than in D0 (fraction 6, with a minor peak at fraction 2), consistent with more engagement in translation “per molecule of Tnnt1 mRNA”; Figure 7B, left). While these differences were prominent when considering Tnnt1 mRNA as a percentage of the total gradient (bottom graph), they were even more accentuated when considering the differences in overall Tnnt1 mRNA abundance in D1 relative to D0 (top graph), together supporting the robust translational engagement of Tnnt1 mRNA with myogenic differentiation.
Figure 7.
FUS inhibits Tnnt1 mRNA cytoplasmic export and translation. (A, B) C2C12 cells that were either proliferating (D0) or 24 h into differentiation (D1) were collected, and cytoplasmic lysates were fractionated through linear 10–50% sucrose density gradients; the fractionation traces (A) depicted ribosome subunits (40S, 60S), monosomes (80S), and polysomes of low- and high-molecular-weight (LMWP and HMWP, respectively). RNA was isolated from each fraction and the levels of Tnnt1 mRNA and control Actb mRNA were measured in each fraction and represented in two different ways; in one, the Tnnt1 and Actb mRNAs in each gradient (D0 and D1) were normalized to 100% and the levels in each fraction represented as a percentage of the total (bottom graphs); in the other, the relative abundance of Tnnt1 and Actb mRNAs in the D1 gradient relative to the D0 gradient was reflected (top graphs). (C, D) C2C12 cells in which FUS was silenced (siFus-3U) or not (siCtrl) were fractionated and processed as described in (A, B). Similarly, the levels of Tnnt1 and Actb mRNAs were represented by normalizing the total on each gradient to 100% and representing the distribution of the mRNA in each fraction (bottom graphs) as well as by preserving the relative abundance in the siFus-3U transfection group relative to the siCtrl transfection group (top graphs). (E) Schematic of the dual luciferase reporter constructs tested. The psiCHECK2 plasmid (pCtrl) was used to clone on the 3′ end of the Renilla coding region either the Tnnt1-CDS1 (pTnnt1-CDS1) or the Tnnt1-3U (pTnnt1-3U). (F, G) C2C12 cells were transfected with siFus-3U or siCtrl; 24 h later, the three vectors shown in (E) were transfected, and 24 h after that the levels of RL mRNA and FL mRNA (F) as well as the RL and FL activities (G) were measured in each transfection group. Data in (F, G) represent the means ± SD from three independent experiments. Statistical significance (*P < 0.05) was assessed with Student’s t test. Data in A–D are representative of three independent fractionation experiments.
We repeated this analysis after silencing FUS for 48 h in proliferating myoblasts. Here, there were no differences in the polysome traces between the control (siCtrl) and FUS-silenced (siFus-3U) populations (Figure 7C), and silencing FUS did not affect the relative distribution of Actb mRNA (Figure 7D, right), which again peaked at fraction 9, with overlapping distributions whether we examined Actb mRNA levels as a fraction of the total in the gradient (bottom graph) or Actb mRNA after silencing FUS relative to control (top graph). In contrast, silencing FUS in proliferating myoblasts affected the distribution of Tnnt1 mRNA, which shifted from fractions 2 and 7 to a heavier fraction (fraction 8) in this analysis (Figure 7D, left), suggestive of more active engagement in translation. This shift in distribution was once again more prominent when considering the total levels of Tnnt1 mRNA after silencing FUS relative to control (top graph) than when simply considering percent total Tnnt1 mRNA per fraction (bottom graph). Together, the polysome analysis data indicate that in myoblasts that were either differentiating (D1) or expressing silenced levels of FUS (siFus-3U), Tnnt1 mRNA was not only elevated, but also more engaged with the translation machinery on a “per Tnnt1 mRNA molecule” basis than in the corresponding control populations, supporting the notion that FUS silencing promoted translation of Tnnt1 mRNA.
To gain further support for this finding, we sought to test the effect of silencing FUS on the translation of a heterologous reporter bearing the Tnnt1 mRNA regions of interaction (as elucidated in Figure 5). A dual luciferase reporter (psiCHECK2) was used to separately clone the coding region (CDS1) and the 3′UTR of Tnnt1 mRNA to express both an experimental Renilla luciferase reporter mRNA (RL mRNA) and a control constitutive Firefly luciferase mRNA (FL mRNA) from the same vector backbone. The reporter plasmids prepared for this analysis are shown in the schematic (Figure 7E). Forty-eight hours after silencing FUS in C2C12 myoblasts by transfecting siFus-3U (siCtrl in control populations), the three vectors (Figure 7E) were further transfected individually, and 24 h after that the levels of RL and FL activity, as well as the levels of RL mRNA and FL mRNA were measured in each transfection group. As shown, silencing FUS did not affect the relative levels of RL mRNA, with or without Tnnt1 RNA segments (relative to FL mRNA), in any of the three reporter groups, as they all showed relatively similar levels of RL mRNA relative to FL mRNA (Figure 7F); these results recapitulate the lack of influence by FUS on Tnnt1 mRNA stability data shown in Figure 6C. Interestingly, however, silencing FUS derepressed the translation of RL (bearing Tnnt1 CDS1 or 3′UTR), as RL activity levels increased significantly from the two RL chimeric mRNAs in populations with silenced FUS (siFus-3U) relative to siCtrl populations (Figure 7G). Together with the polysome analysis data (Figure 7A to D), these results support the idea that FUS did not lower the levels of RNAs bearing Tnnt1 mRNA regulatory elements, but reduced their translation.
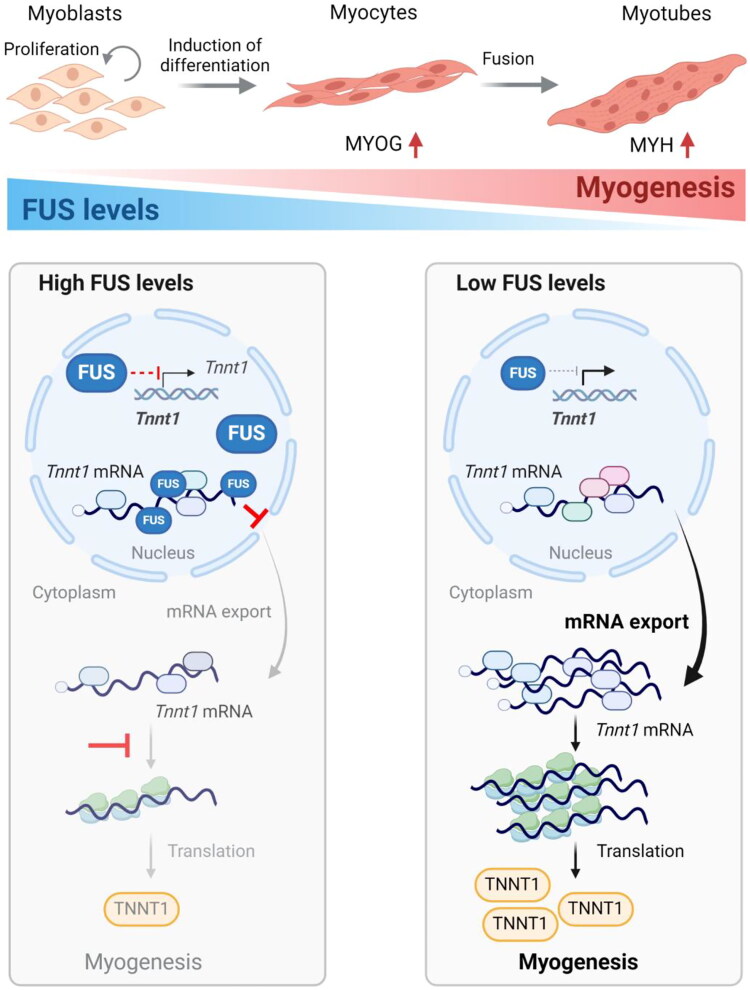
In summary, we propose a model whereby FUS binds both the CDS and 3′UTR of Tnnt1 mRNA and retains it in the nucleus of proliferating C2C12 myoblasts, preventing the untimely production of TNNT1 before myogenesis begins. As myogenic differentiation progresses, FUS levels decline, leading to the release of Tnnt1 mRNA from nuclear sequestration, the increase in Tnnt1 mRNA in the cytoplasm, and an elevated association of Tnnt1 mRNA with polysomes, culminating with increased translation of TNNT1 protein to support the contraction machinery in the differentiated myofiber (Figure 8).
Figure 8.
Proposed function of FUS in myogenesis. In proliferating myoblasts, FUS is highly abundant in the nucleus and retains Tnnt1 mRNA, preventing its export to the cytoplasm. Following the induction of myogenesis, FUS levels decline, allowing Tnnt1 mRNA to be exported to the cytoplasm and to be translated as the myogenic program progresses. In sum, FUS negatively regulates myogenesis by retaining Tnnt1 mRNA in the nucleus, preventing premature TNNT1 production. Model was created using BioRender.
Discussion
In this investigation, we have identified a novel role for FUS in myogenesis. In C2C12 myoblasts, the reduction in FUS levels as myogenesis progressed was associated with an increase in the translation of TNNT1, a contractile protein encoded by the FUS-associated Tnnt1 mRNA. Molecular characterization of this regulatory process revealed that FUS associated with the coding region and 3′UTR of Tnnt1 mRNA, and this association was linked to the increased retention of Tnnt1 mRNA in the nucleus. Upon FUS silencing or a myogenesis-related reduction in FUS, the sequestration of Tnnt1 mRNA in the nucleus was relieved, and the cytoplasmic Tnnt1 mRNA showed increased association with polysomes with a rise in TNNT1 levels at later stages of myogenesis.
Expressed across many tissues, FUS is a multifunctional DNA- and RNA-binding protein involved in transcription, splicing, mRNA stability, and translation.15,16 FUS has been studied extensively in amyotrophic lateral sclerosis (ALS) and frontotemporal dementia (FTD), where it is sometimes mutated, overexpressed, or both.28 In ALS and FTD, much of FUS research has focused on FUS mutations showing mislocalization to cytoplasm of motor neurons or neuromuscular junctions, associated with neurodegeneration.28–30 Interestingly, in ALS-relevant cell paradigms, wild-type and mutant FUS were also shown to sequester other molecules, including mRNAs transcribed from the nuclear and mitochondrial genomes encoding proteins required for mitochondrial respiration.31 This sequestration was sometimes linked to FUS aggregates across the cell, other times to FUS present in mitochondria. Given that ALS and FTD manifestations include muscle weakness and spasms, it will be interesting to test if there is impaired regulation of TNNT1 expression by mutant or aberrantly expressed FUS.
As reported here for skeletal myogenesis, FUS was similarly found to prevent neuronal differentiation and epidermal differentiation. For example, FUS levels were found to be reduced during neurogenic differentiation of neuroblastoma SH-SY5Y cells and during mouse brain development;32 conversely, ectopic overexpression of wild-type FUS reduced neuronal differentiation by maintaining neural stem progenitor cells (NSPCs) in an undifferentiated state.33 In a similar manner, FUS inhibited keratinocyte differentiation, evidenced by the fact that depleting FUS reduced the levels of mRNAs encoding proliferative proteins, while it increased the levels of differentiation markers in epidermal organotypic tissue.34 During keratinocyte differentiation, FUS was reported to function by regulating alternative splicing. Taken together, FUS appears to participate in many differentiation paradigms, often preventing the untimely initiation of differentiation programs.
Here, Tnnt1 mRNA was found to be a major target of FUS in myoblasts (Figure 4). The encoded protein, TNNT1, was found to play a positive role in myogenesis, as silencing TNNT1 reduced myotube formation (Figure 5). Although TNNT1 has been used as a marker protein during myogenesis,35–37 its role in myogenesis was not previously recognized. To study if TNNT1 was elevated by the reduction of FUS and not simply as a consequence of the resulting myogenic progression, the experiments to investigate how FUS regulates TNNT1 expression were performed without inducing differentiation. As observed, silencing FUS increased the levels of Tnnt1 pre-mRNA and overall Tnnt1 mRNA variants, while FUS did not influence the stability of Tnnt1 mRNA or the levels of the different variants (Figure 6C; Supplementary material, Figure S7). Details of how FUS silencing increases the transcription of Tnnt1 mRNA were not studied here, although FUS has been reported to be involved in associating with some promoters and broadly promoting transcription elongation.38–40 Whether FUS binds regulatory regions of the Tnnt1 gene and directly or indirectly inhibits Tnnt1 transcription in proliferating myoblasts, awaits further study.
We focused instead on the robust association of FUS with Tnnt1 mRNA, first identified by RIP-seq analysis, and validated by methods such as RIP followed by RT-qPCR analysis, and biotinylated RNA pulldown followed by Western blot analysis. With a vastly nuclear distribution in myoblasts (Figure 1 and Figure 6), FUS was found to participate in the retention of Tnnt1 mRNA in the nucleus of proliferating myoblasts. The reduction of FUS levels with myogenesis or after silencing released Tnnt1 mRNA, which increased by ∼8-fold in the cytoplasm as myogenesis progressed (Figure 6F) and showed extensive association with polysomes (Figure 7B), to reach the striking levels of TNNT1 production in later stages of myogenesis (Figure 5B and Figure 6B). Although our data support a model whereby FUS mainly suppresses TNNT1 translation indirectly by retaining Tnnt1 mRNA in the nucleus, additional regulatory mechanisms may be linked more directly to the modest presence of FUS in the cytoplasm. Cytoplasmic FUS has been reported to function in mRNA transport, mRNA stabilization, as well as increased and decreased translation of different mRNAs.20,41–45 Given that FUS does not appear to have a uniform influence on mRNA translation, whether the small proportion of cytoplasmic FUS might influence the translation of Tnnt1 mRNA during myogenesis will be explored in a future study dedicated to this specific question.
Other targets of FUS have been identified by cross-linking immunoprecipitation followed by high-throughput sequencing (CLIP-seq) analysis.25,46–49 These earlier studies did not find TNNT1/Tnnt1 mRNA as a target of FUS, likely because TNNT1 is mainly expressed in skeletal muscle cells, and the CLIP-based surveys of FUS target mRNAs were performed in non-muscle cells such as human embryonic kidney HEK293 cells,25 mouse neuroblastoma neuro-2a cells49 and brain tissues.46–48 TNNT1 was also studied in several cancers, where it influenced processes such as cell migration.50–54 Whether FUS regulates TNNT1 expression in these other tissue types is not known at present.
FUS binds across target transcripts—mainly at introns, but also at CDS and 3′UTR.25,47 The previously reported specific RNA motifs where FUS binds include GGUG, GUGGU, and GU-rich stretches,47–49,55 but these sequences did not appear to be FUS binding sites on Tnnt1 mRNA, as determined by biotin pulldown analysis (Figure 5F). Perhaps in myoblasts other RBPs, other molecules, or posttranslational modifications of FUS influence modify the preferred sites where FUS binds Tnnt1 mRNA and possibly also other mRNAs, including those identified in Figure 4D (e.g., Tnni2 and Tnnt3 mRNAs). Additional studies, including CLIP analysis in myoblasts, are needed to elucidate the specific binding requirements between FUS and Tnnt1 mRNA.
It will also be interesting to find out if FUS binds Tnnt1 pre-mRNA, and if it might participate in the splicing of Tnnt1 pre-mRNA. Whether FUS binds Tnnt1 mRNA (or precursor transcripts) as part of FUS aggregates in the nucleus will be examined in the future by single-molecule FISH analysis. An additional possibility that awaits formal testing is if the association of FUS and Tnnt1 mRNA blocks Tnnt1 mRNA export by competing with mRNA export-related proteins or by involving surveillance mechanisms for mRNA export.56 It will be important to establish whether FUS similarly prevents the untimely export of other myogenic mRNAs.
To close, given that TNNT1 functions in muscle contraction, and that depletion or mutation of TNNT1 can diminish muscle function and cause myopathy,21,22,57,58 elucidating the regulators of TNNT1 production can help identify therapeutic targets to intervene in muscle disease. In this regard, a more complete understanding on the role of FUS in human myogenesis, the development of animal models to study FUS regulation of TNNT1 production in vivo, and the discovery of chemical or biological agents to alter FUS function to improve TNNT1 synthesis, will provide tools to combat muscle loss. Whether due to disuse, aging, disease, or neuropathology, we propose that TNNT1 production by FUS is a targetable regulatory paradigm to maintain muscle health.
Materials and Methods
Cell culture, transfection, and myogenic differentiation
Mouse C2C12 myoblasts and immortalized AB678 myoblasts were cultured and differentiated at 37 °C in 5% CO2 as described.59 C2C12 cells were cultured in growth medium (GM), Dulbecco’s modified Eagle’s medium, (DMEM, Life Technologies) supplemented with 20% fetal bovine serum (FBS, Gibco) and 1% antibiotics (Life Technologies). AB678 cells were cultured in GM (equal volume mixture of Ham’s F10 media and Promocell Skeletal Muscle Cell Growth Medium) containing 10% FBS, 1% growth medium supplement Mix (Promocell) and 1% antibiotics. Differentiation of C2C12 and AB678 cells was induced by growth to high density followed by replacement of the GM to differentiation medium (DM, DMEM containing 2% horse serum). For loss-of-function experiments, small interfering RNAs (siRNAs) were transfected into the cells 24 h before induction of differentiation using Lipofectamine 2000 (Invitrogen) for C2C12 with 40 nM siRNA final concentration and Lipofectamine RNAiMAX (Invitrogen) for AB678 with 10 nM siRNA final concentration, following the manufacturer instructions. Control siRNA (siCtrl), mouse siFus, and human siFUS were from Santa Cruz Biotechnology; mouse siFus-3U, mouse siTnnt1, and human siTNNT1 were from IDT. To overexpress FUS, pEGFP-N1 (#60360) and pEGFP-FUS (#60362) were obtained from Addgene; plasmids (2 μg) were transfected into C2C12 cells using Lipofectamine 2000 and differentiation was induced 24 h later.
RNA isolation and RT-qPCR analysis
Cells were lysed using the TriPure™ Isolation Reagent (Sigma-Aldrich) and RNA was isolated using Direct-zol RNA MiniPrep kit (ZYMO RESEARCH) following the manufacturer instructions. Total RNA (1 µg) was subjected to reverse transcription (RT) using the Maxima Reverse Transcriptase (Thermo Fisher) with random hexamers, and the abundance of transcripts was assessed by quantitative PCR (qPCR) analysis with SYBR green master mix (Kapa Biosystems) using gene-specific primer sets (Supplementary material, Table S3). RT-qPCR analysis was conducted using a QuantStudio 5 Real-Time PCR System (Thermo Fisher), the relative RNA levels were quantified by the 2−ΔΔCt method, and were normalized to the levels of 18s rRNA, Gapdh mRNA or Actb mRNA.
Western blot analysis
Cells were lysed in RIPA buffer (10 mM Tris-HCl [pH 7.5], 150 mM NaCl, 1 mM EDTA, 1% NP-40, 0.1% SDS, and 0.5% sodium deoxycholate) containing 1× protease inhibitor cocktail (Roche) and then the cleared lysates were collected after sonication and centrifugation for 15 min at 13,000 × g. Protein concentration was measured using the Bradford assay (Bio-Rad), and protein samples containing 1× SDS Laemmli sample buffer (Bio-Rad) were boiled at 95 °C for 5 min before loading. Proteins were separated by electrophoresis through 4–20% SDS-polyacrylamide gels and transferred onto nitrocellulose membrane using Trans-Blot Turbo transfer system with Trans-Blot Turbo RTA Midi 0.2 µm Nitrocellulose Transfer Kit (Bio-Rad). Membranes were blocked with 5% non-fat milk for 1 h at room temperature and incubated with the primary antibodies for 18 h at 4 °C. After washing with 1× TBS-T, the membranes were incubated with secondary antibodies for 30 min at room temperature. Following additional washing, membranes were briefly incubated with KwikQuant Western blot detection kit (Kindle Biosciences) and chemiluminescent signals on membranes were detected using ChemiDoc system (Bio-Rad). Primary antibodies used for Western blot analysis are listed (Supplementary material, Table S3). The levels of FUS on Western blots were quantified by densitometry analysis of FUS signals using the software ImageJ and normalized to the intensities of GAPDH signals.
Immunofluorescence
After washing twice with PBS, adherent cells were fixed with 4% paraformaldehyde solution (Electron Microscopy Sciences) in PBS for 20 min at room temperature. Cells were washed once and incubated in 0.2% Triton X-100 for 5 min at room temperature for permeabilization. After washing once with PBS, the cell preparations were blocked for 1 h with 10% normal goat serum (Life Technologies), and then primary antibodies were added in blocking buffer and incubated at 4 °C for 18 h. After washing four times with PBS, cells were incubated with diluted secondary antibodies for 1 h. Following additional washes with PBS, cells were incubated in PBS containing DAPI for 20 min at room temperature in the dark; afterward, cells were washed three more times with PBS. Antibodies used for immunofluorescence are listed (Supplementary material, Table S3). Fluorescent micrographs were taken from more than three areas for each experiment using a microscope (KEYENCE BZ-X700). To measure fusion indices and numbers of nuclei per myotube, total numbers of nuclei in a frame were counted using a BZ-Analyzer hybrid macro cell count, and the number of myotubes and the number of nuclei in myotubes were manually counted in at least three fields each.
Ribonucleoprotein immunoprecipitation (RIP) analysis
C2C12 cells in GM were harvested and centrifuged at 500 × g for 5 min at 4 °C. The cell pellet was lysed with RIPA buffer containing protease and RNase inhibitors for 10 min on ice, and the supernatant was collected after centrifugation at 13,000 × g for 15 min at 4 °C. After measuring protein concentration using the Bradford assay, cell lysates (4 mg) were incubated with 8 µg of anti-FUS antibody or control IgG immobilized on Protein A Sepharose beads (Sigma) in NT2 buffer (50 mM Tris [pH 7.4], 150 mM NaCl, 1 mM MgCl2, and 0.05% NP-40) for 2 h with rotation at 4 °C. The remaining cell lysate was used for isolating input RNA. Ribonucleoprotein (RNP) complexes bound to the beads were washed five times with cold NT2 buffer; 50 µL of washed RNP complexes was used for Western blotting, and the remainder was incubated with DNase I and proteinase K. RNA isolated from the RNP complexes and the input using acid phenol-chloroform was used for RNA-seq analysis and validation by RT-qPCR analysis. For the validation, enriched mRNAs were normalized to Gapdh mRNA and quantified with the 2−ΔΔCt method compared to the levels of mRNAs in control IgG IP samples. The antibodies used for RIP are listed (Supplementary material, Table S3).
RNA-seq analysis
RNA isolated after FUS RIP and after silencing FUS in C2C12 cells were subjected to RNA-seq analysis. The quality and quantity of RNA were checked using Agilent RNA Screen Tape on the Agilent Tapestation, and 200 ng of high-quality RNA was used to prepare libraries with the Illumina TruSeq Stranded Total RNA Library prep kit, following the manufacturer protocol. Briefly, after rRNA depletion and cDNA generation, cDNAs were subjected to 3′ end adenylation, adapter ligation, and purification using AMPure beads (Beckman). cDNA products of specific sizes were selected with SPRIselect beads (Beckman), enriched by PCR amplification, and purified again with SPRIselect beads to generate the final libraries. The quality and quantity of the sequencing libraries were checked using the DNA 1000 kit on the Agilent 2100 bioanalyzer for FUS RIP samples, and Agilent DNA 1000 Screen Tape on the Agilent Tapestation for FUS silencing samples. Paired-end sequencing was performed for ∼110 cycles with Illumina Novaseq 6000 sequencer with depth of 50 million reads for FUS RIP samples and 160 million reads for FUS silencing samples.
After sequencing, BCL files were demultiplexed and converted to FASTQ files using the bcl2fastq v2.20.0.422 software. FASTQ files were trimmed for adapter sequences using Cutadapt v. 1.18 and aligned to the mouse genome mm10 Ensembl v. 89 using STAR v. 2.4.0j, and gene counts were created with featureCounts v. 1.6.4. The R package DESeq2 v. 1.30.060 was used for data normalization and differential gene expression analysis. Transcripts were considered as differentially expressed if they met the statistical significance Benjamini–Hochberg adjusted P value < 0.05. Gene Set Enrichment Analysis (GSEA) was performed with clusterProfiler v. 3.17.3.61 To quantify isoforms, STAR v. 2.7.8 was run by setting the quantMode parameter to TranscriptomeSAM, and RSEM v. 1.3.1 was used to quantify isoform expression. The R package tximport v. 1.28.0 was used for loading isoform data generated by RSEM to the R environment. Finally, the package DESeq2 v. 1.4.0 was used for performing differential expression analysis. Adjusted P < 0.05 was considered to be statistically significant. The RNA-seq data are deposited in GSE267276 and GSE267277.
Biotin pulldown assay
To synthesize biotinylated RNA probes, PCR fragments were prepared from C2C12 cDNA using forward primers that contained the T7 RNA polymerase promoter sequence (Supplementary material, Table S3). The primers were designed to amplify each segment on mouse Tnnt1 mRNA (NM_001277903.1); mouse Taf15 mRNA 3′UTR (NM_027427.3), a FUS target,23 was included as a positive control. For the short biotinylated RNA probes corresponding to Tnnt1-CDS1 and Tnnt1-3U, synthesized DNA duplexes containing the T7 RNA polymerase promoter sequence (IDT) were used as templates for in vitro transcription (Supplementary material, Table S3). After purification of the PCR products, biotinylated transcripts were synthesized using the MEGAshortscript™ T7 Transcription Kit (Invitrogen) and Biotin-14-CTP (Invitrogen). C2C12 cells were lysed with RIPA buffer containing protease and RNase inhibitors for 10 min on ice, and the supernatant was collected after centrifugation at 13,000 × g for 15 min at 4 °C. Cell lysates (500 μg) were incubated with 2 μg of purified biotinylated RNAs for 1 h at room temperature, and the complexes were isolated using streptavidin-coupled Dynabeads (Invitrogen). Proteins present in the pulldown material were detected by Western blot analysis.
mRNA stability assay
C2C12 cells were transfected with siRNAs for 48 h in GM and then they were treated with 2.5 μg/mL Actinomycin D to inhibit de novo transcription and collected 2, 4, and 6 h later; untreated cells were harvested at the 0 h time point. Total RNA harvested at each time point was quantified by RT-qPCR analysis as described above and quantified using the 2−ΔΔCt method to calculate the remaining mRNAs compared to time point 0; Ct values were normalized to the levels of 18S rRNA, as it is refractory to Actinomycin D treatment. The labile Myc mRNA was used as a positive control.62
Subcellular fractionation
Forty-eight hours after transfecting proliferating C2C12 cells with siRNAs, cells were harvested by centrifugation at 500 × g for 5 min and used to prepare lysates from whole cells and from nuclei and cytoplasms. Subcellular fractionation was performed using the PARIS kit (Invitrogen) following the manufacturer instructions, and RNAs in nuclear and cytoplasmic fractions were isolated and quantified by RT-qPCR analysis. The abundance was normalized to Gapdh mRNA in each fraction.
Polysome assay
Polysome analysis was performed as previously described.63 Briefly, C2C12 cells in GM were treated with 100 μg/mL of cycloheximide (CHX; Sigma) for 10 min and lysed in polysome extraction buffer (PEB; 20 mM Tris–HCl [pH 7.5], 100 mM KCl, 5 mM MgCl2 and 0.5% NP-40) containing 100 μg/mL CHX, as well as protease and RNase inhibitors. The lysates were further fractionated by ultracentrifugation through linear, 10–50% sucrose gradients, as described.63 After centrifugation, 12 fractions were collected for further analysis. The distribution of mRNAs was quantified by RT-qPCR analysis and plotted as a percentage of the specific mRNA in each fraction relative to the total amount of mRNA in the gradient. Alternative plotting was done by preserving the relative differences in mRNAs between both fractionation groups.
Dual luciferase assay
Reporter plasmids were constructed by cloning mouse Tnnt1-CDS1 and Tnnt1-3U, into the dual luciferase vector psiCHECK2 (Promega). C2C12 cells were first transfected with siRNAs and 24 h later with reporter plasmids in GM; 24 h after that, cells were harvested to prepare RNA to measure RL and FL mRNAs, as well as to prepare protein lysates to measure RL and FL activities using the Dual-Luciferase reporter assay kit (Promega) following the manufacturer instructions with GloMax Discover system (Promega).
Statistical analysis
Data were expressed as the means ± SD of three or more independent experiments. Statistical significance was established by Student’s t test using the GraphPad Prism 9.0 software; *P < 0.05; **P < 0.01; ***P < 0.001.
Supplementary Material
Funding Statement
This work was supported in its entirety by the National Institute on Aging Intramural Research Program, NIH.
Authors’ Contributions
E.J. and M.G. conceived the project, designed experiments, and wrote the manuscript; E.J. performed the majority of the experiments; P.R.P., J.L.M., X.Y., J.H.Y. D.T., C.H.S. contributed experiments; K.M.M., N.B., and S.D. analyzed datasets; Y.P., and J.F. provided expertise and assistance with sequencing. All authors discussed the results and provided comments on the manuscript.
Data Availability Statement
The authors confirm that the data supporting the findings of this study are available within the article and its supplementary materials. The RNA-seq data are deposited at GSE267276 and GSE267277.
Disclosure Statement
No potential conflict of interest was reported by the authors.
References
- 1.Distefano G, Goodpaster BH.. Effects of exercise and aging on skeletal muscle. Cold Spring Harb Perspect Med. 2018;8:a029785. doi: 10.1101/cshperspect.a029785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schmidt M, Schüler SC, Hüttner SS, von Eyss B, von Maltzahn J.. Adult stem cells at work: regenerating skeletal muscle. Cell Mol Life Sci. 2019;76:2559–2570. doi: 10.1007/s00018-019-03093-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McKenna CF, Fry CS.. Altered satellite cell dynamics accompany skeletal muscle atrophy during chronic illness, disuse, and aging. Curr Opin Clin Nutr Metab Care. 2017;20:447–452. doi: 10.1097/mco.0000000000000409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.André LM, Ausems CRM, Wansink DG, Wieringa B.. Abnormalities in skeletal muscle myogenesis, growth, and regeneration in myotonic dystrophy. Front Neurol. 2018;9:368. doi: 10.3389/fneur.2018.00368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Luo H, Lv W, Tong Q, Jin J, Xu Z, Zuo B.. Functional non-coding RNA during embryonic myogenesis and postnatal muscle development and disease. Front Cell Dev Biol. 2021;9:628339. doi: 10.3389/fcell.2021.628339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yu D, Cai Z, Li D, Zhang Y, He M, Yang Y, Liu D, Xie W, Li Y, Xiao W.. Myogenic differentiation of stem cells for skeletal muscle regeneration. Stem Cells Int. 2021;2021:8884283. doi: 10.1155/2021/8884283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Deprez A, Orfi Z, Rieger L, Dumont NA.. Impaired muscle stem cell function and abnormal myogenesis in acquired myopathies. Biosci Rep. 2023;43:BSR20220284. doi: 10.1042/bsr20220284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Koopmans PJ, Ismaeel A, Goljanek-Whysall K, Murach KA.. The roles of miRNAs in adult skeletal muscle satellite cells. Free Radic Biol Med. 2023;209:228–238. doi: 10.1016/j.freeradbiomed.2023.10.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li Y, Chen X, Sun H, Wang H.. Long non-coding RNAs in the regulation of skeletal myogenesis and muscle diseases. Cancer Lett. 2018;417:58–64. doi: 10.1016/j.canlet.2017.12.015. [DOI] [PubMed] [Google Scholar]
- 10.Yang JH, Tsitsipatis D, Gorospe M.. Stoichiometry of long noncoding RNA interactions with other RNAs: insights from OIP5-AS1. Wiley Interdiscip Rev RNA. 2024;15:e1841. doi: 10.1002/wrna.1841. [DOI] [PubMed] [Google Scholar]
- 11.Zhang P, Chao Z, Zhang R, Ding R, Wang Y, Wu W, Han Q, Li C, Xu H, Wang L, et al. Circular RNA regulation of myogenesis. Cells. 2019;8:885. doi: 10.3390/cells8080885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pandey PR, Yang JH, Tsitsipatis D, Panda AC, Noh JH, Kim KM, Munk R, Nicholson T, Hanniford D, Argibay D, et al. circSamd4 represses myogenic transcriptional activity of PUR proteins. Nucleic Acids Res. 2020;48:3789–3805. doi: 10.1093/nar/gkaa035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hinkle ER, Wiedner HJ, Black AJ, Giudice J.. RNA processing in skeletal muscle biology and disease. Transcription. 2019;10:1–20. doi: 10.1080/21541264.2018.1558677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shi DL, Grifone R.. RNA-binding proteins in the post-transcriptional control of skeletal muscle development, regeneration and disease. Front Cell Dev Biol. 2021;9:738978. doi: 10.3389/fcell.2021.738978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Deng H, Gao K, Jankovic J.. The role of FUS gene variants in neurodegenerative diseases. Nat Rev Neurol. 2014;10:337–348. doi: 10.1038/nrneurol.2014.78. [DOI] [PubMed] [Google Scholar]
- 16.Ratti A, Buratti E.. Physiological functions and pathobiology of TDP-43 and FUS/TLS proteins. J Neurochem. 2016;138 Suppl 1:95–111. doi: 10.1111/jnc.13625. [DOI] [PubMed] [Google Scholar]
- 17.Jin JP. Evolution, regulation, and function of N-terminal variable region of troponin T: modulation of muscle contractility and beyond. Int Rev Cell Mol Biol. 2016;321:1–28. doi: 10.1016/bs.ircmb.2015.09.002. [DOI] [PubMed] [Google Scholar]
- 18.Wang DZ, Valdez MR, McAnally J, Richardson J, Olson EN.. The Mef2c gene is a direct transcriptional target of myogenic bHLH and MEF2 proteins during skeletal muscle development. Development. 2001;128:4623–4633. doi: 10.1242/dev.128.22.4623. [DOI] [PubMed] [Google Scholar]
- 19.Zhou Y, Liu S, Liu G, Oztürk A, Hicks GG.. ALS-associated FUS mutations result in compromised FUS alternative splicing and autoregulation. PLoS Genet. 2013;9:e1003895. doi: 10.1371/journal.pgen.1003895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Udagawa T, Fujioka Y, Tanaka M, Honda D, Yokoi S, Riku Y, Ibi D, Nagai T, Yamada K, Watanabe H, et al. FUS regulates AMPA receptor function and FTLD/ALS-associated behaviour via GluA1 mRNA stabilization. Nat Commun. 2015;6:7098. doi: 10.1038/ncomms8098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wei B, Lu Y, Jin JP.. Deficiency of slow skeletal muscle troponin T causes atrophy of type I slow fibres and decreases tolerance to fatigue. J Physiol. 2014;592:1367–1380. doi: 10.1113/jphysiol.2013.268177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mondal A, Jin JP.. Protein structure-function relationship at work: learning from myopathy mutations of the slow skeletal muscle isoform of troponin T. Front Physiol. 2016;7:449. doi: 10.3389/fphys.2016.00449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Colombrita C, Onesto E, Megiorni F, Pizzuti A, Baralle FE, Buratti E, Silani V, Ratti A.. TDP-43 and FUS RNA-binding proteins bind distinct sets of cytoplasmic messenger RNAs and differently regulate their post-transcriptional fate in motoneuron-like cells. J Biol Chem. 2012;287:15635–15647. doi: 10.1074/jbc.M111.333450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ji E, Kim C, Kang H, Ahn S, Jung M, Hong Y, Tak H, Lee S, Kim W, Lee EK.. RNA binding protein HUR promotes autophagosome formation by regulating expression of autophagy-related proteins 5, 12, and 16 in human hepatocellular carcinoma cells. Mol Cell Biol. 2019;39:e00508-18. doi: 10.1128/mcb.00508-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hoell JI, Larsson E, Runge S, Nusbaum JD, Duggimpudi S, Farazi TA, Hafner M, Borkhardt A, Sander C, Tuschl T.. RNA targets of wild-type and mutant FET family proteins. Nat Struct Mol Biol. 2011;18:1428–1431. doi: 10.1038/nsmb.2163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Panda AC, Abdelmohsen K, Yoon JH, Martindale JL, Yang X, Curtis J, Mercken EM, Chenette DM, Zhang Y, Schneider RJ, et al. RNA-binding protein AUF1 promotes myogenesis by regulating Mef2c expression levels. Mol Cell Biol. 2014;34:3106–3119. doi: 10.1128/mcb.00423-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Qian X, Ning H, Zhang J, Hoft DF, Stumpo DJ, Blackshear PJ, Liu J.. Posttranscriptional regulation of IL-23 expression by IFN-gamma through tristetraprolin. J Immunol. 2011;186:6454–6464. doi: 10.4049/jimmunol.1002672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Assoni AF, Foijer F, Zatz M.. Amyotrophic lateral sclerosis, FUS and protein synthesis defects. Stem Cell Rev Rep. 2023;19:625–638. doi: 10.1007/s12015-022-10489-8. [DOI] [PubMed] [Google Scholar]
- 29.Carey JL, Guo L.. Liquid-liquid phase separation of TDP-43 and FUS in physiology and pathology of neurodegenerative diseases. Front Mol Biosci. 2022;9:826719. doi: 10.3389/fmolb.2022.826719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hu L, Mao S, Lin L, Bai G, Liu B, Mao J.. Stress granules in the spinal muscular atrophy and amyotrophic lateral sclerosis: the correlation and promising therapy. Neurobiol Dis. 2022;170:105749. doi: 10.1016/j.nbd.2022.105749. [DOI] [PubMed] [Google Scholar]
- 31.Tsai YL, Coady TH, Lu L, Zheng D, Alland I, Tian B, Shneider NA, Manley JL.. ALS/FTD-associated protein FUS induces mitochondrial dysfunction by preferentially sequestering respiratory chain complex mRNAs. Genes Dev. 2020;34:785–805. doi: 10.1101/gad.335836.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Svetoni F, De Paola E, La Rosa P, Mercatelli N, Caporossi D, Sette C, Paronetto MP.. Post-transcriptional regulation of FUS and EWS protein expression by MIR-141 during neural differentiation. Hum Mol Genet. 2017;26:2732–2746. doi: 10.1093/hmg/ddx160. [DOI] [PubMed] [Google Scholar]
- 33.Stronati E, Biagioni S, Fiore M, Giorgi M, Poiana G, Toselli C, Cacci E.. Wild-type and mutant FUS expression reduce proliferation and neuronal differentiation properties of neural stem progenitor cells. Int J Mol Sci. 2021;22:7566. doi: 10.3390/ijms22147566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Takashima S, Sun W, Otten ABC, Cai P, Peng SI, Tong E, Bui J, Mai M, Amarbayar O, Cheng B, et al. Alternative mRNA splicing events and regulators in epidermal differentiation. Cell Rep. 2024;43:113814. doi: 10.1016/j.celrep.2024.113814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dmitriev P, Barat A, Polesskaya A, O’Connell MJ, Robert T, Dessen P, Walsh TA, Lazar V, Turki A, Carnac G, et al. Simultaneous miRNA and mRNA transcriptome profiling of human myoblasts reveals a novel set of myogenic differentiation-associated mirnas and their target genes. BMC Genomics. 2013;14:265. doi: 10.1186/1471-2164-14-265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ge X, Zhang Y, Park S, Cong X, Gerrard DE, Jiang H.. Stac3 inhibits myoblast differentiation into myotubes. PLoS One. 2014;9:e95926. doi: 10.1371/journal.pone.0095926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tan A, Younis AZ, Evans A, Creighton JV, Coveny C, Boocock DJ, Sale C, Lavery GG, Coutts AS, Doig CL.. PARP1 mediated PARylation contributes to myogenic progression and glucocorticoid transcriptional response. Cell Death Discov. 2023;9:133. doi: 10.1038/s41420-023-01420-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schwartz JC, Ebmeier CC, Podell ER, Heimiller J, Taatjes DJ, Cech TR.. FUS binds the CTD of RNA polymerase II and regulates its phosphorylation at Ser2. Genes Dev. 2012;26:2690–2695. doi: 10.1101/gad.204602.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yang L, Gal J, Chen J, Zhu H.. Self-assembled FUS binds active chromatin and regulates gene transcription. Proc Natl Acad Sci USA. 2014;111:17809–17814. doi: 10.1073/pnas.1414004111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Picchiarelli G, Demestre M, Zuko A, Been M, Higelin J, Dieterlé S, Goy MA, Mallik M, Sellier C, Scekic-Zahirovic J, et al. FUS-mediated regulation of acetylcholine receptor transcription at neuromuscular junctions is compromised in amyotrophic lateral sclerosis. Nat Neurosci. 2019;22:1793–1805. doi: 10.1038/s41593-019-0498-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fujii R, Takumi T.. TLS facilitates transport of mRNA encoding an actin-stabilizing protein to dendritic spines. J Cell Sci. 2005;118:5755–5765. doi: 10.1242/jcs.02692. [DOI] [PubMed] [Google Scholar]
- 42.Zhang G, Chen X, Ma L, Ding R, Zhao L, Ma F, Deng X.. LINC01419 facilitates hepatocellular carcinoma growth and metastasis through targeting EZH2-regulated RECK. Aging (Albany NY). 2020;12:11071–11084. doi: 10.18632/aging.103321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yang Z, Hu T.. Long noncoding RNA HOXC-AS3 facilitates the progression of invasive mucinous adenocarcinomas of the lung via modulating FUS/FOXM1. In Vitro Cell Dev Biol Anim. 2020;56:15–23. doi: 10.1007/s11626-019-00414-8. [DOI] [PubMed] [Google Scholar]
- 44.Yasuda K, Zhang H, Loiselle D, Haystead T, Macara IG, Mili S.. The RNA-binding protein Fus directs translation of localized mRNAs in APC-RNP granules. J Cell Biol. 2013;203:737–746. doi: 10.1083/jcb.201306058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sévigny M, Bourdeau Julien I, Venkatasubramani JP, Hui JB, Dutchak PA, Sephton CF.. FUS contributes to mTOR-dependent inhibition of translation. J Biol Chem. 2020;295:18459–18473. doi: 10.1074/jbc.RA120.013801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ishigaki S, Masuda A, Fujioka Y, Iguchi Y, Katsuno M, Shibata A, Urano F, Sobue G, Ohno K.. Position-dependent FUS-RNA interactions regulate alternative splicing events and transcriptions. Sci Rep. 2012;2:529. doi: 10.1038/srep00529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lagier-Tourenne C, Polymenidou M, Hutt KR, Vu AQ, Baughn M, Huelga SC, Clutario KM, Ling SC, Liang TY, Mazur C, et al. Divergent roles of ALS-linked proteins FUS/TLS and TDP-43 intersect in processing long pre-mRNAs. Nat Neurosci. 2012;15:1488–1497. doi: 10.1038/nn.3230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rogelj B, Easton LE, Bogu GK, Stanton LW, Rot G, Curk T, Zupan B, Sugimoto Y, Modic M, Haberman N, et al. Widespread binding of FUS along nascent RNA regulates alternative splicing in the brain. Sci Rep. 2012;2:603. doi: 10.1038/srep00603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Masuda A, Takeda J, Okuno T, Okamoto T, Ohkawara B, Ito M, Ishigaki S, Sobue G, Ohno K.. Position-specific binding of FUS to nascent RNA regulates mRNA length. Genes Dev. 2015;29:1045–1057. doi: 10.1101/gad.255737.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shi Y, Zhao Y, Zhang Y, AiErken N, Shao N, Ye R, Lin Y, Wang S.. TNNT1 facilitates proliferation of breast cancer cells by promoting G(1)/S phase transition. Life Sci. 2018;208:161–166. doi: 10.1016/j.lfs.2018.07.034. [DOI] [PubMed] [Google Scholar]
- 51.Chen Y, Wang J, Wang D, Kang T, Du J, Yan Z, Chen M.. TNNT1, negatively regulated by miR-873, promotes the progression of colorectal cancer. J Gene Med. 2020;22:e3152. doi: 10.1002/jgm.3152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hao YH, Yu SY, Tu RS, Cai YQ.. TNNT1, a prognostic indicator in colon adenocarcinoma, regulates cell behaviors and mediates EMT process. Biosci Biotechnol Biochem. 2020;84:111–117. doi: 10.1080/09168451.2019.1664891. [DOI] [PubMed] [Google Scholar]
- 53.Huang SC, Huang CC, Ko CY, Huang CY, Liu CH, Lee YK, Chen TY, Hsueh CW, Tzou SJ, Tai MH, et al. Slow skeletal muscle troponin T acts as a potential prognostic biomarker and therapeutic target for hepatocellular carcinoma. Gene. 2023;865:147331. doi: 10.1016/j.gene.2023.147331. [DOI] [PubMed] [Google Scholar]
- 54.Jiang C, Xu F, Yi D, Jiang B, Wang R, Wu L, Ding H, Qin J, Lee Y, Sang J, et al. Testosterone promotes the migration, invasion and EMT process of papillary thyroid carcinoma by up-regulating TNNT1. J Endocrinol Invest. 2024;47:149–166. doi: 10.1007/s40618-023-02132-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lerga A, Hallier M, Delva L, Orvain C, Gallais I, Marie J, Moreau-Gachelin F.. Identification of an RNA binding specificity for the potential splicing factor tls. J Biol Chem. 2001;276:6807–6816. doi: 10.1074/jbc.M008304200. [DOI] [PubMed] [Google Scholar]
- 56.Katahira J. Nuclear export of messenger RNA. Genes (Basel). 2015;6:163–184. doi: 10.3390/genes6020163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ochala J. Thin filament proteins mutations associated with skeletal myopathies: defective regulation of muscle contraction. J Mol Med (Berl). 2008;86:1197–1204. doi: 10.1007/s00109-008-0380-9. [DOI] [PubMed] [Google Scholar]
- 58.Géraud J, Dieterich K, Rendu J, Uro Coste E, Dobrzynski M, Marcorelle P, Ioos C, Romero NB, Baudou E, Brocard J, et al. Clinical phenotype and loss of the slow skeletal muscle troponin T in three new patients with recessive TNNT1 nemaline myopathy. J Med Genet. 2021;58:602–608. doi: 10.1136/jmedgenet-2019-106714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yang JH, Chang MW, Pandey PR, Tsitsipatis D, Yang X, Martindale JL, Munk R, De S, Abdelmohsen K, Gorospe M.. Interaction of OIP5-AS1 with MEF2C mRNA promotes myogenic gene expression. Nucleic Acids Res. 2020;48:12943–12956. doi: 10.1093/nar/gkaa1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Love MI, Huber W, Anders S.. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014;15:550. doi: 10.1186/s13059-014-0550-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yu G, Wang LG, Han Y, He QY.. Clusterprofiler: an R package for comparing biological themes among gene clusters. OMICS. 2012;16:284–287. doi: 10.1089/omi.2011.0118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ratnadiwakara M, Änkö ML.. mRNA stability assay using transcription inhibition by actinomycin D in mouse pluripotent stem cells. Bio Protoc. 2018;8:e3072. doi: 10.21769/BioProtoc.3072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Panda AC, Martindale JL, Gorospe M.. Polysome fractionation to analyze mRNA distribution profiles. Bio Protoc. 2017;7:e2126. doi: 10.21769/BioProtoc.2126. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The authors confirm that the data supporting the findings of this study are available within the article and its supplementary materials. The RNA-seq data are deposited at GSE267276 and GSE267277.