Abstract
Background
Individuals with chronic obstructive pulmonary disease (COPD) are often at risk for or have comorbid cardiovascular disease and are likely to die of cardiovascular-related causes.
Objectives
To prioritize a list of research topics related to the diagnosis and management of patients with COPD and comorbid cardiovascular diseases (heart failure, atherosclerotic vascular disease, and atrial fibrillation) by summarizing existing evidence and using consensus-based methods.
Methods
A literature search was performed. References were reviewed by committee co-chairs. An international, multidisciplinary committee, including a patient advocate, met virtually to review evidence and identify research topics. A modified Delphi approach was used to prioritize topics in real time on the basis of their potential for advancing the field.
Results
Gaps spanned the translational science spectrum from basic science to implementation: 1) disease mechanisms; 2) epidemiology; 3) subphenotyping; 4) diagnosis and management; 5) clinical trials; 6) care delivery; 7) medication access, adherence, and side effects; 8) risk factor mitigation; 9) cardiac and pulmonary rehabilitation; and 10) health equity. Seventeen experts participated, and quorum was achieved for all votes (>80%). Of 17 topics, ≥70% agreement was achieved for 12 topics after two rounds of voting. The range of summative Likert scores was −15 to 25. The highest priority was “Conduct pragmatic clinical trials with patient-centered outcomes that collect both pulmonary and cardiac data elements.” Health equity was identified as an important topic that should be embedded within all research.
Conclusions
We propose a prioritized research agenda with the purpose of stimulating high-impact research that will hopefully improve outcomes among people with COPD and cardiovascular disease.
Keywords: chronic obstructive pulmonary disease, cardiovascular disease, research priorities
Contents
Overview
Introduction
- Methods
- Committee Composition
- Literature Search and Review of Existing Evidence
- Meetings and Modified Delphi Rounds
- Document Development
Results
Discussion
Conclusions
Overview
People with chronic obstructive pulmonary disease (COPD) often have comorbid cardiovascular disease and die of cardiovascular-related causes. Beyond smoking as a shared risk factor, complex pathophysiologic mechanisms are at play between the lungs and the heart. Because people with comorbid cardiovascular conditions are often excluded from COPD trials, our ability to provide evidence-based care to these individuals is hindered. Our goal was to summarize existing evidence and use consensus-based methods to prioritize a list of research topics related to the diagnosis and management of patients with COPD and common cardiovascular diseases, such as heart failure, atherosclerotic cardiovascular disease, and atrial fibrillation. After performing a literature search on comorbid COPD and cardiovascular disease, we convened an international, multidisciplinary committee including a patient member and experts in the field to review the evidence and identify research gaps.
-
•
The research gaps spanned the following 10 domains across the translational science spectrum from basic science to implementation research: 1) mechanisms of disease; 2) epidemiology; 3) subphenotyping; 4) diagnosis and management; 5) clinical trials; 6) care delivery; 7) medication access, adherence, and side effects; 8) risk factor mitigation; 9) cardiac and pulmonary rehabilitation; and 10) health equity.
-
•
A modified Delphi approach was used to prioritize the research topics in real time on the basis of their potential to advance the field and ultimately improve the lives of patients with COPD and comorbid cardiovascular disease. The topic that was voted to be top priority was “Conduct pragmatic clinical trials with patient-centered outcomes that collect both pulmonary and cardiac data elements,” such as real-world effectiveness trials of the polypuff or polypill in the COPD patient population with concurrent cardiovascular disease. In addition, health equity was emphasized by the panel as cross-cutting all the domains and an important gap that should be embedded within all research proposed by the panel.
This research statement sets forth a prioritized research agenda that is based on expert opinion with the purpose of stimulating high-impact research for the optimal management of COPD and cardiovascular disease.
Introduction
The burden of COPD is significant, affecting 479 million individuals worldwide in 2020 (1). The global burden of COPD is projected to increase by 23% between 2020 and 2050 (1). Cardiovascular disease is a leading cause of death worldwide (2). Comorbid cardiovascular conditions are common in patients with COPD (3–9). Compared with the general public, patients with COPD are ∼2.5 times more likely to have cardiovascular disease (7). Across cohorts, the prevalence of heart failure (7–42%), ischemic cardiovascular disease (2–18%), and arrhythmia (3–21%) is consistently high in people with COPD (3–9). Approximately 35% of deaths among patients with COPD are attributed to cardiovascular events (10–12), irrespective of airflow obstruction (13), which means that optimally treating cardiovascular disease in patients with COPD could have significant benefit at the population level.
There is growing recognition of the importance of multimorbidity, which is a shift in clinical medicine and patient care (14), that is, treating the whole patient rather than the individual diseases in isolation. COPD and cardiovascular diseases are intertwined in many complex ways (4, 15–17), including the following:
-
1.
Both share smoking as a significant risk factor (18). Smoking activates the same underlying inflammatory pathways (TNF-α, IL-6, CRP, etc.) and aging pathways (telomere shortening, cellular senescence) in COPD and cardiovascular disease (19, 20). Smoking leads to acute inflammation, oxidative stress, protein imbalance, elastin degradation, hypoxia or hypercapnia, endothelial dysfunction, thrombogenicity, atherosclerosis, and arterial stiffness (19, 21, 22), resulting in direct damage to both lung and heart tissue.
-
2.
The two organs are interconnected anatomically and physiologically, as the pulmonary vasculature is situated between the ventricles, resulting in closely tied structure and function (23). It is well established that hypoxemia induces vasoconstriction in the arteries in the lung, leading to chronic pulmonary hypertension (type 3) and right heart failure. As the right heart chamber and muscle enlarge, there is ventricular interdependence, leading to the obstruction of left ventricular filling and poor cardiac output. This causes pulmonary vascular congestion and pulmonary congestion, which worsen respiratory failure. This interconnection between the lung and heart is cyclical and reciprocal. A more recent example is preliminary evidence showing that certain cardiovascular parameters (oxygen pulse and pulse pressure) improve after lung volume reduction surgery in emphysema (24).
-
3.
Exacerbations in one organ system can perturb the other organ system. The risk of cardiovascular events dramatically increases in the 30 days after a COPD exacerbation and persists up to one year (25–28), which suggests that systemic inflammation caused by one disease process (COPD) directly affects other organs, such as the heart.
-
4.
Inactivity due to fatigue or hypoxemia from COPD can increase the risk of coronary artery disease.
-
5.
Medications to treat one condition can negatively affect the other organ system (19, 29). For example, on the pulmonary side, treating patients with COPD exacerbation with azithromycin is associated with a small increase in the risk of cardiovascular death compared with no antibiotics or amoxicillin, which was most pronounced for patients at highest risk of cardiovascular events (30).
-
6.
The presence of a comorbidity might alter clinicians’ risk:benefit ratio to provide evidence-based care for the first condition. Patients with COPD and heart failure might not be given β-blockers, although it is an evidence-based indication for a β-blocker, out of concern for bronchoconstriction, even though the absolute risk with cardioselective (B1) β-blockers is inconsequential (31–36). At the population level, this practice pattern of not receiving guideline-concordant care for heart failure likely contributes to excess deaths and represents a translation gap.
-
7.
It can be diagnostically challenging to differentiate COPD from cardiovascular disease, because they present with similar symptoms, such as shortness of breath and fatigue, in both the acute and chronic states (15, 37, 38). Without a thorough review of systems, physical examination, laboratory workup (brain natriuretic peptide and differential diagnosis), patients with COPD with worsening shortness of breath might be treated with escalating bronchodilator therapy, when they are in fact developing heart failure and/or arrhythmias. Similarly, patients with COPD who present to acute care with shortness of breath and wheezing may be treated with nebulizers and antibiotics because the suspicion for COPD exacerbation is so high, whereas pulmonary vascular congestion might in fact be the driving etiology.
These complexities have limited our ability to affect the disease course of COPD. Risk factors for cardiovascular disease are well known and modifiable (cholesterol, blood pressure control, etc.), and mortality from ischemic heart disease has trended downward over time. Meanwhile, mortality from COPD has not (39). Trials to treat patients with COPD using new combinations of inhalers have not decreased mortality, which could be because patients are not being treated optimally for all of their comorbid conditions. Understanding the complex interplay between the heart and lungs could open new avenues for population health management and new therapeutics (40). After performing a literature review, we used consensus-based methods to prioritize research topics related to the diagnosis and management of common comorbid cardiovascular conditions that exist in patients with COPD. For the purposes of this workshop, we defined “cardiovascular disease” as heart failure, atherosclerotic cardiovascular disease, and atrial fibrillation, using the clinical framework of pump, ischemia, and rhythm. This research statement is a call to action with the goal of expediting research that will have the greatest impact on patients with COPD and cardiovascular disease.
Methods
Committee Composition
This project was approved by the American Thoracic Society (ATS) Program Review Subcommittee. The co-chairs (L.C.M., M.D., V.G.P.) convened a multidisciplinary, international committee of members representing internal medicine, pulmonology, cardiology, geriatrics, nursing, pharmacy, drug development, quality improvement and policy, learning health systems, patient experience, and patient advocacy. Members represented urban and rural healthcare settings, academic and nonacademic institutions, and professional societies. Before confirming the final roster, potential conflicts of interest were disclosed and managed per the policies and procedures of the ATS.
Literature Search and Review of Existing Evidence
The lead co-chair (L.C.M.) performed a literature search with help from a Kaiser Permanente librarian. The search methodology is described in Figure E1 in the data supplement. Using Medical Subject Headings “Cardiovascular Diseases/therapy”(MAJR) AND “Pulmonary Disease, Chronic Obstructive/therapy”(MAJR) together with filters for adults ≥18 years of age and published in the past 5 years, separate queries were done for articles related to 1) diagnosis, 2) management, and 3) rehabilitation. Articles were reviewed to inform the agenda for Day 1.
Meetings and Modified Delphi Rounds
Two separate meetings were held virtually (September 5, 2023, and September 27, 2023). The first day was a 6-hour session comprising six presentations and four discussions that were led by experts in the field. Presentations focused on existing literature and identifying existing gaps related to three cardiovascular conditions common in COPD (heart failure; atherosclerotic cardiovascular disease, which includes vessels in the heart, brain, and periphery; and atrial fibrillation). These three conditions were chosen using the clinical framework of “cardiovascular pump, ischemia, rhythm.” Content and discussion spanned the outpatient to inpatient care spectrum and addressed the complex relationships that exist between COPD and cardiovascular disease. Discussion of drugs were done at the class level. A patient representative with COPD and cardiovascular disease (C.G.) participated and provided the patient perspective. She stressed the importance of developing new technologies (drugs and devices) to facilitate the care and day-to-day management of COPD, streamline the care of patients with COPD who have multimorbidity to prevent siloed management among specialists, limit polypharmacy, decrease cost for patients, and improve adherence to guideline-based therapies. Twenty-two experts attended the first session. The audio file was transcribed using the TranscribeMe service and loaded into a word cloud that examined the frequency of words and their proximity to one another. The co-chairs generated a list of 10 domains of gaps from Day 1’s discussion that mapped onto the framework for the translational science spectrum, courtesy of the National Heart, Lung, and Blood Institute (41).
The second day was a 2-hour session to review the proposed list of research topics that were generated by the co-chairs and to perform a modified Delphi process in real time to prioritize the list (42, 43). Eighteen experts attended the second session. Seventeen of the 18 experts voted in all polls; the remaining person (co-chair V.G.P.) facilitated the Delphi rounds and was reserved to break a tie (if needed). Participants were given an opportunity to fully read the list of research topics before voting to prevent bias based on the order in which topics were listed. For each topic, participants were asked whether the topic should be considered a top priority, which was defined as holding significant potential for advancing the field and ultimately improving the lives of patients with COPD and cardiovascular disease. Participants voted via Zoom poll using a five-point Likert scale (strongly disagree, disagree, neutral, agree, and strongly agree). Participants were limited to using a vote of strongly agree or agree a maximum of five times to facilitate generating a prioritized list with a gradient. The anonymized results were displayed in real time after each round. If ≥70% agreement was not achieved after the first round (agreeing that the topic is either a priority or not a priority), a short (<10-min) discussion ensued to highlight key strengths and limitations of the proposed topic. A second and final poll was then launched. Additional details about the polling are provided in the data supplement.
Document Development
A co-chair (M.D.) performed the data analysis. The summative Likert score from the final round was calculated, where strongly disagree was assigned a score of −2, disagree was assigned a score of −1, neutral was assigned a score of 0, agree was assigned a score of +1, and strongly agree was assigned a score of +2. The final list of prioritized topics was then ordered by the summative Likert score from the final round. Results were sent to participants electronically for feedback. The lead co-chair (L.C.M.) drafted the initial version of the manuscript, which was then circulated to the full committee and iteratively revised. The ATS Board of Directors approved the final document. This document does not include clinical treatment recommendations.
Results
Committee members were diverse in terms of sex, race and ethnicity, geographic location, medical specialty, clinical discipline, research experience/expertise, and perspective (Table 1). The literature search for COPD and cardiovascular disease yielded 97 articles (34 for diagnosis, 43 for management, and 20 for rehabilitation). The key citations that informed Day 1’s content are highlighted in Table 2.
Table 1.
The International Multidisciplinary Committee for Prioritizing Research Topics in the Field of Chronic Obstructive Pulmonary Disease and Cardiovascular Disease
| Committee Member | Institution | Role | Area of Expertise |
|---|---|---|---|
| Laura Myers, M.D. | Kaiser Permanente Northern California | Co-chair, host | Overlap of COPD and cardiovascular disease, COPD readmissions, patient quality, safety, and policy |
| Valerie G. Press, M.D., M.P.H. | University of Chicago | Co-chair, Delphi facilitator | COPD medications, treatment adherence, health literacy, COPD readmissions |
| Miguel Divo, M.D., M.P.H. | Harvard University | Co-chair, speaker, data analyst | COPD and multimorbidity |
| Jennifer Quint, M.D., Ph.D. | Imperial College London, United Kingdom | Member, speaker | Epidemiology of COPD and cardiovascular disease |
| Peter Lindenauer, M.D. | Baystate Health | Member, speaker | COPD and pulmonary rehabilitation |
| Nirupama Putcha, M.D., M.H.S. | Johns Hopkins Medicine | Member, speaker | Multimorbidity, health disparities |
| Alan Hamilton, Ph.D. | COPD Foundation | Member, speaker | Pharmacological and nonpharmacological interventions for COPD, behavior change for patients with multimorbidity, regulatory drug approval |
| Nathaniel M. Hawkins, M.D., M.P.H. | University of British Columbia, Canada | Member, speaker | Heart failure, cardiac arrhythmias, comorbidities |
| Caroline Gainer | Patient | Member, speaker | Patient experience and advocacy |
| J. Michael Wells, M.D., M.S.P.H. | University of Alabama at Birmingham | Member, discussion moderator | COPD and pulmonary vascular disease; mechanisms of inflammation and vascular remodeling |
| David Mannino, M.D. | COPD Foundation | Member, discussion moderator | Epidemiology of COPD, environmental exposures, inflammation |
| R. Graham Barr, M.D., Dr.P.H. | Columbia University | Member, discussion moderator | Prospective cohort studies, epidemiology, cardiopulmonary interactions |
| Mark Dransfield, M.D. | University of Alabama | Member, discussion moderator | COPD, clinical trials, mechanisms of disease, health system leadership, leadership in multicenter randomized clinical trials |
| Sadiya S. Khan, M.D., M.Sc. | Northwestern University Feinberg School of Medicine | Member | Preventive cardiology, screening for comorbidities, epidemiology, prospective cohort studies, member of the American Heart Association |
| Sagar Shah, M.D. | Kaiser Permanente Northern California | Member | General internal medicine, referrals to specialists, polypharmacy |
| Allan Walkey, M.D. | University of Massachusetts | Member | COPD, practice patterns, health services research, learning health systems |
| Surya P. Bhatt, M.D. | University of Alabama at Birmingham | Member | COPD, clinical trials of medications |
| Andrea S. Gershon, M.D. | Sunnybrook Research Institute, Canada | Member | COPD outcomes, health services research |
| Todd Lee, Pharm.D., Ph.D. | University of Illinois | Member | Pharmacology, COPD medications, polypharmacy |
| Huong Q. Nguyen, R.N., Ph.D. | Kaiser Permanente Southern California | Member | COPD, frailty, patient-centered outcomes |
| Leah Witt, M.D. | University of San Francisco | Member | Geriatrics, multimorbidity |
| Richard Mularski, M.D. | Kaiser Permanente Northwest | Member | COPD and patient-centered outcomes, leadership of clinical trial networks |
Definition of abbreviation: COPD = chronic obstructive pulmonary disease.
Table 2.
Key Citations That Informed the Content of the Committee’s First Meeting
| Topic | Summary of Key Points |
|---|---|
| Burden, impact and complexities of cardiovascular comorbidities in COPD |
|
| Mechanisms and implications of cardiovascular disease in COPD |
|
| Evidence for use of medications in treating coexisting cardiovascular disease in COPD |
|
| Patient-centered outcomes for patients with COPD with cardiovascular disease |
|
| Patient perspective |
|
| Evidence for rehabilitation in patients with COPD with cardiovascular disease |
|
| Health equity in diagnosing and treating patients with COPD and cardiovascular disease |
|
Definition of abbreviations: COPD = chronic obstructive pulmonary disease; ICS = inhaled corticosteroids; IMPACT = Informing the Pathway of COPD Treatment; LABA = long-acting β2-agonist; LAMA = long-acting muscarinic antagonist; SGLT2i = sodium-glucose cotransporter-2 inhibitors; SUMMIT = Study to Evaluate the Effect of Fluticasone Furoate/Vilanterol on Survival in Subjects With Chronic Obstructive Pulmonary Disease.
One important and controversial point of discussion from Day 1 was the concept of COPD as a potential “risk-enhancing factor” for atherosclerotic cardiovascular disease. In the cardiovascular prevention guidelines (44, 45), certain clinical conditions characterized by systemic inflammation, such as rheumatoid arthritis, have been identified as risk-enhancing factors. When a risk-enhancing factor is identified, more intensive primary prevention goals are indicated, because patients’ risk of developing cardiovascular disease is significantly higher. COPD is well known to cause systemic inflammation and increase the risk of cardiovascular disease (46) but is not currently listed as a risk-enhancing factor for primary prevention (45). Experts on the committee believed that research on how to translate existing knowledge about the risk of developing cardiovascular disease in COPD into clinical practice is warranted. They believed that it was a potential missed opportunity not to intensively manage cardiovascular disease risk, which likely explains why so many patients with COPD die of cardiovascular disease.
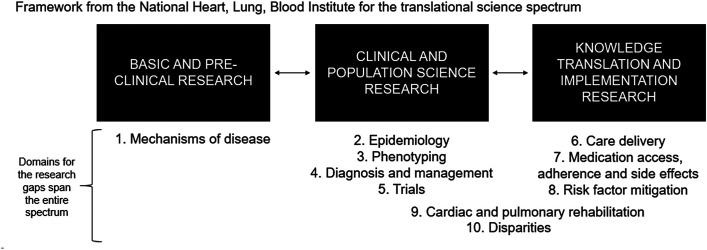
The 10 domains of gaps identified on Day 1 were mapped along the translational science spectrum from basic and preclinical research to clinical and population science research to knowledge translation and implementation science research (Figure 1). These included 1) mechanisms of disease; 2) epidemiology; 3) subphenotyping; 4) diagnosis and management; 5) trials; 6) care delivery; 7) medication access, adherence, and side effects; 8) risk factor mitigation; 9) cardiac/pulmonary rehabilitation; and 10) health equity. A word cloud that reflects the frequency of the words said during the meeting on Day 1 by the size of the font can be seen in Figure 2. The locations of the words in the word cloud reflect how they were used in relation to other words.
Figure 1.
Span of domains across the translational science spectrum. We used the NHLBI’s translational science spectrum as a framework to generate the list of 10 domains between Days 1 and 2. The domains range from basic science about the mechanisms of disease to the implementation of evidence-based treatments into everyday practice.
Figure 2.
Word cloud from transcript of Day 1 committee meeting. This word cloud was generated by feeding the transcript from Day 1 of the committee meeting into an online large language model that examined the frequency of words and their proximity to one another and generated this figure in the shape of the lungs. The words that are larger in size were said more frequently during the committee meeting on Day 1.
On Day 2, the committee unanimously agreed to reposition the two disparity-related topics from the list as ones that cross-cut all other topics. The two specific topics were:1) reduce disparities in the diagnosis and management of patients with COPD and cardiovascular disease (age, gender, race/ethnicity, low literacy, payer), including appropriate and timely diagnosis and adherence to guideline-based recommendations, and 2) expand trial recruitment to include, and potentially focus directly on, patients with COPD and cardiovascular disease, especially those with low socioeconomic status.
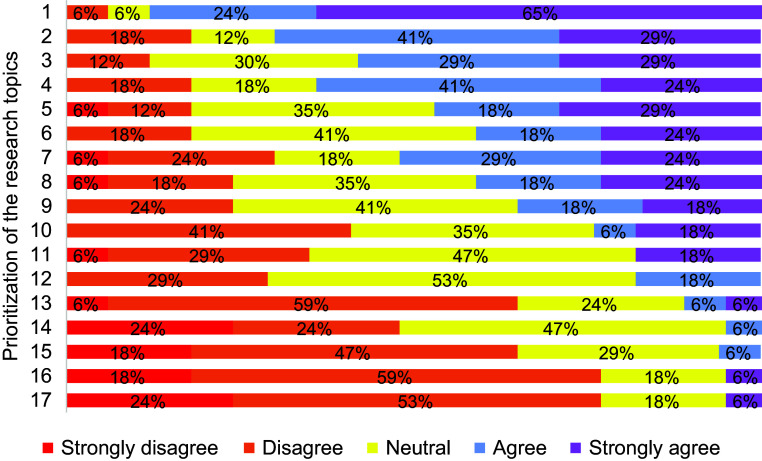
After removing the 2 disparity-related topics, the remaining 17 topics underwent Delphi rounds for prioritization. We achieved >80% to fulfill quorum for all rounds of voting, with no one abstaining from voting. Of the 17 topics that were voted on, agreement of ≥70% was achieved for 10 topics in the first round of voting and 2 additional topics in the second round of voting. Figure 3 shows the distribution of responses for each topic during the final round of voting. Table 3 contains a full description of the prioritized list of topics, ordered from high to low summative Likert score from the final round of voting. The range of summative Likert score was −15 to 25. The topic that was overwhelmingly voted to be the top priority was “Conduct pragmatic clinical trials with patient-centered outcomes that collect both pulmonary and cardiac data elements.” Examples are listed in Table 3 to highlight the types of trials that the committee believed would be most useful.
Figure 3.
Breakdown of responses for prioritization of the research topics in final Delphi round across the five-point Likert scale. If a color/percentage is missing along a row, that means that the value was zero, such as strongly disagree for priority 1. The text of each of the 17 prioritized topics is listed in Table 3. Briefly, they are 1) pragmatic trials with patient-centered outcomes, 2) cost-effectiveness of cardiac and respiratory tests, 3) phenotyping, 4) cardiac and pulmonary rehabilitation, 5) knowledge translation about COPD as a risk-enhancing diagnosis, 6) epidemiology of subgroups, 7) leverage lung cancer screening computed tomography scans, 8) simplified inhaler regimens that are accessible, 9) mechanisms of disease, 10) clinician awareness about evidence-based risks of cardiovascular treatments on lung function, 11) optimal roles of clinicians that prioritizes patients’ needs, 12) leverage technology, 13) longitudinal studies following stable and exacerbated patients with cardiac rhythm monitors, 14) risks and benefits of conservative medication management versus advanced cardiac testing/catheterization in patients with COPD with troponin leak, 15) impact of using active language to describe COPD exacerbation, 16) environmental triggers, and 17) performing spirometry in hospitalized patients. COPD = chronic obstructive pulmonary disease.
Table 3.
Prioritized List of Research Topics to Fill the Most Urgent Gaps in the Field of Chronic Obstructive Pulmonary Disease and Cardiovascular Disease
| Priority | Research Topic | Number of Rounds | Degree of Agreement in Final Round | Summative Likert Score in Final Round |
|---|---|---|---|---|
| 1 | Conduct pragmatic clinical trials with patient-centered outcomes that collect both pulmonary and cardiac data elements. Specifically:
|
1 | 95% | 25 |
| 2 | Determine the cost-effectiveness and other measures of benefit (NNT) of cardiac diagnostic tests (Cardiac Injury Score on ECG, biomarkers [natriuretic peptides, troponin], and echocardiography) in stable and exacerbated patients with COPD. Determine the cost-effectiveness and other measures of benefit (NNT) of spirometry with diffusion capacity to assess lung function among patients with cardiovascular disease. | 1 | 82% | 14 |
| 3 | Refine our knowledge of phenotypes within the COPD population with the goal of understanding patients’ individualized risk of different cardiovascular diseases (i.e., which phenotype predisposes to heart failure, atherosclerotic cardiovascular disease, and atrial fibrillation). Determine the appropriate screening studies and treatments for the different phenotypes. | 2 | 87% | 13 |
| 4 | Identify and further develop the key elements of pulmonary and cardiac rehabilitation programs that specifically help patients with both COPD and cardiovascular disease, potentially aiming for an integrated cardiopulmonary program. | 2 | 83% | 12 |
| 5 | Improve knowledge translation by increasing clinicians’ adherence to guidelines on the primary and secondary prevention of cardiovascular disease in patients with COPD and raise awareness of COPD as a “risk-enhancing diagnosis,” which requires more stringent lipid and blood pressure management. | 2 | 82% | 9 |
| 6 | Define the incidence, prevalence, characteristics, and prognosis of major cardiovascular diseases in patients with COPD using contemporary definitions, including heart failure subtypes (preserved and reduced ejection fraction), acute coronary syndrome subtypes (type 1 and 2 events) and various arrhythmias (both atrial and ventricular). | 2 | 83% | 8 |
| 7 | Determine the best approach to leverage cardiovascular disease seen on lung cancer screening CT scans (coronary calcium, enlarged aorta, pulmonary artery, atherosclerosis, etc.). | 2 | 71% | 7 |
| 8 | Develop and test simplified medication and inhaler regimens that are low cost and accessible to those with low literacy who have COPD and cardiovascular disease. Examine the impact of different pricing strategies of medications on patients’ behavior to be adherent with the goal of changing health policy to improve patients’ access. | 2 | 77% | 6 |
| 9 | Elucidate the mechanisms underlying the relationships between COPD and cardiovascular disease, such as congestive heart failure, atherosclerotic cardiovascular disease, and atrial fibrillation, which are common and affect patients’ long-term trajectory, with the goal of finding targeted therapies that prevent or slow both diseases. | 2 | 77% | 5 |
| 10 | Raise awareness among clinicians about the evidence-based risks of cardiovascular treatments on lung function (such as statins, β-blockers) and pulmonary treatments on cardiovascular function (such as inhaled steroids, β-agonists). | 1 | 59% | 0 |
| 11 | Define the optimal roles of primary care physicians, hospitalists, pulmonologists, and cardiologists in treating patients with COPD and cardiovascular disease in both the outpatient and inpatient settings with the goal of prioritizing patients’ needs. | 1 | 65% | −1 |
| 12 | Leverage technology to improve various aspects of care for patients with COPD and cardiovascular disease, such as delivery of supplemental oxygen that is automatically titrated to oxygen saturation with movement and participation in telerehabilitation programs. | 1 | 71% | −2 |
| 13 | Conduct longitudinal studies following stable and exacerbated patients with cardiac rhythm monitors to examine the risk of undetected or recurrent AF and response to therapy (pharmacologic and ablation). | 1 | 36% | −9 |
| 14 | Determine the risks and benefits of conservative medical management versus advanced cardiac testing or catheterization in patients hospitalized with COPD who have elevated troponin. | 1 | 53% | −11 |
| 15 | Determine the impact of using active language to describe COPD exacerbation (e.g., “lung attack), given the significantly elevated cardiopulmonary risk over time and specifically in the postexacerbation period. | 1 | 35% | −13 |
| 16 | Examine how environmental triggers, such as air pollution, mediate the relationship between COPD and cardiovascular disease. | 1 | 24% | −14 |
| 17 | Evaluate the impact of performing spirometry in hospitalized patients with cardiopulmonary symptoms to differentiate between airflow obstruction and other common conditions that cause dyspnea, such as congestive heart failure. | 1 | 24% | −15 |
Definition of abbreviations: AF = atrial fibrillation; CAT = COPD assessment test; COPD = chronic obstructive pulmonary disease; CT = computed tomography; ECG = electrocardiography; mMRC = Modified Medical Research Council Dyspnea Scale; NNT = number needed to treat; SGLT2i = sodium-glucose cotransporter-2 inhibitors.
Seventeen participants voted on all questions. Participants were allowed to vote strongly agree or agree on only 5 of 17 priorities to facilitate generating a prioritized list with a gradient. The degree of agreement is the percentage of respondents who voted strongly disagree/disagree or neutral/agree/strongly agree about whether the gap should be considered a top priority, which was defined as significant potential for advancing the field and ultimately improving the lives of patients with COPD and cardiovascular disease. The last column is the summative Likert score, from which the final round was calculated, where strongly disagree was assigned −2, disagree was assigned −1, neutral was assigned 0, agree was assigned +1, and strongly agree was assigned +2. The final list of priorities was then ordered by the final-round summative Likert score.
Discussion
Our expert committee identified and prioritized research topics regarding the diagnosis and management of patients with COPD and cardiovascular disease, specifically heart failure, atherosclerotic cardiovascular disease, and atrial fibrillation. Spanning the full translational science spectrum, the gaps encompassed the following 10 domains: 1) mechanisms of disease; 2) epidemiology; 3) subphenotyping; 4) diagnosis and management; 5) clinical trials; 6) care delivery; 7) medication access, adherence, and side effects; 8) risk factor mitigation; 9) cardiac and pulmonary rehabilitation; and 10) health equity. The committee believed strongly that consideration of disparities should be part of research done across all topics. For the remaining 17 topics, we used robust methods (a modified Delphi consensus-building approach) and successfully achieved quorum for all and agreement for the majority (12 of 17 topics after two rounds of Delphi).
The topic with the highest priority score was “Conduct pragmatic clinical trials with patient-centered outcomes that collect both pulmonary and cardiac data elements.” A pragmatic trial is a clinical trial that tests an intervention in a real-world setting (47). Although multimorbidity is common in COPD (5, 48, 49), patients with comorbid cardiovascular diseases are often excluded from trials in the COPD population (50–52), which hinders our ability to provide evidence-based care to patients in the community. Several examples do exist, though, in which both COPD and cardiovascular disease were addressed: 1) One trial comparing inhaler regimens in patients with COPD specifically enrolled patients with and those without stable cardiac disease, which the investigators defined as no myocardial ischemic event in 6 months, no hospitalization for advanced heart failure, and no life-threatening arrhythmia in 12 months (53). 2) An important lesson was gleaned from a post hoc subgroup analysis of a trial that enrolled patients with COPD with cardiovascular disease; the results suggested that among patients with heart failure, those with COPD had similar relative risk reduction with sodium-glucose cotransporter-2 inhibitors compared with those without COPD, but the absolute risk reduction was greater in those with COPD, likely because of the higher frequency of cardiovascular events in this population (54). 3) A trial randomized patients with moderate to severe COPD who did not already have indications for β-blockers to receive metoprolol and showed no difference in time until exacerbation (55).
Future trials should specifically target the enrollment of patients with COPD with cardiovascular disease and collect both pulmonary and cardiovascular-specific data elements, especially on outcomes that are important to patients, such as symptom relief, ability to do activities of daily living, and exacerbation frequency and acuity. In practice, this means that COPD trials should not exclude patients with cardiovascular disease, and cardiovascular disease trials should characterize the respiratory characteristics of patients better. The optimal trial design should be used, whether that is randomization at the individual level or stepped wedge by provider or clinic. The committee believed strongly that the evidence gained in clinical trials should be practical and useful in community settings, that is, apply to the broad population, be developed in conjunction with community organizations, and reflect the real-world effectiveness of the medications. Ideally, trials that address the real-world effectiveness of medications in patients with COPD and cardiovascular disease, especially those that limit the burden of taking multiple pills or inhalers per day (polypill or polypuff), should be prioritized. Pairing qualitative analysis with quantitative analysis to understand patient perspectives is critical, as well as a focus on vulnerable patient populations. Patients with low literacy and English as a second language should be able to access the medications being tested, so designing the trials to successfully engage with diverse populations is crucial. Given that smoking underlies much of the risk of developing COPD and cardiovascular disease, interventions related to smoking cessation are an important target, as well as research that seeks to understand the differential uptake of smoking cessation tools (56). In addition, trials are needed that examine the benefit of newer medications, such as sodium-glucose cotransporter-2 inhibitors, which could have pleiotropic effects on other organs and, therefore, provide benefit in patients with COPD and multimorbidity who have approved indications for the drugs (type 2 diabetes, heart failure, chronic kidney disease).
After some deliberation, the committee believed that the concept of COPD as a risk-enhancing factor was important. Because patients with COPD are so much more likely than the general population to develop atherosclerotic cardiovascular disease (7), the consensus was that screening and aggressive mitigation of atherosclerotic cardiovascular risk factors in the long term (cholesterol, blood pressure, etc.) might improve outcomes. However, a definitive study has not demonstrated this. Given that this research statement is not intended to make clinical recommendations, the committee believed strongly that the statement should at least call for research to quantify the benefit of treating individuals with COPD and concomitant cardiovascular disease with aggressive risk factor control. Trials that examine cardiac risk screening and aggressive risk factor management strategy would likely strengthen the evidence in favor of treating COPD as a risk-enhancing factor and inform future iterations of clinical guidelines. Patients with COPD and cardiovascular disease should not be excluded from receiving guideline-directed primary and secondary prevention for cardiovascular disease (38, 57).
A recent article in the Journal proposes a shift in the guideline-based paradigm of how to choose which inhalers to initiate first in stable patients with COPD (58). Instead of the 2023 Global Initiative for Chronic Obstructive Lung Disease paradigm of A-B-E classification by Modified Medical Research Council Dyspnea Scale score, COPD Assessment Test score, and number of exacerbations, the authors suggest adding a third dimension (E+ and B+), whereby patients with COPD and known cardiovascular disease should be initiated on triple therapy first. This is based on some evidence, albeit not completely consistent (59), that patients with COPD and cardiovascular disease have better outcomes on inhaled corticosteroid-containing therapy (60, 61). Expert clinical consensus is needed to adjudicate whether the body of evidence exists to support this proposed paradigm.
There are policy implications affecting patients with COPD and cardiovascular disease. For instance, the Centers for Medicare and Medicaid Services has a Hospital Readmission Reduction Program that financially penalizes hospitals for excess readmissions after index admissions for diseases including COPD, acute myocardial infarction, and heart failure (62). Since the penalties were implemented, evidence has shown potential inverse relationships between reduced readmissions and mortality for both COPD and heart failure (63, 64). Understanding the interplay between these two diseases and, ultimately, how hospitalizations related to COPD and heart failure can be prevented is important for reimbursement for hospitals (65).
There are several benefits to the modified Delphi approach we took, but there are also limitations. The virtual Delphi approach allows a large number of experts from distant places to participate. Because we made sure that voting results were anonymous, the group could not be swayed by the votes of individuals. However, the ideas presented in this research statement are based on expert opinion. Also, the committee believed that all of the topics identified are important and worthy of further investigation. Because the goal was to prioritize the topics on the basis of urgency and potential impact, we limited to five the number of votes experts could cast for agreeing or strongly agreeing with a topic being a priority to create a gradient for prioritization. We tried to engage as many stakeholders as possible but did not engage payers (insurance agencies) or pharmaceutical representatives.
Conclusions
This research statement sets forth a prioritized research agenda with the purpose of stimulating high-impact research and improving the lives of patients with COPD and cardiovascular disease. Although topics with lower priority ratings were still deemed to be important by the committee, pragmatic clinical trials with patient-centered outcomes that address health disparities were deemed to be the most urgent.
Acknowledgments
Acknowledgment
The authors thank Rachel Kaye from the ATS for facilitating the Zoom meetings, specifically the voting during the Delphi rounds. The authors also acknowledge the Behavioral Science and Health Services Research and Clinical Problems assemblies of the ATS for the opportunity to convene this committee.
This official research statement was prepared by an ad hoc subcommittee of the ATS Assembly on Behavioral Science and Health Services Research.
Members of the subcommittee are as follows:
Laura C. Myers, M.D., M.P.H. (Co-Chair)1
Miguel Divo, M.D. (Co-Chair)2
Valerie G. Press, M.D., M.P.H. (Co-Chair)3
R. Graham Barr, M.D., Dr.P.H.4,5*
Surya P. Bhatt, M.D., M.S.P.H.6‡
Mark Dransfield, M.D.6*
Caroline Gainer7§
Andrea S. Gershon, M.D.8*
Alan Hamilton, Ph.D.7§
Nathaniel M. Hawkins, M.D.9‡§
Sadiya S. Khan, M.D., M.Sc.10*‡
Todd Lee, Pharm.D., Ph.D.11ǁ
Peter Lindenauer, M.D.12§
David M. Mannino, M.D.7*
Richard Mularski, M.D.13ǁ
Huong Nguyen, R.N., Ph.D.14ǁ
Nirupama Putcha, M.D., M.H.S.15§
Jennifer K. Quint, M.D.16‡§
Sagar P. Shah, M.D., M.P.H.1‡
Allan Walkey, M.D.17ǁ
J. Michael Wells, M.D., M.S.P.H.6*
Leah J. Witt, M.D.18,19‡
*Discussant.
‡Writer.
§Speaker.
ǁParticipant.
1Kaiser Permanente Northern California, Oakland, California; 2Brigham and Women’s Hospital, Harvard Medical School, Boston, Massachusetts; 3University of Chicago, Chicago, Illinois; 4Department of Medicine and 5Department of Epidemiology, Columbia University Medical Center, New York, New York; 6University of Alabama at Birmingham, Birmingham, Alabama; 7COPD Foundation, Miami, Florida; 8University of Toronto, Toronto, Ontario, Canada; 9Division of Cardiology, University of British Columbia, Vancouver, British Columbia, Canada; 10Department of Medicine and Preventive Medicine, Feinberg School of Medicine, Northwestern University, Chicago, Illinois; 11Department of Medicine, University of Illinois, Chicago, Illinois; 12Baystate Health, Springfield, Massachusetts; 13Kaiser Permanente Northwest, Portland, Oregon; 14Kaiser Permanente Southern California, Pasadena, California; 15Division of Pulmonary and Critical Care Medicine, Johns Hopkins Medicine, Baltimore, Maryland; 16School of Public Health, Imperial College London, London, United Kingdom; 17University of Massachusetts, Worcester, Massachusetts; and 18Division of Pulmonary, Critical Care, Allergy and Sleep Medicine and 19Division of Geriatrics, Department of Medicine, University of California, San Francisco, San Francisco, California
Footnotes
This official research statement of the American Thoracic Society was approved May 2024
Supported by the American Thoracic Society.
A data supplement for this article is available via the Supplements tab at the top of the online article.
Subcommittee Disclosures: L.C.M. holds stock in Amgen. J.K.Q. served as a consultant for AstraZeneca, Chiesi, GlaxoSmithKline, and Insmed; and received research support from AstraZeneca, Boehringer Ingelheim, GlaxoSmithKline, Health Data Research, Insmed, the Medical Research Council, NIHR, and Sanofi. N.M.H. served as a consultant, speaker, and received research support from AstraZeneca. N.P. served on an advisory board and as a consultant for GlaxoSmithKline and Verona; and received research support from NIH/NIEHS. A.H. served as a consultant for the COPD Foundation. P.L. served as a consultant for Kivo Health. J.M.W. served on an advisory board for GlaxoSmithKline and Takeda; served on an endpoint review committee for AstraZeneca and Bavarian Nordic; holds patent PCT/GB2021/050658 with Mereo BioPharma; received research support from Alpha-1 Foundation, ARCUS-Med, Department of Veteran Affairs, GlaxoSmithKline, Grifols, InhibrX, Medscape, Mereo BioPharma, NIH/NHLBI, Takeda, and Verona Pharma; and holds stock in Alveolus Bio. L.J.W. received honoraria from Curbsiders Podcast, Elsevier, and Medscape/WebMD; received research support from the Health Resources and Services Administration; and received royalties from McGraw Hill. H.N. served on an advisory board for NIH/UCSF; and received research support from NIH and PCORI. A.W. received research support from NIH/NHLBI; and received royalties from UpToDate. D.M.M. served as a consultant for AstraZeneca, Genentech, GlaxoSmithKline, Regeneron, and UpToDate; served as an expert witness for Schlesinger Law Firm; served in a leadership role for the COPD Foundation; received royalties from UpToDate; and holds stock in GlaxoSmithKline. S.P.B. served on an advisory board for GlaxoSmithKline, NIH, and Regeneron; served as a consultant for Apreo, Boehringer Ingelheim, Chiesi, Genentech, GlaxoSmithKline, Regeneron, Sanofi, and Verona; received honoraria from Horizon CME, Integrity CE, and Medscape; received research support from Genentech, NIH, Nuvaira, and Sanofi/Regeneron; and received royalties from Springer. R.G.B. served in a leadership role for the COPD foundation; and received research support from the American Lung Association, the COPD Foundation, and the NIH. R.M. served on an advisory board for the COPD Foundation; and received research support from PCORI, Pfizer, and Sanofi. M.D. served on the board of directors for the COPD Foundation; served as a consultant for Aer Therapeutics, Apreo, AstraZeneca, the COPD Foundation, Genentech, GlaxoSmithKline, Novartis, Pulmonx, and Teva; received research support from the American Lung Association, the NIH, and the U.S. Department of Defense; received royalties from UpToDate; and received travel support from GlaxoSmithKline. S.S.K. received research support from the NHLBI. A.S.G. served on an advisory committee for the Canadian Thoracic Society, the Chest Foundation, and the European Respiratory Society; served as a mentor for Novartis; and received research support from the Canadian Institute of Health Research and the Ontario Ministry of Economic Development and Innovation. M.D. served as a consultant for Regeneron and Sanofi. V.G.P. served as a consultant for Humana and Vizient; served on the data safety and monitoring board for NIH and PCORI; received honoraria from AboutHealth COPD Collaborative, Dell, KUMC, and UCLA; served in a leadership role for the Chest Foundation, Journal of COPD Foundation, and Journal of General Internal Medicine; received research support from the NIH/NHLBI and the Agency for Health Care Research and Quality; and received travel support from the American College of Physicians and the Society of Hospital Medicine. S.P.S., T.L., and C.G. reported no commercial or relevant non-commercial interests from ineligible companies.
References
- 1. Boers E, Barrett M, Su JG, Benjafield AV, Sinha S, Kaye L, et al. Global burden of chronic obstructive pulmonary disease through 2050. JAMA Netw Open . 2023;6:e2346598. doi: 10.1001/jamanetworkopen.2023.46598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Vaduganathan M, Mensah George A, Turco Justine V, Fuster V, Roth Gregory A. The global burden of cardiovascular diseases and risk. J Am Coll Cardiol . 2022;80:2361–2371. doi: 10.1016/j.jacc.2022.11.005. [DOI] [PubMed] [Google Scholar]
- 3. Divo M, Cote C, de Torres JP, Casanova C, Marin JM, Pinto-Plata V, et al. BODE Collaborative Group Comorbidities and risk of mortality in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med . 2012;186:155–161. doi: 10.1164/rccm.201201-0034OC. [DOI] [PubMed] [Google Scholar]
- 4. Balbirsingh V, Mohammed AS, Turner AM, Newnham M. Cardiovascular disease in chronic obstructive pulmonary disease: a narrative review. Thorax . 2022 doi: 10.1136/thoraxjnl-2021-218333. [DOI] [PubMed] [Google Scholar]
- 5. Fabbri LM, Celli BR, Agustí A, Criner GJ, Dransfield MT, Divo M, et al. COPD and multimorbidity: recognising and addressing a syndemic occurrence. Lancet Respir Med . 2023;11:1020–1034. doi: 10.1016/S2213-2600(23)00261-8. [DOI] [PubMed] [Google Scholar]
- 6. Zhu Z, Wang X, Li X, Lin Y, Shen S, Liu C-L, et al. International COPD Genetics Consortium Genetic overlap of chronic obstructive pulmonary disease and cardiovascular disease-related traits: a large-scale genome-wide cross-trait analysis. Respir Res . 2019;20:64. doi: 10.1186/s12931-019-1036-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chen W, Thomas J, Sadatsafavi M, FitzGerald JM. Risk of cardiovascular comorbidity in patients with chronic obstructive pulmonary disease: a systematic review and meta-analysis. Lancet Respir Med . 2015;3:631–639. doi: 10.1016/S2213-2600(15)00241-6. [DOI] [PubMed] [Google Scholar]
- 8. Ioannides AE, Tayal U, Quint JK. Spirometry in atrial fibrillation: what’s the catch? Expert Rev Respir Med . 2023;17:937–950. doi: 10.1080/17476348.2023.2279236. [DOI] [PubMed] [Google Scholar]
- 9. Morgan AD, Rothnie KJ, Bhaskaran K, Smeeth L, Quint JK. Chronic obstructive pulmonary disease and the risk of 12 cardiovascular diseases: a population-based study using UK primary care data. Thorax . 2018;73:877–879. doi: 10.1136/thoraxjnl-2017-210865. [DOI] [PubMed] [Google Scholar]
- 10. Vilkman S, Keistinen T, Tuuponen T, Kivela SL. Survival and cause of death among elderly chronic obstructive pulmonary disease patients after first admission to hospital. Respiration . 1997;64:281–284. doi: 10.1159/000196687. [DOI] [PubMed] [Google Scholar]
- 11. McGarvey LP, John M, Anderson JA, Zvarich M, Wise RA, TORCH Clinical Endpoint Committee Ascertainment of cause-specific mortality in COPD: operations of the TORCH Clinical Endpoint Committee. Thorax . 2007;62:411–415. doi: 10.1136/thx.2006.072348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Brassington K, Selemidis S, Bozinovski S, Vlahos R. New frontiers in the treatment of comorbid cardiovascular disease in chronic obstructive pulmonary disease. Clin Sci (Lond) . 2019;133:885–904. doi: 10.1042/CS20180316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Whittaker H, Rothnie KJ, Quint JK. Cause-specific mortality in COPD subpopulations: a cohort study of 339 647 people in England. Thorax . 2024;79:202–208. doi: 10.1136/thorax-2022-219320. [DOI] [PubMed] [Google Scholar]
- 14. Wilson KC, Gould MK, Krishnan JA, Boyd CM, Brozek JL, Cooke CR, et al. ATS Guideline Methodology Working Group An official American Thoracic Society workshop report: a framework for addressing multimorbidity in clinical practice guidelines for pulmonary disease, critical illness, and sleep disorders. Ann Am Thorac Soc . 2016;13:S12–S21. doi: 10.1513/AnnalsATS.201601-007ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Roversi S, Fabbri LM, Sin DD, Hawkins NM, Agusti A. Chronic obstructive pulmonary disease and cardiac diseases: an urgent need for integrated care. Am J Respir Crit Care Med . 2016;194:1319–1336. doi: 10.1164/rccm.201604-0690SO. [DOI] [PubMed] [Google Scholar]
- 16. Ghoorah K, De Soyza A, Kunadian V. Increased cardiovascular risk in patients with chronic obstructive pulmonary disease and the potential mechanisms linking the two conditions: a review. Cardiol Rev . 2013;21:196–202. doi: 10.1097/CRD.0b013e318279e907. [DOI] [PubMed] [Google Scholar]
- 17. Shnoda M, Gajjar K, Ivanova V. COPD and cardiovascular disease: a review of association, interrelationship, and basic principles for integrated management. Crit Care Nurs Q . 2021;44:91–102. doi: 10.1097/CNQ.0000000000000342. [DOI] [PubMed] [Google Scholar]
- 18. Barnes PJ, Celli BR. Systemic manifestations and comorbidities of COPD. Eur Respir J . 2009;33:1165–1185. doi: 10.1183/09031936.00128008. [DOI] [PubMed] [Google Scholar]
- 19. Morgan AD, Zakeri R, Quint JK. Defining the relationship between COPD and CVD: what are the implications for clinical practice? Ther Adv Respir Dis . 2018;12:1753465817750524. doi: 10.1177/1753465817750524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Maclay JD, MacNee W. Cardiovascular disease in COPD: mechanisms. Chest . 2013;143:798–807. doi: 10.1378/chest.12-0938. [DOI] [PubMed] [Google Scholar]
- 21. Trinkmann F, Saur J, Borggrefe M, Akin I. Cardiovascular comorbidities in chronic obstructive pulmonary disease (COPD)—current considerations for clinical practice. J Clin Med . 2019;8:69. doi: 10.3390/jcm8010069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Romiti GF, Corica B, Pipitone E, Vitolo M, Raparelli V, Basili S, et al. AF-COMET International Collaborative Group Prevalence, management and impact of chronic obstructive pulmonary disease in atrial fibrillation: a systematic review and meta-analysis of 4,200,000 patients. Eur Heart J . 2021;42:3541–3554. doi: 10.1093/eurheartj/ehab453. [DOI] [PubMed] [Google Scholar]
- 23. Barr RG, Bluemke DA, Ahmed FS, Carr JJ, Enright PL, Hoffman EA, et al. Percent emphysema, airflow obstruction, and impaired left ventricular filling. N Engl J Med . 2010;362:217–227. doi: 10.1056/NEJMoa0808836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lammi MR, Ciccolella D, Marchetti N, Kohler M, Criner GJ. Increased oxygen pulse after lung volume reduction surgery is associated with reduced dynamic hyperinflation. Eur Respir J . 2012;40:837–843. doi: 10.1183/09031936.00169311. [DOI] [PubMed] [Google Scholar]
- 25. Dransfield MT, Criner GJ, Halpin DMG, Han MK, Hartley B, Kalhan R, et al. Time-dependent risk of cardiovascular events following an exacerbation in patients with chronic obstructive pulmonary disease: post hoc analysis from the IMPACT trial. J Am Heart Assoc . 2022;11:e024350. doi: 10.1161/JAHA.121.024350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hawkins NM, Nordon C, Rhodes K, Talukdar M, McMullen S, Ekwaru P, et al. Heightened long-term cardiovascular risks after exacerbation of chronic obstructive pulmonary disease. Heart . 2024;110:702–709. doi: 10.1136/heartjnl-2023-323487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Rothnie KJ, Connell O, Müllerová H, Smeeth L, Pearce N, Douglas I, et al. Myocardial infarction and ischemic stroke after exacerbations of chronic obstructive pulmonary disease. Ann Am Thorac Soc . 2018;15:935–946. doi: 10.1513/AnnalsATS.201710-815OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Graul EL, Nordon C, Rhodes K, Marshall J, Menon S, Kallis C, et al. Temporal risk of non-fatal cardiovascular events post COPD exacerbation: a population-based study. Am J Respir Crit Care Med . 2024;209:960–972. doi: 10.1164/rccm.202307-1122OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Rabe KF, Hurst JR, Suissa S. Cardiovascular disease and COPD: dangerous liaisons? Eur Respir Rev . 2018;27:180057. doi: 10.1183/16000617.0057-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ray WA, Murray KT, Hall K, Arbogast PG, Stein CM. Azithromycin and the risk of cardiovascular death. N Engl J Med . 2012;366:1881–1890. doi: 10.1056/NEJMoa1003833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Papiris SA, Triantafillidou C, Kolilekas L, Markoulaki D, Manali ED. Amiodarone: review of pulmonary effects and toxicity. Drug Saf . 2010;33:539–558. doi: 10.2165/11532320-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 32. Hawkins NM, Petrie MC, Macdonald MR, Jhund PS, Fabbri LM, Wikstrand J, et al. Heart failure and chronic obstructive pulmonary disease the quandary of beta-blockers and beta-agonists. J Am Coll Cardiol . 2011;57:2127–2138. doi: 10.1016/j.jacc.2011.02.020. [DOI] [PubMed] [Google Scholar]
- 33. Salpeter S, Ormiston T, Salpeter E. Cardioselective beta-blockers for chronic obstructive pulmonary disease. Cochrane Database Syst Rev . 2005;2005:CD003566. doi: 10.1002/14651858.CD003566.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hawkins NM, MacDonald MR, Petrie MC, Chalmers GW, Carter R, Dunn FG, et al. Bisoprolol in patients with heart failure and moderate to severe chronic obstructive pulmonary disease: a randomized controlled trial. Eur J Heart Fail . 2009;11:684–690. doi: 10.1093/eurjhf/hfp066. [DOI] [PubMed] [Google Scholar]
- 35. Agusti A, Böhm M, Celli B, Criner GJ, Garcia-Alvarez A, Martinez F, et al. GOLD COPD document 2023: a brief update for practicing cardiologists. Clin Res Cardiol . 2023;113:195–204. doi: 10.1007/s00392-023-02217-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Axson EL, Bottle A, Cowie MR, Quint JK. Relationship between heart failure and the risk of acute exacerbation of COPD. Thorax . 2021;76:807–814. doi: 10.1136/thoraxjnl-2020-216390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Celli BR, Fabbri LM, Aaron SD, Agusti A, Brook RD, Criner GJ, et al. Differential diagnosis of suspected chronic obstructive pulmonary disease exacerbations in the acute care setting: best practice. Am J Respir Crit Care Med . 2023;207:1134–1144. doi: 10.1164/rccm.202209-1795CI. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Rothnie KJ, Smeeth L, Herrett E, Pearce N, Hemingway H, Wedzicha J, et al. Closing the mortality gap after a myocardial infarction in people with and without chronic obstructive pulmonary disease. Heart . 2015;101:1103–1110. doi: 10.1136/heartjnl-2014-307251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Rana JS, Khan SS, Lloyd-Jones DM, Sidney S. Changes in mortality in top 10 causes of death from 2011 to 2018. J Gen Intern Med . 2021;36:2517–2518. doi: 10.1007/s11606-020-06070-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Bhatt SP, Wells JM, Dransfield MT. Cardiovascular disease in COPD: a call for action. Lancet Respir Med . 2014;2:783–785. doi: 10.1016/S2213-2600(14)70197-3. [DOI] [PubMed] [Google Scholar]
- 41.National Heart, Lung, and Blood Institute. Bethesda, MD: National Heart, Lung, and Blood Institute; 2023. https://www.nhlbi.nih.gov/science/research-spectrum [Google Scholar]
- 42. Nair R, Aggarwal R, Khanna D. Methods of formal consensus in classification/diagnostic criteria and guideline development. Semin Arthritis Rheum . 2011;41:95–105. doi: 10.1016/j.semarthrit.2010.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Williamson K. In: Research methods for students, academics and professionals. Williamson K, editor. New York: Elsevier Science and Technology; 2002. The Delphi method; pp. 209–220. [Google Scholar]
- 44. Arnett DK, Goodman RA, Halperin JL, Anderson JL, Parekh AK, Zoghbi WA. AHA/ACC/HHS strategies to enhance application of clinical practice guidelines in patients with cardiovascular disease and comorbid conditions: from the American Heart Association, American College of Cardiology, and US Department of Health and Human Services. Circulation . 2014;130:1662–1667. doi: 10.1161/CIR.0000000000000128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Arnett DK, Blumenthal RS, Albert MA, Buroker AB, Goldberger ZD, Hahn EJ, et al. 2019 ACC/AHA guideline on the primary prevention of cardiovascular disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation . 2019;140:e596–e646. doi: 10.1161/CIR.0000000000000678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Walter RE, Wilk JB, Larson MG, Vasan RS, Keaney JF, Lipinska I, et al. Systemic inflammation and COPD: the Framingham Heart Study. Chest . 2008;133:19–25. doi: 10.1378/chest.07-0058. [DOI] [PubMed] [Google Scholar]
- 47. Ford I, Norrie J. Pragmatic trials. N Engl J Med . 2016;375:454–463. doi: 10.1056/NEJMra1510059. [DOI] [PubMed] [Google Scholar]
- 48. Divo M, Celli BR. Multimorbidity in patients with chronic obstructive pulmonary disease. Clin Chest Med . 2020;41:405–419. doi: 10.1016/j.ccm.2020.06.002. [DOI] [PubMed] [Google Scholar]
- 49. Divo MJ, Casanova C, Marin JM, Pinto-Plata VM, de-Torres JP, Zulueta JJ, et al. BODE Collaborative Group COPD comorbidities network. Eur Respir J . 2015;46:640–650. doi: 10.1183/09031936.00171614. [DOI] [PubMed] [Google Scholar]
- 50. Papi A, Vestbo J, Fabbri L, Corradi M, Prunier H, Cohuet G, et al. Extrafine inhaled triple therapy versus dual bronchodilator therapy in chronic obstructive pulmonary disease (TRIBUTE): a double-blind, parallel group, randomised controlled trial. Lancet . 2018;391:1076–1084. doi: 10.1016/S0140-6736(18)30206-X. [DOI] [PubMed] [Google Scholar]
- 51. Singh D, Papi A, Corradi M, Pavlišová I, Montagna I, Francisco C, et al. Single inhaler triple therapy versus inhaled corticosteroid plus long-acting beta2-agonist therapy for chronic obstructive pulmonary disease (TRILOGY): a double-blind, parallel group, randomised controlled trial. Lancet . 2016;388:963–973. doi: 10.1016/S0140-6736(16)31354-X. [DOI] [PubMed] [Google Scholar]
- 52. Vogelmeier C, Hederer B, Glaab T, Schmidt H, Rutten-van Mölken MPMH, Beeh KM, et al. POET-COPD Investigators Tiotropium versus salmeterol for the prevention of exacerbations of COPD. N Engl J Med . 2011;364:1093–1103. doi: 10.1056/NEJMoa1008378. [DOI] [PubMed] [Google Scholar]
- 53. Wise RA, Anzueto A, Cotton D, Dahl R, Devins T, Disse B, et al. TIOSPIR Investigators Tiotropium Respimat inhaler and the risk of death in COPD. N Engl J Med . 2013;369:1491–1501. doi: 10.1056/NEJMoa1303342. [DOI] [PubMed] [Google Scholar]
- 54. Dewan P, Docherty KF, Bengtsson O, de Boer RA, Desai AS, Drozdz J, et al. Effects of dapagliflozin in heart failure with reduced ejection fraction and chronic obstructive pulmonary disease: an analysis of DAPA-HF. Eur J Heart Fail . 2021;23:632–643. doi: 10.1002/ejhf.2083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Dransfield MT, Voelker H, Bhatt SP, Brenner K, Casaburi R, Come CE, et al. BLOCK COPD Trial Group Metoprolol for the prevention of acute exacerbations of COPD. N Engl J Med . 2019;381:2304–2314. doi: 10.1056/NEJMoa1908142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Smith CE, Hill SE, Amos A. Impact of population tobacco control interventions on socioeconomic inequalities in smoking: a systematic review and appraisal of future research directions. Tob Control . 2020;30:e87–e95. doi: 10.1136/tobaccocontrol-2020-055874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Hawkins NM, Peterson S, Ezzat AM, Vijh R, Virani SA, Gibb A, et al. Control of cardiovascular risk factors in patients with chronic obstructive pulmonary disease. Ann Am Thorac Soc . 2022;19:1102–1111. doi: 10.1513/AnnalsATS.202104-463OC. [DOI] [PubMed] [Google Scholar]
- 58. Kostikas K, Gogali A, Hillas G. Cardiovascular disease and COPD: adding a third dimension to the ABE GOLD 2023 COPD classification. Am J Respir Crit Care Med . 2023;208:502–504. doi: 10.1164/rccm.202304-0691LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Vestbo J, Anderson JA, Brook RD, Calverley PMA, Celli BR, Crim C, et al. SUMMIT Investigators Fluticasone furoate and vilanterol and survival in chronic obstructive pulmonary disease with heightened cardiovascular risk (SUMMIT): a double-blind randomised controlled trial. Lancet . 2016;387:1817–1826. doi: 10.1016/S0140-6736(16)30069-1. [DOI] [PubMed] [Google Scholar]
- 60. Lipson DA, Barnhart F, Brealey N, Brooks J, Criner GJ, Day NC, et al. IMPACT Investigators Once-daily single-inhaler triple versus dual therapy in patients with COPD. N Engl J Med . 2018;378:1671–1680. doi: 10.1056/NEJMoa1713901. [DOI] [PubMed] [Google Scholar]
- 61. Gadhvi K, Kandeil M, Raveendran D, Choi J, Davies N, Nanchahal S, et al. Inhaled corticosteroids and risk of cardiovascular disease in chronic obstructive pulmonary disease: a systematic review and meta-regression. Chronic Obstr Pulm Dis . 2023;10:317–327. doi: 10.15326/jcopdf.2022.0386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Centers for Medicare and Medicaid Services. Baltimore, MD: Centers for Medicare and Medicaid Services; 2019. Hospital Readmissions Reduction Program.https://www.cms.gov/medicare/medicare-fee-for-service-payment/acuteinpatientpps/readmissions-reduction-program.html [Google Scholar]
- 63. Puebla Neira DA, Hsu ES, Kuo YF, Ottenbacher KJ, Sharma G. Readmissions Reduction Program, mortality and readmissions for chronic obstructive pulmonary disease. Am J Respir Crit Care Med . 2020;203:437–446. doi: 10.1164/rccm.202002-0310OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Wadhera RK, Joynt Maddox KE, Wasfy JH, Haneuse S, Shen C, Yeh RW. Association of the Hospital Readmissions Reduction Program with mortality among Medicare beneficiaries hospitalized for heart failure, acute myocardial infarction, and pneumonia. JAMA . 2018;320:2542–2552. doi: 10.1001/jama.2018.19232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Press VG, Myers LC, Feemster LC. Preventing COPD readmissions under the hospital readmissions reduction program: how far have we come? Chest . 2021;159:996–1006. doi: 10.1016/j.chest.2020.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Groenewegen A, Zwartkruis VW, Rienstra M, Hollander M, Koffijberg H, Cramer MJM, et al. Improving early diagnosis of cardiovascular disease in patients with type 2 diabetes and COPD: protocol of the RED-CVD cluster randomised diagnostic trial. BMJ Open . 2021;11:e046330. doi: 10.1136/bmjopen-2020-046330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Amegadzie JE, Gao Z, Quint JK, Russell R, Hurst JR, Lee TY, et al. QRISK3 underestimates the risk of cardiovascular events in patients with COPD. Thorax . 2024;79:718–724. doi: 10.1136/thorax-2023-220615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Lenoir A, Whittaker H, Gayle A, Jarvis D, Quint JK. Mortality in non-exacerbating COPD: a longitudinal analysis of UK primary care data. Thorax . 2023;78:904–911. doi: 10.1136/thorax-2022-218724. [DOI] [PubMed] [Google Scholar]
- 69. Gayle AV, Minelli C, Quint JK. Respiratory-related death in individuals with incident asthma and COPD: a competing risk analysis. BMC Pulm Med . 2022;22:28. doi: 10.1186/s12890-022-01823-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Damarla M, Celli BR, Mullerova HX, Pinto-Plata VM. Discrepancy in the use of confirmatory tests in patients hospitalized with the diagnosis of chronic obstructive pulmonary disease or congestive heart failure. Respir Care . 2006;51:1120–1124. [PubMed] [Google Scholar]
- 71. Press VG, Cifu AS, White SR. Screening for chronic obstructive pulmonary disease. JAMA . 2017;318:1702–1703. doi: 10.1001/jama.2017.15782. [DOI] [PubMed] [Google Scholar]
- 72. Spero K, Bayasi G, Beaudry L, Barber KR, Khorfan F. Overdiagnosis of COPD in hospitalized patients. Int J Chron Obstruct Pulmon Dis . 2017;12:2417–2423. doi: 10.2147/COPD.S139919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Prieto Centurion V, Huang F, Naureckas ET, Camargo CA, Charbeneau J, Joo MJ, et al. Confirmatory spirometry for adults hospitalized with a diagnosis of asthma or chronic obstructive pulmonary disease exacerbation. BMC Pulm Med . 2012;12:73. doi: 10.1186/1471-2466-12-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Gulea C, Zakeri R, Kallis C, Quint JK. Impact of COPD and asthma on in-hospital mortality and management of patients with heart failure in England and Wales: an observational analysis. BMJ Open . 2022;12:e059122. doi: 10.1136/bmjopen-2021-059122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Gulea C, Zakeri R, Quint JK. Differences in outcomes between heart failure phenotypes in patients with coexistent chronic obstructive pulmonary disease: a cohort study. Ann Am Thorac Soc . 2022;19:971–980. doi: 10.1513/AnnalsATS.202107-823OC. [DOI] [PubMed] [Google Scholar]
- 76. Angelini ED, Yang J, Balte PP, Hoffman EA, Manichaikul AW, Sun Y, et al. Pulmonary emphysema subtypes defined by unsupervised machine learning on CT scans. Thorax . 2023;78:1067–1079. doi: 10.1136/thorax-2022-219158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Martinez CH, Mannino DM, Divo MJ. Defining COPD-related comorbidities, 2004–2014. Chronic Obstr Pulm Dis . 2014;1:51–63. doi: 10.15326/jcopdf.1.1.2014.0119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Whittaker HR, Bloom C, Morgan A, Jarvis D, Kiddle SJ, Quint JK. Accelerated FEV1 decline and risk of cardiovascular disease and mortality in a primary care population of COPD patients. Eur Respir J . 2021;57:2000918. doi: 10.1183/13993003.00918-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Bhatt SP, Wells JM, Kinney GL, Washko GR, Budoff M, Kim Y-I, et al. COPDGene Investigators β-Blockers are associated with a reduction in COPD exacerbations. Thorax . 2016;71:8–14. doi: 10.1136/thoraxjnl-2015-207251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Dransfield MT, Rowe SM, Johnson JE, Bailey WC, Gerald LB. Use of beta blockers and the risk of death in hospitalised patients with acute exacerbations of COPD. Thorax . 2008;63:301–305. doi: 10.1136/thx.2007.081893. [DOI] [PubMed] [Google Scholar]
- 81. Axson EL, Sundaram V, Bloom CI, Bottle A, Cowie MR, Quint JK. Temporal trends in the incidence of heart failure among patients with COPD and its association with mortality. Ann Am Thorac Soc . 2020;17:939–948. doi: 10.1513/AnnalsATS.201911-820OC. [DOI] [PubMed] [Google Scholar]
- 82. Simons SO, Elliott A, Sastry M, Hendriks JM, Arzt M, Rienstra M, et al. Chronic obstructive pulmonary disease and atrial fibrillation: an interdisciplinary perspective. Eur Heart J . 2021;42:532–540. doi: 10.1093/eurheartj/ehaa822. [DOI] [PubMed] [Google Scholar]
- 83. Bermingham M, O’Callaghan E, Dawkins I, Miwa S, Samsudin S, McDonald K, et al. Are beta2-agonists responsible for increased mortality in heart failure? Eur J Heart Fail . 2011;13:885–891. doi: 10.1093/eurjhf/hfr063. [DOI] [PubMed] [Google Scholar]
- 84. Criner GJ, Connett JE, Aaron SD, Albert RK, Bailey WC, Casaburi R, et al. Canadian Institutes of Health Research Simvastatin for the prevention of exacerbations in moderate-to-severe COPD. N Engl J Med . 2014;370:2201–2210. doi: 10.1056/NEJMoa1403086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Young RP, Hopkins RJ, Agusti A. Statins as adjunct therapy in COPD: how do we cope after STATCOPE? Thorax . 2014;69:891–894. doi: 10.1136/thoraxjnl-2014-205814. [DOI] [PubMed] [Google Scholar]
- 86. Andell P, James SK, Cannon CP, Cyr DD, Himmelmann A, Husted S, et al. PLATO Investigators Ticagrelor versus clopidogrel in patients with acute coronary syndromes and chronic obstructive pulmonary disease: an analysis from the Platelet Inhibition and Patient Outcomes (PLATO) trial. J Am Heart Assoc . 2015;4:e002490. doi: 10.1161/JAHA.115.002490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Harrington RL, Hanna ML, Oehrlein EM, Camp R, Wheeler R, Cooblall C, et al. Defining patient engagement in research: results of a systematic review and analysis. Report of the ISPOR Patient-Centered Special Interest Group. Value Health . 2020;23:677–688. doi: 10.1016/j.jval.2020.01.019. [DOI] [PubMed] [Google Scholar]
- 88. Lindenauer PK, Stefan MS, Pekow PS, Mazor KM, Priya A, Spitzer KA, et al. Association Between initiation of pulmonary rehabilitation after hospitalization for COPD and 1-year survival among Medicare beneficiaries. JAMA . 2020;323:1813–1823. doi: 10.1001/jama.2020.4437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Rochester CL, Alison JA, Carlin B, Jenkins AR, Cox NS, Bauldoff G, et al. Pulmonary rehabilitation for adults with chronic respiratory disease: an official American Thoracic Society clinical practice guideline. Am J Respir Crit Care Med . 2023;208:e7–e26. doi: 10.1164/rccm.202306-1066ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Spitzer KA, Stefan MS, Priya A, Pack QR, Pekow PS, Lagu T, et al. Participation in pulmonary rehabilitation following hospitalization for COPD among Medicare beneficiaries. Ann Am Thorac Soc . 2019;16:99–106. doi: 10.1513/AnnalsATS.201805-332OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Ejike CO, Woo H, Galiatsatos P, Paulin LM, Krishnan JA, Cooper CB, et al. Contribution of individual and neighborhood factors to racial disparities in respiratory outcomes. Am J Respir Crit Care Med . 2021;203:987–997. doi: 10.1164/rccm.202002-0253OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. McCormack MC, Balasubramanian A, Matsui EC, Peng RD, Wise RA, Keet CA. Race, lung function, and long-term mortality in the National Health and Nutrition Examination Survey III. Am J Respir Crit Care Med . 2022;205:723–724. doi: 10.1164/rccm.202104-0822LE. [DOI] [PubMed] [Google Scholar]
- 93. Gaffney AW, Hawks L, White AC, Woolhandler S, Himmelstein D, Christiani DC, et al. Health care disparities across the urban-rural divide: a national study of individuals with COPD. J Rural Health . 2022;38:207–216. doi: 10.1111/jrh.12525. [DOI] [PubMed] [Google Scholar]
- 94. Zarrabian B, Mirsaeidi M. A trend analysis of chronic obstructive pulmonary disease mortality in the United States by race and sex. Ann Am Thorac Soc . 2021;18:1138–1146. doi: 10.1513/AnnalsATS.202007-822OC. [DOI] [PubMed] [Google Scholar]
- 95. Malla G, Bodduluri S, Sthanam V, Sharma G, Bhatt SP. Access to pulmonary rehabilitation among Medicare beneficiaries with chronic obstructive pulmonary disease. Ann Am Thorac Soc . 2023;20:516–522. doi: 10.1513/AnnalsATS.202204-318OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Lee H, Shin SH, Gu S, Zhao D, Kang D, Joi YR, et al. Racial differences in comorbidity profile among patients with chronic obstructive pulmonary disease. BMC Med . 2018;16:178. doi: 10.1186/s12916-018-1159-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Almagro P, López García F, Cabrera FJ, Montero L, Morchón D, Díez J, et al. Grupo Epoc de la Sociedad Española de Medicina Interna Comorbidity and gender-related differences in patients hospitalized for COPD: the ECCO study. Respir Med . 2010;104:253–259. doi: 10.1016/j.rmed.2009.09.019. [DOI] [PubMed] [Google Scholar]
- 98. Krishnan JK, Mallya SG, Nahid M, Baugh AD, Han MK, Aronson KI, et al. Disparities in guideline concordant statin treatment in individuals with chronic obstructive pulmonary disease. Chronic Obstr Pulm Dis . 2023;10:369–379. doi: 10.15326/jcopdf.2023.0395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Quint JK, Herrett E, Bhaskaran K, Timmis A, Hemingway H, Wedzicha JA, et al. Effect of β blockers on mortality after myocardial infarction in adults with COPD: population based cohort study of UK electronic healthcare records. BMJ . 2013;347:f6650. doi: 10.1136/bmj.f6650. [DOI] [PMC free article] [PubMed] [Google Scholar]