Summary
Maintaining cognitive integrity is crucial during underwater operations, which can significantly impact work performance and risk severe accidents. However, the cognitive effects of underwater operations and their underlying mechanism remain elusive, posing great challenges to the medical protection of professionals concerned. Here, we found that a single underwater operation session affects cognition in a time-dependent model. Prolonged exposure elicits significant cognitive impairment and hippocampal dysfunction, accompanied by increased neuroinflammation. Furthermore, RNA sequencing (RNA-seq) analysis revealed the involvement of neuroinflammation and highlighted the critical role of CCR3. Knockdown of CCR3 significantly rescued cognitive impairment and hippocampal dysfunction and reversed the upregulation of pro-inflammatory cytokines, by switching the activated microglia from a pro-inflammatory to a neuroprotective phenotype. Taken together, these results highlighted the time-dependent effects of a single underwater operation session on cognitive function. Knocking down CCR3 can attenuate neuroinflammation by regulating polarization of activated microglia, thereby alleviating prolonged underwater operations-induced cognitive impairment.
Subject areas: Biological sciences, Neuroscience, Systems neuroscience, Sensory neuroscience
Graphical abstract
Highlights
-
•
A single prolonged underwater operation induces cognitive impairment
-
•
CCR3 is crucial in prolonged underwater operation-induced neuroinflammation
-
•
CCR3 knockdown regulates polarization of activated microglia
-
•
CCR3 provides a target for the protection of cognition in special environments
Biological sciences; Neuroscience; Systems neuroscience; Sensory neuroscience
Introduction
Underwater operations are increasingly widely used in various fields such as ocean exploration, military missions, and underwater infrastructure development and maintenance.1,2 These operations involve individuals performing physical tasks in unique and challenging underwater environments. In addition to physical fitness, intact cognitive function is indispensable for the successful completion of operations and the guarantee of personal safety in underwater environments, where mistakes might result in severe consequences.3 Nevertheless, mounting evidence has suggested that cognitive function may be influenced by underwater operations,4,5 which not only affects individuals’ work performance and training effects but can also result in operational errors that lead to accidents. Therefore, exploring how underwater operations affect cognitive function and elucidating the underlying molecular mechanism to develop effective countermeasures are significant for the protection of the brain in underwater environment.
In fact, underwater operations entail physical exercise performed in special underwater environments.6 Over the past decade, research investigating the relationship between exercise and cognition has grown tremendously. A positive interaction between moderate-intensity exercise and cognitive function has been established in conventional environments7,8, indicating that moderate-intensity exercise increases the release of neurotransmitters9 and brain-derived neurotrophic factor (BDNF)10 as well as cerebral blood flow (CBF),11 thus improving cognitive performance. However, studies have also shown that high-intensity or excessive exercise may induce cognitive impairment.12 Recent studies have provided new shreds of evidence to prove the connection between learning-memory declines and acute excessive exercise,13,14 but still precise pathophysiological mechanisms are not completely revealed. In addition, when it comes to the effect of exercise on cognition, besides intensity, duration is another important factor that cannot be ignored. Previous studies found that a 30 min exercise improves cognition, whereas shorter or longer durations of moderate exercise have negligible benefits,15 and exercise exceeding 50 min even causes adverse effects.16,17 As we all know, individuals engaged in special underwater operations face challenges in controlling their operating time. Sometimes the work can be completed within a very short period, while, in other instances, it may extend to several hours. Several studies have investigated the cognitive effects of underwater exercise, yielding varying conclusions, with underwater exercise causing both positive18 and negative effects.4,19,20 Studies also showed that increased stress or physical exercise may additionally influence cognitive function in special environments.21,22 However, so far, the profile of exercise-cognition interaction and the underlying mechanisms in special environments are not fully elucidated.
Special operation divers represent a relatively niche and unique group, resulting in limited research and knowledge. The current study aimed to investigate the effects of underwater operations on their cognitive function and to explore the possible underlying mechanisms. By combining human trial with animal study, we demonstrated that the cognitive effect of a single underwater operation session is time dependent and prolonged exposure causes significant cognitive impairment. In addition, our findings indicated that microglia-mediated neuroinflammation and upregulation of C-C motif chemokine receptor 3 (CCR3) play a crucial role in cognitive impairment induced by prolonged underwater operations. Using adeno-associated virus (AAV) virus, we found that knockdown of CCR3 can alleviate impaired performance in cognitive test and rescue hippocampal dysfunction. Furthermore, inhibiting CCR3 can reduce the upregulation of pro-inflammatory factors and regulation of microglial polarization may be one of the underlying mechanisms. Collectively, our study might provide valuable insights for future interventions to protect the brain of underwater operators.
Results
Underwater operations affect cognitive function and prolonged exposure leads to cognitive decline
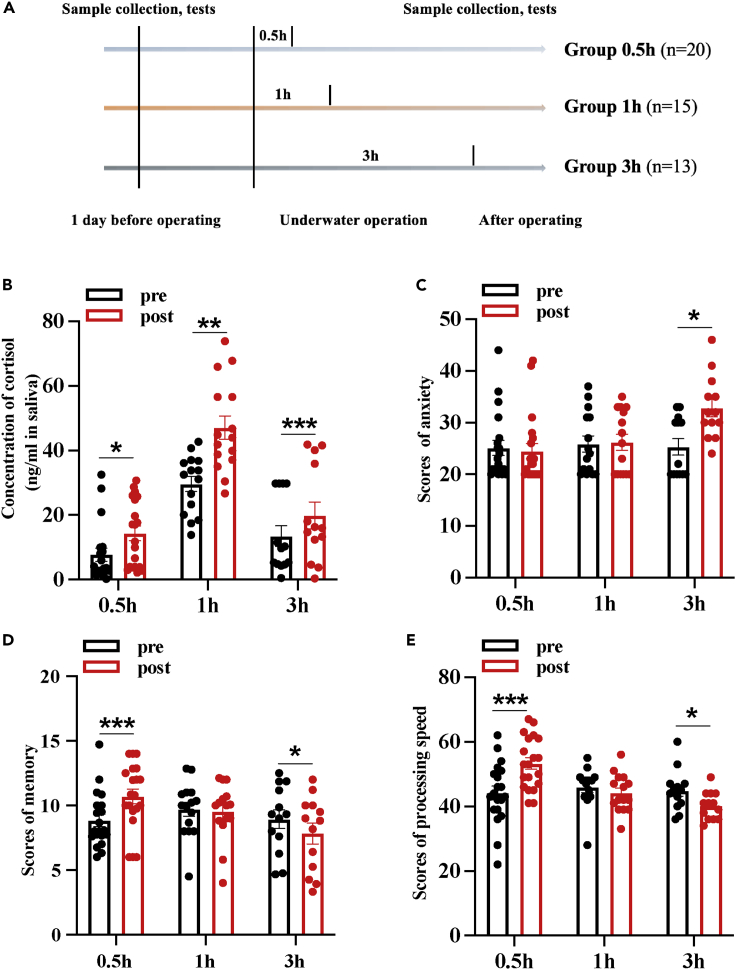
Three groups of divers aged 18–32 years with diving experience of 2–12 years were recruited to perform underwater operations, and a self-controlled trial was conducted. Before and after three operation sessions that last 0.5 h, 1 h, and 3 h, respectively, divers’ stress response, anxiety, and cognitive performance were measured and analyzed (Figure 1A). Stress response was evaluated by concentration of salivary cortisol determined by enzyme-linked immunosorbent assay (ELISA) method. Results showed that salivary cortisol concentration was significantly elevated after three operation sessions (Figure 1B), indicating that 0.5-h, 1-h, and 3-h underwater operations were acute stressors for divers, and they all induced significant acute stress response and physical arousal. The large differences in baseline salivary cortisol levels among the three groups before training were due to the large individual differences in salivary cortisol and its susceptibility to the influence of circadian rhythms, sleep, and other factors. Anxiety is the most common emotional response of adverse stress. The State-Trait Anxiety Inventory (STAI-S) was used to evaluate anxiety of divers, and there were no significant differences in baseline levels of anxiety among the 3 groups. 0.5-h and 1-h underwater operations caused no change in anxiety, whereas a 3-h exposure resulted in significant elevation of anxiety (Figure 1C), indicating the emergency of adverse stress response. The Primary Cognitive Functions Test Battery was applied to assess divers’ alterations in processing speed and memory ability before and after the operations. There were no significant differences in baseline levels among the 3 groups. Divers’ performance improved after the 0.5-h underwater operation, remained unchanged after the 1-h underwater operation, and significantly decreased after the 3-h underwater operation (Figures 1D and 1E). The aforementioned results indicated that a single underwater operation session, acting as an acute stressor, affects divers’ cognitive function in a time-dependent model. A single underwater operation session lasting less than 1 h may not cause obvious adverse effects, but longer exposure can lead to significant cognitive decline.
Figure 1.
Underwater operations affect cognitive function and prolonged exposure leads to cognitive decline
(A) Schematic of the human experimental flowchart. Stress response, anxiety, and cognitive performance of special operation divers were evaluated before and after 3 single underwater operations of different durations (0.5 h, 1 h, 3 h respectively).
(B) Concentration of cortisol in saliva before and after 3 single underwater operations.
(C) Scores of anxiety before and after 3 single underwater operations.
(D) Scores of memory before and after 3 single underwater operations.
(E) Scores of processing speed before and after 3 single underwater operations. Data are expressed as mean ± SEM, statistical analysis is performed using paired two-tailed Student’s t test. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001.
Prolonged underwater exercise elicits significant cognitive impairment in rats
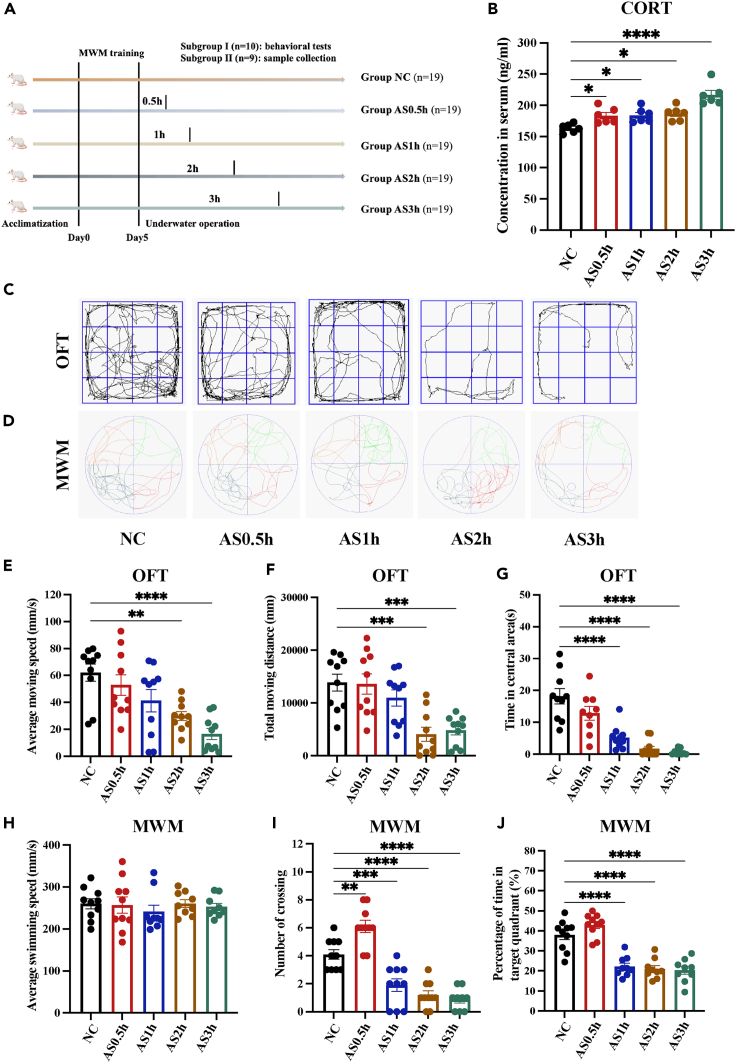
To explore the mechanism, we conducted animal experiments. Rats were exposed to swimming in a 2.0 atmosphere absolute (ATA) hyperbaric environment for 0.5 h, 1 h, 2 h, and 3 h to simulate different durations of underwater operations, lablled as the Acute Stress (AS) 0.5 h group, the AS1 h group, the AS2 h group, the AS3 h group, respectively (Figure 2A). Serum concentrations of corticosterone (CORT) were detected by ELISA to evaluate the stress response. As shown in Figure 2B, compared to the Normal Control (NC) group, CORT concentration was significantly elevated in all underwater swimming rats, indicating the occurrence of an acute stress response and physical arousal. In the open field test (OFT), the average moving speed, the total moving distance, and the time spent in the central area were used to indicate the activity level and exploratory behaviors, which can reflect the level of anxiety of rats. The results showed that rats swimming for more than 2 h exhibited significant anxious behaviors (Figures 2C and 2E–2G). These results suggested that underwater exercise was an acute stressor and elicited an acute stress response and that prolonged exposure caused anxiety in rats, which was consistent with the results of divers. The effect of underwater exercise on cognitive function in rats was measured by the Morris water maze (MWM) test and was indexed by the frequency of crossing the platform and the percentage of time spent in target quadrant. Firstly, the average swimming speed was not different between 5 groups, excluding the effect of motor impairment on cognitive performance (Figures 2D and 2H). There was no significant difference between rats in the NC, AS0.5 h, and AS1 h groups. In contrast, in the simulated underwater environment, rats swimming for more than 1 h crossed platform significantly less and spent a significantly lower percentage of time in the target quadrant compared to the NC group (Figures 2D, 2I, and 2J), indicating the emergency of cognitive impairment. These results suggested that, consistent with divers, exercising in the simulated underwater environment affected rats’ cognitive function in a time-dependent model and prolonged exposure induced significant cognitive impairment.
Figure 2.
Prolonged underwater exercise elicits significant cognitive impairment in rats
(A) Schematic of the animal experimental protocol.
(B) The effect of underwater exercise on the concentration of CORT in serum of rats was detected using ELISA method. (n = 6 in each group).
(C and E–G) The effect of underwater exercise on anxiety of rats was detected using OFT. (n = 10 in each group). Movement trajectory of rats in the OFT (C). Average moving speed of rats in the OFT (E). Total distance traveled by rats in the OFT (F). Time rats stayed in central areas in the OFT (G).
(D and H–J) The effect of underwater exercise on cognitive performance of rats was detected using MWM. (n = 10 in each group). Movement trajectory of rats in the MWM (D). Average swimming speed of rats in the MWM (H). Numbers rats crossing the platform in the MWM (I). Percentage of time rats stayed in the target quadrant in the MWM (J). Data are expressed as mean ± SEM, statistical analysis is performed using one-way ANOVA followed by least significant difference (LSD) multiple comparisons test; compared with the group NC. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, ∗∗∗∗p < 0.0001.
Prolonged underwater exercise leads to hippocampal dysfunction and neuroinflammation
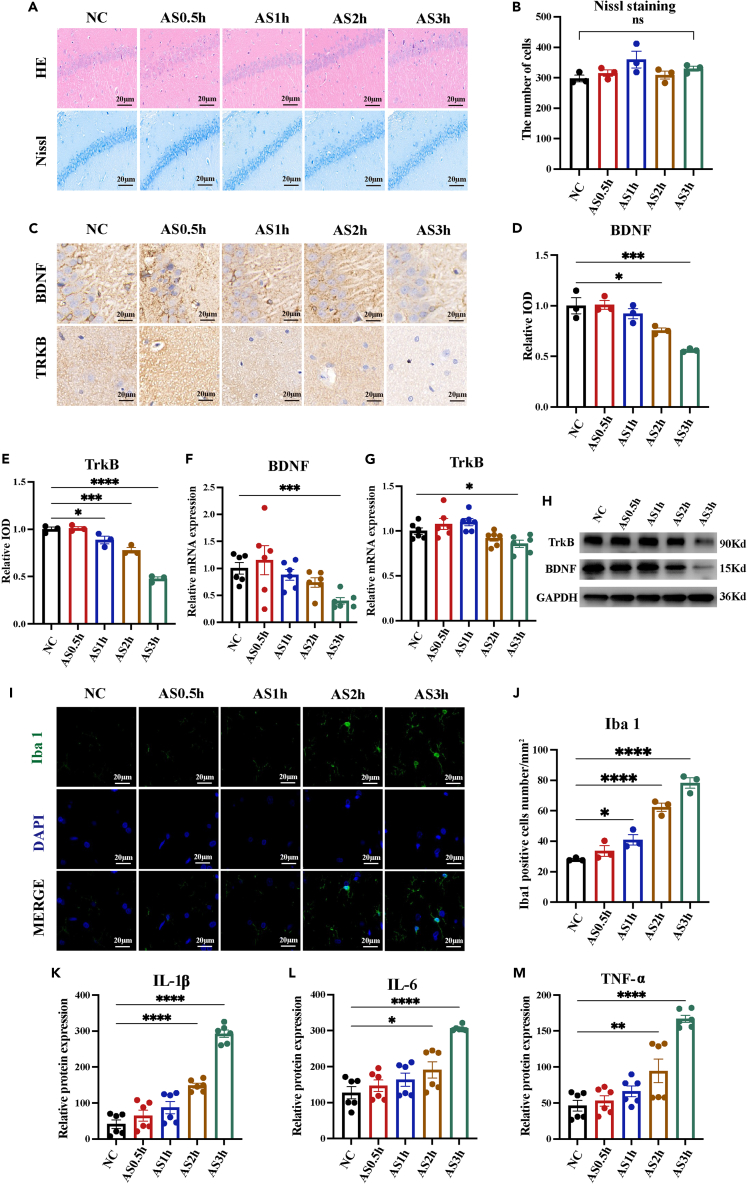
The hippocampus plays a crucial role in cognitive function and is particularly sensitive to stress, making it a key focus in animal experiments to investigate changes in cognitive function at both structural and molecular levels. To investigate whether the hippocampal morphology and function were impaired after underwater exercise, we observed the histopathological changes in the hippocampus. H&E and Nissl staining showed that, compared with the NC group, the hippocampal neurons in the NC, AS0.5 h, and AS1 h groups were arranged in a regular and compact manner, and the Nissl bodies were clearly visible. Rats exposed for more than 1 h had several neurons damaged (wrinkled neurons and blurred nuclear staining), irregular and sparse distribution, and disintegration of Nissl body compared with the NC group (Figure 3A), but not to a significant level. In addition, results of Nissl staining showed that there were no significant differences in the number of positive cells between 5 groups (Figure 3B). Overall, there was little change in hippocampal morphology. It was concluded that a single simulated underwater exercise session failed to cause irreversible morphological damage to the hippocampus. BDNF and its receptor Tropomyosin receptor kinase B (TrkB) plays important roles in neurogenesis, synaptogenesis, regulation of neural plasticity, and learning and memory.10,23 Detection of BDNF and TrkB reveals hippocampal function. Immunohistochemistry (IHC) staining and analysis showed that protein expressions of BDNF and TrkB were downregulated in the hippocampus of rats in the AS2 h and AS3 h groups, with a significant decrease in positive regions (Figures 3C–3E). Consistently, quantitative reverse-transcription PCR (RT-qPCR) showed that BDNF/TrkB transcripts were significantly downregulated in rats in the group AS3 h compared to the NC group (Figures 3F and 3G). Collectively, these aforementioned results suggested that a single simulated underwater exercise session may not cause morphological damage to the hippocampus, whereas prolonged exposure led to significant impairment of hippocampal function.
Figure 3.
Prolonged underwater exercise led to hippocampal dysfunction and neuroinflammation
(A and B) The effect of underwater exercise on the morphology of hippocampus was detected using H&E and Nissl staining. (n = 3 in each group. Scale bar, 50μm).
(C–E) The effect of underwater exercise on the protein expression of BDNF/TrkB in the hippocampus was detected using IHC staining. (n = 3 in each group. Scale bar, 20μm).
(F and G) The effect of underwater exercise on the mRNA expression of BDNF/TrkB in the hippocampus was detected using RT-qPCR. (n = 6 in each group).
(H) The effect of underwater exercise on the protein expression of BDNF/TrkB in the hippocampus was detected using western blotting (n = 3 in each group).
(I and J) The effect of underwater exercise on the microglia activation was detected using IF staining. (n = 3 in each group. Scale bar, 20μm).
(K–M) The effect of underwater exercise on the concentration of IL-1β, IL-6, and TNF-α in the hippocampus was detected using ELISA. (n = 6 in each group). Data are expressed as mean ± SEM, statistical analysis is performed using one-way ANOVE followed by LSD multiple comparisons test; compared with the group NC. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, ∗∗∗∗p < 0.0001.
To date, neuroinflammation has dominated research on cognitive impairment caused by various acute stressors,24,25,26 raising the possibility of common pathophysiological mechanisms in cognitive impairment induced by prolonged underwater exercise. Microglia are the predominant immune cells in the brain. Mounting evidence has suggested that microglia are sensitive to even minor changes in the microenvironment within the CNS27,28 and that activated microglia may trigger subsequent inflammatory cascades, leading to neuroinflammation.24,29 Activation of microglia and secretion of inflammatory factors are hallmarks of neuroinflammation. To investigate the effect of underwater exercise on the activation and morphological changes of microglia, we stained coronal brain slices of rats for the microglia-specific calcium-binding protein, Ionized calcium-binding adaptor molecule 1 (Iba1), as a marker for activated microglia. A robust increase in the number of Iba1-positive microglia in the hippocampus was detected by immunofluorescence (IF) staining in rats exercising for more than 1 h, indicating obvious microglial activation, which escalated with exercising time (Figures 3H and 3I). In addition, the expression of several pro-inflammatory cytokines was detected by ELISA (Figures 3J–3L). The concentrations of interleukin (IL)-1β, IL-6, and tumor necrosis factor alpha (TNF-α) in the hippocampus were not significantly changed in the NC, AS0.5 h, and AS1 h groups, while, compared with the NC group, the concentrations of IL-1β, IL-6, and TNF-α were significantly increased in the AS2 h and AS3 h groups. Taken together, prolonged underwater exercise led to significant neuroinflammation, which might be the underlying mechanism of cognitive impairment.
RNA-seq identifies the involvement of neuroinflammation and the critical role of CCR3
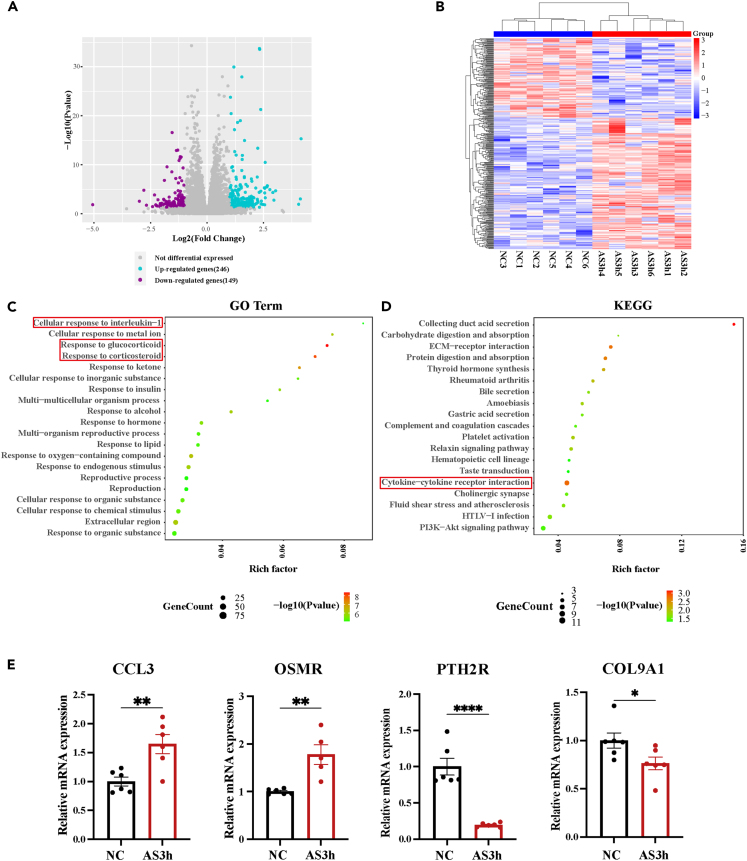
Based on these results, the AS3h group exhibited the most significant cognitive and hippocampal dysfunction and was therefore selected for further mechanism studies. We investigated differentially expressed genes (DEGs) between the NC and AS3 h groups using RNA sequencing (RNA-seq), aiming to uncover the underlying molecular mechanisms. RNA-seq of hippocampal tissues from the NC and AS3 h groups showed the alterations induced by prolonged underwater exercise. DEGs were screened using |log2FoldChange| > 1 and a p value <0.05 as thresholds. Volcano plots showed 395 DEGs between the two groups, of which 149 were downregulated and 246 were upregulated (Figure 4A). By hierarchical clustering analysis, significant DEGs were plotted as a heatmap (Figure 4B), suggesting that there were significant differences in the expression of DEGs in two groups. To verify the accuracy of RNA-seq, four genes including C-C motif chemokine ligand 3 (CCL3), Collagen type IX alpha 1 chain (Col9a1), Parathyroid hormone 2 receptor (Pth2r), and Oncostatin M receptor (Osmr) were randomly selected for RT-qPCR validation. As a result, the expression levels of CCL3 and Osmr were upregulated while the expression levels of Col9a1 and Pth2r were downregulated in the AS3 h group (Figure 4E), which were consistent with the result of RNA-seq. To identify the underlying biological processes and pathways affected by prolonged underwater exercise, the Gene Ontology (Go) annotations and Kyoto Encyclopedia of Genes and Genomes (KEGG) database were used to analyze the biological functions of DEGs. The top 20 most significant processes and pathways were presented as scatterplots (Figures 4C and 4D). GO enrichment analysis revealed that the DEGs were mainly involved in biological processes such as response to CORT and cellular response to IL-1 (Figures 4C and S1A). This validated our earlier hypothesis that neuroinflammation played an important role in cognitive impairment induced by prolonged underwater exercise and was consistent with the increased microglial activation and expression of pro-inflammatory factors in the hippocampus. Furthermore, KEGG analysis revealed that DEGs were enriched in pathways such as cytokine-cytokine receptor interaction, extracellular matrix-receptor interaction, complement and coagulation cascades, and PI3K-Akt signaling pathway (Figures 4D and S1B). The cytokine-cytokine receptor interaction signaling pathway was particularly closely associated with both cognitive function and inflammatory response, thus attracting our attention. Among the 11 DEGs enriched in this pathway, the gene with the most significant expression difference was CCR3. Collectively, the RNA-seq data indicated that the prolonged underwater exercise-induced cognitive impairment is closely associated with neuroinflammation, in which CCR3 plays an important role.
Figure 4.
RNA-seq identifies the involvement of neuroinflammation and the critical role of CCR3
(A) Clustered heatmap for DEGs from NC group and AS3h group (n = 6 in each group).
(B) Volcano plot of differentially expressed genes in NC and AS3h group (purple, downregulation; blue, upregulation; gray, no change).
(C) GO analysis of DEGs in NC and AS3h group.
(D) KEGG analysis of DEGs in NC and AS3h group.
(E) RT-qPCR of 4 selected DEGs to verify the results of RNA-seq. Data are expressed as mean ± SEM, statistical analysis is performed using unpaired two-tailed Student’s t test; compared with the group NC. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, ∗∗∗∗p < 0.0001.
CCR3 expression level was increased in the hippocampus after prolonged underwater exercise
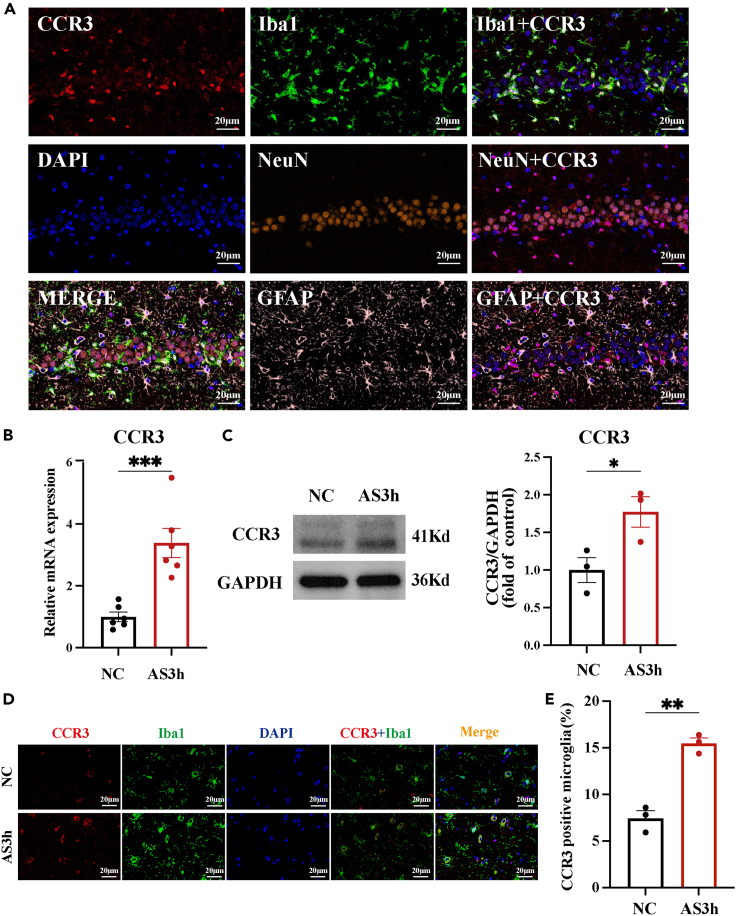
CCR3 is a chemokine receptor that can be expressed by a variety of cells, including eosinophils, basophils, T cells, and monocytes.30 CCR3 binds to several chemokine ligands including CCL11, CCL24, CCL2, etc. and is involved in many functions such as cell migration, allergic response, and parasitic infection.31 Recently, an increasing number of studies have focused on the role of CCR3 in the brain. It has been reported that, in the CNS, CCR3 is predominantly expressed in microglia.32,33,34 In addition, in the inflammatory state, CCR3 expression in microglia is significantly increased.35,36,37 Our IF co-staining results also showed that, in the hippocampal tissue, CCR3 was expressed mainly by microglia and to a lesser extent by neurons and astrocytes (Figure 5A). To study the potential effects of CCR3 on cognitive impairment induced by prolonged underwater exercise, we firstly determined whether CCR3 expression was altered in group AS3 h compared to group NC. RT-qPCR revealed that the mRNA expression of CCR3 increased after prolonged underwater exercise (Figure 5B), and further assessment of the protein expression of CCR3 by western blotting also indicated its marked upregulation in the group AS3h (Figure 5C). Furthermore, to detect changes in the level of CCR3 expression in microglia, we conducted IF staining. The result showed that the percentage of CCR3-positive microglia was significantly increased in group AS3h (Figures 5D and 5E). Collectively, the aforementioned results demonstrated that prolonged underwater exercise increased CCR3 expression, and, moreover, the expression of CCR3 increased dramatically in microglia.
Figure 5.
CCR3 expression level was increased in the hippocampus after prolonged underwater exercise
(A) Co-staining of CCR3 (red) and Iba1 (green), NeuN (orange), and GFAP (pink) in the hippocampus using IF staining (n = 3 in each group, Scale bar, 20μm).
(B) The effect of prolonged underwater exercise on the mRNA expression of CCR3 in the hippocampus was detected using RT-qPCR (n = 6 in each group).
(C) The effect of prolonged underwater exercise on the protein expression of CCR3 in the hippocampus was detected using western blotting (n = 3 in each group).
(D and E) The effect of prolonged underwater exercise on the protein expression of CCR3 in microglia in the hippocampus was detected using IF staining (n = 3 in each group, Scale bar, 20μm). Data are expressed as mean ± SEM, statistical analysis is performed using unpaired two-tailed Student’s t test; compared with the group NC. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, ∗∗∗∗p < 0.0001.
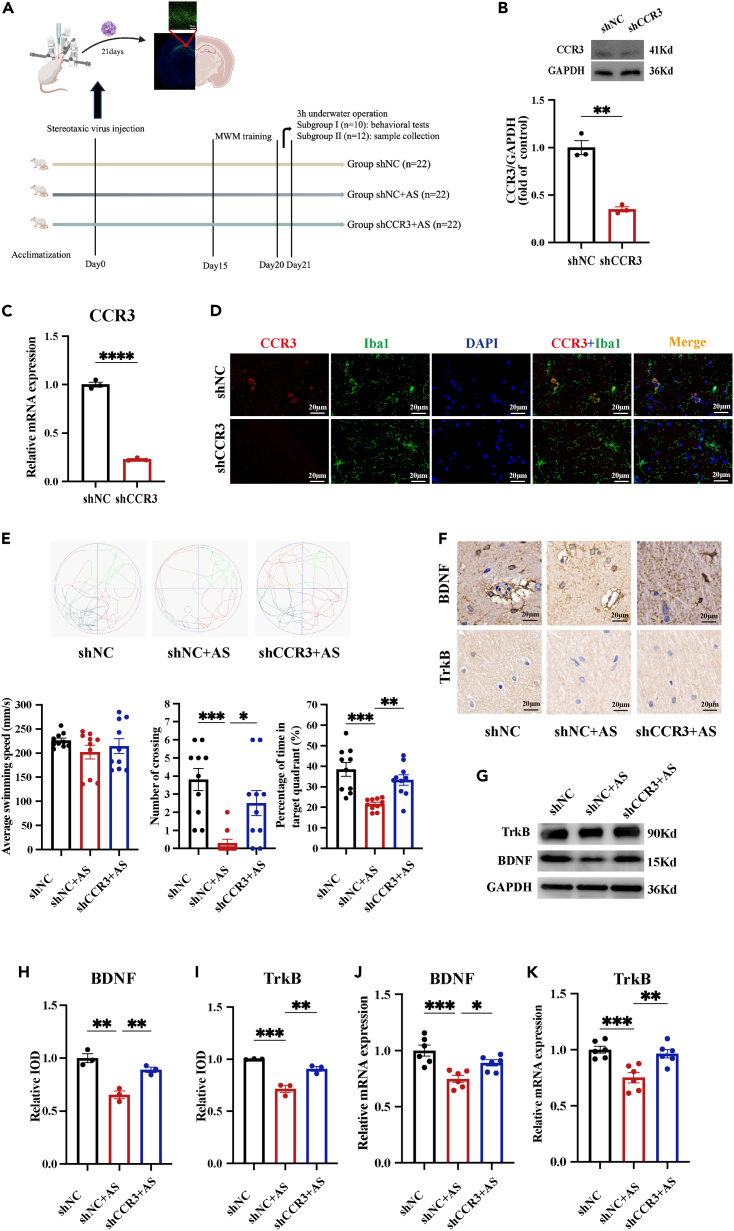
CCR3 knockdown attenuates prolonged underwater exercise-induced cognitive impairment and hippocampal dysfunction
To further investigate the role of CCR3 in cognitive impairment induced by prolonged underwater exercise, AAV carrying AAV2/9-m-EGFP-short hairpin RNA (shRNA) was injected into the hippocampus of rats. The flowchart of the experiment is shown in Figure 6A. Hippocampal tissues were collected 3 weeks after AAV injection to check the accuracy of injection location and the efficiency of transfection. The results showed that the virus was mainly confined to the hippocampal area (Figure 6A), and both mRNA and protein expression of CCR3 was significantly downregulated in rats injected with shCCR3 virus compared with those injected with shNC virus (Figures 6B and 6C), indicating that CCR3 was successfully transfected. In addition, IF staining showed a significant reduction of CCR3 expressed in microglia after shCCR3 virus injection (Figure 6D). To investigate the neuroprotective effects of CCR3 inhibition against prolonged underwater exercise, we examined cognitive and hippocampal function of rats after CCR3 knockdown. MWM training was conducted in rats 15 days after AAV injection, and the probe test and sample collection were conducted after prolonged underwater exercise on day 21 after AAV injection. The MWM results showed an increased platform crossing numbers and a prolonged time in the target quadrant in rats injected with shCCR3 virus compared to those injected with shNC virus (Figure 6E). These data indicated that CCR3 knockdown counteracted the detrimental effects of prolonged underwater exercise on cognitive impairment. In addition, CCR3 knockdown rescued the decreased mRNA and protein expression of BDNF/TrkB (Figures 6F–6K). Altogether, these data further demonstrated the critical role of CCR3 in prolonged underwater exercise-induced cognitive impairment, and inhibition of its expression ameliorated the cognitive impairment and hippocampal dysfunction in rats.
Figure 6.
CCR3 knockdown alleviates prolonged underwater exercise-induced cognitive impairment
(A) Schematic of the knockdown experiment protocol. shNC and shCCR3 virus were injected into the hippocampus of rats and transfection efficiency was detected 3 weeks after injection. MWM training was conducted 15 days after virus injection and MWM test and sample collection were conducted after 3 h underwater exercise at the day 21 after virus injection.
(B and C) The effect of AAV-CCR3 on the mRNA and protein levels of CCR3 in the hippocampus was detected by RT-qPCR (B) and western blot (C). (n = 3 in each group).
(D) The effect of AAV-CCR3 on the protein levels of CCR3 in microglia in the hippocampus was detected by IF (Co-staining of CCR3 (red) and Iba1 (green)). (n = 3 in each group, Scale bar, 20μm).
(E) The effect of AAV-CCR3 on cognitive performance of rats was detected by the MWM test. Movement trajectories of rats in the MWM. (n = 10 in each group).
(F, H, and I) The effect of AAV-CCR3 on the protein expression of BDNF/TrkB of rats was detected by IHC. (n = 3 in each group, Scale bar, 20μm).
(G) The effect of underwater exercise on the protein expression of BDNF/TrkB in the hippocampus was detected using western blotting (n = 3 in each group).
(J and K). The effect of AAV-CCR3 on the mRNA expression of BDNF/TrkB of rats was detected by RT-qPCR. (n = 6 in each group). Data are expressed as mean ± SEM, statistical analysis is performed using unpaired two-tailed Student’s t test (B-C), or one-way ANOVA followed by Tukey’s multiple comparisons test (F–K); p < 0.05, p < 0.01, p < 0.001, p < 0.0001; “#” compared with the group shNC, “∗” compared with the group shNC+AS.
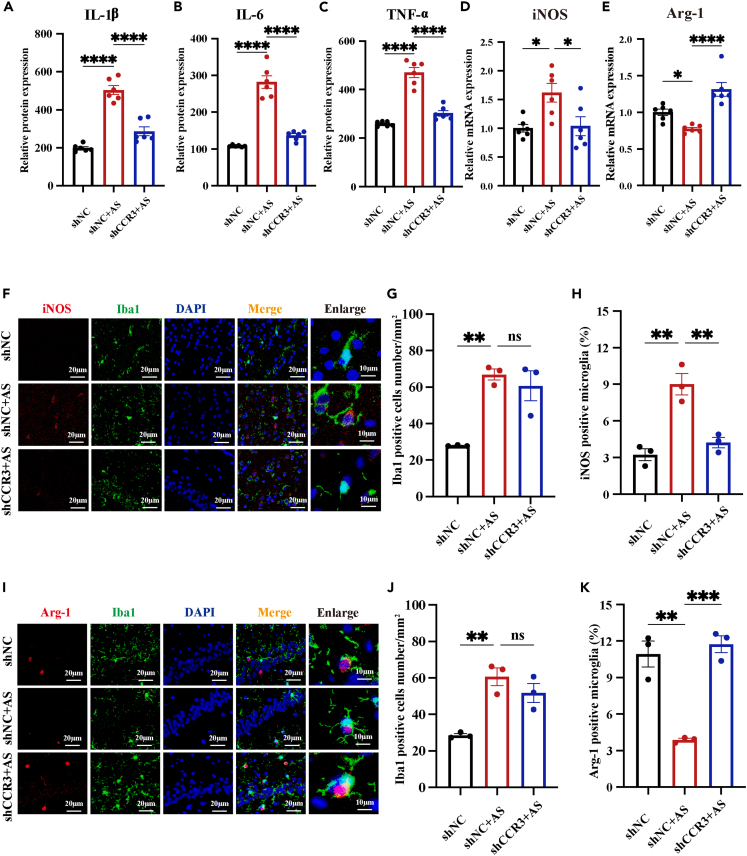
CCR3 knockdown ameliorates neuroinflammation through modulating microglia polarization
Previous studies showed that CCR3 activation accelerates inflammation31,38 while inhibiting or antagonizing CCR3 has potential to alleviate inflammatory response.31 In our study, results of RNA-seq revealed the critical role of neuroinflammation in cognitive impairment induced by prolonged underwater exercise. Therefore, we were finally driven to investigate whether CCR3 knockdown could alleviate neuroinflammation caused by prolonged underwater exercise. We found that knocking down CCR3 reversed the increased expression of pro-inflammatory factors in the hippocampus induced by prolonged underwater exercise (Figures 7A–7C). Next, we detected microglia activation using IF staining. Results showed that Iba1-positive microglia increased significantly in the shNC+AS group compared to the shNC group. However, the shCCR3+AS group and the shNC+AS group did not show any significant change (Figures 7G and 5J), indicating that CCR3 knockdown did not affect the activation of microglia, which prompted us to explore how CCR3 knockdown alleviated the expression of pro-inflammatory cytokines. Activated microglia polarize to the classical M1 pro-inflammatory type and the alternative M2 anti-inflammatory type.39 M1 microglia secrete pro-inflammatory cytokines and neurotoxic substances that damage synapses, induce apoptosis, inhibit neurogenesis, and ultimately cause various symptoms.24 Conversely, M2 microglia release anti-inflammatory cytokines and neurotrophic factors that protect and repair the nervous system and ultimately alleviate various symptoms.40 Inducible nitric oxide synthase (iNOS) and Arginase-1 (Arg-1) are two key enzymes in the arginine metabolic pathway, which are commonly used to reflect the polarization phenotype of microglia at the metabolic level.41 It was hypothesized that CCR3 regulates microglial polarization and thus neuroinflammation. We examined the iNOS and Arg-1 expression in the hippocampus. As expected, the expression of iNOS mRNA significantly increased in the shNC+AS group compared to the shNC group, and the expression of Arg-1 mRNA significantly decreased in the shNC+AS group compared to the shNC group (Figures 7D and 7E). Furthermore, we detected the protein expression of iNOS and Arg-1 in microglia in the hippocampus by IF staining, and the results were consistent with the RT-qPCR results (Figures 7F–7K), indicating that CCR3 knockdown upregulated Arg-1 expression while it downregulated iNOS expression. Overall, the aforementioned results showed that prolonged underwater exercise activated the microglia and promoted the microglia polarization toward M1 pro-inflammatory phenotype, leading to the secretion of pro-inflammatory cytokines. Knockdown of CCR3 did not inhibit the induced microglia activation in the hippocampus. Instead, it shifted activated microglia from a pro-inflammatory to a neuroprotective phenotype and ultimately alleviated the neuroinflammation induced by prolonged underwater exercise.
Figure 7.
CCR3 knockdown alleviated neuroinflammation induced by prolonged underwater exercise through regulating microglia polarization
(A–C) The effect of AAV-CCR3 on the Concentration of IL-1β, IL-6, and TNF-α in the hippocampus was detected by ELISA (n = 6 in each group).
(D and E) The effect of AAV-CCR3 on the mRNA levels of Arg-1 and iNOS in the hippocampus was detected by RT-qPCR (n = 6 in each group).
(F–K) The effect of AAV-CCR3 on the protein levels of Arg-1 and iNOS in microglia in the hippocampus was detected by IF staining (n = 3 in each group, Scale bar, 20μm). Co-staining of iNOS (red) and Iba1 (green) in the hippocampus (F). Iba1 positive cells number in the hippocampus (G). Percentage of iNOS positive microglia in the hippocampus (H). Co-staining of Arg-1 (red) and Iba1 (green) in the hippocampus (I). Iba1 positive cells number in the hippocampus (J). Percentage of Arg-1positive microglia in the hippocampus (G). Data are expressed as mean ± SEM, statistical analysis is performed using one-way ANOVA followed by Tukey’s multiple comparisons test; p < 0.05, p < 0.01, p < 0.001, p < 0.0001; # compared with the group shNC, ∗ compared with the group shNC+AS.
Discussion
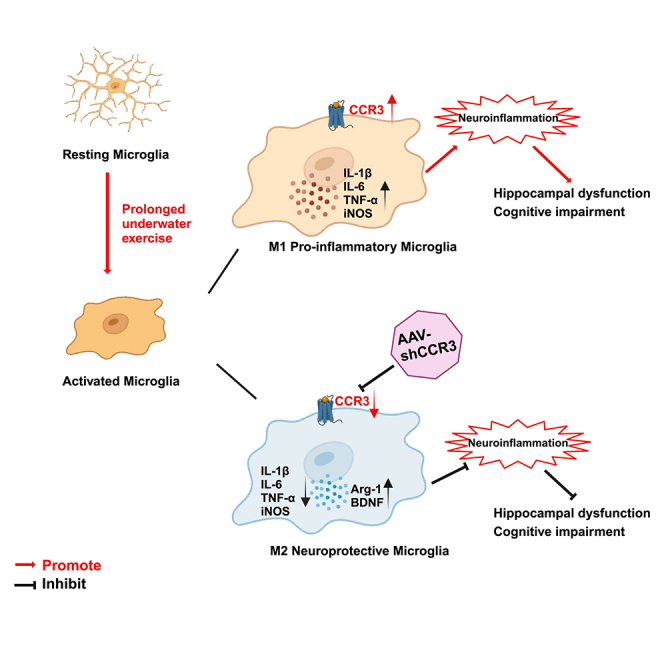
Our findings suggested that cognitive effects of underwater operations were time dependent and that prolonged exposure induced significant cognitive decline. Animal studies showed that prolonged underwater exercise impaired cognitive and hippocampal function through neuroinflammation, in which CCR3 played an important role. We further found that knockdown of CCR3 alleviated cognitive impairment, hippocampal dysfunction, and neuroinflammation caused by prolonged underwater exercise. Mechanistically, inhibition of CCR3 expression could switch the activated microglia from a pro-inflammatory to neuroprotective phenotype. Collectively, our findings demonstrated for the first time that CCR3-mediated neuroinflammation plays a critical role in prolonged underwater exercise-induced cognitive impairment and this gene may provide a potential intervention target for brain protection during underwater operations.
For divers, underwater operation is an acute stressor. In human studies, we found that underwater operations induced significant acute stress response and physical arousal, which agrees with a pervious study reporting that only a 20 min scuba diving could increase the concentration of salivary cortisol.42 Anxiety is the most common emotional response resulting from adverse stress. Our results showed that 0.5 h and 1 h of underwater operation did not cause anxiety, but 3 h of underwater operation resulted in a significant increase in anxiety levels, indicating the emergence of an adverse stress response. Classical research on stress has shown that the effects of stress on cognition are related to its intensity and duration. Moderate stress can improve an individual’s alertness, attention and memory, which is an adaptive response that helps the individual to cope better with emergencies. However, excessive intensity or prolonged duration of stress can have detrimental effects on cognitive performance.43 Our further study found that a 0.5-h underwater operation improved cognitive function in divers, a 1-h underwater operation displayed no significant change, and a 3-h underwater operation induced significant cognitive decline. Growing evidence has supported the inverted-U relationship between exercise and cognition, whereby exercise-induced neurophysiological changes, such as increases in arousal and catecholamine levels, lead to cognition improvement only at an optimal point.44,45 Beyond this point, further increases in exercise workload result in neural noise and deteriorate cognition.46 Most of the research on exercise and cognition has been conducted in conventional environments, and our findings suggest that the inverted-U-shaped relationship between exercise and cognition holds even in underwater environments. Consistently, one study also reported that the well-known positive relationship between moderate exercise and cognition exists in a unique underwater environment and suggested that 20 min of physical exercise in 4-meter-deep underwater environment improved cognitive function.47 The current study provides further evidence that 30 min of acute moderate exercise improves cognitive performance even in some specialized environments. However, there have been no studies investigating how prolonged exercise in an underwater environment affects cognitive function. Previous studies suggested that, in ordinary environments, a critical threshold for significant effects on cognitive function may be reached if exercise lasts at least 15 min,48 and optimal effects of exercise on cognitive function occur when exercise lasts 30 min.49 However, exercise lasting 45 min or longer may lead to decreased cognitive function.17 In the current study, we found that, similar to the conventional environment, exercise lasting 30 min in the underwater environment could improve cognitive performance, while exercise lasting more than 1 h caused cognitive decline.
In animal experiments, underwater exercise lasting less than 1 h did not affect cognitive performance of rats, whereas those lasting 1 h or longer induced significant acute stress response and cognitive decline. This differed slightly from the first part of the human trials which suggested that 1-h underwater operation did not cause significant cognitive decline, possibly because the animals’ nervous systems are more sensitive to external stimuli, which leads to a more pronounced decline in cognitive function. Furthermore, we found that a single simulated underwater exercise session did not cause obvious morphological changes in the hippocampus. As has been introduced before, BDNF/TrkB play crucial roles in brain development, synaptic plasticity, and cognitive function. The relationship between BDNF/TrkB and cognitive impairment has been extensively studied, and there is numerous evidence suggesting that alterations in BDNF/TrkB signaling can partly reflect the changes of cognitive function from the molecular level. Downregulation of BDNF/TrkB has been seen in many neurodegenerative diseases.50 Previous studies have shown that external stimulus such as surgery or trauma can cause neuroinflammation and further downregulate BDNF/TrkB, leading to inhibition of its downstream signaling pathways, which are important for learning and memory.51,52 In our study, the MWM test and changes in BDNF/TrkB levels were combined to reflect alterations in cognitive function in rats at both behavioral and molecular levels. The results showed that prolonged underwater exercise elicited significant worse performance in MWM and downregulation of BDNF/TrkB, indicating the occurrence of cognitive impairment. Growing evidence has demonstrated that neuroinflammation plays a critical role in cognitive impairment induced by various acute stressors.53,54 We hypothesized that neuroinflammation also played a crucial role in the cognitive impairment caused by prolonged underwater exercise. Indeed, prolonged exercise in underwater environment significantly activated microglia and upregulated the expression of pro-inflammatory cytokines including IL-1β, IL-6, and TNF-α in the hippocampus. The aforementioned results suggested that short periods of operations are relatively safe for underwater operators, whereas operations lasting more than 1 h are of concern because they may induce cognitive decline and threaten the safety of operators. Therefore, studies exploring the underlying mechanism of prolonged underwater exercise-induced cognitive impairment are urgent.
Next, the group exhibiting the most severe cognitive impairment and the NC group were chosen to further investigate the mechanism using RNA-seq, and the results indicated the involvement of neuroinflammation, with a key role of CCR3. CCR3 has been shown to play a critical role in the onset and progression of various neuroinflammation and neurodegenerative diseases, such as experimental autoimmune encephalomyelitis,55 Alzheimer's Disease (AD),31,56,57 and Human Immunodeficiency Virus (HIV)-associated dementia.58,59,60 In agreement with previous studies, we found that CCR3 is mainly expressed in microglia in the hippocampus. Moreover, CCR3 expression in microglia was significantly increased after prolonged underwater exercise. In the present study, we found that increased expression of CCR3 was consistent with the fewer platform crossing times and shorter target quadrant time in the MWM test. These results indicated that CCR3 might play a critical role in cognitive impairment induced by prolonged underwater exercise. According to previous studies, antagonizing CCR3 with the CCR3-specific antagonist (GW766944) reversed the pathological changes and symptoms of AD.56 In addition, inhibition of CCR3 by AKST4290 obviously improved learning and memory in the Barnes maze and radial arm water.31 These findings suggested that CCR3 may be a potential target for alleviation of cognitive impairment induced by prolonged underwater exercise. To further explore the role of CCR3, we utilized CCR3 shRNA virus to inhibit the expression of CCR3 in the hippocampus. Unsurprisingly, we found that CCR3 knockdown significantly improved the performance of rats in the MWM and reversed the downregulation of BDNF/TRKB. Moreover, CCR3 knockdown significantly reduced the expression of pro-inflammatory cytokines, indicating that neuroinflammation was alleviated. However, results of IF staining suggested that CCR3 knockdown did not attenuate the activation of microglia, which prompted us to investigate how CCR3 knockdown reduced neuroinflammation. It has been reported that neuroinflammation is associated with microglia polarization, which refers to the specific functions and phenotypes exhibited by microglia in the activated state.61 Microglia activate when stimuli are sensed, and activated microglia can polarize toward the classical M1 pro-inflammatory type and the alternative M2 anti-inflammatory type.62 Accordingly, we hypothesized that CCR3 knockdown might play a role in regulating microglia polarization. As expected, CCR3 knockdown downregulated the expression of iNOS and upregulated the expression of Arg-1, indicating the transition of the activated microglia from a pro-inflammatory to neuroprotective phenotype. Previous studies have found that the decrease in proportion of M1 microglia and the increase in proportion of M2 microglia reduce the synthesis and release of pro-inflammatory cytokines and increase the expression of BDNF.63,64,65 In this study, we also found that knockdown of the CCR3 converted the activated microglia from M1 to M2 phenotype, and consistently the exertion of pro-inflammatory factor was inhibited and the expression of BDNF/TrkB was increased after prolonged exposure to underwater exercise. Nevertheless, this study only localized CCR3 and investigated its role in regulating microglia polarization and cognitive impairment induced by prolonged underwater exercise. The underlying mechanism by which CCR3 functions remains unclear, and these remained to be investigated in the future.
In summary, evidence from this study demonstrates that the cognitive effects of underwater operations are time dependent and that prolonged exposure leads to significant cognitive decline. The underlying molecular mechanism may involve neuroinflammation, and CCR3 plays a critical role. Knocking down CCR3 can alleviate neuroinflammation and cognitive impairment through switching activated microglia from a pro-inflammatory to neuroprotective phenotype. In the future, CCR3 may be applied as a novel intervention target for protecting the brain of people exposed to prolonged underwater operations.
Limitations of the study
In this study, on the cognitive effect of underwater operations, we mainly focused on durations of exposure. As a matter of fact, there are many other factors affecting cognitive performance in underwater operations, such as depth, water temperature, breathing gas, etc. Future studies are needed to construct other types of animal models to comprehensively explore the cognitive effect of underwater operations. In addition, the current study screened for neuroinflammation according to RNA-seq data, and therefore we mainly explored the regulation of CCR3 on microglial cells. However, our current data cannot exclude the role of CCR3 on other cell types in the hippocampus, such as neurons and astrocytes. We plan to address this gap in the near future by breeding animals with specific gene types in specific cells to ensure a rigorous experimental design. Considering the critical role of CCR3 in neuroinflammation and cognitive impairment, it would be important to enhance relative knowledge to appropriately guide the application of interventions for protecting the brain underwater.
STAR★Methods
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| Mouse anti-Iba1 (IF:1:500) | Abcam | Cat#ab283319; RRID: AB_2924797 |
| Rabbit anti-CCR3 (Western blotting:1:1000; IF:1:500) | Affinity | Cat#DF10205-100; RRID: AB_2840784 |
| Rabbit anti-BDNF (Western blotting:1:1000; IHC:1:500) | Abcam | Cat#Ab108319; RRID: AB_10862052 |
| Rabbit anti-TrkB (Western blotting:1:5000; IHC:1:1000) | Abcam | Cat#ab187041; RRID: AB_2892613 |
| Rabbit anti-Arg-1 (IF:1:500) | Cell Signaling Technology | Cat#93668S; RRID: AB_2800207 |
| Mouse anti-iNOS (IF:1:500) | Santa Cruz | Cat#sc-7271; RRID: AB_627810 |
| Rabbit anti-GAPDH (Western blotting:1:1000) | Cell Signaling Technology | Cat#5174S; RRID: AB_10622025 |
| Bacteria and virus strains | ||
| pHBAAV-U6-MCS-CMV-EGFP-CCR3 shRNA | Hanbio Tech | N/A |
| Chemicals, peptides, and recombinant proteins | ||
| TRIzol reagent | Invitrogen | Cat# 15596018 |
| Protease and phosphatase inhibitor cocktail | NCM Biotech | Cat# P002 |
| RIPA lysate buffer | Beyotime | Cat# P0013B |
| BCA kit | Thermo | Cat# 23250 |
| Stripping buffer | Service bio | Cat# G2078 |
| Critical commercial assays | ||
| Human Cortisol ELISA kit | Elabscience Biotechnology | Cat# E-OSEL-H0006 |
| Rat IL-1 beta ELISA Kit | Servicebio | Cat# GER0002 |
| Rat IL-6 ELISA Kit | Servicebio | Cat# GER0001 |
| Rat TNF-alpha ELISA Kit | Servicebio | Cat# GER0004 |
| Rat Corticosterone ELISA kit | Elabscience Biotechnology | Cat# E-OSEL-R0002 |
| First Strand cDNA synthesis Kit | Invitrogen | Cat# K1622 |
| QuantityNova SYBR Green PCR Kit | Qiagen | Cat# 208054 |
| Oligonucleotides | ||
| RT-qPCR primers, see Table S1 | This paper | N/A |
| shCCR3 5′-CAGCTGGAAGCGTTTCCATGCTCTA-3′ | Hanbio Tech | N/A |
| Deposited data | ||
| Raw bulk RNAseq data | This paper | GSE247651 |
| shCCR3 5′-CAGCTGGAAGCGTTTCCATGCTCTA-3′ | Hanbio Tech | N/A |
| Morris Water Maze Experiment Video System | Ji’liang Technology Co., Ltd. | N/A |
| Open Field Test Experiment Video System | Ji’liang Technology Co., Ltd. | N/A |
| NovaSeq 6000 platform | Illumina | N/A |
| ImageJ | National Institute of Health | N/A |
| Prism 9.0 | GraphPad | N/A |
Resource availability
Lead contact
Further information and request for resources and reagents should be directed to and will be fulfilled by the lead contact, Houyu Zhao (Zhaohouyuecho@163.com).
Materials availability
This study did not generate new unique reagents.
Data and code availability
-
•
Raw RNAseq data have been deposited at NCBI and are publicly available. Accession number is listed in the key resources table. All data reported in this paper will be shared by the lead contact upon reasonable request.
-
•
This paper does not report original code.
-
•
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.
Experimental model and study participant details
Participants
Between June 2021 and June 2023, three groups of divers, male, aged 18–32 years old with diving experience of 2–12 years were recruited to perform underwater operations. All participants were individuals self-identified as Han Chinese. They were certified, healthy adult divers with normal visual acuity, either corrected or uncorrected. Exclusion criteria: (1) a history of neurological, pulmonary, cardiovascular, or metabolic disease; (2) use of recreational drugs, psychoactive medication, excessive alcohol or over five caffeine-containing beverages a day. They slept at least 24 h before the underwater operations and measurement. All experimental procedures were performed in accordance with the Declaration of Helsinki and approved by the Naval Special Medical Center of PLA (No 2021070201). All subjects provided written informed consent. Health status and previous diving experience were self-assessed. All divers met the “Medical Examination Standards for Professional Divers” (China National Standard, GB 20827-2007, 2007.08.01). Group I included 20 divers to work underwater at a depth of 10 m for 0.5 h. Group II included 15 divers to work at a depth of 10 m underwater for 1 h. Group III included 13 divers to work at a depth of 10 m underwater for 3 h.
Animals
SD rats (male, 8 weeks of age, 235–265 g body weight) were provided by Laboratory Animal Management Department, Shanghai Institute of Family Planning Science. All rats were housed five animals per cage at a temperature of 21°C–25°C and a relative humidity of 40–60%, with 10 h of light per day and air exchange at 20 times per hour. Food and water were freely available. A 7-day acclimatization for rats was needed before experiments began. All animals used in our study were humanely cared for according to Institutional Animal Care and Use Guidelines of the Naval Special Medical Center of PLA. All animal experiments conformed to relevant regulatory standards and were approved by the Naval Special Medical Center of PLA Animal Ethical Committee (No 2021021).
Method details
Underwater operation
Divers perform their tasks in the open ocean with water temperatures around 25°C and operating depths of around 10 m. The divers were required to complete a conveyor boat delivery task. Three groups of subjects worked underwater for 0.5h, 1h, and 3h respectively.
Saliva collection and assessment
As shown in Figure 1A, unstimulated saliva samples were collected 1 day before operation and immediately after operation. To avoid the effects of physiologic rhythms, saliva collection was set at around 12 p.m. Subjects were required to rinse their mouths with clean water 30 min before saliva collection. The tongue was pressed against the palate to enrich the saliva, and the saliva was spat into a sterile polypropylene transfer pipette (2 mL). After sample collection (before and after operation, separately), samples were immediately transported to the laboratory on dry ice and centrifuged at 4,000 rpm for 10 min at 4°C. The supernatant was dispensed into freeze-driedtubes and stored in the refrigerator at −80°C until analysis. Salivary cortisol was measured using an enzyme-linked immunosorbent assay (ELISA) kit (Elabscience, E-OSEL-H0006, Wuhan, China).
Cognitive function test
A primary cognitive functions test battery designed by Institute of Psychology, Chinese Academy of Sciences (Beijing, China) was used to evaluate subjects’ memory performance and processing speed. Processing speed was evaluated by symbol search test, a revised subtest of the Wechsler Adult Intelligence Scale of the same name. The task is to determine whether one of the five symbols below the horizontal line is one of the two symbols above the horizontal line. Participants must solve this task correctly as quickly as possible within 120 s. The more symbols a subject correctly selected, the higher the score achieved. Memory performance was assessed using operation span test, which consists of two tasks: a mental arithmetic task and an animal recall task. In this test, participants must remember the animals presented and their order, and in the meantime judge whether the following formula is correct or not. The more genera the subject has remembered correctly and the more formulas he has calculated correctly, the higher the score obtained.
State-Trait Anxiety Inventory (STAI-S)
The STAI-S of the State-Trait Anxiety Inventory consists of 20 items. The items are answered on a 4-point Likert scale ranging from 1 (not at all) to 4 (very strongly). The total scores range from 20 to 80, with higher scores indicating higher levels of anxiety. The STAI-S has been shown to be a valid and reliable scale for measuring anxiety. The internal consistency as measured by Cronbach’s alpha ranged from 0.94 to 0.95.
Animal model and experimental design
The animal model was established in a simulated hyperbaric chamber (Yantai Hongyuan Oxygen Industry, Shandong, China) at a pressure of 2.0 ATA. Compression was performed within 3 min and decompression were performed gradually within 10 min by a professional engineer. The rats were placed in a home-made water tank and swam without weight. The water temperature was kept around 23°C–25°C. Laboratory staff overserved the state of animals closely and took them out immediately if the animal is exhausted and put them back into the pool after 3–5 min of rest.
The animal study was conducted into two experiments. In the first study, after 7 days of acclimatization, 95 rats were randomly divided into 5 groups (n = 19 for each): (1) the normal control group (NC); (2) the exercise in hyperbaric environment for 0.5 h group (AS0.5 h); (3) the exercise in hyperbaric environment for 1 h group (AS1 h); (4) the exercise in hyperbaric environment for 2 h group (AS2 h); (5) the exercise in hyperbaric environment for 3 h group (AS3 h). Each group was divided into 2 subgroups: subgroup I for behavioral tests (n = 10) and subgroup II (n = 9) for bioinformatic analysis. In our study, the Morris water maze (MWM) training started the first day after the 7 days’ acclimatation and was conducted in a fixed time between 14:00-18:00 every day for 5 days. On the day 6, after animal model building, the Open-field test (OFT) and MWM probe test was performed between 18:00-20:00 for subgroup I after a 30 min rest and subgroup II were sacrificed immediately. The experiment flowchart is shown in Figure 2A. In the second experiment, to explore the role of CCR3 in cognitive impairment induced by prolonged underwater exercise, 66 animals were randomly divided into 3 groups (n = 22 in each group): the shNC group, the shNC + AS group, the shCCR3 + AS group. Rats in group shNC + AS and group shCCR3 + AS were allowed to swim in the same environment as the first experiment for 3 h. The experiment flowchart is shown in Figure 6A.
Morris water maze (MWM)
The MWM test was conducted using a Morris water maze (Jiliang Technology Co., Ltd., Shanghai, China) to evaluate the cognitive performance of rats. Rats were single-caged and brought into the testing room prior to the beginning of the experiment. The MWM test was divided into the acquisition training and the probe test and was conducted in a circular tank (diameter, 150 cm; height, 60 cm) in a dimly lit room. The water temperature was kept at 23°C–25°C to inhibit the rats from floating. A submerged escape platform (10 cm × 10 cm) was equipped 1.5 cm below the black water surface in one of the quadrants. Spatial cues of different geometry were decorated by the pool sides to help the rats recognize the platform position. The rats were trained 5 consecutive days with four trials per day per rat. In each trial, the rat facing the pool wall were slowly placed into the water from each quadrant to search for the submerged platform. The trial was completed as soon as the rat found the platform or when 60 s had elapsed. If the rat fails to locate the submerged platform during a given trial, it is guided to the submerged platform and allowed to stay there for a duration of 20 s. On day 6, the platform was removed, and a single probe test was performed to measure the integrity and strength of spatial memory 24 h after the last trial of the acquisition phase. Rats were dropped from the opposite quadrant into water and allowed to explore freely for 120 s. Times of crossing the platform and percentage of time spending in the target quadrant were recorded and analyzed for measuring cognitive function.
Open-field test (OFT)
The OFT was employed for evaluation of stress response and anxiety of rats in the Subgroup I. Rats were placed in the center of an open field (100 cm × 100 cm × 60cm) (Jiliang Technology Co., Ltd., Shanghai, China), and exploration was assessed for 4 min. Cages were cleaned with 75% alcohol following each session. The total movement distance, time in central areas and number of crossings were recorded and analyzed.
Histological analysis
In the first experiment, on day 6, after model building, 3 rats in Subgroup II were sacrificed after deep anesthesia with sodium pentobarbital (40 mg/kg, i.p.), and the brain tissues were collected and fixed in 4% paraformaldehyde and then dehydrated and subsequently embedded in paraffin. Paraffin sections of approximately 5 μm thickness were prepared and needed to be dewaxed and rehydrated until staining. In the second experiment, considering the virus was EGFP labeled, before staining, immunohistochemistry and immunofluorescence, the original florescence signal was thoroughly removed using a tissue quencher to avoid interference.
Hematoxylin-eosin (HE) staining
The sections were put into a hematoxylin staining solution for 10 min and then rinsed with distilled water. The sections were added to eosin staining solution for 15 s and washed with distilled water. The sections were dehydrated by gradients of 75%, 85%, 95%, and 100% ethanol for 2 min sequence, transparent by xylene, sealed by neutral gum, then removed from the water at room temperature. The slices were sealed with neutral gum, and the water was removed at room temperature. Using optical microscopy to examine the pathological changes in the hippocampus.
Nissl staining
The sections were stained with 1% cresyl violet solution at room temperature for 30 min and then washed with distilled water, followed by differentiation with 70% alcohol. Finally, immersing the sections in a graded alcohol series and xylene for 2 min each. Observing the sections using an optical microscope to examine the pathological changes in the hippocampus after neutral balsam mounting.
Immunohistochemistry (IHC) and immunofluorescence (IF) staining
IHC and IF staining was performed on 5 μm paraffin-embedded sections of hippocampal tissue. After dehydration, slides were incubated in Antigen Unmasking Solution Citrate Buffer pH7.4 for 20 mwa steamer for antigen retrieval and then blocked for 30 min at room temperature in 3% BSA. Then slides were incubated with primary antibodies at 4°C overnight. For IF staining, after being washed with PBS, the sections were incubated with secondary antibodies (CY3-conjugated goat anti-Rabitt IgG, Servicebio, 1:300 and Alexa Fluor 488-conjugated goat anti-mouse IgG, Servicebio, 1:400) at RT for 1 h without light. Finally, brain slices were then washed again in PBS and cover-slipped with DAPI Fluoromount-G mounting medium. Images of the stained sections were obtained by confocal microscopy (Nikon, Japan). For IHC staining, after being washed with PBS, the sections were incubated with biotinylated IgG at room temperature for 50min. Next, sections incubated with DAB (0.2 mg/mL) for 10 min in room temperature, and then mounted onto slides, dehydrated, and cover slipped. The primary antibodies dilution ratios are provided in the key resources table.
Enzyme-linked immunosorbent assay (ELISA)
On day 6, after model building, 6 rats in Subgroup II were sacrificed after deep anesthesia with sodium pentobarbital (40 mg/kg, i.p.), blood samples and hippocampus tissues were collected. Corticosterone (CORT) (Elabscience Biotechnology Co.,Ltd, E-OSEL-H0006, Wuhan, China) concentrations in serum and IL-1β, TNF-α and IL-6 (Servicebio, GER0002; GER0004; GER0001, Wuhan, China) concentrations in the hippocampus were determined by ELISA kits. All tests were performed according to the manufacturer’s instructions. 6 samples were measured in each group.
Quantitative real-time PCR (RT-qPCR)
Hippocampal tissues from rats in each Subgroup II were isolated and 30mg were kept for RT-PCR (n = 6). Total RNA was extracted from rat hippocampal tissue using trizol reagent (Invitrogen,USA) and purified according to the manufacturer’s instructions. The concentration of each RNA sample was determined by measuring the absorbances at 260 and 280 nm by a spectrophotometer (Thermo, USA). Total RNA was converted to cDNA using a one-step reverse transcription polymerase chain reaction kit (Invitrogen,USA). Following the instructions, a reverse transcription polymerase chain reaction was performed using the kit and a real-time system, and this cDNA was subjected to qPCR amplification conducted on a CFX96 RT PCR detection system (Thermo, USA). The reaction conditions: the first step 95°C for 2 min; the second step 95°C for 5 s; the third step 60°C for 30 s; the second and third steps were cycled 40 times. Each sample was repeated on three different 96-well plates. Finally, we used the 2−ΔΔCT method to assess the mRNA levels of BDNF, TrkB, iNOS, Arg-1 relative to GAPDH, respectively. Primers were synthesized by Sangon Biotech (Shanghai, China). Primer sequences used in this study were shown in Table S1.
Transcriptomic analysis
Hippocampal tissues from rats in each Subgroup II of group NC and group AS3h were isolated and 60mg were kept for RNA-sequencing (n = 6). The total RNA of rat hippocampal tissue was extracted using the RNAmini kit (Qiagen, Germany). RNA quality was examined by gel electrophoresis and with Qubit (Thermo, USA). The mRNAs with PolyA structure were enriched using oligo (dT) magnetic beads and then were fragmented to approximately 300 bp. After the construction of the cDNA library, PCR amplification was employed to enrich the library fragments with a size of 450 bp. The library was quantified by Agilent 2100 Bioanalyzer. The library was then sequenced on a NovaSeq 6000 platform (Illumina, USA) by Nier Biotechnology, Co., Ltd. (Shanghai, China). Taking read counts as the original expression amount of the gene, the differentially expressed genes (DEGs) were identified by DESeq with the screening criteria as follows: |log2FoldChange| > 1, significance p-value <0.05. Subsequently, the Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) enrichment analysis of all DEGs was conducted using the R software. The significantly enriched pathways were determined when p < 0.05 and at least two affiliated genes were included. Four DEGs including Parathyroid Hormone 2 Receptor (Pth2r), Collagen Type IX Alpha 1 Chain (Col9a1), C-C Motif Chemokine Ligand 3 (Ccl3), Oncostatin M Receptor (Osmr) were chosen to verify the precision of the results of transcriptomic analysis using RT-qPCR and primer sequence are shown in Table S1.
Western blotting (WB)
For hippocampus tissues, total protein was extracted with RIPA lysate buffer complemented with protease and phosphatase inhibitor cocktail and centrifuged at 12,000 rpm for 15 min at 4°C (Eppendorf, Germany). The upper liquid layer was assayed for protein concentration according to the BCA kit instructions. The homogenate supernatant was mixed with the loading buffer in a 1:5 ratio to produce samples for electrophoresis on 10% sodium dodecyl sulfate–polyacrylamide (SDS-PAGE) gels, which were then transferred to polyvinylidene difluoride (PVDF) membranes (Millipore, USA) with pore size of 0.22 μm by wet blotting. After blocking the protein with 5% skim milk powder, the blots were incubated with primary antibodies. Following three washes with TBST, secondary anti-rabbit HRP-conjugated antibodies were incubated for 2 h at RT. Protein expression levels were visualized by enhanced chemiluminescence (Shanghai Peiqing Science & Technology Co., Ltd, China). GAPDH served as a loading control. The results were analyzed using ImageJ software. The antibody dilution ratios are provided in the key resources table.
Viral packaging and stereotaxic virus injection
In the second experiment, the CCR3 shRNA adeno-associated virus (AAV) was constructed and packaged by Hanbio Tech (Shanghai, China). For CCR3 shRNA viral packaging, the shRNA sequence of rat CCR3 (5′-CAGCTGGAAGCGTTTCCATGCTCTA-3′) was synthesized and cloned into pHBAAV-U6-MCS-CMV-EGFP plasmid to produce pHBAAV-U6-MCS-CMV-EGFP-CCR3 shRNA. HBAAV2/9- EGFP NC was regarded as the control virus. The shCCR3 and shNC virus were EGFP labeled. The genomic titer was 1.3∗10ˆ12 vg/mL determined by quantitative PCR. For viral injection, rats were anesthetized with sodium pentobarbital (40 mg/kg, i.p.) and then placed on a stereotaxic apparatus (RWD Life Science, Shenzhen, China). Virus was injected using Nanoject II (Drummond Scientific, PA) via a micro pipette. When the rats were in deep anesthesia, 4 μL AAV2/9-m-EGFP-shRNA (CCR3) or AAV2/9-m-EGFP-shRNA (NC) was bilaterally injected into the hippocampus (200 nL/min) (2μL for each side). After waiting for 10 min to allow absorption of AAV, the pipette was slowly withdrawn within 5 min. The coordinates to inject virus into bilateral hippocampus in our study was: −4.24 mm anteroposterior and ±2 mm mediolateral from the bregma, and −3.5 mm dorsoventral from the surface of dura mater. Penicillin was injected to prevent infection (40000 U/kg, ip). Then, keeping rats in a heater about 30min to keep them warm during surgery. For transfection verification, hippocampal tissues were collected 3 weeks after virus injection for gene and protein analysis. For exploration of the role of CCR3, MWM was conducted 2 weeks after virus injection, animal model was built, and hippocampal tissues were collected 3 weeks after virus injection.
Quantification and statistical analysis
GraphPad Prism 9.0 was employed to analyze the data statistically. The results were displayed as the mean ± SEM. The two-tailed Student’s paired t-test was used to compare the divers’ status before and after underwater operations. For animal studies, unpaired two-tailed Student’s t test was used for two-group comparisons, one-way ANOVA followed by LSD or Tukey’s multiple comparisons test was used for three- or more-group comparisons. A p-value <0.05 means statistical significance.
Acknowledgments
This study was supported by Application Promotion Plan (21AH0103), Logistics Research Projects of PLA (BHJ22J020), Shanghai Municipal Health Commission Project (2024QN057, 20204Y0326), and the Sailing Talent Program of Naval Medical University.
Author contributions
H.Z. designed and performed most experiments, analyzed data, and prepared the manuscript; K.L., Z.Y., T.Z., and Y.W. helped with the behavioral test and model preparation; Z.Y. helped with mice stereotaxic injection of lentivirus; J.X. helped with the preparation of the figures; X.Y. helped with the revision of the manuscript; Y.F. and X.Z. helped with the design of experiments and the acquisition of funding. All authors read and approved the final manuscript.
Declaration of interests
The authors declare no competing interests.
Published: July 9, 2024
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.isci.2024.110379.
Contributor Information
Xianpeng Zu, Email: zuxianpeng@163.com.
Yiqun Fang, Email: 1287225836@qq.com.
Supplemental information
References
- 1.de Jong F.J.M., Wingelaar T.T., van Hulst R.A. Pulmonary oxygen toxicity in occupational diving. Occup. Med. 2023;73:231–232. doi: 10.1093/occmed/kqad043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kelly K.R., Palombo L.J., Jensen A.E., Bernards J.R. Efficacy of closed cell wet-suit at various depths and gas mixtures for thermoprotection during military training dives. Front. Physiol. 2023;14 doi: 10.3389/fphys.2023.1165196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Diamond A. Executive functions. Annu. Rev. Psychol. 2013;64:135–168. doi: 10.1146/annurev-psych-113011-143750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Steinberg F., Doppelmayr M. Executive Functions of Divers Are Selectively Impaired at 20-Meter Water Depth. Front. Psychol. 2017;8:1000. doi: 10.3389/fpsyg.2017.01000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hammerton Z. Determining the variables that influence SCUBA diving impacts in eastern Australian marine parks. Ocean Coast Manag. 2017;142:209–217. [Google Scholar]
- 6.Sharma R.I., Marcinkowska A.B., Mankowska N.D., Waśkow M., Kot J., Winklewski P.J. Cognitive Functions in Scuba, Technical and Saturation Diving. Biology. 2023;12 doi: 10.3390/biology12020229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Verburgh L., Königs M., Scherder E.J., Oosterlaan J. Physical exercise and executive functions in preadolescent children, adolescents and young adults: a meta-analysis. Br. J. Sports Med. 2014;48:973–979. doi: 10.1136/bjsports-2012-091441. [DOI] [PubMed] [Google Scholar]
- 8.Skriver K., Roig M., Lundbye-Jensen J., Pingel J., Helge J.W., Kiens B., Nielsen J.B. Acute exercise improves motor memory: exploring potential biomarkers. Neurobiol. Learn. Mem. 2014;116:46–58. doi: 10.1016/j.nlm.2014.08.004. [DOI] [PubMed] [Google Scholar]
- 9.Basso J.C., Suzuki W.A. The Effects of Acute Exercise on Mood, Cognition, Neurophysiology, and Neurochemical Pathways: A Review. Brain Plast. 2017;2:127–152. doi: 10.3233/bpl-160040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hötting K., Schickert N., Kaiser J., Röder B., Schmidt-Kassow M. The Effects of Acute Physical Exercise on Memory, Peripheral BDNF, and Cortisol in Young Adults. Neural Plast. 2016;2016 doi: 10.1155/2016/6860573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Smith J.C., Paulson E.S., Cook D.B., Verber M.D., Tian Q. Detecting changes in human cerebral blood flow after acute exercise using arterial spin labeling: implications for fMRI. J. Neurosci. Methods. 2010;191:258–262. doi: 10.1016/j.jneumeth.2010.06.028. [DOI] [PubMed] [Google Scholar]
- 12.Moreau D., Chou E. The Acute Effect of High-Intensity Exercise on Executive Function: A Meta-Analysis. Perspect. Psychol. Sci. 2019;14:734–764. doi: 10.1177/1745691619850568. [DOI] [PubMed] [Google Scholar]
- 13.Weinstein A., Szabo A. Exercise addiction: A narrative overview of research issues. Dialogues Clin. Neurosci. 2023;25:1–13. doi: 10.1080/19585969.2023.2164841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hsieh S.S., Chueh T.Y., Huang C.J., Kao S.C., Hillman C.H., Chang Y.K., Hung T.M. Systematic review of the acute and chronic effects of high-intensity interval training on executive function across the lifespan. J. Sports Sci. 2021;39:10–22. doi: 10.1080/02640414.2020.1803630. [DOI] [PubMed] [Google Scholar]
- 15.Cian C., Barraud P.A., Melin B., Raphel C. Effects of fluid ingestion on cognitive function after heat stress or exercise-induced dehydration. Int. J. Psychophysiol. 2001;42:243–251. doi: 10.1016/s0167-8760(01)00142-8. [DOI] [PubMed] [Google Scholar]
- 16.Chang Y.K., Chu C.H., Wang C.C., Wang Y.C., Song T.F., Tsai C.L., Etnier J.L. Dose-response relation between exercise duration and cognition. Med. Sci. Sports Exerc. 2015;47:159–165. doi: 10.1249/mss.0000000000000383. [DOI] [PubMed] [Google Scholar]
- 17.Hacker S., Banzer W., Vogt L., Engeroff T. Acute Effects of Aerobic Exercise on Cognitive Attention and Memory Performance: An Investigation on Duration-Based Dose-Response Relations and the Impact of Increased Arousal Levels. J. Clin. Med. 2020;9 doi: 10.3390/jcm9051380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brebeck A.K., Deussen A., Schmitz-Peiffer H., Range U., Balestra C., Cleveland S., Schipke J.D. Effects of oxygen-enriched air on cognitive performance during SCUBA-diving - an open-water study. Res. Sports Med. 2017;25:345–356. doi: 10.1080/15438627.2017.1314289. [DOI] [PubMed] [Google Scholar]
- 19.Dalecki M., Bock O., Schulze B. Cognitive impairment during 5 m water immersion. J. Appl. Physiol. 2012;113:1075–1081. doi: 10.1152/japplphysiol.00825.2012. [DOI] [PubMed] [Google Scholar]
- 20.Lafère P., Hemelryck W., Germonpré P., Matity L., Guerrero F., Balestra C. Early detection of diving-related cognitive impairment of different nitrogen-oxygen gas mixtures using critical flicker fusion frequency. Diving Hyperb. Med. 2019;49:119–126. doi: 10.28920/dhm49.2.119-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pontifex M.B.M., Chandler A.L., Gwizdala M.C., Parks K.L., Fenn A.C., KimberlyKamijo K. A primer on investigating the after effects of acute bouts of physical activity on cognition. Psychol. Sport Exerc. 2019;40:1–22. [Google Scholar]
- 22.Strangman G.E., Sipes W., Beven G. Human cognitive performance in spaceflight and analogue environments. Aviat Space Environ. Med. 2014;85:1033–1048. doi: 10.3357/asem.3961.2014. [DOI] [PubMed] [Google Scholar]
- 23.Balakrishnan R., Kim Y.S., Kim G.W., Kim W.J., Hong S.M., Kim C.G., Choi D.K. Standardized extract of Glehnia Littoralis abrogates memory impairment and neuroinflammation by regulation of CREB/BDNF and NF-κB/MAPK signaling in scopolamine-induced amnesic mice model. Biomed. Pharmacother. 2023;165 doi: 10.1016/j.biopha.2023.115106. [DOI] [PubMed] [Google Scholar]
- 24.Yang Y., García-Cruzado M., Zeng H., Camprubí-Ferrer L., Bahatyrevich-Kharitonik B., Bachiller S., Deierborg T. LPS priming before plaque deposition impedes microglial activation and restrains Aβ pathology in the 5xFAD mouse model of Alzheimer's disease. Brain Behav. Immun. 2023;113:228–247. doi: 10.1016/j.bbi.2023.07.006. [DOI] [PubMed] [Google Scholar]
- 25.Zhang J., He H., Qiao Y., Zhou T., He H., Yi S., Zhang L., Mo L., Li Y., Jiang W., You Z. Priming of microglia with IFN-γ impairs adult hippocampal neurogenesis and leads to depression-like behaviors and cognitive defects. Glia. 2020;68:2674–2692. doi: 10.1002/glia.23878. [DOI] [PubMed] [Google Scholar]
- 26.Zhang X., He Q., Huang T., Zhao N., Liang F., Xu B., Chen X., Li T., Bi J. Treadmill Exercise Decreases Aβ Deposition and Counteracts Cognitive Decline in APP/PS1 Mice, Possibly via Hippocampal Microglia Modifications. Front. Aging Neurosci. 2019;11:78. doi: 10.3389/fnagi.2019.00078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Geraghty A.C., Gibson E.M., Ghanem R.A., Greene J.J., Ocampo A., Goldstein A.K., Ni L., Yang T., Marton R.M., Paşca S.P., et al. Loss of Adaptive Myelination Contributes to Methotrexate Chemotherapy-Related Cognitive Impairment. Neuron. 2019;103:250–265.e8. doi: 10.1016/j.neuron.2019.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gibson E.M., Nagaraja S., Ocampo A., Tam L.T., Wood L.S., Pallegar P.N., Greene J.J., Geraghty A.C., Goldstein A.K., Ni L., et al. Methotrexate Chemotherapy Induces Persistent Tri-glial Dysregulation that Underlies Chemotherapy-Related Cognitive Impairment. Cell. 2019;176:43–55.e13. doi: 10.1016/j.cell.2018.10.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zheng H., Webster M.J., Weickert C.S., Beasley C.L., Paulus M.P., Yolken R.H., Savitz J. Cytomegalovirus antibodies are associated with mood disorders, suicide, markers of neuroinflammation, and microglia activation in postmortem brain samples. Mol. Psychiatry. 2023;28:5282–5292. doi: 10.1038/s41380-023-02162-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sokol C.L., Luster A.D. The chemokine system in innate immunity. Cold Spring Harb. Perspect. Biol. 2015;7 doi: 10.1101/cshperspect.a016303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rege S.V., Teichert A., Masumi J., Dhande O.S., Harish R., Higgins B.W., Lopez Y., Akrapongpisak L., Hackbart H., Caryotakis S., et al. CCR3 plays a role in murine age-related cognitive changes and T-cell infiltration into the brain. Commun. Biol. 2023;6:292. doi: 10.1038/s42003-023-04665-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang Y., Chen K., Sloan S.A., Bennett M.L., Scholze A.R., O'Keeffe S., Phatnani H.P., Guarnieri P., Caneda C., Ruderisch N., et al. An RNA-sequencing transcriptome and splicing database of glia, neurons, and vascular cells of the cerebral cortex. J. Neurosci. 2014;34:11929–11947. doi: 10.1523/jneurosci.1860-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bennett M.L., Bennett F.C., Liddelow S.A., Ajami B., Zamanian J.L., Fernhoff N.B., Mulinyawe S.B., Bohlen C.J., Adil A., Tucker A., et al. New tools for studying microglia in the mouse and human CNS. Proc. Natl. Acad. Sci. USA. 2016;113:E1738–E1746. doi: 10.1073/pnas.1525528113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang Y., Sloan S.A., Clarke L.E., Caneda C., Plaza C.A., Blumenthal P.D., Vogel H., Steinberg G.K., Edwards M.S., Li G., et al. Purification and Characterization of Progenitor and Mature Human Astrocytes Reveals Transcriptional and Functional Differences with Mouse. Neuron. 2016;89:37–53. doi: 10.1016/j.neuron.2015.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Guttenplan K.A., Weigel M.K., Prakash P., Wijewardhane P.R., Hasel P., Rufen-Blanchette U., Münch A.E., Blum J.A., Fine J., Neal M.C., et al. Neurotoxic reactive astrocytes induce cell death via saturated lipids. Nature. 2021;599:102–107. doi: 10.1038/s41586-021-03960-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hasel P., Rose I.V.L., Sadick J.S., Kim R.D., Liddelow S.A. Neuroinflammatory astrocyte subtypes in the mouse brain. Nat. Neurosci. 2021;24:1475–1487. doi: 10.1038/s41593-021-00905-6. [DOI] [PubMed] [Google Scholar]
- 37.Sadick J.S., O'Dea M.R., Hasel P., Dykstra T., Faustin A., Liddelow S.A. Astrocytes and oligodendrocytes undergo subtype-specific transcriptional changes in Alzheimer's disease. Neuron. 2022;110:1788–1805.e1710. doi: 10.1016/j.neuron.2022.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhu L.P., Xu M.L., Yuan B.T., Ma L.J., Gao Y.J. Chemokine CCL7 mediates trigeminal neuropathic pain via CCR2/CCR3-ERK pathway in the trigeminal ganglion of mice. Mol. Pain. 2023;19 doi: 10.1177/17448069231169373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lawrence T., Natoli G. Transcriptional regulation of macrophage polarization: enabling diversity with identity. Nat. Rev. Immunol. 2011;11:750–761. doi: 10.1038/nri3088. [DOI] [PubMed] [Google Scholar]
- 40.Zhang J., Xie X., Tang M., Zhang J., Zhang B., Zhao Q., Han Y., Yan W., Peng C., You Z. Salvianolic acid B promotes microglial M2-polarization and rescues neurogenesis in stress-exposed mice. Brain Behav. Immun. 2017;66:111–124. doi: 10.1016/j.bbi.2017.07.012. [DOI] [PubMed] [Google Scholar]
- 41.Hsu C.H., Pan Y.J., Zheng Y.T., Lo R.Y., Yang F.Y. Ultrasound reduces inflammation by modulating M1/M2 polarization of microglia through STAT1/STAT6/PPARγ signaling pathways. CNS Neurosci. Ther. 2023;29:4113–4123. doi: 10.1111/cns.14333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pourhashemi S.F., Sahraei H., Meftahi G.H., Hatef B., Gholipour B. The Effect of 20 Minutes Scuba Diving on Cognitive Function of Professional Scuba Divers. Asian J. Sports Med. 2016;7 doi: 10.5812/asjsm.38633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lazarus R., Deese J., Osler S.F. The effects of psychological stress upon performance. Psychol. Bull. 1952;49:293–317. doi: 10.1037/h0061145. [DOI] [PubMed] [Google Scholar]
- 44.Davey C.P. Physical exertion and mental performance. Ergonomics. 1973;16:595–599. doi: 10.1080/00140137308924550. [DOI] [PubMed] [Google Scholar]
- 45.Yerkes R.M., Dodson J.D. The relation of strength of stimulus to rapidity of habit-formation. J. Comp. Neurol. Psychol. 1908;18:459–482. [Google Scholar]
- 46.Kashihara K., Maruyama T., Murota M., Nakahara Y. Positive effects of acute and moderate physical exercise on cognitive function. J. Physiol. Anthropol. 2009;28:155–164. doi: 10.2114/jpa2.28.155. [DOI] [PubMed] [Google Scholar]
- 47.Möller F., Hoffmann U., Dalecki M., Dräger T., Doppelmayr M., Steinberg F. Physical Exercise Intensity During Submersion Selectively Affects Executive Functions. Hum. Factors. 2021;63:227–239. doi: 10.1177/0018720819879313. [DOI] [PubMed] [Google Scholar]
- 48.Li L., Men W.W., Chang Y.K., Fan M.X., Ji L., Wei G.X. Acute aerobic exercise increases cortical activity during working memory: a functional MRI study in female college students. PLoS One. 2014;9 doi: 10.1371/journal.pone.0099222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pontifex M.B., Hillman C.H., Fernhall B., Thompson K.M., Valentini T.A. The effect of acute aerobic and resistance exercise on working memory. Med. Sci. Sports Exerc. 2009;41:927–934. doi: 10.1249/MSS.0b013e3181907d69. [DOI] [PubMed] [Google Scholar]
- 50.Ge C., Chen W., Zhang L., Ai Y., Zou Y., Peng Q. Chemogenetic activation of the HPC-mPFC pathway improves cognitive dysfunction in lipopolysaccharide -induced brain injury. Theranostics. 2023;13:2946–2961. doi: 10.7150/thno.82889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Qiu L.L., Pan W., Luo D., Zhang G.F., Zhou Z.Q., Sun X.Y., Yang J.J., Ji M.H. Dysregulation of BDNF/TrkB signaling mediated by NMDAR/Ca(2+)/calpain might contribute to postoperative cognitive dysfunction in aging mice. J. Neuroinflammation. 2020;17:23. doi: 10.1186/s12974-019-1695-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Li Z., Wang H., Xiao G., Du H., He S., Feng Y., Zhang B., Zhu Y. Recovery of post-stroke cognitive and motor deficiencies by Shuxuening injection via regulating hippocampal BDNF-mediated Neurotrophin/Trk Signaling. Biomed. Pharmacother. 2021;141 doi: 10.1016/j.biopha.2021.111828. [DOI] [PubMed] [Google Scholar]
- 53.Wang L.Y., Wang X.P., Lv J.M., Shan Y.D., Jia S.Y., Yu Z.F., Miao H.T., Xin Y., Zhang D.X., Zhang L.M. NLRP3-GABA signaling pathway contributes to the pathogenesis of impulsive-like behaviors and cognitive deficits in aged mice. J. Neuroinflammation. 2023;20:162. doi: 10.1186/s12974-023-02845-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Xu J., Yang C., Zeng S., Wang X., Yang P., Qin L. Disturbance of neuron-microglia crosstalk mediated by GRP78 in Neuropsychiatric systemic lupus erythematosus mice. J. Neuroinflammation. 2023;20:150. doi: 10.1186/s12974-023-02832-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shou J., Peng J., Zhao Z., Huang X., Li H., Li L., Gao X., Xing Y., Liu H. CCL26 and CCR3 are associated with the acute inflammatory response in the CNS in experimental autoimmune encephalomyelitis. J. Neuroimmunol. 2019;333 doi: 10.1016/j.jneuroim.2019.576967. [DOI] [PubMed] [Google Scholar]
- 56.Zhu C., Xu B., Sun X., Zhu Q., Sui Y. Targeting CCR3 to Reduce Amyloid-β Production, Tau Hyperphosphorylation, and Synaptic Loss in a Mouse Model of Alzheimer's Disease. Mol. Neurobiol. 2017;54:7964–7978. doi: 10.1007/s12035-016-0269-5. [DOI] [PubMed] [Google Scholar]
- 57.Ahmad M.A., Kareem O., Khushtar M., Akbar M., Haque M.R., Iqubal A., Haider M.F., Pottoo F.H., Abdulla F.S., Al-Haidar M.B., Alhajri N. Neuroinflammation: A Potential Risk for Dementia. Int. J. Mol. Sci. 2022;23 doi: 10.3390/ijms23020616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.van der Meer P., Ulrich A.M., Gonźalez-Scarano F., Lavi E. Immunohistochemical analysis of CCR2, CCR3, CCR5, and CXCR4 in the human brain: potential mechanisms for HIV dementia. Exp. Mol. Pathol. 2000;69:192–201. doi: 10.1006/exmp.2000.2336. [DOI] [PubMed] [Google Scholar]
- 59.He J., Chen Y., Farzan M., Choe H., Ohagen A., Gartner S., Busciglio J., Yang X., Hofmann W., Newman W., et al. CCR3 and CCR5 are co-receptors for HIV-1 infection of microglia. Nature. 1997;385:645–649. doi: 10.1038/385645a0. [DOI] [PubMed] [Google Scholar]
- 60.Agrawal L., Maxwell C.R., Peters P.J., Clapham P.R., Liu S.M., Mackay C.R., Strayer D.S. Complexity in human immunodeficiency virus type 1 (HIV-1) co-receptor usage: roles of CCR3 and CCR5 in HIV-1 infection of monocyte-derived macrophages and brain microglia. J. Gen. Virol. 2009;90:710–722. doi: 10.1099/vir.0.006205-0. [DOI] [PubMed] [Google Scholar]
- 61.Bivona G., Iemmolo M., Agnello L., Lo Sasso B., Gambino C.M., Giglio R.V., Scazzone C., Ghersi G., Ciaccio M. Microglial Activation and Priming in Alzheimer's Disease: State of the Art and Future Perspectives. Int. J. Mol. Sci. 2023;24 doi: 10.3390/ijms24010884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Fan Z., Zhang W., Cao Q., Zou L., Fan X., Qi C., Yan Y., Song B., Wu B. JAK2/STAT3 pathway regulates microglia polarization involved in hippocampal inflammatory damage due to acute paraquat exposure. Ecotoxicol. Environ. Saf. 2022;234 doi: 10.1016/j.ecoenv.2022.113372. [DOI] [PubMed] [Google Scholar]
- 63.Hooten K.G., Beers D.R., Zhao W., Appel S.H. Protective and Toxic Neuroinflammation in Amyotrophic Lateral Sclerosis. Neurotherapeutics. 2015;12:364–375. doi: 10.1007/s13311-014-0329-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Phillips C. Brain-Derived Neurotrophic Factor, Depression, and Physical Activity: Making the Neuroplastic Connection. Neural Plast. 2017;2017 doi: 10.1155/2017/7260130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Luo H., Zhang C., He L., Lin Z., Zhang J.C., Qi Q., Chen J.X., Yao W. 18β-glycyrrhetinic acid ameliorates MPTP-induced neurotoxicity in mice through activation of microglial anti-inflammatory phenotype. Psychopharmacology (Berl) 2023;240:1947–1961. doi: 10.1007/s00213-023-06415-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
-
•
Raw RNAseq data have been deposited at NCBI and are publicly available. Accession number is listed in the key resources table. All data reported in this paper will be shared by the lead contact upon reasonable request.
-
•
This paper does not report original code.
-
•
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.