Abstract
AIM
To describe the surgical procedure of fusiform penetrating keratoplasty (FPK) using multiple trephines of different sizes for treating patients with severe infectious keratitis.
METHODS
Fourteen eyes underwent FPK, and 15 eyes received conventional penetrating keratoplasty (PK) were included in the study. The best-corrected visual acuity (BCVA), refractive outcomes, endothelial cell density, and postoperative complications were recorded.
RESULTS
The FPK group was followed for an average of 15.3±2.1mo, whereas the PK group was followed for 16.1±1.9mo. The corneal ulcers were elliptical-shaped in all 14 eyes in the FPK group. The mean BCVA (logMAR, 0.26±0.13) showed no statistically significant differences from that in the PK group (logMAR, 0.21±0.12, P>0.05) at 1y after surgery. But the mean curvature, mean astigmatism, and mean spherical equivalent in the FPK group were lower than those in the PK group (P<0.05). Peripheral anterior synechia was observed in one patient in the FPK group, whereas 6 patients in the PK group. Suture loosening and neovascularization were observed in 4 and 5 eyes in the PK group, respectively. No graft immune rejection or elevation of intraocular pressure was observed in the two groups.
CONCLUSION
For patients with elliptical-shaped corneas or corneal ulcers, FPK can avoid disrupting of corneal limbus, reduce the risk of postoperative complications, and can result in satisfactory visual quality.
Keywords: fusiform penetrating keratoplasty, multiple trephines, infectious keratitis, cornea
INTRODUCTION
In current standard penetrating keratoplasty (PK), the graft is circular and is generally located centrally in the cornea, away from the vascular-rich corneal limbus[1]–[3]. In practice, however, the human cornea has a horizontally elliptical shape with a decreased vertical width of the clear area by a vascularized pannus. Given this configuration, conventional round corneal transplantation may inevitably disrupt the corneal limbus, and consequently, the corneal graft which is sutured to the corneal limbus may be at an increased risk of immune rejection. In the case where the corneal lesion is oval in shape, for example, the long diameter of the lesion is 9 mm and the short diameter is only 7 mm, in order to completely remove the diseased tissue, a circular trephine with a diameter of about 10 mm is usually used. However, this will result in a large area of corneal tissue being cut, which may involve or even destroy the limbus. Therefore, researchers have attempted to perform fusiform corneal transplantation that conforms to the morphology of corneal lesions. Initially, researchers used excimer laser to perform the surgery while avoiding the corneal limbus site[4]–[7]. However, the excimer laser cannot be applied to patients with infectious keratitis. In order to address this, we innovatively used multiple trephines of different sizes to create fusiform corneal grafts, and then created matching recipient beds with the assistance of an artificial anterior chamber for the treatment of patients with severe infectious keratitis, achieving good surgical results.
SUBJECTS AND METHODS
Ethical Approval
This retrospective study was approved by the Ethics Committee of Eye Hospital of Shandong First Medical University (No.SDSYKYY201805-1). This study was conducted in compliance with the tenets of the Declaration of Helsinki. Each patient gave written informed consent to participate in this research after the risks and possible adverse consequences had been explained.
Subjects
A consecutive series of 14 eyes (14 patients) underwent fusiform penetrating keratoplasty (FPK) for infectious keratitis at the Eye Hospital of Shandong First Medical University from June 2018 to June 2019. The inclusion criteria were as follows: 1) patients were suggested to undergo penetrating keratoplasty (PK), with over 4/5 of the corneal thickness infected or infiltrated as observed by slit-lamp microscopy and anterior segment optical coherence tomography (AS-OCT), and anti-infectious medication as reported in our previous studies was given for at least 2wk but was ineffective; 2) the long diameter of the corneal ulcers is 1 mm larger than the short one; 3) completing a follow-up of at least 12mo. To compare the postoperative outcomes, 15 patients (15 eyes) who received conventional PK for infectious keratitis by the same surgeon (Gao H) were included in the PK group. In the meanwhile, the age, sex, diagnosis, and preoperative best-corrected visual acuity (BCVA, logMAR) of the 15 patients were comparable with those who underwent FPK.
Surgical Procedure
Preparation of recipient eye
All surgeries were performed under peribulbar anesthesia. First, a suitable trephine (Storz) was selected according to the size of the corneal ulcer, with the diameter of the trephine being 0.5–1 mm larger than the longest diameter of the ulcer. Then, two methylene blue marks were made with the ulcer included inside using the trephine, and the intersection of the two arcs was shown in a fusiform shape. Finally, remove the diseased cornea manually along the marks with a corneal scissor. The Castroviejo circle gauge was used to measure the longest and the shortest diameters of the fusiform recipient bed.
Preparation of donor tissue
The donor tissue was mounted onto an artificial anterior chamber, and Optisol corneal storage medium was injected to stabilize the pressure in the artificial anterior chamber. The longest and the shortest diameter of the graft were set to be 0.5 mm larger than those of the recipient bed. First, a Castroviejo circle gauge was used to mark the longest and the shortest diameter on the graft. Then, the trephine was used to make indentations according to the marked longest and shortest diameter. The intersection of the two indentations was also fusiform. The puncture knife was then used perpendicularly-oriented to the anterior chamber with the viscoelastic agent being injected to support the anterior chamber, and the fusiform graft being cut along the indentation using corneal scissors.
Having prepared the recipient bed and the graft, the surgeon performed conventional PK, and the detailed surgical procedure was introduced in our previous report[1],[8]. The donor cornea was sutured to the recipient bed with 16–24 interrupted 10/0 nylon sutures (Figure 1).
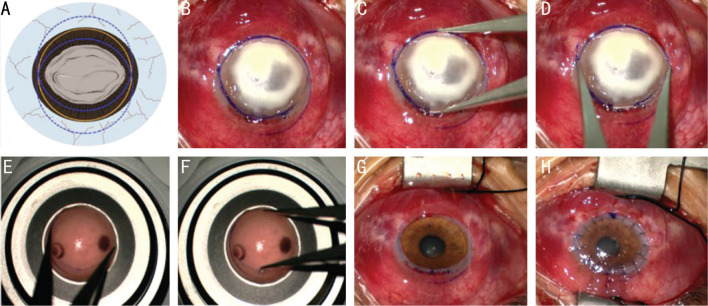
Figure 1. The diagram and surgical procedures of FPK.
A, B: Picture A is the diagram of picture B. A suitable trephine (the yellow circle) is larger than the longest diameter of the ulcer. Two methylene blue marks (the solid lines of the two blue circle) were made with the ulcer included inside using the trephine, and the intersection of the two arcs was shown in a fusiform shape. C, D: The Castroviejo circle gauge was used to measure the longest and the shortest diameter of the fusiform recipient bed. E, F: The Castroviejo circle gauge was used to mark the longest and the shortest diameter of the graft, then a fusiform graft was cut along the indentation using corneal scissors. G: The diseased cornea was dissected along the indentation of the recipient bed. H: The donor cornea was sutured to the recipient bed with 16 interrupted 10/0 nylon sutures. FPK: Fusiform penetrating keratoplasty.
Postoperative Treatment
Anti-infective treatment was administered based on the preoperative or intraoperative pathology results according to our previous report[8]–[13]. In patients with fungal and Acanthamoeba corneal ulcers, glucocorticoids were not used for 3wk after surgery[14]–[15]. Afterwards, 0.1% fluorometholone was used 4 times daily for 6mo and tapered to 0.02% fluorometholone three times daily for at least 1y, tobramycin and dexamethasone ophthalmic ointment was administered every night for 6mo and tapered to twice weekly. The 0.1% tacrolimus eye drops were given four times a day after surgery for 1mo and tapered to three times for 6mo and twice for at least 1y.
Main Outcome Measures
All patients were observed daily during the first week after surgery, weekly during the next 2mo, and monthly thereafter. All sutures were removed at 1y after surgery. BCVA, intraocular pressure, healing time of the epithelium, corneal astigmatism, spherical equivalent, mean corneal curvature, endothelial cell density, and perioperative complications were evaluated 1y after surgery and 3mo after suture removal.
Statistical Analysis
Statistical analysis was performed using SPSS 24.0, with the significance level set at P<0.05. The Kolmogorov-Smirnow test was used to check the normal distribution of data. The two-sample t test was used to analyze independent continuous variables, and the Mann-Whitney U test was instead for non-normally distributed continuous variables. Visual acuity was recorded in international standard eye chart and converted to logMAR for statistical analysis. The related-samples Wilcoxon signed-rank test was used to evaluate the postoperative improvement in BCVA. The Chi-square test and Fisher exact test were used to analyze categorical variables. The graft survival rates were analysed with the Kaplan-Meier survival curves, and the differences between groups were compared using the log-rank test.
RESULTS
Patient Information
The FPK group was followed for an average of 15.3±2.1mo, whereas the PK group was followed for an average of 16.1±1.9mo. The intergroup comparisons of age, sex, diagnosis, and preoperative BCVA showed no significant differences (Table 1).
Table 1. Preoperative basic information of patients.
| Parameter | FPK group (n=14) | PK group (n=15) | t/χ2 | P |
| Age (y) | 54.9±13.7 | 56.3±10.9 | 0.292 | 0.77 |
| Sex (male/female) | 4/10 | 3/12 | 0.281a | 0.61 |
| Diagnoses | 0.492a | |||
| Bacterial keratitis | 6 (42.9) | 5 (33.3) | - | 0.611 |
| Fungal keratitis | 5 (35.7) | 6 (40.0) | - | 0.53 |
| Herpes simplex keratitis | 2 (14.3) | 4 (26.7) | - | 0.81 |
| Acanthamoeba keratitis | 1 (7.14) | 0 | - | 0.30 |
| Preop. BCVA (logMAR) | 2.23±0.40 | 2.36±0.20 | 0.592a | 0.29 |
| Hypopyon | 7 (50.0) | 11 (73.3) | 0.421a | 0.09 |
| Depth of hypopyon (mm) | 1.92±1.19 | 2.40±1.40 | 0.329a | 0.345 |
PK: Penetrating keratoplasty; FPK: Fusiform penetrating keratoplasty; BCVA: Best corrected visual acuity. aFisher's exact test; Others: t test.
n (%)
In the FPK group, the primary diagnoses included bacterial corneal ulcers in 6 eyes, fungal corneal ulcers in 5 eyes, herpes simplex keratitis in 2 eyes, and Acanthamoeba keratitis in 1 eye. The corneal ulcers were elliptical-shaped in all 14 eyes, the long and short diameters measured by Castroviejo circle gauge were 7 mm×5 mm in 5 eyes, and 9 mm×7 mm in 9 eyes. Hypopyon was present in 7 eyes. The diameter of corneal recipient bed/graft included 7.5/8.0 mm in 5 eyes, and 10/10.5 mm in 9 eyes (Table 1).
The primary diagnoses included bacterial corneal ulcers in 5 eyes, fungal corneal ulcers in 6 eyes, and herpes simplex keratitis in 4 eyes in the PK groups. The size of corneal ulcers included 8 mm×8 mm in 7 eyes, and 10 mm×10 mm in 8 eyes. The diameter of corneal recipient bed/graft included 8.5/9.0 in 7 eyes, and 10.5/11.0 in 8 eyes (Table 1).
Visual Acuity and Refractive Outcomes
The mean healing time of the epithelium was 4.3±3.4d and 4.5±2.1d in the FPK and PK groups, respectively (P>0.05).
At 1y after surgery, all eyes maintained a clear graft in the two groups (Figure 2). The mean BCVA (logMAR) was 0.26±0.13 in the FPK group, which showed no statistically significant differences from that in the PK group, which was 0.21±0.12 (P=0.110; Figure 3). The mean curvature was 43.3±3.4 and 40.6±5.7 D in the FPK and PK groups, respectively (P=0.032). The mean astigmatism and mean spherical equivalent in the FPK group were lower than those in the PK group (P<0.05; Table 2).
Figure 2. Slit-lamp and ultrasonic biomicroscopy examination of the FPK group and PK group.
A: Preoperative slit-lamp photograph showed a patient with Acanthamoeba keratitis; B: A clear graft was observed at 1y after FPK; C: Ultrasonic biomicroscopy showed peripheral anterior angle is open; D: Preoperative slit-lamp photograph showed a patient with bacterial keratitis; E: Corneal neovascularization was observed at 1y after PK; F: Extensive peripheral anterior synechia was observed by ultrasonic biomicroscopy. PK: Penetrating keratoplasty; FPK: Fusiform penetrating keratoplasty.
Figure 3. Cumulative BCVA (logMAR) of the FPK group and PK group.
A: Patients' cumulative BCVA preoperative, 1y after surgery and 3mo after suture removal in the FPK group; B: Patients' cumulative BCVA preoperative, 1y after surgery and 3mo after suture removal in the PK group. PK: Penetrating keratoplasty; FPK: Fusiform penetrating keratoplasty; BCVA: Best corrected visual acuity.
Table 2. Postoperative refractive status of the FPK and PK groups.
| Parameters | 1y after surgery |
3mo after suture removal |
||||||
| FPK group | PK group | t | P | FPK group | PK group | t | P | |
| BCVA (logMAR) | 0.26±0.13 | 0.21±0.12 | 4.750 | 0.110 | 0.28±0.10 | 0.23±0.12 | 4.158 | 0.145 |
| Curvature (D) | 43.3±3.4 | 40.6±5.7 | 6.815 | 0.032 | 43.6±2.1 | 40.8±2.3 | 7.054 | 0.028 |
| Astigmatism (D) | 2.01±1.3 | 4.08±1.3 | 7.401 | 0.026 | 2.28±1.1 | 4.21±1.6 | 8.062 | 0.022 |
| Equivalent spherical radius | 2.20±1.1 | 4.15±0.8 | 5.615 | 0.043 | 2.83±1.1 | 3.96±1.2 | 6.172 | 0.038 |
FPK: Fusiform penetrating keratoplasty; PK: Penetrating keratoplasty; BCVA: Best corrected visual acuity.
mean±SD
At 3mo after suture removal, the mean BCVA (logMAR) was 0.28±0.10 in the FPK group, which showed no statistically significant differences from that in the PK group, which was 0.23±0.12 (P=0.145; Figure 3). The mean curvature was 43.6±2.1 and 40.8±2.3 D in the FPK and PK groups, respectively (P=0.028). The mean astigmatism and mean spherical equivalent in the FPK group were lower than those in the PK group (P<0.05; Table 2).
Intraocular Pressure
No elevation of intraocular pressure was detected during the follow-up in the two groups.
Loss of Corneal Endothelial Cells
The mean endothelial cell density was 1783±165 cells per square millimeter at 1y after surgery in the FPK group, with no statistically significant differences compared with the density in the PK group, which was 1801±197 cells per square millimeter (P>0.05; Figure 4).
Figure 4. Attenuation of ECD in the FPK and PK group.

ECD: Endothelial cell density; PK: Penetrating keratoplasty; FPK: Fusiform penetrating keratoplasty.
Ultrasonic Biomicroscopy
Ultrasonic biomicroscopy was used to examine the structures of the anterior chamber angle after keratoplasty. Peripheral anterior synechia was observed in one patient in the FPK group, whereas 6 patients in the PK group (Figure 2).
Intraoperative Complications
No cases of hyphema, iris damage, and lens dislocation were observed during the surgery in the two groups.
Postoperative Complications
No cases of disease recurrence, or graft immune rejection were observed during the follow-up period in the two groups. In the PK group, suture loosening and neovascularization were observed in 4 and 5 eyes, respectively (Figure 2), while no eyes in the FPK group.
DISCUSSION
Under normal circumstances, the horizontal diameter of the cornea is 1 mm larger than the vertical diameter. For patients with total corneal infection or corneal ulcers with large diameters and elliptical shapes, the corneal limbus is bound to be involved if it is trephined with a conventional circular trephine. Due to the rich vascular and lymphatic network in the corneal limbus, the closer the corneal graft is to the corneal limbus, the higher the chance of postoperative immune rejection[16]–[19]. In addition, cutting and suturing at the corneal limbus site not only easily causes intraoperative bleeding, affecting the surgical field and increasing the difficulty of surgery, but it also destroys the structure of anterior chamber angle, increasing the risk of postoperative anterior synechia and secondary glaucoma. In order to address these issues, we proposed the method of manual fusiform corneal transplantation using multiple trephines of different sizes, thus decreasing the risks for corneal limbus destruction secondary to surgery, postoperative secondary glaucoma, and immune rejection.
The core method of FPK is to use an appropriately sized circular trephine to indent each side of the long axis of the corneal ulcer, with the intersection of the two indentations taking on a fusiform shape. The key point is to choose a trephine diameter that is 0.5–1 mm longer than the long diameter of the ulcer, thus ensuring complete coverage of the ulcer area. After the indentation, the Castroviejo gauge is used to measure the long and short diameters of the recipient bed, and then a fusiform-shaped graft is created, with both the long and short diameters of the graft requiring to be 0.5 mm longer than the recipient bed. After the graft is successfully created, the diseased cornea is removed, thus reducing the time of the open-skylight procedure and reducing the risk of prolapse of the ocular contents[20]–[22]. Other surgical procedures were the same as PK with no increase in surgical difficulty, and the mean endothelial cell density was 1783±165 cells per square millimeter at 1y after surgery, with no statistically significant differences compared with the density in the PK group which was 1801±197 cells per square millimeter (P>0.05). The consumables in this study included the routinely applied Storz trephines and an artificial anterior chamber, which did not impose additional financial burden on the patients, making this procedure suitable for clinical promotion and application[23]–[24].
In the PK group, because the corneal limbus was rich in blood vessels, the sutures closed at the corneal limbus were prone to loosening and tended to be tight, resulting in a mean astigmatism and mean spherical equivalent higher than that of the FPK group postoperatively. The visual quality was subsequently affected, despite the fact that the mean BCVA (logMAR) showed no statistically significant differences between the two groups. In addition, the sutures at the corneal limbus of five eyes had signs of inward neovascularization, which would increase the risk of immune rejection. In addition, trephining the cornea at the limbus could easily damage the structure of the chamber angle and lead to anterior synechia after surgery[25]. Ultrasonic biomicroscopy found that six patients in the PK group had anterior synechia[26]–[27], while only one patient in the FPK group had anterior synechia. Although no elevation of intraocular pressures was detected during the follow-up, peripheral anterior synechia was found to be a high-risk factor for the development of glaucoma after PK[28]–[30], which requires long-term follow-up and observation.
Despite these findings, this study had shortcomings including a small number of enrolled patients and short follow-up times, which may affect the comparison of incidences of secondary glaucoma and immune rejection between the two groups. In addition, when preparing the implant bed during the operation, first of all, the surgeon selects a trephine with appropriate diameter according to the size of the lesion, then marks it, and finally cuts it. Follow the same steps to prepare grafts. Compared with conventional circular keratoplasty, the operation time may be longer, which may be a waste of time for new surgeons.
In patients with elliptical-shaped corneas or corneal ulcers, FPK using multiple trephines of different sizes can effectively avoid disruption of the corneal limbus by trephination, with minimal postoperative astigmatism and satisfactory visual quality, while reducing the risk of postoperative complications such as secondary glaucoma, suture loosening, suture neovascularization, and immune rejection.
Footnotes
We sincerely thank our colleagues at Eye Hospital of Shandong First Medical University for their valuable discussions and help. We thank Tong Liu, Eye Hospital of Shandong First Medical University, for her linguistic assistance.
Foundations: Supported by the National Natural Science Foundation of China (No.81900907; No.81870639); Taishan Scholar Program (No.20231255).
Conflicts of Interest: Qi XL, None; Wang LC, None; Wang ML, None; Gao H, None.
REFERENCES
- 1.Gao H, Huang T, Pan ZQ, et al. Survey report on keratoplasty in China: a 5-year review from 2014 to 2018. PLoS One. 2020;15(10):e0239939. doi: 10.1371/journal.pone.0239939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Galvis V, Tello A, Laiton AN, Salcedo SLL. Indications and techniques of corneal transplantation in a referral center in Colombia, South America (2012–2016) Int Ophthalmol. 2019;39(8):1723–1733. doi: 10.1007/s10792-018-0994-z. [DOI] [PubMed] [Google Scholar]
- 3.Pluzsik MT, Seitz B, Flockerzi FA, Langenbucher A, Tóth G, Bohle RM, Szentmáry N. Changing trends in penetrating keratoplasty indications between 2011 and 2018—histopathology of 2123 corneal buttons in a single center in Germany. Curr Eye Res. 2020;45(10):1199–1204. doi: 10.1080/02713683.2020.1737716. [DOI] [PubMed] [Google Scholar]
- 4.Szentmáry N, Langenbucher A, Kus MM, Naumann GOH, Seitz B. Long-term refractive results of elliptical excimer laser penetrating keratoplasty (EELPK) Curr Eye Res. 2007;32(11):953–959. doi: 10.1080/02713680701689781. [DOI] [PubMed] [Google Scholar]
- 5.Lang GK, Naumann GO, Koch JW. A new elliptical excision for corneal transplantation using an excimer laser. Arch Ophthalmol. 1990;108(7):914–915. doi: 10.1001/archopht.1990.01070090016004. [DOI] [PubMed] [Google Scholar]
- 6.Alió JL, Agdeppa MCC, Uceda-Montanes A. Femtosecond laser-assisted superficial lamellar keratectomy for the treatment of superficial corneal leukomas. Cornea. 2011;30(3):301–307. doi: 10.1097/ICO.0b013e3181eeb0c1. [DOI] [PubMed] [Google Scholar]
- 7.Lang GK, Schroeder E, Koch JW, Yanoff M, Naumann GO. Excimer laser keratoplasty. Part 2: elliptical keratoplasty. Ophthalmic Surg. 1989;20(5):342–346. [PubMed] [Google Scholar]
- 8.Chatterjee S, Agrawal D. Recurrence of infection in corneal grafts after therapeutic penetrating keratoplasty for microbial keratitis. Cornea. 2020;39(1):39–44. doi: 10.1097/ICO.0000000000002044. [DOI] [PubMed] [Google Scholar]
- 9.Roy A, Kamra D, Murthy SI, Mohamed A, Chaurasia S, Fernandes M, Das S, Sharma S. Intermediate outcomes of therapeutic penetrating keratoplasty for severe microbial keratitis using glycerol-preserved donor corneas during the COVID-19 pandemic. Indian J Ophthalmol. 2021;69(10):2812–2817. doi: 10.4103/ijo.IJO_1183_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang T, Xie LX, Dong YL, Cheng J. Therapeutic keratoplasty for severe Acanthamoeba keratitis: risk factors, clinical features, and outcomes of postoperative recurrence. Graefes Arch Clin Exp Ophthalmol. 2023;261(5):1299–1309. doi: 10.1007/s00417-022-05883-4. [DOI] [PubMed] [Google Scholar]
- 11.Chen XN, Li XF, Zhang XY, Guo XT, Qi XL, Li SX, Shi WY, Gao H. Comparison of complications and visual outcomes between big-bubble deep anterior lamellar keratoplasty and penetrating keratoplasty for fungal keratitis. Clin Exp Ophthalmol. 2021;49(6):550–559. doi: 10.1111/ceo.13951. [DOI] [PubMed] [Google Scholar]
- 12.Friehmann A, Myerscough J, Giannaccare G, Mazzoni M, Bovone C, Busin M. Successful descemet membrane endothelial keratoplasty in proven herpetic endothelial decompensation requires intensive antiviral therapy. Cornea. 2020;39(2):196–199. doi: 10.1097/ICO.0000000000002215. [DOI] [PubMed] [Google Scholar]
- 13.Gurnani B, Kaur K, Lalgudi VG, Tripathy K. Risk factors for descemet membrane endothelial keratoplasty rejection: current perspectives-systematic review. Clin Ophthalmol. 2023;17:421–440. doi: 10.2147/OPTH.S398418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang T, Li SX, Gao H, Shi WY. Therapeutic dilemma in fungal keratitis: administration of steroids for immune rejection early after keratoplasty. Graefes Arch Clin Exp Ophthalmol. 2016;254(8):1585–1589. doi: 10.1007/s00417-016-3412-0. [DOI] [PubMed] [Google Scholar]
- 15.Hos D, Matthaei M, Bock F, et al. Immune reactions after modern lamellar (DALK, DSAEK, DMEK) versus conventional penetrating corneal transplantation. Prog Retin Eye Res. 2019;73:100768. doi: 10.1016/j.preteyeres.2019.07.001. [DOI] [PubMed] [Google Scholar]
- 16.Kiel M, Bu JB, Gericke A, Vossmerbaeumer U, Schuster AK, Pfeiffer N, Wasielica-Poslednik J. Comparison of DMEK and DSAEK in eyes with endothelial decompensation after previous penetrating keratoplasty. Cornea. 2021;40(9):1218–1224. doi: 10.1097/ICO.0000000000002786. [DOI] [PubMed] [Google Scholar]
- 17.Li SQ, Li M, Gu L, Peng LL, Deng YQ, Zhong J, Wang BW, Wang Q, Xiao YC, Yuan J. Risk factors influencing survival of acellular porcine corneal stroma in infectious keratitis: a prospective clinical study. J Transl Med. 2019;17(1):434. doi: 10.1186/s12967-019-02192-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li SQ, Deng YQ, Tian BS, Huang HX, Zhang HN, Yang RH, Zhong J, Wang BW, Peng LL, Yuan J. Healing characteristics of acellular porcine corneal stroma following therapeutic keratoplasty. Xenotransplantation. 2020;27(2):e12566. doi: 10.1111/xen.12566. [DOI] [PubMed] [Google Scholar]
- 19.Li SX, Li L, Zhou QJ, Gao H, Liu MN, Shi WY. Blood vessels and lymphatic vessels in the cornea and iris after penetrating keratoplasty. Cornea. 2019;38(6):742–747. doi: 10.1097/ICO.0000000000001922. [DOI] [PubMed] [Google Scholar]
- 20.Bafna RK, Kalra N, Asif MI, Agarwal R, Lata SM, Titiyal JS, Sharma N, Vikas SJ. Novel technique of tetra trephination for elliptical-shaped tectonic patch grafts in peripheral sterile keratolysis. Eur J Ophthalmol. 2021;31(5):2769–2775. doi: 10.1177/1120672121998955. [DOI] [PubMed] [Google Scholar]
- 21.Kate A, Vyas S, Bafna RK, Sharma N, Basu SY. Tenon's patch graft: a review of indications, surgical technique, outcomes and complications. Semin Ophthalmol. 2022;37(4):462–470. doi: 10.1080/08820538.2021.2017470. [DOI] [PubMed] [Google Scholar]
- 22.Kusumesh R, Ambastha A, Singh A, Kumari D, Mohan N, Sinha BP, Arya LK. Clinical outcome and course of Tenon's patch graft in corneal perforation and descemetocele. Indian J Ophthalmol. 2022;70(12):4257–4262. doi: 10.4103/ijo.IJO_1279_22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang HO, Jhanji V, Ye C, Ren YP, Zheng QX, Li JY, Zhao ZL, Chen W. Elliptical deep anterior lamellar keratoplasty in severe Acanthamoeba keratitis. Indian J Ophthalmol. 2023;71(3):999–1004. doi: 10.4103/ijo.IJO_1018_22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Di Zazzo A, Varacalli G, De Gregorio C, Coassin M, Bonini S. Therapeutic corneal transplantation in acanthamoeba keratitis: penetrating versus lamellar keratoplasty. Cornea. 2022;41(3):396–401. doi: 10.1097/ICO.0000000000002880. [DOI] [PubMed] [Google Scholar]
- 25.Maier AK, Gundlach E, Gonnermann J, Klamann MK, Eulufi C, Joussen AM, Bertelmann E, Rieck P, Torun N. Anterior segment analysis and intraocular pressure elevation after penetrating keratoplasty and posterior lamellar endothelial keratoplasty. Ophthalmic Res. 2015;53(1):36–47. doi: 10.1159/000365252. [DOI] [PubMed] [Google Scholar]
- 26.Yang YJ, Xiang J, Xu JJ. Anterior synechiae after penetrating keratoplasty in infants and children with Peters' anomaly. BMC Ophthalmol. 2022;22(1):259. doi: 10.1186/s12886-022-02473-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rajagopal RN, Fernandes M. Peters anomaly: novel non-invasive alternatives to penetrating keratoplasty. Semin Ophthalmol. 2023;38(3):275–282. doi: 10.1080/08820538.2023.2176238. [DOI] [PubMed] [Google Scholar]
- 28.Chen JL, Elhusseiny AM, Khodeiry MM, et al. Clinical factors impacting outcomes from failed trabeculectomy leading to glaucoma drainage device implantation and subsequent penetrating keratoplasty. J Glaucoma. 2023;32(9):800–806. doi: 10.1097/IJG.0000000000002239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Karadag O, Kugu S, Erdogan G, Kandemir B, Eraslan Ozdil S, Dogan OK. Incidence of and risk factors for increased intraocular pressure after penetrating keratoplasty. Cornea. 2010;29(3):278–282. doi: 10.1097/ICO.0b013e3181b6eb9e. [DOI] [PubMed] [Google Scholar]
- 30.Wang P, Gao QQ, Su GY, Wang W, Xu LJ, Li GG. Risk factors of urrets-zavalia syndrome after penetrating keratoplasty. J Clin Med. 2022;11(5):1175. doi: 10.3390/jcm11051175. [DOI] [PMC free article] [PubMed] [Google Scholar]