Abstract
Retinal degenerative diseases were a large group of diseases characterized by the primary death of retinal ganglion cells (RGCs). Recent studies had shown an interaction between autophagy and nucleotide-binding oligomerization domain-like receptor 3 (NLRP3) inflammasomes, which may affect RGCs in retinal degenerative diseases. The NLRP3 inflammasome was a protein complex that, upon activation, produces caspase-1, mediating the apoptosis of retinal cells and promoting the occurrence and development of retinal degenerative diseases. Upregulated autophagy could inhibit NLRP3 inflammasome activation, while inhibited autophagy can promote NLRP3 inflammasome activation, which leaded to the accelerated emergence of drusen and lipofuscin deposition under the neurosensory retina. The activated NLRP3 inflammasome could further inhibit autophagy, thus forming a vicious cycle that accelerated the damage and death of RGCs. This review discussed the relationship between NLRP3 inflammasome and autophagy and its effects on RGCs in age-related macular degeneration, providing a new perspective and direction for the treatment of retinal diseases.
Keywords: autophagy, age-related macular degeneration, NLRP3 inflammasome, retinal degeneration, retinal ganglion cells
INTRODUCTION
Inflammation is a defensive response initiated by the human body in response to external stimulation. Recent studies have demonstrated a close connection between inflammation and aging, suggesting that inflammation drives the development of many age-related diseases[1]. Neurodegenerative diseases, such as Alzheimer's disease, Parkinson's disease, muscular dystrophy, lateral sclerosis, and multiple sclerosis, were primarily characterized by the damage and loss of ganglion cells, which were triggered by the increased involvement of damaged DNA. This damage stimulated cells to produce inflammatory cascade reactions, ultimately leading to the degeneration and death of ganglion cells[2]. The retinal nerve fibers were the only neurons in the body that directly connect the outside world with the brain, transmitting light sensing signals to the brain to form visual sensations[3].
Retinal degeneration was a group of chronic fundus diseases, included age-related macular degeneration (AMD), characterized by the progressive loss of cells, such as retinal ganglion cells (RGCs), retinal pigment epithelial (RPE) cells, and structural damage to the retinal layers, ultimately leading to the loss of visual function[3]. Research suggested that chronic inflammation is an important initiating mechanism for the degeneration and death of RGCs[4]. The nucleotide-binding oligomerization domain-like receptor 3 (NLRP3) inflammasome may be involved in multiple inflammatory responses throughout the body and has become a potential therapeutic target for many diseases. Relevant evidence had also been found in ocular inflammatory diseases[5]. NLRP3 played a pivotal role in immune regulation, specifically participating in the formation and signaling of the inflammasome. The abnormal triggering of NLRP3 had been linked to the development of several inflammatory condition. NLRP3 in the inflammatory process emerged as a potential new therapeutic target for treating inflammatory disorders[6]. In neurodegenerative diseases, autophagy had been observed to have a strong correlation with inflammatory reactions. Specifically, mitochondrial autophagy had been shown to trigger the inhibition of the NLRP3 inflammasome, subsequently mitigating neuroinflammation[7]. Autophagy was a physiological process in which eukaryotes self-phagocytose and maintain homeostasis, and played a certain role in pyroptosis caused by inflammation of retinal cells[8]. Therefore, the purpose of this review was to explore the possible regulatory mechanisms in the inflammatory response of RGCs, including the NLRP3 inflammasome and autophagy pathway, and to identify possible therapeutic strategies and potential therapeutic targets.
Search Strategy
The PubMed database, Google scholar and Ovid technologies (OVID) medicine was selected for literature search, and the following key words were used: ‘ganglion cells’ AND ‘inflammation’; ‘retinal degeneration’ AND ‘NLRP3’; ‘NLRP3’ AND ‘autophagy’; ‘retinal degeneration’ AND ‘autophagy’; ‘retinal ganglion cell’ AND ‘NLRP3’; ‘inflammation’ AND ‘autophagy’. After conducting preliminary retrieval, in case of an overwhelming number of documents, utilize a combination of over three keywords to refine the search scope. Conversely, if the retrieved documents are scarce, broaden the search by decreasing the number of keywords. We selected literatures published in English between January 2000 and November 2023, including clinical and basic studies.
Inflammatory Factors Related to Retinal Ganglion Cell Inflammation
AMD are ocular neurodegenerative diseases. The irreversible degeneration of RGCs was the primary cause of vision loss[9]. There were currently no effective and reversible therapies to improve neuroretinal degeneration[10]–[11]. The retina and choroid were the most energy-consuming eye tissues, requiring a large amount of adenosine triphosphate (ATP) to maintain normal physiological functions[12]. Some external factors could induce the body to produce an oxidative stress response. When the excessive oxidative stress load was unable to be digested, oxidative stress damage to the RGCs and photoreceptors would occur[13]. Some intrinsic factors, such as drusen, were also involved in oxidative stress in AMD[14]–[15]. Oxidative stress leaded to an increase in reactive oxygen species (ROS) in retinal cells, causing the death of photoreceptor cells, RGC and RPE cells by inducing changes in autophagy levels[16]. Under harmful stimulation, retinal tissue could develop a chronic inflammatory state, contributing to the degeneration and death of RGCs and other cells in AMD[17]. Autophagy also played a critical role in maintaining the homeostasis of the intracellular environment[18]. Typically, autophagy-related proteins expressed in RGCs and RPE cells maintain normal visual function. However, if the autophagy protein gene was mutated, autophagy would be activated, leading to the development of retinal diseases[19]. Autophagy could effectively reduce the toxic effects of cells[20].
Aging was closely associated with cell death, which in turn stimulated inflammation and triggered a series of cascade reactions[21]. RGCs were the only neurocytes could directly receive external light stimulation. When the body's functions declined, such as chronic light stimulation, metabolic disorders, or other external factors, these could lead to the damage and degeneration of RGC. This in turn caused the release of cytokines, affecting the surrounding cellular environment[22]. The cytokines involved in the inflammation of RGCs and RPE cells are listed in Table 1[23]–[44].
Table 1. Inflammatory cytokines of retinal ganglion cells and RPE cells in AMD.
| Categories | Cytokines | Functional mechanisms | References |
| Interleukin | IL-1β | Involved in proliferation, differentiation, and apoptosis, inflammatory factor | Natoli et al[23], 2017 |
| IL-4 | Regulated humoral and adaptive immunization | Zhou et al[24], 2022 | |
| IL-6 | Involved in cell growth and differentiation | Coorey et al[25], 2015 | |
| IL-8 | Stimulated angiogenesis and capillary leakage | Hautamäki et al[26], 2014 | |
| IL-10 | Increase retinal ganglion cell survival similar to IL-2 | Colares et al[27], 2021 | |
| IL-18 | Upregulated through NLRP3 pathway, inflammatory factor | Peng et al[28], 2020 | |
| Interferon | IFN-γ | Inflammatory factor, immune stimulation | Yu et al[29], 2016 |
| Tumor necrosis factor | TNF-α | Induces retinal ganglion cell degeneration, inflammatory factor | Ko et al[30], 2020 |
| Transforming growth factor-β family | TGF-β1 | Regulated cell proliferation, differentiation and activation | Tarallo et al[31], 2012 |
| TGF-β2 | Growth inhibiting factor, immunosuppressive effect | Llorián-Salvador et al[32], 2022 | |
| Growth factor | VEGF | Promoted angiogenesis and vascular permeability, and stimulated the proliferation and survival of endothelial cells | Liberski et al[33], 2022 |
| HGF | Promoted the proliferation of vascular endothelial cells and the formation of neovascularization | Chu et al[34], 2006 | |
| IGF-1 | Stimulated cell growth and differentiation, reduce protein breakdown | Subramani et al[35], 2023 | |
| Chemokine family | MCP-1/CCL2 | Involved in chronic inflammation, body defense, and anti-tumor | Lechner et al[36], 2017 |
| Other factors | CFH | Regulated the complement-mediated immune system | Hayashi et al[37], 2010 |
| CRP | Activated complement and enhances phagocytosis | Colak et al[38], 2012 | |
| ApoE | Involved in nerve regeneration, immune regulation, lipid metabolism, and enzyme activation | Vessey et al[39], 2022 | |
| tPA | Activated the conversion of endogenous plasminogen to plasmapause | Iglicki et al[40], 2024 | |
| Bcl-2 | Decreased Bax activity and cell apoptosis | Nir et al[41], 2000 | |
| MMP-2 | Zinc-dependent enzyme, cuts extracellular matrix components | De Groef et al[42], 2015 | |
| MMP-9 | Degradation and remodeling of extracellular matrix homeostasis | Descamps et al[43], 2008 | |
| NLRP3 | Lead to the occurrence of autoinflammatory diseases | Weinberger et al[44], 2023 |
ApoE: Apolipoprotein E; Bcl-2: B-cell lymphoma-2; CFH: Complement factor H; CRP: C-reactive protein; HGF: Hepatocyte growth factor; IFN-γ: Interferon-γ; IGF-1: Insulin-like growth factor-1; IL: Interleukin; MCP-1/CCL2: Monocyte chemoattractant protein-1/chemokine (C-C motif) ligand 2; MMP: Matrix metalloproteinase; NLRP3: NOD-like receptor family pyrin domain containing 3; TGF: Transforming growth factor; TNF-α: Tumor necrosis factor-alpha; tPA: Tissue-type plasminogen activator; VEGF: Vascular endothelial growth factor; RPE: Retinal pigment epithelial; AMD: Age-related macular degeneration.
Interleukins
Interleukins (ILs) were classic cytokines which primary functions included transmitting signals, regulating the immune system, participating in inflammation and mediating the activation, proliferation[45]. Among them, IL-1β, IL-4, IL-6, IL-8, and IL-18 were particularly relevant to ophthalmology. IL-1β is involved in a variety of immune and inflammatory responses and cellular activities, including cell proliferation, differentiation, and apoptosis[33]. IL-1β, in conjunction with IL-18, collaborates to regulate immune responses through various downstream mechanisms, which were associated with pain, inflammation, neuroprotection[33]. IL-4 was an immunomodulatory cytokine that regulated the survival and differentiation of nerve cells, while stimulating the cholinergic differentiation of retinal cells[24],[46]. The application of IL-6-like cytokines or the activation of their corresponding downstream signaling pathways could promote neuroprotection and optic nerve regeneration[25]. Signal transduction of IL-8 could be enhanced by inflammatory signals, ROS and death receptors, which may be related to primary or secondary ocular inflammation[26]. The inflammatory response of RGCs could be reduced by decreasing the expression of IL-8[26]. IL-10 was an anti-inflammatory cytokine that could downregulate inflammation and antagonize inflammatory mediators in neuroretina[47]–[48].
Interferons
Interferons (IFNs) were immune system regulators, with IFN-γ being particularly relevant to the eye. It could induce microglia and astrocytes to produce nitric oxide synthase, which was related to the occurrence or protection of certain diseases in the central nervous system. If the expression of IFN-γ increases in response to stimuli in the retina, it may lead to the death of RGCs[49]–[50]. Du et al[51] found that in vitro, IFN-γ-stimulated retinal microglia increased the release of various pro-inflammatory factors, which then further affected RGCs and photoreceptors. Therefore, IFN-γ could produce a variety of cellular effects.
Tumor necrosis factor-alpha
Tumor necrosis factor-alpha (TNF-α), a multifunctional molecule within cells, played roles in inflammation, apoptosis, and immune responses. TNF-α could promote local inflammatory reactions through cell death[51]–[52]. TNF-α exerted a harmful effect on RGCs. By binding to the TNFR1 receptor, TNF-α significantly enhanced the function and expression of Nav1.6 channels on the surface of RGCs, leading to their hyper-excitability and, ultimately, apoptosis[53]. By utilizing antagonists to regulate TNF-α, the inflammatory cellular response could be effectively down-regulated, ultimately mitigating local chronic inflammation within the body[54].
Transforming growth factor-beta family
Transforming growth factor-beta (TGF-β) regulated the growth, differentiation, and apoptosis of retinal cells. Additionally, TGF-β also played an important role in some retinal diseases. TGF-β exhibited a protective effect on RGCs, as it could inhibit the apoptosis of RGCs through certain signaling pathways, thus playing a protective role[55]. Through the regulation of the TGF-α-related signaling pathway, the inflammatory response of RGCs can be mitigated, thereby minimizing local damage to the retina[56].
Growth factors
Vascular endothelial growth factor (VEGF) promoted the survival and synaptic growth of RGCs, suggesting that VEGF may play a positive role in the growth and survival of RGCs[57]. Hepatocyte growth factor has a protective effect on RGCs, and it may exert its protective function by promoting the survival of RGCs and inhibiting cell apoptosis[58]. The main protective mechanism of insulin-like growth factor-1 on RGCs included activating certain intracellular signaling pathways to promote cell survival; promoting the growth of synapses in RGCs helping to maintain the normal structure and function of the retina[51].
Chemokine family
Monocyte chemoattractant protein-1 (MCP-1) was a chemokine that attracts monocytes, macrophages, and other immune cells to the site of inflammation. In the retina, MCP-1 expression may increase when inflammation or injury occurs, attracting more immune cells to the retina[59]. The infiltration and activation of these immune cells could release inflammatory mediators and cytokines, exacerbating the inflammatory response and causing toxic effects on RGCs. This inflammatory environment may lead to damage, apoptosis, or dysfunction of RGCs[60]. Thus, MCP-1 may indirectly affect RGCs, and its specific mechanism may also involve the participation of other factors and signaling pathways, which need further research to clarify.
NLRP3 Inflammasone and Associated Upstream and Downstream Molecules
NLRP3 was an important role in the inflammatory response of RGCs. Other inflammatory factors included complement factor H (CFH), C-reactive protein (CRP)[61]–[62], apolipoprotein E (APoE), tissue-type plasminogen activator (tPA). The activation of the inflammasome was a crucial component of the innate immune response, among which the NLRP3 inflammasome was the well-studied (Table 2, Figure 1). NLRP3 was associated with various diseases throughout the body (Figure 2) and also closely linked to eye disease. It belonged to the NOD-like receptor family, whose members all possess nucleotide-binding and oligomerization domain (NACHT) with similar structures. In addition, NLRP3 included an N-terminal pyrin domain (PYD) and a C-terminal leucine-rich repeat (LRR). The NLRP3 inflammasome was primarily composed of NLRP3, apoptosis-associated spot-like protein (ASC) and caspase-1. The inflammasome activated caspase-1, which promoted the maturation and secretion of IL-1β and IL-18, thereby causing an inflammatory response leading to apoptosis[63]. Several potential mechanisms of NLRP3 activation have been proposed: 1) It was activated by various stimuli, such as uric acid crystals, amyloid-beta fibril, and exogenous ATP; 2) It was activated by the damage associated molecular patterns (DAMPs) mode driven by extracellular ATP and amyloid-β protein; 3) It could be activated by pathogen-associated molecular patterns (PAMP) driven by secretions from pathogens; 4) Under the action of multiple stimuli, the extracellular ATP of pathogenic microorganisms activated the surface receptor P2X7 which may be the upstream signaling event of the activation of the NLRP3 inflammasome[64]; 5) The lysosome was destroyed, which activated the NLRP3[38]; 6) ROS act as a co-signal to activate the NLRP3[65]; 7) The calcium-dependent signaling pathway triggered the NLRP3[66]. NLRP3 upregulation in RPE cells or RGCs could trigger the activation of NLRP3, resulting in the release of IL-1β in AMD[67]. When the function of retinal cells were impaired or their metabolites were not efficiently excreted, the cells experience swelling, vacuolar degeneration and death, which activated the NLRP3 and promoted cytokine secretion[68], leading to the death of surrounding RGCs[69] and creating an inflammatory cascade. Apolipoprotein C3 (ApoC3) had been discovered to trigger the activation of the NLRP3 inflammasome via particular mechanisms, ultimately resulting in immune inflammation[28]. C5 potentially enhanced the NLRP3-mediated inflammatory response[70]. The activation of the NLRP3 inflammasome was shown in Figure 3.
Table 2. Timeline of the development of the NLRP3 inflammasome.
| Year | Event | Findings | Significance | Team and contributions |
| 2002 | NLRP3 was discovered for the first time | The gene responsible for encoding the NLRP3 protein was identified as being a member of the NOD-like receptor family. | This initial understanding of NLRP3 serves as a foundation for further investigations and advances in the field. | Research team of the Immunity Center in Marseille, France. |
| 2004 | Structure analysis of NLRP3 | The researchers studied the structural properties of NLRP3 and elucidated its connection to the disease. | A clear understanding of the structural and functional aspects of NLRP3 is essential for developing effective therapeutic strategies aimed at targeting this critical protein. | The three-dimensional structure of the NLRP3 protein has been resolved through X-ray crystallography. Researchers have genetically engineered mice to investigate the effects of either lacking or excessively expressing NLRP3. |
| 2008 | Relationship between NLRP3 and inflammation | NLRP3 was discovered to participate in inflammatory responses, and a connection to autoimmune diseases was confirmed. | The critical importance of NLRP3 in the inflammatory response has been well documented, paving the way for NLRP3 to serve as a promising new target for anti-inflammatory treatments. | There is an expert consensus on the critical role of NLRP3 in the inflammatory pathway. |
| 2012 | NLRP3 and crystal structure | The crystal structure of NLRP3 in complex with its ligand was published, providing further insight into its activation mechanism. | This understanding helps clarify how NLRP3 is activated, providing a critical foundation for the design of drugs targeting NLRP3. | Gene editing technology was used in the NLRP3 study. |
| 2016 | NLRP3 and the study of disease | NLRP3 had been associated with a wide spectrum of conditions, including gout, diabetes, and neurodegenerative disorders. | The pivotal role of NLRP3 in a diverse array of diseases has been firmly established, making it a crucial target for the treatment of various medical conditions. | Through large-scale genetic studies such as genome-wide association analysis (GWAS). |
| 2020 to present | Study on NLRP3 inhibitors | Inhibitors targeting NLRP3 were currently undergoing clinical trials to evaluate their efficacy and safety. | The research on NLRP3 inhibitors has unveiled novel treatment strategies for inflammatory diseases in the future. | The Inflammation and Host Defense Research Center at Harvard Medical School conducts clinical research. |
NLRP3: NOD-like receptor family pyrin domain containing 3.
Figure 1. The timeline for NLRP3 research progress.

NLRP3: NLR family pyrin domain containing 3 protein.
Figure 2. Schematic diagram of NLRP3 inflammasome and its involvement in systemic related diseases.
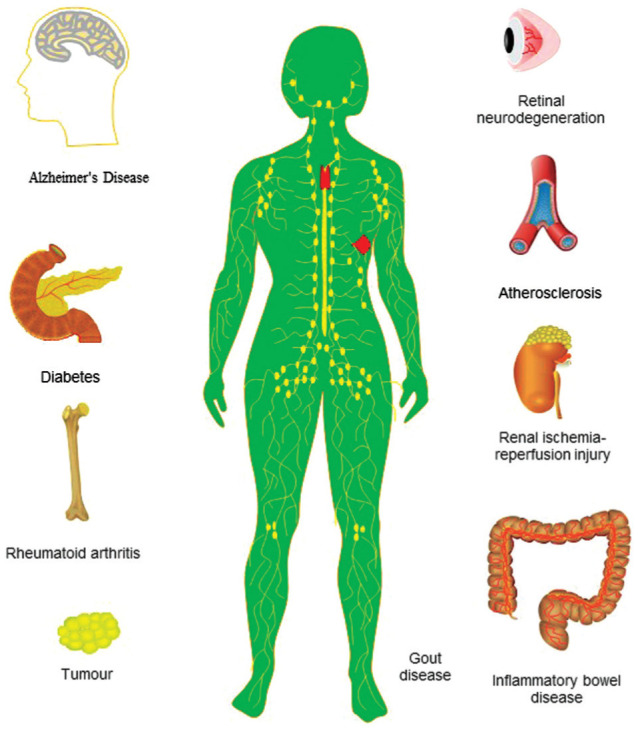
NLRP3 was involved in various systemic diseases, including rheumatoid arthritis, atherosclerosis, diabetes, and inflammatory bowel disease. Moreover, NLRP3 was linked to neurological related diseases such as Alzheimer's disease. In addition to these diseases, NLRP3 was also linked to gout, tumors, and other diseases. The NLRP3 inflammasome was also associated with renal ischemia/reperfusion injury. NLRP3: NLR family pyrin domain containing 3 protein.
Figure 3. Schematic diagram of NLRP3 inflammasome activation.

When cells were stimulated by external factors, NLRP3 was activated. NLRP3 then binded to ASC through its PYD domain, which activated pro-caspase-1 through its CARD domain, leading to its conversion into mature caspase-1. Active caspase-1 then stimulated the cleavage of pro-IL-1β and pro-IL-18 into IL-1β and IL-18, which further stimulated the inflammatory response. IL: Interleukin; NLRP3: NLR family pyrin domain containing 3 protein; ASC: Apoptosis-associated speck-like protein; PYD: Pyrin domain; CRAD: Caspase activation and recruitment domain; PAMP: Pathogen-associated molecular patterns.
Relationship Between NLRP3 and Inflammatory Diseases Related to RGCs
The NLRP3 inflammasome played a decisive role in various diseases, including Alzheimer's disease, autoinflammatory diseases and various neurodegenerative diseases[71]. Ocular neurodegeneration also encompasses AMD, retinitis pigmentosa, optic nerve atrophy, and retinitis neuritis. In diseases like AMD, RGCs undergo degenerative changes, leading to retinal dysfunction and vision loss. NLRP3 was an essential component of the inflammasome. Upon activation of NLRP3, it triggered a cascade of inflammatory responses. RGCs were primarily responsible for receiving and transmitting visual signals. When RGCs were stimulated by long-term chronic inflammation, inflammatory responses occur, causing cellular damage and dysfunction[72]. NLRP3 activation was closely associated with the onset and development of diseases related to RGCs inflammation[73]. In AMD, NLRP3 activation could lead to an increased inflammatory response in RGCs, further aggravating the symptoms and progression of the disease[74]. Therefore, targeting the regulation of NLRP3 may become a novel strategy for the treatment of inflammatory diseases related to RGCs. NLRP3 expression was significantly up-regulated in the microinflammatory environment of the retina, and activation of the NLRP3 could promote the maturation and secretion of IL-1β and IL-18, ultimately leading to a chain inflammatory response in peripheral RGCs and other cells[23]. Targeting NLRP3 or IL-18 could inhibit RPE degeneration, thereby reducing local inflammation and reducing RGCs damage[29]. The X-linked apoptosis suppressor protein could negatively regulate the secretion of IL-1β and IL-18. Located downstream of caspase-1, the X-linked apoptosis suppressor protein was reduced by the activation of NLRP3[30]. The secretion of IL-1β and IL-18 depended on the NLRP3[75]. When the NLRP3 was stimulated and activated, it promoted the conversion of pro-caspase-1 to active caspase-1, which then participated in mediating the programmed death of RPE cells and RGCs, ultimately promoting the development of AMD and leading to visual impairment due to the degeneration and death of RGCs and photoreceptors[24]. TRIM31 also interacted with NLRP3. TRIM31 inhibited the formation of NLRP3 by promoting NLRP3 ubiquitination, thus inhibiting the death of RPE cells[76]. Hwang and Chung[77] found that salazosulfapyridine could reduce the apoptosis of RPE cells induced by tamoxifen by inhibiting caspase-1-mediated inflammation, which proved that the NLRP3 was associated with the onset of AMD.
Relationship Between Autophagy and Inflammatory Diseases Related to RGCs
Autophagy was a cellular self-degradation process that helped remove damaged proteins, organelles, and more to maintain cellular homeostasis and balance. Autophagy also played an important protective role in RGCs. When RGCs were stimulated by inflammation, autophagy could be activated to help the cells clear damaged proteins and organelles, reduce the inflammatory response, and promote cell repair. However, in some cases, the activation of autophagy may not be sufficient to respond to a strong inflammatory response, resulting in increased damage to RGCs. At this point, regulating the autophagy process may help reduce the inflammatory response and protect RGCs from further damage. Pathological changes in AMD include RPE degeneration, drusen, lipofuscin deposition, and more. Autophagy had a certain regulatory effect on these pathological changes, which could reduce the damage of these pathological changes to RGCs[78]–[79]. The presence of ROS was also a critical factor. In RGCs, a delicate balance between ROS production and clearance was essential for maintaining the normal function of the cells. However, in certain circumstances, such as inflammation, oxidative stress, and other pathological conditions, ROS production may increase, resulting in elevated intracellular ROS levels. This state of oxidative stress could cause harmful effects on RGCs, leading to cell damage and dysfunction. Prolonged or excessive oxidative stress may accelerate the degeneration of RGCs, exacerbating vision loss. RGCs were particularly sensitive to ROS. The retina consumes vast amounts of oxygen during normal human eye activity and generates a significant amount of ROS. Under normal conditions, ROS was eliminated by intracellular antioxidant enzymes, maintaining an intracellular ROS balance. If ROS accumulation occurs in an emergency, it could cause cell damage. The chronic oxidative stress response can impair the autophagy ability of retinal cells, leading to retinal damage, including that of RGCs. Therefore, improving autophagy could prevent retinal damage caused by oxidative stress[80]. However, acute oxidative stress can stimulate autophagy activity, while chronic stress may reduce it, as confirmed in an experiment. It was found that the severity of oxidative stress had different effects on autophagy[31]. Oxidative stress could also stimulate the formation of lipofuscins, the main component of N-retinylidene-N-retinylidene ethanolamine (A2E). Autophagy could be induced in ARPE-19 cells when they are incubated with A2E. Autophagy inhibitors combined with A2E can promote cell death, while A2E alone cannot cause cell death[81], and this reduced the release of inflammatory factors that affect RGCs. Chronic inflammation was also another important cause of RGCs damage in AMD. In AMD, chronic inflammation could lead to pathological changes in RGCs and RPE cells under the continuous action of RGCs[82]. When drusen form and deposit between the RPE basement membrane and the Bruch membrane, they could lead to chronic damage and apoptosis of RPE cells, photoreceptors, and RGCs, as drusen contain various inflammatory components, including immunoglobulin, CRP, and complement components[83]. Complement was a protein which activation could attack RPE cells and participated in RGCs death, which were the main processes involved in the pathogenesis of dry AMD[84]. Autophagy could regulate inflammation, and inflammatory factors could also affect autophagy[85]. AMD was mainly characterized by the degeneration of RPE cells and the damage of photoreceptor cells in the macula. Autophagy played a crucial role in maintaining cellular homeostasis and removing damaged organelles. However, with aging, autophagy gradually weakens, resulting in a decrease in the efficiency of intracellular waste removal and damaged organelle degradation[86]. This decrease in autophagy function was closely related to AMD. In the retinal cells of patients with AMD, autophagy activity was reduced, leading to the accumulation of intracellular waste, further exacerbating cell damage and degradation[87]. Activating the autophagy pathway could promote waste removal and cells repairment, potentially slowing the progression and reducing symptoms of AMD[88]. In dry AMD, oxidative stress and inflammation play crucial roles in its development, and autophagy could influence the disease course by regulating these factors. Enhancing autophagy function therefore offered new possibilities for the treatment of AMD.
Effect of NLRP3 Interaction with Autophagy on RGC Inflammation
When NLRP3 was activated, it stimulated inflammatory responses, leading to the infiltration of inflammatory cells and the release of inflammatory mediators, exacerbating the damage and dysfunction of RGCs. Autophagy, as a cytoprotective mechanism, could reduce inflammatory responses by removing damaged proteins and organelles. In RGCs, activation of autophagy could help remove intracellular waste and promote cell repair and regeneration. When NLRP3 interacted with autophagy, the balance between them was critical for the inflammatory response of RGCs. On one hand, if NLRP3 was overactivated and the inflammatory response was intensified, autophagy may not be able to effectively clear the damaged cell components, resulting in increased cellular inflammatory damage. On the other hand, by enhancing autophagy activity, it could promote the remission of inflammatory response and reduce the damage of RGCs. Therefore, regulating the interaction between NLRP3 and autophagy may protect RGCs from inflammatory damage by inhibiting NLRP3 overactivation or enhancing autophagy activity. The mechanisms of NLRP3 and autophagy inflammasome in different ocular diseases were shown in Table 3[89]–[95]. The schematic representation of the interaction between NLRP3 and autophagy inflammasome in RGCs is shown in Figure 4.
Table 3. NLRP3 inflammasome and autophagy in different ocular neuropathic diseases.
| Diseases | Involved in mechanism | References |
| Retinal degeneration | Cyn protects against blue light-induced retinal degeneration by modulating autophagy and decreasing the NLRP3 inflammasome | Feng et al[89], 2021 |
| AMD | Chronic inflammation was regulated by autophagy degradation of NLRP3 inflammasomes in AMD | Piippo et al[90] , 2018 |
| Dry eye | Melatonin inhibited the activated NLRP3 inflammation cascade and maintains normal levels of autophagy. | Wang et al[91], 2021 |
| Alzheimer's disease | Autophagy cleared the extracellular Aβ fibrils by microglia and regulating the Aβ-induced NLRP3 inflammasome. | Cho et al[92], 2014 |
| Corneal transplantation rejection | Blocking NLRP3 inflammasome signaling had been shown to prolong the survival of corneal allografts. | Wei et al[93], 2022 |
| Endophthalmitis | It could reduce the bacterial inflammatory response by inhibiting NLRP3 inflammasome and promote the degradation of ocular bacterial infections by autophagosomes | Singh et al[94], 2023 |
| Diabetic retinopathy | High glucose had been found to increase autophagy, while treatment with 3-MA leaded to the accumulation of ROS and the activation of NLRP3 inflammasome. | Shi et al[95], 2015 |
AMD: Age-related macular degeneration; NLRP3: NOD-like receptor family pyrin domain containing 3; ROS: Reactive oxygen species; 3-MA: 3-Methyladenine.
Figure 4. Relationship between NLRP3 inflammasome and autophagy.

As shown in the figure, when cells were subjected to long-term, chronic harmful stimuli from the outside world, mitochondria could be damaged, leading to the release of ROS. This ROS could then activate NLRP3 through the NF-κB and other pathways. Meanwhile, autophagy could reduce the production of NLRP3 by directly degrading damaged mitochondria, and could also reduce the production of ubiquitinated NLRP3 by recognizing it. This could target pro-IL-1β and pro-IL-18 to reduce their inflammatory products, IL-1β and IL-18, respectively, thereby reducing inflammation. The nucleus could be stimulated by ROS to activate the complement pathway. IL: Interleukin; NLRP3: NLR family pyrin domain containing 3 protein; NF-κB: Nuclear factor kappa-B; ROS: Reactive oxygen species; ASC: Apoptosis-associated speck-like protein.
The NLRP3 inflammasome primarily drived the production and secretion of inflammatory factors, while autophagy regulated and affectd the inflammatory response. AMD was a degenerative disease of the retina, which primarily associated with the functional degeneration of RGCs, RPE cells, and choroidal blood vessels[96]. Autophagy could maintain the homeostasis of the internal environment of RGCs and RPE cells. If autophagy was disrupted, it would lead to the death of RGCs, which may accelerate the pathological process of AMD and vision loss[97]. Inhibition of autophagy in the retina may lead to aggregation of lipofuscin and activation of the NLRP3 inflammasome, which then promoted chronic inflammation of RPE cells and ultimately resulted in the senescence and death of peripheral inflammatory ganglion cells[98]. The interaction between the NLRP3 inflammasome and autophagy was a complex and precise process. On one hand, autophagy could inhibit the activation of NLRP3 and the inflammation-mediated processes by clearing some receptor molecules, signaling molecules, or other proteins involved in the inflammatory response process[99]. On the other hand, autophagy could also promote the formation of the NLRP3 inflammasome under certain conditions[100]. This interaction may be regulated by various intracellular and extracellular factors, such as the state of intracellular signaling pathways, cell type, and environmental conditions. The regulation of this balance was essential for maintaining the normal operation of the inflammatory response and preventing tissue damage caused by excessive inflammatory response. The interaction between NLRP3 and autophagy was manifested in the following aspects: 1) Autophagy could remove inflammasome activators, such as cellular endogenous activators, NLRP3 inflammasome components, and cytokines, thereby reducing inflammasome activation and inflammatory response through the aforementioned pathways[101]; 2) Autophagy could reduce the activation of inflammasome by removing defective mitochondria[102]; 3) The inflammasome could inhibit autophagy. Lipopolysaccharide-induced inflammation leaded to an increase in NLRP3 inflammasome protein levels. Melatonin played an anti-inflammatory role by restoring the aforementioned blocked autophagy pathway, which was due to impaired autophagy flux[89]. A2E had been shown to cause significant autophagic damage and upregulate the negative regulator of autophagy in RPE cells, suggesting that lipofuscin in the retina may inhibit autophagy[90]. In vitro cultures of retinitis pigmentosa cells revealed that lipofuscin-like substances make them more sensitive to lysosomal phototoxicity, leading to the activation of NLRP3 inflammasome and the secretion of inflammatory factors[91]. The autophagy agonist resveratrol possesses antioxidant and anti-inflammatory properties[92]. It could effectively prevent the production and increase of ROS, IL-6, and IL-8 in retinal cells, and reduce the expression of lipofuscin, demonstrating that autophagy could inhibit the activation of NLRP3 inflammasome[93]. Rapamycin could inhibit the activation of NLRP3 inflammasome by inducing autophagy, and neclear factor erythroid 2-related factor 2 (NrF2) could partially mediate the inhibitory effect of rapamycin on ROS, thus regulating the activation of NLRP3 inflammasome[94]. Amyloid-β (Aβ) deposition could drive microglial activation and trigger the NLRP3 inflammasome, which in turn could further exacerbate Aβ deposition, leading to damage to RGCs. Inhibition of microglial autophagy enhances NLRP3 inflammasome activation[95]. Conversely, promoting autophagy inhibited NLRP3 inflammasome activation, suggesting that autophagy may reduce Aβ deposition and vision loss caused by RGC death by inhibiting the NLRP3 inflammasome[103].
Potential Therapeutic Agents for NLRP3 and Autophagy
Currently, small molecule drugs targeting NLRP3 and autophagy in cells had been developed, mainly based on cell experiments, animal experiments, and some clinical trials. Therapeutic agents for NLRP3 in RGCs included MCC950, a small molecule inhibitor that specifically inhibits the activation of NLRP3 inflammasome and can protect RGCs from death[104]. By directly inhibiting NLRP3, CY-09 may help protect RGCs from inflammation-mediated damage and maintain the normal function of nerve cells[105]. NLRP3-in-18 was a novel and highly selective NLRP3 inhibitor, and its effect was still under further study. Pyrazine derivatives could be used as NLRP3 inhibitors for the treatment of inflammation-related and neurodegenerative diseases such as asthma, chronic obstructive pulmonary disease, Parkinson's disease, and Alzheimer's disease[106] inhibited the inflammatory response in macrophages by blocking the NLRP3/caspase-1 pathway. Oxidized ATP could also inhibit NLRP3 by targeting P2X7R, thereby reducing nervous system inflammation[107]. JT002 was a novel NLRP3 inhibitor that effectively reduced the production of NLRP3-dependent pro-inflammatory cytokines and prevents pyrosis[108]. The functional roles of NLRP3 and autophagy and their potential in the treatment of RGC inflammation were summarized in Table 4[109]–[114].
Table 4. Summary of small molecule drugs for NLRP3 in the treatment of inflammatory diseases.
| Names | Targets | Functions | Cell lines/diseases | References |
| MCC950 | P2X7R | Inhibit NLRP3 | Retinal microglia | Zhang et al[109], 2019 |
| CY-09 | Directly binded to the ATP-binding motif of the NLRP3 NACHT domain and inhibited the activity of NLRP3 ATPase | Inhibit NLRP3 | Macrophage | Shen et al[110], 2021 |
| Orlistat | Blocked the assembly and activation of NLRP3 inflammasome | Inhibit NLRP3 | Macrophage | Xu et al[111], 2022 |
| NLRP3-IN-18 | Pyrazine compounds | Inhibit NLRP3 | Still on study | |
| Pyridazine derivatives | Direct inhibitor | Inhibit NLRP3 | Alzheimer's disease | Sabnis [112], 2023 |
| JT002 | Decrease NLRP3-dependent proinflammatory cytokine production | Inhibit NLRP3 | Neuroinflammation | Ambrus-Aikelin et al[113], 2023 |
| Oxidized ATP | P2X7R | Inhibit NLRP3 | Endothelial cells | Fang et al[114], 2011 |
NLRP3: NOD-like receptor family pyrin domain containing 3; 3-MA: 3-Methyladenine ; ATP: Adenosine Triphosphate; NACHT: Nucleotide-binding and oligomerization domain.
Autophagy had a variety of inhibitors and activators. Inhibitors included 3-Methyladenine (3-MA), chloroquine, and bafilomycin A1[115]–[117]. Autophagy agonists included rapamycin, Earle's equilibrium salt solution, and lithium chloride, which could activate the autophagy process and promote the degradation and recycling of intracellular substances[118]–[119]. The functional effects of autophagy and its potential in the treatment of RGC inflammation were summarized in Table 5[120]–[125].
Table 5. Summary of small molecule drugs targeting autophagy in the treatment of inflammatory diseases.
| Names | Targets | Functions | Cell lines/diseases | References |
| 3-MA | PI3K | Inhibited the formation of autophagosomes | HeLa cell | Kosic et al[120], 2021 |
| Chloroquine | Autophagy and toll-like receptors | Inhibited activity of autophagy and TLRs | Macrophage | Chen et al[121], 2018 |
| Bafilomycin A1 | Autophagosome and lysosome | Blocking autophagy flow and lysosomal degradation pathway | Neurodegenerative diseases | Lee et al[122], 2021 |
| Rapamycin | mTOR | Autophagy agonists | Neurodegenerative diseases | Querfurth et al[123], 2021 |
| EBSS | Regulates various ions and osmotic pressure during cell growth and metabolism | Maintained intracellular and intracellular balance, positively regulates autophagy | Nerve cell | Mohamed et al[124], 2014 |
| Lithium chloride | GSK3β, regulates neurotransmitters | Positively regulated autophagy | Nerve cell | Grandjean et al[125], 2009 |
NLRP3: NOD-like receptor family pyrin domain containing 3; 3-MA: 3-Methyladenine ; PI3K: Phosphatidylinositide 3-kinase; mTOR: Mammalian target of rapamycin; GSK3β: Glycogen synthase kinase-3β; EBSS: Earle's balanced salt solution.
FUTURE DIRECTION
RGC inflammation was a pathological process in retinal diseases that could lead to vision loss and retinal dysfunction. During this process, the activation of the NLRP3 inflammasome played a key role, which promoteed the occurrence and development of the inflammatory response[126]. The NLRP3 gene was first identified and characterized in 2002, and it was considered to be a member of the NOD-like receptor family. Subsequently, the structure and function of NLRP3 were studied[127], and its relationship with inflammation was initially explored[128]. Over the next few years, the association between NLRP3 and inflammation was confirmed, and NLRP3 was found to play a crucial role in the inflammatory response, particularly in autoimmune diseases[129]. This led to an expert consensus that NLRP3 was a key molecule in the inflammatory pathway[109]. Subsequently, the crystal structure of NLRP3 and its ligand was published[110], revealing its specific activation mechanism. One study utilized gene-editing technology to create a mouse model lacking NLRP3[112], suggesting the crucial role of NLRP3 in inflammation during animal research[111]. Since 2016, large-scale genetic research methods such as GWAS have been used to further confirm the association of NLRP3 with a variety of diseases[130]. Potential treatments targeting NLRP3 were entering clinical trials to assess their effectiveness and safety[113]. However, an excessive inflammatory response could lead to further damage and degeneration of RGCs. Autophagy, as a cytoprotective mechanism, had potential therapeutic significance in RGCs inflammation. By enhancing autophagy activity, it could promote the removal of intracellular waste, reduce inflammatory responses, and promote cell repair and regeneration[121]–[122]. Therefore, regulating the autophagy process had the potential to become a new therapeutic strategy to protect RGCs from inflammatory damage. The interaction between NLRP3 and autophagy also provided a new perspective for the treatment of RGC inflammation. By regulating the balance between NLRP3 and autophagy, it may be possible to control the extent and timing of the inflammatory response, thereby reducing the damage of inflammation to RGCs[120]. AMD was characterized by the degeneration and death of RGCs and RPE cells and was the main cause of vision loss in these patients. Chronic inflammation played an important role in the pathogenesis of AMD, among which the NLRP3 inflammasome played a driving role in inflammation in various diseases, and autophagy was also involved in the process[130]. The synergistic action of these two factors may lead to the degeneration and necrosis of RGCs. In the chronic inflammatory state of RGCs stimulated by aging, the NLRP3 inflammasome and its related inflammatory factors, as well as the participation of retinal cell autophagy, all produce a cascade reaction, leading to further damage of normal ganglion cells and RPE cells around the retina[124]. Therefore, this paper reviews the two important molecular mechanisms of RGC inflammation, namely NLRP3 and autophagy, and the relationship between NLRP3 and autophagy as well as their potential significance in treatment. At the same time, it summarized the possible inflammatory mechanisms and other mechanisms of AMD, a representative irreversible disease of retinal degenerative diseases. This was a comprehensive analysis of basic research on diseases that severely impair vision. The schematic diagram of NLRP3 targeted therapy and autophagy was shown in Figure 5.
Figure 5. Schematic diagram of NLR family pyrin domain containing 3 protein (NLRP3) inflammasome and autophagy in retinal ganglion cell targeted therapy.

When retinal ganglion cells were subjected to harmful stimulation, they could simultaneously activate NLRP3 to produce inflammation and activate the autophagy pathway. Inhibition of NLRP3 could control the inflammation of retinal ganglion cells and protect ganglion cells. At the same time, excessive autophagy activated by inflammation could damage ganglion cells through degradation and other functions. The addition of autophagy inhibitors could reduce autophagy to a certain extent, reduce inflammatory response and the activation of NLRP3, and autophagy agonists could also inhibit inflammation in some cases. The molecular formulas shown in the picture were from the official PubChem website.
AMD remained a difficult-to-treat disease, with numerous factors involved in its entire progression, including external and internal factors, and the timing of interventions further complicated its study. NLRP3 and autophagy were merely fragments of the intricate internal milieu of the body, and their interventions and the effects on their upstream and downstream pathways cannot completely prevent complications from arising. In addition, compared with wet AMD, dry AMD was more related to the apoptosis of RGCs, photoreceptors, and RPE cells, which was an irreversible process, so there were still many things to explore.
CONCLUSION
In summary, the NLRP3 inflammasome was closely linked to autophagy, and their interaction affected the degree of pyroptosis in RGCs, which in turn impacts the occurrence and development of RGC inflammation. The primary challenges of conducting in vitro research on RGCs encompass the significantly reduced survival rate and proliferation capacity of these cells, as well as the potential loss of their inherent physiological traits and functions. Furthermore, accurately replicating the natural growth environment of the cells poses a significant hurdle in such studies. The NLRP3 inflammasome promoted the onset and development of inflammatory responses. Conversely, pathological changes such as drusen and lipofuscin deposition generated in related diseases could further activate the NLRP3 inflammasome, promoting the secretion of inflammatory factors. Autophagy could affect the course of disease by inhibiting or activating the NLRP3 inflammasome. The interaction between autophagy and NLRP3 and its relationship with AMD may provide new insights into exploring the treatment of AMD, opening up new hopes for clinical practice. In the future, further research could explore specific drugs or therapies targeting NLRP3 and autophagy to achieve effective treatment of RGC inflammation.
Footnotes
Authors' contributions: All authors contributed to study conception and design and definition of intellectual content. Wang XL was responsible for data analysis and manuscript preparation. Gao YX, Yuan QZ, and Zhang M revised the article critically for important intellectual content. Zhang M is guarantor of this work, who had full access to all the data in this study and take responsibility for the integrity and accuracy of the data. All authors approved the final version of the manuscript.
Foundations: Supported by the Project of Sichuan Medical Association (No.S22058); National Key R&D Project (No.2018YFC1106103).
Conflicts of Interest: Wang XL, None; Gao YX, None; Yuan QZ, None; Zhang M, None.
REFERENCES
- 1.Zhao Y, Simon M, Seluanov A, Gorbunova V. DNA damage and repair in age-related inflammation. Nat Rev Immunol. 2023;23(2):75–89. doi: 10.1038/s41577-022-00751-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hou YJ, Dan XL, Babbar M, Wei Y, Hasselbalch SG, Croteau DL, Bohr VA. Ageing as a risk factor for neurodegenerative disease. Nat Rev Neurol. 2019;15(10):565–581. doi: 10.1038/s41582-019-0244-7. [DOI] [PubMed] [Google Scholar]
- 3.Do MTH, Yau KW. Intrinsically photosensitive retinal ganglion cells. Physiol Rev. 2010;90(4):1547–1581. doi: 10.1152/physrev.00013.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kaur G, Singh NK. The role of inflammation in retinal neurodegeneration and degenerative diseases. Int J Mol Sci. 2021;23(1):386. doi: 10.3390/ijms23010386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guo M, Zhu YF, Shi Y, Meng XD, Dong X, Zhang HK, Wang XH, Du M, Yan H. Inhibition of ferroptosis promotes retina ganglion cell survival in experimental optic neuropathies. Redox Biol. 2022;58:102541. doi: 10.1016/j.redox.2022.102541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fu JN, Wu H. Structural mechanisms of NLRP3 inflammasome assembly and activation. Annu Rev Immunol. 2023;41:301–316. doi: 10.1146/annurev-immunol-081022-021207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang L, Cai J, Zhao X, et al. Palmitoylation prevents sustained inflammation by limiting NLRP3 inflammasome activation through chaperone-mediated autophagy. Mol Cell. 2023;83(2):281–297.e10. doi: 10.1016/j.molcel.2022.12.002. [DOI] [PubMed] [Google Scholar]
- 8.Zhang YM, Zhao ZZ, Zhao XH, Xie H, Zhang CY, Sun XD, Zhang JF. HMGB2 causes photoreceptor death via down-regulating Nrf2/HO-1 and up-regulating NF-κB/NLRP3 signaling pathways in light-induced retinal degeneration model. Free Radic Biol Med. 2022;181:14–28. doi: 10.1016/j.freeradbiomed.2022.01.018. [DOI] [PubMed] [Google Scholar]
- 9.Boya P, Esteban-Marttínez L, Serrano-Puebla A, Gómez-Sintes R, Villarejo-Zori B. Autophagy in the eye: development, degeneration, and aging. Prog Retin Eye Res. 2016;55:206–245. doi: 10.1016/j.preteyeres.2016.08.001. [DOI] [PubMed] [Google Scholar]
- 10.Amadoro G, Latina V, Balzamino BO, Squitti R, Varano M, Calissano P, Micera A. Nerve growth factor-based therapy in Alzheimer's disease and age-related macular degeneration. Front Neurosci. 2021;15:735928. doi: 10.3389/fnins.2021.735928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hollyfield JG, Bonilha VL, Rayborn ME, Yang XP, Shadrach KG, Lu L, Ufret RL, Salomon RG, Perez VL. Oxidative damage-induced inflammation initiates age-related macular degeneration. Nat Med. 2008;14(2):194–198. doi: 10.1038/nm1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shaw PX, Stiles T, Douglas C, Ho D, Fan W, Du HJ, Xiao X. Oxidative stress, innate immunity, and age-related macular degeneration. AIMS Mol Sci. 2016;3(2):196–221. doi: 10.3934/molsci.2016.2.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yu B, Ma J, Li J, Wang DZ, Wang ZG, Wang SS. Mitochondrial phosphatase PGAM5 modulates cellular senescence by regulating mitochondrial dynamics. Nat Commun. 2020;11(1):2549. doi: 10.1038/s41467-020-16312-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen YH, Zeng JX, Zhao C, et al. Assessing susceptibility to age-related macular degeneration with genetic markers and environmental factors. Arch Ophthalmol. 2011;129(3):344–351. doi: 10.1001/archophthalmol.2011.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Blasiak J, Szaflik J, Szaflik JP. Implications of altered iron homeostasis for age-related macular degeneration. Front Biosci (Landmark Ed) 2011;16(4):1551–1559. doi: 10.2741/3804. [DOI] [PubMed] [Google Scholar]
- 16.Datta S, Cano M, Ebrahimi K, Wang L, Handa JT. The impact of oxidative stress and inflammation on RPE degeneration in non-neovascular AMD. Prog Retin Eye Res. 2017;60:201–218. doi: 10.1016/j.preteyeres.2017.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mitter SK, Song CJ, Qi XP, et al. Dysregulated autophagy in the RPE is associated with increased susceptibility to oxidative stress and AMD. Autophagy. 2014;10(11):1989–2005. doi: 10.4161/auto.36184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen M, Luo C, Zhao JW, Devarajan G, Xu HP. Immune regulation in the aging retina. Prog Retin Eye Res. 2019;69:159–172. doi: 10.1016/j.preteyeres.2018.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lin W, Xu GX. Autophagy: a role in the apoptosis, survival, inflammation, and development of the retina. Ophthalmic Res. 2019;61(2):65–72. doi: 10.1159/000487486. [DOI] [PubMed] [Google Scholar]
- 20.Chai PW, Ni HY, Zhang H, Fan XQ. The evolving functions of autophagy in ocular health: a double-edged sword. Int J Biol Sci. 2016;12(11):1332–1340. doi: 10.7150/ijbs.16245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ratnayaka JA, Serpell LC, Lotery AJ. Dementia of the eye: the role of amyloid beta in retinal degeneration. Eye (Lond) 2015;29(8):1013–1026. doi: 10.1038/eye.2015.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fiotti N, Pedio M, Battaglia Parodi M, Altamura N, Uxa L, Guarnieri G, Giansante C, Ravalico G. MMP-9 microsatellite polymorphism and susceptibility to exudative form of age-related macular degeneration. Genet Med. 2005;7(4):272–277. doi: 10.1097/01.gim.0000159903.69597.73. [DOI] [PubMed] [Google Scholar]
- 23.Natoli R, Fernando N, Madigan M, Chu-Tan JA, Valter K, Provis J, Rutar M. Microglia-derived IL-1β promotes chemokine expression by Müller cells and RPE in focal retinal degeneration. Mol Neurodegener. 2017;12(1):31. doi: 10.1186/s13024-017-0175-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhou T, Yang ZQ, Ni BY, et al. IL-4 induces reparative phenotype of RPE cells and protects against retinal neurodegeneration via Nrf2 activation. Cell Death Dis. 2022;13(12):1056. doi: 10.1038/s41419-022-05433-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Coorey NJ, Shen WY, Zhu L, Gillies MC. Differential expression of IL-6/gp130 cytokines, jak-STAT signaling and neuroprotection after Müller cell ablation in a transgenic mouse model. Invest Ophthalmol Vis Sci. 2015;56(4):2151–2161. doi: 10.1167/iovs.14-15695. [DOI] [PubMed] [Google Scholar]
- 26.Hautamäki A, Kivioja J, Seitsonen S, Savolainen ER, Liinamaa MJ, Luoma A, Järvelä I, Immonen I. The IL-8, VEGF, and CFH polymorphisms and bevacizumab in age-related macular degeneration. Ophthalmology. 2014;121(4):973–973.e1. doi: 10.1016/j.ophtha.2013.11.035. [DOI] [PubMed] [Google Scholar]
- 27.Colares TG, de Figueiredo CS, de Oliveira Jesus Souza L, dos Santos AA, Giestal-de-Araujo E. Increased retinal ganglion cell survival by exogenous IL-2 depends on IL-10, dopamine D1 receptors, and classical uIL-2/IL-2R signaling pathways. Neurochem Res. 2021;46(7):1701–1716. doi: 10.1007/s11064-021-03313-1. [DOI] [PubMed] [Google Scholar]
- 28.Peng DJ, Li JY, Deng Y, et al. Sodium para-aminosalicylic acid inhibits manganese-induced NLRP3 inflammasome-dependent pyroptosis by inhibiting NF-κB pathway activation and oxidative stress. J Neuroinflammation. 2020;17(1):343. doi: 10.1186/s12974-020-02018-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yu Y, Ren XR, Wen F, Chen H, Su SB. T-helper-associated cytokines expression by peripheral blood mononuclear cells in patients with polypoidal choroidal vasculopathy and age-related macular degeneration. BMC Ophthalmol. 2016;16:80. doi: 10.1186/s12886-016-0251-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ko KW, Milbrandt J, DiAntonio A. SARM1 acts downstream of neuroinflammatory and necroptotic signaling to induce axon degeneration. J Cell Biol. 2020;219(8):e201912047. doi: 10.1083/jcb.201912047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tarallo V, Hirano Y, Gelfand BD, et al. DICER1 loss and Alu RNA induce age-related macular degeneration via the NLRP3 inflammasome and MyD88. Cell. 2012;149(4):847–859. doi: 10.1016/j.cell.2012.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Llorián-Salvador M, Byrne EM, Szczepan M, Little K, Chen M, Xu H. Complement activation contributes to subretinal fibrosis through the induction of epithelial-to-mesenchymal transition (EMT) in retinal pigment epithelial cells. J Neuroinflammation. 2022;19(1):182. doi: 10.1186/s12974-022-02546-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liberski S, Wichrowska M, Kocięcki J. Aflibercept versus faricimab in the treatment of neovascular age-related macular degeneration and diabetic macular edema: a review. Int J Mol Sci. 2022;23(16):9424. doi: 10.3390/ijms23169424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chu R, Zheng X, Chen D, Hu DN. Blue light irradiation inhibits the production of HGF by human retinal pigment epithelium cells in vitro. Photochem Photobiol. 2006;82(5):1247–1250. doi: 10.1562/2006-04-19-RA-880. [DOI] [PubMed] [Google Scholar]
- 35.Subramani M, Van Hook MJ, Ahmad I. Reproducible generation of human retinal ganglion cells from banked retinal progenitor cells: analysis of target recognition and IGF-1-mediated axon regeneration. Front Cell Dev Biol. 2023;11:1214104. doi: 10.3389/fcell.2023.1214104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lechner J, Chen M, Hogg RE, Toth L, Silvestri G, Chakravarthy U, Xu HP. Peripheral blood mononuclear cells from neovascular age-related macular degeneration patients produce higher levels of chemokines CCL2 (MCP-1) and CXCL8 (IL-8) J Neuroinflammation. 2017;14(1):42. doi: 10.1186/s12974-017-0820-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hayashi H, Yamashiro K, Gotoh N, Nakanishi H, Nakata I, Tsujikawa A, Otani A, Saito M, Iida T, Matsuo K, Tajima K, Yamada R, Yoshimura N. CFH and ARMS2 variations in age-related macular degeneration, polypoidal choroidal vasculopathy, and retinal angiomatous proliferation. Invest Ophthalmol Vis Sci. 2010;51(11):5914–5919. doi: 10.1167/iovs.10-5554. [DOI] [PubMed] [Google Scholar]
- 38.Colak E, Majkic-Singh N, Zoric L, Radosavljevic A, Kosanovic-Jakovic N. The role of CRP and inflammation in the pathogenesis of age-related macular degeneration. Biochem Med. 2012;22(1):39–48. doi: 10.11613/bm.2012.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vessey KA, Jobling AI, Tran MX, Wang AY, Greferath U, Fletcher EL. Treatments targeting autophagy ameliorate the age-related macular degeneration phenotype in mice lacking APOE (apolipoprotein E) Autophagy. 2022;18(10):2368–2384. doi: 10.1080/15548627.2022.2034131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Iglicki M, Khoury M, Donato L, Quispe DJ, Negri HP, Melamud JI. Comparison of subretinal aflibercept vs ranibizumab vs bevacizumab in the context of PPV, pneumatic displacement with subretinal air and subretinal tPA in naïve submacular haemorrhage secondary to nAMD. The Submarine Study. Eye (Lond) 2024;38(2):292–296. doi: 10.1038/s41433-023-02676-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nir I, Kedzierski W, Chen J, Travis GH. Expression of Bcl-2 protects against photoreceptor degeneration in retinal degeneration slow (rds) mice. J Neurosci. 2000;20(6):2150–2154. doi: 10.1523/JNEUROSCI.20-06-02150.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.De Groef L, Salinas-Navarro M, Van Imschoot G, Libert C, Vandenbroucke RE, Moons L. Decreased TNF Levels and Improved Retinal Ganglion Cell Survival in MMP-2 Null Mice Suggest a Role for MMP-2 as TNF Sheddase. Mediators Inflamm. 2015;2015:108617. doi: 10.1155/2015/108617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Descamps FJ, Kangave D, Cauwe B, Martens E, Geboes K, Abu El-Asrar A, Opdenakker G. Interphotoreceptor retinoid-binding protein as biomarker in systemic autoimmunity with eye inflictions. J Cell Mol Med. 2008;12(6A):2449–2456. doi: 10.1111/j.1582-4934.2008.00264.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Weinberger Y, Budnik I, Nisgav Y, Palevski D, Ben-David G, Fernández JA, Margalit SN, Levy-Mendelovich S, Kenet G, Weinberger D, Griffin JH, Livnat T. 3K3A-activated protein C inhibits choroidal neovascularization growth and leakage and reduces NLRP3 inflammasome, IL-1β, and inflammatory cell accumulation in the retina. Int J Mol Sci. 2023;24(13):10642. doi: 10.3390/ijms241310642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chau KY, Sivaprasad S, Patel N, Donaldson TA, Luthert PJ, Chong NV. Plasma levels of matrix metalloproteinase-2 and-9 (MMP-2 and MMP-9) in age-related macular degeneration. Eye(Lond) 2008;22(6):855–859. [PubMed] [Google Scholar]
- 46.Granja MG, Braga LE, Carpi-Santos R, et al. IL-4 induces cholinergic differentiation of retinal cells in vitro. Cell Mol Neurobiol. 2015;35(5):689–701. doi: 10.1007/s10571-015-0164-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Balamurugan S, Das D, Hasanreisoglu M, et al. Interleukins and cytokine biomarkers in uveitis. Indian J Ophthalmol. 2020;68(9):1750–1763. doi: 10.4103/ijo.IJO_564_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lopez-Castejon G, Brough D. Understanding the mechanism of IL-1β secretion. Cytokine Growth Factor Rev. 2011;22(4):189–195. doi: 10.1016/j.cytogfr.2011.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Li YX, Huang H, Liu B, Zhang Y, Pan XB, Yu XY, Shen ZY, Song YH. Inflammasomes as therapeutic targets in human diseases. Signal Transduct Target Ther. 2021;6(1):247. doi: 10.1038/s41392-021-00650-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Huyghe J, Priem D, Bertrand MJM. Cell death checkpoints in the TNF pathway. Trends Immunol. 2023;44(8):628–643. doi: 10.1016/j.it.2023.05.007. [DOI] [PubMed] [Google Scholar]
- 51.Du X, Penalva R, Little K, Kissenpfennig A, Chen M, Xu HP. Deletion of Socs3 in LysM+ cells and Cx3cr1 resulted in age-dependent development of retinal microgliopathy. Mol Neurodegener. 2021;16(1):9. doi: 10.1186/s13024-021-00432-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zheng WW, Zhou T, Zhang YX, Ding J, Xie JQ, Wang SQ, Wang ZY, Wang K, Shen LY, Zhu Y, Gao CY. Simplified α2-macroglobulin as a TNF-α inhibitor for inflammation alleviation in osteoarthritis and myocardial infarction therapy. Biomaterials. 2023;301:122247. doi: 10.1016/j.biomaterials.2023.122247. [DOI] [PubMed] [Google Scholar]
- 53.Fischer D. Hyper-IL-6: a potent and efficacious stimulator of RGC regeneration. Eye (Lond) 2017;31(2):173–178. doi: 10.1038/eye.2016.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wu Q, Miao XM, Zhang JJ, et al. Astrocytic YAP protects the optic nerve and retina in an experimental autoimmune encephalomyelitis model through TGF-β signaling. Theranostics. 2021;11(17):8480–8499. doi: 10.7150/thno.60031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ghasemi H, Ghazanfari T, Yaraee R, Faghihzadeh S, Hassan ZM. Roles of IL-8 in ocular inflammations: a review. Ocul Immunol Inflamm. 2011;19(6):401–412. doi: 10.3109/09273948.2011.618902. [DOI] [PubMed] [Google Scholar]
- 56.Goczalik I, Ulbricht E, Hollborn M, et al. Expression of CXCL8, CXCR1, and CXCR2 in neurons and glial cells of the human and rabbit retina. Invest Ophthalmol Vis Sci. 2008;49(10):4578–4589. doi: 10.1167/iovs.08-1887. [DOI] [PubMed] [Google Scholar]
- 57.Sauter MM, Brandt CR. Primate neural retina upregulates IL-6 and IL-10 in response to a herpes simplex vector suggesting the presence of a pro-/anti-inflammatory axis. Exp Eye Res. 2016;148:12–23. doi: 10.1016/j.exer.2016.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Husain S, Abdul Y, Webster C, Chatterjee S, Kesarwani P, Mehrotra S. Interferon-gamma (IFN-γ)-mediated retinal ganglion cell death in human tyrosinase T cell receptor transgenic mouse. PLoS One. 2014;9(2):e89392. doi: 10.1371/journal.pone.0089392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cheng S, Wang HN, Xu LJ, Li F, Miao YY, Lei B, Sun XH, Wang ZF. Soluble tumor necrosis factor-alpha-induced hyperexcitability contributes to retinal ganglion cell apoptosis by enhancing Nav1.6 in experimental glaucoma. J Neuroinflammation. 2021;18(1):182. doi: 10.1186/s12974-021-02236-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fernández-Albarral JA, Salazar JJ, de Hoz R, et al. Retinal molecular changes are associated with neuroinflammation and loss of RGCs in an experimental model of glaucoma. Int J Mol Sci. 2021;22(4):2066. doi: 10.3390/ijms22042066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Foxton R, Osborne A, Martin KR, Ng YS, Shima DT. Distal retinal ganglion cell axon transport loss and activation of p38 MAPK stress pathway following VEGF-A antagonism. Cell Death Dis. 2016;7(5):e2212. doi: 10.1038/cddis.2016.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wong WK, Cheung AW, Yu SW, Sha O, Cho EY. Hepatocyte growth factor promotes long-term survival and axonal regeneration of retinal ganglion cells after optic nerve injury: comparison with CNTF and BDNF. CNS Neurosci Ther. 2014;20(10):916–929. doi: 10.1111/cns.12304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Fujita K, Nishiguchi KM, Shiga Y, Nakazawa T. Spatially and temporally regulated NRF2 gene therapy using Mcp-1 promoter in retinal ganglion cell injury. Mol Ther Methods Clin Dev. 2017;5:130–141. doi: 10.1016/j.omtm.2017.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Dong N, Xu B, Shi H, Tang X. Baicalein inhibits amadori-glycated albumin-induced MCP-1 expression in retinal ganglion cells via a microRNA-124-dependent mechanism. Invest Ophthalmol Vis Sci. 2015;56(10):5844–5853. doi: 10.1167/iovs.15-17444. [DOI] [PubMed] [Google Scholar]
- 65.Chirco KR, Whitmore SS, Wang K, Potempa LA, Halder JA, Stone EM, Tucker BA, Mullins RF. Monomeric C-reactive protein and inflammation in age-related macular degeneration. J Pathol. 2016;240(2):173–183. doi: 10.1002/path.4766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Molins B, Romero-Vázquez S, Fuentes-Prior P, Adan A, Dick AD. C-reactive protein as a therapeutic target in age-related macular degeneration. Front Immunol. 2018;9:808. doi: 10.3389/fimmu.2018.00808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kayagaki N, Warming S, Lamkanfi M, et al. Non-canonical inflammasome activation targets caspase-11. Nature. 2011;479(7371):117–121. doi: 10.1038/nature10558. [DOI] [PubMed] [Google Scholar]
- 68.Li J, Tian SJ, Wang HL, et al. Protection of hUC-MSCs against neuronal complement C3a receptor-mediated NLRP3 activation in CUMS-induced mice. Neurosci Lett. 2021;741:135485. doi: 10.1016/j.neulet.2020.135485. [DOI] [PubMed] [Google Scholar]
- 69.Yao M, Li G, Pu PM, et al. Neuroinflammation and apoptosis after surgery for a rat model of double-level cervical cord compression. Neurochem Int. 2022;157:105340. doi: 10.1016/j.neuint.2022.105340. [DOI] [PubMed] [Google Scholar]
- 70.Seil M, El Ouaaliti M, Fontanils U, Etxebarria IG, Pochet S, Dal Moro G, Marino A, Dehaye JP. Ivermectin-dependent release of IL-1beta in response to ATP by peritoneal macrophages from P2X(7)-KO mice. Purinergic Signal. 2010;6(4):405–416. doi: 10.1007/s11302-010-9205-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Duewell P, Kono H, Rayner KJ, et al. NLRP3 inflammasomes are required for atherogenesis and activated by cholesterol crystals. Nature. 2010;464(7293):1357–1361. doi: 10.1038/nature08938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhou RB, Tardivel A, Thorens B, Choi I, Tschopp J. Thioredoxin-interacting protein links oxidative stress to inflammasome activation. Nat Immunol. 2010;11(2):136–140. doi: 10.1038/ni.1831. [DOI] [PubMed] [Google Scholar]
- 73.Lee GS, Subramanian N, Kim AI, et al. The calcium-sensing receptor regulates the NLRP3 inflammasome through Ca2+ and cAMP. Nature. 2012;492(7427):123–127. doi: 10.1038/nature11588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tseng WA, Thein T, Kinnunen K, Lashkari K, Gregory MS, D'Amore PA, Ksander BR. NLRP3 inflammasome activation in retinal pigment epithelial cells by lysosomal destabilization: implications for age-related macular degeneration. Invest Ophthalmol Vis Sci. 2013;54(1):110–120. doi: 10.1167/iovs.12-10655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ding ZF, Liu SJ, Wang XW, Dai Y, Khaidakov M, Deng XY, Fan YB, Xiang D, Mehta JL. LOX-1, mtDNA damage, and NLRP3 inflammasome activation in macrophages: implications in atherogenesis. Cardiovasc Res. 2014;103(4):619–628. doi: 10.1093/cvr/cvu114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Porciatti V, Chou TH. Modeling retinal ganglion cell dysfunction in optic neuropathies. Cells. 2021;10(6):1398. doi: 10.3390/cells10061398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hwang N, Chung SW. Sulfasalazine attenuates tamoxifen-induced toxicity in human retinal pigment epithelial cells. BMB Rep. 2020;53(5):284–289. doi: 10.5483/BMBRep.2020.53.5.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.D'Souza S, Lang RA. Retinal ganglion cell interactions shape the developing mammalian visual system. Development. 2020;147(23):dev196535. doi: 10.1242/dev.196535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Chen H, Deng Y, Gan XL, et al. NLRP12 collaborates with NLRP3 and NLRC4 to promote pyroptosis inducing ganglion cell death of acute glaucoma. Mol Neurodegener. 2020;15(1):26. doi: 10.1186/s13024-020-00372-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Malsy J, Alvarado AC, Lamontagne JO, Strittmatter K, Marneros AG. Distinct effects of complement and of NLRP3- and non-NLRP3 inflammasomes for choroidal neovascularization. Elife. 2020;9:e60194. doi: 10.7554/eLife.60194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Gao JY, Cui JZ, Wang AK, Chen HHR, Fong A, Matsubara JA. The reduction of XIAP is associated with inflammasome activation in RPE: implications for AMD pathogenesis. J Neuroinflammation. 2019;16(1):171. doi: 10.1186/s12974-019-1558-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bhattarai N, Korhonen E, Mysore Y, Kaarniranta K, Kauppinen A. Hydroquinone induces NLRP3-independent IL-18 release from ARPE-19 cells. Cells. 2021;10(6):1405. doi: 10.3390/cells10061405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Voigt AP, Mulfaul K, Mullin NK, et al. Single-cell transcriptomics of the human retinal pigment epithelium and choroid in health and macular degeneration. Proc Natl Acad Sci U S A. 2019;116(48):24100–24107. doi: 10.1073/pnas.1914143116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Huang PR, Liu WJ, Chen JQ, Hu YF, Wang YW, Sun JR, Feng JY. TRIM31 inhibits NLRP3 inflammasome and pyroptosis of retinal pigment epithelial cells through ubiquitination of NLRP3. Cell Biol Int. 2020;44(11):2213–2219. doi: 10.1002/cbin.11429. [DOI] [PubMed] [Google Scholar]
- 85.Moniruzzaman M, Ghosal I, Das D, Chakraborty SB. Melatonin ameliorates H2O2-induced oxidative stress through modulation of Erk/Akt/NFkB pathway. Biol Res. 2018;51(1):17. doi: 10.1186/s40659-018-0168-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Baek A, Yoon S, Kim J, Baek YM, Park H, Lim D, Chung H, Kim DE. Autophagy and KRT8/keratin 8 protect degeneration of retinal pigment epithelium under oxidative stress. Autophagy. 2017;13(2):248–263. doi: 10.1080/15548627.2016.1256932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Saadat KA, Murakami Y, Tan X, Nomura Y, Yasukawa T, Okada E, Ikeda Y, Yanagi Y. Inhibition of autophagy induces fcell damage by the lipofuscin fluorophore A2E. FEBS Open Bio. 2014;4:1007–1014. doi: 10.1016/j.fob.2014.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Netea MG, Balkwill F, Chonchol M, et al. A guiding map for inflammation. Nat Immunol. 2017;18(8):826–831. doi: 10.1038/ni.3790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Feng JH, Dong XW, Yu HL, Shen W, Lv XY, Wang R, Cheng XX, Xiong F, Hu XL, Wang H. Cynaroside protects the blue light-induced retinal degeneration through alleviating apoptosis and inducing autophagy in vitro and in vivo. Phytomedicine. 2021;88:153604. doi: 10.1016/j.phymed.2021.153604. [DOI] [PubMed] [Google Scholar]
- 90.Piippo N, Korhonen E, Hytti M, et al. Hsp90 inhibition as a means to inhibit activation of the NLRP3 inflammasome. Sci Rep. 2018;8(1):6720. doi: 10.1038/s41598-018-25123-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wang BW, Zuo X, Peng LL, et al. Melatonin ameliorates oxidative stress-mediated injuries through induction of HO-1 and restores autophagic flux in dry eye. Exp Eye Res. 2021;205:108491. doi: 10.1016/j.exer.2021.108491. [DOI] [PubMed] [Google Scholar]
- 92.Cho MH, Cho K, Kang HJ, Jeon EY, Kim HS, Kwon HJ, Kim HM, Kim DH, Yoon SY. Autophagy in microglia degrades extracellular β-amyloid fibrils and regulates the NLRP3 inflammasome. Autophagy. 2014;10(10):1761–1775. doi: 10.4161/auto.29647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wei C, Ma L, Xiang DM, Huang CX, Wang HJ, Wang X, Zhang S, Qi XL, Shi WY, Gao H. Enhanced autophagy alleviated corneal allograft rejection via inhibiting NLRP3 inflammasome activity. Am J Transplant. 2022;22(5):1362–1371. doi: 10.1111/ajt.16968. [DOI] [PubMed] [Google Scholar]
- 94.Singh S, Singh PK, Kumar A. Butyrate ameliorates intraocular bacterial infection by promoting autophagy and attenuating the inflammatory response. Infect Immun. 2023;91(1):e0025222. doi: 10.1128/iai.00252-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Shi HQ, Zhang Z, Wang XD, Li RS, Hou WW, Bi WJ, Zhang XM. Inhibition of autophagy induces IL-1β release from ARPE-19 cells via ROS mediated NLRP3 inflammasome activation under high glucose stress. Biochem Biophys Res Commun. 2015;463(4):1071–1076. doi: 10.1016/j.bbrc.2015.06.060. [DOI] [PubMed] [Google Scholar]
- 96.Buschini E, Piras A, Nuzzi R, Vercelli A. Age related macular degeneration and drusen: neuroinflammation in the retina. Prog Neurobiol. 2011;95(1):14–25. doi: 10.1016/j.pneurobio.2011.05.011. [DOI] [PubMed] [Google Scholar]
- 97.Fan JW, Shen WY, Lee SR, Mathai AE, Zhang R, Xu GZ, Gillies MC. Targeting the Notch and TGF-β signaling pathways to prevent retinal fibrosis in vitro and in vivo. Theranostics. 2020;10(18):7956–7973. doi: 10.7150/thno.45192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Groß CJ, Mishra R, Schneider KS, et al. K+ efflux-independent NLRP3 inflammasome activation by small molecules targeting mitochondria. Immunity. 2016;45(4):761–773. doi: 10.1016/j.immuni.2016.08.010. [DOI] [PubMed] [Google Scholar]
- 99.Brough D, le feuvre RA, Wheeler RD, Solovyova N, Hilfiker S, Rothwell NJ, Verkhratsky A. Ca2+ stores and Ca2+ entry differentially contribute to the release of IL-1 beta and IL-1 alpha from murine macrophages. J Immunol. 2003;170(6):3029–3036. doi: 10.4049/jimmunol.170.6.3029. [DOI] [PubMed] [Google Scholar]
- 100.Lin QS, Li S, Jiang N, et al. PINK1-parkin pathway of mitophagy protects against contrast-induced acute kidney injury via decreasing mitochondrial ROS and NLRP3 inflammasome activation. Redox Biol. 2019;26:101254. doi: 10.1016/j.redox.2019.101254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Hyttinen JMT, Blasiak J, Felszeghy S, Kaarniranta K. MicroRNAs in the regulation of autophagy and their possible use in age-related macular degeneration therapy. Ageing Res Rev. 2021;67:101260. doi: 10.1016/j.arr.2021.101260. [DOI] [PubMed] [Google Scholar]
- 102.Kaarniranta K, Blasiak J, Liton P, Boulton M, Klionsky DJ, Sinha D. Autophagy in age-related macular degeneration. Autophagy. 2023;19(2):388–400. doi: 10.1080/15548627.2022.2069437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kosmidou C, Efstathiou NE, Hoang MV, et al. Issues with the specificity of immunological reagents for NLRP3: implications for age-related macular degeneration. Sci Rep. 2018;8(1):461. doi: 10.1038/s41598-017-17634-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Wooff Y, Fernando N, Wong JHC, et al. Caspase-1-dependent inflammasomes mediate photoreceptor cell death in photo-oxidative damage-induced retinal degeneration. Sci Rep. 2020;10(1):2263. doi: 10.1038/s41598-020-58849-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Kauppinen A, Paterno JJ, Blasiak J, Salminen A, Kaarniranta K. Inflammation and its role in age-related macular degeneration. Cell Mol Life Sci. 2016;73(9):1765–1786. doi: 10.1007/s00018-016-2147-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Liu T, Wang LQ, Liang PP, et al. USP19 suppresses inflammation and promotes M2-like macrophage polarization by manipulating NLRP3 function via autophagy. Cell Mol Immunol. 2021;18(10):2431–2442. doi: 10.1038/s41423-020-00567-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Han XJ, Xu TS, Fang QJ, Zhang HJ, Yue LJ, Hu G, Sun LY. Quercetin hinders microglial activation to alleviate neurotoxicity via the interplay between NLRP3 inflammasome and mitophagy. Redox Biol. 2021;44:102010. doi: 10.1016/j.redox.2021.102010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Biasizzo M, Kopitar-Jerala N. Interplay between NLRP3 inflammasome and autophagy. Front Immunol. 2020;11:591803. doi: 10.3389/fimmu.2020.591803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Zhang YJ, Xu Y, Sun Q, Xue SD, Guan HJ, Ji M. Activation of P2X7R- NLRP3 pathway in retinal microglia contribute to retinal ganglion cells death in chronic ocular hypertension (COH) Exp Eye Res. 2019;188:107771. doi: 10.1016/j.exer.2019.107771. [DOI] [PubMed] [Google Scholar]
- 110.Shen K, Jiang WZ, Zhang CY, Cai LL, Wang Q, Yu HY, Tang ZY, Gu ZF, Chen BH. Molecular mechanism of a specific NLRP3 inhibitor to alleviate seizure severity induced by pentylenetetrazole. Curr Mol Pharmacol. 2021;14(4):579–586. doi: 10.2174/1874467213666200810140749. [DOI] [PubMed] [Google Scholar]
- 111.Xu T, Sheng LP, Guo XW, Ding Z. Free fatty acid increases the expression of NLRP3-Caspase1 in adipose tissue macrophages in obese severe acute pancreatitis. Dig Dis Sci. 2022;67(6):2220–2231. doi: 10.1007/s10620-021-07027-w. [DOI] [PubMed] [Google Scholar]
- 112.Sabnis RW. Pyridazine derivatives as NLRP3 inhibitors for treating asthma, COPD, Parkinson's disease, and Alzheimer's disease. ACS Med Chem Lett. 2023;14(8):1047–1048. doi: 10.1021/acsmedchemlett.3c00311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Ambrus-Aikelin G, Takeda K, Joetham A, et al. JT002, a small molecule inhibitor of the NLRP3 inflammasome for the treatment of autoinflammatory disorders. Sci Rep. 2023;13(1):13524. doi: 10.1038/s41598-023-39805-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Fang KM, Wang YL, Huang MC, Sun SH, Cheng H, Tzeng SF. Expression of macrophage inflammatory protein-1α and monocyte chemoattractant protein-1 in glioma-infiltrating microglia: involvement of ATP and P2X7 receptor. J Neurosci Res. 2011;89(2):199–211. doi: 10.1002/jnr.22538. [DOI] [PubMed] [Google Scholar]
- 115.Li PP, Li S, Wang L, Li HM, Wang Y, Liu HB, Wang X, Zhu XD, Liu ZS, Ye FL, Zhang Y. Mitochondrial dysfunction in hearing loss: oxidative stress, autophagy and NLRP3 inflammasome. Front Cell Dev Biol. 2023;11:1119773. doi: 10.3389/fcell.2023.1119773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Farré-Alins V, Narros-Fernández P, Palomino-Antolín A, et al. Melatonin reduces NLRP3 inflammasome activation by increasing α7 nAChR-mediated autophagic flux. Antioxidants. 2020;9(12):1299. doi: 10.3390/antiox9121299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Ando S, Hashida N, Yamashita D, et al. Rubicon regulates A2E-induced autophagy impairment in the retinal pigment epithelium implicated in the pathology of age-related macular degeneration. Biochem Biophys Res Commun. 2021;551:148–154. doi: 10.1016/j.bbrc.2021.02.148. [DOI] [PubMed] [Google Scholar]
- 118.Brandstetter C, Mohr LK, Latz E, Holz FG, Krohne TU. Light induces NLRP3 inflammasome activation in retinal pigment epithelial cells via lipofuscin-mediated photooxidative damage. J Mol Med. 2015;93(8):905–916. doi: 10.1007/s00109-015-1275-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Lv SY, Wang HG, Li XT. The role of the interplay between autophagy and NLRP3 inflammasome in metabolic disorders. Front Cell Dev Biol. 2021;9:634118. doi: 10.3389/fcell.2021.634118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Kosic M, Paunovic V, Ristic B, et al. 3-Methyladenine prevents energy stress-induced necrotic death of melanoma cells through autophagy-independent mechanisms. J Pharmacol Sci. 2021;147(1):156–167. doi: 10.1016/j.jphs.2021.06.003. [DOI] [PubMed] [Google Scholar]
- 121.Chen DG, Xie J, Fiskesund R, et al. Chloroquine modulates antitumor immune response by resetting tumor-associated macrophages toward M1 phenotype. Nat Commun. 2018;9(1):873. doi: 10.1038/s41467-018-03225-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Lee H, Koh JY. Roles for H+/K+-ATPase and zinc transporter 3 in cAMP-mediated lysosomal acidification in bafilomycin A1-treated astrocytes. Glia. 2021;69(5):1110–1125. doi: 10.1002/glia.23952. [DOI] [PubMed] [Google Scholar]
- 123.Querfurth H, Lee HK. Mammalian/mechanistic target of rapamycin (mTOR) complexes in neurodegeneration. Mol Neurodegener. 2021;16(1):44. doi: 10.1186/s13024-021-00428-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Mohamed NV, Plouffe V, Rémillard-Labrosse G, Planel E, Leclerc N. Starvation and inhibition of lysosomal function increased tau secretion by primary cortical neurons. Sci Rep. 2014;4:5715. doi: 10.1038/srep05715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Grandjean EM, Aubry JM. Lithium: updated human knowledge using an evidence-based approach: part III: clinical safety. CNS Drugs. 2009;23(5):397–418. doi: 10.2165/00023210-200923050-00004. [DOI] [PubMed] [Google Scholar]
- 126.Lee JJ, Chang-Chien GP, Lin SF, Hsiao YT, Ke MC, Chen A, Lin TK. 5-lipoxygenase inhibition protects retinal pigment epithelium from sodium iodate-induced ferroptosis and prevents retinal degeneration. Oxid Med Cell Longev. 2022;2022:1792894. doi: 10.1155/2022/1792894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Ko JH, Yoon SO, Lee HJ, Oh JY. Rapamycin regulates macrophage activation by inhibiting NLRP3 inflammasome-p38 MAPK-NFκB pathways in autophagy- and p62-dependent manners. Oncotarget. 2017;8(25):40817–40831. doi: 10.18632/oncotarget.17256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Barczuk J, Siwecka N, Lusa W, Rozpędek-Kamińska W, Kucharska E, Majsterek I. Targeting NLRP3-mediated neuroinflammation in Alzheimer's disease treatment. Int J Mol Sci. 2022;23(16):8979. doi: 10.3390/ijms23168979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Zhu R, Luo YY, Li SG, Wang ZB. The role of microglial autophagy in Parkinson's disease. Front Aging Neurosci. 2022;14:1039780. doi: 10.3389/fnagi.2022.1039780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Bian F, Yang XY, Xu G, Zheng T, Jin S. CRP-induced NLRP3 inflammasome activation increases LDL transcytosis across endothelial cells. Front Pharmacol. 2019;10:40. doi: 10.3389/fphar.2019.00040. [DOI] [PMC free article] [PubMed] [Google Scholar]


