Abstract
Introduction:
This investigation assessed the clinical characteristics of patients who received care through telemedicine and the clinical impact telemedicine service had on breast cancer patients in a low-income country.
Materials and Methods:
This natural experimental study assessed the impact of telemedicine service on cancer outcomes among breast cancer patients at Shaukat Khanum Memorial Trust (SKMT), Pakistan, between January 1st, 2018, to December 31st, 2022. The study group (hybrid group) consisted of patients that had both face-to-face and telemedicine appointments, and the control group (physical group) included patients with only face-to-face encounters.
Results:
A total of 3,205 patients were included in the analysis. Among those included in the analysis, 3,188 (99.5 %) were females, and the mean age of the cohort was 48.10 ± 11.94 years. Statistically significant differences were observed between the two groups in age, demographic distribution, disease stage, average number of emergency room visits, mean length of stay in the Intensive care unit, and the final patient status (alive at the end of observation period). However, the binary logistic regression model (forward-LR) suggested that the final patient outcome was related to disease relapse, COVID-19 infection, and age.
Conclusion:
Telemedicine clinics, when conducted in parallel with physical clinics (hybrid setup), are safe and have a clinical impact similar to having just physical encounters among breast cancer patients in a low-income country.
Keywords: Breast cancer, low-middle income countries (LMIC), oncology, telehealth, telemedicine
Introduction
Telemedicine uses telecommunication technology to deliver remote health-care services to patients.[1] It has been in practice for decades. However, the recent COVID-19 pandemic has been a catalyst for popularizing and integrating this into the routine standard for delivery of care.[1-3] Telemedicine may include audio, video, or text, alone or in various combinations depending on the mode of communication.[1] Prior investigations have shown telemedicine to improve access to care by decreasing commuting time, distance, and costs; reducing geographical inequities; and increasing follow-up rates among patients.[1,3,4]
Similar observations have been made in investigations assessing the impact of telemedicine on cancer care.[2,4] It has been shown to have a high satisfaction rate among patients and health-care practitioners, and the literature suggests that it improves the preventive and palliative aspects of cancer care.[3-5] Nonetheless, the literature on the impact and safety of telemedicine on cancer care is scarce.[6,7]
Shaukat Khanum Memorial Trust (SKMT), a not-for-profit organization with a network of cancer centers across Pakistan, launched a telemedicine service in April 2020 early in the COVID-19 pandemic. This investigation aims to review the clinical characteristics of patients who received care through telemedicine and to assess the clinical impact of initiating telemedicine services on cancer care among breast cancer patients in a low-income country.
Materials and Methods
Study design and setting
This investigation is a retrospective natural experiment that assesses the impact of telemedicine service on cancer outcomes among breast cancer patients who received care at SKMT, Pakistan, between January 1, 2018, and December 31, 2022. The local institutional review board approved this study (EX-13-01-23-01), and it was exempted from obtaining informed consent from the patients.
The SKMT operates two hospitals and an ambulatory care facility in Pakistan. In 2022, as an indicative example, nearly 300,000 patient encounters occurred in the outpatient setting, 70,000 sessions of chemotherapy were delivered, and almost 78,000 sessions of radiation therapy sessions were conducted. All sites of SKMT use a custom-built electronic medical record system consisting of patient registration, clinical information, order entry, and results viewing modules.
Subjects were identified from an electronic medical database. To be included in the study, subjects had to be adults (male or female) and have received care for breast cancer at one of the facilities of the Trust, between January 1, 2018, and December 31, 2022. Patients where primary cancer could not be assessed or those with carcinoma in situ diagnosis, according to the American Joint Committee on Cancer (AJCC), were excluded.[8]
Physical and telemedicine consultations
Before the COVID-19 pandemic, only physical (face-to-face) clinics were offered to patients. However, in April 2020, a telemedicine service was started, wherein patients on follow-up were seen in telemedicine clinics, while patients on active treatment or those who developed red flag symptoms, such as a new lesion, weight loss, neurological symptoms pain, or worsening of initial symptoms, were seen in person.
All patients were instructed to visit the hospital emergency assessment room (EAR) in case of any medical emergency. A set of clinical guidelines was developed for health-care practitioners conducting telemedicine clinics, which defined procedures for patient identification, awareness of red flags, documentation, prescription entry, and the procedure for ordering investigations. The guidelines also provided guidance on conducting culturally appropriate limited examination. Physicians were advised to limit the examination to the body parts that are typically visible and uncovered and not to prompt patients to remove any clothing other than to roll up a sleeve as far as the elbow or a trouser/pyjama leg as far as the knee.
The study (hybrid) group was composed of patients who had face-to-face appointments as well as telemedicine appointments. These individuals were seen at our hospitals for initiation of breast cancer treatment between January 2021 and December 2021. On the other hand, the control (physical) group consisted of patients who only had face-to-face encounters. They came to our hospitals for breast cancer treatment between January 2018 and December 2018, as shown in Figure 1.
Figure 1.
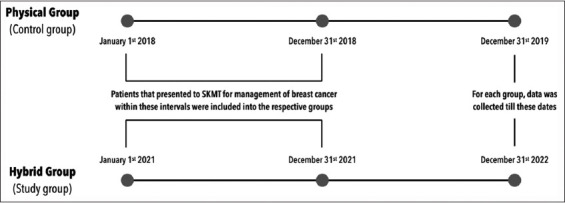
Illustration of study design. For both groups, the recruitment phase was 12 months, followed by observance for outcomes till a fixed term
To be accepted for breast cancer treatment at SKMT, all patients had to have undergone physical examination, biopsy, pathological and laboratory testing (including estrogen and progesterone receptor status and HER2 status), and imaging studies to allow for accurate TNM disease staging according to the AJCC TNM system.[8] All patients are discussed in a multidisciplinary team meeting before initiation of treatment and treated accordingly. For all patients, the initial appointment was physical. In the hybrid group, follow-up appointments were a combination of telemedicine and face-to-face clinics.
At a routine physical encounter (consultations and follow-ups), all patients had assessment of vital signs (blood pressure, pulse and respiratory rate and pain, and if indicated, temperature), anthropometric measurements (height and weight), and a structured history and physical examination. While most telemedicine appointments were conducted primarily through audio-only communication, by telephone, audiovisual consultation was performed when required, such as when an inspection of a surgical wound or lesion was needed, so long as the patient had a “smart” device. Audiovisual consultations were made through the video call function of the WhatsApp application. During telemedicine encounters, all patients had a structured history and review of any pathological or imaging investigations.
Study variables and data collection
The data of all participants were de-identified. Demographic information, age, gender, body mass index, education, and diagnostic and therapeutic characteristics of breast cancer (staging, recurrence, number of chemotherapy regimens, number of surgical procedures, and radiation therapy plans) were extracted. Similarly, information on hospital admissions, including length of stay (total and in the intensive care unit), number of telemedicine and physical clinic appointments, number of visits to the EAR, history of relapse, and history of COVID-19 infection, were recorded. The endpoint of interest, final patient status, was defined as the percentage of patients alive at the end of the observation period. This variable was used as a surrogate of the clinical impact. Data were collected till December 31, 2019, for the physical group and December 31, 2022, for the hybrid group [Figure 1].
Statistical analysis
Data analysis was performed using the Statistical Package for the Social Sciences (SPSS) version 28.0 software (SPSS Inc. Chicago, IL, USA). Descriptive statistics were computed for each variable. Chi-square and t-tests were used for analysis between independent and dependent variables. However, if assumptions for the Chi-square test were not met, Fisher’s exact test was used. The logistic regression analysis model was used to assess the simultaneous impact of all the variables on the final patient status (alive at the end of the observation period). P < 0.05 was considered statistically significant.
Results
A total of 3,303 patients with breast cancer were identified and met the inclusion criteria. However, 98 patients had carcinoma in situ and were excluded from further analysis.
Among those included in the analysis, 3,188 (99.5%) were females, and the mean age of the cohort was 48.10 ± 11.94 years. The age of patients in the physical group was significantly higher than that of participants in the hybrid group (P < 0.001). No statistical difference in gender distribution was noted between the two groups. The proportion of patients from Sindh and Baluchistan was higher in the physical group (7.3% and 1.3%) than in the hybrid group (5% and 0.6%). These differences in demographic distribution were statistically significant (P < 0.05). The majority of the patients in both cohorts had stage II disease. However, in the hybrid group, nearly a quarter (24.7%) of the patients had advanced-stage disease (stages III and IV). On the contrary, 18.4% of the patients had advanced-stage disease in the physical group. These differences were statistically significant [Table 1].
Table 1.
Breakdown of demographic and diagnostic characteristics of the study population

The average number of visits to the EAR in the physical group (1.24 ± 2.28) was significantly lower than in the hybrid group (2.74 ± 3.96). No difference in the average length of stay in the hospital was observed between the two groups. However, the mean length of stay in the intensive care unit was significantly higher for the patients in the hybrid group [Table 2]. There was no difference between the two groups in disease relapse. However, the proportion of patients who were alive at the end of the observation period was significantly higher for the physical group (97.5%) than the hybrid group (95.9%).
Table 2.
Breakdown of hospital visits and medical and surgical history of the study population (N=3205)

A binary logistic regression model (forward LR) suggested that the final patient status was statistically related to disease relapse, COVID-19 infection, and age [Table 3].
Table 3.
Forward logistic regression analysis-generated model for final patient status (alive at the end of the observation period)

Discussion
This study aimed to review clinical characteristics and the clinical impact of initiating telemedicine services on cancer care in breast cancer patients in a low-income country. In 2020, the COVID-19 pandemic, with its attendant lockdowns, need for quarantine, and social distancing, compelled health-care organizations worldwide to employ telemedicine to deliver patient care. The modes of telemedicine offered differed depending on the availability of resources, patient and physician technical proficiency, regional health-care system, regulatory environment, and local culture. At SKMT, telemedicine clinics were started in April 2020. These clinics ran in parallel with the physical (face-to-face) meetings. In nearly all situations, the mode of telemedicine consultation was audio alone due to poor technical quotient and indigent population. By policy, these encounters were reserved for follow-up appointments.
The average age of the study cohort at the time of diagnosis was 48.10 ± 11.94 years, much younger than the average global age of onset of breast cancer.[9] Prior investigations have shown that patients of South Asian descent tend to have an earlier age of breast cancer presentation than the rest of the world.[9,10] The exact reason for this is unknown but may be related to environmental factors.[10]
We found a statistical difference in age between the two groups, the average age of patients in the physical group being older than those seen in the hybrid group. Recently, some studies investigating the impact of the COVID-19 pandemic on the presentation of cancer patients have reported similar observations,[11,12] while others have reported no difference or opposite findings.[13,14] This disparity in findings is likely associated with the impact of the disease on the local population, local COVID-19-related regulations and restrictions, education, ethno-cultural differences, socioeconomics, health-care environment, and variance in screening and treating guidelines.[2,15,16]
The percentage of patients with advanced-stage disease (stages III and IV) was much higher in the hybrid group. The hybrid group consisted of patients who presented during the pandemic. Other investigators found similar results, where patients who presented during the pandemic tended to have more advanced-stage disease.[12,17] This variance in stage composition of the two groups may exist due to limited access to health-care facilities secondary to COVID-19-related travel restrictions, deferment and reduction in screening appointments, and avoidance of visiting hospitals for fear of catching the infection.
The number of emergency room visits was higher among the hybrid group participants. This could be due to multiple reasons, including but not limited to physicians asking patients with sinister symptoms to visit EAR for physical assessment. Nearly 10% of the patients in the hybrid group acquired COVID-19 infection, which could have contributed to them visiting EAR for care or secondary to chemotherapy or radiotherapy-related side effects (hybrid group patients had a higher number of chemotherapy cycles, and a greater percentage received radiation therapy). Similarly, patients may have presented to the EAR if any symptoms were missed during the telemedicine consultation, if they felt the care they received in the telemedicine clinic was sub-optimal, or if they craved the human touch.
The relapse rate was nearly the same in both groups, but the proportion of patients who remained alive differed significantly between the two groups. Further analysis disclosed no effect of the type of arrangement (physical or hybrid groups) in which these patients were seen. The logistic regression model suggested that the occurrence of disease relapse reduced the odds of remaining alive by 6.5 times, and COVID-19 infection decreased the odds by 2.8 times. The model also suggested a statistical correlation between age and the outcome of interest. With each increase in age by 1 year, the odds of survival were lowered by 1.04 times. Even though these odds were statistically significant, they are unlikely to have a noticeable clinical impact. Others have reported similar results.[18-23] The mortality rate has been shown to increase after recurrence. However, the risk varies among patients. Factors such as node positivity, progesterone receptor negativity, younger age at recurrence, and short time from diagnosis to recurrence tend to increase the odds of death in patients with disease relapse.[21,24] Acquiring COVID-19 infection reduced the percentage of patients who remained alive among the study cohorts. COVID-19 infection can cause chemotherapy resistance development in patients with breast cancer, and tamoxifen can induce susceptibility to catching the infection.[23]
This study has some limitations. It was not possible to account for all the variables that may have influenced breast cancer outcomes, such as a prior family history of breast cancer, age of menarche, medical history, or reproductive history. In addition, as a retrospective analysis, there is an inherent risk of bias, false-positive association, or magnification of responses. Nevertheless, this is a natural experimental study. The circumstances (COVID-19) surrounding the implementation of the intervention (hybrid model) were not under the control of the authors. A randomized control design represents the most robust experimental design when inferring causation from an intervention. However, when such a design is not possible (in the case of the natural experimental study), a basic quasi-experimental design, such as the one practiced in the present study, provides a robust alternative.[25] Similarly, a regression-based analytical modeling method was followed. This addressed the lack of randomization, which substantially reduces potential individual-level and confounder bias and improves the internal validity of this study. Finally, data were sourced from the electronic health-care system, using automated extraction analysis, to prevent any potential inaccuracies stemming from reporting or recall bias.
After the onset of the COVID-19 pandemic, like other health-care networks, we were pushed to institute telemedicine service for our patients. The service mode was primarily audio alone, as the cohort of patients SKMT serves are predominantly from a low socioeconomic background. These clinics ran in parallel with physical (face-to-face) meetings and were predominantly reserved for follow-up appointments. In low-income countries like ours, patients often travel hours or thousands of kilometers with family members to seek care. Although over 75% of all cancer patients treated at our hospitals receive all treatment completely free of charge, travel and accommodation costs are still high and can act as a barrier to care. Telemedicine has been of great benefit in lifting geographical barriers and economic restrictions. Our findings suggest that this hybrid setup was safe and had an impact similar to just conducting physical encounters among breast cancer patients.
Acknowledgment
None.
Footnotes
Funding: None.
Author Contributions
Conceived and designed the analysis: KSN, FB, and MAY; Collected the data: KSN and FB; Contributed data or analysis tools: KSN and FB; Performed the analysis: KSN and FB; Wrote the paper: KSN and MAY.
Conflict of Interest:
The author(s) declare(s) that there are no conflict of interest.
References
- 1.Sirintrapun SJ, Lopez AM. Telemedicine in cancer care. Am Soc Clin Oncol Educ Book. 2018;38:540–5. doi: 10.1200/EDBK_200141. [DOI] [PubMed] [Google Scholar]
- 2.Royce TJ, Sanoff HK, Rewari A. Telemedicine for cancer care in the time of COVID-19. JAMA Oncol. 2020;6:1698–9. doi: 10.1001/jamaoncol.2020.2684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yadav K, Ginsburg O, Basu P, Mehrotra R. Telemedicine and cancer care in low- and middle-income countries during the SARS-CoV-2 pandemic. JCO Glob Oncol. 2021;7:1633–8. doi: 10.1200/GO.21.00249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gondal H, Abbas T, Choquette H, Le D, Chalchal HI, Iqbal N, et al. Patient and physician satisfaction with telemedicine in cancer care in Saskatchewan:A cross-sectional study. Curr Oncol. 2022;29:3870–80. doi: 10.3390/curroncol29060309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Roque K, Ruiz R, Otoya-Fernandez I, Galarreta J, Vidaurre T, de Mello RA, et al. The impact of telemedicine on cancer care:Real-world experience from a Peruvian institute during the COVID-19 pandemic. Future Oncol. 2022;18:3501–8. doi: 10.2217/fon-2022-0239. [DOI] [PubMed] [Google Scholar]
- 6.Novara G, Checcucci E, Crestani A, Abrate A, Esperto F, Pavan N, et al. Telehealth in urology:A systematic review of the literature. How much can telemedicine be useful during and after the COVID-19 pandemic? Eur Urol. 2020;78:786–811. doi: 10.1016/j.eururo.2020.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Panet F, Tétreault-Langlois M, Morin V, Sultanem K, Melnychuk D, Panasci L. The risks associated with the widespread use of telemedicine in oncology:Four cases and review of the literature. Cancer Rep (Hoboken) 2022;5:e1531. doi: 10.1002/cnr2.1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Edge SB, Compton CC. The American joint committee on cancer:The 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol. 2010;17:1471–4. doi: 10.1245/s10434-010-0985-4. [DOI] [PubMed] [Google Scholar]
- 9.Bidoli E, Virdone S, Hamdi-Cherif M, Toffolutti F, Taborelli M, Panato C, et al. Worldwide age at onset of female breast cancer:A 25-year population-based cancer registry study. Sci Rep. 2019;9:14111. doi: 10.1038/s41598-019-50680-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Satagopan JM, Stroup A, Kinney AY, Dharamdasani T, Ganesan S, Bandera EV. Breast cancer among Asian Indian and Pakistani Americans:A surveillance, epidemiology and end results-based study. Int J Cancer. 2021;148:1598–607. doi: 10.1002/ijc.33331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alrahawy M, Johnson C, Aker M, Eltyeb HA, Green S. Impact of COVID-19 on the mode of presentation and stage at diagnosis of colorectal cancer. Cureus. 2022;14:e32037. doi: 10.7759/cureus.32037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.İlgün AS, Özmen V. The impact of the COVID-19 pandemic on breast cancer patients. Eur J Breast Health. 2022;18:85–90. doi: 10.4274/ejbh.galenos.2021.2021-11-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nyante SJ, Benefield TS, Kuzmiak CM, Earnhardt K, Pritchard M, Henderson LM. Population-level impact of coronavirus disease 2019 on breast cancer screening and diagnostic procedures. Cancer. 2021;127:2111–21. doi: 10.1002/cncr.33460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Miller MM, Meneveau MO, Rochman CM, Schroen AT, Lattimore CM, Gaspard PA, et al. Impact of the COVID-19 pandemic on breast cancer screening volumes and patient screening behaviors. Breast Cancer Res Treat. 2021;189:237–46. doi: 10.1007/s10549-021-06252-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Elkaddoum R, Haddad FG, Eid R, Kourie HR. Telemedicine for cancer patients during COVID-19 pandemic:Between threats and opportunities. Future Oncol. 2020;16:1225–7. doi: 10.2217/fon-2020-0324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shirke MM, Shaikh SA, Harky A. Implications of telemedicine in oncology during the COVID-19 pandemic. Acta Biomed. 2020;91:e2020022. doi: 10.23750/abm.v91i3.9849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Greene G, Griffiths R, Han J, Akbari A, Jones M, Lyons J, et al. Impact of the SARS-CoV-2 pandemic on female breast, colorectal and non-small cell lung cancer incidence, stage and healthcare pathway to diagnosis during 2020 in Wales, UK, using a national cancer clinical record system. Br J Cancer. 2022;127:558–68. doi: 10.1038/s41416-022-01830-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhou Y, Yang Q, Ye J, Wu X, Hou X, Feng Y, et al. Clinical features and death risk factors in COVID-19 patients with cancer:A retrospective study. BMC Infect Dis. 2021;21:760. doi: 10.1186/s12879-021-06495-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lafourcade A, His M, Baglietto L, Boutron-Ruault MC, Dossus L, Rondeau V. Factors associated with breast cancer recurrences or mortality and dynamic prediction of death using history of cancer recurrences:The French E3N cohort. BMC Cancer. 2018;18:171. doi: 10.1186/s12885-018-4076-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mauguen A, Rachet B, Mathoulin-Pélissier S, Lawrence GM, Siesling S, MacGrogan G, et al. Validation of death prediction after breast cancer relapses using joint models. BMC Med Res Methodol. 2015;15:27. doi: 10.1186/s12874-015-0018-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dent R, Valentini A, Hanna W, Rawlinson E, Rakovitch E, Sun P, et al. Factors associated with breast cancer mortality after local recurrence. Curr Oncol. 2014;21:e418–25. doi: 10.3747/co.21.1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.San Miguel Y, Gomez SL, Murphy JD, Schwab RB, McDaniels-Davidson C, Canchola AJ, et al. Age-related differences in breast cancer mortality according to race/ethnicity, insurance, and socioeconomic status. BMC Cancer. 2020;20:228. doi: 10.1186/s12885-020-6696-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vatansev H, Kadiyoran C, Cumhur Cure M, Cure E. COVID-19 infection can cause chemotherapy resistance development in patients with breast cancer and tamoxifen may cause susceptibility to COVID-19 infection. Med Hypotheses. 2020;143:110091. doi: 10.1016/j.mehy.2020.110091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bhatti AB, Jamshed A, Shah M, Khan A. Breast conservative therapy in Pakistani women:Prognostic factors for locoregional recurrence and overall survival. J Cancer Res Ther. 2015;11:300–4. doi: 10.4103/0973-1482.140828. [DOI] [PubMed] [Google Scholar]
- 25.Leatherdale ST. Natural experiment methodology for research:A review of how different methods can support real-world research. Int J Soc Res Methodol. 2019;22:19–35. [Google Scholar]


