Abstract
Drug addiction is considered a worldwide concern and one of the most prevailing causes of death globally. Opioids are highly addictive drugs, and one of the most common opioids that is frequently used clinically is fentanyl. The potential harmful effects of chronic exposure to opioids on the heart are still to be elucidated. Although β-lactam antibiotics are well recognized for their ability to fight bacteria, its protective effect in the brain and liver has been reported. In this study, we hypothesize that β-lactam antibiotic, ceftriaxone, and the novel synthetic non-antibiotic β-lactam, MC-100093, are cardioprotective against fentanyl induced-cardiac injury by upregulating xCT expression. Mice were exposed to repeated low dose (0.05 mg/kg, i.p.) of fentanyl for one week and then challenged on day 9 with higher dose of fentanyl (1 mg/kg, i.p.). This study investigated cardiac histopathology and target genes and proteins in serum and cardiac tissues in mice exposed to fentanyl overdose and β-lactams. We revealed that fentanyl treatment induced cardiac damage as evidenced by elevated cardiac enzymes (troponin I). Furthermore, fentanyl treatment caused large aggregations of inflammatory cells and elevation in the areas and volumes of myocardial fibers, indicating hypertrophy and severe cardiac damage. Ceftriaxone and MC-100093 treatment, However, induced cardioprotective effects as evidenced by marked reduction in cardiac enzymes (troponin I) and changes in histopathology. Furthermore, ceftriaxone and MC-100093 treatment decreased the levels of hypertrophic genes (α-MHC & β-MHC), apoptotic (caspase-3), and inflammatory markers (IL-6 & NF-κB). This study reports for the first time the cardioprotective effect of β-lactams against fentanyl-induced cardiac injury. Further studies are greatly encouraged to completely identify the cardioprotective properties of ceftriaxone and MC-100093.
Keywords: Cardiotoxicity, Apoptosis, Fentanyl, Ceftriaxone, MC-100093, xCT, Glutamate transporter-1
1. Introduction
Despite the recent therapeutic approaches for cardiovascular diseases (CVDs), these diseases remain the major cause of death, accounting for approximately 30 % of deaths globally (Di Cesare et al., 2024). CVDs are considered the major disease burden globally, with the total medical costs of CVDs were almost $555 billion in 2015 and predicted to increase to nearly $1 trillion by 2035 (Mathers and Loncar, 2006, Dunbar et al., 2018 WHO, 2020). These disturbing statistics showing multiplied rates of morbidity and mortality for CVDs, together with the increased medical costs for CVDs patients, are the most critical healthcare issues these days.
Drug addiction, particularly opioid addiction, continues to be of epidemic proportions across the world (Nakhaee et al., 2020). Millions of people die annually as a consequence of addiction and drug misuse (D'Souza 2015). Drug misuse alters normal glutamate levels, forcing the brain to make adjustments that can negatively impact cognitive function (D'Souza 2015). The United Nations (UN) stated that nearly 6 % of individuals between the ages of 15 and 64 had used drugs in 2021 around the world (United Nations, 2023). It is documented that the number of addicted people multiplied from nearly 240 million in 2011 to over 295 million in 2021 (United Nations, 2023). In Saudi Arabia, the 1-year prevalence of psychological disorders linked to drug abuse was almost 2 % (Altwaijri et al., 2020). Recent report stated that opioids continue to be responsible for death from overdoses (United Nations, 2023). In 2021, about 32 million people consumed opiates, chiefly heroin, and more than 59 million individuals used opioids for nonmedical reasons (United Nations, 2023). The health impact of drugs differs depending on the frequency of use and the tendency for addiction. Opioids are the main addicted drugs in most regions of Europe and some regions in Asia, while the major drugs of abuse in Latin America is cocaine, methamphetamines in East and Southeast Asia, and cannabis in some areas of Africa (United Nations, 2023). Although drug addiction can be fatal and causes catastrophic medical and economic issues, CVDs continue to be the most common causes of death globally (Dimmeler 2011).
Fentanyl is considered one of the most commonly used opioids clinically (Badar et al., 2022). It is an exceptionally synthetic opioid that has greater analgesic effects compared to morphine (Suzuki and El-Haddad, 2017, Ziesenitz et al., 2018, Ramos-Matos et al., 2024). This powerful pharmacological substance usually has a potency 50 to 100 times that of morphine (Suzuki and El-Haddad, 2017, Ziesenitz et al., 2018, Ramos-Matos et al., 2024). Fentanyl has extremely distinct characteristics and pharmacokinetics profile than morphine. Like other opioids, fentanyl produces its effects through binding to mu-opioid receptor. Fentanyl primarily binds to a sub-class of opioid receptors in the brain and contributes to the control of emotions and pain. Nonetheless, it also has the capability to activate other opioid receptors, including the delta (δ) and, possibly, the kappa (κ) receptors (Suzuki and El-Haddad, 2017, Ziesenitz et al., 2018, Ramos-Matos et al., 2024). Respiratory arrest, which resulted from respiratory depression, and subsequent cardiac death, is a predictable complication of the use of opiates. Moreover, it has been determined that opioids prevent the potassium (K+) and sodium (Na+) channels that are required in pacemaker potential (Riley et al., 2020, Badar et al., 2022). This may lead to cardiac death since it blocks repolarization, develops numerous cardiac intervals, and extends several action potential phases, a condition that is recognized to be opioid cardiotoxicity (Riley et al., 2020, Badar et al., 2022).
Opioids have powerful effects on the nervous systems and cardiovascular system, and also have the ability to control the heart rate and blood pressure. The response of the cardiovascular system to an opioid receptor’s agonist is complex and significantly impacted by the conditions of exposure to the opioid and the agonist (Krantz et al., 2021). However, acute opioid receptor activation is well-known for mediating cardiovascular symptoms such as bradycardia, orthostasis, and hypotension (Krantz et al., 2021). It is documented that respiratory and cardiac depression are the mechanisms by which opioids lead to cardiac arrest. Respiratory depression is an ordinary consequence of addiction to opiates, as it disrupts the potassium (K+) and sodium (Na+) channels essential for the pacemaker potential (Ritter et al., 2022). This process could lead to cardiac death since it blocks repolarization, develops numerous cardiac intervals, and extends several action potential phases. It has been documented that the xCT sub-unit of the xc− system is decreased in content and function in opioid withdrawal, which participates significantly in the induction of cardiotoxicity in response to opioids exposure (McClure et al., 2014).
Ceftriaxone is a third-generation, broad-spectrum, β-lactam, antibiotic that is indicated for the treatment of various bacterial infections (Lamb et al., 2002). Lately, several reports demonstrated that GPCRs (G-protein–coupled receptors), for instance glutamate receptors, are influenced by ceftriaxone and that ceftriaxone can offer neuroprotection through improving glutamate uptake and glutamate transporter-1 (GLT-1) expression, thus diminish glutamate toxicity (D'Souza, 2015, Wilkie et al., 2021, Abulseoud et al., 2022). Moreover, it was documented that ceftriaxone significantly induced xCT expression, which could justify the protective effect of ceftriaxone (Rao et al., 2015, Smaga et al., 2020, Das et al., 2022). Cardioprotective medications, for example ceftriaxone, are essential in treating patients at risk for or with recognized cardiovascular disorders as they have minimal levels of harmfulness and an absolute safety profile. Nevertheless, the defined signaling mechanisms via which ceftriaxone produces its protective effect are yet to be explained. Less is described about the signaling mechanisms engaged in cardioprotective effects of ceftriaxone or novel synthetic β-lactam, MC-100093, against fentanyl-induced cardiac toxicity. Therefore, we hypothesized that ceftriaxone and MC-100093 may induce cardioprotection against fentanyl-induced cardiac toxicity by upregulating xCT expression. Results of the current study may provide ample information to produce new but effective medical approaches to avoid cardiac injury and death.
2. Materials and methods
2.1. Materials
Ceftriaxone was obtained from the Sandoz Pharmaceutical Company (Switzerland) (purity > 99 %) and MC-100093 was provided by Temple University, USA (purity: >99 %). CK-MB and cardiac troponin I ELISA kits were purchased from ELK Biotechnology Company (USA). TRIzol reagent was obtained from Ambion (USA). Kits for cDNA reverse transcription and the SYBR Green PCR Master Mix were obtained from ThermoFisher Scientific (USA) and BIMAKE (USA), respectively. Primers for mice (i.e., IL-6, α-MHC, β-MHC, SOD, GLT-1, xCT, Cas-3, and NF-κB) were manually designed and purchased from Integrated DNA Technologies (IDT; Belgium). For western blot reagents, primary and secondary antibodies were purchased from ELK Biotechnology Company (USA) and Fine Biotech Company (China). RIPA lysis buffer and cocktail protease inhibitors were obtained from ThermoFisher Scientific (USA).
2.2. Experimental models
Male 8–10-week-old BALB/c mice weighing 25 to 30 g were selected for our study. The mice were acquired from the Animal Care Center of the College of Pharmacy at King Saud University, Riyadh, Saudi Arabia. Animals were maintained in an air-conditioned room under standard laboratory conditions: 50 ± 5 % humidity, 23 ± 2 °C temperature, with a 12 h–12 h light–dark cycle in the Laboratory Animal Care Center, College of Pharmacy, King Saud University, and were granted unrestricted access to standard rodent chow and water ad libitum. At 12 h preceding the experiment, food was forbidden for all the animals, but they were allowed free access to water. All the defined protocols and procedures implemented in this study were approved by the IACUC committee at KSU (reference number: KSU-SE-22–48).
2.3. Study design
The animals were randomly placed in four groups of 10 mice each. A summary of the experimental study design is shown in Fig. 1.
Fig. 1.
Experimental Study Design.
Group 1 (control): Mice in this group were treated daily via intraperitoneal (i.p.) injection with 0.9 % NaCl on days 1, 3, 5, 6, 7, 8, and 9.
Group 2 (Fen): Mice in this group were given fentanyl i.p. (0.05 mg/kg) on days 1, 3, 5, and 7, and then on day 9, the mice were given a fentanyl overdose of 1 mg/kg i.p. (Uddin et al., 2021, Alasmari et al., 2023).
Group 3 (Fen + MC): Mice in this group were given fentanyl (0.05 mg/kg i.p.) on days 1, 3, 5, and 7, and then on day 9, the mice were given a fentanyl overdose of 1 mg/kg i.p. They were also given MC-100093 from days 5 to 9 at a dosage of 50 mg/kg i.p. (Uddin et al., 2021, Alasmari et al., 2023).
Group 4 (Fen + Cef): Mice in this group were given fentanyl (0.05 mg/kg i.p.) on days 1, 3, 5, and 7, and then on day 9, the mice were given a fentanyl overdose of 1 mg/kg i.p.
They were also given ceftriaxone from days 5 to 9 in a dosage of 200 mg/kg i.p. (Hill et al., 2020, Knackstedt et al., 2021, Uddin et al., 2021, Alasmari et al., 2023).
To carefully mimic the human opioid exposure in our study, we implemented the dosing protocol of intermittent exposure to fentanyl as described previously (Uddin et al., 2021). No signs of toxicity were observed during the duration of treatment.
On day 9, mice were anesthetized using ketamine/xylazine (i.p.) (Wellington et al., 2013). Blood samples from all mice were collected, and the serum was extracted to conduct various cardiac enzyme measurements. Subsequently, heart tissues from all animals were dissected for various experiments. Three heart tissues from each group were fixed in 4 % formaldehyde solution for histopathological analyses, while the other heart tissues were stored for other experiments. The heart to body weight ratio (HW/BW) was utilized in this study as an index of myocardial mass (Tracy and Sander, 2011, Katare et al., 2017).
2.4. Measuring cardiac enzymes
To isolate the serum from the blood samples, coagulated blood was centrifuged at 2000 g for 10 min at 4 °C. Enzyme-linked immunosorbent assay (ELISA) kits were used to assess the cardiac troponin I (cTn-I) and creatine kinase MB isoenzyme (CK-MB) according to the manufacturer’s protocol.
2.5. Histopathological assessments
Heart tissues were dissected and washed three-times with PBS (phosphate-buffered saline), fixed in 10 % formalin, and embedded in paraffin. The embedded samples were cut into thin cross-sections of 3 mm using a microtome and stained with Masson’s trichrome stain and hematoxylin and eosin (H&E) to examine the heart morphology. The tissues were analyzed and imaged using the Olympus light microscope (BX-50; Olympus, Tokyo, Japan). Sections stained by H&E and Masson’s trichrome were subjected to the histopathological scoring system of cardiac muscles based on the following criteria: disorganization, inflammation, degeneration, vasocongestion, and fibrosis and scored as 0, normal; 1, mild; 2, moderate; or 3, severe; the minimum score was 0 and the maximum score was 15 (Demirel et al., 2023).
2.6. Gene expression study
Following the manufacturer’s directions, the total cellular RNA was isolated from the cardiac tissues using the TRIzol reagent. The level of purity and the quantity of the extracted RNA were confirmed using a NanoDrop 8000 spectrophotometer. After that, 1 µg of the extracted RNA was reverse transcribed to cDNA using a reverse transcription kit. The expressions of various genes were quantified using manually designed primers (Table 1) via the 7500 Fast Real-Time PCR system with SYBR Green Master Mix. The housekeeping gene β-actin was used to standardize the data. To determine the relative levels of mRNA expression, the ΔΔCt method was used.
Table 1.
List of primers for different genes used in the gene analysis.
| Gene | Primer sequence (5′-3′) | Product length (bp) |
Accession number |
|---|---|---|---|
| SOD | Forward: GGTGAACCAGTTGTGTTGTCAGGA Reverse: TGCGCAATCCCAATCACTCCA |
383 | NM_011434.2 |
| xCT | Forward: TGTAGAGCCAGTCGGTGATAGCAA Reverse: GAGATAATATGCAGGGACCCCAGTCA |
1566 | NM_011990.2 |
| GLT-1 | Forward: AGGTAGAAGTGCGCATGCATGA Reverse: GGTGGCTGTAAGGCTTACAGTCACAA |
1259 | NM_001077514.4 |
| NF-κB | Forward: CTTATGTGGAGATCATCGAACAGCCG Reverse: ACAGATGCCAGGTCTGTGAACACT |
1348 | NM_001402548.1 |
| IL-6 | Forward: GCCCACCAAGAACGATAGTCAATTCCAG Reverse: GAATTGGATGGTCTTGGTCCTTAGCC |
612 | NM_031168.2 |
| α-MHC | Forward: GAGTTTGAGTGACAGAATGACGGACG Reverse: TTGATGCGTGTCACCATCCAGTTG |
1350 | NM_001164171.1 |
| β-MHC | Forward: TTGAGAATCCAAGGCTCAGCCATG Reverse: ACGCATAATCGTAGGGGTTGTTGG |
955 | NM_001361607.1 |
| β-Actin | Forward: CATGGCATTGTTACCAACTGGGACG Reverse: TTGGTCTCAAGTCAGTGTACAGGCC |
1565 | NM_007393.5 |
2.7. Protein expression study
The expression levels of various proteins were assessed by western blot analysis. In brief, the heart tissues from the different groups were homogenized in ice-cold RIPA lysis buffer enhanced with phosphatase and a protease cocktail inhibitor. Next, a corresponding volume of proteins was electrophoresed using a SDS-PAGE gel and transferred to a PVDF membrane. The membranes were gently rocked while being blocked at room temperature with 5 % non-fat dry milk. The membranes were kept in a primary antibody at 4 °C overnight at a dilution of 1:1000. The primary antibodies used were mouse anti-nuclear factor kappa B (NF-κB) antibody, mouse anti-superoxide dismutase (SOD) antibody, mouse anti-cleaved caspase-3 (CAS-3) antibody, mouse anti-interleukin 6 (IL-6) antibody, mouse anti-cystine/glutamic acid reverse transporter (xCT), mouse anti-glutamate transporter-1 (GLT-1), rabbit anti-Toll-like receptor 4 (TLR-4), mouse anti-Toll-like receptor 2 (TLR-2), and mouse anti-β-actin antibody. The membranes were kept at room temperature with the appropriate HRP-conjugated secondary antibody (1:5000). The membranes were visualized and imaged using a chemiluminescence reagent and Bio-Rad gel-imaging system, respectively.
2.8. Measurement of glutamate level
To measure the glutamate level, a commercially available kit was used as per the manufacturer’s protocol (MyBioSource, USA). In brief, the heart tissues were homogenized with 1 ml of the extraction buffer and centrifuged at 8000 g for 10 min at room temperature, and the supernatant was measured at a wavelength of 570 nm. The results were expressed as nanomoles of glutamate formed per milligram of protein.
2.9. Measurement of lipid peroxidation
The lipid peroxidation was measured using a specialized kit (MyBioSource) according to the described protocol. A mixture of thiobarbituric acid, trichloroacetic acid, and tissue homogenates was incubated for 30 min at 90 °C in a shaking water bath. The samples were kept on ice for 10 min and then centrifuged in a refrigerated centrifuge for 15 min at 3000 g. The wavelength was measured at 540 nm. The results were expressed as nanograms of malondialdehyde (MDA) generated per milligram of protein.
2.10. Measurement of reduced glutathione
To estimate the glutathione (GSH) content in the heart tissue, 1 ml of postmitochondrial supernatant (PMS) was precipitated with sulphosalicylic acid (4 %) as per the manufacturer’s protocol (MyBioSource). The samples were kept at 4 °C for 1 h and then centrifuged at 3000 rpm for 15 min at 4 °C. The absorbance of the assay mixture was measured within 5 min of the addition of DTNB at 412 nm using a UV-spectrophotometer. The GSH content was expressed as nanomoles of GSH per gram of tissue.
2.11. Measurement of catalase activity
The PMS from the heart tissue was used to estimate the catalase (CAT) activity according to the guided protocol (MyBioSource). Briefly, the absorbance of the reaction mixture of phosphate buffer, hydrogen peroxide, and PMS was measured at intervals of 1 min for 5 min at 240 nm.
2.12. Statistical analysis
Data were displayed as mean ± standard deviation (SD). Data were evaluated using a one-way analysis of variance (ANOVA), followed by Tukey’s post-hoc test, to compare the means of the various experimental groups with the mean of the control group using GraphPad Prism version 6.01 (Prism, USA). The level of significance was a p-value of 0.05 or lower.
3. Results
3.1. Effect of ceftriaxone treatment on cardiac enzyme levels
It has been established that heart damage alters levels of the cardiac enzymes troponin I (cTn-I) and creatine kinase-MB (CK-MB). Therefore, we measured cTn-I and CK-MB as markers of cardiac damage. Remarkably, we observed that fentanyl significantly increased the serum levels of cTn-I compared with the control group; this is considered an indicator of cardiac damage (Fig. 2A). Surprisingly, the groups receiving fentanyl and then ceftriaxone or MC-100093 had significantly reduced cTn-I levels compared with the group given fentanyl only (hereinafter known as the fentanyl group; see Fig. 2A). However, no substantial changes were observed in CK-MB levels among the various groups (see Fig. 2B). These findings suggested that ceftriaxone and MC-100093 could mitigate cardiac injury caused by fentanyl.
Fig. 2.
Serum levels of cardiac enzymes. Serum obtained from all groups were examined to determine cTnI (A) & CK-MB (B) levels. Data are showed as mean ± SD (n = 4). *significant against control & # significant against Fen group, where *p < 0.05, #p < 0.05, ##p < 0.01. Fen, Fentanyl; MC, MC-100093; Cef, Ceftriaxone; cTn-I, Troponin I cardiac muscle; CK-MB, Creatine kinase-MB.
3.2. Effect of β-Lactams on cardiac architecture
Several diseases and toxicities have been shown to be associated with modification of and damage to the morphology of cardiac cells. Therefore, we studied how the heart architecture responded to a fentanyl overdose. We found that the section obtained from the myocardium of mice in the control group had normal, healthy myocardial fibers and regular (i.e., oval, centrally located) nuclei, with no verification of necrosis or inflammation (Fig. 3A). However, a section of myocardium obtained from the fentanyl group had signs of severe damage to the cardiac muscles, as manifested by the presence of large aggregations of inflammatory cells (see Fig. 3B). Additionally, the areas and volumes of myocardial fibers were significantly increased, indicating hypertrophy (see Fig. 3B and Table 2). Surprisingly, sections of myocardial fibers from the myocardium of the third group, who were given fentanyl plus MC-100093, had a nearly complete absence of pathological signs and a significant reduction in both areas and volumes of fibers compared to fentanyl group (see Fig. 3C and Table 2). The myocardial sections obtained from the fourth group, given fentanyl plus ceftriaxone, also had a complete absence of pathological signs, such as inflammation and necrosis, and also had a noteworthy reduction in both areas and volumes of fibers compared to fentanyl group (see Fig. 3D and Table 2). Staining with Masson’s trichrome stain showed normal myocardial tissue without depositions of collagenous fibers, except for small amounts of connective tissue in the intermediate spaces in cardiac cells of the control group (Fig. 4A and Table 3, Table 4). Animals given fentanyl exhibited strong depositions of collagenous fibers and a raised cardiac histopathological score, signs that indicated severe pathological alterations (see Fig. 4B and Table 3, Table 4). Meanwhile, the groups given MC-100093 or ceftriaxone had fewer depositions of collagenous fibers and a lower pathological score for the cardiac cells because they had fewer pathological signs (see Fig. 4C to D and Table 3, Table 4).
Fig. 3.
Photomicrographs of cardiac tissue using H&E stain. (A) control cardiac cells showing normal view. (B) Animals treated with fentanyl revealing great inflammation (black arrows), vacuolar degeneration (green arrow). (C) Animals treated with fentanyl plus MC-100093 posting no pathological signs. (D) Animals treated with fentanyl and ceftriaxone showing no pathological features. (F) means myocardial fibers (H&E-400X). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Table 2.
Morphometric analysis of myocardial fibers showing increasing of diameters, areas and volumes of experimental groups compared to control group.
| Groups | Diameter x 107 µm | Area x 105 µm2 | Volume x 107 µm3 |
|---|---|---|---|
| Control | 20 | 158 | 186 |
| Fen | 38* | 370* | 669* |
| Fen + MC | 22# | 185# | 236*# |
| Fen + Cef | 20# | 185# | 236*# |
Fig. 4.
Photomicrographs of cardiac muscles using Masson’s trichrome stain. (A) Control cardiac muscles showing normal depositions of connective tissue of intermediate spaces. (B) Cardiac cells of animals treated with fentanyl exhibiting great fibrosis (F) of bundles of collagenous fibers (green arrows). (C) Cardiac cells of animals treated with fentanyl and MC-compound revealing less depositions of collagenous fibers. (D) Cardiac cells of animals treated with fentanyl plus ceftriaxone posting more less depositions. (Masson’s trichrome-400X). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Table 3.
Percentage and optical density of Masson’s trichrome stain.
| Groups | % | OD |
|---|---|---|
| Control | 8 ± 0.4 | 0.03 ± 0 |
| Fen | 42 ± 9* | 0.25 ± 0.04* |
| Fen + MC | 13 ± 1# | 0.08 ± 0# |
| Fen + Cef | 13 ± 1# | 0.03 ± 0.01# |
Data = mean ± S.D. p ≤ 0.05 considered significant, *significant against control, # significant against Fen group. Fen, Fentanyl; MC, MC-100093; Cef, Ceftriaxone.
Table 4.
Percentage Histopathologic Score of Cardiac Muscle in Control and experimental groups.
| Groups | Disorganization | Inflammation | Degeneration | Vaso-congestion | fibrosis | Total score |
|---|---|---|---|---|---|---|
| Control | 0 | 0 | 0 | 0 | 0 | 0 |
| Fen | 2 | 3 | 3 | 2 | 3 | 13 |
| Fen + MC | 1 | 2 | 1 | 0 | 1 | 5 |
| Fen + Cef | 1 | 1 | 1 | 0 | 1 | 4 |
Data = mean ± S.D. p ≤ 0.05 considered significant, *significant against control, # significant against Fen group. Fen, Fentanyl; MC, MC-100093; Cef, Ceftriaxone.
3.3. β-Lactam attenuated fentanyl-induced cardiac hypertrophy
It has been reported that cardiac hypertrophy can be a pathological change against various insults. Thus, we assessed the heart weight (HW), body weight (BW), and HW/BW ratio to determine whether cardiac hypertrophy was present. Moreover, we determined the expression of two known genes that are reported to be modulated in this process, the α-MHC gene and β-MHC gene. No meaningful changes were noticed in the BW in any of the groups (Fig. 5A). However, the fentanyl group had a substantial rise in HW in comparison to control group (see Fig. 5B). Moreover, we observed a significant increase in the HW/BW ratio in animals given fentanyl compared with the control animals (see Fig. 5C). The groups that received ceftriaxone or MC100093 had a significant reduction in the HW and HW/BW ratio compared with the fentanyl group (see Fig. 5B to C). Furthermore, we demonstrated an increase in the level of expression of the α-MHC gene in the groups given ceftriaxone or MC-100093 compared with the fentanyl group (see Fig. 5D). However, the level of expression of the β-MHC gene decreased significantly in the groups given one of the β-lactams compared with the fentanyl group (see Fig. 5E to F). Overall, these results indicated that ceftriaxone and MC-100093 could mitigate or prevent cardiac hypertrophy caused by a fentanyl overdose.
Fig. 5.
β-Lactams attenuated fentanyl-induced cardiac hypertrophy. Body weight (A), heart weight (B), and heart weight to body weight ratio (C). (D) mRNA levels of α-MHC. (E) mRNA levels of β-MHC. (F) mRNA levels of β-MHC: α-MHC ratio. Data are displayed as mean ± SD. *significant against control & # significant against Fen group, where *p < 0.05, ****p < 0.0001, #p < 0.05, ##p < 0.01, ###p < 0.001, ####p < 0.0001. Fen, Fentanyl; MC, MC-100093; Cef, Ceftriaxone; α −MHC, alpha Myosin heavy chain; β-MHC, beta Myosin heavy chain.
3.4. β-Lactam mitigation of cardiac inflammation that had been induced by fentanyl
Accumulated data have shown that inflammation plays a central and inciting role in processes that cause cardiac damage. So, we determined the levels of expression of genes and proteins for different markers to better understand the cardioprotective role of β-lactams against fentanyl overdoses. We observed that the gene and protein expression levels of NF-κB and IL-6 were significantly elevated in the fentanyl group compared with the control group. This provided an important insight into the involvement of inflammation in fentanyl-induced cardiac toxicity (Fig. 6A to B). Nonetheless, the levels of gene and protein expression of these markers were significantly reduced in the groups given ceftriaxone or MC-100093 compared with the fentanyl group (see Fig. 6C to D). These results indicated that ceftriaxone and MC-100093 could diminish cardiac inflammation induced by fentanyl.
Fig. 6.
β-Lactams inhibited inflammation induced by Fentanyl in the heart. (A) & (B) mRNA levels of NF-κB and IL-6, respectively. (C) & (D) Representative western blot analysis of protein levels of NF-κB and IL-6, respectively. Data are presented as mean ± SD (n = 4). *significant against control & # significant against Fen group, where *p < 0.05, **p < 0.01, #p < 0.05, ##p < 0.01, ###p < 0.001. Fen, Fentanyl; MC, MC-100093; Cef, Ceftriaxone; NF-κB, Nuclear factor kappa-B; IL-6, Interleukin-6; β-actin, Beta actin.
3.5. β-Lactam attenuation of oxidative stress and apoptosis in the heart
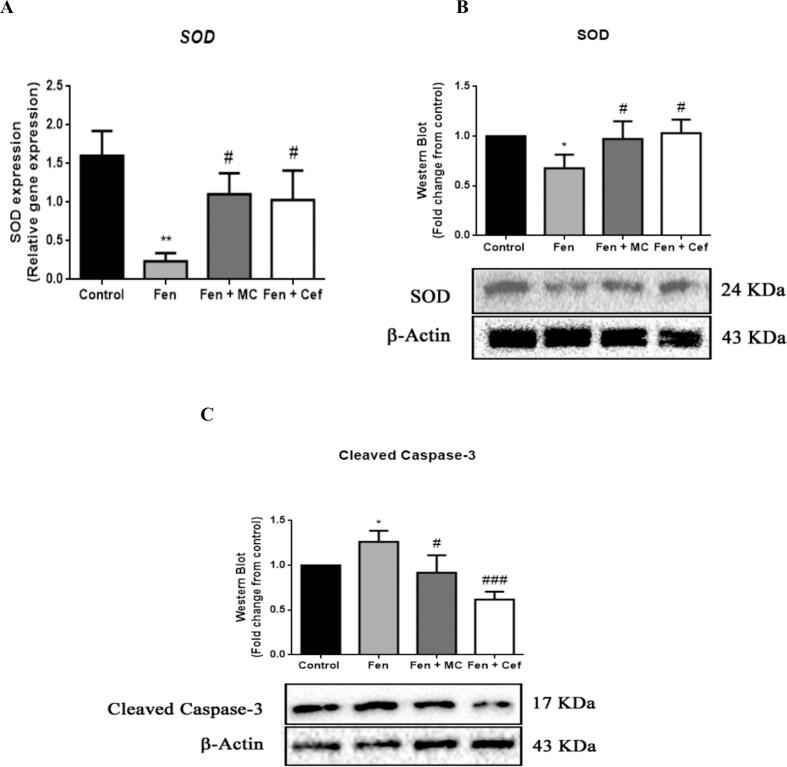
It has been established that oxidative stress and apoptosis are the leading mechanisms of cardiac injury. Therefore, and to better explore the association of oxidative stress and apoptosis with fentanyl overdoses, we measured the levels of expression of genes and proteins when oxidative stress and apoptotic markers were present. The fentanyl group had a significant reduction in levels of the antioxidant protein superoxide dismutase (SOD) and a remarkable elevation of the apoptotic protein cleaved caspase-3 (CAS-3) (Fig. 7A to C). The groups given ceftriaxone or MC-100093 had remarkably induced SOD levels and reduced cleaved CAS-3 levels compared with the fentanyl group (see Fig. 7A to C).
Fig. 7.
β-Lactams reduce oxidative stress and apoptosis in the heart. (A) Relative gene expression of SOD were measured by RT-PCR. (B) & (C) Representative western blot analysis of protein levels of SOD and CAS-3, respectively. Data are presented as mean ± SD (n = 4). *significant against control & # significant against Fen group, where *p < 0.05, **p < 0.01, #p < 0.05, ###p < 0.001. Fen, Fentanyl; MC, MC-100093; Cef, Ceftriaxone; SOD, Superoxide dismutase; CAS-3, Cleaved Caspase-3; β-actin, Beta actin.
3.6. Effect of β-lactams on oxidative stress status
Many cardiotoxic drugs generate reactive oxygen species (ROS), and this is a contributing factor to their cardiotoxicity. ROS be able to induce oxidative damage to numerous essential cell components, including proteins. Increases in lipid peroxidation have been related to induction of cardiotoxicity. Furthermore, reduced cardiac antioxidant capacity has been linked to the generation of ROS and cardiac damage. Thus, we evaluated levels of catalase (CAT), lipid peroxidation, and glutathione (GSH). Results demonstrated that the fentanyl group experienced drastically induced MDA levels compared with the control group (Fig. 8A). The levels of CAT and GSH were significantly decreased in the fentanyl group compared with the control group (see Fig. 8B to C). Conversely, we found that the groups given ceftriaxone or MC-100093 had restored levels of CAT and GSH compared with the fentanyl group (see Fig. 8B to C). Furthermore, the groups given one of the β-lactams had drastically reduced MDA levels compared with the fentanyl group (see Fig. 8A). These findings demonstrated that ceftriaxone and MC-100093 diminished oxidative stress.
Fig. 8.
Effect of β-lactams on oxidative stress level. Biochemical measurements of MDA (A), CAT (B), and GSH (C). Data are showed as mean ± SD (n = 4). *significant against control & # significant against Fen group, where *p < 0.05, **p < 0.01, #p < 0.05, ##p < 0.01, ###p < 0.001. Fen, Fentanyl; MC, MC-100093; Cef, Ceftriaxone; MDA, Malondialdehyde; GSH, Glutathione.
3.7. Effect of β-lactams on xCT and GLT-1 expression in the heart
It has been reported that xCT upregulation has cardioprotective effects through its inhibition of extracellular glutamate and induction of cystine uptake by the xc– system. This process has been demonstrated to enhance the synthesis of intracellular GSH and increase the antioxidant capacity of cells, leading to a reduction in ROS formation and the prevention of cardiac damage. Consequently, we measured the gene and protein expression levels of GLT-1 and xCT and the content of the amino acid glutamate to mechanistically investigate the protective effect of β-lactams. We found that the gene and protein expression levels of xCT were extensively reduced in the fentanyl group compared to control group (Fig. 9A to B). However, the groups given ceftriaxone or MC-100093 had restored gene and protein levels of xCT compared with the fentanyl group (see Fig. 9A to B). Furthermore, the gene and protein expression levels of GLT-1 were unexpectedly increased in the fentanyl group compared with the control group (see Fig. 9C to D). Fentanyl significantly increased the glutamate content, but when ceftriaxone or MC-100093 was given following fentanyl, the glutamate content was remarkably reduced compared with the content in the fentanyl group (see Fig. 9E). These results suggested that ceftriaxone and MC-100093 provided protection through the upregulation of xCT expression and the inhibition of extracellular glutamate content in the heart.
Fig. 9.
Effect of β-lactams on xCT and GLT-1 expression in the heart. (A) & (B) mRNA levels of xCT and GLT-1, respectively. (C) & (D) Representative western blot analysis of protein levels of xCT and GLT-1, respectively. (E) Biochemical analysis of glutamate content. Data are presented as mean ± SD (n = 4). *significant against control & # significant against Fen group, where *p < 0.05, **p < 0.01, #p < 0.05, ##p < 0.01. Fen, Fentanyl; MC, MC-100093; Cef, Ceftriaxone; xCT, Cystine/glutamic acid reverse transporter; GLT-1, Glutamate transporter-1; β-actin, Beta actin.
4. Discussion
Our results in the current study showed that fentanyl intake caused cardiac damage as demonstrated by histopathological examinations. Moreover, we demonstrated that the group given fentanyl developed cardiac damage that led to a substantial elevation in cardiac enzymes. Furthermore, fentanyl triggered cardiotoxicity through the downregulation of xCT, thus leading to the induction of genes that are involved in heart damage (i.e., α-MHC and β-MHC). Additionally, fentanyl provoked cardiotoxicity through inducing the gene and protein expression of inflammatory markers and apoptotic markers and reducing antioxidant markers. However, treatment with ceftriaxone or MC-100093 mitigated these effects and protected the heart via xCT activation.
The xc− system is made up of the controlling heavy chain component 4F2hc (SLC3A2), that exchanges cystine/glutamate with cystine, and the light subunit xCT (SLC7A11), that exchanges extracellular cystine for intracellular glutamate (Martis et al., 2020). The preservation of intracellular GSH levels, that is crucial for defending cells from oxidative damage, is eventually carried out by cysteine taken into the cell by xCT (Fang et al., 2020). A recent report demonstrated that MC-100093 and ceftriaxone reduced neurological and psychiatric manifestations through the upregulation of expression of xCT (Abulseoud et al., 2022). Another study suggested that the overexpression of xCT can diminish cardiac hypertrophy (Zhang et al., 2022). Therefore, we hypothesized that ceftriaxone would protect against fentanyl-induced cardiac injury by upregulating xCT expression. This would lead to the enhancement of the synthesis of intracellular GSH and an increase in the antioxidant capacity of cardiomyocytes, thus reducing the formation of ROS and preventing cardiac damage. We believe that no other preclinical studies had investigated the cardioprotective effects of ceftriaxone or MC-100093. Hence, in the current study we explored the protective effects of ceftriaxone and MC-100093 on the heart and examined the underlying molecular mechanisms for these effects.
Cardiac damage can be effectively assessed by measuring levels of cardiac enzymes, including cTn-I, as well as by the histopathological analysis of cardiac tissues; these analyses have been shown to be key indicators for the effectiveness of cardiotoxic drugs (Shah et al., 2017). In the current study, we observed that the fentanyl group had increased levels of cTn-I than the control group. The histological analysis of cardiac tissues from mice given fentanyl revealed severe pathological signs of cardiac damage manifested by the accumulation of large aggregations of inflammatory cells. These histopathological signs further demonstrated that fentanyl damages cardiomyocytes in mice. However, ceftriaxone and MC-100093 significantly reduced cTn-I levels and led to the nearly complete absence of pathological signs in histopathological findings. These findings suggested that ceftriaxone and MC-10009 can mitigate or prevent heart damage.
Cardiac hypertrophy occurs when cardiomyocytes respond to a raised afterload resulting from a volume or pressure overload. The process makes myocytes more productive, and this enhances the function of the heart. However, cardiac damage can occur when biomechanical stress destroys this compensating system (Hill et al., 2000). The HW/BW ratio has been used for characterizing myocardial hypertrophy (Hagdorn et al., 2019). In the current study, we reported a significant rise in the HW/BW ratio in animals given fentanyl compared with the control animals. However, the groups given a β-lactam had a substantial reduction in the HW/BW ratio compared with the fentanyl group. Furthermore, accumulated data have shown that an alteration in the α-MHC gene is an indicator of cardiac hypertrophy (Molkentin et al., 1996). In our study, we showed that the expression level of the α-MHC gene was decreased in the fentanyl group. However, we also found that the expression level of the α-MHC gene had increased in the group given ceftriaxone and the group given MC-100093 compared with the fentanyl group. These results reflected the toxic damage to the heart that fentanyl can cause and indicated that ceftriaxone and MC-100093 can mitigate or prevent cardiac hypertrophy.
The loss of cardiomyocytes in drug-induced cardiotoxicity can be significantly influenced by apoptosis (Gustafsson and Gottlieb 2007). The most significant markers in the terminal apoptotic pathway are caspases, since they are essential for the initiation and completion of apoptosis (Teringova and Tousek 2017). Therefore, it is reasonable to assume that a small amount of caspase or a malfunctioning caspase may result in a reduction in apoptosis. A previous study using an animal model of cardiac damage found that downregulating CAS-3 reduced the cardiomyocyte apoptotic index and improved cardiac function (Liu 2014). In the current study, we found that fentanyl led to cardiomyocyte injury, as demonstrated by increased levels of CAS-3. However, a substantial decline in the protein expression of CAS-3 was observed in the groups given ceftriaxone or MC-100093.
Several biological functions, including cell death and the response to inflammation, are impacted by oxidative stress, that can lead to the excess formation of ROS and chronic inflammation (Biswas 2016). Various types of cardiovascular diseases have been associated with oxidative stress-induced inflammation, including atherosclerosis, cardiomyopathy, and heart failure. Transcription factors, such as NF-κB, can be activated by oxidative stress, and this can result in the differential expression of specific proteins associated with inflammatory pathways, including IL-6 (Biswas 2016). Our findings demonstrated that fentanyl caused a reduction in antioxidants and an induction of inflammation that caused cardiomyocyte apoptosis; this was verified by the induction of the protein and gene expressions of NF-κB and the decreased protein and gene levels of SOD. Additionally, the induction of IL-6 gene and protein expressions supplied additional evidence of the cardiotoxicity of fentanyl. The levels of gene and protein expression of NF-κB and IL-6 were significantly reduced in the groups given ceftriaxone or MC-100093 compared with the fentanyl group. Furthermore, we demonstrated a significant induction in the gene and protein levels of SOD in the groups given one of the β-lactams compared with the fentanyl group. These results indicated that ceftriaxone and MC-100093 reduced the cardiac inflammation induced by fentanyl by diminishing the oxidative stress.
To attempt to find further evidence of oxidative stress in the present study, we assessed the levels of lipid peroxidation, catalase (CAT), and glutathione (GSH). It has been widely acknowledged that cellular lipids, which produce a range of aldehydes, including MDA, are the main targets of free radicals. Therefore, MDA is frequently used as a biomarker of oxidative stress. Additionally, antioxidants, including CAT and GSH, have been widely investigated to see if they could prevent the cardiac damage resulting from oxidative stress. In the current study, we found that the group given fentanyl had induced MDA levels compared with the control group. Moreover, the levels of CAT and GSH were significantly decreased in the fentanyl group compared with the control group. We also found that the groups given ceftriaxone or MC-100093 had restored levels of CAT and GSH compared with the fentanyl group. Furthermore, the groups given a β-lactam had drastically reduced MDA levels compared with the fentanyl group. These findings validated the results we previously established showing that ceftriaxone and MC-100093 reduced the oxidative stress induced by fentanyl.
It has been reported that GLT-1 and xCT are diminished in content and function in response to opioid withdrawal and that these reductions are potentially involved in the cardiac damage that can develop from the continuous use of opioids (McClure et al., 2014). Furthermore, the enhancement of xCT directly in cardiac myocytes increased GSH levels and also increased the antioxidant capacity of the cells, leading to a reduction in ROS formation and decreased cardiac damage, as mentioned previously. This indicated that enhancing GSH levels through xCT overexpression is a promising approach (Fang et al., 2020). Therefore, we analyzed the gene and protein expression of xCT and GLT-1 and the glutamate content to mechanistically investigate the protective effect of β-lactams. We found that the gene and protein expression levels of xCT were drastically reduced in the fentanyl group compared with the control group. However, the groups given ceftriaxone or MC-100093 had restored gene and protein levels of xCT in comparison to the fentanyl group. Levels of protein and gene expression of GLT-1 were enhanced in the fentanyl group, while the groups given β-lactams displayed a meaningful reduction in the gene and protein expression levels of GLT-1 compared with the fentanyl group. We also found that the fentanyl group had significantly increased glutamate content compared with the other groups, while the groups given ceftriaxone or MC-100093 had remarkably reduced glutamate content compared with the fentanyl group. In a previous study, researchers reported that ethanol intake caused GLT-1 downregulation and that ceftriaxone treatment increased GLT-1 expression in the brain (Sari et al., 2013). A recent study explored the impact of chronic ethanol drinking on the liver and found a substantial enhance in GLT-1 expression in the group given ethanol compared to control group. Additionally, both of the groups given a β-lactam (i.e., ceftriaxone or MC-100093) showed no meaningful alteration in GLT-1 expression compared with the control group and the group given ethanol (Alhaddad et al., 2022). The researchers studied the cardiomyocytes and found that GLT-1 expression was upregulated in the hypertrophic rat heart compared with the normal rat heart; this could explain the observed elevation of GLT-1 expression in the fentanyl group (King et al., 2004, King et al., 2006). The effect of GLT-1 expression on cardiomyocytes is still debatable, but these findings could shed light on the future treatment of cardiac damage. Therefore, our results indicated that ceftriaxone and MC-100093 provided a cardioprotective effect mainly through the upregulation of xCT expression in the heart.
5. Conclusion
In conclusion, we reported, for the first time, that ceftriaxone and MC-100093 offered cardioprotective in response to fentanyl-induced cardiac damage mainly through the upregulation of xCT expression. The cardioprotective effect of ceftriaxone and MC-100093 was evidenced via histopathological restoration, reduction in levels of cTn-I, and the restoration of altered hypertrophic markers. Moreover, we revealed that ceftriaxone and MC-100093 reduced apoptosis, cardiac inflammation, and oxidative stress (see Fig. 10). We propose that these beneficial effects of ceftriaxone and MC-100093 are attributed to the upregulation of xCT expression in the heart. Future studies are warranted to determine the exact mechanism of cardioprotective effect of these β-lactams.
Fig. 10.
Schematic representation of cardioprotective
Institutional Review Board Statement: The study protocol on animals was consented by KSU Local Institutional Study Ethics Committee (REC) (protocol code KSU-SE-22–48, 2023).
CRediT authorship contribution statement
Abdullah F. AlAsmari: Writing – original draft, Validation, Supervision, Resources, Project administration, Investigation, Funding acquisition, Conceptualization. Mohammed M. Alshehri: Writing – review & editing, Writing – original draft, Visualization, Software, Methodology, Data curation. Nemat Ali: Writing – review & editing, Resources, Project administration, Methodology, Investigation, Formal analysis, Data curation. Fawaz AlAsmari: Writing – review & editing, Validation, Formal analysis, Conceptualization. Youssef Sari: Writing – review & editing, Validation, Supervision, Project administration, Conceptualization. Wayne E. Childers: Writing – review & editing, Resources. Magid Abou-Gharbia: Writing – review & editing, Resources. Metab Alharbi: Writing – review & editing, Software, Methodology, Formal analysis. Doaa M. Elnagar: Writing – review & editing, Resources, Methodology. Wejdan S. AL-Qahtani: Writing – review & editing, Software, Methodology.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgment
Authors are thankful to the Researchers Supporting Project number (RSP2024R335), King Saud University, Riyadh, Saudi Arabia.
References
- Abulseoud O.A., Alasmari F., Hussein A.M., et al. Ceftriaxone as a Novel Therapeutic Agent for Hyperglutamatergic States: Bridging the Gap Between Preclinical Results and Clinical Translation. Front Neurosci. 2022;16 doi: 10.3389/fnins.2022.841036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alasmari F., Alasmari M.S., Assiri M.A., et al. Liver Metabolomics and Inflammatory Profiles in Mouse Model of Fentanyl Overdose Treated with Beta-Lactams. Metabolites. 2023;13 doi: 10.3390/metabo13080965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alhaddad H., Wong W., Abou-Gharbia M., et al. Effects of a Novel Beta Lactam Compound, MC-100093, on the Expression of Glutamate Transporters/Receptors and Ethanol Drinking Behavior of Alcohol-Preferring Rats. J Pharmacol Exp Ther. 2022;383:208–216. doi: 10.1124/jpet.122.001147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altwaijri Y.A., Al-Habeeb A., Al-Subaie A.S., et al. Twelve-month prevalence and severity of mental disorders in the Saudi National Mental Health Survey. Int J Methods Psychiatr Res. 2020;29:e1831. doi: 10.1002/mpr.1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badar F., Ashraf A., Bhuiyan M.R., et al. A Peculiar Case of Fentanyl-Induced Cardiomyopathy. Cureus. 2022;14:e27708. doi: 10.7759/cureus.27708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswas S.K. Does the Interdependence between Oxidative Stress and Inflammation Explain the Antioxidant Paradox? Oxid Med Cell Longev. 2016;2016:5698931. doi: 10.1155/2016/5698931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das S.C., Althobaiti Y.S., Hammad A.M., et al. Role of suppressing GLT-1 and xCT in ceftriaxone-induced attenuation of relapse-like alcohol drinking in alcohol-preferring rats. Addict Biol. 2022;27:e13178. doi: 10.1111/adb.13178. [DOI] [PubMed] [Google Scholar]
- Demirel A., Basgoze S., Cakilli K., et al. Histopathological Changes in the Myocardium Caused by Energy Drinks and Alcohol in the Mid-term and Their Effects on Skeletal Muscle Following Ischemia-reperfusion in a Rat Model. Anatol J Cardiol. 2023;27:12–18. doi: 10.14744/AnatolJCardiol.2022.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Cesare M., Perel P., Taylor S., et al. The Heart of the World. Glob Heart. 2024;19:11. doi: 10.5334/gh.1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimmeler S. Cardiovascular disease review series. EMBO Mol Med. 2011;3:697. doi: 10.1002/emmm.201100182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Souza M.S. Glutamatergic transmission in drug reward: implications for drug addiction. Front Neurosci. 2015;9:404. doi: 10.3389/fnins.2015.00404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunbar S.B., Khavjou O.A., Bakas T., et al. Projected Costs of Informal Caregiving for Cardiovascular Disease: 2015 to 2035: A Policy Statement From the American Heart Association. Circulation. 2018;137:e558–e577. doi: 10.1161/CIR.0000000000000570. [DOI] [PubMed] [Google Scholar]
- Fang X., Cai Z., Wang H., et al. Loss of Cardiac Ferritin H Facilitates Cardiomyopathy via Slc7a11-Mediated Ferroptosis. Circ Res. 2020;127:486–501. doi: 10.1161/CIRCRESAHA.120.316509. [DOI] [PubMed] [Google Scholar]
- Gustafsson A.B., Gottlieb R.A. Bcl-2 family members and apoptosis, taken to heart. Am J Physiol Cell Physiol. 2007;292:C45–C51. doi: 10.1152/ajpcell.00229.2006. [DOI] [PubMed] [Google Scholar]
- Hagdorn Q.A.J., Bossers G.P.L., Koop A.C., et al. A novel method optimizing the normalization of cardiac parameters in small animal models: the importance of dimensional indexing. Am J Physiol Heart Circ Physiol. 2019;316:H1552–H1557. doi: 10.1152/ajpheart.00182.2019. [DOI] [PubMed] [Google Scholar]
- Hill J.A., Karimi M., Kutschke W., et al. Cardiac hypertrophy is not a required compensatory response to short-term pressure overload. Circulation. 2000;101:2863–2869. doi: 10.1161/01.cir.101.24.2863. [DOI] [PubMed] [Google Scholar]
- Hill R., Santhakumar R., Dewey W., et al. Fentanyl depression of respiration: Comparison with heroin and morphine. Br J Pharmacol. 2020;177:254–266. doi: 10.1111/bph.14860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katare P.B., Bagul P.K., Dinda A.K., et al. Toll-Like Receptor 4 Inhibition Improves Oxidative Stress and Mitochondrial Health in Isoproterenol-Induced Cardiac Hypertrophy in Rats. Front Immunol. 2017;8:719. doi: 10.3389/fimmu.2017.00719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King N., Lin H., McGivan J.D., et al. Aspartate transporter expression and activity in hypertrophic rat heart and ischaemia-reperfusion injury. J Physiol. 2004;556:849–858. doi: 10.1113/jphysiol.2004.060616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King N., Lin H., McGivan J.D., et al. Expression and activity of the glutamate transporter EAAT2 in cardiac hypertrophy: implications for ischaemia reperfusion injury. Pflugers Archiv : European Journal of Physiology. 2006;452:674–682. doi: 10.1007/s00424-006-0096-z. [DOI] [PubMed] [Google Scholar]
- Knackstedt L.A., Wu L., Rothstein J., et al. MC-100093, a Novel beta-Lactam Glutamate Transporter-1 Enhancer Devoid of Antimicrobial Properties, Attenuates Cocaine Relapse in Rats. J Pharmacol Exp Ther. 2021;378:51–59. doi: 10.1124/jpet.121.000532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krantz M.J., Palmer R.B., Haigney M.C.P. Cardiovascular Complications of Opioid Use: JACC State-of-the-Art Review. J Am Coll Cardiol. 2021;77:205–223. doi: 10.1016/j.jacc.2020.11.002. [DOI] [PubMed] [Google Scholar]
- Lamb H.M., Ormrod D., Scott L.J., et al. Ceftriaxone: an update of its use in the management of community-acquired and nosocomial infections. Drugs. 2002;62:1041–1089. doi: 10.2165/00003495-200262070-00005. [DOI] [PubMed] [Google Scholar]
- Liu Q. Lentivirus mediated interference of Caspase-3 expression ameliorates the heart function on rats with acute myocardial infarction. European Review for Medical and Pharmacological Sciences. 2014;18:1852–1858. [PubMed] [Google Scholar]
- Martis R.M., Knight L.J., Donaldson P.J., et al. Identification, Expression, and Roles of the Cystine/Glutamate Antiporter in Ocular Tissues. Oxid Med Cell Longev. 2020;2020:4594606. doi: 10.1155/2020/4594606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathers C.D., Loncar D. Projections of global mortality and burden of disease from 2002 to 2030. PLoS Med. 2006;3:e442. doi: 10.1371/journal.pmed.0030442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClure E.A., Gipson C.D., Malcolm R.J., et al. Potential role of N-acetylcysteine in the management of substance use disorders. CNS Drugs. 2014;28:95–106. doi: 10.1007/s40263-014-0142-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molkentin J.D., Jobe S.M., Markham B.E. Alpha-myosin heavy chain gene regulation: delineation and characterization of the cardiac muscle-specific enhancer and muscle-specific promoter. J Mol Cell Cardiol. 1996;28:1211–1225. doi: 10.1006/jmcc.1996.0112. [DOI] [PubMed] [Google Scholar]
- Nakhaee S., Ghasemi S., Karimzadeh K., et al. The effects of opium on the cardiovascular system: a review of side effects, uses, and potential mechanisms. Subst Abuse Treat Prev Policy. 2020;15:30. doi: 10.1186/s13011-020-00272-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos-Matos, C. F., K. G. Bistas and W. Lopez-Ojeda, 2024. Fentanyl. StatPearls. Treasure Island (FL) ineligible companies. Disclosure: Karlyle Bistas declares no relevant financial relationships with ineligible companies. Disclosure: Wilfredo Lopez-Ojeda declares no relevant financial relationships with ineligible companies.
- Rao P.S., Saternos H., Goodwani S., et al. Effects of ceftriaxone on GLT1 isoforms, xCT and associated signaling pathways in P rats exposed to ethanol. Psychopharmacology (berl). 2015;232:2333–2342. doi: 10.1007/s00213-015-3868-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riley E.D., Vittinghoff E., Wu A.H.B., et al. Impact of polysubstance use on high-sensitivity cardiac troponin I over time in homeless and unstably housed women. Drug Alcohol Depend. 2020;217 doi: 10.1016/j.drugalcdep.2020.108252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritter M.L., Bohr A.D., McQueen M.B. Association of Cardiac Arrest With Opioid Overdose in Transport. Subst Abuse. 2022;16 doi: 10.1177/11782218221103582. 11782218221103582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sari Y., Sreemantula S.N., Lee M.R., et al. Ceftriaxone treatment affects the levels of GLT1 and ENT1 as well as ethanol intake in alcohol-preferring rats. Journal of Molecular Neuroscience : MN. 2013;51:779–787. doi: 10.1007/s12031-013-0064-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah K.S., Yang E.H., Maisel A.S., et al. The Role of Biomarkers in Detection of Cardio-toxicity. Current Oncology Reports. 2017;19:42. doi: 10.1007/s11912-017-0602-9. [DOI] [PubMed] [Google Scholar]
- Smaga I., Fierro D., Mesa J., et al. Molecular changes evoked by the beta-lactam antibiotic ceftriaxone across rodent models of substance use disorder and neurological disease. Neurosci Biobehav Rev. 2020;115:116–130. doi: 10.1016/j.neubiorev.2020.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki J., El-Haddad S. A review: Fentanyl and non-pharmaceutical fentanyls. Drug Alcohol Depend. 2017;171:107–116. doi: 10.1016/j.drugalcdep.2016.11.033. [DOI] [PubMed] [Google Scholar]
- Teringova E., Tousek P. Apoptosis in ischemic heart disease. Journal of Translational Medicine. 2017;15:87. doi: 10.1186/s12967-017-1191-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tracy R.E., Sander G.E. Histologically measured cardiomyocyte hypertrophy correlates with body height as strongly as with body mass index. Cardiology Research and Practice. 2011;2011 doi: 10.4061/2011/658958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uddin O., Jenne C., Fox M.E., et al. Divergent profiles of fentanyl withdrawal and associated pain in mice and rats. Pharmacol Biochem Behav. 2021;200 doi: 10.1016/j.pbb.2020.173077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The United Nations Office on Drugs and Crime. World Drug Report 3: Executive Summary, 2023, n.d.. UNODC Research.
- Wellington D., Mikaelian I., Singer L. Comparison of ketamine-xylazine and ketamine-dexmedetomidine anesthesia and intraperitoneal tolerance in rats. J Am Assoc Lab Anim Sci. 2013;52:481–487. [PMC free article] [PubMed] [Google Scholar]
- World Health Organization (WHO), 2020. Global health estimates: Leading causes of death. The Global Health Observatory, Explore a World of Health Data.
- Wilkie C.M., Barron J.C., Brymer K.J., et al. The Effect of GLT-1 Upregulation on Extracellular Glutamate Dynamics. Front Cell Neurosci. 2021;15 doi: 10.3389/fncel.2021.661412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X., Zheng C., Gao Z., et al. SLC7A11/xCT Prevents Cardiac Hypertrophy by Inhibiting Ferroptosis. Cardiovasc Drugs Ther. 2022;36:437–447. doi: 10.1007/s10557-021-07220-z. [DOI] [PubMed] [Google Scholar]
- Ziesenitz V.C., Vaughns J.D., Koch G., et al. Pharmacokinetics of Fentanyl and Its Derivatives in Children: A Comprehensive Review. Clin Pharmacokinet. 2018;57:125–149. doi: 10.1007/s40262-017-0569-6. [DOI] [PMC free article] [PubMed] [Google Scholar]