Abstract
Background
Breastfeeding has many benefits for mothers and infants. Lactogenesis II is one of the key steps in the implementation of breastfeeding. If lactogenesis II occurs more than 72 h after delivery, it is termed delayed onset of lactation (DOL). DOL is associated with decreased milk production, shortened breastfeeding time, and pathological neonatal weight loss. A comprehensive summary of the incidence and factors influencing DOL is needed to provide a basis for improving breastfeeding practices and health outcomes.
Methods
Studies on the incidence and factors influencing DOL were retrieved from 13 Chinese and English databases (PubMed, Embase, Web of Science, Cochrane Library, CINAHL, etc.) from database inception to August 2023. Two researchers independently conducted the study screening, data extraction and quality evaluation. Stata 16.0 SE software was used for data analysis, and sensitivity analysis and publication bias tests were also performed. The qualitative description method was used to analyse studies that could not be combined quantitatively.
Results
A total of 35 studies involving 19,176 parturients, including 4,922 who had DOL, were included. The mean Newcastle‒Ottawa scale score of the included studies was ≥ 6, indicating that the quality was relatively high. Finally, the incidence of DOL was 30%, and 13 factors influencing DOL with robust results and no publication bias were obtained: prepregnancy body mass index (overweight or obesity), gestational diabetes, gestational hypertension, thyroid disease during pregnancy, serum albumin levels (< 35 g/L), parity, (unscheduled) caesarean section, caesarean section history, daily sleep duration, gestational age, birth weight (< 2.5 kg), breastfeeding guidance and daily breastfeeding frequency. However, there were still six influencing factors with undetermined associations: age, gestational weight gain, birth weight (≥ 4 kg), anxiety, time of first breastfeeding session (maternal separation) and breast massage or treatment.
Conclusions
The incidence of DOL is high. Clinicians should pay attention to parturients at high risk of DOL and formulate targeted prevention strategies according to the influencing factors to reduce the occurrence of DOL and promote better maternal and infant outcomes.
Trial registration
PROSPERO (ID: CRD42023458786), September 10, 2023.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13006-024-00666-5.
Keywords: Delayed onset of lactation, Incidence, Influencing factor, Meta-analysis, Systematic review
Background
Lactation involves four stages: secretory differentiation, secretory activation, reaching volume, and maintenance of established lactation [1, 2]. Among these stages, secretory activation (lactogenesis II) is triggered by a decrease in progesterone levels after delivery of the placenta and involves changes in prolactin and cortisol (glucocorticoid) secretion and the closure of paracellular pathways [3], indicating that a large amount of milk is being secreted by the mother [4]. Delayed onset of lactation (DOL) is defined as the occurrence of lactogenesis II 72 h after birth [4]. The most commonly used evaluation method for the onset of lactation (OL) is maternal perception of milk coming in [1]. Importantly, studies have shown that the time of OL is negatively correlated with the amount of milk produced on the 14th day postpartum [5]. DOL independently increases the risk of the cessation of any or exclusive breastfeeding at 4 weeks postpartum by 62% [6], thereby shortening the duration of breastfeeding [7, 8] and reducing the rate of exclusive breastfeeding [7, 9]. Moreover, DOL can increase the risk of pathological neonatal weight loss (more than 10% of the birth weight) by 7.1-fold [10, 11]. Consequently, actively taking effective intervention measures to prevent DOL has an important impact on improving maternal and child health outcomes and breastfeeding practices.
Liu et al. [12] and Miao et al. [13] published systematic reviews on the prevalence and factors influencing DOL in Chinese women in 2021 and 2023, respectively. The results revealed that the prevalence of DOL was 24% [12] and 31% [13], respectively, and an increasing trend of DOL, which should attract the attention of clinical workers. In addition, the existing systematic reviews may not be sufficiently comprehensive in literature retrieval and statistical analysis strategies, and their reporting of results may also be inadequate [12–14], potentially affecting the comprehensiveness and consistency of the findings. Therefore, this study focused on the global perspective and prospective research to determine the incidence of DOL and analyse the factors influencing DOL quantitatively through meta-analysis and to summarize the influencing factors that cannot be quantitatively analysed via qualitative description, to provide evidence supporting the development of effective evaluation and intervention measures for preventing DOL.
Methods
This review was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) 2020 statement [15] and was registered in the International Prospective Register of Systematic Reviews (PROSPERO) (ID: CRD42023458786).
Inclusion and exclusion criteria
Studies were considered eligible if they met all of the following criteria: (1) included women who chose to breastfeed after delivery; (2) studied the incidence or factors influencing DOL; (3) included the occurrence of DOL as the outcome and used the time of obvious breast tenderness or the sensation of milk coming in more than 72 h after delivery as the diagnostic criteria; and (4) used a prospective observational design.
Studies that met one of the following exclusion criteria were excluded: (1) studies with incomplete or erroneous data on variables; (2) studies for which data could not be directly or indirectly extracted; (3) studies for which the original text could not be obtained or the type of article was a review, conference paper, correspondence, comments, or study protocol; (4) studies published in different articles including the same participants: (a) for multiple studies of the same research object, the study with the most abundant research content or the most detailed description of the data was included; (b) for multiple studies with overlapping samples, the study with the longest study period was included; otherwise, the most recent study was included; 5) studies not published in Chinese or English; 6) studies with a sample size < 60; 7) nonhuman studies; or 8) studies for which the literature quality was low (quality assessment score ≤ 5).
Systematic search and strategy
Three researchers (YJP, KZ, and YH) jointly developed the search strategy and comprehensively searched the following databases for all relevant Chinese and English studies from database inception to August 2023: (1) Chinese databases: China National Knowledge Infrastructure (CNKI), Wanfang Database, China Science and Technology Journal Database (Weipu Database), and Chinese Biomedical Database (CBM); (2) English databases: PubMed, Ovid-Embase, Web of Science (via Web of Science Core Collection), Cochrane Library (the Cochrane central register of controlled trials, CENTRAL), CINAHL Plus (via EBSCOhost), APA Psycinfo (via EBSCOhost), Scopus, OpenGrey, and ProQuest Dissertations and Theses database (see full search strategy in Supplementary Material 1). To prevent the omission of relevant research, we reviewed the references of the included studies and relevant reviews. After the search was completed, duplicates were automatically removed by Endnote X9, followed by manual screening.
Study selection process and data extraction
Two researchers (YJP and KZ) independently screened the studies and extracted the data, and a third senior researcher (YH) independently reviewed and discussed the differences. Preliminary screening was performed by reading the title and abstract, followed by rescreening by reading the full text. If the title and abstract were not sufficient to make a decision, a decision was done by reading the full text. After the screening process was completed using Endnote X9, data were extracted from the final included studies, including the title, first author, year, study design, population characteristics, number of cases of DOL, sample size, study period, country, incidence, follow-up method and endpoint. In addition, all the influencing factors mentioned in the original study were extracted, and the influencing factors mentioned in two or more studies with the same definition were then identified. Finally, the exposure and outcome data of these influencing factors were extracted. For exposure variables, the number of cases for categorical variables and the mean ± standard deviation or median [interquartile range] for continuous variables were extracted.
Quality assessment
Prospective observational studies were included, so the Newcastle‒Ottawa Scale (NOS) [16] was used to evaluate the quality of the included studies. The NOS includes three columns and eight items. The three columns specifically include the selection of the research population, comparability between groups, and the measurement of results or exposure factors, and the total score ranges from 0 to 9 points. Because the data extracted were the number of cases, the influence of confounding factors could not be controlled for, which might lead to the deterioration of ‘comparability between groups’. The diagnostic criteria for DOL were based on maternal self-reported breast distension, which would lead to an insufficient evaluation; therefore, two points were deducted for all included studies. On this basis, studies with a score ≥ 6 points were considered high-quality studies. Two researchers (YJP and KZ) independently evaluated the quality of the included studies according to the evaluation criteria of the NOS [17]. When the opinions of the two researchers were inconsistent, the study was assigned to a third senior researcher (YH) for independent evaluation and discussion.
Statistical analysis
The extracted data and quality evaluation results were collated into Microsoft Excel 2021, and the data that could be used for quantitative analysis were entered into Stata 16.0 SE software for statistical analysis. In this study, a combined analysis or qualitative description of the influencing factors was performed only for variables included in at least two or more original studies. Because these were prospective observational studies, the risk ratio (RR) was used to combine the effect values for categorical variables, and the weighted mean difference (WMD) was used to combine the effect values for continuous variables. Cochran’s Q test and the I2 statistic were used to quantitatively analyse the heterogeneity between studies: (1) if I2 < 50% and p > 0.05, the heterogeneity between studies was considered low, then a fixed effect model was used; (2) if I2 ≥ 50% or p ≤ 0.05, the heterogeneity between studies was considered high, then a random effect model was used for more conservative statistical analysis. Subgroup analysis was performed to explore the source of heterogeneity.
When a certain influencing factor was included in three or more original studies, sensitivity analysis was carried out by eliminating the studies one by one and merging the remaining studies to test whether the results of the meta-analysis were robust, and we explored the reasons for nonrobust results. If not, the meta-analysis was abandoned, and a qualitative description was carried out instead. When the number of original studies including a certain influencing factor was ≥ 10, a funnel plot was drawn, and Egger’s test was performed to explore whether publication bias existed. If so, the clipping method was used to correct the asymmetry of the funnel plot and the combined effect caused by publication bias. p < 0.05 was considered statistically significant.
Results
Search results and selection
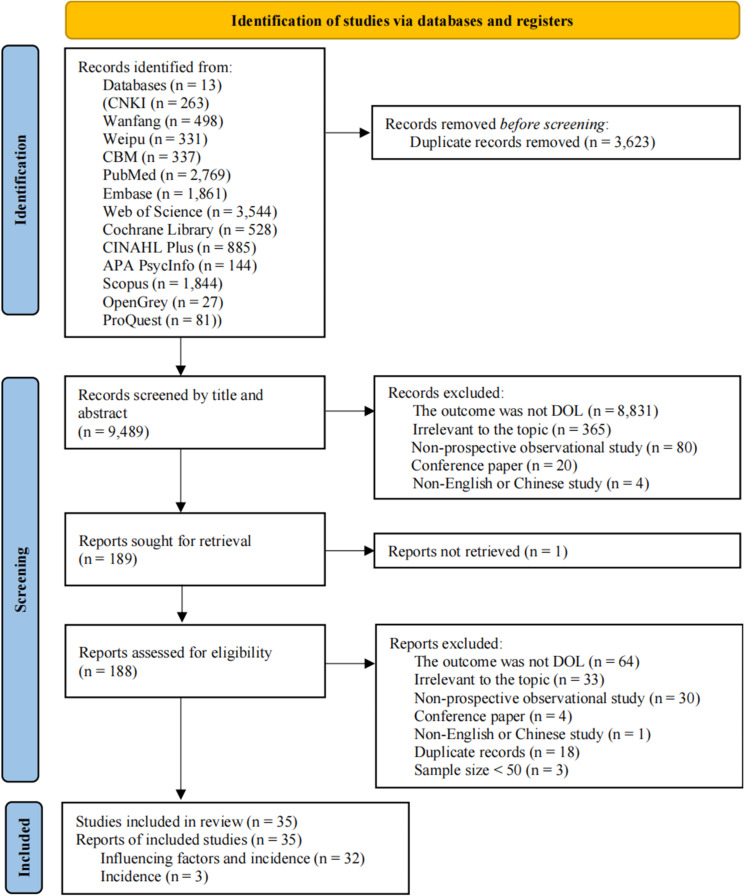
We retrieved 13,112 studies from Chinese and English databases conducted before August 2023, and 9,489 studies were obtained by removing duplicate studies through Endnote and manual methods. After title and abstract screening, 189 studies were included. After rescreening by reading the full text, 32 studies that reported both the incidence and factors influencing DOL and 3 studies that reported only the incidence were ultimately included. No new studies were found after reviewing the references of the included studies and related reviews. The study screening process is shown in Fig. 1.
Fig. 1.
PRISMA flowchart for the identification of studies
Characteristics and quality evaluation of the included studies
A total of 35 included studies were conducted from 1999 to 2023 in 8 countries, including China (n = 23), the United States of America (USA, n = 6), Canada (n = 1), Peru (n = 1), India (n = 1), Australia (n = 1), Brazil (n = 1), and Ghana (n = 1). A total of 19,176 women were included in these studies, 4,922 of whom had DOL. The methods of follow-up involved medical records, questionnaires, or interviews. The mean NOS score of all the studies was ≥ 6 points, indicating that these studies had good methodological quality. The general characteristics and NOS scores of the included studies, sorted alphabetically by author name, are summarized in Table 1.
Table 1.
Characteristics and quality of the included studies
| Study | Maternal characteristic | DOL/total | Period | Country | Incidence (%) | Follow-up | NOS score | |
|---|---|---|---|---|---|---|---|---|
| Method | Endpoint | |||||||
| Bai [18] | General | 112/553 | NR | China | 20.3 | ITV | OL | 7 |
| Chapman [19] | Full-term | 68/192 | Dec. 1996-May 1997 | USA | 35.4 | MR, ITV | OL | 7 |
| Dewey [11] | General | 62/271 | Feb.-Dec. 1999 | USA | 22.9 | ITV | 3 d pp | 7 |
| Ding [20] | Full-term | 107/340 | Jan. 2018-Jan. 2019 | China | 31.5 | QTN, ITV | 72 h-7 d pp | 7 |
| Ding J [21] | Caesarean delivery | 104/330 | Sept. 2021-Jan. 2022 | China | 31.5 | MR, ITV | 7 d pp | 7 |
| Ding QY [22] | Age ≥ 35 years | 106/277 | Mar. 2021-Oct. 2021 | China | 38.3 | MR, QTN, ITV | 72 h pp | 7 |
| Dong [23] | Vaginal delivery | 241/622 | 6 Nov. 2020-16 Jan. 2021 | China | 38.8 | MR, QTN, ITV | OL | 7 |
| Haile [24] | General | 487/2,053 | 2005–2007 | USA | 23.7 | QTN | 21 d pp* | 7 |
| Hilson [25] | General | 28/114 | Aug.-Nov. 1998 | USA | 24.6 | MR, QTN, ITV | 8–12 m pp* | 7 |
| Huang [26] | General | 604/3,282 | started in Jan. 2013 | China | 18.4 | MR, QTN, ITV | OL | 6 |
| Kong, 2008 [27] | General | 29/75 | Oct.-Nov. 2005 | Australia | 38.7 | MR, ITV | NR | 6 |
| Li [28] | MS, Premature | 107/300 | Feb. 2020-May 2021 | China | 35.7 | QTN, ITV | OL | 7 |
| Lian [29] | Caesarean delivery | 156/468 | 9 Oct. 2021-17 May 2022 | China | 33.3 | MR, ITV | 7 d pp | 7 |
| Lu [30] | MS | 56/154 | Jan.-Jun. 2021 | China | 36.4 | QTN, ITV | 7 d pp | 7 |
| Luan [5] | Premature | 36/100 | Feb. 2016-Dec. 2016 | China | 36.0 | QTN | 14 d pp | 7 |
| Luo [31] | GDM | 99/388 | Jan. 2015-Dec. 2018 | China | 25.2 | MR, QTN, ITV | 3 d pp or OL | 7 |
| Matias [32] | Primipara | 30/171 | Nov. 2005-Jun. 2006 | Peru | 17.5 | MR, QTN, ITV | 72–96 h pp | 7 |
| Mullen [33] | Non-GDM | 98/177 | Apr. 2009-Jul. 2010 | Canada | 55.4 | MR, QTN, ITV | 7 d pp | 7 |
| Nommsen-Rivers [4] | Primipara | 190/431 | Jan. 2006-Dec. 2007 | USA | 44.0 | MR, ITV | 7 d pp | 7 |
| Otoo [34] | General | 19/425 | Jan. 2004-Jun. 2007 | Ghana | 4.5 | QTN | OL | 7 |
| Preusting [35] | General | 114/219 | 27 Aug. 2014-15 Oct. 2015 | USA | 52.1 | MR, QTN, ITV | 14 d pp* | 7 |
| Quan [36] | General | 268/1,185 | 1 Mar. 2021-27 Feb. 2022 | China | 22.6 | QTN | NR | 7 |
| Rocha [37] | Primipara | 42/224 | 19 Jan. 2017-23 May 2017 | Brazil | 18.8 | MR, ITV | 7 d pp | 7 |
| Salahudeen [38] | General | 50/200 | Jun. 2011-Dec. 2011 | India | 25.0 | MR, ITV | 7 d pp | 6 |
| Si [39] | GDM | 96/284 | Mar.-Oct. 2016 | China | 33.8 | MR, QTN, ITV | 72 h pp | 7 |
| Wang [40] | General | 68/240 | Jan.-Apr. 2018 | China | 28.3 | MR, ITV | 7 d pp | 6 |
| Wei [41] | General | 722/2,109 | Mar. 2019-Feb. 2021 | China | 34.2 | QTN | NR | 7 |
| Xie [42] | Admitted to MICU | 97/229 | May-Oct. 2019 | China | 42.4 | MR, QTN | OL | 7 |
| Xu [43] | General | 91/269 | Dec. 2019-Nov. 2020 | China | 33.8 | QTN, ITV | NR | 7 |
| Xue [44] | General | 58/204 | Sept. 2013-May 2014 | China | 28.4 | MR, QTN, ITV | 72 h pp | 7 |
| Zhang [45] | Primipara | 86/300 | Jan.-Jun. 2022 | China | 28.7 | MR, QTN, ITV | NR | 6 |
| Zhang YY [46] | General | 64/334 | Mar.-Jun. 2020 | China | 19.2 | MR, QTN, ITV | 72 h pp | 7 |
| Zhang ZY [47] | General | 110/281 | Jan.-Dec. 2019 | China | 39.1 | MR, ITV | OL | 6 |
| Zhao [48] | General | 119/358 | Oct. 2020-Nov. 2021 | China | 33.2 | QTN, ITV | 3 d pp | 7 |
| Zhu [49] | General | 198/2,017 | Mar.-Nov. 2008 | China | 9.8 | MR, QTN, ITV | 2 m pp* | 7 |
MS, maternal separation; GDM, gestational diabetes mellitus; MICU, maternal intensive care unit; MR, medical record; QTN, questionnaire; ITV, interview; NR, not reported; pp, postpartum; m, months; d, days; h, hours
*For the studies with longer follow-up times, the dropout rates were 19.65% (Haile 2017 [24]), 16.79% (Hilson 2004 [25]), 4.00% (Preusting 2017 [35]), and 20.58% (Zhu 2013 [49]), respectively
Meta-analysis and systematic review results
The combined incidence of DOL was obtained via meta-analysis. The meta-analysis results of factors influencing DOL are summarized in Table 2 according to the order of reporting. Combined with the qualitative description of the influencing factors, all the influencing factors involved could be divided into three categories: maternal-related factors, infant-related factors, and breastfeeding-related factors.
Table 2.
Meta-analysis results of factors influencing DOL
| Influencing factors | No. of studies | Heterogeneity test | Test model | Meta-analysis | Sensitivity analysis (Robust) | Egger’s test (t/p-value) | |||
|---|---|---|---|---|---|---|---|---|---|
| p-value | I² (%) | Effect size | Value (95% CI) | p-value | |||||
| Age | |||||||||
| Age (y) | 4 | 0.788 | 0.0 | F | WMD | -0.30 (-0.57, -0.04) | 0.022 | No | - |
| ≥ 35 y | 9 | 0.000 | 94.9 | R | RR | 1.40 (0.96, 2.04) | 0.081 | Yes | - |
| ≥ 30 y | 5 | 0.007 | 71.9 | R | RR | 1.33 (0.98, 1.80) | 0.064 | Yes | - |
| Prepregnancy BMI | |||||||||
| ≥ 25.0 kg/m² | 8 | 0.001 | 71.9 | R | RR | 1.47 (1.17, 1.84) | 0.001 | Yes | - |
| ≥ 24.0 kg/m² | 3 | 0.228 | 32.4 | F | RR | 1.41 (1.14, 1.74) | 0.001 | Yes | - |
| BMI (kg/m²) | 3 | 0.000 | 96.6 | R | WMD | 1.26 (-1.22, 3.75) | 0.319 | Yes | - |
| GWG (excessive)* | 7 | 0.000 | 81.5 | R | RR | 1.38 (1.07, 1.77) | 0.013 | No | - |
| GDM | 14 | 0.010 | 53.0 | R | RR | 1.32 (1.18, 1.49) | 0.000 | Yes | 1.63/0.129 |
| HDP | 13 | 0.000 | 82.1 | R | RR | 1.66 (1.30, 2.12) | 0.000 | Yes | -1.61/0.136 |
| Thyroid disorders during pregnancy | 6 | 0.262 | 22.9 | F | RR | 1.18 (1.05, 1.32) | 0.005 | Yes | - |
| Serum albumin level (g/L) < 35 | 2 | 0.294 | 9.3 | F | RR | 1.57 (1.12, 2.20) | 0.008 | - | - |
| Primipara | 23 | 0.000 | 60.2 | R | RR | 1.40 (1.25, 1.56) | 0.000 | Yes | 1.60/0.125 |
| Caesarean delivery | 20 | 0.000 | 77.0 | R | RR | 1.33 (1.17, 1.52) | 0.000 | Yes | 0.43/0.675 |
| Unscheduled caesarean delivery | 2 | 0.749 | 0.0 | F | RR | 1.24(1.02, 1.51) | 0.033 | - | - |
| Caesarean delivery history | 2 | 0.673 | 0.0 | F | RR | 0.75 (0.60, 0.93) | 0.009 | - | - |
| Duration of labour (h) | 2 | 0.000 | 96.4 | R | WMD | 1.97 (-2.21, 6.16) | 0.356 | - | - |
| Vaginal delivery, stage 2 labour duration > 1 h | 2 | 0.023 | 80.8 | R | RR | 1.41(0.73, 2.72) | 0.308 | - | - |
| Sleep duration (h/d) | 2 | 0.585 | 0.0 | F | WMD | -0.24 (-0.45, -0.02) | 0.029 | - | - |
| EPDS score ≥ 9 | 2 | 0.046 | 75.0 | R | RR | 1.24 (0.80, 1.93) | 0.340 | - | - |
| Education level | |||||||||
| ≥ high school | 2 | 0.511 | 0.0 | F | RR | 1.04 (0.70, 1.55) | 0.848 | - | - |
| ≥ junior college | 3 | 0.098 | 56.9 | R | RR | 1.22 (0.84, 1.77) | 0.291 | Yes | - |
| ≥ undergraduate | 7 | 0.030 | 57.0 | R | RR | 1.07 (0.90, 1.27) | 0.472 | Yes | - |
| > 9 years | 2 | 0.069 | 69.8 | R | RR | 1.00 (0.62, 1.62) | 0.998 | - | - |
| Occupational status (Yes) | 5 | 0.192 | 34.4 | F | RR | 0.99 (0.87, 1.14) | 0.927 | Yes | - |
| Mean monthly household income | |||||||||
| ≤ 5,000 RMB/per person | 3 | 0.952 | 0.0 | F | RR | 1.13 (0.92, 1.37) | 0.238 | Yes | - |
| ≥ 10,000 RMB/per person | 3 | 0.365 | 0.9 | F | RR | 1.00 (0.91, 1.10) | 0.940 | Yes | - |
| Nationality | |||||||||
| Hispanic | 3 | 0.268 | 24.1 | F | RR | 1.10 (0.94, 1.29) | 0.236 | Yes | - |
| White | 2 | 0.150 | 51.8 | R | RR | 0.97 (0.72, 1.30) | 0.841 | - | - |
| Han Chinese | 5 | 0.957 | 0.0 | F | RR | 0.90 (0.72, 1.13) | 0.352 | Yes | - |
| Prenatal smoking status | 4 | 0.622 | 0.0 | F | RR | 1.02 (0.83, 1.24) | 0.872 | Yes | - |
| Prenatal alcohol consumption status | 5 | 0.038 | 60.7 | R | RR | 1.41 (0.88, 2.26) | 0.157 | Yes | |
| ART | 2 | 0.225 | 32.1 | F | RR | 1.10 (0.81, 1.49) | 0.547 | - | - |
| Planned pregnancy | 2 | 0.000 | 97.8 | R | RR | 0.51 (0.11, 2.39) | 0.392 | - | - |
| Insulin treatment | 2 | 0.483 | 0.0 | F | RR | 1.15 (0.71, 1.86) | 0.571 | - | - |
| Fluid infusion (ml/d) | 2 | 0.211 | 36.0 | F | WMD | -21.31 (-72.93, 30.30) | 0.418 | - | - |
| Gestational weeks | |||||||||
| <37 w | 7 | 0.019 | 60.4 | R | RR | 1.29 (1.06, 1.57) | 0.012 | Yes | - |
| Weeks (premature, w) | 2 | 0.726 | 0.0 | F | WMD | -0.47 (-0.89, -0.06) | 0.027 | - | - |
| ≥ 39 w | 2 | 0.656 | 0.0 | F | RR | 1.11 (0.86, 1.43) | 0.432 | - | - |
| Weeks (term, w) | 3 | 0.080 | 60.4 | R | WMD | -0.04(-0.26, 0.19) | 0.744 | Yes | - |
| Birth weight | |||||||||
| <2.5 kg | 2 | 0.590 | 0.0 | F | RR | 1.34 (1.07, 1.67) | 0.011 | - | - |
| ≥ 4 kg | 3 | 0.271 | 23.5 | F | RR | 1.29 (1.07, 1.56) | 0.009 | No | - |
| Weight (kg) | 3 | 0.000 | 94.5 | R | WMD | -0.36 (-0.86, 0.14) | 0.160 | Yes | - |
| Premature infants (g) | 2 | 0.862 | 0.0 | F | WMD | -17.09 (-102.28, 68.09) | 0.694 | - | - |
| Neonatal sex (male) | 9 | 0.897 | 0.0 | F | RR | 0.97 (0.89, 1.05) | 0.462 | Yes | - |
| Apgar score | |||||||||
| < 7 (1 min) | 3 | 0.721 | 0.0 | F | RR | 1.29 (0.92, 1.81) | 0.141 | Yes | - |
| < 8 (1 min) | 3 | 0.018 | 75.1 | R | RR | 1.51 (0.78, 2.93) | 0.218 | Yes | - |
| Breastfeeding guidance (Yes) | 7 | 0.116 | 41.3 | F | RR | 0.72 (0.64, 0.81) | 0.000 | Yes | - |
| Breastfeeding information sources ≥ 3 | 2 | 0.000 | 95.0 | R | RR | 0.50 (0.15, 1.65) | 0.256 | - | - |
| Breastfeeding frequency | |||||||||
| Frequency (t) | 5 | 0.000 | 84.7 | R | WMD | -0.63 (-1.10, -0.16) | 0.008 | Yes | - |
| Postoperative day 1 ≤ 2 t | 2 | 0.581 | 0.0 | F | RR | 1.92 (1.36, 2.72) | 0.000 | - | - |
| Postoperative day 2 ≤ 2 t | 2 | 0.583 | 0.0 | F | RR | 1.71 (1.34, 2.20) | 0.000 | - | - |
| Day 1 < 8 t | 2 | 0.904 | 0.0 | F | RR | 1.00 (0.78,1.28) | 0.970 | - | - |
| History of breastfeeding | 3 | 0.007 | 79.6 | R | RR | 0.73 (0.46, 1.17) | 0.191 | Yes | - |
| Previous insufficient lactation | 2 | 0.916 | 0.0 | F | RR | 0.89 (0.64, 1.24) | 0.491 | - | - |
| Prenatal breast enlargement (No or a little) | 2 | 0.041 | 75.9 | R | RR | 1.35 (0.86, 2.11) | 0.192 | - | - |
| Nipples (flat or sunken) | 4 | 0.009 | 74.2 | R | RR | 0.99 (0.62, 1.58) | 0.956 | Yes | - |
| Bra cup size ≥ D | 2 | 0.080 | 67.3 | R | RR | 1.32 (0.74, 2.34) | 0.347 | - | - |
BMI, body mass index; GWG, gestational weight gain; GDM, gestational diabetes mellitus; HDP, hypertensive disorders of pregnancy; EPDS, Edinburgh Postnatal Depression Scale; RMB, renminbi; ART, assisted reproductive technology; y, years; w, weeks; h, hours; t, times; ‘R’, random; ‘F’, fixed; ‘-‘, not applicable
* Classification according to the recommendations of Weight Gain During Pregnancy: Reexamining the Guidelines [50]
DOL incidence
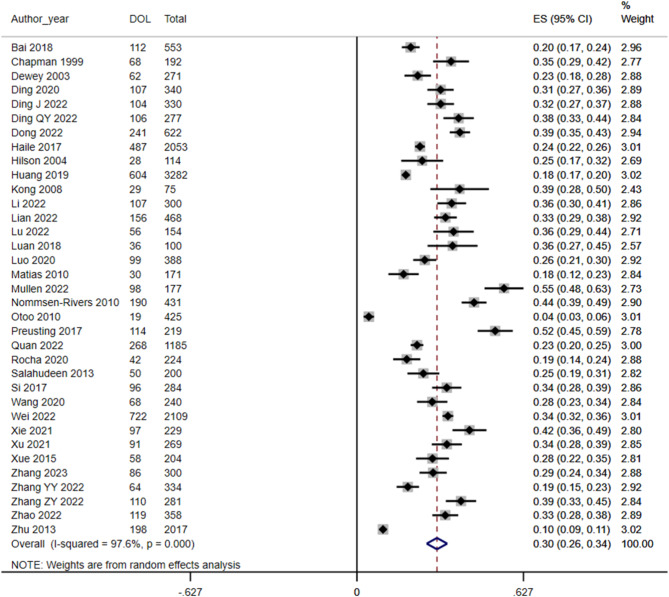
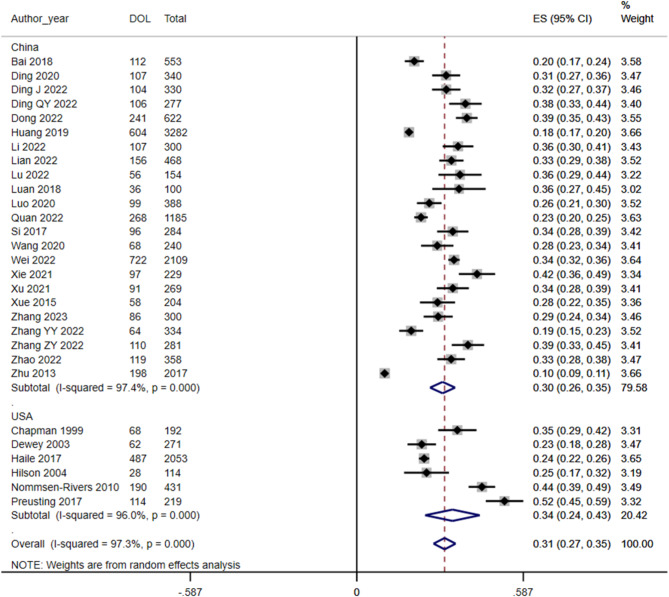
A random-effects model was used to assess the incidence of DOL in 35 studies, and the result was 30% (95% CI 26, 34) (Fig. 2). Subgroup analysis by country category, combined with at least two or more studies, revealed an incidence of DOL of 30% in China (95% CI 26, 35) and 34% in the USA (95% CI 24, 43). The incidence of DOL in the USA was slightly higher than that in China (Fig. 3).
Fig. 2.
Forest plot of DOL incidence
Fig. 3.
Forest plot of the subgroup analysis of DOL incidence
Maternal-related influencing factors
There was a statistically significant difference in age [5, 26, 28, 33] between the DOL and non-DOL groups (WMD =-0.30; 95% CI -0.573, -0.40), but the sensitivity analysis result was not robust. The combined results of 9 studies [24, 31, 36, 41–43, 45, 46, 48] and 5 studies [4, 20, 30, 40, 49], respectively, could not determine the association between a maternal age ≥ 35 years (RR = 1.40; 95% CI 0.96, 2.04) and ≥ 30 years (RR = 1.33; 95% CI 0.98, 1.80) and DOL.
The pooled results of 8 studies [20, 21, 23, 24, 37, 40, 45, 48] and 3 studies [27, 29, 46], respectively, revealed that a prepregnancy BMI ≥ 25.0 kg/m2 (RR = 1.47; 95% CI 1.17, 1.84) and a prepregnancy BMI ≥ 24.0 kg/m2 (RR = 1.41; 95% CI: 1.14, 1.74) were risk factors for DOL. However, the correlation between prepregnancy BMI [26, 30, 33] (WMD = 1.26; 95% CI -1.22, 3.75) and DOL was uncertain. Excessive gestational weight gain (GWG) [24, 37, 40, 41, 45, 46, 48] (RR = 1.38; 95% CI 1.07, 1.77) was a risk factor for DOL, but the sensitivity analysis result was not robust.
The combined results of 14 [20, 21, 24, 26, 28–30, 33, 36–38, 41, 45, 48], 13 [5, 20, 21, 27–30, 36–38, 41, 42, 45], 6 [21, 29, 35, 38, 41, 47] and 2 [5, 29] studies, respectively, revealed that gestational diabetes mellitus (GDM) (RR = 1.32; 95% CI 1.18, 1.49), hypertensive disorders of pregnancy (HDP) (RR = 1.66; 95% CI 1.30, 2.12), thyroid disease during pregnancy (RR = 1.18; 95% CI 1.05, 1.32), and a serum albumin level < 35 g/L (RR = 1.57; 95% CI 1.12, 2.20) were risk factors for DOL. The descriptive analysis could not determine whether anaemia [21, 29, 38] was associated with DOL, and there might be no association between ovarian cysts during pregnancy [21, 29] and DOL.
The pooled results of 23 studies [5, 19–23, 25–29, 31, 33, 35, 36, 38–41, 43, 44, 46, 48] revealed that primiparity (RR = 1.40; 95% CI 1.25, 1.56) was a risk factor for DOL. The combined results of 20 [4, 5, 24, 26, 28, 30–33, 35–38, 40, 41, 44–46, 48, 49] and 2 [21, 29] studies, respectively, revealed that caesarean delivery (RR = 1.33; 95% CI 1.17, 1.52) and unscheduled caesarean delivery (RR = 1.24; 95% CI 1.02, 1.51) were risk factors for DOL. A history of caesarean delivery [21, 29] (RR = 0.75; 95% CI 0.60, 0.93) was a protective factor against DOL. The correlations between the duration of labour [23, 45] (WMD = 1.97; 95% CI -2.21, 6.16), vaginal delivery, and the duration of the second stage of labour > 1 h [4, 19] (RR = 1.41; 95% CI 0.73, 2.72) and DOL were undefined.
Daily sleep duration [5, 28] (WMD =-0.24; 95% CI -0.45, -0.02) was a protective factor against DOL. The correlation between an Edinburgh Postnatal Depression Scale (EPDS) score ≥ 9 points [35, 48] (RR = 1.24; 95% CI 0.80, 1.93) and DOL was unknown. Moreover, descriptive analysis revealed that the relationship between depression [4, 31, 45] and DOL remained unknown, but there might be a correlation between anxiety [22, 31, 45] and DOL.
The results of the meta-analysis revealed that the relationships between the following variables and DOL could not be determined: education level (≥ high school [22, 32], ≥junior college [20, 41, 44], ≥junior undergraduate [4, 21, 23, 24, 37, 46, 48], and > 9 years [29, 49]), occupational status [21, 23, 29, 44, 48], mean monthly household income per person (≤ 5000 RMB [29, 31, 44], and ≥ 10000 RMB [21, 26, 41]), nationality (Hispanic [4, 24, 35], White [33, 35], and Han Chinese [20, 22, 26, 44, 45]), prenatal smoking status [4, 24, 26, 35], prenatal alcohol consumption status [21, 26, 27, 35, 37], assisted reproductive technology (ART) use [21, 41], planned pregnancy [22, 43], insulin treatment [21, 31], and fluid infusion [5, 28]. The descriptive results revealed that height [4, 26], intraoperative or delivery blood loss [4, 21, 29], and drug-induced labour [4, 35, 37] might not be related to DOL, whereas the relationships between stressful life events [22, 45, 49] during pregnancy, exercise during pregnancy [20, 48], and anaesthesia or painkiller use [4, 21, 24, 27, 29, 35, 37] and DOL remained unclear.
Infant-related influencing factors
The pooled results of 7 [29–31, 40–42, 49] and 2 [5, 28] studies, respectively, revealed that a gestational age < 37 weeks (RR = 1.29; 95% CI 1.06, 1.57) and a young gestational age at birth (WMD =-0.47; 95% CI -0.89, -0.06) were risk factors for DOL. Nevertheless, whether gestational age ≥ 39 weeks [4, 32] (RR = 1.11; 95% CI 0.86, 1.43) and gestational age (full-term) [26, 45, 46] (WMD =-0.04; 95% CI -0.26, 0.19) were associated with DOL could not be determined.
A birth weight < 2.5 kg [29, 31] (RR = 1.34; 95% CI 1.07, 1.67) was a risk factor for DOL. A birth weight ≥ 4 kg [22, 26, 48] (RR = 1.29; 95% CI 1.07, 1.56) was also a risk factor, but the sensitivity analysis result was not robust. However, the correlations between neonatal birth weight [30, 33, 45] (WMD =-0.36; 95% CI -0.86, 0.14) and preterm birth weight [5, 28] (WMD =-17.09; 95% CI -102.28, 68.09) and DOL were unclear.
The meta-analysis results could not determine whether neonatal sex [4, 21, 22, 26, 29, 32, 46, 48, 49] and the 1-min Apgar score (< 7 points [21, 28, 38] and < 8 points [4, 32, 35]) were associated with DOL. The descriptive results revealed that the relationships between maternal separation [21, 36, 43] and skin-to-skin contact [4, 35–37] and DOL were unknown.
Breastfeeding-related influencing factors
The combined results of 7 studies [21, 22, 29, 36, 37, 46, 48] revealed that receiving breastfeeding guidance (RR = 0.72; 95% CI 0.64, 0.81) was a protective factor against DOL. The correlation between ≥ 3 breastfeeding information sources [22, 43] (RR = 0.50; 95% CI 0.15, 1.65) and DOL was unknown. Descriptive analysis revealed that breast massage or treatment [22, 36] might be associated with DOL.
The descriptive analysis results revealed that there might be a relationship between a first breastfeeding session after maternal separation [5, 28, 42] and DOL, but it was not clear whether the first breastfeeding session of general mothers [22, 29, 32, 33, 35, 37, 45] was related to DOL. The combined results of 5 studies [5, 28, 42, 45, 46] revealed that breastfeeding frequency (WMD =-0.63; 95% CI -1.10, -0.16) was a protective factor against DOL. Similarly, a breastfeeding frequency ≤ 2 times on the first day [22, 44] (RR = 1.92; 95% CI 1.36, 2.72) and the second day after surgery [22, 44] (RR = 1.71; 95% CI 1.34, 2.20) was a risk factor for DOL. The correlation between a breastfeeding frequency < 8 times [4, 32] from 0 to 24 h after birth (RR = 1.00; 95% CI 0.78, 1.28) and DOL was unknown.
The meta-analysis results revealed that the relationships between a history of breastfeeding [5, 22, 28], previous insufficient lactation [21, 29], prenatal breast enlargement [4, 19], flat or sunken nipples [4, 21, 32, 35], and a bra cup size ≥ D [4, 46] and DOL could not be confirmed. The descriptive results revealed that the relationships between formula milk use (within 24 h [4, 32, 46] or 48 h [4, 32, 35, 48]), LATCH score [26, 35, 46] and nipple pain during lactation [4, 22, 35] and DOL remained unknown.
Sensitivity and publishing bias analysis
The sensitivity analysis results for age (continuous variable), GWG and neonatal birth weight ≥ 4 kg were not robust, whereas the results for the remaining variables were robust. An analysis of the full texts of the studies including these three variables and the sensitivity analysis results revealed that when studies with relatively small samples (< 400 participants) were excluded one by one, the sensitivity analysis results were robust; however, when studies with relatively large samples (> 1,000 participants) were excluded, the sensitivity analysis results became not robust (see Supplementary Material 2).
The results of Egger’s test indicated that there was no publication bias for GDM (p = 0.129), HDP (p = 0.136), primipara (p = 0.125), and caesarean delivery (p = 0.675) (see Table 2). The funnel plots for these four variables also exhibited a basically symmetrical distribution, further suggesting the absence of significant publication bias (see Supplementary Material 3, 4, 5, and 6).
Discussion
The total incidence of DOL was 30% among the 35 included studies. Subgroup analysis revealed that the incidence of DOL was 30% in China and 34% in the USA. Both China and the USA have made many efforts to support breastfeeding and have introduced policies related to breastfeeding, which focus on the positive role of baby-friendly hospitals and policy support, as well as the ‘Ten Steps to Successful Breastfeeding’ framework, breastfeeding clinics and human milk donation programs [51, 52]. Additionally, the American Academy of Pediatrics policy mentions relevant content regarding OL [52]; however, attention to the important impact of OL on breastfeeding is lacking, and China’s policy does not consider OL [51], which may be the reason for the high DOL incidence. Moreover, Patel et al.’s systematic review [53] revealed the effectiveness of dedicated certified lactation consultants or counsellors in promoting breastfeeding, which suggests that they may also have a positive effect on OL support, but this remains to be verified.
The analysis of potential factors influencing DOL revealed statistically significant correlations between DOL and 15 factors: maternal age, prepregnancy BMI (overweight or obesity), GWG, GDM, HDP, thyroid disease during pregnancy, serum albumin levels (< 35 g/L), parity, (unscheduled) caesarean section, caesarean section history, daily sleep duration, gestational age, birth weight (< 2.5 kg or ≥ 4 kg), breastfeeding guidance and daily breastfeeding frequency. However, the sensitivity analysis results for age, GWG and birth weight ≥ 4 kg were not robust. Through descriptive analysis, three factors were found to be likely related to DOL: anxiety, time of first breastfeeding session (maternal separation), and breast massage or treatment.
Maternal-related influencing factors
Combined with the meta-analysis and sensitivity analysis results, although there was a correlation between age and DOL, the result was not robust. A relationship between a maternal age ≥ 35 years or ≥ 30 years and DOL was not found. This suggests that the relationship between maternal age and DOL is still controversial, and more research is needed.
Although the WHO [54] and China [55] have slightly different BMI classification criteria, when the prepregnancy BMI reaches the overweight or obese range, the risk for DOL increases. Studies have shown that women who are overweight or obese before pregnancy have a lower response to prolactin stimulated by sucking [56]. Animal experiments have shown that obesity may impair lactation performance by inducing prolactin resistance [57]. Obesity is an important risk factor for insulin resistance and impaired insulin secretion; insulin is now thought to play a direct role in lactation, including secretory differentiation, secretory activation and mature milk production [58]. The results of this study also revealed that a high prepregnancy BMI was a risk factor for DOL after the standard of overweight or obesity was reached. High GWG may increase the risk of DOL. Although the sensitivity analysis results were not robust, considering the adverse effects of overweight or obesity on DOL, these findings still suggest that GWG has a potentially dangerous effect on DOL, which still requires exploration and verification in further research.
Our analysis revealed that GDM, HDP and thyroid disease during pregnancy were risk factors for DOL. De Bortoli et al.’s systematic review [59] also supports that GDM is a risk factor for DOL. The possible mechanism is that insulin resistance and/or insulin secretion disorders in β cells lead to GDM, of which insulin resistance is the main cause [60], and insulin resistance affects lactation [61]. The ratio of insulin to glucose and adiponectin may also be related to the start time of lactation [62]. Combined with the relationship between obesity and insulin resistance, GDM, obesity and insulin resistance may be associated with DOL in some way [63]. HDP can affect the initiation and duration of breastfeeding [64], and the treatment of HDP may also affect lactation; for example, diuretics may reduce milk production [65]. HDP may also lead to placental dysfunction and decreased prolactin secretion, thereby affecting lactation [66]. Endothelial dysfunction caused by preeclampsia may lead to hypoalbuminaemia in women [67], and lower serum albumin levels indicate poor nutritional status, which may be the cause of DOL [29]. Consistently, this study also revealed that low serum albumin levels were a risk factor for DOL. Animal experiments have shown that hypothyroidism may hinder the ability of the breast to achieve normal milk synthesis and excretion, leading to lactation disorders in pregnant women with thyroid dysfunction [68]. Similarly, hyperthyroidism can also induce impaired release of oxytocin, resulting in milk deposition, apoptosis of glandular cells that secrete milk, and lactation effects [69].
This study revealed that primiparity and (unscheduled) caesarean section were risk factors for DOL, whereas a history of caesarean section was a protective factor against DOL. Compared with multiparas, primiparas may experience longer delivery times, resulting in higher cortisol levels [70, 71], and multiparas may have more prolactin receptors than primiparas [72]. Multiparas may also have better breastfeeding skills than primiparas [73]; hence, primiparas are more likely to experience DOL than multiparas. Similarly, women with a history of caesarean section could have certain breastfeeding experiences; their fear of childbirth in late pregnancy is relatively low [74], and the pressure of childbirth seems to be related to DOL [71]. These factors may be why a history of caesarean section is a protective factor against DOL. Compared with vaginal delivery, (unscheduled) caesarean section may lead to lower levels of oxytocin and prolactin secretion [75, 76], thus increasing the risk of DOL.
Sleep and emotional state may affect the occurrence of DOL. Prolactin secretion has circadian rhythm changes, and sleep deprivation may lead to decreased levels of prolactin secretion [77]. Anxiety and depression are associated with lower oxytocin during feeding [78], and mothers with depression may have insufficient confidence in their ability to breastfeed [79]. In addition, studies have shown that poor sleep quality is associated with depression and anxiety [80, 81]. However, combined with the results of the meta-analysis and descriptive analysis of this study, the relationship between depression and DOL needs further exploration.
Infant-related influencing factors
This study revealed that young gestational age and low birth weight were risk factors for DOL. The shortening of pregnancy may lead to insufficient prenatal breast preparation, and the immature sucking skills of premature infants can lead to insufficient milk discharge [82]. A low birth weight may mean that an infant’s motor development is deficient, which may also affect the infant’s sucking skills, subsequently increasing the risk of DOL [73, 83]. Colostrum is produced before OL (paracellular pathway closure) [84]. Unlike mature milk formed after OL, colostrum is rich in immune factors and cytokines, and the concentration of these substances is inversely proportional to the duration of gestation [84]. Newborns have immature immune systems, especially premature infants whose immune substance transport through the placenta is interrupted prematurely, and colostrum can address this lack of development by providing many bioactive substances [85]. In addition, preterm birth may trigger delayed closure of the paracellular pathway to prolong the supply of protective substances in colostrum [84], although this can lead to DOL. These findings also suggest that medical staff should pay attention to the special therapeutic effect of colostrum on premature infants. For example, oropharyngeal colostrum administration, as proposed by Rodriguez et al. [84] in 2009, has been shown to have a positive effect on the outcomes of preterm infants [86]. Therefore, in clinical practice, it is necessary to help mothers start breastfeeding early and express colostrum, especially for mothers with premature or low-birth-weight infants, to facilitate the use of colostrum for neonatal immune protection.
Breastfeeding-related influencing factors
The WHO recommends that breastfeeding counselling be provided to all pregnant women and women with babies to help enhance their skills, abilities and confidence in breastfeeding [87]. McFadden et al.’s systematic review [88] also revealed that breastfeeding counselling has a positive effect on breastfeeding. Consistent with the results of our study, breastfeeding counselling had a positive protective effect on OL. This study revealed that the frequency of breastfeeding was a protective factor against DOL and should not be less than 2 times/day. Sucking stimulation can trigger the pituitary to release oxytocin, which may be beneficial for uterine involution [89], and frequent breastfeeding and effective milk emptying have positive effects on milk secretion [90, 91]. These findings suggest that in the case of maternal separation, due to the lack of infant sucking, it is necessary to start hand expressing or using a breast pump as soon as possible to mechanically stimulate the areola to promote the release of oxytocin [91], thereby reducing the risk of DOL; moreover, informing mothers of the potential benefits of frequent sucking on uterine involution is recommended to improve their compliance. However, whether the time of the first breastfeeding session of general parturients is related to DOL still needs further exploration. This study revealed that breast massage or treatment might be a protective factor against DOL, and the protective effect may be achieved by simulating sucking and dredging the mammary duct [36].
Strengths and limitations
In the original studies included, different researchers might use different criteria for the same influencing factor. Therefore, this study combined quantitative and qualitative analyses to comprehensively summarize the available studies on the incidence and factors influencing DOL. However, this study inevitably has several limitations: (1) Since most of the original studies reported only statistically significant multivariate analysis results and the multivariate analysis methods used were inconsistent, we chose to extract the exposure and outcome data corresponding to the influencing factors after weighing the effects of bias and confounding on the results; regrettably, few studies reported only statistically significant univariate analysis results, but no publication bias was found by funnel plots or Egger’s tests. (2) There were three factors for which the sensitivity analysis results were not robust. A review of the original studies included revealed that some studies had relatively small sample sizes, which might have resulted in insufficient statistical power. When large-sample studies are eliminated, the results might be affected by the combination of small-sample studies; moreover, sensitivity analysis is not applicable to factors that sourced from only two original studies, so robustness cannot be evaluated. Accordingly, larger samples and higher-quality studies are needed to improve the accuracy and robustness of the results. (3) At present, there are no objective and unified diagnostic criteria for DOL, and the most commonly used method is still the subjective perception of maternal breast distension; however, this method may have a large bias. Therefore, it is still necessary to research milk biomarkers to develop an objective and standard evaluation method for use in clinical practice. (4) The selection of the qualitative description method for some influencing factors was due to the high degree of heterogeneity among the studies, so the results of the qualitative description only have implications, and exact conclusions cannot be drawn. (5) As Chinese researchers, considering the accessibility of the Chinese language, we searched Chinese databases, which may have resulted in the inclusion of many Chinese studies. The studies included were mainly from China and the USA, owing to differences in culture and policy, the results concerning the incidence and factors influencing DOL may vary greatly across countries and even within individual countries. Nevertheless, the results may play a role in the implementation of DOL incidence and influencing factor research by researchers from other countries.
Conclusions
This study revealed that the incidence of DOL was 30%, and the factors influencing DOL may include prepregnancy BMI (overweight or obesity), GDM, HDP, thyroid disease during pregnancy, serum albumin levels (< 35 g/L), parity, (unscheduled) caesarean section, caesarean section history, daily sleep duration, gestational age, birth weight (< 2.5 kg), breastfeeding guidance and daily breastfeeding frequency; however, the relationships between age, GWG, birth weight (≥ 4 kg), anxiety, time of first breastfeeding session (maternal separation) and breast massage or treatment and DOL remain unknown. Considering the adverse effects of DOL, policymakers should pay more attention to OL, a critical period of breastfeeding, and formulate corresponding supportive policies. Researchers are advised to explore and verify objective diagnostic criteria for DOL and the influencing factors for which the associations with DOL remain unknown. In addition, establishing breastfeeding support teams in hospitals is recommended, and clinicians should conduct targeted assessments, risk stratification management, health education and interventions for mothers according to the influencing factors to reduce the occurrence of DOL in the case of rational medical resource use.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
Not applicable.
Abbreviations
- ART
Assisted reproductive technology
- BMI
Body mass index
- DOL
Delayed onset of lactation
- EPDS
Edinburgh Postnatal Depression Scale
- GDM
Gestational diabetes mellitus
- GWG
Gestational weight gain
- HDP
Hypertensive disorders of pregnancy
- MICU
Maternal intensive care unit
- MS
Maternal separation
- NOS
Newcastle‒Ottawa Scale
- OL
Onset of lactation
- RMB
Renminbi
- RR
Risk ratio
- USA
United States of America
- WHO
World Health Organization
- WMD
Weighted mean difference
Author contributions
YJP, KZ, and YH conceptualized and designed this review. YJP, KZ, and YH conducted the literature search, literature screening, data extraction, quality evaluation, and statistical analysis. YJP and KZ wrote the manuscript. YH reviewed and modified the manuscript. All the authors read and approved the final manuscript.
Funding
Not applicable.
Data availability
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Yijuan Peng and Ke Zhuang contributed equally to this study and should be considered co-first authors.
References
- 1.Medina Poeliniz C, Engstrom JL, Hoban R, Patel AL, Meier P. Measures of secretory activation for research and practice: an integrative review. Breastfeed Med. 2020;15(4):191–212. 10.1089/bfm.2019.0247 [DOI] [PubMed] [Google Scholar]
- 2.Meier PP, Patel AL, Hoban R, Engstrom JL. Which breast pump for which mother: an evidence-based approach to individualizing breast pump technology. J Perinatol. 2016;36(7):493–9. 10.1038/jp.2016.14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McManaman JL, Neville MC. Mammary physiology and milk secretion. Adv Drug Deliv Rev. 2003;55(5):629–41. 10.1016/S0169-409X(03)00033-4 [DOI] [PubMed] [Google Scholar]
- 4.Nommsen-Rivers LA, Chantry CJ, Peerson JM, Cohen RJ, Dewey KG. Delayed onset of lactogenesis among first-time mothers is related to maternal obesity and factors associated with ineffective breastfeeding. Am J Clin Nutr. 2010;92(3):574–84. 10.3945/ajcn.2010.29192 [DOI] [PubMed] [Google Scholar]
- 5.Luan D. [The research on milk volume and influencing factors in mothers with preterm infants hospitalized in NlCU] [Thesis]: Binzhou Medical University; 2018.
- 6.Brownell E, Howard CR, Lawrence RA, Dozier AM. Delayed onset lactogenesis II predicts the cessation of any or exclusive breastfeeding. J Pediatr. 2012;161(4):608–14. 10.1016/j.jpeds.2012.03.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huang L, Xu S, Chen X, Li Q, Lin L, Zhang Y, et al. Delayed lactogenesis is associated with suboptimal breastfeeding practices: a prospective cohort study. J Nutr. 2020;150(4):894–900. 10.1093/jn/nxz311 [DOI] [PubMed] [Google Scholar]
- 8.Chapman DJ, Pérez-Escamilla R. Does delayed perception of the onset of lactation shorten breastfeeding duration? J Hum Lactation. 1999;15(2):107–11. 10.1177/089033449901500207 [DOI] [PubMed] [Google Scholar]
- 9.Hruschka DJ, Sellen DW, Stein AD, Martorell R. Delayed onset of lactation and risk of ending full breast-feeding early in rural Guatemala. J Nutr. 2003;133(8):2592–9. 10.1093/jn/133.8.2592 [DOI] [PubMed] [Google Scholar]
- 10.Michel MP, Gremmo-Féger G, Oger E, Sizun J. [Pilot study of early breastfeeding difficulties of term newborns: incidence and risk factors]. Archives de Pédiatrie. 2007;14(5):454–60. 10.1016/j.arcped.2007.01.005 [DOI] [PubMed] [Google Scholar]
- 11.Dewey KG, Nommsen-Rivers LA, Heinig MJ, Cohen RJ. Risk factors for suboptimal infant breastfeeding behavior, delayed onset of lactation, and excess neonatal weight loss. Pediatrics. 2003;112(3 Pt 1):607–19. 10.1542/peds.112.3.607 [DOI] [PubMed] [Google Scholar]
- 12.Liu J, Liu M, Jiang P, Chen H, Hu S, Fu J. [Incidence and influencing factors of delayed onset of lactogenesis II in Chinese parturient women: a systematic review]. Chin J Mod Nurs. 2021;27(10):1300–5. [Google Scholar]
- 13.Miao Y, Zhao S, Liu W, Jiang H, Li Y, Wang A, et al. Prevalence and risk factors of delayed onset lactogenesis II in China: a systematic review and meta-analysis. J Maternal-Fetal Neonatal Med. 2023;36(1):2214833. 10.1080/14767058.2023.2214833 [DOI] [PubMed] [Google Scholar]
- 14.Hu S, Liu J, Jiang P, Sun Z, Zhu Q, Fu J. [A systematic review of the incidence and influencing factors of delayed onset of lactogenesis II]. Chin Gen Pract. 2021;24(24):3110–5. [Google Scholar]
- 15.Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. 10.1136/bmj.n71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25(9):603–5. 10.1007/s10654-010-9491-z [DOI] [PubMed] [Google Scholar]
- 17.Zeng X, Liu H, Chen X, Leng W. [Meta-analysis series (IV): quality assessment tools for observational studies]. Chin J Evidence-Based Cardiovasc Med. 2012;4(04):297–9. [Google Scholar]
- 18.Bai T, Yang Y, Fu X, Zhou Y, Wei X, Zhang F. [Analysis of the impact of postpartum fatigue on the onset of lactation]. Chin J Nurs. 2018;53(4):438–42. [Google Scholar]
- 19.Chapman DJ, Perez-Escamilla R. Identification of risk factors for delayed onset of lactation. J Am Diet Assoc. 1999;99(4):450–512. 10.1016/S0002-8223(99)00109-1 [DOI] [PubMed] [Google Scholar]
- 20.Ding P, Zhao M, Zhang F, Wang L, Yao J, Qiu J, et al. [Nutrients intake in the third trimester and associated factors of delayed onset of lactogenesis II in maternal women]. Chin Gen Pract. 2020;23(5):534–9. [Google Scholar]
- 21.Ding J, Lian W, Ma X. [Construction and validation of risk prediction model for delayed onset of lactogenesis stage II following cesarean section]. Chin J Perinat Med. 2022;25(9):661–9. [Google Scholar]
- 22.Ding Q. [Analysis of factors influencing delayed onset of lactogenesis and construction of a predictive model in advanced cesarean section mothers] [Thesis]: Guangxi University of Chinese Medicine; 2022.
- 23.Dong F, Li L, Zhu K, Zhang S, Guan Y, Han J, et al. [Analysis of current status and influencing factors of lactation initiation delay in women with vaginal delivery]. Chin J Practical Nurs. 2022;38(19):1496–502. [Google Scholar]
- 24.Haile ZT, Chavan BB, Teweldeberhan A, Chertok IR. Association between gestational weight gain and delayed onset of lactation: the moderating effects of race/ethnicity. Breastfeed Med. 2017;12:79–85. 10.1089/bfm.2016.0134 [DOI] [PubMed] [Google Scholar]
- 25.Hilson JA, Rasmussen KM, Kjolhede CL. High prepregnant body mass index is associated with poor lactation outcomes among white, rural women independent of psychosocial and demographic correlates. J Hum Lactation. 2004;20(1):18–29. 10.1177/0890334403261345 [DOI] [PubMed] [Google Scholar]
- 26.Huang L, Chen X, Zhang Y, Sun G, Zhong C, Wang W, et al. Gestational weight gain is associated with delayed onset of lactogenesis in the TMCHC study: a prospective cohort study. Clin Nutr. 2019;38(5):2436–41. 10.1016/j.clnu.2018.11.001 [DOI] [PubMed] [Google Scholar]
- 27.Kong M, Bajorek B. Medications in pregnancy: impact on time to lactogenesis after parturition. J Pharm Pract Res. 2008;38(3):205–8. 10.1002/j.2055-2335.2008.tb00839.x [DOI] [Google Scholar]
- 28.Li J, Yu X, Wang Y, Liu W, Kong H. [The influencing factors of delayed onset of lactogenesis II in preterm parturient women separated from their infants]. Chin J Nurs Educ. 2022;19(4):368–73. [Google Scholar]
- 29.Lian W, Ding J, Xiong T, Liuding J, Nie L. Determinants of delayed onset of lactogenesis II among women who delivered via cesarean section at a tertiary hospital in China: a prospective cohort study. Int Breastfeed J. 2022;17:81. 10.1186/s13006-022-00523-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lu Y, Yin Y, Jiang H. [Influence of pregnancy stress and coping style on delayed onset of lactogenesis in maternal separation]. Chin Nurs Res. 2022;36(23):4148–53. [Google Scholar]
- 31.Luo F, Bao N, Li S. [Establishment and validation of a predictive model for the risk of delayed lactation initiation in pregnant women with gestational diabetes mellitus]. J Clin Pathological Res. 2020;40(6):1394–400. [Google Scholar]
- 32.Matias SL, Nommsen-Rivers LA, Creed-Kanashiro H, Dewey KG. Risk factors for early lactation problems among Peruvian primiparous mothers. Matern Child Nutr. 2010;6(2):120–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mullen AJ, O’Connor DL, Hanley AJ, Piedimonte G, Wallace M, Ley SH. Associations of metabolic and obstetric risk parameters with timing of lactogenesis II. Nutrients. 2022;14(4):876. 10.3390/nu14040876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Otoo GE, Marquis GS, Sellen DW, Chapman DJ, Pérez-Escamilla R. HIV-negative status is associated with very early onset of lactation among Ghanaian women. J Hum Lactation. 2010;26(2):107–17. 10.1177/0890334409348214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Preusting I, Brumley J, Odibo L, Spatz DL, Louis JM. Obesity as a predictor of delayed lactogenesis II. J Hum Lactation. 2017;33(4):684–91. 10.1177/0890334417727716 [DOI] [PubMed] [Google Scholar]
- 36.Quan Y, Liu S, Zhou H. [First lactation time and delayed lactation of parturient in Zhumadian area]. South China J Prev Med. 2022;48(9):1037–40. [Google Scholar]
- 37.Rocha BO, Machado MP, Bastos LL, Barbosa Silva L, Santos AP, Santos LC, et al. Risk factors for delayed onset of lactogenesis II among primiparous mothers from a Brazilian baby-friendly hospital. J Hum Lactation. 2020;36(1):146–56. 10.1177/0890334419835174 [DOI] [PubMed] [Google Scholar]
- 38.Salahudeen MS, Koshy AM, Sen S. A study of the factors affecting time to onset of lactogenesis-II after parturition. J Pharm Res. 2013;6(1):68–72. [Google Scholar]
- 39.Si M. [Correlation between gestational diabetes mellitus (GDM) and delayed onset of lactogenesis (DOL) and risk factors of DOL among GDM puerperae] [Thesis]: Nanjing Medical University; 2017.
- 40.Wang S, Guo N, Jiang H. [Correlations of pre-pregnancy body mass index and gestational weight gain with delayed onset of lactogenesis]. Mod Clin Nurs. 2020;19(9):1–6. [Google Scholar]
- 41.Wei Z, Zhao Z, Meng T, Ye Q, Wan Y, Liu Y. [Occurrence and related factors of delayed lactation initiation of parturients in Tongzhou District, Beijing]. South China J Prev Med. 2022;48(7):817–21. [Google Scholar]
- 42.Xie X, Zhao M. [Current status of delayed onset of lactogenesis II of high-risk pregnant women in maternal intensive care unit and its influence factors: a 229-case study]. J Nursing(China). 2021;28(7):49–53. [Google Scholar]
- 43.Xu H. [A study on the correlation between delayed onset of lactogenesis and breastfeeding self-efficacy and analysis of the factors influencing them] [Thesis]: Soochow University; 2021.
- 44.Xue Y, Xu Q, Liu L, Liu Z, Huang X, Ma J. [Dietary intake and factors influencing delayed onset of lactation among postpartum women in Guangzhou]. South China J Prev Med. 2015;41(3):218–23. [Google Scholar]
- 45.Zhang L, Li Z, Wang F, Du Q. [Analysis of influencing factors of postpartum delayed onset of lactation in primiparas in family delivery room]. J Med Theory Pract. 2023;36(14):2483–6. [Google Scholar]
- 46.Zhang Y, Zhou H, Wang J, Zhang J, Cai Q. [Effects of pre-pregnancy body mass index, gestational weight gain and early feeding behavior on lactogenesis stage II: a prospective study]. Chin J Perinat Med. 2022;25(7):504–12. [Google Scholar]
- 47.Zhang Z, Liu J, Hu S, Jiang P, Liu M, Zhou Y. [Correlation between pregnancy women with hypothyroidism and delayed lactation initiation]. J Mod Med Health. 2022;38(15):2536–9. [Google Scholar]
- 48.Zhao J. [Effect of physical activity of pregnant women in the third trimester on the delayed onset of lactogenesis and exclusive breastfeeding] [Thesis]: Anhui Medical University; 2022.
- 49.Zhu P, Hao J, Jiang X, Huang K, Tao F. New insight into onset of lactation: mediating the negative effect of multiple perinatal biopsychosocial stress on breastfeeding duration. Breastfeed Med. 2013;8(2):151–8. 10.1089/bfm.2012.0010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Institute of Medicine (US) and National Research Council (US) Committee to Reexamine IOM Pregnancy Weight Guidelines. Rasmussen KM, Yaktine AL, editors. Weight gain during pregnancy: reexamining the guidelines. Washington (DC): National Academies Press (US); 2009. Summary. https://www.ncbi.nlm.nih.gov/books/NBK32799/. Accessed 25 Feb 2024. [PubMed]
- 51.[Circular on issuing the breastfeeding promotion action plan(. 2021-2025)]. Gazette of the National Health Commission of the People’s Republic of China. 2021(11):12 – 5.
- 52.Meek JY, Noble L. Policy statement: breastfeeding and the use of human milk. Pediatrics. 2022;150(1). [DOI] [PubMed]
- 53.Patel S, Patel S. The effectiveness of lactation consultants and lactation counselors on breastfeeding outcomes. J Hum Lactation. 2016;32(3):530–41. 10.1177/0890334415618668 [DOI] [PubMed] [Google Scholar]
- 54.Obesity: preventing and managing the global epidemic. Report of a WHO consultation. World Health Organization Technical Report Series. 2000;894:i-xii, 1-253. [PubMed]
- 55.Zhou B. [Predictive values of body mass index and waist circumference to risk factors of related diseases in Chinese adult population]. Chin J Epidemiol. 2002;23(1):5–10. [PubMed] [Google Scholar]
- 56.Buonfiglio DC, Ramos-Lobo AM, Freitas VM, Zampieri TT, Nagaishi VS, Magalhães M, et al. Obesity impairs lactation performance in mice by inducing prolactin resistance. Sci Rep. 2016;6:22421. 10.1038/srep22421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rasmussen KM, Kjolhede CL. Prepregnant overweight and obesity diminish the prolactin response to suckling in the first week postpartum. Pediatrics. 2004;113(5):e465–71. 10.1542/peds.113.5.e465 [DOI] [PubMed] [Google Scholar]
- 58.Nommsen-Rivers LA. Does insulin explain the relation between maternal obesity and poor lactation outcomes? An overview of the literature. Adv Nutr. 2016;7(2):407–14. 10.3945/an.115.011007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.De Bortoli J, Amir LH. Is onset of lactation delayed in women with diabetes in pregnancy? A systematic review. Diabet Med. 2016;33(1):17–24. 10.1111/dme.12846 [DOI] [PubMed] [Google Scholar]
- 60.Powe CE, Allard C, Battista MC, Doyon M, Bouchard L, Ecker JL, et al. Heterogeneous contribution of insulin sensitivity and secretion defects to gestational diabetes mellitus. Diabetes Care. 2016;39(6):1052–5. 10.2337/dc15-2672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lemay DG, Ballard OA, Hughes MA, Morrow AL, Horseman ND, Nommsen-Rivers LA. RNA sequencing of the human milk fat layer transcriptome reveals distinct gene expression profiles at three stages of lactation. PLoS ONE. 2013;8(7):e67531. 10.1371/journal.pone.0067531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Nommsen-Rivers LA, Dolan LM, Huang B. Timing of stage II lactogenesis is predicted by antenatal metabolic health in a cohort of primiparas. Breastfeed Med. 2012;7(1):43–9. 10.1089/bfm.2011.0007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ramanjaneya M, Butler AE, Alkasem M, Bashir M, Jerobin J, Godwin A, et al. Association of complement-related proteins in subjects with and without second trimester gestational diabetes. Front Endocrinol. 2021;12:641361. 10.3389/fendo.2021.641361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Leeners B, Rath W, Kuse S, Neumaier-Wagner P. Breast-feeding in women with hypertensive disorders in pregnancy. J Perinat Med. 2005;33(6):553–60. 10.1515/JPM.2005.099 [DOI] [PubMed] [Google Scholar]
- 65.Vest AR, Cho LS. Hypertension in pregnancy. Curr Atheroscler Rep. 2014;16(3):395. 10.1007/s11883-013-0395-8 [DOI] [PubMed] [Google Scholar]
- 66.Garrido-Gomez T, Quiñonero A, Dominguez F, Rubert L, Perales A, Hajjar KA, et al. Preeclampsia: a defect in decidualization is associated with deficiency of annexin A2. Am J Obstet Gynecol. 2020;222(4):376. e1-e17. 10.1016/j.ajog.2019.11.1250 [DOI] [PubMed] [Google Scholar]
- 67.Saitou T, Watanabe K, Kinoshita H, Iwasaki A, Owaki Y, Matsushita H, et al. Hypoalbuminemia is related to endothelial dysfunction resulting from oxidative stress in parturients with preeclampsia. Nagoya J Med Sci. 2021;83(4):741–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Campo Verde Arboccó F, Sasso CV, Nasif DL, Hapon MB, Jahn GA. Effect of hypothyroidism on the expression of nuclear receptors and their co-regulators in mammary gland during lactation in the rat. Mol Cell Endocrinol. 2015;412:26–35. 10.1016/j.mce.2015.05.026 [DOI] [PubMed] [Google Scholar]
- 69.Varas SM, Muñoz EM, Hapon MB, Aguilera Merlo CI, Giménez MS, Jahn GA. Hyperthyroidism and production of precocious involution in the mammary glands of lactating rats. Reproduction. 2002;124(5):691–702. 10.1530/rep.0.1240691 [DOI] [PubMed] [Google Scholar]
- 70.Chen DC, Nommsen-Rivers L, Dewey KG, Lönnerdal B. Stress during labor and delivery and early lactation performance. Am J Clin Nutr. 1998;68(2):335–44. 10.1093/ajcn/68.2.335 [DOI] [PubMed] [Google Scholar]
- 71.Dimitraki M, Tsikouras P, Manav B, Gioka T, Koutlaki N, Zervoudis S, et al. Evaluation of the effect of natural and emotional stress of labor on lactation and breast-feeding. Arch Gynecol Obstet. 2016;293(2):317–28. 10.1007/s00404-015-3783-1 [DOI] [PubMed] [Google Scholar]
- 72.Zuppa AA, Tornesello A, Papacci P, Tortorolo G, Segni G, Lafuenti G, et al. Relationship between maternal parity, basal prolactin levels and neonatal breast milk intake. Biol Neonate. 1988;53(3):144–7. 10.1159/000242775 [DOI] [PubMed] [Google Scholar]
- 73.Doucet S, Soussignan R, Sagot P, Schaal B. An overlooked aspect of the human breast: areolar glands in relation with breastfeeding pattern, neonatal weight gain, and the dynamics of lactation. Early Hum Dev. 2012;88(2):119–28. 10.1016/j.earlhumdev.2011.07.020 [DOI] [PubMed] [Google Scholar]
- 74.Zhang T, Liu M, Min F, Wei W, Liu Y, Tong J, et al. Fear of childbirth and its determinants in pregnant women in the third trimester: a cross-sectional study. BMC Psychiatry. 2023;23:574. 10.1186/s12888-023-05070-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Nissen E, Uvnäs-Moberg K, Svensson K, Stock S, Widström AM, Winberg J. Different patterns of oxytocin, prolactin but not cortisol release during breastfeeding in women delivered by caesarean section or by the vaginal route. Early Hum Dev. 1996;45(1–2):103–18. 10.1016/0378-3782(96)01725-2 [DOI] [PubMed] [Google Scholar]
- 76.Wang Y, Tao F, Zhu P, Jiang X, Yao Y, Xu Y. [The level of serum prolactin, self-estimated milk yield and growth factor level in colostrum of pregnant women adopting different delivery modes]. Maternal Child Health Care China. 2010;25(10):1411–4. [Google Scholar]
- 77.Fu M, Zhang L, Ahmed A, Plaut K, Haas DM, Szucs K, et al. Does circadian disruption play a role in the metabolic-hormonal link to delayed lactogenesis II? Front Nutr. 2015;2:4. 10.3389/fnut.2015.00004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Stuebe AM, Grewen K, Meltzer-Brody S. Association between maternal mood and oxytocin response to breastfeeding. J Women’s Health. 2013;22(4):352–61. 10.1089/jwh.2012.3768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zubaran C, Foresti K. The correlation between breastfeeding self-efficacy and maternal postpartum depression in southern Brazil. Sex Reproductive Healthc. 2013;4(1):9–15. 10.1016/j.srhc.2012.12.001 [DOI] [PubMed] [Google Scholar]
- 80.Khadka R, Hong SA, Chang YS. Prevalence and determinants of poor sleep quality and depression among postpartum women: a community-based study in Ramechhap district, Nepal. Int Health. 2020;12(2):125–31. 10.1093/inthealth/ihz032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Okun ML, Mancuso RA, Hobel CJ, Schetter CD, Coussons-Read M. Poor sleep quality increases symptoms of depression and anxiety in postpartum women. J Behav Med. 2018;41(5):703–10. 10.1007/s10865-018-9950-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Krebs NF, Belfort MB, Meier PP, Mennella JA, O’Connor DL, Taylor SN, et al. Infant factors that impact the ecology of human milk secretion and composition-a report from Breastmilk Ecology: Genesis of Infant Nutrition (BEGIN) Working Group 3. Am J Clin Nutr. 2023;117:S43–60. 10.1016/j.ajcnut.2023.01.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Canals J, Fernández-Ballart J, Esparó G. Evolution of neonatal behavior assessment scale scores in the first month of life. Infant Behav Dev. 2003;26(2):227–37. 10.1016/S0163-6383(03)00019-5 [DOI] [Google Scholar]
- 84.Rodriguez NA, Meier PP, Groer MW, Zeller JM. Oropharyngeal administration of colostrum to extremely low birth weight infants: theoretical perspectives. J Perinatol. 2009;29(1):1–7. 10.1038/jp.2008.130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Garofoli F, Civardi E, Pisoni C, Angelini M, Ghirardello S. Anti-inflammatory and anti-allergic properties of colostrum from mothers of full-term and preterm babies: the importance of maternal lactation in the first days. Nutrients. 2023;15(19):4249. 10.3390/nu15194249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Fu ZY, Huang C, Lei L, Chen LC, Wei LJ, Zhou J, et al. The effect of oropharyngeal colostrum administration on the clinical outcomes of premature infants: a meta-analysis. Int J Nurs Stud. 2023;144:104527. 10.1016/j.ijnurstu.2023.104527 [DOI] [PubMed] [Google Scholar]
- 87.Guideline: counselling of women to improve breastfeeding practices. Geneva: World Health Organization. 2018. Evidence and Recommendations. https://www.ncbi.nlm.nih.gov/books/NBK539310/. Accessed 25 Feb 2024. [PubMed]
- 88.McFadden A, Siebelt L, Marshall JL, Gavine A, Girard LC, Symon A, et al. Counselling interventions to enable women to initiate and continue breastfeeding: a systematic review and meta-analysis. Int Breastfeed J. 2019;14:42. 10.1186/s13006-019-0235-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Neville MC. Anatomy and physiology of lactation. Pediatr Clin North Am. 2001;48(1):13–34. 10.1016/S0031-3955(05)70283-2 [DOI] [PubMed] [Google Scholar]
- 90.Weaver SR, Hernandez LL. Autocrine-paracrine regulation of the mammary gland. J Dairy Sci. 2016;99(1):842–53. 10.3168/jds.2015-9828 [DOI] [PubMed] [Google Scholar]
- 91.Truchet S, Honvo-Houéto E. Physiology of milk secretion. Best Pract Res Clin Endocrinol Metab. 2017;31(4):367–84. 10.1016/j.beem.2017.10.008 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.