Abstract
Purpose
Head and neck lymphedema (HNL) following radiation therapy for head and neck cancer (HNC) causes patient morbidity. Predicting individual patients’ risk of HNL after treatment is challenging. We aimed to identify the demographic, disease-related, and treatment-related factors associated with external and internal HNL following treatment of HNC with definitive or adjuvant radiation therapy.
Methods and Materials
Relevant clinical, pathologic, and dosimetric data for 76 consecutive patients who received definitive or adjuvant radiation ± chemotherapy were retrospectively collected from a single institution. Multivariable models predictive of external and internal lymphedema using clinicopathologic variables alone and in combination with dosimetric variables were constructed and optimized using competing risk regression.
Results
After median follow-up of 550 days, the incidence of external and internal HNL at 360 days was 70% and 34%, respectively. When evaluating clinical and treatment-related factors alone, number of lymph nodes removed and advanced adenopathy status were predictive of external lymphedema. With incorporation of dosimetric variables, the optimized model included the percentage volume of the contralateral lymph node level VII receiving 30Gy V30 ≥50%, number of lymph nodes removed, and advanced adenopathy status. For internal lymphedema, our clinicopathologic model identified both adjuvant radiation, as opposed to definitive radiation, and advanced adenopathy status. With inclusion of a dosimetric variable, the optimized model included larynx V45 ≥50% and advanced adenopathy.
Conclusions
HNL following HNC treatment is common. For both external and internal lymphedema, nodal disease burden at diagnosis predicts increased risk. For external lymphedema, increasing extent of lymph node dissection prior to adjuvant therapy increases risk. The contralateral level VII lymph node region is also predictive of external lymphedema when radiation dose to V30 is ≥50%, meriting investigation. For internal lymphedema, we confirm that increasing radiation dose to the larynx is the most significant dosimetric predictor of mucosal edema when larynx V45 is ≥50%.
Introduction
Treatment of head and neck cancer (HNC) with definitive or adjuvant radiation ± chemotherapy may cause external and internal head and neck lymphedema (HNL) due to disruption of lymphatic pathways. This disruption prevents lymphatic fluid flow from the interstitium of head and neck soft tissue into the right lymphatic duct and left thoracic duct.1, 2, 3, 4, 5 External HNL occurs when lymph fluid accumulates within the skin and soft tissues of the face and neck, often manifesting as fullness and swelling in the submandibular region and cervical neck. Internal HNL occurs when lymph fluid accumulates within the mucosa and soft tissue structures of the pharynx and larynx, frequently affecting the epiglottis, arytenoids, interarytenoid space, and epiglottic folds.6,7
HNL often occurs after HNC treatment. Across several studies, the prevalence of external HNL is estimated to be between 30% and 90% (median, 54%), and the prevalence of internal lymphedema is estimated to be between 52% and 97% (median, 68%). Simultaneous external and internal lymphedema occurs with an estimated frequency between 29% and 81% (median, 56%).8, 9, 10, 11, 12, 13, 14 The wide range in HNL frequency following HNC treatment across studies is due to not only heterogeneous patient populations and treatments but also differences in lymphedema evaluation methods, lymphedema grading scales, and time at which lymphedema is assessed.11,15, 16, 17
Following HNC treatment, external or internal lymphedema may resolve with time but can also become chronic with a negative impact on patient quality of life.10,13,18, 19, 20, 21 Furthermore, HNL is associated with the presence or development of other HNC treatment sequalae. These include reduced swallowing dysfunction, weight loss, voice changes, difficulty speaking, difficulty breathing, decreased cervical neck range of motion, decreased hearing, body dysmorphia, and diminished quality of life.6,7,10,11,22, 23, 24
In the last 10 years, several groups have discovered risk factors associated with HNL development after HNC treatment, including demographic, tumor, treatment, and dosimetric factors.6,14,21,25,26 Despite these studies, the elevation in risk of HNL specific to radiation treatment is undefined, particularly for external lymphedema. In addition, it is unknown whether radiation dose to cervical neck lymphatic subregions confers increased risk of HNL in the presence of other risk factors. To address these unknowns, we evaluated a single-institution data set.
Methods and Materials
This retrospective study was approved by an institutional review board. Patients treated consecutively with definitive-intent or adjuvant radiation therapy ± chemotherapy for HNC between November 2016 and September 2019 by a single radiation oncologist specializing in treatment of HNC were included. Recorded outcomes were locked in the database for analysis on August 10, 2020. Patients were most frequently treated with intensity modulated radiation therapy on a standard linear accelerator, and a minority of patients were treated using proton radiation therapy. Treatments were delivered at 2 sites within a single health system. In total, 81 patients were eligible for inclusion.
Medical records were reviewed for lymphedema outcome and clinical, pathologic, treatment, and dosimetric data were recorded. Tumor staging was performed according to the American Joint Committee on Cancer Eighth Edition using p16-negative staging for all patients to compare across subsites. External lymphedema occurrence was defined as lymphedema noted on physical exam or upon patient referral to physical therapy specifically for lymphedema, which occurred only after lymphedema manifested. Internal lymphedema occurrence was defined as edematous laryngeal structures visualized during flexible fiber-optic laryngoscopies. Patients who developed lymphedema after surgery and prior to radiation therapy were excluded. Time to lymphedema was calculated from last radiation treatment and cumulative incidence of external lymphedema and internal lymphedema were calculated. Competing events were defined as tumor recurrence and death from any cause. Patients were censored at last oncology follow-up appointment, recurrence, or death. Cumulative incidence functions in the presence of competing events were generated for outcomes and risk groups. Gray's test was used for comparisons between cumulative incidence functions.27
Volumes were contoured using MIM radiation planning software (MIM Software Inc) on computed tomography simulation images used for treatment planning. After volumes were defined for 20 patients, a custom database and workflow was created for autoregistration and autogeneration of contours on subsequent computed tomography scans. These autogenerated contours served as a starting point for subsequent patient volumes, but significant adjustments and recontouring were performed on all patients.
Dosimetry information was obtained for cervical lymph node (LN) regions as defined by consensus contouring guidelines.28 LN levels evaluated included bilateral levels Ib, IIa, IIb, III, IVa, IVb, V (posterior triangle group), Vc (lateral supraclavicular group), and VII [a and b combined, “retropharyngeal” [RP]) as well as midline LN levels Ia and VIa. The bilateral parotid glands and the larynx were also delineated. Bilateral structures were distinguished by location ipsilateral to or contralateral to the primary tumor.
Competing risk regression was used to determine significant clinical, pathologic, and treatment-related variables for lymphedema outcomes using methods previously described.27,29,30 Univariate analysis selected significant variables from models that converged and these variables were considered for multivariable regression. Variables with several levels were determined significant using a Wald test.31 Variables were considered significant if P value was <.05. Given the small sample size, retrospective analysis, and exploratory nature of this study, no corrections were made for multiple hypothesis testing. Clinicopathologic multivariable models were constructed with a forward approach and selected based on minimization of the Akaike information criterion (AIC).32 Hazard ratios (HRs) with 95% CIs and P values are reported for significant factors.
Dose-volume histogram (DVH) data for each structure volume were calculated from the DICOM, structure, and plan files using an open-source software interface DVH Analytics.33 Dosimetry parameters evaluated included minimum dose, mean dose, maximum dose, and V5 to V75 by increments of 5 Gy. Several composite structures were created, including combined bilateral LN levels II to IV, bilateral LN level Ib to VII, and all structures combined. Volume-average dosimetry data were derived for these composite structures from the previously defined LN levels.
DVH-derived dosimetric variables for each volume were iteratively combined with the clinicopathologic variables included in the multivariable models. Dosimetric variables were evaluated if maximally selected rank statistical analysis found significant cut points according to lymphedema outcome. A combined multivariable model including both dosimetric and clinicopathologic variables was constructed using a forward approach and selected based on minimization of the AIC, as previously described.34 This approach selected the combination of clinicopathologic and dosimetric factors that optimized model performance. HRs with 95% CIs and P values are reported for significant multivariable factors. Factors were considered significant for a P value <.05. As before, no corrections were made for multiple hypothesis testing.
Analyses were conducted in R 4.0.3.35 Cumulative incidence and competing risk regression were performed using the package cmprsk,27 and competing risk regression covariate selection was performed using the package crrstep.32 Maximally selected rank statistics were performed using the package maxstat.36 Nomograms were generated using the package rms using published methods.37,38 All plots were created in R using packages ggplot2 and ggpubr.39,40
Results
Of 81 patients treated consecutively for HNC at a single institution by an individual radiation oncologist, 5 (6.2%) were excluded for the development of lymphedema between surgery and the start of radiation treatment. A total of 76 patients were included in the analysis of lymphedema following definitive-intent or adjuvant radiation treatment. The median follow-up time was 550 days (IQR, 276-854 days).
As described in Table 1, the cohort of 76 patients had a median age of 63 years (range, 19-86 years), had a median body mass index of 24.3 (range, 17.3-34.4), were majority men (75%), were majority White (65%), and were often former smokers (58%). Oropharyngeal cancer accounted for 61% of cases, the majority of which were human papillomavirus–mediated (89%). Slightly more than half of the patients had ≤T2 tumors (55%) and ≤N2b nodal disease (56%). LN metastases were common, with metastases present unilaterally (54%), bilaterally (25%), or not present (21%). Sixty-three percent of patients received definitive-intent radiation therapy and 72% received concurrent chemotherapy. The most common regimen was weekly cisplatin (86%) or cisplatin every 3 weeks (7%). Most patients received radiation therapy to the bilateral neck (78%) with photon-based treatment (92%) using a simultaneous integrated boost (75%). The median equivalent dose in 2-Gy fractions prescription dose received was 7000 cGy (range, 5200-7066 cGy). The median number of days of radiation treatment was 46. Table 1 separately reports characteristics for patients with oropharynx primaries.
Table 1.
Patient, tumor, and treatment characteristics
| Variable | All patients (N = 76) | Oropharynx (n = 46) |
|---|---|---|
| Patient characteristics | ||
| Age at completion of treatment (y), median (range) | 63 (19-86) | 62 (30-77) |
| Sex, n (%) | ||
| Female | 19 (25) | 8 (17.4%) |
| Male | 57 (75) | 38 (82.6%) |
| Race/ethnicity, n (%) | ||
| African American | 15 (19.7) | 9 (19.6) |
| Asian | 4 (5.3) | 0 |
| Caucasian | 49 (64.5) | 32 (69.6) |
| Hispanic | 6 (7.9) | 3 (6.5) |
| Not specified | 2 (2.7) | 2 (4.3) |
| BMI, median (range) | 24.3 (17.3-34.4) | 24.3 (17.3-33.2) |
| Smoking status, n (%) | ||
| Current | 2 (2.6) | 0 |
| Former | 44 (57.9) | 25 (54.3) |
| Never | 30 (39.5) | 21 (45.7) |
| Tumor characteristics, n (%) | ||
| Subsite | ||
| Hypopharynx | 2 (2.6) | |
| Larynx | 14 (18.4) | |
| Nasopharynx | 5 (6.6) | |
| Oral cavity | 5 (6.6) | |
| Oropharynx | 46 (60.5) | 46 (100) |
| Other (parotid, sinus, and paranasal sinus) | 4 (5.3) | |
| T stage | ||
| Tis | 2 (2.6) | 0 |
| T1 | 20 (26.3) | 17 (37.0) |
| T2 | 22 (28.9) | 14(30.4) |
| T3 | 13 (17.1) | 5 (10.9) |
| T4 | 19 (25) | 10 (21.8) |
| N stage | ||
| N0 | 16 (21.1) | 5 (10.9) |
| N1 | 10 (13.2) | 3 (6.5) |
| N2 (nasopharynx only) | 2 (2.6) | |
| N2a | 8 (10.3) | 8 (17.4) |
| N2b | 9 (11.8) | 7 (15.2) |
| N2c | 10 (13.2) | 8 (17.4) |
| N3 (nasopharynx only) | 1 (1.3) | 0 |
| N3a | 0 | 0 |
| N3b | 20 (26.3) | 15 (32.6) |
| Advanced adenopathy* | ||
| No | 43 (56.6) | 23 (50.0) |
| Yes | 33 (43.4) | 23 (50.0) |
| Location of lymph node metastases | ||
| None | 16 (21.1) | 5 (10.9) |
| Unilateral | 41 (53.9) | 29 (63.0) |
| Bilateral | 19 (25) | 12 (26.1) |
| Treatment characteristics | ||
| Surgery variables | ||
| Surgical resection, n (%) | ||
| No | 48 (63.2) | 29 (63.0) |
| Yes | 26 (34.2) | 17 (37.0) |
| Laryngectomy | 2 (2.63) | 0 |
| Neck dissection, n (%) | ||
| None | 50 (65.8) | 29 (63.0) |
| Unilateral (ipsilateral) | 20 (26.3) | 4 (8.7) |
| Bilateral | 6 (7.9) | 13 (28.3) |
| Number of lymph nodes removed, average (IQR) | 11.5 (0-20) | 12.2 (0-21) |
| Radiation variables | ||
| Radiation type, n (%) | ||
| Adjuvant | 28 (36.8) | 17 (37.0) |
| Definitive | 48 (63.2) | 29 (63.0) |
| Radiation treatment of neck, n (%) | ||
| None | 6 (7.9) | 0 |
| Unilateral (ipsilateral) | 11 (14.5) | 6 (13.1) |
| Bilateral | 59 (77.6) | 40 (87.0) |
| Radiation modality, n (%) | ||
| Photon | 70 (92.1) | 42 (91.3) |
| Proton | 6 (7.9) | 4 (8.7) |
| Radiation delivery, n (%) | ||
| 2-field | 6 (7.9) | 0 |
| SIB | 57 (75) | 38 (82.6) |
| Sequential | 7 (9.2) | 4 (8.7) |
| Proton | 6 (7.9) | 4 (8.7) |
| EQD2, median (range) | 7000 (5200-7066) | 7000 (5600-7066) |
| Days of radiation, median (range) | 46 (28-70) | 48 (37-54) |
| Chemotherapy variables, n (%) | ||
| Induction chemotherapy | ||
| No | 71 (93.4) | 42 (91.3) |
| Yes | 5 (6.6) | 4 (8.7) |
| Concurrent chemotherapy | ||
| No | 21 (27.6) | 8 (17.4) |
| Yes | 55 (72.4) | 38 (82.6) |
| Adjuvant chemotherapy | ||
| No | 72 (94.7) | 46 (100) |
| Yes | 4 (5.3) | 0 |
Abbreviations: BMI = body mass index; EQD2 = equivalent dose in 2-Gy fractions; SIB = simultaneous integrated boost.
Advanced adenopathy defined as N2c or greater, or N2 and greater for nasopharynx.
External lymphedema in the entire patient cohort
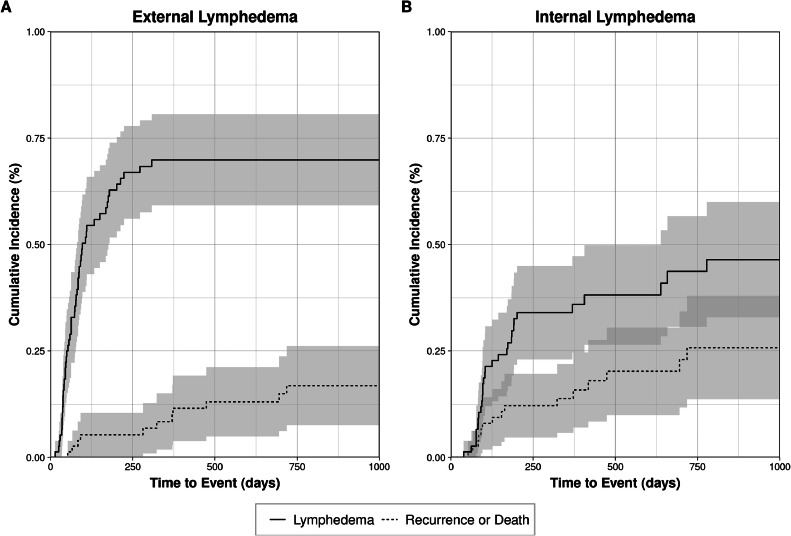
The cumulative incidence of external lymphedema at 60, 180, 360, and 720 days was 28.9%, 62.8%, 69.9%, and 69.9%, respectively. The cumulative incidence of competing events, including tumor recurrence or death, at the same time points was 1.3%, 5.3%, 8.4% and 16.9% (Fig. 1A). Among patients who developed external lymphedema, the median time to its development was 73.5 days (range, 14-308).
Figure 1.
Cumulative incidence of external lymphedema and competing risk events among 76 patients treated with definitive or adjuvant radiation therapy following head and neck cancer treatment. Shaded regions indicate 95% CIs.
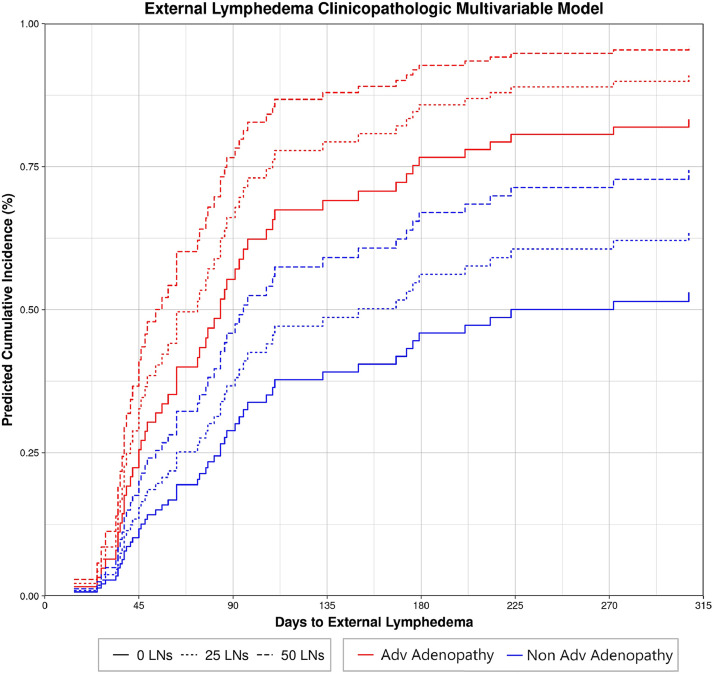
A univariate competing risk regression for external lymphedema found significant clinicopathologic risk factors among models that converged (Table E1): race/ethnicity (P < .001), smoking status (P = .0043), nodal stage (P < .001), advanced adenopathy (P = .0012), location of LN metastases (P = .0015), surgical resection (P = .037), neck dissection (P < .001), and number of LNs removed (P = .0026). Note that advanced adenopathy, for the purposes of this study, indicates ≥N2c disease, or ≥N2 disease for nasopharyngeal primaries, to dichotomize the extent and size of LN metastases. Next, a multivariable competing risk regression model was created using race/ethnicity, smoking status, location of LN metastases, neck dissection, number of LNs removed, and advanced adenopathy status. Nodal stage was excluded as it correlated closely with advanced adenopathy status, leading to failure of model convergence when both variables were included. Using a forward selection method with minimization of the AIC, the best-performing model included number of LNs removed (HR, 1.01 per LN removed; 95% CI, 1.0-1.02; P = .024) and advanced adenopathy status (yes vs no; HR, 2.37; 95% CI, 1.32-4.23; P = .0038). Predicted cumulative incidence for patients with these features is shown in Fig. 2.
Figure 2.
Clinicopathologic multivariable model for external lymphedema depicting increasing risk of external lymphedema with advanced (Adv) adenopathy status at diagnosis and more extensive lymph node (LN) dissections.
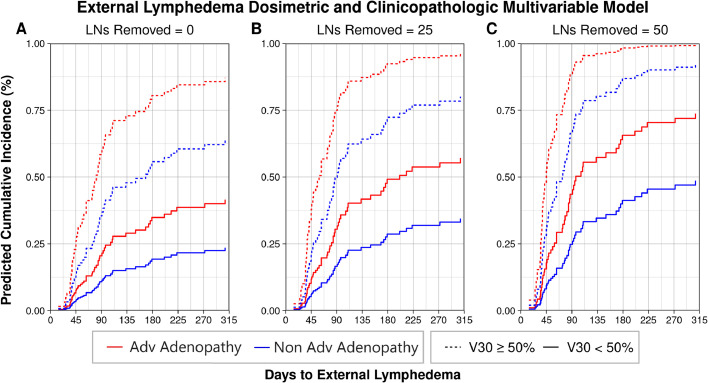
Subsequently, DVH data was extracted for bilateral LN levels, bilateral parotid glands, LN level Ia, LN level VIa, and the larynx. Maximally selected rank statistical analysis selected dosimetric factors with significant cut points on external lymphedema outcome. Of 513 dosimetric variables, 166 had significant cut points for external lymphedema (Table E1). Competing risk regression models incorporating the significant dosimetric variables along with the variables from the clinicopathologic model were iteratively evaluated using forward selection with minimization by AIC. The optimized model included contralateral RP LN level V30 ≥50% (HR, 3.81; 95% CI, 1.59-9.16; P = .0027), number of LNs removed (HR, 1.02; 95% CI, 1.01-1.03; P = .0018), and advanced adenopathy status (HR, 2.01; 95% CI, 1.10-3.64; P = .022). Performing the same procedure to incorporate a second dosimetric variable failed to improve the selected model. Plotting the predictive cumulative incidence of the combined dosimetric and clinicopathologic competing risk regression model reveals an increased risk of external lymphedema with contralateral RP LN level V30 ≥50%, increasing number of dissected LNs, and advanced adenopathy status at diagnosis. (Fig. 3A-C). A nomogram is provided in Fig. E1.
Figure 3.
Combined dosimetric and clinicopathologic multivariable model for external lymphedema depicting increasing risk of external lymphedema with advanced (Adv) adenopathy status at diagnosis, more extensive lymph node (LN) dissections, and a radiation dose to the contralateral retropharyngeal (level VII) LN region V30 ≥50%.
To assess the ability of the combined competing risk regression model risk factors to separate patients into external lymphedema risk groups, the patient cohort was separated into high- and low-risk groups. High-risk patients had a nomogram-derived 50% cumulative incidence of external lymphedema at 6 months and consisted of patients with contralateral RP V30 ≥50%, those with ≥65 LNs dissected, and those with both ≥28 LNs removed and advanced adenopathy status at diagnosis. All other patients were deemed low risk (Fig. E2A). Using Gray's test, the subdistributions of the cumulative incidence curves for external lymphedema risk groups were significantly different (P < .001). At 180 days, the estimated cumulative incidences in the high-risk and low-risk groups were 72% and 22%, respectively.
Internal lymphedema in the entire patient cohort
The cumulative incidence of internal lymphedema at 60, 180, 360 and 720 days was 1.3%, 26.9%, 34%, and 43.7%. The cumulative incidence of recurrence or death was 1.3%, 12.2%, 13.8%, 25.8%, respectively (Fig. 1B).
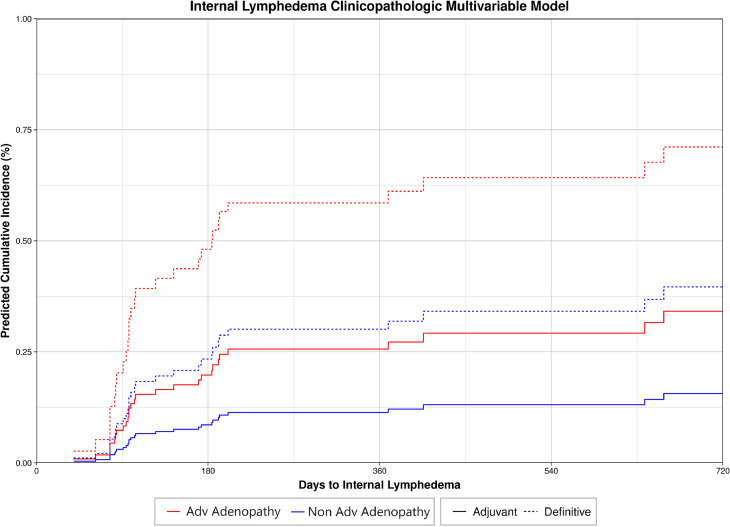
A univariate competing risk regression for internal lymphedema found significant clinicopathologic variables among models that converged (Table E1): adjuvant versus definitive radiation (P = .025), surgical resection (P = .025), and advanced adenopathy status (P = .026). A multivariable competing risk regression model was created for internal lymphedema. Because type of radiation (adjuvant vs definitive-intent) and surgical resection (yes vs no) correlated perfectly, type of radiation was chosen for inclusion with advanced adenopathy status in the tested model. Using a forward selection method with minimization of the AIC, the optimized model included both type of radiation (adjuvant vs definitive-intent; HR, 0.34; 95% CI, 0.15-0.77; P = .010) and advanced adenopathy status (yes vs no; HR, 2.46; 95% CI, 1.22-4.96; P = .012). Predicted cumulative incidence for patients with these features are shown in Fig. 4.
Figure 4.
Clinicopathologic multivariable model for internal lymphedema depicting increasing risk of internal lymphedema with advanced (Adv) adenopathy status at diagnosis and definitive as opposed to adjuvant radiation treatment.
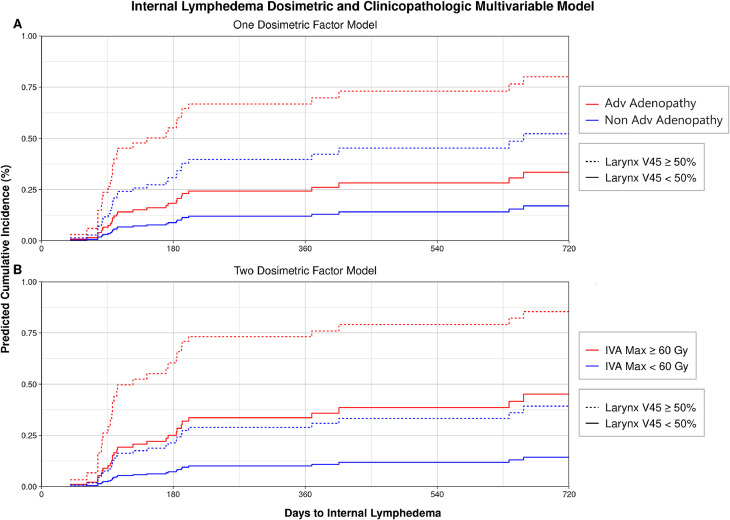
Performing the same maximally selected rank statistical analysis for internal lymphedema, 71 of 513 dosimetric variables had significant cut points for internal lymphedema outcome (Table E1). Upon iteratively combining these significant dosimetric variables with the clinicopathologic factors to create a combined competing risk regression multivariable model, the selected model included larynx V45 ≥50% (HR, 3.96; 95% CI, 1.84-8.53; P < .001) and advanced adenopathy status (HR, 2.18; 95% CI, 1.05-4.50; P = .036). Predicted cumulative incidence for patients with these features is shown in Fig. 5A. The same analysis was performed to assess predictive power of a second dosimetric variable, and the selected model included larynx V45 ≥50% (HR, 3.21; 95% CI, 1.43-7.20; P = .0046) and ipsilateral level IVA maximum dose ≥60 Gy (HR, 3.86; 95% CI, 1.73-8.60; P < .001). Note that in this 2 dosimetric variable model, advanced adenopathy status was excluded in the optimized model, as shown in Fig. 5B. Nomograms are provided in Fig. E3 and Fig. E4.
Figure 5.
Combined dosimetric and clinicopathologic multivariable model for internal lymphedema depicting (A) a multivariable model incorporating only 1 dosimetric factor demonstrating increasing risk of internal lymphedema with advanced (Adv) adenopathy status at diagnosis and a radiation dose to the larynx V45 ≥50% and (B) a multivariable model incorporating 2 dosimetric factors depicting increasing risk of internal lymphedema with a radiation dose to the larynx V45 ≥50% and a maximum (Max) radiation dose to the ipsilateral level IVA ≥60 Gy.
To assess the ability of risk factors to separate patients into risk groups for internal lymphedema, the entire cohort of patients was separated into high- and low-risk groups. Based on a nomogram-derived risk of 50% cumulative incidence of internal lymphedema at 6 months, the high-risk group included patients with larynx V45 ≥50% and ipsilateral level IVA maximum dose ≥60 Gy. Patients not meeting both dosimetric criteria were considered low risk (Fig. E2B). Using Gray's test, the subdistributions of the cumulative incidence curves for internal lymphedema risk groups were significantly different (P < .001). At 180 days, the estimated cumulative incidences in the high-risk and low-risk groups were 58% and 17%, respectively.
Discussion
This institutional analysis attempts to refine the risk factors for development of HNL in HNC patients undergoing curative radiation therapy, either as part of definitive or adjuvant radiation ± chemotherapy. Our results suggest a combination of LN involvement at diagnosis, extent of LN dissection during surgery, and radiation dose to specific substructures are most highly associated with the cumulative incidence to first diagnosis of external or internal lymphedema. To our knowledge, this study, along with our recently published work using AI analysis,41 represents the first attempt at a comprehensive analysis to simultaneously evaluate the clinical, treatment, and dosimetric factors that predict for both external and internal HNL in a time-to-event analysis. Furthermore, by including sequentially treated head and neck primary malignancies at various stages, the inherent variation in radiation doses to cervical lymphatic subregions allowed us to resolve the effect of increasing radiation dose to these subregions and their contribution to HNL.
In our multivariable model for external lymphedema, advanced adenopathy—defined as ≥N2c disease, or ≥N2 disease for nasopharyngeal primaries—appears to double the probability of external lymphedema. In terms of treatment, each additional LN removed during primary surgical resection contributes an additional 2% risk, whereas radiation doses of at least 30 Gy involving ≥50% of the contralateral level VII nodal volume (contralateral RP V30 ≥50%) increase the risk of external lymphedema by 3.8-fold. Several factors found to be significantly associated with lymphedema on univariate analysis were not significant in the multivariable model, including race/ethnicity, current smoking status, laryngectomy, and need for bilateral neck dissection.
Other authors have associated surgical resection and radiation ± chemotherapy treatment with HNL development. Using a cross-sectional patient sample and logistic regression, Deng et al25 revealed a significant increase in the number of treatment modalities (a combination of surgery, radiation, or chemotherapy) in patients with versus without external, internal, and combined (simultaneous external and internal) HNL. HNL was also significantly associated with total radiation dose and days of radiation therapy. In another cross-sectional study of patients evaluating external lymphedema, Sember et al14 found that surgical resection of the primary tumor site and/or neck dissection was associated with external HNL, whereas absence of surgical intervention had significantly decreased rates of external HNL. In a prospective evaluation of external HNL after surgical resection followed by risk-adapted radiation ± chemotherapy, Tribius et al21 found that increasing extent of treatment to the bilateral versus ipsilateral neck with either neck dissection or radiation therapy increased the odds of lymphedema. Our study adds to the evidence that radiation treatment to bilateral LN regions as part of a definitive or adjuvant strategy, as well as increasing extent of LN dissection, increases external lymphedema risk.
Tribius et al21 performed similar multivariable time-to-event analyses evaluating risk factors for external HNL, specifically evaluating for grade 2 lymphedema according to Radiation Therapy Oncology Group (RTOG)/European Organization for Research and Treatment of Cancer late radiation toxicity criteria. At the last follow-up, body mass index (≥25), RT modality (TomoTherapy vs linear accelerator–based intensity modulated radiation therapy), and extracapsular extension were significant on multivariable Cox proportional hazard regression. In our study, advanced adenopathy status, including all patients with N3b disease, is a significant risk factor for external HNL on multivariable competing risk regression. Together, these data suggest that more extensive neck disease at diagnosis as a significant risk factor for external HNL development.
In this study, increasing dose to the contralateral level VII is significantly associated with increased risk of external HNL. As defined by Grégoire et al,28 the level VII LN level includes both the RP (VIIa) and retrostyloid (VIIb) LN sublevels. This region includes the superior carotid sheath from the base of the skull to the caudal edge of the C1 vertebra transverse process, in continuity with the space medial to the carotid sheath between the level of the superior edge of the C1 vertebral body and the cranial edge of the body of the hyoid. In aggregate, the level VII LN level defined this way represents a bilateral anatomic region encompassing the jugulodigastric nodal region superiorly, extending in a superolateral-to-inferomedial direction within the RP and retrostyloid spaces. For simplicity and to emphasize its anatomic location, we refer to the level VII LN level as the RP LN level.
The explanation for why radiation therapy to the contralateral RP LN region may contribute to external lymphedema is not immediately clear. It may be that the dosimetric factor of contralateral RP LN level V30 ≥50% is simply a proxy measurement correlating with extent of diffuse radiation to the contralateral neck. This would imply that general avoidance or sparing of radiation to the contralateral neck could decrease external HNL risk, presumably owing to the ability of the contralateral neck lymphatics to compensate for impaired drainage ipsilateral to the tumor where treatment intensity is maximized.
Alternatively, the pharyngeal lymphatics delineated within this region may be uniquely critical to proper lymphatic fluid drainage from the soft tissues of the bilateral face and neck, representing a defined lymphatic drainage pathway that permits lymphatic fluid flow across midline. In this proposed model of lymphatic physiology, anatomic disruption of ipsilateral lymphatic fluid drainage due to intense multimodality treatment nearest to the tumor causes lymphatic fluid to accumulate in the ipsilateral soft tissues. Under some circumstances, lymphatic channels within the contralateral RP LN level permit compensatory lymphatic fluid flow across midline into the contralateral internal jugular chain lymphatics, thus blunting the development of external lymphedema. However, upon delivery of moderate dosages of radiation to this compensatory pathway, the resulting radiation injury impairs this compensatory pathway, preventing ipsilateral to contralateral lymphatic fluid flow and manifesting as clinically apparent external lymphedema. Thus, our finding that increased radiation dose to the contralateral RP nodal region is associated with increased external HNL may be revealing the existence of a specific compensatory pathway or mode of lymphatic drainage. Lending support to this hypothesis, we note that the top 7 dosimetric and clinicopathologic multivariable models for external lymphedema ranked by AIC incorporate contralateral RP LN level dosimetric factors (mean, V25, V30, V35, V40, V45, and V55).
In reviewing the literature, there are surprisingly few modern anatomic studies of the head and neck lymphatic system, most frequently describing the lymphatic drainage of the dermis and connective tissue.2,42, 43, 44 However, in 1 study, the authors describe a case in which bilateral lymphatic pathways originating in the contralateral lateral hypopharynx (laryngopharynx) ascend in a superolateral direction, cross midline, and drain into the opposite jugular chain.45 One reason for the sparse description of midline lymphatic vessels may be that most anatomic studies often use bisected specimens that would disrupt these connections. As a result, future dedicated anatomic and physiologic studies are needed to confirm functional connections between the bilateral deep cervical lymphatics across the RP space to support or refute the existence of an interconnected RP lymphatic network capable of compensating lymphatic fluid drainage from either side of the neck.
In our patient cohort, external HNL appears to develop most rapidly between approximately 2 and 6 months following treatment, with an increase in cumulative incidence from the end of radiation therapy from 60 days to 180 days of 28.9% to 62.8%. The incidence then stabilizes after 1 year following treatment at a maximum of approximately 70%. A notable limitation of our study is that we were unable to assess the prevalence of lymphedema throughout the follow-up period. Therefore, we are unable to comment on the kinetics of onset to resolution of external lymphedema, only the factors associated with time to first development of HNL.
However, other investigators have evaluated the evolution of external lymphedema at several time points, and their data suggest that external HNL is an acute toxicity of HNC treatment that resolves in a sizable proportion of patients depending on initial severity. Specifically, a prospective study evaluating the prevalence and severity of external HNL by RTOG/European Organization for Research and Treatment of Cancer toxicity criteria after surgery and risk-adapted chemoradiation for locally advanced HNC demonstrated frequent resolution of HNL as evaluated by grade of lymphedema severity at initial follow-up.21 In that study, 3 months following completion of (chemo)radiation therapy, 55% of patients displayed grade 2 lymphedema, 25% grade 1 lymphedema, and 20% no lymphedema. Among patients presenting with grade 2 lymphedema, 57.5% experienced complete resolution of lymphedema, 22% experienced improvement to grade 1 lymphedema, and 20.3% had persistent grade 2 lymphedema at the last follow-up. In patients with grade 1 lymphedema at initial follow-up, 62.3% experienced complete resolution, 30.4% had persistent grade 1 lymphedema, and only 7.2% experienced progression to grade 2 lymphedema. Finally, in patients with no lymphedema at initial follow-up, 72.4% remained without lymphedema, 18.9% developed grade 1 lymphedema, and 8.6% developed grade 2 lymphedema. Although median follow-up was not reported and the variability of follow-up time among patients appears large, the data suggest that many patients will experience an improvement in the severity of lymphedema after their standard initial 30-day follow-up.
A study by Ridner et al13 confirms a trend toward improvement over time in a separate cohort of prospectively evaluated patients, 60% of which underwent definitive chemoradiation with or without induction chemotherapy. These authors used the American Cancer Society Lymphedema of the Head and Neck staging criteria and defined 2 trajectories of lymphedema outcome. For patients experiencing a posttreatment maximum of none-to-mild lymphedema, the severity of external and internal lymphedema remains stable or improves over the 18-month follow-up period. In contrast, for patients experiencing moderate-to-severe lymphedema, lymphedema severity appears to worsen from 6 to 12 months followed by a slight improvement from 12 to 18 months. Finally, in a cross-sectional study, the time since the end of HNC treatment at which evaluating for HNL is performed is inversely associated with the presence of external lymphedema and combined lymphedema, with a median follow-up time of 6.8 months versus 24.5 months in patients with and without lymphedema, respectively, suggesting that longer follow-up times are associated with lower prevalence of lymphedema because of its resolution with additional time since radiation therapy.25
Taken together, patients experiencing external HNL are likely to have variable outcomes, with those with more extensive lymphedema at first follow-up likely to experience worse long-term outcomes. Importantly, it is not clear the degree of utilization and the effect of lymphedema physical therapy or speech and swallowing rehabilitation may have had on these results. In most studies, early aggressive intervention for external HNL appears to lead to improvement in external lymphedema outcomes.11,23,46, 47, 48, 49, 50
In our multivariable model for internal lymphedema, advanced adenopathy status and presence of a radiation dose of 45 Gy to ≥50% of the larynx (larynx V45 ≥50%) were selected as risk factors. When asked to incorporate a second dosimetric factor for inclusion into the multivariable model, the final model contained only the dosimetric factors of larynx V45 ≥50% and ipsilateral level IVA maximum dose ≥60 Gy, excluding advanced adenopathy status. Our interpretation for the substitution of advanced adenopathy status for ipsilateral level IVA maximum dose ≥60 Gy is that in the setting of advanced adenopathy, the radiation dose delivered to inferior nodal levels is apt to increase. Therefore, the ipsilateral level IVA maximum dose ≥60 Gy is an appropriate proxy or substitute for increasing extent of LN involvement at diagnosis. As a result, the multivariable model including advanced adenopathy status in combination with larynx V45 ≥50% is probably most useful when evaluating an individual patient's risk for internal lymphedema. Alternatively, the maximum dose to the ipsilateral level IVA could represent a serial injury to converging lymphatics near the region where the lymphatic ducts enter the venous circulation.
Our internal lymphedema multivariable model agrees with Sanguineti et al's8 retrospective analysis of dosimetric predictors of laryngeal edema in patients receiving primary radiation for squamous cell carcinoma HNC with grossly uninvolved larynxes. When evaluating dosimetry to the larynx in the presence of other demographic, tumor, and treatment factors, their multivariable model demonstrated that the larynx V50 and presence of positive nodes were significant predictors for RTOG grade 2 laryngeal edema. To minimize the risk of edema, larynx constraints of a V50 ≤27% and mean dose ≤43.5 Gy were proposed. It is therefore unsurprising that among all LN levels and organs at risk evaluated in our study, our multivariable model selected the larynx as the structure most predictive for internal lymphedema. In our study, the larynx V50 ≥36% and mean larynx ≥45 Gy appeared correlated with lymphedema outcome. It is also worth highlighting the absence of surgical factors in either multivariable model, whereas in Deng et al,25 both radiation to the surgical bed and number of treatment modalities were associated with internal lymphedema on logistic regression. In our view, it is therefore likely that internal lymphedema is more often mediated by diffuse radiation injury to parallel mucosal lymphatics of laryngeal structures, as opposed to discrete injury such as disruption of lymphatic fluid flow due to surgical disruption.9
Limitations
A major limitation of our study is that no formal assessment of lymphedema was performed such as the MD Anderson Lymphedema Scale. Instead, lymphedema was identified by exam according to subjective assessment by a limited number of providers, or otherwise when a subjective assessment triggered a referral for lymphedema directed physical therapy. As a result, we recorded only whether lymphedema was present or not based on physical exam, but not objective measurement, and no inference about the magnitude of lymphedema was attempted. In addition, different types or locations of lymphedema were not distinguished. For example, in external lymphedema, fluid accumulation predominantly lateralized to 1 side of the face is not distinguished from submental lymphedema. Consequently, it remains unknown whether different expressions of external lymphedema are subject to different risk factors. Similarly for internal lymphedema, subregions of the pharyngeal or laryngeal soft tissues affected by lymphedema were not specified. We also did not record resolution of lymphedema symptoms once it was identified and cannot comment on the proportion of patients in whom lymphedema resolved. For the purposes of our study, we excluded a small subset of patients who developed lymphedema following surgical resection but before radiation treatment. Our findings therefore apply only to those patients who both undergo surgery and receive adjuvant radiation therapy but not to patients who undergo surgery alone. Finally, owing to the sparse literature on dosimetric predictors of external lymphedema, we comprehensively evaluated bilateral LNs individually and in aggregate, and in doing so created a large data set susceptible to type I statistical error. As a result, our conclusions require validation through independent data sets and analyses.
Conclusions
The development of external and internal lymphedema following definitive or adjuvant radiation therapy ± chemotherapy is common. For external lymphedema, risk factors include more advanced nodal disease at diagnosis, increasing extent of LN dissection, and increasing doses of radiation delivered to the contralateral RP LN region. The latter factor is a novel finding that suggests that this region has an important role in the development of external HNL. For internal lymphedema, risk factors include more advanced nodal disease at diagnosis and increasing radiation dose to the larynx.
Disclosures
Kevin R. Rogacki reports departmental support for attendance of conferences as a radiation oncology resident at McGaw Medical Center at Northwestern University. P. Troy Teo received fellowship funding from the Canadian Institute of Health Research (Grant: CIHR-472392). Suvidya Lakshmi Pachigolla reports Swiss Government Excellence Scholarship and is recipient of the TL1 WashU Grant (TL1 TR002344) from National Institutes of Health. Mohamed E. Abazeed reports grants from National Cancer Institute, National Institutes of Health (R37CA222294 and P30CA060553); consulting fees from Mirati Therapeutics, Inc; payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing, or educational events from the American Society for Clinical Pathology; patents pending on a decision support system for individualizing radiation therapy dose; and software support from Siemens Healthcare. Indra Das is Associate editor of The British Journal of Radiology, Journal of Radiation Research, Frontiers in Oncology, and Medical Physics).
Acknowledgments
We gratefully acknowledge the statistical expertise in the form of conversations with Irene Helenowski and Borko D. Jovanovic.
Footnotes
Sources of support: This work had no specific funding.
The data sets generated and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.adro.2024.101545.
Contributor Information
Kevin R. Rogacki, Email: krogacki@roanetwork.com.
P. Troy Teo, Email: peng.teo1@northwestern.edu.
Appendix. Supplementary materials
References
- 1.Beninson J. Lymphedema: patho-physiologic and clinical concepts. Angiology. 1975;26:661–664. doi: 10.1177/000331977502600903. [DOI] [PubMed] [Google Scholar]
- 2.Pan W-R, Suami H, Taylor GI. Lymphatic drainage of the superficial tissues of the head and neck: anatomical study and clinical implications. Plast Reconstr Surg. 2008;121:1614–1624. doi: 10.1097/PRS.0b013e31816aa072. [DOI] [PubMed] [Google Scholar]
- 3.Deng J, Ridner SH, Murphy BA. Lymphedema in patients with head and neck cancer. Oncol Nurs Forum. 2011;38:E1–E10. doi: 10.1188/11.ONF.E1-E10. [DOI] [PubMed] [Google Scholar]
- 4.Ridner SH. Pathophysiology of lymphedema. Semin Oncol Nurs. 2013;29:4–11. doi: 10.1016/j.soncn.2012.11.002. [DOI] [PubMed] [Google Scholar]
- 5.Földi M, Földi E, Strößenreuther R, Kubik S. Földi’s Textbook of Lymphology: For Physicians and Lymphedema Therapists. Urban & Fischer Verlag; 2012. [Google Scholar]
- 6.Queija DDS, Dedivitis RA, Arakawa-Sugueno L, et al. Cervicofacial and pharyngolaryngeal lymphedema and deglutition after head and neck cancer treatment. Dysphagia. 2020;35:479–491. doi: 10.1007/s00455-019-10053-6. [DOI] [PubMed] [Google Scholar]
- 7.Jeans C, Ward EC, Brown B, et al. Association between external and internal lymphedema and chronic dysphagia following head and neck cancer treatment. Head Neck. 2021;43:255–267. doi: 10.1002/hed.26484. [DOI] [PubMed] [Google Scholar]
- 8.Sanguineti G, Adapala P, Endres EJ, et al. Dosimetric predictors of laryngeal edema. Int J Radiat Oncol Biol Phys. 2007;68:741–749. doi: 10.1016/j.ijrobp.2007.01.010. [DOI] [PubMed] [Google Scholar]
- 9.Rancati T, Fiorino C, Sanguineti G. NTCP modeling of subacute/late laryngeal edema scored by fiberoptic examination. Int J Radiat Oncol Biol Phys. 2009;75:915–923. doi: 10.1016/j.ijrobp.2009.04.087. [DOI] [PubMed] [Google Scholar]
- 10.Deng J, Murphy BA, Dietrich MS, et al. Impact of secondary lymphedema after head and neck cancer treatment on symptoms, functional status, and quality of life. Head Neck. 2013;35:1026–1035. doi: 10.1002/hed.23084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Smith BG, Hutcheson KA, Little LG, et al. Lymphedema outcomes in patients with head and neck cancer. Otolaryngol Head Neck Surg. 2015;152:284–291. doi: 10.1177/0194599814558402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Deng J, Ridner SH, Dietrich MS, et al. Prevalence of secondary lymphedema in patients with head and neck cancer. J. Pain Symptom Manage. 2012;43:244–252. doi: 10.1016/j.jpainsymman.2011.03.019. [DOI] [PubMed] [Google Scholar]
- 13.Ridner SH, Dietrich MS, Niermann K, et al. A prospective study of the lymphedema and fibrosis continuum in patients with head and neck cancer. Lymphat Res Biol. 2016;14:198–205. doi: 10.1089/lrb.2016.0001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sember A, Pranskevich C, Scott S, Hutchinson I, Hoffman R. Prehabilitation for lymphedema in head and neck cancer patients at a community cancer center. J Community Support Oncol. 2017;15:e127–e134. [Google Scholar]
- 15.Cox JD, Stetz J, Pajak TF. Toxicity criteria of the Radiation Therapy Oncology Group (RTOG) and the European Organization for Research and Treatment of Cancer (EORTC) Int J Radiat Oncol Biol Phys. 1995;31:1341–1346. doi: 10.1016/0360-3016(95)00060-C. [DOI] [PubMed] [Google Scholar]
- 16.Patterson JM, Hildreth A, Wilson JA. Measuring edema in irradiated head and neck cancer patients. Ann Otol Rhinol Laryngol. 2007;116:559–564. doi: 10.1177/000348940711600801. [DOI] [PubMed] [Google Scholar]
- 17.Deng J, Ridner SH, Wells N, Dietrich MS, Murphy BA. Development and preliminary testing of head and neck cancer related external lymphedema and fibrosis assessment criteria. Eur J Oncol Nurs. 2015;19:75–80. doi: 10.1016/j.ejon.2014.07.006. [DOI] [PubMed] [Google Scholar]
- 18.McGarvey AC, Osmotherly PG, Hoffman GR, Chiarelli PE. Lymphoedema following treatment for head and neck cancer: impact on patients, and beliefs of health professionals. Eur J Cancer Care (Engl) 2014;23:317–327. doi: 10.1111/ecc.12134. [DOI] [PubMed] [Google Scholar]
- 19.Jeans C, Ward EC, Cartmill B, et al. Patient perceptions of living with head and neck lymphoedema and the impacts to swallowing, voice and speech function. Eur J Cancer Care (Engl) 2019;28:e12894. doi: 10.1111/ecc.12894. [DOI] [PubMed] [Google Scholar]
- 20.Deng J, Sinard RJ, Murphy B. Patient experience of head and neck lymphedema therapy: a qualitative study. Support Care Cancer. 2019;27:1811–1823. doi: 10.1007/s00520-018-4428-2. [DOI] [PubMed] [Google Scholar]
- 21.Tribius S, Pazdyka H, Tennstedt P, et al. Prognostic factors for lymphedema in patients with locally advanced head and neck cancer after combined radio(chemo)therapy- results of a longitudinal study. Oral Oncol. 2020;109 doi: 10.1016/j.oraloncology.2020.104856. [DOI] [PubMed] [Google Scholar]
- 22.Murphy BA, Gilbert J. Dysphagia in head and neck cancer patients treated with radiation: assessment, sequelae, and rehabilitation. Semin Radiat Oncol. 2009;19:35–42. doi: 10.1016/j.semradonc.2008.09.007. [DOI] [PubMed] [Google Scholar]
- 23.Jackson LK, Ridner SH, Deng J, et al. Internal lymphedema correlates with subjective and objective measures of dysphagia in head and neck cancer patients. J Palliat Med. 2016;19:949–956. doi: 10.1089/jpm.2016.0018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mathew JM, Mukherji A, Saxena SK, et al. Change in dysphagia and laryngeal function after radical radiotherapy in laryngo pharyngeal malignancies - a prospective observational study. Rep Pract Oncol Radiother. 2021;26:655–663. doi: 10.5603/RPOR.a2021.0078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Deng J, Ridner SH, Dietrich MS, et al. Factors associated with external and internal lymphedema in patients with head-and-neck cancer. Int J Radiat Oncol Biol Phys. 2012;84:e319–e328. doi: 10.1016/j.ijrobp.2012.04.013. [DOI] [PubMed] [Google Scholar]
- 26.Kim D, Nam J, Kim W, et al. Radiotherapy dose–volume parameters predict facial lymphedema after concurrent chemoradiation for nasopharyngeal carcinoma. Radiat Oncol. 2021;16:172. doi: 10.1186/s13014-021-01901-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gray B. cmprsk: subdistribution analysis of competing risk. Available at: https://cran.r-project.org/web//packages/cmprsk/cmprsk.pdf. Accessed January 15, 2023.
- 28.Grégoire V, Ang K, Budach W, et al. Delineation of the neck node levels for head and neck tumors: a 2013 update. DAHANCA, EORTC, HKNPCSG, NCIC CTG, NCRI, RTOG, TROG consensus guidelines. Radiother Oncol. 2014;110:172–181. doi: 10.1016/j.radonc.2013.10.010. [DOI] [PubMed] [Google Scholar]
- 29.Scrucca L, Santucci A, Aversa F. Competing risk analysis using R: an easy guide for clinicians. Bone Marrow Transplant. 2007;40:381–387. doi: 10.1038/sj.bmt.1705727. [DOI] [PubMed] [Google Scholar]
- 30.Scrucca L, Santucci A, Aversa F. Regression modeling of competing risk using R: an in depth guide for clinicians. Bone Marrow Transplant. 2010;45:1388–1395. doi: 10.1038/bmt.2009.359. [DOI] [PubMed] [Google Scholar]
- 31.Lesnoff M, Lancelot R. aod: analysis of overdispersed data. https://cran.r-project.org/web/packages/aod/aod.pdf. Accessed January 15, 2023.
- 32.Varadhan R, Kuk D. crrstep: stepwise covariate selection for the fine & gray competing risks regression model. https://cran.r-project.org/web/packages/crrstep/crrstep.pdf. Accessed January 15, 2023.
- 33.Cutright D, Gopalakrishnan M, Roy A, Panchal A, Mittal BB. DVH analytics: a DVH database for clinicians and researchers. J Appl Clin Med Phys. 2018;19:413–427. doi: 10.1002/acm2.12401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gross JP, Lynch CM, Flores AM, et al. Determining the organ at risk for lymphedema after regional nodal irradiation in breast cancer. Int J Radiat Oncol Biol Phys. 2019;105:649–658. doi: 10.1016/j.ijrobp.2019.06.2509. [DOI] [PubMed] [Google Scholar]
- 35.R Core Team . R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing; Vienna, Austria: 2020. https://www.r-project.org/ [Google Scholar]
- 36.Hothorn T. maxstat: maximally selected rank statistics. https://cran.r-project.org/web//packages/maxstat/maxstat.pdf. Accessed January 15, 2023.
- 37.Harrell Jr FE. rms: regression modeling strategies. https://cran.r-project.org/web/packages/rms/rms.pdf. Accessed January 15, 2023.
- 38.Zhang Z, Kattan MW. Drawing nomograms with R: applications to categorical outcome and survival data. Ann Transl Med. 2017;5:211. doi: 10.21037/atm.2017.04.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wickham H. Springer Cham; 2016. Ggplot2: Elegant Graphics for Data Analysis. [Google Scholar]
- 40.Kassambara A. ggpubr: ‘ggplot2’ based publication ready plots.https://cran.r-project.org/web/packages/ggpubr/ggpubr.pdf. Accessed January 15, 2023.
- 41.Teo PT, Rogacki K, Gopalakrishnan M, et al. Determining risk and predictors of head and neck cancer treatment-related lymphedema: A clinicopathologic and dosimetric data mining approach using interpretable machine learning and ensemble feature selection. Clin Transl Radiat Oncol. 2024;46:100747. doi: 10.1016/j.ctro.2024.100747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pan WR, Suami H, Corlett RJ, Ashton MW. Lymphatic drainage of the nasal fossae and nasopharynx: preliminary anatomical and radiological study with clinical implications. Head Neck. 2009;31:52–57. doi: 10.1002/hed.20926. [DOI] [PubMed] [Google Scholar]
- 43.Pan W-R, Le Roux CM, Levy SM, Briggs CA. The morphology of the human lymphatic vessels in the head and neck. Clin Anat. 2010;23:654–661. doi: 10.1002/ca.21004. [DOI] [PubMed] [Google Scholar]
- 44.Pan W-R, Le Roux CM, Briggs CA. Variations in the lymphatic drainage pattern of the head and neck: further anatomic studies and clinical implications. Plast Reconstr Surg. 2011;127:611–620. doi: 10.1097/PRS.0b013e3181fed511. [DOI] [PubMed] [Google Scholar]
- 45.Saito H, Sato T, Yamashita Y, Amagasa T. Topographical analysis of lymphatic pathways from the meso- and hypopharynx based on minute cadaveric dissections: possible application to neck dissection in pharyngeal cancer surgery. Surg Radiol Anat. 2002;24:38–49. doi: 10.1007/s00276-002-0015-8. [DOI] [PubMed] [Google Scholar]
- 46.Smith BG, Lewin JS. Lymphedema management in head and neck cancer. Curr Opin Otolaryngol Head Neck Surg. 2010;18:153–158. doi: 10.1097/MOO.0b013e32833aac21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Doke KN, Bowman L, Shnayder Y, et al. Quantitative clinical outcomes of therapy for head and neck lymphedema. Adv Radiat Oncol. 2018;3:366–371. doi: 10.1016/j.adro.2018.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gutiérrez C, Mayrovitz HN, Naqvi SHS, Karni RJ. Longitudinal effects of a novel advanced pneumatic compression device on patient-reported outcomes in the management of cancer-related head and neck lymphedema: a preliminary report. Head Neck. 2020;42:1791–1799. doi: 10.1002/hed.26110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ridner SH, Dietrich MS, Deng J, Ettema SL, Murphy B. Advanced pneumatic compression for treatment of lymphedema of the head and neck: a randomized wait-list controlled trial. Support. Care Cancer. 2021;29:795–803. doi: 10.1007/s00520-020-05540-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Deng J, Lukens JN, Swisher-McClure S, et al. Photobiomodulation therapy in head and neck cancer-related lymphedema: a pilot feasibility study. Integr Cancer Ther. 2021;20 doi: 10.1177/15347354211037938. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.