Summary
Little is known about the physiological and biomechanical factors that determine individual preferences in lying posture during sleep. This study investigated relationships between position preference and position‐specific arousals, awakenings, limb movements and limb movement arousals to explore the mechanisms by which biomechanical factors influence position preference. Forty‐one mature‐aged adults underwent 2 nights of at‐home polysomnography ~2 weeks apart, on a standardised firm foam mattress, measuring nocturnal sleep architecture and position. The lateral supine ratio and restlessness indices specific to lateral and supine positions including limb movement index, limb movement arousal index, arousal index, wake index, respiratory arousal index and apnea–hypopnea index were calculated and analysed via linear mixed‐effects regression. In the supine position, all restlessness indices were significantly increased compared with the lateral position, including a 379% increase in respiratory arousals (β = 7.0, p < 0.001), 108% increase in arousal index (β = 10.3, p < 0.001) and 107% increase in wake index (β = 2.5, p < 0.001). Wake index in the supine position increased significantly with more lateral sleep (β = 1.9, p = 0.0013), and significant correlation between lateral supine ratio polysomnography 1 and lateral supine ratio polysomnography 2 (β = 0.95, p < 0.001) indicated strong consistency in sleep preference. Overall, the findings suggest that some individuals have low tolerance to supine posture, represented by a comparatively high wake index in the supine position, and that these individuals compensate by sleeping a greater proportion in the lateral position.
Keywords: arousals, awakenings, lateral position, positional therapy, sleep, sleep apnea, sleep position, sleep posture, supine position
1. INTRODUCTION
Sleep is critical for maintenance of physiological function, and frequent sleep disruption inhibits crucial mechanisms for maintaining cognitive performance and long‐term metabolic health. Sleep is a complex non‐homogeneous process, with an architecture comprised of sleep stages with unique neurological characteristics and physiological functions. Polysomnography (PSG) remains the gold‐standard in assessment of sleep architecture, where electroencephalogram (EEG), electrocardiogram (ECG), electrooculography (EOG) and electromyography (EMG) are recorded by various sensors around the body and interpreted by a trained sleep technician in accordance with AASM scoring criterion (Berry, 2012). Sleep can be categorised into rapid eye movement (REM) or non‐rapid eye movement (NREM) sleep with progressively deepening sleep stages (N1, N2 and N3). N3, or formerly slow‐wave sleep, is the deepest sleep stage and plays a role in immune function (Lange et al., 2003, 2011), declarative memory (Ackermann & Rasch, 2014; Rasch & Born, 2013), growth hormone secretion (Gronfier et al., 1996; Sassin et al., 1969; Takahashi et al., 1968; Van Cauter et al., 1992) and appetite regulation (Theorell‐Haglöw et al., 2010). REM plays a role in promoting emotional processing and memory (Groch et al., 2013; Wassing et al., 2019), preventing cognitive decline (Pase et al., 2017; Song et al., 2015), improving procedural memory (De Koninck et al., 1989; Guerrien, 1994; Karni et al., 1994; Mandai et al., 1989; Scrima, 1982; Verschoor & Holdstock, 1984), and regulating appetite (Rao et al., 2009; Theorell‐Haglöw et al., 2010). According to the AASM, arousals are an abrupt shift in EEG frequency > 3 s in duration, which may be caused by endogenous or exogenous stimuli, or occur spontaneously. Awakenings are characterised by an excess of 15 s of arousal within a 30‐s epoch (Berry, 2012). Arousals and awakenings are a healthy feature of sleep and tend to increase in frequency with age, with ~40 awakenings per night, and ~19 arousals per hour for adults older than 60 years (Boulos et al., 2019; Della Monica et al., 2018; Dijk et al., 2001; Bonnet & Arand, 2007). Excessive arousal and awakening frequency is associated with hypertension (Carrington & Trinder, 2008) and performance detriments, as well as disruption of deep sleep stages and their capacity to perform restorative functions in completeness (Della Monica et al., 2018). Sleep apnea (Ren et al., 2022), excessive road or aircraft noise (Babisch et al., 1999; Jarup et al., 2008; Lercher et al., 2000), and stress (Ekstedt et al., 2004) are known contributors to high awakening and arousal frequencies. There is some evidence that overly firm mattresses are associated with increased arousal frequencies (Chen et al., 2014; Park et al., 2009), demonstrating an interaction between arousals and biomechanical effects of the underlying mattress.
Individuals tend to reposition during sleep, with the majority of sleep in the lateral position (73%), followed by supine (22%) and prone positions (5%; Gordon et al., 2004).The lateral position is associated with decreased likelihood of spinal pain symptoms (Cary et al., 2019; Skarpsno et al., 2017). Furthermore, lateral lying is associated with improved stiffening and dilating of pharyngeal muscles during sleep, and reducing sleep‐disordered breathing symptoms compared with supine sleep (Joosten et al., 2015; Lee & Choi, 2023). However, the interaction between proportion of sleep in each position, and arousal or awakening rates remains unclear. Zhang et al. (2022) measured sleep quality and sleep position of 13 subjects using in‐lab wearable sleep and instrumental position monitors, and found that predominant right lateral lying was associated with improved sleep quality, including decreased awakenings and awakening duration, and there was little difference in sleep quality between predominant left lateral and supine lying. However, position‐specific awakening and arousal rates were not reported, making it unclear if participants slept in the right lateral position because that particular posture had a sleep‐enhancing effect.
There are two key biomechanical parameters of interest for their relationship with sleep parameters: body–mattress interface pressure (Chen et al., 2014) and spinal distortion (Hong et al., 2022; Verhaert et al., 2011). These two biomechanical parameters are dependent on mattress mechanics, individual anatomical characteristics and sleeping position, where sleeping position is the only factor that can be actively adjusted in sleep of healthy individuals on a typical mattress. Little prior research has explored an individual person's tendency to modify sleeping position to promote more favourable lying biomechanics, and the overall effects of individual self‐adjustment of sleep position on sleep continuity remains unclear.
The present study aims to explore the relationship between sleep continuity and sleeping position by examining effects of position‐specific restlessness indices on proportion of sleep in each position, to identify mechanisms that motivate turnover and sleep position preference. This involves statistical analysis of relationships between proportion of sleep in lateral or supine lying, and position‐specific frequency of arousal, awakening, limb movement, limb movement arousal, respiratory arousal, and apnea–hypopnea on a standardised firm mattress. Furthermore, qualitative case studies were conducted to gain insights into interactions between sleeping position, sleep architecture and sleep continuity, and propose further research avenues.
2. METHODS
Forty‐one mature‐aged adults between 54 and 75 years (mean ± standard deviation: 61.6 ± 5.9 years), including 25 females and 16 males, with body mass index (BMI) 18–31 kg m−2 (25.0 ± 2.9 kg m−2) participated in the study. Participants had no recent orthopaedic surgery, no history of acute spinal conditions and no diagnosed sleep pathology. This study was part of a larger study exploring interactions between sleep quality and lying biomechanics. To isolate effects of sleeping position from differences in mattress mechanics, participants were provided a standardised “firm” mattress, which they slept on at home for 4 weeks. To test for a time‐dependent adaptation in sleep position on the firm mattress, participants underwent two at‐home ambulatory PSG studies, the first occurring 1–2 weeks (11.5 ± 4.0 days) and the second occurring 3–4 weeks (26.5 ± 5.0 days) from receipt of the mattress. Ambulatory PSG studies were conducted using the Nox A1s (Nox Medical, Reykjavik, Iceland) portable PSG device, which allows sleep measurement in the home environment, reducing first‐night effects associated with sleep in unfamiliar conditions. Participants underwent level 2 ambulatory PSG set‐up by trained researchers (authors: LR, SH, JP), with electrodes placed to measure EOG, EEG, ECG and EMG on the right and left arms, and left leg (Figure 1). Respiratory bands and a nasal cannula were provided to measure respiration, and a wrist‐worn pulse oximeter measured heart rate and blood oxygen saturation. All sensors were connected to a chest‐worn Nox A1s central unit, with integrated accelerometry that detects sleep position (left, right, supine, prone, upright, unknown). Participants returned home for sleep, returning the device the next morning for data download and analysis in Noxturnal 6.1 (Nox Medical, Reykjavik, Iceland) by a trained sleep technician in accordance with 2023 AASM standards.
FIGURE 1.
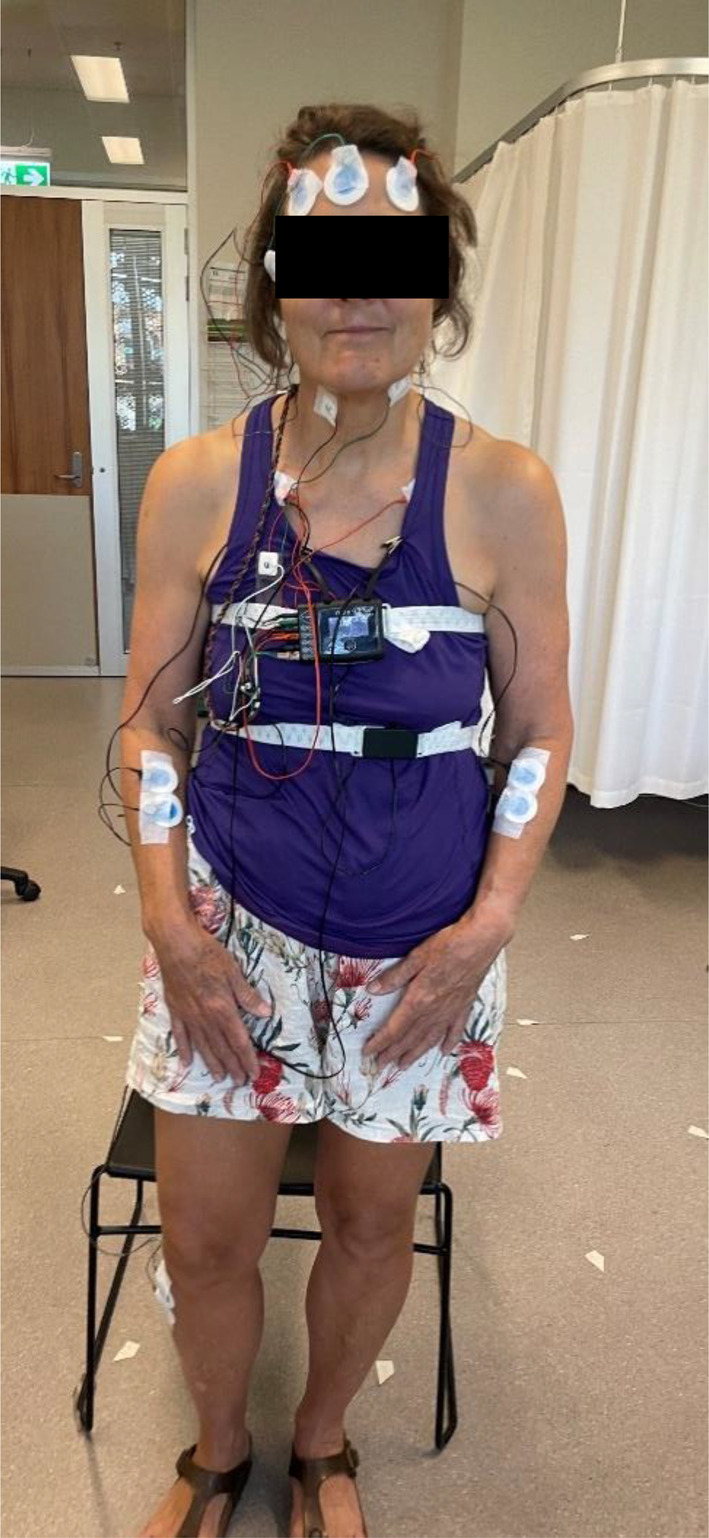
Level 2 ambulatory polysomnography (PSG) set‐up with Nox A1s.
2.1. Analysis
Sleep scoring data including start and end times of all sleep events were exported from Noxturnal to CSV format. A MATLAB algorithm (MATLAB R2023B, Mathworks, Portola Valley, USA) was implemented to calculate positional measures such as turnover frequency (TOF), duration of sleep in lateral and supine positions, and lateral supine ratio (LSR), calculated as:
Restlessness indices were categorised into respective sleep events, including wake index (WI), arousal index (AI), limb movement index supine (LMI), limb movement arousal index (LMAI), respiratory arousal index (RAI) and apnea–hypopnea index (AHI).
Each restlessness indices category was calculated for the whole night (RI), and for sleep in the supine position (RI‐S) and lateral position (RI‐L).
Statistical analysis was performed in R 4.3.0 (R Core Team, 2023), using linear mixed‐effects package “lme4” (Kuznetsova et al., 2017). Statistical differences in restlessness indices between lateral and supine were analysed, where positional restlessness index (RIposition) was defined as the response, position (lateral or supine) as fixed effect, and participant ID as random effect to account for the repeat measure of 2 nights of PSG for each participant:
To analyse relationships between restlessness indices and proportion of sleep in lateral or supine positions, linear mixed‐effects regression was used. LSR was the response, predicted by restlessness index in lateral (FL) and supine (FS) as fixed effects, and participant ID as the random effect:
Relationships between LSR and overall restlessness indices were similarly investigated:
Linear regression was performed using the “lm” function (R Core Team, 2023) to measure the effect of LSR on the 1st night of PSG (LSR PSG1) and on the 2nd night of PSG (LSR PSG2):
Furthermore, intra‐class correlation was calculated to determine the level of agreement between LSR PSG1 and PSG2 in accordance with Koo and Li (2016).
Case studies were conducted for Participant 82, chosen for greatest difference in LSR between night 1 and 2, and Participant 61, chosen for near equal distribution of lateral and supine sleep on both night 1 and 2. For each case study, night 1 and night 2 hypnogram and restlessness histograms were visualised using MATLAB R2023b.
3. RESULTS
3.1. Overall sleep position preference
Overall, participants slept on average 34.4 ± 22.8% supine, 61.8 ± 22.6% lateral, and 3.9 ± 8.7% prone (Table 1). Because of the small proportion of prone sleep, frequencies in prone were not analysed further. No side preference was observed, with 30.1 ± 17.4% left side and 31.7 ± 21.3% right side.
TABLE 1.
Summary of sleep position distribution measures including TOF and TST.
| Sleep position measures | |
|---|---|
| TST (hr) | 7.6 ± 1.1 |
| TOF (turns per hour) | 2.5 ± 1.8 |
| LSR (%) | 64.5 ± 23.2 |
| Lateral (% of TST) | 61.8 ± 22.6 |
| Left (% of TST) | 30.1 ± 17.4 |
| Right (% of TST) | 31.7 ± 21.3 |
| Supine (% of TST) | 34.4 ± 22.8 |
| Prone (% of TST) | 3.9 ± 8.7 |
LSR, lateral supine ratio; TOF, turnover frequency; TST, total sleep time.
3.1.1. Comparison of restlessness indices in lateral and supine positions
Across all restlessness indices, including respiratory indices, events were significantly more frequent in the supine position compared with lateral (Table 2). The average RAI increased 379% from lateral to supine, and AHI increased 297%. The WI and AI increased by 108% and 107%, respectively. The proportion of arousals associated with respiratory events increased from 19% to 44% from lateral to supine positions, and the proportion of arousals association with limb movements decreased from 34% to 22%. Averaging over both nights, one participant had an AHI over 15, and 11 participants had supine AHI above 15, while no participants had a lateral AHI over 15.
TABLE 2.
Differences in restlessness indices between supine and lateral positions.
| Average event index for each restlessness measure by position (events per hr) | Effect of position (lateral = 0, supine = 1) on event index | ||||||
|---|---|---|---|---|---|---|---|
| Overall | Lateral | Supine | β | 2.5% CI | 97.5% CI | p | |
| WI | 2.6 ± 1.2 | 2.3 ± 1.0 | 4.8 ± 3.9 | 2.5 | 1.7 | 3.3 | < 0.001 |
| AI | 12.4 ± 5.0 | 9.7 ± 4.4 | 20.0 ± 5.0 | 10.3 | 7.5 | 13.1 | < 0.001 |
| LMI | 23.0 ± 18.7 | 19.9 ± 18.6 | 28.4 ± 20.0 | 8.5 | 3.6 | 13.4 | < 0.001 |
| LMAI | 3.3 ± 1.6 | 3.3 ± 1.7 | 4.3 ± 3.9 | 1.0 | 0.15 | 1.9 | 0.024 |
| RAI | 4.0 ± 3.4 | 1.9 ± 2.7 | 8.9 ± 8.5 | 7.0 | 5.1 | 8.9 | < 0.001 |
| AHI | 5.6 ± 4.5 | 2.9 ± 3.0 | 11.4 ± 9.9 | 8.5 | 6.2 | 10.7 | < 0.001 |
Lateral, supine and overall restlessness indices are reported as mean ± standard deviation.
Statistical analysis of differences between lateral and supine restlessness indices was performed using linear mixed‐effects modelling (LMER), with participant ID assigned as a random effect, and position assigned as fixed effect (lateral = 0, supine = 1), and restlessness index as outcome. β indicates the difference from lateral to supine, confidence for β is given by CIs (lower: 2.5% CI; upper: 97.5% CI). The p‐value represents statistical significance of the regression model, where p < 0.05 is statistically significant. It was found that restlessness indices had statistically significant differences between lateral and supine positions.
AHI, apnea–hypopnea index; AI, arousal index; CI, confidence interval; LMAI, limb movement arousal index; LMI, limb movement index; RAI, respiratory arousal index; WI, wake index.
3.2. Time adaptation in sleep position preference
There was no evidence of adaptation in sleep position preference while on the firm mattress. Modelling of the effect of the number of days on the mattress on LSR indicated a weak effect towards increased supine sleep over time (β = −0.16, p = 0.26); however, the relationship was insignificant with wide confidence intervals (2.5% CI: −0.43, 97.5% CI: 0.12; Table 3).
TABLE 3.
Relationships between LSR and days sleeping on the mattress, TOF, and other restlessness indices.
| Effect of overall and position‐specific restlessness indices on LSR | ||||
|---|---|---|---|---|
| Β | 2.5% CI | 97.5% CI | p | |
| Days | −0.16 | −0.43 | 0.12 | 0.26 |
| TOF | −0.29 | −2.4 | 1.8 | 0.79 |
| WI | −0.64 | −4.1 | 2.9 | 0.71 |
| WI‐S | 1.9 | 0.68 | 3.1 | 0.0013 |
| WI‐L | −1.1 | −5.1 | 2.9 | 0.60 |
| AI | −0.71 | −1.5 | 0.0001 | 0.054 |
| AI‐S | 0.18 | −0.098 | 0.47 | 0.22 |
| AI‐L | 0.18 | −0.67 | 1.0 | 0.66 |
| LMI | −0.054 | −0.24 | 0.13 | 0.56 |
| LMI‐S | 0.29 | 0.029 | 0.56 | 0.035 |
| LMI‐L | −0.21 | −0.48 | 0.047 | 0.12 |
| LMAI | 1.1 | −1.0 | 3.4 | 0.31 |
| LMAI‐S | 0.34 | −0.52 | 1.2 | 0.45 |
| LMAI‐L | 1.3 | −0.71 | 3.3 | 0.21 |
| RAI | −0.96 | −2.2 | 0.23 | 0.11 |
| RAI‐S | 0.26 | −0.14 | 0.66 | 0.21 |
| RAI‐L | 0.64 | −0.66 | 1.90 | 0.32 |
| AHI | −0.84 | −1.8 | 0.01 | 0.048 |
| AHI‐S | 0.15 | −0.2 | 0.51 | 0.40 |
| AHI‐L | 0.42 | −0.86 | 1.70 | 0.51 |
Linear mixed‐effect models were developed in R 4.3.0 using lme4 package. Participant ID was assigned as a random effect to account for repeat measure associated with both nights of sleep, restlessness indices were assigned as fixed effect, and LSR as outcome. β is the coefficient representing the slope, where negative β indicates trend towards lower LSR and more supine sleep, and positive indicates greater LSR and more lateral sleep. Confidence for β is given by lower and upper 95% CIs. The p‐value represents statistical significance of the regression model, where p < 0.05 is statistically significant.
3.3. LSR night 1 vs night 2
The difference in LSR between PSG on night 1 and night 2 was −2.7 ± 13.4%, indicating a trend towards more supine sleep on the second night (Figure 2b). Similarly, there was a strong significant correlation between LSR PSG1 and LSR PSG2 (β = 0.95, p < 0.001) with good agreement (intra‐class correlation: 0.83), indicating individual participants had strong consistency in sleep position preference between nights (Figure 2a).
FIGURE 2.
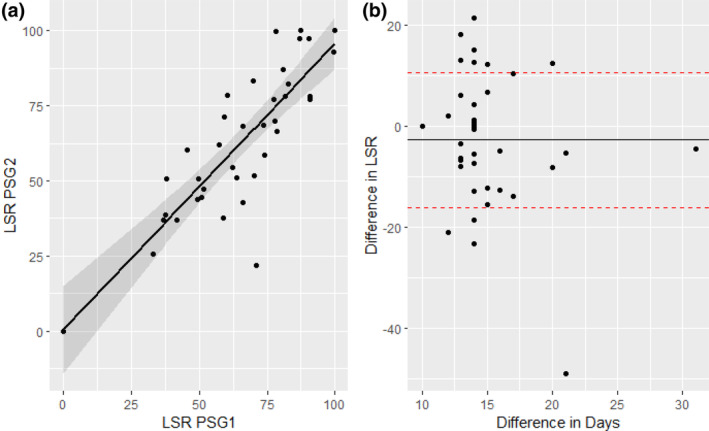
Relationship between first and second polysomnography (PSG) lateral supine ratio (LSR). (a) Scatter plot of LSR on the 1st night of PSG (LSR PSG1) on the x‐axis, and LSR PSG2 on the y‐axis. The solid line shows the linear regression model (β = 0.95, p < 0.001). (b) Scatter plot of the difference in LSR between PSG1 and 2 on the y‐axis, and the days between PSG1 and 2 on the x‐axis.
AHI, apnea–hyponea index; AI, arousal index; CI, confidence interval; LMAI, limb movement arousal index; LMI, limb movement index; LSR, lateral supine ratio; RAI, respiratory arousal index; TOF, turnover frequency; WI, wake index.
3.4. Position preference and positional restlessness
Linear regression modelling of effects of restlessness indices on LSR, including both nights of sleep, demonstrated that wakefulness in the supine position was associated with increased lateral lying, but lateral wakefulness had less effect on sleep position (Table 3). WI‐S had greater standard deviation (WI‐S: 4.80 ± 3.87 wakes per hr) than WI‐L (WI‐L: 2.31 ± 1.05 wakes per hr), and a strong significant relationship between LSR and WI‐S was found (β = 1.9, p = 0.0013), while WI‐L had a weaker effect on LSR and was not statistically significant (β = −1.1, p = 0.60; Figure 3). Similarly, LMI in the supine position (LMFS) had a significant relationship with LSR (β = 0.29, p = 0.035), while LMI in the lateral position (LMFL) had a weaker effect and was not significant (β = −0.21, p = 0.12). Positional AI, positional LMAI, positional respiratory arousals and positional AHI had no significant relationship with LSR.
FIGURE 3.
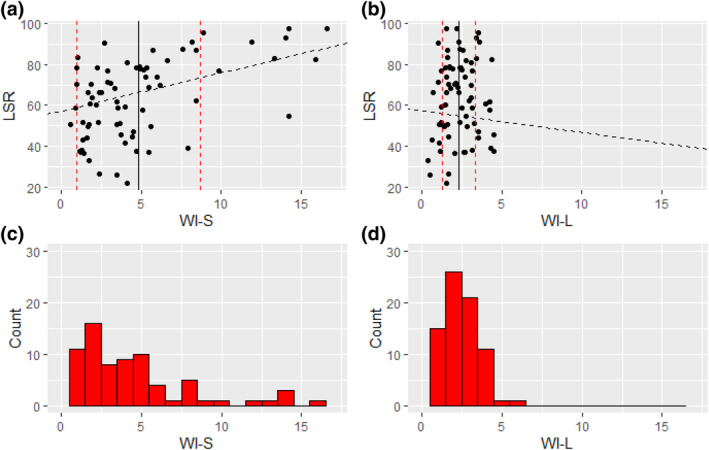
Differences in the relationship with lateral supine ratio (LSR) between wake index in the supine position (WI‐S) and wake index in the lateral position (WI‐L). Shown are scatter plots demonstrating the relationship between LSR and WI‐S (a) and WI‐L (b), where the black solid line shows the mean positional WI, and red dotted lines show standard deviations. The black dashed line shows the linear mixed‐effects regression model described by intercept and coefficient (β). Histograms are shown that demonstrate the difference in distribution of WI‐S (c) and WI‐L (d). It can be seen that the plurality of participants have WI‐L or WI‐S within 1.5–2.5 awakenings per hour; however, WI‐S is more distributed with wider standard deviations.
The AHI significantly decreased with more lateral sleep (β = −0.84, p = 0.048). Although not statistically significant, CIs indicate strong confidence in a decrease in respiratory arousals with more lateral lying (2.5% CI: −2.2; 97.5% CI: 0.23). Similarly, CIs indicated strong confidence in a decrease in overall arousals (β = −0.71, p = 0.054) with more lateral lying (2.5% CI: −1.45; 97.5% CI: 0.0001).
3.4.1. Case studies
Participant 82 (age: 60 years; BMI: 18.9 kg m−2; gender: female) had the greatest change in LSR across PSG night 1 and night 2 (49.0%; Figure 4). Comparing sleep architecture on the first and second night of PSG, the first night right‐sided lateral lying was preferential (59.2%), with no occurrence of left‐sided lying, the sleep was rich in REM (23.2%) and N3 (14.6%). N3 sleep and the supine position never coincided. On the second night almost no right‐lying sleep occurred, with some left‐lying sleep (17.6%) and more supine sleep (63.3%). An increased N3 latency was observed, with an increase in arousals and limb movements in the first bout of N2 from 23:00 hours to 24:00 hours, prohibiting progression through to N3, and overall decreasing both N3% (3.8%) and REM% (8.4%). Overall, increased supine sleep on the second night of PSG coincided with difficulties entering N3 sleep.
FIGURE 4.
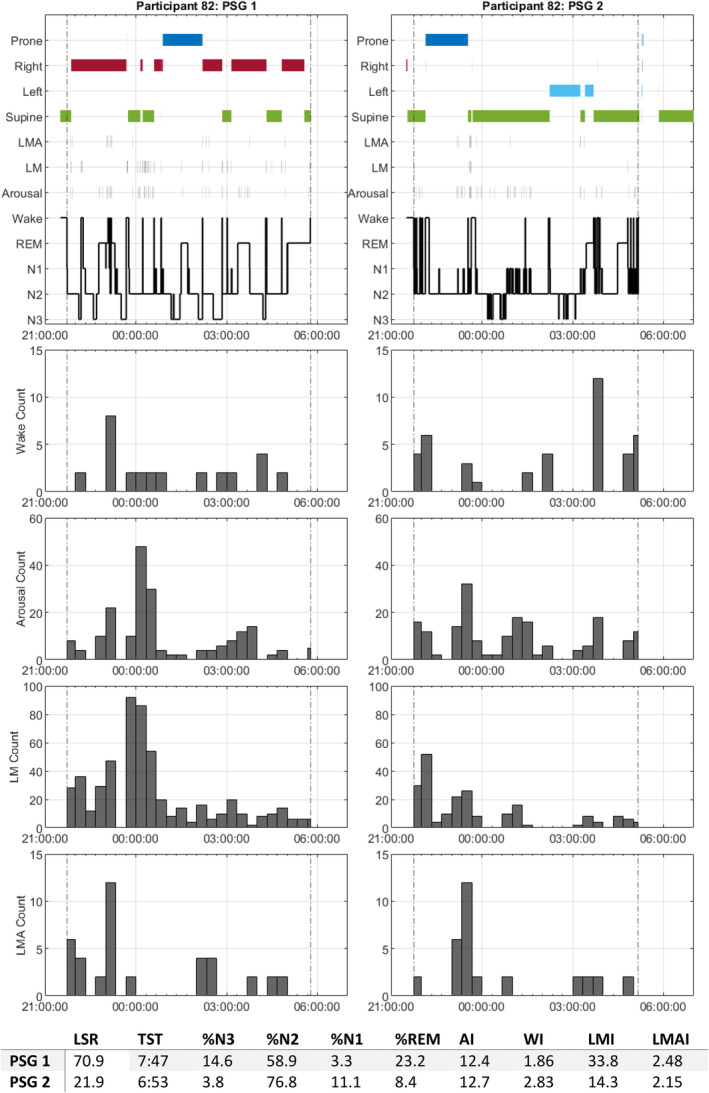
Participant 82 polysomnography (PSG)1 and PSG2 sleep event timelines. PSG1 timeline is shown on the left, and PSG2 on the right. Dotted vertical lines represent sleep onset and sleep offset. The top hypnogram shows continuous sleep event data, including sleep posture (supine in green, left in cyan, right in red, prone in blue), limb movement arousal (LMA), limb movement (LM), arousal and sleep stages, including wake, rapid eye movement (REM), N1, N2 and N3. Histograms are shown representing the count of wakes, arousals, LM and LMA within 20‐min epochs starting from the hour. The bottom table shows sleep statistics relating to each night of sleep for the participant, total sleep time (TST) is expressed as hh:mm, % N3, N2, N1 and REM calculated as proportion of TST.
Participant 61 (age: 66 years; BMI: 21.1 kg m−2; gender: male) was of interest due to both nights consistently close to LSR = 50 (Figure 5). Comparison of the first and second nights showed a relatively similar pattern of sleep onset in the supine position continuing through the first bout of deep sleep, followed by intermittent bouts of left lateral and supine sleep, with relatively sparse prone and right‐lying sleep. All restlessness indices were greater on the first night of sleep, with LMI 359% greater on the 1st night, largely due to a period of wakefulness from 03:20 hours to 03:40 hours with 120 limb movements; however, this had no effect on N3 or total sleep time (TST). Overall, both deep sleep and position preference was consistent despite large changes in restlessness.
FIGURE 5.
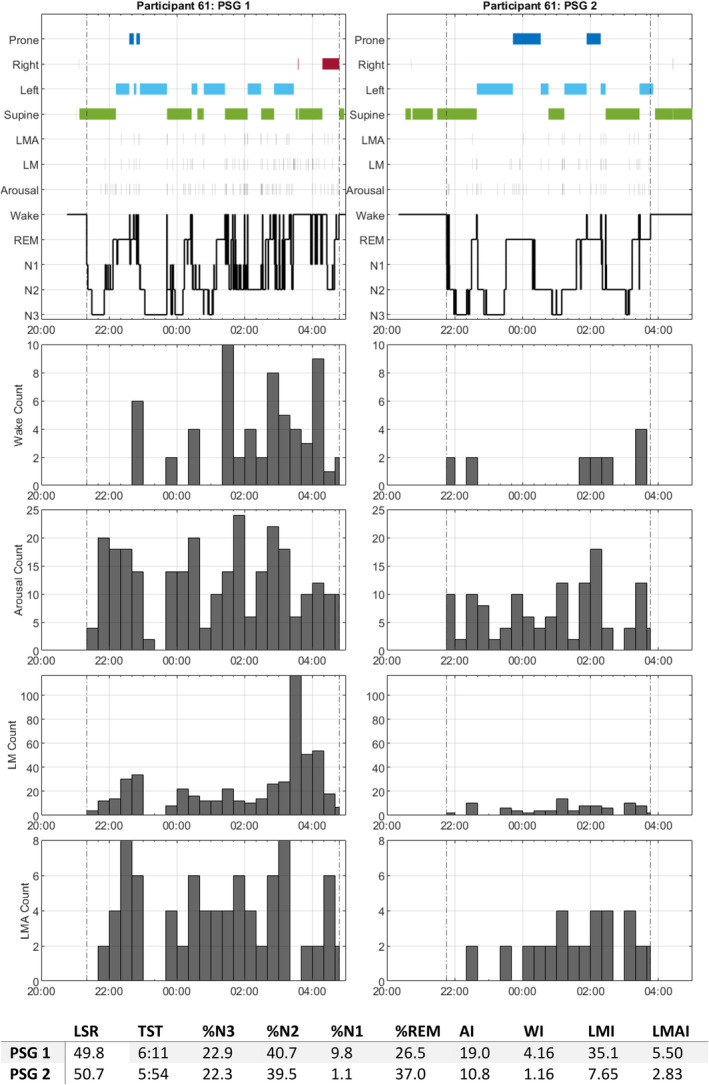
Participant 61 polysomnography (PSG)1 and PSG2 sleep event timelines. Similar layout to Figure 4.
4. DISCUSSION
This study is the first to investigate relationships between sleep position preference and positional arousals, awakenings, limb movements and limb movement arousals, and respiratory events in healthy individuals, to elucidate the physiological motivation for repositioning during sleep. All restlessness indices were significantly greater in supine compared with lateral position, with a particularly strong increase in frequency of respiratory events. The WI in the supine position had the strongest association with lateral position preference, suggesting that increased vigilance with awakening in the supine position provides greater opportunity for turnover to supine, while more transient subconscious arousals have little effect on sleep position preference. Increases in lateral sleep reduced AHI and AIs, supporting the role of position therapy in reducing effects of sleep apnea; however, wide standard deviations in restlessness indices across both positions warn against generalised recommendations to sleep more laterally.
In agreement with current literature, the current study finds the lateral position to be most prevalent in healthy sleep, followed by supine and then prone positions (Gordon et al., 2004; Skarpsno et al., 2017; Zhang et al., 2022). We found sleep position to be a strongly ingrained habit, with a strong relationship between first‐ and second‐night sleep position distribution, with no evidence that sleep position distribution changed over time to adapt to a new firm mattress. This agrees with Verhaert et al. (2011), who found that individuals were consistent in sleep position even when underlying mattress mechanics changed between nights. The case study of participant 82, chosen due to a large change in sleep preference from lateral to supine between nights, showed signs of a deep sleep‐inhibiting effect of the supine position. Lorrain and Koninck (1998) found no clear relationship between sleep position and sleep stage across the 25 healthy participants. However, further research should investigate the effects of deviation from natural position preference on sleep architecture.
We find that all restlessness metrics, including respiratory events, limb movements, arousals and awakenings were more frequent in the supine position. In agreement with this, Zhang et al. (2022) found awakenings were more frequent in the supine position in a healthy cohort, but did not investigate effects of arousals or AHI. We find that AHI and RAI had the greatest percentage increase in the supine position, and this is likely a significant contributor to overall increased arousals and awakenings in the supine position. This finding is in line with a meta‐analysis by Menon and Kumar (2013), who found AHI and respiratory disturbance to be approximately twice as great in the supine position compared with the lateral position in adult cohorts with sleep apnea. Interestingly, this association was greater in patients with less severe sleep apnea, which is supported by our current findings, where our cohort with no prior sleep apnea diagnosis had a three times increase in AHI and four times increase in RAI in the supine position.
We find awakenings and arousal rates in the supine position twice as frequent as in the lateral position; however, the increase in respiratory arousals does not fully account for this increase in arousals. Basner et al. (2011) investigated effects of exogenous arousals on sleep architecture, and found increased noise‐related awakenings and arousals corresponded with decreases in spontaneous arousals and awakenings, partially dampening effects of noise on sleep architecture. We do not observe this effect in response to increased respiratory arousals. The increase in RAI accounted for 68% of the overall increase in AI, suggesting that disturbance of respiratory pathways does not fully explain increase in overall arousals, and points towards the contribution of other posture‐related mechanisms.
Another potential contributor is a susceptibility to spinal discomfort in the supine position. During supine sleep on a firm mattress there is a flattening of the lordotic curve and forward rotation of the pelvis, while in lateral lying natural lordosis is largely maintained (Haex, 2004; Verhaert et al., 2011). A cross‐sectional study by Gordon et al. (2007) found that the lateral sleep position is more effective for alleviating cervical spine pain, as well as scapular and arm pain. Abanobi et al. (2015) studied the habits of welders and panel beaters, and found that supine sleep preference was associated with lower back pain. Chronic low back pain has been linked to increases in sleep disturbance (Kelly et al., 2011). While chronic low back pain sufferers were excluded from this study, flattening of the lordotic curve and subsequent deformation of intervertebral discs may create discomfort over a sustained period, where some participants may tolerate far longer periods of supine sleep than others.
The novel finding of this study is that WI in the supine position was closely related to sleep position preference, but not any other restlessness indices with the exception of LMI. We speculate that greater awakenings in the supine position induced by increased arousals creates an overall heightened vigilance in supine, representing greater awareness, and the ability to respond to discomfort and adjust sleeping position. While more frequent supine awakenings were associated with increased lateral position, there was no clear effect of lateral awakenings on sleep position preference. This may be explained by the considerably greater variation in wake frequencies in the supine position, relative to very low variation in wake frequencies in the lateral position. According to AASM rules, limb movements may be scored during wakeful periods, while arousals do not occur during wakefulness (Berry et al., 2012). Therefore, this association may be a result of frequent limb movements during the wakeful periods.
At‐home PSG was utilised as it represents the gold‐standard in characterising sleep stages, arousals and awakenings in the home environment. However, this set‐up utilises wires around the body, a nasal cannula and central unit, which disturbs the usual pre‐bed routine and sleep environment. In particular, the chest‐worn central unit likely deters lying in the prone position, and so this study focusses on the proportion of lateral and supine sleep where the PSG set‐up is likely to have similar effect.
5. CONCLUSION
Overall, this study explores if individuals adapt sleep position to encourage more continuous sleep. We found strong consistency in sleep position preference between nights of sleep. Awakenings were more frequent in the supine position with greater standard deviation, and increased awakening index in the supine position strongly predicted preference towards the lateral position. However, increased arousals, including respiratory or limb movement arousals in supine did not predict preference towards the lateral position. These findings indicate that individuals are effective at adapting sleep position to promote decreased awakening, but are not effective at adapting sleep position to reduce more subconscious disruptions to sleep architecture. These findings support the role of position therapy in cases where there are large relative differences in AHI between lateral and supine positions; however, large deviations in restlessness indices suggest that personalised assessment of sleep position‐specific restlessness indices is still required to inform recommendations.
AUTHOR CONTRIBUTIONS
Lionel Rayward: Conceptualization; methodology; data curation; software; investigation; writing – original draft; writing – review and editing; project administration; visualization. Selina W. K. Ho: Data curation; project administration; writing – review and editing. Daniel Green: Conceptualization; supervision; funding acquisition. J. Paige Little: Conceptualization; data curation; supervision; resources; project administration; writing – review and editing; funding acquisition.
CONFLICT OF INTEREST STATEMENT
The authors acknowledge the research support provided by Sealy Australia. This support has provided: salary for Dr Lionel Rayward, A/ Prof. Paige Little and Ms Selina W. K Ho. Research datasets for healthy adults (including PSG data).
ACKNOWLEDGEMENTS
The authors acknowledge sleep study support, including sleep scoring, provided by Corey‐Ann Roberts, Mater Sleep Unit. Open access publishing facilitated by Queensland University of Technology, as part of the Wiley ‐ Queensland University of Technology agreement via the Council of Australian University Librarians.
Rayward, L. , Ho, S. W. K. , Green, D. , & Little, J. P. (2025). Sleep disruption and sleep position: Increased wake frequency in supine predicts lateral position preference. Journal of Sleep Research, 34(1), e14325. 10.1111/jsr.14325
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
REFERENCES
- Abanobi, O. C. , Ayeni, G. O. , Ezeugwu, C. C. , & Ayeni, O. A. (2015). Risk‐disposing habits of Lowback pain amongst welders and panel beaters in Owerri, South‐East Nigeria. Indian Journal of Public Health, 6(3), 187. 10.5958/0976-5506.2015.00164.3 [DOI] [Google Scholar]
- Ackermann, S. , & Rasch, B. (2014). Differential effects of non‐REM and REM sleep on memory consolidation? Current Neurology and Neuroscience Reports, 14(2), 430. 10.1007/s11910-013-0430-8 [DOI] [PubMed] [Google Scholar]
- Babisch, W. , Ising, H. , Gallacher, J. E. J. , Sweetnam, P. M. , & Elwood, P. C. (1999). Traffic noise and cardiovascular risk: The Caerphilly and speedwell studies, third Phase‐10‐year follow up. Archives of Environmental Health: An International Journal, 54(3), 210–216. 10.1080/00039899909602261 [DOI] [PubMed] [Google Scholar]
- Basner, M. , Müller, U. , & Elmenhorst, E.‐M. (2011). Single and combined effects of air, road, and rail traffic noise on sleep and recuperation. Sleep, 34(1), 11–23. 10.1093/sleep/34.1.11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry, R. B. (2012). The AASM manual for the scoring of sleep and associated events: Rules, terminology and technical specifications, version 2.0. American Academy of Sleep Medicine. [Google Scholar]
- Berry, R. B. , Brooks, R. , Gamaldo, C. E. , Harding, S. M. , Marcus, C. L. , Vaughn, B. V. , & Tangredi, M. M. (2012). The AASM manual for the scoring of sleep and associated events: Rules. Terminology and Technical Specifications. Version 2.0. www.aasmnet.org [Google Scholar]
- Bonnet, M. H. , & Arand, D. L. (2007). EEG arousal norms by age. Journal of Clinical Sleep Medicine, 3(3), 271–274. 10.5664/jcsm.26796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulos, M. I. , Jairam, T. , Kendzerska, T. , Im, J. , Mekhael, A. , & Murray, B. J. (2019). Normal polysomnography parameters in healthy adults: A systematic review and meta‐analysis. The Lancet Respiratory Medicine, 7(6), 533–543. 10.1016/S2213-2600(19)30057-8 [DOI] [PubMed] [Google Scholar]
- Carrington, M. J. , & Trinder, J. (2008). Blood pressure and heart rate during continuous experimental sleep fragmentation in healthy adults. Sleep, 31(12), 1701–1712. 10.1093/sleep/31.12.1701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cary, D. , Briffa, K. , & McKenna, L. (2019). Identifying relationships between sleep posture and non‐specific spinal symptoms in adults: A scoping review. BMJ Open, 9(6), e027633. 10.1136/bmjopen-2018-027633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, Z. , Li, Y. , Liu, R. , Gao, D. , Chen, Q. , Hu, Z. , & Guo, J. (2014). Effects of Interface pressure distribution on human sleep quality. PLoS One, 9(6), e99969. 10.1371/journal.pone.0099969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Koninck, J. , Lorrain, D. , Christ, G. , Proulx, G. , & Coulombe, D. (1989). Intensive language learning and increases in rapid eye movement sleep: Evidence of a performance factor. International Journal of Psychophysiology, 8(1), 43–47. 10.1016/0167-8760(89)90018-4 [DOI] [PubMed] [Google Scholar]
- Della Monica, C. , Johnsen, S. , Atzori, G. , Groeger, J. A. , & Dijk, D. J. (2018). Rapid eye movement sleep, sleep continuity and slow wave sleep as predictors of cognition, mood, and subjective sleep quality in healthy men and women, aged 20‐84 years. Frontiers in Psychiatry, 9, 255. 10.3389/fpsyt.2018.00255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dijk, D.‐J. , Duffy, J. F. , & Czeisler, C. A. (2001). Age‐related increase in awakenings: Impaired consolidation of NonREM sleep at all circadian phases. Sleep, 24(5), 565–577. 10.1093/sleep/24.5.565 [DOI] [PubMed] [Google Scholar]
- Ekstedt, M. , Åkerstedt, T. , & Söderström, M. (2004). Microarousals during sleep are associated with increased levels of lipids, cortisol, and blood pressure. Psychosomatic Medicine, 66(6), 925–931. 10.1097/01.psy.0000145821.25453.f7 [DOI] [PubMed] [Google Scholar]
- Gordon, S. , Grimmer, K. , & Trott, P. (2007). Sleep position, age, gender, sleep quality and waking Cervico‐thoracic symptoms. Internet Journal of Allied Health Sciences and Practice, 5(1), 1–8. 10.46743/1540-580X/2007.1134 [DOI] [Google Scholar]
- Gordon, S. J. , Grimmer, K. A. , & Trott, P. (2004). Self‐reported versus recorded sleep position: An observational study. Internet Journal of Allied Health Sciences and Practice, 2(1), 7. [Google Scholar]
- Groch, S. , Wilhelm, I. , Diekelmann, S. , & Born, J. (2013). The role of REM sleep in the processing of emotional memories: Evidence from behavior and event‐related potentials. Neurobiology of Learning and Memory, 99, 1–9. 10.1016/j.nlm.2012.10.006 [DOI] [PubMed] [Google Scholar]
- Gronfier, C. , Luthringer, R. , Follenius, M. , Schaltenbrand, N. , Macher, J. P. , Muzet, A. , & Brandenberger, G. (1996). A quantitative evaluation of the relationships between growth hormone secretion and Delta wave electroencephalographic activity during Normal sleep and after enrichment in Delta waves. Sleep, 19(10), 817–824. 10.1093/sleep/19.10.817 [DOI] [PubMed] [Google Scholar]
- Guerrien, A. (1994). Paradoxical sleep and memory processes in humans. Acta Psychiatrica Belgica, 94(2), 75–87. [PubMed] [Google Scholar]
- Haex, B. (2004). Back and Bed: Ergonomic Aspects of Sleeping.
- Hong, T. T.‐H. , Wang, Y. , Wong, D. W.‐C. , Zhang, G. , Tan, Q. , Chen, T. L.‐W. , & Zhang, M. (2022). The influence of mattress stiffness on spinal curvature and intervertebral disc stress—An experimental and computational study. Biology, 11(7), 1030. 10.3390/biology11071030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarup, L. , Babisch, W. , Houthuijs, D. , Pershagen, G. , Katsouyanni, K. , Cadum, E. , Dudley, M.‐L. , Savigny, P. , Seiffert, I. , Swart, W. , Breugelmans, O. , Bluhm, G. , Selander, J. , Haralabidis, A. , Dimakopoulou, K. , Sourtzi, P. , Velonakis, M. , & Vigna‐Taglianti, F. (2008). Hypertension and exposure to noise near airports: The HYENA study. Environmental Health Perspectives, 116(3), 329–333. 10.1289/ehp.10775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joosten, S. A. , Edwards, B. A. , Wellman, A. , Turton, A. , Skuza, E. M. , Berger, P. J. , & Hamilton, G. S. (2015). The effect of body position on physiological factors that contribute to obstructive sleep apnea. Sleep, 38(9), 1469–1478. 10.5665/sleep.4992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karni, A. , Tanne, D. , Rubenstein, B. S. , Askenasy, J. J. , & Sagi, D. (1994). Dependence on REM sleep of overnight improvement of a perceptual skill. Science, 265(5172), 679–682. 10.1126/science.8036518 [DOI] [PubMed] [Google Scholar]
- Kelly, G. A. , Blake, C. , Power, C. K. , O'Keeffe, D. , & Fullen, B. M. (2011). The association between chronic low Back pain and sleep. The Clinical Journal of Pain, 27(2), 169–181. 10.1097/AJP.0b013e3181f3bdd5 [DOI] [PubMed] [Google Scholar]
- Koo, T. K. , & Li, M. Y. (2016). A guideline of selecting and reporting Intraclass correlation coefficients for reliability research. Journal of Chiropractic Medicine, 15(2), 155–163. 10.1016/j.jcm.2016.02.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuznetsova, A. , Brockhoff, P. B. , & Christensen, R. H. B. (2017). lmerTest package: Tests in linear mixed effects models. Journal of Statistical Software, 82(13), 1–26. 10.18637/jss.v082.i13 [DOI] [Google Scholar]
- Lange, T. , Dimitrov, S. , Bollinger, T. , Diekelmann, S. , & Born, J. (2011). Sleep after vaccination boosts immunological memory. Journal of Immunology, 187(1), 283–290. 10.4049/jimmunol.1100015 [DOI] [PubMed] [Google Scholar]
- Lange, T. , Perras, B. , Fehm, H. L. , & Born, J. (2003). Sleep enhances the human antibody response to hepatitis A vaccination. Psychosomatic Medicine, 65(5), 831–835. 10.1097/01.psy.0000091382.61178.f1 [DOI] [PubMed] [Google Scholar]
- Lee, K.‐I. , & Choi, J. H. (2023). Positional therapy for obstructive sleep apnea: Therapeutic modalities and clinical effects. Sleep Medicine Research, 14(3), 129–134. 10.17241/smr.2023.01837 [DOI] [Google Scholar]
- Lercher, P. , Widmann, U. , & Kofler, W. (2000). Transportation noise and blood pressure: The importance of modifying factors. In Proceedings of the 29th international congress and exhibition on noise control engineering (Cassereau D, Ed). InterNoise. (Vol. 4, pp. 2071–2075). [Google Scholar]
- Lorrain, D. , & Koninck, J. D. (1998). Sleep position and sleep stages: Evidence of their Independence. Sleep, 21(4), 335–340. 10.1093/sleep/21.4.335 [DOI] [PubMed] [Google Scholar]
- Mandai, O. , Guerrien, A. , Sockeel, P. , Dujardin, K. , & Leconte, P. (1989). REM sleep modifications following a Morse code learning session in humans. Physiology & Behavior, 46(4), 639–642. 10.1016/0031-9384(89)90344-2 [DOI] [PubMed] [Google Scholar]
- Menon, A. , & Kumar, M. (2013). Influence of body position on severity of obstructive sleep apnea: A systematic review. ISRN Otolaryngology, 2013, 1–7. 10.1155/2013/670381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park, S. J. , Kim, J. S. , & Kim, C. (2009). Comfort evaluation and bed adjustment according to sleeping positions. Human Factors and Ergonomics in Manufacturing & Service Industries, 19(2), 145–157. [Google Scholar]
- Pase, M. P. , Himali, J. J. , Grima, N. A. , Beiser, A. S. , Satizabal, C. L. , Aparicio, H. J. , Thomas, R. J. , Gottlieb, D. J. , Auerbach, S. H. , & Seshadri, S. (2017). Sleep architecture and the risk of incident dementia in the community. Neurology, 89(12), 1244–1250. 10.1212/wnl.0000000000004373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Core Team . (2023). R: A language and environment for statistical computing (R 4.3.2). R Foundation for Statistical Computing. [Google Scholar]
- Rao, M. N. , Blackwell, T. , Redline, S. , Stefanick, M. L. , Ancoli‐Israel, S. , Stone, K. L. , & Osteoporotic Fractures in Men (MrOS) Study Group . (2009). Association between sleep architecture and measures of body composition. Sleep, 32(4), 483–490. 10.1093/sleep/32.4.483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasch, B. , & Born, J. (2013). About Sleep's role in memory. Physiological Reviews, 93(2), 681–766. 10.1152/physrev.00032.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren, R. , Zhang, Y. , Yang, L. , Somers, V. K. , Covassin, N. , & Tang, X. (2022). Association between arousals during sleep and hypertension among patients with obstructive sleep apnea. Journal of the American Heart Association, 11(1), e022141. 10.1161/JAHA.121.022141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sassin, J. F. , Parker, D. C. , Mace, J. W. , Gotlin, R. W. , Johnson, L. C. , & Rossman, L. G. (1969). Human growth hormone release: Relation to slow‐wave sleep and sleep‐walking cycles. Science, 165(3892), 513–515. 10.1126/science.165.3892.513 [DOI] [PubMed] [Google Scholar]
- Scrima, L. (1982). Isolated REM sleep facilitates recall of complex associative information. Psychophysiology, 19(3), 252–259. [DOI] [PubMed] [Google Scholar]
- Skarpsno, E. S. , Mork, P. J. , Nilsen, T. I. L. , & Holtermann, A. (2017). Sleep positions and nocturnal body movements based on free‐living accelerometer recordings: Association with demographics, lifestyle, and insomnia symptoms. Nature and Science of Sleep, 9, 267–275. 10.2147/NSS.S145777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song, Y. , Blackwell, T. , Yaffe, K. , Ancoli‐Israel, S. , Redline, S. , Stone, K. L. , & Osteoporotic Fractures in Men (MrOS) Study Group . (2015). Relationships between sleep stages and changes in cognitive function in older men: The MrOS sleep study. Sleep, 38(3), 411–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi, Y. , Kipnis, D. M. , & Daughaday, W. H. (1968). Growth hormone secretion during sleep. The Journal of Clinical Investigation, 47(9), 2079–2090. 10.1172/jci105893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theorell‐Haglöw, J. , Berne, C. , Janson, C. , Sahlin, C. , & Lindberg, E. (2010). Associations between short sleep duration and central obesity in women. Sleep, 33(5), 593–598. [PMC free article] [PubMed] [Google Scholar]
- Van Cauter, E. , Kerkhofs, M. , Caufriez, A. , Van Onderbergen, A. , Thorner, M. O. , & Copinschi, G. (1992). A quantitative estimation of growth hormone secretion in normal man: Reproducibility and relation to sleep and time of day. The Journal of Clinical Endocrinology and Metabolism, 74(6), 1441–1450. 10.1210/jcem.74.6.1592892 [DOI] [PubMed] [Google Scholar]
- Verhaert, V. , Haex, B. , De Wilde, T. , Berckmans, D. , Verbraecken, J. , de Valck, E. , & Vander Sloten, J. (2011). Ergonomics in bed design: The effect of spinal alignment on sleep parameters. Ergonomics, 54(2), 169–178. 10.1080/00140139.2010.538725 [DOI] [PubMed] [Google Scholar]
- Verschoor, G. J. , & Holdstock, T. L. (1984). REM bursts and REM sleep following visual and auditory learning. South Africa Journal of Psychology, 14(3), 69–74. [Google Scholar]
- Wassing, R. , Lakbila‐Kamal, O. , Ramautar, J. R. , Stoffers, D. , Schalkwijk, F. , & Van Someren, E. J. W. (2019). Restless REM sleep impedes overnight amygdala adaptation. Current Biology, 29(14), 2351–2358.e4. 10.1016/j.cub.2019.06.034 [DOI] [PubMed] [Google Scholar]
- Zhang, Y. , Xiao, A. , Zheng, T. , Xiao, H. , & Huang, R. (2022). The relationship between sleeping position and sleep quality: A flexible sensor‐based study. Sensors (Basel, Switzerland), 22(16), 6220. 10.3390/s22166220 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.


