Abstract
Reverse remodeling, the overarching concept behind myocardial recovery, describes the process in which the maladaptive cardiac structural and functional alterations are reversed by removing the underlying etiology or by therapy. This review addresses different imaging modalities and biomarkers as possible predictors for reverse remodeling in patients with chronic heart failure. Although echocardiography remains the imaging modality of choice in daily practice, the presence and amount of fibrosis on cardiac magnetic resonance is a better predictor and inversely correlated with the likelihood for reverse remodeling. A decrease in NT-proBNP levels and serum soluble ST3 during follow-up is associated with better clinical and structural outcomes. The role of troponins and galectine-3 is less clear. There is a promising role for microRNAs in the future, although more research is necessary. Accurate predictors of reverse remodeling could help identify patients with an increased likelihood for reverse remodeling and, in turn, improve patient-tailored medicine.
Keywords: myocardial recovery, reverse remodeling, heart failure, biomarkers, imaging, echocardiography, cardiac magnetic resonance
Introduction
Heart failure is characterized as a heterogenous syndrome with different clinical presentations, different underlying etiologies, and different treatment options. Over time, the disease can either remain stable, deteriorate into end-stage heart failure, or improve with even complete recovery.1 Novel guideline-directed medical therapies have demonstrated improved morbidity and survival in patients with heart failure but could also improve underlying structural and functional abnormalities. Myocardial recovery is the result of reverse remodeling due to removal of the underlying etiological trigger or therapeutic interventions (drugs, cardiac resynchronization therapy, or left ventricular assist devices). On a macroscopic level, there is (partial) normalization of the cardiac function and morphological structure. Both structural and functional recovery result in improved clinical outcomes with a reduction in morbidity and mortality. These patients probably have different phenotypes than patients with heart failure with reduced ejection fraction without myocardial recovery despite initiated therapies. In order to improve personalized medicine, it would be beneficial if the occurrence of myocardial recovery could be predicted and these patients could be identified. Nonischemic cardiomyopathies, shorter disease course, female gender, and nonfamilial cardiomyopathies are known to have a higher likelihood for cardiac remodeling.2,3 In addition to these clinical parameters, biomarkers and imaging modalities, and especially the evolution over time, could help to identify patients prone to experience cardiac recovery. Myocardial recovery implies the complete normalization of both cardiac structure and function without clinical heart failure events. In contrast, the term “reverse remodeling” is more broadly used to describe any degree of structural recovery, including myocardial recovery. Reverse remodeling is the result of regression of underlying molecular, cellular, and tissue adaptations, which leads to normative changes in cardiac function, dimensions, and geometry of a previously failing heart. However, it does not imply complete normalization, and heart failure symptoms may still occur. Reverse remodeling is assessed by different imaging modalities, but there is a large heterogeneity in the definition. Overall, there is a general agreement that reverse remodeling may be defined as more than 10% improvement in left ventricular ejection fraction (LVEF) or its normalization (LVEF > 50%) in combination with an index LV diameter reduction of more than 10% or its normalization (< 33 mm/m2). It is important to recognize that patients with reverse remodeling might still have abnormal cardiac features. In contrast, myocardial recovery represents a successful and complete recovery of the heart with freedom from future heart failure events. This can only be achieved if the heart did not yet suffer from irreversible damage.4,5,6 This review discusses the role of different biomarkers and imaging modalities in predicting reverse remodeling and myocardial recovery in patients with heart failure (Table 1). Both terms are used throughout the review since most studies use the term “reverse remodeling” rather than “myocardial recovery” as an end point.
Table 1.
Overview of imaging modalities and biomarkers to predict reverse remodeling. CMR: cardiac magnetic resonance; GLS: global longitudinal strain; LA: left atrial; LGE: late gadolinium enhancement; LV: left ventricular; LVEDV: left ventricular end diastolic volume; LVEF: left ventricular ejection fraction; LVESV: left ventricular end systolic volume; NT-proBNP: N-terminal pro-B-type natriuretic peptide; ST2: suppression of tumorigenicity 2; RR: reverse remodeling
|
| ||
|---|---|---|
| MARKER | (PATHO)PHYSIOLOGY | PREDICTOR |
|
| ||
| Imaging modalities | ||
|
| ||
| Echocardiography | ||
|
| ||
| LV GLS | Deformation characteristics of the myocardium; limit dependence on LV volumes | GLS ≤ 16%: increased risk for decrease in LVEF during follow-up |
|
| ||
| LA GLS | Increased LV filling pressures are transmitted backwards and will lead to LA enlargement, dysfunction | LA GLS > 10.8%; increased incidence of RR |
|
| ||
| Myocardial work quantification | Incorporation of strain with left ventricular pressure; less loading dependent | Good correlation with fibrosis on CMR. More research necessary |
|
| ||
| Cardiac magnetic resonance | ||
|
| ||
| LGE | Marker to assess the degree of myocardial fibrosis | Low LGE (cut-off < 7-8% of LV mass) and higher degree of myocardial edema; higher incidence of RR No role in follow-up |
|
| ||
| Contractile reserve | Change in LVEF after dobutamine administration | Larger change; higher incidence of RR No correlation with degree of fibrosis |
|
| ||
| Biomarkers | ||
|
| ||
| NT-proBNP | Marker of myocardial stress and stretch |
|
|
| ||
| Troponins | Marker of myocyte injury and necrosis |
|
|
| ||
| Soluble ST2 | Marker of fibrosis and hypertrophy |
|
|
| ||
| Galectin-3 | Marker of macrophage activity, part of fibrosis process | Levels < 20 ng/mL during follow-up; higher incidence of RR No independent predictive value |
|
| ||
| Big endothelin-1 | Marker of vasoconstriction, hypertrophy and fibrosis resulting in adverse remodeling | Lower levels; higher likelihood of RR |
|
| ||
Imaging Modalities
Echocardiography
In daily clinical practice, echocardiography remains the imaging modality of choice to assess cardiac function and structure due to its low cost, safety, broad availability, and short time consumption. Some basic standard echocardiographic parameters are associated with reverse remodeling, such as mild mitral regurgitation, higher tricuspid annular plane systolic excursion, and smaller left atrial volume. An increase in LVEF, LV end systolic volume, or LV end diastolic volume are more easily achieved during follow-up in a more dysfunctional ventricle as this is expressed as a relative change. However, these changes are less likely to lead to an improvement in ejection fraction above 40%, and patients with a more dilated ventricle still have a higher risk for adverse events. Therefore, deformation characteristics of the myocardium evaluated through speckle tracking and global longitudinal strain (GLS) is a more sensitive method to assess contractility and systolic function of the LV as it is less dependent on ventricular volumes.2,7
GLS is already widely used as a follow-up imaging modality in cardio-oncology and might drive therapeutic decisions because a decline in GLS is accepted as an early predictor of chemotherapy induced cardiotoxicity.8 In a study of 160 dilated cardiomyopathy patients, LV GLS was the sole predictor for reverse remodeling.9 In addition, in patients with recovered LVEF, a decreased GLS (≤ 16%) was a predictor for a decrease in LVEF during follow-up (sensitivity 88%, specificity 46%), while patients with a GLS > 16% were more likely to have a stable LVEF (sensitivity 47%, specificity 83%).10
Left atrial GLS might also help predict myocardial recovery. The left atrium has an elastic reservoir function for the pulmonary venous inflow during LV systole; it is a passive conduit during LV diastole and has an active filling function during the late ventricular diastole. In addition, LV filling pressures are transmitted backwards, so increased LV filling pressures may lead to left atrial enlargement.11 Improvements in LV function may consequently result in left atrial reverse remodeling.12 A left atrial GLS above 10.8% was highly sensitive and predictive for reverse remodeling (sensitivity 96%, specificity 82%).13 Therefore, both absolute values and changes in GLS might be useful in the early prediction of myocardial recovery and play a role in monitoring heart failure patients.
Similar to LVEF, GLS measurements are to some extent loading dependent.14 Myocardial work quantification has been developed as a noninvasive tool to assess myocardial function. By incorporating strain with LV pressure, this method overcomes the limitations associated with LVEF and GLS measurements.15,16 The indices calculated with myocardial work quantification correlate better than GLS and LVEF with fibrosis on cardiac magnetic resonance (CMR).17 Since this is a relatively novel technique, additional research is necessary to evaluate its role as a monitoring tool for reverse remodeling.18
Cardiac resynchronization therapy (CRT) has proven to be beneficial in patients with heart failure with a reduced ejection fraction and a left bundle branch block. Because restoration of mechanical synchronicity is the main goal, several echocardiographic parameters of dyssynchrony prior to implantation have been postulated as predictors for CRT response. The Predictors of Response to CRT (PROSPECT) study evaluated several baseline echocardiographic parameters to determine whether they could predict the clinical and echocardiographic response to CRT. However, the study demonstrated that no single echocardiographic parameter (beyond baseline LVEF in current guidelines) should be used to improve patient selection.19,20
Cardiac Magnetic Resonance
Despite its limited availability, duration, and possible contraindications, CMR has a lower intra-observer variation, better contrast resolution, and is not limited by a poor acoustic window. Late gadolinium enhancement (LGE) is a marker to assess the degree of myocardial fibrosis (Figure 1). The absence of LGE is a strong predictor for myocardial recovery and improved clinical outcomes (sensitivity 55.6%, specificity 92.3%).21,22,23,24 The degree of LGE is inversely related to the likelihood for reverse remodeling, irrespective of underlying LVEF and LV end diastolic volume (LVEDV).25,26 A cut-off of 7% to 8% (expressed as percentage of LV mass) has been proposed to predict reverse remodeling (sensitivity 97%, specificity 61%).21,27 Another cohort confirmed that patients with a low extent of LGE had a higher incidence of reverse remodeling. In addition, they demonstrated that this, in combination with a higher myocardial edema ratio, was a stronger predictor for reverse remodeling than endomyocardial biopsy, biomarkers, and conventional echocardiography.28 There is no direct indication for serial follow-up of CMR images and LGE measurements since a change in the presence of LGE is not expected.25
Figure 1.

Late gadolinium enhancement on cardiac magnetic resonance. Late gadolinium enhancement (LGE) cardiac magnetic resonance image obtained in a patient with ischemic cardiomyopathy. Severe reduced left ventricular ejection fraction (LVEF 17%) with approximately 40% LGE in the left ventricle. Despite optimal medical therapy, no myocardial recovery was expected. Four years later, due to recurrent sustained ventricular tachycardia, the patient was referred for left ventricular assist device.
A cohort study demonstrated that a poor contractile reserve (change in LVEF assessed with CMR after dobutamine administration) could identify patients with dilated cardiomyopathy at a low likelihood of reverse remodeling after 12 months. In addition, a large contractile reserve with dobutamine was a predictor for a greater improvement in LVEF (1% increase associated with 0.4% increase in LVEF at 12 months). The amount of myocardial fibrosis did not correlate with the degree of contractile reserve. Due to the accuracy and reproducibility, the authors highlight the importance of using CMR for the assessment of contractile reserve. 29
To conclude, GLS measurements provide better prognostication than standard LV end diastolic diameter (LVEDD) and LVEF in echocardiography. Myocardial work quantification is a promising novel technique, although more research is necessary prior to implementation as a standard screening tool. The absence of LGE on CMR is a strong predictor for myocardial recovery, especially in the presence of myocardial edema. An overview of the most relevant articles are depicted in Table 2.
Table 2.
Highlight of research articles supporting different imaging modalities for the prediction of reverse remodeling. DCMP: dilated cardiomyopathy; LA: left atrial; LGE: late gadolinium enhancement; LVEDD: left ventricular end diastolic dimension; LVEDDi: left ventricular end diastolic dimension index; LVEF: left ventricular ejection fraction; RR: reverse remodeling; GLS: global longitudinal strain; LVESD: left ventricular end-systolic diameter
|
| |||
|---|---|---|---|
| AUTHOR, YEAR | STUDY POPULATION | DEFINITION RR | OUTCOME/FINDING |
|
| |||
| Echocardiography | |||
|
| |||
| Ikeda et al. 201530 | N = 207, DCMP |
|
LVEDDi decrease during the first 6 months was predictive for RR later on |
|
| |||
| Adamo et al. 201731 | N = 96, LVEF < 50% |
|
Abnormal LV GLS (≤ 16%): predictor for LVEF decrease. Normal LV GLS (> 16%): stable LVEF |
|
| |||
| Swat et al. 201832 | N = 166, DCMP |
|
Baseline LV GLS > 8% in patients with LVESD > 43.5 mm associated with RR |
|
| |||
| Jung et al. 20209 | N = 160, DCMP |
|
Baseline LV GLS (cut-off 10%) independent predictor RR in sinus rhythm |
|
| |||
| Torii et al. 202113 | N = 100, new onset HF |
|
LA strain (cut-off 10.8%) independent predictor for RR |
|
| |||
| CMR | |||
|
| |||
| Kubanek et al. 201328 | N = 44, DCMP |
|
Baseline lower extent of LGE and greater myocardial edema ratio are independent predictors of RR |
|
| |||
| Masci et al. 201325 | N = 58, DCMP |
|
LGE absence at baseline strong predictor for RR at 2 years follow-up |
|
| |||
| Kida et al. 201323 | N = 31, DCMP |
|
LGE absence at baseline strong predictor for RR at 6 months follow-up |
|
| |||
| Ishii et al. 201627 | N = 66, DCMP |
|
LGE mass < 8% predicts RR |
|
| |||
| Chimura et al. 201726 | N = 129, DCMP |
|
LGE absence and GLS predict independently RR |
|
| |||
| Barison et al. 201821 | N = 71, DCMP |
|
LGE absence predicts RR |
|
| |||
Biomarkers
Biomarkers have the advantage over imaging modalities to demonstrate changes in myocardial function prior to structural and functional alterations. Biomarkers defining different biological processes are useful to identify the different underlying pathological change, such as myocardial stress and stretch (natriuretic peptides), myocyte injury (troponins), fibrosis (soluble ST2), and inflammation (galectin 3) (Figure 2).1,7,33,34
Figure 2.
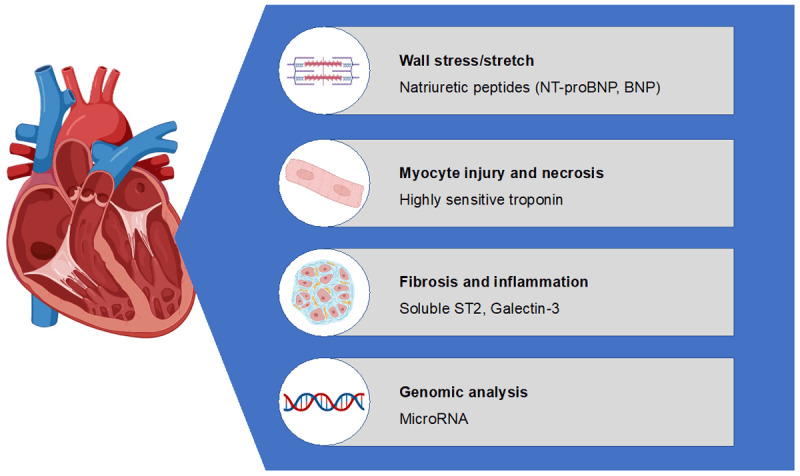
Different biomarkers according to their pathophysiological action.
Natriuretic Peptides
Natriuretic peptides are commonly used biomarkers in patients with heart failure and are produced in the presence of myocardial wall stretch and stress due to volume or pressure overload. B-type natriuretic peptide (BNP) and N-terminal pro-B-type natriuretic peptide (NT-proBNP) are the cleavage products of pro B-type natriuretic peptide. As NT-proBNP relies on renal excretion, their circulating levels are higher than BNP, which is also cleared by peripheral receptors and enzymatic breakdown. The role of natriuretic peptide in monitoring myocardial recovery is solely examined for NT-proBNP.
The PROTECT (ProBNP Outpatient Tailored Chronic Heart Failure) study was done to evaluate the use of NT-proBNP as a treatment goal in chronic heart failure management. A sub-study of 116 patients with echocardiographic follow-up evaluated the interplay between NT-proBNP and LV remodeling. They demonstrated that the change in NT-proBNP over 10 months was positively correlated with the change in LVEDV and LVESV and negatively correlated with the change in LVEF. In addition, there was a reduction in mitral regurgitation and improvement in diastolic function and right ventricular function.35 This was confirmed by the GUIDE-IT (Guiding Evidence Based Therapy Using Biomarker Intensified Treatment in Heart Failure) echo sub-study, highlighting that a reduction in NT-proBNP levels over time might function as a predictor for improvement in LV function and volumes.1 However, no correlation was found between the initial NT-proBNP value and change in myocardial recovery.7 In the Prospective Study of Biomarkers, Symptom Improvement, and Ventricular Remodeling During Sacubitril/Valsartan Therapy for Heart Failure (PROVE-HF) study, a reduction in NT-proBNP values was seen 2 weeks after therapy initiation while echocardiographic improvements were observable after 6 months, supporting the hypothesis that serial NT-proBNP follow-up can function as a predictor and monitoring tool for myocardial recovery. These echocardiographic improvements correlated with a better clinical outcome with regard to heart failure hospitalizations and mortality.36 In contrast to NT-proBNP, the role of BNP is less clear, especially with the current treatment modalities and use of sacubitril/valsartan since BNP is a substrate of neprilysin.
Troponin
Cardiac troponins are a marker of myocyte injury and necrosis and typically used in the setting of coronary artery disease. Cardiac troponin can be measured through a conventional or highly sensitive assay. If measured through the latter assay, cardiac troponins are almost always elevated in patients with heart failure.34
Elevated levels are associated with adverse remodeling and poor prognosis.33 A low initial value (< 11 ng/L) of highly sensitive troponin T is correlated with a higher incidence of myocardial recovery compared to patients with an initial higher level of troponin T.7,37
The evidence with regard to cardiac troponin change as a predictor for cardiac remodeling is more ambiguous. A small study in 60 patients with dilated cardiomyopathy demonstrated that a decrease in troponin T during follow-up was associated with improved echocardiographic parameters and reduced cardiovascular events.38 However, the change in troponin T did not correlate with the change in LV dimension or function. A subanalysis of the PROTECT study found the same observation for change in cardiac troponin I.39 In patients after a myocardial infarction, higher peak levels of cardiac troponins were associated with a larger infarct size, larger reduction in LVEF, and worse clinical outcomes.40,41
Soluble Suppression of Tumorigenicity 2
Suppression of tumorigenicity 2 (ST2) is a transmembrane receptor in cardiomyocytes, fibroblasts, and endothelial cells and a member of the interleukin-1 receptor family. It consists of two isoforms, a transmembrane (ST2L) and soluble (sST2) isoform. The latter is expressed in cases of pressure overload, taking part in the generation of fibrosis. The binding of interleukin-33 (IL-33) with ST2L blocks the hypertrophic pathway in the cardiomyocyte, thereby taking part in the cardioprotective signaling system of the heart (Figure 3). However, in response to pressure overload, sST2 is produced and functions as a decoy receptor by binding IL-33. The cardioprotective mechanisms preventing fibrosis are partially mitigated, rendering sST2 a marker of fibrosis and hypertrophy.42,43,44,45 Therefore, higher levels are associated with a reduction in ejection fraction, worse right ventricular function, and increased LVEDD and LVEDV.46,47
Figure 3.

sST2 and galectin-3 as markers of fibrosis. As a response to cardiac damage, macrophage activation results in the production of galectin-3, which leads to fibroblast activation and the release of IL-33. Normally, IL-33 binds to ST2L, by which it initiates an antihypertrophic an antifibrotic pathway. sST2 works as a decoy receptor, preventing the binding of IL-33 to ST2L. IL-33: interleukin-33; sST2: soluble suppression of tumorigenicity 2; ST2L: suppression of tumorigenicity 2 ligand
In addition, patients with higher levels had a worse prognosis, with an even higher predictive power when combined with NT-proBNP levels.48,49 A multivariate analysis on 304 outpatients with heart failure with reduced ejection fraction pointed out that sST2 was the only independent marker correlating with reverse remodeling. Over a follow-up of 1 year, more time spend with sST2 levels below 35 ng/mL was associated with a higher likelihood of reverse remodeling.37 Based on this observation, the ST2-R2 score has been proposed, in which soluble ST2 in combination with other clinical parameters predict reverse remodeling. An sST2 level above 48 ng/mL is associated with a reduced likelihood of reverse remodeling due to the excess of myocardial fibrosis.50
Galectin-3
Galectin-3 is secreted by cardiac macrophages during phagocytosis. It stimulates fibroblast proliferation and alters collagen deposition, by which it takes part in the fibrosis process (Figure 3). Increased levels are associated with adverse outcomes in patients with heart failure. In contrast to sST2, galectin-3 does not provide additional predictive information when combined with NT-proBNP values.37 Data for galectin-3 as a predictor for reverse remodeling are conflicting. A substudy of the PROTECT trial demonstrated that lower levels during serial follow-up are associated with an increase in LVEF during follow-up.51 Another study demonstrated that a low baseline value was associated with a decrease in LVEDV.52 Serial measurements could also be useful as a predictor since the time spent with galectine-3 concentration below 20 ng/mL was an independent predictor for fewer cardiovascular events and better improvement in LVEF.53 However, the relationship between galectin-3 and echocardiographic indices could not be confirmed in other trials.54,55 The marker is not included in the ST2-R2 score as it has no independent predictive value.50
Big Endothelin-1
Big endothelin-1 (ET-1) is a precursor of ET-1, which activates cardiomyocytes and fibroblasts by binding on the ET receptor. This leads to several processes, such as vasoconstriction, hypertrophy, and fibrosis resulting in adverse remodeling. Big ET-1 is released in response to neurohormonal activation and cytokine release. Lower levels of big ET-1 are associated with less-severe neurohormonal stimulation and lower pressure overload and therefore have a higher likelihood of reverse remodeling and lower incidence of adverse events.56
Extracellular Matrix Proteins
Alterations in extracellular matrix composition and fibroblast proliferation and activation and a dysregulated collagen homeostasis lead to reduced myocardial compliance, ventricular remodeling, and fibrosis.57 Some of the key enzymes/inhibitors of this are now recognized, such as matrix metalloproteinase 9, tissue inhibitors of metalloproteinase 1, bone morphogenetic protein 1, and procollagen. However, further investigation is necessary to elucidate their use in monitoring and predicting cardiac remodeling.34
MicroRNAs
MicroRNAs are small, non-coding RNAs taking part in the regulation of gene expression by binding to complementary messenger RNA. In heart failure, different microRNAs are expressed, and some are thought to be cardiac specific that are released in response to myocardial injury or wall stress.58 Specific microRNAs have been identified that could predict reverse remodeling and myocardial recovery with LVAD, CRT, or neurohormonal therapy.59 While the field of genomic analysis remains promising, it is relatively novel and needs further investigations to evaluate its exact role as prognostic biomarkers. These biomarkers might have the potential to identify patients early in their disease course and will boost patient-tailored therapy.
Overall, NT-proBNP and troponins are the most commonly known and used biomarkers to date (Table 3). Serial follow-up of NT-proBNP can be used to monitor patients, since a decrease is associated with improved LVEF over time. In addition, patients with an initial low troponin level (< 11 ng/L) have higher chances for reverse remodeling. Fibrosis is associated with irreversible damage. Markers of fibroblast activation are sST2 and galectin-3, of which a low sST2 level (< 35 ng/mL) is an independent predictor of reverse remodeling. MicroRNAs are promising as they might improve the ability to identify patients benefiting from a specific therapy, although more research is needed.
Table 3.
Highlights of research articles supporting different biomarkers for the prediction of reverse remodeling. CHF: chronic heart failure; HFrEF: heart failure with reduced ejection fraction, LV: left ventricle; LVEF: left ventricular ejection fraction, LVEDVi: left ventricular end-diastolic volume index, LVESVi: left ventricular end-systolic volume index; RR: reverse remodeling
|
| |||
|---|---|---|---|
| AUTHOR, YEAR | STUDY POPULATION (N) | END POINT | OUTCOME/FINDING |
|
| |||
| NT-proBNP | |||
|
| |||
| Weiner et al. 201335 | HFrEF (LVEF < 40%) (N = 116) |
Improvement in LVEF, LVEDVi, LVESVi | NT-proBNP measurement associated with RR |
|
| |||
| Gaggin et al. 201437 | HFrEF (N = 151) |
Clinical outcome | Baseline NT-proBNP predicts clinical outcome |
|
| |||
| Cho et al. 201860 | DCM and AHF | LVEF ≥ 50% | Decrease in NT-proBNP between initial presentation and discharge (> 1633.5 pg/mL), predictor for RR at 6 months |
|
| |||
| Daubert et al. 201961 | HFrEF (LVEF ≤ 40%) (N = 268) |
Improvements in LVESVi, LVEDVi, EF, | NT-proBNP < 1,000 pg/mL associated with RR |
|
| |||
| Januzzi et al. 201962 |
HFrEF (LVEF ≤ 40%) with elevated natriuretic peptides (N = 654) |
Improvement in LVEF, LVEDVi, LVESVi | A decrease in NT-proBNP over time is associated with RR |
|
| |||
| Troponin | |||
|
| |||
| Sato et al. 200138 | DCM (N = 60) |
Improvement in LVEF, LVDd | TnT levels during follow-up < 0.02 ng/mL are associated with RR |
|
| |||
| Chia et al. 200840 | STEMI (N = 378) |
Functional and clinical outcome | TnI at 72 hours > 55 ng/mL was associated with a large infarct size and low LVEF |
|
| |||
| Miller et al. 200963 | CHF (N = 172) |
Clinical outcome | Elevated cTnT (> 0.01 ng/mL) are associated with increased risk of events |
|
| |||
| O’Connor et al. 201139 | AHF (N = 288) |
Clinical outcome | Positive cTnT (> 0.03 ng/mL) are associated with a worse outcome |
|
| |||
| Felker et al. 201264 | ADHF (N = 808) |
Clinical outcome | cTnI above 99% percentile predicts in-hospital outcome |
|
| |||
| Gaggin et al. 201437 | HFrEF (N = 151) |
Clinical outcome | Baseline Hs-TnT predicts outcome |
|
| |||
| Felker et al. 201565 | AHF (N = 1074) |
Clinical outcome | Hs-cTnT are associated with worse outcome |
|
| |||
| Brooks et al. 201641 | LVEF ≤ 35% post-myocardial infarction (N = 231) |
Improvement in LVEF | Peak troponin levels are associated with RR |
|
| |||
| Soluble ST2 | |||
|
| |||
| Weinberg et al. 200345 | LVEF ≤ 30% (N = 161) |
Clinical outcome | Change in sST2 was associated with clinical outcome |
|
| |||
| Daniels et al. 201047 | Heart failure history, symptoms or risk factors (N = 588) | 1 year mortality | sST2 (> 28.25 ng/mL) independent predictor for 1 year mortality |
|
| |||
| Bayes-Genis et al. 201248 | CHF (N = 891) |
Clinical outcome | sST2 associated with mortality |
|
| |||
| Gaggin et al. 201437 | HFrEF (N = 151) |
Improvement in LVEF, LVESVi, LVEDVi | Serial sST2 predicts RR |
|
| |||
| Ky et al. 201149 | HFrEF (N = 1141) |
Clinical outcome | sST2 (> 36.3 ng/mL) associated with adverse outcome |
|
| |||
| Lupon et al. 201566 | LVEF < 40% (N = 304) |
LVEF increase with > 15% LVEF increase with 10% + reduction of LVESDi > 20% or LVESVi ≥ 40% |
sST2 levels < 48 ng/mL was associated with RR |
|
| |||
| Galectin-3 | |||
|
| |||
| Tang et al. 201154 | HFrEF (LVEF ≤ 35%) (N = 178) | Functional and clinical outcome | No relation between baseline levels galectin-3 and echocardiographic indices; high levels associated with poor clinical outcome |
|
| |||
| Motiwala et al. 201351 | HFrEF (N = 151) |
Functional and clinical outcome | Serial follow-up with galectin-3 < 20 ng/L: associated with lower event rate and increase in LVEF |
|
| |||
| Lok et al. 201352 | HFrEF (N = 240) |
Functional and clinical outcome | Galectin-3 levels are associated with change in LVEDV and predictor of mortality |
|
| |||
| Weir et al. 201355 | HFrEF (N = 100) |
LV remodeling | No correlation between galectin-3 and RR |
|
| |||
Conclusion
Myocardial recovery and the broader concept of reverse remodeling describe the regression of maladaptive functional and structural cardiac changes in the presence of heart failure. Early identification through biomarkers and imaging modalities could help to identify patients with a higher likelihood for reverse remodeling, improving personalized medicine and consequently better prognosis.
Key Points
Myocardial recovery, and the broader concept of reverse remodeling, describe the regression of cardiac structural and function changes due to neurohormonal activation, pressure, and volume overload in patients with heart failure.
As relative improvements in left ventricular ejection fraction (LVEF) and left ventricular dimensions are more easily obtainable in a more dysfunctional heart, the assessment of global longitudinal strain (GLS) is more reliable to predict structural changes over time. By incorporating the afterload, myocardial work quantification was even better in predicting fibrosis on cardiac magnetic resonance imaging than GLS or LVEF alone. However, more research with regard to myocardial work quantification is necessary.
A decrease in NT-proBNP and troponin levels over time are associated with structural and function improvements with a better clinical outcome.
Serum soluble ST3, a decoy receptor for IL-33, is a marker of fibrosis and hypertrophy. In a multivariate analysis, this was the sole predictor for myocardial fibrosis, which led to the development of the ST2-R2 score.
Cardio-specific microRNAs are identified that could help phenotyping heart failure patients with a higher likelihood for reverse remodeling. However, more research is necessary.
Competing Interests
The authors have no competing interests to declare.
References
- 1.Yan CL, Grazette L. A review of biomarker and imaging monitoring to predict heart failure recovery. Front Cardiovasc Med. 2023. Apr 6:10:1150336. doi: 10.3389/fcvm.2023.1150336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aimo A, Gaggin HK, Barison A, Emdin M, Januzzi JL Jr. Imaging, Biomarker, and Clinical Predictors of Cardiac Remodeling in Heart Failure With Reduced Ejection Fraction. JACC Heart Fail. 2019. Sep;7(9):782-794. doi: 10.1016/j.jchf.2019.06.004 [DOI] [PubMed] [Google Scholar]
- 3.Lupón J, Gavidia-Bovadilla G, Ferrer E, et al. Dynamic Trajectories of Left Ventricular Ejection Fraction in Heart Failure. J Am Coll Cardiol. 2018. Aug 7;72(6):591-601. doi: 10.1016/j.jacc.2018.05.042 [DOI] [PubMed] [Google Scholar]
- 4.Kim GH, Uriel N, Burkhoff D. Reverse remodelling and myocardial recovery in heart failure. Nat Rev Cardiol. 2018. Feb;15(2):83-96. doi: 10.1038/nrcardio.2017.139 [DOI] [PubMed] [Google Scholar]
- 5.Chrysakis N, Xanthopoulos A, Magouliotis D, et al. Myocardial Recovery. Diagnostics (Basel). 2023. Apr 21;13(8):1504. doi: 10.3390/diagnostics13081504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hnat T, Veselka J, Honek J. Left ventricular reverse remodelling and its predictors in non-ischaemic cardiomyopathy. ESC Hear Fail. 2022. Aug;9(4):2070-2083. doi: 10.1002/ehf2.13939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chudý M, Goncalvesová E. Prediction of Left Ventricular Reverse Remodelling: A Mini Review on Clinical Aspects. Cardiology. 2022;147(5–6):521-528. doi: 10.1159/000526986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thavendiranathan P, Negishi T, Somerset E, et al. Strain-Guided Management of Potentially Cardiotoxic Cancer Therapy. J Am Coll Cardiol. 2021. Feb 2;77(4):392-401. doi: 10.1016/j.jacc.2020.11.020 [DOI] [PubMed] [Google Scholar]
- 9.Jung IH, Park JH, Lee JA, et al. Left Ventricular Global Longitudinal Strain as a Predictor for Left Ventricular Reverse Remodeling in Dilated Cardiomyopathy. J Cardiovasc Imaging. 2020. Apr;28(2):137-149. doi: 10.4250/jcvi.2019.0111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Adamo L, Perry A, Novak E, Makan M, Lindman B, Mann DL. Abnormal Global Longitudinal Strain Predicts Future Deterioration of Left Ventricular Function in Heart Failure Patients With a Recovered Left Ventricular Ejection Fraction. Circ Heart Fail. 2017. Jun;10(6):e003788. doi: 10.1161/CIRCHEARTFAILURE.116.003788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hoit BD. Left atrial size and function: role in prognosis. J Am Coll Cardiol. 2014. Feb 18;63(6):493-505. doi: 10.1016/j.jacc.2013.10.055 [DOI] [PubMed] [Google Scholar]
- 12.Shiba M, Kato T, Morimoto T, et al. Left atrial reverse remodeling improves risk stratification in patients with heart failure with recovered ejection fraction. Sci Rep. 2022. Mar 16;12(1):4473. doi: 10.1038/s41598-022-08630-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Torii Y, Kusunose K, Hirata Y, et al. Left Atrial Strain Associated with Functional Recovery in Patients Receiving Optimal Treatment for Heart Failure. J Am Soc Echocardiogr. 2021. Sep;34(9):966-975.e2. doi: 10.1016/j.echo.2021.03.016 [DOI] [PubMed] [Google Scholar]
- 14.Yingchoncharoen T, Agarwal S, Popović ZB, Marwick TH. Normal ranges of left ventricular strain: a meta-analysis. J Am Soc Echocardiogr. 2013. Feb;26(2):185-91. doi: 10.1016/j.echo.2012.10.008 [DOI] [PubMed] [Google Scholar]
- 15.Ilardi F, D’Andrea A, D’Ascenzi F, et al. Myocardial Work by Echocardiography: Principles and Applications in Clinical Practice. J Clin Med. 2021. Sep 29;10(19):4521. doi: 10.3390/jcm10194521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moya A, Buytaert D, Penicka M, Bartunek J, Vanderheyden M. State-of-the-Art: Noninvasive Assessment of Left Ventricular Function Through Myocardial Work. J Am Soc Echocardiogr. 2023. Oct;36(10):1027-1042. doi: 10.1016/j.echo.2023.07.002 [DOI] [PubMed] [Google Scholar]
- 17.Cui C, Li Y, Liu Y, et al. Association Between Echocardiographic Non-invasive Myocardial Work Indices and Myocardial Fibrosis in Patients With Dilated Cardiomyopathy. Front Cardiovasc Med. 2021. Aug 16:8:704251. doi: 10.3389/fcvm.2021.704251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marzlin N, Hays AG, Peters M, et al. Myocardial Work in Echocardiography. Circ Cardiovasc Imaging. 2023. Feb;16(2):e014419. doi: 10.1161/CIRCIMAGING.122.014419 [DOI] [PubMed] [Google Scholar]
- 19.Chung ES, Leon AR, Tavazzi L, et al. Results of the Predictors of Response to CRT (PROSPECT) trial. Circulation. 2008. May 20;117(20):2608-16. doi: 10.1161/CIRCULATIONAHA.107.743120 [DOI] [PubMed] [Google Scholar]
- 20.Marsan NA, Breithardt OA, Delgado V, Bertini M, Tops LF. Predicting response to CRT. The value of two- and three-dimensional echocardiography. Europace. 2008. Nov:10 Suppl 3:iii73-9. doi: 10.1093/europace/eun219 [DOI] [PubMed] [Google Scholar]
- 21.Barison A, Aimo A, Ortalda A, et al. Late gadolinium enhancement as a predictor of functional recovery, need for defibrillator implantation and prognosis in non-ischemic dilated cardiomyopathy. Int J Cardiol. 2018. Jan 1:250:195-200. doi: 10.1016/j.ijcard.2017.10.043 [DOI] [PubMed] [Google Scholar]
- 22.Becker MAJ, Cornel JH, van de Ven PM, van Rossum AC, Allaart CP, Germans T. The Prognostic Value of Late Gadolinium-Enhanced Cardiac Magnetic Resonance Imaging in Nonischemic Dilated Cardiomyopathy: A Review and Meta-Analysis. JACC Cardiovasc Imaging. 2018. Sep;11(9):1274-1284. doi: 10.1016/j.jcmg.2018.03.006 [DOI] [PubMed] [Google Scholar]
- 23.Kida K, Yoneyama K, Kobayashi Y, Takano M, Akashi YJ, Miyake F. Late gadolinium enhancement on cardiac magnetic resonance images predicts reverse remodeling in patients with nonischemic cardiomyopathy treated with carvedilol. Int J Cardiol. 2013. Sep;168(2):1588-9. doi: 10.1016/j.ijcard.2013.01.043 [DOI] [PubMed] [Google Scholar]
- 24.Ikeda Y, Inomata T, Fujita T, et al. Cardiac fibrosis detected by magnetic resonance imaging on predicting time course diversity of left ventricular reverse remodeling in patients with idiopathic dilated cardiomyopathy. Heart Vessels. 2016. Nov;31(11):1817-1825. doi: 10.1007/s00380-016-0805-2 [DOI] [PubMed] [Google Scholar]
- 25.Masci PG, Schuurman R, Andrea B, et al. Myocardial fibrosis as a key determinant of left ventricular remodeling in idiopathic dilated cardiomyopathy: a contrast-enhanced cardiovascular magnetic study. Circ Cardiovasc Imaging. 2013. Sep;6(5):790-9. doi: 10.1161/CIRCIMAGING.113.000438 [DOI] [PubMed] [Google Scholar]
- 26.Chimura M, Onishi T, Tsukishiro Y, et al. Longitudinal strain combined with delayed-enhancement magnetic resonance improves risk stratification in patients with dilated cardiomyopathy. Heart. 2017. May;103(9):679-686. doi: 10.1136/heartjnl-2016-309746 [DOI] [PubMed] [Google Scholar]
- 27.Ishii S, Inomata T, Fujita T, et al. Clinical significance of endomyocardial biopsy in conjunction with cardiac magnetic resonance imaging to predict left ventricular reverse remodeling in idiopathic dilated cardiomyopathy. Heart Vessels. 2016. Dec;31(12):1960-1968. doi: 10.1007/s00380-016-0815-0 [DOI] [PubMed] [Google Scholar]
- 28.Kubanek M, Sramko M, Maluskova J, et al. Novel predictors of left ventricular reverse remodeling in individuals with recent-onset dilated cardiomyopathy. J Am Coll Cardiol. 2013. Jan 8;61(1):54-63. doi: 10.1016/j.jacc.2012.07.072 [DOI] [PubMed] [Google Scholar]
- 29.Tayal U, Wage R, Newsome S, et al. Predictors of left ventricular remodelling in patients with dilated cardiomyopathy – a cardiovascular magnetic resonance study. Eur J Heart Fail. 2020. Jul;22(7):1160-1170. doi: 10.1002/ejhf.1734 [DOI] [PubMed] [Google Scholar]
- 30.Ikeda Y, Inomata T, Iida Y, et al. Time course of left ventricular reverse remodeling in response to pharmacotherapy: clinical implication for heart failure prognosis in patients with idiopathic dilated cardiomyopathy. Heart Vessels. 2016. Apr;31(4):545-54. doi: 10.1007/s00380-015-0648-2 [DOI] [PubMed] [Google Scholar]
- 31.Adamo L, Perry A, Novak E, Makan M, Lindman BR, Mann DL. Abnormal Global Longitudinal Strain Predicts Future Deterioration of Left Ventricular Function in Heart Failure Patients With a Recovered Left Ventricular Ejection Fraction. Circ Heart Fail. 2017. Jun;10(6):e003788. doi: 10.1161/CIRCHEARTFAILURE.116.003788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Swat SA, Cohen D, Shah SJ, et al. Baseline Longitudinal Strain Predicts Recovery of Left Ventricular Ejection Fraction in Hospitalized Patients With Nonischemic Cardiomyopathy. J Am Heart Assoc. 2018. Oct 16;7(20):e09841. doi: 10.1161/JAHA.118.009841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.González A, Richards AM, de Boer RA, et al. Cardiac remodelling – Part 1: From cells and tissues to circulating biomarkers. A review from the Study Group on Biomarkers of the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail. 2022. Jun;24(6):927-943. doi: 10.1002/ejhf.2493 [DOI] [PubMed] [Google Scholar]
- 34.Motiwala SR, Gaggin HK. Biomarkers to Predict Reverse Remodeling and Myocardial Recovery in Heart Failure. Curr Heart Fail Rep. 2016. Oct;13(5):207-218. doi: 10.1007/s11897-016-0303-y [DOI] [PubMed] [Google Scholar]
- 35.Weiner RB, Baggish AL, Chen-Tournoux A, et al. Improvement in structural and functional echocardiographic parameters during chronic heart failure therapy guided by natriuretic peptides: mechanistic insights from the ProBNP Outpatient Tailored Chronic Heart Failure (PROTECT) study. Eur J Heart Fail. 2013. Mar;15(3):342-51. doi: 10.1093/eurjhf/hfs180 [DOI] [PubMed] [Google Scholar]
- 36.Mohebi R, Liu Y, Piña IL, et al. Dose-Response to Sacubitril/Valsartan in Patients With Heart Failure and Reduced Ejection Fraction. J Am Coll Cardiol. 2022. Oct 18;80(16):1529-1541. doi: 10.1016/j.jacc.2022.08.737 [DOI] [PubMed] [Google Scholar]
- 37.Gaggin HK, Szymonifka J, Bhardwaj A, et al. Head-to-head comparison of serial soluble ST2, growth differentiation factor-15, and highly-sensitive troponin T measurements in patients with chronic heart failure. JACC Heart Fail. 2014. Feb;2(1):65-72. doi: 10.1016/j.jchf.2013.10.005 [DOI] [PubMed] [Google Scholar]
- 38.Sato Y, Yamada T, Taniguchi R, et al. Persistently increased serum concentrations of cardiac troponin t in patients with idiopathic dilated cardiomyopathy are predictive of adverse outcomes. Circulation. 2001. Jan 23;103(3):369-74. doi: 10.1161/01.cir.103.3.369 [DOI] [PubMed] [Google Scholar]
- 39.O’Connor CM, Fiuzat M, Lombardi C, et al. Impact of serial troponin release on outcomes in patients with acute heart failure: analysis from the PROTECT pilot study. 2011. Nov;4(6):724-32. doi: 10.1161/CIRCHEARTFAILURE.111.961581 [DOI] [PubMed] [Google Scholar]
- 40.Chia S, Senatore F, Raffel OC, Lee H, Wackers FJT, Jang IK. Utility of cardiac biomarkers in predicting infarct size, left ventricular function, and clinical outcome after primary percutaneous coronary intervention for ST-segment elevation myocardial infarction. JACC Cardiovasc Interv. 2008. Aug;1(4):415-23. doi: 10.1016/j.jcin.2008.04.010 [DOI] [PubMed] [Google Scholar]
- 41.Brooks GC, Lee BK, Rao R, et al. Predicting Persistent Left Ventricular Dysfunction Following Myocardial Infarction: The PREDICTS Study. J Am Coll Cardiol. 2016. Mar 15;67(10):1186-1196. doi: 10.1016/j.jacc.2015.12.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bartunek J, Delrue L, Van Durme F, et al. Nonmyocardial production of ST2 protein in human hypertrophy and failure is related to diastolic load. J Am Coll Cardiol. 2008. Dec 16;52(25):2166-74. doi: 10.1016/j.jacc.2008.09.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sanada S, Hakuno D, Higgins LJ, Schreiter ER, McKenzie ANJ, Lee RT. IL-33 and ST2 comprise a critical biomechanically induced and cardioprotective signaling system. J Clin Invest. 2007. Jun;117(6):1538-49. doi: 10.1172/JCI30634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Seki K, Sanada S, Kudinova AY, et al. Interleukin-33 prevents apoptosis and improves survival after experimental myocardial infarction through ST2 signaling. Circ Heart Fail. 2009. Nov;2(6):684-91. doi: 10.1161/CIRCHEARTFAILURE.109.873240 [DOI] [PubMed] [Google Scholar]
- 45.Weinberg EO, Shimpo M, Hurwitz S, Tominaga S, Rouleau JL, Lee RT. Identification of serum soluble ST2 receptor as a novel heart failure biomarker. Circulation. 2003. Feb 11;107(5):721-6. doi: 10.1161/01.cir.0000047274.66749.fe [DOI] [PubMed] [Google Scholar]
- 46.Shah RV, Chen-Tournoux AA, Picard MH, van Kimmenade RRJ, Januzzi JL. Serum levels of the interleukin-1 receptor family member ST2, cardiac structure and function, and long-term mortality in patients with acute dyspnea. Circ Heart Fail. 2009. Jul;2(4):311-9. doi: 10.1161/CIRCHEARTFAILURE.108.833707 [DOI] [PubMed] [Google Scholar]
- 47.Daniels LB, Clopton P, Iqbal N, Tran K, Maisel AS. Association of ST2 levels with cardiac structure and function and mortality in outpatients. Am Heart J. 2010. Oct;160(4):721-8. doi: 10.1016/j.ahj.2010.06.033 [DOI] [PubMed] [Google Scholar]
- 48.Bayes-Genis A, de Antonio M, Galán A, et al. Combined use of high-sensitivity ST2 and NTproBNP to improve the prediction of death in heart failure. Eur J Heart Fail. 2012. Jan;14(1):32-8. doi: 10.1093/eurjhf/hfr156 [DOI] [PubMed] [Google Scholar]
- 49.Ky B, French B, McCloskey K, et al. High-sensitivity ST2 for prediction of adverse outcomes in chronic heart failure. Circ Heart Fail. 2011. Mar;4(2):180-7. doi: 10.1161/CIRCHEARTFAILURE.110.958223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lupón J, Gaggin HK, De Antonio M, et al. Biomarker-assist score for reverse remodeling prediction in heart failure: The ST2-R2 score. Int J Cardiol. 2015. Apr 1:184:337-343. doi: 10.1016/j.ijcard.2015.02.019 [DOI] [PubMed] [Google Scholar]
- 51.Motiwala SR, Szymonifka J, Belcher A, et al. Serial measurement of galectin-3 in patients with chronic heart failure: results from the ProBNP Outpatient Tailored Chronic Heart Failure Therapy (PROTECT) study. Eur J Heart Fail. 2013. Oct;15(10):1157-63. doi: 10.1093/eurjhf/hft075 [DOI] [PubMed] [Google Scholar]
- 52.Lok DJ, Lok SI, Bruggink-André de la Porte PW, et al. Galectin-3 is an independent marker for ventricular remodeling and mortality in patients with chronic heart failure. Clin Res Cardiol. 2013. Feb;102(2):103-10. doi: 10.1007/s00392-012-0500-y [DOI] [PubMed] [Google Scholar]
- 53.Motiwala SR, Szymonifka J, Belcher A, et al. Measurement of novel biomarkers to predict chronic heart failure outcomes and left ventricular remodeling. J Cardiovasc Transl Res. 2014. Mar;7(2):250-61. doi: 10.1007/s12265-013-9522-8 [DOI] [PubMed] [Google Scholar]
- 54.Tang WHW, Shrestha K, Shao Z, Borowski AG, Troughton RW, Thomas JD, et al. Usefulness of plasma galectin-3 levels in systolic heart failure to predict renal insufficiency and survival. Am J Cardiol. 2011. Aug 1;108(3):385-90. doi: 10.1016/j.amjcard.2011.03.056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Weir RAP, Petrie CJ, Murphy CA, et al. Galectin-3 and cardiac function in survivors of acute myocardial infarction. Circ Heart Fail. 2013. May;6(3):492-8. doi: 10.1161/CIRCHEARTFAILURE.112.000146 [DOI] [PubMed] [Google Scholar]
- 56.Feng J, Liang L, Chen Y, et al. Big Endothelin-1 as a Predictor of Reverse Remodeling and Prognosis in Dilated Cardiomyopathy. J Clin Med. 2023. Feb 8;12(4):1363. doi: 10.3390/jcm12041363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Travers JG, Kamal FA, Robbins J, Yutzey KE, Blaxall BC. Cardiac Fibrosis: The Fibroblast Awakens. Circ Res. 2016. Mar 18;118(6):1021-40. doi: 10.1161/CIRCRESAHA.115.306565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Corsten MF, Dennert R, Jochems S, et al. Circulating MicroRNA-208b and MicroRNA-499 reflect myocardial damage in cardiovascular disease. Circ Cardiovasc Genet. 2010. Dec;3(6):499-506. doi: 10.1161/CIRCGENETICS.110.957415 [DOI] [PubMed] [Google Scholar]
- 59.Shah P, Bristow MR, Port JD. MicroRNAs in Heart Failure, Cardiac Transplantation, and Myocardial Recovery: Biomarkers with Therapeutic Potential. Curr Heart Fail Rep. 2017. Dec;14(6):454-464. doi: 10.1007/s11897-017-0362-8 [DOI] [PubMed] [Google Scholar]
- 60.Cho JY, Kim KH, Song JE, et al. Predictors of Left Ventricular Functional Recovery and Their Impact on Clinical Outcomes in Patients With Newly Diagnosed Dilated Cardiomyopathy and Heart Failure. Heart Lung Circ. 2018. Jan;27(1):41-49. doi: 10.1016/j.hlc.2017.02.013 [DOI] [PubMed] [Google Scholar]
- 61.Daubert MA, Adams K, Yow E, et al. NT-proBNP Goal Achievement Is Associated With Significant Reverse Remodeling and Improved Clinical Outcomes in HFrEF. JACC Heart Fail. 2019. Feb;7(2):158-168. doi: 10.1016/j.jchf.2018.10.014 [DOI] [PubMed] [Google Scholar]
- 62.Januzzi JL Jr, Prescott MF, Butler J, et al. Association of Change in N-Terminal Pro-B-Type Natriuretic Peptide Following Initiation of Sacubitril-Valsartan Treatment With Cardiac Structure and Function in Patients With Heart Failure With Reduced Ejection Fraction. JAMA. 2019. Sep 17;322(11):1085-1095. doi: 10.1001/jama.2019.12821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Miller WL, Hartman KA, Burritt MF, Grill DE, Jaffe AS. Profiles of serial changes in cardiac troponin T concentrations and outcome in ambulatory patients with chronic heart failure. J Am Coll Cardiol. 2009. Oct 27;54(18):1715-21. doi: 10.1016/j.jacc.2009.07.025 [DOI] [PubMed] [Google Scholar]
- 64.Felker GM, Hasselblad V, Tang WHW, et al. Troponin I in acute decompensated heart failure: insights from the ASCEND-HF study. Eur J Heart Fail. 2012. Nov;14(11):1257-64. doi: 10.1093/eurjhf/hfs110 [DOI] [PubMed] [Google Scholar]
- 65.Felker GM, Mentz RJ, Teerlink JR, et al. Serial high sensitivity cardiac troponin T measurement in acute heart failure: insights from the RELAX-AHF study. Eur J Heart Fail. 2015. Dec;17(12):1262-70. doi: 10.1002/ejhf.341 [DOI] [PubMed] [Google Scholar]
- 66.Lupón J, Gaggin HK, de Antonio M, et al. Biomarker-assist score for reverse remodeling prediction in heart failure: The ST2-R2 score. Int J Cardiol. 2015. Apr 1:184:337-343. doi: 10.1016/j.ijcard.2015.02.019 [DOI] [PubMed] [Google Scholar]


