Abstract
α-Synuclein (αSyn) aggregates, detected in the biofluids of patients with Parkinson’s disease (PD), have the ability to catalyze their own aggregation, leading to an increase in the number and size of aggregates. This self-templated amplification is used by newly developed assays to diagnose Parkinson’s disease and turns the presence of αSyn aggregates into a biomarker of the disease. It has become evident that αSyn can form fibrils with slightly different structures, called “strains” or polymorphs, but little is known about their differential reactivity in diagnostic assays. Here, we compared the properties of two well-described αSyn polymorphs. Using single-molecule techniques, we observed that one of the polymorphs had an increased tendency to undergo secondary nucleation and we showed that this could explain the differences in reactivity observed in in vitro seed amplification assay and cellular assays. Simulations and high-resolution microscopy suggest that a 100-fold difference in the apparent rate of growth can be generated by a surprisingly low number of secondary nucleation “points” (1 every 2000 monomers added by elongation). When both strains are present in the same seeded reaction, secondary nucleation displaces proportions dramatically and causes a single strain to dominate the reaction as the major end product.
Keywords: α-synuclein, Parkinson’s disease, single-molecule detection, strains, preformed fibrils, RT-QuIC, PMCA, seed amplification assay
Introduction
α-Synuclein (αSyn) aggregation is a hallmark of Parkinson’s disease (PD).1 Intracellular accumulation of aggregated αSyn in neurons is the main histopathological marker of PD and other synucleinopathies such as multiple system atrophy (MSA), and dementia with Lewy bodies (DLB). αSyn aggregates propagate from cell to cell, inducing protein aggregation in neighboring neurons in a prion-like manner.2
αSyn aggregation has been well characterized in vitro.(3) αSyn monomers are mainly disordered in solution and aggregation happens de novo through misfolding. This leads to the formation of oligomers that will ultimately convert to cross-β-sheet structures that form the amyloid core of αSyn fibrils. These fibrils have the unique ability to propagate by recruiting monomers and incorporating them into the amyloid structure. As shown in Figure 1A, the recruitment of monomers can occur at the end of the fibrils, causing linear growth of the fibrils in a mechanism called elongation. But the recruitment of monomers can also occur along the surface of the fibrils and cause the formation of new fibrils growing as side branches; a mechanism termed secondary nucleation.4
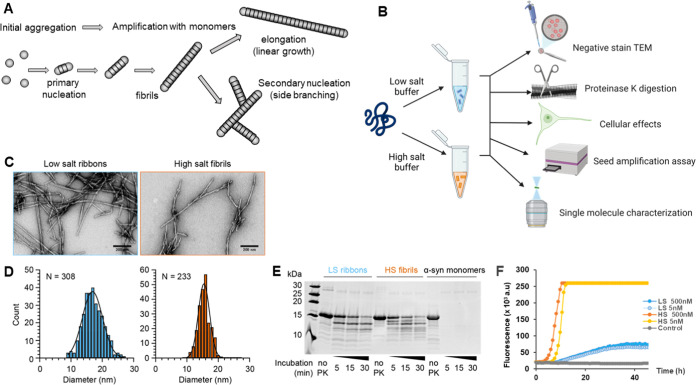
Figure 1.
Characterization of in vitro recombinant αSyn assemblies. (A) Schematic of αSyn aggregation and the different mechanisms at play: The initial formation of aggregates by primary nucleation is followed by the growth of the fibrils by two mechanisms: pure elongation (linear growth) or secondary nucleation (creation of a new fibril on the surface of the first fibril). (B) Schematic of the generation and characterization of recombinant αSyn polymorphs from monomers aggregated in low-salt (sky blue) and high-salt buffer (HSB) (vermilion), using different assays. (C) Transmission electron micrographs showing assemblies of recombinant αSyn under low-salt and high-salt buffer conditions, producing low-salt ribbons and high-salt fibrils, respectively. Scale bar 200 nm. (D) Histogram plot of the diameter of negatively stained low-salt ribbons (17.1 ± 3.3 nm, mean ± standard deviation (SD), N = 308) and high-salt fibrils (15.5 ± 1.9 nm, mean ± standard deviation, N = 233). (E) Proteinase K digestion profile of ribbons, fibrils, and αSyn monomers. Substrates were digested by the addition of proteinase K (1.5 μg/mL), quenched at 5, 15, and 30 min with loading buffer, heated at 90 °C prior to resolving using reducing SDS-PAGE. (F) Typical data obtained from a real-time quaking-induced conversion (RT-QuIC) assay on ribbons (LS) and fibrils (HS) at two different concentrations of seeds. Amplification was carried out in phosphate buffer saline (PBS), in the presence of 7 μM wild-type (WT) αSyn monomer, 10 μM thioflavin (ThT), and 6 glass beads. Fluorescence was recorded every 45 min. Control is an unseeded reaction.
This ability to convert a monomer into amyloids in solution is exploited by the seed amplification assays (SAAs).5 SAAs were initially developed to detect misfolded cellular prion proteins (PrPC) in prion diseases and have been extended to identify the presence of other amyloids.6 αSyn SAA is becoming a crucial diagnostic tool in PD research. A series of studies have demonstrated the sensitivity and specificity of αSyn SAAs to detect αSyn aggregates in the cerebrospinal fluid of patients and to accurately identify PD.5,7,8 Positive responses to αSyn SAA correlated with an increased probability of developing a synucleinopathy disease, both for patients with REM sleep behavior disorder (RBD)9,10 or mild cognitive impairment11 later developing PD and DLB, respectively. αSyn SAA was also recently applied to the study of a large longitudinal cohort of >1000 patients and found to be around 87.7% sensitive and >95% specific for PD.7 Importantly, however, this study also revealed molecular heterogeneity between PD patients, with patients bearing a mutation in the PD risk gene leucine-rich repeat kinase 2 (LRRK2) showing less of an αSyn response than patients with sporadic PD. Taken together, these studies have established that αSyn aggregation is a diagnostic biomarker for PD and may allow for the earlier diagnosis of PD and other related synucleinopathies.7,12,13
Another important development in the field of PD and synucleinopathies is the emerging hypothesis that distinct αSyn strains characterize and perhaps dictate pathology.3,14,15 The concept of strains was first established in prion research16 and refers to the fact that different prion polymorphs propagate specific disease phenotypes when injected into animal models. The concept has subsequently been extended to other amyloid-forming proteins, such as amyloid β,17 Tau,18 and TDP-43.19 In the case of αSyn, structural differences were observed in aggregates from the brain of PD/DLB20 patients compared to MSA patients21,22 and there is growing evidence that αSyn strains induce different disease phenotypes.3,15
αSyn strains (or polymorphs) can be generated in vitro by modifying the conditions in which αSyn aggregation occurs. Bousset and co-workers characterized two αSyn polymorphs, known as ribbons and fibrils,23 by limited proteolysis and electron microscopy. These polymorphs were then shown to induce distinct toxicities in cells.23 Intracerebral inoculation in rats showed that fibrils induced more neuronal death while ribbons induced more Lewy-body-like inclusions.24 In wild-type mice, increased αSyn phosphorylation and ubiquitination were observed upon injection of ribbons into the striatum or olfactory bulb, compared to fibrils.25 Increased αSyn deposition also correlated with ribbon injection in a mouse MSA model, while fibrils resulted in increased cellular death.26 Finally, in nonhuman primates, intraputaminal injection of ribbons and fibrils induced strain-specific patterns of αSyn aggregation and phosphorylation.27
Here, we investigate the amplification propensity of these two polymorphs using single-molecule spectroscopy28,29 and different structural, biochemical, and functional assays, as summarized in Figure 1B. We unexpectedly found that the mechanism of amplification differs dramatically between the two polymorphs, with the ribbons undergoing mainly elongation while the fibrils supported secondary nucleation. We show that this difference in the amplification mechanism explains the differences in response in αSyn SAA. As SAA is becoming essential in PD diagnostics, the strain-dependent response and mechanisms of amplification should be taken into consideration in future studies.
Results
Generation and Characterization of Ribbon and Fibril Polymorphs
Ribbons and fibrils were generated as previously described.3,23 Ribbons are obtained in low ionic conditions (5 mM Tris, pH 7.5, 0.02% w/v NaN3) while aggregation in high ionic conditions (50 mM Tris, pH 7.5, 150 mM KCl, 0.02% w/v NaN3) produced fibrils.23 Well-established assays were performed to characterize these different polymorphs (Figure 1B).
Transmission electron microscopy revealed that monomeric αSyn effectively assembled to produce long filamentous structures after 7 days and 2 days of incubation for ribbons and fibrils, respectively (Figure 1C). Ribbons displayed a flattened morphology with occasional twists and had a measured diameter of 17.1 ± 3.3 nm (Figure 1C,D). Fibrils produce thinner cylindrical filaments with twists also observed, but they had a more consistent diameter of 15.5 ± 1.9 nm (Figure 1C,D). These measurements were consistent with previously published data.23
Proteinase K digestion was then performed to assess the profile of the ribbons and fibrils obtained. A time course of proteinase K digestion was performed for different batches of αSyn polymorphs. The β-sheet folded core of assembled αSyn filaments was highly resistant to proteinase while, as expected, monomers were completely digested after 5 min of exposure to the enzyme. Digestion of the ribbons and fibrils revealed a distinct banding pattern, with more bands observed on digested fibrils compared to ribbons (Figure 1E). This demonstrates that these two assemblies were structurally different from each other. Notably, the banding patterns for our reference ribbons and fibrils were similar to those reported in Van der Perren et al.3 indicating that the ribbons and fibrils were successfully recreated in the current study.
Ribbons and Fibrils Have Different Profiles in SAA
We then tested the amplification potential of these two polymorphs in αSyn SAA. Seeded amplification was conducted in a real-time quaking-induced conversion (RT-QuIC) assay format, with intermittent shaking in a 96-well plate. Aggregation was monitored by following thioflavin T (ThT) fluorescence over time, as ThT recognizes β-sheet structure aggregates.30 Ribbons or fibrils, at different concentrations, were seeded in monomeric WT αSyn in PBS and shaken every 15 min at 300 rpm for 60 s. The kinetics were much faster for fibrils compared to ribbons (Figure 1F). We also observed that seeding with fibrils consistently led to a higher fluorescence plateau than ribbons at all concentrations tested. Therefore, fibrils are more efficient at templating aggregation, with ribbons and fibrils producing different profiles of amplification in RT-QuIC.
Ribbons and Fibrils Induced Distinct Inclusions in SH-SY5Y Cells
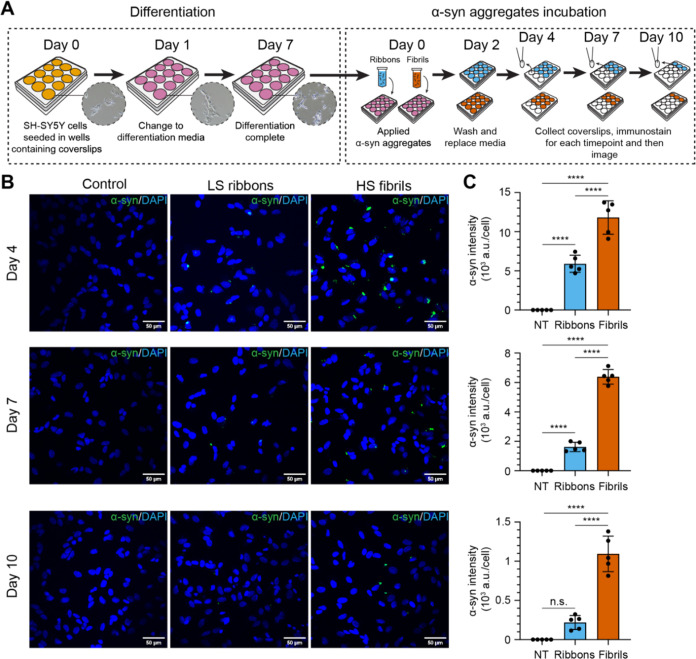
To evaluate the impact of the two different polymorphs on cells, differentiated SH-SY5Y neuroblastoma cells were treated with 5 μg/mL (0.35 μM) ribbons or fibrils, or PBS as a control. The formation of intracellular αSyn inclusions was then measured over time at days 4, 7, and 10 post-inoculation (Figure 2A). Both ribbon- and fibril-treated cells exhibited visible inclusions and/or puncta of αSyn, while no such structures were observed in the control group (Figure 2B). Our previous results had shown that the number of αSyn inclusions reached the local maxima at days 4–6 post-fibril treatment before gradually declining, likely due to autophagic degradation.31 Consistent with this, inclusions induced by both ribbons and fibrils reached their maximum on day 4 and steadily decreased subsequently on days 7 and 10 (Figure 2C). However, we observed significantly higher numbers of cytoplasmic inclusions induced by fibrils compared to ribbons at all three time points. Specifically on day 4, the intensity of αSyn inclusions induced by fibrils was approximately 2-fold greater than those induced by ribbons, while by days 7 and 10, this difference had increased to more than four times. Meanwhile, cytotoxicity was not observed when we compared the extracellular release of lactate dehydrogenase between ribbon, fibril-treated cells, and the control group (Supporting Figure S1). Note that the lack of cytotoxicity and cell death is in contrast to what Bousset et al.23 observed in their cell model.
Figure 2.
Characterization of recombinant αSyn assembly incubation on SH-SY5Y cells. (A) SH-SY5Y neuroblastoma cell culture scheme. Cells were seeded on coverslips at the bottom of a 12-well plate and imaged using brightfield microscopy (inset images). SH-SY5Y cells were differentiated for 7 days before incubation with αSyn ribbons or fibrils in differentiation media. Effects of αSyn assemblies on cells were assessed by immunostaining and confocal microscopy at 4, 7, and 10 days postaddition of the assemblies. (B) Differentiated SH-SY5Y cells were treated with or without 5 μg/mL different αSyn polymorphs and subsequently fixed for immunofluorescence staining of total αSyn (green punctae) at indicated time points. DAPI staining is represented in blue. Scale bar is 50 μm. (C) Bar graphs quantifying the average intensity of immunostained αSyn per cell. NT, no treatment, ribbons (sky blue) and fibrils (vermilion), respectively. Each symbol represents a field of view (FOV). Total αSyn intensity was normalized by the number of cells based on the DAPI signal. Error bars represent mean ± standard deviation. Statistics used ordinary one-way ANOVA Tukey’s comparison test with a single pooled variance, p ≤ 0.0001 (****), nonsignificant (n.s.).
Single-Molecule Seed Amplification Assay (smSAA) Revealed Differential Growth Mechanism between Ribbons and Fibrils
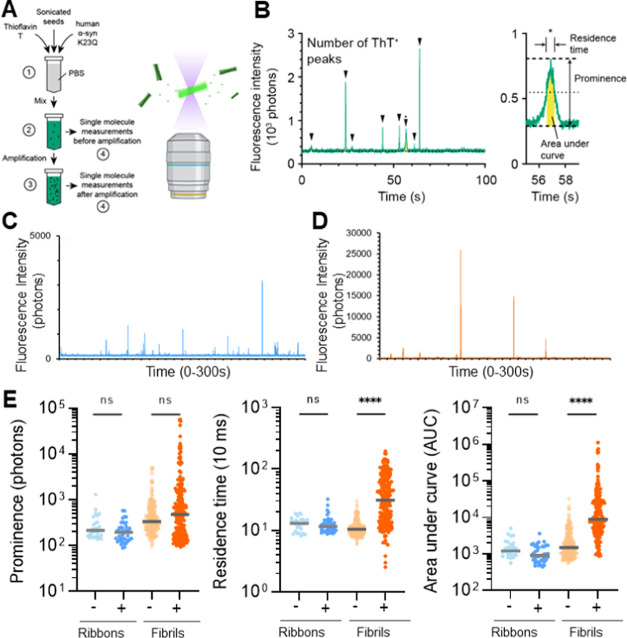
Cell model data suggested that the high-salt fibrils were more seeding competent with respect to their ability to generate bright aggregates in the cytosol and RT-QuIC data showed that fibrils amplify faster and are more abundant than ribbons. We then performed single-molecule seed amplification assay (smSAA) measurements to examine the molecular properties of the amplified products, as previously described.28,29 Sonicated seeds, either ribbons or fibrils, were diluted to a single aggregate concentration in a solution of phosphate buffer saline (PBS) containing 10 μM ThT and 20 μM of monomeric human αSyn K23Q. We then characterized the aggregates before and after amplification (Figure 3A). As in RT-QuIC, the excess of monomeric αSyn promotes the growth of the fibrils in a prion-like manner. The main difference with RT-QuIC is that our protocol uses senescent conditions, as we want to avoid fragmentation to track the growth of the aggregates. In this work, we use a very short amplification time (5 h) as we have previously demonstrated that the technique remains sufficient to observe microscopic changes for individual aggregates.28,29 To observe individual aggregates, we used our three-dimensional (3D)-printed AttoBright instrument28,29,32 as it was specifically designed to quantify and characterize ThT-positive events. The obtained fluorescence traces are the real-time acquisition of the fluctuations of fluorescence in the small detection volume. As shown in Figure 3B, when a single aggregate diffuses through the detection volume, a burst of fluorescence above the background is observed in the trace. These peaks are counted automatically using our algorithm and “fingerprinted”. For each peak, we determine its respective maximal intensity (prominence) and residence time (duration of the burst, which is linked to its physical size). The scatter plots of prominence against residence time provide a visualization of different αSyn subspecies in the sample, as we introduced previously.29 The area under the curve (AUC) for each peak is also calculated and the total intensity measured in all peaks of a given trace is an equivalent of the “bulk” readout of the RT-QuIC assay.
Figure 3.
Single-molecule measurement on recombinant αSyn polymorphs. (A) Schematic of the single-molecule seed amplification assay (smSAA). (1) αSyn polymorphs are seeded in a PBS solution containing thioflavin T (ThT, 10 μM) and human αSyn K23Q (20 μM). (2) The solution is mixed, and half of the reaction is measured using single-molecule spectroscopy to quantify the types of assemblies before an amplification step. (3) Sample is then amplified and measured again. Amplification enhances the signal of existing seeds by promoting αSyn assemblies. (4) Schematic of the microscope setup. The inset shows ThT stained αSyn assemblies diffusing across the confocal volume. (B) Typical fluorescence traces. Fluorescence traces are analyzed to report the total number of ThT+ peaks (events) per measurement and (inset) the prominence of individual peaks, residence time (full-width half-maximum), and area under the curve (yellow). The inset shows a region denoted by (*) in the trace. (C) Typical raw trace obtained for amplified ribbons shows sharp peaks (fast-diffusing) (D) The amplified fibrils show a very different profile at the single-molecule level, with very intense and slow-diffusing species. (E) Logarithmic scatter plot comparing peak prominence, residence time, and area under the curve before (− sign, light) and after amplification (+ sign, dark) of LS ribbons and HS fibrils. Gray horizontal line is the median value. Each symbol represents an individual event and statistics used the Kruskall–Wallis test, p ≤ 0.0001 (****), nonsignificant (n.s.).
Before amplification, ribbons and fibrils were sonicated to reduce the size of the seeds, which was then confirmed by electron microscopy (Supporting Figure S2). The single-molecule fluorescent traces show that before amplification, both ribbons and fibrils appear as fast-diffusing aggregates. Amplification was first performed in conditions that were optimized for quiescent reactions: in previous work, we determined that reactions at 55 °C led to significant amplification of recombinant fibrils and CSF samples from patients.29 In this case, we use the mutant K23Q that was developed by the Caughey laboratory,8 as we found it to be more stable at higher temperatures.29 After only 5 h of amplification, ribbons still behave as fast-diffusing species (Figure 3C) while we observed a very large change in the properties of fibrils, as the raw traces show very wide and intense peaks (Figure 3D). The fingerprinting of the intensity, residence time, and area under the curve for the ribbons and fibrils show two very distinct signatures (Figure 3E). The most differentiating feature is the large increase in the residence time for the fibrils, and this suggests two very different mechanisms of growth for the two polymorphs.
High-Salt Fibrils Grew by Secondary Nucleation
The large shift in residence time observed for amplified fibrils reveals that their apparent size has greatly increased. We therefore turned to high-resolution microscopy to observe these large aggregates. Confocal microscopy images show that within 5 h, nanometer-sized sonicated fibrils have grown to sizes up to 5 μm (Figure 4). The 3D reconstruction of the aggregates clearly shows the growth of dense networks of fibrils, with branching structures and not pure linear elongation of a long and single amyloid core (Supporting Figure S3). This aligns perfectly with the idea of secondary nucleation and fibril growth catalyzed on the surface of the fibrils.
Figure 4.
High-resolution confocal imaging of the amplified fibrils. Confocal microscopy was performed on the fibrils after 5 h of amplification, on a Zeiss LSM880 microscope with an AiryScan unit (see the SI for details). Z-stacks were recorded and analyzed using Zen software to generate the final high-resolution images. In the same setup, amplified ribbons could not be detected, as expected for smaller, fast-diffusing particles.
It should be noted that in general, secondary nucleation is proposed to release new fibrils into solution, but our images suggest that the new fibrils do not detach spontaneously. Confirming this idea, the number of peaks measured by the single-molecule technique did not change significantly during amplification. This could be easily explained by our amplification conditions, as the solution is not shaken, and we do not use any mechanical disruption (beads or sonication) that could break the assemblies.
Impact of Secondary Nucleation on Seed Amplification Assays
Having observed that fibrils amplify much faster than ribbons, we wanted to see if those differences could be explained by the addition of secondary nucleation and determine what rate of secondary nucleation would be sufficient to dominate the kinetics. We turned to mathematical modeling following the approach described in Knowles et al.33 In this work, the authors laid the foundations for a global fit of the kinetics found in seed amplification assays.
We considered three elementary reactions in the model: (1) elongation—adding a monomer on a growth point, (2) secondary nucleation—adding a new growth point in a polymer, and (3) fragmentation—breaking a polymer into two and creating two new growth points. Those three processes are schematized in Figure 5A. In this model, we did not consider primary nucleation as seed amplification assays are designed to eliminate the spontaneous formation of seeding competent αSyn nuclei in the absence of aggregates. The detailed equations can be found in the Supporting Information.
Figure 5.
Mathematical modeling of seed amplification and competition between strains. (A) Visual representation of the different parameters used for simulation: M—the polymer mass concentration, N—the concentration of growth points, and m—the concentration of free monomers. The diagram on the right shows the elementary reactions that we considered in this model. ke (respectively, ks, kf) is the rate of the elongation (respectively, secondary nucleation, fragmentation) reaction. (B) Numerical fit of experimental RT-QuIC data seeded with ribbons or fibrils at 5, 50, or 500 nM using the numerical solution derived from panel (A). (C) Numerical solutions for the time evolution of the relative polymer mass concentration, with different secondary nucleation to elongation ratios denoted by λ where λ = 10–3 means that one new branch is created for 1000 monomers added through elongation. The relative polymer mass reaches 1 when all monomers have been incorporated into the polymers. The fragmentation rate is set to 0. (D) Numerical modeling of competition between different strains seeded in the same solution. Fragmentation and elongation rates were set to the same value, and a rate of secondary nucleation was applied only for fibrils. The rate constants were extracted from the fit in panel (B). The initial composition of polymer mass was set to 100, 99.9999999, 99.99999, 99.999, 99.9, and 99% of ribbons. (E) Final content of ribbons in the polymer mass at the end of simulation (80 h) is displayed for low seed concentration (0.1% of monomer equivalent) or high seed concentration (1% monomer equivalent). (F) Experimental competition between ribbons and fibrils. The 4 curves correspond to 4 different ratios of ribbons and fibrils in the seeds. Each curve is obtained by averaging the results of 2 RT-QuIC experiments with the same parameters.
We simulated the RT-QuIC responses obtained for ribbons and fibrils, using the same elongation rate for the two species, and fitted the experimental curves by adjusting fragmentation and secondary nucleation rates. However, as detailed in the Supporting Information, the fragmentation and secondary nucleation have a similar impact on the equation: physically, both phenomena increase the number of nucleation points. Instead of fitting ks and kf independently, we consider a combined rate of fragmentation and secondary nucleation k*f. As shown in Figure 5B, the values obtained for ribbons (k*f = 5.31 × 10–6 s–1) and fibrils (k*f = 5.65 × 10–4 s–1) differ by 100-fold. As the samples in the 96-well plate are shaking at the same speed with the same number of beads, it would be surprising that the rate of fragmentation would be extremely different, and we can assume that a large contribution to the kinetics comes from the presence of secondary nucleation.
To go further, we decided to simulate
the effect of secondary nucleation
and generated growth curves for different values of ks and ke. To simplify, we
consider the parameter 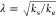 that describes the number of branching
events. For example, λ = 10–4 means that approximately
one point of secondary nucleation is created every 10,000 elongation
events. The curves are presented in Figure 5C. The insert demonstrates that for λ
= 10–4, the effect of secondary nucleation is barely
visible at short time scales, but significant deviations are observed
for λ = 5 × 10–4. With λ = 10–3 i.e., one nucleation every 1000 monomers, the apparent
growth doubled within 2 h, and the reaction proceeded extremely rapidly.
that describes the number of branching
events. For example, λ = 10–4 means that approximately
one point of secondary nucleation is created every 10,000 elongation
events. The curves are presented in Figure 5C. The insert demonstrates that for λ
= 10–4, the effect of secondary nucleation is barely
visible at short time scales, but significant deviations are observed
for λ = 5 × 10–4. With λ = 10–3 i.e., one nucleation every 1000 monomers, the apparent
growth doubled within 2 h, and the reaction proceeded extremely rapidly.
These simulations matched the data observed for fibrils and ribbons and enabled us to estimate the number of secondary nucleation events. The equations suggest that the 100-fold difference in the rate of growth can be generated by as little as one secondary nucleation “point” every 2000 monomers added by elongation (see the Supporting Information for details of these estimations). Considering a typical interstrand distance of 0.5 nm in amyloid fibrils, this suggests that branching would occur every 1 μm; the 3D images of the aggregates after amplification shown in Figure 4 are consistent with this spacing between secondary branches.
Such a large difference in kinetics prompted us to explore competition between strains and see whether there is a selection of specific strains during amplification. Indeed, recent publications have shown that different αSyn strains are produced in the different synucleinopathies. The question now is whether the assays can replicate the seeds with high fidelity, or if they can be strongly biased toward strains with high secondary nucleation propensity.
We first simulated the amplification of samples where ribbons and fibrils would be present simultaneously. We considered a constant total number of “seeds” (ribbons + fibrils) and only changed the ratio of the two polymorphs. We simulated the growth of the seeds and plotted the proportion of ribbons as a function of time. Figure 5D shows that extremely small amounts of fibrils present in solution would dominate the response. The effect is so significant that even with an initial proportion of ribbons close to 100% (99.99999%), fibrils become the dominant polymorph at the end of the reaction.
It is interesting to note that when seeds are present in high numbers (up to 1% of seeds) the replication has higher fidelity (Figure 5E). This is relevant for polymorph propagation in vitro (when reamplifying a stock of highly concentrated fibrils) but in seed amplification assays from biofluids, the initial concentration of aggregates is likely to be in the picomolar range (i.e., 106 lower concentrations than the monomer used for amplification.)
Finally, we tested the competition between polymorphs experimentally, by setting up RT-QuIC reactions with different ratios of fibrils to ribbons. The data obtained in Figure 5F confirm that small amounts of fibrils (as low as 0.01% of ribbons) dramatically change the overall response of the RT-QuIC assays.
Assessment of Growth Mechanisms under Different Experimental Conditions
The mechanism of amyloid growth is known to be highly dependent on experimental conditions and we wanted to examine whether the difference between fibrils and ribbons amplification was conserved in other amplification buffers. We next performed smSAA using different protocols and assessed their single-molecule profiles.
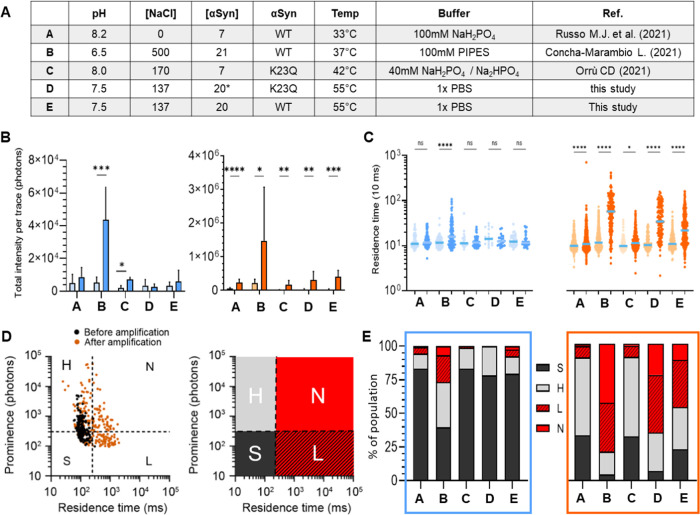
First, we performed smSAA for 5 h using different buffer conditions outlined in Figure 6A. These conditions were selected based on their use across different laboratories to perform RT-QuIC on patient samples.5 Amplification (measured as the increase in total ThT fluorescence) occurred in most conditions (Figure 6B), and again a strong difference was observed between ribbons and fibrils for efficiency of growth. Fibrils were amplified strongly in all buffers, with at least a 7-fold increase in total ThT fluorescence per trace (Figure 6B). As with our reference conditions (condition D, PBS, K23Q αSyn monomer), all conditions created a significant increase in residence time (Figure 6C). Ribbons on the other hand generally failed to increase in residence time but rather showed higher prominence. One notable exception is condition B. Condition B provided the best conditions to promote the growth of ribbons, with an 8.5-fold change in total fluorescence per trace. Notably, this condition was the only one that led to the appearance of high molecular weight αSyn “ribbons” which shifted the median residence time from 116 to 157 ms (Figure 6C). The distinctive buffer constituents of B include its mild acidic conditions (pH 6.5) coupled with high ionic strength (500 mM NaCl). Acidic conditions have been shown to accelerate the aggregation of αSyn due to the enhancement of secondary nucleation.4,34 The effect of the high concentration of NaCl could enhance the rate of aggregation because the high-salt buffer produced significantly more yield and faster assembly kinetics compared to ribbons.23 However, some studies demonstrated that very high ionic strength significantly reduces the aggregation of αSyn due to screening of electrostatic repulsion by salt.35,36 Condition B also produced the largest change in median residence time for fibrils, from 123 to 628 ms. These data strongly suggest that the residence time can be used as a signature of secondary nucleation.
Figure 6.
Single-molecule profiling of ribbons and fibrils amplified in different conditions. (A) Table of the different conditions used: A (32 °C, 7 μM αSyn WT), B (37 °C, 21 μM αSyn WT), (42 °C, 7 μM αSyn K23Q), D (55 °C, 20 μM αSyn K23Q), and E (55 °C, 20 μM αSyn WT). (B) Bar graph showing the total fluorescence intensity of the trace for low-salt ribbons (blue) and high-salt fibrils (vermilion) before (light) and after 5 h of amplification (bright) in different conditions. Error bar is the standard deviation. (C) Logarithmic scatter plot comparing the residence time of ThT+ peaks (events) detected in fluorescence traces before (light) and after amplification (bright), for ribbons (blue) and fibrils (vermilion). Each symbol represents an individual event and statistics used the Kruskall–Wallis test, p ≤ 0.05 (*), p ≤ 0.0001 (****), nonsignificant (n.s.). (D) The events before (black) and after (vermilion) amplification (here for fibrils amplified in conditions D) can be visualized on a scatter plot of prominence vs residence time. We defined 4 quadrants to separate fast-diffusing and slow-diffusing species, and high-intensity vs low-intensity species. The four species are defined as S (dark gray, <300 photons, <250 ms), H (light gray, >300 photons, <250 ms), L (hashed red, <300 photons, >250 ms), and N (red, >300 photons, >250 ms). (E) Distribution of the different species detected after amplification for the different buffers. Results for the ribbons and fibrils are presented in the blue and vermilion frame, respectively.
We then analyzed the “fingerprints” of the aggregates by plotting the intensity of the peak vs residence time, similar to FACS (fluorescence-activated cell sorting). We “gated” at different thresholds of intensity and residence time to define 4 subspecies, as shown in Figure 6D. For ribbons, the relatively low abundance of slow-diffusing particles (L and N quadrants) indicates that growth mainly occurs through elongation except in acidic conditions where secondary nucleation could be observed. Fibrils produced large assemblies (L and N) when amplified in PBS WT and K23Q (conditions D and E), similarly to B, indicating a predominance of secondary nucleation. We also observed that conditions A and C produced a similar profile with a majority of H events, suggesting that these two conditions partially suppressed secondary nucleation while promoting elongation to maintain the strong amplification of fibrils (Figure 6E). These experiments are performed in a mildly basic buffer (phosphate buffer, pH ≥ 8.0) which is indeed thought to limit secondary nucleation.4
We next examined the effects of other experimental parameters on the mechanism of amplification. Changing the amplification conditions from 3 h at 55 °C to 5 h at 37 °C did not significantly change the efficiency or mechanism of growth (data not shown). SmSAA was then performed in PBS with various concentrations of αSyn WT to determine the minimum concentration required to observe secondary nucleation. With ribbons, some increase in total ThT fluorescence was observed with substrate concentration ≥7 μM but all conditions failed to increase the median residence time after amplification, even at the highest concentration of 28 μM monomer (Supporting Figure S4). Fibrils showed an observable change in total ThT fluorescence at 3.5 μM monomers, emphasizing their stronger seeding potential. However, changes in residence time were observed as the basis of the growth mechanism only when ≥7 μM monomers were supplied in the reaction.
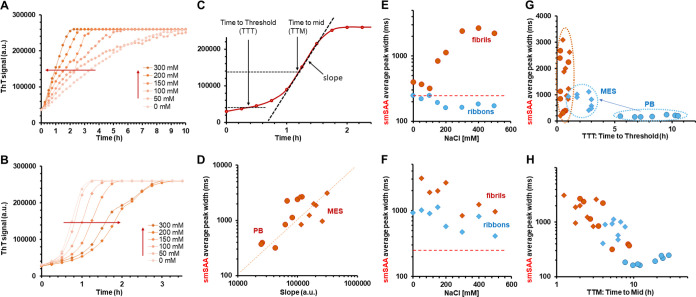
We then paid particular interest to the effect of salt concentration on amplification (Figure 7). Contrary to other amyloids, it was reported that increased salt concentration reduced the kinetics of amplification of αSyn fibrils.35,36 We performed two series of RT-QuIC and smSAA experiments, varying the salt (NaCl) concentration from 0 to 500 mM in neutral (phosphate buffer, pH 7.4) and acidic (MES, pH 6.0) conditions. In Figure 7A,B, the results of the RT-QuIC are shown for amplification of the fibrils in different salt concentrations at low pH and neutral pH. In neutral pH, increasing salt concentration leads to faster reactions (Figure 7A) while we observe the reverse effect for low pH (Figure 7B). The RT-QuIC curves can be quantified by measuring the slope of the reaction, the time-to-initial threshold (TTT), and the time to reach midreaction (TTM), as described in Figure 7C. We then conducted smSAA on fibrils and ribbons in these conditions and studied correlations between the smSAA and RT-QuIC. To take into account the faster dynamics of secondary nucleation, single-molecule traces were acquired after 1.5 and 4.5 h, for acidic and neutral conditions, respectively.
Figure 7.
Correlation between RT-QuIC and single-molecule profiling and effect of salt concentration. (A) RT-QuIC curves obtained for reactions seeded with fibrils in phosphate buffer (PB, pH 7.5) with different concentrations of NaCl. The curves show an increase in reactivity with increasing salt concentrations. (B) RT-QuIC curves obtained for reactions seeded with fibrils in MES buffer (pH 6.0) with different NaCl concentrations; the reactions appear slower when [NaCl] increases. (C) Schematic of the RT-QuIC curve and definition of different parameters for quantification: slope of the reaction (calculated around the midpoint), time-to-threshold (TTT, time point when reactivity reaches 10% of the final value), and time-to-midpoint (TTM, time point when ThT signal has increased to 50% of the final value). (D) From experiments conducted in quiescent conditions and analyzed using our single-molecule method, we determined the smSAA average peak width. The peak width correlates strongly with the slope of the RT-QuIC reaction, as shown here, for fibrils in PB (round) and MES (diamond) buffers. Data for ribbons are color-coded in blue, and in red for fibrils; data are displayed as round dots for phosphate buffer, and diamonds for MES buffer. These colors are used for panels (E–H). (E) smSAA diffusion time as a function of salt concentration for ribbons (blue) and fibrils (vermilion) in phosphate buffer pH 7.5. The red dotted line indicates the arbitrary threshold for slow-diffusing particles (250 ms). (F) SmSAA diffusion times for ribbons (blue) and fibrils (vermilion) in MES buffer pH 6.0; the data show that both fibrils and ribbons have been converted to aggregates with high diffusion times. (G) Scatter plot of smSAA average peak width vs time-to-threshold (TTT), showing that low pH leads to much faster reactions for ribbons and higher diffusion times. (H) Scatter plot of time-to-mid (TTM) vs smSAA average peak width for ribbons and fibrils in all conditions of pH and salt, showing a general trend of decreasing diffusion times with increasing reaction times.
As shown in Figure 7D, the slope of the RT-QuIC reactions depends very strongly on the diffusion times measured in smSAA. When we explore into more detail the salt-dependence of the diffusion times, we observe two different behaviors. At neutral pH, fibrils amplified significantly better than ribbons, as observed before; we observe a clear salt-dependent increase in the diffusion times, suggesting that secondary nucleation is increasing as a function of salt concentration (Figure 7E). For low pH, we observe higher average diffusion times for both ribbons and fibrils and a decrease of diffusion times with increasing salt concentrations (Figure 7F). As shown in Figure 7G, the switch to low pH increases the reactivity of ribbons, as we observe a shift to lower time-to-threshold for all salt conditions; at the same time, we detect a large shift in diffusion times. This suggests that acidic conditions could boost the apparent RT-QuIC reactivity of ribbons by increasing secondary nucleation. This matches well with published observations.4 In Figure 7H, the overall data for ribbons and fibrils, in low and neutral pH, show a clear trend of faster reactions with higher smSAA diffusion times, reinforcing the idea that secondary nucleation plays a major role in the apparent reactivity in RT-QuiC.
αSyn Polymorphs Maintained Their Properties upon Amplification
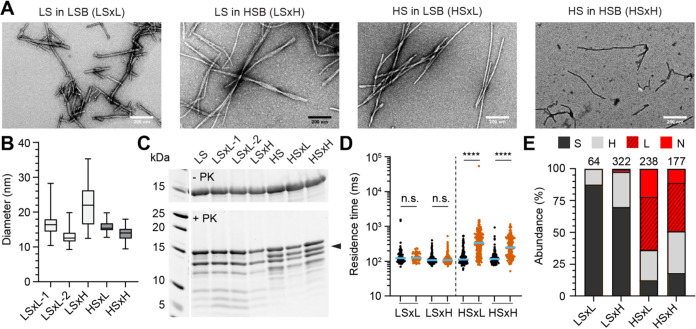
Previous results by Bousset et al.23 demonstrated that the low-salt ribbons and high-salt fibrils gave rise to different phenotypes when seeded in different buffers, pointing to a loss of properties upon amplification. To re-explore this aspect, ribbons were seeded in a high-salt buffer and fibrils were seeded in a low-salt buffer (LSB) in a cross-buffer seeding experiment to produce new assemblies. The polymorphs generated this way were denoted as LSxH and HSxL respectively. Ribbons seeded in low buffer (LSxL) and fibrils in high buffer (HSxH) were also employed as controls. The cross-seeded species were assessed by transmission electron microscopy, proteinase K digestion, and smSAA. LSxL retained the same phenotype as its parental ribbon seeds, and no variation was observed between two independent batches LSxL-1 and LSxL-2 (Figure 8B,C). These products produce filaments of similar width, had identical proteinase K digestion patterns and identical single-molecule amplification phenotype in its poor ability to undergo secondary nucleation to its parental strain as expected (Figure 8B–E). LSxH also resembles its parental LS strain and does not have the same PK digestion profile as fibrils, which is recognized by a discriminatory band at approximately 13 kDa (Figure 8C, black arrow). This indicates that free monomers do not sample the high ionic buffer to generate high-salt fibrils de novo when ribbon seeds are present in the solution. Surprisingly, cross-seeded HSxL also retained the PK profile and secondary nucleation-prone phenotype in smSAA of the parent fibrils. HSxL was indeed also highly seeding competent and able to grow via secondary nucleation (Figure 8D,E). HSxL PK digestion did not reveal any new bands of de novo assembly of low-salt ribbons in solution.
Figure 8.
Characterization of αSyn polymorphs obtained by cross-buffer seeded reactions. (A) Ribbons (LS) were seeded in low-salt buffer (LSB) or high-salt buffer (HSB) yielding the products LSxL and LSxH, respectively. Similarly, fibrils (HS) were seeded in low-salt buffer (LSB) or high-salt buffer (HSB) giving rise to HSxL and HSxH products. Representative electron micrographs of negatively stained assemblies after amplification. (B) Box plot quantifying the diameter of filaments from electron micrographs; LSxL-1 (16.4 ± 2.7 nm, mean ± standard deviation, N = 244), LSxL-2 (13.0 ± 2.2 nm, N = 42), LSxH (21.9 ± 6.0 nm, N = 40), HSxL (15.8 ± 1.6 nm, N = 76), HSxH (13.7 ± 2.0, N = 28). Whiskers represent min-max values. (C) Proteinase K digestion profile of seeded products from recombinant αSyn polymorphs in the absence (top) and after the addition of proteinase K (bottom, PK). Black arrow points at a band that discriminates ribbons from fibrils. (D) Logarithmic scatter plot comparing the residence time of events in seeded reaction before (black) and after (vermilion) amplification with αSyn K23Q. The median value is highlighted in blue. Each symbol represents an individual event and statistics used the Kruskall–Wallis test, p ≤ 0.0001 (****), nonsignificant (n.s.). (E) Distribution of ThT+ events detected after amplification in cross-seeded reactions. S (dark gray, <300 photons, <250 ms), H (light gray, >300 photons, <250 ms), L (hashed red, <300 photons, >250 ms) and N (red, >300 photons, >250 ms). The number of events detected and used for quantification is indicated above each bar.
These findings indicate that the amyloid core, at least with the recombinant in vitro polymorphs used in the current study, can propagate structural information irrespective of the environment in which they are amplified.
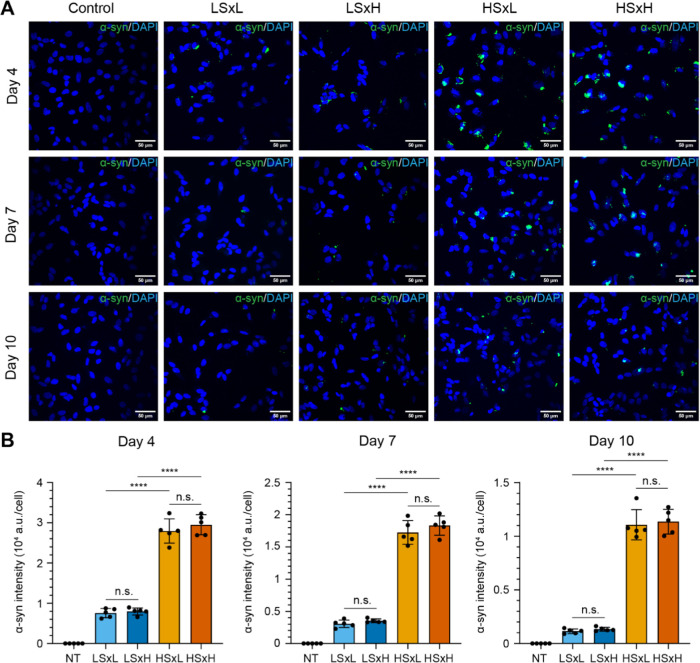
As a final validation strategy, we sought to explore the potential variations of the pathological properties of these cross-seeded polymorphs in SH-SY5Y neuroblastoma cells. Large batches of cross-buffer seeded polymorphs LSxL, HSxH, LSxH, and HSxL were prepared and characterized by single-molecule amplification and proteinase K digestion (Supporting Figure S5). These cross-seeded polymorphs were added to differentiated SH-SY5Y cells to assess their ability to induce αSyn inclusions in the cytosol using immunostaining and confocal microscopy. The density, count, and intensity of αSyn of LSxH were not significantly different from cells treated with LSxL (Figure 9A,B). LSxH produced low αSyn intensities per cell, similar to the parental strain (Figure 9B). Inversely, HSxL was able to produce very bright and large αSyn punctae when seeded in cells, indistinguishable from HSxH (Figure 9A,B). Notably, it was found that cells exposed to both fibrils HSxL or HSxH exhibited significantly stronger cytoplasmic αSyn intensity than those exposed to ribbons on days 4, 7, or 10. The cross-buffer seeded polymorphs were not cytotoxic to the cells, similar to the parent strains (Supporting Figure S6). Overall, these results were consistent with the previous data (Figure 8), as there was no disparity in cytoplasmic inclusions between ribbons or fibrils seeded in their parental buffer or cross-seeded in high-salt or low-salt buffer, respectively.23
Figure 9.
Temporal changes of cytoplasmic αSyn inclusions following treatment of SH-SY5Y cells with cross-seeded αSyn aggregates. (A) Confocal images of differentiated SH-SY5Y cells treated without (NT, no treatment) or with 5 μg/mL of diverse αSyn aggregates: ribbons seeded in low-salt buffer (LSxL), ribbons seeded in high-salt buffer (LSxH), fibrils in low-salt buffer (HSxL) and fibrils seeded in high-salt buffer (HSxH). Cells were subsequently fixed for immunofluorescence staining to quantify total αSyn (green) at day 4, 7, and 10 post-incubation with the aggregates. DAPI staining is represented in blue. Scale bar of 50 μm. (B) Bar graphs quantifying the average intensity of immunostained αSyn per cell upon treatment with different αSyn aggregates (NT: not treated, light blue: LSxL, dark blue: LSxH, light vermilion: HSxL, dark vermilion: HSxH). Each symbol represents a FOV. Total αSyn intensity was normalized by the number of cells based on a DAPI signal. Error bars represent mean ± standard deviation. Statistics used ordinary one-way ANOVA Tukey’s comparison test with a single pooled variance, p ≤ 0.0001 (****), nonsignificant (n.s.).
Discussion
Generating Fibrils and Ribbons
Here, we have successfully produced ribbons and fibrils as described in the literature.3,23 Of note, we observed that different batches of assemblies obtained in the low-salt conditions displayed different digestion patterns and only some of them corresponded to the reported ribbon fold (Supporting Figure S7). This was not the case for the fibrils, which reproducibly gave the same PK digestion pattern and structural characteristics. This is consistent with recent studies that showed that different polymorphs can in some cases be generated stochastically in the same experimental conditions.37−39 Some low-salt polymorphs had a more fibril-like profile, based on the PK digestion pattern, but remained less susceptible to undergo secondary nucleation compared to fibrils (see Supporting Figure S8).
The fact that we never observed a banding pattern corresponding to the fibrils upon PK digestion of the LSxH polymorphs (ribbons seeded in buffer-promoting fibrils) was unexpected. Indeed, fibrils form faster than ribbons de novo and fibrils also amplify more efficiently than ribbons. We would therefore expect that primary nucleation in high-salt would produce some fibrils that would quickly represent a sizable proportion of the final product (see below). Our findings indicate that the presence of seeds is very efficient at blocking primary nucleation given the ability of fibrils to become the dominant species upon amplification (Figure 5). Note that in these cross-seeding experiments, the amount of preformed HS and LS strains added to the reaction is relatively high (seeds represent 5% of the total proteins); this could explain the high fidelity of strain replication. Our results for amplification in LS buffer differ from the initial results from Bousset et al.,23 where “ribbons are generated irrespective of the nature of the seeds, fibrils or ribbons”.
Cellular Effects of Fibrils and Ribbons
We tested the effects of the generated polymorphs on cells. As reported,23,34 fibrils strongly induce aggregation in treated cells compared to ribbons, however, we did not notice differences in toxicity between the polymorphs, based on measuring the levels of lactate dehydrogenase at days 4, 7, and 10. There was no significant difference in cytotoxicity between HS/LS treated cells and nontreated cells, indicating that HS/LS did not induce cytotoxicity on D4/7/10. Bousset et al.23 reported that fibrils were noticeably more toxic than ribbons in their assay, which examined cytotoxicity at early time points (24–48 h treatment). In their case, there was a clear dose-dependent decrease in cell survival measured by cell counting and 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay, in undifferentiated SH-SY5Y cells. They further showed induction of apoptosis in differentiated cells, within 48 h. As our first time point is at day 4, it is possible that we did not observe this early apoptotic phase, which might be linked to the interaction between the αSyn aggregates and the membrane. Based on our data, it seems that once αSyn fibrils and ribbons have been taken up by the cells, they induce endogenous αSyn aggregation without a noticeable effect on toxicity.31
Fibrils Undergo Secondary Elongation More Readily than Ribbons
Our single-molecule data revealed a striking difference in the mechanism of amyloid growth between fibrils and ribbons. Our hypothesis that the observed increase in the residence time was due to the formation of 3D aggregates was validated using high-resolution microscopy, showing branched networks of filaments upon amplification of fibrils. The propensity of fibrils to grow by secondary nucleation was not limited to our set of experimental conditions. Rather, the same properties were seen using other sets of experimental conditions (monomer, buffer, temperature). The conditions of amplification could however strongly influence the mechanism of growth. In conditions known to promote secondary nucleation (e.g., low pH, high-salt in condition B) both polymorphs amplified through 3D growth. On the other hand, amplification at low concentrations of monomers prevented secondary nucleation, as expected. Yet, in both cases, fibrils remained more efficient at amplifying than ribbons.
Buell et al.4 analyzed in detail how solution conditions affect the kinetics of these steps. They showed that in acidic quiescent conditions (pH < 6), seeded aggregation of αSyn was much faster than at physiological pH, due to a dramatic increase in the rate of secondary nucleation.34 Peduzzo et al.40 then studied the seeded amplification of ribbons and fibrils in acidic conditions (pH 6.5 or 5.5). They found that at low seed concentration (which favors secondary nucleation), fibrils and ribbons showed different amplification profiles, suggesting different relative contributions of elongation and secondary nucleation to the growth of αSyn assemblies. Amplified fibrils also looked more branched on atomic force spectroscopy images than amplified ribbons that remain mainly linear. The branching phenotype was lost in the reaction where higher seed concentrations were used, which limits secondary nucleation. The authors concluded that ribbons and fibrils may be different “in the relative ability to act as templates for fibril elongation and secondary nucleation”. Our data support this conclusion and show that this difference in behavior holds true for a range of conditions.
Aggregate Conformation as a Determinant of Secondary Nucleation
The nucleation and growth processes of αSyn aggregation have been well characterized. Different studies have established that secondary nucleation is the leading process of amplification for αSyn at pH < 6.04−34,40 as well as for other amyloid-forming proteins (insulin, islet amyloid polypeptide (IAPP) or Amyloid β). Secondary nucleation is a multistep process where monomers initially attach to the surface then convert into an aggregation-competent fold (nucleus formation) and elongate. Kumari et al.41 showed that the positively charged N-terminus of αSyn monomers could interact with the negatively charged C-terminal domain of the aggregated αSyn. Removing the electrostatic charges by truncations of the C-terminus of αSyn allows secondary nucleation to become the main growth mechanism at neutral pH.42 It has been reported that the physical size of the seed could influence its ability to undergo secondary nucleation43 but in our hands, sonicated ribbons were longer than the sonicated fibrils.
As a surface-catalyzed process, it is not surprising in retrospect that strains would be prone to different growth mechanisms. Cross-reactivity between Aβ40 and Aβ42 has been studied, and despite their high identity, Aβ40 and Aβ42 form strikingly different structures.44 Separate studies have shown that Aβ40 fibrils could not only induce the formation of Aβ42 fibrils by surface-catalyzed secondary nucleation while Aβ42 fibrils did support this cross-nucleation.45,46 In the case of αSyn, polymorphic assemblies possess different core structures and expose different residues at their surface, evidenced by variation in conformational antibody recognition, for example.47 NMR shows that the N-terminus of ribbons tends to be more structured than in fibrils,48 making it less solvent-accessible and therefore resistant to proteolysis.47 In these studies, the C-terminal domain of αSyn remained dynamic and unresolved, but it is likely that the structure of the amyloid core will have a long-range influence on the rest of the protein as well. A recent study by Pálmadóttir et al.49 illustrates the influence of polymorphs on monomer–aggregate interactions. Polymorphs generated in the same conditions (10 mM Tris, pH 7.0) could adopt different morphologies and display different binding modes to αSyn monomers, as assessed by NMR. One polymorph bound to the N-terminus of the monomer as previously described,34,41 while the other interacted with a large part of the monomer. A similar mechanism could be at play here.
Impact of Secondary Nucleation on Strain Retention
With the development of αSyn SAA for the study of synucleinopathies,50 the question of whether structural characteristics of the seeds are retained upon amplification becomes very relevant. More studies are still needed to get a definitive answer, but it is thought for now that the assemblies generated by secondary nucleation are more influenced by the reaction conditions than by the parent seed. This was shown for αSyn, insulin, or the mouse prion protein.46 The case of Aβ is less clear, as the assemblies’ surface selectively interacts with some isomers that are structurally compatible,51 yet the secondary nuclei do not necessarily inherit the properties of the seed.45 Here, we used our model to ask whether the difference of growth mechanism could have an impact on the final product of the amplification, irrespective of strain retention. Indeed, this is relevant if we are to use in vitro amplification to study the heterogeneity of αSyn strains in patients. To do this, we simulated a competition between different strains, with two different dynamics of growth (as calculated in Figure 5B). The initial conditions were varied by changing the ratio of ribbons to fibrils and growth was simulated for up to 80 h (Figure 5D). Adding as little as 10 parts per billion (0.0000001%) of fibrils would already have a significant impact on the final composition of the solution (55.9% of ribbons final). Adding >0.1% of fibrils leads to final solutions where the presence of ribbons becomes almost insignificant (<5%). Obviously, this simulation did not account for many other variables and processes that are at play during in vitro amplification and is based on overly contrasted differences of mechanisms between the strains. However, when we performed RT-QuIC assays with ribbon and fibril seeds at the same time, minute amounts of fibrils dramatically changed the response of the assay (Figure 5F). This suggests that differences in mechanisms of growth between strains should be kept in mind for future studies of pathological αSyn aggregates relying on amplification.
In conclusion, we demonstrated that αSyn polymorphism could impact the mode of amyloid amplification. The ability of fibrils, but not ribbons, to support secondary nucleation at neutral pH leads to faster amplification in vitro as well as the formation of larger aggregates in cells. This should be considered when comparing different αSyn polymorphs and strains linked to different pathologies that would presumably have notable disparities in properties and conformations.
Materials and Methods
Expression and Purification of Human αSyn Wild-Type and K23Q
The expression and purification of untagged recombinant human αSyn wild-type (WT) was performed as described in Lau et al.29 Purified αSyn were checked by reducing SDS-PAGE and had its concentration determined spectroscopically at 280 nm absorbance with ε = 5960 M–1 cm–1. The expression and purification of recombinant human αSyn K23Q was performed as described in Groveman et al.8 Purified proteins were aliquoted, flash-frozen with liquid nitrogen for storage at −80 °C.
Production Recombinant αSyn Aggregates
Purified recombinant human αSyn WT was exchanged into a low-salt buffer (LSB, 5 mM Tris, pH 7.5, 0.02% w/v NaN3) using Zeba spin columns (Thermo Scientific, 89890).
Production of recombinant low-salt and high-salt αSyn aggregates was performed in screw-top microtubes (SSIbio, tubes 2340-00 and caps 2001-00) with a final volume of 600 μL.
Generation of low-salt ribbons was initiated by diluting human αSyn wild-type (250 μM) in the low-salt buffer (5 mM Tris, pH 7.5, 0.02% w/v NaN3). Generation of high-salt fibrils was initiated by diluting human αSyn wild-type (250 μM) in the high-salt buffer (HSB, 50 mM Tris, pH 7.5, 150 mM KCl, 0.02% w/v NaN3). The tubes were shaken horizontally at 600 rpm, 37 °C inside a falcon tube strapped on a vortexer. Ribbons and fibrils αSyn assemblies were collected after 7 and 2 days, respectively. Ribbons and fibrils were sonicated on the M220 focused-ultrasonicator (Covaris) with a set point of 12 °C with peak power of 75 W, duty factor of 25, 200 cycles/burst, delay 5 s, 60 cycles. Nonsonicated and sonicated materials were frozen in liquid nitrogen for storage at −80 °C. Samples were thawed on tap water and kept at room temperature to prevent disassembly at 4 °C.
Cross-Seeding αSyn Aggregates in Low-Salt and High-Salt Buffers
Ribbons and fibril aggregates (250 μM) were sonicated for 5 min at room temperature on a Branson sonicator with setting 3, 30% duty cycle. Seeding reactions were initiated by adding 5 μL of the sonicated materials to low-salt buffer (LSB, 5 mM Tris, pH 7.5, 0.02% w/v NaN3) or high-salt buffer (HSB, 50 mM Tris, pH 7.5, 150 mM KCl, 0.02% w/v NaN3) containing human αSyn wild-type (100 μM) with a final volume of 100 μL as the first batch. Assembly was induced by shaking at 180 rpm, 37 °C for 8 days. Ribbons seeded in LSB (LSxL), ribbons seeded in HSB (LSxH), fibrils seeded in LSB (HSxL) and fibrils seeded in HSB (HSxH) were sonicated on the M220 focused-ultrasonicator (Covaris) with a set point of 12 °C with peak power of 75 W, duty factor of 25, 200 cycles/burst, delay 5 s, 60 cycles. The second batch of cross-seeded aggregates used for cell studies were reprepared at a final volume of 500 μL and sonicated (Qsonica, Q500) at 50% strength, 1 min sonication time with 3 s on/off. Nonsonicated and sonicated materials were frozen in liquid nitrogen for storage at −80 °C. Samples were thawed on tap water and kept at room temperature to prevent disassembly at 4 °C. Two batches of ribbon seeded in LSB (LSxL-1 and LSxL-2) were produced.
Negative Staining Electron Microscopy
αSyn ribbons, fibrils, and cross-seeded αSyn aggregates were diluted to 12.5 μM with sterile Milli-Q water. Samples were negatively stained by adapting a published protocol.29 A copper grid (200 mesh, coated with carbon and Formvar, Ted Pella, 01811) was cleaned by glow discharge and the sample was applied onto with 1 min incubation at room temperature. The grid was wicked dry before staining uranyl acetate (2% w/v) for 30 s. This process was repeated two more times and the grid was air-dried. Micrographs were collected using a FEI Tecnai G2 20 electron microscope at 19,500-fold magnification mounted with a Gatan Orius SC1000A CCD camera. Particle diameters were measured using ImageJ.
Proteinase K Digestion and Reducing SDS-PAGE
Nonsonicated ribbons and fibrils (40 μL of 250 μM) were centrifuged at 50,000 g for 30 min at 25 °C (S100AT3, Eppendorf Himac). The concentration of αSyn in the supernatant was determined spectroscopically at 280 nm to estimate the concentration of αSyn in the pellet. Ribbons and fibrils were diluted to 40 μM (monomer equivalent) in the digestion buffer to a final volume of 10 μL. Cross-seeded αSyn aggregates (100 μM) had the assemblies diluted to a final concentration of 40 μM without centrifugation to a final volume of 10 μL for digestion. Monomeric αSyn WT (40 μM) was used as the control to ensure the proteinase K was functional. For each reaction, 5 μL was withdrawn and quenched in a Tricine SDS sample buffer (Thermo Scientific, LC1676) containing β-mercaptoethanol (4% v/v). The remaining solution was digested by adding 0.21 μL of 37.5 μg/mL of proteinase K (final 1.5 μg/mL), incubated at 37 °C for exactly 5, 15, or 30 min at 37 °C and quenched by adding an equal volume of Tricine running buffer containing β-mercaptoethanol (4% v/v). All samples were then heated at 90 °C for 10 min and resolved using reducing SDS-PAGE with Tricine gels (Thermo Scientific, EC66955BOX) at 120 V for 1 h 50 min. Gels were fixed with a fixing solution (50% v/v methanol, 7% v/v acetic acid) for 15 min, stained with SYPRO Ruby stain (Thermo Scientific, S12000) overnight at 4 °C in the dark. Gel was destained with a wash-solution (10% v/v methanol, 7% v/v acetic acid) for 15 min and washed twice with Milli-Q water before imaging using a GelDoc (Bio-Rad). Gel was analyzed using ImageJ. Three independent repeats were performed for time course proteinase K digestion of ribbons and fibrils. Two independent repeats were performed for the proteolytic digestion of cross-seeded aggregates.
Single-Molecule Seed Amplification Assay Measurements and Analysis
Single-molecule seed amplification assay (smSAA) of αSyn aggregates followed a previously established protocol.28 Human αSyn K23Q were filtered using a 100k MWCO Amicon filter (Merck Millipore, UFC510096) to remove aggregates. Concentrations were determined spectroscopically with absorbance at 280 nm and ε = 5960 M–1 cm–1. Sonicated ribbons (6.3 nM) and fibrils (1 nM) were diluted in phosphate buffer saline (PBS, 137 mM NaCl, 2.7 mM KCl, 10 mM Na2HPO4, and 1.8 mM KH2PO4), filtered human αSyn K23Q (20 μM) and thioflavin T (10 μM) to a final volume of 40 μL. This volume was separated into two equal-volume reactions. One volume was amplified at 55 °C for 5 h in a thermocycler and the other volume was measured on the 3D confocal microscope immediately as control (before amplification). Samples were loaded to a custom polydimethylsiloxane (PDMS) plate adhered to a glass coverslip (ProSciTech, G425-4860) and observed using the inverted 3D printed confocal microscope, equipped with a 450 nm laser and water immersion 40×/1.2 NA objective (Zeiss).28 Emitted fluorescence from ThT was filtered by a dichroic mirror (488 nm) and a long-pass filter (500 nm) before focusing onto a single photon avalanche diode (Micro Photon Devices). Fluorescence spectroscopy traces were recorded for 10 min, 100 s/trace in 10 ms bins. Traces acquired on different days were pooled together for analysis. Traces were analyzed using a custom Python script on Spyder version 4.0.1 to extract single-molecule parameters in an automated and unbiased manner as previously described.29 SmSAA of sonicated ribbons (25 nM) and sonicated fibrils (1 nM) using different concentrations of human αSyn WT (0.88, 1.75, 3.5, 7, 14, 28 μM) were performed in PBS at 55 °C for 5 h.
Sonicated ribbons seeded in LSB (LSxL), ribbons seeded in HSB (LSxH), fibrils seeded in LSB (HSxL), and fibrils seeded in HSB (HSxH) were diluted to 20, 20, 5, and 0.5 nM respectively in PBS containing filtered human αSyn K23Q (20 μM) and thioflavin T (10 μM) for smSAA. Reactions were amplified at 55 °C for 5 h. Sonicated cross-seeded aggregates used for cell studies were amplified using the same protocol with an adjustment of final concentration of aggregates of 20, 10, 2, and 2 nM for LSxL, LSxH, HSxL, and HSxH, respectively. Traces were pooled together for analysis from at least two independent amplification experiments.
Confocal Microscopy on Amplified αSyn Fibrils
High-salt fibrils were amplified using smSAA with PBS containing ThT (10 μM) and human αSyn K23Q at 55 °C for 3 h. Two reactions were set up. A small volume of amplified reaction (5 μL) was transferred between two clean coverslips and mounted onto a Chamlide chamber (Live Cell Instrument, CMB) for imaging using confocal microscopy. Confocal microscopy was performed on an LSM880 laser scanning confocal microscope with AiryScan (Zeiss, Oberkochen, Germany) using a 63×/1.4 oil immersion objective. The ThT dye bound onto the amplified fibrils was excited with a 488 nm Argon laser using powers setting 20 and 30%. Fluorescence emission was cleaned up through a dual-band emission filter (420–475/500–545 nm) and detected by an Airyscan GaAsP photomultiplier tube array at a voxel-size of 0.04 × 0.04 × 0.17 μm3. The Z-stacks of sizes between 8 and 10 μm were recorded to capture the whole structure of the fibrillar aggregate. Raw image stacks were AiryScan processed using Zen Black 2.3 (3D processing, auto filter). Movies and images were rendered using Imaris Microscopy Image Analysis Software (Oxford Instruments) and the length and width of each aggregate were measured by ImageJ.
SH-SY5Y Cell Culture and Treatment with Recombinant αSyn Aggregates
Protein concentrations of αSyn aggregates were remeasured by Pierce bicinchoninic acid assay (Thermo Fisher Scientific, 23227) with BSA as standard to normalize the treatment to differentiated SH-SY5Y cells. SH-SY5Y human neuroblastoma cells were cultured in DMEM/Ham’s F-12 (Thermo Fisher Scientific, 11320033) supplemented with 10% w/v low endotoxin fetal bovine serum (Thermo Fisher Scientific, 16000069), 2 mM l-glutamine (Thermo Fisher, 25030081) and 1% w/v penicillin–streptomycin (Thermo Fisher Scientific, 10378016). Cells were seeded on coverslips in 12-well plates (2.5 × 104 cells/well) and incubated overnight at 37 °C, 5% v/v CO2 and 95% humidity. Differentiation was induced in DMEM/Ham’s F-12 containing 1% fetal bovine serum, 1% w/v penicillin–streptomycin, and 10 μM of retinoic acid (Sigma Aldrich, R2625-100MG) for 7 days and was verified using brightfield microscopy. Immediately before treating the cells, αSyn aggregates were diluted in sterile Dulbecco’s PBS (DPBS, Thermo Fisher Scientific, 14040133) to 0.1 mg/mL (6.9 μM), sonicated for 30 s at 40% amplitude and 1 s on/off pulse (Qsonica, Q125). Cells were then treated with 1 mL of different strains of αSyn at 5 μg/mL (0.35 μM) and washed with DPBS after 2 days. Cells were further maintained for up to 10 days with media changed every 3 days.
Confocal Fluorescence Microscopy of SH-SY5Y Cells Treated with Recombinant αSyn Aggregates
Cells on coverslips were washed with PBS (Thermo Fisher Scientific, 18912014) and fixed with 4% w/v paraformaldehyde (Sigma Alrich, 158127-3KG) for 20 min. After washing again with PBS, cells were permeabilized with 0.3% v/v triton (Sigma Alrich, X100-500 ML) for 15 min. Blocking was performed with 3% w/v BSA (Sigma Alrich, A9647-1KG) and 0.1% v/v Triton for 1 h. The primary antibody used was mouse monoclonal anti-αSyn (BD Biosciences, 610787; 1:400 dilution) diluted in 3% w/v BSA and 0.1% v/v triton and incubated overnight at 4 °C. After washing with PBS, donkey antimouse Alexa Fluor 488 secondary antibodies (Invitrogen, A21202; 1:400 dilution) were added for 1 h in the dark. Following another wash with PBS, DAPI (Sigma Alrich, D9542-5MG) was added to the last wash at a 1:10,000 dilution and incubated for 15 min. Finally, coverslips were mounted with fluorescent mounting medium (Dako, S3023) and allowed to dry for 1 day before imaging using a Nikon C2 confocal microscope taken at 40× magnification. Images were analyzed for α-synuclein inclusions using ImageJ. The threshold tool was used to highlight stained areas and measure integrated density. Cell number was obtained based on DAPI staining. Cells close to the field of view (FOV) edges were excluded from analysis. Five images (30–100 cells per image) were analyzed per treatment condition. Fluorescent intensity was normalized to DAPI count for the number of SH-SY5Y cells. Statistical analysis was conducted using Prism software (GraphPad Software). Comparisons between two groups were performed using Student’s t-test, while one-way ANOVA with Tukey’s posthoc test was used for comparisons among multiple groups. A p-value <0.05 was considered statistically significant, and all figures show mean ± standard deviation (SD) of the data.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acschemneuro.4c00185.
Author Contributions
∥ D.L. and Y.T. contributed equally to this study; D.L. and Y.T. executed most of the experiments, production and purification of monomeric αSyn proteins, biochemical and single-molecule characterization of the polymorphs, electron microscopy, cell studies, wrote the initial draft, and reviewed manuscript; V.K. performed RT-QuIC experiments; T.C. developed and refined the mathematical model; D.K. and M.B. performed the confocal imaging of the aggregates; E.J. and N.J.G. performed in vitro amplification experiments and controls; C.L.M. and N.D. supervised V.K. and Y.T. respectively and contributed to the writing and review of the manuscript; E.S. and Y.G. conceptualized, obtained funding, supervised the work, and wrote and reviewed the manuscript.
This work was funded by the Michael J Fox Foundation (grant MJFF-021286 to E.S., Y.G., N.D., and C.L.M.) and by an NHMRC idea grant APP2028330 (to E.S. and Y.G.).
The authors declare the following competing financial interest(s): ES and YG are the inventors of the AttoBright instrument, which is patented; ES and YG are the founders of AttoQuest Pty Ltd, an official spin-off from UNSW that commercializes the AttoBright instruments.
Supplementary Material
References
- Goedert M.; Jakes R.; Spillantini M. G. The Synucleinopathies: Twenty Years On. J. Parkinson’s Dis. 2017, 7 (s1), S51–S69. 10.3233/JPD-179005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma J.; Gao J.; Wang J.; Xie A. Prion-Like Mechanisms in Parkinson’s Disease. Front. Neurol. 2019, 13, 552 10.3389/fnins.2019.00552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Perren A.; Gelders G.; Fenyi A.; Bousset L.; Brito F.; Peelaerts W.; Van den Haute C.; Gentleman S.; Melki R.; Baekelandt V. The structural differences between patient-derived α-synuclein strains dictate characteristics of Parkinson’s disease, multiple system atrophy and dementia with Lewy bodies. Acta Neuropathol. 2020, 139 (6), 977–1000. 10.1007/s00401-020-02157-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buell A. K.; Galvagnion C.; Gaspar R.; Sparr E.; Vendruscolo M.; Knowles T. P.; Linse S.; Dobson C. M. Solution conditions determine the relative importance of nucleation and growth processes in α-synuclein aggregation. Proc. Natl. Acad. Sci. U.S.A. 2014, 111 (21), 7671–7676. 10.1073/pnas.1315346111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo M. J.; Orru C. D.; Concha-Marambio L.; Giaisi S.; Groveman B. R.; Farris C. M.; Holguin B.; Hughson A. G.; LaFontant D. E.; Caspell-Garcia C.; Coffey C. S.; Mollon J.; Hutten S. J.; Merchant K.; Heym R. G.; Soto C.; Caughey B.; Kang U. J. High diagnostic performance of independent alpha-synuclein seed amplification assays for detection of early Parkinson’s disease. Acta Neuropathol. Commun. 2021, 9 (1), 179 10.1186/s40478-021-01282-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira N. D. C.; Caughey B. Proteopathic Seed Amplification Assays for Neurodegenerative Disorders. Clin. Lab. Med. 2020, 40 (3), 257–270. 10.1016/j.cll.2020.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siderowf A.; Concha-Marambio L.; Lafontant D. E.; Farris C. M.; Ma Y.; Urenia P. A.; Nguyen H.; Alcalay R. N.; Chahine L. M.; Foroud T.; Galasko D.; Kieburtz K.; Merchant K.; Mollenhauer B.; Poston K. L.; Seibyl J.; Simuni T.; Tanner C. M.; Weintraub D.; Videnovic A.; Choi S. H.; Kurth R.; Caspell-Garcia C.; Coffey C. S.; Frasier M.; Oliveira L. M. A.; Hutten S. J.; Sherer T.; Marek K.; Soto C. Assessment of heterogeneity among participants in the Parkinson’s Progression Markers Initiative cohort using α-synuclein seed amplification: a cross-sectional study. Lancet Neurol. 2023, 22 (5), 407–417. 10.1016/S1474-4422(23)00109-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groveman B. R.; Orrù C. D.; Hughson A. G.; Raymond L. D.; Zanusso G.; Ghetti B.; Campbell K. J.; Safar J.; Galasko D.; Caughey B. Rapid and ultra-sensitive quantitation of disease-associated α-synuclein seeds in brain and cerebrospinal fluid by αSyn RT-QuIC. Acta Neuropathol. Commun. 2018, 6 (1), 7 10.1186/s40478-018-0508-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iranzo A.; Fairfoul G.; Ayudhaya A. C. N.; Serradell M.; Gelpi E.; Vilaseca I.; Sanchez-Valle R.; Gaig C.; Santamaria J.; Tolosa E.; Riha R. L.; Green A. J. E. Detection of α-synuclein in CSF by RT-QuIC in patients with isolated rapid-eye-movement sleep behaviour disorder: a longitudinal observational study. Lancet Neurol. 2021, 20 (3), 203–212. 10.1016/S1474-4422(20)30449-X. [DOI] [PubMed] [Google Scholar]
- Miglis M. G.; Adler C. H.; Antelmi E.; Arnaldi D.; Baldelli L.; Boeve B. F.; Cesari M.; Dall’Antonia I.; Diederich N. J.; Doppler K.; Dušek P.; Ferri R.; Gagnon J. F.; Gan-Or Z.; Hermann W.; Högl B.; Hu M. T.; Iranzo A.; Janzen A.; Kuzkina A.; Lee J. Y.; Leenders K. L.; Lewis S. J. G.; Liguori C.; Liu J.; Lo C.; Ehgoetz Martens K. A.; Nepozitek J.; Plazzi G.; Provini F.; Puligheddu M.; Rolinski M.; Rusz J.; Stefani A.; Summers R. L. S.; Yoo D.; Zitser J.; Oertel W. H. Biomarkers of conversion to α-synucleinopathy in isolated rapid-eye-movement sleep behaviour disorder. Lancet Neurol. 2021, 20 (8), 671–684. 10.1016/S1474-4422(21)00176-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi M.; Baiardi S.; Teunissen C. E.; Quadalti C.; van de Beek M.; Mammana A.; Maserati M. S.; Van der Flier W. M.; Sambati L.; Zenesini C.; Caughey B.; Capellari S.; Lemstra A.; Parchi P. Diagnostic Value of the CSF α-Synuclein Real-Time Quaking-Induced Conversion Assay at the Prodromal MCI Stage of Dementia With Lewy Bodies. Neurology 2021, 97 (9), e930–e940. 10.1212/WNL.0000000000012438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood H. A seed amplification assay to detect Parkinson disease pathology. Nat. Rev. Neurol. 2023, 19, 324 10.1038/s41582-023-00814-1. [DOI] [PubMed] [Google Scholar]
- Berg D.; Klein C. α-synuclein seed amplification and its uses in Parkinson’s disease. Lancet Neurol. 2023, 22 (5), 369–371. 10.1016/S1474-4422(23)00124-2. [DOI] [PubMed] [Google Scholar]
- Ayers J. I.; Lee J.; Monteiro O.; Woerman A. L.; Lazar A. A.; Condello C.; Paras N. A.; Prusiner S. B. Different α-synuclein prion strains cause dementia with Lewy bodies and multiple system atrophy. Proc. Natl. Acad. Sci. U.S.A. 2022, 119 (6), e2113489119 10.1073/pnas.2113489119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holec S. A. M.; Woerman A. L. Evidence of distinct α-synuclein strains underlying disease heterogeneity. Acta Neuropathol. 2021, 142 (1), 73–86. 10.1007/s00401-020-02163-5. [DOI] [PubMed] [Google Scholar]
- Carta M.; Aguzzi A. Molecular foundations of prion strain diversity. Curr. Opin. Neurobiol. 2022, 72, 22–31. 10.1016/j.conb.2021.07.010. [DOI] [PubMed] [Google Scholar]
- Lau H. H. C.; Ingelsson M.; Watts J. C. The existence of Aβ strains and their potential for driving phenotypic heterogeneity in Alzheimer’s disease. Acta Neuropathol. 2020, 142 (1), 17–39. 10.1007/s00401-020-02201-2. [DOI] [PubMed] [Google Scholar]
- Narasimhan S.; Guo J. L.; Changolkar L.; Stieber A.; McBride J. D.; Silva L. V.; He Z.; Zhang B.; Gathagan R. J.; Trojanowski J. Q.; Lee V. M. Y. Pathological Tau Strains from Human Brains Recapitulate the Diversity of Tauopathies in Nontransgenic Mouse Brain. J. Neurosci. 2017, 37 (47), 11406–11423. 10.1523/JNEUROSCI.1230-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porta S.; Xu Y.; Lehr T.; Zhang B.; Meymand E.; Olufemi M.; Stieber A.; Lee E. B.; Trojanowski J. Q.; Lee V. M. Distinct brain-derived TDP-43 strains from FTLD-TDP subtypes induce diverse morphological TDP-43 aggregates and spreading patterns in vitro and in vivo. Neuropathol. Appl. Neurobiol. 2021, 47 (7), 1033–1049. 10.1111/nan.12732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y.; Shi Y.; Schweighauser M.; Zhang X.; Kotecha A.; Murzin A. G.; Garringer H. J.; Cullinane P. W.; Saito Y.; Foroud T.; Warner T. T.; Hasegawa K.; Vidal R.; Murayama S.; Revesz T.; Ghetti B.; Hasegawa M.; Lashley T.; Scheres S. H. W.; Goedert M. Structures of α-synuclein filaments from human brains with Lewy pathology. Nature 2022, 610 (7933), 791–795. 10.1038/s41586-022-05319-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahnawaz M.; Mukherjee A.; Pritzkow S.; Mendez N.; Rabadia P.; Liu X.; Hu B.; Schmeichel A.; Singer W.; Wu G.; Tsai A. L.; Shirani H.; Nilsson K. P. R.; Low P. A.; Soto C. Discriminating α-synuclein strains in Parkinson’s disease and multiple system atrophy. Nature 2020, 578 (7794), 273–277. 10.1038/s41586-020-1984-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweighauser M.; Shi Y.; Tarutani A.; Kametani F.; Murzin A. G.; Ghetti B.; Matsubara T.; Tomita T.; Ando T.; Hasegawa K.; Murayama S.; Yoshida M.; Hasegawa M.; Scheres S. H. W.; Goedert M. Structures of α-synuclein filaments from multiple system atrophy. Nature 2020, 585 (7825), 464–469. 10.1038/s41586-020-2317-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bousset L.; Pieri L.; Ruiz-Arlandis G.; Gath J.; Jensen P. H.; Habenstein B.; Madiona K.; Olieric V.; Böckmann A.; Meier B. H.; Melki R. Structural and functional characterization of two alpha-synuclein strains. Nat. Commun. 2013, 4, 2575 10.1038/ncomms3575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peelaerts W.; Bousset L.; Van der Perren A.; Moskalyuk A.; Pulizzi R.; Giugliano M.; Van den Haute C.; Melki R.; Baekelandt V. α-Synuclein strains cause distinct synucleinopathies after local and systemic administration. Nature 2015, 522 (7556), 340–344. 10.1038/nature14547. [DOI] [PubMed] [Google Scholar]
- Suzuki G.; Imura S.; Hosokawa M.; Katsumata R.; Nonaka T.; Hisanaga S. I.; Saeki Y.; Hasegawa M. α-synuclein strains that cause distinct pathologies differentially inhibit proteasome. eLife 2020, 9, e56825 10.7554/eLife.56825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torre-Muruzabal T.; Van der Perren A.; Coens A.; Gelders G.; Janer A. B.; Camacho-Garcia S.; Klingstedt T.; Nilsson P.; Stefanova N.; Melki R.; Baekelandt V.; Peelaerts W. Host oligodendrogliopathy and α-synuclein strains dictate disease severity in multiple system atrophy. Brain 2023, 146 (1), 237–251. 10.1093/brain/awac061. [DOI] [PubMed] [Google Scholar]
- Fayard A.; Fenyi A.; Lavisse S.; Dovero S.; Bousset L.; Bellande T.; Lecourtois S.; Jouy C.; Guillermier M.; Jan C.; Gipchtein P.; Dehay B.; Bezard E.; Melki R.; Hantraye P.; Aron Badin R. Functional and neuropathological changes induced by injection of distinct alpha-synuclein strains: A pilot study in non-human primates. Neurobiol. Dis. 2023, 180, 106086 10.1016/j.nbd.2023.106086. [DOI] [PubMed] [Google Scholar]
- Bhumkar A.; Magnan C.; Lau D.; Jun E. S. W.; Dzamko N.; Gambin Y.; Sierecki E. Single-Molecule Counting Coupled to Rapid Amplification Enables Detection of α-Synuclein Aggregates in Cerebrospinal Fluid of Parkinson’s Disease Patients. Angew. Chem., Int. Ed. 2021, 60 (21), 11874–11883. 10.1002/anie.202014898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau D.; Magnan C.; Hill K.; Cooper A.; Gambin Y.; Sierecki E. Single Molecule Fingerprinting Reveals Different Amplification Properties of α-Synuclein Oligomers and Preformed Fibrils in Seeding Assay. ACS Chem. Neurosci. 2022, 13 (7), 883–896. 10.1021/acschemneuro.1c00553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeVine H. III Thioflavine T interaction with synthetic Alzheimer’s disease beta-amyloid peptides: detection of amyloid aggregation in solution. Protein Sci. 1993, 2 (3), 404–410. 10.1002/pro.5560020312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao J.; Perera G.; Bhadbhade M.; Halliday G. M.; Dzamko N. Autophagy activation promotes clearance of α-synuclein inclusions in fibril-seeded human neural cells. J. Biol. Chem. 2019, 294 (39), 14241–14256. 10.1074/jbc.RA119.008733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown J. W. P.; Bauer A.; Polinkovsky M. E.; Bhumkar A.; Hunter D. J. B.; Gaus K.; Sierecki E.; Gambin Y. Single-molecule detection on a portable 3D-printed microscope. Nat. Commun. 2019, 10 (1), 5662 10.1038/s41467-019-13617-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knowles T. P. J.; Waudby C. A.; Devlin G. L.; Cohen S. I.; Aguzzi A.; Vendruscolo M.; Terentjev E. M.; Welland M. E.; Dobson C. M. An analytical solution to the kinetics of breakable filament assembly. Science 2009, 326 (5959), 1533–1537. 10.1126/science.1178250. [DOI] [PubMed] [Google Scholar]
- Tittelmeier J.; Druffel-Augustin S.; Alik A.; Melki R.; Nussbaum-Krammer C. Dissecting aggregation and seeding dynamics of α-Syn polymorphs using the phasor approach to FLIM. Commun. Biol. 2022, 5 (1), 1345 10.1038/s42003-022-04289-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramis R.; Ortega-Castro J.; Vilanova B.; Adrover M.; Frau J. Unraveling the NaCl Concentration Effect on the First Stages of α-Synuclein Aggregation. Biomacromolecules 2020, 21 (12), 5200–5212. 10.1021/acs.biomac.0c01292. [DOI] [PubMed] [Google Scholar]
- Gaspar R.; Lund M.; Sparr E.; Linse S. Anomalous Salt Dependence Reveals an Interplay of Attractive and Repulsive Electrostatic Interactions in α-synuclein Fibril Formation. QRB Discov. 2020, 1, e2 10.1017/qrd.2020.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Giorgi F.; Laferrière F.; Zinghirino F.; Faggiani E.; Lends A.; Bertoni M.; Yu X.; Grélard A.; Morvan E.; Habenstein B.; Dutheil N.; Doudnikoff E.; Daniel J.; Claverol S.; Qin C.; Loquet A.; Bezard E.; Ichas F. Novel self-replicating α-synuclein polymorphs that escape ThT monitoring can spontaneously emerge and acutely spread in neurons. Sci. Adv. 2020, 6 (40), eabc4364 10.1126/sciadv.abc4364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pálmadóttir T.; Waudby C. A.; Bernfur K.; Christodoulou J.; Linse S.; Malmendal A. Morphology-Dependent Interactions between α-Synuclein Monomers and Fibrils. Int. J. Mol. Sci. 2023, 24 (6), 5191 10.3390/ijms24065191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh B. P.; Morris R. J.; Kunath T.; MacPhee C. E.; Horrocks M. H. Lipid-induced polymorphic amyloid fibril formation by α-synuclein. Protein Sci. 2023, 32, e4736 10.1002/pro.4736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peduzzo A.; Linse S.; Buell A. K. The Properties of α-Synuclein Secondary Nuclei Are Dominated by the Solution Conditions Rather than the Seed Fibril Strain. ACS Chem. Neurosci. 2020, 11 (6), 909–918. 10.1021/acschemneuro.9b00594. [DOI] [PubMed] [Google Scholar]
- Kumari P.; Ghosh D.; Vanas A.; Fleischmann Y.; Wiegand T.; Jeschke G.; Riek R.; Eichmann C. Structural insights into α-synuclein monomer-fibril interactions. Proc. Natl. Acad. Sci. U.S.A. 2021, 118 (10), e2012171118 10.1073/pnas.2012171118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Wateren I. M.; Knowles T. P. J.; Buell A. K.; Dobson C. M.; Galvagnion C. C-terminal truncation of α-synuclein promotes amyloid fibril amplification at physiological pH. Chem. Sci. 2018, 9 (25), 5506–5516. 10.1039/C8SC01109E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakunthala A.; Datta D.; Navalkar A.; Gadhe L.; Kadu P.; Patel K.; Mehra S.; Kumar R.; Chatterjee D.; Devi J.; Sengupta K.; Padinhateeri R.; Maji S. K. Direct Demonstration of Seed Size-Dependent α-Synuclein Amyloid Amplification. J. Phys. Chem. Lett. 2022, 13 (28), 6427–6438. 10.1021/acs.jpclett.2c01650. [DOI] [PubMed] [Google Scholar]
- Cukalevski R.; Yang X.; Meisl G.; Weininger U.; Bernfur K.; Frohm B.; Knowles T. P. J.; Linse S. The Aβ40 and Aβ42 peptides self-assemble into separate homomolecular fibrils in binary mixtures but cross-react during primary nucleation. Chem. Sci. 2015, 6 (7), 4215–4233. 10.1039/C4SC02517B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brännström K.; Islam T.; Gharibyan A. L.; Iakovleva I.; Nilsson L.; Lee C. C.; Sandblad L.; Pamrén A.; Olofsson A. The Properties of Amyloid-β Fibrils Are Determined by their Path of Formation. J. Mol. Biol. 2018, 430 (13), 1940–1949. 10.1016/j.jmb.2018.05.001. [DOI] [PubMed] [Google Scholar]
- Hadi Alijanvand S.; Peduzzo A.; Buell A. K. Secondary Nucleation and the Conservation of Structural Characteristics of Amyloid Fibril Strains. Front. Mol. Biosci. 2021, 8, 669994 10.3389/fmolb.2021.669994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landureau M.; Redeker V.; Bellande T.; Eyquem S.; Melki R. The differential solvent exposure of N-terminal residues provides ″fingerprints″ of alpha-synuclein fibrillar polymorphs. J. Biol. Chem. 2021, 296, 100737 10.1016/j.jbc.2021.100737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gath J.; Bousset L.; Habenstein B.; Melki R.; Böckmann A.; Meier B. H. Unlike twins: an NMR comparison of two α-synuclein polymorphs featuring different toxicity. PLoS One 2014, 9 (3), e90659 10.1371/journal.pone.0090659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pálmadóttir T.; Waudby C. A.; Bernfur K.; Christodoulou J.; Linse S.; Malmendal A. Morphology-Dependent Interactions between alpha-Synuclein Monomers and Fibrils. Int. J. Mol. Sci. 2023, 24 (6), 5191 10.3390/ijms24065191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Standke H. G.; Kraus A. Seed amplification and RT-QuIC assays to investigate protein seed structures and strains. Cell Tissue Res. 2023, 392 (1), 323–335. 10.1007/s00441-022-03595-z. [DOI] [PubMed] [Google Scholar]
- Thacker D.; Sanagavarapu K.; Frohm B.; Meisl G.; Knowles T. P. J.; Linse S. The role of fibril structure and surface hydrophobicity in secondary nucleation of amyloid fibrils. Proc. Natl. Acad. Sci. U.S.A. 2020, 117 (41), 25272–25283. 10.1073/pnas.2002956117. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.